Abstract
Rho GDP-dissociation inhibitor (GDIα) inhibits glucose-stimulated insulin secretion (GSIS) in part by locking Rho GTPases in an inactive GDP-bound form. The onset of GSIS causes phosphorylation of GDIα at Ser174, a critical inhibitory site for GDIα, leading to the release of Rho GTPases and their subsequent activation. However, the kinase regulator(s) that catalyzes the phosphorylation of GDIα in islet β cells remains elusive. We propose that SAD-A, a member of AMP-activated protein kinase–related kinases that promotes GSIS as an effector kinase for incretin signaling, interacts with and inhibits GDIα through phosphorylation of Ser174 during the onset GSIS from islet β cells. Coimmunoprecipitation and phosphorylation analyses were carried out to identify the physical interaction and phosphorylation site of GDIα by SAD-A in the context of GSIS from INS-1 β cells and primary islets. We identified GDIα directly binds to SAD-A kinase domain and phosphorylated by SAD-A on Ser174, leading to dissociation of Rho GTPases from GDIα complexes. Accordingly, overexpression of SAD-A significantly stimulated GDIα phosphorylation at Ser174 in response to GSIS, which is dramatically potentiated by glucagonlike peptide-1, an incretin hormone. Conversely, SAD-A deficiency, which is mediated by short hairpin RNA transfection in INS-1 cells, significantly attenuated endogenous GDIα phosphorylation at Ser174. Consequently, coexpression of SAD-A completely prevented the inhibitory effect of GDIα on insulin secretion in islets. In summary, glucose and incretin stimulate insulin secretion through the phosphorylation of GDIα at Ser174 by SAD-A, which leads to the activation of Rho GTPases, culminating in insulin exocytosis.
SAD-A reverses the adverse effect of GDIα on insulin secretion in islets by binding and phosphorylating GDIα at Ser174 site.
Glucose-stimulated insulin secretion (GSIS) requires coordinated signaling events that regulate trafficking of insulin-laden secretory granules for their docking and fusion with the plasma membrane (1). Recent studies have implicated an essential role of Rho GTPases in regulating the exocytosis of insulin. Rho GTPases comprise a family of molecular switches including RhoA, RAC1, and CDC42 that undergo a conformational change upon binding GTP. The binding of GTP to Rho GTPases triggers their interaction with target (effector) proteins to promote a wide range of cellular activities, including cytoskeleton remodeling, which is required for insulin exocytosis. Hence, the onset of GSIS is associated with the recycling of Rho GTPases, including RhoA, CDC42, and RAC1, from the inactive GDP-bound form to the active GTP-bound form in islet β cells (2–6). The recycling process is delicately controlled by GDP-dissociation inhibitors (GDIs), including GDIα, which sequesters RhoA, CDC42, and RAC1 in an inactive form in the cytoplasm (7, 8). Prevention of the recycling process leads to inhibition of GSIS (4), whereas overexpression of a dominant active form of RAC1 impairs GSIS concurrently with disruption to F-actin remodeling (4, 9). Hence, overexpression of GDIα has been shown to inhibit GSIS (10, 11). Additionally, the onset of GSIS also results in stimulation of the phosphorylation of GDIα at Ser174, a negative regulatory site of GDIα, leading to the activation and translocation of CDC42 and RAC1 from the cytoplasm to the plasma membrane in islet β cells (10). However, the kinase regulator(s) responsible for the phosphorylation remains to be identified.
SAD-A, also referred to as BRSK2, is a member of the AMP-activated protein kinase (AMPK)–related kinases under the control of LKB1. LKB1 is a tumor suppressor kinase responsible for the inherited familial cancer disorder Peutz-Jeghers syndrome (12). The AMPK-related kinases have recently been implicated in the regulation of dynamic islet function, including islet morphology, β-cell polarity, energy homeostasis, and GSIS (13), as evidenced by the phenotype of multiple mouse models of LKB1 deletion in pancreas and islet β cells (14–18). SAD-A is most closely related to AMPK among the 12 members of AMPK-related kinases, implicating an important role in energy metabolism. SAD-A and its highly conserved isoform, SAD-B, are predominantly expressed in the brain, where they regulate various neuronal function, such as neuronal polarity, axon formation and specification, maturation of nerve terminals, and neurotransmitter release, presumably by regulating cytoskeletal remodeling (19–23). SAD-B kinase also regulates cell division by mediating γ-tubulin phosphorylation and centrosome duplication (24).
In contrast to SAD-B, whose expression is restricted to the brain, SAD-A is exclusively expressed in both the brain and pancreas, the primary targeting tissue for incretin hormone action. The term “incretin effect” was coined from early observations that oral administration of glucose enhances insulin secretion to a greater extent than that seen with isoglycemic intraperitoneal administration (25), which led to the identification of glucagonlike peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). Both GLP-1 and GIP greatly potentiate GSIS in response to oral glucose load. Indeed, SAD-A is activated by protein kinase A (PKA) and CaMKKα-mediated signaling pathways (26, 27). Both cAMP and calcium are powerful second messengers that potentiate GSIS and mediate GLP-1’s effect. Our recent studies have identified a crucial role of SAD-A in regulating GSIS as a mediator of incretin response in islet β cells (28, 29). SAD-A is activated in response to stimulation with glucose or incretin hormones and is required for GLP-1 receptor-mediated signal transduction in islet β cells (29). Accordingly, overexpression SAD-A greatly enhanced GSIS and significantly potentiated GLP-1’s effect (30). Conversely, targeted deletion of SAD-A in mice leads to inadequate incretin response and glucose intolerance (29). Additionally, SAD-A regulates islet β-cell size and mass as a downstream effector protein of mTORC1 signaling (31). Furthermore, SAD-A governs insulin exocytosis in part by promoting F-actin remodeling in response to glucose stimulation through phosphorylation of p21-activated kinase 1 (PAK1) kinase, the effector of Rho GTPases (30). However, whether SAD-A directly regulates Rho GTPases activation remains elusive. In the present studies, we investigated the molecular mechanism by which SAD-A regulates Rho GTPases activation. We show that SAD-A activates Rho GTPases by inhibiting GDIα activity through direct binding and phosphorylation of GDIα at Ser174, culminating in insulin exocytosis.
Material and Methods
Ethics
All animals were maintained in an environmentally controlled facility with diurnal light cycle and free access to water and a standard rodent chow (Teklad 2018; Harlan, Madison, WI). All experiments involving animals were performed in compliance with approved institutional animal care and use protocols according to National Institutes of Health guidelines (publication no. 86-23, 1985).
Cells and reagents
INS-1 832/13 cells (RRID: CVCL_7226, INS-1) from Dr. Christopher B. Newgard’s laboratory (Duke University Medical Center, Durham, NC) were cultured according to the protocol reported before (32) and routinely tested for mycoplasma. The 293T cells (RRID: CVCL_0045) purchased from the ATCC were cultured according to standard methods and routinely tested for mycoplasma. For experiments, INS-1 cells were seeded at a density of 2.5 × 104 cells/cm2 and incubated for 18 hours before proceeding with transfection.
Transient transfections were performed by using X-tremeGENE HP DNA Transfection Reagent (Sigma-Aldrich, St. Louis, MO). The pcDNA3.1-Flag-SAD-A and adenovirus expression of Flag-SAD-A or SAD-A mutants were generated and described previously (30). Site-directed mutagenesis of plasmids was performed by using the Quickchange multisite kit (Stratagene, San Diego, CA). The plasmids of pcDNA-myc-PAK1 and pcDNA-myc-GDIα were used as previously described (33). Reagents or key resources are listed in Table 1. Traditional western blot was performed as previously described (30). Capillary western blot analyses were performed using the ProteinSimple Wes System (San Jose, CA). Samples were prepared according to Wes kit protocol (Wes-Rabbit; 12-230 kDa; catalog no. PS-MK-14). A biotinylated ladder provided molecular weight standards for each assay. Serial dilution of samples and antibodies was performed to validate the detection of target protein. GDIα antibody was purchased from Santa Cruz Biotechnology (Dallas, TX) (34). Anti-RhoGDI (phospho S174) antibody was purchased from Abcam (35). c-Myc antibody (36), Rho A antibody (37), and Cdc42 antibody were purchased from Santa Cruz Biotechnology (38). Rac1 antibody was purchased from Cytoskeleton (Colorado Springs, CO) (39). Flag antibody (40) and β-Actin antibody were purchased from Sigma-Aldrich (41). SAD-A antibody was purchased from Proteintech Group (Rosemont, IL) (42). Mouse IgG horseradish peroxidase (43), rabbit IgG horseradish peroxidase (44), and anti-Flag® M2 Affinity Gel were purchased from Sigma-Aldrich (45).
