INTRODUCTION
Virus must engage with host cells to replicate and spread, which warrants profound changes in the cellular signaling pathways, especially the instigation of host immune responses. Host defenses are complex systemic immune responses at different levels, including intrinsic, innate and adaptive immunity. Recent studies show_that the tripartite motif (TRIM) containing proteins play a versatile role in all layers of host immune defenses. TRIM proteins are a family of ubiquitin E3 ligases which share a characteristic N-terminal tripartite motif (also known as RBCC motif) consisting of a highly conserved order of three domains: the really interesting new gene (RING) domain, one or two B-box domains and a coiled coil region [1]. The RING domain confers TRIM proteins the ubiquitin E3 ligase activity. Ubiquitination is a post-translational modification that alters target protein stability, trafficking, subcellular localization, enzymatic activation and protein recruitment [2]. TRIM proteins have been found to promote host immune response by ubiquitination of critical signaling molecules in immunity and interfere with viral infection by directly targeting viral proteins for degradation.
TRIM proteins are known for a long time to participate in various biological processes, such as development and carcinogenesis; however, we are just now discovering their importance in recent years. Notably, the study of TRIM proteins in host defense is one of the fastest growing subjects in the field of immunity. Thus, systematic and comprehensive understanding of the role of TRIM proteins in multiple layers of host defense will not only help elucidate the antiviral mechanisms of TRIM proteins but also provide potentials for developing new antiviral therapeutics. In this review, we focus on the role and the antiviral mechanisms of TRIM proteins in each layer of host immune defense, elaborate the viral evasion strategy by targeting TRIM proteins and discuss the future study direction.
TRIM PROTEIN STRUCTURE, CLASSIFICATION, AND EXPRESSION
TRIM protein family consists of 76 members, which all share the RBCC motif. RBCC domain comprises three motifs which are RING, B-box and coiled coil [3]. The RING is a 40–60 amino acid long zinc finger motif, which is essential for the ubiquitin E3 ligase activity and the binding with the E2 conjugating enzyme. RING domain plays a crucial role in TRIM proteins-mediated ubiquitination. B-box domain presents in either single or paired form and plays a role in self-assembly of TRIM proteins and interaction with their substrates. Coiled coil is a common hyper-helical structure that involves protein-protein interaction, including protein dimerization. Similarly, the coiled coil of TRIM proteins mediates dimerization and self-association of TRIM proteins. Additionally, the coiled coil sequesters TRIM proteins in the cytoplasm or the nuclear bodies [4].
The RBCC motif is followed by one or more domains located on the C-terminus of TRIM proteins. The TRIM superfamily is classified into eleven groups according to the C-terminal domains (Fig. 1). Group 1 is featured with multiple domains, including the C-terminal subgroup one signature (COS), fibronectin type 3 (FN3) and SPRY domain. COS box domain is responsible for microtubule binding. FN3 binds heparin and DNA. The SPRY domain (also termed as B30.2) is involved in protein-protein interaction and RNA binding. Group 2 has a single COS domain whereas Group 3 has both COS and FN3 domains. Group 4 is the largest subfamily of TRIM proteins characterized by a SPRY domain at the C terminus. Group 5 has no defined motif in the C-terminal region. Group 6 has the PHD-BROMO domains while group 7 possesses the NHL repeats. Bromodomains bind acetylated lysine residue on the N-terminal of histones and repress transcription. NHL repeats mediate protein-protein interaction. Groups 8–10 are featured with MATH, ARF and filamin domain, respectively. Filament type immunoglobulin domains are responsible for dimerization and actin crosslinking whereas ADP ribosylation factor-like (ARF) domains stimulate vesicular trafficking. MATH domain is essential for receptor-TRAF protein interaction. Group 11 comprises a transmembrane domain (TM) in their C-terminal region. Taken together, the variable C-terminal domains of TRIM proteins contribute to the versatile binding ability to their substrate.
Figure 1. The schematics of the subgroups of TRIM proteins.
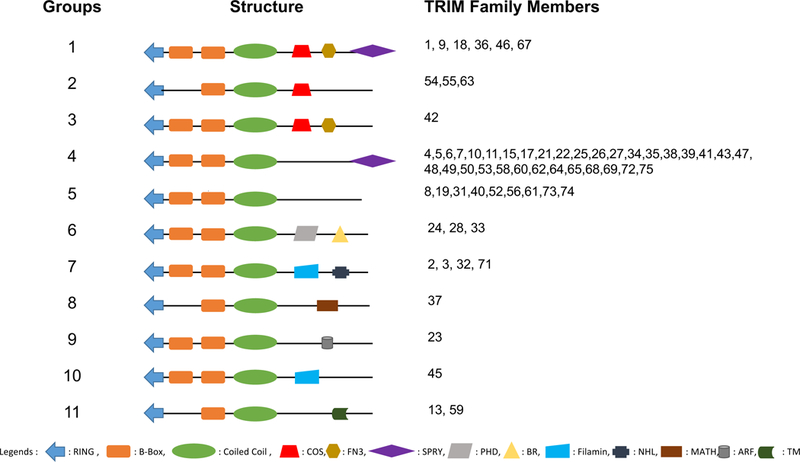
Each number in the column of TRIM Family Members is corresponding to the designated TRIM protein number. For example, 1 stands for TRIM1.
TRIM proteins are found in many types of cells, such as immune cells, epithelial cells, endothelial cells, fibroblasts, and neuronal cells. Interestingly, TRIM proteins are expressed differentially in B-cells, T-cells, dendritic cells, mast cells, NK-cells, and macrophages, suggesting a regulatory mechanism for TRIM. In addition, TRIM proteins show distinct subcellular localizations. Many of them form cytoplasmic bodies when overexpressed. Mutations in the RING, coiled coil domain or B-box have been reported to cause aberrant localization. More importantly, viral infection results in cytosolic TRIM proteins trafficking into the nucleus [5].
TRIM PROTEINS AND UBIQUITINATION
Ubiquitin is a highly conserved and ubiquitously expressed 76-amino acid protein [6]. The ubiquitination process is a reversible covalent conjugation of ubiquitin to substrate by a three-stepwise enzymatic system (Fig. 2). In the first step, ubiquitin is activated by a ubiquitin-activating enzyme (E1) in an ATP-dependent reaction, in which the C-terminus of ubiquitin links to the E1 via a thioester bond. In the second step, the activated ubiquitin is transferred to a ubiquitin-conjugating enzyme (E2), forming an E2-Ub thioester. The E2 then co-opts with the ubiquitin ligase (E3) to access to the substrate protein. The E2-E3 enzyme complex conjugates the ubiquitin to the substrate via an isopeptide bond between the C-terminal glycine of ubiquitin and the lysine of the substrate protein. To date, two E1s (UBA1 and UBA6), about 35 E2s and more than 600 E3 ligases have been identified in humans, suggesting a single E2 can interact with multiple E3s. Regarding TRIM proteins, the Cys3-His-Cys4 zinc finger in the RING domain binds several E2s, including UBE2D and UBE2E.
Figure 2. The process of ubiquitination.
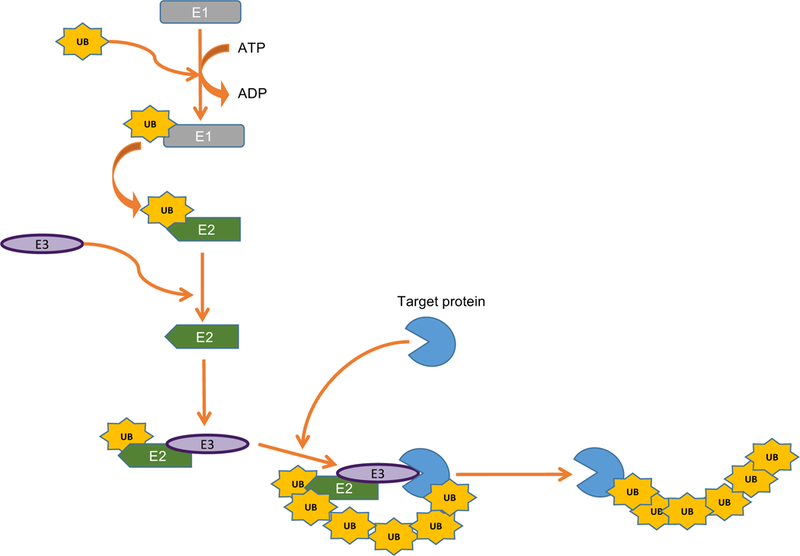
Ubiquitin is first activated by a ubiquitin-activating enzyme (E1) in an ATP-dependent reaction. The activated ubiquitin is then transferred to a ubiquitin-conjugating enzyme (E2). Subsequently, the E2-E3 enzyme complex conjugates one or more ubiquitin proteins to the substrate.
