Abstract
Objective
Hospital environmental surfaces are frequently contaminated by microorganisms. However, the causal mechanism of bacterial contamination of the environment as a source of transmission is still debated. This prospective study was performed to characterize the nature of multidrug-resistant organism (MDRO) transmission between the environment and patients using standard microbiological and molecular techniques.
Setting
Prospective cohort study at 2 academic medical centers.
Design
A prospective multicenter study to characterize the nature of bacterial transfer events between patients and environmental surfaces in rooms that previously housed patients with 1 of 4 ‘marker’ MDROs: methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Clostridium difficile, and MDR Acinetobacter baumannii. Environmental and patient microbiological samples were obtained on admission into a freshly disinfected inpatient room. Repeat samples from room surfaces and patients were taken on days 3 and 7 and each week the patient stayed in the same room. The bacterial identity, antibiotic susceptibility, and molecular sequences were compared between organisms found in the environment samples and patient sources.
Results
We enrolled 80 patient–room admissions; 9 of these patients (11.3%) were asymptomatically colonized with MDROs at study entry. Hospital room surfaces were contaminated with MDROs despite terminal disinfection in 44 cases (55%). Microbiological Bacterial Transfer events either to the patient, the environment, or both occurred in 12 patient encounters (18.5%) from the microbiologically evaluable cohort.
Conclusions
Microbiological Bacterial Transfer events between patients and the environment were observed in 18.5% of patient encounters and occurred early in the admission. This study suggests that research on prevention methods beyond the standard practice of room disinfection at the end of a patient’s stay is needed to better prevent acquisition of MDROs through the environment.
Hospital environmental surfaces are frequently contaminated by microorganisms.1 When contaminated, such surfaces can potentially act as vectors for transmission of bacteria that can lead to healthcare-associated infections (HAIs).2 Although contaminated surfaces have been hypothesized to play an important role in the causal pathway of HAIs, the nature, direction, persistence, and quantity of bacterial transfer between surfaces and patients remain poorly understood.
Nevertheless, the concept of bacterial contamination of the environment as a source of transmission is still debated. Flaws with previous studies have included (1) studies taking place during an outbreak setting, (2) suboptimal study design,3 and (3) lack of molecular epidemiology to show correlation between isolates from the environmental and those from patients. However, our group recently reported results from a large, multicenter randomized controlled trial on interventions to improve disinfection practices.4 Although our study only focused on strategies that improve terminal room disinfection, the results suggest that the environment is responsible for at least 10%–30% of MDRO acquisitions.
Thus, we undertook this prospective multicenter study as a substudy of our large trial to characterize the nature of MDRO transmission between the environment and patients using a combination of standard microbiological and molecular techniques. The objective of this study was to determine whether, when, and in what direction epidemiologically important pathogens transfer between patients and surfaces within hospital rooms.
Methods
We performed a prospective cohort study at 2 hospitals: Duke University Hospital (a 921-bed tertiary-care academic medical center in Durham, North Carolina) and Duke Regional Hospital (a 250-bed community hospital in Durham, North Carolina). The study was designed to characterize the baseline and temporal profile of microorganisms on environmental surfaces of acute-care hospital rooms and on patients admitted to these newly disinfected rooms. We sought to characterize the nature of bacterial transfer events between patients and environmental surfaces using 4 ‘marker’ MDROs: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), Clostridium difficile, and multidrug-resistant (MDR) Acinetobacter baumannii. These organisms were chosen due to their importance as pathogens in HAIs and their propensity to contaminate and persist on hospital surfaces.2
The study was approved by the Duke University Health System Institutional Review Board and was registered on ClinicalTrials.gov (trial no. ). Additionally, the current study was performed in the study hospitals contemporaneously with the BETR Disinfection study (trial no. ),4 the first controlled, cluster-randomized multicenter study investigating the efficacy of reducing incidence of HAIs with use of enhanced terminal cleaning strategies. In short, participating hospitals were randomized to terminally clean all patient rooms with (1) reference (quaternary ammonium disinfectant except for C. difficile, for which bleach was used); (2) UV (quaternary ammonium disinfectant and disinfecting ultraviolet [UV-C] light except for C. difficile, for which bleach and UV-C were used); (3) bleach only; or (4) bleach and UV-C. Every strategy was used at each hospital in 4 randomly assigned consecutive 7-month periods. Thus, each participating hospital would implement all 4 cleaning strategies for 6 months with 1 month of washout between different cleaning methods. The routine daily cleaning of the patient rooms continued during the study with quaternary ammonium for all rooms or bleach for rooms that had housed patients with Clostridium difficile infection (CDI) according to standard practice at participating hospitals. We enrolled 20 subjects for each type of terminal disinfection strategy.
