Abstract
The conserved Mediator co-activator complex plays an essential role in regulation of RNA polymerase II transcription in all eukaryotes. Understanding Mediator’s structure and interactions is critical for determining how the complex influences initiation and conveys regulatory information to the basal transcription machinery. Here we present a 4.4 Å resolution cryo-EM map of Schizosaccharomyces pombe Mediator in which conserved Mediator subunits are individually resolved. The essential Med14 subunit works as a central backbone that connects the Head, Middle, and Tail Mediator modules. Comparison with a 7.8 Å resolution cryo-EM map of a Mediator-RNA polymerase II holoenzyme reveals that changes in the structure of Med14 facilitate a large-scale Mediator rearrangement essential for holoenzyme formation. Our study suggests that access to different conformations and crosstalk between structural elements are essential to the Mediator regulation mechanism and could explain Mediator’s capacity to integrate multiple regulatory signals
The 25–30 protein (>1MDa) Mediator complex plays an essential role in regulation of transcription initiation across eukaryotes− it influences preinitiation (PIC) complex formation and stimulates phosphorylation of the RNA polymerase II (RNAPII) carboxy-terminal domain (CTD) by TFIIH, and it interacts with activators and repressors to convey regulatory information to the basal transcription machinery1,2. Mediator’s minimal catalytic activity (it includes a single kinase), similarity among low-resolution EM structures of Mediators from various organisms3–5, and reports of Mediator rearrangements upon interaction with activators, repressors, or RNAPII6–8, suggest that understanding Mediator structure, conformational dynamics, and interactions will be essential to discern its mechanism.
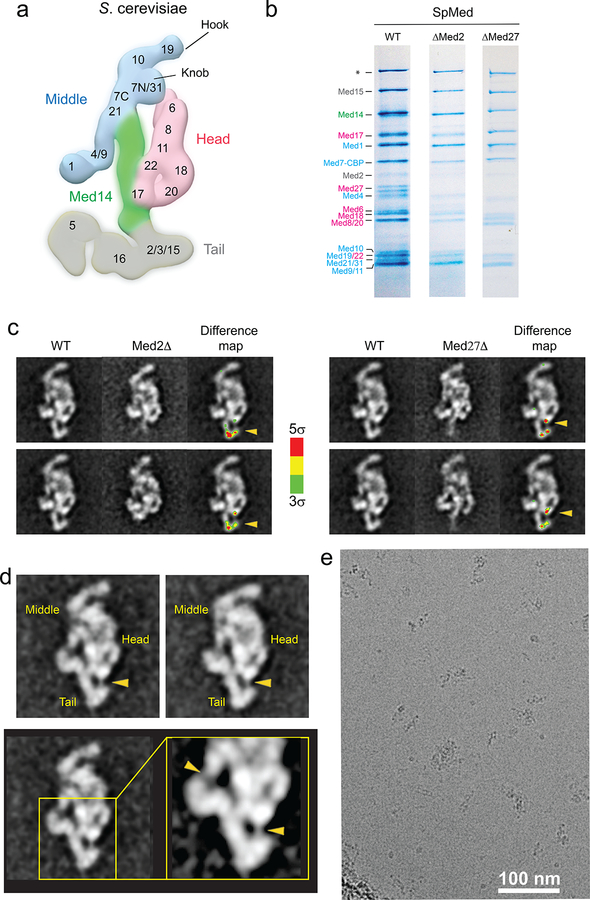
We previously provided a molecular description of the Saccharomyces cerevisiae Mediator (ScMED) by defining boundaries between conserved Head, Middle, Tail, and CDK8 modules, and localizing all 25 Mediator subunits in low resolution cryo-EM maps8,9 (Extended Data Fig 1a). The modular organization of the Schizosaccharomyces pombe and human Mediators were found to be homologous to that of ScMED8,10, and even subunit interactions within modules appear to be conserved from yeast to humans8. Portions of the Mediator structure, notably the 7-subunit Head, have been characterized at high-resolution by X-ray crystallography11–15. However, until now, a detailed molecular understanding of the entire Mediator and its interaction with RNAPII were not available. Crucial details of the Mediator-RNAPII interaction were revealed by a ~10Å resolution map of a core of recombinant Mediator subunits interacting with a minimal preinitiation complex (PIC)16. Extrapolation from this structure by matching to low-resolution cryo-EM maps of the entire Mediator8 and a human PIC17 suggested how interaction with Mediator could stabilize dynamic portions of the PIC.
Here we present a near-atomic (4.4 Å) resolution cryo-EM map of Schizosaccharomyces pombe Mediator (SpMED) and a corresponding atomic model including all subunits (complete Head and Middle [minus Med1], and Med14) essential for viability and basal transcription regulation. The cryo-EM structure provides a detailed description of the complete Head, of the previously unresolved Middle and Med14, and of their complex interactions. All module and subunit contacts are consistent with published results from cross-linking/mass spectrometry (XL/MS) analyses of yeast and human Mediators16,18,19. Med14 mediates all interactions between modules and a unique structure, comprising repeating structural motifs, allows Med14 to function as a backbone that enables large-scale Mediator rearrangements. We also present a 7.8 Å resolution cryo-EM map of the Mediator-RNAPII holoenzyme. Comparison of the holoenzyme and Mediator structures shows that holoenzyme formation is contingent on concerted Mediator rearrangements in which Med14 plays an essential role. In the holoenzyme, Mediator establishes multiple contacts with RNAPII, holding it in a specific orientation. The polymerase conformation remains unchanged after interaction with Mediator, but considering the structure of the yeast preinitiation complex (PIC) suggests that Mediator’s holoenzyme conformation could stabilize dynamic portions of the PIC. We found that the structure of Mediator is geared to enable changes required for RNAPII interaction and PIC stabilization, and that the capacity to access different conformational states is essential for the Mediator mechanism.
Structure of SpMED
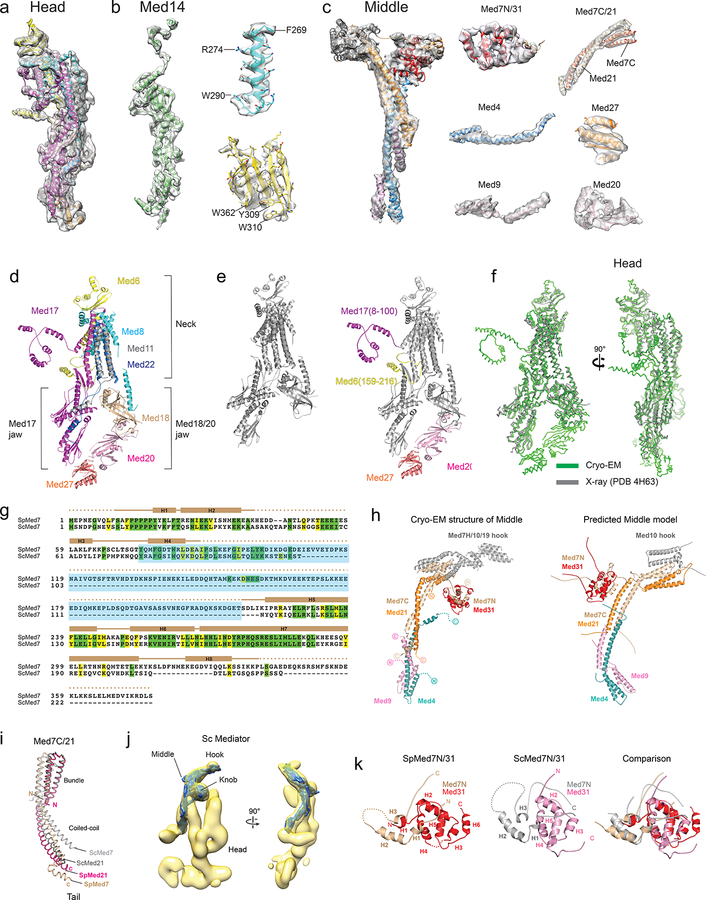
SpMED was immuno-purified through an affinity tag, SpMED-specific subunits were localized, and specimens were prepared for cryo-EM analysis (Supplemental Information, Extended Data Table 1a, and Extended Data Figs 1 and 2a–b). Analysis of free SpMED images resulted in a 4.4 Å resolution cryo-EM map (Fig 1a–b, Extended Data Fig 2c–e, and Extended Data Table 1b). The best-resolved portions of the map were the central Med14 and the Head (Fig 1c and Extended Data Fig 2f). High mobility resulted in blurring of the hook and Med1 (at the top and bottom ends of the Middle, respectively), and also of the Tail. Local refinement improved the resolution of the hook to ~8 Å (Extended Data Fig 2g), but could not improve Med1 or the Tail. Secondary structure elements were visible throughout the SpMED cryo-EM map. Individual subunits could be resolved and some side-chain densities were apparent in the highest-resolution portions of the map (Med14 and the Head) (Extended Data Fig 3a–c). Various α-helices were apparent around the base and main body of the hook, but we could not assign them to specific subunits. An atomic model including all core Mediator subunits could be built based on the Mediator cryo-EM map and published X-ray structures of Mediator subcomplexes (Fig 1d).
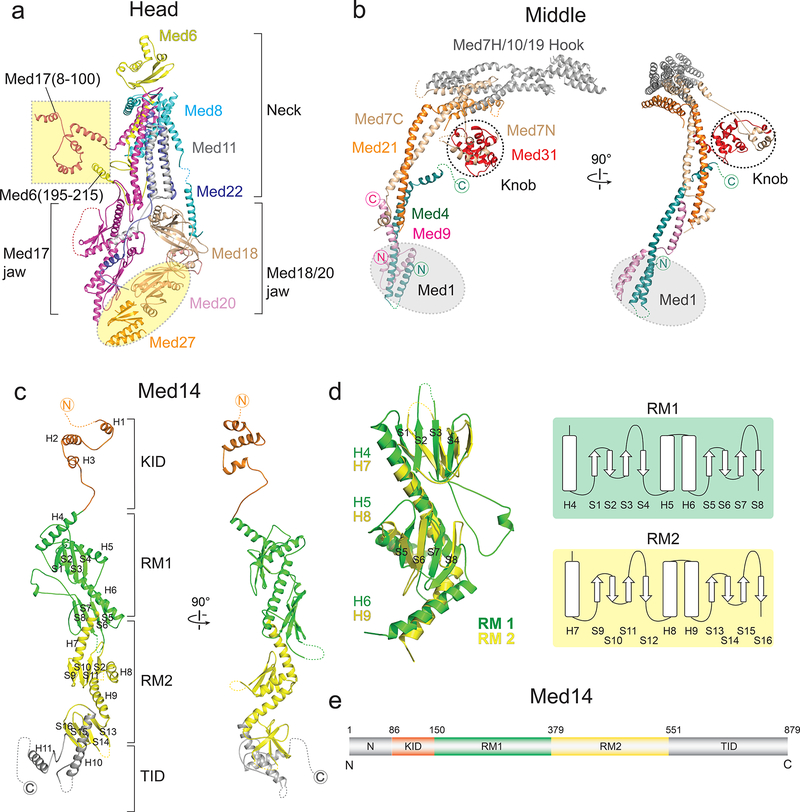
Figure 1. Mediator cryo-EM map and structure.
a, SpMED cryo-EM map at 4.4 Å resolution (Head magenta, Middle blue, Med14 green). b, Local resolution of SpMED cryo-EM map. c, Partial X-ray structure of the SpMED Head [PDB 4H63] docked into SpMED cryo-EM map. d, Atomic model of SpMED including all conserved core Mediator subunits (except Med1).
