ABSTRACT
Background
Polyunsaturated fatty acids (PUFAs) are associated with a lower risk of multiple diseases. Fatty acid desaturase 1 gene (FADS1) polymorphisms and dietary PUFA intake are both established determinants of circulating PUFA proportions.
Objective
We explored the joint effects of FADS1 polymorphisms and dietary PUFA intake on circulating PUFA proportions.
Design
We studied 2288 participants from a nested case-control study of coronary artery disease among participants who provided blood samples in the Nurses’ Health Study and the Health Professionals Follow-Up Study. Dietary PUFA intake was obtained from semiquantitative food-frequency questionnaires. FADS1 rs174546 was genotyped by using the Affymetrix 6.0 platform, and circulating PUFA proportions were measured with gas-liquid chromatography. Linear regression models were used to examine the associations between rs174546 and circulating proportions of each fatty acid. Gene-diet interactions were tested by including a cross-product term of dietary intake of each PUFA by rs174546 genotype in the linear regression models.
Results
After adjustment for sex and ancestry, each copy of the C allele of rs174546 was associated with higher circulating proportions of arachidonic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) and lower proportions of linoleic acid and α-linolenic acid. The magnitude of positive association between higher consumption of dietary EPA or DHA and circulating proportions of EPA increased with each copy of the rs174546_T allele (P-interaction = 0.01 and 0.007, respectively). Each 1-SD increment in EPA intake was associated with an average 3.7% increase in circulating EPA proportions among participants with the rs174546_CC genotype and an average 7.8% increase among participants with the TT genotype.
Conclusions
Carriers of the T allele at FADS1 rs174546 may need higher doses of dietary EPA and DHA to achieve the same circulating proportions of EPA as carriers of the C allele. The implications of these findings on disease risk and dietary guidelines require further study.
Keywords: gene-diet interaction, FADS1, dietary PUFA intake, EPA, DHA
INTRODUCTION
PUFAs are associated with the risk of multiple diseases, including cardiovascular disease (CVD) (1–5), neurodegenerative disease (6–8), and cancer (9, 10).
Longer-chain PUFAs can be synthesized endogenously from 18-carbon PUFAs. Long-chain n–3 PUFAs, such as EPA (20:5n–3), can be derived from α-linolenic acid (ALA; 18:3n–3) by elongation and desaturation, regulated by the Δ5 desaturase enzyme (D5D) (11, 12). The fatty acid desaturase 1 gene (FADS1), located on chromosome 11(11q12–13.1), encodes D5D (13). Recent genomewide association studies have identified associations between single nucleotide polymorphisms (SNPs) in FADS1 and PUFA biosynthesis and circulating fatty acid proportions (14–16). These genetic variants of FADS1 may play an important role in determining the efficiency of D5D in converting 18-carbon PUFAs to longer-chain fatty acids (13, 17–25). The SNP rs174546 in FADS1 is highly associated with plasma fatty acid concentrations at the genomewide significance level and in high linkage disequilibrium (LD) (r2 > 0.8) with other variants within FADS1; thus, this lead SNP can tag previously reported SNPs in FADS1 with genomewide significant associations with circulating fatty acids (15, 22–24).
Circulating fatty acid proportions are determined not only by genetics and metabolism but also by dietary fatty acid intake amounts (26, 27). Circulating PUFA proportions are influenced directly by the consumption of a marine diet or foods rich in PUFAs (18, 28–30). Plasma EPA and DHA (22:6n–3) proportions have been used as reliable biomarkers of fatty acid intake from marine products such as fish and fish-oil supplements in many studies (31, 32).
SNPs in FADS1 and dietary PUFA intakes are both established determinants of circulating PUFA proportions (14, 18, 30). However, little is known about how FADS1 rs174546 and dietary EPA or DHA intake jointly influence circulating PUFA proportions in plasma. Understanding the FADS1–dietary PUFA interaction may help promote diet modifications based on genetic makeup.
Therefore, in the current study, we aimed to explore the joint effects of FADS1 polymorphisms and dietary PUFA intake on circulating fatty acid proportions in a case-control study nested within 2 population-based cohorts: the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). Evaluation of these effect modifiers provides important implications for individuals with genetic susceptibility and promotes personalized recommendations for the consumption of a marine diet (33).
METHODS
Study population
A nested case-control study of coronary artery disease (CAD) was conducted in participants who provided blood samples in the HPFS and the NHS (34). Details of the study design were described elsewhere (35). Briefly, the NHS is a prospective cohort study in 121,700 female registered nurses from 11 US states who were aged 30–55 y at study initiation in 1976. The HPFS cohort consists of 51,529 male health professionals from all US states who were aged 40–75 y at baseline in 1986. A subset of 32,826 NHS participants provided blood samples between 1989 and 1990, and a subset of 18,225 HPFS participants provided blood samples between 1993 and 1995. Among individuals who were free of CVD and cancer at the time of blood draw in both the HPFS and NHS, we prospectively identified incident CAD cases and selected 1–2 controls for each case with the use of the risk-set sampling from those who remained free of CAD events when the case was diagnosed. Cases and controls were matched on age (±2 y), smoking status (never smoker; past smoker; or current smoker: 1–14 or ≥15 cigarettes/d), fasting status at blood draw (fasting for 10 h or not fasting), and time of blood draw. A total of 2288 participants (720 CAD cases and 1568 CAD-free controls) were included in our study. The participant flow chart is shown in Supplemental Figure 1.
