Abstract
Hepatitis D is the most severe form of viral hepatitis associated with a more rapid progression to cirrhosis and an increased risk of hepatocellular carcinoma and mortality compared with hepatitis B mono-infection. Although once thought of as a disappearing disease, hepatitis D is now becoming recognized as a serious worldwide issue due to improvement in diagnostic testing and immigration from endemic countries. Despite these concerns, there is currently only one accepted medical therapy (pegylated-interferon-α) for the treatment of hepatitis D with less than desirable efficacy and significant side effects. Due to these reasons, many patients never undergo treatment. However, increasing knowledge about the virus and its life cycle has led to the clinical development of multiple promising new therapies that hope to alter the natural history of this disease and improve patient outcome. In this article, we will review the literature from discovery to the current investigational therapies.
Keywords: Hepatitis D, hepatitis B, epidemiology, natural history, outcome, treatment, novel therapies
Introduction
Hepatitis D is a rare form of viral hepatitis that was first described in 1977 by Rizzetto et al. through immunofluorescence detection of the delta antigen and anti-delta antibody in the serum and liver tissues of hepatitis B surface antigen (HBsAg) carriers [1]. While it has been estimated to affect 15–20 million people worldwide, more recent data suggest that the global disease burden may be closer to 62–72 million [2]. Hepatitis D virus (HDV) is an incomplete RNA virus that requires the assistance of the hepatitis B virus (HBV), specifically the HBsAg, to be infectious in humans [3]. Once chronicity is established, HDV has been described as the most severe form of viral hepatitis, with progression to cirrhosis in 10%–15% of patients within 2 years and in 70%–80% of patients within 5–10 years [4, 5]. Despite this, a lack of adequate treatment options currently exists for HDV. Current international guidelines suggest the use of pegylated-interferon-α (peg-INF-α), although sustained virological response (SVR) rates with this treatment are reported to be only 20%–30% [6–8]. In addition, even after SVR is achieved, late relapse remains an issue [9]. Recent advances in our understanding of the HDV viral cycle have led to the development of several promising investigative therapies that are under investigation.
In this piece, we review the virology, epidemiology, diagnosis, clinical presentation, natural history, outcomes and the available and investigative treatments pertaining to this disease.
Viral structure and life cycle
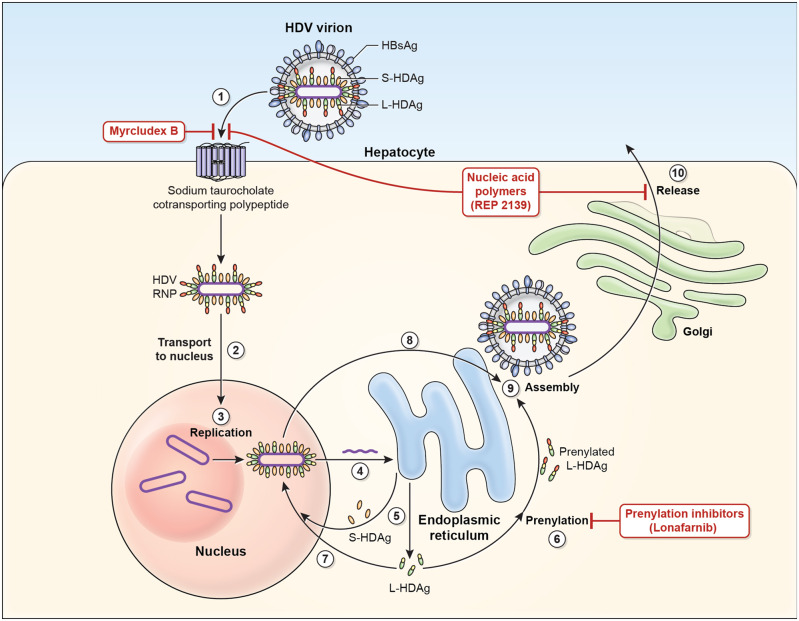
HDV is a small, spherical RNA virus measuring ∼36 nm in diameter with an inner nucleocapsid that consists of a short (∼1.7-kb) single-stranded, circular RNA and approximately 200 molecules of hepatitis D antigen (HDAg) [10, 11]. This viral genome is the smallest among known mammalian viruses and shares structural similarity to viroid RNAs [12–14]. It encodes for a single protein, the HDAg, which exists in two forms: the small HDAg (S-HDAg) and large HDAg (L-HDAg). Structurally, the two forms are identical except that the L-HDAg has an additional 19 amino acid sequence at the C-terminus [15]. The outer coat of the HDV virion consists of components taken from HBV, thus mandating a co-infective process with HBV, which includes the small, medium and large HBsAg [3]. The isoforms of HBsAg are embedded in a lipid envelope surrounding the HDV genome and HDV antigen isoforms. The large HBsAg then undergoes a process called myristoylation at the N-terminus to prepare for cell entry [16–18]. The life cycle of HDV is depicted in Figure 1. First, the HDV virion binds to the human hepatocyte through an interaction between the myristoylated N-terminus of the pre-S1 domain of the large HBsAg and the host receptor, which has been identified as the multiple transmembrane receptor sodium taurocholate cotransporting polypeptide (NTCP) located on the basolateral membrane of hepatocytes [19, 20]. After entry, the HDV genome is translocated to the nucleus via the HDAg [21]. Upon arriving in the nucleus, HDV commandeers host RNA polymerase II, a DNA-directed RNA polymerase, for transcription of HDV RNA [22]. Replication of the HDV occurs through a rolling-circle mechanism [12, 23]. The initial step is synthesis of multimeric linear transcripts from the circular genomic template. Afterwards, these multimeric linear transcripts are cleaved into monomers by autocatalytic self-cleaving sequences called ribozymes. Subsequently, monomer RNAs are ligated by the ribozyme into an antigenomic, monomeric, circular RNA that serves as a template for another round of rolling-circle replication. The finished product is a circular genomic HDV RNA [13].
Figure 1.
Hepatitis D virus viral life cycle and sites of drug target. 1. Hepatitis D virus (HDV) virion attaches to the hepatocyte via interaction between hepatitis B surface antigen proteins and the sodium taurocholate cotransporting polypeptide (NTCP), a multiple transmembrane transporter. 2. HDV ribonucleoprotein (RNP) is translocated to nucleus mediated by the hepatitis D antigen (HDAg). 3. HDV genome replication occurs via a ‘rolling-circle’ mechanism. 4. HDV antigenome is transported out of the nucleus to the endoplasmic reticulum (ER). 5. HDV antigenome is translated in the ER into small HDAg (S-HDAg) and large HDAg (L-HDAg). 6. L-HDAg undergoes prenylation prior to assembly. 7. S-HDAg is transported back to the nucleus where it supports HDV replication. 8. New HDAg molecules are associated with new transcripts of genomic RNA to form new RNPs that are exported to the cytoplasm. 9. New HDV RNP associates with hepatitis B virus (HBV) envelop proteins and assembled into HDV virions. 10. Completed HDV virions are released from the hepatocyte via the trans-Golgi network.
Three different RNAs are generated during the replication process: the HDV genome, the antigenome that is its exact complement and a third smaller antigenome that contains the open reading frame (ORF) that codes for the HDAg [22]. During replication, the HDV genome and antigenome collapses into a characteristic unbranched rod-like structure [24]. Both forms of HDAg are translated in the endoplasmic reticulum from the ORF located on the antigenomic HDV RNA strand [25]. The S-HDAg plays a significant role in replication, since it is required for RNA synthesis, while the L-HDAg inhibits RNA synthesis and is required for packaging. Cellular adenosine deaminase acting on RNA (ADAR) editing of the amber codon on the viral antigenome RNA enables the HDV to switch from replication to packaging [24].
The HDV RNA and HDAg proteins interact to form a ribonucleoprotein (RNP) particle at a molar ratio of 200 [12, 26]. The modification of post-translation HDAg proteins is essential for this process but is also crucial for HDV replication and HDV-virion assembly [27, 28]. One of these post-translational modification processes is called farnesylation, which is crucial for HDV-virion assembly and serves as a target for drug action. At the C-terminal of the L-HDAg is a 19-amino acid polypeptide that includes the C-terminal CXXX-box motif (where C = cysteine and X = any amino acid), which is the substrate for prenyltransferases to add a prenyl lipid group [15, 29]. Farnesyl is one of the prenyl groups that can be added via the enzyme farnesyltransferase in a process specifically called farnesylation. Farnesylation anchors the RNP to the endoplasmic reticulum membrane where HBV envelop proteins are made and then makes the RNP more lipophilic and more amenable to interactions with HBsAg. This C-terminal 19-amino acid residue region, which is not well conserved between different HDV genotypes, plays a key role in the varied assembly efficiencies between genotypes, which likely has pathologic implications [30–32]. Interestingly, HBV envelope proteins are made in excess so, even when HBV is suppressed, additional HDV virions can still be assembled [33].
Finally, once the RNP interacts with the envelop protein of HBV and the HDV is assembled, the HDV virion is now ready for release. The HDV virion is released via the trans-Golgi network, where it can go on to infect other hepatocytes. However, the exact mechanism of HDV-virion release remains unknown [15].
Viral genotypes
To date, eight distinct HDV genotypes have been recognized, with two to four subtypes per genotype characterized by >90% similarity over the entire genome sequence [2, 34, 35]. The geographical distributions of the HDV genotypes have changed over time, most probably due to human migration patterns. Currently, genotype 1 is the most prevalent worldwide and the predominant genotype in Europe and North America [36]. Genotype 2 was previously confined to Asia but has now emerged in Middle Eastern countries including Iran [37] and Egypt [38]. Genotype 3 is mainly found in the Amazon Basin in South America and is the most different of all the genotypes exhibiting ∼40% divergence at the nucleic acid level [39]. Genotype 4 is predominately found in Taiwan, China and Japan [35]. Meanwhile, genotypes 5, 6, 7 and 8 have traditionally been found only in Africa, but recent reports describe the migration genotypes 5, 6 and 7 to various parts of Europe [40–42]. Notably, central Africa is thought to be main site of HDV diversification, with the presence of genotypes 1, 5, 6, 7 and 8 [35].
It is well known that specific HDV genotypes influence clinical outcomes. HDV genotype 3 appears to be the most pathogenic of all the HDV genotypes [43, 44]. HDV genotype 1 patients have lower rates of remission, more aggressive disease and worse outcomes than HDV genotype 2 patients [45, 46]. For example, in a study from Taiwan, Su et al. reported significantly lower rates of remission in HDV genotype 1 compared to HDV genotype 2 (15.2% vs 40.2%, P = 0.007) and more adverse outcomes (cirrhosis, hepatocellular carcinoma [HCC] or mortality) (52.2% vs 25.0%, P = 0.005) [45]. This is likely due to HDV genotype 1 being a more efficient genotype in terms of virion assembly and RNA editing than genotype 2, resulting in the secretion of more viral particles [31, 32].
Epidemiology and risk factors
The seroprevalence of HDV among HBsAg-positive carriers has substantial variations worldwide. These are depicted in Table 1. Interestingly, more recent data have shown that 8% of the general Mongolian population is estimated to be positive for HDV [2]. Notably, in the USA, the prevalence of HDV among HBV carriers has been reported to range from 2% to 50%, depending on the patient population [63–66]. A large study of the US Veteran’s Affairs medical system more recently reported an HDV seroprevalence of 3.4% among patients with chronic HBV who are tested for HDV [78]. A study using the National Health and Nutrition Examination Survey reported a HDV seroprevalence of 42% among HBV carriers [65]. Finally, the highest estimation of HDV seroprevalence came from a study of HBV-positive intravenous drug users (IVDU), which showed that the seroprevalence of HDV increased from 29% in 1988–1989 to 50% in 2005–2006 [66]. However, a general lack of HDV RNA validation in these studies prevents estimation of true HDV prevalence.