Subject enrollment
We prospectively identified subjects for enrollment using the admissions and transfer data from of the health system electronic medical record. All patients admitted to newly cleaned rooms at participating hospitals were eligible. To enhance the ability to detect and document bacterial transmission events between patients and hospital environments, study personnel specifically sought out (1) patients housed in rooms whose antecedent patient was placed on contact precautions for any reason and (2) patients with anticipated hospital stay of≥48 hours. Informed consent was obtained from all subjects enrolled in the study. Patients were excluded if they had already been placed in the newly cleaned room prior to screening procedures, baseline sampling, or informed consent.
Specimen collection
Study personnel made study visits to collect specimens from the patient and environmental room surfaces at the time of enrollment (day 0) and at defined intervals thereafter (ie, study days 3 and 7 and each week after study enrollment). Importantly, the environmental specimens were obtained on day 0 after terminal disinfection but prior to subject entry into the room. Where possible, a final set of specimens was collected from the patient and environmental room surfaces on the day of discharge from the room. Study personnel performed hand hygiene and donned contact isolation equipment prior to entering the room and taking microbiological specimens to reduce introduction of microorganisms.
Study personnel obtained 2 microbiological swabs from 4 body sites (nares, oropharynx, axilla, and perineum) at each study visit5, 6 and a fecal specimen if available on the day of the visit.7, 8 Microbiological samples were also collected from 7 high-frequency touch surfaces in the hospital room of the enrolled subject; these surfaces included the bed rail, overbed table, top of the nearest bedside table, arm rest of chair, sink, toilet seat, and the floor of the shower bloc.9 Each surface area was sampled repeatedly using 10 individual Rodac plates (5 for aerobic and 5 for anaerobic culture) to enhance microbiological yield and to reduce sampling error.10
Outcomes
We identified 2 primary outcomes of interest: (1) The baseline and subsequent patterns of patient colonization and hospital surface contamination, and (2) the number of microbiological and molecularly proven bacterial transfer events between hospital surfaces and patients. We also identified 2 secondary outcomes of interest: (1) the direction and timing of bacterial transfer events and (2) the clonal relatedness of bacterial isolates involved in transfer events. We defined microbiological bacterial transfer (MBT) events as the detection of microorganisms from patients and environmental surfaces of the same genus, species, and antibiotic susceptibility (for MRSA and VRE). The likely direction of bacterial transmission was surmised based on the sequence of detection. For example, if an organism was found on environmental surfaces prior to identification in patient specimens, we categorized the MBT event as an environment-to-patient transmission. If an organism was detected on patient and environmental specimens at the same study visit, the direction of the MBT was defined as indeterminate.
Microbiological methods for patient-derived specimens, specimens from environmental sampling and the molecular analysis and relatedness testing are described in detail in the supplemental appendix.
