Abstract
Objective:
The present study tested whether improvements in sleep and circadian problems mediate the effect of a novel transdiagnostic sleep and circadian intervention (TranS-C) on improvements in five health domains (emotional, cognitive, behavioral, social, and physical) in community-residing, evening chronotype adolescents who were at risk for problems in these five health domains.
Method:
Participants were 176 adolescents (age mean [SD] = 14.77 [1.84] years; 58% female), who were randomized to receive 6-sessions of TranS-C or psychoeducation (PE). Putative mediators tested were eveningness, weekday-weekend discrepancy in total sleep time and waketime, daytime sleepiness, Pittsburgh Sleep Quality Index score, and parent-reported sleep-wake problems. Risk in five health domains was measured using adolescent self-reported questionnaires, parent-reported Child Behavior Checklist, and ecological momentary assessment (EMA) of problems in the five health domains.
Results:
Reduced eveningness mediated the effects of TranS-C on reducing both self-reported and parent-reported risk in the five health domains. Reduction in daytime sleepiness mediated the effects of TranS-C on parent-reported risk in the five health domains. Reduction in parent-reported sleep-wake problems mediated the effects of TranS-C on self-reported, parent-reported, and EMA-assessed risk in the five health domains. Results did not support the other hypothesized mediators.
Conclusions:
TranS-C exerts effects on reducing risk in multiple mental and physical health domains through reducing sleep and circadian problems in evening chronotype adolescents. Further research of TranS-C in other samples to assess its benefits for sleep and circadian problems as well as mental and physical health is warranted.
Keywords: adolescence, sleep, mental health, intervention, transdiagnostic
Introduction
Adolescence can be a period of risk for a variety of adverse mental and physical outcomes. Domains of risk include mental disorders such as depression and anxiety (Polanczyk, Salum, Sugaya, Caye, & Rohde, 2015), problem behaviors such as substance use (Jessor, Donovan, & Costa, 2017), school failure and social problems (Dupéré et al., 2017; Obradović, Burt, & Masten, 2010), and lack of physical exercise (Carlson et al., 2016). Eveningness chronotype and the associated sleep and circadian problems are important contributors to these adverse outcomes in youth (e.g., Carskadon, 2011; Gregory & Sadeh, 2016). This sleep-risk pathway has been highlighted as a research priority in adolescent sleep health. In particular, there is a lack of treatment research that examines if reducing sleep and circadian problems is a pathway to improving broad health outcomes in youth (Parthasarathy et al., 2016).
Eveningness chronotype is defined as the tendency to increase activity later in the day and going to bed and getting up later. Eveningness impacts about 40% of adolescents (Gradisar, Gardner, & Dohnt, 2011). Eveningness is associated with multiple domains of functioning. In the emotional domain, eveningness is associated with depression, anxiety, emotional instability, and suicidality (Gau et al., 2007). In the cognitive domain, it is associated with school problems, attention problems, and cognitive performance (Goldstein, Hahn, Hasher, Wiprzycka, & Zelazo, 2007; Short, Gradisar, Lack, & Wright, 2013). In the behavioral domain, it is associated with substance use (e.g., caffeine, alcohol, nicotine, drugs), impulsivity, and poor self-regulation (Adan, Natale, Caci, & Prat, 2010; Digdon & Howell, 2008; Negriff, Dorn, Pabst, & Susman, 2011; Randler, 2008; Susman et al., 2007). It is also associated with greater social problems, less physical activities, and poor physical health (Miller, Lumeng, & LeBourgeois, 2015; Schaal, Peter, & Randler, 2010). Much of this evidence is based on cross-sectional data; however, in a few longitudinal studies eveningness predicts increased risk for psychopathology. Overall, eveningness is a well-documented risk factor for a wide range of adverse mental and physical outcomes.
Eveningness is thought to arise from a confluence of a biological shift in the circadian system at puberty (Roenneberg et al., 2004) along with social changes (e.g., less parental control, more technology use) and academic demands (e.g., homework, early school start time) during adolescence (Carskadon, 2011; Viner et al., 2012). The consequences are sleep deprivation and attempts to catch-up on sleep which contribute to variability in sleep schedule from school days to weekends (Crowley, Acebo, & Carskadon, 2007; Gradisar et al., 2013). The latter is referred to as “social jetlag” (Wittmann, Dinich, Merrow, & Roenneberg, 2006). Both sleep deprivation and sleep variability are highly prevalent in adolescents.
Unlike the extensive support for cognitive behavioral interventions for sleep problems in adults (for recent reviews, see Trauer, Qian, Doyle, Rajaratnam, & Cunnington, 2015; van Straten et al., 2018), fewer studies have examined psychosocial interventions for adolescent sleep problems. Nevertheless, the preliminary evidence thus far is promising. A recent meta-analysis summarizing 9 trials (n = 357), out of which 4 were randomized controlled trials (RCTs) and 2 included active control, provided preliminary support suggesting that adolescent cognitive behavioral sleep interventions are effective in improving sleep duration and sleep quality as well as reducing daytime sleepiness and symptoms of depression and anxiety (Blake, Sheeber, Youssef, Raniti, & Allen, 2017). However, only one RCT was a fully-powered study comparing a group sleep improvement intervention with cognitive-behavioral and mindfulness-based components to study skill group (Blake et al., 2016). A unique contribution of the current study is that it is drawn from a fully-powered RCT that tested a novel, individual sleep intervention for youth–the Transdiagnostic Sleep and Circadian Intervention (TranS-C; Harvey, 2016; Harvey & Buysse, 2017)–against an active control condition.
TranS-C was developed to target modifiable psychosocial, behavioral, and cognitive contributors to adolescent sleep problems, including the impact of eveningness chronotype such as sleep deprivation and sleep variability (Harvey, 2016; Harvey & Buysse, 2017). TranS-C is designed to address sleep and circadian dysfunctions, which is a theoretically and biologically plausible transdiagnostic process across psychiatric disorders (Harvey, Murray, Chandler, & Soehner, 2011). A transdiagnostic process is defined as a process common in more than one psychiatric disorder. TranS-C is transdiagnostic intervention in two ways. First, it targets a variety of sleep and circadian problems, such as insomnia, delayed and advanced sleep phase, difficulty waking up or getting out of bed, too much time in bed, and irregular sleep-wake schedules. Second, it is designed to be useful across psychological and health problems such as major depression, anxiety disorders, and bipolar disorder. Note that although a shift towards evening circadian preference is partly triggered by the onset and progression of puberty, there are contributors that can be modified through a psychosocial intervention such as TranS-C. Examples of TranS-C treatment targets include implementing an earlier bedtime, regularizing bedtime and wake-up time, not napping after school, getting homework done earlier, and addressing excessive worries around bedtime.
A recent RCT reported that TranS-C, compared to psychoeducation (PE) about sleep and health, was associated with reduced eveningness and improvements in selected sleep and circadian outcomes as well as some specific measures within the five health domains in evening chronotype adolescents (Harvey et al., 2018). Specifically, TranS-C, relative to PE, was associated with greater reduction in evening circadian preference, earlier endogenous circadian phase, less weeknight-weekend discrepancy in total sleep time and wakeup time, less daytime sleepiness, and better self-reported sleep via youth and parent report. TranS-C was not associated with significant improvement in total sleep time or significant advancing of bedtime, relative to PE. TranS-C was also not associated with significantly greater reduction of the risk in the five health domains (i.e., emotional, cognitive, behavioral, social, and physical health), relative to PE. The only exception was that TranS-C, relative to PE, was associated with greater reduction in the parent-reported risk composite score for the cognitive domain (Harvey et al., 2018).
The present study takes the next step in this program of research. Specifically, the goal is to establish whether reductions in eveningness and sleep problems mediate the effect of TranS-C, relative to PE, on reducing risk in five health domains (i.e., emotional, cognitive, behavioral, social, and physical) in eveningness chronotype adolescents. This is an important next step because sleep and circadian dysfunction is hypothesized as a transdiagnostic process underlying multiple mental disorders (Harvey et al., 2011). There are three aims. The first aim is to test whether changes in sleep mediate the effects of treatment condition (TranS-C vs. PE) on the youth self-reported risk in five health domains. The second aim was to test whether changes in sleep mediate the effects of treatment condition (TranS-C vs. PE) on the parent-reported risk in the five health domains. The third aim was to test whether changes in sleep mediate the effects of treatment condition (TranS-C vs. PE) on risk in the five health domains measured by ecological momentary assessment (EMA). We hypothesized that pre- to post-treatment reduction in eveningness and sleep problems will mediate the effect of TranS-C on pre- to post-treatment improvements in the five health domains as measured by youth self-report (Aim 1), parent-report (Aim 2), and EMA (Aim 3). Note that we considered constructing a single latent variable of health indicated by youth-report, parent-report, and EMA-assessed outcome variables. However, this model is considered too complex for the current sample size of 176 (Herzog, Boomsma, & Reinecke, 2007; Moshagen, 2012). Another reason for examining youth-report, parent-report, and EMA-assessed outcomes separately was to be consistent with the trial registry and main report of post-treatment effects (Harvey et al., 2018).
Methods
Participants and Procedures
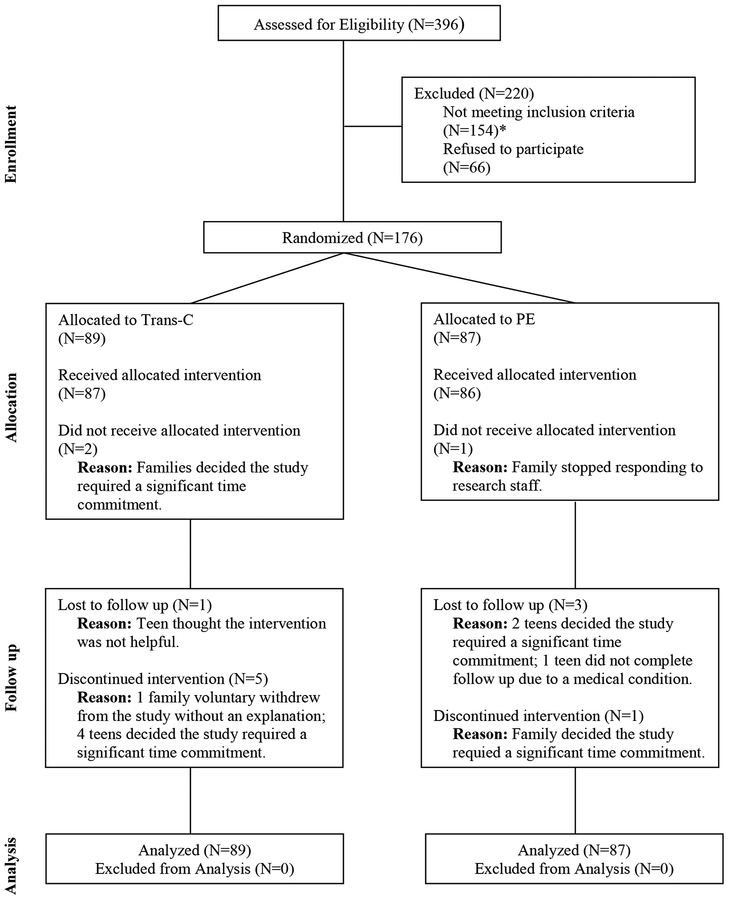
Participants were 176 adolescents who participated in a randomized controlled trial in a university clinic. They were recruited through clinician referrals and advertisements from January 2013 to February 2016. Briefly, participants and parents/guardians were first screened for eligibility via a telephone interview, and potentially eligible individuals participated in an in-person assessment to determine eligibility. Parents or guardians of all participants provided informed consent and participants provided informed assent. The participants were randomly assigned, stratified by age (10–14 vs. 15–18 years) and sex, in a 1:1 parallel group design, to receive either TranS-C or PE for 6 weekly sessions. The assessors were blind to treatment allocation. Sequentially numbered, opaque, sealed envelopes were used based on a computer-generated random number list. A project coordinator conducted the randomization procedure after all eligibility assessments were completed. Participants were assessed at baseline and at the end of treatment. The Committee for the Protection of Human Subjects at the University of California Berkeley approved the study. The trial protocol and outcomes were preregistered (https://clinicaltrials.gov; ). Figure 1 shows the participant flow. A detailed description of the study procedure can be found elsewhere (Harvey et al., 2018).
