Abstract
Objective
To prospectively examine the association between low-density lipoprotein (LDL) cholesterol (LDL-C) concentrations and intracerebral hemorrhage (ICH) risk.
Methods
The current cohort study included 96,043 participants (mean age 51.3 years) who were free of stroke, myocardial infarction, and cancer at baseline (2006). Serum LDL-C concentrations were assessed in 2006, 2008, 2010, and 2012. Cumulative average LDL-C concentrations were calculated from all available LDL-C data during that period. Incident ICH was confirmed by review of medical records.
Results
We identified 753 incident ICH cases during 9 years of follow-up. The ICH risk was similar among participants with LDL concentrations of 70 to 99 mg/dL and those with LDL-C concentrations ≥100 mg/dL. In contrast, participants with LDL-C concentrations <70 mg/dL had a significantly higher risk of developing ICH than those with LDL-C concentrations of 70 to 99 mg/dL; adjusted hazard ratios were 1.65 (95% confidence interval [CI] 1.32–2.05) for LDL-C concentrations of 50 to 69 mg/dL and 2.69 (95% CI 2.03–3.57) for LDL-C concentrations <50 mg/dL.
Conclusions
We observed a significant association between lower LDL-C and higher risk of ICH when LDL-C was <70 mg/dL, and the association became nonsignificant when LDL-C ≥70 mg/dL. These data can help determination of the ideal LDL range in patients who are at increased risk of both atherosclerotic disease and hemorrhagic stroke and guide planning of future lipid-lowering studies.
Intracerebral hemorrhage (ICH) is the second most common stroke type, with a high mortality and disability rate.1–3 Although ICH is of special concern in developing countries4 such as China,5 the prevalence of hemorrhagic stroke is high in developed countries (particularly for blacks and Hispanics in the United States).6 Because cholesterol has a key role in the structural formation of cell membranes, low cholesterol concentration has been proposed as a potential risk factor for ICH.7 Some,8,9 but not all,10–17 epidemiologic studies reported an inverse association between low-density lipoprotein cholesterol (LDL-C) concentrations and risk of ICH. However, with 1 exception,14 studies on this topic have been limited by a small number of incident ICH cases (<300). Furthermore, these studies were based on a single baseline LDL-C measurement, which could underestimate the association between LDL-C and ICH risk because of normal fluctuations and changes in LDL-C concentrations over time, particularly due to the initiation of lipid-lowering therapy. Because many older adults are at risk of hemorrhagic strokes, it is important to clarify the association between LDL-C concentration and ICH risk, knowing that lipid lowering is recommended for ischemic stroke prevention by all relevant guidelines. In particular, the 2018 American Heart Association/American College of Cardiology multisociety guideline on the management of blood cholesterol emphasized using maximally tolerated lipid-lowering medications to reduce LDL-C levels for patients with atherosclerotic cardiovascular disease.18 However, long-term safety such as risk of hemorrhagic stroke after the more intensive lipid-lowering remains uncertain to date.18
To better understand the relationship between LDL-C and ICH, we prospectively examined the association between LDL-C concentrations, which were repeatedly assessed every 2 years, and subsequent risk of ICH among >95,000 adults.
Methods
Participants
As detailed elsewhere,19 the Kailuan study is a community-based multicenter prospective cohort study in Tangshan, an industrial city in China, designed to investigate risk factors of chronic disease. From June 2006 to October 2007 (the cohort baseline, referred to as 2006 survey), a total of 101,510 participants (81,110 men and 20,400 women) from 11 centers underwent a standardized questionnaire, physical examination, and laboratory tests. Follow-up assessments were conducted biannually to update participant status on the aforementioned parameters. We excluded 1,306 participants due to missing baseline LDL-C concentration data and 4,161 participants due to a diagnosis of stroke, myocardial infarction, or cancer at the baseline. A total of 96,043 participants were included in the current analyses.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Ethics Committee of the Kailuan General Hospital. The Kailuan study was registered at International Clinical Trials Registry Platform (apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TNRC-11001489) with study identifying number ChiCTR-TNRC-11001489. Informed consent was obtained from the participants. Deidentified data were used for analyses.
