Abstract
The glycoprotein FSH, a product of pituitary gonadotrope cells, regulates ovarian follicle development in females and spermatogenesis in males. FSH is a heterodimer of the common α gonadotropin subunit and the hormone-specific FSHβ subunit (a product of the Fshb gene). Using a conditional knockout approach (Cre-lox), we previously demonstrated that Fshb expression in mice depends on the transcription factors forkhead box L2 (FOXL2) and SMAD4. Deletion of Foxl2 or Smad4 alone led to FSH deficiency, female subfertility, and oligozoospermia in males. Simultaneous deletion of the two genes yielded a greater suppression of FSH and female sterility. The Cre-driver used previously was first active during embryonic development. Therefore, it is unclear whether FOXL2 and SMAD4 play important roles in the development or adult function of gonadotropes, or both. To address this question, we developed a tamoxifen-inducible Cre-driver line, which enabled Foxl2 and Smad4 gene deletions in gonadotropes of adult mice. After tamoxifen treatment, females with previously demonstrated fertility exhibited profound reductions in FSH levels, arrested ovarian follicle development, and sterility. FSH levels were comparably reduced in males 1 or 2 months after treatment; however, spermatogenesis was unaffected. These data indicate that (1) FOXL2 and SMAD4 are necessary to maintain FSH synthesis in gonadotrope cells of adult mice, (2) FSH is essential for female reproduction but appears to be unnecessary for the maintenance of spermatogenesis in adult male mice, and (3) the inducible Cre-driver line developed here provides a powerful tool to interrogate gene function in gonadotrope cells of adult mice.
Conditional deletion of the Foxl2 and Smad4 genes in gonadotrope cells of adult mice leads to suppression of FSH production and female sterility.
FSH, a product of pituitary gonadotrope cells, plays fundamental roles in mammalian reproduction (1, 2). In females, FSH acts on ovarian granulosa cells to regulate the latter stages of follicle development and estrogen biosynthesis before ovulation (3). Purified and recombinant forms of the hormone are the foundation of ovarian stimulation protocols in assisted reproduction and in vitro fertilization (4). Although FSH is also used to treat hypogonadism in men, its necessity in male reproduction is controversial. The hormone targets testicular Sertoli cells, stimulating their proliferation during development (5, 6). In adulthood, FSH regulates androgen-binding protein synthesis and thereby intratesticular testosterone (T) concentrations (7). Through these two mechanisms, FSH can regulate spermatogenesis. However, whether FSH is necessary for sperm production is less clear (8–10).
FSH is a dimeric protein, composed of the chorionic gonadotropin α subunit and the hormone-specific FSHβ (FSHB) subunit. FSH synthesis is stimulated by hypothalamic GnRH and by intrapituitary activins (or related ligands in the TGFβ superfamily). Mechanisms through which GnRH regulates FSH are incompletely described. In contrast, activins are proposed to stimulate FSH by promoting transcription of the FSHβ subunit gene (Fshb) (11). Upon binding to type I and II receptor serine/threonine kinases, activins stimulate the formation of complexes of SMAD proteins (SMAD3 and SMAD4) and forkhead box L2 (FOXL2). SMAD-FOXL2 complexes then bind the proximal Fshb promoter to regulate gene transcription (12–19). This model, based principally on in vitro observations, has been largely substantiated via conditional knockout (cKO) approaches in mice. Gonadotrope-specific deletion of Smad3, Smad4, and Foxl2, alone or in combination, leads to profound reductions in pituitary Fshb mRNA levels and FSH secretion (19–21). Depending on the model, this further leads to subfertility (small litters) or sterility in females and to oligozoospermia in males. The phenotypes are reminiscent of those seen in mice deficient in the Fshb subunit (1).
In these mouse models, the Cre recombinase enzyme was expressed from the GnRH receptor (Gnrhr) locus, conferring gonadotrope-specific gene deletion in the anterior pituitary (22). Cre activity is first detected in these mice at approximately embryonic day 13 (23). Thus, although phenotypes were observed in adult animals, the previous analyses did not allow investigators to conclude whether FSH deficiency derived from losses of the SMAD and FOXL2 proteins in development, adulthood, or some combination of the two. To address this confound, we developed a mouse model by using the tamoxifen-inducible Cre-lox recombination system that enables the deletion of genes in adult murine gonadotropes. We used these animals to simultaneously recombine floxed Foxl2 and Smad4 loci in gonadotrope cells of adult mice. As was found with the developmentally expressed Cre-driver (20), inducible knockout mice exhibited selective reductions in Fshb expression after treatment. Females were rendered subfertile or sterile by a block in ovarian follicle development at the early antral stage. In contrast to the case with developmental knockouts, testis size and spermatogenesis in males were unaffected in adult knockout mice, despite profound decreases in FSH levels.
Materials and Methods
Generation of control and experimental animals
The Foxl2fx/fx;Smad4fx/fx mice were described previously (20). Inducible GnRHR-IRES-Cre (GRIC) mice (Gnrhrtm1.1(cre/ERT2)Djb; MGI:5908151; iGRIC for simplicity) were generated as described in Supplemental Fig. 1. Foxl2fx/fx;Smad4fx/fx mice were crossed with iGRIC/iGRIC animals to generate iGRIC/+;Foxl2fx/+;Smad4fx/+ progeny, which were then crossed to Foxl2fx/fx;Smad4fx/fx mice to produce inducible knockouts (iGRIC/+;Foxl2fx/fx;Smad4fx/fx) and controls (Foxl2fx/fx;Smad4fx/fx). G26Sortm1(EYFP)Cos/J (Rosa26YFP/YFP) mice were ordered from Jackson Laboratories (004077) and were crossed with iGRIC/iGRIC mice to generate iGRIC/+;Rosa26YFP/+ animals (24). In the fluorescence-activated cell sorting experiment, the Rosa26YFP allele was introduced to iGRIC/+;Foxl2fx/fx;Smad4fx/fx mice to generate iGRIC/+;Foxl2fx/fx;Smad4fx/fx;Rosa26YFP/+ animals. Control mice in the sorting experiment had the iGRIC/+;Foxl2+/+;Smad4+/+;Rosa26YFP/+ genotype but were subjected to the same tamoxifen treatment protocol. Mice were genotyped by PCR of genomic DNA using the primers listed in Supplemental Table 1. All animal experiments were performed in accordance with institutional and federal guidelines and were approved by the McGill University and Goodman Cancer Centre Facility Animal Care Committee (protocol 5204).
Tamoxifen injection
Animals received either 2 mg tamoxifen (Sigma-Aldrich T5648; prepared in 100 μL corn oil) or oil vehicle once daily IP, every other day, four times in total unless otherwise specified (e.g., in Fig. 1B).
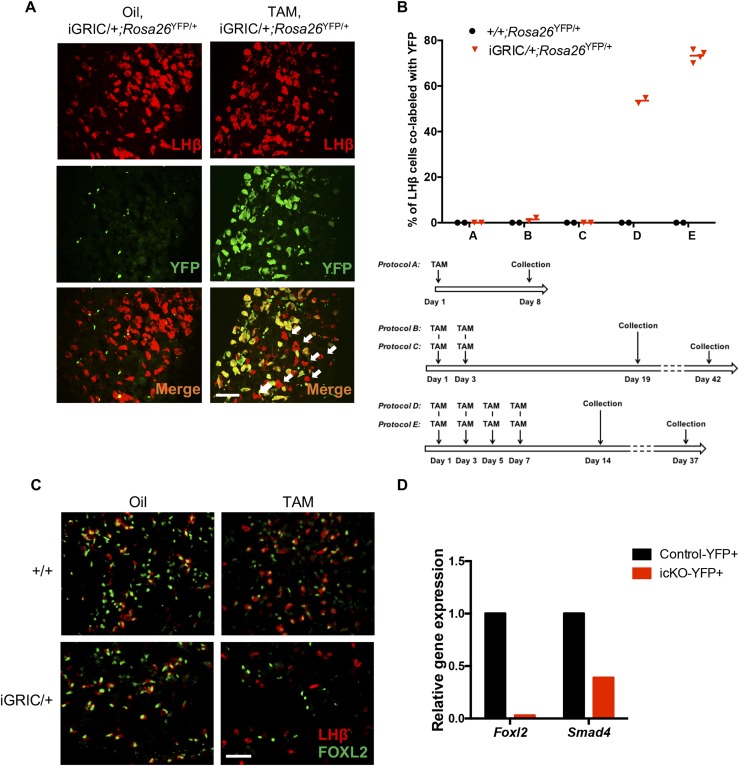
Figure 1.
