Abstract
Increased bone resorption is considered to explain why intermittent PTH is anabolic for bone but continuous PTH is catabolic. However, when cyclooxygenase-2 (COX2) is absent in mice, continuous PTH becomes anabolic without decreased resorption. In murine bone marrow stromal cells (BMSCs), serum amyloid A (SAA)3, induced in the hematopoietic lineage by the combination of COX2-produced prostaglandin and receptor activator of nuclear factor κB ligand (RANKL), suppresses PTH-stimulated osteoblast differentiation. To determine whether SAA3 inhibits the anabolic effects of PTH in vivo, wild-type (WT) and SAA3 knockout (KO) mice were infused with PTH. In WT mice, continuous PTH induced SAA3 and was catabolic for bone. In KO mice, PTH was anabolic, increasing trabecular bone, serum markers of bone formation, and osteogenic gene expression. In contrast, PTH increased all measurements associated with bone resorption, as well as COX2 gene expression, similarly in KO and WT mice. SAA1 and SAA2 in humans are likely to have analogous functions to SAA3 in mice. RANKL induced both SAA1 and SAA2 in human bone marrow macrophages in a COX2-dependent manner. PTH stimulated osteogenesis in human BMSCs only when COX2 or RANKL was inhibited. Addition of recombinant SAA1 or SAA2 blocked PTH-stimulated osteogenesis. In summary, SAA3 suppresses the bone formation responses but not the bone resorption responses to PTH in mice, and in the absence of SAA3, continuous PTH is anabolic. In vitro studies in human bone marrow suggest that SAA may be a target for enhancing the therapeutic effects of PTH in treating osteoporosis.
SAA3 suppressed bone anabolic effects of PTH infusion in mice and osteogenic effects of PTH in murine bone marrow stromal cells (BMSCs). SAA1/SAA2 suppressed osteogenic effects of PTH in human BMSCs.
PTH is a major stimulator of bone resorption. PTH acts via type 1 PTH/PTH-related peptide (PTH/PTHrP) receptor (PTH1R), a G protein–coupled receptor, which is highly expressed by osteoblast lineage cells and activates both Gαs and Gαq signaling pathways (1–3). PTH stimulates bone resorption by acting on osteoblast lineage cells to increase receptor activator of nuclear factor κB ligand (RANKL), required for osteoclast differentiation, and decrease osteoprotegerin (OPG), a decoy receptor for RANKL (4). After the completion of skeletal development, bone is continuously remodeled by bone turnover, that is, local cycles of bone resorption followed by bone formation. Turnover may be balanced, resulting in no change in bone mass, or unbalanced, resulting in bone gain or loss. When PTH is injected intermittently, bone formation is increased more than resorption, resulting in bone gain. Intermittent PTH was the first anabolic agent approved for osteoporosis therapy in the United States (5, 6).
In contrast to the anabolic effects of intermittently injected PTH, continuous PTH is catabolic, and bone is lost (7–9). The paradoxical effect of intermittent vs continuous PTH is generally attributed to greater stimulation of resorption with continuous PTH compared with intermittent PTH (10). However, this argument does not take into account the suppression of osteoblastic differentiation in response to continuous PTH in vitro (11). Others have suggested that suppression of PTH-stimulated bone formation is largely responsible for the bone loss associated with continuous PTH (12). We found that the suppression of PTH-stimulated osteoblast differentiation in vitro was dependent on COX2-produced prostaglandin E2 (PGE2) (11) and that continuous PTH in vivo was catabolic in wild-type (WT) mice but anabolic in mice with absent COX2 (9). The switch from bone loss to gain was due to increased formation with no change in resorption.
PTH is a potent inducer of COX2, the major enzyme responsible for regulating PGE2 production in osteoblastic lineage cells (13, 14). PTH-stimulated RANKL can also induce COX2 and PGE2 production in bone marrow macrophages (BMMs). Although COX2/PGE2 was required for the in vitro inhibition of osteoblastic differentiation in response to PTH, the inhibition was not a direct effect of PGE2 on osteoblastic cells but instead was due to a factor secreted by BMMs in response to PGE2 and RANKL (11). That factor was subsequently identified in BMMs as serum amyloid A (SAA)3 (15).
SAA is a family of highly conserved apolipoproteins in vertebrates, whose levels can rise 1000-fold in the circulation during episodes of inflammation, injury, or infection (16–19). Four genes exist in mice (Saa1 to Saa4) and in humans (SAA1 to SAA4). The individual family members are acutely or constitutively expressed. In mice Saa1, Saa2, and Saa3 are acute phase inducible genes, whereas Saa4 is constitutively expressed. In humans, SAA1 and SAA2 are inducible, SAA4 is constitutive, and SAA3 may be a pseudogene (20, 21). Although liver is thought to be the source of most inducible SAA in serum, all inducible isoforms are also produced extrahepatically by a variety of tissues, including macrophages and adipocytes (19, 22–27). All serum SAAs are associated with high-density lipoproteins, SAA3 less than SAA1 and SAA2, and effects are probably modulated by this association. SAA proteins have been implicated in many diseases associated with inflammation but their exact functions and mechanisms of action are still being determined (19).
Our studies to date have shown that SAA3 is secreted by murine BMMs in response to RANKL and COX2/PGE2 and is likely the inhibitor of PTH-stimulated differentiation in murine osteoblast cultures. Our major goal for this study was to determine whether SAA3 mediated the switch from catabolic to anabolic of continuous PTH infusion in mice, as suggested by the in vitro studies. A second goal was to determine whether continuous PTH had similar effects on human osteoblastic cells to those seen in murine osteoblastic cells and whether SAA might play a role in these effects.
Materials and Methods
Materials
Human PTH (hPTH; hPTH1–34, H-4835) was purchased from Bachem Bioscience (Torrance, CA). ALZET micro-osmotic pumps (model 1004) were from Durect (Cupertino, CA). NS398 (70590), a selective COX2 activity blocker, was from Cayman Chemical (Ann Arbor, MI). Recombinant murine (rm) macrophage colony-stimulating factor (rmM-CSF, 416-ML), recombinant human (rh)M-CSF (216-MC), rmRANKL (462-TEC), rhRANKL (390-TN), rmOPG/Fc-chimera (459-MO), and rhOPG (185-OS) were purchased from R&D Systems (Minneapolis, MN). rhSAA1 (30053) was from PeproTech (Rocky Hill, NJ). rhSAA2 (TP304977) was from Origene (Rockville, MD). All other chemicals were from Sigma-Aldrich (St. Louis, MO), unless otherwise stated.
Generation and genotyping of SAA3 knockout mice
The SAA3 knockout (KO) mouse in the CD-1 outbred background was generated at the Center for Mouse Genome Modification of UConn Health (Supplemental Fig. 1). The mouse line was generated by CRISPR/Cas9-mediated gene editing by microinjection of Saa3 single-guided RNA, Cas9 mRNA, and targeting single-stranded oligo-directed nucleotides into the pronucleus of CD-1 one-cell embryos. The single-stranded oligo-directed nucleotide, bearing two in-frame tandem termination codons (TAA and TGA) and an SalI restriction enzyme digestion site, was introduced (knockin) into the first coding exon of the Saa3 gene to generate the SAA3 L14X mutation. The knockin was first identified by PCR followed by SalI digestion and then confirmed by sequencing of the PCR product. We obtained a positive heterozygous (HET) founder male mouse with one WT allele and one knockin allele. This founder male was bred with WT CD-1 females to establish the mouse lines. For subsequent genotyping, the following primers were used: Saa3nE2Reverse, 5′-CCATCTTTGGCTGTCAACTCCC-3′ (specific to WT allele); Saa3nSReverse, 5′-CCATCTTTGGCTGTCgACTTAT-3′ (specific to knockin allele); and Saa3nE1Forward, 5′-GAGTTGCCAGAACTTTCCAG-3′ (specific to WT and knockin alleles). Genotyping was performed in tail DNA extracts by PCR with conditions as follows: 33 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and elongation at 72°C for 45 seconds. For the experiments, WT and SAA3 KO mice were generated by HET × HET mating. The HET colonies were refreshed twice a year by mating HET mice with WT mice bought from Charles River (Wilmington, MA).
Continuous PTH infusion protocol
WT and SAA3 KO male mice (3.5 months old; n = 6 per group) were infused with vehicle (0.001 N hydrochloric acid/acidified 0.1% BSA in 1× PBS) or PTH (hPTH1–34, 40 µg/kg/d) for 12 days. The ALZET micro-osmotic pumps (flow rate 0.11 µL/h and delivery time 4 weeks) were filled with 100 µL of vehicle or PTH and surgically implanted, under isoflurane (1.5% to 2% with 250 mL/min O2) anesthesia, into the subcutaneous cavity of the midscapular region of mice following the manufacturer’s instructions (http://www.alzet.com). Prior to implantation, the filled pumps were primed in sterile saline for 6 hours at 37°C. After implantation, mice were examined regularly for any signs of distress and weighed every third day.
Serum measurements
At the end of the infusion, blood was collected by heart puncture after euthanasia with gaseous carbon dioxide. The blood was allowed to clot for 30 minutes at room temperature, and sera were collected after centrifugation for 10 minutes at 5000 rpm. Tartrate-resistant acid phosphatase (TRAP) 5b, C-terminal telopeptide (CTX), and N-terminal propeptide of type I procollagen (PINP) were assayed using the MouseTRAP enzyme immunoassay (EIA; SB-TR103), Mouse/RatLaps CTX-1 EIA (AC-06F1) and rat/mouse PINP EIA (AC-33F1) kits from Immunodiagnostic Systems (Fountain Hills, AZ). Osteocalcin was measured by a mouse osteocalcin ELISA kit (60-1305) from Immutopics (San Clemente, CA). Calcium was measured by a kit (2400-1) from Eagle Diagnostics (De Soto, TX). SAA3 was measured by a mouse SAA3 ELISA kit (EZMSAA3-12K) from Millipore EMD (Billerica, MA). For TRAP, CTX, and PINP kits, the company reports an intra-assay coefficient of variation (CV) of <6.5%, 5.8% to 9.2% and 5.0% to 6.4%, respectively, and the lowest detection limit as 0.1 U/L, 2 ng/mL, and 0.7 ng/mL, respectively. For the osteocalcin (BGLAP) kit, the company reports an intra-assay CV of 2.3% to 3.7% and the lowest detection limit as 0.4 ng/mL. For the SAA3 kit, the company reports an intra-assay CV of 3% to 14% and the lowest detection limit as 0.078 µg/mL.
Dual-energy X-ray absorptiometry
In vivo femur bone mineral density (BMD) was measured by PIXImus2 densitometer (GE Medical Systems, Madison, WI). Mice were anesthetized with isoflurane (1.5% to 2% with 250 mL/min O2) and BMD of right femur was measured 1 day prior to infusion (start BMD) and at the end of infusion. The percentage change in BMD was calculated relative to start BMD for each mouse. Lean mass, percentage body fat, and bone mineral content (BMC) were also measured by dual-energy X-ray absorptiometry (DXA). The region of interest (ROI) for percentage body fat was set to exclude the head and was from cervical vertebra C3 to sacral vertebra S3.