Table 1.
Key Resources
| Reagent or Resource | Source | Catalog |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| RhoGDI-GST protein (human recombinant) | Cytoskeleton | GDI01 |
| Human GDIα oligopeptides | Peptide 2.0 Inc. | Custom synthesis |
| Exendin-4 | Eli Lilly and Company | BYETTA® (exenatide) |
| X-tremeGENE™ HP DNA transfection reagent | Sigma-Aldrich | 6366546001 |
| ATP, [γ-32P]- 3000 Ci/mmol 10 mCi/mL lead, 250 µCi | PerkinElmer | NEG002A250UC |
| Coomassie stain | Bio-Rad | 1610786 |
| P81 phosphocellulose paper | Millipore | 20-134 |
| shRNA SAD-A | Franck Polleux | PMID: 17482548 |
| Collagenase XI | Sigma-Aldrich | C7657 |
| Histopaque®-1077 | Sigma-Aldrich | 10771 |
| Protease inhibitor cocktail tablets | Millipore | PICT |
| Protein G sepharose | GE Healthcare | 17061805 |
| Experimental models: organisms/stains | ||
| 8-week-old male C57BL/6J mice | The Jackson Laboratory | Stock No.: 000664 |
| Critical commercial assays | ||
| Pierce BCA protein assay kit | Thermo Fisher | 23225 |
| Pierce™ ECL | Thermo Fisher | 32106 |
| Pierce™ ECL Plus | Thermo Fisher | 32132 |
| Insulin radioimmunoassay kit | Millipore | RI-13K |
| Other | ||
| IKA® RW 20 digital | IKA® Works, Inc. | RW 20 digital |
Abbreviations: BCA, bicinchoninic acid assay; ECL, enhanced chemiluminescence; PMID, PubMed Identifier; shRNA, short hairpin RNA.
Mass spectrometry
The coimmunoprecipitation (co-IP) analyses were carried out in MIN6 β cells (RRID: CVCL_0431) and stably overexpressed Flag-tagged SAD-A (30) by using Flag antibody as the bait. The immunocomplex was treated with trypsin and processed by Penn State College of Medicine’s Mass Spectrometry and Proteomics Core facility.
In vitro phosphorylation assays
In vitro SAD-A kinase assays were carried out using recombinant SAD-A proteins described previously (30). The recombinant SAD-A protein overexpressed in Sf9 cells were partially purified by anti-FLAG M2 affinity resin (Sigma-Aldrich). To reduce the autophosphorylation signals of SAD-A kinase, the recombinant SAD-A protein resin was washed once with 1× kinase buffer (50 mM HEPES, pH 7.5, 10 mM MgCl2, and 2 mM MnCl2), and incubated with 25 µM ATP for 10 minutes at 37°C. Evaluation of SAD-A kinase activity was determined by incubating the pretreated recombinant human Flag-SAD-A protein resin with recombinant human glutathione S-transferase (GST)–GDIα protein (Cytoskeleton) or 0, 1, 10, or 30 µg human GDIα oligopeptides (Peptide 2.0 Inc., Chantilly, VA) in 1× kinase buffer and 10 µCi of [γ-32P] ATP in a final volume of 30 µL for 20 minutes at 37°C. Incorporation of 32P-phosphate into the oligopeptide substrates was determined as described previously (30). The GDIα oligopeptides used in current studies, which covered all the predicated or previously identified phosphorylation sites of human GDIα, were chosen from the Phosphosite Web site (https://www.phosphosite.org) and synthesized from Peptide 2.0 Inc. (Chantilly, VA): T7: MAEQEPTAEQLAQIA; S24:ENEEDEHSVNYKPPA; S34:YKPPAQKSIQEIQEL; S96:DLTGDLESFKKQSFV; S101:LESFKKQSFVLKEGV; S148:KTDYMVGSYGPRAEE; and S174:KGMLARGSYSIKSRF.
Immunoprecipitation and GST fusion protein pull-down assay
The protocol was described in a previous study (30). Briefly, the cells were lysed in 500 µL lysis buffer (40 mM HEPES, pH 7.4, 100 mM NaCl, 1% Triton X-100, 25 mM NaF, 1 mM sodium orthovanadate, 10 µg/mL leupeptin, and 10 µg/mL aprotinin). The indicated antibodies were used in immunoprecipitation. The binding protein was eluted by boiling in sample loading buffer, separated by SDS-PAGE, and detected by western blot analysis.
Subcellular fractionation assay
INS-1 cells were transiently transfected in a 10-cm dish for 48 hours. The cells were collected and resuspended 150 µL of homogenizer buffer (0.25 M sucrose, 0.01 M Tris/HCl, pH 7.4, 1 mM EDTA) and then homogenized in a cell mix with IKA® RW 20 Digital at 370 rpm/min in a glass rotor and glass tube for 15 minutes on ice, fractionated by centrifugation at 800g for 10 minutes at 4°C, and followed by centrifugation at 100,000g for 60 minutes at 4°C. In the second centrifugation, the supernatant was a cytosolic fraction, and the pellets were a membrane fraction. The membrane fraction was resuspended with an equal volume of cytosolic fraction. Western blot was performed by using the same amount of membrane and cytosolic fraction fractions.
Islet isolation and insulin secretion assay
Islet isolation was described in a previous study (30). Briefly, islets were isolated from 8-week-old C57BL/6J mice by collagenase XI (Sigma-Aldrich) perfusion and Histopaque (Sigma-Aldrich) separation from acinar and ductal tissues. Insulin secretion studies were performed 48 hours after adenovirus infection at a multiplicity of infection equal to 100 and were analyzed in six repeated samples with 10 islets per sample. Insulin secretion levels were determined by an insulin radioimmunoassay kit (Millipore, Burlington, MA), and were normalized to total insulin content. For protein isolation, islets were lysed in 50 µL of lysis buffer with a protease inhibitor cocktail (Calbiochem, San Diego, CA); protein was quantified by standard techniques. A total of 50 µg of protein was resolved on a 10% SDS gel for western blot analysis.
Statistical analysis
Results are shown as averages and SEMs. Student t test, nonparametric Mann-Whitney U test, or ANOVA was used to calculate differences between groups where appropriate. *P < 0.05, **P < 0.01, and ***P < 0.001 were considered significant. All in vitro experiments were performed at least three times. Power analysis was conducted to determine the sample size. Prism, version 7.0 (GraphPad Software, San Diego, CA), was used for these analyses.