Statistical analysis
We used standard descriptive statistics, including medians and interquartile ranges (IQRs) for nonnormally distributed continuous variables. For quantitative analyses of data from Rodac plates, culture results were aggregated to obtain the number of colony-forming units (CFUs) per environmental site, not per plate.10
Results
Demographics
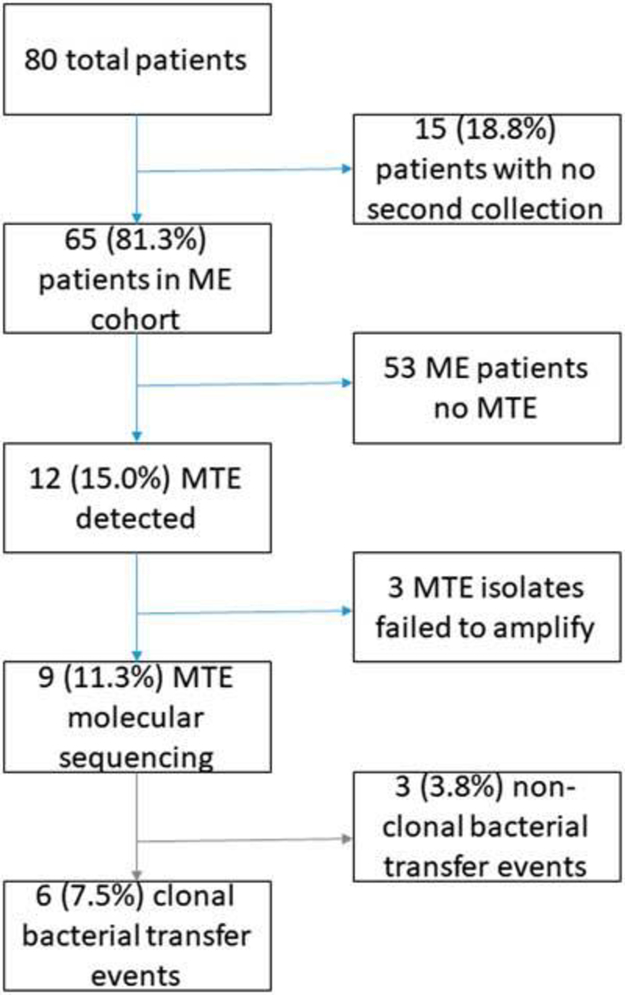
We enrolled and collected data on 80 patient–room encounters that occurred in 68 general ward rooms; 67 of the patients (84%) were white, and 54 (68%) were female (Table 1). Collectively, 79% of the enrolled patients were admitted under 2 medical services: general medicine and oncology/hematology. The median length of hospital stay for enrolled patients was 4.9 days. Of the 80 patients, 15 (18.8%) were discharged before the day 3 study visit and provided only baseline specimens (Fig. 1). The remaining 65 patient–room encounters (81.3%) provided the baseline and at least 1 other pair of patient–environmental specimens on subsequent study visits for comparison; this group of 65 patients were considered the microbiologically evaluable (ME) cohort.
Table 1.
Demographic and Admission Characteristics of Subjects Enrolled in the Current Study
| Variable | Full Cohort (n=80), No. (%) |
|---|---|
| Age, y, median (IQR) | 60 (55–69) |
| Female | 54 (68) |
| Caucasian | 67 (84) |
| General medicine service | 22 (28) |
| Oncology | 29 (36) |
| Only 1 collection | 15 (19) |
| 2 collections | 48 (60) |
| ≥ 3 collections | 17(21) |
| Length of hospital stay, d median (IQR) | 4.9 (3.1–12.0) |
Note. IQR, interquartile range.
Fig. 1.
Included and excluded study patients and results of bacterial transfer events. Percentages of the total population displayed. MTE, microbiological transfer event; ME, microbiologically evaluable.
Baseline and temporal pattern of patient colonization and surface contamination
In total, 9 patients (11.3%) were asymptomatically colonized with MDROs at study entry: MRSA colonization was observed in 6 encounters (7.5%), VRE colonization was observed in 2 encounters (2.5%), and C. difficile colonization was found in 2 encounters (2.5%). Notably, 1 of these patients (1.25%) was concurrently colonized with MRSA and C. difficile.
Hospital room surfaces were contaminated with MDROs despite terminal disinfection in 44 of 80 patient rooms (55%) at time of study enrollment. Clostridium difficile was detected in 21 rooms (26.3%); VRE was detected in 18 rooms (22.5%); MRSA was detected in 15 rooms (18.8%); and MDR Acinetobacter was detected in 9 rooms (11.3%). Contamination with multiple MDROs was observed in 19 (23.8%) rooms; 2 MDROs were identified in 17 rooms (21.3%), and 3 MDROs (2.5%) were identified in 2 rooms.
The bioburden of MDROs on tested hospital surfaces was generally low at enrollment (median, 6 CFU of MDROs/cm2; interquartile range [IQR], 2–16 CFU/cm2). Notably, the bioburden was similarly low regardless of the organism detected (Table 2).
Table 2.
Baseline Contamination of Hospital Surfaces at Enrollment by Pathogen
| Variable | MRSA | VRE | Clostridium difficile | Acinetobacter |
|---|---|---|---|---|
| Rooms with contamination, no. (%) | 15 (18.8) | 18 (22.5) | 21 (26.3) | 9(11.3) |
| Median CFU/cm2 (IQR) | 6(3–13) | 8 (5–38) | 3 (1–11) | 4 (1–9) |
Note. MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; CFU, colony-forming units; IQR, interquartile range.