Head module
We docked the partial crystal structure of the SpMED Head14 into our cryo-EM map, built a model for subunit Med20 starting from the crystal structure of the ScMED Med20, and built a partial model of a putative portion of Med27 ab initio based on the cryo-EM map (Fig 2a and Extended Data Fig 3d). Med27, which is conserved in metazoan Mediator20 extends the Med18/20 jaw, connecting it to Med17 and the central Med14. The SpMED cryo-EM map also revealed the Med17 N-terminus (Med17N) and Med6 C-terminus (Med6C), which were missing in all published crystal structures of the Head (Extended Data Fig 3e). Helical segments at the N-termini of both Med17N and Med6C are involved in inter-module connections. The conformation of the SpMED Head is not altered by interaction with other Mediator modules (Extended Data Fig 3f).
Figure 2. Structures of the Head, Middle, and Med14.
a, Head module. Med20, Med27, Med17(8–100) and Med6(195–215), absent in a previous X-ray structure, are highlighted in yellow. b, Middle module. Non-Middle subunits in the knob (indicated by dashed circle) are not shown. Med1 position highlighted in gray. c, Med14 (81–686) includes two repeats of a structural domain (RM1 and RM2; in green and yellow), a C-terminal Tail interaction domain (TID; in gray), and an extended N-terminal knob interaction domain (KID; in light orange) that forms part of the Middle’s knob. Alpha helices and beta sheets are labeled. d, Overlay of Med14’s RM1 and RM2 and corresponding topology diagrams. Alpha helices and beta sheets labeled as in (c). RMSD values between 136 atom pairs in the RM1 and RM2 portions of Med14 is 5.4 Å. e, Med14 domain architecture.
Middle module
We considered the SpMED cryo-EM map, ScMED Middle module subunit localization results8, sequence alignment between ScMED and SpMED Med7 subunits (Extended Data Fig 3g), published results from XL/MS analysis of ScMED16,19, and a proposed model for Middle organization (based on X-ray structures of Med7 C-terminus (Med7C)/Med21 and Med7 N-terminus (Med7N)/Med31 complexes, secondary structure predictions, and XL/MS results)21 to decipher the structure of the Middle (Fig 2b). The elongated structure of the Middle, largely composed of α-helical elements, and with a “knob” formed by Med7N/Med31 and the N-termini of Med14 and Med17, is generally similar to the previously proposed model21, but differs considerably from it in the conformation and relative orientation of common elements (Extended Data Fig 3h). The overall structure of SpMED Med7C/Med21 is very similar to the X-ray structure of ScMED Med7C/Med2115, but its conformation is different (Extended Data Fig 3i). The overall structures of the SpMED and ScMED Middles are very similar (Extended Data Fig 3j), consistent with considerable sequence homology among their component subunits.
The structure of the SpMED Med7 N-terminus (Med7N)/Med31 knob is also similar to the published X-ray structure of the ScMED knob11 (Extended Data Fig 3k), but Sp Med7N is ~100 residues longer than Sc Med7N (Extended Data Fig 3g) and includes additional helices around the base of the SpMED hook. At the bottom end of the Middle, the N-terminal portions of Med4 and Med9 form a 4-helix bundle adjacent to Med1. A longer Med4 helix extends upwards and contacts Med14 near the knob (Fig 2b). We could not identify density corresponding to the C-terminal portion of Med4, which appears to be disordered (consistent with this, we could not detect an MBP tag engineered into the C-terminus of ScMED Med48). The long Med9 α-helix that starts next to Med1 runs parallel to the longer Med4 helix and both overlap with the bottom end of Med7C/Med21. The extended, overlapping Med7C/Med21 and Med4/Med9 helical bundles explain the elongated and rigid structure of the Middle module.
Med14
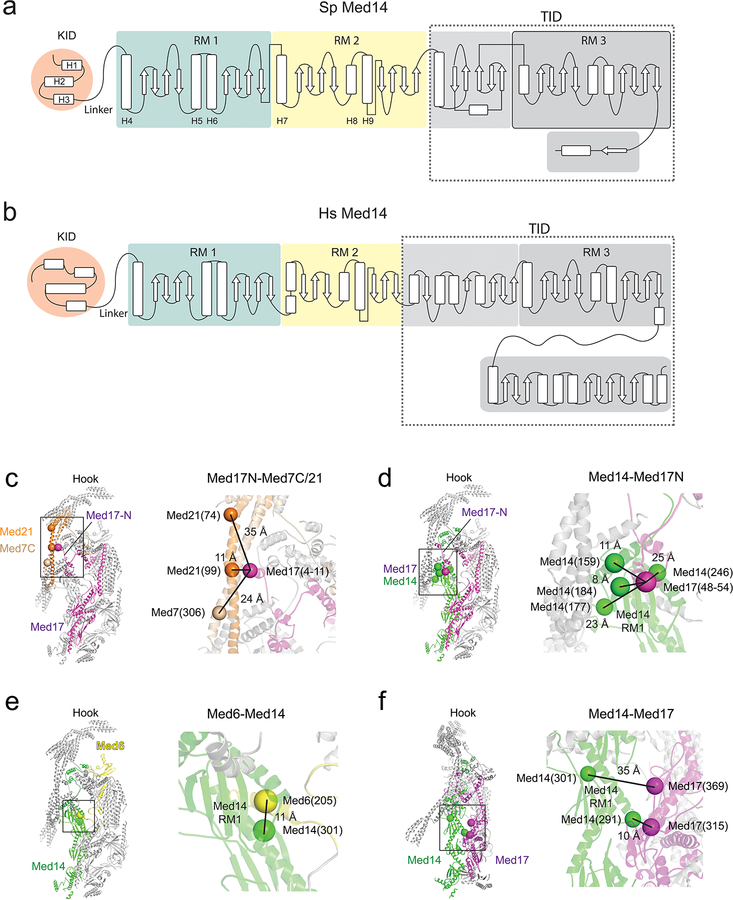
The central Med14 was the best-resolved portion of the SpMED cryo-EM map. We could identify bulky side-chains (Extended Data Fig 3b) and build ab initio an atomic model including most of the Med14 density (~90 N-terminal residues and ~200 C-terminal residues that interact with Med2 and Med15 in the Tail were not well resolved in the cryo-EM map). Although Med14 has a narrow, elongated structure like the Middle, it lacks the large helical bundles that give the Middle its rigidity. An extended portion near the N-terminus forms part of the knob (KID, Knob-interaction domain; residues ~90–150). The KID is followed by two repeats of a motif (RM1 and RM2) in which 3 α-helices and 8 β-sheets are interspersed to form most of Med14 (Fig 2c–d). Secondary structure predictions suggest that a slightly larger third copy of the repeating motif might be present in the C-terminal portion of Med14 and interact with Tail module (TID, Tail interaction domain; residues ~550–879) (Fig 2e and Extended Data Fig 4a). Med14 functions as a backbone, spanning >200 Å down the center of Mediator, connecting the Head, Middle, and Tail modules, and playing a key role in determining Mediator conformation (described below). Conservation of secondary structure organization between S pombe and human Med14s (Extended Data Fig 4b) suggests that the overall structure and function of Med14 should also be conserved.
A combined atomic model of the Head, Middle, and Med14 (Extended Data Video 1) includes 14 SpMED subunits and secondary structure elements corresponding to 2 additional ones (Med10, and Med19). Only Med1 and Tail subunits Med2 and Med15 remain unresolved. This amounts to a near-atomic description of 16 out of 19 core Mediator subunits conserved in Mediator complexes across all eukaryotes. Subunit interactions and critical inter-module contacts revealed by the Mediator atomic model agree with interactions detected by XL/MS analyses of ScMED16,19 (Extended Data Fig 4c–f).
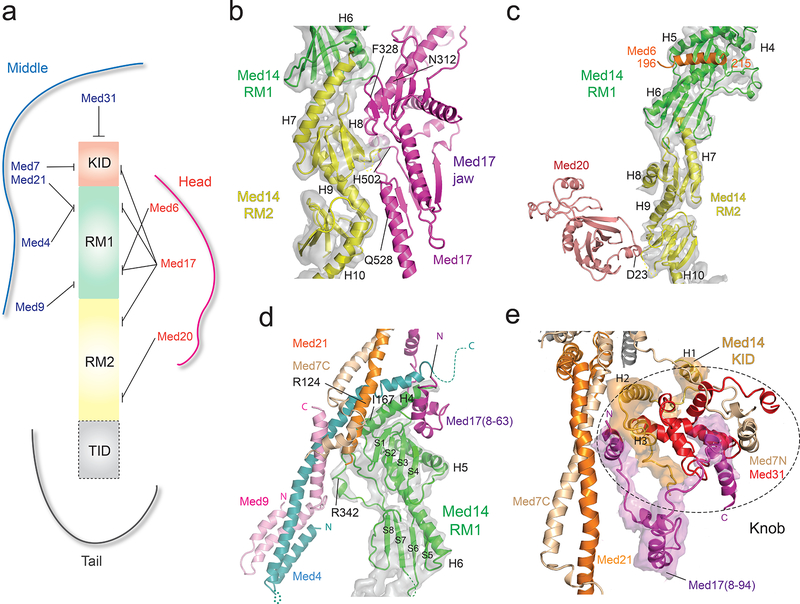
Mediator module interfaces
Consistent with the critical importance of Med14 for Mediator function and interaction with RNAPII18, virtually all Mediator module interactions involve Med14 (Fig 3a). Shape complementarity between Med14 and Med17 results in an extended (~3000 Å2) Med14-Head interface (Fig 3b). Two additional Med14-Head contacts, involving Med20 and the Med6 C-terminus (Fig 3c), are conserved8,19. The Med14-Med20 contact is critical to stabilize the Head. In S cerevisiae, deletion of Med18 and concomitant loss of Med20 results in increased Head’s neck mobility and increased RNAPII interaction8. The top portion of Med14 (RM1) forms a complex Med14-Middle interface, which involves three Middle subunits (Med4/9/21) (Fig 3d). Further work will be required to understand how the C-terminal (bottom) portion of Med14 (TID) interfaces with Med2-Med15 in the Tail.