The study was approved by the Institutional Review Board of Brigham and Women's Hospital and the Human Subjects Committee Review Board of Harvard TH Chan School of Public Health, Boston, Massachusetts. Informed consent was obtained from all participants.
Assessment of circulating fatty acid proportions
The blood sample collection and measurement of fatty acids were described elsewhere (36, 37). Briefly, fatty acids, including linoleic acid (LA; 18:2n–6), ALA, arachidonic acid (AA; 20:4n–6), EPA, and DHA, were extracted from plasma with the use of a hexane-isopropanol mixture and esterified with methanol and acetyl chloride. After esterification, the methanol and acetyl chloride were evaporated, and the fatty acid methyl esters were re-dissolved in isooctane. The concentrations of methyl ester were analyzed by gas-liquid chromatography. Peak retention times and area percentages of total fatty acids were identified by injecting known standards. The proportions of these fatty acids were expressed as percentages of total fatty acid content (Nu-Chek-Prep). Both technicians and laboratory personnel were blinded to case-control status of the samples. Blinded quality-control samples were analyzed along with case-control samples. CVs were generally <10% for these assays.
Assessment of dietary fatty acid intake
The intakes of total fat and 5 specific dietary fatty acids, including EPA, DHA, LA, ALA, and AA, were obtained and estimated from semiquantitative food-frequency questionnaires (sFFQs) every 4 y in the NHS and HPFS. In our study, the dietary intake amounts of fatty acids around the time at which blood samples provided were used to avoid recall bias (NHS: 1986, 1990; HPFS: 1990, 1994). The detailed description, validity, and reproducibility of the sFFQs have been published elsewhere (38–40). For each food item, a standard portion size was specified, and the participants were asked how often, on average, they consumed foods of that specified amount during the previous year. There were 9 possible responses listed in the sFFQs: never or <1 time/mo, 1–3 times/mo, 1 time/wk, 2–4 times/wk, 5–6 times/wk, 1 time/d, 2–3 times/d, 4–5 times/d, or ≥6 times/d. The intake of fatty acids was calculated by multiplying the frequency of consumption of each food by the fatty acid composition in the specified amount of that food. The food composition was primarily based on USDA food data. Dietary fatty acid intake was energy-adjusted by using the nutrient residual method (41). The validity and reproducibility of the sFFQs showed that the correlation coefficients between intakes assessed by the sFFQs and dietary records were 0.57 for total fat and 0.48 for total PUFAs (39, 40, 42). Total energy intake was calculated by summing up energy intake from all foods. An alternative Healthy Eating Index (aHEI) score was calculated by summarizing the consumption of 11 foods and nutrients associated with chronic disease risk, including fruit, vegetables, nuts and legumes, red and processed meat, whole grains, sugar-sweetened beverages and fruit juice, alcohol, sodium, trans fat, long-chain n–3 fatty acids, and polyunsaturated fat as an indicator of adherence to healthy eating behavior (43).
Genotyping
Genotyping was performed among the individuals with the use of the Affymetrix 6.0 platform. Quality control was conducted on the basis of minor allele frequency, call rate, and departure from Hardy-Weinberg equilibrium (44). Additional SNPs were imputed with the use of MACH and the 1000 Genomes Project Phase 3 v5 reference panel. We selected rs174546 in FADS1, which is highly associated with plasma fatty acid proportions at the genomewide significance level and in high LD (r2 > 0.8) with other variants in or near the FADS1 gene; thus, this lead SNP tagged SNPs in FADS1 with previouslyreported genomewidesignificant associations with circulating fatty acids (15, 22–24).
Statistical analysis
The proportions of plasma fatty acids and intakes of dietary PUFAs were summarized with the use of means and SDs. Nonnormally distributed plasma fatty acids with D > 0.05 in a goodness-of-fit test (Kolmogorov-Smirnov test) were log-transformed to improve normality before further analysis (variables that were log-transformed were ALA, EPA, and DHA). Differences between cases and controls were compared with the use of t tests or Wilcoxon’s Signed Rank tests. To minimize the effects of the scale of dietary factors in the interaction analyses, we rescaled dietary exposures by using mean-centered and standardized dietary variables, which were used as continuous variables. For FADS1 SNP rs174546, an additive genetic model was used (0 copy, 1 copy, or 2 copies of the C allele). Linear regression models were used to examine the marginal association between rs174546 and circulating proportions of each fatty acid. Gene-diet interactions were tested via likelihood ratio tests by including a cross-product term of dietary intake of each fatty acid by rs174546 genotype. We adjusted for sex, the top 4 principal components (PCs) of ancestry, and total energy intake in the multivariable models. [Details of the PCs of ancestry have been described by Price et al. (45).] Briefly, PCs are calculated by using ∼10,000 representative SNPs with the use of EIGENSTRAT via PC analysis to control for population stratification. The top 4 PCs that captured the majority of heterogeneity were used (44, 45). We further adjusted for aHEI in our sensitivity analysis. All of the statistical analyses were conducted using SAS statistical package, version 9.4 (SAS Institute). A 2-sided P value <0.05 indicated significance.