The ubiquitin moiety can be conjugated to the substrate by a single molecule (mono-ubiquitination) or by adding polyubiquitin chain (polyubiquitination). The polyubiquitin chains are grouped into different linkages determined by the conjugation via one of the seven lysines (K6, K11, K27, K29, K33, K48, K63) between ubiquitins. The linkage of polyubiquitin can influence the fate of the substrate, adding another layer of complexity to the outcome of ubiquitination. The majority of TRIM proteins mediate either K48- or K63-linked polyubiquitination of target proteins. The K48-linked ubiquitination usually directs proteins for proteasome-dependent degradation while the K63-linked ubiquitination involves non-proteolytic processes, such as subcellular localization, signalosome stability and activation, and trafficking. For example, TRIM32 targets influenza PB1 protein for K48-linked polyubiquitination, which results in PB1 protein degradation and viral restriction [7]. In contrast, TRIM25 mediates K63-linked ubiquitination of RIG-I, which facilitates RIG-I multimerization and activation [8].
Although most TRIM proteins are ubiquitin E3 ligases, several TRIM proteins can act as an E3 ligase for ubiquitin-like molecules, such as SUMO and ISG15. For instance, TRIM28 mediates sumoylation of IRF7, which leads to inhibition of IRF7 transactivation and IFN expression [9]. It is also reported that TRIM25 acts as an ISG15 E3 ligase for 14–3-3 sigma protein; however, the biological consequence is not clear [10]. These TRIM proteins, including TRIM25 and TRIM28, are well-known ubiquitin E3 ligases. But how these TRIM proteins work on two or more different post-translational modifications is unknown. Future direction of this field is to pinpoint the underlying molecular mechanisms, which will deepen our understanding of the role of TRIM proteins in host defense.
ROLE OF TRIM PROTEINS IN HOST DEFENSE
1. TRIM proteins and intrinsic immunity
Intrinsic immunity is the instant antiviral response by a group of constitutively expressed host factors through diverse mechanisms. The “preexisted” expression of these host factors guarantees the rapid and direct inhibition of viruses at almost every step of viral life cycle. A group of TRIM proteins belongs to these intrinsic factors that have been reported to curb viral infection through direct interaction with viral proteins. These TRIM proteins employ distinct mechanisms to inhibit viral entry, replication or dissemination. The intrinsic antiviral mechanism of each of these TRIM proteins will be discussed below (Table 1).
Table 1.
Summary of the role of TRIM proteins in intrinsic immunity
| TRIM protein | Mode of action | Cell line | Method | Reference |
|---|---|---|---|---|
| TRIM5α | SPRY domain ofTRIM5α interacts with the capsid of HIV and restricts the viral uncoating and replication. | HT1080 CELLS | overexpression | 13 |
| TRIM11 | TRIM11 restricts HIV-1 reverse transcription by accelerating premature viral uncoating | HEK293, THP-1 | overexpression, RNAi | 14 |
| TRIM14 | RINGless TRIM14 interacts with HCV NS5A protein through SPRY domain and mediates its k48 polyubiquitination and degradation. | JFH,THP-1,HEK293,A549,Huh-7 | overexpression, RNAi, CRISPR | 32 |
| TRIM19 | TRIM19/PML blocks HIV viral replication at two distinct steps. First is at early post entry stage and it also causes silencing of trancription | HEK293,MEF and CRFK cells | overexpression, RNAi, CRISPR | 17 |
| TRIM22 | TRIM22 blocks the intracellular trafficking of the HIV viral structural protein Gag to the surface of the cell through its E3 ligase activity | HOS-CD4/CXCR4 cells, HeLa,HEK293 | overexpression, RNAi | 16 |
| TRIM22 | TRIM22 interacts with class II interactor and sp1, thus inhibits HIV viral gene transcription initiation and elongation | HEK293T,COS-1,HeLa, U937 and macrophages | overexpression, RNAi | 18 |
| TRIM22 | TRIM22 tragets Influenza nucleoprptein and mediates its k48 polyubiquitination and degradation | HEK293, A549,MDCK | overexpression, RNAi | 20 |
| TRIM22 | TRIM22 inhibits the activity of HBV core promoter (CP) by interacting throgh C-terminal SPRY domain and acting as a E3 ligase. | HeLa,HepG2 Cells | overexpression | 23 |
| TRIM22 | TRIM22 interacts with HCV NS5A protein through SPRY domain and mediates its k48 polyubiquitination and degradation | HEK293T, Huh-7 | overexpression, RNAi | 24 |
| TRIM22 | TRIM22 interacts with the viral 3C protease (3C(PRO)) of EMCV and mediates its ubiquitination and degradation | HEK293,HeLa | overexpression | 25 |
| TRIM25 | TRIM25 inhibits viral RNA synthesis results from its binding to viral ribonucleoproteins (vRNPs) of Influenza. | CRFK,A549 | Overexpression, CRISPR | 22 |
| TRIM28 | TRIM28 binds to acetylated integrase and indced deacetylation of it which restricts integration of HIV viral cDNA into the host genome | HEK293,HeLa | overexpression, RNAi | 15 |
| TRIM32 | TRIM32 interacts with the PB1 of the Influenza RNA polymerase complex and mediates its ubiquitination and proteasomal degradation | HEK293,A549, MEF | overexpression, RNAi, CRISPR | 7 |
| TRIM41 | TRIM41 tragets Influenza nucleoprptein through its SPRY domain and mediates its k48 polyubiquitination and degradation | HEK293,A549 | overexpression, RNAi, CRISPR | 19 |
| TRIM52 | TRIM52 act against Japanese Encephalitis Virus infection by targeting and degrading viral NS2A through ubiquitination | BHK21,HEK293T | overexpression | 33 |
| TRIM56 | TRIM56 inhibits BVDV replication by targeting an intracellular viral RNA replication | HEK293,Huh-7,THP-1, | overexpression, RNAi | 27 |
| TRIM56 | TRIM56 restricts YFV and DENV2 through E3 ligase activity and c-terminal domain interaction | HEK293, HeLa, mosquito C6/36 | overexpression, RNAi | 28 |
| TRIM56 | TRIM56 specifically impede intracellular influenza virus RNA synthesis without acting as a E3 ligase. | HEK293, MDBK | overexpression, RNAi | 21 |
1.1. TRIM proteins-mediated intrinsic immunity to HIV-1
TRIM5α is the prototype of TRIM proteins in intrinsic immunity. TRIM5α is a well-known cytosolic host restriction factor to the cross-species transmission of retroviruses, which protects the Old World monkeys including rhesus macaques from infection of HIV-1 [11]. The restriction specificity of TRIM5α is dependent on its C-terminal PRY/SPRY domain that mediates the interaction with HIV-1 capsid [12]. On the HIV-1 capsid surface, TRIM5 proteins assemble into a hexagonal net with the SPRY domains centered on the edges and the B-box and RING domains at the vertices. The conformational flexibility of the hexagonal net allows TRIM5α to accommodate the variable curvature of retroviral capsids. TRIM5α-capsid interaction results in premature uncoating, thereby halting reverse transcription and transport to the nucleus of the viral genome [12] (Fig. 3). The ubiquitin E3 ligase activity of TRIM5α is required for restriction; however, TRIM5α-mediated ubiquitination of HIV-1 capsid has not been detected. TRIM5α also binds and restricts N tropic mouse leukemia virus (N-MLV) [13], but the precise mechanism is yet to be determined. In addition, it also reported that TRIM5α inhibits retroviral infection by activating innate immune signaling. We discuss it in the below section of TRIM proteins and Innate Immunity.
Figure 3. The role of TRIM proteins in intrinsic immunity.
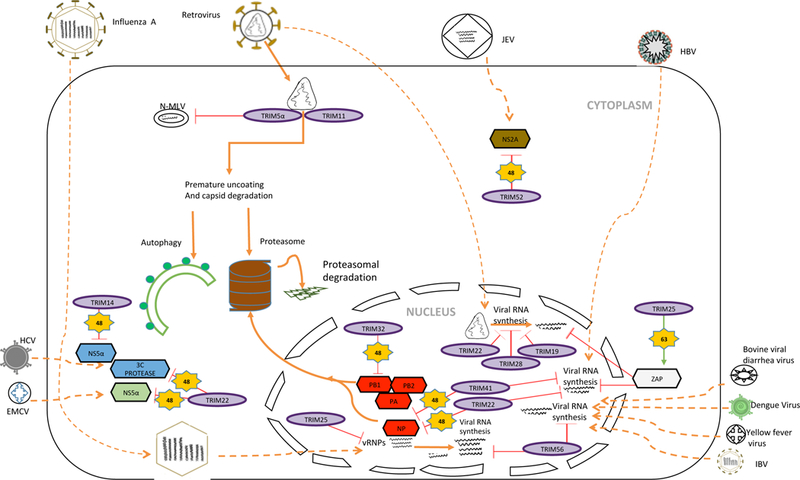
This figure illustrates that TRIM proteins employ distinct mechanisms to inhibit viral entry, replication or dissemination by interacting with viral proteins.
