Abstract
Serial biopsy of pancreatic ductal adenocarcinoma (PDAC), to chart tumour evolution presents a significant challenge. We examined the utility of circulating free DNA (cfDNA) as a minimally invasive approach across a cohort of 55 treatment-naïve patients with PDAC; 31 with metastatic and 24 with locally advanced disease. Somatic mutations in cfDNA were detected using next generation sequencing in 15/24 (62.5%) and 27/31 (87%) of patients with locally advanced and metastatic disease, respectively. Copy number changes were detected in cfDNA of 10 patients of whom 7 exhibited gain of chromosome 12p harbouring KRAS as well as a canonical KRAS codon 12 mutation. In multivariable Cox Regression analysis, we show for the first time that patients with KRAS copy number gain and KRAS mutation have significantly worse outcomes, suggesting that this may be linked to PDAC progression. The simple cfDNA assay we describe will enable determination of the presence of KRAS copy number gain and KRAS mutations in larger studies and clinical trials.
Subject terms: Tumour biomarkers, Cancer genomics
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease with <7% 5-year survival1 and increasing worldwide incidence2. Poor patient outcomes are attributed to several factors, including late diagnosis, chemotherapy resistance and the absence of druggable targets to improve patient outcomes3. Obtaining tumour biopsies is challenging and carbohydrate antigen 19-9 (CA 19-9), the only approved circulating biomarker for routine clinical management of PDAC (National Comprehensive Cancer Network [NCCN] guidelines) is limited by sub-optimal sensitivity and specificity. More recently, circulating cell free DNA (cfDNA) has been proposed as a minimally invasive alternative to traditional blood-based protein biomarkers and invasive tissue biomarkers for many solid cancer types, including PDAC4,5. A previous study detected KRAS mutations in cfDNA of 58.9% of patients with PDAC with distant metastasis and 18.2% of patients with locally advanced disease6. In this pilot study, we evaluated targeted KRAS sequencing and broad next-generation sequencing (NGS) analysis of 641 cancer-associated genes in the cfDNA of 55 patients with PDAC to evaluate the potential clinical utility of cfDNA in PDAC (Fig. 1A).
Figure 1.
(A) Sample Workflow. This flowchart explains the workflow used in this study starting from whole blood samples collected in this study to the analysis performed. (B) Combined copy number and mutational analysis of cfDNA. Combined mutational and copy number plot for the 85 genes that were positive for at least 1 mutation across all 55 cfDNA samples analysed. Boxes coloured orange represent mutation and a copy number gain (CNG), yellow mutation and copy number loss (CNL), green mutation, red CNG, and blue for CNL. (C) Kaplan-Meier analysis of the overall survival according to KRAS mutation alone (7/55), KRAS mutation and copy number gain (7/55), along with KRAS wild-type (41/55) in 55 patients revealed best prognosis for patients with KRAS wild-type, with a median survival of 10.6 months, followed by patients with KRAS mutation alone with a median survival of 5.6 months. Patients with both the KRAS mutation and copy number gain had the worst prognosis with a median survival of 2.5 months (overall Log-rank test p-value = < 0.0001). KRAS WT vs KRAS MUT only Log-rank p-value = 0.0610, KRAS WT vs KRAS CN gain + MUT Log-rank p = value = 0.0012 and KRAS MUT only vs KRAS CN gain + MUT Log-rank p-value = < 0.0001. (D) Hazard ratios for the factors that were used in a multivariable analysis.
Results
A total of 55 treatment-naïve patients with PDAC were identified (between Feb 2011 to Apr 2014); 24 with locally advanced disease and 31 with metastatic disease. The clinical details including age, gender, performance status and metastatic sites are provided in the Supplementary Table 1.
