Short abstract
Suboptimal conditions during prenatal ontogeny can impair development of several physiological systems and result in cardiometabolic diseases in adulthood. The kidney has been identified as one of the most sensitive organs for developmental programming, but underlying mechanisms are not fully understood. Therefore, in our study we explored the consequences of prenatally increased angiotensin II (Ang II) on the structural development of the kidney and its damage by infiltrated immune cells under normal diet and after an increased salt intake, as a second insult representing a lifestyle factor in humans. Female rats were implanted with osmotic mini-pumps continuously releasing Ang II of dose 2 µg/kg/h during last two weeks of pregnancy, whereas control females were sham operated. Immunohistological and ultrastructural evaluations of the kidneys and their infiltration with immune cells were performed in mature male progeny kept either on a standard or increased salt (2% NaCl) diet. Glomerular volume decreased and the cortical tubulointerstitial injury increased in the offspring prenatally exposed to Ang II with no additional effect of increased salt. Ultrastructural examination demonstrated degenerative changes in proximal tubules, mainly fewer and shorter microvilli in the brush border, enlarged mitochondria, and an increased number of lysosomes in the epithelial cells in the progeny prenatally exposed to Ang II. Moreover, the treatment resulted in increased infiltration of T-cells and macrophages in the renal cortex compared to controls. These changes paralleled with reduced numbers of cytotoxic T-cells in circulation and higher oxidative burst of neutrophils in the progeny of Ang II-treated mothers compared to controls. Altogether, results suggest that prenatally increased Ang II promoted infiltration of immune cells in the kidney and subsequent oxidative stress, which induced a damage of renal glomerular and tubular system entailing negative consequences on the cardiovascular system.
Impact statement
Suboptimal prenatal conditions can contribute to development of cardiovascular diseases and an altered renin-angiotensin-aldosterone system (RAAS) can be involved in the process. In our study, increased angiotensin II in pregnant female rats resulted in renal cortical interstitial damage, and renal ultrastructural changes in the glomeruli, the brush border of proximal tubules and mitochondria in mature male offspring. The treatment promoted infiltration of T cells and macrophages in the kidneys and primed an oxidative burst of circulating neutrophils, indicating a pro-inflammatory state in the progeny of angiotensin II-treated mothers. Deregulated RAAS of mothers is involved in developmental programming of hypertension in adult male offspring via damaging kidney morphology and function. These findings suggest that preventing the activation of RAAS and oxidative stress during perinatal development might protect against hypertension development in adult male progeny.
Keywords: Renin-angiotensin-aldosterone system, development, prenatal programming, hypertension, immune cell infiltration, kidney ultrastructure
Introduction
Inadequate environment during ontogeny can induce changes in the development of several organs involved in blood pressure (BP) regulation and an increased incidence of hypertension and ischemic heart disease in adulthood.1,2 The kidney plays an important role in BP control in adulthood and is highly affected by a subnormal intrauterine environment.3 Several environmental variables, such as malnutrition, hypoxia, and stress during pregnancy can lead to lower nephron numbers and a decreased filtration rate and can result in increased BP in offspring.4,5
Effective functioning of the kidney depends highly on events occurring during the organ development, which requires the coordinated spatiotemporal expression of genes and signaling pathways.6 One of the key regulatory systems is the renin-angiotensin-aldosterone system (RAAS), which components are highly expressed in the developing kidney. RAAS influences nephrogenesis and vascularization and guarantees the structural and functional development of the kidney.7 These effects are primarily mediated by the angiotensin receptors AT1 and AT2. AT1 receptors are present in the crust of developing glomeruli, proximal tubules and vasculature, and their expression is the highest during the kidney maturation.8 These receptors stimulate growth and proliferation of embryonic renomedullary interstitium,9 ensure proper communication between the cells and the extracellular matrix,10 and induce production of different growth factors.11 AT2 receptors are much more abundant in the fetal kidney than in adulthood. During nephrogenesis, AT2 receptors are mainly expressed in undifferentiated mesenchyme12 and can mediate apoptosis, which is an important mechanism to counterbalance growth-stimulatory effects of AT1 receptors.11
Up-regulation of RAAS can be observed in response to different maternal conditions such as dehydration,13 reduced salt intake,14 gestational diabetes,15 or disordered renal function.16 Deregulation of RAAS during pregnancy and early postnatal period can alter fluid and ion homeostasis resulting in a deregulation of BP and subsequent hypertension in mature offspring. For example, experimentally induced gestational hypertension has been shown to sensitize male offspring to Ang II-elicited hypertension and upregulate the central RAAS components and proinflammatory markers in the brain.17 Moreover, in our previous study we demonstrated that exposure of pregnant rats to increased Ang II altered postnatal development and increased BP in offspring.18 These changes were associated with increased aldosterone levels and decreased renin activity in the circulation,18 but physiological mechanisms behind this phenomenon are not fully explained. Angiotensin II increases expression of cytokines, inflammatory and fibrotic factors that affect renal hemodynamics, and leads to the development of glomerular damage. In addition, Ang II directly stimulates the accumulation of macrophages in glomerular and tubular cells.19 Hypertensive individuals exhibit an increased incidence of renal fibrosis, glomerulosclerosis as well as T-lymphocyte and macrophage infiltration compared to normotensive individuals.20 Thus, we hypothesize that dysregulated RAAS during pregnancy induces pro-inflammatory conditions in the progeny, infiltration of immune cells into the kidney and subsequent inflammation, which can at least partially explain negative maternal effects on hypertension development in adulthood.