Several other TRIM proteins also exhibit intrinsic anti-retroviral activity, including TRIM11 [14], TRIM28 [15], TRIM22 [16] and TRIM19 [17] (Fig. 3). Like TRIM5α, TRIM11 also interacts with HIV-1 capsid. Similarly to TRIM5α, TRIM11 restricts HIV-1 reverse transcription by promoting premature viral uncoating [14]. TRIM28 (also known as KAP1) limits HIV-1 by binding the acetylated integrase [15]. The acetylation of integrase is essential for the integration of HIV-1 cDNA into the host genome. TRIM28 mediates the deacetylation of viral integrase through the formation of a protein complex that includes the deacetylase HDAC1 [15]. TRIM22 restricts HIV infection by two distinct mechanisms. First, TRIM22 controls the trafficking of the gag protein to the plasma membrane and interferes with the assembly of new viral particles [16]. Secondly, TRIM22 co-opts with class II transactivator (CIITA) and Sp1 to inhibit viral gene transcription initiation and elongation [18]. TRIM19, also known as promyelocytic leukemia protein (PML), restricts the HIV-1 by reducing the reverse transcription products in the host and interfering with viral transcription [17]. It is not surprising that multiple TRIM proteins restrict HIV-1 as each TRIM protein employs a different mode of action. However, most work is based on cell lines and lack of animal models. Further animal work will elucidate the in vivo role of these TRIM proteins.
1.2. TRIM proteins-mediated intrinsic immunity to influenza A virus
Similar to HIV-1, influenza A virus (IAV) is targeted by multiple TRIM proteins. TRIM32 and TRIM41 are host intrinsic immune factors to IAV (Fig. 3). TRIM32 senses and targets IAV PB1 polymerase protein for ubiquitination and protein degradation [7]. TRIM41 inhibits the IAV by its interaction with viral nucleoprotein through the SPRY domain, which leads to K48-linked polyubiquitination and subsequent protein degradation [19]. TRIM22, an interferon-stimulated gene, also ubiquitinates and degrades IAV nucleoprotein[20] (Fig. 3). TRIM56 inhibits the replication of influenza viruses by specifically targeting viral RNA synthesis through its C-terminal tail [21] (Fig. 3). Furthermore, TRIM25, a critical TRIM in innate immunity (discussed in below section), inhibits IAV through a direct mechanism that is independent of its ubiquitin ligase activity and the interferon pathway. TRIM25 binds IAV ribonucleoproteins and inhibits viral RNA synthesis [22] (Fig. 3). These recent studies show that TRIM proteins either target viral proteins for protein degradation or inhibit viral RNA synthesis. It is clear that TRIM proteins mediate viral protein degradation through K48-linked polyubiquitination; however, the mechanism for the inhibition of viral RNA synthesis is elusive. As the ubiquitin E3 ligase activity is dispensable [22], these TRIM proteins might disrupt IAV polymerase complex by directly binding NP or other subunit of viral RNP.
1.3. TRIM proteins-mediated intrinsic immunity to other viruses
In addition to IAV, TRIM22 restricts many other viruses (Fig. 3). First, TRIM22 inhibits HBV by suppressing the core promoter responsible for viral pre-genomic RNA synthesis [23]. Secondly, TRIM22 also restricts hepatitis C virus (HCV) by targeting NS5α for ubiquitination and degradation [24]. Lastly, TRIM22 inhibits encephalomyocarditis virus (EMCV) by targeting viral 3C protease for ubiquitination and degradation [25]. These studies indicate that TRIM22 is a broad-spectrum antiviral protein by mediating K48-linked ubiquitination and subsequent proteasomal degradation of viral proteins; however, whether these viral proteins share a common motif for TRIM22 interaction needs further clarification.
Like TRIM22, several other TRIM proteins are reported to have broad-spectrum antiviral activity (Fig. 3). For example, TRIM19 inhibits HIV-1 (discussed above), VSV, herpes virus and adeno-associated virus [26]. TRIM56 restricts influenza B virus, yellow fever virus and dengue virus by reducing the vRNA levels through its C-terminal domain [27, 28], however, the mechanism is not clear. Additionally, TRIM25 mediates K63-linked ubiquitination of ZAP (zinc finger antiviral protein) to promote ZAP antiviral activity to retroviruses, alphaviruses, filoviruses and HBV [29, 30]. ZAP has been shown to recruit RNA exosome to degrade viral RNA, thereby limiting viral infection, which suggests a general antiviral mechanism.
In addition, several viruses are targeted by TRIM proteins through ubiquitination-mediated protein degradation (Fig. 3). TRIM41 inhibits HBV enhancer II activity, which is dependent on its E3 ubiquitin ligase activity and the integrity of the C-terminal domain [31]. Similarly, TRIM14 inhibits the HCV infection by targeting the NS5α protein for degradation [32]. Japanese encephalitis virus (JEV), another virus from the family Flaviviridae, is inhibited by TRIM52. TRIM52 interacts with the nonstructural protein 2A of JEV and mediates NS2A ubiquitination and protein degradation [33].
2. TRIM proteins and innate immunity
Innate immune responses are triggered by exogenous microbial products that are common to many pathogens but are absent in the host [34]. These microbial products, also known as pathogen-associated molecular patterns (PAMPs), engage with the pattern-recognition receptors (PRRs) which either present on the cell surface or inside the cells [34]. The engagement activates a series of signal cascades to stimulate the expression of a cohort of chemokines, cytokines, and genes critical for host defense. The PRRs consist of several subgroups, including Toll-like receptor (TLR), RIG-I-like receptor (RLR), cyclic GMP-AMP synthase (cGAS) and NOD-like receptor (NLR). TLR, RLR, and cGAS signaling pathways converge to the hub, TANK-binding kinase 1 (TBK1) and IKK epsilon (IKKε). TBK1 and IKKε phosphorylate the interferon regulatory factors (IRFs). For example, TBK1 phosphorylates serines in the C-terminal domain of IRF3, which triggers the dimerization and nuclear translocation of IRF3. In the nucleus, IRFs form active transcriptional complexes that bind to interferon stimulation response elements (ISRE) and activate type I IFN genes expression [34]. Type I IFN is the master cytokine in innate immunity and induces mRNA expression of > 300 IFN-stimulated genes (ISGs), such as Myxovirus resistance protein 1 (Mx1). Interestingly, some TRIM proteins are ISGs, including TRIM22, TRIM25, and TRIM5. In this section, we discuss the role of TRIM proteins in the following innate immune signaling pathways (Table 2).
Table 2.
