Abstract
Estrogens bind to two nuclear estrogen receptor (ER) subtypes, ERα and ERβ, which are expressed in differing amounts in various tissues. The endogenous estrogen, 17β-estradiol (E2), binds to both subtypes with nearly equal affinity and is the prototypical agonist. Selective estrogen receptor modulators (SERMs) may bind to both subtypes with equivalent affinities but have agonist activities in some tissues while having antagonist activities in others. In the present study, we demonstrate that the first reported endogenous SERM, 27-hydroxycholesterol (27-OHC), binds preferentially (>100-fold) to ERβ over ERα. Furthermore, 27-OHC is not able to fully compete with E2 binding, suggesting the two may bind at different sites. We provide an allosteric ternary complex model for the simultaneous binding of 27-OHC and E2 to ERβ, which accurately describes the binding data we have observed. We conclude that 27-OHC is a negative allosteric modifier of E2 binding, with an inhibitor constantof 50 nM and cooperativity factor (α) of 0.036. We also propose an in silico three-dimensional model of the simultaneous binding to guide future experiments. Further study of this unique binding model may allow for the discovery of novel ERβ-selective ligands and potentially explain the lack of effectiveness of ERβ-selective agonists in humans vs preclinical models.
Negative allosteric modification of 17β-estradiol binding to estrogen receptor β by 27-hydroxycholesterol -was identified and verified by analysis of various types of receptor-ligand binding assays.
Estrogens exert diverse actions on distinct target tissues. The classic actions of estrogens are mediated by two nuclear estrogen receptor (ER) subtypes, ERα and ERβ (1–4). Knockout studies in rodents have shown that the ERs are important for the proper function of reproductive tissues (5, 6). These studies, along with others using ER-selective ligands, have also demonstrated important roles for the ERs in the bone, cardiovascular system, breast, prostate, brain, immune system, and inflammatory processes (4–11). In tissues where ERα and ERβ are both expressed, they often have opposing roles. ERα is often implicated in the stimulation of proliferation and inflammation, whereas ERβ inhibits these processes (10–14). Several exogenous ERβ-selective agonists with antiproliferative and anti-inflammatory properties have been discovered and/or synthesized (10, 11, 15). Another class of molecules, known as selective estrogen receptor modulators (SERMs), has similar affinities for both receptors but mixed agonist/antagonist properties, which are tissue, cell, and promoter dependent (16). Multiple exogenous SERMs have been tested clinically, for example, for their ability to have positive effects similar to those of hormone replacement therapy in postmenopausal women, while attempting to avoid the side effects observed with hormone replacement therapy in the Women’s Health Initiative study (17–20). Interestingly, the oxysterol, 27-hydroxycholesterol (27-OHC), has been identified as the first endogenous SERM (21–24).
27-OHC is the most abundant oxysterol in circulation, with a concentration of 150 to 730 nM (10% in an unesterified form) found in normal adult human serum (25–27). 27-OHC is synthesized, from cholesterol, by the cytochrome p450 enzyme CYP27A1 and metabolized by CYP7B1. In humans, mutations in CYP27A1 cause the autosomal-recessive disease cerebrotendinous xanthomatosis (28–30). The symptoms of this disease include brain and tendon xanthomas, dementia, epileptic seizures, mental retardation, and premature atherosclerosis. CYP7B1 mutations in humans result in the autosomal-recessive disease spastic paraplegia type 5 (31–33). The most common symptoms of this disease are weakness and spasticity of the lower limbs. At least two cases of liver failure in infants have also been associated with mutations in CYP7B1 (34, 35). 27-OHC is an agonist for both liver X receptor (LXR) subtypes, LXRα and LXRβ, which regulate cholesterol and fatty acid homeostasis (36). As a SERM, 27-OHC acts as an ER agonist in MCF7 breast cancer cells and as an antagonist in the bone and vasculature (21–23). Although the transcriptional activity of 27-OHC has recently been under investigation by many laboratories, the details of ER binding have not. In this report, we demonstrate the binding selectivity of 27-OHC for ERβ over ERα and present an allosteric binding model for ERβ.
Materials and Methods
Reagents
Tritiated 17β-estradiol (3H-E2) was purchased from American Radiolabeled Chemicals (St. Louis, MO) and PerkinElmer (Waltham, MA). 17β-estradiol (E2) was purchased from Sigma-Aldrich (St. Louis, MO). 27-OHC and additional oxysterols were purchased from Avanti Polar Lipids (Alabaster, AL). Purified human full-length recombinant ERα (P2187) and ERβ (P2466) proteins (PanVera) were purchased from Invitrogen/Life Technologies (Grand Island, NY). ICI 182,780 was a gift from Dr. Rex Hess (University of Illinois, Urbana-Champaign). For critical micelle concentration assays, 1-(2-pyridylazo)-2-naphthol (PAN) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and absorbance at 470 nm was determined by following the methods of Furton and Norelus (37) using Synergy 2 (Biotek, Winooski, VT).
In vitro transcription/translation
Human ERα and ERβ proteins were synthesized by using the in vitro rabbit reticulocyte lysate coupled transcription/translation (TnT) kit from Promega (Madison, WI). PCDNA3.1 vectors containing the ERs were incubated with the components of the kit, including the T7-polymerase, and incubated for 90 minutes at 30°C; they were then separated into aliquots, frozen, and stored at −20°C.
Competitive binding assays
Initial experiments were performed with TnT translation mixes, diluted to a final concentration of 1% volume-to-volume ratio in a buffer containing 10 mM Tris, 1 mM EDTA, and 10% volume-to-volume ratio glycerol (reagents from Fisher Scientific, Fairlawn, NJ). 3H-E2 was added to a final concentration of 1 nM in these assays. Final experiments were performed with PanVera purified ERs, diluted to 0.3–0.5 nM in the same buffer as the TnT-derived ER protein with the addition of 1 mM EGTA (Sigma-Aldrich), 1 mM phenylmethanesulfonyl fluoride (Sigma-Aldrich), 1 mM dithiothreitol (Fisher Scientific), and 0.01% weight-to-volume ratio bovine serum albumin (Fisher Scientific). 3H-E2 was added to a final concentration of 3 nM in these assays. All assays were done on ice and incubated overnight at 4°C. Separation of free and bound ligand was done via addition of dextran-coated charcoal (DCC) at a final concentration of 1% weight-to-volume ratio. DCC was a mixture of 10% weight-to-weight ratio dextran (Sigma-Aldrich) to activated charcoal powder (Sigma-Aldrich) in a final volume of assay buffer, yielding a 5% weight-to-volume ratio. DCC was incubated for 15 minutes with shaking every 5 minutes, followed by centrifugation for 10 minutes. The supernatant was removed and added to Scintisafe 30% scintillation cocktail (Fisher Scientific), vortexed, and measured on a MicroBeta Trilux (PerkinElmer) or Beckman scintillation counter (Beckman Coulter, Brea, CA).