Therefore, the aim of our study was (1) to evaluate consequences of prenatally increased Ang II on development and ultrastructure of the kidneys; (2) to examine the degree of renal damage due to infiltration of immune cells into tubular interstitium in adult male offspring; (3) to analyze whether the effects of prenatally increased Ang II on kidneys can be exacerbated by an increased salt intake, as a secondary insult.
Materials and methods
Parental Wistar rats were obtained from Velaz (Prague, Czech Republic). Animals were housed in a temperature controlled room (21 ± 2°C) under a light:dark regime 12:12 h with lights on at 7:00 h. Upon confirmation of mating by the presence of sperm in vaginal smears, females were housed in separate cages. Pregnant female rats were fed with standard laboratory chow and weighed weekly. On day 6 of pregnancy, osmotic mini-pumps (ALZET, Cupertino, California, USA, model 2002) were implanted in five females (group ANG), releasing Ang II continuously for two weeks with a dosage of 2 µg/kg/h (Angiotensin II, Calbiochem, San Diego, California, USA). The vehicle for Ang II was saline. Mini-pumps were implanted interscapulary under ketamine/xylazine anesthesia. Four females were sham operated at the same time and served as a control group (CONT). Sham operated rats were implanted with an inert object of the same size and weight as the mini-pumps. After delivery, the litter size, birth weight, and sex ratio of the offspring were assessed. Litters were culled to eight animals (four males, four females) per dam. Offspring were fed with standard laboratory chow (0.5% NaCl) until week 15 of age. Afterwards, half of the animals were provided with chow containing increased salt (2% NaCl) until the end of the experiment. Therefore, we had four groups and each consisted of at least of one and maximally two rats from the same litter per treatment: CONTc (offspring from sham-operated females on standard diet; n = 6); CONTs (offspring from sham-operated females on diet with increased salt content; n = 6); ANGc (offspring from females exposed to Ang II on standard diet; n = 7); ANGs (offspring from females exposed to Ang II on diet with increased salt content; n = 7). Data for BP, body weight, relative organ weights, plasma aldosterone levels, and plasma renin activity were published in our previous paper.18 In the present study, we focused especially on the renal morphology and an evaluation of the renal damage in males, since we found that Ang II treatment of pregnant rats caused a more pronounced increase of BP in the male progeny. At the end of the experiment (age of 18 weeks), rats were anaesthetized with carbon dioxide and killed by decapitation. For histological and immunofluorescence analyses, the kidneys were removed, fixed in 4% paraformaldehyde, and embedded in paraffin. Renal coronal sections of 4 μm were prepared and deparaffinized before further processing.
Histological analyses
On deparaffinized sections stained with hematoxylin-eosin, the total number of glomeruli was determined in 15 images per section and calculated per mm2.21 The mean glomerular volume was estimated according to the Weibel-Gomez method as previously published.22,23 The glomerulosclerosis index (GSI) and the cortical tubulointerstitial injury (CTI) were evaluated on sections stained with Mowry's combined Alcian blue-periodic acid-Schiff method24 according to the published protocol.25 For GSI, 50 glomeruli per kidney were examined and graded on a scale: Grade 0 – normal glomeruli; Grade 1 – sclerotic area up to 25%; Grade 2 – sclerotic area 25–50%; Grade 3 – sclerotic area 50–75% and Grade 4 – sclerotic area 75–100%. Cortical tubulointerstitial injury was evaluated as inflammatory cell infiltration, tubular dilatation, atrophy or interstitial fibrosis and analyzed on 15 non-overlapping fields in the renal cortex using a scale; Grade 0 – no abnormal findings; Grade 1 – mild (<25% of the cortex); Grade 1 – moderate (25–50% of the cortex) and Grade 3 – severe (> 50% of the cortex).
Immunofluorescence analyses
Deparaffinized sections were boiled at 95°C for 15 min in 0.01 M citrate buffer for antigen retrieval and blocked in 5% donkey serum in phosphate-buffered saline (PBS) with 0.05% saponin. Sections were stained with anti-CD43 (AbD Serotec, Bio-Rad Laboratories, Hercules, California, USA) to evaluate T-cell infiltration and with anti-CD68 (Abcam, Cambridge, UK) to detect renal infiltration by macrophages. Overnight incubation with primary antibodies (1:100 diluted in PBS) was at 4°C. Thereafter, the sections were incubated with the secondary antibody (Rhodamine TRITC, Jackson Immunoresearch, Philadelphia, USA) diluted 1:1000 in PBS for 1 h at room temperature. The nuclei were counterstained with DAPI (1:10,000, Roche, Indianapolis, USA). Analysis of CD43 and CD68 positive cells was performed using the fluorescence microscope Zeiss Axioscope (Carl Zeiss, Zaventem, Belgium). The cells were counted in 20 randomly selected images of the renal cortex and averaged from two sections per each individual. Cell numbers are calculated per mm2.