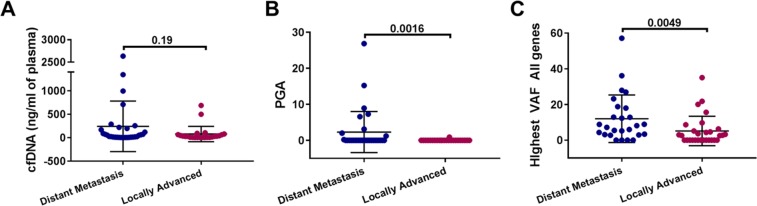
No somatic mutations or copy number alterations were detected in 16 non-cancer controls (Table 1). No significant differences were observed in yield of cfDNA detected between the 31 patients with metastatic and 24 with locally advanced PDAC (p-value = 0.19; Fig. 2). From cfDNA NGS, both CNA and somatic mutations were elevated in the patients with metastatic disease compared to the patients with locally advanced disease (p-values of 0.0164 and 0.0049, respectively, Fig. 2B,C). Somatic mutations were detected in 87% (27/31) and 54% (13/24) of the samples from patients with metastatic and locally advanced disease, respectively. Known non-synonymous activating KRAS mutations, confirmed by ddPCR, were detected in 35% (11/31) and 12.5% (3/24) of samples from patients with metastatic and locally advanced disease respectively. In addition to the 14 mutations detected by NGS, a further seven KRAS mutations (four metastatic, three locally advanced) were detected using ddPCR, which were below the 2.5% VAF (Variant Allele frequency) threshold used for NGS analysis (Fig. 1B). In keeping with previous studies, NGS of cfDNA from the patiens with metastatic disease also identified canonical TP53 and KMT2D mutations at frequencies of 29% (9/31) and 16% (5/31) respectively6 (Fig. 1B).
Table 1.
Univariate and multivariable Cox regression analysis for prediction of OS.
| Parameter | At risk group | HR [95% CI] | p-value | |
|---|---|---|---|---|
| Univariate analysis | ||||
| Gender | Male (vs Female) | 1.68 [0.97, 2.89] | 0.064 | |
| Age | Continuous | 0.99 [0.97, 1.02] | 0.545 | |
| ECOG Performance status | 1 (vs 0) | 1.77 [0.77, 4.08] | 0.179 | |
| >=2 (vs)) | 4.34 [1.72, 10.96] | 0.002 | ||
| ORR | Response (vs no response) | 0.31 [0.13, 0.76] | 0.010 | |
| Metastasis | Number of metastatic sites | >=2 sites (vs <2 sites) | 1.31 [0.66, 2.62] | 0.44 |
| Liver metastasis | Yes (vs No) | 3.05 [1.70, 5.51] | <0.001 | |
| Lung metastasis | Yes (vs No) | 0.77 [0.35, 1.67] | 0.505 | |
| Other sites | Yes (vs No) | 1.61 [0.68, 3.83] | 0.283 | |
| WCC$ | Continuous | 1.18 [1.11, 1.25] | <0.001 | |
| Neutrophils$ | Continuous | 1.19 [1.11, 1.26] | <0.001 | |
| Lymphocytes | Continuous | 0.72 [0.46, 1.12] | 0.143 | |
| LDH | Continuous | 1.001 [1.000, 1.003] | 0.003 | |
| Ca 19.9 (Log2) | Continuous | 1.07 [0.99, 1.16] | 0.091 | |
| CNA | Continuous | 7.09 [3.19, 15.78] | <0.001 | |
| Mutational burden | Number of mutations$ | Continuous | 1.06 [1.01, 1.12] | 0.028 |
| Number of KRAS mutations$ | Continuous | 1.11 [1.07, 1.16] | <0.001 | |
| KRAS mutations present | Yes (vs No) | 3.46 [1.76, 6.77] | <0.001 | |
| KRAS copy number gain | Yes (vs No) | 10.94 [3.85, 31.08] | <0.001 | |
| Highest VAF | Continuous | 1.07 [1.04, 1.10] | <0.001 | |
| Multivariable analysis | ||||
| ECOG Performance status | 1 (vs 0) | 2.51 [0.98, 6.38] | 0.053 | |
| >=2 (vs 0) | 4.20 [1.48, 11.94] | 0.007 | ||
| Metastasis | Liver metastasis | Yes (vs No) | 2.83 [1.28, 6.24] | 0.010 |
| Mutational burden | KRAS copy number gain | Yes (vs No) | 3.47 [1.19, 10.17] | 0.023 |
| Highest VAF | Continuous | 1.05 [1.01, 1.08] | 0.005 | |
Abbreviations: ORR, objective response rate (clinical outcome variable); VAF: variant allele frequency; ECOG, Eastern Cooperative Oncology Group; WCC: white cell count; $, Excluded from stepwise model building due to collinearity.
Figure 2.
Comparison between patients with distant metastasis and locally advanced disease for (A) Yield of cf DNA in ng/ml of plasma (B). Percent Genome Amplified (PGA) and (C) highest VAF.
Measurable copy number alterations (CNA) were detected in 10 of the 55 patients’ cfDNA samples (nine metastatic, one locally advanced), of whom seven exhibited a gain in chromosome 12p that harbours KRAS (Fig. 1B). All seven PDAC cfDNA samples with copy number gain (CNG) of KRAS also exhibited non-synonymous somatic mutations in KRAS (Fig. 1B and Supplementary Table 1).