Validation of the efficiency and specificity of tamoxifen-induced recombination in gonadotropes. (A) Male iGRIC/+;Rosa26YFP/+ mice were treated with oil (vehicle) or tamoxifen with one of five different treatment protocols [see schematic in panel (B)]. Cells in pituitary sections were labeled for YFP (green) and LHβ (red). Arrows indicate LHβ+/YFP− cells. (B) Top: quantification of LHβ-positive cells colabeled with YFP after different vehicle or tamoxifen treatments. Bottom: schematic representation of the different tamoxifen treatment protocols. (C) Immunofluorescence staining for FOXL2 (green) and LHβ (red) in pituitary sections from males in the four treatment groups. (D) RT-qPCR analyses of relative Foxl2 and Smad4 gene expression in YFP+ cells purified from control (black) and inducible KO (red) pituitaries 4 weeks after the last tamoxifen injection. Gene expression levels were normalized to the housekeeping gene Rpl19. Scale bars in panels (A) and (C), 50 µm. icKO, inducible conditional knockout; TAM, tamoxifen.
Pituitary immunofluorescence
Pituitary sections were prepared as described previously (25) and were then blocked with normal serum and incubated with primary antibody rabbit anti-GFP (1:500; Bioss Antibodies; bs-2194R; RRID: AB_10881247), rabbit anti-rat FSHβ (1:500; National Institute of Diabetes and Digestive and Kidney Diseases; AFP-7798_1289P), or rabbit anti-FOXL2 (1:8000; Dr. Dagmar Wilhelm, Department of Anatomy and Neuroscience, University of Melbourne, Melbourne, Australia; RRID: AB_2687958) with goat anti-LHβ (1:500; Santa Cruz Biotechnology; sc-7824; RRID: AB_2249859) overnight at 4°C. After three washes with PBS, sections were incubated with Alexa Fluor 488–conjugated donkey anti-rabbit (1:600; Life Technologies; A-21206; RRID: AB_141708) and Alexa Fluor 594-conjugated donkey anti-goat (1:600; Life Technologies; A-11058; RRID: AB_142540); or Alexa Fluor 594-conjugated donkey anti-rabbit (1:600; Life Technologies; A-21207; RRID: AB_141637) and Alexa Fluor 488-conjugated donkey anti-goat (1:600; Life Technologies; A-11055; RRID: AB_142672) secondary antibodies for 1 hour at room temperature. After another three washes with PBS, coverslips were mounted with ProLong Gold antifade reagent 4′,6-diamidino-2-phenylindole (Life Technologies; P36966; RRID: AB_2629482) and dried at 37°C for 15 minutes in the dark. Slides were analyzed on a Zeiss Axio Imager M2 microscope. Images were acquired with an Axiocam 506 mono camera (Zeiss) using ZEN 2.3 pro (Zeiss) software.
Pituitary cell counting
Total numbers of LHβ+/YFP+ and LHβ+/YFP− cells were counted in each field of merged images. An average of 80 LHβ+ cells were counted per field. Each data point represents the mean of 3 to 5 fields from one section. Samples were collected from two to four animals per genotype per treatment.
Fluorescence-activated cell sorting
Cell sorting was performed as described previously (25). Briefly, pituitary cell suspensions were prepared from seven iGRIC/+;Foxl2fx/fx;Smad4fx/fx;Rosa26YFP/+ mice (both sexes combined) and nine iGRIC;Rosa26YFP/+ mice (both sexes combined). We obtained 1.08 × 104 YFP+ cells for the controls and 8.81 × 103 YFP+ cells for the inducible knockout mice. Total RNA was extracted and relative gene expression levels were assessed as described previously (25).
Ovarian histology
Ovarian histology was performed as described previously (25). Images were acquired with a Leica Microsystems DFC310 FX 1.4-megapixel digital color camera with a Leica Microsystems DM1000 light-emitting diode microscope using Leica Application Suite version 4.0.0 software or with a Zeiss Axio Imager M2 microscope.
Fertility assessment
To assess fertility, we first paired females of both genotypes with wild type C57BL6 males (Charles River; 000664) until they produced two litters. We removed male partners upon visual observation of the second pregnancy. One week after the second litter was born, females were randomly assigned to one of the two treatment groups. Oil vehicle or tamoxifen was injected once daily, every other day, four times in total in each female (as described earlier). Four weeks after the last injection, females were repaired with fertility-proven, wild type males for a period of 3 months or until they produced three litters. Date of birth and litter size were recorded, as described previously (25). Pups were removed from the breeding couples at postnatal day 15.
Testicular immunohistochemistry
Testes were isolated from male mice ≥1 month after the last injection (oil or tamoxifen), fixed overnight in Bouin buffer at room temperature, and then dehydrated in 70% ethanol for 3 days. Tissues were paraffin-embedded and cut into 4-μm sections. After deparaffinization, antigen unmasking was performed by submerging the sections in boiling 10 mM sodium citrate (pH 6.0) for 22 minutes. After two washes in 0.025% PBS-Triton (BioShop, TRX777), endogenous peroxidase activity was quenched in 3% hydrogen peroxide for 10 minutes. After three washes with PBS with Tween 20 (PBST), nonspecific binding was blocked with 5% normal donkey serum (Vector Laboratories, S-1000) prepared in PBST for 1 hour at room temperature, then incubated with primary rabbit anti–sex-determining region Y–box 9 (SOX9) (1:250; Cell Signaling, 82630; RRID: AB_2665492) overnight at 4°C. After three washes with PBST, sections were incubated with biotinylated secondary antibody (goat anti-rabbit; 1:200; Vector Laboratories; PK-6101; RRID: AB_2336820) for 1 hour at room temperature. The signals were then amplified via the avidin-biotin complex method (Vector Laboratories; PK-6101) according to the manufacturer’s instructions. After another three washes with PBST, peroxidase substrate solution (DAB; Vector Laboratories; SK-4100 plus 1% hydrogen peroxide) was added and left to react for 4 minutes at room temperature. Sections were counterstained with hematoxylin solution (Sigma-Aldrich; GHS232) and dehydrated in successive baths of ethanol, then toluene, and mounted with PermountTM (Fisher Chemical; SP15-100).
Sertoli cell counts
Sertoli cell counts were performed by a blinded observer. Tubules that appeared either round or nearly round in transverse sections were selected randomly. SOX9-positive cells (i.e., Sertoli cells) were counted at ×400 magnification with ZEN software (ZEN 2012 blue edition; Zeiss). Data are from counts of 30 seminiferous tubules (two counts of 15 seminiferous tubules per slide separated by 50 µm).
Sperm counting and motility analyses
Epididymal sperm were counted with a hemocytometer as described previously (21, 25). Sperm motility was analyzed via computer-assisted sperm analysis (2), as described previously (25, 26).
Hormone analyses
Blood was collected from the submandibular vein or by cardiac puncture and serum obtained as described previously (25). Serum FSH levels were assessed by Luminex assay (Millipore; MPTMAG-49K) according to the manufacturer’s instructions. Serum LH was measured with an in-house sandwich ELISA, as described previously (27). To measure pituitary FSH and LH content, we prepared pituitary lysates as described in Li et al. (25), and total protein concentration was measured by bicinchoninic acid protein assay kit (Thermo Fisher; 23225). Pituitary FSH content was measured by radioimmunoassay (28) at the Ligand Assay and Analysis Core of the Center for Research in Reproduction at the University of Virginia (Charlottesville, VA). The reportable ranges were 2.3 to 60.0 ng/mL or 1.8 to 60 ng/mL for females and males, respectively; intra-assay coefficients of variation were <20%. Female samples were diluted 10 times and male samples were diluted 40 times for assessment. Pituitary LH content was measured with the in-house sandwich ELISA, as described previously (29). Samples were diluted 10,000 times for analysis.
Quantitative RT-PCR
Pituitaries were extracted, immediately frozen in liquid nitrogen, and stored at −80°C. Control females were euthanized in the morning of random days of the estrous cycle. Induced knockout females, most of which did not cycle, were euthanized the same day as control littermates. Total RNA was extracted as described previously (25). Quantitative RT-PCR (RT-qPCR) analyses were performed as described previously (30). Primers used in RT-qPCR analysis are listed in Supplemental Table 1.