Table 1.
Epidemiology of hepatitis D
| Endemic country | Seroprevalence | Non-endemic country | Seroprevalence |
|---|---|---|---|
| Brazil | 41.9% [47] | Australia | 4.1%–4.8% [48, 49] |
| China | 3.5%–13.1% [50–52] | England | 2.6%–8.5% [53, 54] |
| Egypt | 9.9% [38] | Greece | 4.2% [55] |
| Germany | 8%–10.9% [56, 57] | Japan | 6% [58] |
| India | 10.6%–37.5% [59, 60] | Jordan | 2% [61] |
| Iran | 17%–48% [37, 62] | United States | 2%–50% [63–66] |
| Italy | 8.3%–23.0% [67] | ||
| Mongolia | 56.5% [68] | ||
| Pakistan | 16.6%–58.6% [69] | ||
| Saudi Arabia | 8.6% [70] | ||
| Taiwan | 4.4%–15.3% [71, 72] | ||
| Thailand | 21.8%–65.5% [73, 74] | ||
| Tunisia | 17.7% [75] | ||
| Turkey | 7%–45.5% [76, 77] |
Despite the various HDV epidemiological reports, there continues to be insufficient data on the true global prevalence of HDV [2]. This is attributable to several factors. First, reported rates are likely to be underestimations, stemming from incomplete population testing because of a lack of clinician awareness resulting in a lack of diagnosis along with a lack of availability of HDV clinical tests. In a nationwide study in the USA of 25 603 HBV patients, only 8.5% of patients were ever tested for HDV [78]. Manesis et al. reported that only a third of Greek patients with chronic hepatitis B (CHB) were tested for HDV [55]. El Bouzidi et al. similarly reported that only 40% of British chronic HBV patients were tested for anti-HDV in patients with positive HBsAg [40]. Special focus also needs to be made for HDV testing among patients with hepatitis C virus (HCV) and human immunodeficiency virus (HIV) due to a high rate of co-infection because of shared risk factors such as transfusions, tattooing, IVDU and high-risk sexual practices [79]. For example, reported co-infection rates with HCV have ranged from 26%–73% [42, 48, 53, 68] and HIV of 12.7% [42].
Second, studies from the same country have reported discrepant numbers that may be due to significant geographical variations even within the same country. For example, a meta-analysis from Turkey described that the HDV seroprevalence of west Turkey was 4.8%, while the seroprevalence of HDV in southeast Turkey was 46.3% [80]. In addition, most of the epidemiologic studies on the seroprevalence of HDV among chronic HBV patients did not require HDV polymerase chain reaction (PCR) confirmation as an inclusion factor, further preventing the estimation of the true prevalence of HDV. Finally, the timing of the publication is also important because the seroprevalence of HDV declined drastically after the late 1980s due to the implementation of HBV vaccine programs around the world [81–83].
Despite an earlier notion that HDV was a disappearing disease in many parts of the world, this has proven to be no longer the case. In fact, more recent data have shown that the prevalence of HDV has remained stable or has increased in many endemic and non-endemic countries due to an increase in associated risk factors such as immigration, IVDU, men-who-have-sex-with men (MSM) and intra-familial spread [41, 67, 71, 79, 84, 85]. For example, a collection of studies from Italy reported a decline in the prevalence of HDV among HBsAg-positive patients from 23% in 1987 to 14% in 1992 to 8.3% in 1997, which was attributed to HBV-vaccination programs [67, 86, 87]. However, more recent data have suggested that the prevalence of HDV in Italy has either remained stable at 8.1% [85] or has rebounded to 11.3% [84]. Lin et al. showed that the prevalence of HDV increased from 38.5% to 89.8% among HIV-infected IVDU patients with HBV from 2001 through 2012 [71]. Furthermore, outbreaks of HDV have been especially rampant among areas of with low social-economic levels [79, 80]. Due to immigration from endemic countries, multiple studies have reported an increase in the prevalence of HDV in countries in which HDV was previously uncommon [48, 49, 53–55]. In a study by Coghill et al. from Australia, those born in Africa were shown to have a higher risk ratio (RR) for HDV infection (RR, 1.55; 95% confidence interval [95% CI], 1.14–2.09) [48]. Cross et al. reported in a study from England that over half of their HDV patients were from regions of the world where HDV is endemic including Southern or Eastern Europe (28.1%), Africa (26.8%) and the Middle East (7.3%) [53]. Similarly, Manesis et al. reported that immigrants represented over half of the HDV burden in Greece [55].
Due to these reasons, HDV is now becoming a serious issue worldwide. A recently published systematic review and meta-analysis reported a worldwide HDV seroprevalence of 14.6% among HBV-positive patients compared with prior estimates of approximately 5%, with a substantially higher seroprevalence in the IVDU and high-risk sexual-behavior populations [2, 88]. These findings stress the importance of HDV awareness regardless of country of origin. Indeed, HDV needs to be especially considered in high-risk populations such as among patients who are immigrants from endemic countries, IVDU, MSM and with positive family members.
Diagnostics
This section will focus on the different diagnostic modalities and tests that are available (Table 2). When HDV was first discovered, the diagnosis of HDV relied on immunohistochemical staining of HDAg in liver tissue that could only be obtained through liver biopsy [1]. Testing for serum immunoglobulin M (IgM)/immunoglobulin G (IgG) anti-HDV quickly became available, which, in addition to liver chemistries and the patient’s clinical picture, enabled classification of patients into the two stages of HDV infection: acute and chronic HDV [89]. The use of serum IgM/IgG anti-HDV for this purpose was not perfect, however, as anti-HDV IgG is not 100% specific to chronic HDV and can be found in acute HDV. Likewise, anti-HDV IgM is not specific to acute HDV and can frequently be found in chronic HDV [61, 90, 91].
Table 2.
Diagnostic tests for hepatitis D
| Diagnostic test | Detection | Significance | Comments |
|---|---|---|---|
| Liver HDAg | Detects HDV antigen on liver histology via immunohistochemical staining | Indicates active infection | Lack of availability. Poor sensitivity |
| Serum HDAg | Detects HDV antigen in the serum | Indicates active infection but disappears quickly | Rarely performed. May be undetectable in chronic HDV |
| Anti-HDV IgM | Detects the presence of IgM antibodies against HDV in the serum | Indicates active infection, usually found in acute but can be found in chronic HDV | Often negative in chronic HDV but can be positive during periods of increased HDV replication |
| Anti-HDV IgG | Detects the presence of IgG antibodies | Usually indicates previous infection or chronic HDV | Appears late in acute HDV but persistent in chronic HDV |
| HDV RNA PCR (Qualitative) | Detects HDV RNA in the serum | Indicates active infection, can be found in acute or chronic HDV | LLOD depends on the assay. Useful for diagnosis |
| HDV RNA PCR (Quantitative) | Quantifies HDV RNA in the serum | Indicates active infection, can be found in acute or chronic HDV | LLOQ depends on the assay. Useful for treatment monitoring |
| HDV genotyping | Determines HDV genotype | Distinguish specific HDV genotype (1–8) with possible prognostic significance | Not commercially available |
HDAg, hepatitis D antigen; HDV, hepatitis d virus; RNA, ribonucleic acid; PCR, polymerase chain reaction; LLOD, lower limits of detection; LLOQ, lower limits of quantification.
For more than a decade after the discovery of HDV, testing for the presence of HDV RNA remained sub-optimal, with techniques such as the slot-blot hybridization technique [92]. In the early 1990s, serum HDV RNA PCR techniques were developed that enabled efficient and accurate testing of HDV in HBsAg-positive patients [92–94]. However, these early assays only enabled qualitative testing of HDV and were not able to quantify the virus in the serum. The emergence of quantification PCR assays in the early 2000s finally enabled measurement of HDV RNA viremia in the serum [95, 96]. Despite these advances, there continued to be a high degree of variation in the sensitivity and specificity of the HDV assays due to a lack of standardization. Recent measures by the World Health Organization (WHO) resulting in the first international external quality control for HDV quantification have led to substantial improvements in this area but significant assay heterogeneity of the available assays remains [97, 98].
HDV should be highly suspected in HBV patients with persistently elevated liver chemistries despite suppressed HBV viral loads with or without nucleos(t)ide analog (NA) therapy without a history of significant alcohol intake or metabolic syndrome. This is due to direct suppression of HBV replication by HDV itself [48]. Not surprisingly, HBV viral loads do not appear to have any correlation with serum alanine aminotransferase (ALT) levels in chronic HDV [99]. However, this scenario most often applies to hepatitis B envelope antigen (HBeAg)-negative and patients who are HBeAg-positive may still have a high HBV viral load despite the presence of HDV. The American Association for the Study of Liver Diseases (AASLD) currently recommends that HBsAg-positive patients with low or undetectable HBV DNA but high ALT levels be considered for HDV testing while the European Association for the Study of the Liver (EASL) and Asian Pacific Association for the Study of the Liver do not offer specific details regarding which patients with HBV should be tested [6–8]. The recommended screening test for HDV is serum anti-HDV IgG followed by HDV RNA PCR if positive. Screening is also recommended in HIV-positive patients, IVDU patients, MSM, those at risk for sexually transmitted diseases and immigrants from endemic areas [6]. Nonetheless, when present, HDV is usually found in the setting of acute hepatitis or chronic liver disease and rarely in the setting of an asymptomatic HBV carrier [61].
Acute hepatitis D
Acute HDV occurs after an incubation period of 3–7 weeks and may present with non-specific flu-like symptoms with high levels of ALT and aspartate aminotransferase (AST) followed possibly by jaundice [100]. The presentation of acute HDV can mirror that of acute HBV infection, making it important to test for both processes in a patient with acute hepatitis. Acute HDV can either represent co-infection of HBV and HDV or super-infection of HDV. Co-infection usually leads to an acute self-limited hepatitis that cannot be distinguished clinically from acute HBV. Co-infection induces a cellular immune response followed by down-regulation of HBV replication and elimination of infected hepatocytes by cytotoxic cells [101]. Meanwhile, super-infection presents as an acute exacerbation of pre-existing chronic HBV or elevations in liver chemistries in an asymptomatic HBsAg carrier [100]. Acute co-infection of HBV and HDV rarely progresses to a chronic HDV infection, although acute HDV due to super-infection of HDV in a patient with HBV progresses to chronic HDV 80%–100% of the time [82, 101].
Clinically, patients may appear the same as in co-infection with elevated ALT levels, but the acute phase of HDV super-infection is characterized by more active HDV replication and suppression of HBV [101]. In addition to HBV viral loads being markedly suppressed, anti-hepatitis B core (HBc) IgM are usually negative or with low titer [46, 100]. Although anti-HDV IgM levels may be a useful indicator of an acute HDV infection, it has only been described to be positive in 41% of acute cases and can also been found during flares of chronic HDV infection [61, 102]. Anti-HDV IgG may develop late in acute HDV and may persist for a lifetime even after the patient clears the virus [61, 101, 102].
Acute HDV can vary in severity from mild to fulminant hepatitis. Fulminant hepatic failure has been reported to occur in 1% of HBV/HDV co-infected patients and in 5% of HDV super-infected patients [103]. Genotype 1 HDV appears to be a risk factor for fulminant hepatic failure in acute HDV due a higher efficiency of editing, assembly and replication compared with genotype 2 [31, 32, 45, 104]. HDV may cause hepatic failure through direct cytotoxicity caused by the S-HDAg or inducing an exaggerated immune response leading to the destruction of hepatocytes by cytotoxic T cells [101, 105, 106].