Ex vivo microcomputed tomography
Lumbar vertebrae (L1 to L6) and right femurs, dissected of connective tissue, were fixed in 70% ethanol. Trabecular morphometry within the centrum of L4 vertebrae and the metaphyseal region of distal femurs and the cortical morphometry at mid-diaphysis of femurs were measured using cone-beam microfocus X-ray CT (µCT40; ScanCo Medical, Bassersdorf, Switzerland). Serial tomographic images were acquired at 55 kV and 145 µA, collecting 1000 projections per rotation at 300 ms integration time. Three-dimensional images were reconstructed in a 16-mm field of view using the standard convolution back-projection algorithms at a discrete density of 244,141 voxels/mm3 (16-µm cubic voxels). Segmentation of bone from marrow was performed in conjunction with constrained Gaussian filter, applying hydroxyapatite-equivalent density thresholds of 285 and 740 mg/cm3 for trabecular and cortical compartments, respectively. For trabecular morphometry in the distal femur, the ROI was defined within the endosteal borders and located 1 mm proximally from the midline of the growth plate. In the vertebral bodies, ROI included the central 80% of vertebral height.
Static and dynamic histomorphometry
For dynamic histomorphometry, mice were injected IP with calcein (20 mg/kg) and demeclocycline (50 mg/kg) at 7 and 2 days, respectively, before euthanasia. Right femurs were used for the dynamic and left femurs for the static histomorphometry. Both measurements were performed on the OsteoMeasure analysis software (OsteoMetrics, Atlanta, GA). The terminology and units used are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (28).
For static histomorphometry, femurs were fixed in 10% buffered formalin, decalcified in 15% EDTA, dehydrated in grades of ethanol, and cleared in xylene and embedded in paraffin. Five-micrometer-thick longitudinal sections were cut on a microtome (Microm, Richards-Allan Scientific, Kalamazoo, MI) and stained for TRAP and hematoxylin. Static parameters were measured in a defined area between 200 and 2000 µm from the end of the growth plate, encompassing an area of 2.25 mm2.
For dynamic histomorphometry, 10-μm-thick longitudinal sections were cut from femurs fixed in 70% ethanol and embedded undecalcified in methyl methacrylate. The ROI for measurements was the same as used for static analysis. Mineralizing surface per bone surface (MS/BS) and mineral apposition rate (MAR), calculated as the distance between the midpoints of double labels over time interval, were measured on unstained sections under ultraviolet light, using a 4′,6-diamidino-2-phenylindole/fluorescein/Texas Red triple-band filter. Bone formation rate per bone surface (BFR/BS) was calculated as the product of MAR and MS/BS. Bright-field microscopic images were acquired at ×20 (2/0.08× objective) and ×200 (20/0.50× objective) magnifications and fluorescent images were acquired at ×400 (40/0.75× objective) magnification using Olympus DP73 microscope with cellSens Entry software.
Cell cultures
All cell cultures were maintained in a humidified atmosphere of 5% carbon dioxide at 37°C. The basic medium used was α-minimal essential medium (12571063) with 10% heat-inactivated fetal bovine serum (10082147) and 100 U/mL penicillin-streptomycin (15140148) from Invitrogen (Carlsbad, CA). Medium was changed every 3 days. The solvents used for the preparation of various treatments were: 0.1% BSA in 1× PBS for RANKL, OPG, M-CSF, SAA1, and SAA2; 0.001 N hydrochloric acid/acidified 0.1% BSA in 1× PBS for PTH; dimethyl sulfoxide for isobutyl methylxanthine (IBMX); and ethanol for NS398. The concentration of all solvents in the culture medium was kept at ≤0.1%.
Murine cell cultures
Bone marrow stromal cell (BMSC) cultures, for osteoblast differentiation studies, were prepared as described previously (11). Whole marrow from long bones of 12- to 14-week-old WT and SAA3 KO male mice was plated in six-well tissue culture dishes at 106 nucleated cells per well and cultured in osteoblast differentiation medium containing basic medium plus 50 µg/mL phosphoascorbate (Wako Pure Chemical Industry, Osaka, Japan) from the beginning and with every medium change. All treatments were given from the beginning and with every medium change. To induce mineralization, 5 mM β-glycerol phosphate was added on day 7. At the time of medium change, the spent medium was collected, centrifuged at 800 rpm for 5 minutes at 4°C to remove debris, and stored frozen at −80°C for PGE2 measurements.
BMMs were prepared as described previously (11, 15). Whole marrow from long bones of 12- to 14-week-old male WT and SAA3 KO mice was plated at 107 nucleated cells per well in 150-mm Petri dishes (Fisher Scientific, Pittsburg, PA) in basic medium plus 100 ng/mL rmM-CSF. BMMs were expanded in rmM-CSF one to three times, for 3 days, before being used for the experiments. Bone marrow cultures, to quantify the number of osteoclasts, were prepared by plating the whole marrow from long bones of 12- to 14-week-old WT and SAA3 KO male mice in 24-well tissue culture dishes at 2 × 106 nucleated cells per well in basic medium and grown up to 8 days in the presence of vehicle or PTH.
Primary osteoblasts (POBs) from calvariae of 1-week-old WT pups were obtained as described previously (11, 15) and plated at 5 × 104 cells per well in six-well tissue culture dishes in osteoblast differentiation medium.
For conditioned medium (CM) studies, BMMs were plated at 6 × 104 cells per well in 12-well tissue culture dishes in basic medium plus rmM-CSF (30 ng/mL) and rmRANKL (30 ng/mL) for 1 day. CM was collected and centrifuged at 800 rpm for 5 minutes at 4°C and kept frozen until use. SAA3 accumulated in CM was assessed by an SAA3 ELISA kit (EZMSAA3-12K). For cAMP studies with CM, POBs were grown for 5 days in osteoblast differentiation medium and then treated with 3 parts CM and 1 part osteoblast differentiation medium plus 0.5 mM IBMX, to block phosphodiesterase activity, 1 hour prior to the addition of vehicle or PTH for 20 minutes. For osteoblast differentiation studies with CM, POBs were cultured for 14 days with 3 parts CM and 1 part osteoblast differentiation medium plus vehicle or PTH from the beginning and with every medium change. β-Glycerol phosphate (5 mM) was added on day 7. All POB cultures were also given rmOPG (50 ng/mL) at the time when CM was added to block any osteoclastogenesis, developing from the contaminating marrow cells present in the POBs, by RANKL, in the CM, or induced by PTH treatment.
Human cell cultures
Bone marrow aspirates were obtained from the proximal humeri of de-identified consenting patients undergoing arthroscopic rotator cuff repair as described previously (29). The patients included six males and one female of average age 55 ± 2 years (range, 46 to 60 years). None of the patients had a history of diabetes, steroids intake, radiotherapy, chemotherapy, hepatitis, HIV/AIDS, or any health conditions affecting bone or soft-tissue growth. In brief, the bone marrow aspirate was obtained by inserting a 14-guage aspiration needle into the medullary cortex, at the bone cartilage junction, of the proximal head of the humerus, where the first suture anchor of the repair was placed later during the surgery. The aspirate was aseptically transferred to the laboratory, where it was centrifuged and the pellet was suspended in basic medium.
For BMSC cultures, 4 × 106 nucleated cells per well were plated in six-well dishes and cultured up to 21 days in osteoblast differentiation medium. Otherwise, the protocols were similar to those for murine BMSCs.
Human BMMs were prepared similar to murine BMMs. For osteoclastogenesis, gene expression, and CM experiments, human BMMs were plated in 12-well dishes at 10 × 104 cells per well and cultured in basic medium with rhM-CSF (30 ng/mL) or rhM-CSF plus rhRANKL (30 ng/mL each) in the absence or presence of NS398 (100 nM) for 8 days. On one set of cells, total RNA was extracted on day 3. The other set of cells was continued as cultures and was fixed every day thereafter for TRAP staining. Medium was collected from each well every day after day 3 and centrifuged at 800 rpm for 5 minutes at 4°C and stored frozen at −80°C for SAA1 and SAA2 ELISA and for CM experiments.
For CM studies, frozen medium from days 4 to 6 of rhM-CSF plus rhRANKL with and without NS398-treated cultures was used. For osteoblast differentiation studies, 4 × 106 nucleated whole bone marrow cells were plated per well in six-well dishes and cultured up to 21 days in 3 parts CM and 1 part osteoblast differentiation medium. rhOPG (100 ng/mL) was added to prevent RANKL from CM, or induced by PTH, from stimulating osteoclastogenesis. Vehicle or PTH was given from the beginning of culture and with every medium change, and 5 mM β-glycerol phosphate was added on day 7 of culture.
Intracellular cAMP measurement
Murine POBs were scraped in 500 µl per well of ice-cold ethanol. The ethanolic cell suspensions were collected and centrifuged at 4°C for 10 minutes at 1500g. Supernatants were evaporated to dryness using a lyophilizer. cAMP was measured with an ELISA kit from Cayman Chemical (581001). The company reports an intra-assay CV of 6.7% to 11.6% and the lowest detection limit as 3.1 pmol/mL.
PGE2 measurement
PGE2 accumulated in the medium of human BMSCs was measured with a monoclonal PGE2 ELISA kit (514010) from Cayman Chemical. The company reports an intra-assay CV of 4.2% to 30.4% and the lowest detection limit of 15 pg/mL. PGE2 accumulated in the medium of murine BMSCs was measured by the Parameter™ PGE2 ELISA kit (KGE0048) from R&D Systems. The company reports an intra-assay CV of 5.0% to 9.0% and the lowest detection limit is 39 pg/mL.
SAA1 and SAA2 measurement
SAA1 protein accumulated in the medium of human BMMs was measured by an SAA1 human ELISA kit (KA2108; Abnova Corporation, Walnut, CA). The company reports an intra-assay CV of 3.1% to 4.0% and the lowest detection limit is 0.06 µg/mL. SAA2 protein was measured by an SAA2 human ELISA kit (EKU07300; Biomatik, Wilmington, DE). The company reports an intra-assay CV of <10% and the lowest detection limit is 0.279 ng/mL.
Quantitative real-time PCR
Total RNA was extracted from cells and freshly dissected tissues, including tibia, kidney, and liver. For tibial RNA extraction, both ends of each tibia were cut off to remove the growth plates and the marrow was not flushed. Both tibiae from a mouse were combined and homogenized in TRIzol (Invitrogen) for one sample of RNA. For kidney RNA, the left kidney from each mouse was halved (∼100 mg) and homogenized in TRIzol. For liver RNA, a small piece from the right liver lobe (∼70 to 80 mg) was homogenized in TRIzol. Integrity of RNA was checked on BioAnalyzer RNA NanoChips (Agilent Technologies, Santa Clara, CA) at the UConn Health molecular core facility. Four or 5 µg of total RNA was DNase treated (Ambion, Austin, TX) and converted to cDNA by a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). PCR was performed in 96-well plates. Each sample was amplified in duplicate. Primers for PCR (Supplemental Table 1) were either Assay-on-Demand Gene Expression TaqMan® probes (Applied Biosystems) or validated SYBR Green primers (http://pga.mgh.harvard.edu/primerbank). The TaqMan gene assay ID Mm04208126_mH for Saa2 detected both Saa2.1 (RefSeq NM_011314.2) and Saa1.1 (RefSeq NM_009117.3) transcripts, whereas the gene assay ID Mm00656927_g1 for Saa1 detected only Saa1.1 transcript (RefSeq NM-009117.3). Gapdh or GAPDH or Actb (actin, β) served as endogenous control. All primers were checked for equal efficiency over a range of target gene concentrations. PCR reaction mixture was run in an Applied Biosystems QuantStudio Flex 6 instrument. Data were analyzed by comparative Ct (ΔΔCt) method, and results reported as relative quantification (RQ) values. The RQ values were calculated as follows: ΔCt target = Ct target in the sample − Ct endogenous control in the sample; ΔCt calibrator = Ct target in the calibrator − Ct endogenous control in the calibrator; ΔΔCt = ΔCt target − ΔCt calibrator. RQ = 2−ΔΔCt. As per the manufacturer’s recommendation, Ct values >35 were considered undetectable.