Primary pituitary cultures
Primary pituitary cultures were prepared from control and induced knockout animals as previously described (31). In the in vitro induced gene recombination experiment, cells were treated with 1.5 μM 4-hydroxytamoxifen (4-OHT; Sigma-Aldrich; H7904) prepared in M199 culture media supplemented with 10% fetal bovine serum for 24 hours. Cells were then treated with activin A or medium alone as described previously (25).
Statistical analysis
Reproductive organ weights, sperm counts, sperm motility, serum hormonal levels, pituitary and ovarian gene expression, and pituitary protein levels were analyzed by one-way ANOVA followed by Dunn multiple comparisons tests. Primary culture experiments were analyzed by one-way ANOVA as indicated earlier, Student t test, or two-way ANOVA followed by Sidak correction, where applicable. All statistical analyses were performed with Prism 6 (GraphPad Software). Data are presented as mean (scatter graph) or mean + SEM (bar graph). P < 0.05 was considered significant.
Results
Generation of gonadotrope-specific tamoxifen-inducible Cre mice
Since 2008, GRIC mice have been used to conditionally delete genes in murine gonadotrope cells. The mice express the Cre recombinase enzyme from the endogenous Gnrhr locus beginning around embryonic day 13 (23). Here, we modified the GRIC targeting vector by replacing the Cre expression cassette with Cre-ERT2 (32), which enables tamoxifen-inducible Cre activity (Supplemental Fig. 1). iGRIC mice were generated by conventional gene targeting in embryonic stem cells. We then crossed the mice to a reporter strain (Rosa26YFP) to determine the specificity and efficiency of tamoxifen-induced recombination (24). iGRIC/+;Rosa26YFP/+ mice exhibited YFP in LHβ-expressing pituitary gonadotropes after tamoxifen but not oil vehicle treatment (Fig. 1A). After testing a variety of treatment conditions, we found that four injections of 2 mg tamoxifen delivered every other day was highly efficient, leading to YFP in 55% and 75% of LHβ+ gonadotropes 2 or 4 weeks, respectively, after the final injection (Fig. 1B). In contrast, nearly all LHβ+ gonadotropes expressed YFP when the original GRIC allele was used (GRIC/+;Rosa26YFP/+ mice; Supplemental Fig. 2). No other cell lineages expressed YFP in either the GRIC or iGRIC model, and no YFP was observed in the latter without previous tamoxifen treatment. Thus, the new iGRIC model conferred both specific and efficient recombination in gonadotropes, though slightly less efficient than with the original GRIC model.
Tamoxifen efficiently induces recombination of floxed Foxl2 and, to a lesser extent, Smad4 alleles in gonadotropes of adult mice
We previously showed that the GRIC model can efficiently recombine floxed Foxl2 and Smad4 alleles in murine gonadotropes, leading to severe FSH deficiency, female sterility, and male oligozoospermia (20). Here, we examined the efficiency of recombination of these alleles using the new iGRIC model in adult mice. One month after the fourth tamoxifen injection, FOXL2 protein expression was ablated in essentially all LHβ+ gonadotropes (Fig. 1C and 1D; Supplemental Fig. 3A). The remaining FOXL2+ cells were mostly thyrotropes (Supplemental Fig. 3B). The depletion of pituitary Foxl2 mRNA expression was roughly equivalent to that seen with the GRIC model (Fig. 1D) (20, 21). Immunofluorescent staining for SMAD4 yielded ambiguous results (data not shown). Therefore, we measured Smad4 and Foxl2 mRNA expression in purified gonadotropes. Consistent with the protein expression data, Foxl2 mRNA was essentially absent in gonadotropes from inducible knockout mice (Fig. 1D). In contrast, Smad4 mRNA levels were reduced by ~60% in purified gonadotrope cells after tamoxifen-induced recombination compared with controls (Fig. 1D). These data indicate that iGRIC mice could be used to recombine several different loci (at least four at a time) in adult mice, although the efficiency of recombination differed between floxed alleles.
Impaired fertility and arrested follicular development in induced Foxl2/Smad4 knockout females
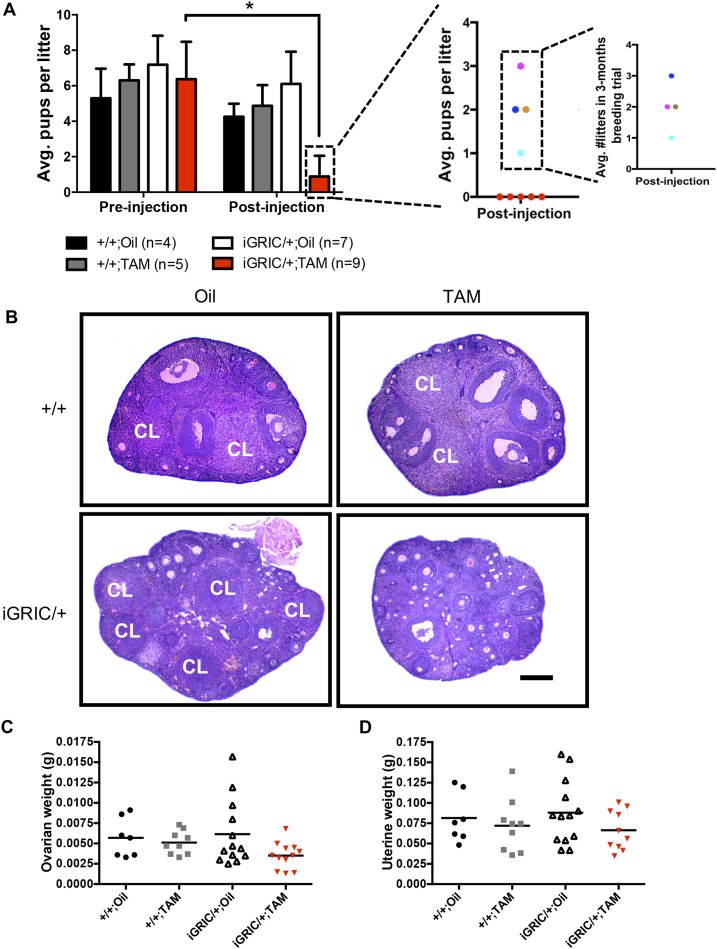
A powerful feature of the inducible system is that each animal can serve as its own control. We therefore assessed fertility in experimental (iGRIC/+;Foxl2fx/fx;Smad4fx/fx) and control (Foxl2fx/fx;Smad4fx/fx) females before and after tamoxifen injections. Eight-week-old animals were paired with wild type males and allowed to produce up to two litters. All females, regardless of genotype, showed normal puberty onset (data not shown) and fertility, indicating that there was no precocious Cre activity under physiological conditions (Fig. 2A). One month after tamoxifen injections, experimental females (hereafter induced F2S4 cKO) became subfertile (4 of 9) or infertile (5 of 9; Fig. 2A). The four animals that maintained some fertility produced small litters of one to three pups, and three of four animals produced two or fewer litters in 3 months (Fig. 2A, right panels). In contrast, fertility remained intact in all the other groups, including tamoxifen-treated females with the control genotype (Fig. 2A, gray bars). Ovarian histology revealed an arrest in follicle development at the early antral stage and the absence of corpora lutea in most of the induced F2S4 cKO females (Fig. 2B). Indeed, many females that ovulated before tamoxifen-induced recombination did not do so after treatment (Supplemental Fig. 4). Ovarian and uterine weights were comparable in all groups, although there was a nonsignificant reduction in ovarian weight in the induced F2S4 cKO mice (Fig. 2C and 2D).
Figure 2.
Impaired fertility and ovarian follicular development in induced F2S4 cKO females. (A) Fertility assessment before and after oil or tamoxifen injections in control and induced F2S4 cKO females. The panels at the right indicate individual fertility data (litter size) for sterile (red dots) and subfertile females (cyan, royal blue, brown, and pink dots). The number of litters produced by subfertile mice is also shown. (B) Representative ovarian tissue sections in control and induced F2S4 cKO mice. Scale bar, 200 μm. (C) Ovarian and (D) uterine weights of control and induced F2S4 cKO females 1 month after oil or tamoxifen injections. Horizontal lines reflect group means (+SEM). Each data point represents one animal. Fertility of each group before injection was analyzed by one-way ANOVA. Fertility before and after treatment in iGRIC/+;TAM group was analyzed by Student t test. *P < 0.05. Avg., average; CL, corpus luteum; TAM, tamoxifen.