Kaplan-Meier analysis of overall survival (OS) based on KRAS mutation alone (7/55), KRAS mutation and CNG (7/55) and with KRAS wild-type (34/55), revealed best prognosis for patients with KRAS wild-type (median survival 10.6 months), followed by patients with KRAS mutation without CNG (median survival 5.5 months). The worst prognosis was associated with the combination of a KRAS mutation and CNG (median survival 2.5 months, Log-rank p-value < 0.0001; Fig. 1C,D). Univariate analysis identified highest VAF (any gene), KRAS CNG, performance status (PS) and presence of liver metastases as significant factors for shorter survival with a p-value < 0.05. Stepwise multivariable analysis (Table 1) identified KRAS CNG and mutation as an independent predictor for shorter survival.
Discussion
In this pilot study of 55 patients with PDAC, we applied NGS and ddPCR to cfDNA to establish which readouts, if any, are linked to clinical outcomes. Although we see a relatively short median survival of 7.99 months compared to the 19.77 months reported in a TCGA study7, this most likely reflects differences in staging with the TCGA cohort comprising operable localised disease whereas our cohort includes patients with locally advanced and metastatic disease, resulting in a shorter median survival, in line with those reported by other groups8,9. Analysis of cfDNA from each patient revealed the presence of a canonical KRAS somatic mutation, which was determined by ddPCR and was found to be 38% (21/55) overall; 48% (15/31) in metastatic disease and 25% (6/24) in locally advanced disease, in keeping with other published studies6. Although our detection rate of 38% for the presence of a KRAS mutation in patient cfDNA is in line with other reports, there is considerable variation in reported frequencies (27~93%)10,11 which may reflect the methodologies employed, as well as the variability of KRAS allelic ratios in the tumour10 and the low ctDNA burden associated with pancreatic cancer12. Analysis of a larger cohort with a consistent specified cfDNA methodology is required to assess the affect of KRAS variation on the accuracy of prognosis.
As expected, from the threshold of detection used for the targeted NGS in this study (2.5%)13, only 14/21 ddPCR positive samples were found to harbour targeted NGS somatic KRAS mutations (Supplementary Table 1). However, by extending the NGS analysis to an additional 640 genes, somatic mutations were detected in 71% (39/55) in all samples; 84% (26/31) in metastatic disease and 50% (12/24) in locally advanced disease. The most striking novel observation that emerged from this study was that >10% of patients with PDAC harboured both a KRAS mutation and a KRAS CNG, and that the latter correlated with a worsened prognosis. Although amplified mutated KRAS has been reported in non-small cell lung cancer (NSCLC) and is also associated with poor clinical outcome14, this is the first report in PDAC. In addition to identifying CNG of KRAS, we also noted four cases where TP53 mutations were accompanied by copy number loss (CNL), suggesting that further analysis of a larger patient group may also identify CNL as a prognostic biomarker.
We now have the opportunity to verify these initial results by examining additional patient cfDNAs from the on-going Precision-Panc clinical trial, and serial measurements may inform response to treatment15.
Our results demonstrate cfDNA analysis can be used in advanced disease to identify patients with worse prognosis who may benefit from more aggressive chemotherapy. In addition, the identification of KRAS CNG and mutation as a poor prognostic factor, could also help to identify patients with resectable disease with higher risk of early tumour relapse, who may benefit from additional staging imaging before surgery (i.e. Magnetic resonance imaging of the liver or 18 fluorodeoxyglucose (FDG)-positron emission tomograph) or potential neo-adjuvant.
Materials and Methods
Non-Cancer volunteer and patient blood sample collection
Patients diagnosed with advanced treatment-naïve PDAC were prospectively recruited. Baseline blood samples (before treatment initiation) were collected in Cell-Free™ DNA BCTs (Streck, Omaha, NE), or BD Vacutainer® K2EDTA tubes, following receipt of informed consent in compliance with the Declaration of Helsinki and Good Clinical Practice under ethics approval number 07/H1014/96 (approved by Internal Review and Ethics Board of the Manchester Cancer Research Centre BioBank).
Circulating cell free DNA preparation
Plasma and cfDNA were isolated as previously described16. Germline DNA was isolated from EDTA whole blood, using QIAmp Blood Mini Kit (Qiagen, Hilden, Germany) as per manufacturer’s instructions.
NGS library preparation and sequencing
Whole genome sequencing (WGS) of cfDNA and corresponding germline DNA from the patients as well as non-cancer controls were carried out using the Accel-NGS® 2 S Plus DNA Library Kit as previously described16.
Targeted NGS analysis
Targeted NGS of 641 cancer-associated genes was carried out using Agilent SureSelectXT as described previously13.