Summary of the role of TRIM proteins in innate immunity
| TRIM protein | Mode of action | Cell line | Method |
|---|---|---|---|
| TRIM4 | TRIM4 induces the Lys 63-linked ubiquitination of CARD domain RIG-I, which is crucial for the cytosolic RIG-I signalling pathway | HEK293,THP-1,U937 | Overexpression, RNAi |
| TRIM5 | TRIM5 couples innate viral sensing and CD8+ T-cell activation to increase species barriers against retrovirus infection. | U937,HLA-B*2705-restricted CD8+ T | Overexpression |
| TRIM6 | TRIM6 synthesize unanchored K48-linked poly-ubiquitin chains, which activate IKKε for subsequent STAT1 phosphorylation. | HEK293T,A549,Human DCs | Overexpression, RNAi |
| TRIM11 | TRIM11 inhibits RIG-I-mediated IFNβ production by targeting the TBK1 signaling complex. Interaction is dependent on CC domain of TRIM11 | HEK293, HeLa | Overexpression, RNAi |
| TRIM13 | TRIM13 enhances RIG-I activation and positively regulate signaling pathway | HEK293,A549 | overexpression |
| TRIM13 | TRIM13 regulates the type I IFN response through inhibition of MDA5 activity | HEK293,MEF,L929,BMDM | Knockout, overexpression |
| TRIM14 | TRIM14 interacts with MAVS through its SPRY domain and undergoes k63 linked autoubiquitination and recruits NEMO to the MAVS complex. | HEK293,A549,HeLa | Overexpression, RNAi |
| TRIM21 | The IRF association domain of IRF5 interacts with TRIM21 via its PRY/SPRY domain and mediates its K48 and k63 ubiquitination & regulation. | HEK293T,HEK293-TLR7,TBH-1 | Overexpression |
| TRIM21 | TRIM21 interacts with IRF3 through its SPRY domain and inhibits IRF3 ubiquitination and positively regulates it. | HEK293T, HEK293 | Overexpression, RNAi |
| TRIM21 | TRIM21 acts as a E3 ligase and mediates Monoubiquitination of IKKβ Down-regulates NF-κB Signalling | HEK293T, HEK293, HeLa | Overexpression |
| TRIM21 | TRIM21 interacts with Fc fragment of swine immunoglobulin G (sFc) fused VP1 of FMDV and thereby causing its K48-linked degradation. | PK-15,BHK21,HEK293 | Overexpression |
| TRIM23 | TRIM23 through its ARF domain Interacts with NEMO resulting in its K27-linked ubiquitination and positively regulate downstream pathway | HEK293,HeLa,MEF | Overexpression, RNAi |
| TRIM24 | T-Cell–Intrinsic TRIM24 Is Required for IL-1β–Mediated Activation of TH2 Cells | CD4+ T cells | Knockout, overexpression |
| TRIM25 | TRIM25 induces the Lys 63-linked ubiquitination of CARD domain RIG-I, which is crucial for the cytosolic RIG-I signalling pathway | HEK293T, MEF and Hela | Overexpression, RNAi |
| TRIM25 | Short, Unanchored, K63-Linked Ubiquitin Chains mediated by TRIM25 Activate RIG-I | HEK293T | overexpression |
| TRIM25 | TRIM25 mediates k48 linked polyubiquitination of MAVS and positively regulates IRF3 pathway through regulating oligomerization | HEK293, MEF,HeLa | Overexpression, RNAi |
| TRIM26 | Autoubiquitination of TRIM26 helps TBK1-NEMO interaction, and recruits TBK1 to the MAVS signalsome. | HEK293, THP-1,HeLa, MEF | Overexpression, RNAi |
| TRIM27 | TRIM27 negatively regulates signaling involved in the antiviral response and inflammation by targeting the IKKs | HEK293 | Overexpression, RNAi |
| TRIM27 | TRIM27 mediates K-48 polyubiquitination of PI3KC2β, leading to a decrease in PI3K activity and negatively regulate CD4 T cells | Jurkat-KCa3.1 T, human CD4 T | overexpression, knockout mice |
| TRIM27 | TRIM27 through its SPRY domain interacts with NDB domain of NOD2 and targets it for K48-linked ubiquitination proteasomal degradation | HEK293.HeLa,SW480 | Overexpression, RNAi |
| TRIM28 | TRIM28 acts as a SUMO E3 ligase and negatively regulates IRF7 by SUMOylation | HEK293,HEK293T,A549 | Overexpression, RNAi |
| TRIM29 | TRIM29 induces NEMO ubiquitination through K-48 linkage which results in proteasomal degradation and supression of downstream pathway | MH-S,HEK293 alveolar macrophages | Knockout, overexpression, RNAi |
| TRIM29 | TRIM29 targets STING for K48 ubiquitination and degradation and negatively regulates STING dependent pathway | HEK293, BMDM, MEF,BMDC | Knockout, overexpression, RNAi |
| TRIM30α | TRIM30α interacts with TAK1-TAB2-TAB3 and promotes the k48 linked ubiquitination of degradation of TAB2 and TAB3 thus supressing signaling | J774, HEK293T, HeLa,BMDM | Knockout, overexpression, RNAi |
| TRIM30α | TRIM30α prevents TRAF6 autoubiquitination, diminishes IκBα phosphorylation and negatively regulates the NF-kB pathway | J774, HEK293T, HeLa,BMDM | Knockout, overexpression, RNAi |
| TRIM30α | TRIM30α promotes the degradation of STING via K48-linked ubiquitination and negatively regulates STING dependent pathway | HEK293,MEF,L929,BMDC,D2SC | CRISPR, overexpression, RNAi |
| TRIM30α | TRIM30 negatively regulates NLRP3 inflammasome activation by modulating and inhibiting ROS production | J774, HEK 293T, BMDM | Knockout, overexpression, RNAi |
| TRIM31 | TRIM31 interacted with MAVS and catalyzed the Lys63 (K63)-linked polyubiquitination and regulates downstream pathway positively | HEK293,MEF | Knockout, overexpression, RNAi |
| TRIM31 | TRIM31 directly binds to NLRP3, promotes K48-linked polyubiquitination and proteasomal degradation of NLRP3. | HEK293T,THP-1 | Knockout, overexpression, RNAi |
| TRIM32 | TRIM32 targets STING for K63-linked ubiquitination through its E3 ligase activity, which promoted the interaction of STING with TBK1. | HEK293, THP-1 | Overexpression, RNAi |
| TRIM33 | TRIM33 targets DHX33 for K63 linked ubiquitination which activates the formation of the DHX33-NLRP3 inflammasome complex. | HEK293T,THP-1 | Overexpression, RNAi |
| TRIM38 | TRIM38 interacts with and mediates k48 linked ubiquitination and degradation of TRIF and negatively regulates TLR¾ dependent pathway | BMDM,BMDC | Knockout, overexpression |
| TRIM38 | TRIM38 activates MDA5- and RIG-I through their SUMOylation which enhances dephosphorylation and activation and inhibits its degradation | HEK293,MEF,BMDM | Knockout, overexpression, RNAi |
| TRIM38 | TRIM38 targets NAP1 for ubiquitination and subsequent proteasome-mediated degradation and supresses IFNβ response. | HEK293,HEK293 -TLR¾, RAW cell | Overexpression, RNAi |
| TRIM38 | TRIM38 Interacts with TAB2/3 Through Its PRY-SPRY Domain and mediates k48-linked ubiqutination, degradation and translocation | HEK293,HeLa, | CRISPR, overexpression, RNAi |
| TRIM38 | TRIM38 sumoylates STING during the early phase of viral infection, promoting both STING activation and protein stability | HEK293,HEK293T, MEF | Knockout, overexpression |
| TRIM38 | TRIM38 binds to TRAF6 and promotes its K48-linked polyubiquitination and proteasomal degradation thus supresses downstream pathway | HEK293,RAW cells | Overexpression, RNAi |
| TRIM44 | TRIM44 acts as a deubiquitinase enzyme and prevents k48 linked degradation of MAVS positively regulate MAVS dependent pathway | HEK293,HEK293T,L939,HeLa | Overexpression, RNAi |
| TRIM56 | TRIM56 interacts with TRIF through its C-terminal domain and enhances TLR3 pathway in a E3 ligase independent manner. | HEK293,HEK293-TLR3,Vero,Huh-7.5 | Overexpression, RNAi |
| TRIM56 | TRIM56 interacts with STING and targets it for K63-linked ubiquitination which initiates dimerization and facilitates STING -TBK1 interaction | HEK293,HeLa,human lung fibroblast | Overexpression, RNAi |
| TRIM56 | TRIM56 induces the Lys335 monoubiquitination of cGAS which increases of its dimerization, DNA-binding activity, and cGAMP production. | HEK293T, L929, and Vero | Knockout, overexpression |
| TRIM65 | TRIM65 interacts and promotes K63-linked ubiquitination of MDA5 at lysine 743, which is critical for MDA5 oligomerization and activation. | HEK293, BMDM, MEF HeLa | Overexpression, RNAi |
| TRIM9s | TRIM9s undergoes Lys-63-linked auto-polyubiquitination and serves as a platform to bridge GSK3β to TBK1, activation of IRF3 signaling. | HEK293,A549,THP-1 | Overexpression, RNAi |
2.1. TRIM proteins and TLR signaling
TLRs are membrane proteins residing on cell surface or endosomes. The TLRs on endosomes recognize viral nucleic acids [34]. For example, TLR3 detects viral double-strand RNA while TLR7 and TLR8 sense viral single-strand RNA. TLR9 recognizes unmethylated CpG motifs within viral DNA. Although all TLRs activate TBK1/IKKε and downstream IRF signaling, they recruit different adaptors. TLR3 recruits TRIF whereas TLR7, 8 and 9 relay signals through MyD88.
Several TRIM proteins regulate TLR signaling pathways (Fig. 4). TRIM21 (also known as Ro52) has been reported to negatively regulate IFN production by targeting IRFs, including IRF3, IRF7, and IRF5 for ubiquitination and degradation [35]. However, another study presented an opposite result by showing that TRIM21 interferes with the interaction between Pin1 (peptidyl-prolyl cis/trans isomerase, NIMA-interacting 1) and IRF3, thus preventing IRF3 ubiquitination and degradation [36]. It will need further investigation to clarify the role of TRIM21 in IRF3 signaling although these observations are context-dependent. TRIM protein also regulates TLR adaptors. For example, TRIM56 promotes TLR3 activation by interacting with TRIF, but its E3 ligase activity is not required [37]. By contrast, TRIM38 impairs TLR3 signaling by targeting TRIF for proteasomal degradation [38]. The discovery of TRIM protein as a negative regulator of host immune response is important because it represents a new self-control mechanism to prevent over-activation of immune response.
Figure 4. The role of TRIM proteins in innate immunity.