Spermatogenesis is unaffected in induced Foxl2/Smad4 knockout mice
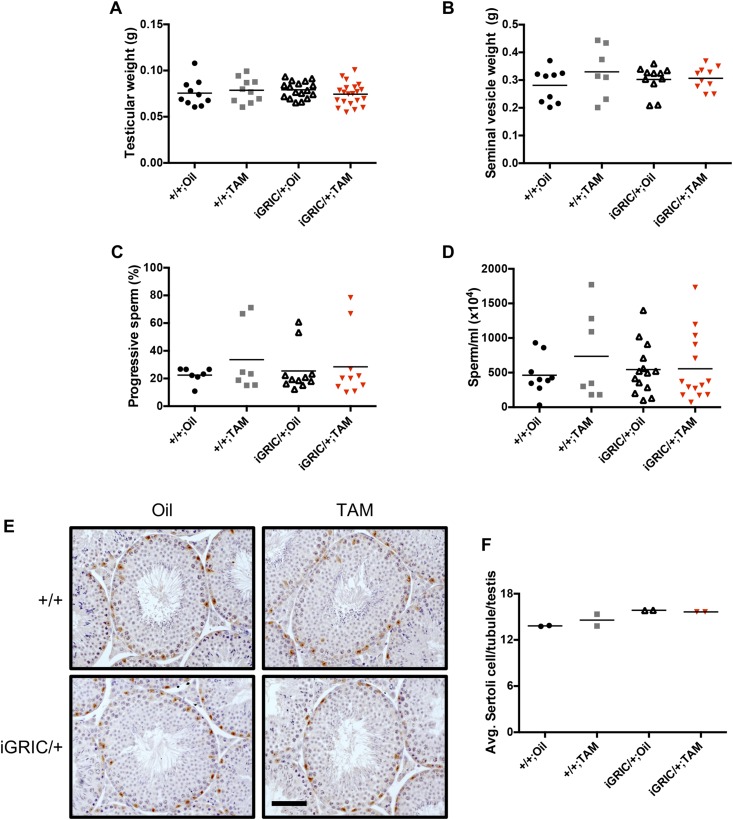
In contrast to the situation in females, induced recombination of the Foxl2 and Smad4 loci did not affect reproductive organs in adult males. Testicular and seminal vesicle weights were normal (Fig. 3A and 3B). Notably, sperm motility (Fig. 3C), sperm counts (Fig. 3D), testicular morphology (Fig. 3E), and Sertoli cell numbers (Fig. 3F) in induced cKO males were indistinguishable from those of controls 2 months after tamoxifen treatment. A complete spermatogenic cycle is estimated at 34.5 days in the mouse (33). We did not assess fertility in males because spermatogenesis was unaffected and because previous models of FSH deficiency did not show fertility defects in males (1).
Figure 3.
Intact testicular weights and spermatogenesis in induced cKO males. (A) Testicular and (B) seminal vesicle weights from control and induced F2S4 cKO males 2 months after oil or tamoxifen injections. (C) Progressive sperm motility from caudal epididymides. (D) Sperm counts from snap frozen epididymides. (E) Representative testicular tissue sections from control and induced F2S4 cKO mice. Sertoli cells were identified with SOX9 using immunohistochemistry (brown stain). (F) Average Sertoli cell numbers per tubule per testis 2 months after oil or tamoxifen injection. Scale bar, 100 µm. There were no statistically significant differences between groups. TAM, tamoxifen.
Induced knockout mice are FSH deficient
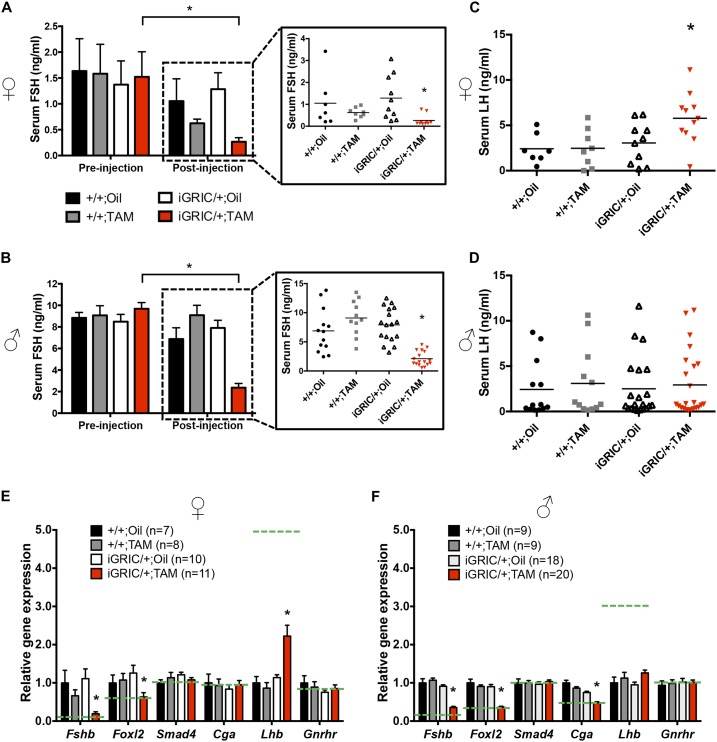
The phenotype in females was consistent with FSH deficiency, which we investigated in detail. Before tamoxifen treatment, FSH levels were equivalent between genotypes in randomly cycling females (Fig. 4A, left). However, 1 month after the last tamoxifen injection, FSH was significantly lower in induced F2S4 cKO females than in control groups and lower than their own serum FSH levels before injection (~83% reduction; Fig. 4A, right). Similarly, pituitary FSH content was significantly lower after tamoxifen treatment, as assessed by RIA (Fig. 5A) or immunofluorescence (Fig. 5E). Reductions in FSH protein derived from a significant decrease (~80%) in pituitary Fshb mRNA expression in induced F2S4 cKO mice (Fig. 4E). Serum estradiol levels could not be reliably measured, because many of the samples were at the detection limit of the assay (Supplemental Fig. 5A). Ovarian aromatase (Cyp19a1) mRNA expression mirrored the serum FSH levels, although the differences between groups were not statistically significant (Supplemental Fig. 5B).
Figure 4.
Reduced FSH production and secretion in induced F2S4 cKO mice. Serum FSH levels in (A) females and (B) males before (left) and after (right) tamoxifen or oil treatment. Individual serum FSH levels after injection are shown in the boxes at the right. Serum LH levels in (C) females and (D) males after treatment. (E and F) RT-qPCR analysis of relative mRNA levels of pituitary Fshb, Foxl2, Smad4, Cga, Lhb, and Gnrhr in females and males after treatment with oil or tamoxifen. All gene expression was normalized to the housekeeping gene Rpl19. Dashed lines represent mRNA levels in mice lacking the Foxl2 and Smad4 genes during development (20). Relative gene expression and serum FSH levels before and after treatment were analyzed separated by one-way ANOVAs followed by Dunn multiple comparisons test. Serum FSH levels before and after treatment in the same group were analyzed by Student t test. There were no significant differences between the control groups. *P < 0.05. TAM, tamoxifen.