Bacterial transfer events
We detected 12 microbiological bacterial transfer (MBT) events (18.5%) among the 65 patients of the ME cohort: 2 (16%) were associated with MRSA, 5 (42%) were associated with VRE, and 5 (42%) were related to C. difficile (Tables 3). We categorized these 12 MBT events into 3 categories based on likely direction of bacterial transfer (Table 3): 4 MBT events (33%) occurred from patient to environment; 4 events (33%) occurred from environment to patient; and in 2 environment-to-patient transfer events (50%), a molecularly similar organism was detectable on hospital room surfaces at baseline. The other 2 apparent environment-to-patient MBT events involved molecularly dissimilar organisms of the same genus and species. Notably, 4 MBT events (33%) were of indeterminate direction because a marker organism was detectable in both the patient and the environment at the same post-baseline visit.
Table 3.
Description of 12 Cases of Potential Microbiological Bacterial Transfer Eventsa
| Patient | Target MDRO | Terminal Clean Protocol | Patient- Environment | Environment-Patient | Indeterminate | Presence of Molecularly Related Isolates | Presence of Molecularly Discordant MDRO Isolatesb |
|---|---|---|---|---|---|---|---|
| A | MRSA | Bleach | X | X | |||
| B | MRSA | Bleach | X | X | X | ||
| C | VRE | Quat. | X | X | |||
| D | VREc | Bleach + UV | X | ||||
| E | VREc | Bleach + UV | X | ||||
| F | VREc | Bleach + UV | X | ||||
| G | VRE | Bleach | X | X | |||
| H | CDI | Bleach | X | X | X | ||
| I | CDI | Bleach | X | X | |||
| J | CDI | Quat. | X | X | X | ||
| K | CDI | Quat. | X | X | X | ||
| L | CDI | Quat. + UV | X | X | |||
| Total | 12 | 4 (33%) | 4 (33%) | 4 (33%) | 6 (50%) | 7(58%) |
Note. MRSA, methicillin resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; MDR ABC, multidrug-resistant Acinetobacter baumanii complex; Quat., quaternary ammonium; CDI, Clostridium difficile infection.
Terminal cleaning protocol used for disinfecting the room prior to admission: bleach, bleach+UV irradiation, Quat., Quat.+UV irradiation.
Subjects could have both molecularly related and molecularly discordant transfer events if>1 ribotype of a target MDRO species was identified.
Molecular relatedness unknown due to noninterpretable pulsed-field gel electrophoresis (PFGE).
We attempted to perform molecular relatedness testing on patient and environmental isolates obtained from these 12 MBT events; however, 3 patient-derived VRE isolates failed to amplify despite repeated attempts. Thus, complete clonal relatedness data were only available for 9 (75%) of 12 MBT events (Table 3). Molecular sequencing of isolates captured in MBT events showed that MDRO transmission frequently involved both molecularly related and molecularly dissimilar isolates of the same organism. For instance, 3 MBT events (33%) involved molecularly dissimilar isolates (ie, not true transmission events), and 4 other MBT events (44%) involved a combination of molecularly dissimilar and molecularly related isolates. Only 2 MBT events (22%) involved strictly molecularly related isolates.
Moreover, 4 distinct MRSA pulsotypes were identified in the 2 MBT events (Supplemental Fig. 1). None of the pulsotypes matched control MRSA types tested (ie, USA 100, 200, 300, 400, 500, 600, 700, 800, 1000, or 1100). In 1 patient-to-environmental MBT event, the environmental isolates were identical to the patient-derived isolates and all isolates belonged to pulsotype group III. In a second patient-environmental set in which the direction of MBT was classified as indeterminate, the patient isolates belonged to MRSA pulsogroup I and matched 8 environmental isolates. Interestingly, 4 other types of MRSA were also encountered in the environment, including 2 other distinct pulsotype groups (MRSA groups II and IV) and 2 MRSA singleton isolates (ie, without a molecular match).
Five MBT events were VRE related (Tables 3); 1 event was from patient to environment, 1 event from environment to patient, and 3 other events were indeterminate. While 7 patient isolates were available, they produced only 2 interpretable PFGE patterns, and 91 environmental isolates produced 58 PFGE patterns. Among all isolates analyzed, 4 were major PFGE pulsotypes of VRE with at least 2 isolates of>80% similarity, but 14 singleton isolates were identified (Supplemental Fig. 2). Furthermore, the VRE pulsotypes were not shared between different patient–environment sets.
