Abstract
Bovine mastitis is a common inflammatory disease caused by multiple factors in early lactation or dry period. Genome wide association studies (GWAS) can provide a convenient and effective strategy for understanding the biological basis of mastitis and better prevention. 2b-RADseq is a high-throughput sequencing technique that offers a powerful method for genome-wide genetic marker development and genotyping. In this study, single nucleotide polymorphisms (SNPs) of the immune-regulated gene correlative with mastitis were screened and identified by two stage association analysis via GWAS-2b-RADseq in Chinese Holstein cows. We have screened 10,058 high quality SNPs from 7,957,920 tags and calculated their allele frequencies. Twenty-seven significant SNPs were co-labeled in two GWAS analysis models [Bayesian (P < 0.001) and Logistic regression (P < 0.01)], and only three SNPs (rs75762330, C > T, PIC = 0.2999; rs88640083, A > G, PIC = 0.1676; rs20438858, G > A, PIC = 0.3366) were annotated to immune-regulated genes (PTK2B, SYK, and TNFRSF21). Identified three SNPs are located in non-coding regions with low or moderate genetic polymorphisms. However, independent sample population validation (Case-control study) data showed that three important SNPs (rs75762330, P < 0.025, OR > 1; rs88640083, P < 0.005, OR > 1; rs20438858, P < 0.001, OR < 1) were significantly associated with clinical mastitis trait. Importantly, PTK2B and SYK expression was down-regulated in both peripheral blood leukocytes (PBLs) of clinical mastitis cows and in vitro LPS (E. coli)–stimulated bovine mammary epithelial cells, while TNFRSF21 was up-regulated. Under the same conditions, expression of Toll-like receptor 4 (TLR4), AKT1, and pro-inflammatory factors (IL-1β and IL-8) were also up-regulated. Interestingly, network analysis indicated that PTK2B and SYK are co-expressed in innate immune signaling pathway of Chinese Holstein. Taken together, these results provided strong evidence for the study of SNPs in bovine mastitis, and revealed the role of SYK, PTK2B, and TNFRSF21 in bovine mastitis susceptibility/tolerance.
Keywords: SNPs, bovine mastitis, protein tyrosine kinase 2 (PTK2B), spleen tyrosine kinase (SYK), TNF receptor superfamily member 21 (TNFRSF21), immune responses
Introduction
Bovine mastitis is the most complex and costly inflammatory disease with high incidence, which seriously affects developing dairy industry worldwide (1–3). Although the breeding conditions of dairy cows have improved, mastitis is still a concern. Dairy cow mastitis causes direct economic losses in many ways, including decreased milk production, increased treatment cost, antibiotic residues, bacterial contamination, and even increased elimination rate due to cow death (4–7). Previous studies have shown that cow mastitis has complex traits (clinical and subclinical mastitis) and is affected by multiple factors, including genetic features, nutrition, and hygiene standard, pathogen infections, and seasonal changes. Among them, pathogen infection (Gram-negative, such as Escherichia coli; Gram-positive, such as Staphylococcus aureus and Streptococcus uberis) is the main cause of mastitis (8–13). Therefore, rapid identification of pathogens is critical to determine the symptoms of infection (14). However, mastitis-related immune response is a complex biological process involving immune cells, mammary epithelial cells, and endothelial cells (8). It is well-known that mastitis causes a dramatic increase in bovine milk somatic cell counts (SCC), mainly neutrophils (8, 15, 16). It is also known that somatic cell score (SCS) is the primary trait for detection of mastitis with high hereditary capacity (17). Thus, screening and identifying susceptible or resistant genes associated with SCC or SCS will provide novel strategies to reduce the incidence of mastitis and improve the quality of dairy cows populations (17–19).
It has been recognized that better understanding of the genetic and biological basis of a complex environment will facilitate genome prediction and the development of appropriate control strategies (20–24). In the past decade, different research strategies, including single nucleotide polymorphism (SNP) in a candidate gene, quantitative trait loci (QTL) and GWAS (12, 25, 26), were successfully used to identify the genes significantly associated with the mastitis traits. GWAS has achieved unprecedented success in identifying gene regions and candidate gene variations that are closely related to clinical phenotypes and disease susceptibility (chromosome and gene level, in term of the association between SNPs and traits) (27–29). In addition, identification of associated gene mutations can help reveal the pathogenesis of disease, provide an entry point for treatment, and analyze common genetic variations to identify multiple risk loci for complex diseases (30, 31). GWAS is widely regarded as a potent method to identify SNPs in dairy cattle mastitis traits (17, 32). GWAS data showed that Bos Taurus autosome 2, 4, 6, 10, 14, 18, and 20 associated with clinical mastitis were significantly correlated with SCS in cows (33–35). Additionally, two clinical mastitis candidate genes [vitamin D-binding protein precursor (GC) and neuropeptide FF receptor 2 (NPFFR2)] were detected using high-density single nucleotide polymorphic array and genomic sequencing (18, 26). Wu et al. detected five mastitis susceptibility genes (NPFFR2, SLC4A4, DCK, LIFR, and EDN3) in Danish Holsteins (36). In 2015, Wang et al. identified another two mastitis susceptibility genes (TRAPPC9 and ARHGAP39) in Chinese Holstein (17). In the same year, Wu et al. confirmed the differential expression of TLR4/NF-κB signaling pathway-related genes (up-regulation genes: TLR4, MyD88, IL-6, and IL-10; down-regulation genes: CD14, TNF-α, MD-2, IL-1β, NF-κB, and IL-12) in the mammary gland of Chinese Holstein mastitis cows by Gene-Chip microarray (37). Genetic variations in immune response, specific pathogen (LY75, DPP4, ITGB6, and NR4A2) and lymphocyte antigen-6 complex genes (LY6K, LY6D, LYNX1, LYPD2, SLURP1, and PSCA) might lead to clinical mastitis in American Holstein cows (38). Additionally, single gene polymorphisms (CXCR1, MAP4K4) and their signaling pathways (TLR4/NF-κB) served as genetic markers for mastitis in different cow populations (12, 39). However, there is some inconsistency in genetic variation or polymorphism in genes associated with mastitis traits. Therefore, genetic mutations and polymorphisms in mastitis-associated genes should be screened and validated in different populations.
Type IIB restriction site-associated DNA sequencing (2b-RADseq) is a high-throughput genotyping-by-sequencing (GBS) method based on type IIB restriction endonuclease (for instance, BsaXI and AlfI), which provides a powerful method for identifying gene SNP in the population genome (40, 41). It has strong technical repeatability, uniform depth of sequencing, high cost-effectiveness and genome wide coverage (40, 42). Furthermore, 2b-RADseq technique successfully predicted multi-locus sequence typing (MLST) and provided more details on the population information than MLST technique (41). In addition, this method is also suitable for creating high-density genetic or linkage maps of genomic region or locus markers and revealing the regions associated with related traits by QTL mapping and association analysis (43, 44). More importantly, 2b-RAD can identify key SNPs associated with traits by deep sequencing in fewer samples, thereby preliminary labeling candidate genes (45). Therefore, 2b-RAD may be an ideal genomic screening and labeling platform for detecting mastitis resistance or susceptibility genes in dairy cattle.
In this study, GWAS-2b-RADseq was used to screen and identify mastitis susceptibility or resistance SNPs in Chinese Holstein, and to validate the reliability of key significant SNPs in another independent sample population. Then, immune-regulated or inflammatory associated genes were marked based on the results of significant differential SNPs Gene Ontology (GO) annotation. Finally, the expression levels of candidate genes were evaluated both in vitro (peripheral blood leukocytes, PBLs) and in vivo (bovine mammary epithelial cells, bMECs). Understanding the genetic and biological pathways of bovine mastitis susceptibility or resistance candidate genes is a worthwhile strategy for anti-mastitis research and application.
Materials and Methods
Sample Libraries and Preparation
The procedures involving animals were approved by the Animal Welfare Committee of Nanjing Agricultural University, Nanjing, China, and approval No.20160615. And all animal experiments were carried out in strict accordance with the guidelines and rules established by the committee.
Chinese Holstein cows used for 2b-RADseq library and independent sample population validation came from two different pastures of the Nanjing Weigang Dairy Co., Ltd. At 2b-RADseq stage, forty dairy cows, daughters of ten bulls, were selected from 596 lactating Chinese Holstein in the third early-lactation period (15–60 d), which were further divided into two subgroups according to their clinical mastitis phenotypes [mammary gland (visual observation): red, swollen, fever, pain, and milk flocculation, etc., and laboratory diagnosis (mainly SCC)] (46, 47): case group [20 cows, clinical mastitis appeared in all three lactation periods, and SCC values [(2100.3 ± 891.1) × 104) > 100 × 104 cells/ml] and control group [20 cows, no mastitis during the three lactation periods, and SCC values [(22.4 ± 6.8) × 104) < 30 × 104 cells/ml]. At the independent sample population validation stage, 383 cows (73 in case group and 310 in control group), daughters of thirty-one bulls, were selected from 886 lactating cows in the third early-lactation period (15–60 d) in another pasture for population genetics verification. All experimental cows' mastitis clinical traits were diagnosed by pasture veterinarians, and SCC were obtained from pasture Dairy Herd Improvement (DHI). In their respective pastures, all animals have the same growth, feeding environment, and similar production levels (305 days correction of milk yield, 305D).
Blood samples were aseptically collected from the bovine coccygeal vein to a disposable (EDTA-K2) vacuum anticoagulation tube. And blood samples were collected from the case group during the presence of clinical mastitis based on SCC and clinical findings. Genomic DNA was extracted from whole blood using TIANamp Genomic DNA Kit (Cat#DP304-03, TIANGEN Biotech). The quality of genomic DNA was detected by NanoDrop and agarose gel methods (extracted 3 μL of genomic DNA, loaded on 1% agarose gel, 100 V CV 25 min, viewed under ultraviolet light and photographed). Genomic DNA with a quality (OD260/OD280 = 1.7–1.8) that meets the experimental requirements was used for subsequent 2b-RADseq analysis.
2b-RAD Library, Sequencing, and Raw Reads Quality Control
Forty sample libraries set up met a protocol developed by 2b-RAD sequencing needs with a little change and five-label tandem technique (40, 48). The Bos Taurus genomes (Bos taurus UMD 3.1.1) was used as the reference for predicting electronic-enzyme-cut digestion of genomic DNA. Finally, Bael restriction endonuclease, a commonly used restriction endonuclease that specifically recognize the AC(N)4GTAYC (NNNNNNNNNNACNNNNGTAYCNNNNNNNNNNNN) and GRTAC(N)4GT (NNNNNNNNNNNNNNNTGNNNNCATRGNNNNNNN) sites, and has the advantages of high stability, good repeatability, and uniform distribution in the genome, was selected to digest genomic DNA. The restriction enzyme digested DNA fragment tags of each sample were linked by standard 5′-NNN-3′ connector. Paired-end sequencing was carried out on the Illumina Hiseq Xten (https://support.illumina.com/downloads/sequencing-analysis-viewer-software-v2-1-8.html) platform. Construction the library and steps of the modeling followed Wang et al. with a minor change (41); Supplemental statistical model (Figure S1).
SNPs RAD Typing, Linkage Disequilibrium (LD) and Genetic Diversity Analysis
Single nucleotide polymorphism marker typing (RAD typing) was performed on Enzyme Reads using the maximum likelihood (ML) method in SOAP software. The statistic SNP typing results, using R Package cluster analysis of the differences between sample SNPs. SNPs were annotated using SnpEff software (Version: 4.1g) (http://snpeff.sourceforge.net/). Plink software was used to calculate the r2 value of the pairwise SNPs, with the main parameters set to: -r2-Id-window-kb 1000-Id-window 50-Id-window-r2 0.2. According to the median of the software, the work F(x) = 1/(log10 ((x+10(7−C))/107) + C) was used to fit, and map by chromosome/grouping. To find the LD block in the case-control group, we added the parameters “block output GAB-pair wise Tagging' to R package runner.
Principal component analysis (PCA) method was used to evaluate the population. Then a correlation test for each subgroup, including the first five PC selected as covariate analysis signs for population division adjustment (Table S2). And the first two influential feature vectors were selected to draw the correlation between the samples. To assess experimental samples' genetic diversity, polymorphic information content (PIC), observed heterozygosity (Ho) and expected heterozygosity (He) values were also calculated for each SNP loci. In addition, the genetic differentiation coefficient between the subgroups was statistical.
Association Analysis Between SNPs and Clinical Mastitis Traits in Chinese Holstein
We considered a GWAS for the quality traits of dairy cow mastitis, and 2b-RAD markers genotype for each individual SNP loci. To ensure the accuracy of the analysis, we used multi-stage GWAS for identification of important SNPs associated with mastitis traits. GWAS analysis was performed using Bayesian and Logistic regression analysis model to compare significant SNPs between case-control. Quantile-Quantile Plot (QQ-plot) evaluated the rationality of the two analysis models. GO enrichment analysis performed on all genes with SNPs, and their functions described in conjunction with GO annotations. Hypergeometric Distribution Test (Cytoscape software 3.6) used to calculate significant gene enrichment in each GO entry. Protein and protein interaction networks were predicted and constructed in the string 10.5 database (Bos Taurus) (49).