Chronic hepatitis D
Chronic HDV is defined as the presence of HDV infection for greater than 6 months. Clinically, patients may be asymptomatic or have non-specific symptoms of fatigue, malaise and anorexia [100]. Three distinct chronic HBV/HDV patterns have been described. Most patients will have predominant HDV replication with suppressed HBV replication and are HBeAg-negative. This is because HDV tend to suppress HBV transcription as well as HBV virion release, which is hypothesized to be mediated by HDAg [107]. A similar phenomenon is seen in those with HCV/HDV co-infection as well in which HDV suppresses HCV replication [56]. In many patients with predominant HDV replication, HBV viral loads may be even undetectable, depending on the sensitivity of the assay. In a cohort of 126 chronic HDV patients, HBV viral loads could not be detected in 67% of patients [91]. Some HDV patients, however, can have similar viral loads of both viruses and rarely can patients have predominant HBV replication [108]. These patients are often HBeAg-positive. HBeAg has been reported to be positive in 9.0%–22.8% of HDV cases [44, 45, 90, 109]. Still, HBV viral loads are not suppressed as much as in the acute hepatitis stage and may reactivate in certain patients [46].
Chronic hepatitis D outcomes
It is well described that patients who are chronically infected with HBV/HDV have significantly worse liver disease compared to those with chronic HBV mono-infection [46, 101]. Table 3 illustrates the increased risks associated with chronic HDV compared with HBV mono-infection. Patients co-infected with chronic HBV/HDV have an accelerated progression to cirrhosis [58, 90, 110], an increased risk of decompensation [111], an increased risk of HCC [58, 61, 78, 90, 111–113], are more likely to undergo liver transplantation [48] and are at an increased risk for mortality [42, 78, 90, 111] compared with HBV alone. Co-infections of HCV in addition to HBV/HDV, however, is not a risk factor for more advanced disease [56]. In the Hepatitis Delta International Network (HDIN) registry of 1576 patients with HDV, cirrhosis was described to be present in 48.7%, HCC developed in 2.5% and 3.6% required liver transplantation [44]. After cirrhosis develops, the first dominant complication that tends to occur is clinical cirrhotic decompensation and not HCC [90, 111].
Table 3.
Associated risks of chronic hepatitis D
| Clinical outcome | Approximate relative risk increase* |
|---|---|
| Cirrhosis [58, 90, 110] | 2- to 3-fold |
| Hepatocellular carcinoma [58, 61, 78, 90, 111–113] | 3- to 6-fold |
| Liver transplantation [48] | 2-fold |
| Hepatic decompensation [111] | 2-fold |
| Mortality [42, 78, 90, 111] | 2-fold |
Compared with hepatitis B mono-infection.
Interestingly, HBV/HDV-related HCC may be different from HBV mono-infection HCC. Abbas et al. compared HCC in HBV/HDV co-infected patients to those in HBV alone [114]. HBV mono-infected patients with HCC were more likely to have multifocal tumors, elevations in alpha-fetoprotein level >1000 IU/mL and higher TNM-based staging [114]. This may be due to a different set of underlying pathogenesis mechanisms driving HBV/HDV HCC compared with HBV HCC. It is thought that HBV causes HCC through mechanisms such as chronic inflammation, integration of HBV DNA into host cellular DNA that disrupt or promote the expression of cellular genes and the direct effect of HBV proteins on cellular functions [115]. Meanwhile, the underlying drivers of HCC in chronic HDV are thought to be severe necroinflammation resulting in oxidative stress as well as epigenetic mechanisms such as DNA methylation and histone modifications induced by the L-HDAg [113]. The L-HDAg may also be a mediator in the oxidative stress pathways [27]. Overall, the risk of HCC in chronic HDV has been described to be increased ∼3- to 6-fold compared with HBV alone [58, 78, 111, 112]. Like HBV, cirrhotic HDV patients who lose HBsAg and seroconvert to becoming positive for antibody to hepatitis B surface antigen (anti-HBs) with interferon-α (IFN-α) therapy continue to be at risk for HCC [90].
Although initial studies did not show an association between HDV and mortality [61], more recent studies have described an increased mortality risk in chronic HDV [42, 78, 90, 111]. Fattovich et al. demonstrated, in cohort of 2000 Western European HBV-positive patients, that anti-HDV-positive patients were at a 2-fold increased risk of mortality relative to anti-HDV negative HBV patients [111]. This effect was also seen in a large cohort of HBV/HIV ± HDV patients followed for a median of 8.7 years. In this study, Beguelin et al. reported a ∼2-fold increased risk of mortality due to HDV [42]. The primary cause of death in HDV is most often liver failure [90]. This finding was supported by Niro et al., who found that over half of chronic HDV patients who become cirrhotic will advance to liver failure [91].
Predictors of worse outcomes in chronic HDV have generally varied between studies but have included cirrhosis at presentation [82, 91], age [44, 45, 82], male sex [91], HDV genotype 1 infection [45], country of origin [44] and positivity for serum anti-HDV IgM [116]. Multiple studies have confirmed that the HBV viral load has no impact on meaningful outcomes, although this is possibly due to the suppressive effect of HDV on HBV replication, which prevents any correlation from being obvious [45, 53, 108]. Although HBeAg-positive HDV patients typically have higher biochemical disease activity, HBsAg levels and HBV viral loads compared to HBeAg-negative HDV, long-term clinical outcomes do not differ [109]. This contrasts with studies in HBV mono-infection that have demonstrated significant associations between HBV viral loads with clinical outcomes such as histology, subsequent risk of cirrhosis and HCC [117, 118].
Meanwhile, anti-HDV IgM and HDV RNA levels have been shown to have disease activity and prognostic implications. Anti-HDV IgM levels correlate with higher biochemical activity indicators such as ALT levels as well as total histological activity index [90, 116]. In a study of 78 patients from the Hep-Net-International-Delta-Hepatitis-Intervention Trial-2 (HIDIT-2) study with long-term follow-up, only 1 out of 11 (9%) anti-HDV IgM-negative patients developed clinical decompensation compared with 26 of 67 (39%) anti-HDV IgM-positive patients [116]. Similarly, high HDV RNA levels also correlate with disease activity [96] and are associated with an increase in the risk of HCC as well as progression to cirrhosis in non-cirrhotic patients [90]. In genotype 3, HDV RNA levels have been shown to positively correlate with necroinflammatory activity and fibrosis stage and negatively correlate with platelet counts. Patients with an HDV viral load of ≥2log10 had a significantly increased risk of advanced fibrosis (odds ratio of 6.47; 95% CI, 1.79–23.37; P = 0.004) [108].
Therapies for HDV
Although there is currently no US Food and Drug Administration-approved therapy for HDV infection, IFN-α and peg-IFN-α can be used in chronic HDV, although both induce low rates (∼20%–30%) of SVR, defined as an undetectable HDV RNA viral load at 6 months post cessation of treatment [36, 119, 120]. The low rates of response may be due to the interference of HDV with IFN-α intracellular signaling [121]. Notably, the interpretation of the SVR results of older therapeutic studies in HDV, especially prior to the standardization of the HDV assays by the WHO, should be made with caution, as there are wide variations between the different assays to detect and quantify HDV RNA [36, 97]. Additionally, although SVR has been used as the primary outcome in various clinical trials [122, 123], there remains uncertainty as to whether this outcome is adequate in HDV due to reported rates of late post-treatment relapse [9].
A major limitation of IFN-α-based therapies is the known side effects that accompany this therapy, which include; flu-like symptoms, myalgias, arthralgias, exacerbation of psychiatric illness, hematologic toxicity and elevations in transaminases [124]. Furthermore, the efficacy of IFN-α-based therapy is decreased in cirrhosis and is not recommended for use in patients with advanced cirrhosis (Childs B and C) due to fear of decompensation [36]. Because of these limitations, many patients with HDV never undergo treatment [48, 90]. In a study from Italy, only 30% of patients received interferon therapy with a mean follow-up of 233 months [90].
Nonetheless, IFN-α-based therapy has been shown to favorably affect the natural history of chronic HDV with substantial benefits that may become evident even years after treatment cessation [125]. These benefits include improvement of liver histology (specifically activity grade and fibrosis), clearance of HDV, decreased risk of liver decompensation or need for liver transplantation and survival [125–127]. Prospective data from the Hep-Net Greece Cohort Study showed that interferon-based treatment significantly decreased disease progression in HDV-positive patients (hazard ratio [HR] = 0.14; 95% CI, 0.02–0.86; P = 0.033) [55]. This finding has been collaborated by several other studies that have shown that a lack of interferon therapy was an independent predictor of worse outcome [91]. Thus, the AASLD recommends peg-IFN-α for 12 months for those with elevated HDV RNA levels and ALT elevation [6], while EASL recommends peg-IFN-α for at least 48 weeks for those with compensated liver disease [7].
Interferon-α monotherapy
In chronic HDV infection, two types of IFN-α therapy have been explored: IFN-α2a and IFN-α2b. IFN-α is usually given as a subcutaneous injection three times a week. SVR rates with IFN-α are ∼17% [128]. In a landmark early study that randomized 42 chronic HDV patients to either IFN-α 9 million units or 3 million units three times a week or no treatment for 48 weeks, 7/14 (50%) of the patients receiving the higher dose of IFN-α had normalization of alanine aminotransferase and clearance of HDV RNA. Treatment with the higher dose was associated with histologic improvement [129]. However, the high rate of HDV RNA clearance must be interpreted with caution in this study due to the low sensitivities of the assays used at the time as well as the size of the trial.
To improve compliance, a pegylated group was added to IFN-α to form peg-IFN-α that allowed once-a-week subcutaneous dosing [128]. Like IFN-α, there are two types of peg-IFN-α that have been investigated in trials: peg-IFN-α2a and peg-IFN-α2b, which appear to have similar efficacy. The recommended dose and duration of peg-IFN-α are 180 μg/week for 48 weeks for chronic HDV [130]. Clinical trials investigating the efficacy of peg-IFN-α are shown in Table 4. Recent studies have reported SVR rates of approximately 20%–30% after 1 year of therapy with peg-IFN-α [122]. However, as previously mentioned, relapse is common, even among patients who achieve SVR. In a study evaluating the long-term follow-up of the HIDIT-1 trial, 9 of the 16 patients who achieved SVR tested positive for HDV RNA at least once during post-SVR follow-up [9].
Table 4.
Clinical trials on the use of pegylated-interferon-α
| Publication | Publication year | Dose and delivery | Study arms and duration | Number of patients | VR | SVR |
|---|---|---|---|---|---|---|
| Erhardt et al. [123] | 2006 | 1.5 mcg/kg SC/wk | peg-IFN-α for 48 weeks | 12 | NR | 17% |
| Niro et al. [131] | 2006 | 1.5 mcg/kg SC/wk | peg-IFN-α for 72 weeks ± ribavirin for 48 weeks | 38 | 13% | 21% |
| Castelnau et al. [132] | 2006 | 1.5 mcg/kg SC/wk | peg-IFN-α for 48 weeks | 14 | 57% | 43% |
| Wedemeyer et al. [122] | 2011 | 180 mcg SC/wk | peg-IFN-α ± adefovir vs placebo for 48 weeks | 90 | 23% | 28% |
| Gheorghe et al. [133] | 2011 | 1.5 mcg/kg SC/wk | peg-IFN-α for 52 weeks | 49 | 33% | 25% |
Peg-IFN-α, pegylated-interferon-α; VR, virological response; SVR, sustained virological response; NR, not reported.
The optimal treatment duration of interferon therapy has continued to be undefined and treatment beyond 1 year is controversial. Although there have been reports of patients who have cleared chronic HDV infection after long-term IFN-α therapy of up to 12 years [126, 134, 135] and a study by Karaca et al., who reported that more than half of their patients who received peg-IFN-α for 2 years achieved SVR [136], further studies have shown a lack of substantial improvement in SVR rates or rates of post-treatment relapse [135, 137, 138]. In contrast, a recent retrospective study reported that maintained virological response rates, defined as remaining HDV RNA-negative 2 years after treatment discontinuation, increased with treatment duration and reached 50% at 5 years of treatment [127].