Figure 3. Mediator inter-module contacts (Med14 density segmented from the SpMED cryo-EM map is shown in semi-transparent gray in panels b-d).
a, Contacts between Med14 and subunits in the Head, Middle and Tail modules. b, Med14-Med17 jaw interface. Selected Med17 residues labeled for reference. c, Contacts between Med14 and Head subunits Med6 and Med20. d, Med14-Middle interface. The N-terminal portion of Med14’s RM1 interacts with portions of Med4, Med7, Med9, and Med21, all of which converge near the center of the Middle module. The N-terminal portion of Med17 also contributes to the Med14-Middle interface. e, Knob structure. The knob’s central core is formed by Med31 (in red). The Med14 KID and Med17N domains cross module boundaries to converge on the knob, whose intricate structure includes elements from the Head, Middle, and Med14. Densities corresponding to Med14 KID and to Med17N were segmented from the SpMED cryo-EM map and are shown in semi-transparent orange and magenta, respectively.
Three inter-module contacts near the top of the Mediator complex show a distinctive type of domain swapping in which two portions of a subunit connected by a flexible linker are bound to different modules. The first such contact involves the previously undetected Med17 N-terminus. From the point where X-ray models of the Head stop, the Med17 N-terminus extends to form 4 short α-helices that interact with the Med7N/Med31 “knob” in the Middle and with the top portion of Med14 (Fig 3e). This Head-Middle connection, detected in XL/MS studies of ScMED16,19 explains the ability of the Head and Middle module to associate in the absence of Med1418 and appears to be destabilized by deletion of Med31, as evidenced by changes in the position of the Head module in class averages of ΔMed31 ScMED8. Second, the Med6 C-terminus, also absent in X-ray models of the Head, is seen extending towards Med14 and forming a helix that is nestled near the top of Med14 (Fig 3c). Third, the Med14 KID (residues ~90–150) extends from the top of Med14 to form three alpha helices that sit between the Middle’s knob and the “hinge” of the Med7C/Med21 helix bundle (Fig 3e).
The complexity of this portion of the SpMED structure likely extends to the hook, because deletion of Med19 (which forms the distal end the hook) results not only in loss of hook density, but also in high variability in the position of the Middle module8. Correspondence between intermodule contacts revealed by the SpMED atomic model and those detected in ScMED XL/MS studies, despite considerable sequence divergence between Sc and Sp Mediators, suggests that intermodule contacts play a critical role in Mediator organization.
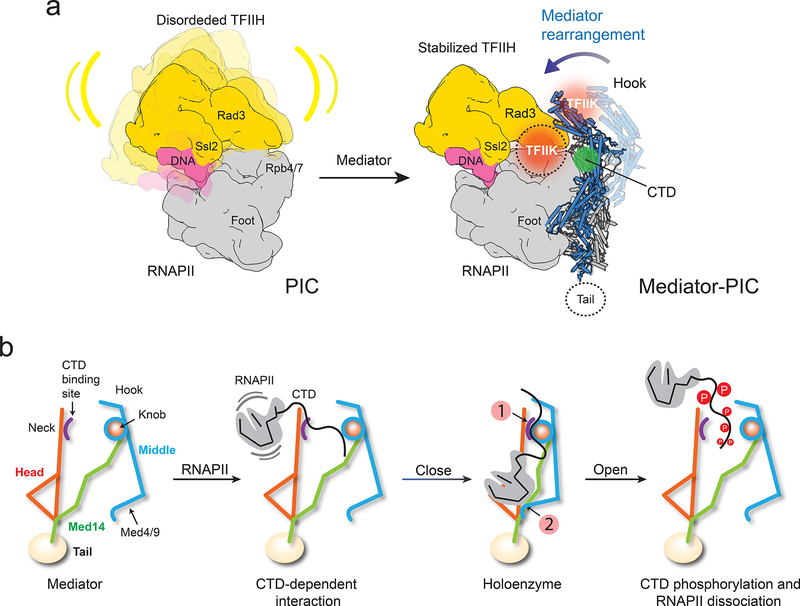
Mediator rearrangement in the holoenzyme
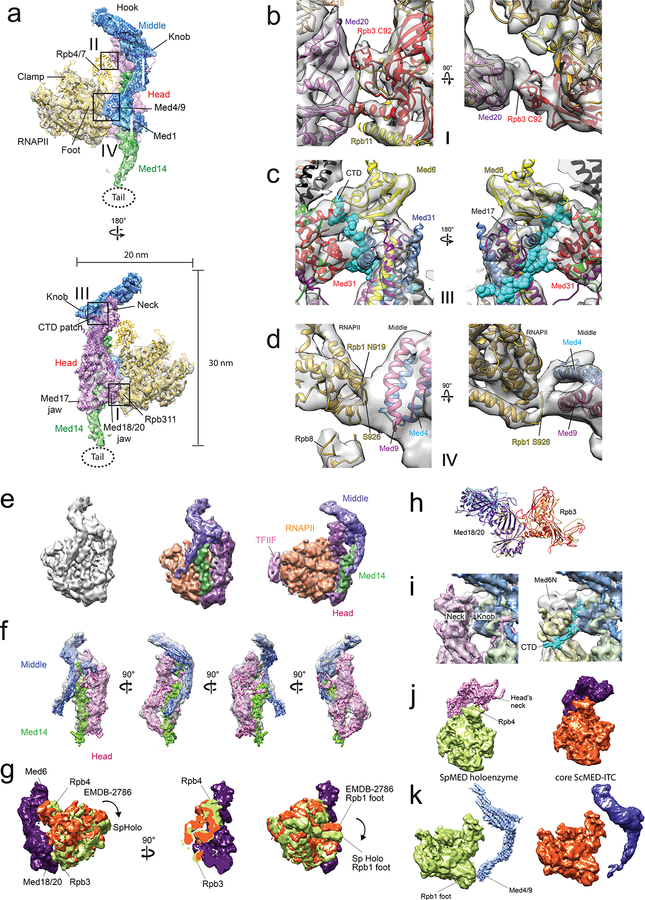
RNAPII and all core Mediator subunits were well-resolved in a 7.8 Å resolution cryo-EM map calculated from images of holoenzyme particles (Supplemental Information, Fig 4a, and Extended Data Fig 5a–d). An atomic model of RNAPII (PDB-3H0G) could be directly fitted into the polymerase portion of the holoenzyme map (Extended Data Fig 5e–h). Individual Mediator modules were also fitted into the Mediator portion of a holoenzyme map and refined to obtain a holoenzyme atomic model (Fig 4a and Extended Data Video 2). The S pombe holoenzyme shows the same overall structure observed in a ~10Å resolution cryo-EM map of a recombinant S cerevisiae core Mediator subcomplex interacting with RNAPII and a minimal set of basal factors (Sc core MED-ITC)16, and in a low resolution cryo-EM map of a S cerevisiae Mediator-preinitiation complex (Sc MED-PIC)22 (Extended Data Fig 5i–k). However, there are differences in detail (Extended Data Fig 6). Most notably, our holoenzyme shows a tight interaction between the four-helix bundle formed by the N-termini of Med4 and Med9 in the Middle module and the polymerase Rpb1 foot (Supplementary Information and Extended Data Fig 6d). It has been reported that deletion of the N-terminal portion of ScMED Med9 (~80 aa, likely corresponding to the two short Med9 helices in the Med4/Med9 4-helix bundle that contacts Rpb1) leaves Mediator intact, but results in a marked decrease in PIC assembly and transcriptional activity23.
Figure 4. Mediator-RNAPII holoenzyme and Mediator rearrangements upon holoenzyme formation.
a, Cryo-EM map of the holoenzyme at 7.8 Å resolution and corresponding atomic model (Head in magenta, Middle in blue, Med14 in green, RNAPII in yellow). Contacts between Mediator and RNAPII highlighted by squares. b, Free Mediator (in yellow) and holoenzyme (Head in magenta, Middle and Med14 in blue, RNAPII in gray) structures compared after matching their Head modules (holoenzyme map in semi-transparent gray). Changes in the position of the Middle’s hook and Med4/9 indicated by arrows. c, Comparing Med14 in free Mediator (yellow) and holoenzyme (blue) shows a large rotation between helices H7 and H9 upon holoenzyme formation. d, Model showing how RNAPII (in gray) would interact with the free form of Mediator (Head in red and Middle/Med14 in yellow). Repositioning of the Middle in the holoenzyme is essential to establish contacts between the CTD-binding patch on the Head’s neck (expected position of the CTD’s main chain interacting with the Head in green) and the knob, and between Med4/9 and polymerase’s Rpb1 foot.
Despite multiple contacts with Mediator, the conformation of RNAPII is not altered in the holoenzyme. Instead, holoenzyme formation is contingent on a substantial change in Mediator conformation (Fig 4b and Extended Data Video 3). In the holoenzyme, the structures of the Head and Middle remain constant (Extended Data Fig 7a–b), but their relative orientation is very different due to considerable changes in the conformation of Med14. The lower portion of Med14 remains constant, likely due to extensive interaction with Med17 in the Head (Fig 3b). However, the unique structure of Med14 allows its top portion to move considerably, resulting in a large change in the position of Med14’s RM1 and KID domains (Fig 4c and Extended Data Fig 7c). The structure of the Med14-Middle interface remains unchanged (Extended Data Fig 7d), causing the position of the Middle to track changes in the position of the top half of Med14. Importantly, the extended Med17N and Med6C that interact with the top portion of Med14 move with it (Extend Data Fig 7e). This organization of module junctions in and around the knob provides an important illustration of the way in which Mediator structure determines its conformational behavior: changes in Mediator conformation observed upon holoenzyme formation are limited to what can be enabled by Med14 and accommodated by flexible inter-module linkers.
Repositioning of the Middle in the holoenzyme causes the hook to move by ~50 Å, decreasing the distance between the Head’s neck and the Middle’s knob from ~25 Å to ~10 Å. At the opposite end of the Middle, the N-terminal portions of Med4 and Med9 move to contact the polymerase Rpb1 foot (Fig 4d and Extended Data Fig 7f). Therefore, the rigidity of the Middle connects changes at two critical Mediator-RNAPII contacts (we have never observed a Mediator conformation in which the Head-Middle gap was closed and the Rpb1 contact open, or vice versa). Analogous changes in Mediator conformation take place upon formation of the S cerevisiae holoenzyme (Extended Data Fig 7g–i), but were perhaps missed in other studies16 due to the lack of a higher resolution map of free ScMED.