Transmission electron microscopy
Renal tissue samples (1–2 mm3) were fixed in 3% glutaraldehyde (Sigma Aldrich, Germany) in 0.2 M phosphate buffer (pH 7.3). Samples were then rinsed three times in 0.1 M sodium phosphate-buffered solution (pH 7.3) and post-fixed in 1% osmium tetroxide (Serva, Germany) at 4°C. Dehydration was performed using a graded ethanol series, followed by tissue clearing in epoxypropane. Tissues were placed in a mixture of resin (Durcupan ACM, Fluka, Switzerland) and epoxypropane (1:1) at room temperature for several hours. Samples were embedded in Durcupan ACM and polymerized at 60°C for 72 h. After polymerization, the embedded samples were cut into sections of 0.7–1.0 µm and stained with 1% toluidine blue dissolved in 1% of sodium borate. Ultrathin sections (600–900 nm thick) were cut with the Reichert ultramicrotome, placed onto copper meshes, and contrasted with uranyl acetate and lead acetate (Serva, Germany) for 10 min. Samples were then imaged on an FEI Morgagni 268D transmission electron microscope (100 kV tungsten filament, Czech Republic) and imaged using a 4 MPxCCD digital camera.
Blood collection and flow cytometry analyses
Rats were immobilized in a restraint chamber and blood was withdrawn from a lateral tail vein. Fluorochrome-conjugated monoclonal antibodies used for immunophenotyping were as follows: PE-Cy5.5 anti-rat CD45 (clone OX-1; Invitrogen); FITC anti-rat CD3 (clone G4.18; eBiocience); APC anti-rat CD4 (clone OX-35; eBiocience); PE anti-rat CD8a (clone OX-8; eBiocience); PE anti-rat CD45RA (clone OX-33; Invitrogen); APC anti-rat NKR-P1A (clone 10/78; Invitrogen) and FITC anti-rat Granulocytes (clone HIS48; eBiocience). Briefly, aliquots of whole blood (50 µL) were quadruple- or double-stained with a mixture of antibodies for 30 min at 4°C in the dark. Thereafter, erythrocytes were lysed with 0.5 mL of lysis buffer (eBioscience) for 10 min at room temperature. The lysis was stopped by adding 1 mL of PBS supplemented with 0.5% bovine serum albumin and 0.1% sodium azide and samples were immediately analyzed on the flow cytometer. Rat leukocyte subsets were identified on the base of their surface markers in the gate for total leukocytes (CD45+): T-cells (CD3+), helper T-cells (CD3+CD4+), cytotoxic T cells (CD3+CD8a+), B cells (CD45RA+), NK cells (CD3-NKR-P1A+), neutrophils, and monocytes (HIS48+ and side scatter gating). To analyze the functional activity of neutrophils, 50 µL of whole blood was diluted 20 times with PBS containing 20 µM of 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCF-DA, Sigma, USA) and incubated for 30 min at 37°C in the dark. Thereafter, each sample was divided into two equal parts; one part was stimulated with 1 µM of phorbol-12-myristate-13-acetate (PMA; Sigma, USA) and one served as unstimulated control. After incubation (for 30 min at 37°C in the dark), cells were washed with PBS and erythrocytes were lysed as described above. After the erythrocyte lysis, cells were washed, resuspended in 0.3 mL of PBS, and analyzed on the flow cytometer. Within the cell, H2-DCF-DA is converted to 2′,7′-dichlorofluorescein (DCF) and fluorescence of this product represents a measure of H2O2 production and this is linearly related to the oxidative burst of stimulated neutrophils.26 Functional activity of neutrophils was expressed as a fold increase of mean DCF fluorescence between unstimulated and PMA-stimulated samples. Flow cytometry was performed on the BD Accuri C6 cytometer and data were analyzed using FlowJo v10.0.6 software (TreeStar Inc., Ashland, USA).
Statistical analyses
Statistical analysis was performed using STATISTICA 7.0 (Statsoft Inc., USA). All data fit a normal distribution as evaluated with Kolmogorov–Smirnov test. Data for renal morphometric parameters and renal infiltration were examined by two-way analysis of variance (fixed factors: prenatal treatment, type of postnatal diet and interaction between both factors) followed by Fisher least significant post hoc tests if the interaction was significant. Data for blood leukocytes were compared between CONT and ANG rats by Student t-tests.
Results
Renal structure and ultrastructure
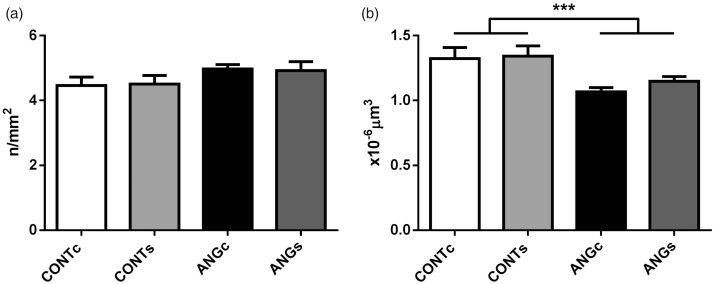
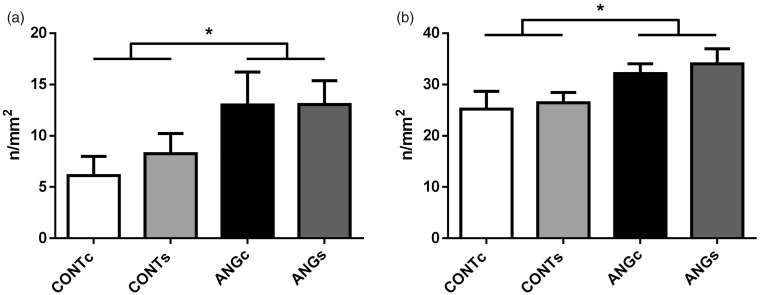
The total number of glomeruli was not affected either by prenatally elevated Ang II (F(1,22)=3.73, P = 0.066) or increased salt intake in adulthood (F(1,22)=0.00; P = 0.990; Figure 1(a)). Mean glomerular volume decreased in the progeny of Ang II-treated mothers compared to controls (F(1,22)=14.28, P = 0.001) and was not affected by salt diet (F(1,22)=0.77, P = 0.391; Figure 1(b)).