For C. difficile, we identified 5 C. difficile-related MBT events; they produced interpretable ribotype patterns from 8 patient isolates and 23 environmental isolates. Three MBT events involved molecularly related isolates: 2 events were due to environmental to patient transfer and 1 event was due to patient to environment transfer. Also, 2 MBT events involved molecularly dissimilar isolates of C. difficile (ie, not true transmissions). Environmental isolates of C. difficile also showed the most variability in ribotype patterns (Supplemental Fig. 3). All patient–environment sets included environmental isolates that had ribotype patterns different from the patient isolates.
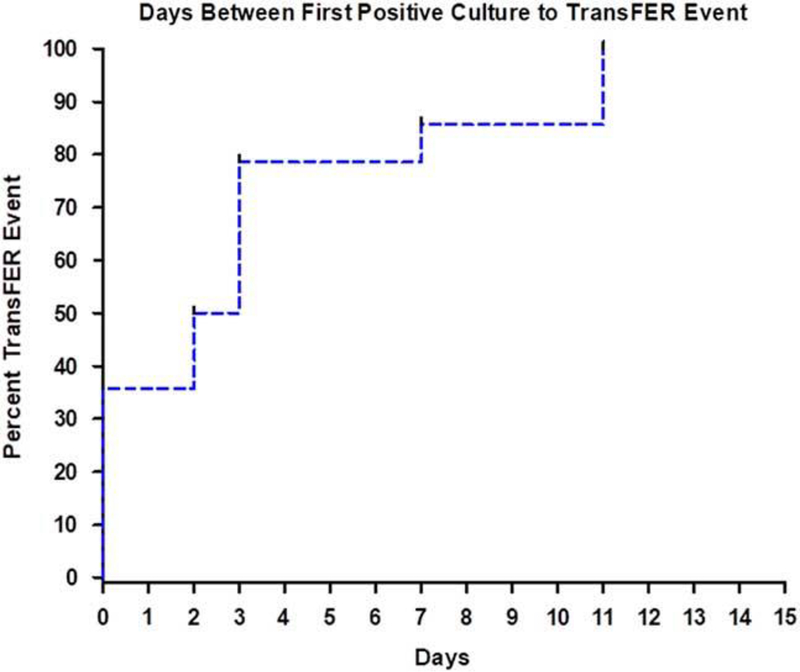
Of the 12 MBT events, time-to-event analyses showed that 80% of the documented transmission, regardless of direction, occurred within 3 days of identifying a target MDRO from any patient or environmental site (Fig. 2).
Fig. 2.
Time-to-event analysis showing time from first positive bacterial culture from any source to documented transfer of clonally identical bacteria between patients and room surfaces.
Specific examples of bacterial transmission events
Several of the observed MBTs were complex and deserve specific narrative beyond aggregated statistical information.
Patient to environment
Patient A was colonized with MRSA in the oropharynx and the perineum at study enrollment. At the same initial study visit, none of the environmental surfaces in the patient’s room were contaminated with an MDRO. On study day 3, the oropharyngeal carriage of MRSA was again detected. In addition, a MRSA with identical PFGE pulsotype was also detected from environmental samples obtained from bed rails. This patient did not have a documented HAI with MRSA.
Patient B had no MDRO colonization at enrollment. Enrollment samples from recently terminally cleaned environment revealed C. difficile on bathroom floor surfaces. On day 3, the same C. difficile was still detected on the bathroom floor. Surprisingly, the patient was asymptomatically colonized with a second and different strain of C. difficile at the same study visit on day 3 (strain B). Patient B developed symptoms of CDI on day 7, and the second strain of C. difficile was detected in stool specimens. Subsequently, we found evidence of environmental contamination with the second strain of C. difficile on chair arm and in the patient room sink 7 and 14 days following the onset of CDI, respectively. Without sequential patient and environmental sampling and the molecular confirmation, the environment would have been blamed as the source for C. difficile acquisition and infection.
Environment to patient
Neither Patient C nor the environmental surfaces were colonized with any of the marker MDROs at enrollment. On day 3, C. difficile isolates were detected on the sink and the bed rail. On day 14, the patient’s perineal specimen showed the same clonal type of CDI as that recovered on day 3 from the environmental specimen. The patient remained asymptomatic for CDI throughout the duration of the study and the hospital stay.