Luciferase assays
Ishikawa uterine cancer cells were passaged in Eagle minimal essential medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum and maintained at 37°C in the presence of 5% CO2. Cells were removed from flasks with trypsin and plated at 5 × 104 cells per well in 24-well plates, or 8 × 103 cells perwell in 96-well plates, for 18 to 24 hours in phenol red free Eagle minimal essential medium (Sigma-Aldrich) supplemented with DCC-stripped 10% fetal bovine serum. Cells were then transfected with the vitellogenin estrogen response element luciferase reporter plasmid, the SV40-renilla expression plasmid as an internal control, and either the ERα or the ERβ expression plasmid and incubated for 18 to 24 hours. Cells were treated with compounds for an additional 18 to 24 hours and then lysed. Luciferase and renilla activity were measured on a Biotek Synergy 2 plate reader using the Stop & Glo Dual Luciferase Kit from Promega.
Statistical analysis and in silico data
Nonlinear regressions, response and binding parameter determinations, and statistical analyses were performed by using Prism 6.0 (GraphPad, La Jolla, CA). The one-site competitive binding calculations in GraphPad software use the Cheng-Prusoff equation (38). The allosteric binding parameter calculations use equation 6 from Christopoulos and Kenakin (39), and additional information is available at https://www.graphpad.com/guides/prism/7/curve-fitting/index.htm?reg_allosteric_competition.htm. Ligand docking was performed by using Rosetta Ligand on the Rosetta Online Server that Includes Everyone (40, 41). Three-dimensional (3D) conformers of 27-OHC were created by using Frog2 version 2.14 (42). The top 10 models from each docking attempt were evaluated, and publication images were generated by using UCSF Chimera (UCSF Resource for Biocomputing, Visualization, and Informatics, San Francisco, CA) (43).
Results
One-site competitive binding analysis
Using a competitive radioligand binding assay, we measured the ability of 27-OHC to bind to the ERs. Concentrations of receptor and 3H-E2 were used that kept the specific binding to <15% of the total radioligand concentration to prevent receptor-induced ligand depletion. We chose to use two different sources of receptor in preliminary experiments to rule out the possibility of source-specific results. Data shown are with the purified ERs because this allowed us to be consistent with receptor concentration. Half maximal inhibitory concentrations (IC50s) and inhibitor constant (Ki) values were obtained via analysis with Prism 6.0 software (GraphPad) by using the formula for one-site competitive binding.
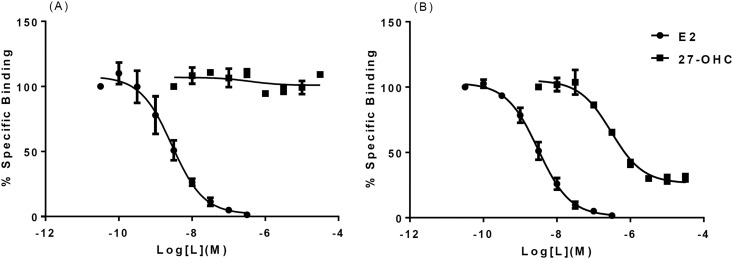
27-OHC bound to ERβ, but not ERα, in the presence of 3 nM 3H-E2 [Fig. 1(A) and 1(B)]. The affinity of 27-OHC binding to ERα was previously reported as 1.32 μM (22). Initial experiments with 1 nM 3H-E2 suggested a Ki > 3.3 μM without reaching saturation. However, little to no competition was observed in our final experiments with purified ERα and 3nM 3H-E2, even at the highest concentrations of 27-OHC tested [Fig. 1(A)]. For ERβ we observed an IC50 of 300 nM for 27-OHC and 3 nM for nontritiated E2 [Fig. 1(B)]. The calculated Ki for 27-OHC binding to ERβ was∼30 nM by using the one-site formula. Interestingly, saturating concentrations of 27-OHC inhibited E2 binding only to ∼35% above baseline. Our results indicate that 27-OHC binds ERβ at least 100-fold better than it does ERα.
Figure 1.
Competitive binding of 27-OHC with purified full-length human ERα and ERβ proteins. (A) 27-OHC does not compete with tritiated E2 (3 nM) for binding to ERα. IC50 for cold E2, ∼3 nM. IC50 for 27-OHC, not determined. (B) 27-OHC appears to compete with tritiated E2 (3 nM) for binding to ERβ, but not to baseline. IC50 for unlabeled E2, ∼3 nM; IC50 for 27-OHC, ∼300 nM; Ki, ∼27 nM. Error bars indicate standard deviation.
On the basis of additional screening (Figs. 2 and 3), 24(S)-hydroxycholesterol [24(S)-OHC] was also tested for its ability to bind to ERβ. We obtained an IC50 of 1.8 μM, corresponding to a Ki of ∼170 nM in these experiments [Supplemental Fig. 1(A)]. Also of note was that the saturating concentrations of 24(S)-OHC inhibited the binding of 3nM 3H-E2 by only 50%.
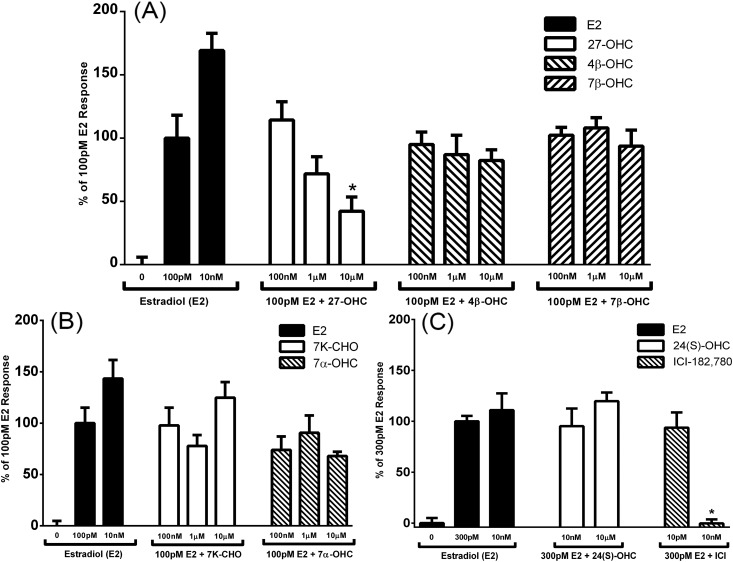
Figure 2.