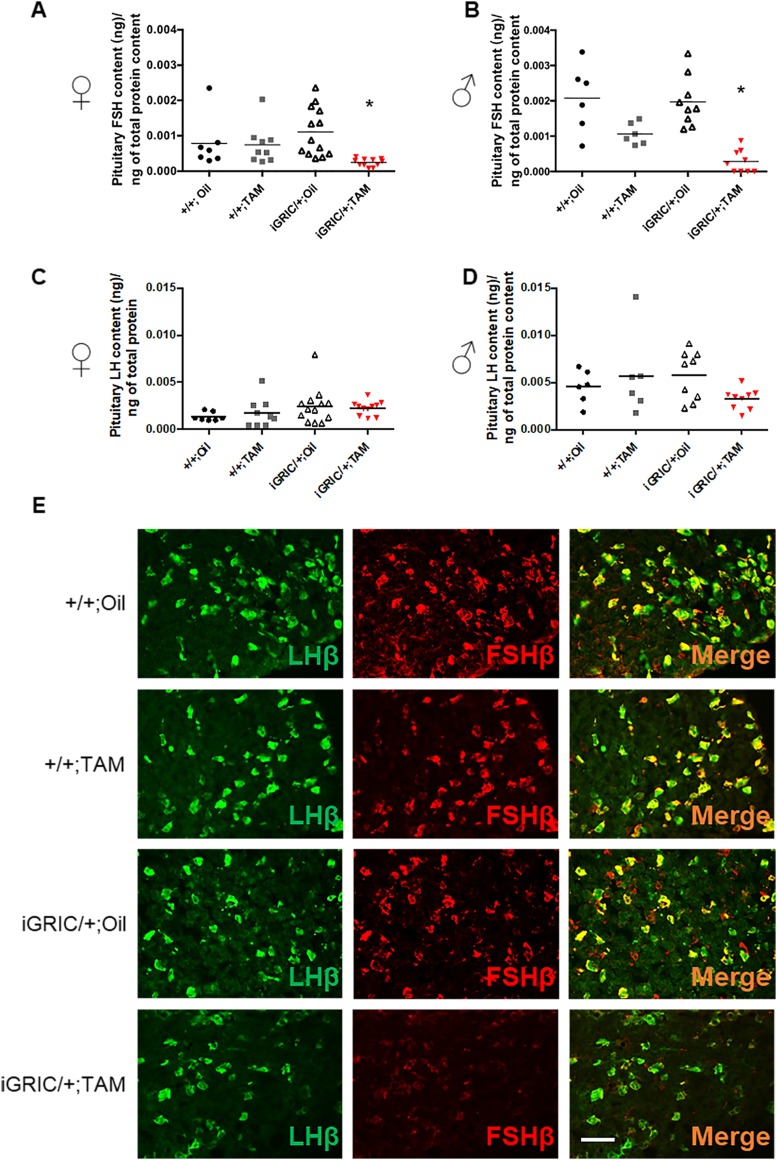
Figure 5.
Reduced pituitary FSH protein content in induced F2S4 cKO mice. Pituitary FSH content in (A) females and (B) males after oil or tamoxifen treatment. Control female pituitaries were collected at random estrous cycle stages; cKO pituitaries were collected at the same time as their control littermates. (C and D) Pituitary LH content in (C) females and (D) males after treatment. (E) Immunofluorescence staining for LHβ (green) and FSHβ (red) in pituitaries of male control and induced F2S4 cKO mice. Data were analyzed by one-way ANOVA followed by Dunn multiple comparisons test. Scale bar, 50 µm. *P < 0.05. TAM, tamoxifen.
Despite the absence of a spermatogenesis phenotype, induced F2S4 cKO males similarly showed normal serum FSH before treatment (Fig. 4B, left) but significantly reduced serum FSH (~76%) (Fig. 4B, right) and pituitary FSH protein content (Fig. 5B) 2 months after the last tamoxifen injection. Pituitary Fshb mRNA levels were similarly reduced (~70%) 1 (Supplemental Fig. 6) or 2 months (Fig. 4F) after treatment.
Induced F2S4 cKO females showed significantly higher serum LH compared with controls (Fig. 4C), which was correlated with increases in pituitary Lhb mRNA levels (Fig. 4E) but not pituitary LH content (Fig. 5C). In contrast, serum LH, pituitary Lhb mRNA, and pituitary LH content were comparable across all groups of males 2 months after tamoxifen treatment (Fig. 4D and 4F and Fig. 5D). Gnrhr mRNA levels did not differ between genotypes, but Cga was lower in induced F2S4 cKO males than in the other groups (Fig. 4F).
In both sexes, pituitary Foxl2 expression was significantly reduced (Fig. 4E and 4F). Note that, in addition to gonadotropes, FOXL2 is expressed in thyrotropes in the pituitary gland (34). Therefore, the remaining Foxl2 expression was probably contributed by thyrotropes (Supplemental Fig. 3B). In fact, the residual pituitary Foxl2 expression levels in both males and females was comparable to that seen with the original GRIC model (Fig. 4E and 4F) (21). In contrast, Smad4 mRNA expression did not differ between groups (Fig. 4E and 4F). This was not unexpected given the low abundance of gonadotropes in the pituitary (~5% to 10% of cells), the broad expression of Smad4 in all pituitary lineages (35), and the incomplete recombination of the floxed Smad4 alleles in gonadotropes of these mice (Fig. 1D).
Tamoxifen effects on pituitary gene expression are transient
The absence of any effects in control mice treated with tamoxifen argues strongly that it was induced gene recombination rather than tamoxifen itself that produced the observed phenotypes in experimental mice. However, we treated adult wild type males with one or four injections of tamoxifen and then collected their pituitaries 6 hours, 1 day, 1 week, or 2 weeks later to assess the acute and lasting effects of the drug. Tamoxifen suppressed Gnrhr mRNA maximally at 6 hours (Supplemental Fig. 7A). Levels increased but still remained below control values at 1 day and 1 week. By 2 weeks after injection, Gnrhr mRNA levels had returned to baseline. In contrast, Fshb, Foxl2, Lhb, and Cga mRNA levels were generally stable after tamoxifen injections (Supplemental Fig. 7B–7E).
Induced deletion of Foxl2 and Smad4 impairs activin-regulated Fshb expression in culture
FOXL2 and SMAD4 are implicated in the stimulatory actions of activins on FSH (19). To determine whether activin regulation of Fshb was impaired in induced F2S4 cKO mice, we compared the behavior of their pituitaries in culture with those of the three control groups. Basal Fshb expression in cultured murine pituitaries depends on endogenous activins or related TGFβ ligands (20, 25, 36–40). Pituitary cultures from control mice, of both sexes, showed equivalent levels of basal and activin A–stimulated Fshb mRNA expression (Fig. 6A and 6B). In contrast, basal and activin A–induced Fshb expression were reduced to nearly undetectable levels in pituitaries from induced F2S4 cKO mice. These effects were gene-specific, as expression of Gnrhr, Lhb, and Cga did not differ between the four groups (Supplemental Fig. 8).
Figure 6.
Impaired basal and activin A–stimulated Fshb expression in cultured pituitary cells of induced F2S4 cKO mice. Primary pituitary cultures were prepared from control and induced F2S4 cKO (A) females and (B) males at least 1 month after oil or tamoxifen injection. Cells were treated with vehicle or 1 nM activin A. (C) Primary pituitary cultures were prepared from male and female (combined) iGRIC/+;Foxl2fx/fx;Smad4fx/fx mice or from (D) wild-type mice. Cells were then treated with 1.5 µM of 4-OHT or vehicle. Twenty-four hours later, cells were treated with vehicle or 1 nM activin A for 24 hours. In all panels, RNA was extracted and gene expression was assessed by RT-qPCR. Bars represent the means (+SEM) of three independent experiments. In (A) and (B), basal Fshb expression levels (black bars) were analyzed by one-way ANOVA followed by Dunn multiple comparisons test. Data in (C) and (D) were analyzed by two-way ANOVA followed by Sidak post hoc tests. Bars with different symbols (^, &, or *) differ significantly. #Significantly different from the other groups; activin A stimulation on Fshb expression in each group was analyzed by Student t test (red vs black bars). *P < 0.05. n.s., not significant; TAM, tamoxifen.
In a final experiment, we determined the ability of tamoxifen to induce gonadotrope-specific gene recombination in vitro. We cultured pituitary cells from GRIC/+;Foxl2fx/fx;Smad4fx/fx mice that were previously untreated in vivo. We then added oil vehicle or the active metabolite 4-OHT to half of the wells. 4-OHT treatment led to a significant suppression of Fshb mRNA expression and a complete block of the activin A response (Fig. 6C). 4-OHT also reduced levels of Foxl2 and Gnrhr mRNA but not those of Smad4, Lhb, or Cga (Supplemental Fig. 9A–9E). It was 4-OHT–induced recombination in gonadotropes, and not 4-OHT itself, that led to decreases in Fshb expression, because 4-OHT did not affect the expression of Fshb or any of the other genes investigated, with the exception of Gnrhr, when applied to pituitary cultures from wild type mice (Fig. 6D and Supplemental Fig. 9F–9J).