A critical implication of our holoenzyme structure is that narrowing of the gap between the knob and a conserved patch14 on the Head’s neck creates a CTD-binding site. It was reported that deletion of the N-terminal portion of Med7 (Med7N), deletion of Med31, or deletion of both Med7N and Med31 result in slow-growth phenotypes11. A possible connection between knob components and the CTD was also implied by the observation that deletion of Med31 is synthetically lethal with a truncation of the CTD to only 11 heptad repeats24. It was suggested16,22, based on consideration of the crystal structure of a Head-CTD peptide complex13, that the CTD could bind between the Head and the knob in the holoenzyme. However, the dependence on a Mediator rearrangement was overlooked and the proposition could not be tested without determining the precise orientation of the Med7N/Med31 knob in the holoenzyme and the knob’s contribution to CTD-interaction. We found that deletion of Med31 (expected to disrupt interaction of the CTD between the knob and the Head’s neck) resulted in a considerable decrease in Mediator-RNAPII interaction (Extended Data Fig 8a). Matching the X-ray structure of the S cerevisiae Med7N/Med31 subcomplex to the corresponding portion of our holoenzyme structure we identified conserved Med31 residues expected to be in close contact with the CTD (Extended Data Fig 8b–c) and investigated the effect of mutating those residues. Three different point mutations targeting conserved residues in a short Med31 helix (or immediately adjacent to it) that our holoenzyme model predicts should contact the CTD (Extended Data Fig 8b) resulted in decreased Mediator-RNAPII interaction (Extended Data Fig 8d). In contrast, mutation of two residues in a loop following the short helix, or mutation of a residue far removed from the CTD (Extended Data Fig 8b) had no effect on Mediator-RNAPII interaction (Extended Data Fig 8d). None of the Med31 point mutations had an effect on knob stability (Extended Data Fig 8e).
Mediator initiation regulation mechanism
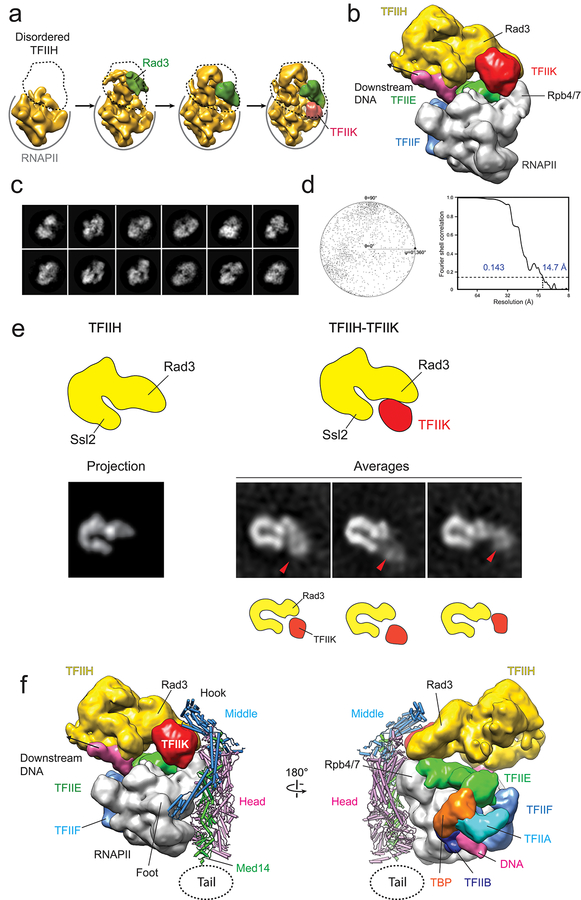
How Mediator can enhance basal transcription and facilitate CTD phosphorylation is explained by considering the holoenzyme-PIC interaction. A striking characteristic of PICs revealed by cryo-EM studies is high mobility of TFIIH, consistent with previous reports of high TFIIH flexibility25. Full engagement of TFIIH with TFIIE and promoter DNA is observed in only a fraction of PIC particles17,26 (Extended Data Fig 9a), and is facilitated by the presence of the TFIIK kinase responsible for CTD phosphorylation26,27.
Through 3D image clustering of a published S cerevisiae PIC cryo-EM dataset26, we were able to identify a subset of PIC particles with well-ordered TFIIH, and in which previously undetected TFIIK density is immediately adjacent to the Rad3 TFIIH helicase (Extended Data Fig 9b–d). This TFIIK position is consistent with reported Rad3-TFIIK interaction25 and is observed in images of TFIIH particles preserved in stain (Extended Data Fig 9e).
Matching the holoenzyme and PIC-TFIIK cryo-EM maps by overlapping the unchanged RNAPII portions of both reveals a remarkable shape complementarity. The hook contacts the Rad3 helicase and TFIIK kinase (Extended Data Fig 9f). Consistent with this model, it has been reported that the CTD kinase activity of TFIIK is not enhanced by an otherwise intact Mediator lacking Med1928.
Previous suggestions about the way in which Mediator could stabilize TFIIH conformation and exert an effect on basal transcription and CTD phosphorylation16,29 are bolstered by analysis of the holoenzyme structure and its anticipated interaction with a complete PIC (Fig 5a). Comparing the Mediator and holoenzyme structures indicates that Mediator-TFIIH interaction and PIC stabilization are entirely dependent on a rearrangement of the Mediator structure to its holoenzyme conformation. Further studies will be needed to determine if interaction with Mediator could direct TFIIK to an alternative position on the opposite side of the hook, as observed in some TFIIH averages (Extended Data Fig 9e).
Figure 5. Mediator stabilization of the PIC and rearrangements that orchestrate RNAPII interaction.
a, TFIIH (yellow) is highly mobile in the PIC (left). Repositioning of the Middle upon holoenzyme formation (Mediator Middle in light blue, holoenzyme Middle in dark blue) would stabilize TFIIH (right) by bringing Mediator’s hook into direct contact with TFIIH’s Rad3 and TFIIK. TFIIH stabilization would explain enhancement of basal transcription and CTD phosphorylation by Mediator. A cryo-EM map of the PIC and TFIIH 2D class averages show TFIIK as indicated (orange circle). An alternate TFIIK position on the opposite side of the hook (faded orange circle) observed in some TFIIH 2D averages could be favored upon Mediator interaction. Either placement would put TFIIK immediately adjacent to its CTD target (CTD position indicated by green circle). b, CTD binding at a site accessible in free Mediator would initiate RNAPII interaction, help trigger a change to the holoenzyme conformation (with a closed CTD-binding gap and the CTD engaged between the Head’s neck and the knob (indicated by number 1 in a red circle), enable a Mediator-Rpb1 foot contact (indicated by number 2 in a red circle), stabilize RNAPII/PIC interaction and position the CTD for phosphorylation by TFIIK. After initiation, CTD phosphorylation by TFIIK would help revert Mediator to its free conformation, with an open CTD-binding gap and movement of Med4/9 away from Rpb1. This would disrupt Mediator interaction with RNAPII/PIC and facilitate the transition to elongation.
Conclusions
Near-atomic resolution cryo-EM maps of Mediator and holoenzyme reveal critical details of their structures, as well as Mediator rearrangements essential for holoenzyme formation. A pliable Med14 enables large-scale changes in Mediator conformation. Repositioning of the Middle module upon holoenzyme formation links two polymerase contacts (Rpb1 foot and the CTD) and is expected to enable interactions with the Rad3 helicase and TFIIK kinase of TFIIH in the PIC. Conformational changes, and structural crosstalk that allows various Mediator-PIC contacts to reinforce one another, are likely essential to Mediator’s ability to control initiation and integrate regulatory signals. Conservation of the Mediator and PIC structures8,26,29,30 suggests that this should hold true across eukaryotes.
We found two Mediator-RNAPII interaction modes. Variable RNAPII binding around the hook is consistent with an essential CTD requirement for Mediator interaction31–33 and likely represents the initial Mediator-RNAPII contact mode. A transition to the holoenzyme Mediator conformation, perhaps facilitated by basal factors that enter the PIC early on, would enable the multiple Mediator-RNAPII contacts required for stable Mediator-RNAPII interaction, facilitate TFIIE and TFIIH recruitment34, stabilize the PIC, and enhance CTD targeting by the TFIIK kinase1. Crosstalk between Middle-RNAPII contacts could be important for the transition to elongation33. CTD phosphorylation would reverse binding at the CTD-binding gap and help destabilize the Rpb1 contact, facilitating RNAPII release from the PIC (Fig 5b).
We expect that the ability to access different conformational states is essential for the Mediator mechanism. Factors involved in regulation of activated transcription are likely to act by exploiting further Mediator conformational changes, for example through internal flexibility of the Head or through Tail rearrangements facilitated by Med14’s C-terminal domain.
Methods
S. pombe strains.
The gene manipulations necessary to generate fission yeast strains required for mass spectrometry and EM analyses were carried out using standard protocols. A PCR-based genomic epitope-tagging method was used to construct subunit deletion strains. The strains used in this study, all derived from the standard 972h- S. pombe strain, are listed in Extended Data Table 1a. Strains used for MudPIT analysis were C-terminally FLAG-tagged on Med4, Med7, Med2(SPCC4F11.03c), or Med15 by replacing the stop codons of each gene with sequences encoding 3x FLAG followed by a stop codon and the NatMX6 marker as described35. Strains used for EM studies were derived from a med7-TAP-med13Δ strain (CG27, med7+::TAP-kanMX6 med13::kanMX436. Deletion of Med13 prevented interaction of core Mediator with the dissociable CDK8 module, which would have interfered with EM imaging. For subunit localization, Med2 or Med27 were deleted from the CG27 strain by replacing the coding region of the appropriate gene with the NatMX6 marker.
Mass spectrometry.
S. pombe Mediator preparations used for MudPIT mass spectrometry were purified by anti-FLAG immunopurification from 972h- derivatives expressing FLAG-tagged Mediator subunits. FLAG-immunopurified proteins were treated with benzonase, TCA precipitated, denatured, reduced and alkylated, digested with endoproteinase Lys-C and trypsin, and analyzed by MudPIT mass spectrometry as described37,38. 972h- cells or 972h- derivatives expressing FLAG-tagged Mediator subunits were grown at 32 °C in rich medium (YES) supplemented with adenine, histidine, leucine, and uracil (225 μg/ml). Cells were harvested at mid-log phase, washed in cold H2O, and washed in buffer containing 50 mM Tris-HCl (pH7.5), 150 mM NaCl, 0.1% NP40, and 10% glycerol with protease inhibitor cocktail (Sigma cat. no. P8849). Cells were pulverized in liquid nitrogen using a mortar and pestle and then re-suspended in the same buffer. Whole cell lysates were centrifuged at 35,000 x g to remove cell debris. Supernatants were incubated overnight at 4 °C with anti-FLAG agarose beads (Sigma, 500 μl beads / 108 starting cells) that had been pre-equilibrated in 50 mM Tris-HCl (pH7.5), 150 mM NaCl, 0.1% NP40, and 10% glycerol with protease inhibitor cocktail. Beads were washed four times with approximately 10 bead volumes of buffer containing 50 mM Tris-HCl (pH 7.5), 300 mM NaCl, and 0.1% NP40, and bound proteins were eluted using 0.2 mg/ml 3X Flag peptide (Sigma) in 50mM Tris-HCl, 300 mM NaCl, and 0.1% NP40. FLAG-immunopurified proteins were treated with benzonase and TCA precipitated prior to analysis by multidimensional protein identification technology (MudPIT), as described previously.