Figure 1.
Number of glomeruli (a) and glomerular volume (b) in the adult male progeny of control (CONT) and angiotensin II treated (ANG) rat mothers. Both groups were provided either with standard chow (CONTc, n = 6 and ANGc, n = 7) or increased salt diet (CONTs, n = 6 and ANGs, n = 7) for three weeks. Data are given as means ± SEM. ***P < 0.001.
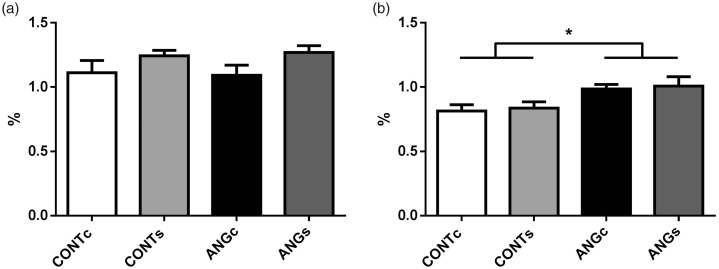
The progeny of Ang II-treated mothers did not differ in glomerular sclerosis compared to control group (F(1,18)=0.00, P = 0.993; Figure 2(a)). On the other hand, the CTI index was higher in rats prenatally exposed to Ang II compared to controls (F(1,18)=8.02, P < 0.05). Increased salt intake did not affect both parameters (F(1,18)=3.07, P = 0.097 for GSI and F(1,18)=0.15, P = 0.704 for CTI).
Figure 2.
Renal glomerulosclerosis index (a) and index of tubulointerstitial injury (b) in the adult male progeny of control (CONT) and angiotensin II-treated (ANG) rat mothers. Both groups were provided either with standard chow (CONTc, n = 5 and ANGc, n = 4) or increased salt diet (CONTs, n = 6 and ANGs, n = 7) for three weeks. Data are given as means ± SEM. *P < 0.05.
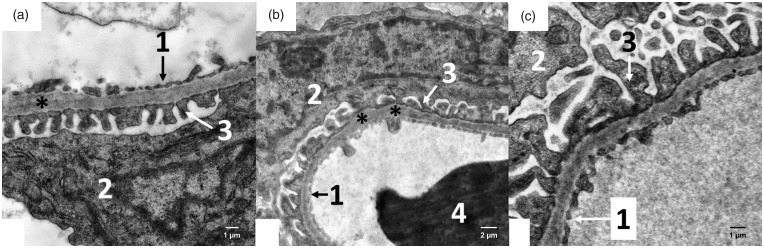
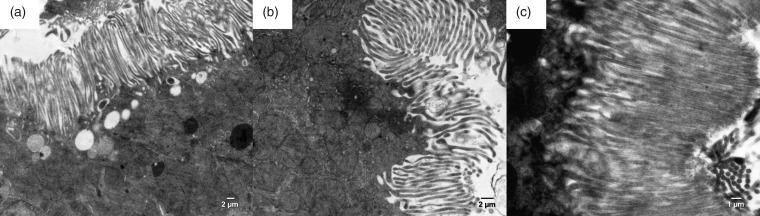
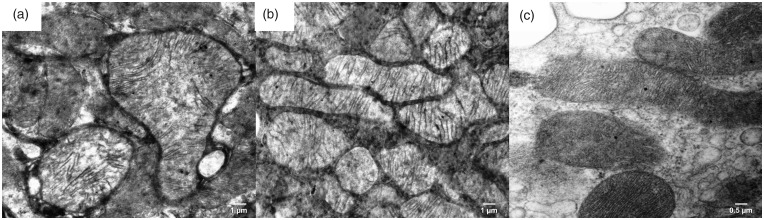
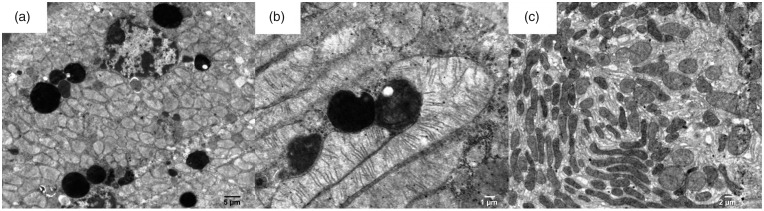
The filtration membrane of the Malpighian renal corpuscle of the ANG group was nonuniform, as documented by a variable size at certain loci, where the fused basal laminae of endothelial cells and podocytes were considerably thicker in comparison to the control group (Figure 3). The apical domain of the proximal tubule epithelial cells of CONT rats contained abundant, densely packed, and regularly organized microvilli with a length up to 3–4 µm. Rats of the ANG group had markedly less developed microvilli, which were also shorter (Figure 4). Mitochondria in the cytoplasm of the proximal tubule epithelial cells of ANG group were enlarged with irregularly arranged and less abundant cristae (Figure 5). Moreover, the cytoplasm of ANG rats contained a considerably higher number of lysosomes and dense residual bodies. In ANG rats, we frequently observed the process of fusion between lysosomes and mitochondria, resulting in the formation of autophagosomes. On the contrary, the CONT group showed only a sporadic distribution of lysosomes within the cytoplasm of the proximal tubule epithelial cells (Figure 6).