Figure 1.
CONSORT Diagram Illustrating the Flow of Participants Through the Study
*Out of 154 who were ineligible, 87 did not meet criteria for eveningness chronotype, 6 had eveningness chronotype but no risk, and 61 did not meet criteria for inclusion due to medical reasons, substance use, suicidality, trauma, or other.
Inclusion criteria were: 1) 10 to 18 years olds living with a parent or guardian, and attending a class/job by 9 AM at least 3 days per week; 2) fluent in English; 3) were able and willing to give informed assent; 4) reported eveningness as demonstrated by scoring within the lowest quartile (27 or lower) of the Children’s Morningness-Eveningness Preferences Scale (CMEP; Dagys et al., 2012) and had a 7-day sleep diary showing a sleep onset time of 10:40 PM or later for 10–13 year olds, 11:00 PM or later for 14–16 year olds, and 11:20 PM or later for 17–18 year olds at least 3 nights per week (note that a sleep diary was collected to confirm late sleep onset, and this was separated from the sleep diary collected at pre-treatment assessment and used in the analysis). In addition, this sleep pattern had to have been present for the past three months per youth self-report. These age-group cutoffs were derived from prior research (Giannotti, Cortesi, Sebastiani, & Ottaviano, 2002; Maslowsky & Ozer, 2014) and reflect developmentally derived norms in sleep among adolescents; 5) participants must fall into an ‘at risk’ range on measures of at least one of the five health domains (see Table 1).
Table 1.
Inclusion Criteria Operationalizing ‘At Risk’ for the Five Health Domains
| Risk Domain | Criteria for Inclusion |
|---|---|
| Emotional | ≥ 4 on any of the following items on the CDRS: Difficulty Having Fun, Social Withdrawal, Irritability, Depressed Feelings, Excessive Weeping, or a T-score of 61 or above on the MASC-10, based on age group (10–11 years, 12–15 year, 16–19 years) using the MASC-10 Profile. |
| Behavioral | A SSS score greater than 3.93 for males aged 10–13, greater than 3.19 for females aged 10–13, greater than 4.07 for males aged 14–18, or greater than 3.19 for females aged 14–18 or taking ADHD medication or the KSADS indicating a diagnosis of ADHD or current alcohol or substance abuse assessed with the KSADS. |
| Social | A parent rating their child as “worse” than others the participants age on one or more of the social behavior items (Section VI) from the CBCL. |
| Cognitive | A parent rating their child as “failing” in one or more academic class from CBCL Section VII. |
| Physical | A score of 4 or above on the PHQ-15, six or more days of school absences, or a BMI above the 85th percentile for the participant’s sex and age. |
Note. CDRS = Child Depression Rating Scale (Poznanski et al., 1984), the cutoff is commensurate with “clinical symptoms.” MASC-10 = Multidimensional Anxiety Scale for Children, the cutoff T-score was selected to capture the ‘slightly elevated’ through to the ‘very elevated’ range (March, Sullivan, & Parker, 1999). SSS = Sensation Seeking Scale (Russo et al., 1993), the cutoff correspond to at or above one standard deviation over the normative average (Stephenson, Hoyle, Palmgreen, & Slater, 2003). K-SADS = Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., 1997). CBCL = Child Behavior Checklist (Becker, Ramsey, & Byars, 2015), which asks the parent if their child does “worse”, “average”, or “better” than other teens their age or if the teen is “failing”, “below average”, “average” or “above average”. PHQ-15 = Physical Health Questionnaire-15, the cutoff corresponds to ‘minimal somatic symptom severity’ through to the ‘high somatic symptom severity’ range (Kroenke, Spitzer, & Williams, 2002). BMI = Body Mass Index, the cutoff corresponds to 1 standard deviation above the mean.
Exclusion criteria were: 1) an active, progressive physical illness or neurological degenerative disease directly related to the onset and course of the sleep disturbance; 2) evidence of obstructive sleep apnea, restless legs syndrome, or periodic limb movement disorder; 3) a significantly impairing pervasive developmental disorder based on parent/caregiver self-report during phone screening; 4) bipolar disorder, schizophrenia, or another current Axis I disorder if there was a risk of harm if treatment was delayed; 5) taking medications that alter sleep (e.g., hypnotics) within 4 weeks of the assessment (2 weeks for melatonin); 6) history of substance dependence in the past six months or current suicide risk sufficient to preclude treatment on an outpatient basis. Sleep disorders were assessed using the Duke Structured Interview for Sleep Disorders (Edinger et al., 2009). Axis I disorder and risk of harm questions were assessed during the phone screening procedure and answered by both the parent/caregiver and the adolescent. A full assessment of the Axis I disorders was made during the pre-treatment assessment using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997). In addition, participants were not excluded if they were stable on a medication for at least 4 weeks prior to the pre-treatment assessment. One exception was participants using hypnotics who were automatically excluded, unless they had stopped using hypnotics 4 weeks prior (2 weeks for melatonin) to the pre-treatment assessment.
Treatment Conditions
Both treatment conditions involve 6 individual, weekly 50-minute sessions during school year delivered by doctoral or master’s level therapists. A key difference between the two treatment conditions was that TranS-C facilitates behavior change whereas PE only provides information on sleep and health.
Transdiagnostic Sleep and Circadian Intervention for Youth (TranS-C; Harvey, 2009, 2016; Harvey & Buysse, 2017) was derived from studies of sleep treatments for youth that were disorder-focused (e.g., treating insomnia for depressed adolescents). Sources for TranS-C include Cognitive Behavioral Therapy for Insomnia (Morin et al., 2006; Morin & Espie, 2003; Perlis, Aloia, & Kuhn, 2011), Interpersonal and Social Rhythm Therapy (Frank et al., 2005), Chronotherapy (Wirz-Justice, Benedetti, & Terman, 2009), and Motivational Interviewing (Miller & Rollnick, 2002). TranS-C took a transdiagnostic approach to address real-life sleep and circadian problems as these problems are often not neatly categorized. TranS-C adopts a modular approach and targets the maintaining psychosocial, behavioral, and cognitive processes. It includes 4 cross-cutting modules (i.e., functional analysis and case formulation, goal setting, motivational interviewing, sleep and circadian education), 4 core modules (e.g., establishing regular sleep-wake times, improving daytime functioning, correcting unhelpful sleep-related beliefs, relapse prevention), and 7 optional modules (e.g., improving sleep efficiency, reducing time in bed, reducing sleep-related worry and vigilance, dealing with circadian rhythms problems). All TranS-C participants completed the cross-cutting and core modules TranS-C modules. Optional modules were administered if needed based on the case-formulation.
Psychoeducation (PE) is an active comparison group that has been associated with sleep improvement (Harvey et al., 2015). PE sessions provide information on the inter-relationship between sleep, stress, diet, health, exercise, accidents, and mood. Participants also sampled meditation, yoga, and/or outdoor appreciation. The emphasis was on providing information rather than facilitating behavior change.
Treatment Integrity and Credibility.
Cognitive Therapy Rating Scale (Young & Beck, 1980) scores for TranS-C (n = 69, M = 45.43, SD = 4.45) and treatment integrity scores for PE (n = 77, M = 92.44, SD = 10.13) indicate that both treatments were delivered with fidelity. There were no group differences (all p > 0.05) on the Credibility and Expectation Questionnaire (Devilly & Borkovec, 2000).
Measures of Mediators
Putative mediators tested were evening chronotype as measured by Children’s Morningness-Eveningness Preferences Scale, weekday-weekend discrepancy in total sleep time and waketime derived by 7 days sleep diary, sleepiness as measured by Sleepiness Scale, sleep quality as measured by the Pittsburgh Sleep Quality Index, and parent-reported sleep wake problems as measured by CBCL Sleep Composite.
Children’s Morningness-Eveningness Preferences Scale (CMEP).
Adolescent’s chronotype was assessed using the CMEP (Carskadon, Vieira, & Acebo, 1993), on which a high score of 43 indicates an extreme morning preference and a low score of 10 indicates an extreme evening preference. Prior studies have reported good reliability coefficients (α = 0.78 to 0.82) for CMEP scores in multiple adolescent samples (Carskadon, Seifer, & Acebo, 1991; Díaz‐Morales, de Leon, & Sorroche, 2007). CMEP scores also have moderate to strong convergent validity with other sleep measures (Crowley, Bushnell, Acebo, & Carskadon, 2006; Kim, Dueker, Hasher, & Goldstein, 2002). A higher score indicates greater preference for morningness. This includes endorsing statements such as it is “easy to get up in the morning,” “preferable to take a two-hour test earlier in the day, and having “most energy in the morning to do favorite things.”
Sleep Diary.
A one week (7-day) Sleep Diary (Carney et al., 2012) was collected during the pre- and post-treatment assessment period by trained research assistants over the phone. Another 7-day sleep diary was collected at the eligibility assessment to confirm late sleep onset. Calls were made every morning for the week leading up to treatment, and again for the week after receiving treatment. Good-to-excellent reliability has been reported for bedtime, sleep onset latency, and sleep duration from five weekday nights of sleep diary entries in multiple adolescent samples (Short, Arora, Gradisar, Taheri, & Carskadon, 2017). Total sleep time (TST) and waketime discrepancy from weekday-to-weekend were derived from the sleep diaries. TST was calculated as “time in bed – sleep onset latency - wake after sleep onset - terminal wakefulness” based on Buysse et al. (2006) definition. Waketime discrepancy from weekday-to-weekend was calculated as “weeknight average - weekend average for wake time.”
Sleepiness Scale.