SpMED proteins were identified using a modification of the MudPIT procedure38. TCA-precipitated proteins were urea-denatured, reduced, alkylated and digested with endoproteinase Lys-C (Roche) followed by modified trypsin (Roche) as described37. Peptide mixtures were loaded onto 100 μm fused silica microcapillary columns packed with 5-μm C18 reverse phase (Aqua, Phenomenex), strong cation exchange particles (Partisphere SCX, Whatman), and reverse phase (McDonald et al., 2004). Loaded microcapillary columns were placed in-line with an LCQ or LTQ ion trap mass spectrometer equipped with a nano-LC electrospray ionization source (ThermoFinnigan). Fully automated MudPIT runs were carried out on the electrosprayed peptides38. Tandem mass (MS/MS) spectra were interpreted using SEQUEST39 against a database of a database of S. pombe proteins (downloaded from NCBI on 2012-01-23), and complemented with 177 sequences from usual contaminants (human keratins, IgGs, proteolytic enzymes). To estimate false positive discovery rates, each sequence was randomized keeping amino acid composition and length the same, and the resulting “shuffled” sequences were added to the “normal” database (doubling its size) and searched at the same time.
Peptide/spectrum matches were sorted and selected using DTASelect40 with the following criteria set: spectra/peptide matches were only retained if they had a DeltaCn of at least 0.08, and minimum XCorr of 1.8 for singly-, 2.0 for doubly-, and 3.0 for triply-charged spectra. In addition, peptides had to be fully-tryptic and at least 7 amino acids long. Combining all runs, proteins had to be detected by at least 2 such peptides or by 1 peptide with 2 independent spectra. Under these criteria, the overall false discovery rate was less than 0.2%. Peptide hits from multiple runs were compared using CONTRAST40. To estimate relative protein levels, distributed Normalized Spectral Abundance Factors (dNSAFs) were calculated for each detected protein41–44.
S. pombe Mediator purification for EM studies.
All of the Mediator used for EM studies was purified essentially as described before9. To purify SpMED for EM studies, each TAP-tagged fission yeast strain was grown at 31°C to an OD600 of ~3 in 2xYPD media. After harvesting by low-speed centrifugation, cells were washed with double deionized water and frozen in liquid nitrogen. Frozen cells were broken by blending with dry-ice under liquid nitrogen. Whole-cell extract was prepared as previously described45 starting from 100g of broken cell powder. The whole-cell extract was precipitated in 55% ammonium sulfate and, after spinning down, the pellet was resuspended using buffer A (20mM Hepes pH 7.4, 300mM KOAc, 0.5mM EDTA, 10% Glycerol, 0.05% NP-40, 5mM betamercaptoethanol, and protease inhibitors). After the suspension was clarified by centrifugation, the supernatant was incubated for 2 hr at 4°C with 0.5 ml of IgG-sepharose resin beads (GE Healthcare). After incubation, the beads were washed with buffer A (including a dose of fresh protease inhibitors) and then with buffer A including 1mM DTT. 100 units of AcTEV protease (Invitrogen) was added to the beads and they were incubated with the protease overnight at 4°C. SpMED was then eluted with three column volumes of buffer A including 1mM DTT. For the next purification step, the IgG eluate fractions were diluted 5-fold with buffer B (20mM Hepes pH 7.4, 300mM KOAc, 10% Glycerol, 0.05% NP-40, 5mM β-ME, 1mM imidazole, 1mM MgCl2) containing 1.5mM CaCl2 and incubated with 200μL of calmodulin affinity resin (STRATAGENE) for 2 hr at 4°C. The resin beads were washed with 10 mL of buffer B including 1.5mM CaCl2. The purified SpMED complex was eluted with buffer B containing 5mM EGTA at room temperature and flash-frozen in liquid nitrogen.
SpMED subunit localization experiments.
Stained specimens of ΔMed2 and ΔMed27 SpMED (~10–25 μg/ml, in a buffer containing 20 mM HEPES buffer (pH 7.4), 100 mM potassium acetate, 3mM β-Mercaptoethanol, 2% glycerol, and 0.01% NP-40) were preserved with 0.75% (w/v) uranyl formate. Images of stained specimens were recorded at a magnification of 52,000X on a 4,096 × 4,096 CMOS detector (Tietz, Inc.) using a Tecnai Spirit electron microscope (FEI) equipped with an LaB6 filament and operating at an acceleration voltage of 120 kV. Images were automatically acquired with Leginon46. Particle picking was carried out using the DoG Picker program included in Appion47. Two-fold pixel binning of the stained particle images resulted in a final pixel size of 4.1 Å. Image alignment and classification were carried out using the Iterative Stable Alignment and Clustering (ISAC) program implemented in the SPARX software package48.
Cryo-EM data collection and image analysis.
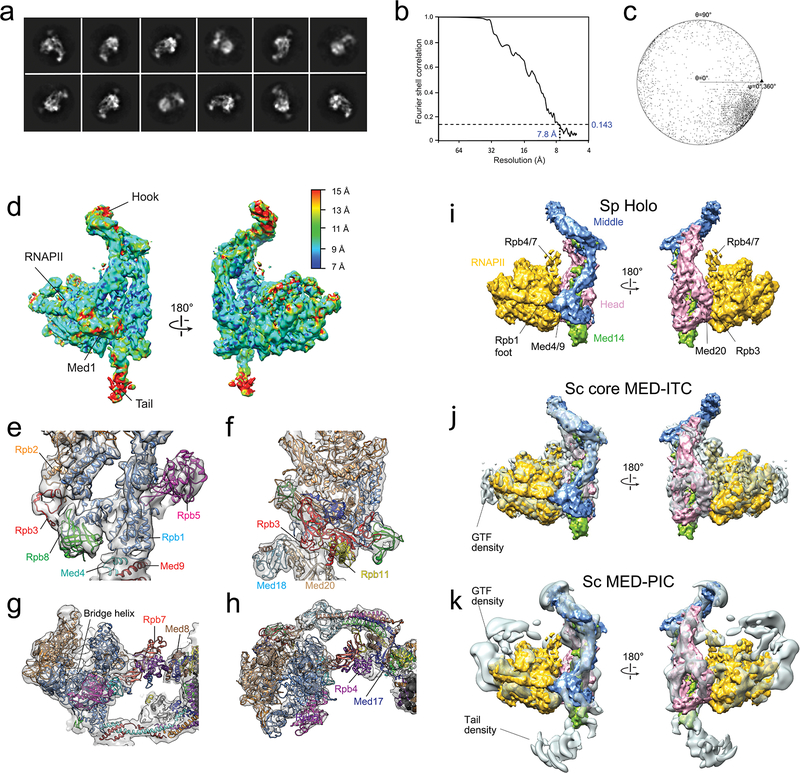
For preparation of cryo-EM samples, 3 uL of ΔMed13 SpMED (~1 mg/ml, in a buffer containing 20 mM HEPES buffer (pH 7.4), 200 mM potassium acetate, 5mM β-Mercaptoethanol, and 0.01% NP-40) were applied to plasma-cleaned 400 mesh C-flat carbon grids with 2.0μm holes (EM Sciences) using a Solarus plasma cleaner (Gatan), incubated on the grid for 30 s at 4°C, and then manually blotted and vitrified in liquid ethane49. Images were automatically acquired with Leginon46 and recorded on a K2 Summit direct electron detector (Gatan, Inc.) operating in counting mode, using a Titan Krios electron microscope (FEI) operating at a 300 kV accelerating voltage. Images were recorded at 1.0–4.0 μm underfocus values and a magnification of 22,500X, which resulted on a pixel size of 1.31 Å. Total electron dose for each image was ~40 electrons Å−2 over a 7 s exposure time, fractionated into 35 frames. Dose-fractioned frames were aligned50 and contrast transfer function parameters for each image were determined using the programs CTFFIND351. Particle picking was carried out using template-based picking as described for stained specimens, except that a larger (~10,000) initial image set was used to generate separate template image sets for Mediator, holoenzyme, and RNAPII. An initial SpMED 3D map was obtained using as reference an Sc MED cryo-EM map8 low-pass filtered to 60 Å and masked to hide the Tail module. The image analysis procedure included repeated rounds of 2D clustering and 3D classification to eliminate non-particle images, separate images into free Mediator, free RNAPII, Mediator-RNAPII holoenzyme (multiple Mediator-RNAPII contacts), and Mediator with RNAPII loosely bound near the hook. Further 3D classification was used to select subsets of Mediator, free RNAPII, and holoenzyme images that would result in the highest-resolution cryo-EM maps (Extended Data Fig 2a). For example, for SpMED, a total of 235,337 automatically-selected cryo-images were screened using multi-variate statistical analysis and multi-reference analysis tools implemented in Appion47. After particle screening a final stack with ~96,374 SpMED images was separated into 4 classes using 3D classification with Relion52. Two of those classes were merged to obtain a set of 67,462 images that were again screened by 2D clustering to obtain a final set of 42,484 SpMED images (70% of the initial 96,000 Mediator images identified by sorting) that were run through 3D refinement and movie processing in Relion to obtain the final 4.4 Å resolution SpMED cryo-EM map using a mask that blocked out the flexible hook, Tail, and Med1 (Extended Data Fig 2f). A similar procedure identified a set of 11,433 RNAPII images (57% of the initial 19,000 polymerase images identified by sorting) used to calculate a map at 4.3 Å resolution (not shown). Classification of holoenzyme images followed by calculation of 3D maps shows that the orientation of RNAPII in the holoenzyme is always the same, but that there are slight variations in overall conformation of the holoenzyme that limit the resolution of the cryo-EM maps (Extended Data Fig 2b). The most homogeneous subset of holoenzyme images included 3,862 holoenzyme images (35% of the initial 11,000 holoenzyme images identified by sorting) that resulted in a map at 7.8 Å resolution. All resolutions reported here correspond to gold-standard Fourier shell correlation (FSC) values using the 0.143 criterion53. Due to variability in the position of RNAPII loosely associated with the hook, we calculated 2D class averages but did not try to obtain a 3D map.