Figure 3.
Electron micrograph of the rat glomerulus in the male progeny of angiotensin II-treated rat mothers (a + b) and controls (c). For the filtration membrane, asterisks indicate thickenings of glomerular basement membranes in experimental groups of rats. In the control group, the glomerular basement membrane is without thickenings. 1 – fenestrated endothelia; 2 – body of the podocyte with euchromatic nucleus, rough endoplasmic reticulum and Golgi; 3 – numerous pedicles of podocytes abutting the glomerular basement membrane; 4 – erythrocyte.
Figure 4.
Electron micrograph of the rat proximal tubule in the male progeny of angiotensin II-treated rat mothers (a + b) and controls (c). In experimental group, microvilli at the apical surface of epithelial cells of proximal tubules are less numerous in comparison to control group of rats.
Figure 5.
Electron micrograph of mitochondria from the rat proximal tubule in the male progeny of angiotensin II-treated rat mothers (a + b) and controls (c). The experimental group mitochondria are bulged (enlarged) with irregularly arranged and less abundant cristae in comparison with the control group.
Figure 6.
Electron micrograph of the cytoplasm of the rat proximal tubule in the male progeny of angiotensin II-treated rat mothers (a + b) and controls (c). The cytoplasm of experimental groups contains a high number of lysosomes (electron dense, black, round structures on electron micrographs). Fusion of lysosomes with mitochondria is present on Figure b.
Renal infiltration
Prenatal exposure to Ang II significantly increased renal infiltration of T-cells (F(1,18)=6.04, P < 0.05; Figure 7(a)) and macrophages (F(1,18)=6.53, P < 0.05; Figure 7(b)) compared to control groups. No effects of increased salt intake on renal infiltration were found for both T-cells (F(1,18)=0.21, P = 0.655; Figure 7(a)) and macrophages (F(1,18)=0.30, P = 0.588; Figure 7(b)).
Figure 7.
Renal infiltration of T-cells (CD43) (a) and macrophages (CD68) (b) in the adult male progeny of control (CONT) and angiotensin II-treated (ANG) rat mothers. Both groups were provided either with standard chow (CONTc, n = 5 and ANGc, n = 4) or increased salt diet (CONTs, n = 6 and ANGs, n = 7) for three weeks. Data are given as means ± SEM. *P < 0.05.
Number and functional activity of blood leukocytes
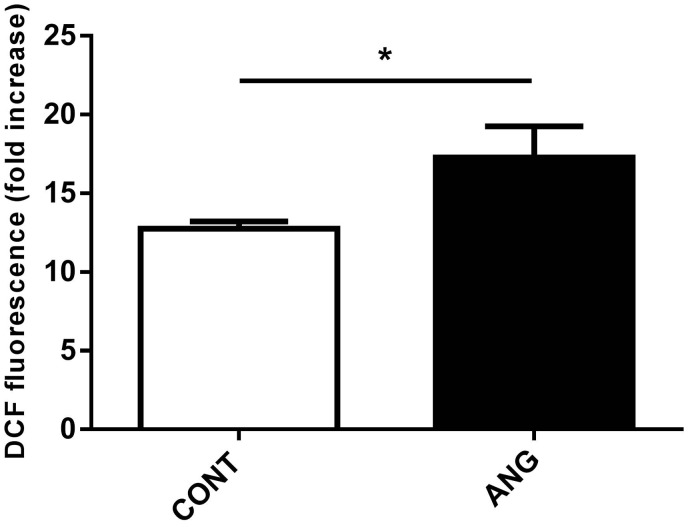
Reduced numbers of cytotoxic CD8+ T-cells were found in the circulation of ANG compared to CONT rats, while no differences between ANG and CONT rats were recorded in total count of leukocytes and in both numbers and percentages of other immune-cell subsets as shown in Table 1. Progeny of Ang II-treated mothers displayed a higher oxidative burst of neutrophils in response to PMA stimulation compared to CONT rats (t = 2.241, P < 0.05, df = 10; Figure 8).
Table 1.
Total count of leukocytes and both counts and percentages of individual leukocyte populations in the male progeny of control (CONT, n = 6) and angiotensin II-treated (ANG, n = 6–7) rat mothers.
|
Counts (No/ µL) |
Percentages (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONT | ANG | t-test | P-value | df | CONT | ANG | t-test | P-value | df | |
| Leukocytes | 9551 ± 623 | 8899 ± 763 | −0.646 | 0.531 | 11 | |||||
| Neutrophils | 1289 ± 65 | 1267 ± 114 | −0.162 | 0.874 | 10 | 13.8 ± 1.1 | 15.4 ± 1.7 | 0.800 | 0.442 | 10 |
| Monocytes | 1262 ± 40 | 1138 ± 157 | −0.770 | 0.459 | 10 | 12.9 ± 1.1 | 13.8 ± 1.5 | 0.447 | 0.664 | 10 |
| T cells | 4347 ± 462 | 3558 ± 349 | −1.385 | 0.193 | 11 | 46.1 ± 1.8 | 40.7 ± 3.2 | −1.418 | 0.184 | 11 |
| Th cells | 2987 ± 343 | 2568 ± 260 | −0.989 | 0.344 | 11 | 69.3 ± 1.4 | 72.4 ± 1.8 | 1.311 | 0.216 | 11 |
| Tc cells | 1248 ± 104 | 930 ± 83* | −2.417 | <0.05 | 11 | 29.6 ± 1.4 | 26.6 ± 1.8 | −1.259 | 0.234 | 11 |
| NK cells | 326 ± 47 | 320 ± 70 | −0.071 | 0.944 | 11 | 3.4 ± 0.4 | 3.5 ± 0.6 | 0.170 | 0.868 | 11 |
| B cells | 1937 ± 171 | 1650 ± 240 | −0.942 | 0.367 | 11 | 20.7 ± 1.2 | 18.8 ± 2.5 | −0.641 | 0.534 | 11 |
Note: Data are presented as means ± SEM. Percentages are calculated as proportions of total number of leukocytes, except helper (Th) and cytotoxic (Tc) T cells, which are calculated as proportions of the number of T cells. Differences between CONT and ANG rats are compared by Student t-tests; df: degrees of freedom.