Assessment of incident ICH
The first occurrence of ICH was considered as the outcome, either the first nonfatal ICH or ICH death without a preceding nonfatal event. Ascertainment of incident ICH was described previously.20 Briefly, all participants were linked to the Municipal Social Insurance Institution database and Hospital Discharge Register to identify potential cases of incident ICH, which cover all the Kailuan study participants. ICD-9 and ICD-10 were used to identify potential cases of ICH. Additional information on medical history of ICH was collected via questionnaire biennially in the Kailuan study since 2006. Information on death was collected from provincial vital statistics offices. For potential cases of ICH identified by the ICD code or questionnaire, a panel of 3 physicians examined their medical records. Fatal ICH was confirmed by medical records, autopsy reports, or death certificates with ICH listed as the cause of death. ICH events were diagnosed according to the World Health Organization criteria and either brain CT or MRI for confirmation. A total of 429 (57%) patients with incident ICH had clear information on parenchymal location of hematomas, as reviewed by an experienced radiologist.21 The hematomas were categorized into 2 groups based on location: deep (i.e., hemorrhages that originated in the thalamus, basal ganglia, cerebellum, or brainstem) and nondeep (i.e., lobar) hematomas. Because the incident cases of epidural, subdural, and subarachnoid hemorrhages were rare (<0.02%), the ICH was the intraparenchymal hemorrhage in the current study.
Assessment of lipid profile
Blood samples were collected in the morning of the survey after an overnight fast at the baseline (2006) and subsequent survey visits in 2008, 2010, and 2012. LDL-C, high-density lipoprotein cholesterol (HDL-C), triglycerides, and total cholesterol (TC) were measured with the enzymatic colorimetric method (Mind Bioengineering Co Ltd, Shanghai, China). The interassay coefficient of variation for each measurement was <10% with the use of an autoanalyzer (Hitachi 747, Hitachi, Tokyo, Japan) at the central laboratory of the Kailuan General Hospital.
Assessment of potential covariates
Information on age, sex, education level, income level, occupation, physical activity, smoking status, alcohol intake, and medical history (e.g., hypertension, diabetes mellitus, and active treatment such as hypoglycemic, antihypertensive, lipid-lowering agents, and aspirin) was collected via questionnaires, as detailed elsewhere.20 Weight and height were measured by trained fieldworkers during surveys. Body mass index (BMI) was calculated as body weight (kilograms) divided by the square of height (meters squared). Systolic and diastolic blood pressures were measured twice from the seated position with a mercury sphygmomanometer. The average of the 2 readings was used for analysis. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications in the last 2 weeks regardless of blood pressure status. Prehypertension was classified as systolic blood pressure between 120 and 139 mm Hg or diastolic blood pressure between 80 and 89 mm Hg. Fasting blood glucose was measured with the hexokinase/glucose-6-phosphate dehydrogenase method. Plasma high-sensitivity C-reactive protein (hs-CRP) concentrations were measured with high-sensitivity particle-enhanced immunonephelometry assay. Alanine aminotransferase was measured with an enzymatic rate method. Serum creatinine was assessed with the sarcosine oxidase assay method (Creatinine kit, BioSino Bio-Technology and Science Inc, Beijing, China). Estimated glomerular filtration rate (eGFR) was calculated with a modified 4-variable Chronic Kidney Disease Epidemiology Collaboration formula and adjusted for the Chinese population.22 All the plasma samples were measured with the aforementioned autoanalyzer (Hitachi 747). The definition of diabetes mellitus was laboratory based and combined with medication use, that is, a concentration of fasting blood glucose ≥7.0 mmol/L or use of oral hypoglycemic agent or during active treatment with insulin, and prediabetes was defined as a concentration of fasting blood glucose between 5.6 and 6.9 mmol/L.