Interferon combination therapy
Due to the limited efficacy of interferon monotherapy in chronic HDV, several studies have evaluated the utility of combining interferon-based therapy with antiviral medications. Medications that have been studied include: ribavirin [131, 139], lamivudine [140, 141], famciclovir [142], tenofovir [138] and adefovir [122]. Overall, these studies have not been demonstrated to provide any added benefit such as improvement in virological response rates. The AASLD recommends that, since NAs have no efficacy against HDV, they should not be used in patients with suppressed or low HBV replication except in those with cirrhosis [6]. Meanwhile, EASL recommends that NA therapy should be considered in HBV/HDV co-infected patients with evidence of ongoing HBV DNA replication (>2000 IU/mL) and can be considered in those with advanced liver disease [7].
Monitoring for response during and after interferon therapy
During IFN-α or peg-IFN-α therapy, patients should be monitored monthly with complete blood counts and liver chemistries. In addition, patients should be tested for HBsAg and their HDV RNA and HBV DNA should be quantified at baseline and at 3-month intervals during treatment [143, 144]. After initiation of peg-IFN-α therapy, HDV RNA begins to decrease at around 1 week [145]. A negative or greater than 2log10 IU/mL decline in HDV RNA at 6 months of interferon therapy has been described to be predictive of SVR [132, 146]. Early viral kinetics may also predict the likelihood of response to interferon-α-based therapies. Mathematical modeling of HDV viral kinetic responses to peg-IFN-α therapy has demonstrated that HDV declines in a biphasic manner and the presence of a flat second-phase response is associated with non-response [145]. Additionally, patients with HDV replication greater than 1log10 higher than HBV replication may have a delayed HDV RNA response to peg-IFN-α therapy [147]. Finally, HBsAg titers are often correlated with HDV RNA levels and its decline in the serum may be an important early biomarker of HDV response. A HBsAg of less than 1000 IU/mL at 6 months is predictive of HDV loss [135, 148].
Investigational therapies
Due to the current limitations of interferon-α-based therapies, there has been substantial recent interest in novel therapies for this devastating disease. Current investigational therapeutic pipelines can be separated into the following classes: (i) interferon-lambda (IFN-λ), (ii) prenylation inhibitors (Lonafarnib [LNF]), (iii) entry inhibitors (Myrcludex B) and (iv) nucleic acid polymers (NAPs). These new therapies target various stages of the HDV life cycle and are currently in various stages of clinical development, with promising results thus far (Figure 1 and Table 5). RNA-interference (RNAi)-based therapies that target post-transcriptional gene silencing are also on the horizon for HDV but have yet to undergo clinical testing [158, 159]. Of note, clinical trials with the new investigational therapies have explored a variety of new outcomes compared with older trials. A decline of greater than 2log10 IU/mL of HDV RNA was recently proposed as a surrogate marker for initial treatment efficacy [160].
Table 5.
Clinical trials on investigative treatments for hepatitis D
| Therapeutic agents | Publication | Trial phase | Dose and delivery | Study arms and duration | Number of patients | HDV change (log10 IU/L) | HDV negative |
|---|---|---|---|---|---|---|---|
| Pegylated- interferon-λ† | Hamid et al. [149] | 2 | peg-IFN-λ 120/180 mcg SC/wk | peg-IFNλ for 48 weeks | 33 | NR | 3 of 11 |
| Lonafarnib | Koh et al. [150] | 2 | LNF 100/200 mg PO/BID | Lonafarnib for 4 weeks vs placebo | 14 | 100 mg (–0.73) 200 mg (–1.54) | NR |
| Lonafarnib ritonavir (LOWR-1) | Yurdaydin et al. [151] | 2 | LNF 100/200/300 mg PO/BIDRTV 100 mg PO/BID | LNF ± RTV ± peg-IFN-α for 5-12 weeks | 15 | LNF 100 mg BID + RTV (–3.2) LNF 100 mg BID + peg-IFN-α (–3.0) | LNF monotherapy (2 of 6)* |
| Lonafarnib ritonavir pegylated-interferon-α (LOWR-2)† | Yurdaydin et al. [152] | 2 | LNF 25/50/75/100 mg PO/BIDRTV 100 mg PO/BIDpeg-IFN-α 180 mcg SC/wk | LNF + RTV ± peg-IFN-α for 12–24 weeks | 58 | LNF 25 mg BID + RTV + peg-IFN-α (–5.57) | LNF 25 mg BID + RTV + peg-IFN-α (3 of 5) |
| Lonafarnib ritonavir (LOWR-3)† | Koh et al. [153] | 2 | LNF 50/75/100 mg PO/dailyRTV 100 mg PO/daily | LNF + RTV for 12–24 weeks | 21 | LNF 50 mg (–1.93) LNF 75 mg (–1.3) LNF 100 mg (–0.29) | NR |
| Lonafarnib ritonavir (LOWR-4)† | Wedemeyer et al. [154] | 2 | LNF 50/75/100 mg PO/BID | LNF + RTV for 24 weeks | 15 | –1.87 | NR |
| Myrcludex B | Bogomolov et al. [155] | 1b/2a | MB 2 mg SC/daypeg-IFN-α 180 mcg SC/wk | peg-IFN-α for 48 weeks or Myrcludex B ± peg-IFN-α for 24 weeks followed by peg-IFN-α for 24–48 weeks | 24 | Myrcludex B (–1.67) Myrcludex B + peg-IFN-α (-2.6) | Myrcludex B (2 of 8) Myrcludex B + peg-IFN-α (5 of 7) |
| Myrcludex B | Wedemeyer et al. [156] | 2b | MB 2/5/10 mg SC/dayTDF 245 mg PO/day | TDF ± Myrcludex B | 120 | Myrcludex B 2 mg (–1.7) Myrcludex B 5 mg (–1.6) Myrcludex B 10 mg (–2.7) | NR |
| Nucleic acid polymer (REP2139) | Bazinet et al. [157] | 2 | REP 500/250 mg IV/weekpeg-IFN-α 180 mcg SC/wk | REP 2139 for 15 weeks followed by peg-IFN-α + REP2139 for 15 weeks followed by peg-IFN-α for 33 weeks | 12 | –5.34 | 9 of 12 |
Post-treatment result.
Interim results; peg-IFN-λ, pegylated-interferon-λ; HDV, hepatitis D virus; LNF, lonafarnib; TDF, tenofovir disoproxil fumarate; NR, not reported; RTV, ritonavir; LOWR, LOnfarnib With and without Ritonavir; MB, Myrcludex B; pegylated-interferon-α, peg-IFN-α.
Pegylated-interferon-lambda
Pegylated-interferon-lambda-1a (peg-IFN-λ) is a conjugate of the recombinant human type-III IFN, IL-29 and a linear polyethylene glycol chain that has antiviral activity against HBV and HCV like peg-IFN-α, which is a type-I IFN [161]. However, unlike type-I IFN receptors, which are ubiquitously expressed in all tissues and cells, type-III IFN receptors are only restricted to cells of epithelial origin and theoretically more heavily concentrated in the liver. Due to this, IFN-λ has been described to cause fewer systemic interferon side effects such as myalgias, flu-like symptoms and arthralgias, thus improving tolerability [162]. However, the two interferons act on the same interferon-stimulated-genes pathway that acts on the JAK/STAT signal-transduction cascade, leading to antiviral activities and reduction of HDV viremia as well as intrahepatic genomic and antigenomic HDV RNA [161, 163].
Since peg-IFN-λ has a better tolerability profile compared with peg-IFN-α, it has been investigated in HBV (with comparable efficacy to peg-IFN-α) [164], HCV [165] and now HDV [149]. In HDV, the LIMT-HDV (Lambda Interferon MonoTherapy in Hepatitis Delta Virus) study [NCT02765802] is a phase 2, open-label, randomized study evaluating two doses of peg-IFN-λ (120 and 180 mcg weekly) in 33 patients with HDV for 48 weeks. Interim results reporting data on the first 20 patients have been encouraging. By Week 8, 3 of 11 patients had become HDV RNA-negative and 5 of 11 had a greater than –2log10 IU/mL decline in HDV RNA [149]. End-of-trial data from that study are pending, although another clinical trial called the LIFT (Lambda InterFeron combo Therapy) trial [NCT03600714] recently started enrolling at the National Institutes of Health. This trial is investigating combination therapy with peg-IFN-λ, LNF and ritonavir (RTV) in 26 patients for 24 weeks.
Prenylation inhibitors (Lonafarnib)
As described earlier, prenylation is the process of adding a prenyl lipid group (either farnesyl or geranylgeranyl) to the C-terminal cysteine of the target protein [29]. In HDV, a farnesyl group is added to the C-terminus of the L-HDAg in a process called farnesylation catalysed by the enzyme farnesyltransferase [15]. This host process is critical to viral assembly, as it causes the L-HDAg to become lipophilic and join with the HBsAg to form a virus particle that is ready to be secreted and infect other hepatocytes [166]. LNF is an oral prenylation inhibitor that acts on the enzyme farnesyltransferase (Figure 1) [167]. In mouse and hepatic tissue-culture studies, it has been shown to inhibit the secretion of virions and leads to intracellular accumulation of viral RNA, prevent production of infectious HDV particles and enhance the innate immune response [166, 168]. LNF has been studied in various malignancies including progeria and thus has a proven safety record [169, 170]. To date, LNF has been studied in over 100 patients with chronic HDV [150–154].
In a seminal proof-of-concept, phase 2 A double-blinded, randomized, placebo-controlled study, LNF was tested in 14 patients with chronic HDV infection at doses of 100 mg (Group 1) and 200 mg (Group 2) twice daily for 28 days with 6 months of post-therapy follow-up [150]. Patients experienced a mean log HDV RNA decrease of 0.73log10 IU/mL (Group 1) and 1.54log10 IU/mL (Group 2). Half of the patients in Group 2 achieved a decrease from baseline to nadir HDV RNA of greater than –2log10 IU/mL. Serum LNF concentrations correlated with HDV RNA changes (r2 = 0.78; P < 0.001). This study provided the first evidence that another therapy, other than interferon, had efficacy in chronic HDV. However, gastrointestinal side effects such as diarrhea, nausea, anorexia, dyspepsia and weight loss were common, especially among patients in the higher-dose group, and the HDV RNA suppression was not sustained after discontinuation of therapy.
Subsequently, this trial was followed by four dose finding/optimization clinical trials termed LOWR-HDV (LOnafarnib With Ritonavir in HDV) 1, 2, 3 and 4 [151–154]. Since LNF is extensively metabolized by CYP3A4, these clinical trials included the addition of RTV—a CYP3A4 inhibitor that has been extensively used in HIV [171]. By utilizing RTV, LNF levels could be boosted in the blood while the gastrointestinal tract was exposed to lower doses of LNF. This allowed lower doses of lonafarnib, resulting in better tolerability while simultaneously increasing post-absorption LNF levels and antiviral efficacy [172].
LOWR-1 [NCT02430181] was a single-center, phase 2 pilot study performed in Ankara, Turkey that investigated varying doses of LNF ± RTV ± peg-IFN-α for 5–12 weeks in five groups of patients (three patients per group) [151]. The most impressive antiviral and biochemical responses were achieved in the LNF 100 mg twice daily (BID) + RTV 100 mg daily (HDV decline 3.2log10 IU/L) and LNF 100 mg BID + peg-IFN-α groups (HDV decline 3.0log10 IU/L) after 8 weeks of therapy. The rate of decline was more rapid compared with prior studies with peg-IFN-α alone [122]. Although no patients cleared the virus during treatment, two patients in the LNF monotherapy group achieved an undetectable HDV viral load post treatment after a transient ALT flare [151].