SAA3 immunostaining
Murine BMMs, at 104 cells per well in eight-well chamber slides (Nunc® Laboratory-Tek®, Thermo Fisher Scientific, Waltham, MA), were grown for a day in basic medium plus 30 ng/mL each of rmM-CSF and rmRANKL. Cells were fixed in 4% formaldehyde in 1× PBS for 15 minutes at room temperature. Cells were rinsed three times with 1× PBS and blocked in 5% normal goat serum in 1× PBS containing 0.3% Triton X-100 for 60 minutes. Cells were then incubated with rabbit polyclonal SAA (H-84; sc-20651; Santa Cruz Biotechnology, Dallas, TX) antibody (30) at 1:100 dilution in 1% BSA in 1× PBS containing 0.3% Triton X-100 overnight at 4°C. Cells were rinsed three times in 1× PBS and then incubated with goat anti-rabbit IgG-Texas Red–conjugated secondary (sc-2780; Santa Cruz Biotechnology) antibody (31) at 1:200 dilution for 2 hours at room temperature in the dark. After rinsing in 1× PBS, cells were covered with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (8961; Cell Signaling Technology, Danvers, MA). Fluorescent microscopic images were acquired at ×200 magnification (20/0.40× objective) using Leica Microsystems LAS X applications.
TRAP staining
Cell cultures were fixed with 2.5% glutaraldehyde in 1× PBS for 30 minutes at room temperature and stained with a leukocyte acid phosphatase kit (TRAP kit, Sigma-Aldrich). TRAP+ multinucleated cells (MNCs) per well were counted under the microscope. Bright-field images were acquired using a Nikon Eclipse TE 2000-U, RT Slider Spot diagnostic microscope with Spot advanced software.
Alizarin red staining
To stain mineralized nodules, cells were washed with 1× PBS, fixed in 100% methanol on ice for 30 minutes at 4°C, and stained with 40 mM solution of alizarin red S (pH 4.2) for 10 minutes at room temperature. Dishes were washed with water, air dried, and scanned for images.
Statistical analysis
All data are presented as mean ± SEM. Statistical analyses were performed using SigmaPlot 11 (Systat Software). Normally distributed data comparing two groups were analyzed by a two-tailed unpaired t test. Normally distributed data with more than two groups were analyzed by one- or two-way ANOVA and post hoc Bonferroni pairwise multiple comparisons. When data for one-way ANOVA were not normally distributed, they were examined by a Kruskal–Wallis ANOVA on ranks with a Tukey post hoc comparison. When data for two-way analyses were not normally distributed, they were log10 transformed before analysis.
Study approval
All animal studies were approved by the Institutional Animal Care and Use Committee of UConn Health (Farmington, CT). All human studies were approved by the Institutional Review Board of UConn Health. Written informed consent statements were received from all the patients prior to inclusion in the study.
Results
Basal skeletal phenotypes of WT and SAA3 KO mice were similar
We used CRISPR/Cas9-mediated gene editing to generate a knockin mouse unable to produce the SAA3 gene product, called the SAA3 KO mouse (Supplemental Fig. 1). The SAA3 KO mice were obtained in the expected Mendelian ratios (Supplemental Table 2). At 3.5 months of age, male SAA3 KO mice weighed 4% more than WT mice, associated with 4% higher body fat by DXA (Table 1). There was no difference in body weight or fat between female WT and KO mice. There were no differences between WT and KO mice in lean mass, femoral BMD, or BMC.
Table 1.
Phenotyping of 3.5-mo-Old WT and SAA3 KO Male and Female Mice
| Parameter | WT Male (n = 24) | KO Male (n = 24) | WT Female (n = 19) | KO Female (n = 19) |
|---|---|---|---|---|
| Weight, g | 31.5 ± 0.17 | 32.8 ± 0.18a | 25.7 ± 0.23 | 25.8 ± 0.18 |
| BMD, mg/cm2 | 78.6 ± 0.99 | 81.1 ± 1.05 | 70.7 ± 0.60 | 71.0 ± 0.68 |
| BMC, mg | 31.6 ± 0.22 | 32.2 ± 0.24 | 25.6 ± 0.34 | 25.7 ± 0.32 |
| Body fat, % | 15.9 ± 0.23 | 16.6 ± 0.24b | 14.6 ± 0.21 | 14.5 ± 0.22 |
| Lean mass, g | 23.7 ± 0.09 | 23.9 ± 0.10 | 19.7 ± 0.25 | 19.9 ± 0.20 |
Femoral BMD, femoral BMC, body fat, and lean mass were measured in vivo by DXA. Data are means ± SEM for 19 to 24 mice.
P < 0.01, determined by two-tailed, unpaired t test.
P < 0.05, determined by two-tailed, unpaired t test.
PTH infusion increased SAA3 and decreased BMD in WT mice
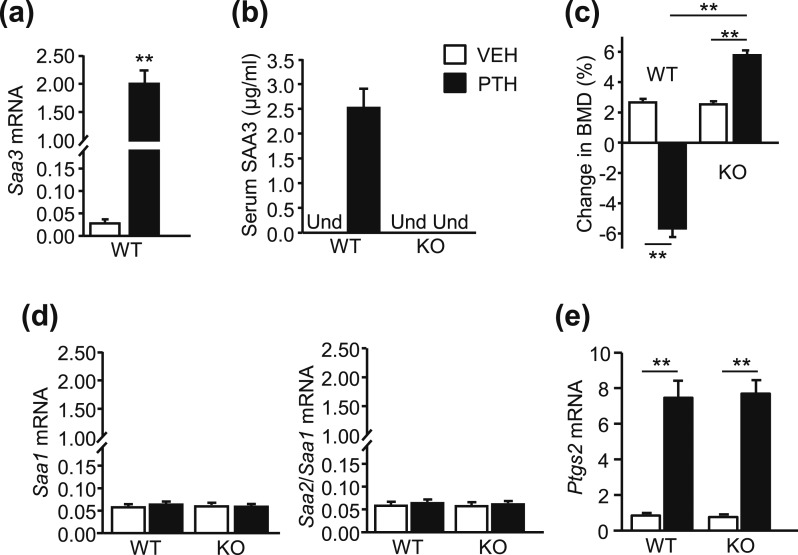
Male mice, 3.5 months old, were infused for 12 days with vehicle or PTH (40 µg/kg/d). WT and SAA3 KO mice became hypercalcemic by the end of infusion but did not lose weight (Table 2). In WT vehicle-treated mice, Saa3 mRNA expression in the tibiae was very low (Ct ≥ 34, mean ΔCt = 15.8 ± 0.34) and SAA3 protein in serum was undetectable. We did not measure Saa3 mRNA in the KO mice because the transcript would be similar to WT except for the knockin mutation. As expected, serum SAA3 was undetectable in vehicle- and PTH-treated KO mice. Both Saa3 mRNA (mean ΔCt = 9.5 ± 0.17) and serum SAA3 were markedly increased after PTH infusion in WT mice [Fig. 1(a) and 1(b)]. Femoral BMD was measured at the beginning and end of infusion to calculate the percentage change [Fig. 1(c)]. Mice were still in the growth phase and femoral BMD increased 2.5% in both vehicle-infused WT and KO mice. PTH infusion decreased BMD 5.6% in WT mice or 8% relative to vehicle-treated WT mice. In SAA3 KO mice, infusion increased BMD 5.5% or 3% relative to vehicle-treated KO mice.
Table 2.
Body Weight and Serum Calcium of 3.5-mo-Old WT and SAA3 KO Male Mice Infused with Vehicle or PTH (40 µg/kg/d) for 12 d
| Parameter | WT Mice | SAA3 KO Mice | ||
|---|---|---|---|---|
| Vehicle (n = 6) | PTH (n = 6) | Vehicle (n = 6) | PTH (n = 6) | |
| Start weight, g | 31.6 ± 0.43 | 31.6 ± 0.32 | 33.2 ± 0.26 | 32.9 ± 0.51 |
| End weight, g | 32.8 ± 0.42 | 32.8 ± 0.26 | 34.3 ± 0.26 | 34.1 ± 0.48 |
| Serum calcium at end, mg/dL | 9.76 ± 0.07 | 11.4 ± 0.21a | 9.62 ± 0.14 | 11.5 ± 0.25a |
Data are means ± SEM for six mice.
P < 0.01, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
Figure 1.
PTH infusion increased SAA3 expression and decreased femoral BMD in WT mice. WT and SAA3 KO male mice (3.5 mo old) were infused with vehicle (VEH) or PTH (40 µg/kg/d) for 12 d. (a) Saa3 mRNA expression in tibiae of WT mice. Both ends of each tibia were cut off to remove the growth plates and the marrow was not flushed. mRNA expression was measured by quantitative real-time PCR (qPCR) and data were reported as RQ values. (b) SAA3 protein in serum of WT and SAA3 KO mice by ELISA. Und, undetectable. (c) Change in in vivo femoral BMD calculated as: (BMD at end of infusion − BMD at start of infusion)/BMD at start of infusion. (d) Saa1, Saa2/Saa1, and (e) Ptgs2 (COX2) mRNA in WT and KO tibiae. Both ends of each tibia were cut off to remove the growth plates and the marrow was not flushed. mRNA expression was measured by qPCR and data were reported as RQ values. Bars are means ± SEM for n = 6 mice per genotype and treatment group. For (a), **P < 0.01, determined by two-tailed, unpaired t test. For (c) and (e), **P < 0.01, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
To determine whether other SAA isoforms compensated for absent SAA3, we measured expression of Saa1 and Saa2 transcripts in tibiae. The primers for Saa1 detected transcript for Saa1 only, whereas the primers for Saa2 detected transcripts for both Saa1 and Saa2. The expression of Saa1 mRNA was low in tibiae from vehicle-infused WT mice (Ct ≥ 33, mean ΔCt = 14.2 ± 0.11) and vehicle-infused KO mice (Ct ≥ 33, mean ΔCt = 14.1 ± 0.11). The corresponding values for Saa2/Saa1 mRNA were also low for vehicle-infused WT (Ct ≥ 33, mean ΔCt = 14.1 ± 0.18) and vehicle-infused KO (Ct ≥ 33, mean ΔCt = 14.1 ± 0.17) mice. The expression of both Saa1 and Saa2/Saa1 was unaffected by PTH infusion [Fig. 1(d)]. PTH infusion increased Ptgs2 (COX2) mRNA similarly in tibiae of WT and SAA3 KO mice [Fig. 1(e)], consistent with COX2 being upstream from SAA3 and unaffected by SAA3 KO.