Discussion
Here, we describe the development of a tamoxifen-inducible Cre-driver line for the murine gonadotrope cell lineage. Using these mice, we were able to selectively and efficiently delete two genes, Foxl2 and Smad4, simultaneously in gonadotropes of adult mice. These animals display normal reproductive physiology before treatment with the inducing agent tamoxifen. One month after treatment, both female and male cKO mice showed profound suppression of FSH levels, secondary to a selective reduction of pituitary Fshb mRNA expression. This FSH deficiency leads to subfertility (reduced litter size), but more often sterility, in female mice that were previously fertile. The principal defect appears to be in FSH-stimulated ovarian follicle development, which is arrested at the early antral stage in most cases. This phenotype is comparable to that of mice lacking a functional Fshb subunit gene from conception or of mice with gonadotrope-specific deletions of Foxl2 and Smad4 or Smad3 and Smad4 beginning around embryonic day 13 (1, 20, 25). Males similarly show profound FSH deficiency after tamoxifen treatment. However, in contrast to global Fshb or conditional Foxl2/Smad4 and Smad3/Smad4 knockout mice, which show profound oligozoospermia (20, 25), reduction of FSH in adulthood had no measurable effect on spermatogenesis.
The results of this work provide important insights into the control of FSH synthesis and FSH actions in the reproductive axis. First, they show that FOXL2 and SMAD4 are essential for Fshb expression in gonadotropes of adult mice. Using GRIC mice to ablate these genes during embryonic life leads to a similar FSH deficiency as described here (20). Despite the fact that LH production was largely intact in these animals, it was at least formally possible that the gene deletions somehow affected gonadotrope development, indirectly causing FSH deficiency (20). The results with the iGRIC strain put this possibility to rest, conclusively demonstrating a necessity for FOXL2 and, perhaps, SMAD4 to maintain Fshb expression in adult gonadotropes. Second, the data show that FSH is fundamentally necessary for the later stages of ovarian follicle development. Regardless of whether FSH is eliminated during embryonic life or is reduced in adulthood, follicle growth is arrested at the preantral or early antral stage. Third, the results strongly suggest that FSH regulates spermatogenesis during development but not in adulthood in mice.
FSH regulation of spermatogenesis
It is well known that FSH stimulates Sertoli cell proliferation during early postnatal development (5, 41, 42). The number of Sertoli cells determines spermatogenic capacity postpubertally. Fshb and Fshr knockout mice have lower Sertoli cell numbers and lower sperm counts than wild type mice (1, 7–9). We did not previously measure Sertoli cell number in gonadotrope-specific Foxl2/Smad4 or Smad3/4 cKO (by using the GRIC Cre-driver), but these animals similarly show reduced testis size and sperm counts (20, 25). Mice lacking Foxl2 alone in gonadotropes exhibit decreases in FSH levels, testis size, sperm counts, and, importantly, Sertoli cell numbers (21). Therefore, it seems likely that loss of both Foxl2 and Smad4 during gonadotrope development would lead to a similar reduction in Sertoli cell number. In contrast, the inducible knockout mice developed here have normal size testes, sperm counts, and Sertoli cell numbers. Therefore, FSH appears to regulate Sertoli cell proliferation only during a critical developmental time window in mice.
However, impaired spermatogenesis in these mice might still have been anticipated given other actions of FSH in the testis, including stimulation of androgen-binding protein (ABP) levels (7, 43–45). ABP increases intratesticular T concentrations, which are important for spermatogenesis. That said, the absolute levels of T necessary are not defined and may differ across species. In hypogonadal (hpg; GnRH-deficient) mice, for example, exogenous T or DHT alone (in the absence of FSH) is sufficient to induce spermatogenesis, even when intratesticular androgen levels remain very low (46, 47). Thus, provided LH is available to stimulate T production above a needed threshold, reduced FSH stimulation of ABP may be of little consequence in mice. In contrast, in humans and nonhuman primates, it is clear that FSH plays important roles in both the initiation and maintenance of spermatogenesis. For example, FSH can stimulate resumption of spermatogenesis in men rendered gonadotropin-deficient with T enanthate (48). Similarly, in cynomolgus monkeys treated with a GnRH antagonist, exogenous FSH can stimulate spermatogenesis (49).
One could argue that the extent of FSH suppression (~76%) achieved in our study may have been insufficient to affect spermatogenesis. However, an equivalent extent of FSH suppression was sufficient to profoundly impair ovarian function in females. It is also possible that FSH was not suppressed for an adequate period of time to affect spermatogenesis. However, animals were investigated 2 months after the initiation of gene recombination, which is roughly the length of two complete spermatogenic cycles (33). Collectively, the data suggest that FSH does not regulate spermatogenesis in adult mice, although a complete abrogation of FSH production may be needed to firmly reach this conclusion.
Differences in effects of Foxl2/Smad4 deletion during development and in adulthood
Ablation of Foxl2 and Smad4 in developing (GRIC) and adult (iGRIC) gonadotropes produced very similar but not identical phenotypes (20). In addition to the differences in spermatogenesis discussed earlier, there are other notable discrepancies between the two models. In both males and females, the extent of FSH suppression was greater with GRIC than with iGRIC mice (20). This difference probably reflects the extent of gene recombination, because inducible systems are inherently incomplete or mosaic (50, 51), and our data show that Smad4 mRNA levels were reduced by only 60%. Another notable difference is in uterine weight, which was reduced in mice with the GRIC driver but not with the iGRIC driver. The uterus is highly sensitive to estrogens (52–54). It is therefore possible that estrogen suppression is greater with the GRIC than iGRIC model. Given the role of FSH in estrogen biosynthesis and the somewhat higher FSH levels in the iGRIC mice, this seems a distinct possibility. Unfortunately, RIAs and ELISAs for 17β-estradiol in mice may lack the necessary sensitivity to make this determination (55). Indeed, the estradiol data collected here were often at the limit of detection of the assay. However, we did note a nonsignificant reduction in ovarian aromatase (Cyp19a1) mRNA expression in the inducible knockouts relative to the control groups (Supplemental Fig. 5B), suggesting that estradiol levels may be lower in these mice. Finally, in males with the GRIC allele, serum LH and pituitary Cga mRNA levels were significantly reduced despite a significant increase in pituitary Lhb mRNA levels (20). With the iGRIC model, LH levels were normal in males 2 months after tamoxifen treatment. Pituitary Cga levels were again reduced but to a lesser extent than with the GRIC driver, whereas Lhb was unaffected. We currently lack a definitive explanation for these differences between the two models, but the relative extents of Smad4 recombination between the two models should be considered.
Generation of a tamoxifen-inducible Cre-driver for murine gonadotrope cells
The mouse model described here, iGRIC, will be of broad utility to investigators interested in interrogating gene function in adult gonadotropes. Thus far, we have demonstrated that with four 2-mg tamoxifen injections every other day, we achieve >75% recombination with two different floxed alleles (Foxl2 and Rosa26YFP) after 4 weeks. We were also able to effectively recombine up to five floxed alleles in individual gonadotropes (2 × Foxl2, 2 × Smad4, and 1 × Rosa26YFP). Based on our experience, it seems likely that recombination efficiency will differ between alleles and that some optimization may be necessary. Indeed, after we noted that YFP labeling increases between 2 and 4 weeks after the last of four tamoxifen injections in our standard protocol and that Gnrhr expression is transiently reduced by tamoxifen (Supplemental Fig. 7A), we asked whether modifying the injection protocol might further improve recombination of the Rosa26YFP reporter allele. Recall that Cre is expressed from the Gnrhr locus in the iGRIC model, so it is possible, if not likely, that tamoxifen simultaneously induces Cre activity from existing Cre-ERT2 fusion proteins in the gonadotrope while attenuating production of new Cre-ERT2. Therefore, we injected iGRIC/+;Rosa26YFP/+ reporter mice with 2 mg tamoxifen twice (with 1 day between injections) and then waited 1 week before administering two more injections of tamoxifen. Four weeks after the last injection, we observed YFP in >87% of LHβ+ cells (Supplemental Fig. 10). Thus, we are confident that with some optimization, other investigators can achieve high levels of recombination with their floxed genes of interest. Indeed, we are currently varying the protocol to determine whether we can increase the extent of Smad4 knockdown when using this model to better determine SMAD4’s role in adulthood. In preliminary analyses, we observed that the four-injection protocol described here had no effect on FSH production in males and variable effects on fertility in females with the iGRIC/+;Smad4fx/fx genotype (G.S., unpublished data, 2018). This result suggests that the phenotypes described here may derive more from the loss of FOXL2 than from the combined loss of FOXL2 and SMAD4. However, we will be able to make this determination only when we ablate the genes independently and to equivalent extents.