The apparent competitive transcriptional activity of various oxysterols on human ERα with E2 in an estrogen response element (ERE) luciferase reporter inhibition screen in Ishikawa cells. (A) Antagonist activity of 100 nM, 1 μM, and 10 μM 27-OHC, 4β-OHC, and 7β-OHC on 100 pM E2 stimulation of ERE-driven luciferase expression. Error bars indicate standard error of the mean. *P < 0.05 compared with 100 pM E2-only control. (B) Antagonist activity of 100 nM, 1 μM, and 10 μM 7-ketocholesterol (7K-CHO), and 7α-OHC on 100 pM E2 stimulation of ERE-driven luciferase expression. (C) Antagonist activity of 10 nM, and 10 μM, 24(S)-OHC, and Faslodex (ICI-182-780) on 300 pM E2 stimulation of ERE-driven luciferase expression. Error bars indicate standard error of the mean. *P < 0.05 compared with 300 pM E2-only control.
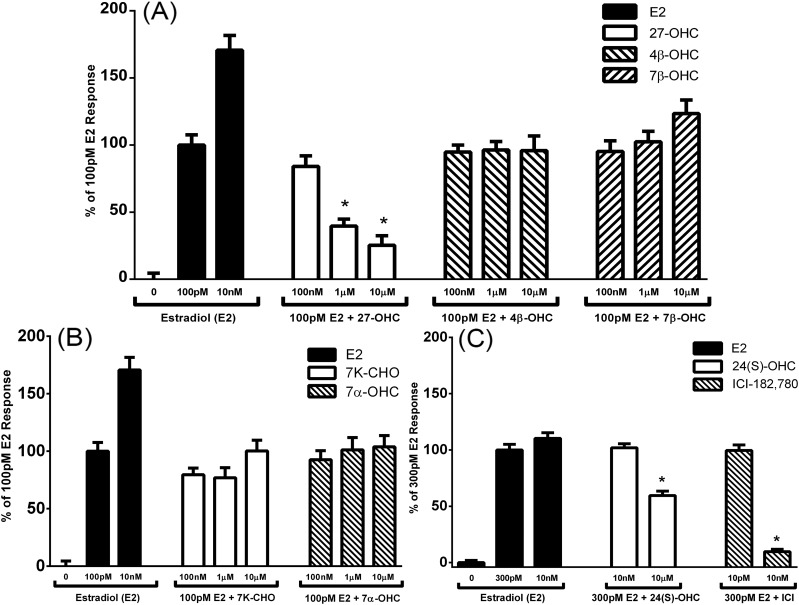
Figure 3.
The apparent competitive transcriptional activity of various oxysterols on ERβ with E2 in an estrogen response element (ERE) luciferase reporter inhibition screen in Ishikawa cells. (A) Antagonist activity of 100 nM, 1 μM, and 10 μM 27-OHC, 4β-OHC, and 7β-OHC on 100 pM E2 stimulation of ERE-driven luciferase expression. Error bars indicate standard error of the mean. *P < 0.05 compared with 100 pM E2-only control. (B) Antagonist activity of 100 nM, 1 μM, and 10 μM 7-ketocholesterol (7K-CHO) and 7α-OHC on 100 pM E2 stimulation of ERE-driven luciferase expression. (C) Antagonist activity of 10 nM and 10 μM 24(S)-OHC and ICI-182,780 on 300 pM E2 stimulation of ERE-driven luciferase expression. Error bars indicate standard error of the mean. *P < 0.05 compared with 300 pM E2-only control.
Inhibition of ER transcriptional activity by oxysterols
We used an in vitro luciferase transcription assay to screen additional oxysterols for their ability to interact with the ERs selectively and similarly to 27-OHC in the presence of E2 in the Ishikawa uterine cancer cell line. Ishikawa uterine cancer cells were transfected with the vitellogenin estrogen response element–driven luciferase reporter, a renilla expression plasmid as a cell number control, and either one of the expression plasmids for the ERs. The fold-change in luciferase expression over renilla expression in treated vs untreated controls was used to determine transcriptional activity. Of the oxysterols tested, only 27-OHC inhibited the transcriptional activity of both receptors at 10 μM [Fig. 2 and Fig. 3(A)]. 24(S)-OHC significantly inhibited ERβ transcriptional activity only at the highest dose tested [Fig. 3(C)].
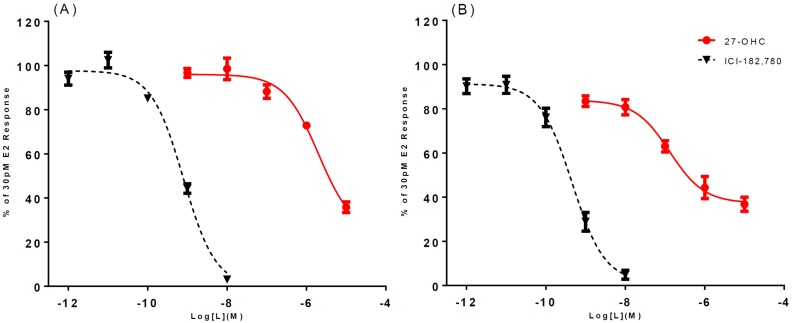
Full-dose curves for 27-OHC and 24(S)-OHC were then performed to obtain IC50s via analysis with Prism 6.0 software (GraphPad). 27-OHC is an antagonist for both receptors, but 27-OHC inhibited ERβ with better potency than it did ERα [Fig. 4(A) and 4(B)]. Also of note is that (similar to the binding observations) saturating concentrations of 27-OHC inhibited ERβ to only ∼35% of maximum stimulated activity. For ERα, 27-OHC had an IC50 > 2 μM [Fig. 4(A)]. However, for ERβ, 27-OHC had an IC50 of ∼130 nM in the Ishikawa cell line [Fig. 4(B)]. 27-OHC is a selective ERβ antagonist that displays >10-fold selectivity over ERα in this assay. Similar results for 27-OHC were also obtained in the C4-12-5 cell line (Supplemental Fig. 2).
Figure 4.
The apparent competitive transcriptional activity dose curves of 27-OHC in an estrogen response element luciferase reporter inhibition screen in Ishikawa cells. (A) 27-OHC inhibits human ERα stimulation by E2 (30 pM); IC50 > 2 μM. (B) 27-OHC inhibits ERβ stimulation by E2 (30 pM); IC50, ∼130 nM. ICI-182-780, Faslodex.