Because PTH1R is expressed in other tissues than bone (32, 33), and because liver is a major source of SAA3 (27), we examined Saa3 mRNA expression in kidney and liver to determine whether it was regulated by PTH (Table 3). There was no regulation of Saa3 mRNA by PTH in these tissues. PTH did increase Ptgs2 (COX2) mRNA in kidney tissue from both WT and KO mice but had no effect on Ptgs2 mRNA in liver tissue.
Table 3.
Saa3 and Ptgs2 (COX2) mRNA Expression in Kidneys and Livers of 3.5-mo-Old Male WT and SAA3 KO Mice Infused With Vehicle or PTH (40 µg/kg/d) for 12 Days
| Gene | Saa3 mRNA (RQ Values) | Ptgs2 mRNA (RQ Values) | ||||||
|---|---|---|---|---|---|---|---|---|
| WT | SAA3 KO | WT | SAA3 KO | |||||
| Vehicle | PTH | Vehicle | PTH | Vehicle | PTH | Vehicle | PTH | |
| Kidney | 0.98 ± 0.05 | 1.14 ± 0.22 | N/A | N/A | 0.05 ± 0.01 | 0.25 ± 0.08a | 0.05 ± 0.01 | 0.29 ± 0.09a |
| Liver | 58.0 ± 3.00 | 52.7 ± 5.33 | N/A | N/A | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.01 |
Data are means ± SEM for three mice.
Abbreviation: N/A, not applicable.
P < 0.05, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
PTH infusion increased trabecular bone in SAA3 KO mice but not in WT mice
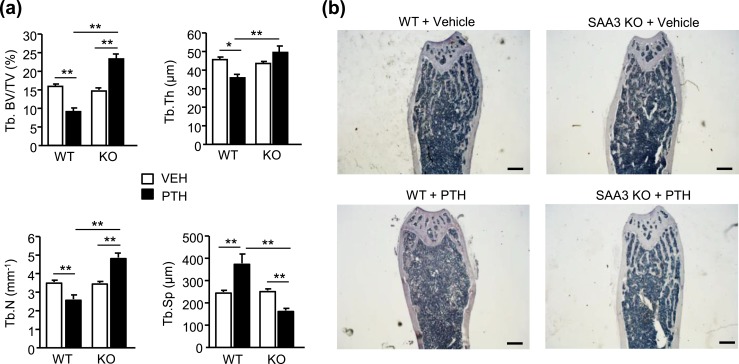
BMD measures contributions from both trabecular and cortical bone. We used microcomputed tomography (µCT) to measure morphometry of trabecular bone in both the distal femur and the L4 vertebra (Fig. 2). There was no significant difference in any of the parameters measured for femurs between vehicle-infused WT and SAA3 KO mice [Fig. 2(a) and 2(b)]. Compared with the vehicle controls, PTH infusion decreased femoral trabecular bone volume/total volume (BV/TV) 49% in WT mice. This loss of BV/TV was associated with decreased trabecular thickness (Tb.Th) of 15%, decreased trabecular number (Tb.N) of 28%, increased trabecular spacing (Tb.Sp) of 46%, and decreased connectivity density (Conn.Dens) of 52% in PTH-infused WT mice compared with vehicle-infused WT mice [Fig. 2(b)]. In contrast, PTH infusion increased femoral BV/TV 40% in SAA3 KO mice. This increase in BV/TV was associated with no change in Tb.Th and increased Tb.N of 17% in PTH-infused KO mice compared with vehicle-infused KO mice. There was a small but nonsignificant decrease in Tb.Sp and increase in Conn.Dens in PTH-infused KO mice compared with vehicle-infused KO mice. However, when PTH-infused KO mice were compared with PTH-infused WT mice, femoral BV/TV was 2.4-fold greater, Tb.Sp was decreased by 55%, and Conn.Dens was increased by 3.2-fold. The structure model index (SMI) in vehicle-infused WT and KO femurs was 1.99 ± 0.06 and 2.18 ± 0.07, respectively, and in PTH-infused WT and KO femurs was 2.50 ± 0.22 and 1.74 ± 0.16, respectively. The decrease in SMI in femurs from PTH-infused KO mice compared with femurs from PTH-infused WT mice was significant (P < 0.01), suggesting that the trabeculae became more platelike in PTH-infused KO femurs.
Figure 2.
PTH infusion increased trabecular bone in SAA3 KO mice but not in WT mice. WT and SAA3 KO male mice (3.5 mo old) were infused with vehicle (VEH) or PTH (40 µg/kg/d) for 12 d. (a–d) µCT analyses of trabecular bone in distal femurs and L4 vertebrae of WT and SAA3 KO male mice. (a) Representative longitudinal images (scale bars, 400 µm) of trabecular bone in distal femurs and (b) corresponding morphometry for trabecular BV/TV, Tb.Th, Tb.N, Tb.Sp, and Conn.Dens. (c) Representative longitudinal images (scale bars, 400 µm) and (d) corresponding morphometry of trabecular bone in L4 vertebrae. Bars are means ± SEM for n = 6 mice per genotype and treatment group. *P < 0.05, **P < 0.01, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
µCT morphometry of trabecular bone in L4 vertebrae showed no significant difference in any of the parameters measured between vehicle-infused WT and KO mice [Figure 2(c) and 2(d)]. PTH infusion decreased vertebral BV/TV 28% in WT mice compared with vehicle-infused mice. There was no significant change in Tb.Th, Tb.N, and Tb.Sp. Conn.Dens was decreased 38% by PTH in WT mice compared with vehicle-infused WT mice. PTH infusion increased vertebral BV/TV 70% in KO mice compared with vehicle-infused mice. This increase was associated with an increase in Tb.Th of 29%, an increase of 31% in Tb.N, a decrease in Tb.Sp of 23%, and a nonsignificant increase in Conn.Dens in PTH-infused KO mice compared with vehicle-infused mice. When PTH-infused KO mice were compared with PTH-infused WT mice, vertebral BV/TV was 2.3-fold greater and Conn.Dens was 80% greater. The SMI in vertebrae from vehicle-infused WT and KO mice was 1.80 ± 0.14 and 1.78 ± 0.06, respectively. The SMI in vertebrae from PTH-infused WT and KO mice was 2.24 ± 0.09 and 0.82 ± 0.18, respectively. The decrease in SMI in vertebrae from PTH-infused KO mice was significant (P < 0.01) compared with vertebrae from vehicle-infused KO and PTH-infused WT mice, suggesting that similar to femurs, trabeculae become more platelike in KO vertebrae with PTH.
Cortical morphometry measured by µCT at femur mid-diaphysis did not show any significant differences between vehicle- or PTH-infused WT and KO mice (Table 4).
Table 4.
Cortical Morphometry by µCT in the Femoral Midshaft Region of WT and SAA3 KO Male Mice Infused with Vehicle or PTH
| Parameter | WT Mice | SAA3 KO Mice | ||
|---|---|---|---|---|
| Vehicle (n = 6) | PTH (n = 6) | Vehicle (n = 6) | PTH (n = 6) | |
| Femur length, mm | 16.1 ± 0.06 | 16.2 ± 0.14 | 15.7 ± 0.21 | 15.7 ± 0.15 |
| Cortical area, mm2 | 1.17 ± 0.04 | 1.05 ± 0.08 | 1.03 ± 0.04 | 1.08 ± 0.05 |
| Cortical thickness, mm | 0.25 ± 0.00 | 0.22 ± 0.01 | 0.22 ± 0.00 | 0.22 ± 0.00 |
| Cortical porosity, % | 0.29 ± 0.03 | 0.24 ± 0.03 | 0.22 ± 0.03 | 0.48 ± 0.24 |
| Subperiosteal area, mm2 | 2.33 ± 0.04 | 2.23 ± 0.11 | 2.26 ± 0.09 | 2.30 ± 0.09 |
| Subendosteal area, mm2 | 1.15 ± 0.04 | 1.18 ± 0.05 | 1.22 ± 0.05 | 1.22 ± 0.05 |
Data are means ± SEM for six mice.
PTH infusion increased bone formation parameters only in SAA3 KO mice but increased resorption parameters similarly in both WT and SAA3 KO mice
Static histomorphometry permits the measurement of the percentage trabecular bone surface covered by the bone-forming osteoblasts [osteoblast surface per bone surface (Ob.S/BS)] and the bone-resorbing osteoclasts [osteoclast surface per bone surface (Oc.S/BS)]. We first confirmed that the trabecular bone changes in the distal femur resulting from PTH infusion in WT and KO mice as determined by histomorphometry were similar to those observed by µCT [Fig. 3(a) and 3(b)]. PTH infusion increased Ob.S/BS 5.7-fold in femoral trabecular bone from SAA3 KO mice and only 1.5-fold in WT mice [Fig. 4(a)]. Plump, cuboidal osteoblasts were seen around trabeculae in PTH-infused KO femoral trabecular bone but not in PTH-infused WT [Fig. 4(b)]. In contrast, PTH increased Oc.S/BS and percentage eroded surface per bone surface similarly in WT and KO femurs [Fig. 4(a)].
Figure 3.
Histomorphometric analyses of trabecular bone in distal femurs were similar to µCT analyses. WT and SAA3 KO male mice (3.5 mo old) were infused with vehicle (VEH) or PTH (40 µg/kg/d) for 12 d. (a) Histomorphometric parameters for trabecular BV/TV, Tb.Th, Tb.N, and Tb.Sp. (b) Representative hematoxylin-stained ×20 original magnification (scale bars, 500 µm) microscopic images of femurs. Bars are means ± SEM for n = 6 mice per genotype and treatment group. *P < 0.05, **P < 0.01, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
Figure 4.
PTH infusion increased trabecular bone formation parameters only in SAA3 KO mice but increased resorption parameters similarly in both WT and SAA3 KO mice. WT and SAA3 KO male mice were infused with vehicle (VEH) or PTH. (a) Static histomorphometry of trabecular bone in distal femurs for Ob.S/BS, Oc.S/BS, and eroded surface per bone surface (ES/BS). (b) Representative TRAP-stained (red) and counterstained hematoxylin ×200 original magnification (scale bars, 100 µm) microscopic images of trabecular bone in distal femurs. Plump cuboidal osteoblasts around trabeculae are marked with yellow arrows. (c) Dynamic histomorphometry of trabecular bone in distal femurs for MS/BS, MAR, and BFR/BS. (c) Representative calcein-labeled (green) and demeclocycline-labeled (orange-brown) ×400 original magnification (scale bars, 25 µm) microscopic images of trabeculae. (e and f) Measurement of (e) serum bone formation markers, PINP, and BGLAP (osteocalcin) and (f) serum bone resorption markers, CTX and TRAcP5b, measured by ELISA. (g and h) mRNA expression of Runx2, Bglap (osteocalcin), Tnfsf11 (RANKL), and Tnfrsf11b (OPG) in the tibiae measured by quantitative real-time PCR and reported as RQ values. Both ends of each tibia were cut off to remove the growth plates and the marrow was not flushed. Bars are means ± SEM for n = 6 mice per genotype and treatment group. *P < 0.05, **P < 0.01, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
Dynamic histomorphometry compares the uptake of bone-seeking labels, injected at two different times, at mineralizing surfaces [Figure 4(c) and 4(d)]. PTH infusion increased the percentage MS/BS 11.1-fold in KO femurs and only 2.5-fold in WT femurs. MAR was increased 3.1-fold in KO femurs by PTH. BFR/BS was increased 31.8-fold in KO femurs and only 3.6-fold in WT femurs by PTH. Hence, PTH increased the number of actively mineralizing osteoblasts more in SAA3 KO femurs than in WT femurs.