Somatic mutation detection from targeted re-sequencing data
Three mutation callers were used: MuTect (version 1.1.5); VarScan (version 2.3.9) and Biomedical Genomics Workbench 4.1 (CLC Bio, Qiagen). Single nucleotide variant (SNV) calls were accepted, if identified by both MuTect and Biomedical Genomics Workbench and indels accepted if identified by both VarScan and Biomedical Genomics Workbench (Fig. 1B). HMMcopy (version 1.8.0) was used to call regions as gained or lost from WGS16.
Droplet digital PCR
Droplet digital PCR (ddPCR) was carried out using a QX200 ddPCR system (Bio-Rad) with ddPCRTM KRAS Screening multiplex kit17.
Statistical analyses
Mann-Whitney t-tests were used to compare cfDNA metrics (cfDNA in ng/ml of plasma, Percent genome amplified [PGA] and Highest VAF) between patients with locally advanced disease and patients with distant metastases. Factors associated with mutational burden and standard clinical and biochemical factors were subjected to Kaplan-Meier survival analysis and univariate Cox proportional hazards regression to predict overall survival (OS), considering the proportionality and linearity assumptions. OS was defined as the time in months between date of first diagnosis of malignancy and time of death. Univariately significant parameters (5% significance level) were included in a multivariable Cox regression analysis (bidirectional stepwise selection based on Akaike information criterion; exclusion of collinear parameters and clinical outcome variable). Statistical analysis was performed using the computing environment R (R Development Core Team, 2005).
Ethics approval and consent to participate
Blood samples were collected from patients with PDAC following receipt of informed consent in compliance with the Declaration of Helsinki and Good Clinical Practice under ethics 07/H1014/96, after approval from the Internal Review and the Ethics Boards of The Manchester Cancer Research Centre BioBank.
Supplementary information
Acknowledgements
We sincerely thank the patients and their families for provision of blood samples for research. Funding for this study was from Cancer Research UK (CRUK) via the core CRUK Manchester Institute grant (C5759/A27412), the CRUK Manchester Centre (C5759/A25254), the CRUK Manchester Experimental Cancer Medicines Centre (A20465) and the NIHR Manchester Biomedical research Centre. Sumitra Mohan’s salary and consumable costs were funded via CRUK Precision Panc grant C480/A25235. Sample collection and analysis was undertaken via the Pancreatic Cancer Research Fund (PCRF) 2012 Project Grant. AL received funding from European Society for Medical Oncology (ESMO) Fellowship Programme, Spanish Society of Medical Oncology (SEOM) Fellowship Programme, American Society of Clinical Oncology (ASCO) Conquer Cancer Foundation Young Investigator Award and The Christie Charity.
Author Contributions
G.B., C.D., J.W.V. and A.L. designed the study. J.W.V., A.L., R.H. and MMN. recruited and consented the patients, and collected blood samples. A.L. provided clinical data. M.A., D.G.R., G.B. and C.D. conceived and designed the experiments. M.A., S.M., N.S. and A.H. performed the experiments. M.A., S.M., G.B., A.L., H.S.L., S.S, P.S, B.K. and S.G. analysed the data. A.L., T.D. and C.Z., performed statistical analyses. S.M, M.A., G.B., C.D., A.L. and J.W.V. interpreted the data. S.M., M.A., G.B, C.D., A.L. and J.W.V. prepared the manuscript. All authors reviewed the manuscript.
Data Availability
All the data generated or analysed during this study are included in this published article, or are available from the corresponding author upon reasonable request.
Competing Interests
AL received travel and educational support from Ipsen, Pfizer, Bayer, AAA, Sirtex Medical, Novartis, Mylan and Delcath Systems; speaker honoraria from Merck, Pfizer and Ipsen; advisory honoraria from EISAI and Nutricia; she is a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen. JV reports Consulting or Advisory role for Ipsen, Novartis, AstraZeneca, Merck, Delcath Systems, Agios, Pfizer, PCI Biotech, Incyte, Keocyt, QED, Pieris Pharmaceuticals, Genoscience Pharma, Mundipharma EDO; Honoraria from Ipsen; and Speakers’ Bureau for Novartis, Ipsen, Nucana and Imaging Equipment Limited; all outside the scope of this work. CD acts in a consultant or advisory role for Biocartis and AstraZeneca and receives research grants/support from AstraZeneca, Astex Pharmaceuticals, Bioven, Amgen, Carrick Therapeutics, Merck AG, Taiho Oncology, GSK, Bayer, Boehringer Ingelheim, Roche, BMS, Novartis, Celgene, Epigene Therapeutics Inc; all outside the scope of work. MMN has received research grant support from Servier, Ipsen and NuCana. She has received travel and accommodation support from Bayer and Ipsen and speaker honoraria from Pfizer, Ipsen and NuCana. She has served on advisory boards for Celgene, Ipsen, Sirtex and Baxalta; all outside the scope of this work. Other authors have no conflict of interests to declare.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juan W. Valle, Caroline Dive and Ged Brady jointly supervised this work.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-47489-7.