The full dose curve of 24(S)-OHC confirmed that inhibition was only observed at the highest dose tested (10 μM) for ERβ [Supplemental Fig. 1(B)]. Despite the interesting observation that 24(S)-OHC inhibited E2 binding to ERβ to a maximum of 50%, the Ki of 170 nM is at the upper limits of physiological relevance for this oxysterol, and it did not have a corresponding effect in our activity assay (26, 27). For these reasons, we decided to focus on 27-OHC.
Examining the incomplete inhibition of E2 binding to ERβ by 27-OHC
27-OHC binding effects are on the specific binding of the receptor. The nonspecific binding did not significantly increase in the presence of high concentrations of 27-OHC, ruling out the possibility of a simple artifact caused by a lack of sufficient adsorption by the dextran-coated charcoal (Supplemental Fig. 3). We also analyzed the ability of 27-OHC to form micelles, which would limit its effective concentration, using a dye incorporation assay, and found no evidence of a critical micellar concentration from 0.1 to 30 μM (Supplemental Fig. 4). However, it was noted that adding 27-OHC to final concentrations of 100 to 150 μM, in assay buffer, caused the solution to become homogeneously opaque, and concentrations of ≥300μM were visibly insoluble. For this reason, we chose to use no higher than 30 μM in our experiments.
Multiple competition experiments were performed at various doses of fixed 3H-E2 to determine the effect of E2 concentration on the resulting competition curves of 27-OHC. Strikingly, across a 30-fold range of 3H-E2 concentrations, the maximum binding inhibition observed at saturating concentrations of 27-OHC decreased from ∼90% to 25% whereas the IC50s were shifted less than threefold [Supplemental Fig. 5(A)–5(D)]. These data offer additional evidence against the possibility of 27-OHC being a competitive ligand that is merely solubility limited. If it were, it would inhibit binding competitively until it hits its solubility point (or critical micellar concentration), after which any additional ligand would not be available for binding: that is, any higher concentrations tested would show no additional influence on the binding of the tracer. Although our curves do mostly begin to bottom out at ∼3 μM, the 10-μM and 30-μM concentrations almost always further inhibit binding [Supplemental Fig. 5(A)–5(C)].
27-OHC is a negative allosteric modulator of E2 binding to ERβ
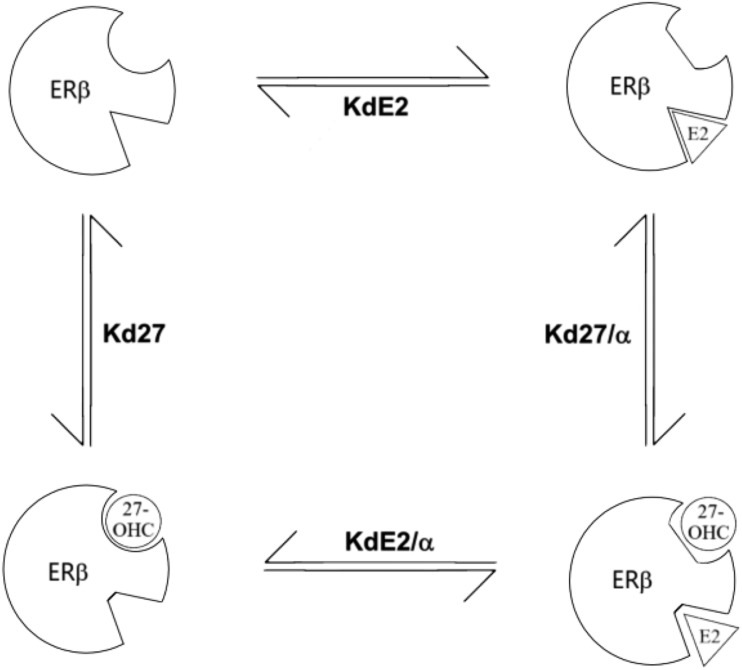
The above evidence clearly demonstrated that 27-OHC was not a classically competitive ligand with E2 for binding to ERβ, nor were the unique curves the result of various artifacts. We hypothesized that the lack of complete competition was the result of allosteric, rather than competitive, binding. Running an allosteric binding analysis on our original binding data from Fig. 1(B), in GraphPad we obtained a Ki of 35 nM for 27-OHC and a cooperativity factor (α) of 0.029. We used a standard allosteric ternary complex model to obtain theoretical equilibrium dissociation constant (Kd)/Ki values for all possible receptor-ligand combinations. This predicts that when the receptor is saturated by both E2 and 27-OHC, each would have an apparent Kd or Ki that was shifted ∼30-fold, ∼5 nM, and ∼1 μM, respectively (Fig. 5).
Figure 5.
Predicted allosteric ternary complex model for E2 and 27-OHC binding to human ERβ. The affinities of E2 (KdE2) and 27-OHC (Kd27) for ERβ are modified by the allosteric effects [cooperativity factor (α)] of each other’s binding. When both ligands are binding simultaneously, their affinities for ERβ are given by the functions KdE2/α and Kd27/α.
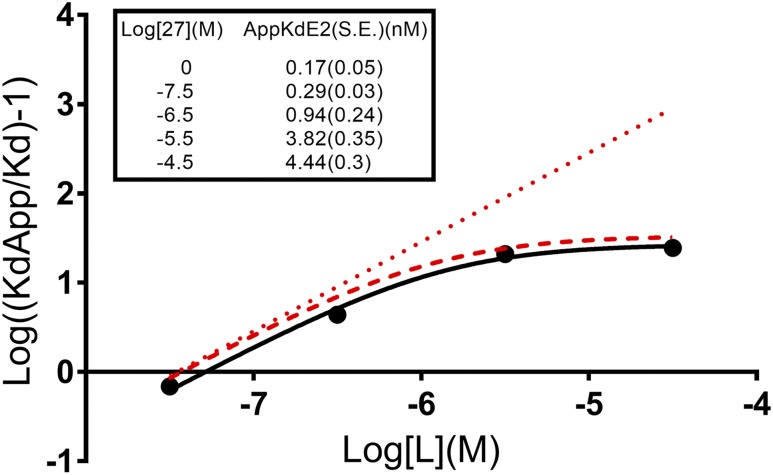
To test our model, we created an affinity ratio plot, which is a classic diagnostic plot for negative allosteric modifiers (44, 45). The affinity ratio plot was constructed from the results of multiple saturation binding assays. Each saturation binding assay was done in the presence of various doses of a fixed concentration of 27-OHC to determine its effect on tritiated E2 saturation binding. Across a 1000-fold range of 27-OHC concentrations, the apparent Kd (KdApp) of E2 only increased to a maximum of 4.4 nM (Fig. 6, insert).
Figure 6.