Serum markers of bone formation and bone resorption were measured after the 12 days of infusion. There was no difference in any of these markers between vehicle-infused WT and SAA3 KO mice [Fig. 4(e) and 4(f)]. PTH infusion increased markers of formation, P1NP and osteocalcin (BGLAP), fivefold and 6.8-fold, respectively, in KO mice but did not increase either in WT mice [Fig. 4(e)]. In contrast, the markers of bone resorption, CTX and TRAP 5b, were increased similarly in both WT and KO mice [Fig. 4(f)]. Hence, absence of SAA3 increased the bone formation response to PTH without affecting the bone resorption response to PTH.
We also measured the mRNA expression of genes associated with bone formation [Fig. 4(g)] and resorption [Fig. 4(h)] in tibiae from WT and KO mice at the end of infusion. There was no difference in gene expression between vehicle-infused WT and KO mice. Runx2 and Bglap (osteocalcin), genes associated with osteoblast differentiation, were increased by PTH infusion only in SAA3 KO tibiae. Tnfsf11 (RANKL), whose product is critical for osteoclastogenesis, and Tnfrsf11b (OPG), whose product is the decoy receptor for RANKL, are combined in the ratio Tnfsf11/Tnfrsf11b to determine bone resorption (34). The expression of Tnfsf11 was increased similarly in both WT and KO tibiae by PTH, and the expression of Tnfrsf11b was decreased similarly in both WT and KO tibiae by PTH. The ratio of Tnfsf11/Tnfrsf11b was increased similarly in both WT (3.1-fold) and KO (2.9-fold) tibiae by PTH. Hence, all measures of factors associated with bone resorption were affected similarly by PTH infusion in both WT and KO mice.
PTH infusion increased expression of genes that mediate anabolic effects and decreased expression of genes that inhibit anabolic effects only in SAA3 KO mice
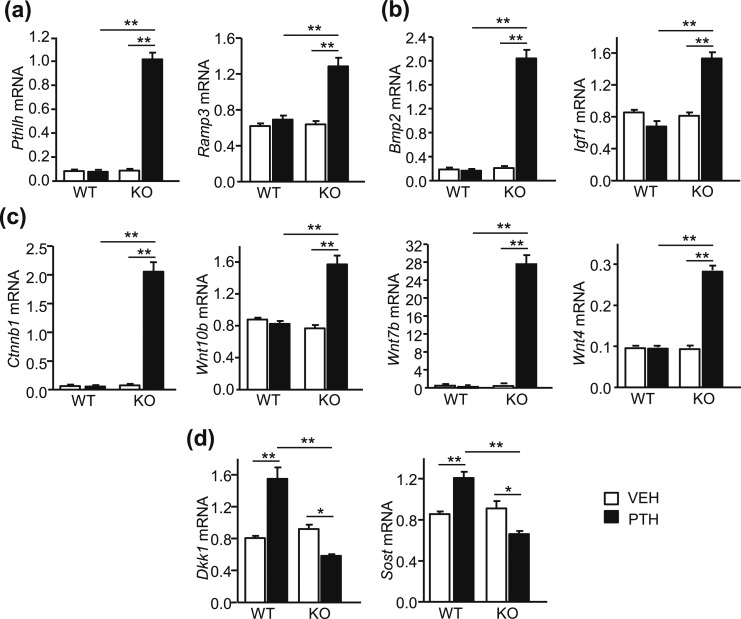
In vivo studies comparing effects of injected PTH (1–34) and PTH analogs in mice concluded that the anabolic effects of PTH occurred predominantly via Gαs/cAMP-initiated pathways (35). Gene expression for two proteins whose expression is mediated via cAMP, PTHrP (Pthlh) and receptor activity modifying protein 3 (Ramp3), were measured in the tibiae at the end of infusion. PTH increased the expression of both only in bone from SAA3 KO mice [Fig. 5(a)].
Figure 5.
PTH infusion increased expression of genes that mediate anabolic effects and decreased expression of genes that inhibit anabolic effects in SAA3 KO but not WT mice. mRNA expression measured in tibiae of WT and SAA3 KO male mice infused with vehicle (VEH) or PTH. Both ends of each tibia were cut off to remove the growth plates and the marrow was not flushed. mRNA expression was measured by quantitative real-time PCR and reported as RQ values. mRNA expression of (a) cAMP-regulated genes, Pthlh (PTHrP) and Ramp3, (b) growth factor genes, Bmp2 and Igf1, (c) genes promoting Wnt signaling, Ctnnb1 (catenin, β1), Wnt10b, Wnt7b, and Wnt4, and (d) genes inhibiting Wnt signaling, Dkk1 and Sost. Bars are means ± SEM for n = 6 mice per genotype and treatment group. *P < 0.05, **P < 0.01, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
Growth factors, such as bone morphogenetic protein 2 (BMP2) and IGF1, are proposed to mediate the anabolic effects of PTH (36, 37). PTH increased the gene expression of both Bmp2 and Igf1 only in bone from SAA3 KO mice [Fig. 5(b)]. Wnt signaling is also considered to be important for the anabolic effects of PTH (38–40). Ctnnb1 (catenin, β1), Wnt4, Wnt7b, and Wnt10b were all elevated by PTH only in tibial bone from SAA3 KO mice [Fig. 5(c)]. In contrast, gene expression for inhibitors of Wnt signaling, Dkk1 and Sost, was increased in WT bone by PTH but inhibited in SAA3 KO bone [Fig. 5(d)]. Thus, continuous PTH in vivo increased expression of genes associated with positive effects on bone formation, while decreasing expression of genes associated with negative effects, in SAA3 KO mice but not in WT mice.
In vitro results from continuously treated murine bone marrow cultures paralleled in vivo results from infusion studies
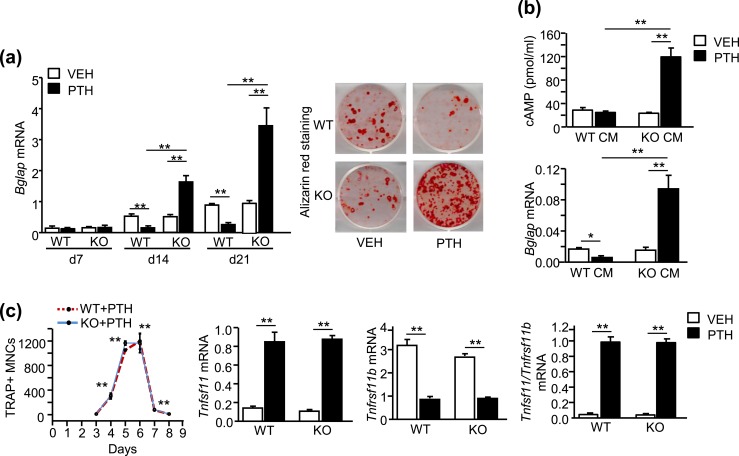
PTH was given to WT and SAA3 KO BMSC cultures with every medium change. This protocol provides continuous exposure to PTH, as PTH is stable in these cultures up to 72 hours between medium changes (41). PTH increased Bglap (osteocalcin) mRNA and alizarin red staining for mineralization in SAA3 KO BMSC cultures but not in WT BMSC cultures [Fig. 6(a)]. Thus, PTH stimulated osteoblastic differentiation in the absence of SAA3, despite the presence of PTH-induced PGE2 in WT and SAA3 KO cultures (Table 5).
Figure 6.
In vitro, the presence of SAA3 blocked PTH-stimulated osteoblast differentiation and cAMP production but had no effect on PTH-stimulated osteoclast-like cell differentiation. (a) Measurement of mRNA (Bglap) for the osteoblast marker, osteocalcin, and alizarin red staining for mineralized nodules at day 21 in BMSCs following treatment with vehicle (VEH) or continuous PTH (10 nM). (b) PTH-stimulated cAMP production and Bglap mRNA in POBs. CM from M-CSF plus RANKL (30 ng/mL each)–treated WT and SAA3 KO BMMs was added to POBs (3 parts CM and 1 part differentiation medium). OPG (50 ng/mL) was added to prevent osteoclastogenesis by any marrow remaining in POB cultures. For cAMP, POBs were grown for 5 d before treatment with CM, OPG, and 0.5 mM IBMX to block phosphodiesterase activity. One hour later, VEH or PTH was added for 20 min. For Bglap, POBs were treated with CM, OPG and VEH, or PTH for 14 d. (c) WT and SAA3 KO bone marrow cultures were treated with VEH or PTH. TRAP+ MNCs were counted per well. Gene expression for Tnfsf11 (RANKL) and Tnfrsf11b (OPG) was measured by quantitative real-time PCR on day 4 and reported as RQ values. Data are means ± SEM for n = 3 to 4 independent samples. For osteoclast count in (c), **P < 0.01, significantly different from day 3, determined by one-way ANOVA, and for (a), (b), and the rest of (c), *P < 0.05, **P < 0.01, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
Table 5.
Continuous PTH Stimulated Similar PGE2 (nM) Accumulation in the Culture Medium of WT and SAA3 KO BMSCs
| Days | WT | SAA3 KO | ||
|---|---|---|---|---|
| Vehicle | PTH | Vehicle | PTH | |
| 0–3 | Und | 2.84 ± 0.39 | Und | 2.93 ± 0.69 |
| 3–7 | Und | 9.42 ± 1.83a | Und | 10.34 ± 2.23b |
| 7–10 | Und | 5.40 ± 0.58 | Und | 5.01 ± 0.82 |
| 10–14 | Und | 1.45 ± 0.12 | Und | 1.23 ± 0.14 |
| 14–18 | Und | Und | Und | Und |
| 18–21 | Und | Und | Und | Und |
BMSCs were treated with vehicle or PTH (10 nM). Medium was collected at every medium change and assessed for PGE2 by ELISA. Data are means ± SEM for n = 3 independent samples.
Abbreviation: Und, undetectable.
P < 0.05, significantly different from days 0 to 3, determined by one-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
P < 0.01, significantly different from days 0 to 3, determined by one-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
BMSC cultures contain both osteoblastic and osteoclastic lineages. In previous in vitro studies, we demonstrated that osteoclast lineage cells were responsible for the inhibition of PTH-stimulated osteoblast differentiation in BMSCs (11) and that BMMs, stimulated by RANKL to become preosteoclasts, produced SAA3 as long as COX2 activity was present (15). As expected, RANKL-treated WT, but not SAA3 KO, BMMs expressed and secreted SAA3 (Supplemental Fig. 2A). CM from RANKL-treated WT, but not SAA3 KO, BMMs added to POBs blocked the PTH stimulation of cAMP production and osteoblast differentiation [Fig. 6(b)]. Thus, SAA3 blocked PTH-stimulated cAMP and osteoblast differentiation in vitro, supporting the in vivo observations.