Independent Sample Population Validated Significant SNPs
The independent sample population validation had the same screening conditions as the 2b-RADseq stage. The case-control study was used for the population validation of significant SNPs. The genomic DNA from peripheral blood of cows was extracted by Rapid Blood Genomic DNA Isolation Kit (#B518223, Sangon Biotech). The whole blood genomic DNA quantified (OD260/OD280 = 1.7–1.8) by the SparkTM multimode microplate reader (Tecan, Swizerland). Table S7 lists the PCR primer sequence details containing significant SNPs. The volume of the final PCR reaction system was 50 μl, including 5 μl 10 × Buffer (Mg2+), 1 μl dNTPs, 1.4 upstream, and 1.2 μl downstream primer, 0.4 μl Taq-DNA polymerase, 200 ng DNA template, and 35 μl ddH2O. PCR reaction conditions were as follows: denaturation 95°C for 5 min, 32 cycles of 95°C for 40 s, 58°C for 30 s, and 72°C for 50 s; and extension at 72°C for 7 min. PCR products detected by 2% agarose gel electrophoresis, then used for direct sequencing after passing the quality test.
Blood Total RNA Extraction and Transcriptional Analysis
Total RNA extracted from peripheral of cow blood samples and bMECs using the Whole Blood Total RNA Isolation Kit (#B518623, Sangon Biotech). The concentration of RNA was quantified (OD260/OD280 = 1.9–2.0, 2 μg/μl) by the SparkTM multimode microplate reader. The gDNA removal reaction system was 10 μl, including gDNA Buffer 2 μl, total RNA 4 μl, and RNase-free water 4 μl, 42°C for 5 min. The first strand cDNA extracted from total RNA (4 μl, ~3 μg) using RevertAid First Strand cDNA Synthesis Kit (#K1621, Thermo Scientific). RT-qPCR reaction performed on the Bio-RadCFX ManagerTM (United States) using the SuperReal (SYBR Green) fluorescence quantification kit (#FP205, TIANGEN Biotech). The primer sequence details for RT-qPCR shown in Table S8. The protocol as followed: 95.0°C for 15.0 min; 95.0°C for 20 s, 57.0°C for 20 s, 72.0°C for 30 s, 40 cycles; Melt Curve 65.0°C for 5 s, 95.0°C increment 50 s; 4.0°C forever.
Isolation and Primary Culture of Bovine Mammary Epithelial Cells (bMECs) From Control Group
The steps of isolation of bMECs were as follows (50, 51): (1) Three healthy Chinese Holstein cows in the third early-lactation stage were selected and slaughtered. Then breast tissues (including mammary acinar tissue) were collected and cleaned with 75% ethanol for two times (5 min/times), and placed in Dulbecco's Modified Eagle's Medium/Nutrient Mixture F-12 Ham (DMEM/F12) complete medium supplemented with 15% Fetal Bovine Serum (FBS, Lot#42F1376K, Gibco, USA), 1% Penicillin-Streptomycin Solution (Double-antibiotics, Cat#C0222, Beyotime, China), 1% Hydrocortisone (Cat#CS-2226, MedChemExpress, USA), and 1% Estradiol (Cat#HY-B0141, MedChemExpress, USA). The breast tissues were brought back to the aseptic processing room within 2 h. (2) Rinse the breast tissue with 1 × PBS buffer (containing double-antibiotics) under sterile conditions until clarified. (3) The mammary acinar tissues were isolated under aseptic conditions, and washed with DMEM/F12 medium until clarification, then centrifuged at 800 rpm for 2 min. (4) Mammary acinar tissues were cut into 1–2 mm3 tissue pieces with sterile scissors, rinsed to clear with DMEM/F12 medium, and centrifuged at 800 rpm for 2 min. (5) The tissue pieces were uniformly inoculated into a cell culture flask, placed in a 37°C, 5% CO2 incubator, inverted for 30–60 min, and the culture solution was removed. (6) The cell culture flask was placed upright and DMEM/F12 complete medium was added. The culture medium was changed every 48 h, and the bMECs were purified when the cell adhering density reached 90%. (7) Primary culture of bMECs: The purified cell suspension was inoculated into new culture flask, placed in 37°C, 5% CO2 cell incubator, and the culture medium was changed every 48 h. (8) Identification of bMECs: Epithelial cells are sensitive to Cytokeratin-18 (C-04, Cat#sc-51582, SANTA CRUZ, USA), and immunofluorescence staining is used to identify bMECs (Figure S7B). bMECs were sub-cultured (primary cells within 20 generations).
Lipopolysaccharides (LPS, Cat#D8437, Sigma-Aldrich, USA) From E. coli Stimulates bMECs for in vitro Validation
The post-transcriptional analysis validation experiment was performed by qRT-PCR using β-actin as an internal reference. Total RNA was extracted by All-In-One DNA/RNA/protein Mini-Preps Kit (#BS88003, Sangon Biotech, China) and stored at −80 °C. Three micrograms of total RNA was used for qRT-PCR using the same protocol. bMECs (5 × 105) were sub-cultured in 6-well-plates for 24 h and then stimulated with LPS. First, bMECs were stimulated with different LPS concentrations (100, 50, 25, 12.5, and 0 μg/ml) for 30 min. Then, specific LPS concentration (25 μg/ml) was selected, based on the SYK expression level, and used in time course (control (0 min), 15, 30, 45, 60, and 120 min) to stimulate bMECs (5 × 105).
Statistical Model Analysis
Linear models are commonly methods for analyzing phenotypic and genotype correlations. Strict quality control is used to remove SNP sites that are not performing well in RAD typing. In this study, Bayesian and Logistic regression models were used to detect significant SNP loci in clinical mastitis traits in dairy cows (Supplemental statistical models). Case-control study validated the population genetics of important SNP loci associated with mastitis traits in dairy cows. Differential expressions of genes relative quantitative Analysis (2−ΔΔCt) are based on qRT-PCR results via Student's t-test and one-way ANOVA (and non-parametric).
Results
High Quality SNPs Acquisition, RAD Typing and Population Genetic Differentiation
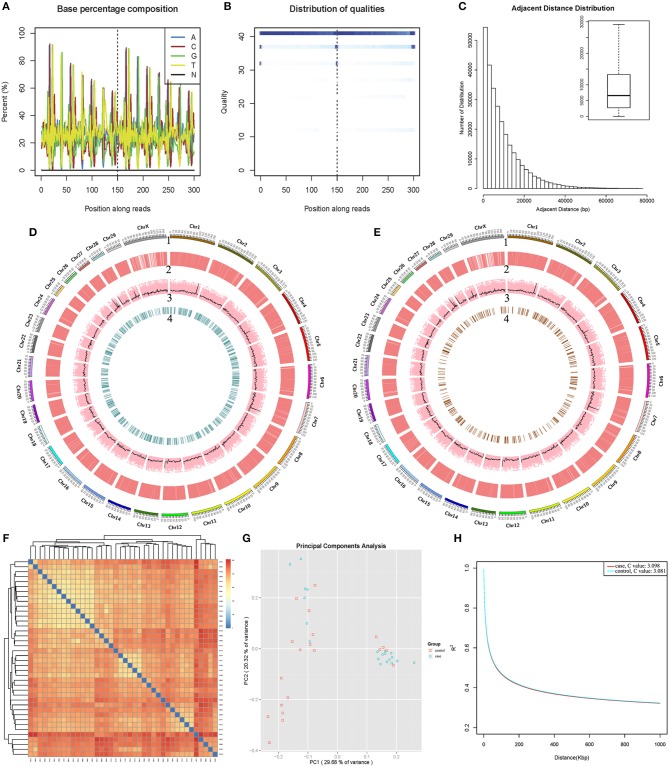
In this project, the genomic DNAs of 40 Chinese Holstein cows digested with Bael restriction endonuclease and the total of 7,957,920 unique tags were obtained. On Clean Reads, the proportion of A/C/G/T/N bases appeared in each position were shown in Figures 1A,B was quality values of the base sequencing results at each position and the base recognition accuracy rate were 99.99%. The average distance between adjacent tags was 9,589 bp (Figure 1C), and the unique tag alignment ratio for all samples was 59.69%−72.71%. 10,058 high quality SNPs from 7,957,920 unique tags were selected for RAD typing, and the distribution of SNPs on chromosomes was analyzed by sliding window (Figure S2). All SNPs and their average sequencing depth (17.43 × ) were shown in Figure 1D (Bayesian analysis) and Figure 1E (logistic regression analysis). Differential SNP cluster analysis showed that data pattern similarity within group was higher; while the similarity between groups was lower. The genetic relationship between the samples was also shown in Figure 1F. PCA was performed on the obtained SNP markers to obtain the two most influential feature vectors (Figure 1G).
Figure 1.
2b-RADseq and biological information analysis of Chinese Holstein cows. (A) Proportion of A/C/G/T/N bases at each position: the abscissa was Reads base position, and ordinate were the bases' proportion; different colors represent base types, and the base that was not recognized in sequencing were labeled as N. (B) Sequencing base mass value at each position: the darker the color, the higher the base ratio of the mass value in the data. (C) Average spacing between adjacent tags: The horizontal line in the middle of the box plot is the average spacing between the labels. (D) Bayesian and (E) Logistic regression model analysis of SNPs quality traits in all samples (1–4): chromosome scale; SNPs for all samples; sequencing depth for each SNPs; significant SNPs (P < 0.05). (F) Differential SNP cluster analysis: The color difference indicates the number of difference SNPs between samples and the near-far relationship of clusters between samples. (G) Principal Component Analysis and RAD typing of SNPs: Abscissa represented principal component 1 (PC1); ordinate represented principal component 2 (PC2); each point was a sample with different shapes and colors representing different groups. (H) The Linkage Disequilibrium attenuation curve of case-control SNPs.
The LD coefficients between pairwise markers of SNPs in the genome were calculated, and average LD coefficients between the molecular markers of the case and control group were shown in Figure 1H, respectively. Their LD decay rates also appeared to be the same, and C value were 3.098 (case) and 3.081 (control). Here, since heterozygote risk assessment was between two homozygotes, this line fit the data reasonably which matched to additive genotype risk. In this case there was no deviation, and the test was convincing (Figure S3A, Table S3). We also calculated the genetic differentiation coefficient (Wright's fixation index, Fst) between the two groups. The value was 0.01869, indicating that the genetic differentiation between case-control groups was smaller.
Genome Wide Association Analysis
The Quantile-Quantile Plot (QQ-plot) data showed that the P-value was consistent with expected values at all SNP site, indicated that the two analysis models matched well (Figures S3B,C). Bayesian analysis has identified 42 significant SNPs with P < 0.001 (Table S4), while logistic regression analysis model has selected 51 SNPs with P < 0.01 (Table S5). As expected, significant SNPs screened by two analytical models would vary. Total of 27 significant SNPs appeared simultaneously in both analytical models, except these loci with odds ratio (OR) and 95% Confidence Interval (CI) value were not “Na,” and Upper bounds of 95% confidence interval (U95) value were not “Nan” (Table 1). We also noticed that the OR values of the eight SNPs (rs114843903, rs5881560, rs17514753, rs17518215, rs22015301, rs9704351, rs20438858, and rs5088452) were < 1, indicating that these SNPs are associated with mastitis susceptibility traits. However, the OR values of other 19 SNPs, including rs75762330 and rs88640083, were >1, indicating that these SNPs were associated with mastitis resistance.
Table 1.