Although we demonstrated the high efficiency and specificity of this Cre-driver line, some may be wary of using tamoxifen in the context of reproductive studies. However, our experience should allay such fears. As we show, the effects of tamoxifen itself on pituitary gene expression are both modest and short-lived. One or 2 months after the final injection, control males treated with tamoxifen were indistinguishable from control mice treated with oil vehicle. Females may be more sensitive to the effects of tamoxifen, however, because FSH levels appeared to be lower (though not significantly) in tamoxifen-treated than oil-treated controls 4 weeks after the final injection (Fig. 4A and data not shown). Moreover, tamoxifen-treated controls exhibited normal fertility (Fig. 2A), and their FSH levels were comparable to those of oil-treated controls at the end of the fertility trial (data not shown). If the apparent reductions in FSH remain a concern for some, we recommend waiting 8 weeks after injection to begin fertility or hormone assessments. However, 4 weeks seems sufficient in our experience. This amount of time is also sufficient for gene recombination and its downstream effects to occur. Finally, because gonadotropes are generally considered to be a stable cell population, one can expect the effects of recombination to be durable without any recovery of function, as we observed over a 3-month period in females.
Others may wonder whether there is Cre activity beyond gonadotrope cells in these mice. Although we did not investigate this question systematically, one can assume that extrapituitary sites of Cre-ERT2 expression would mirror those of Cre expression in GRIC mice, namely the male germline and some (still undefined) neurons (23, 56–59). Induced recombination in these cells must still be determined, but given that tamoxifen can cross both the blood-brain (60) and blood-testis barriers (61), at least some recombination in these tissues should be expected. The effects in testis, though, should be short lived, because Cre activity is observed only at the round spermatid stage in GRIC mice (23). Indeed, preliminary analyses suggest that recombination of the Foxl2 allele is observable in the pituitary but not testes 2 months after tamoxifen treatment (Supplemental Fig. 11).
In summary, we developed an inducible Cre-driver line for murine gonadotrope cells. Deletion of the Foxl2 and Smad4 genes in adult gonadotropes leads to FSH deficiency and impaired female reproduction. Sperm counts and motility remain intact, suggesting that FSH may not be not essential for the maintenance of spermatogenesis in adult male mice. Our studies provide a valuable mouse model and a roadmap for investigations of gene function in adult gonadotropes.
Supplementary Material
Acknowledgments
The authors thank Dr. Bernard Robaire for providing access to the CASA system; Dr. Alfredo Ribeiro-da-Silva for providing access to the Zeiss Axio Imager M2 microscope; McGill University Life Science Complex Advanced BioImaging Facility for providing access to the confocal microscope; and Dr. Dagmar Wilhelm for generously providing the FOXL2 antibody.
Financial Support: This study was supported in part by Canadian Institutes of Health Research operating Grants MOP-133394 and 123447 and Natural Sciences and Engineering Research Council of Canada Discovery Grant 2015-05178 (to D.J.B.). Y.L. and G.S. received fellowships from the McGill Centre for Research in Reproduction and Development and Dr. Samuel Solomon Fellowships in Endocrinology. G.S. also received scholarship support from Fonds de Recherche du Québec - Santé (31338) and Canadian Institutes of Health Research (152308). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD28934 and Janice Penney and Jacinthe Sirois from the transgenic facility at the McGill Life Sciences Complex.
Disclosures: The authors have nothing to disclose.
Glossary
Abbreviations:
- 4-OHT
4-hydroxytamoxifen
- ABP
androgen-binding protein
- cKO
conditional knockout
- FOXL2
forkhead box L2
- FSHB
FSHβ
- Gnrhr
GnRH receptor
- GRIC
GnRHR-IRES-Cre
- iGRIC
inducible GnRHR-IRES-Cre
- PBST
PBS with Tween 20
- RT-qPCR
quantitative RT-PCR
- SOX9
sex-determining region Y–box 9
- T
testosterone
References
- 1. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204. [DOI] [PubMed] [Google Scholar]
- 2. Matthews CH, Borgato S, Beck-Peccoz P, Adams M, Tone Y, Gambino G, Casagrande S, Tedeschini G, Benedetti A, Chatterjee VK. Primary amenorrhoea and infertility due to a mutation in the beta-subunit of follicle-stimulating hormone. Nat Genet. 1993;5(1):83–86. [DOI] [PubMed] [Google Scholar]
- 3. Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15(6):725–751. [DOI] [PubMed] [Google Scholar]
- 4. Couzinet B, Lestrat N, Brailly S, Forest M, Schaison G. Stimulation of ovarian follicular maturation with pure follicle-stimulating hormone in women with gonadotropin deficiency. J Clin Endocrinol Metab. 1988;66(3):552–556. [DOI] [PubMed] [Google Scholar]
- 5. Singh J, Handelsman DJ. Neonatal administration of FSH increases Sertoli cell numbers and spermatogenesis in gonadotropin-deficient (hpg) mice. J Endocrinol. 1996;151(1):37–48. [DOI] [PubMed] [Google Scholar]
- 6. Simoni M, Weinbauer GF, Gromoll J, Nieschlag E. Role of FSH in male gonadal function. Ann Endocrinol (Paris). 1999;60(2):102–106. [PubMed] [Google Scholar]
- 7. Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O’Shaughnessy PJ. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145(1):318–329. [DOI] [PubMed] [Google Scholar]
- 8. Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141(5):1795–1803. [DOI] [PubMed] [Google Scholar]
- 9. Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance [published correction appears in PNAS. 1999;96(2):795] Proc Natl Acad Sci USA. 1998;95(23):13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sairam MR, Krishnamurthy H. The role of follicle-stimulating hormone in spermatogenesis: lessons from knockout animal models. Arch Med Res. 2001;32(6):601–608. [DOI] [PubMed] [Google Scholar]
- 11. Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don’t, and why you should care. Fertil Steril. 2010;93(8):2465–2485. [DOI] [PubMed] [Google Scholar]
- 12. Bailey JS, Rave-Harel N, McGillivray SM, Coss D, Mellon PL. Activin regulation of the follicle-stimulating hormone beta-subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol Endocrinol. 2004;18(5):1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suszko MI, Balkin DM, Chen Y, Woodruff TK. Smad3 mediates activin-induced transcription of follicle-stimulating hormone beta-subunit gene. Mol Endocrinol. 2005;19(7):1849–1858. [DOI] [PubMed] [Google Scholar]
- 14. Lamba P, Wang Y, Tran S, Ouspenskaia T, Libasci V, Hébert TE, Miller GJ, Bernard DJ. Activin A regulates porcine follicle-stimulating hormone beta-subunit transcription via cooperative actions of SMADs and FOXL2. Endocrinology. 2010;151(11):5456–5467. [DOI] [PubMed] [Google Scholar]
- 15. Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK. Regulation of the rat follicle-stimulating hormone beta-subunit promoter by activin. Mol Endocrinol. 2003;17(3):318–332. [DOI] [PubMed] [Google Scholar]
- 16. Corpuz PS, Lindaman LL, Mellon PL, Coss D. FoxL2 Is required for activin induction of the mouse and human follicle-stimulating hormone beta-subunit genes. Mol Endocrinol. 2010;24(5):1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB. Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-beta gene. Mol Endocrinol. 2005;19(1):237–254. [DOI] [PubMed] [Google Scholar]
- 18. Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone beta subunit transcription. Mol Endocrinol. 2009;23(7):1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tran S, Lamba P, Wang Y, Bernard DJ. SMADs and FOXL2 synergistically regulate murine FSHbeta transcription via a conserved proximal promoter element. Mol Endocrinol. 2011;25(7):1170–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. FASEB J. 2014;28(8):3396–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, Boehm U, Treier M, Boerboom D, Bernard DJ. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27(3):407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701–2711. [DOI] [PubMed] [Google Scholar]