LOWR-2 [NCT02430194] was a phase 2, dose-ranging study that attempted to optimize dosing by evaluating 58 patients treated with 10 separate treatment arms of LNF (25 mg, 50 mg, 100 mg BID) + RTV 100 mg BID ± peg-IFN-α for 12–24 weeks [173]. This study showed impressive synergistic activity of lower-dose LNF/RTV with peg-IFN-α resulting in an HDV decline of 5.57log10 IU/L at 24 weeks. Three of five patients became HDV RNA-negative and ALT-normalized, and the regimen was well tolerated [152]. Interestingly, from the LOWR-1 and LOWR-2 studies, 5 of 27 (18.5%) patients treated with LNF who had a detectable HDV RNA at the end of treatment subsequently experienced a post-treatment ALT flare followed by rapid clearance of HDV, suggesting immune restoration [174].
LOWR-3 [NCT02511431] is a phase 2, double-blind, randomized, placebo-controlled study that evaluated once-daily dosing in 21 patients for 12–24 weeks [153]. Patients were randomized to doses of LNF at 50, 75 or 100 mg plus RTV 100 mg once a day. Final data are pending but patients experienced the highest HDV decline with LNF 50 mg (1.93log10 IU/L) compared with LNF 75 mg (1.3log10 IU/L) and LNF 100 mg (0.29log10 IU/L) at 24 weeks. One patient became HDV RNA-negative during the study and 6/21 (28.6%) patients achieved a HDV RNA below 250 IU/mL [153].
Finally, LOWR-4 [NCT02527707] is a phase 2, open-label, dose-titration study that treated 15 chronic HDV patients for 24 weeks [154]. Patients were started on LNF 50 mg BID and RTV 100 mg BID initially. LNF was then increased to a maximum of 100 mg BID per protocol as tolerated. Interim results have been reported describing that dose escalation was feasible and patients experienced a mean HDV decline of 1.87log10 IU/L at Week 8 [154].
In summary, the regimen that appears to have the highest efficacy in these LOWR-HDV trials is that of low-dose LNF 25 or 50 mg twice a day and ritonavir 100 mg twice a day with peg-IFN-α from LOWR-2. Three of five patients were HDV RNA-negative at Week 24—a response that persisted to Week 48 in the two patients who stopped treatment at Week 24 [175]. Although these results are promising and LNF has been well tolerated in the recent LOWR trials, long-term follow-up regarding therapy efficacy and safety are needed. It is encouraging to note that there has not been any evidence of viral resistance in the LNF studies thus far [151–154]. As mentioned, the LIFT trial [NCT03600714] evaluating the combination of peg-IFN-λ, LNF and RTV is ongoing. A phase 3 trial called the D-LIVR (Delta Liver Improvement and Virologic Response) study [NCT03719313] studying the efficacy and safety of LNF + RTF ± peg-INF-α will begin recruiting soon.
Entry inhibitor (Myrcludex B)
Myrcludex B is a myristoylated peptide of 47-amino acids derived from the preS1-domain of the L-HBsAg protein and first-in-class HBV/HDV entry inhibitor that works by inactivating the host receptor, NTCP [20]. This receptor is crucial for viral entry and inactivation leads to a decrease in the number of infected cells (Figure 1). Entry inhibitors as a class may also have a role in eliminating intrahepatic HBV/HDV genome [176]. Initial mice studies of Myrcludex B showed that Myrcludex B significantly inhibited HDV-infection establishment. However, continuous drug administration was necessary to prevent HDV spread [177].
In a phase 1 b/2a, randomized, three-arm, parallel, open-label, proof-of-concept study [NCT02637999] evaluating 24 patients with chronic HDV randomized (1:1:1) to Myrcludex B, peg-IFN-α or combination, Myrcludex B for 24 weeks showed efficacy as both a monotherapy as well as exhibiting a synergistic effect with peg-IFN-α. Myrcludex B was administered as a 2-mg subcutaneous injection once daily. Although the primary endpoint—a change in HBsAg levels—was not met, both Myrcludex B monotherapy and combination therapy with peg-IFN-α led to significant declines in HDV RNA and ALT levels. At 24 weeks, mean HDV declines were as follows: 1.67log10 IU/L (Myrcludex B), 2.6log10 IU/L (Myrcludex B + peg-IFN-α) and 2.2log10 IU/L (peg-IFN-α). Two out of the eight patients in both monotherapy groups became HDV RNA-negative, while combination therapy led to five of the seven patients becoming HDV RNA-negative [155]. Myrcludex was generally well tolerated.
This study was followed by a phase 2 b, multicenter, open-label study [NCT03546621] conducted in 120 patients with chronic HDV who were randomized to tenofovir ± Myrcludex B (2, 5, 10 mg) for 24 weeks. At 24 weeks, HDV declines were the most impressive at 5 and 10 mg: 1.6log10 IU/L (Myrcludex B 5 mg) and 2.7log10 IU/L (Myrcludex B 10 mg) [156, 178]. Efficacy was dose-dependent, with the best results seen at the 10-mg dose, with 76.6% of the patients in that arm achieving the primary endpoint (a decrease in HDV RNA by 2log10 or HDV RNA negativity). However, relapse occurred in most responders (60%–83%, depending on group) after cessation of therapy [156]. A phase 2, randomized, open-label study [NCT02637999] evaluating Myrcludex B ± peg-IFN-α for 24 weeks followed by peg-IFN-α for 48 weeks vs peg-IFN-α for 48 weeks has been completed, with results pending.
Although these results are promising, the appearance of antibodies to Myrcludex B in some patients has been described and is likely to be concerning. Although no correlation between the appearance of antibodies to safety or efficacy has been seen, attention to this finding has to be made in future studies [155]. In addition, HDV has been shown in ‘in vitro’ and ‘in vivo’ studies to propagate during liver regeneration through clonal cell division despite the presence of Myrcludex B [179]. Furthermore, Blank et al. reported that Myrcludex B significantly increases total plasma bile-acid exposure by 19.2-fold and conjugated bile acids by 124-fold in healthy human volunteers because NTCP is also a physiological bile-acid transporter [180]. Although the patients in that study remained asymptomatic, the long-term effects of elevated bile acids in the setting are unknown [180].
Nucleic acid-based polymer (REP2139)
NAPs are phosphorothioated oligonucleotides with broad-spectrum antiviral activity against many different types of viral infections including HCV and HIV [181–184]. NAPs continue to be investigated in HBV monotherapy due to a potent effect on HBsAg secretion [185, 186]. The leading drug in this class of HDV medication is REP2139 (Replicor©), which is administered as a once-a-week intravenous infusion. In HDV, the exact mechanisms of antiviral effects of NAPs have yet to be fully elucidated, but they appear to inhibit the secretion of HBsAg envelope proteins as well as being active at viral entry (Figure 1) [185, 187]. The antiviral effects of NAPs appear to be dependent on the size and amphipathicity of the polymer. The inhibition of viral entry of NAPs appears to be at least partly due to the NAPs binding to HDV particles and preventing attachment of the virus to the cell-surface glycosaminoglycans [181].
The initial proof-of-concept study, REP301 [NCT02233075], was an open-label, non-randomized study evaluating 12 patients with chronic HDV [157]. Patients received 500 mg intravenous REP2139 weekly for 15 weeks followed by combination therapy of 250 mg intravenous REP2139 and peg-IFN-α for 15 weeks and then peg-IFN-α monotherapy for 33 weeks. REP2139 demonstrated potent efficacy on HBV and HDV. There was a mean 3.5log10 IU/L decline in HBsAg from baseline and 5 of 12 patients became HBsAg-negative, which was maintained to 1 year post treatment. Nine of 12 patients became HDV RNA-negative by the time of treatment, with 7 of 9 patients remaining HDV RNA-negative at 1.5 years of post-therapy follow-up. Mean HDV RNA decline was 5.34log10 IU/L [157, 188]. Durability of the response is being evaluated in a long-term follow-up study [NCT02876419].
REP2139 appears to be the only one of the new investigative drugs that results in a fast reduction in HBsAg [143]. Although REP2139 was well tolerated, substantial ALT flares did occur in some patients, which may limit its use in cirrhosis [187]. Other concerns with NAPs include: side effects such as hair loss, dysphagia and dysgeusia that were seen during a prior HBV trial, which was attributed to heavy-metal exposure at the trial site, as well as the possibility of late relapses, which were seen during a prior HBV trial [157, 185].
Conclusion
HDV is a rapidly progressive viral hepatitis that increases the risk of cirrhosis, HCC, hepatic decompensation, liver transplantation and mortality compared with HBV mono-infection. Due to immigration and improvements in diagnosis through the development and standardization of diagnostic tests, HDV is now becoming increasingly recognized as a problem worldwide. However, there continues to be a lack of efficacious treatment options. Interferon therapy with peg-IFN-α remains the only regularly used therapy outside of clinical trials. However, recent improvements in our understanding of the virus have led to the investigation of several new and promising therapies—one or more of which may eventually be approved for use in HDV.
Authors’ contributions
B.D. and C.K. conceived of and designed the manuscript. B.D. and C.K. interpreted the available studies on the subject. B.D., T.H. and C.K. drafted and revised the manuscript. All authors reviewed and approved the final manuscript.
Funding
This study was supported by [grant number Z99-DK-999999] from the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases.
Acknowledgements
None.
Conflict of interest
None declared.