Discussion
We used a prospective study design and molecular techniques to study the transmission of MDROs between patients and surfaces of hospital rooms. The most important finding from the current study is the demonstration of microbiological bacterial transfer events in 12 patient encounters (18.5%) from the ME cohort (Fig. 1). Molecular testing of specimens showed that 6 encounters (66.7% of the 9 ME cohort with molecular data and 9.2% of the 65 evaluable patients) involved molecularly identical strains of MDRO. Indeed, 7.5% of all hospital-room encounters showed transfer of clonally identical MDROs. Perhaps most importantly, we identified 2 encounters (3%) in which the patient acquired an MDRO present in the environment at the time of admission; both events were confirmed environment-to-patient transmissions involving C. difficile.
We believe that these observed rates of MDRO transmission are underestimates of the true bacterial transfer phenomenon for 2 primary reasons: (1) limitations in sampling and (2) lack of sensitivity of current microbiological methods. Furthermore, we only tracked bacterial transmission using 4 ‘marker’ MDROs; we hypothesize that bacterial transmission occurs at a larger scale in real-life healthcare settings, involving wild-type species and organisms of varying drug-resistances, such as those that have extended-spectrum β-lactamase (ESBL), TEM and SHV, or AmpC resistance determinants.
Our baseline microbiological samples from enrolled patients provided a representative glimpse into the prevalence of MDRO colonization in our subjects. Indeed, 11.3% of the enrolled patients were asymptomatically colonized with at least 1 type of MDRO, a finding consistent with other studies.11 This prevalence underscores the importance of understanding the local antimicrobial susceptibility patterns of organisms to guide appropriate and effective antibiotic choice.
The baseline microbiological cultures from environmental sources showed that 55% of hospital patient rooms still had at least 1 surface with detectable microbial growth of MDROs at time of patient admission despite terminal disinfection procedures. The average level of surface contamination was low but was clearly sufficient for documented transmission to patients. These environmental microbiological data from our study add weight to recent investigations showing that the carrier status of a room’s prior occupant can increase risk of MDRO acquisition for the subsequent occupant.12, 13 These results support the urgency of investigating and implementing enhanced terminal-cleaning procedures to further reduce residual microbial contamination during patient room turnover and to minimize the risk of bacterial transmission.4 Finally, these findings occurred despite concurrent application of enhanced terminal room disinfection strategies and high rates of compliance of surface cleaning.4
Our observation of low-level bacterial contamination after terminal cleaning highlights another important limitation in current literature; there is no consensus method for assessing or defining a surface as “clean.”14 The establishment of a definition or target of “clean” surface is difficult, but it is needed for future technologies and real-world interventions to reduce risk for pathogen transmission.14
Throughout the hospital stay, we observed that hospital room surfaces became contaminated with MDROs. We hypothesized that these new environmental isolates were introduced during the hospitalization through one or a combination of the following sources: importation through healthcare staff, contaminated fomites brought into the room (eg, trays, medical equipment, etc.), visitors, or unmasking of prior bacterial colonization of the patient through triggers such as antibiotic selection pressure. Where bacterial-transmission events were noted, most occurred early (within 3 days) into the admission of a newly cleaned room. Furthermore, bacterial transmission from the environment resulted in both asymptomatic carriage and symptomatic infection among the patients. The early transmission of MDRO between the environment and patient is an important observation and points to the opportunity for development of effective prevention strategies of bacterial transmission.15, 16
Our study was limited by the modest number of patients and rooms we could feasibly enroll and study using microbiological and molecular techniques. The representativeness of our study of usual clinical practice was also potentially lowered because the study targeted rooms that previously housed patients on contact precautions. Second, our microbiological sampling was not always timed to occur before daily cleaning by environmental services staff; thus, some surfaces may have been freshly cleaned prior to sampling. Furthermore, we recognize that external vectors could introduce organisms to the hospital room environment and the patient throughout the study period (eg, healthcare staff or visitors). However, other factors also counterbalance these external forces and reduce detection of transferred organisms, such as treatment with concurrent antibiotic and/or a high hand hygiene performance rate that is greater than published literature (>90% compliance).17 We believe that these limitations suggest that our findings represent the minimum impact of the environment on acquisition of MDROs. Furthermore, this endeavor represents the largest prospective study to confirm and quantify clonal bacterial transmission between hospitalized patients and environmental surfaces using molecular techniques. Our microbiological methods were important for distinguishing between potential and definitive transmission events.