Results
SAD-A interaction with GDIα through kinase domain
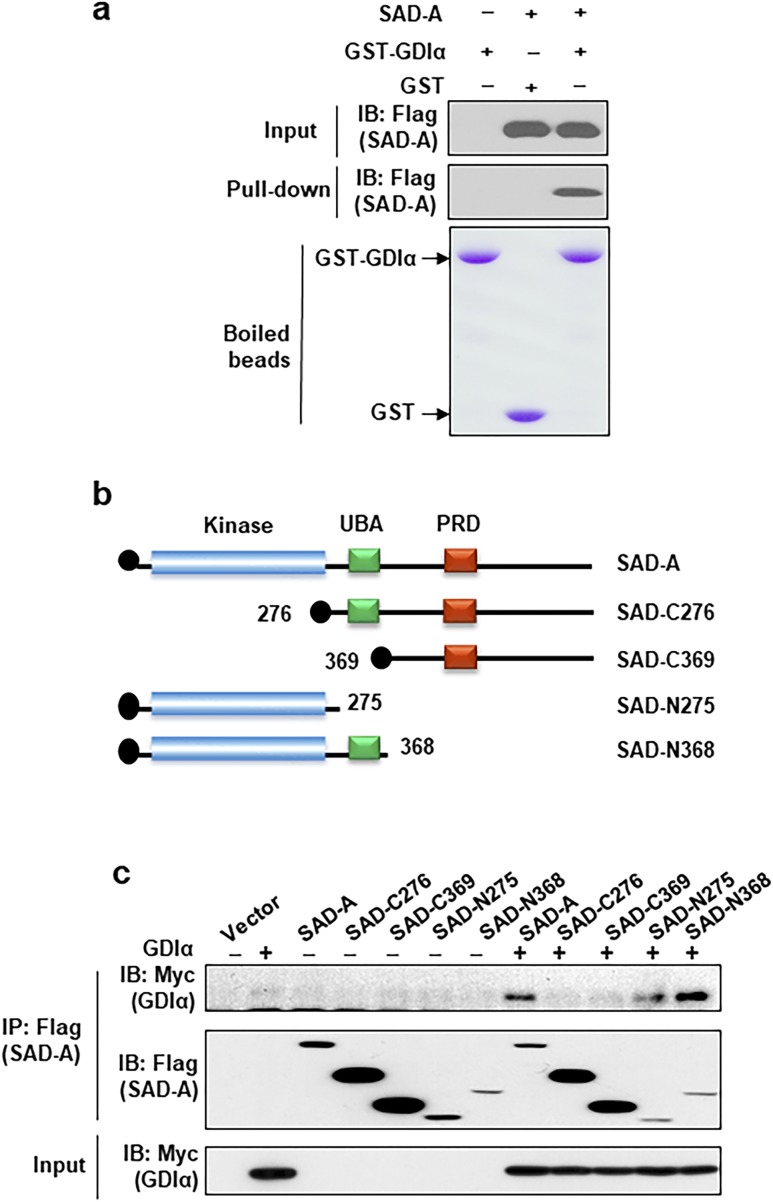
Co-IP analysis using Flag-tagged SAD-A followed by mass spectrometric analysis showed GDIα could be one of the binding proteins with SAD-A kinase (the full results of this screen will be published as a separate study). To verify the specificity of the interaction, we performed GST pull-down assay by using cell lysate from 293T cells and purified GST-GDIα fusion protein and confirmed this binding (Fig. 1a). To identify domains responsible for the interaction of SAD-A with GDIα, we generated a series of deletion mutants of SAD-A (Fig. 1b). These SAD-A mutants carry different domains of SAD-A and were used in the co-IP analysis in 293T cells. Deletion of the C-terminus of SAD-A, including the UBA and PRD domains, did not significantly affect its interaction with GDIα, as shown in Fig. 1c. In contrast, deletion of the kinase domain diminished the interaction of SAD-A with GDIα, suggesting that the kinase domain of SAD-A is required for its interaction with GDIα.
Figure 1.
SAD-A interaction with GDIα through kinase domain. Identification of key regulatory sites and domains required for SAD-A interaction with GDIα. (a) Analysis of SAD-A interaction with GDIα by GST-pull down assay. The lysate from 293T cells, which overexpressed SAD-A or vector, were used in the assay. (b) Diagrams depicting the structures of SAD-A and its deletion mutants generated for the identification of domains required for its interaction with GDIα. (c) Analysis of SAD-A interaction with GDIα by co-IP analysis from 293T cells coexpressing Myc-GDIα and Flag-SAD-A or the indicated SAD-A mutants. The immunocomplexes were pulled down by anti-Flag antibodies and probed by western blot analysis using indicated antibodies. The expression level of Myc-GDIα was verified by western blot analysis from the cell lysates as shown in the lower panel. The N-terminal kinase domain of SAD-A (N368 and N275) was required for its interaction with GDIα as demonstrated by co-IP analysis using deletion mutants shown in panel (b). IB, immunoblotting; IP, immunoprecipitation.
SAD-A phosphorylates GDIα at Ser174 site from an in vitro kinase assay
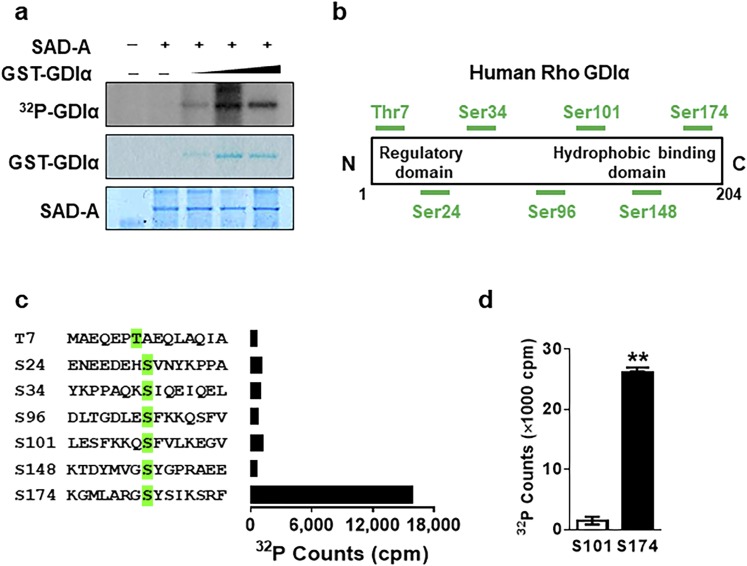
We next postulated that the physical interaction of GDIα with SAD-A would lead to GDIα phosphorylation by SAD-A, which was tested by in vitro phosphorylation analysis of GDIα using purified Flag-SAD-A and GST-GDIα. As shown in Fig. 2a, the purified SAD-A specifically phosphorylated GST-GDIα fusion protein in a dose-dependent manner. To identify the phosphorylation site of GDIα by SAD-A, we next carried out systematic in vitro phosphorylation analysis using synthetic oligopeptides that contain all the known or predicted serine/threonine phosphorylation sites of GDIα as the substrates (Fig. 2b). As shown in Fig. 2c (and quantified in Fig. 2d), SAD-A specifically phosphorylated GDIα at Ser174, a previously identified phosphorylation site required for Rho GTPases activation and the onset of GSIS (10, 11), but not any other potential phosphorylation sites.
Figure 2.
SAD-A specifically phosphorylates GDIα at the Ser174 site. (a) Phosphorylation of the purified GST-GDIα by in vitro kinase assay using [γ-32P] ATP and the purified SAD-A protein overexpressed in Sf9 insect cells. (b) Structures of GDIα and its predicted phosphorylation sites. (c) GDIα phosphorylation by the purified SAD-A protein using GDIα oligopeptides, which contain the predicted phosphorylation sites (highlighted in green) as substrate. (d) The quantification of assay carried out in panel (c) after being repeated three times. **P < 0.01 (t test).
SAD-A binds and phosphorylates GDIα at Ser174 in response to glucose and GLP-1 treatment in β cells
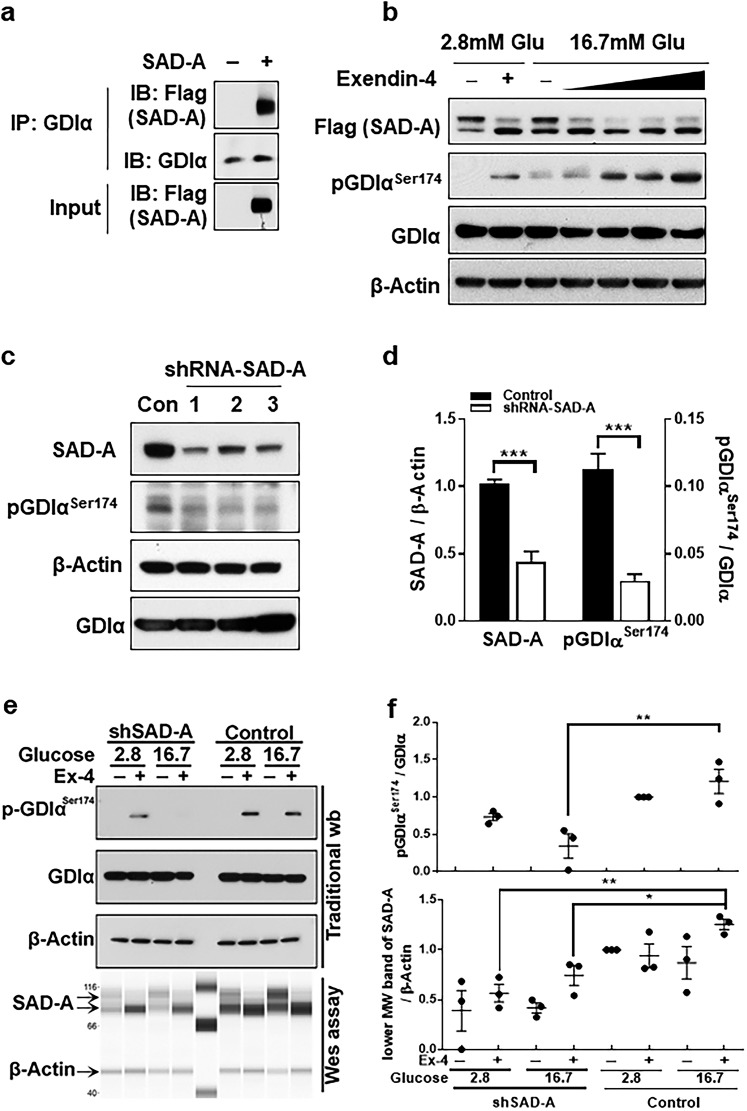
To verify the interaction in β cells, we overexpressed the recombinant SAD-A in isolated islets and carried out a co-IP analysis using Flag-tagged SAD-A as the bait, because no commercial SAD-A antibody is suitable for immunoprecipitation assay. The results show that SAD-A specifically interacted with endogenous GDIα, which was evidenced by specific immunoprecipitation with anti-Flag antibody (Fig. 3a). Using antiphospho-Ser174 antibodies to GDIα, we investigated the role of SAD-A in regulating endogenous GDIα phosphorylation in INS-1 islet β cells. We first examined whether SAD-A overexpression would stimulate endogenous GDIα phosphorylation at Ser174, and, if so, whether the phosphorylation could be regulated by glucose and GLP-1. INS-1 β cells were transiently transfected with a SAD-A expression plasmid, followed by treatment with glucose or glucose plus increasing doses of Exendin-4, a long-acting analog of GLP-1. Consistent with findings from an in vitro phosphokinase assay, SAD-A overexpression significantly stimulated Ser174 phosphorylation in GDIα in response to glucose stimulation and GLP-1 treatment (Fig. 3b). Strikingly, the Ser174 phosphorylation was dramatically enhanced in response to treatment of Exendin-4 in the presence of high glucose concentration. The findings were consistent with our report that SAD-A significantly potentiates GLP-1’s effect on GSIS only in the presence of high glucose (29).