Ten items from the School Sleep Habits Survey comprising the Sleepiness Scale (Wolfson & Carskadon, 1998) was administered during pre- and post-treatment assessment. All items on the Sleepiness Scale used a 4-point scale (0 = no, 1 = struggled to stay awake, 2 = fallen asleep, 3 = both “struggled to stay awake” and “fallen asleep”) to rate participant responses. The Sleepiness Scale scores have adequate reliability (Carskadon et al., 1991; Wolfson & Carskadon, 1998) and have been validated against sleep diary and actigraphy on representative adolescent samples (Giannotti et al., 2002; Russo, Bruni, Lucidi, Ferri, & Violani, 2007; Wolfson et al., 2003).
Pittsburgh Sleep Quality Index (PSQI).
The PSQI (Buysse et al., 1989) was administered at pre- and post-treatment assessment. PSQI is a 19-item questionnaire generating 7 component sub-scores, and the sum of these sub-scores produces one global score. The global score ranges from 0 to 21 with higher scores indicating greater sleep problems in the past month. The PSQI scores have demonstrated adequate reliability and validity data against other sleep measures in adolescent samples (de la Vega et al., 2015; Mollayeva et al., 2016).
Child Behavior Checklist (CBCL) Sleep Composite.
A CBCL sleep composite was derived based on seven items on the parent-report CBCL (Achenbach & Rescorla, 2001) that measure sleep functioning (Becker, Ramsey, & Byars, 2015). Items were rated on a 3-point scale (0 = not true, 1 = somewhat/sometimes true, 2 = very true/often true). The reliability and validity of the CBCL have been well-documented (Achenbach & Rescorla, 2001). The CBCL Sleep Composite has been tested and used in prior literature and have shown strong convergent validity with well-validated measures of children’s sleep (Becker et al., 2015).
Measures of Functioning in Five Health Domains
Three sets of composite scores were used to indicate functioning or risk in five health domains, namely youth self-reported composite risk scores, parent-reported composite risk scores, and ecological momentary assessment (EMA) composite risk scores. Each set of composite scores was composed of measures of emotional, cognitive, behavioral, social, and physical health.
Youth Self-Report Composite Risk Score.
Five composite scores, each composed of measures of emotional, cognitive, behavioral, social, and physical health, were used to indicate functioning in five health domains. The composite scores were calculated for each of the five health domains by standardizing the raw score (i.e., created z-score) of each measure, and then averaged the standardized scores from each respective health domain’s measure. Summary scores for specific measures were reverse coded when necessary so that all scores of specific measures within a domain would have the same direction (i.e., higher score indicates greater risk). Specific measures for each domain are listed below:
Emotional domain.
Emotional domain was assessed by a composite of depressive and anxiety symptoms as measured by the Children’s Depression Rating Scale (CDRS; Poznanski et al., 1984) and the Multidimensional Anxiety Scale for Children (MASC; March, Sullivan, & Parker, 1999) respectively. The CDRS is 17-item rating scale with possible scores ranging from 17 to 113, and higher scores indicating depression. The MASC is a 39-item scale with possible scores ranging from 0 to 117, and higher scores indicating more anxiety.
Cognitive domain.
Cognitive domain was assessed by a composite of the Attention Control Scale (ACS; Derryberry & Reed, 2002) and pertinent school-related items found in the Youth Social Adjustment Scale – Self Report (YSAS; Weissman, Orvaschel, & Padian, 1980). We used six YSAS items related to school and cognitive functioning: 1. How many days of classes did you miss in the last two weeks? 2. Have you been able to keep up with your classwork in the last two weeks? 3. During the last 2 weeks, have you been ashamed of how you do your schoolwork? 4. Have you had any arguments with kids at school in the last two weeks? 5. Have you felt unhappy at school during the last two weeks? 6. Have you found your schoolwork interesting the last two weeks? The ACS is a 20-item questionnaire, whose scores range from 4 to 80, with higher scores demarcating better attention control. To keep the directionality of the present study we reverse coded the ACS so higher scores meant greater risk in the cognitive composite. We used 6 items from the YSAS, where higher scores indicated more cognitive and school problems.
Behavioral domain.
Behavioral domain was measured by a composite of the Brief Sensation Seeking Scale (BSSS8; Hoyle, Stephenson, Palmgreen, Lorch, & Donohew, 2002) and the Alcohol and Substance Use Questionnaire (Johnston, Malley, Bachman, & Schulenberg, 2009). The BSSS is an 8-item questionnaire, rated on a 5-point scale, designed to assess the thrill and sensation seeking, with higher scores indicate higher sensation seeking (range 0 to 40). The Alcohol and Substance Use Questionnaire assess the use alcohol, caffeine, and recreational drugs in the past 30 days; moreover, we added extra questions on caffeine and energy drinks in this study. This measure used a 7-point rating scale (range 0 to 46) with higher scores indicating more frequent use of the aforementioned substances. Greater risk in the behavioral domain is indicated by higher scores.
Social domain.
Social domain was assessed by taking the average of the Youth Social Adjustment Scale – Self-Report (Weissman et al., 1980) subscales on friends, family, and romantic relationships. Higher scores in the social domain indicate more social impairment.
Physical domain.
Physical domain was assessed by deriving a composite of the Physical Health Questionnaire-15(PHQ-15; Kroenke, Spitzer, & Williams, 2002) and the Modifiable Activity Questionnaire for Adolescents (MAQ; Aaron & Kriska, 1997). The PHQ-15 is a 15-item questionnaire with scores ranging from 0 to 30, more physical complaints are indicated by a higher score. MAQ is questionnaire designed to assess activity levels, with scores indicating number of hours active or exercising in a week. A higher score was indicative of a more active and exercise rich life. To maintain directionality of risk in the physical domain the PHQ-15 was reverse coded to combine with MAQ to generate a composite score. Thereafter, the composite was reverse coded as to have higher scores indicate higher risk in the physical domain.
Parent-Reported Composite Risk Score via the Child Behavior Checklist (CBCL).
Parents were given the 113-item CBCL (Achenbach & Rescorla, 2001) questionnaire to rate the behavioral and psychological functioning of their adolescent. Each item of the CBCL was on 3-point scale (0 = not true, 1 = somewhat/sometimes true, 2 = very true/often true), with higher scores signaling more problematic behaviors. The parent-reported composite risk score was derived using seven of the CBCL subscales. Anxious/Depressed and Withdrawn/Depressed subscales comprised the Emotional Domain. Thought and Attention subscales comprised the Cognitive Domain. Rule-Breaking and Aggressive Behavior subscales comprised the Behavioral Domain. Social Problems and Somatic Complaints subscales comprised the Social and Physical Domains respectively.
Youth Ecological Momentary Assessment (EMA) Composite Risk Score.
Trained interviewers called participant at pre- and post-treatment during a one-week (7-day) period to administer the EMA interview. Every call consisted of a structured interview based on Silk et al. (2011) designed to evaluate the five health-relevant risk domains. The interviews were administered twice during weekdays and four times on weekends, for a total of 36 calls per participant for the entire study. Weekday calls took place between 4 and 9 PM as participants were instructed to turn off their cell phones during school hours. Weekend calls were made between 11 AM and 9 PM. Interviewers were instructed to space the calls by at least 30 minutes, and call back after 5 minutes if participant was non-responsive to two initial calls.
Responses from the brief interviews were transcribed into a primary database, which was later used by five certified coders (trained and supervised research assistants) to independently code a subset of the data. To become a certified coder, coders had to match an “expert coder” at over 80% accuracy for at least 54 consecutive calls. The expert coder NBG designed the coding manual and supervised the coding team. Coders independently coded a subset of the database (5%) with a 93.21% agreement with the expert coder. EMA coding was adapted from methods found in Silk et al. (2011). The Youth EMA Composite Risk Score was derived by taking the average scores for the week for each domain.
Emotional Domain.
Emotional health was assessed by employing a short version of the Positive and Negative Affect Schedule for Children (PANAS-C; Laurent et al., 1999). A positivity ratio was calculated using four positive and five negative affect items from the PANAS-C. Subjective well-being can be predicted by a high positive to negative ratio (Diener, 2000). Research has also demonstrated that sleep deprivation and evening preference are associated with a low positivity ratio (Dagys et al., 2012).
Cognitive Domain.
Cognitive health was assessed by averaging adolescent self-ratings (1 to 5 Likert scale) on questions of concertation, levels of distraction and focus related to task-at-hand the moment phone rang. Question asked were modified from previous research (Derryberry & Reed, 2002) and included “At the moment the phone rang, what were you doing?”
Behavioral Domain.
Behavioral health was assessed by calculating the average frequency and consumption of junk food, caffeine, nicotine and other substances the week of the EMA calls. Questions about food intake, drinking, smoking and chewing gum at moment of phone rang was asked from all participants.
Social Domain.
Social health was assessed by calculating the Positivity Ratio when the participant was alone, with family, or with friends at the time of the EMA call.
Physical Domain.
Physical health was assessed by a total score on a binary variable (active = 1 and inactive = 2) (Kanning & Schlicht, 2010) derived by questions developed in past research (Rofey et al., 2010).
Data Analysis
Data analysis was conducted using Mplus version 8 (Muthén & Muthén, 1998–2017). All models controlled for sex and age, the stratification factors used in randomization. Two-sided significance level of 0.05 was used. All models used maximum likelihood estimation. A mediation model was indicated because: 1) TranS-C targets sleep and circadian problems (i.e., shift eveningness preference, improve TST, advance bedtime) with the goal of reducing eveningness, sleep problems, and risk in health-related domains, and 2) sleep and circadian dysfunction is a theoretically and empirically supported transdiagnostic process underlying risk and vulnerability in all five health risk domains. The independent variable for the mediation models was the randomized treatment condition (TranS-C vs. PE). The mediator variables were the primary and secondary sleep and circadian outcomes of this RCT that TranS-C elicited significant pre-post improvement over PE (as reported in Harvey et al., 2018). The mediator variables tested were: CMEP (primary outcome), TST weekday-weekend discrepancy, waketime weekday-weekend discrepancy, sleepiness, PSQI, and CBCL sleep composite (secondary outcomes) measured at pre- and post-treatment. Those sleep and circadian variables that were not associated with treatment condition cannot be mediators of the treatment by definition (Kraemer, Wilson, Fairburn, & Agras, 2002) and thus were not evaluated in the current study. The outcome variables for the mediation models were: 1) a latent variable indicated by emotional, cognitive, behavioral, social, and physical health risk composite scores based on youth self-reported questionnaires (primary outcomes of the trial; Aim 1), 2) a latent variable indicated by parent-reported CBCL subscales that map onto the five health domains (secondary outcomes of the trial; Aim 2), and 3) a latent variable indicated by EMA-based risk in five health domains (secondary outcomes of the trial; Aim 3). Three latent variables were used to represent 5 health domains measured risk by youth-report, parent-report, and EMA to reduce the number of outcomes and tests. All health outcomes were measured at pre- and post-treatment. Percentage of missing data is reported in supplement material 1. Sample scripts are included as supplemental material 2. All estimates reported in tables and figures were standardized on the dependent variable (i.e., STDY standardization), which can be interpreted as the change in standard deviation units of y when x changes from 0 to 1).