Twenty-seven significant SNPs jointly screened by the Bayesian Model and Logical regression analysis model.
| Ref_ID | Chromosome | Bayesian analysis | Logical regression analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P* | CHISQ | OR | L95 | U95 | P** | OR | L95 | U95 | STAT | ||
| rs47045687 | AC_000159.1 | 0.000106 | 15.02 | 14.21 | 2.937 | 68.76 | 0.004718 | 11.36 | 2.106 | 61.32 | 2.826 |
| rs114843903 | AC_000159.1 | 0.000628 | 11.69 | 0.1678 | 0.05817 | 0.4842 | 0.006341 | 0.215 | 0.07128 | 0.6483 | −2.73 |
| rs90835937 | AC_000160.1 | 0.00067 | 11.57 | 5.808 | 2.038 | 16.55 | 0.007839 | 3.838 | 1.424 | 10.34 | 2.659 |
| rs5881560 | AC_000162.1 | 0.000877 | 11.07 | 0.1406 | 0.04076 | 0.4852 | 0.005781 | 0.1412 | 0.03515 | 0.5669 | −2.76 |
| rs8678060 | AC_000162.1 | 0.000465 | 12.25 | 6.929 | 2.196 | 21.86 | 0.00522 | 5.866 | 1.695 | 20.3 | 2.793 |
| rs37588412 | AC_000162.1 | 0.000842 | 11.15 | 6 | 1.994 | 18.06 | 0.009236 | 4.128 | 1.42 | 12 | 2.603 |
| rs75762330 | AC_000165.1 | 0.000259 | 9.078 | 7.07 | 1.78 | 28.08 | 0.00127 | 6.605 | 1.496 | 29.15 | 2.492 |
| rs88640083 | AC_000165.1 | 0.000192 | 5.477 | 9.25 | 1.047 | 81.7 | 0.00314 | 12 | 1.248 | 115.4 | 2.152 |
| rs17176625 | AC_000166.1 | 0.000512 | 12.07 | 6.662 | 2.185 | 20.31 | 0.0069 | 4.749 | 1.534 | 14.71 | 2.702 |
| rs17514753 | AC_000166.1 | 0.000126 | 14.7 | 0.1146 | 0.03528 | 0.3725 | 0.00321 | 0.03618 | 0.003978 | 0.329 | −2.947 |
| rs17518215 | AC_000166.1 | 0.000561 | 11.9 | 0.1605 | 0.05443 | 0.4733 | 0.005858 | 0.05012 | 0.005962 | 0.4213 | −2.756 |
| rs22015301 | AC_000166.1 | 0.000247 | 13.44 | 0.1235 | 0.03669 | 0.4155 | 0.002015 | 0.1079 | 0.02628 | 0.4434 | −3.088 |
| rs98519900 | AC_000166.1 | 0.000538 | 11.98 | 6.25 | 2.131 | 18.33 | 0.007809 | 3.866 | 1.428 | 10.47 | 2.66 |
| rs14802054 | AC_000169.1 | 0.000684 | 11.53 | 6 | 2.058 | 17.5 | 0.005923 | 8.018 | 1.821 | 35.32 | 2.752 |
| rs25949166 | AC_000170.1 | 2.68E-05 | 17.64 | 12.33 | 3.262 | 46.63 | 0.001027 | 12.78 | 2.792 | 58.52 | 3.283 |
| rs33866959 | AC_000171.1 | 0.000366 | 12.7 | 7.222 | 2.309 | 22.59 | 0.00748 | 4.233 | 1.47 | 12.19 | 2.675 |
| rs48577224 | AC_000173.1 | 0.0003884 | 12.59 | 6 | 2.154 | 16.71 | 0.004518 | 4.881 | 1.634 | 14.58 | 2.84 |
| rs49099498 | AC_000173.1 | 7.08E-05 | 15.79 | 14.57 | 3.059 | 69.4 | 0.00866 | 7.677 | 1.676 | 35.16 | 2.625 |
| rs27017918 | AC_000175.1 | 0.000265 | 13.31 | 20.28 | 2.496 | 164.8 | 0.00583 | 21.89 | 2.441 | 196.4 | 2.757 |
| rs32265465 | AC_000175.1 | 0.000341 | 12.83 | 8 | 2.344 | 27.31 | 0.006382 | 5.728 | 1.634 | 20.08 | 2.727 |
| rs9704351 | AC_000177.1 | 0.000628 | 11.69 | 0.09167 | 0.01908 | 0.4404 | 0.001736 | 0.05793 | 0.009745 | 0.3444 | −3.132 |
| rs20438858 | AC_000180.1 | 0.00062 | 11.71 | 0.1515 | 0.04821 | 0.4762 | 0.009494 | 0.2394 | 0.08128 | 0.7052 | −2.594 |
| rs22490040 | AC_000180.1 | 0.000185 | 13.97 | 8.061 | 2.545 | 25.53 | 0.00419 | 6.859 | 1.836 | 25.62 | 2.863 |
| rs28580132 | AC_000180.1 | 0.0005616 | 11.9 | 7.143 | 2.199 | 23.21 | 0.008612 | 4.642 | 1.477 | 14.59 | 2.627 |
| rs3233588 | AC_000181.1 | 0.0006203 | 11.71 | 6.6 | 2.1 | 20.74 | 0.001532 | 12.28 | 2.603 | 57.92 | 3.169 |
| rs1988979 | AC_000182.1 | 8.24E-05 | 15.5 | 11.62 | 2.975 | 45.43 | 0.006471 | 6.124 | 1.662 | 22.57 | 2.723 |
| rs50888452 | AC_000187.1 | 0.000937 | 10.95 | 0.1667 | 0.05543 | 0.5011 | 0.008504 | 0.22 | 0.07121 | 0.6795 | −2.631 |
indicated the P-value calculated by Chi-square (< 0.001);
is the t-statistic P-value of the logical regression model (< 0.01).
CHISQ is Chi-square under Chi-square test. STAT is the t-statistic coefficient under the logistic regression model.
OR: Estimated odds ratio. L95: Lower bound of 95% confidence interval for odds ratio. U95: Upper bound of 95% confidence interval for odds ratio. Nan: meaningless number. Na: missing value or Not available.
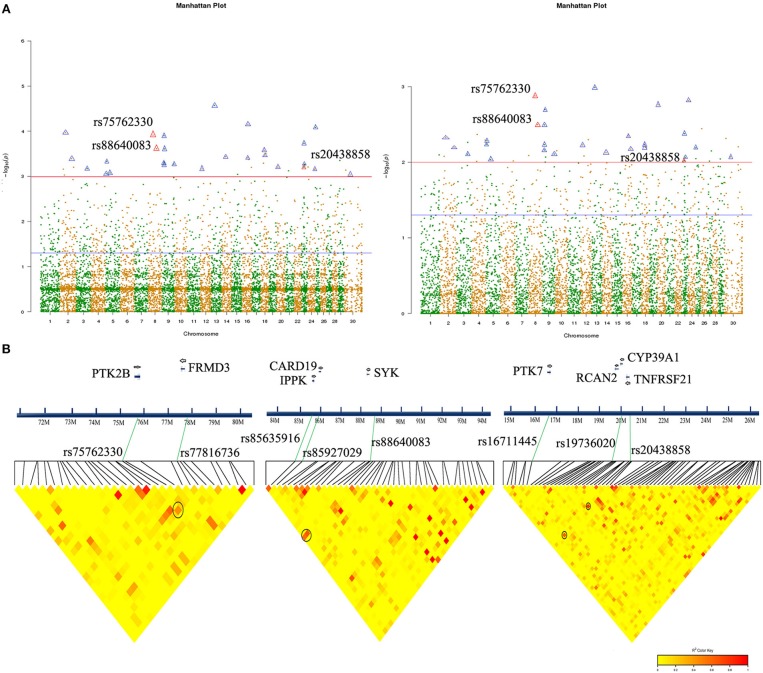
We annotated all 27 significant SNPs to determine their location in the chromosomal genome (Figure 2A, Table 2):14 SNPs located in the intergenic region, 10 in intron, and one in 3′-UTR, one in upstream and one in downstream. Go annotation for 27 significant SNPs revealed that only three SNPs were annotated to immune-related genes, namely SNPs rs75762330, rs88640083, and rs20438858. SNP rs75762330 (C > T, OR > 1, PIC = 0.2999 > 0.25) was annotated to PTK2B gene located on BTA 8 with moderate polymorphism. SNP rs88640083 (A > G, OR > 1, PIC = 0.1676 < 0.25) annotated to SYK gene located on BTA eight with low polymorphism. SNP rs20438858 (G > A, OR < 1, PIC = 0.3366 > 0.25) was annotated to TNFRSF21 located on BTA 23 and was moderate polymorphism. The Fst values of the rs75762330, rs88640083, and rs20438858 were 0.2165, 0.1297 and 0.2459, respectively (Table S9), suggesting that the genetic differentiation of the three SNPs in case-control group was small.
Figure 2.
Significant SNP association analysis and chromosomal localization in Chinese Holstein mastitis. (A) Manhattan map showed significant SNPs associated with Chinese Holstein mastitis traits (co-marked by both models: Bayesian (left), Logistic analysis (right). Triangular markers are significant SNPs that appear in both analytical models (red triangles are SNPs annotated to immune-related genes: rs75762330, rs88640083, and rs20438858). (B) Partial LD block of three significant SNPs, respectively, with a distance interval of 1 Mb. Important SNPs associated with potential candidate functional genes. The redder the LD blocks color, the stronger correlation (black circles).
Table 2.
Twenty-seven significant SNPs genetic diversity and their Go function annotations.
| Ref_ID | SNPs | Variants region | He | Ho | PIC | Gene locus |
|---|---|---|---|---|---|---|
| rs47045687 | C > T | Intergenic | 0.3607 | 0.25 | 0.2957 | id38041-LYPD6B |
| rs114843903 | C > T | Intergenic | 0.5 | 0.3529 | 0.375 | NYAP2-id50263 |
| rs90835937 | C > T | Intergenic | 0.4983 | 0.2941 | 0.3741 | id84954-LOC100138140 |
| rs5881560 | T > C | Intron | 0.4082 | 0.3429 | 0.3249 | OSBPL8 |
| rs8678060 | T > C | Intron | 0.4444 | 0.3333 | 0.3457 | SYT1 |
| rs37588412 | T > C | Intron | 0.4444 | 0.2778 | 0.3457 | LOC786089 |
| rs75762330 | C > T | Intron | 0.3673 | 0.303 | 0.2999 | PTK2BΔ |
| rs88640083 | A > G | Intergenic | 0.1847 | 0.2059 | 0.1676 | SYKΔ-DIRAS2 |
| rs17176625 | C > T | Intergenic | 0.4824 | 0.3125 | 0.3661 | LOC-100295568-HTR1B |
| rs17514753 | T > C | Intergenic | 0.4775 | 0.4848 | 0.3635 | TRNAA-UGC-MEI4 |
| rs17518215 | C > T | Intergenic | 0.4844 | 0.5294 | 0.3671 | TRNAA-UGC-MEI4 |
| rs22015301 | G > C | Downstream | 0.405 | 0.359 | 0.323 | id241467 |
| rs98519900 | G > A | Intergenic | 0.5 | 0.2727 | 0.375 | PARK2-PARK2 |
| rs14802054 | A > G | Intron | 0.4927 | 0.4545 | 0.3713 | TSC22D1 |
| rs25949166 | A > G | Intron | 0.4097 | 0.325 | 0.3258 | PRTFDC1 |
| rs33866959 | A > T | Intron | 0.4824 | 0.25 | 0.3661 | CPA6 |
| rs48577224 | T > C | Intergenic | 0.4965 | 0.3611 | 0.3733 | NPHP4-LOC100297820 |
| rs49099498 | G > A | Intergenic | 0.375 | 0.1842 | 0.3047 | NPHP4-LOC100297820 |
| rs27017918 | G > A | Intergenic | 0.2945 | 0.2564 | 0.2512 | LOC100138242-TRNAW-CCA |
| rs32265465 | T > C | Intergenic | 0.4244 | 0.2778 | 0.3343 | LOC789319-CDH11 |
| rs9704351 | C > G | Intergenic | 0.382 | 0.4 | 0.3091 | MAP1B-CARTPT |
| rs20438858 | G > A | Intron | 0.4284 | 0.2432 | 0.3366 | TNFRSF21Δ |
| rs22490040 | C > T | Intergenic | 0.4761 | 0.3438 | 0.3628 | DEFB112-TFAP2D |
| rs28580132 | A > G | 3′-UTR | 0.4761 | 0.2812 | 0.3628 | LOC785873 |
| rs3233588 | G > A | Upstream | 0.4284 | 0.4595 | 0.3366 | id550612 |
| rs1988979 | G > A | Intron | 0.4269 | 0.2059 | 0.3358 | TBC1D24 |
| rs50888452 | G > A | Intron | 0.4995 | 0.3438 | 0.3748 | SRPX2 |
He, Heterozygosity Expectation; Ho, Heterozygosity Observation; PIC, Polymorphism Information Content.
Three significant SNPs were annotated to the immune-regulated genes, respectively.
Genetic linkage analysis showed that SNPs rs75762330 was not correlated with rs88640083 (r2 = 0.0022) and rs20438858 (r2 = 0.043), while rs20438858 was weakly correlated with rs88640083 (r2 = 0.22). LD block map showed that rs75762330 was associated with rs77816736, while rs88640083 was associated with rs85927029 and rs85635916, and rs20438858 associated with rs19736020 and rs16711445 (Figure 2B). The SNPs rs77816736, rs85927029, rs85635916, rs19736020, and rs16711445 were annotated to FRMD3, CARD19, IPPK, CYP39A1, and RCAN2, and PTK7 gene, respectively. Although the P-values of these five SNPs were >0.05 (Table S6), there was still potential correlation between rs75762330 and FRMD3, rs88640083 and CARD19 and IPPK, rs20438858, and the genes (CYP39A1, RCAN2, and PTK7).
