Abstract
Objective
To investigate whether vitamin D supplementation is associated with lower mortality in adults.
Design
Systematic review and meta-analysis of randomised controlled trials.
Data sources
Medline, Embase, and the Cochrane Central Register from their inception to 26 December 2018.
Eligibility criteria for selecting studies
Randomised controlled trials comparing vitamin D supplementation with a placebo or no treatment for mortality were included. Independent data extraction was conducted and study quality assessed. A meta-analysis was carried out by using fixed effects and random effects models to calculate risk ratio of death in the group receiving vitamin D supplementation and the control group.
Main outcome measures
All cause mortality.
Results
50 trials with a total of 74 655 participants were identified. Vitamin D supplementation was not associated with all cause mortality (risk ratio 0.98, 95% confidence interval 0.95 to 1.02, I2=0%), cardiovascular mortality (0.98, 0.88 to 1.08, 0%), or non-cancer, non-cardiovascular mortality (1.05, 0.93 to 1.18, 0%). Vitamin D supplementation statistically significantly reduced the risk of cancer death (0.85, 0.74 to 0.97, 0%). In subgroup analyses, all cause mortality was significantly lower in trials with vitamin D3 supplementation than in trials with vitamin D2 supplementation (P for interaction=0.04); neither vitamin D3 nor vitamin D2 was associated with a statistically significant reduction in all cause mortality.
Conclusions
Vitamin D supplementation alone was not associated with all cause mortality in adults compared with placebo or no treatment. Vitamin D supplementation reduced the risk of cancer death by 15%. Additional large clinical studies are needed to determine whether vitamin D3 supplementation is associated with lower all cause mortality.
Study registration
PROSPERO registration number CRD42018117823.
Introduction
Vitamin D supplementation has been advocated for maintaining or even improving musculoskeletal health. Evidence from observational studies indicates that low vitamin D status is associated with higher mortality from life threatening conditions such as cancer and cardiovascular disease.1 2 Therefore, supplemental vitamin D has been viewed as a potential strategy for preventing non-skeletal chronic diseases.3 4 5 If adequate vitamin D concentrations were to reduce risk of death from a wide variety of medical conditions, vitamin D supplementation would be a safe, economical, and widely available method to reduce mortality.
Clinical data examining the effect of vitamin D supplementation on mortality reduction are inconsistent. Observational studies have revealed an inverse association of vitamin D status and mortality.6 7 8 9 Previous systemic reviews and meta-analyses of randomised controlled trials suggested that vitamin D supplementation has a small effect on total mortality.5 10 11 Interpretation of these reviews is difficult because they include trials of vitamin D administered with calcium, which has been associated with uncommon but important side effects (eg, cardiovascular events).12 13 14 15 Additionally, these reviews lack sufficient detail (eg, community versus institution settings), and trial sequential analysis showed that the pooled sample size failed to meet the optimum size.10 11
Recently, additional trials16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 assessing the effect of vitamin D supplementation on mortality have become available, which have approximately doubled the number of trial participants. Among these trials, the Vitamin D and Omega 3 Trial (VITAL) did not confirm the benefit of vitamin D supplementation on mortality.31 Because of the conflicting evidence, limitations of previous reviews, and availability of new data, we aimed to conduct a systematic review and meta-analysis of randomised controlled trials to evaluate the effect of vitamin D supplementation on all cause mortality.
Methods
Protocol and guidance
This study was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).33 The protocol for this review was registered with PROSPERO (CRD42018117823).
Inclusion criteria
We considered trials to be eligible if they enrolled adults (age ≥18) with any health condition; if they compared vitamin D supplements at any dose with placebo or no treatment (when other agents were also given (eg, calcium), they had to be the same dosage in all groups); if they provided information on deaths from all causes (non-accidental) or any cause reported separately; and if they were randomised controlled trials (including quasi randomised and cluster randomised trials).
Exclusion criteria
We excluded studies if they were case reports, case series, or observational studies; if all the participants received vitamin D; if they included pregnant or lactating women, or critically ill patients; if they used hydroxylated vitamin D or vitamin D analogues (which could differ from native vitamin D in effect and safety, including lower risk of fall34 and higher risk of hypercalcaemia10 34).
Outcomes
The primary outcome was all cause mortality. Secondary outcomes were cancer mortality, cardiovascular mortality, non-cancer or non-cardiovascular mortality, cerebrovascular disease mortality, and ischaemic heart disease mortality. Supplemental eTable 1 shows the definitions of these outcomes.
Search strategy
One of the authors (PX) conducted the search of several databases: Medline (Ovid), Embase (Ovid), the Cochrane Central Register of Controlled Trials (CENTRAL), from inception to 26 December 2018. We also searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform to identify ongoing or unpublished eligible trials. To maximise the search for relevant articles, we reviewed reference lists of identified trials and systematic reviews. We did not apply language restrictions. Supplemental eTable 2 presents the search strategy.
Study selection
After removal of duplicates, two independent researchers (YZ and LJ) screened all titles and abstracts. They obtained full texts and performed further screening when studies were deemed eligible. Disagreements were resolved by consensus.
Data collection process
Two independent researchers (YZ and LJ) used a standard data extraction form to extract data from the included trials. When randomised controlled trials had more than two arms, we pooled data from the separate treatment arms. When a study mentioned an outcome of interest without providing estimates, we contacted the author for the data. Disagreements were resolved by consensus.
Assessment of risk of bias and quality of evidence
Two researchers (YZ and LJ) independently assessed the quality of all included trials by using the Cochrane Collaboration risk of bias tool.35 They also examined the quality of evidence for outcomes using the grading of recommendations assessment, development, and evaluation (GRADE) approach.36
Data synthesis
We performed statistical analyses using RevMan (version 5.3.3; The Cochrane Collaboration) and the meta package in R (version 3.4.3; R Project for Statistical Computing). Analyses for all outcomes were conducted on an intention to treat basis. We used risk ratios and their associated 95% confidence intervals to assess outcomes, and considered a P value less than 0.05 to be statistically significant. We assessed heterogeneity using the I2 test.37 If significant heterogeneity was not present (I2<50%), we used fixed effects models to pool outcomes; we used random effects models when significant heterogeneity was present (I2≥50%). The possibility of small study effects was assessed qualitatively by visual estimate of the funnel plot and quantitatively by calculation of the Egger test, the Begg test, and the Harbord test.38
Trial sequential analysis
We performed trial sequential analysis to explore whether cumulative data were adequately powered to evaluate outcomes. Trial sequential analysis (version 0.9.5.10)39 was used to maintain an overall 5% risk of type I error and 80% power. We initially anticipated an intervention effect of a 10% relative risk reduction for all cause mortality. In additional analyses, we used progressively smaller thresholds (7.5% and 5%) until the optimum sample size exceeded the actual sample size.