- 23. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci USA. 2010;107(37):16372–16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y, Schang G, Boehm U, Deng CX, Graff J, Bernard DJ. SMAD3 regulates follicle-stimulating hormone synthesis by pituitary gonadotrope cells in vivo. J Biol Chem. 2017;292(6):2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oakes CC, Kelly TL, Robaire B, Trasler JM. Adverse effects of 5-aza-2′-deoxycytidine on spermatogenesis include reduced sperm function and selective inhibition of de novo DNA methylation. J Pharmacol Exp Ther. 2007;322(3):1171–1180. [DOI] [PubMed] [Google Scholar]
- 27. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sánchez-Criado JE, Guelmes P, Bellido C, Gónzalez M, Hernández G, Aguilar R, Garrido-Gracia JC, Bello AR, Alonso R. Tamoxifen but not other selective estrogen receptor modulators antagonizes estrogen actions on luteinizing hormone secretion while inducing gonadotropin-releasing hormone self-priming in the rat. Neuroendocrinology. 2002;76(4):203–213. [DOI] [PubMed] [Google Scholar]
- 29. Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, Parlow AF, Veldhuis JD, Waters MJ, Chen C. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152(8):3165–3171. [DOI] [PubMed] [Google Scholar]
- 30. Bernard DJ. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone beta subunit in mouse gonadotrope cells. Mol Endocrinol. 2004;18(3):606–623. [DOI] [PubMed] [Google Scholar]
- 31. Ho CC, Zhou X, Mishina Y, Bernard DJ. Mechanisms of bone morphogenetic protein 2 (BMP2) stimulated inhibitor of DNA binding 3 (Id3) transcription. Mol Cell Endocrinol. 2011;332(1-2):242–252. [DOI] [PubMed] [Google Scholar]
- 32. Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27(22):4324–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99(3):507–516. [DOI] [PubMed] [Google Scholar]
- 34. Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol Endocrinol. 2006;20(11):2796–2805. [DOI] [PubMed] [Google Scholar]
- 35. Yeung CM, Chan CB, Leung PS, Cheng CH. Cells of the anterior pituitary. Int J Biochem Cell Biol. 2006;38(9):1441–1449. [DOI] [PubMed] [Google Scholar]
- 36. Rejon CA, Ho CC, Wang Y, Zhou X, Bernard DJ, Hébert TE. Cycloheximide inhibits follicle-stimulating hormone β subunit transcription by blocking de novo synthesis of the labile activin type II receptor in gonadotrope cells. Cell Signal. 2013;25(6):1403–1412. [DOI] [PubMed] [Google Scholar]
- 37. Gore AJ, Philips DP, Miller WL, Bernard DJ. Differential regulation of follicle stimulating hormone by activin A and TGFB1 in murine gonadotropes. Reprod Biol Endocrinol. 2005;3(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ho CC, Bernard DJ. Bone morphogenetic protein 2 signals via BMPR1A to regulate murine follicle-stimulating hormone beta subunit transcription. Biol Reprod. 2009;81(1):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ho CC, Bernard DJ. Bone morphogenetic protein 2 acts via inhibitor of DNA binding proteins to synergistically regulate follicle-stimulating hormone beta transcription with activin A. Endocrinology. 2010;151(7):3445–3453. [DOI] [PubMed] [Google Scholar]
- 40. Lee KB, Khivansara V, Santos MM, Lamba P, Yuen T, Sealfon SC, Bernard DJ. Bone morphogenetic protein 2 and activin A synergistically stimulate follicle-stimulating hormone beta subunit transcription. J Mol Endocrinol. 2007;38(1–2):315–330. [DOI] [PubMed] [Google Scholar]
- 41. Rebourcet D, Darbey A, Monteiro A, Soffientini U, Tsai YT, Handel I, Pitetti JL, Nef S, Smith LB, O’Shaughnessy PJ. Sertoli cell number defines and predicts germ and Leydig cell population sizes in the adult mouse testis. Endocrinology. 2017;158(9):2955–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Orth JM. The role of follicle-stimulating hormone in controlling Sertoli cell proliferation in testes of fetal rats. Endocrinology. 1984;115(4):1248–1255. [DOI] [PubMed] [Google Scholar]
- 43. Hansson V, Weddington SC, French FS, McLean W, Smith A, Nayfeh SN, Ritzén EM, Hagenäs L. Secretion and role of androgen-binding proteins in the testis and epididymis. J Reprod Fertil Suppl. 1976;(24 suppl):17–33. [PubMed]
- 44. Ritzen EM, Hagenas L, Hansson V, Frensh FS. In vitro synthesis of testicular androgen binding protein (ABP): stimulation by FSH and androgen. Curr Top Mol Endocrinol. 1975;2:353–366. [DOI] [PubMed] [Google Scholar]
- 45. Tindall DJ, Mena CR, Means AR. Hormonal regulation of androgen-binding protein in hypophysectomized rats. Endocrinology. 1978;103(2):589–594. [DOI] [PubMed] [Google Scholar]
- 46. Singh J, Handelsman DJ. The effects of recombinant FSH on testosterone-induced spermatogenesis in gonadotrophin-deficient (hpg) mice. J Androl. 1996;17(4):382–393. [PubMed] [Google Scholar]
- 47. Singh J, O’Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136(12):5311–5321. [DOI] [PubMed] [Google Scholar]
- 48. Matsumoto AM, Karpas AE, Paulsen CA, Bremner WJ. Reinitiation of sperm production in gonadotropin-suppressed normal men by administration of follicle-stimulating hormone. J Clin Invest. 1983;72(3):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weinbauer GF, Behre HM, Fingscheidt U, Nieschlag E. Human follicle-stimulating hormone exerts a stimulatory effect on spermatogenesis, testicular size, and serum inhibin levels in the gonadotropin-releasing hormone antagonist-treated nonhuman primate (Macaca fascicularis). Endocrinology. 1991;129(4):1831–1839. [DOI] [PubMed] [Google Scholar]
- 50. Guo C, Yang W, Lobe CG. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis. 2002;32(1):8–18. [DOI] [PubMed] [Google Scholar]
- 51. Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2(10):743–755. [DOI] [PubMed] [Google Scholar]
- 52. O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281(36):26683–26692. [DOI] [PubMed] [Google Scholar]
- 53. Winuthayanon W, Lierz SL, Delarosa KC, Sampels SR, Donoghue LJ, Hewitt SC, Korach KS. Juxtacrine activity of estrogen receptor α in uterine stromal cells is necessary for estrogen-induced epithelial cell proliferation. Sci Rep. 2017;7(1):8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Groothuis PG, Dassen HH, Romano A, Punyadeera C. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update. 2007;13(4):405–417. [DOI] [PubMed] [Google Scholar]
- 55. Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152(11):4443–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Skinner DC, Albertson AJ, Navratil A, Smith A, Mignot M, Talbott H, Scanlan-Blake N. Effects of gonadotrophin-releasing hormone outside the hypothalamic-pituitary-reproductive axis. J Neuroendocrinol. 2009;21(4):282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fraser HM, Sellar RE, Illingworth PJ, Eidne KA. GnRH receptor mRNA expression by in-situ hybridization in the primate pituitary and ovary. Mol Hum Reprod. 1996;2(2):117–121. [DOI] [PubMed] [Google Scholar]
- 58. Granger A, Ngô-Muller V, Bleux C, Guigon C, Pincas H, Magre S, Daegelen D, Tixier-Vidal A, Counis R, Laverrière JN. The promoter of the rat gonadotropin-releasing hormone receptor gene directs the expression of the human placental alkaline phosphatase reporter gene in gonadotrope cells in the anterior pituitary gland as well as in multiple extrapituitary tissues. Endocrinology. 2004;145(2):983–993. [DOI] [PubMed] [Google Scholar]
- 59. Anjum S, Krishna A, Sridaran R, Tsutsui K. Localization of gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin and GnRH receptor and their possible roles in testicular activities from birth to senescence in mice. J Exp Zool A Ecol Genet Physiol. 2012;317(10):630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Valny M, Honsa P, Kirdajova D, Kamenik Z, Anderova M. Tamoxifen in the mouse brain: implications for fate-mapping studies using the tamoxifen-inducible Cre-loxP system. Front Cell Neurosci. 2016;10:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sultana T, Hou M, Stukenborg JB, Töhönen V, Inzunza J, Chagin AS, Sollerbrant K. Mice depleted of the coxsackievirus and adenovirus receptor display normal spermatogenesis and an intact blood-testis barrier. Reproduction. 2014;147(6):875–883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.