SpMED and holoenzyme modeling and refinement.
To build the Mediator atomic model, we started by rigid-body fitting the crystal structure of the S. pombe Head module14 (PDB-4H63) and a model of the Middle module54 into the SpMED cryo-EM map using Chimera55. For regions of the Mediator map for which neither a structure nor a model were available, we built the model ab initio based on the density map and results from secondary structure prediction. To build the holoenzyme model, we started by fitting the crystal structure of S. pombe RNAPII56 (PDB-3H0G) and our Mediator atomic model into holoenzyme cryo-EM map using Chimera55. The Head and Middle portions of the Mediator atomic model could be fitted into the holoenzyme map without modification, by simply adjusting their relative orientation. The Med14 portion of the Mediator model could not be fitted directly into the holoenzyme map, but had to be rearranged to account for changes in Med14 structure upon holoenzyme formation. Rigid-body fitting of S. pombe RNAPII crystal structure results in a slight crash between Rpb4 (residues 127–135) and Med17 (residues 138–142). Therefore, the position of Rpb4/Rpb77 was rotated slightly (~10°) to fit into the holoenzyme map. Model adjustments were done using Coot57. Refinement of the Mediator and holoenzyme models against the respective cryo-EM maps was done by using the real space refinement function implemented in Phenix58. Final models were validated using MOLPROBITY59 (Extended Data Table 1b).
Functional validation of Mediator-RNAPII contacts in the Sc holoenzyme.
Budding yeast strains in which Med31 was deleted, or carrying Med31 point mutations, were prepared to test the functional significance of Mediator-RNAPII contacts in the holoenzyme. Med31 point mutant strains were obtained by using a one-step gene replacement method60 to introduce point mutations in the Med31 allele of a Med22-TAP tagged strain. Briefly, Med31 was C-terminally FLAG-tagged by replacing the stop codon with sequences encoding 5xFLAG and the KanMX6 marker. Overlapping PCR fragments bearing Med31 with targeted point mutations and the KanMX6 selection marker were transformed into the Med22-TAP tagged strain. Colonies carrying the mutations were confirmed by DNA sequencing. The resulting strains, all derived from strain BJ2168, are listed in Extended Data Table 1a.
S. cerevisiae Mediator purification.
Wild-type, ΔMed31, and Med31 point mutant Mediator complexes were immunopurified from cell extracts prepared from the corresponding yeast strains through the TAP-tag on Med22 using IgG Sepharose6 and calmodulin resins as described previously8. Briefly, the total protein concentrations of supernatant recovered from an ammonium sulfate precipitation pellet obtained from 4L of yeast culture was estimated using the Bradford protein assay (Bio-Rad) and supernatant containing 600 mg protein was incubated with 80 μl of IgG Sepharose 6 resin for 3 hours at 4°C. The beads were collected by centrifugation at 150xg and then washed a total of six times by rotating for 4 minutes with 5 ml of washing buffer (20mM HEPES-KOH pH 7.6, 0.3M KOAc, 10% Glycerol, 0.5 mM EDTA, 0.05% NP-40, 3mM b-ME; the first 3 times with protease inhibitors and the last 3 times without protease inhibitors) and collected again by centrifugation. After washing, the beads were incubated on a rotating mixer with 400 μl of buffer (20mM HEPES-KOH pH 7.6, 0.3M KOAc, 10% Glycerol, 0.5 mM EDTA, 0.05% NP-40, 1mM b-ME, and 1.5 mM DTT) containing AcTEV protease (20U, ThermoFisher Scientific) at 4°C for 16 hr. The beads were separated by centrifugation from eluted Mediator in the supernatant. The IgG elute proteins were diluted 3-fold with buffer C (20mM Hepes pH 7.4, 300mM KOAc, 10% Glycerol, 0.05% NP-40, 5mM β-ME, 1mM imidazole, 1mM MgCl2) containing 1.5mM CaCl2,. and incubated with 20μL of calmodulin affinity resin (STRATAGENE) for 1 hr at 4°C. The resin beads were washed a total of three times with 1.5 mL of buffer C including 1.5mM CaCl2. The purified complex was eluted with buffer C containing 5mM EGTA. Purified Mediator complexes were resolved in 4–20% gradient SDS-PAGE WedgeWell gels (Thermo Fisher Scientific), along with supernatant recovered from ammonium sulfate precipitation pellets used for normalization, and then transferred onto Pre-Cut Nitrocellulose or PVDF membranes (Thermo Fisher Scientific). Mediator and RNAPII bands were identified by western blotting using 8WG16 (ab817) (AbCam), anti-Med2 (sc-28058) (Santa Cruz Biotechnology), anti-FLAG M2 (F1804) (Sigma-Aldrich), anti-CBP (sc-33000) (Santa Cruz Biotechnology), horseradish peroxidase anti-peroxidase (PAP) (P1291) (Sigma-Aldrich) and HRP-conjugated anti-goat IgG (sc-2768) (Santa Cruz Biotechnology). Protein signals were visualized either by chemiluminescent detection using SuperSignal West Pico or Femto Substrates (ThermoFisher Scientific),
Data availability
Cryo-EM maps and atomic coordinates have been deposited with the Electron Microscopy Data Bank (with accession codes 8479 and 8480) and Protein Data Dank (accession codes 5U0P and 5U0S). The original MudPIT data (raw files, peak files, search files, as well as DTASelect result files, and protein sequences fasta file) can be accessed from the Stowers Original Data Repository at: http://www.stowers.org/research/publications/libpb-1070.
Extended Data
Extended Data Figure 1. S cerevisiae and S pombe Mediator subunit localization.
a, Localization of S cerevisiae Mediator subunits. b. Coomassie blue-stained SDS-PAGE analysis (4–20% gradient gel) of purified wild-type, ΔMed2, and ΔMed27 S pombe Mediator. The Med2 band is comparatively weaker, suggesting that the subunit might be substoichiometric in purified wild-type SpMED. Deletion of Med2 results in loss of Med27, but not the other way around. The band marked with the asterisk corresponds to Rpb1, which was confirmed by mass spectrometry. Subunits are colored by module (Head, pink; Middle, blue; Tail, gray) c. 2D class averages for wild-type, ΔMed2, and ΔMed27 SpMED, and color-coded (by standard deviation values from the average) difference maps indicating the position of Med2 and Med27 (highlighted by yellow arrow heads). d, Wild-type SpMED class averages and a close up showing a Med27-Tail connection (lower-right arrowhead) comparable to the connection between the Med4/Med9 4-helix bundle and the rest of the Middle module (upper-left arrowhead). e, A raw micrograph showing a typical area of a SpMED cryo-EM sample.
Extended Data Figure 2. 2D clustering, 3D classification, and refinement of Mediator and holoenzyme images recorded from SpMED cryo-EM samples.
a, Particle images were clustered into homogeneous classes after automated picking. Clustering and alignment showed that a fraction (~29%, green box) of particle images initially picked as possible “holoenzyme” correspond to images of particles in which RNAPII, loosely tethered to Mediator, dominates alignment and blurs Mediator density (variable Mediator position suggested by pasted semi-transparent Mediator averages), preventing calculation of reliable 3D maps showing the moving RNAPII bound around the Mediator hook. b, Cryo-EM holoenzyme maps calculated from 3 different subsets of holoenzyme images all show the same Mediator and RNAPII conformations and relative orientations, with differences limited to the amount of detail apparent in each map. c, SpMED 2D cryo class averages. d, FSC plot and resolution estimation using the gold-standard 0.143 criteria for the SpMED cryo-EM map. e, Angular distribution plot for the SpMED cryo-EM image set. f, Focused refinement (mask indicated by dashed red line) of the SpMED core by masking out the hook, Med1, and Tail portions. g, Local refinement of the top portion of the Middle module.
Extended Data Figure 3. Cryo-EM map and structure of SpMED Head and Middle modules.
a, Head portion of the SpMED cryo-EM map showing secondary structure elements corresponding to individual subunits. The resolution of the Head portion of the map 4.5–5.0 Å. b, Med14 portion of the EM map and close-ups showing density corresponding to bulky Med14 side chains. The resolution of the Med14 portion of the map 4.0–4.5 Å. c, Middle portion of the SpMED cryo-EM map showing secondary structure elements corresponding to various subunits, and map segments corresponding to individual Middle components. The resolution of the Middle portion of the map and its segments is 5–6 Å. d, Cryo-EM structure of the Head module. Colors as indicated in Fig 1c. e, Partial X-ray structure of the Head module (PDB 4H63; left, in gray) compared with the cryo-EM structure of the Head module (right) with portions not included in the X-ray crystal structure colored and labeled. f, Superposition of the X-ray (gray) and cryo-EM (green) structures of the Head module shows a close correspondence between common elements, indicating that the overall conformation of the Head is not changed by interaction with other Mediator modules. RMSD values between 744 Cα atom pairs in corresponding portions of the X-ray and EM structures of the Head is 2.1 Å. g, Alignment of SpMed7 and ScMed7 protein sequences. The secondary structure evident in the cryo-EM structure of SpMed7 is indicated. Identical and similar residues are highlighted in green and yellow, respectively. Residues in S pombe Med7 expected to be part of the hook are highlighted in light blue. h, Cryo-EM structure of the Middle (left) and a model of the Middle41 based on X-ray structures of Med7C/Med21 and Med7N/31, homology modeling of Med4/9/10, and results from cross-linking mass spec analysis (right). i, Comparison between the X-ray structure of Sc Med7N/21 (gray) and the Sp Med7N/21 from the cryo-EM map. j, Fitting of the Sp Middle module structure (solid blue) into the Sc Mediator cryo-EM map (transparent yellow) shows that the Middle structure is conserved between S cerevisiae and S pombe. k, Comparison between the cryo-EM structure of SpMed7N/31 and the X-ray structure of ScMed7N/31 shows that this portion of the Mediator structure is highly conserved. RMSD values between Cα 84 atom pairs in corresponding portions of the X-ray structure of ScMed 7N/31 (PDB 3FBI) and the EM structure of SpMed 7N/31 is 2.0 Å.
Extended Data Figure 4. Secondary structure predictions for yeast and human Med14 proteins, and correspondence between published XL/MS analysis of ScMED and the atomic model based on the cryo-EM map of SpMED.
a, Secondary structure for S pombe Med14. b, Secondary structure for H sapiens Med14. The C-terminal portion of human Med14 is comparatively larger. c, Med17N cross-links with Med7N-Med21. d, Med14 (RM1) cross-links with Med17N. e, Med6C cross-links with Med14(RM1). f, Med14 (RM1) cross-links with Med17 (Med17 jaw). The residue number/ranges shown in the figure are those corresponding to Sp Mediator subunits. For all residue pairs in the SpMED atomic model corresponding to crosslinked residues in the XL/MS analysis of ScMED, the distance between residues in a pair is below 35 Å (the maximum expected distance between crosslinked residues based on the structure of the crosslinking reagent).