RESULTS
The intakes of dietary fatty acids and plasma fatty acid proportions among NHS and HPFS participants, according to CAD status, are shown in Table 1. Compared with controls, CAD cases had lower plasma proportions of AA (P < 0.05). The plasma LA and EPA proportions and all dietary fatty acids were comparable between cases and controls for both men and women (P > 0.05).
TABLE 1.
Intakes of dietary fatty acids and plasma fatty acid proportions among NHS and HPFS participants, according to CAD status1
| NHS (n = 1142) | HPFS (n = 1146) | |||
|---|---|---|---|---|
| CAD cases (n = 340) | Controls (n = 802) | CAD cases (n = 380) | Controls (n = 766) | |
| Dietary fatty acids intake, mg/d | ||||
| LA | 10,812.4 ± 4247.4 | 10,772.1 ± 3912.5 | 11,302.2 ± 4451.2 | 11,029.8 ± 4175.8 |
| ALA | 987.1 ± 402.6 | 966.2 ± 384.0 | 1084.3 ± 424.6 | 1062.9 ± 397.7 |
| DHA | 152.5 ± 109.6 | 154.5 ± 115.3 | 166.1 ± 136.9 | 168.1 ± 145.3 |
| EPA | 76.0 ± 63.3 | 79.8 ± 102.8 | 99.0 ± 140.7 | 101.6 ± 138.5 |
| AA | 74.1 ± 29.3 | 74.9 ± 29.5 | 81.4 ± 33.0 | 79.5 ± 33.8 |
| Total fat | 65,695.3 ± 21,810.8 | 64,502.2 ± 20,300.3 | 71,567.3 ± 25,406.4 | 69,568.5 ± 25,282.6 |
| Plasma fatty acid proportions, % of total fatty acids | ||||
| LA | 29.93 ± 4.79 | 30.28 ± 4.64 | 30.18 ± 4.59 | 30.58 ± 4.46 |
| ALA | 0.50 ± 0.17 | 0.47 ± 0.15 | 0.64 ± 0.25** | 0.60 ± 0.24 |
| AA | 6.95 ± 1.60*** | 7.53 ± 1.72 | 7.05 ± 1.73* | 7.27 ± 1.71 |
| EPA | 0.46 ± 0.28 | 0.47 ± 0.22 | 0.60 ± 0.37 | 0.64 ± 0.46 |
| DHA | 1.35 ± 0.57** | 1.49 ± 0.62 | 1.67 ± 0.76 | 1.75 ± 0.74 |
1Values are means ± SDs. Differences between cases and controls were compared by using t tests or Wilcoxon's Signed Rank tests. *P < 0.05; **P < 0.01; ***P < 0.0001. AA, arachidonic acid; ALA, α-linolenic acid; CAD, coronary artery disease; HPFS, Health Professionals Follow-Up Study; LA, linoleic acid; NHS, Nurses’ Health Study.
Marginal association between FADS1 rs174546 and plasma fatty acid proportions
Each copy of the C allele of rs174546 was associated with higher circulating proportions of AA (P < 0.0001), EPA (P < 0.0001), and DHA (P = 0.0001) and lower proportions of LA (P < 0.0001) and ALA (P < 0.0001) (Table 2). The associations remained after adjustment for sex and the top 4 PCs of ancestry (P < 0.0001 for LA, ALA, AA, and EPA and P = 0.001 for DHA).
TABLE 2.
Associations of FADS1 SNP rs174546_C with plasma fatty acid proportions1
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Plasma fatty acid biomarkers | β | (95% CI) | P | β | (95% CI) | P |
| LA | −0.85 | (−1.17, −0.54) | <0.0001 | −0.84 | (−1.15, −0.52) | <0.0001 |
| ALA (log-transformed) | −0.03 | (−0.04, −0.01) | <0.0001 | −0.03 | (−0.04, −0.02) | <0.0001 |
| AA | 1.26 | (1.16, 1.36) | <0.0001 | 1.27 | (1.17, 1.37) | <0.0001 |
| EPA (log-transformed) | 0.09 | (0.08, 0.11) | <0.0001 | 0.09 | (0.07, 0.10) | <0.0001 |
| DHA (log-transformed) | 0.02 | (0.01, 0.04) | 0.0001 | 0.02 | (0.01, 0.03) | 0.001 |
1Linear regression models were used to examine the association between rs174546_C and plasma fatty acid proportions. Model 1 was the unadjusted model; model 2 was adjusted for sex and the top 4 principal components of ancestry. AA, arachidonic acid; ALA, α-linolenic acid; FADS1, fatty acid desaturase 1; LA, linoleic acid; SNP, single nucleotide polymorphism.