Figure 3.
SAD-A binds and phosphorylates GDIα at Ser174 during Exendin-4 (Ex-4) and glucose treatment in β cells. (a) Co-IP of SAD-A and endogenous GDIα from islets by using anti-GDIα antibodies. The islets were infected with recombinant adenoviruses overexpressing Flag-SAD-A or empty vector. The immunocomplexes were probed by western blot analysis using anti-Flag antibodies for SAD-A. (b) Ser174-GDIα was dose-dependently increased by SAD-A after stimulated by 16.7 mM glucose and Ex-4. INS-1 cells were transiently transfected with FLAG–SAD-A for 48 hours followed by 1 hour starvation in 1% fetal bovine serum 1640 at 2.8 mM glucose, the media were then changed with 0, 1, 6, 12, and 24 nM of Ex-4 together 16.7 mM glucose, or 12 nM Ex-4together 2.8 mM glucose. Collect cell lysate at 10 minutes and followed by western blot. (c) INS-1 cells were transiently transfected with expression vectors for three different shRNAs (lanes 1 through 3) targeted to endogenous SAD-A or a scrambled form as negative control (Con) followed by western blot analysis for the expression level of SAD-A, GDIα, and Ser174-GDIα. β-actin was used as an internal control for protein loading. (d) Quantification of SAD-A and Ser174-GDIα level in panel (c). *** P < 0.001 (t test). (e) Ser174-GDIα was analyzed after shRNA-mediated knockdown of SAD-A in the presence or absence of high glucose and 24 nM of (Ex-4). (f) Quantification of SAD-A and Ser174-GDIα level in panel (e). [(f), upper panel] Ser174-GDIα density analysis. Two-way repeated-measure ANOVA [column: control and shSAD-A; row: group 1 (2.8 mM Glu + 12 nM Ex-4) and group 2 (16.7 mM Glu + 12 nM Ex-4)] revealed a significant main effect of column [F(1, 8) = 22.98, P = 0.0014] and a nonsignificant effect of row [F(1, 8) = 0.5853, P = 0.4662] and a significant interaction between factors [F(1, 8) = 6.443, P = 0.0348]. ** P < 0.01 (post hoc comparisons with Tukey test correction). [(f), lower panel] 85-Kd (lower MW) band of SAD-A density analysis. Two-way repeated-measure ANOVA [column: control and shSAD-A; row: group 1 (2.8 mM Glu + 12 nM Ex-4) and group 2 (16.7 mM Glu + 12 nM Ex-4)] revealed a significant main effect of column [F(1, 8) = 22.34, P = 0.0015] and a significant effect of row [F(1, 8) = 6.903, P = 0.0303] and nonsignificant interaction between factors [F(1, 8) = 0.5366, P = 0.4847]. *P < 0.05 and **P < 0.01 (post hoc comparisons with Tukey test correction). IB, immunoblotting; IP, immunoprecipitation; wb, western blot.
To provide further support for the role of SAD-A in regulating Ser174 phosphorylation in GDIα, we next determined the effect of endogenous SAD-A deficiency on GDIα phosphorylation in INS-1 cells mediated by short hairpin RNA (shRNA) targeted to SAD-A (20). Transient expression of shRNA in INS-1 cells resulted in significant knockdown of the endogenous SAD-A when compared with the scramble control (Fig. 3c). In support of the findings of SAD-A overexpression in INS-1 cells, endogenous SAD-A deficiency significantly decreased phosphorylation of endogenous GDIα on Ser174 site (Fig. 3c, quantified in Fig. 3d). SAD-A protein contains two bands during western blot detection, a higher molecular band (∼105 KD) that represents a phosphorylated and inactive form of SAD-A, and a lower molecular weight band (∼85 KD), which is the nonphosphorylated and active form of SAD-A (29). shRNA knockdown of lower molecular weight SAD-A reduced Ser174 phosphorylation of GDIα under high glucose (16.7 mM) and Exendin-4–stimulated conditions (Fig. 3e, quantified in Fig. 3f). However, endogenous GDIα phosphorylation at the Ser174 site in response to glucose and Exendin-4 after partial knockdown of SAD-A in INS-1 cells will be seen (Fig. 3e, quantified in Fig. 3f). We carried out capillary electrophoresis in Wes system to detect the SAD-A protein because of the low expression level of endogenous SAD-A in INS-1 cells; the antibody conditions were tested and are shown in Supplemental Fig. 1. Together, the findings demonstrated a crucial role of SAD-A in stimulating Ser174 phosphorylation of GDIα in response to glucose and GLP-1 stimulation.
SAD-A stimulates Rho GTPase activation by promoting its dissociation from GDIα
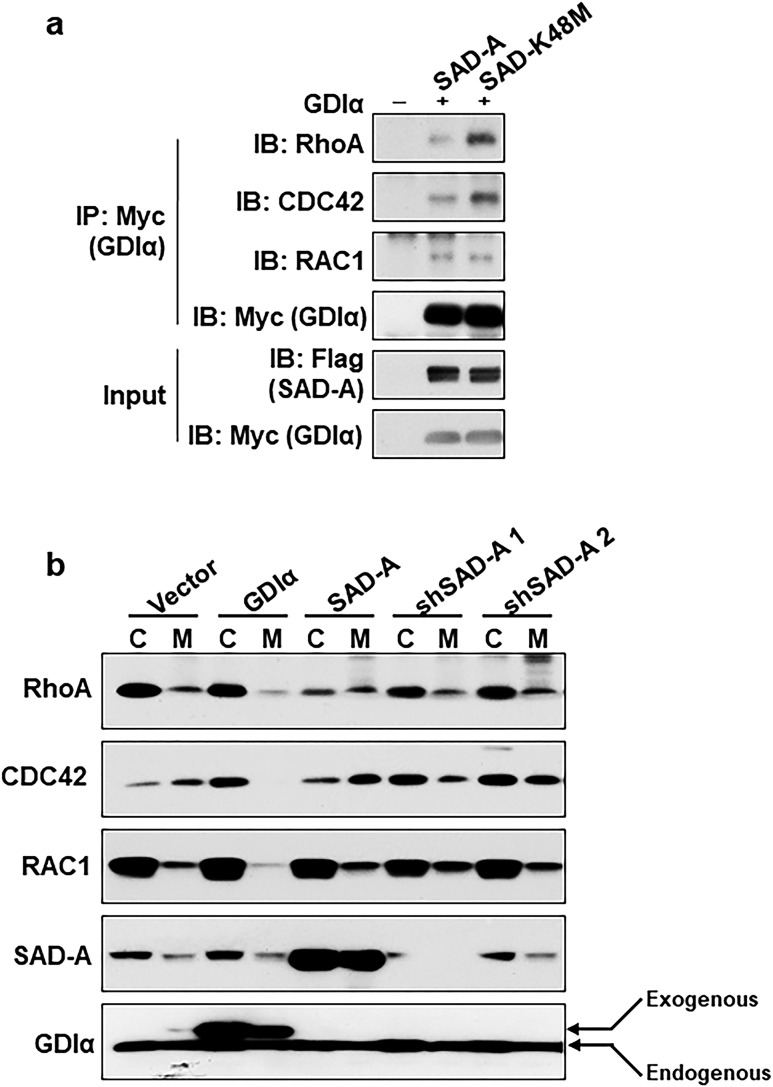
We next investigated whether phosphorylation of Ser174 by SAD-A would lead to Rho GTPase activation by a GDIα dissociation assay. INS-1 cells were transiently transfected with expression vectors for SAD-A or its kinase-dead mutant (SAD-K48M), as previously reported with Myc-GDIα (29). GDIα was used as the “bait” for the co-IP assay, followed by western blot analysis of coimmunoprecipitates of each of the Rho GTPases, including RhoA, CDC42, and RAC1. As shown in Fig. 4a, overexpression of SAD-A, but not the kinase-dead mutant, significantly stimulated the dissociation of RhoA and CDC42 from GDIα when compared with vector control. In contrast, SAD-A affected the binding of Rac1 to GDIα less.