Extended Data Figure 5. Cryo-EM analysis of the Sp holoenzyme and comparison with Sp RNAPII X-ray structure [PDB 3H0G] and Sc core MED-ITC and Sc MED-PIC cryo-EM maps.
a, 2D holoenzyme cryo-EM class averages. b, FSC plot for the holoenzyme cryo-EM map. c, Angular distribution plot for the holoenzyme cryo-EM map. d, Local resolution values in the holoenzyme cryo-EM map. e, The Rpb1 foot portion of PDB-3H0G, and neighboring domains, fitted into the holoenzyme map. f, The Rpb3/Rpb11 portion of PDB-3H0G, and neighboring domains, fitted into the holoenzyme map. g, A slice through the central portion of a front view of PDB-3H0G shows the bridge helix and comparatively weak Rpb4/Rpb7 density making contacts with Med8 and Med17 in the Head. h, A slice through the central portion of a top view of PDB-3H0G shows Rpb4/Rpb7 contacting Med8 and Med17. i, Sp holoenzyme segmented into modules. j, Sc core MED-ITC with modules from Sp holoenzyme fitted in. The gap between Med4/9 and the Rpb1 foot in Sc core MED-ITC is hidden by the fitted Sp holoenzyme Middle. k, Sc MED-PIC (EMDB-8307) with modules from Sp holoenzyme fitted in. There is no gap between Med4/9 and the Rpb1 foot in Sc MED-PIC.
Extended Data Figure 6. Cryo-EM map and model of the Mediator-RNAPII holoenzyme, and comparison with the Sc core MED-ITC cryo-EM map (EMDB-2786).
a, Cryo-EM map of the holoenzyme at 7.8 Å resolution and corresponding atomic model (Head in magenta, Middle in blue, Med14 in green, RNAPII in yellow). Contacts between Mediator and RNAPII highlighted by squares. b, Rpb3/Rpb11 interaction with Med20 in the Head’s movable jaw. Rpb3’s C92, important for RNAPII interaction with Mediator, is indicated. Med20, and Rpb3 are shown in purple and red, respectively. c, Putative CTD density interacting between the Head’s neck and Middle’s knob. Main chain CTD residues (Y10-Y24) are shown in cyan after docking the crystal structure of an ScMED Head-CTD complex [PDB 4GWQ] into the Head portion of the holoenzyme cryo-EM map. d, Rpb1 foot interaction with Med4N and Med9N in the Middle. Selected Rpb1 residues are indicated for reference. e, EMDB-2786 segmented into Head, Middle, Med14, GTFs, and promoter DNA. f, Fitting of Sp holoenzyme Head, Middle, and Med14 (without any changes to relative module orientation) into Mediator portion of EMDB-2786 map. g, Comparison of the RNAPII positions in the Sp holoenzyme and EMDB-2786 cryo-EM maps after matching of the Head modules (EMDB-2786 Head and RNAPII in purple and orange, respectively; Sp holoenzyme RNAPII in light green). Mediator and RNAPII portions of both complexes match, but there is a difference in RNAPII rotation. h, Comparison of the Med20-Rpb3 contact in Sp holoenzyme and EMDMB-2786 after matching of Med20 subunits (EMDB-2786 Med18/20 and Rpb3 in purple and light orange, respectively. Sp holoenzyme Med18, Med20, and Rpb3 in light blue, kakhi, and red, respectively). i, Comparison of putative CTD density in Sp holoenzyme map (left; density in gap between Head’s neck and Middle’s knob) with position of CTD peptide in an X-ray structure of a Head-CTD complex (PDB 4GWQ; molecular model of the Head in 4GWQ in light yellow, CTD peptide density in cyan). The N-terminal domain of 4GWQ’s Med6 (aa 1–120) were not considered for Head module alignment, because the Sc Med6 has a 42 aa insertion starting at residue 65. j, Comparison of Head-Rpb4/Rpb7 contacts (SpMED Head in pink as molecular model). k, Comparison of Med4/9-Rpb1 foot contacts (SpMED Middle in light blue as molecular model).
Extended Data Figure 7. Conserved conformation of Head, Middle, and Med14-Middle interface between Sp Mediator and Sp holoenzyme, and Mediator rearrangements upon holoenzyme formation.
a. The structure of the Head module is very similar in free Mediator (yellow) and holoenzyme (blue), except for the positions of the Med17N and Med6C portions, which connect to the knob and Med14, respectively. RMSD values between 1334 Cα atom pairs in corresponding portions of the Mediator and holoenzyme EM structures of the Head is 1.9 Å (the Med17N and Med14C domains that move with the knob were not included in the RMSD calculation). b, The structure of the Middle module is also very similar in free Mediator (yellow) and holoenzyme (blue). RMSD values between Cα 801 atom pairs in corresponding portions of the Mediator and holoenzyme EM structures of the Middle is 2.7 Å. c, Med14 has a considerable conformational change between free Mediator (yellow) and holoenzyme (blue). d, The structure of the Med14 (RM1)-Middle interface is the same in free Mediator and holoenzyme. e, Med14-interacting Med17N and Med6C move along with the top of Med14. f, Repositioning of the Middle upon holoenzyme formation closes the CTD-binding gap. It also brings Middle subunits Med4 and Med9 into contact with polymerase’s Rpb1 foot (highlighted in light green). The rigidity of the Middle module effectively links the contacts Mediator establishes with Rpb1’s CTD and foot domains. g, Sc core MED-ITC structure with Head, Middle, and Med14 portions colored in purple, blue, and green, respectively. h, Matching the Head portion of Sc core MED-ITC to the Head portion of the published cryo-EM map of free S cerevisiae Mediator1 (semi-transparent teal) shows a large rearrangement of the Middle upon Sc Mediator interaction with RNAPII. i, Matching the Head portion of Sc MED-PIC to the Head portion of the published cryo-EM map of free S cerevisiae Mediator1 (semi-transparent teal) also shows a large rearrangement of the Middle upon Sc Mediator interaction with RNAPII.
Extended Data Figure 8. Functional validation of Mediator-RNAPII contacts.
a, Left, coomassie blue-stained SDS-PAGE analysis (4–20% gradient gel) of purified wild-type, and ΔMed31 S cerevisiae Mediator. Right, effect of Med31 deletion on interaction of Sc Mediator with RNAPII. Wild-type and ΔMed31 ScMED were purified by TAP-tagging. Protein elutes from a calmodulin resin were analyzed. b, The x-ray structure of Sc Med7N/31 (PDB 3FBI) was fitted into Sp holoenzyme cryo-EM map. (Sc Med31 in green and Sc Med7N In orange). ScMED Med31 residues expected to be in close contact with the CTD were mutated as indicated. Head, Middle, and Med14 in the Sp holoenzyme cryo-EM map shown in transparent pink, cyan and green, respectively. c, Med31 sequence conservation in Sc, Sp, and human Mediators. d, Effect of Med31 point mutations on Mediator interaction with RNAPII. Y41E and T44P Med31 mutations resulted in a considerable decrease in RNAPII interaction. Mutation Y38A had a smaller but still considerable effect. Q45–46AA double mutation had no effect on RNAPII binding. A K83A mutation of a lysine located far from the CTD had no effect on RNAPII interaction either. e, 2D class averages showing that Med31 point mutations that affect RNAPII interactions do not result in destabilization of the knob (knob highlighted by yellow arrowhead in magnified insets). WT and ΔMed31 class averages shown for comparison.
Extended Data Figure 9. Variability in conformation of PIC particles and cryo-EM map of a TFIIK-containing PIC.
a, PIC maps showing various degrees of TFIIH organization. b, Cryo-EM map of a TFIIK-containing PIC. TFIIH and TFIIK are colored in yellow and red, respectively. c, 2D cryo-EM class averages of TFIIK-containing PIC. d, FSC plot for the TFIIK-containing PIC cryo-EM map (left; the resolution was estimated using the gold-standard 0.143 criterium), and angular distribution plot for the TFIIK-containing PIC cryo-EM map (right). e, Comparison between projections of the TFIIH portion of the PIC-TFIIK map calculated from cryo-EM data (left), and 2D class averages calculated from negative-stained images of TFIIH-TFIIK (right). In TFIIH-TFIIK 2D averages, TFIIK can be found in three positions, one of which matches the one observed in the PIC-TFIIK map. f. Possible model for TFIIK-containing PIC interaction with the holoenzyme conformation of Mediator. The position of the mobile Tail is indicated by the dashed circle.
Extended Data Table 1.
a, Yeast strains used in this study. b, Cryo-EM data collection, processing and refinement statistics*.
| Strain | Genotype | Source |
|---|---|---|
| TP161 | med7+::TAP-kanMX6 med13::kanMX4 | 18 |
| SG101 | med7+::TAP-kanMX6 med13::kanMX4 med2::clonNAT | This study |
| SG102 | med7+::TAP-kanMX6 med13::kanMX4 med27::clonNAT | This study |
| SG103 | med4+::FLAG-kanMX6 | This study |
| SG104 | med7+::FLAG-kanMX6 | This study |
| SG105 | Med2+::FLAG-kanMX6 | This study |
| SG106 | Med15+::FLAG-kanMX6 | This study |
| KSC101 | BJ2168, Med22-TAP::KlTRP1 | 5 |
| KSC102 | BJ2168, Med22-TAP::KlTRP1 Med31-5xFLAG::KanMX6 | This study |
| KSC103 | BJ2168, Med22-TAP::KlTRP1 Med31(Y38A)-5xFLAG::KanMX6 | This study |
| KSC104 | BJ2168, Med22-TAP::KlTRP1 Med31(Y41E)-5xFLAG::KanMX6 | This study |
| KSC105 | BJ2168, Med22-TAP::KlTRP1 Med31(T44P)-5xFLAG::KanMX6 | This study |
| KSC106 | BJ2168, Med22-TAP::KlTRP1 Med31(Q45A/Q46A)-5xFLAG::KanMX6 | This study |
| KSC107 | BJ2168, Med22-TAP::KlTRP1 Med31(K83A)-5xFLAG::KanMX6 | This study |
| KTB218 | BJ2168, Med22-TAP::KlTRP1 med31Δ::KanMX6 | 5 |
| Data collection and Processing | ||
|---|---|---|
| Microscope | Titan Krios | |
| Voltage (keV) | 300 | |
| Defocus range (μm) | 0.8 to 4.0 | |
| Videos | 11,181 | |
| Frames per movie | 35 | |
| Exposure time per frames (ms) | 200 | |
| Magnification | 22,500x | |
| Dose rate (e−pixel/sec) | 9.8 | |
| Total dose per movie (e−/Å2) | 43 | |
| Refinement | Mediator | Holoenzyme* |
| Particles | 42,484 | 3,862 |
| Map Resolution (Å) | 4.4 | 7.8 |
| RMS angle (°) | 0.01 | 0.01 |
| RMA bonds (Å) | 1.50 | 1.01 |
| Ramachandran outliers (%) | 1.1 | 1.3 |
| Ramachandran allowed (%) | 14.0 | 15.6 |
| Ramachandran favored (%) | 84.9 | 83.1 |
| All-atom clash score | 17.52 | 29.12 |
| Molprobity score | 2.31 | 2.58 |
Model statistics for the holoenzyme are based only on the Mediator portion of the model. Resolution for the Mediator portion of the holoenzyme map is 7.4 Å
Supplementary Material
Acknowledgements
This work was supported by US National Institutes of Health grants R01 GM67167 (F.J.A.) and R01 GM41628 (R.C.C. and J.W.C) and by a grant to the Stowers Institute from the Helen Nelson Medical Research Fund at the Greater Kansas City Community Foundation. We thank Claes Gustafsson for providing the Med7-TAP, Med13Δ S. pombe strain used for the high-resolution EM analysis.