PTH stimulated TRAP+ MNCs by the first week in both WT and KO BMSC cultures. We were not able to count these osteoclast-like cells, as many are covered by a canopy of other cells (Supplemental Fig. 2B). Tnfsf11/Tnfrsf11b mRNA expression was increased similarly in both WT and KO cultures by PTH in these cultures (Supplemental Fig. 2B). To quantify the numbers of osteoclast-like cells formed in response to PTH, we compared bone marrow cultures from WT and SAA3 KO mice. These cultures have mesenchymal lineage supporting cells that produce RANKL, but the differentiation of osteoclasts is predominant. PTH increased the number of TRAP+ MNCs similarly in both WT and KO cultures [Fig. 6(c); Supplemental Fig. 2C]. Expression of Tnfsf11 and Tnfrsf11b was regulated similarly in WT and KO bone marrow cultures [Fig. 6(c)]. Thus, SAA3 deficiency had no apparent effect on the osteoclastogenic potential of PTH, similar to the in vivo observations.
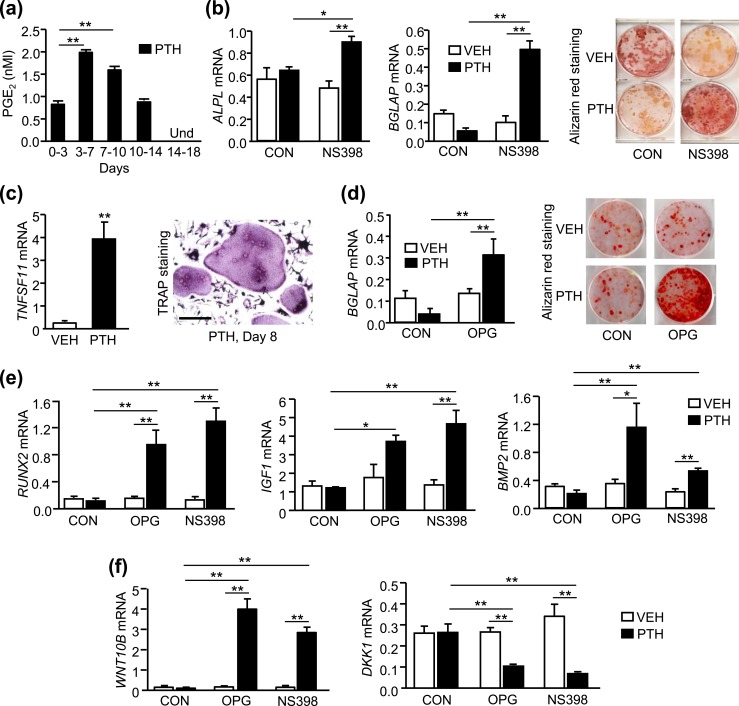
Continuous PTH increased osteoblast differentiation in human BMSCs only when COX2 or RANKL activity was blocked
In murine BMSCs, the inhibition of PTH-stimulated osteoblast differentiation, now shown to be due to SAA3, required both COX2 activity and PTH-stimulated RANKL (11, 15). To determine whether COX2 was required in human BMSCs(hBMSCs), hBMSCs were cultured from bone marrow aspirates of de-identified consenting patients undergoing rotator cuff repair and treated continuously with PTH for up to 21 days. PGE2 was undetectable in the medium of vehicle-treated cultures but accumulated in the medium following PTH treatment [Fig. 7(a); Supplemental Fig. 3]. PTH did not stimulate the expression of ALPL (alkaline phosphatase) or BGLAP (osteocalcin) or increase alizarin red staining for mineralization [Fig. 7(b)]. However, in the presence of NS398, a selective blocker of COX2 activity that blocked the PTH stimulation of PGE2 (Supplemental Fig. 3), PTH stimulated markers of osteoblast differentiation and mineralization [Fig. 7(b)].
Figure 7.
Continuous PTH increased osteoblast differentiation in hBMSCs only when COX2 or RANKL activity was blocked. hBMSCs were cultured with vehicle (VEH) or PTH (10 nM), with or without NS398 (100 nM), a selective inhibitor of COX2, or OPG (100 ng/mL), which blocks RANKL binding to its receptor. (a) Measurement of accumulated medium PGE2 by ELISA. (b) Markers of osteoblast differentiation, ALPL (alkaline phosphatase) and BGLAP (osteocalcin), and alizarin red staining for mineralization at day 21. (c) TNFSF11 (RANKL) mRNA at day 7 and TRAP staining at day 8. Scale bar, 100 µm. (d) BGLAP mRNA and alizarin red staining at day 21. (e) RUNX2 mRNA at day 7 and IGF1 and BMP2 mRNA at day 21. (f) WNT10B and DKK1 mRNA at day 7. mRNA was measured by quantitative real-time PCR and data are reported as RQ values. Bars are means ± SEM for n = 3 independent samples. For (a), **P < 0.01, determined by one-way ANOVA, post hoc Bonferroni pairwise multiple comparisons. For (c), **P < 0.01, determined by two-tailed unpaired t test. For (d)–(f), *P < 0.05, **P < 0.01, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons. Und, undetectable.
Similar to effects seen in murine BMSCs, PTH induced TNFSF11 (RANKL) mRNA expression and formation of TRAP+ MNCs in the first week of hBMSC cultures [Fig. 7(c)]. Similar to murine BMSCs, the osteoclast-like cells were covered by a canopy of other cells. The canopy was removed by trypsin treatment before fixing the cells for TRAP staining. To determine whether RANKL was also required for the inhibitory effect, hBMSCs were treated with OPG, which blocks the interaction of RANKL with RANK receptors. PTH stimulated BGLAP (osteocalcin) mRNA expression and alizarin red staining only in the cultures treated with OPG [Fig. 7(d)]. PTH increased the mRNA expression of RUNX2, an early marker of osteoblastic differentiation, and IGF1 and BMP2, growth factors thought to mediate anabolic effects of PTH, only when cells were treated with OPG or NS398, consistent with the inhibition being dependent on both RANKL and COX2, as seen in murine cells [Fig. 7(e)]. PTH also increased WNT10B and decreased DKK1 mRNA expression only in the presence of OPG or NS398 [Fig. 7(f)].
Similar to the murine experiments, the inhibitory activity could be transferred via CM (Supplemental Fig. 4). CM from RANKL-treated human BMMs (hBMMs) added to hBMSCs, which were treated with OPG to block RANKL effects, blocked the PTH stimulation of BGLAP mRNA expression. In contrast, CM from RANKL-treated hBMMs treated with NS398 to prevent COX2 production of PGE2 in the BMM cultures did not block the PTH stimulation of BGLAP. Hence, similar to murine experiments, the inhibition of PTH-stimulated osteoblast differentiation in BMSC cultures required both COX2 activity and RANKL activity and the inhibitory factor secreted by BMMs required COX2 activity.
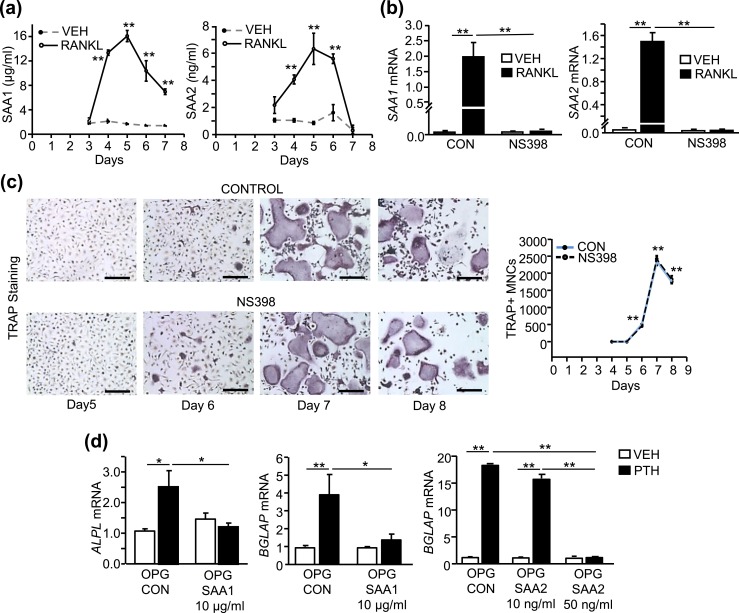
RANKL induced SAA1 and SAA2 in hBMMs, in the presence of COX2 activity, and exogenous SAA1 or SAA2 inhibited PTH-stimulated osteoblastic differentiation
In humans, Saa3 may be a pseudogene, whereas SAA1 and SAA2 are the major inducible proteins (18). The secretion of both SAA1 and SAA2 was induced in hBMMs by RANKL [Fig. 8(a)]. The induction peaked before TRAP+ MNCs were seen in culture, suggesting that SAA1 and SAA2 are produced by preosteoclasts [Fig. 8(c)]. The peak concentration of SAA1 was 16 µg/mL compared with 6 ng/mL for SAA2, or 2.7 × 103 greater than SAA2. The RANKL induction of SAA1 and SAA2 was COX2-dependent [Fig. 8(b)], whereas the RANKL induction of TRAP+ MNCs was not [Fig. 8(c)], as demonstrated by treatment of cultures with NS398.
Figure 8.
NS398 blocked the RANKL induction of SAA1 and SAA2 expression in hBMMs but did not affect osteoclast-like cell numbers, and SAA1 and SAA2 inhibited PTH-stimulated osteoblastic differentiation in hBMSCs. (a–c) hBMMs were treated with M-CSF (30 ng/mL) plus vehicle (VEH) or M-CSF plus RANKL (30 ng/mL each) with/without NS398 (100 nM). (a) SAA1 and SAA2 protein measurement in the culture medium of hBMMs measured by ELISA. (b) SAA1 and SAA2 mRNA at day 3. (c) TRAP-stained microscopic images at ×100 original magnification (scale bars, 200 µm) and TRAP+ MNC counts per well. (d) ALPL (alkaline phosphatase) and BGLAP (osteocalcin) mRNA expression in hBMSCs at day 21 treated with VEH or PTH in presence of OPG (100 ng/mL) to prevent osteoclastogenesis with/without rhSAA1 (10 µg/mL) or rhSAA2 (10 to 50 ng/mL). Data are means ± SEM for n = 3 independent samples. For (a), **P < 0.01, significantly different from VEH treated at same time point, determined by two-tailed unpaired t test. For (c), **P < 0.01, significantly different from day 4, determined by one-way ANOVA, post hoc Bonferroni pairwise multiple comparisons. For (b) and (d), *P < 0.05, **P < 0.01, determined by two-way ANOVA, post hoc Bonferroni pairwise multiple comparisons.