Independent Sample Population Verification Three Significant SNPs
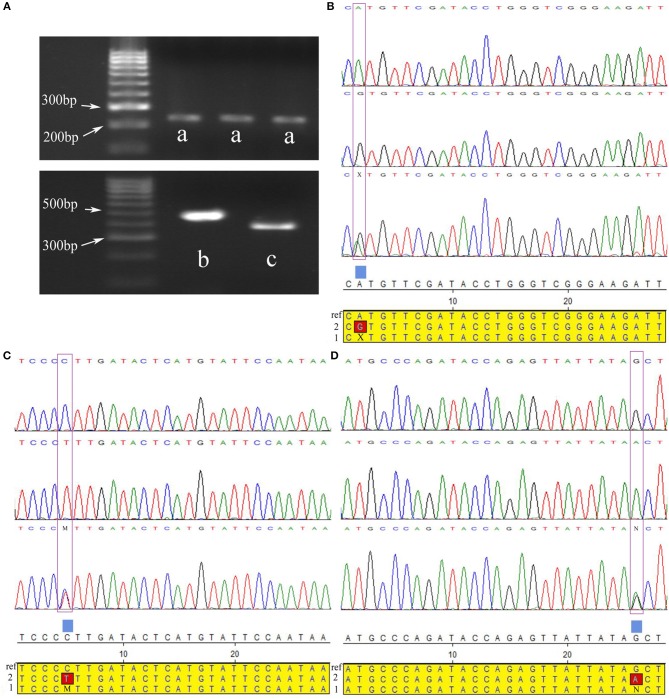
Correlation analyses were performed on three significant SNPs in another larger independent Chinese Holstein dairy population via direct sequencing (Figure 3). The results showed that the p-values of the three SNPs were all < 0.05, indicating that they have significant association with Chinese Holstein mastitis traits (Table 4). The correlation between rs20438858 and risk of mastitis was still statistically significant, with the adjustment allele OR = 0.359 < 1, suggesting that rs20430083 is associated with mastitis susceptibility traits. While, the other two significant SNPs (rs75762330 and rs88640083) with the adjustment allele OR = 2.416 and 1.879 > 1, respectively, were associated with mastitis resistance traits. Population verification data were consistent with the GWAS analysis results. We also noted that AFe (Attributable Fraction) value for rs75762330 and rs88640083 was 0.2489 and 0.2426 > 0, respectively, while rs20438858 AFe value was −1.786 < 0, also indicating that rs75762330 and rs88640083 are associated with mastitis resistance traits, and rs20430083 is associated with mastitis susceptibility traits.
Figure 3.
Three significant SNPs were validated for population genetics using direct sequencing in Chinese Holstein cows. (A) Gel electrophoresis pattern PCR amplified fragments near three significant SNPs, a-c were PCR amplified fragments of SNPs rs88640083, rs75762330, and rs20438858 regions, respectively. (B–D) Directing sequencing results of PCR amplification products near above three important SNPs, and their alignment with reference sequences (ref: reference sequences; 1: heterozygous sequences; 2: variant sequences). The purple boxes were where the three SNPs located. X, M, and N represented the heterozygous types of the three SNPs, respectively.
Table 4.
Population genetics validated three significant SNPs in another individual population via case-control study.
| SNPs | Base | Case | Control | AFe* | OR** | χ2 | P < | Df = |
|---|---|---|---|---|---|---|---|---|
| rs88640083 | G | 47 (a) | 152 (b) | 0.2426 | 1.879 | 5.578 | 0.025 | 1 |
| A | 26 (c) | 158 (d) | ||||||
| rs75762330 | T | 36 (a) | 89 (b) | 0.2489 | 2.416 | 10.279 | 0.005 | 1 |
| C | 37 (c) | 221 (d) | ||||||
| rs20438858 | T | 17 (a) | 142 (b) | −1.786 | 0.359 | 12.34 | 0.001 | 1 |
| C | 56 (c) | 168 (d) |
AFe refers to the proportion of mastitis caused by the SNPs to all mastitis; AFe > 0 indicating that the SNPs positive associated with mastitis susceptibility, while AFe < 0 is negative.
OR is the exposure ratio (Odds ratio); OR > 1 indicating that the SNPs positive associated with mastitis susceptibility, while OR < 1 is negative.
Network Analysis of Three Immune-Regulated Genes and Their Proteins
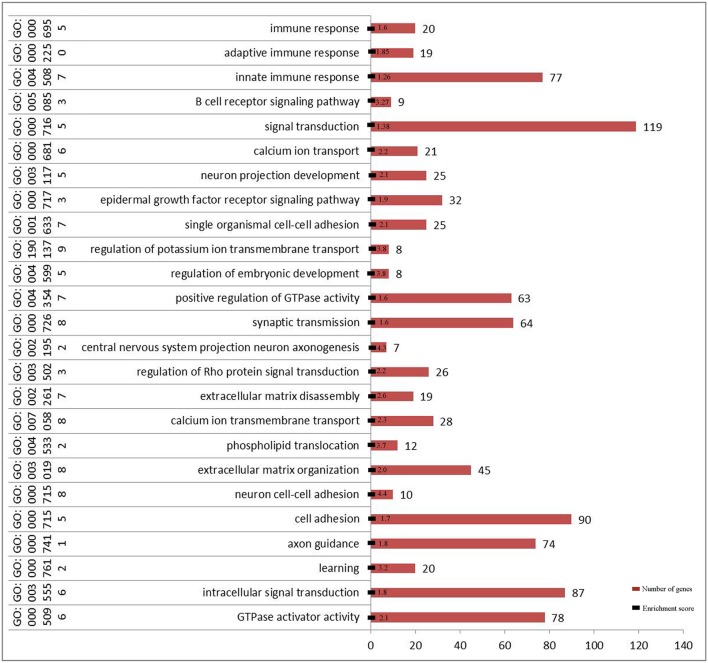
Gene ontology enrichment analysis (biological process, cellular component, and molecular function) indicated that three important genes are associated with adaptive or innate immune response in Chinese Holstein (Figure 4 and Figure S4). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed that seven important pathways related to immune response were detected (False Discovery Rate (FDR) < 0.05, Table 3), suggesting that multiple immune response pathways are involved in Chinese Holstein mastitis (Figure S5A). Network protein interaction analysis showed that SYK and PTK2B are involved in natural killer cell mediated cytotoxicity pathway. SYK and AKT1 are in the PI3K-AKT and B cell receptor signaling pathways. PTK2B and AKT1 are in the chemokine signaling pathway. Co-expression scores of the above two proteins are between 0.074 and 0.314 (Figure S5B). TNFRSF21 is involved in the regulation of cytokine-cytokine receptor interaction (FDR = 1.48E-3).
Figure 4.
Significant function of GO enrichment analysis (biological process) in 2b-RADseq stage (FDR < 0.001).
Table 3.
KEGG enrichment items pathways and regulatory genes of three significant SNPs.
| KEGG ID | KEGG term | False discovery rate (FDR) | Enrichment score | Differentially expressed gene (DEG) |
|---|---|---|---|---|
| Path:ko04064 | NF-kappa B signaling pathway* | 2.16E-4 | 1.398 | CARD11; CHUK; RELB; MAP3K7; CARD14; TAB1; ATM; SYK; IL1B; CSNK2A1; LBP; TRIM25; PLCG2; IKBKB; CD40; TNFRSF11A; TIRAP; CARD10; DDX58; PRKCB |
| Path:ko04662 | B cell receptor signaling pathway* | 2.53E-4 | 1.378 | CARD11; DAPP1; CHUK; PIK3CB; GSK3B; PPP3CA; SYK; VAV3; NFATC2; PTPN6; INPP5D; PLCG2; IKBKB; PIK3R2; AKT2; PRKCB |
| Path:ko04151 | PI3K-Akt signaling pathway* | 5.15E-4 | 1.311 | ITGA8; TNR; THBS1; CDK6; GNB5; PRKAA2; CHUK; ITGB8; GH1; IL2RA; PCK1; FGF13; PDGFA; FGF14; EIF4B; PTK2; CREB1; CDKN1B; PIK3CB; PRKCA; PIK3R5; VEGFC; FN1; PKN1; IL7R; GYS1; EGF; GSK3B; RELN; LAMA4; EFNA3; SYK; LAMB1; FGFR2; ANGPT4; ITGA9; COL4A6; IGF1R; IBSP; MYB; FGF12; CREB5; COL4A2; PGF; COL6A3; RBL2; PHLPP2; RPTOR; IL3RA; LAMA3; LOC104968606; IKBKB; LAMA2; PIK3R2; FLT3; PDPK1; COL9A2; PHLPP1; IFNAR2; AKT2; MET; TNXB; PRLR; ITGA2; LAMC2; PPP2R2B; BCL2L11; PDGFD; THBS4; LPAR1; CSF3R; PPP2CB; LAMA1; LOC530102 |
| Path:ko04650 | Natural killer cell mediated cytotoxicity*, ** | 8.26E-4 | 1.953 | LCP2; LOC100850276; SHC3; PTK2B; PIK3CB; PRKCA; CD247; SH2D1B; PPP3CA; SYK; VAV3; NFATC2; PTPN6; PLCG2; PIK3R2; ITGAL; IFNAR2; BRAF; PRKCB |
| Path:ko04660 | T cell receptor signaling pathway | 3.02E-3 | 1.279 | CARD11; LCP2; CHUK; MAP3K7; PAK6; PIK3CB; NCK2; CD247; PAK7; GSK3B; PPP3CA; VAV3; NFATC2; PTPN6; IKBKB; PIK3R2; PDPK1; IL5; CD28; AKT2; TEC |
| Path:ko04062 | Chemokine signaling pathway** | 7.12E-3 | 1.227 | DOCK2; TIAM1; GNB5; SHC3; CHUK; PTK2B; PTK2; ADCY2; PIK3CB; LOC508666; GRK5; CCL5; CCR8; GSK3B; ADCY5; VAV3; STAT5B; PREX1; STAT3; ADCY1; IKBKB; PIK3R2; ELMO1; AKT2; PLCB1; CCR9; BRAF; PARD3; PRKCB |
| Path:ko04060 | Cytokine-cytokine receptor interaction*** | 9.15E-3 | 1.119 | IL12RB2; IL18; GH1; IL2RA; PDGFA; IFNE; IL1RAP; LOC508666; IL20RA; IL12RB1; VEGFC; TNFSF18; RELT; IL7R; CCL5; IFNL3; CCR8; EGF; IL17C; IL1B; IL1R2; IL18R1; IL3RA; FLT3; CD40; TNFRSF11A; TNFRSF21; BMPR1A; IL5; IFNAR2; IL17B; CCR9; MET; PRLR; IL10RA; ACVR1; PDGFD; IL13RA1; CSF3R |
SYK gene is involved in KEGG enrichment pathway.
PTK2B gene is involved in KEGG enrichment pathway.
TNFRSF21 gene is involved in KEGG enrichment pathway.
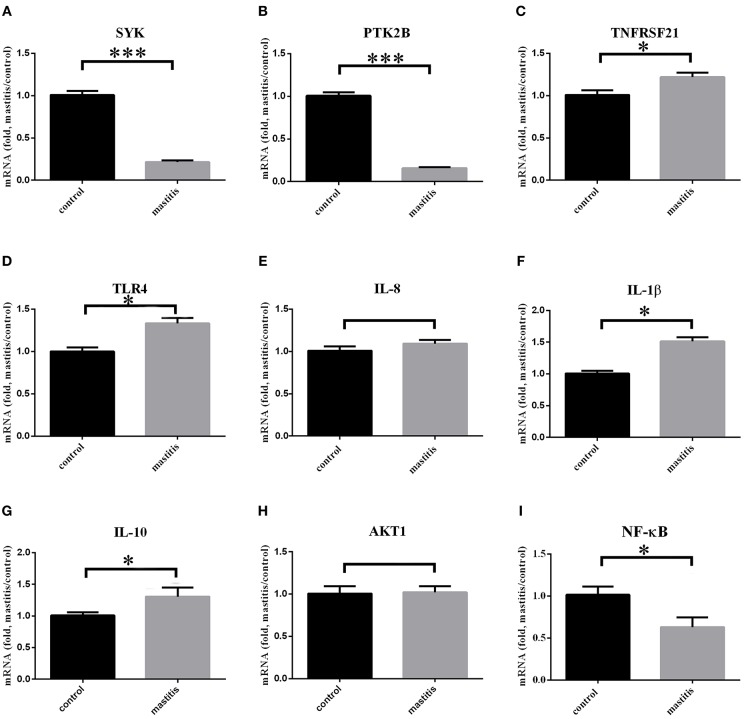
Expression Variation of PTK2B, SYK, TNFRSF21, TLR4, AKT1, NF-κB, IL-1β, IL-8, and IL-10 in Case-Control Group
Total of nine genes (three candidate genes: SYK, PTK2B, and TNFRSF21; TLR4, Toll-like receptor; AKT1, Protein kinase B; NF-κB, Nuclear factor-kappa B; IL-8 and IL-1β, pro-inflammatory factor; IL-10, inflammation and immunosuppressive factor) were selected for qRT-PCR analsysis (Figure 5). It showed that expression of SYK and PTK2B was down-regulated significantly (P < 0.001) in mastitis cows in comparison to the control group, while TNFRSF21 was up-regulated. AKT1 expression level had no significant difference (P > 0.05), while NF-κB down-regulated (P < 0.05). TLR4 (P < 0.05) and two other pro-inflammatory factors (IL-8, P > 0.05; IL-1β, P < 0.01) were also up-regulated. However, IL-8 expression level had no significant difference in peripheral white blood cells (P > 0.05). The expression level of IL-10 were up-regulated (P < 0.05). The mean Ct value with SD of the β-actin in case-control samples were 20 ± 0.2665 and 20.43 ± 0.2789, respectively (Figure S7A).
Figure 5.
Three immune-related candidate genes, TLR4, AKT1, NF-κB, IL-1β, IL-8, and IL-10 mRNA expression levels in peripheral blood leukocytes of experimental cows. ***Represent P < 0.001. *Represent P < 0.05. (A–I) Immune-related gene expression in mastitis-control cows.