Allostery diagnostic plot (affinity ratio plot) for 27-OHC binding with E2 to human ERβ. Dotted line is a model of a purely competitive binding interaction with 27-OHC having a Ki of 35 nM. Dashed line is a modeled allosteric interaction based on 27-OHC having a Ki of 35nM and α of 0.029. Solid line is our observed data for the interaction between 27-OHC and E2 binding on ERβ. Calculated values for 27-OHC are Ki of ∼50 nM and α ∼0.036. (Inset) Observed apparent Kd values (nM) for 3H-E2 in the various saturation assays at each given concentration of 27-OHC [Log (M)]. S.E., standard error.
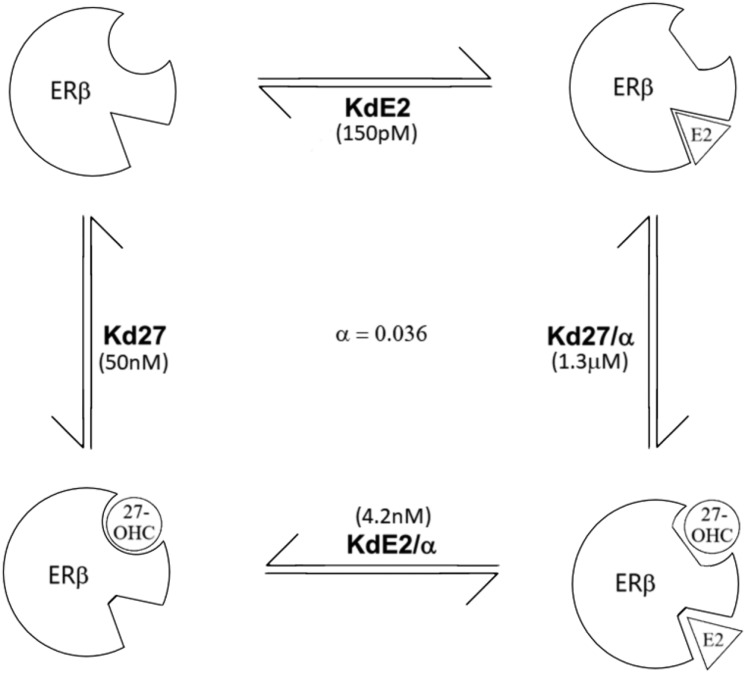
On a standard affinity ratio plot, a strictly competitive ligand will give a straight line when plotted as log[(KdApp/Kd)-1] vs Log [L] (M). Negative allosteric modifiers will yield a curved line with a horizontal asymptote related to the value of α, and x-intercept related to the Ki, when plotted this way. In Fig. 6, the dotted line is an idealized strictly competitive ligand with Kd = 35 nM. The dashed line is an idealized allosteric modifier with our values from our allosteric analysis of Fig. 1(B). The solid line is the best-fit line using nonlinear regression with the formula above and our actual KdApp values experimentally determined from the saturation analyses. Taken together, these experiments give strong evidence that 27-OHC is a negative allosteric modifier of E2 binding to ERβ with an α of 0.036 and a Ki of 50 nM (Fig. 7).
Figure 7.
Final allosteric ternary complex model for observed interactions of E2 and 27-OHC binding to human ERβ. The observed Kd of E2 in the absence of 27-OHC is ∼150 pM. The apparent Kd of E2 in the presence of saturating concentrations of 27-OHC is ∼4.2 nM. Calculated values for Kd of 27-OHC in the absence and in the presence of saturating concentrations of E2 are ∼50 nM and ∼1.3 μM, respectively. The calculated value of the cooperativity factor (α) is 0.036 for the interaction.
Figure 6 also strengthens the argument against 27-OHC being solubility limited. 27-OHC at 300 nM, which is well within observed physiological concentrations and below any suspected solubility point, demonstrates an allosteric effect rather than competitive on the apparent Kd of 3H-E2 in saturation assays (Fig. 6). If 27-OHC were a solubility limited competitor, any concentration below the solubility point would act competitively and lie along the dotted red (competitive) line in Fig. 6, not the dashed red or solid black lines (allosteric). Additionally, 30 μM 27HC inhibits the apparent Kd of E2 by ∼16% more than 3 μM in these assays (Fig. 6, insert).
Docking of 27-OHC and E2 in the 3D structure of ERβ
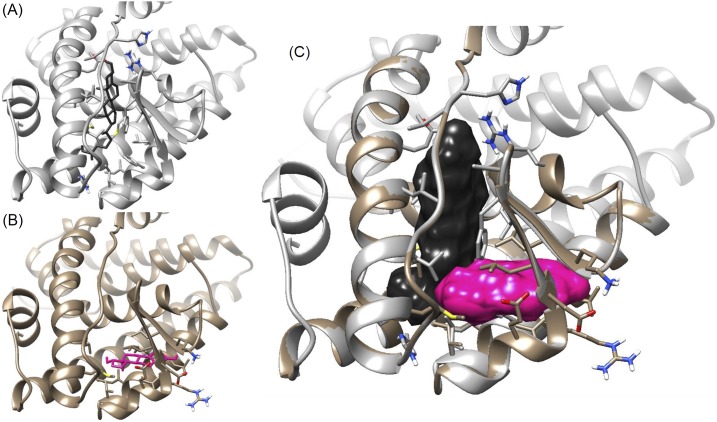
Using in silico docking, we assessed the ability of 27-OHC and E2 to bind in the canonical E2 binding site as well as an alternative site near the small β sheet within the ligand binding pocket of ERβ (PDB: 3OLS). Rosetta ligand, running on the Rosetta Online Server that Includes Everyone, was used to search a 5A radius centered within the canonical site and a 4.5A area centered near the β strands. Interestingly, 27-OHC could not be docked successfully within the alternate site, but E2 could [Fig. 8(A) and 8(B)]. It appears that the D rings of two E2 molecules would overlap if they were both in the pocket at the same time. However, 27-OHC and E2 may fit together, as 27-OHC sits relatively higher in the pocket and its tail extends through the area where the D ring of E2 would be in the canonical site, allowing more space for E2 in the alternate site. There are relatively few conflicting residues when the two models are overlaid, and the only conflict between the two ligands is between the D ring hydroxyl of E2 and the flexible side chain of 27-OHC [Fig. 8(C)].
Figure 8.
3D models for the binding of E2 and 27-OHC to human ERβ. (A) In silico tests of multiple conformers of 27-OHC predicted that 27-OHC could bind in the classic E2 binding site, with the A ring up and the D ring down, in this model. (B) In silico tests predicted that E2 could bind at an alternate site near the β sheet, with the A ring out and the D ring in, in this model. (C) Manual overlay of (A) and (B), showing few overlapping residues with compounds binding together in space-filling mode.