Subgroup analyses
We performed several subgroup analyses to test interactions according to dose (≥2000 and <2000 IU/day); type of vitamin D (vitamin D2 and vitamin D3); timing of treatment (daily and intermittently); baseline 25 hydroxyvitamin D (≥50 and <50 nmol/L); and mean age (≥70 and <70 years). We conducted retrospective subgroup analyses based on length of follow-up (at least three years and less than three years); year of publication (before 2014 and in or after 2014); sex (female and both sexes); residential status (community and institution); bolus (yes and no); intervention (vitamin D and calcium with vitamin D); and latitude (≥40° and <40°).
Sensitivity analyses
We conducted sensitivity analyses by excluding trials with high or unknown risk of bias; excluding trials with high risk or unknown risk of bias of the different domains; excluding quasi randomised or cluster randomised trials; excluding the largest trial; excluding trials with a follow-up of less than one year; using random effect models; adding trials that had been excluded for using vitamin D administered with calcium; adding trials that had been excluded for using hydroxylated vitamin D or vitamin D analogues; and using trial duration rather than long term follow-up.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing of results. The results will be disseminated to a wide audience, including members of the public, patients, health professionals, and experts in the specialty through social media and networks.
Results
Eligible studies and study characteristics
We initially identified 21 425 records, and included 50 eligible trials16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 in the final meta-analysis (fig 1). Table 1 shows a summary of included trials and supplemental eTables 3 and 4 give details of those trials. The trials comprised 74 655 participants, with 7993 all cause deaths, 1331 deaths from cardiovascular disease, 865 deaths from cancer, and 1045 deaths from non-cancer, non-cardiovascular disease. Supplemental eTable 5 summarises the details of three large ongoing randomised trials.
Fig 1.
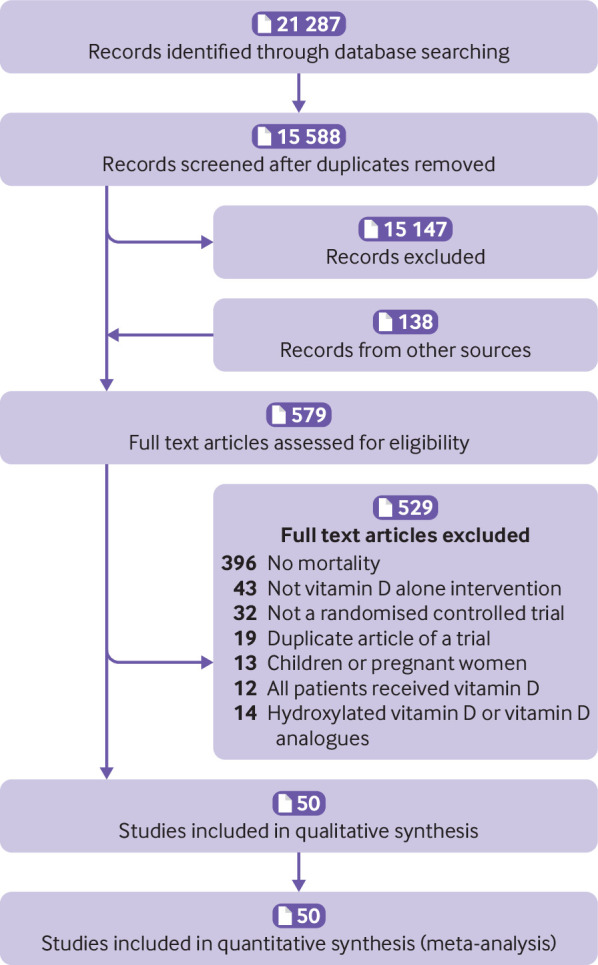
Search strategy and final included and excluded studies
Table 1.
Summary characteristics of included studies
| Characteristics | No of trials (No of participants) |
|---|---|
| Eligible studies: | |
| Total No of trials (No of participants) | 50 (74 655) |
| Median (IQR) follow-up (years) | 1.0 (0.7-3.0) |
| Follow-up at least three years | 12 (57 818) |
| Median (IQR) No of participants | 251 (125-839) |
| Total No of deaths | 7993 |
| Median (IQR) % female | 64(40-100) |
| Median (IQR) age (years) | 74 (65-80) |
| Country: | |
| European | 29 (32 840) |
| American | 10 (31 240) |
| Asian-Pacific | 10 (10 057) |
| International country | 1 (518) |
| Baseline 25 hydroxyvitamin D (nmol/L): | |
| <25 | 4(886) |
| 25-50 | 21 (15 194) |
| 50-75 | 16 (24 408) |
| >75 | 2 (26 058) |
Supplemental eFigures 1 and 2 show risk of bias. Twenty trials had a low risk of bias, 18 trials had an unclear risk, and 12 trials had a high risk of bias. Using the GRADE summary of evidence, the quality of evidence for the primary outcome was high (supplemental eTable 6).