To determine whether exogenous addition of human recombinant SAA1 or SAA2 could inhibit the osteogenic effects of PTH, we treated hBMSCs with concentrations similar to peak concentrations induced by RANKL treatment of hBMMs [Fig. 8(a)]. SAA1 at 10 µg/mL blocked the osteogenic effects of PTH whereas 10 ng/mL of SAA2 did not inhibit the PTH effects [Fig. 8(d)]. However, SAA2 at 50 ng/mL did block the osteogenic effects of PTH. These results suggest that SAA1 is likely to be the major contributor to the inhibition of PTH in the CM due to its greater production.
Discussion
COX2 activity was previously shown to inhibit the anabolic effects of PTH infusion on bone in mice (9). In vitro, COX2-produced PGE2, combined with RANKL, causes preosteoclasts to secrete SAA3, which inhibits PTH-stimulated osteoblast differentiation (11, 15). SAA3 was induced by continuous PTH infusion in vivo in bone and serum of WT but not COX2 KO mice (15). In the present study continuous PTH was anabolic for trabecular bone in vivo and stimulated osteoblastic differentiation in BMSC cultures when SAA3 was absent, even though COX2 was expressed and PGE2 produced. The anabolic responses to 12 days of PTH infusion in SAA3 KO mice were similar to those in COX2 KO mice, with striking increases in distal femur and vertebral trabecular bone volumes in both genotypes (9). As found in our study on COX2 KO mice (9), there was no effect of absent SAA3 on PTH-stimulated bone resorption in vivo or osteoclast differentiation in vitro. These observations are consistent with SAA3 mediating the inhibitory effect of COX2/PGE2 on the anabolic effects of continuous PTH in vivo.
Similar to our previous studies using murine cells, COX2-produced PGE2 inhibited PTH-stimulated osteoblast differentiation in human BMSCs. SAA1 and SAA2 mRNA and protein were induced by RANKL in human BMMs in a PGE2-dependent manner. Addition of recombinant SAA1 and SAA2 inhibited PTH-stimulated osteoblast differentiation in human BMSCs. These results suggest that SAA1 and SAA2 play a similar role in humans to SAA3 in mice and may inhibit the anabolic responses to PTH in humans.
In our in vitro studies, SAA3 inhibited PTH-stimulated cAMP production in osteoblastic cells by activating Gαi/o signaling, which blocks the adenylate cyclase conversion of ATP to cAMP (15). Some studies have suggested that cAMP/protein kinase A signaling mediates both anabolic and catabolic actions of PTH on trabecular bone (35, 42). In SAA3 or COX2 KO mice but not in WT mice, PTH infusion induced the expression of genes reported to be mediated by cAMP/protein kinase A signaling, such as Ramp3 and Ctnnb1 (catenin, β1); genes important for the differentiation of osteoblasts, such as Runx2; as well many genes thought to be essential for the anabolic effects of PTH, such as Igf1 and Bmp2 (9, 15, 40). In contrast, the effects of PTH infusion on gene expression of factors critical for osteoclast differentiation, such as RANKL, OPG, and monocyte chemoattractant protein 1 (43), were not affected by the absence of COX2 or SAA3. These observations suggest that continuous PTH infusion drives trabecular bone formation via a cAMP-dependent signaling pathway and resorption by a cAMP-independent pathway.
Serum SAA3 was measurable only in PTH-infused mice. The traditional targets of PTH, associated with its regulation of calcium metabolism, are bone and kidney. However, PTH1R is expressed in many tissues, including liver, which can be a major source of SAA3 in mice (27, 32, 33, 44, 45). Because we used a global KO of SAA3 and a continuous pharmacological dose of PTH, there may have been contributions to the elevated SAA3 in response to PTH from these other tissues. We did examine Saa3 mRNA in kidney and liver tissues from WT mice, with and without PTH infusion, and found no regulation by PTH. These results suggest that, at least, these two tissues did not contribute to the PTH-stimulated elevation of serum SAA3. In our in vitro model using BMSCs, RANKL produced by osteoblastic lineage cells is required, in addition to COX2/PGE2, to produce SAA3 in myeloid/macrophage cells in response to PTH (11, 15). T lymphocytes also express PTH1R and, in response to PTH, they may secrete RANKL and cytokines, such as IL-17A and TNF-α, which can stimulate RANKL in osteoblasts (46, 47). To better understand the relevant tissues for the effects of SAA3 on PTH responses, we are generating a floxed Saa3 mouse to target Saa3 deletion tissue specifically.
The SAA inhibition of PTH-stimulated cAMP production and bone formation appears to be a new category of function for SAA. SAA1 and SAA2 proteins in humans are acutely and highly elevated in serum in response to tissue injury or inflammation, suggesting that they play important roles in host defense. They can induce, and be induced by, proinflammatory cytokines and have been implicated in many diseases associated with chronic inflammation, including amyloidosis, obesity, diabetes, rheumatoid arthritis, atherosclerosis, and cancer metastasis (19, 26, 48–52). SAA1 and SAA2 are thought to be predominantly produced by the liver but can be produced extrahepatically, as seen in our study of human BMSCs. Although SAA1 and SAA2 can also be acutely induced in mice, we have been unable to regulate their expression with PTH infusion in bones of mice, with continuous PTH in murine POBs, or with RANKL in cultured murine BMMs (15). SAA3 appears to be the major acutely expressed extrahepatic SAA in mice. In contrast to a previous study reporting that SAA3 did not contribute to circulating SAA (53), our results in the current experiments and other published data did find SAA3 to be elevated in the serum of WT mice after PTH infusion (15).
Continuous infusion of a pharmacological dose of PTH is useful as a model because the removal of suppression of osteoblastic differentiation results in very clear and dramatic anabolic effects of PTH. Additionally, in vitro studies, in which treatment with PTH is also continuous, predict the in vivo results. This has enabled us to identify SAA3 in vitro before undertaking in vivo studies. The question now is whether SAA3 is influencing bone mass in more physiologic or therapeutic conditions. SAA3 might suppress the anabolic effects of PTH in situations of high endogenous PTH levels, such as hyperparathyroidism, or when PTH is given intermittently at higher doses as therapy for osteoporosis. Previously we gave intermittent PTH to WT and COX2 KO mice and were surprised to find that it had a greater anabolic effect in COX2 KO mice than in WT mice (54). It was this observation that led eventually to our identifying SAA3 as mediating the inhibitory effects of COX2. Because PTH is rapidly degraded, when it is given as a single daily injection, it persists in the circulation very briefly (55). SAA3 is also transiently induced, and its expression may peak after PTH has produced most of its actions. Thus, we expect to see smaller differences between WT and SAA3 KO mice given intermittent PTH, compared with continuous PTH. We anticipate that these differences will be on the order of those seen in our previous study with COX2 KO mice.
SAA3 might suppress the anabolic effects of PTH in situations where SAA3 levels are elevated, such as inflammation or aging. Although we did not find detectable serum SAA3 in 3- to 4-month-old mice without PTH, serum SAA3 was elevated in 18-month-old mice without external perturbation and was associated with increased bone loss (56). Hence, SAA may be important in the bone loss of aging, which is thought to be associated with chronic inflammation. Although proinflammatory cytokines are generally thought to cause the increased resorption associated with inflammation, our data suggest that a reverse scenario is possible and increased RANKL-induced resorption could increase proinflammatory cytokines via the induction of SAA.
In summary, murine SAA3 is a novel suppressor of the anabolic effects of continuous PTH. Absence of SAA3 results in a PTH-stimulated cAMP-mediated pathway that increases cancellous bone formation without affecting PTH-stimulated resorption, thus uncoupling formation from resorption in this model. Intermittent PTH is an important drug for treating osteoporosis, and the potential to enhance its anabolic effects may be important for those who respond poorly. There are a number of ways that SAA production might be decreased by currently available agents, including denosumab (an inhibitor of RANKL actions), COX2 specific inhibitors, and PGE2 receptor antagonists, and it would be interesting to examine their effects on PTH-stimulated anabolic effects.
Supplementary Material
Acknowledgments
We thank Judith Kalinowski and David Bridgewater for technical help with histology and Renata Rydzik for µCT scanning. We thank Skylar Wright and Michael Frankie for help in performing mouse osteoclast experiments and human PGE2 assays.
Financial Support: This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Award AR060286 (to C.P.) and by a Musculoskeletal Institute Collaborative Internal Award (to A.D.M.).
Disclosure Summary: A.D.M. is a consultant for Orthofix (Lewisville, TX) and for Arthrex Inc. (Naples, FL) and has received grant/research support from Arthrex Inc. The remaining authors have nothing to disclose.