Discussion
As a negative allosteric modifier, 27-OHC allows for a unique fine-tuning of ERβ binding and activity. With respect to its antagonistic response activity, it does not inhibit activity to baseline, with an appearance similar to that of a partial agonist. However, we have shown here that this effect is more likely due to its ability to bind ERβ simultaneously along with E2 and allosterically induce a conformational change in the receptor that reduces the receptor’s affinity for E2. This is different from a classical partial agonist, which binds competitively with a full agonist but does not stimulate to the same extent.
We have been able to explain our ERβ binding data via the use of a standard allosteric ternary complex model. There are many reasons why our data may differ from a previous report (22). In that report, the authors chose to use concentrations of hot E2 that were only ∼twofold higher than the Kd, and we used concentrations that were ∼15-fold higher than our Kd in our initial assays. Our data with various doses of hot E2 clearly show how this affects the observed binding curves, with lower doses yielding curves that appear more like competitive ligands. It is also important to note that the reported total binding in that report is more than the recommended 15% of the total hot E2, which leads to ligand depletion. If the binding is not allosteric, a reasonable amount of ligand depletion can be easily corrected for, so this would not be a major concern. However, ligand depletion has larger and unexpected effects on the binding constants obtained when the unlabeled ligand is an allosteric modifier (46).
We cannot, however, so easily explain the differences obtained with 27-OHC binding to ERα. Certainly, the previously mentioned factors apply, but we would still expect to see some inhibition of binding at the concentrations of hot E2 and 27-OHC tested here if 27-OHC had equal effects on each receptor. In preliminary studies, we saw a small amount of inhibition of 1 nM hot E2 binding to ERα, but it was not enough to accurately determine binding constants. It is possible that 27-OHC binds ERα allosterically as well but has a combination of poorer affinity and allosteric effect (α), relative to ERβ, such that technically it cannot be measured unless very low hot E2, and very high 27-OHC concentrations are used.
The present study demonstrates simultaneous binding of two endogenous ligands to the ER. Previous reports of alternate binding sites in other nuclear receptors include androgen receptor (47, 48), thyroid hormone receptor (49, 50), vitamin D receptor (51, 52), and peroxisome proliferator–activated receptor γ (53, 54). There have also been previous reports of exogenous ligands binding to alternate sites within ERα and ERβ (55–57). Trilostane (a synthetic 3β-hydroxysteroid dehydrogenase inhibitor) was previously reported to be a positive allosteric modulator of E2 binding and a noncompetitive inhibitor of ER function (58). The alternate site in our 3D model is in the same relative location as that proposed by Mizwicki et al. (52), as well as the recently identified alternate binding site in peroxisome proliferator–activated receptor γ (53). Our 3D model is an intriguing starting point, yet many questions remain. It appears in this model that 27-OHC should be able to be completely competed off by E2, but that does not fit with our data. The model we used is based on the structure of the ERβ ligand binding domain in the presence of E2, and the structure may be very different in the presence of 27-OHC because there is a great deal of plasticity in the ligand binding pockets of nuclear receptors, including the ERs (59). It may also be possible that competition is less likely because of the two molecules stabilizing each other in the positions modeled, similar to the recent observation in pregnane X receptor (60).
Recently, we have shown that mice lacking ERβ have a higher incidence of advanced prostate cancer in the TRAMP mouse model (13). The ERβ-selective agonist 8β-VE2 has been shown to cause apoptosis in prostate cancer cells in vitro (15). Because of the high affinity of 27-OHC, we speculate that this may be an important biomarker for the success of treating prostate cancer with ERβ-selective agonists, such as 8β-VE2, in vivo. Those with hypercholesterolemia may be at a higher risk because 27-OHC concentrations are positively correlated with those of total cholesterol, and this has recently been demonstrated in breast cancer (25, 61, 62). In addition, because total cholesterol, and thus 27-OHC, increases with age, we believe this may be a factor in the increased incidence of prostate cancer in the aging population. Interestingly, in patients with prostate cancer, a decrease of CYP27A1 (the enzyme that makes 27-OHC) is associated with increased recurrence and grade in prostate cancer (63).
The status and interplay among LXRs, ERs, and 27-OHC metabolizing enzymes will probably be of great importance to our understanding of estrogen-responsive processes. We hypothesize that 27-OHC will have a substantial impact in many cases where ERβ agonists are used for their positive biological effects. Several ERβ-selective agonists have been tested for their ability to inhibit disease processes, ranging from inflammatory diseases to cancer, but these have not experienced the success in humans that they have preclinically (9, 10, 64, 65). In conclusion, 27-OHC is an ERβ-selective negative allosteric modulator of 17β-estradiol binding. Structural and mutational studies are needed to confirm the site of 27-OHC binding in the presence and absence of E2. 27-OHC’s potentially important role as a biomarker, investigational agent, and drug target in many disease processes warrants continued study.
Supplementary Material
Acknowledgments
The authors thank Drs. Lu Yuan and Wade Welshons for their helpful discussions regarding this work. They also thank Nathan Mahloch for his thoughtful review of the paper.