Figure 4.
SAD-A stimulates Rho GTPase activation by promoting dissociation from GDIα. (a) GDIα dissociation assay were carried out by co-IP in INS-1 β cells. The cells were cotransfected with Myc-GDIα and Flag-SAD-A or the indicated SAD-A mutants. Anti-Myc antibodies were used for immunoprecipitating Myc-GDIα and other proteins. The immunocomplexes were probed by western blot analysis using anti-RhoA, CDC42, and RAC1 antibodies. (b) Subcellular fractionation analysis for cytosolic and membrane translocation of Rho GTPases. The INS-1 cells were transiently transfected with Flag-SAD-A (SAD-A), Myc-GDIα (GDIα), two different shRNAs (1 and 2), or empty vector (vector). The lysates were fractionated by differential centrifugation into cytosolic and membrane fractions. The fractionation was analyzed by western blot analysis using RAC1, CDC42, RhoA, SAD-A, and GDIα antibodies. C, cytosolic; IB, immunoblotting; IP, immunoprecipitation; M, membrane; shSAD-A, short hairpin RNA targeted to SAD-A.
The onset of GSIS involves the recycling of small GTPases between the inactive GDP-bound forms in the cytoplasm and its active GTP-bound conformation associated with plasma membrane. The recycling process is tightly regulated by GDIα, which directly binds to Rho family GTPases (11). We next investigated whether SAD-A overexpression would stimulate the translocation of Rho GTPases from the cytoplasm to the cell membrane, a critical step required for the activation of RhoA or CDC42, by subcellular localization studies. Consistent with the findings from the co-IP analysis, overexpression of SAD-A significantly stimulated the translocation of both RhoA and CDC42 from the cytoplasm to the membrane in INS-1 cells. In contrast, the translocation was entirely inhibited by GDIα overexpression and shRNA–SAD-A groups (Fig. 4b). Again, SAD-A overexpression affected the translocation of Rac1 less.
SAD-A restored glucose responsiveness in islet β cells through inhibition of GDIα
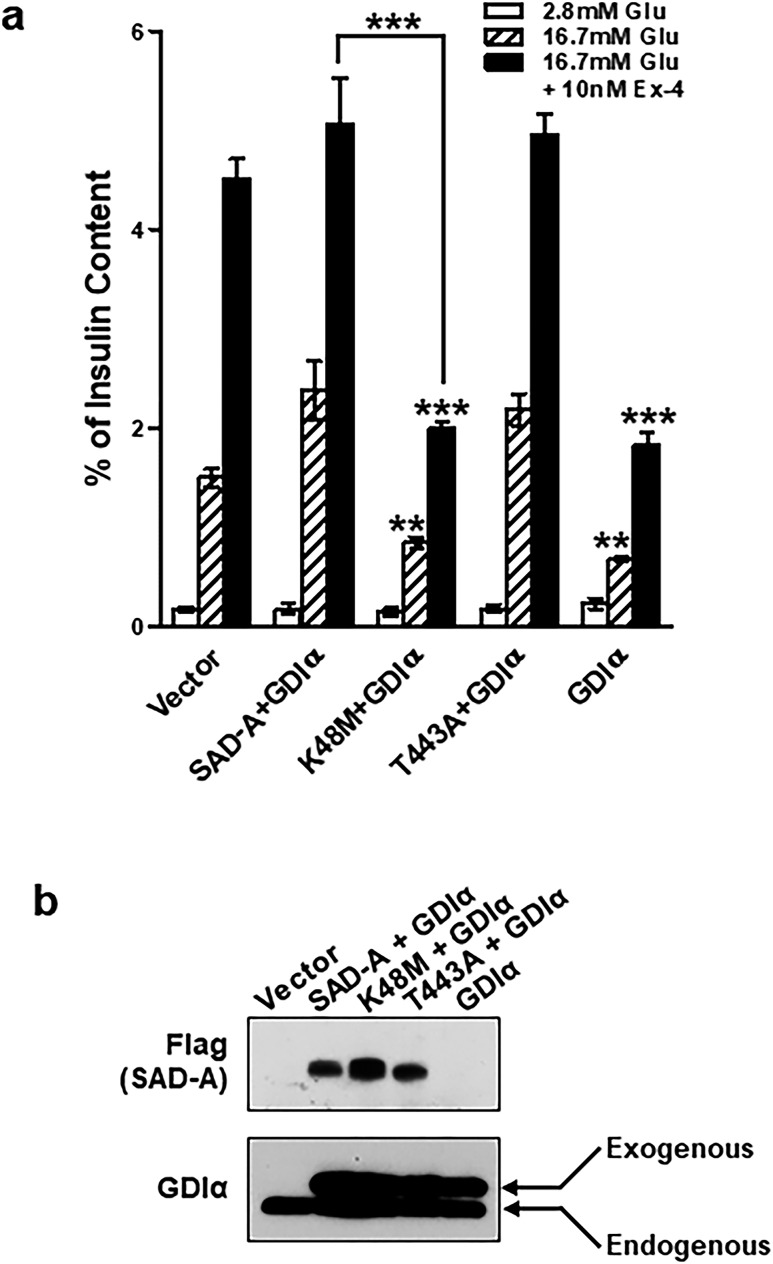
The onset of GSIS is associated with phosphorylation of GDIα at Ser174 in pancreatic islets (10). We postulated that phosphorylation of GDIα at Ser174 by SAD-A would attenuate the inhibitory effect of GDIα on GSIS, which was tested by adenoviral overexpression of GDIα and SAD-A in isolated islets. Overexpression of SAD-A, or the constitutively active SAD-A mutant (SAD-T443A) (29, 30) but not the kinase-inactive form (SAD-K48M), significantly enhanced GSIS and the potentiating effect of forskolin on GSIS (Fig. 5a). In contrast, overexpression of GDIα dramatically inhibited GSIS and abrogated the potentiating effect of GLP-1 on GSIS. In support of a role of SAD-A as the kinase regulator of GDIα, adenoviral expression of SAD-A completely reversed the insulin secretion defect caused by GDIα overexpression (Fig. 5a). These results were corroborated by previous reports that GDIα negatively regulates GSIS through inhibition of Cdc42 and RhoA (10, 46). Phosphorylation of GDIα at Ser174 is required for the onset of GSIS (10). The western blot results showed the expression of each virus during insulin secretion process (Fig. 5b).
Figure 5.
Coexpression of GDIα and SAD-A relieves the inhibitory effects of GDIα on GSIS. (a) Isolated mouse islets were infected or coinfected with recombinant adenoviruses expressing the indicated proteins (from left to right: vector, Flag-SAD-A and Myc-GDIα, kinase-dead SAD-A mutant K48M and Myc-GDIα, constitutively active SAD-A mutant T443A and Myc-GDIα, and Myc-GDIα) and were analyzed for GSIS by radioimmunoassay after 48 hours of infection. (b) Western blots analysis of islets after infected or coinfected with recombinant adenoviruses expressing the indicated proteins. **P < 0.01 and ***P < 0.001 when compared with vector control or indicated groups.