Glossary
Abbreviations
- BFR/BS
bone formation rate per bone surface
- BMC
bone mineral content
- BMD
bone mineral density
- BMM
bone marrow macrophage
- BMP2
bone morphogenetic protein 2
- BMSC
bone marrow stromal cell
- BV/TV
bone volume/total volume
- CM
conditioned medium
- Conn.Dens
connectivity density
- COX2
cyclooxygenase-2
- CTX
C-terminal telopeptide
- CV
coefficient of variation
- DXA
dual-energy X-ray absorptiometry
- EIA
enzyme immunoassay
- hBMM
human bone marrow macrophage
- hBMSC
human bone marrow stromal cell
- HET
heterozygous
- hPTH
human PTH
- IBMX
isobutyl methylxanthine
- KO
knockout
- MAR
mineral apposition rate
- M-CSF
macrophage colony-stimulating factor
- MNC
multinucleated cell
- MS/BS
mineralizing surface per bone surface
- Ob.S/BS
osteoblast surface per bone surface
- Oc.S/BS
osteoclast surface per bone surface
- OPG
osteoprotegerin
- PGE2
prostaglandin E2
- PINP
N-terminal propeptide of type I procollagen
- POB
primary osteoblast
- PTH1R
type 1 PTH receptor
- PTHrP
PTH-related peptide
- Ramp3
receptor activity modifying protein 3
- RANKL
receptor activator of nuclear factor κB ligand
- rh
recombinant human
- rm
recombinant murine
- ROI
region of interest
- RQ
relative quantification
- SAA
serum amyloid A
- SMI
structural model index
- Tb.N
trabecular number
- Tb.Sp
trabecular spacing
- Tb.Th
trabecular thickness
- TRAP
tartrate-resistant acid phosphatase
- WT
wild-type
- μCT
microcomputed tomography
References
- 1. Vilardaga JP, Romero G, Friedman PA, Gardella TJ. Molecular basis of parathyroid hormone receptor signaling and trafficking: a family B GPCR paradigm. Cell Mol Life Sci. 2011;68(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahon MJ. The parathyroid hormone receptorsome and the potential for therapeutic intervention. Curr Drug Targets. 2012;13(1):116–128. [DOI] [PubMed] [Google Scholar]
- 3. Taylor CW, Tovey SC. From parathyroid hormone to cytosolic Ca2+ signals. Biochem Soc Trans. 2012;40(1):147–152. [DOI] [PubMed] [Google Scholar]
- 4. Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Potts JT, Gardella TJ. Progress, paradox, and potential: parathyroid hormone research over five decades. Ann N Y Acad Sci. 2007;1117(1):196–208. [DOI] [PubMed] [Google Scholar]
- 6. Augustine M, Horwitz MJ. Parathyroid hormone and parathyroid hormone-related protein analogs as therapies for osteoporosis. Curr Osteoporos Rep. 2013;11(4):400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iida-Klein A, Lu SS, Kapadia R, Burkhart M, Moreno A, Dempster DW, Lindsay R. Short-term continuous infusion of human parathyroid hormone 1–34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J Endocrinol. 2005;186(3):549–557. [DOI] [PubMed] [Google Scholar]
- 8. Robling AG, Kedlaya R, Ellis SN, Childress PJ, Bidwell JP, Bellido T, Turner CH. Anabolic and catabolic regimens of human parathyroid hormone 1–34 elicit bone- and envelope-specific attenuation of skeletal effects in Sost-deficient mice. Endocrinology. 2011;152(8):2963–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choudhary S, Canalis E, Estus T, Adams D, Pilbeam C. Cyclooxygenase-2 suppresses the anabolic response to PTH infusion in mice. PLoS One. 2015;10(3):e0120164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greenfield EM. Anabolic effects of intermittent PTH on osteoblasts. Curr Mol Pharmacol. 2012;5(2):127–134. [PubMed] [Google Scholar]
- 11. Choudhary S, Blackwell K, Voznesensky O, Deb Roy A, Pilbeam C. Prostaglandin E2 acts via bone marrow macrophages to block PTH-stimulated osteoblast differentiation in vitro. Bone. 2013;56(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horwitz MJ, Tedesco MB, Sereika SM, Prebehala L, Gundberg CM, Hollis BW, Bisello A, Garcia-Ocaña A, Carneiro RM, Stewart AF. A 7-day continuous infusion of PTH or PTHrP suppresses bone formation and uncouples bone turnover. J Bone Miner Res. 2011;26(9):2287–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pilbeam CC, Choudhary S, Blackwell KA, Raisz LG. Prostaglandins and bone metabolism In: Bilezikian JP, Raisz LG, and Martin TJ, eds. Principles of Bone Biology. 3rd ed.San Diego, CA: Elsievier/Academic Press; 2008:1235–1271. [Google Scholar]
- 14. Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21(5):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choudhary S, Goetjen A, Estus T, Jacome-Galarza CE, Aguila HL, Lorenzo J, Pilbeam C. Serum amyloid A3 secreted by preosteoclasts inhibits parathyroid hormone-stimulated cAMP signaling in murine osteoblasts. J Biol Chem. 2016;291(8):3882–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265(2):501–523. [DOI] [PubMed] [Google Scholar]
- 17. Ye RD, Sun L. Emerging functions of serum amyloid A in inflammation. J Leukoc Biol. 2015;98(6):923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun L, Ye RD. Serum amyloid A1: structure, function and gene polymorphism. Gene. 2016;583(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Buck M, Gouwy M, Wang JM, Van Snick J, Opdenakker G, Struyf S, Van Damme J. Structure and expression of different serum amyloid A (SAA) variants and their concentration-dependent functions during host insults. Curr Med Chem. 2016;23(17):1725–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kluve-Beckerman B, Song M. Genes encoding human serum amyloid A proteins SAA1 and SAA2 are located 18 kb apart in opposite transcriptional orientations. Gene. 1995;159(2):289–290. [DOI] [PubMed] [Google Scholar]
- 21. Larson MA, Wei SH, Weber A, Weber AT, McDonald TL. Induction of human mammary-associated serum amyloid A3 expression by prolactin or lipopolysaccharide. Biochem Biophys Res Commun. 2003;301(4):1030–1037. [DOI] [PubMed] [Google Scholar]
- 22. Meek RL, Eriksen N, Benditt EP. Murine serum amyloid A3 is a high density apolipoprotein and is secreted by macrophages. Proc Natl Acad Sci USA. 1992;89(17):7949–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meek RL, Eriksen N, Benditt EP. Serum amyloid A in the mouse. Sites of uptake and mRNA expression. Am J Pathol. 1989;135(2):411–419. [PMC free article] [PubMed] [Google Scholar]
- 24. Upragarin N, Landman WJ, Gaastra W, Gruys E. Extrahepatic production of acute phase serum amyloid A. Histol Histopathol. 2005;20(4):1295–1307. [DOI] [PubMed] [Google Scholar]
- 25. Reigstad CS, Lundén GO, Felin J, Bäckhed F. Regulation of serum amyloid A3 (SAA3) in mouse colonic epithelium and adipose tissue by the intestinal microbiota. PLoS One. 2009;4(6):e5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. den Hartigh LJ, Wang S, Goodspeed L, Ding Y, Averill M, Subramanian S, Wietecha T, O’Brien KD, Chait A. Deletion of serum amyloid A3 improves high fat high sucrose diet-induced adipose tissue inflammation and hyperlipidemia in female mice. PLoS One. 2014;9(9):e108564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tannock LR, De Beer MC, Ji A, Shridas P, Noffsinger VP, den Hartigh L, Chait A, De Beer FC, Webb NR. Serum amyloid A3 is a high density lipoprotein-associated acute-phase protein. J Lipid Res. 2018;59(2):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Arciero RA, Drissi H. Rapid isolation of human stem cells (connective tissue progenitor cells) from the proximal humerus during arthroscopic rotator cuff surgery. Am J Sports Med. 2010;38(7):1438–1447. [DOI] [PubMed] [Google Scholar]
- 30. AB_2182780 RRID:
- 31. AB_649006 RRID:
- 32. Rouleau MF, Warshawsky H, Goltzman D. Parathyroid hormone binding in vivo to renal, hepatic, and skeletal tissues of the rat using a radioautographic approach. Endocrinology. 1986;118(3):919–931. [DOI] [PubMed] [Google Scholar]
- 33. Ureña P, Kong XF, Abou-Samra AB, Jüppner H, Kronenberg HM, Potts JT Jr, Segre GV. Parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acids are widely distributed in rat tissues. Endocrinology. 1993;133(2):617–623. [DOI] [PubMed] [Google Scholar]
- 34. Martin TJ, Sims NA. RANKL/OPG; critical role in bone physiology. Rev Endocr Metab Disord. 2015;16(2):131–139. [DOI] [PubMed] [Google Scholar]
- 35. Yang D, Singh R, Divieti P, Guo J, Bouxsein ML, Bringhurst FR. Contributions of parathyroid hormone (PTH)/PTH-related peptide receptor signaling pathways to the anabolic effect of PTH on bone. Bone. 2007;40(6):1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khan MP, Khan K, Yadav PS, Singh AK, Nag A, Prasahar P, Mittal M, China SP, Tewari MC, Nagar GK, Tewari D, Trivedi AK, Sanyal S, Bandyopadhyay A, Chattopadhyay N. BMP signaling is required for adult skeletal homeostasis and mediates bone anabolic action of parathyroid hormone. Bone. 2016;92:132–144. [DOI] [PubMed] [Google Scholar]
- 37. Tahimic CG, Wang Y, Bikle DD. Anabolic effects of IGF-1 signaling on the skeleton. Front Endocrinol (Lausanne). 2013;4(6):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee M, Partridge NC. Parathyroid hormone signaling in bone and kidney. Curr Opin Nephrol Hypertens. 2009;18(4):298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest. 2011;34(10):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Estus TL, Choudhary S, Pilbeam CC. Prostaglandin-mediated inhibition of PTH-stimulated β-catenin signaling in osteoblasts by bone marrow macrophages. Bone. 2016;85:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rickard DJ, Wang FL, Rodriguez-Rojas AM, Wu Z, Trice WJ, Hoffman SJ, Votta B, Stroup GB, Kumar S, Nuttall ME. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39(6):1361–1372. [DOI] [PubMed] [Google Scholar]
- 42. Kondo H, Guo J, Bringhurst FR. Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J Bone Miner Res. 2002;17(9):1667–1679. [DOI] [PubMed] [Google Scholar]
- 43. Siddiqui JA, Johnson J, Le Henaff C, Bitel CL, Tamasi JA, Partridge NC. Catabolic effects of human PTH (1–34) on bone: requirement of monocyte chemoattractant protein-1 in murine model of hyperparathyroidism. Sci Rep. 2017;7(1):15300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goltzman D, White JH. Developmental and tissue-specific regulation of parathyroid hormone (PTH)/PTH-related peptide receptor gene expression. Crit Rev Eukaryot Gene Expr. 2000;10(2):135–149. [PubMed] [Google Scholar]
- 45. Amizuka N, Lee HS, Kwan MY, Arazani A, Warshawsky H, Hendy GN, Ozawa H, White JH, Goltzman D. Cell-specific expression of the parathyroid hormone (PTH)/PTH-related peptide receptor gene in kidney from kidney-specific and ubiquitous promoters. Endocrinology. 1997;138(1):469–481. [DOI] [PubMed] [Google Scholar]
- 46. Pacifici R. Role of T cells in the modulation of PTH action: physiological and clinical significance. Endocrine. 2013;44(3):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tawfeek H, Bedi B, Li JY, Adams J, Kobayashi T, Weitzmann MN, Kronenberg HM, Pacifici R. Disruption of PTH receptor 1 in T cells protects against PTH-induced bone loss. PLoS One. 2010;5(8):e12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10(11):1349–1355. [DOI] [PubMed] [Google Scholar]
- 49. Eklund KK, Niemi K, Kovanen PT. Immune functions of serum amyloid A. Crit Rev Immunol. 2012;32(4):335–348. [DOI] [PubMed] [Google Scholar]
- 50. Simons JP, Al-Shawi R, Ellmerich S, Speck I, Aslam S, Hutchinson WL, Mangione PP, Disterer P, Gilbertson JA, Hunt T, Millar DJ, Minogue S, Bodin K, Pepys MB, Hawkins PN. Pathogenetic mechanisms of amyloid A amyloidosis. Proc Natl Acad Sci USA. 2013;110(40):16115–16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lu J, Yu Y, Zhu I, Cheng Y, Sun PD. Structural mechanism of serum amyloid A-mediated inflammatory amyloidosis. Proc Natl Acad Sci USA. 2014;111(14):5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Getz GS, Krishack PA, Reardon CA. Serum amyloid A and atherosclerosis. Curr Opin Lipidol. 2016;27(5):531–535. [DOI] [PubMed] [Google Scholar]
- 53. Chiba T, Han CY, Vaisar T, Shimokado K, Kargi A, Chen MH, Wang S, McDonald TO, O’Brien KD, Heinecke JW, Chait A. Serum amyloid A3 does not contribute to circulating SAA levels. J Lipid Res. 2009;50(7):1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu M, Choudhary S, Voznesensky O, Gao Q, Adams D, Diaz-Doran V, Wu Q, Goltzman D, Raisz LG, Pilbeam CC. Basal bone phenotype and increased anabolic responses to intermittent parathyroid hormone in healthy male COX-2 knockout mice. Bone. 2010;47(2):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Satterwhite J, Heathman M, Miller PD, Marín F, Glass EV, Dobnig H. Pharmacokinetics of teriparatide (rhPTH[1–34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif Tissue Int. 2010;87(6):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Choudhary S, Yee SP, Adams D, Rydzik R, Lorenzo J, Pilbeam C. Serum amyloid A3 (SAA3) causes age-related bone loss Available at: www.asbmr.org/education/AbstractDetail?aid=b6b01d6e-3f76-4d1a-8b48-a027a31063a4. Accessed 30 January 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.