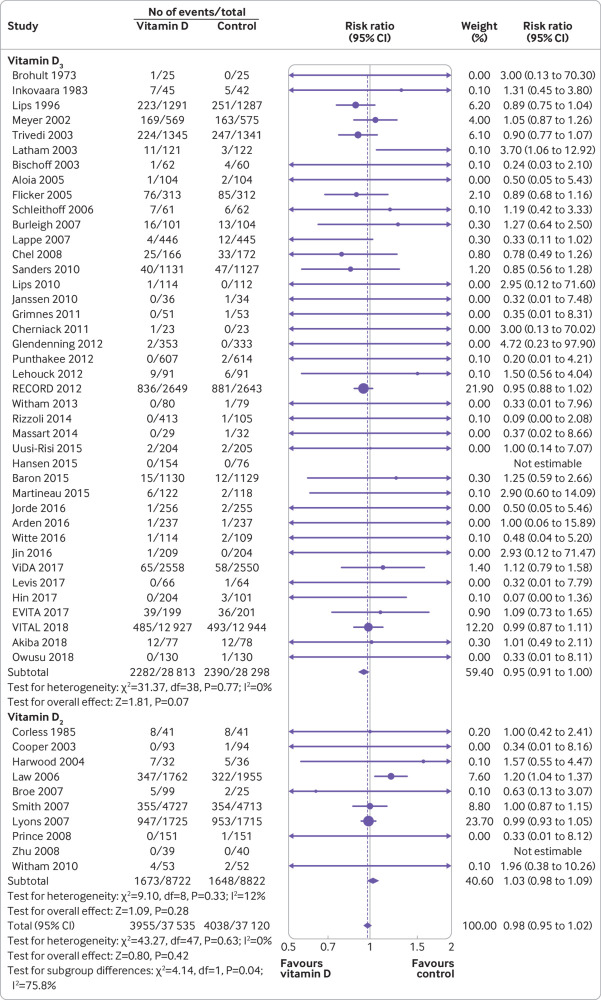
Primary outcome: all cause mortality
All 50 trials reported all cause mortality. There was no statistically significant difference in all cause mortality between the vitamin D supplementation group and the control group (risk ratio 0.98, 95% confidence interval 0.95 to 1.02, I2=0%; fig 2). In trial sequential analysis, the information size of all cause mortality met the required size of 10% and 7.5% relative risk reduction; however, futility was not reached in our additional trial sequential analysis with 5% relative risk reduction (supplemental eFigures 3-5). Funnel plot analysis showed no asymmetry (supplemental eFigure 6); additionally the Egger test (P=0.50), Begg test (P=0.20), and Harbord test (P=0.36) detected no significant small study effects. The meta-analysis results for all cause mortality were robust in sensitivity analyses (supplemental eTable 7).
Fig 2.
Forest plot of all cause mortality of trials evaluating vitamin D3 and vitamin D2 supplementation
Subgroup analyses found that all cause mortality was significantly lower among trials with vitamin D3 supplementation than in trials with vitamin D2 supplementation (P for interaction=0.04; table 2), although neither group was associated with all cause mortality. Meta-regressions found that all cause mortality was significantly lower in trials with longer follow-up (P for interaction=0.04; supplemental eFigures 9 and 10).
Table 2.
Subgroup analysis of the effect of vitamin D on all cause mortality
| Subgroup title | No of trials | No of participants | I2 (%) | Risk ratio (95% CI) | P for interaction |
|---|---|---|---|---|---|
| Overall | 50 | 74 655 | 0 | 0.98 (0.95 to 1.02) | — |
| No of participants: | |||||
| ≥2000 | 11 | 63 793 | 24 | 0.99 (0.95 to 1.03) | 0.65 |
| <2000 | 39 | 10 862 | 0 | 0.95 (0.81 to 1.11) | |
| No of events: | |||||
| ≥200 | 8 | 54 168 | 0 | 0.99 (0.95 to 1.03) | 0.85 |
| <200 | 42 | 20 487 | 0 | 0.97 (0.85 to 1.11) | |
| Age (years): | |||||
| ≥70 | 22 | 39 390 | 0 | 1.00 (0.90 to 1.11) | 0.78 |
| <70 | 28 | 35 265 | 17 | 0.98 (0.94 to 1.02) | |
| Sex: | |||||
| Female | 14 | 17 671 | 20 | 0.95 (0.86 to 1.05) | 0.47 |
| Male and female | 36 | 56 984 | 0 | 0.99 (0.95 to 1.03) | |
| Baseline mean 25 hydroxyvitamin D (nmol/L): | |||||
| ≥50 | 18 | 50 466 | 0 | 1.04 (0.97 to 1.12) | 0.07 |
| <50 | 26 | 16 080 | 19 | 0.95 (0.90 to 1.01) | |
| Year of publication: | |||||
| Before 2014 | 33 | 37 088 | 5 | 0.98 (0.94 to 1.02) | 0.77 |
| In or after 2014 | 17 | 37 567 | 0 | 1.00 (0.90 to 1.11) | |
| Type of vitamin D: | |||||
| Vitamin D3 | 40 | 57 111 | 0 | 0.95 (0.91 to 1.00) | 0.04* |
| Vitamin D2 | 10 | 17 544 | 12 | 1.03 (0.98 to 1.09) | |
| Daily dose equivalent (IU): | |||||
| <2000 | 30 | 39 785 | 0 | 0.98 (0.94 to 1.02) | 0.56 |
| ≥2000 | 16 | 34 116 | 0 | 1.01 (0.91 to 1.13) | |
| Timing: | |||||
| Daily | 31 | 47 931 | 0 | 0.99 (0.94 to 1.04) | 0.79 |
| Intermittently | 15 | 25 815 | 0 | 0.98 (0.93 to 1.03) | |
| Bolus or not: | |||||
| Bolus | 10 | 24 612 | 0 | 0.98 (0.93 to 1.04) | 1.00 |
| Non-bolus | 36 | 49 134 | 0 | 0.98 (0.93 to 1.04) | |
| Residential status: | |||||
| Community | 41 | 62 362 | 0 | 0.97 (0.92 to 1.02) | 0.31 |
| Institution | 9 | 12 293 | 0 | 1.01 (0.96 to 1.06) | |
| Follow-up: | |||||
| At least three years | 12 | 57 818 | 0 | 0.97 (0.93 to 1.01) | 0.26 |
| Less than three years | 38 | 16 837 | 6 | 1.02 (0.95 to 1.11) | |
| Intervention: | |||||
| Vitamin D | 34 | 62 767 | 0 | 1.00 (0.96 to 1.05) | 0.08 |
| Vitamin D plus calcium | 16 | 11 888 | 0 | 0.93 (0.87 to 1.00) | |
| Latitude: | |||||
| ≥40° | 36 | 41 002 | 5 | 0.99 (0.95 to 1.03) | 0.71 |
| <40° | 13 | 33 135 | 0 | 0.97 (0.87 to 1.08) |
Statistically significant.