References
- 1.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.can-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 4.Heitzer E, et al. Tumor-associated copy number changes in the circulation of patients with prostate cancer identified through whole-genome sequencing. Genome Med. 2013;5:30. doi: 10.1186/gm434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohan S, et al. Changes in colorectal carcinoma genomes under anti-EGFR therapy identified by whole-genome plasma DNA sequencing. PLoS Genet. 2014;10:e1004271. doi: 10.1371/journal.pgen.1004271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takai, E. et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Scientific Reports5, 18425, 10.1038/srep18425 [DOI] [PMC free article] [PubMed]
- 7.Nicolle R, et al. Prognostic Biomarkers in Pancreatic Cancer: Avoiding Errata When Using the TCGA Dataset. Cancers. 2019;11:126. doi: 10.3390/cancers11010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peran I, Madhavan S, Byers SW, McCoy MD. Curation of the Pancreatic Ductal Adenocarcinoma Subset of the Cancer Genome Atlas Is Essential for Accurate Conclusions about Survival-Related Molecular Mechanisms. Clin Cancer Res. 2018;24:3813–3819. doi: 10.1158/1078-0432.ccr-18-0290. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 10.Lennerz JK, Stenzinger A. Allelic ratio of KRAS mutations in pancreatic cancer. The oncologist. 2015;20:e8–9. doi: 10.1634/theoncologist.2014-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Calvez-Kelm F, et al. KRAS mutations in blood circulating cell-free DNA: a pancreatic cancer case-control. Oncotarget. 2016;7:78827–78840. doi: 10.18632/oncotarget.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouliere F, et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Science Translational Medicine. 2018;10:eaat4921. doi: 10.1126/scitranslmed.aat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothwell Dominic G., Ayub Mahmood, Cook Natalie, Thistlethwaite Fiona, Carter Louise, Dean Emma, Smith Nigel, Villa Shaun, Dransfield Joanne, Clipson Alexandra, White Daniel, Nessa Kamrun, Ferdous Saba, Howell Matthew, Gupta Avinash, Kilerci Bedirhan, Mohan Sumitra, Frese Kris, Gulati Sakshi, Miller Crispin, Jordan Allan, Eaton Helen, Hickson Nicholas, O’Brien Ciara, Graham Donna, Kelly Claire, Aruketty Sreeja, Metcalf Robert, Chiramel Jaseela, Tinsley Nadina, Vickers Alexander J., Kurup Roopa, Frost Hannah, Stevenson Julie, Southam Siobhan, Landers Dónal, Wallace Andrew, Marais Richard, Hughes Andrew M., Brady Ged, Dive Caroline, Krebs Matthew G. Utility of ctDNA to support patient selection for early phase clinical trials: the TARGET study. Nature Medicine. 2019;25(5):738–743. doi: 10.1038/s41591-019-0380-z. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki H, et al. Evaluation of Kras gene mutation and copy number gain in non-small cell lung cancer. J Thorac Oncol. 2011;6:15–20. doi: 10.1097/JTO.0b013e31820594f0. [DOI] [PubMed] [Google Scholar]
- 15.Graham JS, et al. PRIMUS-001: An adaptive phase II study of FOLFOX-A (FOLFOX and nab-paclitaxel) versus AG (nab-paclitaxel and gemcitabine) in patients with metastatic pancreatic cancer, with integrated biomarker evaluation (ISRCTN75002153) – Part of Precision-Panc. J Clin Oncol. 2018;36:TPS4158–TPS4158. doi: 10.1200/JCO.2018.36.15_suppl.TPS4158. [DOI] [Google Scholar]
- 16.Williamson SC, et al. Vasculogenic mimicry in small cell lung cancer. Nature Communications. 2016;7:13322. doi: 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janku F, et al. Multiplex KRASG12/G13 mutation testing of unamplified cell-free DNA from the plasma of patients with advanced cancers using droplet digital polymerase chain reaction. Ann Oncol. 2017;28:642–650. doi: 10.1093/annonc/mdw670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated or analysed during this study are included in this published article, or are available from the corresponding author upon reasonable request.