Interactions between FADS1 rs174546_C and dietary PUFA intake for circulating fatty acid proportions
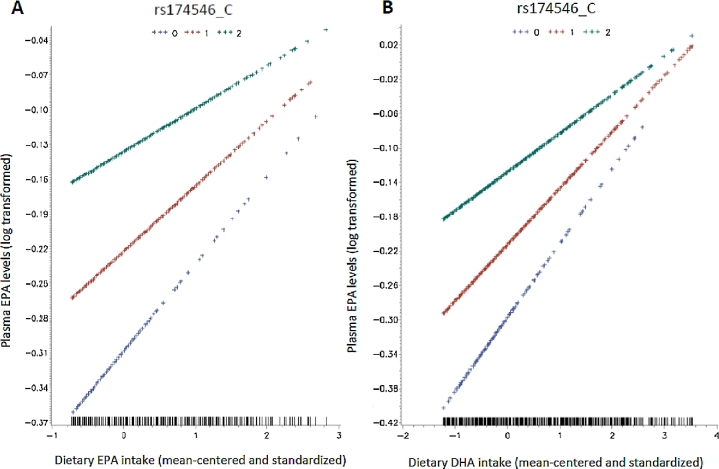
We found that dietary EPA and DHA intakes might modify the association between FADS1 rs174546 and circulating EPA proportions in plasma (P-interaction = 0.01 for dietary EPA and 0.007 for dietary DHA). After adjustment for sex and the top 4 PCs of ancestry, the interactions were slightly attenuated but remainedsignificant (P-interaction = 0.02 for dietary EPA and 0.01 for dietary DHA). Further adjustment for total energy intake did not change the interactions substantially, and the interactions were still significant (P-interaction = 0.04 for dietary EPA and 0.02 for dietary DHA) (Table 3). We plotted the associations between dietary EPA or DHA intake and circulating EPA proportions according to rs174546 genotype in Figure 1. The magnitude of the positive association between higher consumption of dietary EPA or DHA and circulating proportions of EPA increased with each copy of the rs174546_T allele. Each 1-SD increment in EPA intake was associated with an average 3.7% increase in circulating EPA proportions among participants with the rs174546_CC genotype and an average 7.8% increase among participants with the TT genotype. Each 1-SD increment in DHA intake was associated with an average 4.6% increase in circulating EPA proportions among participants with the rs174546_CC genotype and an average 9.0% increase among participants with the TT genotype. In our sensitivity analyses, we further adjusted for aHEI and found no substantial change in the results (data not shown). We did not find any nominally significant interactions between FADS1 polymorphisms and other dietary PUFA intakes on circulating fatty acid proportions (Supplemental Table 1).
TABLE 3.
Interactions between FADS1 SNP rs174546_C and dietary EPA and DHA intakes on plasma EPA proportions1
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma EPA proportions | β | (95% CI) | P | β | (95% CI) | P | β | (95% CI) | P |
| Dietary EPA intake | |||||||||
| rs174546_C | 0.09 | (0.07, 0.11) | <0.0001 | 0.09 | (0.07, 0.10) | <0.0001 | 0.08 | (0.07, 0.10) | <0.0001 |
| Dietary EPA intake | 0.09 | (0.06, 0.12) | <0.0001 | 0.08 | (0.05, 0.10) | <0.0001 | 0.07 | (0.05, 0.10) | <0.0001 |
| rs174546_C × dietary EPA intake | −0.02 | (−0.04, −0.004) | 0.01 | −0.02 | (−0.04, −0.003) | 0.02 | −0.02 | (−0.03, −0.009) | 0.04 |
| Dietary DHA intake | |||||||||
| rs174546_C | 0.09 | (0.07, 0.10) | <0.0001 | 0.08 | (0.07, 0.10) | <0.0001 | 0.08 | (0.07, 0.10) | <0.0001 |
| Dietary DHA intake | 0.10 | (0.07, 0.13) | <0.0001 | 0.09 | (0.06, 0.11) | <0.0001 | 0.09 | (0.06, 0.11) | <0.0001 |
| rs174546_C × dietary DHA intake | −0.02 | (−0.04, −0.006) | 0.007 | −0.02 | (−0.04, −0.005) | 0.01 | −0.02 | (−0.03, −0.003) | 0.02 |
1Gene-diet interactions were tested via likelihood ratio tests by including a cross-product term of dietary EPA and DHA intake by rs174546_C allele numbers in the linear regression models. Model 1 was unadjusted; model 2 was adjusted for sex and the top 4 principal components of ancestry; and model 3 was further adjusted for total energy intake (kilocalories per day). FADS1, fatty acid desaturase 1 gene; SNP, single nucleotide polymorphism.
FIGURE 1.
Interactions between FADS1 SNP rs174546_C and dietary EPA (A) and DHA (B) intakes on circulating EPA proportions were tested via likelihood ratio tests by including a cross-product term of dietary EPA and DHA intake by rs174546_C allele numbers in the linear regression models. The top lines represent those who are homozygous for the C allele (C/C), the middle lines represent those who are heterozygous (C/T), and the bottom lines represent those who are homozygous for the T allele (T/T). The y axis shows the log-transformed plasma EPA proportions. The x axis shows the mean-centered and standardized intake amounts of EPA and DHA. FADS1, fatty acid desaturase 1; SNP, single nucleotide polymorphism.