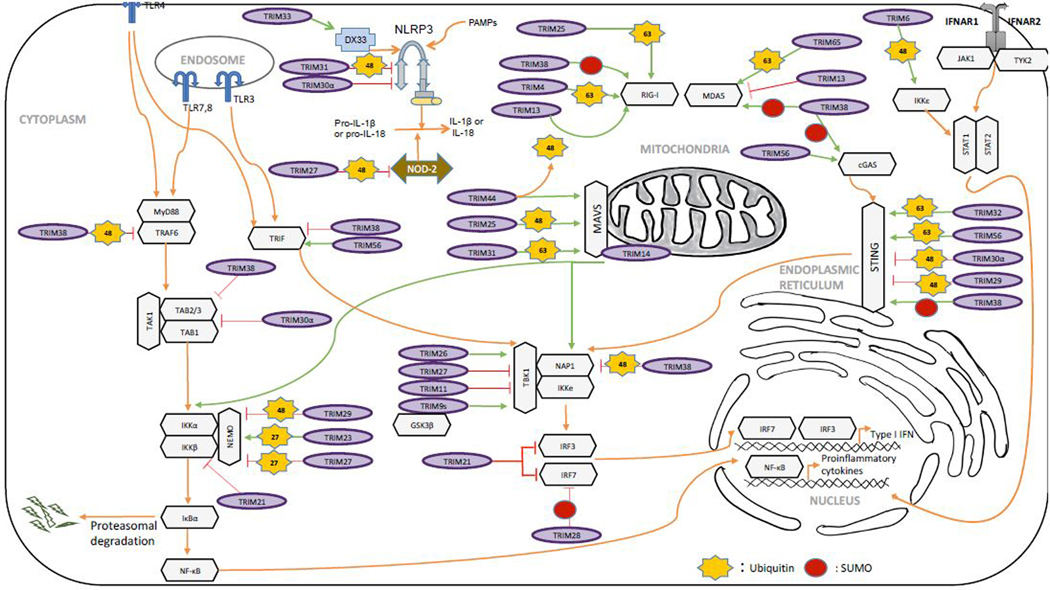
This figure illustrates that TRIM proteins play both positive and negative roles in TLR, RLR and cGAS signaling pathways.
2.2. TRIM proteins and RLR signaling pathway.
The RLRs, including RIG-I, MDA5, and LGP2, recognize the double-stranded RNA (dsRNA) or 5’ triphosphate RNA generated by viral replication of RNA viruses in the cytoplasm. Engagement of viral RNA activates RIG-I and MDA5, which binds the mitochondrial antiviral signaling protein (MAVS) and initiates the TBK1-IRF3 signaling cascade to induce type I IFN expression [34].
2.2.1. TRIM proteins and the RIG-I-like receptors.
TRIM proteins play both positive and negative role in modulating the RLR signaling pathway (Fig. 4). TRIM25 mediates K63-linked ubiquitination of RIG-I [8] and the polyubiquitin chain stabilizes the oligomerization of RIG-I, which promotes MAVS aggregation and subsequent activation. Similarly, TRIM4 conjugates K63-linked polyubiquitin chains the CARD domain of RIG-I [39]. Interestingly, TRIM25 also synthesizes unanchored K63-linked polyubiquitin chain, which helps RIG-I oligomerization, and stability to supports the interaction with MAVS [40]. MDA5, another RLR, also undergoes K63-linked ubiquitination; however, TRIM65 is the cognate ubiquitin E3 ligase. Similar to RIG-I ubiquitination, the K63-linked ubiquitination of MDA5 is critical for MDA5 oligomerization and activation [41]. By contrast, TRIM13 negatively regulate the MDA5-dependent IRF activation although it not clear whether the ubiquitin E3 ligase activity is required [42]. In addition, TRIM38 positively regulates both RIG-I and MDA5 by sumoylation, which prevents K48-linked polyubiquitination and degradation [43]. It is clear that TRIM proteins regulate the activity of the cytosolic RNA sensor, RIG-I and MDA5, however, how these TRIM proteins are regulated upon external signal stimulation, such as RNA virus infection, is elusive. Furthermore, whether there is functional redundancy of these TRIM proteins and how these TRIM proteins coordinate need further investigation.
2.2.2. TRIM proteins and MAVS.
Multiple TRIM proteins have been found to regulate MAVS on the mitochondria with distinct outcomes (Fig. 4). TRIM31 mediates K63-linked polyubiquitination of MAVS and promotes the formation of prion-like aggregates, the active form of MAVS [44]. TRIM14 bridges NEMO to MAVS by the polyubiquitin of TRIM14 to activate IRF3 and NF-kB pathways [45]. Additionally, TRIM44 has been to show to prevent K48-linked polyubiquitination of MAVS [46]. Unexpectedly, one study reported that TRIM25 mediated K48-linked polyubiquitination of MAVS, which permits IRF3 signaling but inhibits NF-kB activity, another branch of MAVS signaling [47]. As TRIM25 is well known as one of the ubiquitin E3 ligases critical for the K63-linked polyubiquitination and subsequent activation of RIG-I, it should require a more careful experimental design to study the role of TRIM25 on MAVS.
2.2.3. TRIM proteins and the TBK1 protein complex.
Several TRIM proteins target the TBK1 kinase complex for IFN regulation (Fig. 4). First, TRIM26 undergoes autoubiquitination upon viral infection and the polyubiquitin of TRIM26 bridges NEMO to TBK1, thus facilitating TBK1 activation [48]. Secondly, the short form of TRIM9 (TRIM9s) also undergoes K63-linked auto-polyubiquitination upon viral infection. The polyubiquitin of TRIM9s serves as a platform to bridge GSK3β to TBK1, leading to the activation of IRF3 signaling [49]. Thirdly, TRIM11 inhibits TBK1 activity by blocking TBK1 interaction with its binding partners, NAP1 and TANK [50]. Lastly, TRIM38 facilitates K48 linked polyubiquitination and degradation of NAP1, resulting in reduced activity of IRF3 [51]. As the TBK1 kinase complex is the hub of various innate immune signaling pathways, whether these TRIM proteins are specific to the RLR signal pathway or also work on other innate signaling pathways need to be clarified in the future.
2.3. TRIM proteins and cGAS signaling
Cytosolic viral DNA is recognized by the recently identified DNA sensor, cyclic GMP-AMP synthase (cGAS, also known as MB21D1) [52]. After binding to DNA, cGAS produces cyclic GMP-AMP (cGAMP). cGAMP is a second messenger and binds to an endoplasmic reticulum membrane protein, the stimulator of interferon genes (STING, also known as TMEM173, MPYS, MITA, and ERIS), which leads the dimerization of STING. Subsequently, STING recruits TBK1 to the endoplasmic reticulum and activates TBK1. Activated TBK1 phosphorylates interferon regulatory factors (IRFs).
TRIM56 and TRIM32 have been reported to catalyze K63-linked polyubiquitination of STING, which is essential for activation of IFNβ expression and restriction of DNA viruses [53, 54] (Fig. 4). In contrast, TRIM30α and TRIM29 promote the degradation of STING via K48-linked ubiquitination through a proteasome-dependent pathway [55, 56] (Fig. 4). Interestingly, TRIM38-mediated sumoylation of STING counteracts the K48-linked ubiquitination of STING and prevents STING from protein degradation [57] (Fig. 4). Similarly, TRIM38 also mediates sumoylation of cGAS, thus preventing cGAS polyubiquitination and degradation [57]. Recently, the ubiquitin E3 ligase for STING, TRIM56, was found to catalyze monoubiquitination of cGAS, which promotes cGAS dimerization, DNA-binding activity, and cGAMP production [58] (Fig. 4). Notably, TRIM38 and TRIM56 regulate both cGAS and STING. However, STING is a membrane protein on the ER while cGAS is a cytosolic and nuclear protein. The subcellular localization of these TRIM proteins and the intracellular trafficking of these proteins are the questions need to be addressed.
2.4. TRIM proteins and NLR signaling
NLRs comprise a family of intracellular sensors that recognize microbial products and mount proinflammatory and antimicrobial immune response. Upon stimulation, NLR forms inflammasomes, a large and highly ordered cytosolic complex, which leads to caspase-mediated proteolytic activation and IL-1β and IL-18 [34]. Several TRIM proteins have been reported to regulate NLR signaling by targeting the signaling proteins for ubiquitination and protein degradation (Fig. 4). For example, TRIM33 binds and ubiquitinates DHX33, a cytosolic receptor upstream of NLRP3. The ubiquitination of DHX33 facilitates inflammasome activation [59]. Coiled Coil domain of TRIM31 interacts with NACHT domain of NLRP3 and mediates K48-linked ubiquitination that leads to the degradation of NLRP3 [60]. TRIM30α negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production [61]. In addition, TRIM27 degrades NOD2 and inhibits the NOD2 receptors during viral infection [62].