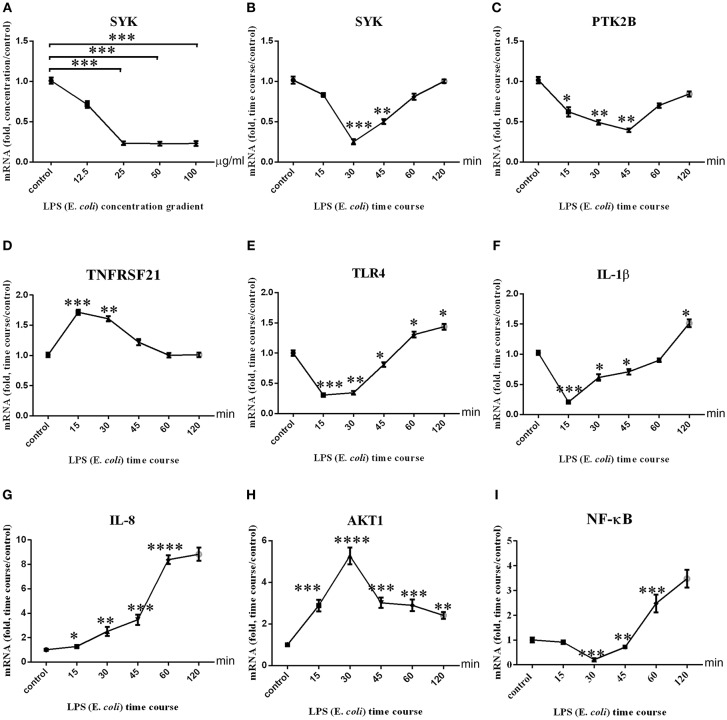
Expression Variation of PTK2B, SYK, TNFRSF21, TLR4, AKT1, NF-κB, IL-8, IL-1β, and IL-10 in bMECs via LPS Stimulation
The expression level of candidate genes and related pro-inflammatory factors was detected in LPS-stimulated bMECs in vitro. The optimal LPS concentration was 25 μg/ml (Figure 6A). We then performed the time course experiment with this concentration (Figures 6B–I). Figure 6B showed that SYK expression was down-regulated, and then rebounded from 30 to 120 min. PTK2B expression level was down-regulated at 0–45 min, and then up from 45 to 120 min (Figure 6C). TNFRSF21 expression level was up-regulated from 15 to 60 min, and then down during 60–120 min (Figure 6D). The expression of TLR4 was down-regulated in 0–45 min and up-regulated in 60–120 min (Figure 6E). IL-1β expression levels were down-regulated within 0–60 min and up-regulated at the 120 min (Figure 6F). IL-8 expression up-regulated and had an upward trend, which was almost the same at 60–120 min (Figure 6G). AKT1 expression was up-regulated, and showed an upward trend at 0–30 min and a downward trend at 30–120 min (Figure 6H). NF-κB expression was down-regulated within 0–45 min, while up-regulated after 60 min (Figure 6I). The expression of IL-10 was up-regulated, and showed an upward trend at 0–15 and 30–45 min and a downward trend at 15–30 and 45–120 min (Figure S6). The mean Ct value with SD of the β-actin in LPS concentration stimulated and time course were shown in Figures S7C,D, respectively.
Figure 6.
Three immune-related candidate genes, TLR4, AKT1, NF-κB, IL-1β, IL-8, and mRNA expression levels in bMECs. (A) The mRNA expression level of SYK after stimulation of BMSCs by LPS concentration gradient. (B–I) The mRNA expression levels of SYK, PTK2B, TNFRSF21, TLR4, AKT1, IL-1β, and IL-8 after stimulation of bMECs by LPS (E. coli, 25 μg/ml) time courses. ****represent P < 0.0001. ***represent P < 0.001. **represent P < 0.01. *represent P < 0.05.
Discussion
Clinical mastitis is a common unavoidable disease that seriously affects the health benefits and breeding profits of dairy cows (3, 38). In the production process, mastitis is still not fully controlled despite thorough hygiene, antibiotic treatment, vaccination, and other measures (52). Therefore, the association between dairy cows' genetic variation and mastitis is receiving more and more attention (18, 35, 53, 54). This study sought to screen for significant differential SNPs associated with clinical mastitis via GWAS-2bRADseq, to identify potential clinical mastitis susceptibility or resistance genes and to verify for their authenticity and role in mastitis resistance/tolerance in vitro and in vivo.
Two-stage correlation analysis for three mastitis significant SNPs and Population genetic verification.
In stage I, a total of 10,058 high quality SNPs were screened by 2b-RADseq. The average LD coefficient of 100 kb on the genome of case-control two Chinese Holstein cows was about 0.5. However, the corresponding LD coefficient was still above 0.3 when the distance was 1,000 kb. It is suggested that the genetic diversity of the population of Chinese Holstein cows decreased after acclimatization and selection, and the linkage degree (LD) between the loci were strengthened. Bayesian model screened out 42 significant SNPs when P < 0.001, while the Logical regression analysis model had no SNPs; however, the Bayesian model had 257 significant SNPs when P < 0.01, while Logical regression analysis model labeled 51 significant SNPs. Therefore, the accuracy of molecular breeding value (MBV) prediction is important for the successful application of genome selection (GS) (55). Therefore, selecting the appropriate analytical model based on genotypic and phenotypic data is critical to achieving accurate results (56). This study, two association analysis models (Bayesian model and logical regression model) were used to process 2b-RAD sequencing data to improve accuracy and reduce false positives. It's obvious that the thresholds values of the two analytical models were significantly different. Giving us hints that when processing data, it must be statistically analyzed according to their respective thresholds. Comparison of results from two analytical models, we reduced the dimensions when processing data and only considered the SNPs associated with immune-related genes and their signaling pathways. Finally, we identified three (rs75762330, rs88640083, and rs20438858) novel bovine mastitis traits significant SNPs in Chinese Holstein cows.
With regarding to stage II, we used a case-control study to verify the association between three significant SNPs and bovine mastitis. We compared the exposure ratios of important SNPs in case and control groups, and only considered the relationship between SNP and mastitis traits under the exclusion of external matching factors (57–59). According to the Pitman efficiency increment formula (2R/(R+1)), we determined the appropriate sample size and gained higher test efficiency (1/4). Here, we validated the association of three important SNPs with cows mastitis. SNP rs75762330 and rs88640083 (OR > 1, AFe > 0) are positive, and SNP rs20438858 is negative (OR < 1, AFe < 0) associated with mastitis susceptibility, respectively.
Three significant mastitis-associated SNPs are located in genomic non-coding sequences and in low or moderate genetic polymorphisms.
Previous researchers found that conserved non-coding regions (CNCs) in introns and near genes show large allelic frequency shifts, similar in magnitude to missense variations, suggesting that CNCs are critical for gene function regulation and evolution in many species, including yeast, fruit flies, and vertebrates (60–64). Our GWAS data provided a statistical list of SNPs associated with mastitis traits in dairy cows, where three significant SNPs (rs75762330, rs88640083, and rs20438858) are annotated to immune-related genes are located in non-coding regions (intron and intergenic) of the genome. GWAS studies also showed that the mastitis traits were controlled by multiple loci in the genome, and the genetic effects of each locus were relatively small (18, 26, 34, 38). SNP rs88640083 (PIC = 0.1676 < 0.25) had low polymorphism, while SNPs rs75762330 (0.25 < PIC = 0.2999 < 0.5) and rs20438858 (0.25 < PIC = 0.3366 < 0.5) were moderately polymorphic. Despite the low or moderately heritability of clinical mastitis, identifying significant SNPs associated with clinical mastitis are extremely important for breeding programs that reduce the incidence of mastitis (36, 65).
LPS Stimulates bMEC in a Dose-and Time-Dependent Manner in vitro
Pathogen-specific mastitis traits are direct indicators of cow mastitis infection. E. coli is the main pathogen of clinical mastitis, while Staphylococcus aureus and Streptococcus uberis mainly cause sub-clinical mastitis (8, 66). Once infected, mammary gland innate immunity is initiated; bMECs act as the first line of defense against exogenous pathogens and secrete several cytokines (e.g., IL-1β, IL-8, and TNF-α) to recruit neutrophils into the mammary gland (8, 67, 68), causing a sharp rise in SCC intra-mammary infection in early- and late-lactation (69–71) and the corresponding mastitis phenotype. Therefore, bMEC is the key for rapid elimination of bacteria and prevention of mastitis (inflammation of bovine mammary gland) (72). Exogenous stimulation of bMEC activity was dose- and time-dependent manner as well (73–75). SYK is a non-receptor tyrosine kinase that plays an essential role in various biological functions (76). SYK-Pyk2 (PTK2B) axis is participated in regulation of TNF activation in human neutrophils (77). Therefore, the optimal LPS stimulation concentration and time was determined with reference to the SYK expression level in bMECs. Then we counted and compared the changes of TLR4, IL-1β, IL-8, and NF-κB expression in LPS-stimulated bMECs time course, and their expected parameters and fold differences serve as positive controls (67), and confirming the reliability of time course bMECs study results: the down-regulation of PTKTB2 and SYK, respectively, and the up-regulation of TNFRSF21.
Three Important Immune-Regulated Genes (PTK2B, SYK, and TNRSF21) Associated With Mastitis Traits in Chinese Holstein Cows
Gene Ontology function analysis annotated three significant SNPs to three immune regulatory genes PTK2B, SYK, and TNFRSF21, respectively. The LD block data also showed that there were strong LD relationship between rs75762330 and PTK2B, rs88640083 and SYK, rs20438858 and TNFRSF21, suggesting that these three immune-regulated genes are important candidate genes associated with mastitis traits in Chinese Holstein cows.
PTK2B is a protein tyrosine kinase that regulates humoral immune response and homeostasis of innate immune cells (78–81). It is involved in regulating TLR4 cascade and IL-10 production in macrophages and affects the migration of dendritic cells (DCs) (78, 80, 82). PTK2B participates in the innate immune response through interaction with IkappaB kinase β (IKKβ) and TANK-binding kinase 1 (TBK 1) (83), and is critical for neutrophil degranulation and host defense against bacterial infection (84). PTK2B expression levels in bPBLs of mastitis cows were significantly down-regulated (P < 0.001) and had a time course difference in LPS-stimulated bEMCs, suggesting that PTK2B plays an important role in clinical mastitis.
SYK is an important regulator factor for TLR4 signaling pathway (85, 86), and regulates the secretion of IL-1β through CARD 9 in dendritic cells (87). In macrophages, SYK, as a downstream regulatory molecule of TLR4 and IL10, plays a dual role in pro-inflammatory and anti-inflammatory responses (88, 89). SYK is also involved in regulating the proliferation of dairy mammary epithelial cells, milking cycles, and milk production (90). The expression levels of SYK in bPBLs and LPS-stimulated bMECs of mastitis cows were significantly down-regulated, indicating that SYK is involved in inflammatory and immune response of clinical mastitis. As shown in Figures 6B,C, the temporal sequences of SYK, PTK2B, TLR4, and IL-1β expression are similar, suggesting that there might be an immune response pathway in bMECs participating in bovine mastitis inflammatory response. However, as the stimulation is prolonged, TLR4, NF-κB, and IL1β are up-regulation at later time points, suggesting that different immune response mechanisms may be initiated, and the phenomenon which needs further study.
TNFRSF21 is a member of the TNF/TNFR family, and plays a critical role in immune response and inflammation (91–93). It regulates the degeneration of the mammary gland and providing protection against infection (94). TNFRSF21 is also involves in NF-κB signaling pathway (95), which is the key pathways to identify exogenous pathogens and induce inflammation and immune response. TNFRSF21 was significantly up-regulated in bPBLs of mastitis cows and LPS-stimulated bMECs, indicating that TNFRSF21 plays an important role in bovine mastitis inflammation (96).