Figure 8.
Oxidative burst of neutrophils in response to phorbol myristate acetate (PMA) stimulation in the male progeny of control (CONT, n = 6) and angiotensin II treated (ANG, n = 6) rat mothers. Oxidative burst was calculated as fold increase of mean dichlorofluorescein (DCF) fluorescence between unstimulated and PMA stimulated samples. Data are given as means ± SEM.* P < 0.05.
Discussion
Our data proved the important effects of prenatally increased Ang II on kidney development and subsequent renal injury in adult offspring. Prenatally increased Ang II led to decreased glomerular volume, higher cortical interstitial damage, and increased infiltration of immune cells into the renal interstitium. Ultrastructural images showed profound changes in the brush border of proximal tubules, glomeruli, and mitochondria.
In our previous paper, we reported increased BP and decreased relative kidney weight of offspring prenatally exposed to elevated Ang II levels.18 Because the kidney has an important role in several developmental models of hypertension,27,28 we investigated in more detail the consequences of prenatally increased Ang II on the structure and function of the kidney in male offspring. In the present study, we focused only on males because previously we showed that male offspring of Ang II treated mothers displayed more distinct increase in BP than females.18 Likewise, published data demonstrate more serious negative effects of maternal programming in males than females.17 RAAS plays an essential role in the development of the kidney in all stages of organogenesis,29,30 and deregulation of RAAS during intrauterine development leads to permanent changes in the kidney, such as reduced total number of the glomeruli.27,28 Reduction of nephron numbers at young age can lead to hyperfiltration in remaining nephrons with subsequent proteinuria, progressive renal damage, and hypertension.31
Increased Ang II levels during the prenatal period may result in changes in the kidney development and increase the susceptibility to hypertension in adulthood. Our morphological analyses revealed decreased mean glomerular volume in the offspring after prenatal exposure to Ang II. Together with the lower relative kidney weight,18 all these changes can result in a reduced filtration area and glomerular filtration rate. The decreased glomerular filtration rate may lead to increased vascular resistance and hypertension.32 Since the increased salt intake in either group induced changes of these parameters, we assume organizational effects of Ang II during the kidney development instead of a direct effect on BP regulation.
We found increased CTI in offspring of ANG mothers with no additional effect of increased salt. Moreover, distinct ultrastructural changes were observed in proximal tubules. Animals prenatally exposed to Ang II had fewer microvilli, which were also often shortened and less distributed along brush border. This phenomenon was also observed in SHR rats33 and suggests impaired reverse resorption of proximal tubules. These findings are consistent with our previous results, which showed an impaired function of renal Na, K-ATPase localized on basolateral membranes of tubules in the progeny of Ang II treated mothers.34 Moreover, Ang II is known to increase glomerular damage in models with genetic up-regulation of RAAS, such as TGR(mRen2)27 rats.35
In our experiment higher salt diet was applied as a second insult to mimic postnatal life style factors in a human population.5 It was expected that such a “second hit” may exacerbate predispositions, which were induced by the prenatal Ang II treatment. However, we did not find significant changes in the kidney after increased salt intake and this finding is in agreement with data on BP in our previous study.18 Surprisingly, the progeny of Ang II treated mothers displayed a decreased sensitivity of BP to salt. The absence of negative effects of higher salt diet was not expected but can be explained by a moderately increased (2%) salt content, which is still in a physiological range. Therefore, we assume that the dose and/or duration of salt treatment were not sufficient for induction of negative changes.
Clinical and experimental studies indicate that Ang II recruits inflammatory cells, induces the release of cytokines, and directly stimulates intracellular signaling mechanisms related to kidney inflammation and fibrosis contributing to the progression of chronic kidney diseases.36 In our experiment, prenatal exposure to Ang II led to a significant increase of infiltrated T-cells and macrophages. This process is related to kidney damage such as nephropathy37 or hypertension.38 Immune cell infiltration can further exacerbate kidney damage because of enhanced production of reactive oxygen species and cytokines, thereby increasing oxidative stress and via the cytokines vasoconstriction of the capillary vessels of the kidney occurs.39
The recruitment of monocytes into the kidney is not a random process and is stimulated by chemoattractant proteins such as monocyte chemoattractant protein-1. Angiotensin II has been shown to induce the production of this protein in glomerular endothelial cells via AT1 receptors and nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase-dependent reactive oxygen species (ROS) generation.40 In the same way, Ang II can stimulate an oxidative burst in neutrophils.41 This is in line with our results as the progeny of Ang II-treated mothers displayed an increased oxidative burst of stimulated neutrophils indicating potential priming effects on these immune cells. Kidney damage and increased ROS production in hypertension are often associated with mitochondrial dysfunction.42 This was suggested also in our study based on ultrastructural changes in the mitochondria in renal tissues of prenatally exposed Ang II animals. Moreover, we found a large number of lysosomes and frequent fusion of lysosomes with mitochondria in epithelial cells of the proximal tubules in ANG rats indicating that damaged mitochondria may be removed by lysosomes. All these morphological changes confirm deregulation of RAAS in animals prenatally exposed to Ang II.