As illustrated in Figure 2, the Analysis of Covariance (ANCOVA) model was used to test the mediated or indirect effects, in which the pre-treatment measurement of each mediator and outcome variable was included as a covariate in the analysis (MacKinnon, 2008). This method performs best in simulation studies over other models (e.g., difference score model) and is recommended for pretest-posttest control group design (Valente & MacKinnon, 2017). A bootstrapping procedure with 5,000 replications was used to test the statistical significance of the indirect effect, which is the product term of am2x and by2m2 illustrated in Figure 2. Bootstrap 95% confidence intervals were reported (MacKinnon, Lockwood, & Williams, 2004). Model fit was evaluated using the combination of the comparative fit index (CFI), Tucker–Lewis index (TLI), the root-mean-square error of approximation (RMSEA), and standardized root mean square residual (SRMR). We used guidelines suggested in the literature: CFI and TLI greater than 0.95 for reasonably good fit (Hu & Bentler, 1999) and values between 0.90 and 0.95 for acceptable model fit (Bentler, 1990); RMSEA ≤ 0.08 for adequate fit and ≤ .05 for close fit (Browne & Cudeck, 1993); SRMR ≤ 0.08 for acceptable fit (Hu & Bentler, 1999).
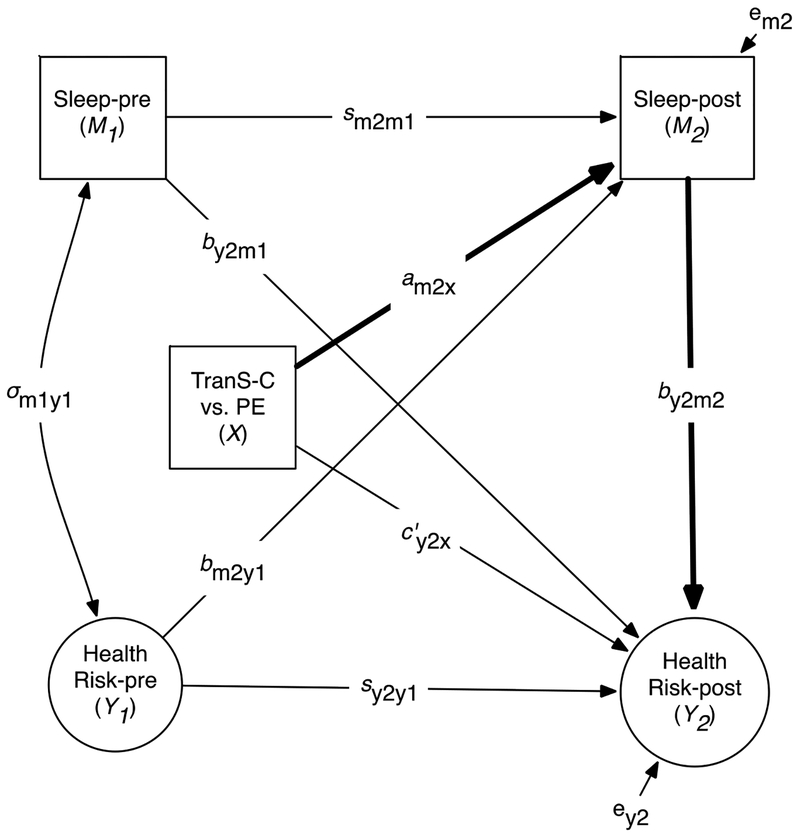
Figure 2.
Conceptual Path Diagram for the Mediation Models
Note. This figure shows how the mediation model is specified. The mediational or indirect effects of interest (bolded) is the product of these two bolded paths: am2xby2m2. Health Risk (indicated as circles) represents a latent variable comprised of composite scores in emotional, cognitive, behavioral, social, and physical (not shown here) domains measured by self-report (Aim 1), parent-report (Aim 2), or EMA (Aim 3). Sex and age (not shown) are added as covariates in this model. X indicates treatment comparisons TranS-C vs. PE. M indicates Sleep/Circadian mediator at baseline (M1) and posttreatment (M2). Y indicates Health Risk outcomes at baseline (Y1) and posttreatment (Y2). The path diagram includes correlation at baseline between mediator and dependent variable σm1y1, stability of mediator (sm2m1) and stability of dependent variable (sy2y1), Y2 cross-lag (by2m1), M2 cross-lag (bm2y1), effect of X on M2 (am2x), effect of X on Y2 (c’y2x), and effect of M2 on Y2 (by2m2). The effect of X on the mediator measured at posttreatment (M2) adjusted for baseline mediator (M1) is expressed as M2 = intercept + am2xX + sm2m1M1 + bm2y2Y1 + eM2. The effect of X on the outcome variable measured at posttreatment (Y2) adjusted for the other variables is expressed as: Y2 = intercept + c’y2xX + sy2y1Y1 + by2m1M1 + by2m2M2 + eY2. The mediated effect of X on Y2 through M2 in this model is assessed by the product of am2x and by2m2 (i.e., am2xby2m2). This figure and Table 5 are connected such that all paths that are relevant to the test of mediation are represented in Table 5 under the heading of each column (e.g., indirect effect: am2xby2m2).
Results
Table 2 presents the demographic variables and sample characteristics. Table 3 presents the descriptive statistics of all study variables. Table 4 presents the model fit statistics and all models achieved acceptable fit. Table 5 presents the results from mediation analysis for youth self-reported risk composite (Aim 1), parent-reported risk composite (Aim 2), and EMA-assessed risk (Aim 3). Significant indirect effects, as shown in Table 5, are illustrated in Figure 3 and supplemental Figures S1–5.
Table 2.
Demographic Variables and Sample Characteristics.
| Characteristic | Whole Sample (N=176) |
TranS-C (N=89) |
PE (N=87) |
|---|---|---|---|
| Female | 102 (58) | 49(55) | 53(61) |
| Hispanic/Latino | 27(15) | 14(16) | 13(15) |
| Race | |||
| Caucasian | 114 (65) | 58 (65) | 56 (64) |
| African American/Black | 12 (7) | 4 (4) | 8 (9) |
| American Indian or Alaska Native | 0 (0) | 0 (0) | 0 (0) |
| Asian | 18 (10) | 11 (12) | 7 (8) |
| Native Hawaiian/Other Pacific Islander | 2 (1) | 2 (2) | 0 |
| Refused to answer | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 0 (0) | 0 (0) | 0 (0) |
| Mixed Race | 30 (17) | 14 (16) | 16 (18) |
| Family annual income | |||
| ≤ 20,000 | 6 (3) | 2 (2) | 4(5) |
| 20,001 – 50,000 | 21 (12) | 11 (12) | 10 (11) |
| 50,001 – 100,000 | 42 (24) | 26 (29) | 16 (18) |
| 100,000+ | 102 (58) | 47 (53) | 55 (63) |
| Refused to answer/missing | 5 (3) | 3 (3) | 2 (2) |
| Current grade at pretreatment | |||
| 5 | 5 (3) | 4 (4) | 1 (1) |
| 6 | 7 (4) | 4 (4) | 3 (3) |
| 7 | 14 (8) | 6 (7) | 8 (9) |
| 8 | 25 (14) | 11(12) | 14 (16) |
| 9 | 28 (16) | 16 (18) | 12 (14) |
| 10 | 46 (26) | 22 (25) | 24 (28) |
| 11 | 25 (14) | 13 (15) | 12 (14) |
| 12 | 25 (14) | 12 (13) | 13 (15) |
| College | 1 (1) | 1 (1) | |
| Any current K-SADS Dx (teen report) | 63/171 (37%) | 34/87 (39%) | 29/84 (35%) |
| Any past K-SADS Dx (teen report) | 77/171 (45) | 40/86 (47) | 37/85 (44) |
| Any current K-SADS Dx (parent report) | 49/168 (29) | 21/85 (25) | 28/83 (34) |
| Any past K-SADS Dx (parent report) | 55/168 (33) | 24/84 (29) | 31/84 (37) |
| M (SD) | M (SD) | M (SD) | |
| Age | 14.77 (1.84) | 14.76 (1.94) | 14.78 (1.74) |
Note. M = Mean. SD = Standard Deviation. K-SADS = Schedule for Affective Disorders and Schizophrenia for School-Age Children. Dx = diagnosis. There was no group difference on any of the demographic variable across treatment conditions (Harvey et al., 2018).
Table 3.