Financial Support: This work was supported by grants from the Department of Defense (DOD), Congressionally Directed Medical Research Programs (Grant PC100455 to D.B.L.); the National Center for Complementary and Integrative Health (NCCIH) (to D.B.L.), the Office of Dietary Supplements (ODS); and the National Cancer Institute (NCI) (Grant P50AT006273). The contents herein are solely the responsibility of the authors and do not necessarily represent the official views of the DOD, NCCIH, ODS, NCI, or National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 3D
three-dimensional
- 3H-E2
tritiated 17β-estradiol
- 24(S)-OHC
24(S)-hydroxycholesterol
- 27-OHC
27-hydroxycholesterol
- DCC
dextran-coated charcoal
- E2
17β-estradiol
- ER
estrogen receptor
- IC50
half maximal inhibitory concentration
- Kd
equilibrium dissociation constant
- KdApp
apparent equilibrium dissociation constant
- Ki
inhibitor constant
- LXR
liver X receptor
- SERM
selective estrogen receptor modulator
- TnT
transcription/translation
References
- 1. Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, et al. . Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA. 1985;82(23):7889–7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murdoch FE, Gorski J. The role of ligand in estrogen receptor regulation of gene expression. Mol Cell Endocrinol. 1991;78(3):C103–C108. [DOI] [PubMed] [Google Scholar]
- 3. Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93(12):5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. [DOI] [PubMed] [Google Scholar]
- 5. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95(26):15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Förster C, Mäkela S, Wärri A, Kietz S, Becker D, Hultenby K, Warner M, Gustafsson JA. Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci USA. 2002;99(24):15578–15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295(5554):505–508. [DOI] [PubMed] [Google Scholar]
- 9. Elloso MM, Phiel K, Henderson RA, Harris HA, Adelman SJ. Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J Endocrinol. 2005;185(2):243–252. [DOI] [PubMed] [Google Scholar]
- 10. Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC. Selective estrogen receptor-beta agonists repress transcription of proinflammatory genes. J Immunol. 2008;180(1):630–636. [DOI] [PubMed] [Google Scholar]
- 11. Minutolo F, Macchia M, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor β ligands: recent advances and biomedical applications. Med Res Rev. 2011;31(3):364–442. [DOI] [PubMed] [Google Scholar]
- 12. Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci. 2009;1155(1):174–186. [DOI] [PubMed] [Google Scholar]
- 13. Slusarz A, Jackson GA, Day JK, Shenouda NS, Bogener JL, Browning JD, Fritsche KL, MacDonald RS, Besch-Williford CL, Lubahn DB. Aggressive prostate cancer is prevented in ERαKO mice and stimulated in ERβKO TRAMP mice. Endocrinology. 2012;153(9):4160–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tekmal RR, Nair HB, Perla RP, Kirma N. HER-2/neu x aromatase double transgenic mice model: the effects of aromatase overexpression on mammary tumorigenesis. J Steroid Biochem Mol Biol. 2007;106(1-5):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McPherson SJ, Hussain S, Balanathan P, Hedwards SL, Niranjan B, Grant M, Chandrasiri UP, Toivanen R, Wang Y, Taylor RA, Risbridger GP. Estrogen receptor-beta activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFalpha mediated. Proc Natl Acad Sci USA. 2010;107(7):3123–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295(5564):2465–2468. [DOI] [PubMed] [Google Scholar]
- 17. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 18. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S; Women’s Health Initiative Steering Committee . Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. [DOI] [PubMed] [Google Scholar]
- 19. Hansdóttir H. Raloxifene for older women: a review of the literature. Clin Interv Aging. 2008;3(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berrodin TJ, Chang KC, Komm BS, Freedman LP, Nagpal S. Differential biochemical and cellular actions of Premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combination. Mol Endocrinol. 2009;23(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DuSell CD, Nelson ER, Wang X, Abdo J, Mödder UI, Umetani M, Gesty-Palmer D, Javitt NB, Khosla S, McDonnell DP. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology. 2010;151(8):3675–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13(10):1185–1192. [DOI] [PubMed] [Google Scholar]
- 23. DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22(1):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Umetani M, Shaul PW. 27-Hydroxycholesterol: the first identified endogenous SERM. Trends Endocrinol Metab. 2011;22(4):130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dzeletovic S, Breuer O, Lund E, Diczfalusy U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal Biochem. 1995;225(1):73–80. [DOI] [PubMed] [Google Scholar]
- 26. Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51(11):3299–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burkard I, Rentsch KM, von Eckardstein A. Determination of 24S- and 27-hydroxycholesterol in plasma by high-performance liquid chromatography-mass spectrometry. J Lipid Res. 2004;45(4):776–781. [DOI] [PubMed] [Google Scholar]
- 28. Cali JJ, Hsieh CL, Francke U, Russell DW. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem. 1991;266(12):7779–7783. [PMC free article] [PubMed] [Google Scholar]
- 29. Leitersdorf E, Reshef A, Meiner V, Levitzki R, Schwartz SP, Dann EJ, Berkman N, Cali JJ, Klapholz L, Berginer VM. Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis in Jews or Moroccan origin. J Clin Invest. 1993;91(6):2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim KS, Kubota S, Kuriyama M, Fujiyama J, Björkhem I, Eggertsen G, Seyama Y. Identification of new mutations in sterol 27-hydroxylase gene in Japanese patients with cerebrotendinous xanthomatosis (CTX). J Lipid Res. 1994;35(6):1031–1039. [PubMed] [Google Scholar]
- 31. Tsaousidou MK, Ouahchi K, Warner TT, Yang Y, Simpson MA, Laing NG, Wilkinson PA, Madrid RE, Patel H, Hentati F, Patton MA, Hentati A, Lamont PJ, Siddique T, Crosby AH. Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am J Hum Genet. 2008;82(2):510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goizet C, Boukhris A, Durr A, Beetz C, Truchetto J, Tesson C, Tsaousidou M, Forlani S, Guyant-Maréchal L, Fontaine B, Guimarães J, Isidor B, Chazouillères O, Wendum D, Grid D, Chevy F, Chinnery PF, Coutinho P, Azulay JP, Feki I, Mochel F, Wolf C, Mhiri C, Crosby A, Brice A, Stevanin G. CYP7B1 mutations in pure and complex forms of hereditary spastic paraplegia type 5. Brain. 2009;132(Pt 6):1589–1600. [DOI] [PubMed] [Google Scholar]
- 33. Schüle R, Brandt E, Karle KN, Tsaousidou M, Klebe S, Klimpe S, Auer-Grumbach M, Crosby AH, Hübner CA, Schöls L, Deufel T, Beetz C. Analysis of CYP7B1 in non-consanguineous cases of hereditary spastic paraplegia. Neurogenetics. 2009;10(2):97–104. [DOI] [PubMed] [Google Scholar]