Footnotes
Online Content Methods, along with any additional Extended Data display items and Supplementary Information are available in the online version of the paper, references unique to these sections appear only in the online paper.
Supplemental Information is available in the online version of the paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Kornberg RD Mediator and the mechanism of transcriptional activation. Trends Biochem Sci 30, 235–239, (2005). [DOI] [PubMed] [Google Scholar]
- 2.Malik S & Roeder RG The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet 11, 761–772, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai G, Imasaki T, Takagi Y & Asturias FJ Mediator structural conservation and implications for the regulation mechanism. Structure 17, 559–567, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taatjes DJ, Schneider-Poetsch T & Tjian R Distinct conformational states of nuclear receptor-bound CRSP-Med complexes. Nat Struct Mol Biol 11, 664–671, (2004). [DOI] [PubMed] [Google Scholar]
- 5.Asturias FJ, Jiang YW, Myers LC, Gustafsson CM & Kornberg RD Conserved structures of mediator and RNA polymerase II holoenzyme. Science 283, 985–987, (1999). [DOI] [PubMed] [Google Scholar]
- 6.Meyer KD, Lin SC, Bernecky C, Gao Y & Taatjes DJ p53 activates transcription by directing structural shifts in Mediator. Nat Struct Mol Biol 17, 753–760, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naar AM, Taatjes DJ, Zhai W, Nogales E & Tjian R Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev 16, 1339–1344, (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai KL et al. Subunit architecture and functional modular rearrangements of the transcriptional mediator complex. Cell 157, 1430–1444, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai KL et al. A conserved Mediator-CDK8 kinase module association regulates Mediator-RNA polymerase II interaction. Nat Struct Mol Biol 20, 611–619, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X et al. Redefining the modular organization of the core Mediator complex. Cell Res 24, 796–808, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koschubs T et al. Identification, structure, and functional requirement of the Mediator submodule Med7N/31. Embo J 28, 69–80, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imasaki T et al. Architecture of the Mediator head module. Nature 475, 240–243, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson PJ, Bushnell DA, Trnka MJ, Burlingame AL & Kornberg RD Structure of the Mediator Head module bound to the carboxy-terminal domain of RNA polymerase II. Proc Natl Acad Sci U S A 109, 17931–17935, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lariviere L et al. Structure of the Mediator head module. Nature, (2012). [DOI] [PubMed] [Google Scholar]
- 15.Baumli S, Hoeppner S & Cramer P A conserved mediator hinge revealed in the structure of the MED7.MED21 (Med7.Srb7) heterodimer. J Biol Chem 280, 18171–18178, (2005). [DOI] [PubMed] [Google Scholar]
- 16.Plaschka C et al. Architecture of the RNA polymerase II-Mediator core initiation complex. Nature 518, 376–380, (2015). [DOI] [PubMed] [Google Scholar]
- 17.He Y et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533, 359–365, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cevher MA et al. Reconstitution of active human core Mediator complex reveals a critical role of the MED14 subunit. Nat Struct Mol Biol 21, 1028–1034, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson PJ et al. Molecular architecture of the yeast Mediator complex. Elife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boube M, Joulia L, Cribbs DL & Bourbon HM Evidence for a mediator of RNA polymerase II transcriptional regulation conserved from yeast to man. Cell 110, 143–151, (2002). [DOI] [PubMed] [Google Scholar]
- 21.Lariviere L et al. Model of the Mediator middle module based on protein cross-linking. Nucleic Acids Res, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson PJ et al. Structure of a Complete Mediator-RNA Polymerase II Pre-Initiation Complex. Cell 166, 1411–1422 e1416, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi H, Kasahara K & Kokubo T Saccharomyces cerevisiae Med9 comprises two functionally distinct domains that play different roles in transcriptional regulation. Genes Cells 14, 53–67, (2009). [DOI] [PubMed] [Google Scholar]
- 24.Fan HY, Cheng KK & Klein HL Mutations in the RNA polymerase II transcription machinery suppress the hyperrecombination mutant hpr1 delta of Saccharomyces cerevisiae. Genetics 142, 749–759, (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons BJ et al. Subunit architecture of general transcription factor TFIIH. Proc Natl Acad Sci U S A 109, 1949–1954, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami K et al. Structure of an RNA polymerase II preinitiation complex. Proc Natl Acad Sci U S A 112, 13543–13548, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami K et al. Architecture of an RNA polymerase II transcription pre-initiation complex. Science 342, 1238724, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baidoobonso SM, Guidi BW & Myers LC Med19(Rox3) regulates Intermodule interactions in the Saccharomyces cerevisiae mediator complex. J Biol Chem 282, 5551–5559, (2007). [DOI] [PubMed] [Google Scholar]
- 29.Louder RK et al. Structure of promoter-bound TFIID and model of human pre-initiation complex assembly. Nature, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plaschka C et al. Transcription initiation complex structures elucidate DNA opening. Nature 533, 353–358, (2016). [DOI] [PubMed] [Google Scholar]
- 31.Myers LC et al. The Med proteins of yeast and their function through the RNA polymerase II carboxy-terminal domain. Genes Dev 12, 45–54, (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranish JA, Yudkovsky N & Hahn S Intermediates in formation and activity of the RNA polymerase II preinitiation complex: holoenzyme recruitment and a postrecruitment role for the TATA box and TFIIB. Genes Dev 13, 49–63, (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svejstrup JQ et al. Evidence for a mediator cycle at the initiation of transcription. Proc. Natl. Acad. Sci 94, 6075–6078, (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esnault C et al. Mediator-dependent recruitment of TFIIH modules in preinitiation complex. Mol Cell 31, 337–346, (2008). [DOI] [PubMed] [Google Scholar]
- 35.Bahler J et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951, (1998). [DOI] [PubMed] [Google Scholar]
- 36.Elmlund H et al. The cyclin-dependent kinase 8 module sterically blocks Mediator interactions with RNA polymerase II. Proc Natl Acad Sci U S A 103, 15788–15793, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Washburn MP, Wolters D & Yates JR 3rd. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19, 242–247, (2001). [DOI] [PubMed] [Google Scholar]
- 38.Florens L & Washburn MP Proteomic analysis by multidimensional protein identification technology. Methods Mol Biol 328, 159–175, (2006). [DOI] [PubMed] [Google Scholar]
- 39.Eng JK, McCormack AL & Yates JR An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5, 976–989, (1994). [DOI] [PubMed] [Google Scholar]
- 40.Tabb DL, McDonald WH & Yates JR 3rd. DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res 1, 21–26, (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Florens L et al. Analyzing chromatin remodeling complexes using shotgun proteomics and normalized spectral abundance factors. Methods 40, 303–311, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paoletti AC et al. Quantitative proteomic analysis of distinct mammalian Mediator complexes using normalized spectral abundance factors. Proc Natl Acad Sci U S A 103, 18928–18933, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zybailov B et al. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J Proteome Res 5, 2339–2347, (2006). [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Wen Z, Washburn MP & Florens L Refinements to label free proteome quantitation: how to deal with peptides shared by multiple proteins. Anal Chem 82, 2272–2281, (2010). [DOI] [PubMed] [Google Scholar]
- 45.Takagi Y, Chadick JZ, Davis JA & Asturias FJ Preponderance of free mediator in the yeast Saccharomyces cerevisiae. J Biol Chem, (2005). [DOI] [PubMed] [Google Scholar]
- 46.Suloway C et al. Automated molecular microscopy: the new Leginon system. J Struct Biol 151, 41–60, (2005). [DOI] [PubMed] [Google Scholar]
- 47.Lander GC et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J Struct Biol 166, 95–102, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohn M et al. SPARX, a new environment for Cryo-EM image processing. J Struct Biol 157, 47–55, (2007). [DOI] [PubMed] [Google Scholar]
- 49.Dubochet J et al. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys 21, 129–228, (1988). [DOI] [PubMed] [Google Scholar]
- 50.Li X et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat Methods 10, 584–590, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mindell JA & Grigorieff N Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol 142, 334–347, (2003). [DOI] [PubMed] [Google Scholar]
- 52.Scheres SH RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol 180, 519–530, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henderson R et al. Outcome of the first electron microscopy validation task force meeting. Structure 20, 205–214, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koschubs T et al. Preparation and topology of the Mediator middle module. Nucleic Acids Res 38, 3186–3195, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pettersen EF et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612, (2004). [DOI] [PubMed] [Google Scholar]
- 56.Spahr H, Calero G, Bushnell DA & Kornberg RD Schizosacharomyces pombe RNA polymerase II at 3.6-A resolution. Proc Natl Acad Sci U S A 106, 9185–9190, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emsley P, Lohkamp B, Scott WG & Cowtan K Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams PD et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen VB et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66, 12–21, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitazono AA, Tobe BT, Kalton H, Diamant N & Kron SJ Marker-fusion PCR for one-step mutagenesis of essential genes in yeast. Yeast 19, 141–149, (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cryo-EM maps and atomic coordinates have been deposited with the Electron Microscopy Data Bank (with accession codes 8479 and 8480) and Protein Data Dank (accession codes 5U0P and 5U0S). The original MudPIT data (raw files, peak files, search files, as well as DTASelect result files, and protein sequences fasta file) can be accessed from the Stowers Original Data Repository at: http://www.stowers.org/research/publications/libpb-1070.