Descriptive Statistics of Study Variables
| TranS-C | PE | |||
|---|---|---|---|---|
| Outcome (range) | Baseline | Post-treatment | Baseline | Post-treatment |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Sleep and Circadian Outcomes | ||||
| SD-TST weeknight-weekend discrepancy in min | −70.39 (113.10) | −31.16 (115.19) | −48.91 (89.28) | −56.46 (106.25) |
| SD-WUP weeknight-weekend discrepancy in min | −1.90 (1.36) | −1.13 (1.29) | −1.42 (1.28) | −1.32 (1.55) |
| Sleepiness (0–30) | 6.20 (4.52) | 4.83 (4.03) | 6.15 (4.01) | 6.37 (4.71) |
| PSQI (0–21) | 7.58 (2.99) | 5.85 (2.56) | 7.58 (3.03) | 6.75 (3.48) |
| CBCL Sleep Composite (0–14) | 3.32 (2.03) | 1.84 (1.86) | 3.24 (2.13) | 2.51 (1.91) |
| CMEP (0–43) | 21.11 (3.79) | 25.08 (4.86) | 21.52 (3.86) | 23.6 1 (4.60) |
| Youth Self-Report Composite Risk Score | ||||
| Emotional health: | ||||
| CDRS (17–113) | 33.90 (9.34) | 27.01 (8.72) | 33.08 (9.90) | 27.00 (8.16) |
| MASC (0–117) | 46.51 (17.73) | 45.45 (17.10) | 45.98 (15.99) | 44.74 (18.03) |
| Composite | 0.25 (0.91) | −0.18 (0.80) | 0.17 (0.82) | −0.21 (0.81) |
| Cognitive health: | ||||
| ACS (4–80) | 50.56 (8.23) | 52.18 (8.09) | 51.24 (7.22) | 51.29 (7.77) |
| YSAS school/cognitive items (0–24) | 11.68 (2.95) | 11.69 (3.14) | 11.90 (2.83) | 12.49 (2.94) |
| Composite | 0.02 (0.85) | −0.09 (0.89) | −0.01 (0.71) | 0.09 (0.87) |
| Behavioral health: | ||||
| Sensation Seeking Scale (0–40) | 27.28 (5.97) | 27.35 (6.61) | 26.36 (6.22) | 27.51 (7.04) |
| Alcohol and Substance Use (0–46) | 5.76 (8.24) | 5.51 (8.10) | 5.67 (6.62) | 6.26 (8.37) |
| Composite | 0.01 (0.81) | −0.01 (0.83) | −0.07 (0.78) | 0.06 (0.92) |
| Social health: | ||||
| YSAS: Friends (9–45) | 18.53 (4.58) | 17.73 (3.69) | 18.81 (4.98) | 18.68 (4.82) |
| YSAS: Family (6–30) | 11.92 (3.50) | 11.33 (3.56) | 12.34 (3.67) | 11.68 (4.17) |
| YSAS: Romantic (2–10) | 7.34 (2.03) | 7.62 (1.78) | 7.59 (1.69) | 7.62 (1.85) |
| Composite | −0.04 (0.60) | −0.09 (0.62) | 0.09 (0.71) | 0.02 (0.69) |
| Physical health: | ||||
| MAQ | 3.36 (5.35) | 4.20 (8.22) | 2.83 (4.31) | 3.40 (5.11) |
| PHQ (0–30) | 9.30 (5.37) | 7.97 (5.01) | 8.58 (4.40) | 7.01 (4.33) |
| Composite | 0.02 (0.77) | 0.04 (0.83) | 0.00 (0.66) | −0.01 (0.56) |
| Parent-Reported Composite Risk Score | ||||
| Emotional Health | ||||
| Anxious/Depressed (0–26) | 3.13 (3.48) | 2.61 (2.97) | 4.11 (3.78) | 3.61 (3.56) |
| Withdrawn/Depressed (0–16) | 2.83 (2.84) | 2.49 (2.54) | 3.14 (2.77) | 2.99 (2.72) |
| Composite | −0.04 (0.93) | −0.18 (0.79) | 0.16 (0.92) | 0.06 (0.87) |
| Cognitive Health | ||||
| Thought problems (0–30) | 3.56 (2.59) | 2.38 (2.31) | 3.75 (2.73) | 3.60 (2.90) |
| Attention problems (0–20) | 4.23 (3.61) | 4.01 (3.85) | 4.17 (4.13) | 4.33 (4.30) |
| Composite | 0.05 (0.81) | −0.20 (0.81) | 0.08 (0.90) | 0.07 (0.97) |
| Behavioral Health | ||||
| Rule-Breaking Behavior (0–34) | 1.91 (2.31) | 1.39 (1.87) | 1.98 (2.16) | 2.31 (2.61) |
| Aggressive Behavior (0–36) | 3.84 (4.02) | 3.62 (4.22) | 4.54 (4.52) | 3.76 (3.73) |
| Composite | −0.01 (0.91) | −0.15 (0.86) | 0.09 (0.88) | 0.07 (0.92) |
| Social Health | ||||
| Social Problems (0–22) | 1.36 (1.52) | 1.24 (1.81) | 1.86 (2.15) | 1.83 (2.49) |
| Composite | −0.10 (0.75) | −0.16 (0.89) | 0.14 (1.06) | 0.13 (1.23) |
| Physical Health | ||||
| Somatic Complaints (0–22) | 2.89 (3.11) | 2.14 (2.75) | 2.49 (2.74) | 2.01 (2.43) |
| Composite | 0.18 (1.12) | −0.09 (0.99) | 0.03 (0.99) | −0.14 (0.87) |
| Youth EMA Composite Risk Score | ||||
| Emotional health (Positivity Ratio) | 1.47 (0.53) | 1.46 (0.65) | 1.45 (0.42) | 1.44 (0.49) |
| Cognitive health | 6.78 (0.98) | 6.86 (0.87) | 7.09 (1.16) | 6.91 (1.06) |
| Behavioral health | 2.82 (2.79) | 2.28 (2.67) | 3.21 (3.15) | 2.34 (2.53) |
| Social health | ||||
| Alone | 1.45 (0.52) | 1.50 (0.66) | 1.40 (0.42) | 1.43 (0.48) |
| With a family member | 1.72 (0.68) | 1.59 (0.85) | 1.67 (0.60) | 1.60 (0.62) |
| With a peer | 2.10 (0.75) | 2.25 (0.84) | 2.01 (0.76) | 1.93 (0.59) |
| Physical health | 1.47 (0.31) | 1.59 (0.35) | 1.44 (0.32) | 1.53 (0.34) |
Note. SD = Standard Deviation. SD-TST = Sleep diary total sleep time. SD-WUP = Sleep diary wakeup time. Sleepiness = Sleepiness subscale from School Sleep Habits Survey. PSQI = Pittsburgh Sleep Quality Index. CBCL = Child Behavior Checklist (parent-report). CMEP = Children’s Morningness–Eveningness Preferences Scale. CDRS = Children’s Depression Rating Scale. MASC = Multidimensional Anxiety Scale for Children. ACS = Attention Control Scale. YSAS = Youth Social Adjustment Scale. MAQ = Modifiable Activity Questionnaire for Adolescents. PHQ = Physical Health Questionnaire-15. EMA = Ecological Momentary Assessment.
Table 4.
Model Fit Statistics for the Mediation Models
| Mediators | χ2 | df | RMSEA | CFI | TLI | SRMR |
|---|---|---|---|---|---|---|
| Youth Self-Reported Risk Composites (outcome) | ||||||
| CMEP | 109.31 | 75 | 0.05 | 0.96 | 0.94 | 0.07 |
| TST discrepancy | 100.82 | 75 | 0.04 | 0.96 | 0.95 | 0.07 |
| Waketime discrepancy | 107.01 | 75 | 0.05 | 0.96 | 0.94 | 0.07 |
| Sleepiness | 112.57 | 75 | 0.05 | 0.95 | 0.94 | 0.08 |
| PSQI | 104.46 | 75 | 0.05 | 0.96 | 0.95 | 0.07 |
| CBCL Sleep Composite | 124.74 | 75 | 0.06 | 0.94 | 0.92 | 0.07 |
| Parent-Reported Risk Composites (outcome) | ||||||
| CMEP | 337.84 | 171 | 0.07 | 0.92 | 0.90 | 0.07 |
| TST discrepancy | 306.69 | 171 | 0.07 | 0.93 | 0.92 | 0.07 |
| Waketime discrepancy | 326.43 | 171 | 0.07 | 0.92 | 0.90 | 0.07 |
| Sleepiness | 338.13 | 171 | 0.08 | 0.92 | 0.90 | 0.07 |
| PSQI | 329.29 | 171 | 0.07 | 0.92 | 0.91 | 0.07 |
| CBCL Sleep Composite* | 345.45 | 171 | 0.08 | 0.92 | 0.90 | 0.07 |
| EMA (outcome) | ||||||
| CMEP | 218.44 | 137 | 0.06 | 0.94 | 0.93 | 0.07 |
| TST discrepancy | 228.89 | 137 | 0.06 | 0.93 | 0.92 | 0.07 |
| Waketime discrepancy | 228.23 | 137 | 0.06 | 0.93 | 0.92 | 0.07 |
| Sleepiness | 227.38 | 137 | 0.06 | 0.93 | 0.92 | 0.07 |
| PSQI | 226.56 | 137 | 0.06 | 0.94 | 0.92 | 0.07 |
| CBCL Sleep Composite | 210.32 | 137 | 0.06 | 0.95 | 0.94 | 0.07 |
Note. RMSEA = Root-Mean-Square Error of Approximation; CFI = Comparative Fit Index; TLI = Tucker–Lewis index; SRMR = Standardized Root Mean Square Residual. Model chi-square was reported but not used in fit evaluation. CMEP = Children’s Morningness-Eveningness Preferences Scale; TST = Total Sleep Time; PSQI = Pittsburgh Sleep Quality Index; CBCL = Child Behavior Checklist.
For this mediator in relation to parent-reported risk composite (as indexed by CBCL subscales) only, we removed the 7 sleep items that comprised the CBCL Sleep Composite from the CBCL subscale scores so that there was no overlap between the mediator and the outcome.
Table 5.
Mediation Analysis Results for the Health Risk Composites
| Mediators |
Indirect effect (am2x*by2m2) Estimate (95% CI) |
Tx -> Mediator (am2x) Estimate (95% CI) |
Mediator -> Health Risk (by2m2) Estimate (95% CI) |
Tx -> Health Risk (c’y2x) Estimate (95% CI) |
|---|---|---|---|---|
| Youth Self-Reported Risk | ||||
| Composite (outcome) | ||||
| CMEP | −0.10 (-0.22, −0.01) | 0.37 (0.09, 0.62) | −0.28 (-0.46, −0.09) | −0.01 (-0.26, 0.26) |
| TST discrepancy | −0.001 (-0.05, 0.05) | 0.27 (-0.04, 0.58) | −0.01 (-0.15, 0.13) | −0.09 (-0.34, 0.19) |
| Waketime discrepancy | −0.002 (-0.05, 0.04) | 0.18 (-0.15, 0.48) | −0.01 (-0.18, 0.14) | -0.10 (-0.18, 0.14) |
| Sleepiness | −0.06 (-0.14, −0.01) | −0.35 (-0.59, −0.09) | 0.17 (0.04, 0.32) | −0.04 (-0.29, 0.24) |
| PSQI | −0.04 (-0.13, 0.03) | −0.27 (-0.50, −0.03) | 0.14 (-0.11, 0.38) | −0.05 (-0.32, 0.25) |
| CBCL Sleep Composite | −0.10 (-0.20, −0.03) | −0.37 (-0.62, −0.12) | 0.28 (0.10, 0.45) | 0.03 (-0.23, 0.30) |
| Parent-Reported Risk | ||||
| Composite (outcome) | ||||
| CMEP | −0.08 (-0.17, −0.01) | 0.37 (0.10, 0.62) | −0.21 (-0.34, −0.06) | 0.10 (-0.34, 0.14) |
| TST discrepancy | −0.04 (-0.12, 0.003) | 0.31 (0.002, 0.62) | −0.13 (-0.25, −0.01) | −0.12 (-0.37, 0.11) |
| Waketime discrepancy | −0.04 (-0.13, 0.01) | 0.23 (-0.12, 0.54) | −0.16 (-0.32, −0.002) | −0.12 (-0.36, 0.12) |
| Sleepiness | −0.04 (-0.11, 0.02) | −0.37 (-0.61, −010) | 0.11 (-0.05, 0.27) | −0.14 (0.38, 0.10) |
| PSQI | 0.01 (-0.04, 0.06) | −0.25 (-0.48, 0.003) | −0.02 (-0.19, 0.16) | −0.18 (-0.46, 0.09) |
| CBCL Sleep Composite* | −0.17 (-0.34, −0.03) | −0.34 (-0.59, −0.09) | 0.49 (0.26, 0.69) | 0.03 (-0. 15, 0.20) |
| EMA (outcome) | ||||
| CMEP | −0.04 (-0.12, 0.01) | 0.35 (0.08, 0.60) | −0.14 (-0.28, 0.02) | 0.07 (-0.17, 0.33) |
| TST discrepancy | 0.001 (-0.04, 0.05) | 0.26 (-0.06, 0.58) | 0.002 (-0.13, 0.14) | 0.05 (-0.19, 0.30) |
| Waketime discrepancy | 0.01 (-0.03, 0.05) | 0.18 (-0.17, 0.49) | 0.05 (-0.08, 0.17) | 0.06 (-0.17, 0.34) |
| Sleepiness | 0.02 (-0.05, 0.10) | −0.35 (-0.60, −0.09) | −0.07 (-0.22, 0.16) | 0.01 (-0.21, 0.27) |
| PSQI | −0.03 (-0.11, 0.03) | −0.28 (-0.52, −0.03) | 0.09 (-0.10, 0.30) | 0.07 (-0.17, 0.33) |
| CBCL Sleep Composite | −0.06 (-0.14, −0.004) | −0.37 (-0.62, −0.12) | 0.17 (0.02, 0.32) | 0.09 (−0.14, 0.35) |
Notes. All models controlled for sex and age. All estimates are standardized on the dependent variable (interpreted as the change in standard deviation units of y when x changes from 0 to 1). 95% CI = bootstrap confidence interval. Significant effects are in bold. Risk is a latent variable indicated by pre- or post-treatment scores in emotional, cognitive, behavioral, social, and physical health composites for youth self-reported risk and EMA and CBCL subscales for parent-reported risk. CMEP = Children’s Morningness-Eveningness Preferences Scale; TST discrepancy = weekday-weekend discrepancy in Total Sleep Time; Waketime discrepancy = weekday-weekend discrepancy in Waketime; PSQI = Pittsburgh Sleep Quality Index; CBCL = Child Behavior Checklist.