Conclusions
Increased Ang II in pregnant female rats resulted in changes of renal morphology and infiltration of immune cells into the kidney of mature male offspring. These processes were accompanied by degenerative changes, especially in proximal tubules of nephron. Our results suggest that the deregulated RAAS in mothers is involved in developmental programming of hypertension in adult male offspring via deterioration of kidney morphology and function.
Authors’ contributions
All authors participated in the design of experiments, analysis of the data and writing of the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Slovak Research and Development Agency (APVV-0291–12 and APVV-17–0178).
References
- 1.Barker DJP, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986; 327:1077–81 [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989; 298:564–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moritz KM, Dodic M, Wintour EM. Kidney development and the fetal programming of adult disease. Bioessays 2003; 25:212–20 [DOI] [PubMed] [Google Scholar]
- 4.Herring CM, Bazer FW, Johnson GA, Wu G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp Biol Med 2018; 243:525–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorey ES, Pantaleon M, Weir KA, Moritz KM. Adverse prenatal environment and kidney development: implications for programing of adult disease. Reproduction 2014; 147:R189–R98 [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Garrett MR. Nephron number, hypertension, and CKD: physiological and genetic insight from humans and animal models. Physiol Genomics 2017; 49:180–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilgers KF, Norwood VF, Gomez RA. Angiotensin's role in renal development. Semin Nephrol 1997; 17:492–501 [PubMed] [Google Scholar]
- 8.Tufro-McRreddie A, Romano LM, Harris JM, Ferder L, Gomez RA. Angiotensin II regulates nephrogenesis and renal vascular development. Am J Physiol 1995; 269:F110–F5 [DOI] [PubMed] [Google Scholar]
- 9.Maric C, Aldred GP, Harris PJ, Alcorn D. Angiotensin II inhibits growth of cultured embryonic renomedullary interstitial cells through the AT2 receptor. Kidney Int 1998; 53:92–9 [DOI] [PubMed] [Google Scholar]
- 10.Cantley LG. Growth factors and the kidney: regulation of epithelial cell movement and morphogenesis. Am J Physiol 1996; 271:F1103–F13 [DOI] [PubMed] [Google Scholar]
- 11.Wolf G. Angiotensin II and tubular development. Nephrol Dial Transplant 2002; 17:48–51 [DOI] [PubMed] [Google Scholar]
- 12.Kakuchi J, Ichiki T, Kiyama S, Hogan BLM, Fogo A, Inagami T, Ichikawa I. Developmental expression of renal angiotensin II receptor genes in the mouse. Kidney Int 1995; 47:140–7 [DOI] [PubMed] [Google Scholar]
- 13.Guan J, Mao C, Xu F, Geng C, Zhu L, Wang A, Xu Z. Prenatal dehydration alters renin-angiotensin system associated with angiotensin-increased blood pressure in young offspring. Hypertens Res 2009; 32:1104–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakuyama H, Katoh M, Wakabayashi H, Zulli A, Kruzliak P, Uehara Y. Influence of gestational salt restriction in fetal growth and in development of diseases in adulthood. J Biomed Sci 2016; 23:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Fan Y, Xia F, Geng C, Mao C, Jiang S, He R, Zhang L, Xu Z. Prenatal water deprivation alters brain angiotensin system and dipsogenic changes in the offspring. Brain Res 2011; 1382:128–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maduwegedera D, Kett MM, Flower RL, Lambert GW, Bertram JF, Wintour EM, Denton KM. Sex differences in postnatal growth and renal development in offspring of rabbit mothers with chronic secondary hypertension. Am J Physiol 2007; 292:R706–R14 [DOI] [PubMed] [Google Scholar]
- 17.Xue B, Beltz TG, Guo F, Johnson AK. Sex differences in maternal gestational hypertension-induced sensitization of angiotensin II hypertension in rat offspring: the protective effect of estrogen. Am J Physiol Regul Integr Comp Physiol 2018; 314:R274–R81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svitok P, Senko T, Panakova Z, Olexova L, Krskova L, Okuliarova M, Zeman M. Prenatal exposure to angiotensin II increases blood pressure and decreases salt sensitivity in rats. Clin Exp Hypertens 2017; 39:489–94 [DOI] [PubMed] [Google Scholar]
- 19.Imig JD, Ryan MJ. Immune and inflammatory role in renal disease. Compr Physiol 2013; 3:957–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wade B, Abais-Battad JM, Mattson DL. Role of immune cells in salt-sensitive hypertension and renal injury. Curr Opin Nephrol Hypertens 2016; 25:22–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertram JF, Soosaipillai MC, Ricardo SD, Ryan GB. Total numbers of glomeruli and individual glomerular cell types in the normal rat kidney. Cell Tissue Res 1992; 270:37–45 [DOI] [PubMed] [Google Scholar]
- 22.Weibel ER. Stereological methods. Practical methods for biological morphometry. London, UK: Academic Press, 1979 [Google Scholar]