Secondary outcome: other mortality
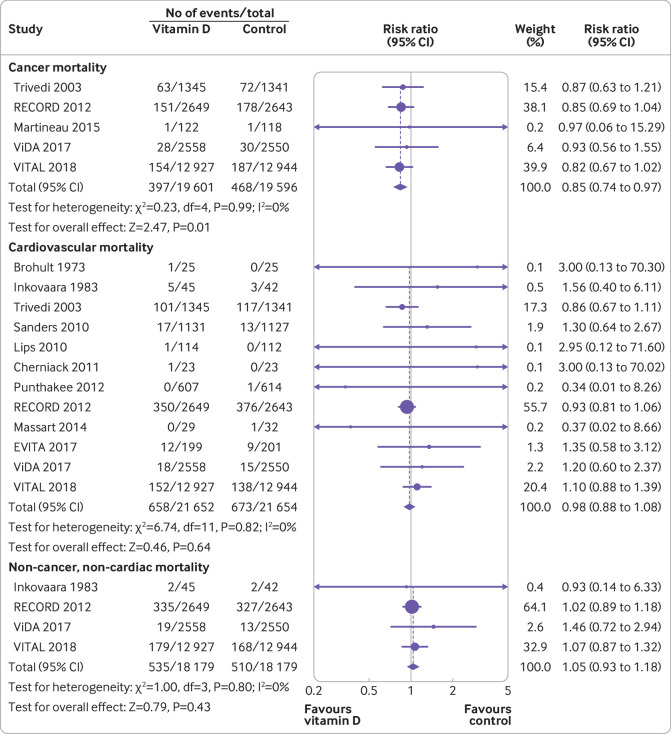
Vitamin D supplementation was associated with significant reduction in cancer mortality (risk ratio 0.85, 95% confidence interval 0.74 to 0.97, I2=0%; fig 3). However, benefit was only seen in participants receiving vitamin D3 supplementation, and no participants received vitamin D2 supplementation (supplemental eTable 8). We found no statistically significant difference between groups in cardiovascular mortality (0.98, 0.88 to 1.08, I2=0%) or non-cancer, non-cardiovascular mortality (1.05, 0.93 to 1.18, I2=0%). Vitamin D supplementation did not reduce the risk of death from cerebrovascular disease (1.04, 0.84 to 1.29, I2=0%; supplemental eTable7) or ischaemic heart disease (0.96, 0.81 to 1.15, I2=0%; supplemental eTable 8).
Fig 3.
Forest plot of cancer mortality, cardiovascular mortality, and non-cancer, non-cardiovascular mortality of trials evaluating vitamin D supplementation
Discussion
In this meta-analysis of 50 randomised controlled trials with a total of 74 655 participants, vitamin D supplementation was not significantly associated with total mortality (risk ratio 0.98, 95% confidence interval 0.95 to 1.02). The findings suggest that vitamin D supplementation reduced cancer mortality by 15% (95% confidence interval 0.74 to 0.97), but not mortality from cardiovascular disease, cerebrovascular disease, or ischaemic heart disease.
Principal findings and comparison with other studies
The results of this study on all cause mortality differ from two previous systematic reviews.5 10 11 A Cochrane review in 2014 found that vitamin D supplementation decreased all cause mortality in analyses of 56 trials with a total of 95 286 participants (relative risk 0.97, 95% confidence interval 0.94 to 0.99, P=0.02).10 In the same year, a systematic review by Bolland and colleagues that included 40 trials with a total of 81 173 participants also suggested a small effect on all cause mortality (0.96, 0.93 to 1.00, P=0.04).11 The previous reviews probably reached more optimistic conclusions as a result of different selection criteria and newly published trials. Compared with these reviews,10 11 we excluded more than 10 trials totalling approximately 50 000 participants of vitamin D administered with calcium, six trials73 74 75 76 77 78 79 of hydroxylated vitamin D or vitamin D analogues, and one trial80 retracted in 2017. To determine whether the null finding was driven by excluding trials which had been included in previous reviews, we performed two sensitivity analyses by adding trials that were originally excluded, and confirmed the results of the overall analysis. Moreover, this study additionally included 17 randomised controlled trials16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 published after 2014, so that the more recent trials accounted for 50.3% (37 568/74 655) of the total number of participants.
In contrast to the results for total mortality, this study found that vitamin D supplementation reduced cancer mortality by 15%. The results of previous reviews on cancer mortality have been inconsistent. In 2014, a Cochrane review by Bjelakovic and colleagues presented low quality evidence that vitamin D supplementation resulted in a decrease in cancer mortality (relative risk 0.88, 95% confidence interval 0.78 to 0.98), but suggested that the required information size was not reached.81 In parallel, two systematic reviews published similar results.11 82 However, their meta-analyses were limited by the number of trials (n≤4), administration of a generally low dose of vitamin D (≤1100 IU/day), and mixed interventions (vitamin D plus calcium). In 2018, a meta-analysis by Goulão and colleagues did not find evidence to suggest that vitamin D supplementation alone reduced cancer mortality (1.03, 0.91 to 1.15).83 After we submitted our current study for initial review by The BMJ, an additional meta-analysis by Keum and colleagues was published.84 Their review found that vitamin D supplementation significantly reduced cancer mortality (0.87, 0.79 to 0.96).84 Our findings on cancer mortality are consistent with those of Keum and colleagues, but some of the methods used in the two studies differ. The study by Keum and colleagues included trials of hydroxylated vitamin D, vitamin D analogues, and vitamin D administered with calcium, which were excluded in our study. Moreover, our study provided absolute and relative risks, evaluated the quality of the evidence by using the GRADE approach, and explored the optimum sample size with trial sequential analysis. More importantly, our study found that reduced cancer mortality was only seen with vitamin D3 supplementation, not with vitamin D2 supplementation.