DISCUSSION
In our study conducted in US male health professionals and female nurses, we observed that FADS1 was related to circulating PUFA proportions. Furthermore, the consumption of EPA and DHA might modulate the relation between FADS1 rs174546 and circulating proportions of EPA. In particular, individuals who carry the T allele at the FADS1 SNP rs174546 may need higher doses of dietary EPA and DHA to achieve the same circulating proportions of EPA as carriers of the C allele.
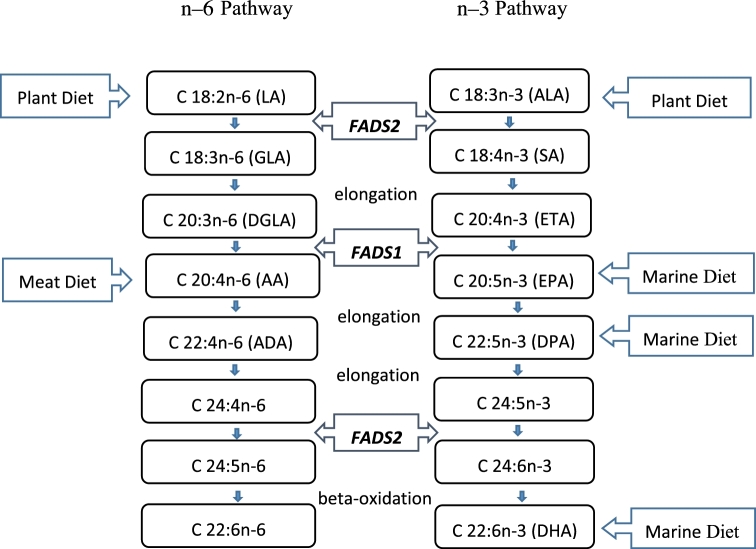
In our study, we found a lower baseline plasma proportion of AA among CAD cases than controls. A study conducted in a Dutch population also reported that a high ratio of baseline C20:4n–6 (AA) to C20:3n–6 dihomo-γ-linolenic acid (reflecting D5D activity) was associated with a reduced CAD risk, which was in line with our study (46). The associations between FADS1 and circulating fatty acid proportions have been well established in previous studies (47–49). With the use of both candidate gene study and the genomewide association study approach, numerous studies have reported that the minor alleles in FADS1 were associated with higher ALA and LA and lower AA and EPA proportions (14, 15, 21, 50). In line with our marginal association results, Bokor et al. (21) also found that the minor allele (T allele) of rs174546 in FADS1 was associated with higher proportions of ALA and lower proportions of EPA. These findings support previous evidence that FADS1 variation may influence the conversion of ALA to EPA and the flow through the biosynthetic pathway from ALA to DHA (Figure 2).
FIGURE 2.
Biological synthesis pathway of n–3 and n–6 PUFAs. AA, arachidonic acid; ADA, adrenic acid; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; ETA, eicosatetraenoic acid; FADS, fatty acid desaturase gene; GLA, γ-linolenic acid; LA, linoleic acid; SA, stearidonic acid.
Few studies, to our knowledge, have evaluated the joint effects of FADS1 polymorphisms and dietary PUFA intake on circulating fatty acid proportions. A recent cross-sectional study conducted by Takkunen et al. (51) found an interaction between the intake of marine PUFAs and FADS1 variant rs174550 (r2 = 1, in complete linkage disequilibrium with FADS1 rs174546 in our study) on circulating proportions of EPA, which was in agreement with our results. However, the study was restricted to 962 men and the statistical power might be limited. We extended the analysis greatly by including both male and female participants and augmented the sample size further to 2288 to validate this gene-diet interaction. Our results are also similar to what has been presented before (52). A meta-analysis of 9 studies (n = 11,668) conducted in the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium found no significant interaction between FADS1 SNPs and dietary fatty acid intake, after adjustment for multiple comparisons (P > 0.002) (53). They highlighted interactions between FADS1 SNPs (rs174548 and rs174538, in strong LD with rs174546 in our study: r2 = 0.81 and 0.91, respectively) and ALA and LA intakes affecting plasma EPA and DHA. Interactions between rs174538 and both ALA and LA for plasma DHA were nominally significant (P = 0.03 and 0.02, respectively). In our independent data set, none of the interactions between rs174546 and dietary ALA or LA were significant (see Supplemental Table 1). (We note that the NHS and HPFS samples only contributed data on erythrocyte fatty acid proportions to the previous CHARGE meta-analysis. The analyses of plasma fatty acids have not been presented before.)