- 34. Setchell KD, Schwarz M, O’Connell NC, Lund EG, Davis DL, Lathe R, Thompson HR, Weslie Tyson R, Sokol RJ, Russell DW. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J Clin Invest. 1998;102(9):1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ueki I, Kimura A, Nishiyori A, Chen HL, Takei H, Nittono H, Kurosawa T. Neonatal cholestatic liver disease in an Asian patient with a homozygous mutation in the oxysterol 7alpha-hydroxylase gene. J Pediatr Gastroenterol Nutr. 2008;46(4):465–469. [DOI] [PubMed] [Google Scholar]
- 36. Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276(42):38378–38387. [DOI] [PubMed] [Google Scholar]
- 37. Furton KG, Norelus A. Determining the critical micelle concentration of aqueous surfactant solutions: using a novel colorimetric method. J Chem Educ. 1993;70(3):254. [Google Scholar]
- 38. Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. [DOI] [PubMed] [Google Scholar]
- 39. Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54(2):323–374. [DOI] [PubMed] [Google Scholar]
- 40. Combs SA, Deluca SL, Deluca SH, Lemmon GH, Nannemann DP, Nguyen ED, Willis JR, Sheehan JH, Meiler J. Small-molecule ligand docking into comparative models with Rosetta. Nat Protoc. 2013;8(7):1277–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lyskov S, Chou FC, Conchúir SO, Der BS, Drew K, Kuroda D, Xu J, Weitzner BD, Renfrew PD, Sripakdeevong P, Borgo B, Havranek JJ, Kuhlman B, Kortemme T, Bonneau R, Gray JJ, Das R. Serverification of molecular modeling applications: the Rosetta Online Server that Includes Everyone (ROSIE). PLoS One. 2013;8(5):e63906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miteva MA, Guyon F, Tuffery P. Frog2: Efficient 3D conformation ensemble generator for small compounds. Nucleic Acids Res. 2010;38(Web Server issue):W622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. [DOI] [PubMed] [Google Scholar]
- 44. Hulme EC, Trevethick MA. Ligand binding assays at equilibrium: validation and interpretation. Br J Pharmacol. 2010;161(6):1219–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stockton JM, Birdsall NJ, Burgen AS, Hulme EC. Modification of the binding properties of muscarinic receptors by gallamine. Mol Pharmacol. 1983;23(3):551–557. [PubMed] [Google Scholar]
- 46. Avlani VA, McLoughlin DJ, Sexton PM, Christopoulos A. The impact of orthosteric radioligand depletion on the quantification of allosteric modulator interactions. J Pharmacol Exp Ther. 2008;325(3):927–934. [DOI] [PubMed] [Google Scholar]
- 47. Buzón V, Carbó LR, Estruch SB, Fletterick RJ, Estébanez-Perpiñá E. A conserved surface on the ligand binding domain of nuclear receptors for allosteric control. Mol Cell Endocrinol. 2012;348(2):394–402. [DOI] [PubMed] [Google Scholar]
- 48. Estébanez-Perpiñá E, Arnold LA, Nguyen P, Rodrigues ED, Mar E, Bateman R, Pallai P, Shokat KM, Baxter JD, Guy RK, Webb P, Fletterick RJ. A surface on the androgen receptor that allosterically regulates coactivator binding [published correction appears in Proc Natl Acad Sci USA. 2007;104(47):18874]. Proc Natl Acad Sci USA. 2007;104(41):16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Estébanez-Perpiñá E, Arnold LA, Jouravel N, Togashi M, Blethrow J, Mar E, Nguyen P, Phillips KJ, Baxter JD, Webb P, Guy RK, Fletterick RJ. Structural insight into the mode of action of a direct inhibitor of coregulator binding to the thyroid hormone receptor. Mol Endocrinol. 2007;21(12):2919–2928. [DOI] [PubMed] [Google Scholar]
- 50. Arnold LA, Estébanez-Perpiñá E, Togashi M, Jouravel N, Shelat A, McReynolds AC, Mar E, Nguyen P, Baxter JD, Fletterick RJ, Webb P, Guy RK. Discovery of small molecule inhibitors of the interaction of the thyroid hormone receptor with transcriptional coregulators. J Biol Chem. 2005;280(52):43048–43055. [DOI] [PubMed] [Google Scholar]
- 51. Menegaz D, Mizwicki MT, Barrientos-Duran A, Chen N, Henry HL, Norman AW. Vitamin D receptor (VDR) regulation of voltage-gated chloride channels by ligands preferring a VDR-alternative pocket (VDR-AP). Mol Endocrinol. 2011;25(8):1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mizwicki MT, Keidel D, Bula CM, Bishop JE, Zanello LP, Wurtz JM, Moras D, Norman AW. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci USA. 2004;101(35):12876–12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hughes TS, Giri PK, de Vera IM, Marciano DP, Kuruvilla DS, Shin Y, Blayo AL, Kamenecka TM, Burris TP, Griffin PR, Kojetin DJ. An alternate binding site for PPARγ ligands. Nat Commun. 2014;5:3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Waku T, Shiraki T, Oyama T, Maebara K, Nakamori R, Morikawa K. The nuclear receptor PPARγ individually responds to serotonin- and fatty acid-metabolites. EMBO J. 2010;29(19):3395–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Hoorn WP. Identification of a second binding site in the estrogen receptor. J Med Chem. 2002;45(3):584–589. [DOI] [PubMed] [Google Scholar]
- 56. Wang Y, Chirgadze NY, Briggs SL, Khan S, Jensen EV, Burris TP. A second binding site for hydroxytamoxifen within the coactivator-binding groove of estrogen receptor beta. Proc Natl Acad Sci USA. 2006;103(26):9908–9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Andruska ND, Zheng X, Yang X, Mao C, Cherian MM, Mahapatra L, Helferich WG, Shapiro DJ. Estrogen receptor α inhibitor activates the unfolded protein response, blocks protein synthesis, and induces tumor regression. Proc Natl Acad Sci USA. 2015;112(15):4737–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Puddefoot JR, Barker S, Glover HR, Malouitre SD, Vinson GP. Non-competitive steroid inhibition of oestrogen receptor functions. Int J Cancer. 2002;101(1):17–22. [DOI] [PubMed] [Google Scholar]
- 59. Nettles KW, Bruning JB, Gil G, O’Neill EE, Nowak J, Guo Y, Kim Y, DeSombre ER, Dilis R, Hanson RN, Joachimiak A, Greene GL. Structural plasticity in the oestrogen receptor ligand-binding domain [ published correction appears in EMBO Rep. 2007;8:6102007]. EMBO Rep. 2007;8(6):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Delfosse V, Dendele B, Huet T, Grimaldi M, Boulahtouf A, Gerbal-Chaloin S, Beucher B, Roecklin D, Muller C, Rahmani R, Cavaillès V, Daujat-Chavanieu M, Vivat V, Pascussi JM, Balaguer P, Bourguet W. Synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds. Nat Commun. 2015;6(1):8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342(6162):1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, Sullivan PM, Kemper JK, Gunn MD, McDonnell DP, Nelson ER. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8(1):864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alfaqih MA, Nelson ER, Liu W, Safi R, Jasper JS, Macias E, Geradts J, Thompson JW, Dubois LG, Freeman MR, Chang CY, Chi JT, McDonnell DP, Freedland SJ. CYP27A1 loss dysregulates cholesterol homeostasis in prostate cancer. Cancer Res. 2017;77(7):1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Paruthiyil S, Cvoro A, Zhao X, Wu Z, Sui Y, Staub RE, Baggett S, Herber CB, Griffin C, Tagliaferri M, Harris HA, Cohen I, Bjeldanes LF, Speed TP, Schaufele F, Leitman DC. Drug and cell type-specific regulation of genes with different classes of estrogen receptor beta-selective agonists. PLoS One. 2009;4(7):e6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.