Statistical analyses
Statistical analyses were conducted with SAS version 9.4 (SAS Institute, Inc, Cary, NC) and Stata 15 (StataCorp LLC, College Station, TX). Formal hypothesis testing was 2 sided with a significance level of 0.05. Person-years for each participant were calculated from the date the 2006 survey was completed to the diagnosed date of ICH or death, loss to follow-up (5%), or December 31, 2015, whichever came first. To represent long-term LDL-C patterns of individuals, we calculated updated cumulative average LDL-C concentrations from all available LDL-C measurement from 2006 to the end of follow up. For example, the incidence of ICH from 2006 to 2008 was related to the 2006 LDL-C concentrations, and the incidence from 2008 to 2010 was related to the average LDL-C concentrations in 2006 and 2008. In secondary analyses, we used a single measure of LDL-C in 2006 and updated LDL-C and cumulative average TC, HDL-C, and triglycerides as the exposure.
LDL-C concentrations were categorized as <50, 50 to 69, 70 to 99, 100 to 129, 130 to 159, and ≥160 mg/dL. TC concentrations were categorized as <120, 120 to 199, 200 to 239, and ≥240 mg/dL. HDL-C concentrations were categorized as ≥60, 40 to 59, and <40 mg/dL. Triglycerides concentrations were categorized as <150, 150 to 199, and ≥200 mg/dL. All categories were based on the definition of hypocholesterolemia/hypercholesterolemia and the current targets for statin treatment.18,23,24 We used the category with LDL-C concentration of 70 to 99 mg/dL, TC concentration of 120 to 199 mg/dL, HDL-C concentration ≥60 mg/dL, and triglycerides concentration <150 mg/dL as the reference groups. The Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of total and subtypes of ICH based on the updated cumulative average LDL-C, after adjustment for potential cofounders.
For more detailed analyses of the dose-response trends, the continuous measure of cumulative average LDL-C was used to fit a restricted cubic spline model25 and to obtain a smooth representation of the hazard ratio as a function of LDL-C concentrations with adjustment for the effects of potential confounders. We used 5 knots defined at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles to divide continuous LDL-C concentration into 5 intervals.
To explore whether the potential LDL-ICH relation was confounded by cholesterol-lowering agents (e.g., statins), we conducted a sensitivity analysis by excluding the participants who used cholesterol-lowering agents. Because anticoagulants would increase the risk of ICH, we excluded participants with atrial fibrillation or flutter, deep venous thrombosis, pulmonary infarction, or heart valve disease, which are major indications for use of anticoagulants. These participants were referred to as anticoagulant users in this report. Because incident cancer, myocardial infarction, incident ischemic stroke, or high-grade inflammation might be associated with low LDL-C and higher risk of ICH, we excluded participants who developed these conditions during follow-up. We also excluded individuals with hypertension and uncontrolled hypertension (i.e., treated hypertension with systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg) during the follow-up. Because the altered LDL-C concentration may be the consequence of impending ICH (i.e., reverse causality), we conducted a 2-year lag analysis by excluding those with ICH onset during the first 2 years of follow-up.
Likelihood ratio tests were conducted to examine statistical interactions between LDL-C concentrations and sex, age, hypertension during the follow-up (yes/no), hypertension in 2006 (yes/no), diastolic blood pressure in 2006 (<90 mm Hg/≥90 mm Hg), BMI (<28/≥28 kg/m2), and drinking status (current drinkers/noncurrent drinkers in 2006) in relation to ICH risk by comparing −2 log likelihood χ2 between nested models with and without the cross-product terms.
Data availability
Deidentified data are available to researchers on request by contacting with Dr. Wu or Dr. Gao.