For this mediator in relations to parent-reported risk composite (as indexed by CBCL subscales) only, we removed the 7 sleep items that comprised the CBCL Sleep Composite from the CBCL subscale scores so that there was no overlap between the mediator and the outcome.
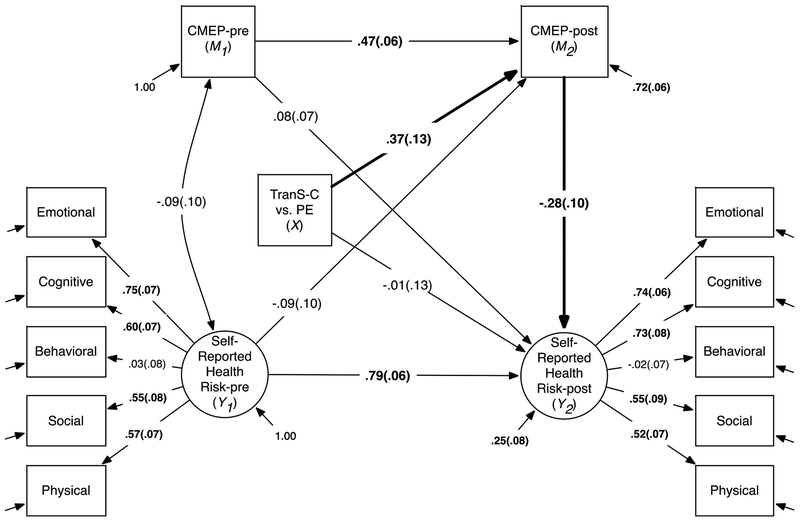
Figure 3.
CMEP Mediates the Effects of TranS-C on Youth Self-Reported Health Risk.
Notes. The indirect paths tested in this model are shown in bold. The indirect effects from TranS-C to Youth Self-Reported Health Risk at posttreatment via CMEP was estimated at −0.10, 95% bootstrap CI: (−0.22, −0.01) as shown in Table 5. CMEP = Children’s Morningness–Eveningness Preferences Scale. Standardized coefficients (STDY standardization) and standard errors are shown on the corresponding paths. Significant coefficients are in bold. Residuals of each indicator variable (e.g., Emotional, Cognitive) for Self-Reported Health Risk are not shown. Sex and age (not shown) are added as covariates in this model.
Aim 1
As shown in Table 5, the indirect effects from TranS-C (vs. PE) to greater reduction in youth self-reported risk in five health-related domains were significant via shifting away from extreme eveningness as indexed by CMEP (indirect effect: −0.10, 95% CI [−0.22, −0.01]), reducing daytime sleepiness (indirect effect: −0.06, 95% CI [−0.14, −0.01]), and reducing parent-reported sleep problems on the CBCL sleep composite (indirect effect: −0.10, 95% CI [−0.20, −0.03]). None of the other indirect effects was significant. Also, there was no evidence of direct effects of treatment condition on any of the health domain outcomes. Significant indirect effects from Table 5 on youth-self-report health risk were illustrated in Figures 3 and supplemental Figures S1–2. Specifically, Figure 3 illustrates the indirect effect of TranS-C on reducing youth self-reported health risk via reducing eveningness as indexed by CMEP. Figure S1 illustrates the indirect effect of TranS-C on reducing youth self-reported health risk via reducing daytime sleepiness. Figure S2 illustrates the indirect effect of TranS-C on reducing youth self-reported health risk via reducing parent-reported sleep problems on the CBCL sleep composite.
Aim 2
As shown in Table 5 and Figure S3, the indirect effect from TranS-C (vs. PE) to reduced parent-reported risk in five health-related domains was significant via shifting away from extreme eveningness as indexed by CMEP (indirect effect: −0.08, 95% CI [−0.17, −0.01]). As shown in Table 5 and Figure S4, the indirect effect from TranS-C (vs. PE) to reduced parent-reported risk in five health-related domains was significant via reducing parent-reported sleep problems on the CBCL sleep composite (indirect effect: −0.17, 95% CI [−0.34, −0.03]). None of the other indirect effects was significant.
Aim 3
As shown in Table 5 and Figure S5, the indirect effect from TranS-C (vs. PE) to reduced EMA-derived risk in five health-related domains was significant via parent-reported sleep problems on the CBCL sleep composite (indirect effect: −0.06, 95% CI [−0.14, −0.004]). None of the other indirect effects was significant.
Discussion
In a sample of 176 adolescents aged 10 to 18 years, the present study examined whether improvements in sleep and circadian problems mediated the effects of the TranS-C, relative to PE, on reducing risk in emotional, cognitive, behavioral, social, and physical health domains. As predicted, we found that 1) shifting away from extreme eveningness preference mediated the effects of TranS-C (vs. PE) on reducing youth self-reported as well as parent-reported risk in the five health domains, 2) a reduction in daytime sleepiness mediated the effects of TranS-C (vs. PE) on reducing parent-reported risk in the five health domains, and 3) a reduction in parent-reported sleep-wake problems mediated the effects of TranS-C (vs. PE) on reducing risks in the five health domains measured by youth self-report, parent-report, and EMA. We note that in a previous report we showed that greater reduction in eveningness associated with TranS-C (vs. PE) was in concordance with evidence of circadian phase advancing measured using dim light melatonin onset from pre to posttreatment (Harvey et al., 2018), suggesting that changes in morningness-eveningness preference may reflect a shift in circadian phase preference.
These findings are in line with previous research suggesting that sleep disturbances can contribute to and exacerbate psychopathology (e.g., Bei, Manber, Allen, Trinder, & Wiley, 2016; McMakin et al., 2016). In addition, improving sleep may reduce psychopathology. Specifically, research shows that treating insomnia not only improves sleep but also reduces mood episodes, and improves functioning in adult bipolar individuals (Harvey et al., 2015). In depressed adolescents, treating sleep issues improves both sleep and depression outcomes (Clarke et al., 2015). In adolescents with insomnia, improvements in insomnia mediated the effects of CBT for insomnia on long-term reduction in self-reported psychological symptoms, including affective, anxiety, somatic, attention deficit and hyperactivity disorder, and oppositional problems (de Bruin et al., 2017). Our results are also consistent with previous research suggesting that sleep intervention improves both sleep and academic performance in children (e.g., Gruber, Somerville, Bergmame, Fontil, & Paquin, 2016). Our results extend the literature by adding evidence for a transdiagnostic intervention for sleep and circadian problems as well as documenting the mediating effects of improved sleep and circadian problems on both mental and physical outcomes in adolescents. In addition, we acknowledge that the relationships between sleep/circadian outcomes and health outcomes are likely to be bi-directional (e.g., Harvey, 2008), which is an important direction for future research.
We were surprised that there was no evidence for the other putative mediators, namely weekday-weekend discrepancy for TST and waketime as well as PSQI. Nevertheless, the indirect effects were in the expected direction but did not reach statistical significance for TST and waketime discrepancy on the parent-reported risk composite and PSQI on the EMA-assessed risk composite. One future direction may be to evaluate potential moderators (e.g., age group, sex, pubertal status) of TranS-C to understand whether the tested mediation was only prominent in certain subgroups. This may be particularly important for TST and waketime discrepancy, because the sleep diary variables exhibited greater variabilities in the current sample. In addition, there was more significant findings of indirect effects for youth self-reported and parent-reported health risk compared to EMA. One possible explanation for the lack of findings from EMA-assessed health outcomes is that averaging 18 assessments over 1-week period might cancel out interesting intra-individual variability. Future studies with larger sample size may consider modeling EMA data using dynamic structural equation modeling rather than simply using the means.
It is worth noting that in our prior report we found no significant direct effects of TranSC, above and beyond the effects of PE, on the composite risk scores in the five health domains (Harvey et al., 2018), though there were direct effects on some specific measures in the five health domains. However, the current mediation results suggest that there were indirect effects of TranS-C (vs. PE) on reducing risk in multiple health domains via selected sleep and circadian variables. Overall, these findings support further studying the use of TranS-C in adolescents and other populations not only on reducing sleep and circadian problems but also on its potential broad benefits in mental and physical health. It is important to note that TranS-C takes a transdiagnostic rather than disorder-focused approach. Thus, it could address complex cooccurring sleep and circadian problems seen in real-world clinical setting and reduce clinician’s burden for training.
Several limitations should be noted. First, this study did not include a no-treatment control condition. Therefore, interpretation of the current results should consider the fact that participants in the PE condition also showed pre-to-post improvement in a number of sleep, circadian, and health outcomes, which has been documented in the literature (e.g., Colom, Vieta, & Scott, 2006). It is possible that we would observe even larger treatment effects of TranS-C, relative to no treatment. Second, the current study only included primary and secondary outcomes listed on the trial registry with one exception. One secondary outcome of the trial, dim light melatonin onset, will be examined along with other biological variables in a separate report. Because we only examined primary and secondary outcomes listed on trial registry, a resultant limitation is that no objective measures of sleep (e.g., actigraphy) were included. Third, the associations between self-report measures could be due to shared method variance. Future research should replicate the current findings using a variety of methods such as objective measures of sleep. The fourth limitation is the number of models and the potential problem of multiple comparisons. However, the mediator and outcome variables were theoretically and empirically correlated constructs; thus the 18 mediation models tested in this paper were correlated. Hence, introducing a correction for multiple comparisons may reduce statistical power. We have also emphasized interpreting the effect size estimates and the confidence intervals, which is consistent with the current recommendations (Cumming, 2012, 2014).
Furthermore, in the current study we were unable to evaluate a comprehensive model whereby multi-informants, multi-methods of assessment are all incorporated due to the limited sample size. Future studies, with adequate sample size, should examine the fit and utility of a measurement model where youth-report, parent-report, EMA, or other measures of health are all incorporated. This model may help consolidate evidence and resolve problems of discrepancies across informants and methods. Relatedly, when interpreting results related to the health outcomes, we note that the specific measures that comprise the health domains may vary across respondents (youth vs. parent-report), particularly in the cognitive and behavioral domains. For example, in the cognitive domain, youth-report includes measures of attention and school-related problems, whereas parent-report includes measures of attention and thought problems; in the behavioral domain, youth-report includes measures of sensation seeking and substance use, whereas parent-report includes measures of rule-breaking and aggressive behaviors. Hence, the discrepancy of results comparing youth versus parent-reported health risk should be interpreted with this limitation in mind.