References
- 1. Rizzetto M, Canese MG, Arico S. et al. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977;18:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen HY, Shen DT, Ji DZ. et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut 2018;68:381–2. [DOI] [PubMed] [Google Scholar]
- 3. Taylor JM. Structure and replication of hepatitis delta virus RNA. Curr Top Microbiol Immunol 2006;307:1–23. [DOI] [PubMed] [Google Scholar]
- 4. Rizzetto M, Verme G, Recchia S. et al. Chronic hepatitis in carriers of hepatitis B surface antigen, with intrahepatic expression of the delta antigen: an active and progressive disease unresponsive to immunosuppressive treatment. Ann Intern Med 1983;98:437–41. [DOI] [PubMed] [Google Scholar]
- 5. Yurdaydin C, Idilman R, Bozkaya H. et al. Natural history and treatment of chronic delta hepatitis. J Viral Hepat 2010;17:749–56. [DOI] [PubMed] [Google Scholar]
- 6. Terrault NA, Lok ASF, McMahon BJ. et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. [DOI] [PubMed] [Google Scholar]
- 8. Sarin SK, Kumar M, Lau GK. et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heidrich B, Yurdaydin C, Kabacam G. et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology 2014;60:87–97. [DOI] [PubMed] [Google Scholar]
- 10. Sureau C. The role of the HBV envelope proteins in the HDV replication cycle. Curr Top Microbiol Immunol 2006;307:113–31. [DOI] [PubMed] [Google Scholar]
- 11. Bonino F, Heermann KH, Rizzetto M. et al. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol 1986;58:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sureau C, Negro F.. The hepatitis delta virus: replication and pathogenesis. J Hepatol 2016;64:S102–S116. [DOI] [PubMed] [Google Scholar]
- 13. Lai MM. The molecular biology of hepatitis delta virus. Annu Rev Biochem 1995;64:259–86. [DOI] [PubMed] [Google Scholar]
- 14. Kamath PS, Wiesner RH, Malinchoc M. et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–70. [DOI] [PubMed] [Google Scholar]
- 15. Glenn JS. Prenylation of HDAg and antiviral drug development. Curr Top Microbiol Immunol 2006;307:133–49. [DOI] [PubMed] [Google Scholar]
- 16. Wang KS, Choo QL, Weiner AJ. et al. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature 1986;323:508–14. [DOI] [PubMed] [Google Scholar]
- 17. Bonino F, Hoyer B, Shih JW. et al. Delta hepatitis agent: structural and antigenic properties of the delta-associated particle. Infect Immun 1984;43:1000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abou-Jaoude G, Molina S, Maurel P. et al. Myristoylation signal transfer from the large to the middle or the small HBV envelope protein leads to a loss of HDV particles infectivity. Virology 2007;365:204–9. [DOI] [PubMed] [Google Scholar]
- 19. Barrera A, Guerra B, Notvall L. et al. Mapping of the hepatitis B virus pre-S1 domain involved in receptor recognition. J Virol 2005;79:9786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan H, Zhong G, Xu G. et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012;1:e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hughes SA, Wedemeyer H, Harrison PM.. Hepatitis delta virus. Lancet 2011;378:73–85. [DOI] [PubMed] [Google Scholar]
- 22. Chang J, Nie X, Chang HE. et al. Transcription of hepatitis delta virus RNA by RNA polymerase II. J Virol 2008;82:1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor JM. Hepatitis delta virus: cis and trans functions required for replication. Cell 1990;61:371–3. [DOI] [PubMed] [Google Scholar]
- 24. Casey JL. Control of ADAR1 editing of hepatitis delta virus RNAs. Curr Top Microbiol Immunol 2012;353:123–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weiner AJ, Choo QL, Wang KS. et al. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol 1988;62:594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gudima S, Chang J, Moraleda G. et al. Parameters of human hepatitis delta virus genome replication: the quantity, quality, and intracellular distribution of viral proteins and RNA. J Virol 2002;76:3709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang IC, Chien CY, Huang CR. et al. Induction of hepatitis D virus large antigen translocation to the cytoplasm by hepatitis B virus surface antigens correlates with endoplasmic reticulum stress and NF-kappaB activation. J Gen Virol 2006;87:1715–23. [DOI] [PubMed] [Google Scholar]
- 28. Li YJ, Stallcup MR, Lai MM.. Hepatitis delta virus antigen is methylated at arginine residues, and methylation regulates subcellular localization and RNA replication. J Virol 2004;78:13325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glenn JS, Watson JA, Havel CM. et al. Identification of a prenylation site in delta virus large antigen. Science 1992;256:1331–3. [DOI] [PubMed] [Google Scholar]
- 30. Radjef N, Gordien E, Ivaniushina V. et al. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J Virol 2004;78:2537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hsu SC, Syu WJ, Sheen IJ. et al. Varied assembly and RNA editing efficiencies between genotypes I and II hepatitis D virus and their implications. Hepatology 2002;35:665–72. [DOI] [PubMed] [Google Scholar]
- 32. Lin FM, Lee CM, Wang TC. et al. Initiation of RNA replication of cloned Taiwan-3 isolate of hepatitis delta virus genotype II in cultured cells. Biochem Biophys Res Commun 2003;306:966–72. [DOI] [PubMed] [Google Scholar]
- 33. Chai N, Chang HE, Nicolas E. et al. Properties of subviral particles of hepatitis B virus. J Virol 2008;82:7812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deny P. Hepatitis delta virus genetic variability: from genotypes I, II, III to eight major clades? Curr Top Microbiol Immunol 2006;307:151–71. [DOI] [PubMed] [Google Scholar]
- 35. Le Gal F, Dziri S, Gerber A. et al. Performance characteristics of a new consensus commercial kit for hepatitis D virus RNA viral load quantification. J Clin Microbiol 2017;55:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rizzetto M, Smedile A.. Pegylated interferon therapy of chronic hepatitis D: in need of revision. Hepatology 2015;61:1109–11. [DOI] [PubMed] [Google Scholar]
- 37. Sadeghian H, Varasteh N, Esmaeelzadeh A. et al. Distribution of hepatitis delta virus genotypes in Mashhad, northeast Iran. Jundishapur J Microbiol 2015;8:e14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fouad R, Abdo M, Eldeen HG. et al. Influence of delta virus infection on the virologic status in Egyptian patients with chronic hepatitis B virus genotype D. J Med Virol 2016;88:837–42. [DOI] [PubMed] [Google Scholar]
- 39. Casey JL, Brown TL, Colan EJ. et al. A genotype of hepatitis D virus that occurs in northern South America. Proc Natl Acad Sci USA 1993;90:9016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. El Bouzidi K, Elamin W, Kranzer K. et al. Hepatitis delta virus testing, epidemiology and management: a multicentre cross-sectional study of patients in London. J Clin Virol 2015;66:33–7. [DOI] [PubMed] [Google Scholar]
- 41. Servant-Delmas A, Le Gal F, Gallian P. et al. Increasing prevalence of HDV/HBV infection over 15 years in France. J Clin Virol 2014;59:126–8. [DOI] [PubMed] [Google Scholar]
- 42. Beguelin C, Moradpour D, Sahli R. et al. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol 2017;66:297–303. [DOI] [PubMed] [Google Scholar]
- 43. Casey JL, Niro GA, Engle RE. et al. Hepatitis B virus (HBV)/hepatitis D virus (HDV) coinfection in outbreaks of acute hepatitis in the Peruvian Amazon basin: the roles of HDV genotype III and HBV genotype F. J Infect Dis 1996;174:920–6. [DOI] [PubMed] [Google Scholar]
- 44. Wranke A, Pinheiro Borzacov LM, Parana R, et al. The hepatitis delta international network (HDIN). Liver Int 2018;38:842–50. [DOI] [PubMed] [Google Scholar]
- 45. Su CW, Huang YH, Huo TI. et al. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology 2006;130:1625–35. [DOI] [PubMed] [Google Scholar]
- 46. Wu JC, Chen TZ, Huang YS. et al. Natural history of hepatitis D viral superinfection: significance of viremia detected by polymerase chain reaction. Gastroenterology 1995;108:796–802. [DOI] [PubMed] [Google Scholar]
- 47. Braga WSM, Castilho M. D C, Borges FG. et al. Hepatitis D virus infection in the Western Brazilian Amazon—far from a vanishing disease. Rev Soc Bras Med Trop 2012;45:691–5. [DOI] [PubMed] [Google Scholar]
- 48. Coghill S, McNamara J, Woods M. et al. Epidemiology and clinical outcomes of hepatitis delta (D) virus infection in Queensland, Australia. Int J Infect Dis 2018;74:123–7. [DOI] [PubMed] [Google Scholar]
- 49. Shadur B, MacLachlan J, Cowie B.. Hepatitis D virus in Victoria 2000–2009. Intern Med J 2013;43:1081–7. [DOI] [PubMed] [Google Scholar]
- 50. Chen X, Xuan M, Yin Y.. [Study of HDV infection in Shandong province]. Zhonghua Liu Xing Bing Xue Za Zhi 1998;19:138–40. [PubMed] [Google Scholar]
- 51. Zhao X. [The seroepidemiological observation on hepatitis delta virus infection]. Zhonghua Liu Xing Bing Xue Za Zhi 1990;11:202–4. [PubMed] [Google Scholar]
- 52. Zhang JY, Jin ZH, Wang CJ.. [A seroepidemiological study on hepatitis D virus (HDV) infection in Henan Province, China]. Zhonghua Liu Xing Bing Xue Za Zhi 1995;16:365–8. [PubMed] [Google Scholar]
- 53. Cross TJ, Rizzi P, Horner M. et al. The increasing prevalence of hepatitis delta virus (HDV) infection in South London. J Med Virol 2008;80:277–82. [DOI] [PubMed] [Google Scholar]
- 54. Kelly V, Kensit J, Barrett A.. Hepatitis D (delta) infection in south-east London. Lancet 1989;1:45.. [DOI] [PubMed] [Google Scholar]
- 55. Manesis EK, Vourli G, Dalekos G. et al. Prevalence and clinical course of hepatitis delta infection in Greece: a 13-year prospective study. J Hepatol 2013;59:949–56. [DOI] [PubMed] [Google Scholar]
- 56. Heidrich B, Deterding K, Tillmann HL. et al. Virological and clinical characteristics of delta hepatitis in Central Europe. J Viral Hepat 2009;16:883–94. [DOI] [PubMed] [Google Scholar]
- 57. Wedemeyer H, Manns MP.. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol 2010;7:31–40. [DOI] [PubMed] [Google Scholar]
- 58. Tamura I, Kurimura O, Koda T. et al. Risk of liver cirrhosis and hepatocellular carcinoma in subjects with hepatitis B and delta virus infection: a study from Kure, Japan. J Gastroenterol Hepatol 1993;8:433–6. [DOI] [PubMed] [Google Scholar]
- 59. Chakraborty P, Kailash U, Jain A. et al. Seroprevalence of hepatitis D virus in patients with hepatitis B virus-related liver diseases. Indian J Med Res 2005;122:254–7. [PubMed] [Google Scholar]
- 60. Banker DD, Desai P, Brawner TA. et al. Hepatitis delta virus infection in Bombay. Trans R Soc Trop Med Hyg 1992;86:424–5. [DOI] [PubMed] [Google Scholar]
- 61. Toukan AU, Abu-el-Rub OA, Abu-Laban SA. et al. The epidemiology and clinical outcome of hepatitis D virus (delta) infection in Jordan. Hepatology 1987;7:1340–5. [DOI] [PubMed] [Google Scholar]
- 62. Bakhshipour A, Mashhadi M, Mohammadi M. et al. Seroprevalence and risk factors of hepatitis delta virus in chronic hepatitis B virus infection in Zahedan. Acta Med Iran 2013;51:260–4. [PubMed] [Google Scholar]
- 63. Weisfuse IB, Hadler SC, Fields HA. et al. Delta hepatitis in homosexual men in the United States. Hepatology 1989;9:872–4. [DOI] [PubMed] [Google Scholar]
- 64. Hershow RC, Chomel BB, Graham DR. et al. Hepatitis D virus infection in Illinois state facilities for the developmentally disabled: epidemiology and clinical manifestations. Ann Intern Med 1989;110:779–85. [DOI] [PubMed] [Google Scholar]
- 65. Patel EU, Thio CL, Boon D. et al. Prevalence of hepatitis B and hepatitis D virus infections in the United States, 2011–2016. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kucirka LM, Farzadegan H, Feld JJ. et al. Prevalence, correlates, and viral dynamics of hepatitis delta among injection drug users. J Infect Dis 2010;202:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gaeta GB, Stroffolini T, Chiaramonte M. et al. Chronic hepatitis D: a vanishing disease? An Italian multicenter study. Hepatology 2000;32:824–7. [DOI] [PubMed] [Google Scholar]