Discussion
Defective insulin secretion from pancreatic β cells is one of the critical hallmarks of type 2 diabetes mellitus (47), but the underlying mechanisms remain elusive. GLP-1 and its long-acting analogs improve islet β-cell function by restoring glucose responsiveness and have been used in the clinics as a popular treatment of type 2 diabetic patients (48, 49). Although GLP-1 receptor activation is known to potentiate GSIS through cAMP/PKA and CaMKK signaling pathways in pancreatic β cells, the distal amplifiers for the stimulus secretion coupling effects of incretin hormones remain to be identified (49). Our recent studies have identified SAD-A as a mediator of GLP-1 receptor-activated signal transduction pathways. SAD-A is activated through phosphorylation by PKA and CaMKK in islet β cells in response to treatment with glucose, GLP-1, and forskolin, a surrogate of incretin action. SAD-A also regulates GSIS in part through phosphorylation and activation of PAK1, the effector protein Rho GTPases. However, whether PAK1 or Rho GTPases activation is required for incretin responses by SAD-A remains elusive. In this report, we identified a crucial role of SAD-A kinase in regulating GDIα activation, implicating a mechanism by which SAD-A governs incretin response in islet β cells.
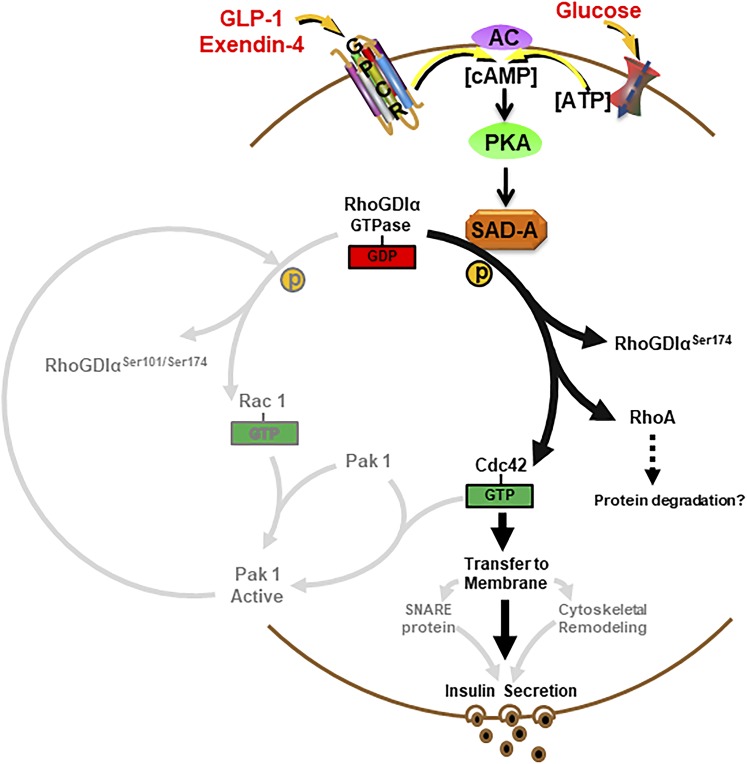
The Rho family of small GTPases acts as intracellular molecular switches that transduce signals from extracellular stimuli to the actin cytoskeleton. Activation of the Rho family of GTPases is required for the GSIS by regulating transport and fusion of insulin-laden secretory granules with the cytoplasmic membrane (10). Yet, signal transduction pathways leading to the activation of Rho GTPases in islet β- cells remain elusive. The Rho GTPases have been associated with glucose stimulate insulin secretion; however, the Rab GTPase protein family (Rab3A, Rab27A, and Rab37) and Ras-like GTPase family (Rap1 and RalA) have been reported to facilitate incretin-mediated insulin secretion in pancreatic β cells (50, 51). Administration of GLP-1 to patients with type 2 diabetes rescues insulin secretion defects in part through cAMP-Epac2-Rap1 signaling (52). Despite these recent findings, the signaling pathways responsible for the beneficial effects of GLP-1 administration in type 2 diabetic patients remain largely unexplored. In this study, we report that GDIα and Rho GTPase were involved in glucose and Exendin-4 stimulated insulin secretion, which were regulated by SAD-A kinase (Fig. 6). The Cdc42 was released from GDIα complex because of the phosphorylation of GDIα at Ser174 by SAD-A kinase and transferred to the membrane to stimulate insulin secretion. In support of a potential role of SAD-A in regulating Rho GTPase activation, SAD-A overexpression leads to significant remodeling of the actin cytoskeleton in islet β cells (30).
Figure 6.
A proposed model for the critical role of SAD-A in regulating insulin secretion through phosphorylation of GDIα at Ser174. An elevated level of cAMP, which was generated from activation of incretin hormone receptors and glucose metabolism, can trigger direct phosphorylation of key regulatory sites of GDIα at Ser174 by SAD-A, leading to full activation of GTPases, which stimulate cytoskeletal remodeling and GSIS. AC, adenylyl cyclase; GPCR, G protein–coupled receptors.
SAD-A kinase reduces the affinity of Rho A for GDIα when overexpressed in islet β cells. Interestingly, SAD-A overexpression also decreases the cytosolic form of Rho A (Fig. 4b). Consistence with this finding, the Rho A and Rho-associated kinase signaling pathway contributes to the stabilization of the actin cytoskeleton and inhibits GSIS (53). Furthermore, GLP-1 has been showed to inhibit pathological upregulated RhoA/Rho-associated kinase (54). Several single-nucleotide polymorphisms in and around RhoA were associated with elevated fasting insulin and homeostasis model assessment index of insulin resistance, suggesting a possible role in metabolic dysregulation (54). Although the molecular mechanism is still unclear, the SAD-A–mediated disassociation of cytosolic Rho A from GDIα may lead to Rho A protein degradation. In line with this idea, Exendin-4 can suppress the activity of RhoA and restore cell viability in human β cells (55, 56).
In this study, we identified SAD-A as an upstream activator of Rho GTPases through modulation of GDIα. GDIα is a negative regulator of GSIS and Rho family GTPases by preventing their binding to the PAK1 effector (7, 8). Currently, the only known kinase that has been described to bind and phosphorylate GDIα is PAK1, a downstream effector of Rac1 and Cdc42. The activated Rac1 or Cdc42 GTPases binds to PAK1 and induces an increase its kinase activity (57, 58). The activated Pak1 then binds and phosphorylates GDIα at Ser101 and Ser174, resulting in positive feed-forward regulation activation of Rac1. Although PAK1 can phosphorylate GDIα to release Rac1, the activation of Cdc42 happened before GDIα phosphorylation by PAK1. Collectively, the dissociation of Cdc42 from GDIα requires other kinases to be involved. An unknown specific kinase may play a pivotal role during the initial phosphorylation of GDIα in islet β-cell insulin secretion pathways. Furthermore, Cdc42 is critical for F-actin remodeling and targeting of insulin-containing granules to the SNARE protein syntaxin 1A and VAMP2, which are two important processes involved in insulin granule movement (2, 59). Our results show that SAD-A binds explicitly to GDIα through its kinase domain, leading to direct phosphorylation of GDIα at Ser174, a key regulatory phosphorylation site in the hydrophobic binding domain which is required for Rho GTPase activation and GSIS (10, 60). The findings were further confirmed by results from a peptide phosphorylation profiling assay that demonstrates that Ser174 is the only site of GDIα specifically phosphorylated by SAD-A among all the predicated or previously identified phosphorylation sites. Consistent with the findings, SAD-A overexpression significantly stimulated GDIα phosphorylation at Ser174, which is dramatically enhanced by GLP-1 treatment in the presence of high glucose. The results are highly reminiscent of the incretin effect because GLP-1 potentiates GSIS only in response to hyperglycemia. In further support for the results, SAD-A deficiency significantly decreased Ser174 phosphorylation of the endogenous GDIα in INS-1 β cells. Consequently, adenoviral expression of SAD-A in isolated mouse islets completely abolished the inhibitory effects of GDIα on GSIS, linking the incretin effect to the activation of Rho GTPases.