- 23.Lane PH, Steffes MW, Mauer SM. Estimation of glomerular volume: a comparison of four methods. Kidney Int 1992; 41:1085–9 [DOI] [PubMed] [Google Scholar]
- 24.Kujal P, Čertíková Chábová V, Škaroupková P, Husková Z, Vernerová Z, Kramer HJ, Walkowska A, Kompanowska-Jezierska E, Sadowski J, Kitada K, Nishiyama A, Hwang SH, Hammock BD, Imig JD, Červenka L. Inhibition of soluble epoxide hydrolase is renoprotective in 5/6 nephrectomized Ren-2 transgenic hypertensive rats. Clin Exp Pharmacol Physiol 2014; 41:227–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano Y, Hirano T, Uehara K, Nishibayashi S, Hattori K, Aihara M, Yamada Y. New rat model induced by anti-glomerular basement membrane antibody shows severe glomerular adhesion in early stage and quickly progresses to end-stage renal failure. Pathol Int 2008; 58:361–70 [DOI] [PubMed] [Google Scholar]
- 26.Elbim C, Lizard G. Flow cytometric investigation of neutrophil oxidative burst and apoptosis in physiological and pathological situations. Cytometry Part A 2009; 75A:475–81 [DOI] [PubMed] [Google Scholar]
- 27.Nuyt AM, Alexander BT. Developmental programming and hypertension. Curr Opin Nephrol Hypertens 2009; 18:144–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koleganova N, Piecha G, Ritz E, Becker LE, Müller A, Weckbach M, Nyengaard JR, Schirmacher P, Gross-Weissmann ML. Both high and low maternal salt intake in pregnancy alter kidney development in the offspring. Am J Physiol 2011; 301:F344–F54 [DOI] [PubMed] [Google Scholar]
- 29.Yosypiv IV. A new role for the renin-angiotensin system in the development of the ureteric bud and renal collecting system. Keio J Med 2008; 57:184–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Lasaitiene D, Gabrielsson BG, Carlsson L, Billig H, Carlsson B, Marcussen N, Sun XF, Friberg P. Neonatal losartan treatment suppresses renal expression of molecules involved in cell-cell and cell-matrix interactions. J Am Soc Nephrol 2004; 15:1232–43 [DOI] [PubMed] [Google Scholar]
- 31.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure less of one, more the other?. Am J Hypertens 1988; 1:335–47 [DOI] [PubMed] [Google Scholar]
- 32.Vääräniemi K, Koskela J, Tahvanainen A, Tikkakoski A, Wilenius M, Kähönen M, Kööbi T, Niemelä O, Mustonen J, Pörsti I. Lower glomerular filtration rate is associated with higher systemic vascular resistance in patients without prevalent kidney disease. J Clin Hypertens 2014; 16:722–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drueke T, Hennessen U, Nabarra B, Ben Nasr L, Lucas PA, Dang P, Thomasset M, Lacour B, Coudrier E, McCarron DA. Ultrastructural and functional abnormalities of intestinal and renal epithelium in the SHR. Kidney Int 1990; 37:1438–48 [DOI] [PubMed] [Google Scholar]
- 34.Jagmasevic-Mezesova L, Svitok P, Kalocayova B, Zeman M, Vrbjar N. Sex-specific response of renal Na,K-ATPase to prenatal angiotensin 2 exposure and increased salt intake in offspring. J Physiol Pharmacol 2018; 69:83–90 [DOI] [PubMed] [Google Scholar]
- 35.Svitok P, Husková Z, Červenková L, Kikerlová S, Vaňourková Z, Sedláková L, Vacková Š, Šutovska H, Zeman M, Kopkan L. The exaggerated salt-sensitive response in hypertensive transgenic rats (TGR mRen-2) fostered by a normotensive female. Hypertens Res 2019; 42:459–468 [DOI] [PubMed] [Google Scholar]
- 36.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 2004; 65:1009–16 [DOI] [PubMed] [Google Scholar]
- 37.Zoja C, Garcia PB, Remuzzi G. The role of chemokines in progressive renal disease. Front Biosci 2009; 14:1815–22 [DOI] [PubMed] [Google Scholar]
- 38.McMaster William G, Kirabo A, Madhur Meena S, Harrison David G. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 2015; 116:1022–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lara LS, McCormack M, Semprum-Prieto LC, Shenouda S, Majid DSA, Kobori H, Navar LG, Prieto MC. AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II-salt hypertension. Am J Physiol 2012; 302:85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Q, Yang XH, Cheng YX. Angiotensin II stimulates MCP-1 production in rat glomerular endothelial cells via NAD(P)H oxidase-dependent nuclear factor-kappa B signaling. Braz J Med Biol Res 2009; 42:531–6 [DOI] [PubMed] [Google Scholar]
- 41.El Bekay R, Álvarez M, Monteseirín J, Álba G, Chacón P, Vega A, Martín-Nieto J, Jiménez J, Pintado E, Bedoya FJ, Sobrino F. Oxidative stress is a critical mediator of the angiotensin II signal in human neutrophils: involvement of mitogen-activated protein kinase, calcineurin, and the transcription factor NF-κB. Blood 2003; 102:662–71 [DOI] [PubMed] [Google Scholar]
- 42.Rubattu S, Pagliaro B, Pierelli G, Santolamazza C, Di Castro S, Mennuni S, Volpe M. Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. Int J Mol Sci 2015; 16:823–39 [DOI] [PMC free article] [PubMed] [Google Scholar]