2.5. TRIM proteins and other innate immune signaling pathways
The NF-κB transcription factors play a critical role in various antiviral immune signaling pathways [34]. A wide range of soluble and membrane-bound extracellular ligands activate NF-κB, such as LPS and IL-1. For example, TLR4 recruits a series of intermediary adaptors after LPS stimulation. These adaptors further recruit and activate the IKK kinase complex consisting of IKKα, IKKβ, and NEMO. The activated IKK complex phosphorylates IκBα, which leads to IκBα protein degradation and subsequent release and activation of NF-ĸB. These pathways are negatively regulated by several TRIM proteins (Fig. 4). For example, TRIM30α and TRIM38 mediate protein degradation of TAB2/3, the adaptors of TAK1, thus inhibiting TAK1 activity and downstream IKK kinase activation [63, 64]. TRIM21 inhibits the NF-ĸB pathway through mono-ubiquitination of IKKβ [65]; however, the molecular mechanism is not clear. TRIM38 catalyzes K48-linked polyubiquitination of TRAF6 (TNF receptor-associated factor 6) to negatively regulate NF-κB signaling pathway [66]. In addition, TRIM27 inhibits NF-ĸB and IRF3 via interaction with IKK, but the mechanism is not clear [67]. TRIM29 also suppresses both pathways by degradation of NEMO [68]. By contrast, TRIM23 binds NEMO through the C terminal ARF domain and targets NEMO for K27-linked ubiquitination, which leads to increased activation of NF-κB signaling [69]. The mechanism for K27-linked ubiquitination-mediated NEMO activation is unknown. The K27-linked polyubiquitin doesn’t lead to NEMO protein degradation. It is plausible that the K27-linked polyubiquitin provides a signaling platform for the recruitment of the downstream signaling molecules or facilitates NEMO dimerization.
IFN binds IFN receptors to activate JAK (Janus kinase)-STAT (signal transducer and activator of transcription) pathway, which stimulates hundreds of ISGs to execute the antiviral actions. TRIM6 is found to catalyze unanchored K48-linked polyubiquitin chains, which activates IKKε to phosphorylate STAT1 (Fig. 4). The phosphorylation results in the induction of a subset of ISGs essential for the antiviral response [70].
3. TRIM proteins and adaptive immunity
Most TRIM proteins are found to regulate intrinsic and innate immunity; however, there are also several TRIM proteins that regulate adaptive immunity. TRIM5 couples innate viral sensing and CD8+ T-cell activation to increase species barriers against retrovirus infection [71]. TRIM8 is a Th17-specific HIV dependency factor and an important anti-HIV factor [72]. TRIM24 regulates the expression of Th2-type cytokines [73]. TRIM27 inhibits the activation of CD4+ cells through K48-linked polyubiquitination of PI3K-C2β [74]. Additionally, TRIM21 plays an important role in the detection of antibody-opsonized viruses, such as the foot and mouth disease virus (FMDV). TRIM21 binds to the highly conserved Fc region of immunoglobulins and targets the virus for degradation by the proteasome [75]. Overall, there are only a few TRIM proteins are discovered to regulate adaptive immunity because of the limits of research tools. Nonetheless, the list will grow as more animal models for TRIM proteins are generated.
TARGETING TRIM PROTEINS: A NEWLY DISCOVERED VIRAL EVASION STRATEGY
• Targeting TRIM proteins by RNA virus
The arms race against host defense has forced viruses to evolve new strategies for evasion. Thus, it is not a surprise to find some viruses that target the TRIM proteins to dampen host defense. Firstly, several RNA viruses target TRIM25, the regulator of RIG-I. The non-structural protein 1 (NS1) of influenza A virus directly interacts with the coiled coil domain of TRIM25, thereby preventing its multimerization which is essential for the E3 ligase activity. The defective TRIM25 fails to catalyze K63-linked ubiquitination of RIG-I, thus blocking type I IFN expression [76]. Similarly, the nucleoprotein of SARS-associated coronavirus (SARS-CoV), MERS-CoV and the severe fever with thrombocytopenia virus (SFTSV) also interact with TRIM25 and prevent TRIM25-RIG-I interaction, thereby inhibiting RIG-I signaling [77]. The Non-coding RNAs of the PR-2b strain of Dengue virus bind to the host TRIM25 and prevents USP15-mediated deubiquitination, which is important for RIG-I activation [78]. Secondly, TRIM6 is the target of two RNA viruses. The matrix protein of Nipah virus induces TRIM6 degradation to suppress TRIM6-mediated IFN response [79] whereas Ebola virus utilizes TRIM6 to ubiquitinate its VP35 protein, thereby promoting VP35 activity and virus replication [80]. Lastly, several RNA viruses utilize TRIM proteins to suppress type I IFN signaling pathway. The NS5 protein of yellow fever virus interacts with TRIM23, which results in K6-linked polyubiquitination of NS5. The polyubiquitination facilitates NS5 interaction with STAT2, thereby inhibiting type I IFN response [81].
• Targeting TRIM proteins by DNA virus
Like RNA viruses, DNA viruses also target TRIM protein to subvert immune response by several distinct mechanisms. The HBV X protein suppresses TRIM22 mRNA expression by CpG methylation of the 5`-UTR of TRIM22 gene [82]. Epstein-Barr Virus (EBV) targets TRIM28 for phosphorylation at serine residue 824, which impairs the antiviral function of TRIM28 [83]. In addition, several DNA viruses target TRIM proteins for ubiquitination. The ICP0 protein of HSV-1 ubiquitinates and degrades TRIM27, resulting in the inhibition of immune response [84]. Similarly, Gammaherpesvirus MHV-68 degrades TRIM19 [85].
PERSPECTIVES
The co-evolution of host and virus has kept shaping the viral survival strategy and host defense system. Although these mechanisms exist for a very long time, many of them await for discovery, including the antiviral mechanism of the TRIM protein family. A recent systematic screening suggests that more TRIM proteins are involved in host immunity and viral restriction [5]. Despite the increasing knowledge of TRIM proteins, there are several questions needed to be addressed or directions may be explored in the future. First, what are the underlying mechanisms for the broad-spectrum antiviral activity and specificity of TRIM proteins? As discussed above, some TRIM proteins, such as TRIM22, TRIM19, and TRIM56, demonstrate broad-spectrum antiviral activity by inhibition of multiple viruses. However, whether there is a common mechanism for these TRIM proteins is not clear. For example, TRIM22 ubiquitinates different viral proteins and restricts the infection of viruses from different families. It will be interesting to know whether the interacting viral proteins share a common motif for TRIM interaction. Conversely, multiple TRIM proteins may attack the same virus. For example, HIV-1 has been shown to be limited by several TRIM proteins. However, detailed biochemical and genetic analyses are further needed to clarify the functional redundancy among TRIM proteins. Secondly, how TRIM proteins are activated and regulated upon viral infection? Most studies focus on the mechanisms by which TRIM proteins modulate host immunity and viral infection. Little is known about the regulatory mechanisms for TRIM proteins. Elucidation of the regulatory mechanism for TRIM proteins will help develop a novel strategy to boost host immunity to viral infection. Thirdly, the in vivo role of most TRIM proteins is unknown. Recently, several TRIM protein transgenic and knockout mice have been generated. These mice are the valuable tools to study the in vivo role of TRIM proteins. However, most of these mouse models have not been examined for viral infection. Lastly, is there any inherent relevance of the genetic mutations of TRIM proteins to human immune diseases? Genetic mutations in the NHL repeats of TRIM32 cause recessive hereditary muscle disorders, including limb girdle muscular dystrophy 2H (LGMD2H) [86]. Recent genome-wide association (GWA) studies implied a link of a group of TRIM proteins to the susceptibility of neuropsychiatric disorders, developmental diseases, cardiovascular and metabolic diseases [86]. However, whether genetic mutations of TRIM proteins are linked to infectious diseases is not clear. Taken all together, studies in the above directions will open new spectrum in anti-viral research and will provide developing strategy for prevention and control of viral infections.
Executive Summary.
What are TRIM proteins?
TRIM proteins are a family that shares a characteristic N-terminal tripartite motif consisting of a highly conserved order of three domains: RING, B-box, and coiled coil.
TRIM proteins are ubiquitin E3 ligases that target substrates for ubiquitination.
TRIM-mediated ubiquitination regulates protein stability, trafficking, subcellular localization, enzymatic activation, and protein recruitment.
TRIM proteins and intrinsic immunity
Multiple TRIM proteins target viral protein directly to limit DNA and RNA virus infection.
TRIM5a and other TRIM proteins restrict HIV-1 infection.
TRIM32, TRIM41 and TRIM22 limit influenza A virus infection by targeting viral protein for ubiquitination-mediated protein degradation.
TRIM proteins and innate immunity
TRIM proteins regulate TLR, RLR, NLR and cGAS-mediated innate immune signaling pathways.
TRIM25 mediates K63-linked ubiquitination of RIG-I, which facilitates RIG-I oligomerization and subsequent activation.
TRIM56 and TRIM32 mediate K63-linked ubiquitination of STING, which is critical for cytosolic DNA-elicited type I interferon expression.