Our results showed that the magnitude of the positive association between higher consumption of dietary EPA or DHA and circulating proportions of EPA increased with each copy of the rs174546_T allele, implying that people with TT and TC genotypes may benefit more from higher EPA and DHA consumption. Mechanisms for our observed interactions remain unclear. However, our findings are biologically plausible. Dietary EPA and DHA may influence n–3 fatty acid biosynthesis, as shown in a previous study conducted in the European Investigation into Cancer and Nutrition (EPIC)–Norfolk cohort in which the conversion of ALA to longer-chain n–3 PUFAs was different in fish consumers and nonconsumers (54). When the consumption of EPA is low, the D5D enzyme (encoded by FADS1) is needed to convert ALA into EPA to elevate the circulating proportions of EPA (15). Those with relatively inefficient D5D enzymes (carriers of the T allele) will consequently have lower EPA proportions in the context of an EPA-poor diet. When the EPA intake is increased, the plasma EPA proportions are elevated and the genetic association of rs174546 with circulating EPA proportions is gradually attenuated (Figure 2). Our findings are consistent with the hypothesis of “differential susceptibility” (55, 56), which supposes that vulnerability genes might function like plasticity genes, whose risk can be attenuated by a favorable environment or amplified by an adverse environment. Our results support the point of view that higher EPA and DHA consumption may confer a greater benefit for TT genotype carriers. Such information could contribute to the targeting of EPA and DHA recommendations, whereby additional long-chain n–3 PUFA intakes could be recommended for those carrying the T allele of FADS1 rs174546. However, to our knowledge, further downstream functional investigations on potential mechanisms are warranted and the ultimate effect on CAD risk remains to be studied. In addition, because the effects shown in our study are modest and most individuals in North America do not consume nearly enough EPA and DHA in their diet, greater consumption of EPA and DHA is still recommended for all people.
Several limitations of the current study should be considered. First, our results might be potentially confounded by unmeasured or unknown factors. However, in our study, the cases and controls were matched on age, smoking status, fasting status, and time of blood draw and the plasma proportions of EPA were comparable among cases and controls. We also carefully adjusted for sex, the top 4 PCs of ancestry, total energy intake, and aHEI in the regression models. In principle, case-control ascertainment might cause the relations between diet, FADS1 SNPs, and circulating fatty acids to differ from those in the general population. In practice, however, assuming the effects of diet and FADS1 are mediated through circulating fatty acid proportions, and considering the modest associations between circulating fatty acids and CAD, ascertainment is unlikely to create an interaction of the magnitude we observed between diet and FADS1 on circulating fatty acid proportions. In particular, there was no significant association between CAD and circulating EPA (5), suggesting that case-control ascertainment is unlikely to account for the interactions we observed. In addition, we conducted several sensitivity analyses. When we stratified by CAD case and control status and combined the results of case-only and control-only samples using an inverse variance–weighted fixed-effects meta-analysis, the pooled results were similar to our main results (data not shown). We also conducted inverse-probability-of-sampling-weighted (IPW) regression to account for case-control ascertainment from the underlying NHS and HPFS blood subcohorts. Point estimates from the IPW analyses did not differ appreciably from those in Table 3; P values for the SNP-EPA interactions were slightly larger (as expected from the robust variance estimate used in the IPW analysis) but smaller for the SNP-DHA analyses (due to the larger interaction effect estimates; Supplemental Table 2). Second, measurement errors in the assessment of dietary fatty acid intake are inevitable; however, our sFFQs have been well validated and reproduced by several previous studies (40, 42). Third, our study was restricted to FADS1 SNP rs174546. As indicated above, the SNP used in the current study shows a strong association with plasma PUFA proportions and tags a cluster of SNPs associated with fatty acid proportions. In addition, rs174546 is in high linkage disequilibrium (r2 > 0.8) in the HapMap CEU population (Utah residents with Northern and Western European ancestry from the CEPH collection) with several SNPs used in previous gene-diet interaction studies (e.g., rs174550, rs174538, rs174548, rs174556, and rs1535) (51, 53). Hence, this SNP is a logical choice for examining FADS1–dietary PUFA interactions. Fourth, we did not adjust for multiple comparisons, and some dietary fatty acids that showed nonsignificant interactions with rs174546 may due to the limited power to detect the relatively small interaction effects conferred by the locus. However, statistical power calculation (conducted by using Quanto, version 1.2.4) showed that we had high (>80%) power at the significance level of 0.05 (2-sided) to detect interaction effects of ≥2% from current sample size.
In summary, carriers of the T allele at the FADS1 SNP rs174546 may need higher doses of dietary EPA and DHA to achieve the same circulating proportions of EPA as carriers of the C allele. The implications of these findings on disease risk and dietary guidelines require further study.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—JJ, MKJ, WCW, and PK: designed the research; JJ and HH: analyzed the data; JJ, XJ, and PK: wrote the manuscript; AVAK, MS, QS, MKJ, and WCW: conducted the research; PK: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
The Nurses’ Health Study and the Health Professionals Follow-Up Study were supported by grants from the National Cancer Institute (CA186107, CA167552, and CA49449) and the National Heart, Lung, and Blood Institute (HL034594, HL088521, HL060712, and HL35464).
Supplemental Figure 1 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AA, arachidonic acid; aHEI, alternative Healthy Eating Index; ALA, α-linolenic acid; CAD, coronary artery disease; CVD, cardiovascular disease; D5D, Δ5 desaturase; FADS1, fatty acid desaturase 1; HPFS, Health Professionals Follow-Up Study; IPW, inverse-probability-of-sampling-weighted; LA, linoleic acid; LD, linkage disequilibrium; NHS, Nurses’ Health Study; PC, principal component; sFFQ, semiquantitative food-frequency questionnaire; SNP, single nucleotide polymorphism.