An important finding from our subgroup analysis was that the effect of vitamin D differs for vitamin D2 and D3 supplementation. We found that all cause mortality was significantly lower among trials with vitamin D3 supplementation than in trials with vitamin D2 supplementation; however neither supplement was associated with statistically significant reduced risk. Similarly, vitamin D3 supplementation reduced the risk of cancer mortality, but vitamin D2 did not. The different effect on mortality of vitamin D2 and D3 might be explained by the diverse effect on raising 25 hydroxyvitamin D concentrations. Historically, vitamin D2 and vitamin D3 were considered to be equally effective at raising 25 hydroxyvitamin D concentrations. Currently, the comparative efficacy of vitamins D2 and D3 has been investigated in several intervention trials, with most indicating that vitamin D3 increases 25 hydroxyvitamin D concentrations more efficiently than vitamin D2.85 86 A Cochrane review in 2014 found that vitamin D3 seemed to reduce total mortality (risk ratio 0.94, 95% confidence interval 0.91 to 0.98), whereas vitamin D2 had no statistically significant beneficial effects on total mortality (1.02, 0.96 to 1.08).10 However, the Cochrane review did not reveal heterogeneity between vitamin D2 and D3. Therefore, we should be cautious about the strength of the evidence that vitamin D3 reduced all cause mortality (0.95, 0.91 to1.00, P=0.07).
Vitamin D3 is the most widely used type of vitamin D supplementation and has a clinically relevant effect of reducing all cause mortality by 5%, with the P value and 95% confidence interval close to the level of formal statistical significance. The current study is not a positive study, but it is also not an unambiguously negative study. In addition, subgroup analyses are observational by nature and are not based on randomised comparisons.87 Therefore, the effect of vitamin D3 on all cause mortality requires additional evidence, preferably gathered by future large randomised controlled trials.
A further important finding from meta-regression was that all cause mortality was statistically significantly lower in trials with longer follow-up. Sensitivity analysis found a potential effect of vitamin D supplementation on all cause mortality after trials with a follow-up of less than one year were excluded (risk ratio 0.97, 95% confidence interval 0.93 to 1.00). However, subgroup analysis did not find a statistically significant difference in the effect of vitamin D supplementation on mortality in trials with a follow-up of less than three years and more than three years (P=0.26). Additionally, the previous meta-analysis did not find a subgroup difference according to the length of follow-up.10 11
The VITAL trial reported increasing benefit over time.31 Although no significant differences relate to cancer mortality (risk ratio 0.83, 95% confidence interval 0.67 to 1.02) or all cause mortality (0.99, 0.87 to 1.12), after excluding the first one and two years of follow-up, the risk ratio was significantly reduced to 0.75 for cancer mortality (95% confidence interval 0.59 to 0.96) and was slightly reduced to 0.96 for all cause mortality (0.84 to 1.11). Therefore, the length of follow-up could modify the effect of vitamin D supplementation on all cause mortality.
Strengths and limitations
This systematic review and meta-analysis has several methodological strengths. We followed the recommendations of the Cochrane Collaboration and PRISMA statement, including a priori protocol. This study also included a rigorous assessment of the quality of evidence using the GRADE approach (the quality for the primary outcome was high) and of the minimum information size required in trial sequential analysis (the study met the optimum size).
Our study has important limitations. The study was based solely on published trials that reported mortality outcomes. However, most trials of vitamin D supplementation did not report mortality, which suggests that substantial selective reporting was likely. Also, all cause mortality reported among all included trials was the secondary outcome of the trials. Data for this secondary outcome might have been collected differently than data for the primary outcome in the trials.
Most included trials allowed personal supplementation with low dose vitamin D in the control group. In the VITAL trial,31 for example, 42.5% of participants in the control group used vitamin D supplementation (≤800 IU/day). The high prevalence of vitamin D supplementation in the control group made it more difficult to distinguish between the treatment and control groups.
The dose of vitamin D used in included trials varied. Our study could not accurately compare equivalent daily vitamin D supplementation dose in the included trials because they all had different treatment regimens and dosing intervals (daily, weekly, monthly, or bolus doses). This might be one of the reasons why this study did not determine an effective daily dose of vitamin D supplementation. Furthermore, the vitamin D status before, during, and after treatment is useful to determine the effectiveness of vitamin D supplementation in improving the actual vitamin D status. Long term vitamin D status is expected to be a much more accurate, reliable, and important clinical parameter compared with a daily dose of vitamin D supplementation. However, previous trials were limited in providing such data. These limitations and uncertainties associated with vitamin D supplementation dose and vitamin D status in treatment and control groups warrant further investigation.
The baseline 25 hydroxyvitamin D concentrations of trial participants have not been low enough, which could partly contribute to the null finding on the association of vitamin D supplementation and all cause mortality. Observational studies have indicated an increased mortality risk only at low 25 hydroxyvitamin D concentrations. An individual participant data meta-analysis of observational studies showed that the adjusted hazard ratio (95% confidence interval) for mortality in the 25 hydroxyvitamin D groups with concentrations less than 30, 30-40, and 40-50 nmol/L were 1.67 (1.44 to 1.89), 1.33 (1.16 to 1.51), and 1.15 (1.00 to 1.29), respectively, compared with participants with 25 hydroxyvitamin D concentrations of 75-100 nmol/L.8 In this study, more than half of participants (50 466/66 546) from trials reported a baseline mean 25 hydroxyvitamin D concentration of more than 50 nmol/L.
Implications
Mortality is the most important clinical outcome. Our study size met the optimum sample size of 7.5% relative risk reduction and the pooled risk ratio was close to 1 with a narrow confidence interval. Our findings suggest that vitamin D supplementation did not have a clinically relevant effect on all cause mortality, and so there is little evidence that vitamin D supplementation reduces all cause mortality. However, vitamin D supplementation reduced cancer mortality by 15%. Therefore, this analysis supports the concept that the risk of cancer death could be reduced by vitamin D supplementation, and a more targeted intervention for this role might be appropriate.
The current study found that all cause mortality was significantly lower among trials with vitamin D3 supplementation than in trials with vitamin D2 supplementation, with a trend towards reduced all cause mortality in those taking vitamin D3 (P=0.07). Similarly, vitamin D3 supplementation reduced the risk of cancer death, but vitamin D2 did not. Another finding from subgroup analysis suggested that all cause mortality was significantly lower in trials with longer follow-up, and that the benefit of reduced cancer mortality was seen in trials with longer follow-up (more than three years) but not in those with a shorter follow-up. According to these findings, supplementation with vitamin D3 for at least three years should be considered. Additional large randomised controlled trials are needed to confirm the results from our subgroup analyses.