Results
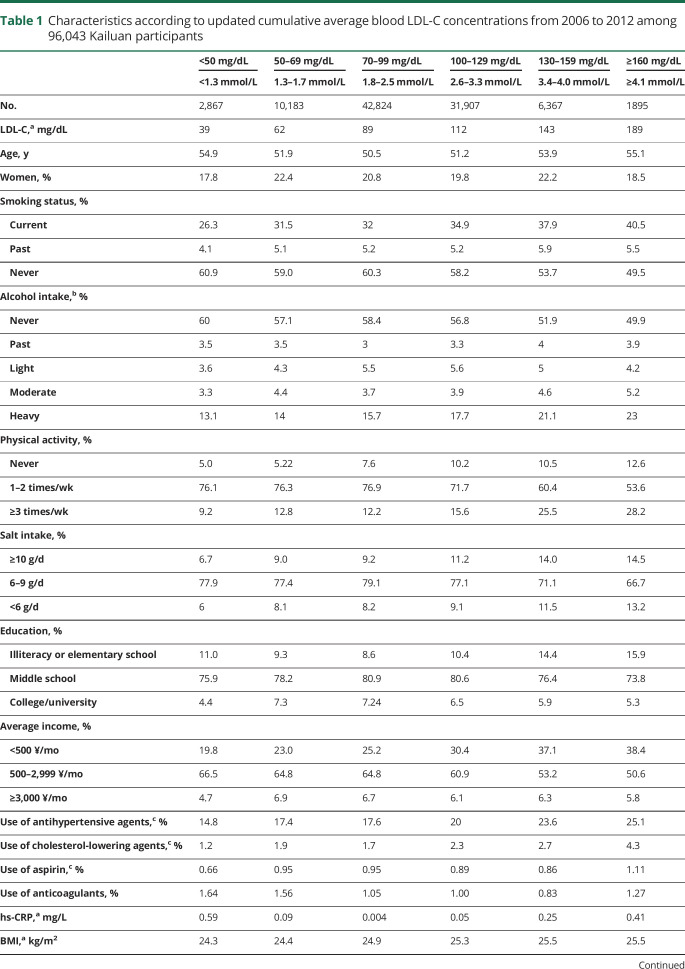
During the 9-year follow-up period (2006–2015), we identified 753 cases of incident ICH. In general, participants with higher LDL-C concentrations were more likely to smoke and report heavy alcohol intake; to engage in more physical activity; to have higher salt intakes, higher BMIs, higher TC concentrations, and lower eGFR levels; and to use antihypertensive, cholesterol-lowering agents, and aspirin (table 1).
Table 1.
Characteristics according to updated cumulative average blood LDL-C concentrations from 2006 to 2012 among 96,043 Kailuan participants
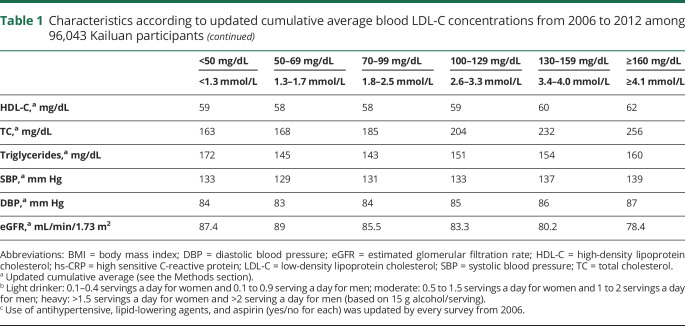
The ICH risk was similar for participants with LDL-C concentrations of 70 to 99 mg/dL and those with LDL-C concentrations >100 mg/dL. In contrast, participants with LDL-C concentrations <70 mg/dL had a significantly higher risk of developing ICH than those with LDL-C concentrations of 70 to 99 mg/dL (adjusted HR 1.65 [95% CI 1.32–2.05] for LDL-C concentrations of 50 to 69 mg/dL and 2.69 [95% CI 2.03–3.57] for LDL-C concentrations of <50 mg/dL) (table 2). In the restricted cubic spline model, we observed a cutoff point of LDL-C at 75.7 mg/dL (HR 1.24, 95% CI 1.00–1.54) at which the association between lower LDL-C and higher risk of ICH became significant (figure). Results did not change materially in the sensitivity analyses by excluding participants who used cholesterol-lowering agents or anticoagulants during the follow-up; participants who developed cancer, myocardial infarction, ischemic stroke, or high-grade inflammation (hs-CRP>10 mg/L) during the follow-up; participants with hypertension or uncontrolled hypertension during the follow-up; or participants with ICH onset during the first 2 years of follow-up. Similar patterns were observed when baseline or updated LDL-C concentration was used as the exposure, although the strength of the associations between low LDL-C concentrations and high ICH risk was attenuated (table 2).
Table 2.