- 68. Tsatsralt-Od B, Takahashi M, Nishizawa T. et al. High prevalence of dual or triple infection of hepatitis B, C, and delta viruses among patients with chronic liver disease in Mongolia. J Med Virol 2005;77:491–9. [DOI] [PubMed] [Google Scholar]
- 69. Mumtaz K, Hamid SS, Adil S. et al. Epidemiology and clinical pattern of hepatitis delta virus infection in Pakistan. J Gastroenterol Hepatol 2005;20:1503–7. [DOI] [PubMed] [Google Scholar]
- 70. Al-Traif I, Ali A, Dafalla M. et al. Prevalence of hepatitis delta antibody among HBsAG carriers in Saudi Arabia. Ann Saudi Med 2004;24:343–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lin HH, Lee SS, Yu ML. et al. Changing hepatitis D virus epidemiology in a hepatitis B virus endemic area with a national vaccination program. Hepatology 2015;61:1870–9. [DOI] [PubMed] [Google Scholar]
- 72. Lu SN, Chen TM, Lee CM. et al. Molecular epidemiological and clinical aspects of hepatitis D virus in a unique triple hepatitis viruses (B, C, D) endemic community in Taiwan. J Med Virol 2003;70:74–80. [DOI] [PubMed] [Google Scholar]
- 73. Louisirirotchanakul S, Wasi C, Uneklabh C. et al. High prevalence of delta virus infection in Thai intravenous drug abusers. Southeast Asian J Trop Med Public Health 1988;19:191–5. [PubMed] [Google Scholar]
- 74. Theamboonlers A, Hansurabhanon T, Verachai V. et al. HDV genotyping by RT-PCR, RFLP and direct sequencing. Infection 2002;30:140–4. [DOI] [PubMed] [Google Scholar]
- 75. Triki H, Said N, Ben Salah A. et al. Seroepidemiology of hepatitis B, C and delta viruses in Tunisia. Trans R Soc Trop Med Hyg 1997;91:11–4. [DOI] [PubMed] [Google Scholar]
- 76. Mese S, Nergiz S, Tekes S. et al. Seroprevalence of serum HBsAg positivity and hepatitis delta virus infection among blood donors in Southeastern Turkey. Clin Ter 2014;165:95–8. [DOI] [PubMed] [Google Scholar]
- 77. Bahcecioglu IH, Aygun C, Gozel N. et al. Prevalence of hepatitis delta virus (HDV) infection in chronic hepatitis B patients in eastern Turkey: still a serious problem to consider. J Viral Hepat 2011;18:518–24. [DOI] [PubMed] [Google Scholar]
- 78. Kushner T, Serper M, Kaplan DE.. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol 2015;63:586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Komas NP, Ghosh S, Abdou-Chekaraou M. et al. Hepatitis B and hepatitis D virus infections in the Central African Republic, twenty-five years after a fulminant hepatitis outbreak, indicate continuing spread in asymptomatic young adults. PLoS Negl Trop Dis 2018;12:e0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Degertekin H, Yalcin K, Yakut M. et al. Seropositivity for delta hepatitis in patients with chronic hepatitis B and liver cirrhosis in Turkey: a meta-analysis. Liver Int 2008;28:494–8. [DOI] [PubMed] [Google Scholar]
- 81. Ciancio A, Rizzetto M.. Chronic hepatitis D at a standstill: where do we go from here? Nat Rev Gastroenterol Hepatol 2014;11:68–71. [DOI] [PubMed] [Google Scholar]
- 82. Buti M, Homs M, Rodriguez-Frias F. et al. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J Viral Hepat 2011;18:434–42. [DOI] [PubMed] [Google Scholar]
- 83. Rosina F, Conoscitore P, Cuppone R. et al. Changing pattern of chronic hepatitis D in Southern Europe. Gastroenterology 1999;117:161–6. [DOI] [PubMed] [Google Scholar]
- 84. Wedemeyer H, Heidrich B, Manns MP.. Hepatitis D virus infection—not a vanishing disease in Europe!. Hepatology 2007;45:1331–2; author reply 1332–3. [DOI] [PubMed] [Google Scholar]
- 85. Gaeta GB, Stroffolini T, Smedile A. et al. Hepatitis delta in Europe: vanishing or refreshing? Hepatology 2007;46:1312–3. [DOI] [PubMed] [Google Scholar]
- 86. Smedile A, Lavarini C, Farci P. et al. Epidemiologic patterns of infection with the hepatitis B virus-associated delta agent in Italy. Am J Epidemiol 1983;117:223–9. [DOI] [PubMed] [Google Scholar]
- 87. Sagnelli E, Stroffolini T, Ascione A. et al. Decrease in HDV endemicity in Italy. J Hepatol 1997;26:20–4. [DOI] [PubMed] [Google Scholar]
- 88. Farci P. Delta hepatitis: an update. J Hepatol 2003;39(Suppl 1):S212–9. [DOI] [PubMed] [Google Scholar]
- 89. Rizzetto M. Hepatitis D virus: introduction and epidemiology. Cold Spring Harb Perspect Med 2015;5:a021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Romeo R, Del Ninno E, Rumi M. et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009;136:1629–38. [DOI] [PubMed] [Google Scholar]
- 91. Niro GA, Smedile A, Ippolito AM. et al. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol 2010;53:834–40. [DOI] [PubMed] [Google Scholar]
- 92. Madejon A, Castillo I, Bartolome J. et al. Detection of HDV-RNA by PCR in serum of patients with chronic HDV infection. J Hepatol 1990;11:381–4. [DOI] [PubMed] [Google Scholar]
- 93. Deny P, Lecot C, Jeantils V. et al. Polymerase chain reaction-based detection of hepatitis D virus genome in patients infected with human immunodeficiency virus. J Med Virol 1993;39:214–8. [DOI] [PubMed] [Google Scholar]
- 94. Jardi R, Buti M, Cotrina M. et al. Determination of hepatitis delta virus RNA by polymerase chain reaction in acute and chronic delta infection. Hepatology 1995;21:25–9. [PubMed] [Google Scholar]
- 95. Le Gal F, Gordien E, Affolabi D. et al. Quantification of hepatitis delta virus RNA in serum by consensus real-time PCR indicates different patterns of virological response to interferon therapy in chronically infected patients. J Clin Microbiol 2005;43:2363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yamashiro T, Nagayama K, Enomoto N. et al. Quantitation of the level of hepatitis delta virus RNA in serum, by real-time polymerase chain reaction—and its possible correlation with the clinical stage of liver disease. J Infect Dis 2004;189:1151–7. [DOI] [PubMed] [Google Scholar]
- 97. Chudy M, Hanschmann K, Bozsayi M, et al. Collaborative study to establish a World Health Organization international standard for hepatitis D virus RNA for nucleic acid amplification technology (NAT)-based assays. WHO Report (WHO/BS/2013.2227); 2013.
- 98. Le Gal F, Brichler S, Sahli R. et al. First international external quality assessment for hepatitis delta virus RNA quantification in plasma. Hepatology 2016;64:1483–94. [DOI] [PubMed] [Google Scholar]
- 99. Sakugawa H, Nakasone H, Nakayoshi T. et al. Hepatitis B virus concentrations in serum determined by sensitive quantitative assays in patients with established chronic hepatitis delta virus infection. J Med Virol 2001;65:478–84. [PubMed] [Google Scholar]
- 100. Farci P, Niro GA.. Clinical features of hepatitis D. Semin Liver Dis 2012;32:228–36. [DOI] [PubMed] [Google Scholar]
- 101. Fiedler M, Roggendorf M.. Immunology of HDV infection. Curr Top Microbiol Immunol 2006;307:187–209. [DOI] [PubMed] [Google Scholar]
- 102. Negro F. Hepatitis D virus coinfection and superinfection. Cold Spring Harb Perspect Med 2014;4:a021550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Abbas Z, Afzal R.. Life cycle and pathogenesis of hepatitis D virus: a review. World J epatol 2013;5:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wu JC, Choo KB, Chen CM. et al. Genotyping of hepatitis D virus by restriction-fragment length polymorphism and relation to outcome of hepatitis D. Lancet 1995;346:939–41. [DOI] [PubMed] [Google Scholar]
- 105. Guilhot S, Huang SN, Xia YP. et al. Expression of the hepatitis delta virus large and small antigens in transgenic mice. J Virol 1994;68:1052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cole SM, Gowans EJ, Macnaughton TB. et al. Direct evidence for cytotoxicity associated with expression of hepatitis delta virus antigen. Hepatology 1991;13:845–51. [PubMed] [Google Scholar]
- 107. Wu JC, Chen PJ, Kuo MY. et al. Production of hepatitis delta virus and suppression of helper hepatitis B virus in a human hepatoma cell line. J Virol 1991;65:1099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Braga WS, de Oliveira CM, de AJ. et al. Chronic HDV/HBV co-infection: predictors of disease stage—a case series of HDV-3 patients. J Hepatol 2014;61:1205–11. [DOI] [PubMed] [Google Scholar]
- 109. Heidrich B, Serrano BC, Idilman R. et al. HBeAg-positive hepatitis delta: virological patterns and clinical long-term outcome. Liver Int 2012;32:1415–25. [DOI] [PubMed] [Google Scholar]
- 110. Fattovich G, Boscaro S, Noventa F. et al. Influence of hepatitis delta virus infection on progression to cirrhosis in chronic hepatitis type B. J Infect Dis 1987;155:931.. [DOI] [PubMed] [Google Scholar]
- 111. Fattovich G, Giustina G, Christensen E. et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B: The European Concerted Action on Viral Hepatitis (Eurohep). Gut 2000;46:420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ji J, Sundquist K, Sundquist J.. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst 2012;104:790–2. [DOI] [PubMed] [Google Scholar]
- 113. Abbas Z, Abbas M, Abbas S. et al. Hepatitis D and hepatocellular carcinoma. World J Hepatol 2015;7:777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Abbas Z, Qureshi M, Hamid S. et al. Hepatocellular carcinoma in hepatitis D: does it differ from hepatitis B monoinfection? Saudi J Gastroenterol 2012;18:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Brechot C. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: old and new paradigms. Gastroenterology 2004;127:S56–61. [DOI] [PubMed] [Google Scholar]
- 116. Wranke A, Heidrich B, Ernst S. et al. Anti-HDV IgM as a marker of disease activity in hepatitis delta. PLoS One 2014;9:e101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chen CJ, Yang HI, Iloeje UH.. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology 2009;49:S72–84. [DOI] [PubMed] [Google Scholar]
- 118. Chen CJ, Yang HI, Su J. et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65–73. [DOI] [PubMed] [Google Scholar]
- 119. Rizzetto M. Chronic hepatitis D: at a standstill? Dig Dis 2016;34:303–7. [DOI] [PubMed] [Google Scholar]
- 120. Elazar M, Glenn JS.. Emerging concepts for the treatment of hepatitis delta. Curr Opin Virol 2017;24:55–9. [DOI] [PubMed] [Google Scholar]
- 121. Pugnale P, Pazienza V, Guilloux K. et al. Hepatitis delta virus inhibits alpha interferon signaling. Hepatology 2009;49:398–406. [DOI] [PubMed] [Google Scholar]
- 122. Wedemeyer H, Yurdaydin C, Dalekos GN. et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011;364:322–31. [DOI] [PubMed] [Google Scholar]
- 123. Erhardt A, Gerlich W, Starke C. et al. Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver Int 2006;26:805–10. [DOI] [PubMed] [Google Scholar]
- 124. Sleijfer S, Bannink M, Van Gool AR. et al. Side effects of interferon-alpha therapy. Pharm World Sci 2005;27:423–31. [DOI] [PubMed] [Google Scholar]
- 125. Wranke A, Serrano BC, Heidrich B. et al. Antiviral treatment and liver-related complications in hepatitis delta. Hepatology 2017;65:414–25. [DOI] [PubMed] [Google Scholar]
- 126. Farci P, Roskams T, Chessa L. et al. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology 2004;126:1740–9. [DOI] [PubMed] [Google Scholar]
- 127. Yurdaydin C, Keskin O, Kalkan C. et al. Interferon treatment duration in patients with chronic delta hepatitis and its effect on the natural course of the disease. J Infect Dis 2018;217:1184–92. [DOI] [PubMed] [Google Scholar]
- 128. Abbas Z, Khan MA, Salih M. et al. Interferon alpha for chronic hepatitis D. Cochrane Database Syst Rev 7;10.1002/14651858.CD006002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Farci P, Mandas A, Coiana A. et al. Treatment of chronic hepatitis D with interferon alfa-2a. N Engl J Med 1994;330:88–94. [DOI] [PubMed] [Google Scholar]