Several large ongoing trials have the potential to corroborate or refute our findings. In the D-Health trial (Australian New Zealand Clinical Trials Registry: ACTRN12613000743763), high dose vitamin D supplementation (60 000 IU/month) is being used to prevent mortality and cancer in Australian adults aged 60-79. The D-Health trial recently completed the recruitment of almost 21 315 participants, with a minimum of five years of follow-up. Using a similar study design, the VIDAL trial (Vitamin D and Longevity trial; ISRCTN46328341) is analysing the effect of intermittent high dose vitamin D supplementation (60 000 IU/month) on all cause mortality in adults aged 65-84 with a corrected serum calcium level of 2.65 mmol/L. The DO-HEALTH trial (Vitamin D3-Omega3-Home Exercise-Healthy Ageing and Longevity Trial; ClinicalTrials.gov identifier: NCT01745263) has recruited 2152 participants from five European countries aged 70 years and older. The specific aim is to establish whether vitamin D will prevent disease at an older age. The final results of the DO-HEALTH trial will be available in autumn 2019. Although none of these trials have screened for low baseline 25 hydroxyvitamin D for eligibility, all trials have used vitamin D3 as the intervention.
Conclusions
Overall, vitamin D supplementation was not associated with all cause mortality, cardiovascular mortality, or non-cancer, non-cardiovascular mortality in adults. However, vitamin D supplementation was associated with a reduced risk of cancer mortality by 15%. There was a trend towards reduced all cause mortality with vitamin D3 supplementation, which warrants further investigation.
What is already known on this topic
Observational studies showed that low vitamin D levels were associated with increased mortality from life threatening conditions such as cancer and cardiovascular disease
Clinical data examining the effect of vitamin D supplementation on mortality reduction are inconsistent
What this study adds
Vitamin D supplementation alone was not associated with all cause mortality in adults compared with placebo or no treatment
Vitamin D supplementation reduced the risk of cancer death
Acknowledgments
We thank L Dade Lunsford (University of Pittsburgh Medical Centre), Jing Li (West China Hospital), and Huiwen Tan (West China Hospital) for their support in preparing the final draft of this paper.
Web extra.
Extra material supplied by authors
Web appendix: Supplemental e-material
Contributors: FF and YZ conceived the study and designed the protocol. PX performed the literature search. YZ and LJ selected the studies and extracted the relevant information. JT, YF, and YZ synthesised the data. YZ wrote the first draft of the paper. All authors critically revised successive drafts of the paper and approved the final version. FF and YZ are the study guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work is supported by the projects of the National Natural Science Foundation of China (No 81100925 and No 81472361) and by the National Key R&D Program of China (No 2018YFA010860004). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Natural Science Foundation of China and the National Key R&D Program of China; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: Additional data available from the corresponding author at fangfang1057@outlook.com.
The lead author (FF) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. Chowdhury R, Kunutsor S, Vitezova A, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014;348:g1903. 10.1136/bmj.g1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang TJ. Vitamin D and cardiovascular disease. Annu Rev Med 2016;67:261-72. 10.1146/annurev-med-051214-025146 [DOI] [PubMed] [Google Scholar]
- 3. Grandi NC, Breitling LP, Vossen CY, et al. Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease. Am Heart J 2010;159:1044-51. 10.1016/j.ahj.2010.03.031 [DOI] [PubMed] [Google Scholar]
- 4. Yin L, Ordóñez-Mena JM, Chen T, Schöttker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Prev Med 2013;57:753-64. 10.1016/j.ypmed.2013.08.026 [DOI] [PubMed] [Google Scholar]
- 5. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol 2017;5:986-1004. 10.1016/S2213-8587(17)30357-1 [DOI] [PubMed] [Google Scholar]
- 6. Johansson H, Odén A, Kanis J, et al. Low serum vitamin D is associated with increased mortality in elderly men: MrOS Sweden. Osteoporos Int 2012;23:991-9. 10.1007/s00198-011-1809-5 [DOI] [PubMed] [Google Scholar]
- 7. Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 2012;95:91-100. 10.3945/ajcn.111.014779 [DOI] [PubMed] [Google Scholar]
- 8. Gaksch M, Jorde R, Grimnes G, et al. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One 2017;12:e0170791. 10.1371/journal.pone.0170791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durazo-Arvizu RA, Dawson-Hughes B, Kramer H, et al. The reverse J-shaped association between serum total 25-hydroxyvitamin D concentration and all-cause mortality: the impact of assay standardization. Am J Epidemiol 2017;185:720-6. 10.1093/aje/kww244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2014;(1):CD007470. 10.1002/14651858.CD007470.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol 2014;2:307-20. 10.1016/S2213-8587(13)70212-2 [DOI] [PubMed] [Google Scholar]
- 12. Anderson JL, May HT, Horne BD, et al. Intermountain Heart Collaborative (IHC) Study Group Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol 2010;106:963-8. 10.1016/j.amjcard.2010.05.027 [DOI] [PubMed] [Google Scholar]
- 13. Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 2010;341:c3691. 10.1136/bmj.c3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewis JR, Zhu K, Prince RL. Adverse events from calcium supplementation: relationship to errors in myocardial infarction self-reporting in randomized controlled trials of calcium supplementation. J Bone Miner Res 2012;27:719-22. 10.1002/jbmr.1484 [DOI] [PubMed] [Google Scholar]
- 15. Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011;342:d2040. 10.1136/bmj.d2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massart A, Debelle FD, Racapé J, et al. Biochemical parameters after cholecalciferol repletion in hemodialysis: results grom the VitaDial randomized trial. Am J Kidney Dis 2014;64:696-705. 10.1053/j.ajkd.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 17. Rizzoli R, Dawson-Hughes B, Kaufman JM, et al. Correction of vitamin D insufficiency with combined strontium ranelate and vitamin D3 in osteoporotic patients. Eur J Endocrinol 2014;170:441-50. 10.1530/EJE-13-0775 [DOI] [PubMed] [Google Scholar]