Adjusted HRs (95% CIs) for risk of ICH according to blood LDL-C concentrations from 2006 to 2012 among 96,043 Kailuan participants
Figure. Hazard ratios for intracerebral hemorrhage according to updated cumulative average blood LDL cholesterol from 2006 to 2012 among 96,043 Kailuan participants.
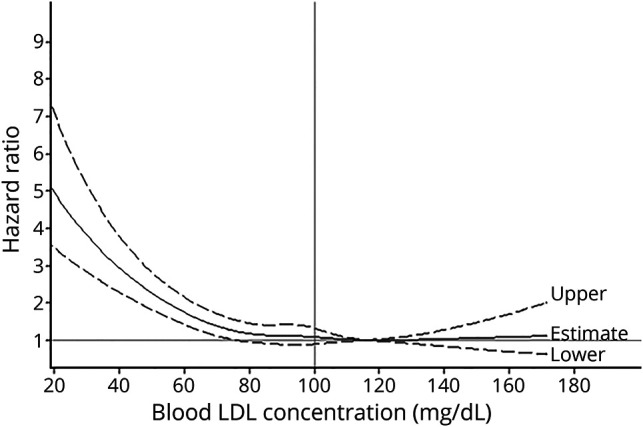
Model was adjusted for age; sex; smoking (current, past or never); alcohol intake (never, past, light, moderate, or heavy); education (illiteracy or elementary school, middle school, or college/university); physical activity (never, sometimes, or active); average monthly income of each family member (<500, 500–2,999, or ≥3,000 ¥); salt intake (≥10.0, 6.0–9.9 or <6.0 g/d); updated diabetes status (no, prediabetes, or diabetes mellitus); use of antihypertensive, lipid-lowering agents, aspirin, and anticoagulants (yes/no for each); updated cumulative average body mass index (≥30.0, 25.0–29.9, or <25.0 kg/m2); triglycerides (<150, 150–199, 200–240, or ≥240 mg/dL), high-density lipoprotein cholesterol (≥60, 40–59, or <40 mg/L), and high-sensitivity C-reactive protein (<1.00, 1.00–2.99, or ≥3.00 mg/mL) concentrations; alanine aminotransferase (for men <47 or ≥47 U/L; for women <36 or ≥36 U/L); systolic blood pressure (quintile); diastolic blood pressure (quintile); and estimated glomerular filtration rate (quintile). Data were fitted by a restricted cubic spline Cox proportional hazards model. The 95% confidence intervals are indicated by the dashed lines. LDL = low-density lipoprotein.
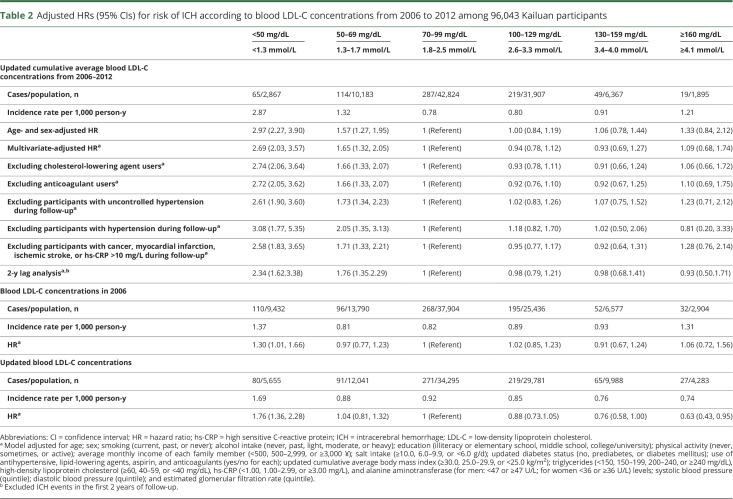
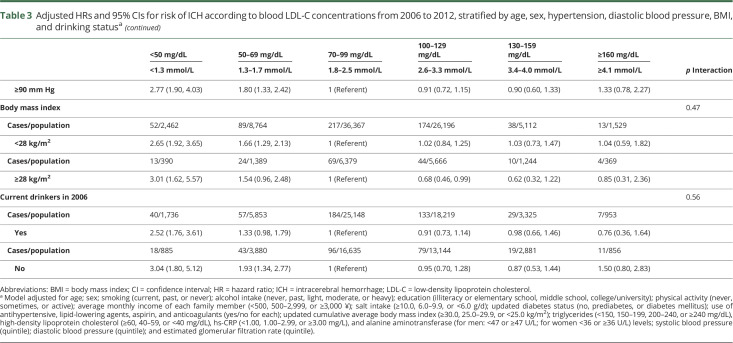
The association between LDL-C concentrations and ICH risk was not significantly modified by age, sex, hypertension status, diastolic blood pressure, BMI, and drinking status (p for interaction ≥ 0.1 for all) (table 3). When we further examined the location of ICH, the association between lower LDL-C concentration and higher risk persisted consistently for both deep and nondeep hematoma (p trend < 0.0001 for both) (table 4).