In sum, the current study conducted a mediation analysis and found evidence for indirect effects of a novel transdiagnostic sleep and circadian treatment TranS-C, relative to PE, on reducing adolescent risk in five health domains via improved sleep and circadian problems. Findings highlight the important role of sleep and circadian problems in adolescent health, and support the use of a short, transdiagnostic sleep and circadian intervention that may improve both sleep and circadian problems as well as reduce risk in multiple health domains.
Supplementary Material
Public Health Significance Statement:
This study reports indirect effects of a novel transdiagnostic sleep and circadian treatment (TranS-C), relative to psychoeducation, on reducing risk in five health domains via improved sleep and circadian problems in adolescents. This study suggests that by addressing sleep and circadian problems TranS-C may improve health in multiple domains. Findings also highlight the important role of sleep and circadian problems in adolescent health.
Acknowledgments
This research was supported by National Institute of Child Health and Human Development R01HD071065. Clinical trial registration: (https://clinicaltrials.gov/show/NCT01828320). The authors declared no conflicts of interest.
Appendix
Data Transparency Statement
The data reported in this manuscript have been previously published and were collected as part of a larger data collection (at one or more points in time). Findings from the data collection have been reported in separate manuscripts. MS 1 (published) focuses on TranS-C treatment effects from pre to posttreatment on outcomes specified in the clinical trial registry; while MS 2 (the current manuscript) focuses on a secondary data analysis of this RCT testing treatment mediators using pre and posttreatment data. Two other manuscripts used the pretreatment/baseline data: MS 3 (published) investigated relationships between endogenous circadian phase and affect using only pretreatment data; MS 4 (published) focuses on the relationship between food choice and sleep improvement using a a small subset of participants (n = 42) on a specific task that is unrelated/non-overlapping with the current submission. Note that due to burden on participants, the food choice task in this paper was cut from the protocol after the 42nd participants. In contrast, the submitted manuscript is based on n = 176; MS 5 (published) focuses on the relationship between a composite measure of sleep health and risks in the five health domains using only pre-treatment data
References
- Aaron DJ, & Kriska AM (1997). Modifiable activity questionnaire for adolescents. Medicine and Science in Sports and Exercise, 29, s79–s82. [Google Scholar]
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA School-Age Forms & Profiles: Child Behavior Checklist for Ages 6 – 18, Teacher’s Report Form, & Youth Self-Report. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families. [Google Scholar]
- Adan A, Natale V, Caci H, & Prat G (2010). Relationship between circadian typology and functional and dysfunctional impulsivity. Chronobiology International, 27(3), 606–619. 10.3109/07420521003663827 [DOI] [PubMed] [Google Scholar]
- Becker SP, Ramsey RR, & Byars KC (2015). Convergent validity of the Child Behavior Checklist sleep items with validated sleep measures and sleep disorder diagnoses in children and adolescents referred to a sleep disorders center. Sleep Medicine, 16(1), 79–86. 10.1016/j.sleep.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Bei B, Manber R, Allen NB, Trinder J, & Wiley JF (2016). Too long, too short, or too variable? Sleep intraindividual variability and its associations with perceived sleep quality and mood in adolescents during naturalistically unconstrained sleep. Sleep, 5(2). 10.1093/sleep/zsw067 [DOI] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238–246. 10.1037/0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- Blake MJ, Sheeber LB, Youssef GJ, Raniti MB, & Allen NB (2017). Systematic Review and Meta-analysis of Adolescent Cognitive-Behavioral Sleep Interventions. Clinical Child and Family Psychology Review, 20(3), 227–249. 10.1007/s10567-017-0234-5 [DOI] [PubMed] [Google Scholar]
- Blake MJ, Waloszek JM, Schwartz O, Raniti M, Simmons JG, Blake L, … Allen NB (2016). The SENSE study: Post intervention effects of a randomized controlled trial of a cognitive–behavioral and mindfulness-based group sleep improvement intervention among at-risk adolescents. Journal of Consulting and Clinical Psychology, 84(12), 1039–1051. 10.1037/ccp0000142 [DOI] [PubMed] [Google Scholar]
- Browne MW, & Cudeck R (1993). Alternative ways of assessing model fit In Bollen KA & Long JS (Eds.), Testing Structural Equation Models (pp. 136–162). Beverly Hills, CA: Sage. [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, & Morin CM (2006). Recommendations for a standard research assessment of insomnia. Sleep, 29(9), 1155–1173. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, R. CF III, … Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carlson JA, Schipperijn J, Kerr J, Saelens BE, Natarajan L, Frank LD, … Sallis JF (2016). Locations of Physical Activity as Assessed by GPS in Young Adolescents. Pediatrics, 137(1), e20152430 10.1542/peds.2015-2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The Consensus Sleep Diary: Standardizing Prospective Sleep Self-Monitoring. Sleep, 35(2), 287–302. 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA (2011). Sleep in Adolescents: The Perfect Storm. Pediatric Clinics of North America, 58(3), 637–647. 10.1016/j.pcl.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Seifer R, & Acebo C (1991). Reliability of six scales in a sleep questionnaire for adolescents. Sleep Research, 20, 421. [Google Scholar]
- Carskadon MA, Vieira C, & Acebo C (1993). Association between puberty and delayed phase preference. Sleep, Vol. 16, pp. 258–262. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8506460 [DOI] [PubMed] [Google Scholar]
- Clarke G, McGlinchey EL, Hein K, Gullion CM, Dickerson JF, Leo MC, & Harvey AG (2015). Cognitive-behavioral treatment of insomnia and depression in adolescents: A pilot randomized trial. Behaviour Research and Therapy, 69, 111–118. 10.1016/j.brat.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom F, Vieta E, & Scott J (2006). Psychoeducation manual for bipolar disorder. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Crowley SJ, Acebo C, & Carskadon MA (2007). Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Medicine, 8(6), 602–612. 10.1016/j.sleep.2006.12.002 [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Bushnell DL, Acebo C, & Carskadon MA (2006). Internal consistency and construct validity of two morningness/eveningness questionnaires in children. Sleep, 29(Supplem, A66. [Google Scholar]
- Cumming G (2012). Understanding the new statistics: Effect sizes, confidence intervals, and meta-analysis. Routledge. [Google Scholar]
- Cumming G (2014). The New Statistics: Why and How. Psychological Science, 25(1), 7–29. 10.1177/0956797613504966 [DOI] [PubMed] [Google Scholar]
- Dagys N, McGlinchey EL, Talbot LS, Kaplan KA, Dahl RE, & Harvey AG (2012). Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. Journal of Child Psychology and Psychiatry and Allied Disciplines, 53(6), 660–667. 10.1111/j.1469-7610.2011.02502.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vega R, Tomé-Pires C, Solé E, Racine M, Castarlenas E, Jensen MP, & Miró J (2015). The Pittsburgh Sleep Quality Index: Validity and factor structure in young people. Psychological Assessment, 27(4), e22–7. 10.1037/pas0000128 [DOI] [PubMed] [Google Scholar]
- Derryberry D, & Reed MA (2002). Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology, 111(2), 225–236. 10.1037/0021-843X.111.2.225 [DOI] [PubMed] [Google Scholar]
- Devilly GJ, & Borkovec TD (2000). Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry, 31(2), 73–86. 10.1016/S0005-7916(00)00012-4 [DOI] [PubMed] [Google Scholar]
- Díaz‐Morales JF, de Leon MCD, & Sorroche MG (2007). Validity of the morningness-eveningness scale for children among Spanish adolescents. Chronobiology International, 24(3), 435–447. [DOI] [PubMed] [Google Scholar]
- Diener E (2000). Subjective well-being: The science of happiness and a proposal for a national index. American Psychologist, 55(1), 34–43. 10.1037/0003-066X.55.1.34 [DOI] [PubMed] [Google Scholar]
- Digdon NL, & Howell AJ (2008). College students who have an eveningness preference report lower self-control and greater procrastination. Chronobiology International, 25(6), 1029–1046. 10.1080/07420520802553671 [DOI] [PubMed] [Google Scholar]
- Dupéré V, Dion E, Nault-Brière F, Archambault I, Leventhal T, & Lesage A (2017). Revisiting the Link Between Depression Symptoms and High School Dropout: Timing of Exposure Matters. Journal of Adolescent Health, 1–7. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wyatt JK, Olsen MK, Stechuchak KM, Carney CE, Chiang A, … Radtke RA (2009). Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening. Sleep, 32, A265–A265. [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, … Monk T (2005). Two-Year Outcomes for Interpersonal and Social Rhythm Therapy in Individuals With Bipolar I Disorder. Archives of General Psychiatry, 62(9), 996 10.1001/archpsyc.62.9.996 [DOI] [PubMed] [Google Scholar]
- Gau SS-F, Shang C-Y, Merikangas KR, Chiu Y-N, Soong W-T, & Cheng AT-A (2007). Association between Morningness-Eveningness and Behavioral/Emotional Problems among Adolescents. Journal of Biological Rhythms, 22(3), 268–274. 10.1177/0748730406298447 [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, & Ottaviano S (2002). Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research, 11(3), 191–199. 10.1046/j.1365-2869.2002.00302.x [DOI] [PubMed] [Google Scholar]
- Goldstein D, Hahn CS, Hasher L, Wiprzycka UJ, & Zelazo PD (2007). Time of day, intellectual performance, and behavioral problems in Morning versus Evening type adolescents: Is there a synchrony effect? Personality and Individual Differences. 10.1016/j.paid.2006.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradisar M, Gardner G, & Dohnt H (2011). Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Medicine, 12(2), 110–118. 10.1016/j.sleep.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, & Czeisler CA (2013). The sleep and technology use of Americans: Findings from the National Sleep Foundation’s 2011 sleep in America poll. Journal of Clinical Sleep Medicine, 9(12), 1291–1299. 10.5664/jcsm.3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, & Sadeh A (2016). Annual Research Review: Sleep problems in childhood psychiatric disorders - a review of the latest science. Journal of Child Psychology and Psychiatry, 57(3), 296–317. 10.1111/jcpp.12469 [DOI] [PubMed] [Google Scholar]
- Gruber R, Somerville G, Bergmame L, Fontil L, & Paquin S (2016). School-based sleep education program improves sleep and academic performance of school-age children. Sleep Medicine, 21, 93–100. 10.1016/j.sleep.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Harvey AG (2008). Insomnia, psychiatric disorders, and the transdiagnostic perspective. Current Directions in Psychological Science, 17(5), 299–303. 10.1111/j.1467-8721.2008.00594.x [DOI] [Google Scholar]