- 130. Terrault NA, Bzowej NH, Chang KM. et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Niro GA, Ciancio A, Gaeta GB. et al. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology 2006;44:713–20. [DOI] [PubMed] [Google Scholar]
- 132. Castelnau C, Le Gal F, Ripault MP. et al. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology 2006;44:728–35. [DOI] [PubMed] [Google Scholar]
- 133. Gheorghe L, Iacob S, Simionov I. et al. Weight-based dosing regimen of peg-interferon alpha-2b for chronic hepatitis delta: a multicenter Romanian trial. J Gastrointestin Liver Dis 2011;20:377–82. [PubMed] [Google Scholar]
- 134. Lau DT, Kleiner DE, Park Y. et al. Resolution of chronic delta hepatitis after 12 years of interferon alfa therapy. Gastroenterology 1999;117:1229–33. [DOI] [PubMed] [Google Scholar]
- 135. Heller T, Rotman Y, Koh C. et al. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther 2014;40:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Karaca C, Soyer OM, Baran B. et al. Efficacy of pegylated interferon-alpha treatment for 24 months in chronic delta hepatitis and predictors of response. Antivir Ther 2012;18:561–6. [DOI] [PubMed] [Google Scholar]
- 137. Yurdaydin C, Bozkaya H, Karaaslan H. et al. A pilot study of 2 years of interferon treatment in patients with chronic delta hepatitis. J Viral Hepat 2007;14:812–6. [DOI] [PubMed] [Google Scholar]
- 138. Wedemeyer H, Yurdaydin C, Ernst S. et al. Prolonged therapy of hepatitis delta for 96 weeks with pegylated-interferon-2a plus tenofovir or placebo does not prevent HDV RNA relapase after treatment: the HIDIT-2 study. J Hepatol 2014;60:S2–3. [Google Scholar]
- 139. Gunsar F, Akarca US, Ersoz G. et al. Two-year interferon therapy with or without ribavirin in chronic delta hepatitis. Antivir Ther 2005;10:721–6. [PubMed] [Google Scholar]
- 140. Yurdaydin C, Bozkaya H, Onder FO. et al. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat 2008;15:314–21. [DOI] [PubMed] [Google Scholar]
- 141. Lau DT, Doo E, Park Y. et al. Lamivudine for chronic delta hepatitis. Hepatology 1999;30:546–9. [DOI] [PubMed] [Google Scholar]
- 142. Yurdaydin C, Bozkaya H, Gurel S. et al. Famciclovir treatment of chronic delta hepatitis. J Hepatol 2002;37:266–71. [DOI] [PubMed] [Google Scholar]
- 143. Farci P, Anna Niro G.. Current and future management of chronic hepatitis D. Gastroenterol Hepatol (NY) 2018;14:342–51. [PMC free article] [PubMed] [Google Scholar]
- 144. Manesis EK, Schina M, Le Gal F. et al. Quantitative analysis of hepatitis D virus RNA and hepatitis B surface antigen serum levels in chronic delta hepatitis improves treatment monitoring. Antivir Ther 2007;12:381–8.. [PubMed] [Google Scholar]
- 145. Guedj J, Rotman Y, Cotler SJ. et al. Understanding early serum hepatitis D virus and hepatitis B surface antigen kinetics during pegylated interferon-alpha therapy via mathematical modeling. Hepatology 2014;60:1902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Keskin O, Wedemeyer H, Tuzun A. et al. Association between level of hepatitis D virus RNA at week 24 of pegylated interferon therapy and outcome. Clin Gastroenterol Hepatol 2015;13:2342–9. e2341–2. [DOI] [PubMed] [Google Scholar]
- 147. Lutterkort GL, Wranke A, Hengst J. et al. Viral dominance patterns in chronic hepatitis delta determine early response to interferon alpha therapy. J Viral Hepat 2018;25:1384–94. [DOI] [PubMed] [Google Scholar]
- 148. Niro GA, Smedile A, Fontana R. et al. HBsAg kinetics in chronic hepatitis D during interferon therapy: on-treatment prediction of response. Aliment Pharmacol Ther 2016;44:620–8. [DOI] [PubMed] [Google Scholar]
- 149. Hamid S, Etzion O, Luri Y. et al. A phase 2 randomized clinical trial to evaluate the safety and efficacy of pegylated interferon lambda monotherapy in patients with chronic hepatitis delta virus infection: interim results from the LIMT HDV Study. Hepatology 2017;66:496A. [Google Scholar]
- 150. Koh C, Canini L, Dahari H. et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis 2015;15:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yurdaydin C, Keskin O, Kalkan C. et al. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: the LOWR HDV-1 study. Hepatology 2018;67:1224–36. [DOI] [PubMed] [Google Scholar]
- 152. Yurdaydin C, Idilman R, Keskin O. et al. A phase 2 dose-optimization study of lonafarnib with ritonavir for the treatment of chronic delta hepatitis-end of treatment results from the LOWR HDV-2 study. J Hepatol 2017;66:S33–S34. [Google Scholar]
- 153. Koh C, Surana P, Han T. et al. A phase 2 study exploring once daily dosing of ritonavir boosted lonafarnib for the treatment of chronic delta hepatitis; end of study results from the LOWR HDV-3 study. J Hepatol 2017;66:S101–S102. [Google Scholar]
- 154. Wedemeyer H, Port K, Deterding K. et al. A phase 2 study of titrating-dose lonafarnib plus Ritonavir in patients with chronic hepatitis D: interim results from the lonafarnib with Ritonavir in HDV-4 (LOWR HDV-4) study. Hepatology 2016;64:121A. [Google Scholar]
- 155. Bogomolov P, Alexandrov A, Voronkova N. et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: first results of a phase Ib/IIa study. J Hepatol 2016;65:490–8. [DOI] [PubMed] [Google Scholar]
- 156. Wedemeyer H, Bogomolov P, Blank A. et al. Final results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with Tenofovir in patients with chronic HBV/HDV co-infection. J Hepatol 2018;68:S3. [Google Scholar]
- 157. Bazinet M, Pantea V, Cebotarescu V. et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2017;2:877–89. [DOI] [PubMed] [Google Scholar]
- 158. Yurdaydin C. Recent advances in managing hepatitis D. F1000Res 2017;6:1596.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Singh S, Gupta SK, Nischal A. et al. Design of potential siRNA molecules for hepatitis delta virus gene silencing. Bioinformation 2012;8:749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yurdaydin C, Abbas Z, Buti M. et al. Treating chronic hepatitis delta: the need for surrogate markers of treatment efficacy. J Hepatol 2019;70:1008–15. [DOI] [PubMed] [Google Scholar]
- 161. Donnelly RP, Kotenko SV.. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res 2010;30:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Muir AJ, Shiffman ML, Zaman A. et al. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology 2010;52:822–32. [DOI] [PubMed] [Google Scholar]
- 163. Giersch K, Homs M, Volz T. et al. Both interferon alpha and lambda can reduce all intrahepatic HDV infection markers in HBV/HDV infected humanized mice. Sci Rep 2017;7:3757.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chan HLY, Ahn SH, Chang TT. et al. Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: a randomized phase 2b study (LIRA-B). J Hepatol 2016;64:1011–9. [DOI] [PubMed] [Google Scholar]
- 165. Foster GR, Chayama K, Chuang WL. et al. A randomized, controlled study of peginterferon lambda-1a/ribavirin +/– daclatasvir for hepatitis C virus genotype 2 or 3. Springerplus 2016;5:1365.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bordier BB, Marion PL, Ohashi K. et al. A prenylation inhibitor prevents production of infectious hepatitis delta virus particles. J Virol 2002;76:10465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Glenn JS, Marsters JC Jr, Greenberg HB.. Use of a prenylation inhibitor as a novel antiviral agent. J Virol 1998;72:9303–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lempp F, Schlund F, Rieble L. et al. Prenylation inhibition of the large hepatitis delta virus antigen leads to intracellular accumulation of viral RNA and enhanced innate immune responses. Hepatology 2018;68:151A–2A. [Google Scholar]
- 169. Gordon LB, Shappell H, Massaro J. et al. Association of lonafarnib treatment vs no treatment with mortality rate in patients with Hutchinson-Gilford progeria syndrome. JAMA 2018;319:1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wong NS, Morse MA.. Lonafarnib for cancer and progeria. Expert Opin Investig Drugs 2012;21:1043–55. [DOI] [PubMed] [Google Scholar]
- 171. Boffito M, Back D, Gatell JM.. Twenty years of boosting antiretroviral agents: where are we today?. Aids 2015;29:2229–33. [DOI] [PubMed] [Google Scholar]
- 172. Canini L, Koh C, Cotler SJ. et al. Pharmacokinetics and pharmacodynamics modeling of lonafarnib in patients with chronic hepatitis delta virus infection. Hepatol Commun 2017;1:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yurdaydin C, Idilman R, Kalkan C. et al. Exploring optimal dosing of lonafarnib with Ritonavir for the treatment of chronic delta hepatitis—interim results from the Lowr Hdv-2 study. Hepatology 2016;64: 910A. [Google Scholar]
- 174. Yurdaydin C, Idilman R, Kalkan C. et al. The prenylation inhibitor lonafarnib can induce post-treatment Alt flares with viral Cle. Hepatology 2016;64:927A. [Google Scholar]
- 175. Glenn JS. Emerging therapeutic targets for hepatitis delta virus infection. Gastroenterol Hepatol (NY) 2018;14:47–9. [PMC free article] [PubMed] [Google Scholar]
- 176. Tu T, Urban S.. Virus entry and its inhibition to prevent and treat hepatitis B and hepatitis D virus infections. Curr Opin Virol 2018;30:68–79. [DOI] [PubMed] [Google Scholar]
- 177. Volz T, Giersch K, Allweiss L. et al. P0530: Myrcludex-B inhibits establishment of HDV super-infection in HBV infected mice and reduces HDV viremia in stably HBV/HDV co-infected mice. J Hepatol 2015;62:S514. [Google Scholar]
- 178. Wedemeyer H, Bogomolov P, Blank A, et al. Interim results of a multicenter, open-label phase 2b clinical trial to assess the safety and efficacy of Mycrludex B in combination with tenofovir in patients with HBV/HDV coinfection [AASLD 2773178]; 2017.
- 179. Giersch K, Bhadra OD, Volz T. et al. Hepatitis delta virus persists during liver regeneration and is amplified through cell division both in vitro and in vivo. Gut 2019;68:150–7. [DOI] [PubMed] [Google Scholar]
- 180. Blank A, Eidam A, Haag M. et al. The NTCP-inhibitor Myrcludex B: effects on bile acid disposition and Tenofovir pharmacokinetics. Clin Pharmacol Ther 2018;103:341–8. [DOI] [PubMed] [Google Scholar]
- 181. Beilstein F, Blanchet M, Vaillant A. et al. Nucleic acid polymers are active against hepatitis delta virus infection in vitro. J Virol 2018;92:e01416–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Guzman EM, Cheshenko N, Shende V. et al. Amphipathic DNA polymers are candidate vaginal microbicides and block herpes simplex virus binding, entry and viral gene expression. Antivir Ther 2007;12:1147–56. [PubMed] [Google Scholar]
- 183. Lee AM, Rojek JM, Gundersen A. et al. Inhibition of cellular entry of lymphocytic choriomeningitis virus by amphipathic DNA polymers. Virology 2008;372:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Matsumura T, Hu Z, Kato T. et al. Amphipathic DNA polymers inhibit hepatitis C virus infection by blocking viral entry. Gastroenterology 2009;137:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Al-Mahtab M, Bazinet M, Vaillant A.. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS One 2016;11:e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Bazinet MP, Placinta G, Update on safety and efficacy in the REP 401 protocol: REP 2139-Mg or REP 2165-Mg used in combination with tenofovir disoproxil fumarate and pegylated-Interferon alpha-2a in treatment naive Caucasian patients with chronic HBeAg negative HBV infection: International Liver Congress. Amsterdam, Netherlands, 2017.
- 187. Alfaiate D, Negro F.. Nucleic acid polymers: much-needed hope for hepatitis D? Lancet Gastroenterol Hepatol 2017;2:841–2. [DOI] [PubMed] [Google Scholar]
- 188. Bazinet M, Pantea V, Cebotarescu V. et al. Establishment of persistent functional remission of HBV and HDV infection following REP 2139 and pegylated interferon alpha 2a therapy in patients with chronic HBV/HDV co-infection: 18 month follow-up results from the REP 301-LTF study. J Hepatol 2018;68:S509. [Google Scholar]