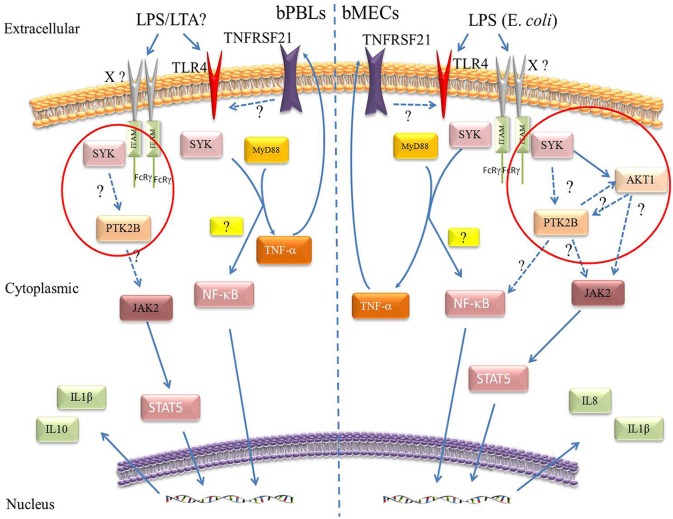
Three Immune-Regulated Genes (PTK2B, SYK, and TNFRSF21) Initiate Different Immune Response in bPBLs and bMECs to Cope With Mastitis
In bPBLs and bMECs, PTK2B, SYK, and TNFRSF21 are involved in different mechanisms of immune responses associated with mastitis (Figure 7). There was no significant difference in the expression level of AKT1 in bPBLs. But interestingly, they were significantly up-regulated in bMECs, and the trends were completely opposite to SYK and PTK2B, suggesting that PTK2B, SYK, and AKT1 participate in the immune cascade response of bMECs. PTK2B-AKT1 is involved in chemokine signaling pathway, while SYK-AKT1 is involved in B cell receptor signaling pathway and PI3K-AKT signaling pathway. However, AKT1, as a key player in the Jak2/STAT5 pathway, plays an important role in the regulation of differentiation, secretion, survival, and proliferation of mammary epithelial cells as well as mammary remodeling and lactation sustainability in dairy cows (90, 97–100). JAK2 and STAT5, two key players in the immune response, are associated with cow mastitis susceptibility (11, 101). Mastitis caused by bacterial infection of the mammary gland induces IL8 expression (102), and IL-8 plays an important role in the recruitment and degranulation of neutrophils in mammary gland (13). However, there were no significantly different in the expression levels of IL-8 in bPBLs of Chinese Holstein, while there were significant differences in LPS-stimulated bMECs, indicating that IL-8 has vital function on innate immunity of bMECs. The expression of IL-1β was significantly up-regulated in both clinical mastitis bPBLs and LPS-stimulated bMECs, suggesting that it is essential in bovine mastitis. IL-10, an anti-inflammatory cytokine involved in adaptive and innate immune regulation, plays a critical role in infection by limiting excess immune response to the pathogens and preventing host damage (103–106). It's regulated by Pyk2 (PTK2B) in LPS-stimulated mouse macrophages, and negatively regulated by SYK in dendritic cells (82, 89). Therefore, up-regulation of IL-10 expression in bPBLs, suggesting that IL-10 may be regulated by PTK2B and SYK, and thus participate in the regulation of inflammatory response in dairy cow mastitis. NF-κB pathway plays a central role in the host innate and acquired immune responses to microbial pathogen infection and is essential for maintaining immune homeostasis (107, 108). NF-κB expression was down-regulated in mastitis cows' bPBLs and time-dependent in LPS-stimulated bMECs. Therefore, we hypothesized that SYK and TNFRSF21 may be involved in the NF-κB pathway, stimulating IL-1β secretion and promote mastitis inflammation in both bPBLs and bMECs. SYK and PTK2B may be involved in the SYK/PTK2B/AKT1/JAK2 pathway in bMECs that stimulates IL-8 secretion and recruits neutrophils to kill pathogenic microorganisms, resulting in the resistance to mastitis inflammation.
Figure 7.
Molecular mechanisms prediction of the three immune-regulated genes in Chinese Holstein cows. Peripheral blood leukocytes of clinical mastitis cows (Left) and LPS (E. coli) stimulated bMECs (Right). Solid arrows represent known mechanisms and dashed arrows represent unknowns.
Conclusions
In this study, we were committed to improve understanding of biogenetic variation of mastitis, and providing a genetic basis for constructing and improving anti-mastitis characteristic in Chinese Holstein cows. First, we used GWAS-2b-RADseq to identify significant SNPs. We used two-stage correlation analysis to find 27 significant SNPs associated with risk of mastitis, among which three SNPs (rs75762330, rs88640083, and rs20438858) were annotated to immune-regulated genes (PTK2B, SYK, and TNFRSF21). Next, population genetic verification of three important SNPs was performed in another individual cow population, confirming that SNPs rs75762330, rs88640083, and rs20438858 were associated with mastitis susceptibility. Transcriptional analysis showed that PTK2B and SYK expression level was down-regulated in both bPBLs of clinical mastitis cows and in vitro LPS (E. coli)–stimulated bMECs indicating that PTK2B and SYK are important candidate genes associated with mastitis resistance traits. TNFRSF21 was up-regulated suggesting that TNFRSF21 is an important candidate gene associated with mastitis susceptibility traits. Network analysis showed that different immune response mechanisms are activated to deal with pathogen (E. coli) invasion. However, their molecular mechanisms for response to bovine mastitis remain unclear. Do SYK and TNFRSF21 participate in the NF-κB pathway? What are their statuses in bMECs? What are the co-expression mechanisms of SYK and PTK2B in bMECs in response to pathogens? Is there a SYK/PTK2B/AKT1/JAK2 pathway in bMECs or bovine mammary gland? Answering these questions will keep scientists interested in the immune response mechanisms caused by bovine mastitis.
Data Availability
The publicly available datasets generated for this study can be found in the NCBI SRA database - PRJNA556499 - https://www.ncbi.nlm.nih.gov/sra/PRJNA556499.
Ethics Statement
The procedures involving animals were approved by the Animal Welfare Committee of Nanjing Agricultural University, Nanjing, China, and approval No.20160615. And all animal experiments were carried out in strict accordance with the guidelines and rules established by the committee.
Author Contributions
YC, JL, HL, and GW co-designed the experiment. FY, FC, LL, LY, TB, CL, DY, MZ, XJ, JL, and LY participated in the screening of experimental animals and blood sample extraction. FY and FC sorted and analyzed 2b-RAD sequencing data. FY wrote and calibrated experiments manuscripts. The final manuscript was read and authorized by all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Nanjing Weigang Dairy Co., Ltd. for providing experimental Chinese Holstein blood samples and Shanghai Oe Biotech Co., Ltd. for providing 2b-RAD genome sequencing technology support.
Footnotes
Funding. This study was supported by Agricultural Innovation fund of Jiangsu Province [grant No. CX(17)1005]; National Key R&D Program of China [grant No. 2018YFC1200201]; Start-up grant from Nanjing Agricultural University (grant No. 804090).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01579/full#supplementary-material
Whole genome-wide sequencing (2B-RADseq) diagram of Chinese Holstein cows.
Distribution of SNP markers on chromosomes: the horizontal axis indicated the coordinates of the physical position of the chromosomes; the vertical axis represented the number of the corresponding SNPs (window size, 20 Kbp; Step length, 10 Kbp).
Genotyping of all SNPs and evaluation of two GWAS analysis models. (1) Genotyping and genotype score of 10058 SNPs (A): 0 (two bases of the type were different from the reference genome); 1 (two bases of typing were the same as one of the reference genomes); 2 (two bases of typing were the same as those of the reference genome). (2) The consistency of Bayesian (B) and logistic regression (C) analysis for SNPs observed and expected value –log10 (P), respectively. The P-value observation is almost the same as the expected, indicated that the analysis model was reasonable. There were several SNPs P-values exceeded expected, which suggested that these locus might be significantly associated with dairy cows' mastitis traits.
Hierarchical Network of candidate Gene function based on go enrichment Analysis. Each circle represented a Go entry; the color indicated the enrichment degree, the deeper the color (yellow), the more genes enriched in the Go entry; the direction of the arrow indicated hierarchic relationship.
Candidate genes interaction network Diagram based on KEGG Database. (A) Protein-protein interaction (93 genes) network map constructed in STRING 10.5 with the three candidate genes as the core (avg. local clustering coefficient: 0.707). (B) Based on co-expression of homologous genes from other species (co-expression scores: 0.074–0.314), predicted association between SYK and PTK2B.
The mRNA expression levels of IL-10 after stimulation of bMECs by LPS (E. coli, 25 μg/ml) time course.
The mean Ct value with SD of the β-actin in bPBLs of case-control samples (A) and bMECs (C,D), and identification of bMECs by fluorescence confocal microscope [(B), 1: DAPI staining of cell nucleus, 2: immunofluorescence staining of Cytokeratin 18, 3: synthetic map of nuclear (1) and cytoplasmic (2) staining].
Illumina base recognition and mass value correspondence table.
The top five principal component data of all SNPs for each sample.
Three genotypic risk estimation data of case-control by Armitage-test.
Forty two important SNPs screened out via Bayesian Analysis Model.
The logistic regression model screened out 51 important SNPs.
Five SNPs associated with three important SNPs screened by the Bayesian Model and Logical regression analysis model.
Three pairs of PCR amplification primers for population genetics validation.
Primers of internal reference gene (β-actin), three immune-regulate genes, TLR4, AKT1, NF-κB, and three interleukin genes for RT-qPCR analysis.
Fst values of 27 significantly different SNPs.
A supplement to the “Materials and Methods” section.
References
- 1.Maunsell FP, Morin DE, Constable PD, Hurley WL, Mccoy GC, Kakoma I, et al. Effects of mastitis on the volume and composition of colostrum produced by Holstein cows. J Dairy Sci. (1998) 81:1291–9. 10.3168/jds.S0022-0302(98)75691-7 [DOI] [PubMed] [Google Scholar]
- 2.Schukken YH, Hertl J, Bar D, Bennett GJ, Gonzalez RN, Rauch BJ, et al. Effects of repeated gram-positive and gram-negative clinical mastitis episodes on milk yield loss in Holstein dairy cows. J Dairy Sci. (2009) 92:3091–105. 10.3168/jds.2008-1557 [DOI] [PubMed] [Google Scholar]
- 3.Welderufael BG, Janss LLG, De Koning DJ, Sorensen LP, Lovendahl P, Fikse WF. Bivariate threshold models for genetic evaluation of susceptibility to and ability to recover from mastitis in Danish Holstein cows. J Dairy Sci. (2017) 100:4706–20. 10.3168/jds.2016-11894 [DOI] [PubMed] [Google Scholar]
- 4.Swinkels JM, Hogeveen H, Zadoks RN. A partial budget model to estimate economic benefits of lactational treatment of subclinical Staphylococcus aureus mastitis. J Dairy Sci. (2005) 88:4273–87. 10.3168/jds.S0022-0302(05)73113-1 [DOI] [PubMed] [Google Scholar]
- 5.Halasa T, Huijps K, Osteras O, Hogeveen H. Economic effects of bovine mastitis and mastitis management: a review. Vet Q. (2007) 29:18–31. 10.1080/01652176.2007.9695224 [DOI] [PubMed] [Google Scholar]
- 6.Halasa T, Nielen M, De Roos AP, Van Hoorne R, De Jong G, Lam TJ, et al. Production loss due to new subclinical mastitis in Dutch dairy cows estimated with a test-day model. J Dairy Sci. (2009) 92:599–606. 10.3168/jds.2008-1564 [DOI] [PubMed] [Google Scholar]
- 7.Hogeveen H, Huijps K, Lam TJ. Economic aspects of mastitis: new developments. N Z Vet J. (2011) 59:16–23. 10.1080/00480169.2011.547165 [DOI] [PubMed] [Google Scholar]
- 8.Rinaldi M, Li RW, Capuco AV. Mastitis associated transcriptomic disruptions in cattle. Vet Immunol Immunopathol. (2010) 138:267–79. 10.1016/j.vetimm.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 9.Hertl JA, Schukken YH, Welcome FL, Tauer LW, Grohn YT. Pathogen-specific effects on milk yield in repeated clinical mastitis episodes in Holstein dairy cows. J Dairy Sci. (2014) 97:1465–80. 10.3168/jds.2013-7266 [DOI] [PubMed] [Google Scholar]
- 10.Moosavi M, Mirzaei A, Ghavami M, Tamadon A. Relationship between season, lactation number and incidence of clinical mastitis in different stages of lactation in a Holstein dairy farm. Vet Res Forum. (2014) 5:13–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Usman T, Wang Y, Liu C, Wang X, Zhang Y, Yu Y. Association study of single nucleotide polymorphisms in JAK2 and STAT5B genes and their differential mRNA expression with mastitis susceptibility in Chinese Holstein cattle. Anim Genet. (2015) 46:371–80. 10.1111/age.12306 [DOI] [PubMed] [Google Scholar]
- 12.Pokorska J, Dusza M, Kulaj D, Zukowski K, Makulska J. Single nucleotide polymorphisms in the CXCR1 gene and its association with clinical mastitis incidence in Polish Holstein-Friesian cows. Genet Mol Res. (2016) 15:gmr7247. 10.4238/gmr.15027247 [DOI] [PubMed] [Google Scholar]
- 13.Kiku Y, Ozawa T, Takahashi H, Kushibiki S, Inumaru S, Shingu H, et al. Effect of intramammary infusion of recombinant bovine GM-CSF and IL-8 on CMT score, somatic cell count, and milk mononuclear cell populations in Holstein cows with Staphylococcus aureus subclinical mastitis. Vet Res Commun. (2017) 41:175–82. 10.1007/s11259-017-9684-y [DOI] [PubMed] [Google Scholar]
- 14.Bannerman DD. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J Anim Sci. (2009) 87:10–25. 10.2527/jas.2008-1187 [DOI] [PubMed] [Google Scholar]
- 15.Negussie E, Stranden I, Mantysaari EA. Genetic association of clinical mastitis with test-day somatic cell score and milk yield during first lactation of Finnish Ayrshire cows. J Dairy Sci. (2008) 91:1189–97. 10.3168/jds.2007-0510 [DOI] [PubMed] [Google Scholar]