Table 3.
Adjusted HRs and 95% CIs for risk of ICH according to blood LDL-C concentrations from 2006 to 2012, stratified by age, sex, hypertension, diastolic blood pressure, BMI, and drinking statusa
Table 4.
Adjusted HRs and 95% CIs for risk of deep and nondeep ICH according to updated cumulative average blood LDL-C concentrations from 2006 to 2012 among 96,043 Kailuan participants
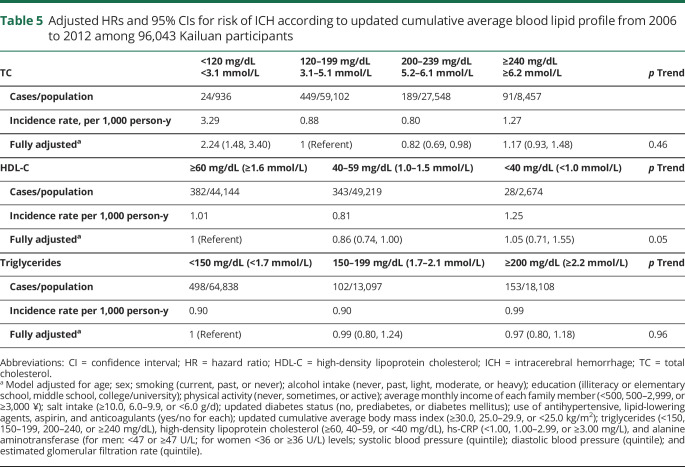
Lower (<120 mg/dL) TC concentrations were associated with higher ICH risk compared with TC concentrations of 120 to 199 mg/dL. In contrast, the associations between HDL-C, triglycerides, and ICH risk were not significant (table 5).
Table 5.
Adjusted HRs and 95% CIs for risk of ICH according to updated cumulative average blood lipid profile from 2006 to 2012 among 96,043 Kailuan participants
Discussion
In this community-based prospective study of 96,043 participants with 9 years of follow-up, individuals with average LDL-C <70 mg/dL had a higher risk of ICH relative to those with LDL-C of 70 to 99 mg/dL, independently of potential codeterminants, including age, sex, BMI, hypertension, and drinking status. Excluding participants using cholesterol-lowering agents and anticoagulants did not change the results. Our study used currently recommended cutoffs in estimating the risk18,23; thus, the findings provide potentially actionable results. In patients at higher ICH risk, the use of a less stringent LDL-C target, that is, 70 to 99 mg/dL, might be more appropriate to obtain a better balance between cardiovascular disease and ischemic and hemorrhagic risks.