These findings have several important implications for future studies and interventions. The observed transmission of selected MDROs are markers of larger-scale bacterial admixing between the microbial flora of the hospital environment and that of the patient. If microbial transmission occurs early, readily, and frequently between patients and the environment, as shown in the study, the standard hospital cleaning practice of performing a detailed room disinfection only at the end of patient stay (ie, “terminal” cleaning) may be inadequate to prevent the acquisition of MDROs through the environment. Indeed, these results should compel us to develop new technologies and interventions to achieve safe continuous environmental disinfection within the healthcare setting. Future effort and research to reduce transmission of MDROs through the healthcare environment must improve upon the status quo approach to environmental disinfection.
Supplementary Material
Financial support
The current study was supported by the US Centers for Disease Control and Prevention (CDC) as part of the Duke University–University of North Carolina Prevention Epicenters Program (grant no. U54CK000164 to Dr Sexton), the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID grant no. K23AI095357 to Dr Anderson). The views expressed in this article are those of the authors and do not represent the views of the CDC or the NIH.
Footnotes
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Footnote: PREVIOUS PRESENTATION: This study was presented in part at the IDWeek 2015 Conference on October 9th, 2015, in San Diego, California.
References
- 1.Weber DJ, Rutala WA. Understanding and preventing transmission of healthcare-associated pathogens due to the contaminated hospital environment. Infect Control Hosp Epidemiol 2013;34:449–452. [DOI] [PubMed] [Google Scholar]
- 2.Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis 2013;26:338–344. [DOI] [PubMed] [Google Scholar]
- 3.Harris AD. How important is the environment in the emergence of nosocomial antimicrobial-resistant bacteria? Clin Infect Dis 2008;46:686–688. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DJ, Chen LF, Weber DJ, et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet 2017;389:805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mermel LA, Cartony JM, Covington P, Maxey G, Morse D Methicillin-resistant Staphylococcus aureus colonization at different body sites: a prospective, quantitative analysis. J Clin Microbiol 2011;49:1119–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitterman Y, Laor A, Itzhaki S, Weber G Characterization of the best anatomical sites in screening for methicillin-resistant Staphylococcus aureus colonization. Eur J Clin Microbiol Infect Dis 2010;29:391–397. [DOI] [PubMed] [Google Scholar]
- 7.Mahida N, Boswell T Improving the detection rate of vancomycin-resistant enterococci colonisation using groin swabs. J Clin Pathol 2014;67:544–545. [DOI] [PubMed] [Google Scholar]
- 8.Gordon NC, Wareham DW. Evaluation of CHROMagar Acinetobacter for detection of enteric carriage of multidrug-resistant Acinetobacter baumannii in samples from critically ill patients. J Clin Microbiol 2009;47:2249–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huslage K, Rutala WA, Sickbert-Bennett E, Weber DJ. A quantitative approach to defining “high-touch” surfaces in hospitals. Infect Control Hosp Epidemiol 2010;31:850–853. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DJ, Gergen MF, Smathers E, et al. Decontamination of targeted pathogens from patient rooms using an automated ultraviolet-C-emitting device. Infect Control Hosp Epidemiol 2013;34:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Agata EM, Horn MA, Ruan S, Webb GF, Wares JR. Efficacy of infection control interventions in reducing the spread of multidrug-resistant organisms in the hospital setting. PLoS One 2012;7:e30170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell BG, Dancer SJ, Anderson M, Dehn E Risk of organism acquisition from prior room occupants: a systematic review and meta-analysis. J Hosp Infect 2015;91:211–217. [DOI] [PubMed] [Google Scholar]
- 13.Shaughnessy MK, Micielli RL, DePestel DD, et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol 2011;32:201–206. [DOI] [PubMed] [Google Scholar]
- 14.Han JH, Sullivan N, Leas BF, Pegues DA, Kaczmarek JL, Umscheid CA. Cleaning hospital room surfaces to prevent health care-associated infections: a technical brief. Ann Intern Med 2015;163:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium ifficile strains among long-term care facility residents. Clin Infect Dis 2007;45:992–998. [DOI] [PubMed] [Google Scholar]
- 16.Muto CA. Asymptomatic Clostridium difficile colonization: Is this the tip of another iceberg? Clin Infect Dis 2007;45:999–1000. [DOI] [PubMed] [Google Scholar]
- 17.Chen LF, Carriker C, Staheli R, et al. Observing and improving hand hygiene compliance: implementation and refinement of an electronic-assisted direct-observer hand hygiene audit program. Infect Control Hosp Epidemiol 2013;34:207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.