- 16.Santman-Berends IM, Lam TJ, Keurentjes J, Van Schaik G. An estimation of the clinical mastitis incidence per 100 cows per year based on routinely collected herd data. J Dairy Sci. (2015) 98:6965–77. 10.3168/jds.2015-9642 [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Ma P, Liu J, Zhang Q, Zhang Y, Ding X, et al. Genome-wide association study in Chinese Holstein cows reveal two candidate genes for somatic cell score as an indicator for mastitis susceptibility. BMC Genet. (2015) 16:111. 10.1186/s12863-015-0263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahana G, Guldbrandtsen B, Thomsen B, Holm LE, Panitz F, Brondum RF, et al. Genome-wide association study using high-density single nucleotide polymorphism arrays and whole-genome sequences for clinical mastitis traits in dairy cattle. J Dairy Sci. (2014) 97:7258–75. 10.3168/jds.2014-8141 [DOI] [PubMed] [Google Scholar]
- 19.Kadri NK, Guldbrandtsen B, Lund MS, Sahana G. Genetic dissection of milk yield traits and mastitis resistance quantitative trait loci on chromosome 20 in dairy cattle. J Dairy Sci. (2015) 98:9015–25. 10.3168/jds.2015-9599 [DOI] [PubMed] [Google Scholar]
- 20.Daetwyler HD, Capitan A, Pausch H, Stothard P, Van Binsbergen R, Brondum RF, et al. Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nat Genet. (2014) 46:858–65. 10.1038/ng.3034 [DOI] [PubMed] [Google Scholar]
- 21.Crispim AC, Kelly MJ, Guimaraes SE, Fonseca E, Silva F, Fortes MR, et al. Multi-trait GWAS and new candidate genes annotation for growth curve parameters in Brahman cattle. PLoS ONE. (2015) 10:e0139906. 10.1371/journal.pone.0139906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selimovic-Hamza S, Boujon CL, Hilbe M, Oevermann A, Seuberlich T. Frequency and pathological phenotype of bovine astrovirus CH13/NeuroS1 infection in neurologically-diseased cattle: towards assessment of causality. Viruses. (2017) 9:12. 10.3390/v9010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varshney RK, Saxena RK, Upadhyaya HD, Khan AW, Yu Y, Kim C, et al. Whole-genome resequencing of 292 pigeonpea accessions identifies genomic regions associated with domestication and agronomic traits. Nat Genet. (2017) 49:1082–8. 10.1038/ng.3872 [DOI] [PubMed] [Google Scholar]
- 24.Visscher PM, Wray NR, Zhang Q, Sklar P, Mccarthy MI, Brown MA, et al. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet. (2017) 101:5–22. 10.1016/j.ajhg.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brondum RF, Su G, Janss L, Sahana G, Guldbrandtsen B, Boichard D, et al. Quantitative trait loci markers derived from whole genome sequence data increases the reliability of genomic prediction. J Dairy Sci. (2015) 98:4107–16. 10.3168/jds.2014-9005 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Guldbrandtsen B, Thomasen JR, Lund MS, Sahana G. Genome-wide association study for longevity with whole-genome sequencing in 3 cattle breeds. J Dairy Sci. (2016) 99:7289–98. 10.3168/jds.2015-10697 [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. (2011) 88:76–82. 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SH, Decandia TR, Ripke S, Yang J, Schizophrenia Psychiatric Genome-Wide Association Study C, International Schizophrenia C et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. (2012) 44:247–50. 10.1038/ng.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross-Disorder Group of the Psychiatric Genomics C. Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. (2013) 45:984. 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Hou Y, Yin X, Bao L, Tang A, Song L, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. (2012) 148:886–95. 10.1016/j.cell.2012.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berndt SI, Gustafsson S, Magi R, Ganna A, Wheeler E, Feitosa MF, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. (2013) 45:501–12. 10.1038/ng.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiggans GR, Sonstegard TS, Vanraden PM, Matukumalli LK, Schnabel RD, Taylor JF, et al. Selection of single-nucleotide polymorphisms and quality of genotypes used in genomic evaluation of dairy cattle in the United States and Canada. J Dairy Sci. (2009) 92:3431–6. 10.3168/jds.2008-1758 [DOI] [PubMed] [Google Scholar]
- 33.Sodeland M, Kent MP, Olsen HG, Opsal MA, Svendsen M, Sehested E, et al. Quantitative trait loci for clinical mastitis on chromosomes 2, 6, 14 and 20 in Norwegian Red cattle. Anim Genet. (2011) 42:457–65. 10.1111/j.1365-2052.2010.02165.x [DOI] [PubMed] [Google Scholar]
- 34.Meredith BK, Kearney FJ, Finlay EK, Bradley DG, Fahey AG, Berry DP, et al. Genome-wide associations for milk production and somatic cell score in Holstein-Friesian cattle in Ireland. BMC Genet. (2012) 13:21. 10.1186/1471-2156-13-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wijga S, Bastiaansen JW, Wall E, Strandberg E, De Haas Y, Giblin L, et al. Genomic associations with somatic cell score in first-lactation Holstein cows. J Dairy Sci. (2012) 95:899–908. 10.3168/jds.2011-4717 [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Lund MS, Sahana G, Guldbrandtsen B, Sun D, Zhang Q, et al. Association analysis for udder health based on SNP-panel and sequence data in Danish Holsteins. Genet Sel Evol. (2015) 47:50. 10.1186/s12711-015-0129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J, Li L, Sun Y, Huang S, Tang J, Yu P, et al. Altered molecular expression of the TLR4/NF-kappaB signaling pathway in mammary tissue of Chinese Holstein cattle with mastitis. PLoS ONE. (2015) 10:e0118458. 10.1371/journal.pone.0118458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiezzi F, Parker-Gaddis KL, Cole JB, Clay JS, Maltecca C. A genome-wide association study for clinical mastitis in first parity US Holstein cows using single-step approach and genomic matrix re-weighting procedure. PLoS ONE. (2015) 10:e0114919. 10.1371/journal.pone.0114919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattarai D, Chen X, Ur Rehman Z, Hao X, Ullah F, Dad R, et al. Association of MAP4K4 gene single nucleotide polymorphism with mastitis and milk traits in Chinese Holstein cattle. J Dairy Res. (2017) 84:76–9. 10.1017/S0022029916000832 [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Meyer E, Mckay JK, Matz MV. 2b-RAD: a simple and flexible method for genome-wide genotyping. Nat Methods. (2012) 9:808–10. 10.1038/nmeth.2023 [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Liu P, Lv J, Li Y, Cheng T, Zhang L, et al. Serial sequencing of isolength RAD tags for cost-efficient genome-wide profiling of genetic and epigenetic variations. Nat Protoc. (2016) 11:2189–200. 10.1038/nprot.2016.133 [DOI] [PubMed] [Google Scholar]
- 42.Guo Y, Yuan H, Fang D, Song L, Liu Y, Liu Y, et al. An improved 2b-RAD approach (I2b-RAD) offering genotyping tested by a rice (Oryza sativa L.) F2 population. BMC Genomics. (2014) 15:956. 10.1186/1471-2164-15-956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiao W, Fu X, Dou J, Li H, Su H, Mao J, et al. High-resolution linkage and quantitative trait locus mapping aided by genome survey sequencing: building up an integrative genomic framework for a bivalve mollusc. DNA Res. (2014) 21:85–101. 10.1093/dnares/dst043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Su K, Wang G, Zhang L, Zhang J, Li J, et al. High-density genetic linkage map construction and quantitative trait locus mapping for hawthorn (Crataegus pinnatifida Bunge). Sci Rep. (2017) 7:5492. 10.1038/s41598-017-05756-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo X, Shi X, Yuan C, Ai M, Ge C, Hu M, et al. Genome-wide SNP analysis using 2b-RAD sequencing identifies the candidate genes putatively associated with resistance to ivermectin in Haemonchus contortus. Parasit Vectors. (2017) 10:31. 10.1186/s13071-016-1959-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam T, Olde Riekerink R, Sampimon O, Smith H. Mastitis diagnostics and performance monitoring: a practical approach. Ir Vet J. (2009) 62(Suppl 4):S34–9. 10.1186/2046-0481-62-S4-S34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jadhav PV, Das DN, Suresh KP, Shome BR. Threshold somatic cell count for delineation of subclinical mastitis cases. Vet World. (2018) 11:789–93. 10.14202/vetworld.2018.789-793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubin CJ, Zody MC, Eriksson J, Meadows JR, Sherwood E, Webster MT, et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature. (2010) 464:587–91. 10.1038/nature08832 [DOI] [PubMed] [Google Scholar]
- 49.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. (2017) 45:D362–8. 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.German T, Barash I. Characterization of an epithelial cell line from bovine mammary gland. In Vitro Cell Dev Biol Anim. (2002) 38:282–92. [DOI] [PubMed] [Google Scholar]
- 51.Basirico L, Morera P, Dipasquale D, Troscher A, Serra A, Mele M, et al. Conjugated linoleic acid isomers strongly improve the redox status of bovine mammary epithelial cells (BME-UV1). J Dairy Sci. (2015) 98:7071–82. 10.3168/jds.2015-9787 [DOI] [PubMed] [Google Scholar]
- 52.Bouchard DS, Seridan B, Saraoui T, Rault L, Germon P, Gonzalez-Moreno C, et al. Lactic acid bacteria isolated from bovine mammary microbiota: potential allies against bovine mastitis. PLoS ONE. (2015) 10:e0144831. 10.1371/journal.pone.0144831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korkmaz FT, Elsasser TH, Kerr DE. Variation in fibroblast expression of toll-like receptor 4 and lipopolysaccharide-induced cytokine production between animals predicts control of bacterial growth but not severity of Escherichia coli mastitis. J Dairy Sci. (2018) 101:10098–115. 10.3168/jds.2017-14372 [DOI] [PubMed] [Google Scholar]
- 54.Windig JJ, Urioste JI, Strandberg E. Integration of epidemiology into the genetic analysis of mastitis in Swedish Holstein. J Dairy Sci. (2013). 96:2617–26. 10.3168/jds.2012-6076 [DOI] [PubMed] [Google Scholar]
- 55.Moser G, Tier B, Crump RE, Khatkar MS, Raadsma HW. A comparison of five methods to predict genomic breeding values of dairy bulls from genome-wide SNP markers. Genet Sel Evol. (2009) 41:56. 10.1186/1297-9686-41-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Resende MF, Jr, Munoz P, Resende MD, Garrick DJ, Fernando RL, Davis JM, et al. Accuracy of genomic selection methods in a standard data set of loblolly pine (Pinus taeda L.). Genetics. (2012) 190:1503–10. 10.1534/genetics.111.137026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagheri M, Moradi-Sharhrbabak M, Miraie-Ashtiani R, Safdari-Shahroudi M, Abdollahi-Arpanahi R. Case-control approach application for finding a relationship between candidate genes and clinical mastitis in Holstein dairy cattle. J Appl Genet. (2016) 57:107–12. 10.1007/s13353-015-0299-0 [DOI] [PubMed] [Google Scholar]
- 58.Weissbrod O, Flint J, Rosset S. Estimating SNP-based heritability and genetic correlation in case-control studies directly and with summary statistics. Am J Hum Genet. (2018) 103:89–99. 10.1016/j.ajhg.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou W, Nielsen JB, Fritsche LG, Dey R, Gabrielsen ME, Wolford BN, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. (2018) 50:1335–41. 10.1038/s41588-018-0184-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haudry A, Platts AE, Vello E, Hoen DR, Leclercq M, Williamson RJ, et al. An atlas of over 90,000 conserved noncoding sequences provides insight into crucifer regulatory regions. Nat Genet. (2013) 45:891–8. 10.1038/ng.2684 [DOI] [PubMed] [Google Scholar]
- 61.Visser M, Palstra RJ, Kayser M. Human skin color is influenced by an intergenic DNA polymorphism regulating transcription of the nearby BNC2 pigmentation gene. Hum Mol Genet. (2014) 23:5750–62. 10.1093/hmg/ddu289 [DOI] [PubMed] [Google Scholar]
- 62.Petibon C, Parenteau J, Catala M, Elela SA. Introns regulate the production of ribosomal proteins by modulating splicing of duplicated ribosomal protein genes. Nucleic Acids Res. (2016) 44:3878–91. 10.1093/nar/gkw140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dickel DE, Ypsilanti AR, Pla R, Zhu Y, Barozzi I, Mannion BJ, et al. Ultraconserved enhancers are required for normal development. Cell. (2018) 172:491–9.e415. 10.1016/j.cell.2017.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patrushev LI, Kovalenko TF. Functions of noncoding sequences in mammalian genomes. Biochemistry. (2014) 79:1442–69. 10.1134/S0006297914130021 [DOI] [PubMed] [Google Scholar]
- 65.Hansen M, Lund MS, Sorensen MK, Christensen LG. Genetic parameters of dairy character, protein yield, clinical mastitis, and other diseases in the Danish Holstein cattle. J Dairy Sci. (2002) 85:445–52. 10.3168/jds.S0022-0302(02)74093-9 [DOI] [PubMed] [Google Scholar]
- 66.Taponen S, Liski E, Heikkila AM, Pyorala S. Factors associated with intramammary infection in dairy cows caused by coagulase-negative staphylococci, Staphylococcus aureus, Streptococcus uberis, Streptococcus dysgalactiae, Corynebacterium bovis, or Escherichia coli. J Dairy Sci. (2017) 100:493–503. 10.3168/jds.2016-11465 [DOI] [PubMed] [Google Scholar]
- 67.Gilbert FB, Cunha P, Jensen K, Glass EJ, Foucras G, Robert-Granie C, et al. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet Res. (2013) 44:40. 10.1186/1297-9716-44-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng L, Xu Y, Lu J, Liu M, Bin D, Miao J, et al. Variant innate immune responses of mammary epithelial cells to challenge by Staphylococcus aureus, Escherichia coli and the regulating effect of taurine on these bioprocesses. Free Radic Biol Med. (2016) 96:166–80. 10.1016/j.freeradbiomed.2016.04.022 [DOI] [PubMed] [Google Scholar]