The AMPK family of kinases and the Rho family of small GTPase share many common regulatory functions, including cytoskeletal remodeling, cellular polarity, vesicle fusion, exocytosis, morphogenesis, axon growth and specification, and cancer (12, 20, 61–66). Yet, little is known about the functional interactions between the two families of signal proteins. The present studies provide strong evidence for a direct link between members of AMPK-related kinases and the Rho GTPase family of proteins, suggesting that many of the previously reported cellular events regulated by LKB1, AMPK, and other AMPK-related kinases may now be better explained in terms of activation of Rho GTPases, as evidenced by multiple regulatory roles of LKB1 in cancer, polarity, and metabolism (67).
Together, based on the available body of evidence, we propose that signaling by SAD-A defines a mechanism of GDIα inhibition in pancreatic β cells that are required for the onset of GSIS. The mechanism entails that SAD-A is activated by PKA and CaMKK in response to an elevated level of [cAMP]i and [Ca2+]i generated from glucose metabolism and activation of G protein–coupled receptors of incretins, such as GLP-1 and GIP. SAD-A directly binds to and phosphorylates GDIα at Ser174, leading to GDIα inactivation. Concurrently, SAD-A also directly binds to and phosphorylates PAK1 at Thr423, as shown by our previous studies (30), leading to the synergistic activation of Rho GTPases that are required for cytoskeletal remodeling, vesicle trafficking, docking, and fusion with the plasma membrane, culminating in GSIS (Fig. 6). In addition to GSIS reported in this study, SAD-A has recently been implicated in a number of biological functions, including neuronal polarity, axon formation, and neurotransmitter release, all of which require cytoskeletal remodeling. Our single-cell transcriptomic results found that SAD-A and GDIα were expressed in the same cell, which was different from PAK1 expression pattern in mouse islets (unpublished data). This result suggests a mechanism for SAD-A to regulation of islet β-cell insulin secretion, which could be independent of the PAK1 signal cascade. Therefore, the present findings will offer a potential mechanism by which SAD-A regulates these biological functions because GDIα has also been implicated in the same biological processes through regulation of Rho GTPase (68–70).
Supplementary Material
Acknowledgments
We are grateful to Dr. J. Sanes (Harvard University, Cambridge, MA) for providing the SAD-A antibodies; Dr. F. Polleux (Columbia University, New York, NY) for DNA vector for SAD-A shRNAs; Dr. B. Stanley (Penn State College of Medicine, Hershey, PA) for mass spectrometry; and Drs. C. Newgard (Duke University Medical Center, Durham, NC), J. Miyazaki (Osaka University Medical School, Osaka, Japan), S. Özcan (University of Kentucky College of Medicine, Lexington, KY), and J. Shao (University of California San Diego, La Jolla, CA) for the islet β-cell lines.
Financial Support: This work was supported in part by National Institutes of Health Grant R01 DK080157 (to Y.S.), American Diabetes Association Grant 1-14-BS-185 (to Y.S.), National Institutes of Health Grants R01 DK80157 and R01 DK089229 (to N.M.), American Diabetes Association (to N.M.), San Antonio Claude D. Pepper Older Americans Independence Center Grant AG044271 (to N.M.), San Antonio Nathan Shock Center Grant AG013319 (to N.M.), and Biology of Aging T32 training Grant T32 AG021890 (to J.N.).
Author Contributions: J.N. conceived and designed the experimental studies, carried out a considerable portion of them, acquired and analyzed the data, wrote and revised the manuscript critically for publication, and led and directed the study. Y.S. assisted in the conception and design of the study and in critically revising the manuscript. N.M. assisted in the analysis and interpretation of the data and in critically revising the manuscript for its intellectual content. C.S. and Z.C. contributed in performing part of the experimental studies, image acquisition, and reading and revising the manuscript. All authors have given their final approval of the version to be published. J.N. is the guarantor of the work.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AMPK
AMP-activated protein kinase
- co-IP
coimmunoprecipitation
- GDIα
GDP-dissociation inhibitor
- GIP
glucose-dependent insulinotropic peptide
- GLP-1
glucagonlike peptide-1
- GSIS
glucose-stimulated insulin secretion
- GST
glutathione S-transferase
- PAK1
p21-activated kinase 1
- PKA
protein kinase A
- shRNA
short hairpin RNA
References
- 1. Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121(6):2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nevins AK, Thurmond DC. Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol. 2003;285(3):C698–C710. [DOI] [PubMed] [Google Scholar]
- 3. Asahara S, Shibutani Y, Teruyama K, Inoue HY, Kawada Y, Etoh H, Matsuda T, Kimura-Koyanagi M, Hashimoto N, Sakahara M, Fujimoto W, Takahashi H, Ueda S, Hosooka T, Satoh T, Inoue H, Matsumoto M, Aiba A, Kasuga M, Kido Y. Ras-related C3 botulinum toxin substrate 1 (RAC1) regulates glucose-stimulated insulin secretion via modulation of F-actin. Diabetologia. 2013;56(5):1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li J, Luo R, Kowluru A, Li G. Novel regulation by Rac1 of glucose- and forskolin-induced insulin secretion in INS-1 beta-cells. Am J Physiol Endocrinol Metab. 2004;286(5):E818–E827. [DOI] [PubMed] [Google Scholar]
- 5. Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, Rabaglia ME, Vadakekalam J, Metz SA. Glucose- and GTP-dependent stimulation of the carboxyl methylation of CDC42 in rodent and human pancreatic islets and pure beta cells. Evidence for an essential role of GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest. 1996;98(2):540–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sidarala V, Veluthakal R, Syeda K, Kowluru A. EHT 1864, a small molecule inhibitor of Ras-related C3 botulinum toxin substrate 1 (Rac1), attenuates glucose-stimulated insulin secretion in pancreatic β-cells. Cell Signal. 2015;27(6):1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15(7):356–363. [DOI] [PubMed] [Google Scholar]
- 8. Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390(Pt 1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem. 2007;282(13):9536–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Z, Thurmond DC. Differential phosphorylation of RhoGDI mediates the distinct cycling of Cdc42 and Rac1 to regulate second-phase insulin secretion. J Biol Chem. 2010;285(9):6186–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kowluru A, Veluthakal R. Rho guanosine diphosphate-dissociation inhibitor plays a negative modulatory role in glucose-stimulated insulin secretion. Diabetes. 2005;54(12):3523–3529. [DOI] [PubMed] [Google Scholar]
- 12. Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75(1):137–163. [DOI] [PubMed] [Google Scholar]
- 13. Rutter GA, Leclerc I. The AMP-regulated kinase family: enigmatic targets for diabetes therapy. Mol Cell Endocrinol. 2009;297(1-2):41–49. [DOI] [PubMed] [Google Scholar]
- 14. Hezel AF, Gurumurthy S, Granot Z, Swisa A, Chu GC, Bailey G, Dor Y, Bardeesy N, Depinho RA. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol. 2008;28(7):2414–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Granot Z, Swisa A, Magenheim J, Stolovich-Rain M, Fujimoto W, Manduchi E, Miki T, Lennerz JK, Stoeckert CJ Jr, Meyuhas O, Seino S, Permutt MA, Piwnica-Worms H, Bardeesy N, Dor Y. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab. 2009;10(4):296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu A, Ng AC, Depatie C, Wijesekara N, He Y, Wang GS, Bardeesy N, Scott FW, Touyz RM, Wheeler MB, Screaton RA. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab. 2009;10(4):285–295. [DOI] [PubMed] [Google Scholar]
- 17. Mountjoy PD, Rutter GA. Glucose sensing by hypothalamic neurones and pancreatic islet cells: AMPle evidence for common mechanisms? Exp Physiol. 2007;92(2):311–319. [DOI] [PubMed] [Google Scholar]