- 18. Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med 2015;373:1519-30. 10.1056/NEJMoa1500409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansen KE, Johnson RE, Chambers KR, et al. Treatment of vitamin D insufficiency in postmenopausal women: a randomized clinical trial. JAMA Intern Med 2015;175:1612-21. 10.1001/jamainternmed.2015.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med 2015;3:120-30. 10.1016/S2213-2600(14)70255-3 [DOI] [PubMed] [Google Scholar]
- 21. Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med 2015;175:703-11. 10.1001/jamainternmed.2015.0225 [DOI] [PubMed] [Google Scholar]
- 22. Arden NK, Cro S, Sheard S, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthritis Cartilage 2016;24:1858-66. 10.1016/j.joca.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin X, Jones G, Cicuttini F, et al. Effect of vitamin D supplementation on tibial cartilage volume and knee pain among patients with symptomatic knee osteoarthritis: a randomized clinical trial. JAMA 2016;315:1005-13. 10.1001/jama.2016.1961 [DOI] [PubMed] [Google Scholar]
- 24. Jorde R, Sollid ST, Svartberg J, et al. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metab 2016;101:1647-55. 10.1210/jc.2015-4013 [DOI] [PubMed] [Google Scholar]
- 25. Witte KK, Byrom R, Gierula J, et al. Effects of vitamin D on cardiac function in patients with chronic HF: the VINDICATE study. J Am Coll Cardiol 2016;67:2593-603. 10.1016/j.jacc.2016.03.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hin H, Tomson J, Newman C, et al. Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos Int 2017;28:841-51. 10.1007/s00198-016-3833-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levis S, Gómez-Marín O. Vitamin D and physical function in sedentary older men. J Am Geriatr Soc 2017;65:323-31. 10.1111/jgs.14510 [DOI] [PubMed] [Google Scholar]
- 28. Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol 2017;2:608-16. 10.1001/jamacardio.2017.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zittermann A, Ernst JB, Prokop S, et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J 2017;38:2279-86. 10.1093/eurheartj/ehx235 [DOI] [PubMed] [Google Scholar]
- 30. Akiba T, Morikawa T, Odaka M, et al. Vitamin D supplementation and survival of patients with non-small cell lung cancer: a randomized, double-blind, placebo-controlled trial. Clin Cancer Res 2018;24:4089-97. 10.1158/1078-0432.CCR-18-0483 [DOI] [PubMed] [Google Scholar]
- 31. Manson JE, Cook NR, Lee IM, et al. VITAL Research Group Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33-44. 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Owusu JE, Islam S, Katumuluwa SS, et al. Cognition and vitamin D in older African-American women: physical performance and osteoporosis prevention with vitamin D in older African Americans trial and dementia. J Am Geriatr Soc 2019;67:81-6. 10.1111/jgs.15607 [DOI] [PubMed] [Google Scholar]
- 33. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 34. Richy F, Dukas L, Schacht E. Differential effects of D-hormone analogs and native vitamin D on the risk of falls: a comparative meta-analysis. Calcif Tissue Int 2008;82:102-7. 10.1007/s00223-008-9102-0 [DOI] [PubMed] [Google Scholar]
- 35. Shinichi A. Cochrane Handbook for Systematic Reviews of Interventions. Online Kensaku 2014;35:154-5. [Google Scholar]
- 36. Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 38. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008;61:763-9. 10.1016/j.jclinepi.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 40. Brohult J, Jonson B. Effects of large doses of calciferol on patients with rheumatoid arthritis. A double-blind clinical trial. Scand J Rheumatol 1973;2:173-6. 10.3109/03009747309097085 [DOI] [PubMed] [Google Scholar]
- 41. Inkovaara J, Gothoni G, Halttula R, Heikinheimo R, Tokola O. Calcium, vitamin D and anabolic steroid in treatment of aged bones: double-blind placebo-controlled long-term clinical trial. Age Ageing 1983;12:124-30. 10.1093/ageing/12.2.124 [DOI] [PubMed] [Google Scholar]
- 42. Corless D, Dawson E, Fraser F, et al. Do vitamin D supplements improve the physical capabilities of elderly hospital patients? Age Ageing 1985;14:76-84. 10.1093/ageing/14.2.76 [DOI] [PubMed] [Google Scholar]
- 43. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med 1996;124:400-6. 10.7326/0003-4819-124-4-199602150-00003 [DOI] [PubMed] [Google Scholar]
- 44. Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res 2002;17:709-15. 10.1359/jbmr.2002.17.4.709 [DOI] [PubMed] [Google Scholar]
- 45. Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res 2003;18:343-51. 10.1359/jbmr.2003.18.2.343 [DOI] [PubMed] [Google Scholar]
- 46. Cooper L, Clifton-Bligh PB, Nery ML, et al. Vitamin D supplementation and bone mineral density in early postmenopausal women. Am J Clin Nutr 2003;77:1324-9. 10.1093/ajcn/77.5.1324 [DOI] [PubMed] [Google Scholar]
- 47. Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID, Fitness Collaborative Group A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS). J Am Geriatr Soc 2003;51:291-9. 10.1046/j.1532-5415.2003.51101.x [DOI] [PubMed] [Google Scholar]
- 48. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326:469. 10.1136/bmj.326.7387.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ, Nottingham Neck of Femur (NONOF) Study A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NONOF) Study. Age Ageing 2004;33:45-51. 10.1093/ageing/afh002 [DOI] [PubMed] [Google Scholar]
- 50. Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med 2005;165:1618-23. 10.1001/archinte.165.14.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Flicker L, MacInnis RJ, Stein MS, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc 2005;53:1881-8. 10.1111/j.1532-5415.2005.00468.x [DOI] [PubMed] [Google Scholar]
- 52. Law M, Withers H, Morris J, Anderson F. Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in elderly people in residential accommodation. Age Ageing 2006;35:482-6. 10.1093/ageing/afj080 [DOI] [PubMed] [Google Scholar]
- 53. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006;83:754-9. 10.1093/ajcn/83.4.754 [DOI] [PubMed] [Google Scholar]
- 54. Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc 2007;55:234-9. 10.1111/j.1532-5415.2007.01048.x [DOI] [PubMed] [Google Scholar]