- 69.Sargeant JM, Leslie KE, Shirley JE, Pulkrabek BJ, Lim GH. Sensitivity and specificity of somatic cell count and California Mastitis Test for identifying intramammary infection in early lactation. J Dairy Sci. (2001) 84:2018–24. 10.3168/jds.S0022-0302(01)74645-0 [DOI] [PubMed] [Google Scholar]
- 70.Dufour S, Dohoo IR. Monitoring herd incidence of intramammary infection in lactating cows using repeated longitudinal somatic cell count measurements. J Dairy Sci. (2013) 96:1568–80. 10.3168/jds.2012-5902 [DOI] [PubMed] [Google Scholar]
- 71.Godden SM, Royster E, Timmerman J, Rapnicki P, Green H. Evaluation of an automated milk leukocyte differential test and the California Mastitis Test for detecting intramammary infection in early- and late-lactation quarters and cows. J Dairy Sci. (2017) 100:6527–44. 10.3168/jds.2017-12548 [DOI] [PubMed] [Google Scholar]
- 72.Alva-Murillo N, Ochoa-Zarzosa A, and Lopez-Meza JE. Sodium octanoate modulates the innate immune response of bovine mammary epithelial cells through the TLR2/P38/JNK/ERK1/2 pathway: implications during Staphylococcus aureus internalization. Front Cell Infect Microbiol. (2017). 7:78. 10.3389/fcimb.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu M, Song S, Li H, Jiang X, Yin P, Wan C, et al. The protective effect of caffeic acid against inflammation injury of primary bovine mammary epithelial cells induced by lipopolysaccharide. J Dairy Sci. (2014) 97:2856–65. 10.3168/jds.2013-7600 [DOI] [PubMed] [Google Scholar]
- 74.Shahid M, Wang J, Gu X, Chen W, Ali T, Gao J, et al. Prototheca zopfii induced ultrastructural features associated with apoptosis in bovine mammary epithelial cells. Front Cell Infect Microbiol. (2017) 7:299. 10.3389/fcimb.2017.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J, Guo C, Wei Z, He X, Kou J, Zhou E, et al. Morin suppresses inflammatory cytokine expression by downregulation of nuclear factor-kappaB and mitogen-activated protein kinase (MAPK) signaling pathways in lipopolysaccharide-stimulated primary bovine mammary epithelial cells. J Dairy Sci. (2016) 99:3016–22. 10.3168/jds.2015-10330 [DOI] [PubMed] [Google Scholar]
- 76.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. (2010) 10:387–402. 10.1038/nri2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han H, Fuortes M, Nathan C. Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation. J Exp Med. (2003) 197:63–75. 10.1084/jem.20021638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Racioppi L, Noeldner PK, Lin F, Arvai S, Means AR. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J Biol Chem. (2012) 287:11579–91. 10.1074/jbc.M111.336032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kremer AN, Van Der Griendt JC, Van Der Meijden ED, Honders MW, Ayoglu B, Schwenk JM, et al. Development of a coordinated allo T cell and auto B cell response against autosomal PTK2B after allogeneic hematopoietic stem cell transplantation. Haematologica. (2014) 99:365–9. 10.3324/haematol.2013.086652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rhee I, Zhong MC, Reizis B, Cheong C, Veillette A. Control of dendritic cell migration, T cell-dependent immunity, and autoimmunity by protein tyrosine phosphatase PTPN12 expressed in dendritic cells. Mol Cell Biol. (2014) 34:888–99. 10.1128/MCB.01369-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Llewellyn RA, Thomas KS, Gutknecht MF, Bouton AH. The nonreceptor protein tyrosine kinase Pyk2 promotes the turnover of monocytes at steady state. J Leukoc Biol. (2017) 102:1069–80. 10.1189/jlb.1A0217-063R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okenwa C, Kumar A, Rego D, Konarski Y, Nilchi L, Wright K, et al. SHP-1-Pyk2-Src protein complex and p38 MAPK pathways independently regulate IL-10 production in lipopolysaccharide-stimulated macrophages. J Immunol. (2013) 191:2589–603. 10.4049/jimmunol.1300466 [DOI] [PubMed] [Google Scholar]
- 83.Liu X, Zhang P, Zhang Y, Wang Z, Xu S, Li Y, et al. Glycolipid iGb3 feedback amplifies innate immune responses via CD1d reverse signaling. Cell Res. (2018) 29:42–53. 10.1038/s41422-018-0122-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamen LA, Schlessinger J, Lowell CA. Pyk2 is required for neutrophil degranulation and host defense responses to bacterial infection. J Immunol. (2011) 186:1656–65. 10.4049/jimmunol.1002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi SH, Gonen A, Diehl CJ, Kim J, Almazan F, Witztum JL, et al. SYK regulates macrophage MHC-II expression via activation of autophagy in response to oxidized LDL. Autophagy. (2015) 11:785–95. 10.1080/15548627.2015.1037061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schweighoffer E, Nys J, Vanes L, Smithers N, Tybulewicz VLJ. TLR4 signals in B lymphocytes are transduced via the B cell antigen receptor and SYK. J Exp Med. (2017) 214:1269–80. 10.1084/jem.20161117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yasukawa S, Miyazaki Y, Yoshii C, Nakaya M, Ozaki N, Toda S, et al. An ITAM-Syk-CARD9 signalling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells. Nat Commun. (2014) 5:3755. 10.1038/ncomms4755 [DOI] [PubMed] [Google Scholar]
- 88.Lin YC, Huang DY, Chu CL, Lin YL, Lin WW. The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci Signal. (2013) 6:ra71. 10.1126/scisignal.2003973 [DOI] [PubMed] [Google Scholar]
- 89.Yin H, Zhou H, Kang Y, Zhang X, Duan X, Alnabhan R, et al. Syk negatively regulates TLR4-mediated IFNbeta and IL-10 production and promotes inflammatory responses in dendritic cells. Biochim Biophys Acta. (2016) 1860:588–98. 10.1016/j.bbagen.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hou X, Lin L, Xing W, Yang Y, Duan X, Li Q, et al. Spleen tyrosine kinase regulates mammary epithelial cell proliferation in mammary glands of dairy cows. J Dairy Sci. (2016) 99:3858–68. 10.3168/jds.2015-10118 [DOI] [PubMed] [Google Scholar]
- 91.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. (2001) 104:487–501. 10.1016/S0092-8674(01)00237-9 [DOI] [PubMed] [Google Scholar]
- 92.Strilic B, Yang L, Albarran-Juarez J, Wachsmuth L, Han K, Muller UC, et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. (2016) 536:215–8. 10.1038/nature19076 [DOI] [PubMed] [Google Scholar]
- 93.Fujikura D, Ikesue M, Endo T, Chiba S, Higashi H, Uede T. Death receptor 6 contributes to autoimmunity in lupus-prone mice. Nat Commun. (2017) 8:13957. 10.1038/ncomms13957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khalil E, Digby MR, Thomson PC, Lefevre C, Mailer SL, Pooley C, et al. Acute involution in the tammar wallaby: identification of genes and putative novel milk proteins implicated in mammary gland function. Genomics. (2011) 97:372–8. 10.1016/j.ygeno.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 95.Klima M, Brouckova A, Koc M, Andera L. T-cell activation triggers death receptor-6 expression in a NF-kappaB and NF-AT dependent manner. Mol Immunol. (2011) 48:1439–47. 10.1016/j.molimm.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 96.Fang L, Hou Y, An J, Li B, Song M, Wang X, et al. Genome-wide transcriptional and post-transcriptional regulation of innate immune and defense responses of bovine mammary gland to Staphylococcus aureus. Front Cell Infect Microbiol. (2016) 6:193. 10.3389/fcimb.2016.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maroulakou IG, Oemler W, Naber SP, Klebba I, Kuperwasser C, Tsichlis PN. Distinct roles of the three Akt isoforms in lactogenic differentiation and involution. J Cell Physiol. (2008) 217:468–77. 10.1002/jcp.21518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen CC, Boxer RB, Stairs DB, Portocarrero CP, Horton RH, Alvarez JV, et al. Akt is required for Stat5 activation and mammary differentiation. Breast Cancer Res. (2010) 12:R72. 10.1186/bcr2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Creamer BA, Sakamoto K, Schmidt JW, Triplett AA, Moriggl R, Wagner KU. Stat5 promotes survival of mammary epithelial cells through transcriptional activation of a distinct promoter in Akt1. Mol Cell Biol. (2010) 30:2957–70. 10.1128/MCB.00851-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arranz A, Doxaki C, Vergadi E, Martinez De La Torre Y, Vaporidi K, Lagoudaki ED, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci USA. (2012) 109:9517–22. 10.1073/pnas.1119038109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Villarino AV, Kanno Y, O'shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. (2017) 18:374–84. 10.1038/ni.3691 [DOI] [PubMed] [Google Scholar]
- 102.Sugimoto M, Fujikawa A, Womack JE, Sugimoto Y. Evidence that bovine forebrain embryonic zinc finger-like gene influences immune response associated with mastitis resistance. Proc Natl Acad Sci USA. (2006) 103:6454–9. 10.1073/pnas.0601015103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saraiva M, O'garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. (2010) 10:170–81. 10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- 104.Garaude J, Acin-Perez R, Martinez-Cano S, Enamorado M, Ugolini M, Nistal-Villan E, et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat Immunol. (2016) 17:1037–45. 10.1038/ni.3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang S, Xia P, Chen Y, Qu Y, Xiong Z, Ye B, et al. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell. (2017) 171, 201–16.e218. 10.1016/j.cell.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 106.Proto JD, Doran AC, Gusarova G, Yurdagul A, Jr, Sozen E, Subramanian M, et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity. (2018) 49:666–77.e666. 10.1016/j.immuni.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rahman MM, Mcfadden G. Modulation of NF-kappaB signalling by microbial pathogens. Nat Rev Microbiol. (2011) 9:291–306. 10.1038/nrmicro2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lv S, Li J, Qiu X, Li W, Zhang C, Zhang ZN, et al. A negative feedback loop of ICER and NF-kappaB regulates TLR signaling in innate immune responses. Cell Death Differ. (2017) 24:492–9. 10.1038/cdd.2016.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Whole genome-wide sequencing (2B-RADseq) diagram of Chinese Holstein cows.
Distribution of SNP markers on chromosomes: the horizontal axis indicated the coordinates of the physical position of the chromosomes; the vertical axis represented the number of the corresponding SNPs (window size, 20 Kbp; Step length, 10 Kbp).
Genotyping of all SNPs and evaluation of two GWAS analysis models. (1) Genotyping and genotype score of 10058 SNPs (A): 0 (two bases of the type were different from the reference genome); 1 (two bases of typing were the same as one of the reference genomes); 2 (two bases of typing were the same as those of the reference genome). (2) The consistency of Bayesian (B) and logistic regression (C) analysis for SNPs observed and expected value –log10 (P), respectively. The P-value observation is almost the same as the expected, indicated that the analysis model was reasonable. There were several SNPs P-values exceeded expected, which suggested that these locus might be significantly associated with dairy cows' mastitis traits.
Hierarchical Network of candidate Gene function based on go enrichment Analysis. Each circle represented a Go entry; the color indicated the enrichment degree, the deeper the color (yellow), the more genes enriched in the Go entry; the direction of the arrow indicated hierarchic relationship.
Candidate genes interaction network Diagram based on KEGG Database. (A) Protein-protein interaction (93 genes) network map constructed in STRING 10.5 with the three candidate genes as the core (avg. local clustering coefficient: 0.707). (B) Based on co-expression of homologous genes from other species (co-expression scores: 0.074–0.314), predicted association between SYK and PTK2B.
The mRNA expression levels of IL-10 after stimulation of bMECs by LPS (E. coli, 25 μg/ml) time course.
The mean Ct value with SD of the β-actin in bPBLs of case-control samples (A) and bMECs (C,D), and identification of bMECs by fluorescence confocal microscope [(B), 1: DAPI staining of cell nucleus, 2: immunofluorescence staining of Cytokeratin 18, 3: synthetic map of nuclear (1) and cytoplasmic (2) staining].
Illumina base recognition and mass value correspondence table.
The top five principal component data of all SNPs for each sample.
Three genotypic risk estimation data of case-control by Armitage-test.
Forty two important SNPs screened out via Bayesian Analysis Model.
The logistic regression model screened out 51 important SNPs.
Five SNPs associated with three important SNPs screened by the Bayesian Model and Logical regression analysis model.
Three pairs of PCR amplification primers for population genetics validation.
Primers of internal reference gene (β-actin), three immune-regulate genes, TLR4, AKT1, NF-κB, and three interleukin genes for RT-qPCR analysis.
Fst values of 27 significantly different SNPs.
A supplement to the “Materials and Methods” section.
Data Availability Statement
The publicly available datasets generated for this study can be found in the NCBI SRA database - PRJNA556499 - https://www.ncbi.nlm.nih.gov/sra/PRJNA556499.