REFERENCES
- 1. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011;58(20):2047–67. [DOI] [PubMed] [Google Scholar]
- 2. Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med 2002;346(15):1113–8. [DOI] [PubMed] [Google Scholar]
- 3. Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010;376(9740):540–50. [DOI] [PubMed] [Google Scholar]
- 4. Sun Q, Ma J, Campos H, Rexrode KM, Albert CM, Mozaffarian D, Hu FB. Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction. Am J Clin Nutr 2008;88(1):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC et al. Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 2016;176(8):1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cederholm T, Salem N Jr., Palmblad J. Omega-3 fatty acids in the prevention of cognitive decline in humans. Adv Nutr 2013;4(6):672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res 2014;53:1–17. [DOI] [PubMed] [Google Scholar]
- 8. Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids 2007;77(5–6):287–93. [DOI] [PubMed] [Google Scholar]
- 9. Khankari NK, Murff HJ, Zeng C, Wen W, Eeles RA, Easton DF, Kote-Jarai Z, Al Olama AA, Benlloch S, Muir K et al. Polyunsaturated fatty acids and prostate cancer risk: a Mendelian randomisation analysis from the PRACTICAL consortium. Br J Cancer 2016;115(5):624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dinwiddie MT, Terry PD, Whelan J, Patzer RE. Omega-3 fatty acid consumption and prostate cancer: a review of exposure measures and results of epidemiological studies. J Am Coll Nutr 2016;35(5):452–68. [DOI] [PubMed] [Google Scholar]
- 11. Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP. Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res 1995;36(12):2471–7. [PubMed] [Google Scholar]
- 12. Burdge GC. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot Essent Fatty Acids 2006;75(3):161–8. [DOI] [PubMed] [Google Scholar]
- 13. Marquardt A, Stohr H, White K, Weber BH. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics 2000;66(2):175–83. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI study. PLoS Genet 2009;5(1):e1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lemaitre RN, Tanaka T, Tang W, Manichaikul A, Foy M, Kabagambe EK, Nettleton JA, King IB, Weng LC, Bhattacharya S et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet 2011;7(7):e1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tintle NL, Pottala JV, Lacey S, Ramachandran V, Westra J, Rogers A, Clark J, Olthoff B, Larson M, Harris W et al. A genome-wide association study of saturated, mono- and polyunsaturated red blood cell fatty acids in the Framingham Heart Offspring Study. Prostaglandins Leukot Essent Fatty Acids 2015;94:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang L, Athinarayanan S, Jiang G, Chalasani N, Zhang M, Liu W. Fatty acid desaturase 1 gene polymorphisms control human hepatic lipid composition. Hepatology 2015;61(1):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al-Hilal M, Alsaleh A, Maniou Z, Lewis FJ, Hall WL, Sanders TA, O'Dell SD. Genetic variation at the FADS1-FADS2 gene locus influences delta-5 desaturase activity and LC-PUFA proportions after fish oil supplement. J Lipid Res 2013;54(2):542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gieger C, Geistlinger L, Altmaier E, Hrabe de Angelis M, Kronenberg F, Meitinger T, Mewes HW, Wichmann HE, Weinberger KM, Adamski J et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet 2008;4(11):e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gillingham LG, Harding SV, Rideout TC, Yurkova N, Cunnane SC, Eck PK, Jones PJ. Dietary oils and FADS1-FADS2 genetic variants modulate [13C]α-linolenic acid metabolism and plasma fatty acid composition. Am J Clin Nutr 2013;97(1):195–207. [DOI] [PubMed] [Google Scholar]
- 21. Bokor S, Dumont J, Spinneker A, Gonzalez-Gross M, Nova E, Widhalm K, Moschonis G, Stehle P, Amouyel P, De Henauw S et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J Lipid Res 2010;51(8):2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, Arnold M, Erte I, Forgetta V, Yang TP et al. An atlas of genetic influences on human blood metabolites. Nat Genet 2014;46(6):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Illig T, Gieger C, Zhai G, Romisch-Margl W, Wang-Sattler R, Prehn C, Altmaier E, Kastenmuller G, Kato BS, Mewes HW et al. A genome-wide perspective of genetic variation in human metabolism. Nat Genet 2010;42(2):137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wagele B, Altmaier E, Deloukas P, Erdmann J, Grundberg E et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011;477(7362):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010;466(7307):707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dias CB, Wood LG, Garg ML. Effects of dietary saturated and n-6 polyunsaturated fatty acids on the incorporation of long-chain n-3 polyunsaturated fatty acids into blood lipids. Eur J Clin Nutr 2016;70(7):812–8. [DOI] [PubMed] [Google Scholar]
- 27. Burdge GC, Finnegan YE, Minihane AM, Williams CM, Wootton SA. Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of [13C]alpha-linolenic acid to longer-chain fatty acids and partitioning towards beta-oxidation in older men. Br J Nutr 2003;90(2):311–21. [DOI] [PubMed] [Google Scholar]