- 55. Burleigh E, McColl J, Potter J. Does vitamin D stop inpatients falling? A randomised controlled trial. Age Ageing 2007;36:507-13. 10.1093/ageing/afm087 [DOI] [PubMed] [Google Scholar]
- 56. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 2007;85:1586-91. 10.1093/ajcn/85.6.1586 [DOI] [PubMed] [Google Scholar]
- 57. Lyons RA, Johansen A, Brophy S, et al. Preventing fractures among older people living in institutional care: a pragmatic randomised double blind placebo controlled trial of vitamin D supplementation. Osteoporos Int 2007;18:811-8. 10.1007/s00198-006-0309-5 [DOI] [PubMed] [Google Scholar]
- 58. Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women--a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford) 2007;46:1852-7. 10.1093/rheumatology/kem240 [DOI] [PubMed] [Google Scholar]
- 59. Chel V, Wijnhoven HA, Smit JH, Ooms M, Lips P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos Int 2008;19:663-71. 10.1007/s00198-007-0465-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med 2008;168:103-8. 10.1001/archinternmed.2007.31 [DOI] [PubMed] [Google Scholar]
- 61. Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. J Clin Endocrinol Metab 2008;93:743-9. 10.1210/jc.2007-1466 [DOI] [PubMed] [Google Scholar]
- 62. Janssen HC, Samson MM, Verhaar HJ. Muscle strength and mobility in vitamin D-insufficient female geriatric patients: a randomized controlled trial on vitamin D and calcium supplementation. Aging Clin Exp Res 2010;22:78-84. 10.1007/BF03324819 [DOI] [PubMed] [Google Scholar]
- 63. Lips P, Binkley N, Pfeifer M, et al. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am J Clin Nutr 2010;91:985-91. 10.3945/ajcn.2009.28113 [DOI] [PubMed] [Google Scholar]
- 64. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303:1815-22. . 10.1001/jama.2010.594 [DOI] [PubMed] [Google Scholar]
- 65. Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail 2010;3:195-201. 10.1161/CIRCHEARTFAILURE.109.907899 [DOI] [PubMed] [Google Scholar]
- 66. Cherniack EP, Florez HJ, Hollis BW, Roos BA, Troen BR, Levis S. The response of elderly veterans to daily vitamin D3 supplementation of 2,000 IU: a pilot efficacy study. J Am Geriatr Soc 2011;59:286-90. 10.1111/j.1532-5415.2010.03242.x [DOI] [PubMed] [Google Scholar]
- 67. Grimnes G, Figenschau Y, Almås B, Jorde R. Vitamin D, insulin secretion, sensitivity, and lipids: results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes 2011;60:2748-57. 10.2337/db11-0650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Avenell A, MacLennan GS, Jenkinson DJ, et al. RECORD Trial Group Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab 2012;97:614-22. 10.1210/jc.2011-1309 [DOI] [PubMed] [Google Scholar]
- 69. Glendenning P, Zhu K, Inderjeeth C, Howat P, Lewis JR, Prince RL. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res 2012;27:170-6. . 10.1002/jbmr.524 [DOI] [PubMed] [Google Scholar]
- 70. Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2012;156:105-14. . 10.7326/0003-4819-156-2-201201170-00004 [DOI] [PubMed] [Google Scholar]
- 71. Punthakee Z, Bosch J, Dagenais G, et al. TIDE Trial Investigators Design, history and results of the Thiazolidinedione Intervention with vitamin D Evaluation (TIDE) randomised controlled trial. Diabetologia 2012;55:36-45. 10.1007/s00125-011-2357-4 [DOI] [PubMed] [Google Scholar]
- 72. Witham MD, Price RJ, Struthers AD, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med 2013;173:1672-9. 10.1001/jamainternmed.2013.9043 [DOI] [PubMed] [Google Scholar]
- 73. Ott SM, Chesnut CH., 3rd Calcitriol treatment is not effective in postmenopausal osteoporosis. Ann Intern Med 1989;110:267-74. 10.7326/0003-4819-110-4-267 [DOI] [PubMed] [Google Scholar]
- 74. Grady D, Halloran B, Cummings S, et al. 1,25-Dihydroxyvitamin D3 and muscle strength in the elderly: a randomized controlled trial. J Clin Endocrinol Metab 1991;73:1111-7. . 10.1210/jcem-73-5-1111 [DOI] [PubMed] [Google Scholar]
- 75. Sato Y, Maruoka H, Oizumi K. Amelioration of hemiplegia-associated osteopenia more than 4 years after stroke by 1 alpha-hydroxyvitamin D3 and calcium supplementation. Stroke 1997;28:736-9. 10.1161/01.STR.28.4.736 [DOI] [PubMed] [Google Scholar]
- 76. Sato Y, Kuno H, Kaji M, Saruwatari N, Oizumi K. Effect of ipriflavone on bone in elderly hemiplegic stroke patients with hypovitaminosis D [retracted]. Am J Phys Med Rehabil 1999;78:457-63. . 10.1097/00002060-199909000-00008 [DOI] [PubMed] [Google Scholar]
- 77. Sato Y, Manabe S, Kuno H, Oizumi K. Amelioration of osteopenia and hypovitaminosis D by 1alpha-hydroxyvitamin D3 in elderly patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1999;66:64-8. 10.1136/jnnp.66.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab 2001;86:3618-28. . 10.1210/jcem.86.8.7703 [DOI] [PubMed] [Google Scholar]
- 79. Dukas L, Bischoff HA, Lindpaintner LS, et al. Alfacalcidol reduces the number of fallers in a community-dwelling elderly population with a minimum calcium intake of more than 500 mg daily. J Am Geriatr Soc 2004;52:230-6. 10.1111/j.1532-5415.2004.52060.x [DOI] [PubMed] [Google Scholar]
- 80. Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial [retracted]. Cerebrovasc Dis 2005;20:187-92. . 10.1159/000087203 [DOI] [PubMed] [Google Scholar]
- 81. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev 2014;(6):CD007469. 10.1002/14651858.CD007469.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer 2014;111:976-80. 10.1038/bjc.2014.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Goulão B, Stewart F, Ford JA, MacLennan G, Avenell A. Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr 2018;107:652-63. . 10.1093/ajcn/nqx047 [DOI] [PubMed] [Google Scholar]
- 84. Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol 2019;30:733-43. 10.1093/annonc/mdz059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr 2012;95:1357-64. 10.3945/ajcn.111.031070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wilson LR, Tripkovic L, Hart KH, Lanham-New SA. Vitamin D deficiency as a public health issue: using vitamin D2 or vitamin D3 in future fortification strategies. Proc Nutr Soc 2017;76:392-9. . 10.1017/S0029665117000349 [DOI] [PubMed] [Google Scholar]
- 87.JPT Higgins SGE. The Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, Oxford 2011; version 5.1.0.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplemental e-material