- 18. Sun G, Tarasov AI, McGinty JA, French PM, McDonald A, Leclerc I, Rutter GA. LKB1 deletion with the RIP2.Cre transgene modifies pancreatic beta-cell morphology and enhances insulin secretion in vivo. Am J Physiol Endocrinol Metab. 2010;298(6):E1261–E1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307(5711):929–932. [DOI] [PubMed] [Google Scholar]
- 20. Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129(3):549–563. [DOI] [PubMed] [Google Scholar]
- 21. Lilley BN, Pan YA, Sanes JR. SAD kinases sculpt axonal arbors of sensory neurons through long- and short-term responses to neurotrophin signals. Neuron. 2013;79(1):39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inoue E, Mochida S, Takagi H, Higa S, Deguchi-Tawarada M, Takao-Rikitsu E, Inoue M, Yao I, Takeuchi K, Kitajima I, Setou M, Ohtsuka T, Takai Y. SAD: a presynaptic kinase associated with synaptic vesicles and the active zone cytomatrix that regulates neurotransmitter release. Neuron. 2006;50(2):261–275. [DOI] [PubMed] [Google Scholar]
- 23. Yasuda T, Shibasaki T, Minami K, Takahashi H, Mizoguchi A, Uriu Y, Numata T, Mori Y, Miyazaki J, Miki T, Seino S. Rim2alpha determines docking and priming states in insulin granule exocytosis. Cell Metab. 2010;12(2):117–129. [DOI] [PubMed] [Google Scholar]
- 24. Alvarado-Kristensson M, Rodríguez MJ, Silió V, Valpuesta JM, Carrera AC. SADB phosphorylation of gamma-tubulin regulates centrosome duplication. Nat Cell Biol. 2009;11(9):1081–1092. [DOI] [PubMed] [Google Scholar]
- 25. Dupré J, Beck JC. Stimulation of release of insulin by an extract of intestinal mucosa. Diabetes. 1966;15(8):555–559. [DOI] [PubMed] [Google Scholar]
- 26. Guo Z, Tang W, Yuan J, Chen X, Wan B, Gu X, Luo K, Wang Y, Yu L. BRSK2 is activated by cyclic AMP-dependent protein kinase A through phosphorylation at Thr260. Biochem Biophys Res Commun. 2006;347(4):867–871. [DOI] [PubMed] [Google Scholar]
- 27. Fujimoto T, Yurimoto S, Hatano N, Nozaki N, Sueyoshi N, Kameshita I, Mizutani A, Mikoshiba K, Kobayashi R, Tokumitsu H. Activation of SAD kinase by Ca2+/calmodulin-dependent protein kinase kinase. Biochemistry. 2008;47(13):4151–4159. [DOI] [PubMed] [Google Scholar]
- 28. Nie J, Han X, Shi Y. SAD-A and AMPK kinases: the “yin and yang” regulators of mTORC1 signaling in pancreatic β cells. Cell Cycle. 2013;12(21):3366–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nie J, Lilley BN, Pan YA, Faruque O, Liu X, Zhang W, Sanes JR, Han X, Shi Y. SAD-A potentiates glucose-stimulated insulin secretion as a mediator of glucagon-like peptide 1 response in pancreatic β cells. Mol Cell Biol. 2013;33(13):2527–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nie J, Sun C, Faruque O, Ye G, Li J, Liang Q, Chang Z, Yang W, Han X, Shi Y. Synapses of amphids defective (SAD-A) kinase promotes glucose-stimulated insulin secretion through activation of p21-activated kinase (PAK1) in pancreatic β-Cells. J Biol Chem. 2012;287(31):26435–26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nie J, Liu X, Lilley BN, Zhang H, Pan YA, Kimball SR, Zhang J, Zhang W, Wang L, Jefferson LS, Sanes JR, Han X, Shi Y. SAD-A kinase controls islet β-cell size and function as a mediator of mTORC1 signaling. Proc Natl Acad Sci USA. 2013;110(34):13857–13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49(3):424–430. [DOI] [PubMed] [Google Scholar]
- 33. Feng Q, Albeck JG, Cerione RA, Yang W. Regulation of the Cool/Pix proteins: key binding partners of the Cdc42/Rac targets, the p21-activated kinases. J Biol Chem. 2002;277(7):5644–5650. [DOI] [PubMed] [Google Scholar]
- 34. RRID:AB_2227516.
- 35. RRID:AB_1524317.
- 36. RRID:AB_627268.
- 37. RRID:AB_632346
- 38. RRID:AB_631213.
- 39. RRID:AB_10709971.
- 40. RRID:AB_259529.
- 41. RRID:AB_476744.
- 42. RRID:AB_2243660.
- 43. RRID:AB_772210.
- 44. RRID:AB_772206.
- 45. RRID:AB_10063035.
- 46. Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev. 2010;31(1):52–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116(7):1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. [DOI] [PubMed] [Google Scholar]
- 49. Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP-GEFII--Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276(49):46046–46053. [DOI] [PubMed] [Google Scholar]
- 50. Regazzi R, Sadoul K, Meda P, Kelly RB, Halban PA, Wollheim CB. Mutational analysis of VAMP domains implicated in Ca2+-induced insulin exocytosis. EMBO J. 1996;15(24):6951–6959. [PMC free article] [PubMed] [Google Scholar]
- 51. Ljubicic S, Bezzi P, Brajkovic S, Nesca V, Guay C, Ohbayashi N, Fukuda M, Abderrhamani A, Regazzi R. The GTPase Rab37 participates in the control of insulin exocytosis [published correction appears in PLoS One. 2013;8(7):10]. PLoS One. 2013;8(6):e68255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kwan EP, Xie L, Sheu L, Ohtsuka T, Gaisano HY. Interaction between Munc13-1 and RIM is critical for glucagon-like peptide-1 mediated rescue of exocytotic defects in Munc13-1 deficient pancreatic beta-cells. Diabetes. 2007;56(10):2579–2588. [DOI] [PubMed] [Google Scholar]
- 53. Hammar E, Tomas A, Bosco D, Halban PA. Role of the Rho-ROCK (Rho-associated kinase) signaling pathway in the regulation of pancreatic beta-cell function. Endocrinology. 2009;150(5):2072–2079. [DOI] [PubMed] [Google Scholar]
- 54. Kong X, Yan D, Sun J, Wu X, Mulder H, Hua X, Ma X. Glucagon-like peptide 1 stimulates insulin secretion via inhibiting RhoA/ROCK signaling and disassembling glucotoxicity-induced stress fibers. Endocrinology. 2014;155(12):4676–4685. [DOI] [PubMed] [Google Scholar]
- 55. Tsukamoto M, Niimi N, Sango K, Takaku S, Kanazawa Y, Utsunomiya K. Neurotrophic and neuroprotective properties of exendin-4 in adult rat dorsal root ganglion neurons: involvement of insulin and RhoA. Histochem Cell Biol. 2015;144(3):249–259. [DOI] [PubMed] [Google Scholar]
- 56. Aly H, Rohatgi N, Marshall CA, Grossenheider TC, Miyoshi H, Stappenbeck TS, Matkovich SJ, McDaniel ML. A novel strategy to increase the proliferative potential of adult human β-cells while maintaining their differentiated phenotype. PLoS One. 2013;8(6):e66131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zenke FT, King CC, Bohl BP, Bokoch GM. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J Biol Chem. 1999;274(46):32565–32573. [DOI] [PubMed] [Google Scholar]
- 58. Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102(3):387–397. [DOI] [PubMed] [Google Scholar]
- 59. Daniel S, Noda M, Cerione RA, Sharp GW. A link between Cdc42 and syntaxin is involved in mastoparan-stimulated insulin release. Biochemistry. 2002;41(30):9663–9671. [DOI] [PubMed] [Google Scholar]
- 60. DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell. 2004;15(1):117–127. [DOI] [PubMed] [Google Scholar]
- 61. Hong-Geller E, Cerione RA. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP(3)/calcium pathway in RBL-2H3 mast cells. J Cell Biol. 2000;148(3):481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev. 2009;89(3):777–798. [DOI] [PubMed] [Google Scholar]
- 63. Kardash E, Reichman-Fried M, Maitre JL, et al. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat Cell Biol. 2010;12(1):47–53. [DOI] [PubMed] [Google Scholar]
- 64. Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6(6):459–471. [DOI] [PubMed] [Google Scholar]
- 65. Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114(2):215–227. [DOI] [PubMed] [Google Scholar]
- 66. Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416(6879):442–447. [DOI] [PubMed] [Google Scholar]
- 67. Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kreis P, Barnier JV. PAK signalling in neuronal physiology. Cell Signal. 2009;21(3):384–393. [DOI] [PubMed] [Google Scholar]
- 69. Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395(6698):194–198. [DOI] [PubMed] [Google Scholar]
- 70. Jacobs T, Causeret F, Nishimura YV, Terao M, Norman A, Hoshino M, Nikolić M. Localized activation of p21-activated kinase controls neuronal polarity and morphology. J Neurosci. 2007;27(32):8604–8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.