TRIM proteins and adaptive immunity
TRIM5 couples innate viral sensing and CD8+ T-cell activation to increase species barriers against retrovirus infection.
TRIM27 inhibits the activation of CD4+ cells through K48-linked polyubiquitination of PI3K-C2β.
Targeting TRIM proteins: a newly discovered viral evasion strategy
Influenza NS1 interacts with TRIM25 and blocks TRIM25-mediated RIG-I activation.
The ICP0 protein of HSV-1 ubiquitinates and degrades TRIM27, resulting in the inhibition of immune response.
ACKNOWLEDGMENT
We apologize to all those colleagues whose important work is not cited because of space and reference limitations. This work was supported by NIH grants R01AI141399 (S.L.), R15AI126360 (S.L.), R21AI137750 (S.L.) and P20GM103648 (S.L.), and OCAST grant HR17–045 (S.L.).
REFERENCE
- 1.Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. BioEssays : news and reviews in molecular, cellular and developmental biology 27(11), 1147–1157 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Yau R, Rape M. The increasing complexity of the ubiquitin code. Nat Cell Biol 18(6), 579–586 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Rajsbaum R, García-Sastre A, Versteeg GA. TRIMmunity: The Roles of the TRIM E3-Ubiquitin Ligase Family in Innate Antiviral Immunity. Journal of Molecular Biology 426(6), 1265–1284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reymond A, Meroni G, Fantozzi A et al. The tripartite motif family identifies cell compartments. The EMBO journal 20(9), 2140–2151 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Versteeg Gijs a, Rajsbaum R, Sánchez-Aparicio Maria t et al. The E3-Ligase TRIM Family of Proteins Regulates Signaling Pathways Triggered by Innate Immune Pattern-Recognition Receptors. Immunity 38(2), 384–398 (2013).* This article is the first systematic study of the role of TRIM proteins in innate immunity. By screening using overexpression and RNAi, the authors identified multiple TRIM proteins that have potential roles in innate signaling pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husnjak K, Dikic I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu Rev Biochem 81 291–322 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Fu B, Wang L, Ding H, Schwamborn JC, Li S, Dorf ME. TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase. PLoS Pathog 11(6), e1004960 (2015).* Demonstrates that TRIM32 translocates into the nucleus after influenza infection. In the nucleus, TRIM32 mediates K48-linked ubiquitination of the PB1, which leads to PB1 protein degradation, thereby disrupting the viral polymerase complex and inhibiting viral replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gack MU, Shin YC, Joo CH et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446(7138), 916–920 (2007).** This is the first study showing that TRIM25 mediates K63-linked ubiquitination of RIG-I. The K63-linked ubiquitination is critical for RIG-I activation and host defense to RNA virus infection. [DOI] [PubMed] [Google Scholar]
- 9.Liang Q, Deng H, Li X et al. Tripartite motif-containing protein 28 is a small ubiquitin-related modifier E3 ligase and negative regulator of IFN regulatory factor 7. Journal of immunology (Baltimore, Md. : 1950) 187(9), 4754–4763 (2011).* Demonstrates that TRIM28 is an E3 ligase for SUMOylation of IRF7 and the SUMOylation negatively regulates IRF7 activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou W, Zhang DE. The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. The Journal of Biological Chemistry 281(7), 3989–3994 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427(6977), 848–853 (2004).** This is a milestone paper that shows, for the first time, TRIM5a is a restriction factor for HIV-1. TRIM5a is the prototype for the TRIM proteins that modulate host intrinsic immunity. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JL, Campbell EM, Wu X, Vandegraaff N, Engelman A, Hope TJ. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. Journal of virology 80(19), 9754–9760 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Current biology : CB 15(1), 73–78 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Yuan T, Yao W, Tokunaga K, Yang R, Sun B. An HIV-1 capsid binding protein TRIM11 accelerates viral uncoating. Retrovirology 13(1), 72 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allouch A, Di Primio C, Alpi E et al. The TRIM family protein KAP1 inhibits HIV-1 integration. Cell host & microbe 9(6), 484–495 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Barr SD, Smiley JR, Bushman FD. The interferon response inhibits HIV particle production by induction of TRIM22. PLoS pathogens 4(2), e1000007 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masroori N, Merindol N, Berthoux L. The interferon-induced antiviral protein PML (TRIM19) promotes the restriction and transcriptional silencing of lentiviruses in a context-specific, isoform-specific fashion. Retrovirology 13 19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forlani G, Accolla RS. Tripartite Motif 22 and Class II Transactivator Restriction Factors: Unveiling Their Concerted Action against Retroviruses. Frontiers in immunology 8 1362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patil G, Zhao M, Song K et al. TRIM41-Mediated Ubiquitination of Nucleoprotein Limits Influenza A Virus Infection. Journal of virology 92(16), (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Pietro A, Kajaste-Rudnitski A, Oteiza A et al. TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation. J Virol 87(8), 4523–4533 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Li NL, Shen Y et al. The C-Terminal Tail of TRIM56 Dictates Antiviral Restriction of Influenza A and B Viruses by Impeding Viral RNA Synthesis. J Virol 90(9), 4369–4382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyerson NR, Zhou L, Guo YR et al. Nuclear TRIM25 Specifically Targets Influenza Virus Ribonucleoproteins to Block the Onset of RNA Chain Elongation. Cell host & microbe 22(5), 627–638.e627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao B, Duan Z, Xu W, Xiong S. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located RING domain. Hepatology (Baltimore, Md.) 50(2), 424–433 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Yang C, Zhao X, Sun D et al. Interferon alpha (IFNalpha)-induced TRIM22 interrupts HCV replication by ubiquitinating NS5A. Cellular & molecular immunology 13(1), 94–102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eldin P, Papon L, Oteiza A, Brocchi E, Lawson TG, Mechti N. TRIM22 E3 ubiquitin ligase activity is required to mediate antiviral activity against encephalomyocarditis virus. The Journal of general virology 90(Pt 3), 536–545 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Geoffroy MC, Chelbi-Alix MK. Role of promyelocytic leukemia protein in host antiviral defense. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 31(1), 145–158 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Liu B, Wang N, Lee YM, Liu C, Li K. TRIM56 is a virus- and interferon-inducible E3 ubiquitin ligase that restricts pestivirus infection. Journal of virology 85(8), 3733–3745 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, Li NL, Wang J et al. Overlapping and distinct molecular determinants dictating the antiviral activities of TRIM56 against flaviviruses and coronavirus. Journal of virology 88(23), 13821–13835 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li MM, Lau Z, Cheung P et al. TRIM25 Enhances the Antiviral Action of Zinc-Finger Antiviral Protein (ZAP). PLoS pathogens 13(1), e1006145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng X, Wang X, Tu F, Wang Q, Fan Z, Gao G. TRIM25 Is Required for the Antiviral Activity of Zinc Finger Antiviral Protein. Journal of virology 91(9), (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Guo JT, Wu JZ, Yang G. Identification and characterization of multiple TRIM proteins that inhibit hepatitis B virus transcription. PLoS One 8(8), e70001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Chen Y, Li C et al. TRIM14 inhibits hepatitis C virus infection by SPRY domain-dependent targeted degradation of the viral NS5A protein. Scientific reports 6 32336 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan W, Wu M, Qian S et al. TRIM52 inhibits Japanese Encephalitis Virus replication by degrading the viral NS2A. Sci Rep 6 33698 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 140(6), 805–820 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Lazzari E, Korczeniewska J, Ni Gabhann J, Smith S, Barnes BJ, Jefferies CA. TRIpartite motif 21 (TRIM21) differentially regulates the stability of interferon regulatory factor 5 (IRF5) isoforms. PLoS One 9(8), e103609 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang K, Shi HX, Liu XY et al. TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. Journal of immunology (Baltimore, Md. : 1950) 182(6), 3782–3792 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Shen Y, Li NL, Wang J, Liu B, Lester S, Li K. TRIM56 is an essential component of the TLR3 antiviral signaling pathway. The Journal of biological chemistry 287(43), 36404–36413 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu MM, Xie XQ, Yang Q et al. TRIM38 Negatively Regulates TLR¾-Mediated Innate Immune and Inflammatory Responses by Two Sequential and Distinct Mechanisms. Journal of immunology (Baltimore, Md. : 1950) 195(9), 4415–4425 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Yan J, Li Q, Mao AP, Hu MM, Shu HB. TRIM4 modulates type I interferon induction and cellular antiviral response by targeting RIG-I for K63-linked ubiquitination. Journal of molecular cell biology 6(2), 154–163 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Zeng W, Sun L, Jiang X et al. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 141(2), 315–330 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lang X, Tang T, Jin T, Ding C, Zhou R, Jiang W. TRIM65-catalized ubiquitination is essential for MDA5-mediated antiviral innate immunity. The Journal of experimental medicine 214(2), 459–473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayan K, Waggoner L, Pham ST et al. TRIM13 is a negative regulator of MDA5-mediated type I interferon production. Journal of virology 88(18), 10748–10757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu MM, Liao CY, Yang Q, Xie XQ, Shu HB. Innate immunity to RNA virus is regulated by temporal and reversible sumoylation of RIG-I and MDA5. The Journal of experimental medicine 214(4), 973–989 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu B, Zhang M, Chu H et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor MAVS through Lys63-linked polyubiquitination. Nature immunology 18(2), 214–224 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Zhou Z, Jia X, Xue Q et al. TRIM14 is a mitochondrial adaptor that facilitates retinoic acid-inducible gene-I-like receptor-mediated innate immune response. Proceedings of the National Academy of Sciences of the United States of America 111(2), E245–254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang B, Wang J, Wang Y et al. Novel function of Trim44 promotes an antiviral response by stabilizing VISA. Journal of immunology (Baltimore, Md. : 1950) 190(7), 3613–3619 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Castanier C, Zemirli N, Portier A et al. MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC biology 10 44 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ran Y, Zhang J, Liu LL et al. Autoubiquitination of TRIM26 links TBK1 to NEMO in RLR-mediated innate antiviral immune response. Journal of molecular cell biology 8(1), 31–43 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Qin Y, Liu Q, Tian S, Xie W, Cui J, Wang RF. TRIM9 short isoform preferentially promotes DNA and RNA virus-induced production of type I interferon by recruiting GSK3beta to TBK1. Cell research 26(5), 613–628 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y, Song B, Park C, Kwon KS. TRIM11 negatively regulates IFNbeta production and antiviral activity by targeting TBK1. PLoS One 8(5), e63255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao W, Wang L, Zhang M et al. Tripartite motif-containing protein 38 negatively regulates TLR¾- and RIG-I-mediated IFN-beta production and antiviral response by targeting NAP1. Journal of immunology (Baltimore, Md. : 1950) 188(11), 5311–5318 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 54(2), 289–296 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Tsuchida T, Zou J, Saitoh T et al. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity 33(5), 765–776 (2010).* Demonstrates that TRIM56 mediates K63-linked ubiquitination of STING. The ubiquitination is critical for cytosolic DNA-elicited STING activation and type I IFN expression. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Hu MM, Wang YY, Shu HB. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem 287(34), 28646–28655 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Lian Q, Yang B et al. TRIM30alpha Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING. PLoS pathogens 11(6), e1005012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Q, Lin L, Tong Y et al. TRIM29 negatively controls antiviral immune response through targeting STING for degradation. Cell discovery 4 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu MM, Yang Q, Xie XQ et al. Sumoylation Promotes the Stability of the DNA Sensor cGAS and the Adaptor STING to Regulate the Kinetics of Response to DNA Virus. Immunity 45(3), 555–569 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Seo GJ, Kim C, Shin WJ, Sklan EH, Eoh H, Jung JU. TRIM56-mediated monoubiquitination of cGAS for cytosolic DNA sensing. Nature communications 9(1), 613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weng L, Mitoma H, Trichot C et al. The E3 ubiquitin ligase tripartite motif 33 is essential for cytosolic RNA-induced NLRP3 inflammasome activation. Journal of immunology (Baltimore, Md. : 1950) 193(7), 3676–3682 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song H, Liu B, Huai W et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nature communications 7 13727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu Y, Mao K, Zeng Y et al. Tripartite-motif protein 30 negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production. Journal of immunology (Baltimore, Md. : 1950) 185(12), 7699–7705 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Zurek B, Schoultz I, Neerincx A et al. TRIM27 negatively regulates NOD2 by ubiquitination and proteasomal degradation. PLoS One 7(7), e41255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi M, Deng W, Bi E et al. TRIM30 alpha negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation. Nature immunology 9(4), 369–377 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Hu MM, Yang Q, Zhang J et al. TRIM38 inhibits TNFalpha- and IL-1beta-triggered NF-kappaB activation by mediating lysosome-dependent degradation of TAB2/3. Proc. Natl. Acad. Sci. U. S. A. 111(4), 1509–1514 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wada K, Niida M, Tanaka M, Kamitani T. Ro52-mediated monoubiquitination of IKK{beta} down-regulates NF-{kappa}B signalling. Journal of biochemistry 146(6), 821–832 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao W, Wang L, Zhang M, Yuan C, Gao C. E3 ubiquitin ligase tripartite motif 38 negatively regulates TLR-mediated immune responses by proteasomal degradation of TNF receptor-associated factor 6 in macrophages. Journal of immunology (Baltimore, Md. : 1950) 188(6), 2567–2574 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Zha J, Han KJ, Xu LG et al. The Ret finger protein inhibits signaling mediated by the noncanonical and canonical IkappaB kinase family members. Journal of immunology (Baltimore, Md. : 1950) 176(2), 1072–1080 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Xing J, Weng L, Yuan B et al. Identification of a role for TRIM29 in the control of innate immunity in the respiratory tract. Nature immunology 17(12), 1373–1380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arimoto K, Funami K, Saeki Y et al. Polyubiquitin conjugation to NEMO by triparite motif protein 23 (TRIM23) is critical in antiviral defense. Proceedings of the National Academy of Sciences of the United States of America 107(36), 15856–15861 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajsbaum R, Versteeg GA, Schmid S et al. Unanchored K48-linked polyubiquitin synthesized by the E3-ubiquitin ligase TRIM6 stimulates the interferon-IKKepsilon kinase-mediated antiviral response. Immunity 40(6), 880–895 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jimenez-Moyano E, Ruiz A, Kloverpris HN et al. Nonhuman TRIM5 Variants Enhance Recognition of HIV-1-Infected Cells by CD8+ T Cells. Journal of virology 90(19), 8552–8562 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cleret-Buhot A, Zhang Y, Planas D et al. Identification of novel HIV-1 dependency factors in primary CCR4(+)CCR6(+)Th17 cells via a genome-wide transcriptional approach. Retrovirology 12 102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez-Lloret J, Okoye IS, Guidi R et al. T-cell-intrinsic Tif1alpha/Trim24 regulates IL-1R expression on TH2 cells and TH2 cell-mediated airway allergy. Proceedings of the National Academy of Sciences of the United States of America 113(5), E568–576 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai X, Srivastava S, Sun Y et al. Tripartite motif containing protein 27 negatively regulates CD4 T cells by ubiquitinating and inhibiting the class II PI3K-C2beta. Proceedings of the National Academy of Sciences of the United States of America 108(50), 20072–20077 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan W, Zhang D, Qian P et al. Swine TRIM21 restricts FMDV infection via an intracellular neutralization mechanism. Antiviral research 127 32–40 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Gack MU, Albrecht RA, Urano T et al. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5(5), 439–449 (2009).* Demonstrates that influenza A virus NS1 protein interacts with TRIM25, which interferes with the function of TRIM25 and impairs RIG-I activation and type I IFN production. This study shows a novel viral evasion strategy by targeting TRIM proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu Y, Li W, Gao T et al. The Severe Acute Respiratory Syndrome Coronavirus Nucleocapsid Inhibits Type I Interferon Production by Interfering with TRIM25-Mediated RIG-I Ubiquitination. J Virol 91(8), (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manokaran G, Finol E, Wang C et al. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science (New York, N.Y.) 350(6257), 217–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bharaj P, Wang YE, Dawes BE et al. The Matrix Protein of Nipah Virus Targets the E3-Ubiquitin Ligase TRIM6 to Inhibit the IKKepsilon Kinase-Mediated Type-I IFN Antiviral Response. PLoS pathogens 12(9), e1005880 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bharaj P, Atkins C, Luthra P et al. The Host E3-Ubiquitin Ligase TRIM6 Ubiquitinates the Ebola Virus VP35 Protein and Promotes Virus Replication. J Virol 91(18), (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laurent-Rolle M, Morrison J, Rajsbaum R et al. The interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell host & microbe 16(3), 314–327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim KH, Park ES, Kim DH et al. Suppression of interferon-mediated anti-HBV response by single CpG methylation in the 5’-UTR of TRIM22. Gut 67(1), 166–178 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Li X, Burton EM, Bhaduri-Mcintosh S. Chloroquine triggers Epstein-Barr virus replication through phosphorylation of KAP1/TRIM28 in Burkitt lymphoma cells. PLoS pathogens 13(3), e1006249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Conwell SE, White AE, Harper JW, Knipe DM. Identification of TRIM27 as a novel degradation target of herpes simplex virus 1 ICP0. J Virol 89(1), 220–229 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ling PD, Tan J, Sewatanon J, Peng R. Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus. Journal of virology 82(16), 8000–8012 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watanabe M, Hatakeyama S. TRIM proteins and diseases. Journal of biochemistry 161(2), 135–144 (2017). [DOI] [PubMed] [Google Scholar]