- 28. Dawczynski C, Massey KA, Ness C, Kiehntopf M, Stepanow S, Platzer M, Grun M, Nicolaou A, Jahreis G. Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: effects on circulating eicosanoids and cardiovascular risk factors. Clin Nutr 2013;32(5):686–96. [DOI] [PubMed] [Google Scholar]
- 29. Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007;86(1):74–81. [DOI] [PubMed] [Google Scholar]
- 30. Chung H, Nettleton JA, Lemaitre RN, Barr RG, Tsai MY, Tracy RP, Siscovick DS. Frequency and type of seafood consumed influence plasma (n-3) fatty acid concentrations. J Nutr 2008;138(12):2422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serra-Majem L, Nissensohn M, Overby NC, Fekete K. Dietary methods and biomarkers of omega 3 fatty acids: a systematic review. Br J Nutr 2012;107(Suppl 2):S64–76. [DOI] [PubMed] [Google Scholar]
- 32. Kuriki K, Nagaya T, Tokudome Y, Imaeda N, Fujiwara N, Sato J, Goto C, Ikeda M, Maki S, Tajima K et al. Plasma concentrations of (n-3) highly unsaturated fatty acids are good biomarkers of relative dietary fatty acid intakes: a cross-sectional study. J Nutr 2003;133(11):3643–50. [DOI] [PubMed] [Google Scholar]
- 33. Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp Biol Med (Maywood) 2010;235(7):785–95. [DOI] [PubMed] [Google Scholar]
- 34. Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 35. Malik VS, Chiuve SE, Campos H, Rimm EB, Mozaffarian D, Hu FB, Sun Q. Circulating very-long-chain saturated fatty acids and incident coronary heart disease in US men and women. Circulation 2015;132(4):260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, Campos H. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol 2005;162(4):373–81. [DOI] [PubMed] [Google Scholar]
- 37. Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr 2002;76(4):750–7. [DOI] [PubMed] [Google Scholar]
- 38. Willett WC. Nutritional epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 39. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 41. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(4 Suppl):1220S–8S; discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 42. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 43. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lindstrom S, Loomis S, Turman C, Huang H, Huang J, Aschard H, Chan AT, Choi H, Cornelis M, Curhan G et al. A comprehensive survey of genetic variation in 20,691 subjects from four large cohorts. PLoS One 2017;12(3):e0173997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006;38(8):904–9. [DOI] [PubMed] [Google Scholar]
- 46. Lu Y, Vaarhorst A, Merry AH, Dolle ME, Hovenier R, Imholz S, Schouten LJ, Heijmans BT, Muller M, Slagboom PE et al. Markers of endogenous desaturase activity and risk of coronary heart disease in the CAREMA cohort study. PLoS One 2012;7(7):e41681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kwak JH, Paik JK, Kim OY, Jang Y, Lee SH, Ordovas JM, Lee JH. FADS gene polymorphisms in Koreans: association with omega6 polyunsaturated fatty acids in serum phospholipids, lipid peroxides, and coronary artery disease. Atherosclerosis 2011;214(1):94–100. [DOI] [PubMed] [Google Scholar]
- 48. Merino DM, Johnston H, Clarke S, Roke K, Nielsen D, Badawi A, El-Sohemy A, Ma DW, Mutch DM. Polymorphisms in FADS1 and FADS2 alter desaturase activity in young Caucasian and Asian adults. Mol Genet Metab 2011;103(2):171–8. [DOI] [PubMed] [Google Scholar]
- 49. Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr 2008;138(11):2222–8. [DOI] [PubMed] [Google Scholar]
- 50. Ameur A, Enroth S, Johansson A, Zaboli G, Igl W, Johansson AC, Rivas MA, Daly MJ, Schmitz G, Hicks AA et al. Genetic adaptation of fatty-acid metabolism: a human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am J Hum Genet 2012;90(5):809–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takkunen MJ, de Mello VD, Schwab US, Kuusisto J, Vaittinen M, Agren JJ, Laakso M, Pihlajamaki J, Uusitupa MI. Gene-diet interaction of a common FADS1 variant with marine polyunsaturated fatty acids for fatty acid composition in plasma and erythrocytes among men. Mol Nutr Food Res 2016;60(2):381–9. [DOI] [PubMed] [Google Scholar]
- 52. Roke K, Mutch DM. The role of FADS1/2 polymorphisms on cardiometabolic markers and fatty acid profiles in young adults consuming fish oil supplements. Nutrients 2014;6(6):2290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith CE, Follis JL, Nettleton JA, Foy M, Wu JH, Ma Y, Tanaka T, Manichakul AW, Wu H, Chu AY et al. Dietary fatty acids modulate associations between genetic variants and circulating fatty acids in plasma and erythrocyte membranes: meta-analysis of nine studies in the CHARGE consortium. Mol Nutr Food Res 2015;59(7):1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Welch AA, Shakya-Shrestha S, Lentjes MA, Wareham NJ, Khaw KT. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non–fish-eating meat-eaters, vegetarians, and vegans and the product-precursor ratio [corrected] of α-linolenic acid to long-chain n–3 polyunsaturated fatty acids: results from the EPIC-Norfolk cohort. Am J Clin Nutr 2010;92(5):1040–51. [DOI] [PubMed] [Google Scholar]
- 55. Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry 2009;14(8):746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull 2009;135(6):885–908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.