- Harvey AG (2009). A Transdiagnostic Approach to Treating Sleep Disturbance in Psychiatric Disorders. Cognitive Behaviour Therapy, 38(sup1), 35–42. 10.1080/16506070903033825 [DOI] [PubMed] [Google Scholar]
- Harvey AG (2016). A Transdiagnostic Intervention for Youth Sleep and Circadian Problems. Cognitive and Behavioral Practice, 23(3), 341–355. 10.1016/j.cbpra.2015.06.001 [DOI] [Google Scholar]
- Harvey AG, & Buysse DJ (2017). Treating Sleep Problems: A Transdiagnostic Approach. New York, NY: The Guilford Press. [Google Scholar]
- Harvey AG, Hein K, Dolsen MR, Dong L, Rabe-Hesketh S, Gumport NB, … Blum DJ (2018). Modifying the Impact of Eveningness Chronotype (“Night-Owls”) in Youth: A Randomized Controlled Trial. Journal of the American Academy of Child & Adolescent Psychiatry, 57(10), 742–754. 10.1016/j.jaac.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Murray G, Chandler RA, & Soehner A (2011). Sleep disturbance as transdiagnostic: Consideration of neurobiological mechanisms. Clinical Psychology Review, 31(2), 225–235. 10.1016/j.cpr.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Soehner AM, Kaplan K. a., Hein K, Lee J, Kanady J, … Buysse DJ (2015). Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: A pilot randomized controlled trial. Journal of Consulting and Clinical Psychology, 83(3), 564–577. 10.1037/a0038655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W, Boomsma A, & Reinecke S (2007). The model-size effect on traditional and modified tests of covariance structures. Structural Equation Modeling, 14(3), 361–390. 10.1080/10705510701301602 [DOI] [Google Scholar]
- Hoyle RH, Stephenson MT, Palmgreen P, Lorch EP, & Donohew RL (2002). Reliability and validity of a brief measure of sensation seeking. Personality and Individual Differences, 32(3), 401–414. 10.1016/S0191-8869(01)00032-0 [DOI] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Jessor R, Donovan JE, & Costa F (2017). Problem Behavior Theory and Behavioral Health in Adolescence In Problem Behavior Theory and Adolescent Health : The Collected Works of Richard Jessor, Volume 2 (pp. 449–475). 10.1007/978-3-319-51349-2_22 [DOI] [Google Scholar]
- Johnston LD, Malley PMO, Bachman JG, & Schulenberg JE (2009). Monitoring the Future: National Results on Adolescent Drug Use. Overview of Key Findings, 2008. National Institute on Drug Abuse (NIDA), 1–90. [Google Scholar]
- Kanning M, & Schlicht W (2010). Be Active and Become Happy: An Ecological Momentary Assessment of Physical Activity and Mood. Journal of Sport and Exercise Psychology, 32(2), 253–261. 10.1123/jsep.32.2.253 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kim S, Dueker GL, Hasher L, & Goldstein D (2002). Children’s time of day preference: age, gender and ethnic differences. Personality and Individual Differences, 33(7), 1083–1090. [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, & Agras WS (2002). Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry, 59(10), 877 10.1001/archpsyc.59.10.877 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JBW (2002). The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine, 64(2), 258–266. 10.1097/00006842-200203000-00008 [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE, Rudolph KD, Potter KI, Lambert S, … Gathright T (1999). A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment, 11(3), 326–338. 10.1037/1040-3590.11.3.326 [DOI] [Google Scholar]
- MacKinnon DP (2008). Introduction to statistical mediation analysis. New York, NY: Lawrence Erlbaum Associates Taylor & Francis Group. [Google Scholar]
- MacKinnon DP, Lockwood CM, & Williams J (2004). Confidence Limits for the Indirect Effect: Distribution of the Product and Resampling Methods. Multivariate Behavioral Research, 39(1), 99–128. 10.1207/s15327906mbr3901_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Sullivan K, & Parker J (1999). Test-Retest Reliability of the Multidimensional Anxiety Scale for Children. Journal of Anxiety Disorders, 13(4), 349–358. 10.1016/S0887-6185(99)00009-2 [DOI] [PubMed] [Google Scholar]
- Maslowsky J, & Ozer EJ (2014). Developmental trends in sleep duration in adolescence and young adulthood: Evidence from a national United States sample. Journal of Adolescent Health, 54(6), 691–697. 10.1016/j.jadohealth.2013.10.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Dahl RE, Buysse DJ, Cousins JC, Forbes EE, Silk JS, … Franzen PL (2016). The impact of experimental sleep restriction on affective functioning in social and nonsocial contexts among adolescents. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57(9), 1027–1037. 10.1111/jcpp.12568 [DOI] [PubMed] [Google Scholar]
- Miller AL, Lumeng JC, & LeBourgeois MK (2015). Sleep patterns and obesity in childhood. Current Opinion in Endocrinology & Diabetes and Obesity, 22(1), 41–47. 10.1097/MED.0000000000000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (2002). Motivational interviewing: Preparing people for change. New York: Guilford Press. [Google Scholar]
- Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, & Colantonio A (2016). The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Medicine Reviews, 25, 52–73. 10.1016/j.smrv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, & Lichstein KL (2006). Psychological And Behavioral Treatment Of Insomnia: Update Of The Recent Evidence (1998–2004). Sleep, 29(11), 1398–1414. 10.1093/sleep/29.11.1398 [DOI] [PubMed] [Google Scholar]
- Morin CM, & Espie CA (2003). Insomnia: A clinical guide to assessment and treatment. New York, NY: Kluwer Academic/Plenum Publishers. [Google Scholar]
- Moshagen M (2012). The model size effect in SEM: Inflated goodness-of-fit statistics are due to the size of the covariance matrix. Structural Equation Modeling, 19(1), 86–98. 10.1080/10705511.2012.634724 [DOI] [Google Scholar]
- Muthén LK, & Muthén BO (2017). Mplus User’s Guide (Eighth). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Negriff S, Dorn LD, Pabst SR, & Susman EJ (2011). Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Research, 185(3), 408–413. 10.1016/j.psychres.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović J, Burt KB, & Masten AS (2010). Testing a dual cascade model linking competence and symptoms over 20 years from childhood to adulthood. Journal of Clinical Child and Adolescent Psychology, 39(1), 90–102. 10.1080/15374410903401120 [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Carskadon MA, Jean-Louis G, Owens J, Bramoweth A, Combs D, … Buysse D (2016). Implementation of Sleep and Circadian Science: Recommendations from the Sleep Research Society and National Institutes of Health Workshop. Sleep, 39(12), 2061–2075. 10.5665/sleep.6300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Aloia MS, & Kuhn B (2011). Behavioral Treatment for Sleep Disorders: A Comprehensive Primer of Behavioral Sleep Medicine. Amsterdam: Elsevier. [Google Scholar]
- Polanczyk GV, Salum GA, Sugaya LS, Caye A, & Rohde LA (2015). Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. Journal of Child Psychology and Psychiatry and Allied Disciplines, 56(3), 345–365. 10.1111/jcpp.12381 [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, & Gibbons R (1984). Preliminary studies of the reliability and validity of the children’s depression rating scale. Journal of the American Academy of Child Psychiatry, 23(2), 191–197. 10.1097/00004583-198403000-00011 [DOI] [PubMed] [Google Scholar]
- Randler C (2008). Differences between smokers and nonsmokers in morningness-eveningness. Social Behavior and Personality, 36(5), 673–680. 10.2224/sbp.2008.36.5.673 [DOI] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, & Merrow M (2004). A marker for the end of adolescence. Current Biology, 14(24), R1038–R1039. 10.1016/j.cub.2004.11.039 [DOI] [PubMed] [Google Scholar]
- Rofey DL, Hull EE, Phillips J, Vogt K, Silk JS, & Dahl RE (2010). Utilizing Ecological Momentary Assessment in Pediatric Obesity to Quantify Behavior, Emotion, and Sleep. Obesity, 18(6), 1270–1272. 10.1038/oby.2009.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo PM, Bruni O, Lucidi F, Ferri R, & Violani C (2007). Sleep habits and circadian preference in Italian children and adolescents. Journal of Sleep Research, 16(2), 163–169. [DOI] [PubMed] [Google Scholar]
- Schaal S, Peter M, & Randler C (2010). Morningness-eveningness and physical activity in adolescents. International Journal of Sport and Exercise Psychology, 8(2), 147–159. 10.1080/1612197X.2010.9671939 [DOI] [Google Scholar]
- Short MA, Arora T, Gradisar M, Taheri S, & Carskadon MA (2017). How Many Sleep Diary Entries Are Needed to Reliably Estimate Adolescent Sleep? Sleep, 40(3). 10.1093/sleep/zsx006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, & Wright HR (2013). The impact of sleep on adolescent depressed mood, alertness and academic performance. Journal of Adolescence, 36(6), 1025–1033. 10.1016/j.adolescence.2013.08.007 [DOI] [PubMed] [Google Scholar]
- Silk JS, Forbes EE, Whalen DJ, Jakubcak JL, Thompson WK, Ryan ND, … Dahl RE (2011). Daily emotional dynamics in depressed youth: A cell phone ecological momentary assessment study. Journal of Experimental Child Psychology, 110(2), 241–257. 10.1016/j.jecp.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, & Dorn LD (2007). Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Developmental Psychology, 43(4), 811–822. 10.1037/0012-1649.43.4.811 [DOI] [PubMed] [Google Scholar]
- Trauer JM, Qian MY, Doyle JS, Rajaratnam SMW, & Cunnington D (2015). Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Annals of Internal Medicine, 163(3), 191–204. 10.7326/M14-2841 [DOI] [PubMed] [Google Scholar]
- Valente MJ, & MacKinnon DP (2017). Comparing Models of Change to Estimate the Mediated Effect in the Pretest–Posttest Control Group Design. Structural Equation Modeling: A Multidisciplinary Journal, 24(3), 428–450. 10.1080/10705511.2016.1274657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, & Lancee J (2018). Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Medicine Reviews, 38, 3–16. 10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Viner RM, Ozer EM, Denny S, Marmot M, Resnick M, Fatusi A, & Currie C (2012). Adolescence and the social determinants of health. The Lancet, 379(9826), 1641–1652. 10.1016/S0140-6736(12)60149-4 [DOI] [PubMed] [Google Scholar]
- Weissman MM, Orvaschel H, & Padian N (1980). Children’s symptom and social functioning self-report scales: Comparison of mothers’ and children’s reports. The Journal of Nervous and Mental Disease, Vol. 168, pp. 736–740. 10.1097/00005053-198012000-00005 [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, & Terman M (2009). Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light & Wake Therapy. Basel: Karger. [Google Scholar]
- Wittmann M, Dinich J, Merrow M, & Roenneberg T (2006). Social Jetlag: Misalignment of Biological and Social Time. Chronobiology International, 23(1–2), 497–509. 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- Wolfson AR, & Carskadon MA (1998). Sleep Schedules and Daytime Functioning in Adolescents. Child Development, 69(4), 875–887. 10.1111/j.1467-8624.1998.tb06149.x [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, … Martin JL (2003). Evidence for the validity of a sleep habits survey of adolescents Evidence for the Validity of a Sleep Habits Survey for Adolescents. Sleep, 26(2), 9–12. [DOI] [PubMed] [Google Scholar]
- Young J, & Beck AT (1980). Cognitive Therapy Scale: Rating Manual. Philadelphia, PA: University of Pennsylvania. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.