Unlike our study, previous studies on this topic were based on a single-time measurement of LDL-C at baseline. Nevertheless, most of them showed a similar trend between low LDL-C concentrations and high ICH incidence or ICH mortality, although the results frequently did not reach a level of statistical significance. For example, in a pooled prospective cohort study with 135 cases of incident ICH (n = 21,680), the adjusted HR was 0.50 (95% CI 0.29–0.87) for the highest vs the lowest quartile of LDL-C.8 In another prospective cohort study, participants with LDL-C concentration ≥140 mg/dL had a 50% lower risk of ICH mortality relative to those with an LDL-C <80 mg/dL.9
Because hypercholesterolemia is directly related to increased cardiovascular morbidity and mortality,26 statins are recommended by the American Heart Association/American College of Cardiology and European Society of Cardiology/European Atherosclerosis Society for the primary and secondary prevention of atherosclerotic cardiovascular disease.18,23 A recent analysis of National Health and Nutrition Examination Survey (NHANES) data from 2005 to 2010 reported that the number of people eligible for statin therapy would rise from 43.2 million US adults (37.5%) to 56.0 million (48.6%) on the basis of the aforementioned guidelines for management of LDL-C.27 The net number of new statin prescriptions could potentially raise by 12.8 million, including 10.4 million for primary prevention.28 Clinical trials such as Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT), Reversal of Atherosclerosis With Aggressive Lipid Lowering (REVERSAL), A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID), and Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) showed that lowering LDL-C to <70 mg/dL halted or even reversed the development of atherosclerotic plaque and reduced heart attack and stroke rates.29–32 However, concerns persist that a reduction of LDL-C might entail some risk. A post hoc analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) clinical trial indicated that atorvastatin use was associated with an increased risk of ICH in patients enrolled after experiencing a hemorrhagic stroke.33 Furthermore, the latest meta-analysis of statin clinical trials showed that there was an insignificant association between statin therapy and the risk of hemorrhagic stroke (pooled relative risk 1.14 for per 1.0-mmol/L LDL-C reduction, 95% CI 0.96–1.36).34 A latest meta-analysis of nonstatin clinical trials of further lowering of LDL-C in patients starting with very low levels showed a nonsignificant positive association between LDL-C lowering and increased risk of hemorrhagic stroke, with 250 total cases (pooled relative risk 1.11, 95% CI 0.87–1.43).35 Meanwhile, a recent pooled analysis including 2 trials of anti-proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors did not find evidence for the use of PCSK9 and altered subsequent risk of ICH; those investigators did not identify any hemorrhagic stroke cases in either study.36 Similar results of insignificant association between LDL-C and hemorrhagic stroke were also observed in the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) trial (92 incident ICH cases)37 and the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial (53 incident ICH case).38 However, these currently available limited data should be interpreted with caution because of the short follow-up durations (1.0–6.0 years) and small sample size of hemorrhagic stroke incident cases. As suggested by another meta-analysis of PCSK9 trials, the potential effects of PCSK9 “remain inconclusive for rarer CVD [cardiovascular disease] and non-CVD events such as haemorrhagic stroke,”39 which could be due to lack of statistical power. In the current study with ≈96,000 participants, we observed that ICH risk was inversely associated with LDL-C concentration <70 mg/dL. This observation suggests that maintaining LDL-C concentration in a range of 70 to 100 mg/dL might be optimal for ICH prevention. A recent meta-analysis of 34 randomized clinical trials showed that more intensive LDL-C–lowering therapy was associated with a reduced total mortality only for participants with higher baseline LDL-C concentrations, not for those with baseline LDL-C levels <100 mg/dL.40
Studies supported the notion that low TC concentration was associated with increased ICH risk. For example, in a prospective study with 77 cases of ICH (n = 23,867), participants in the lowest quintile of TC (≤188 mg/dL) had a significantly higher ICH incidence rate relative to those with higher TC concentrations (p trend = 0.001).41 A similar pattern was observed in the current study. The possibility cannot be ruled out that the association between TC and ICH risk could be, at least in part, driven by LDL-C. This is suggested by an insignificant relationship between HDL-C/triglycerides and ICH risk in the current analyses.
Although the mechanisms explaining how low LDL-C could promote hemorrhagic stroke are unclear, there are some possible explanations. These include erythrocyte fragility due to low cholesterol in erythrocyte membrane,42 LDL-related platelet activation and tissue factor expression,43 and impaired coagulation function.43,44 Furthermore, low LDL-C concentrations were strongly related to a higher number of microbleeds,45 a well-known risk factor for ICH.46 Previous studies also demonstrated that the APOE ε4 genotype was associated with cerebral amyloid angiopathy–related ICH (i.e., nondeep ICH),47,48 and APOE ε4 carriers manifested higher rates of decline in LDL-C concentration over time.49 Collectively, these data suggest that low LDL-C concentration and elevated ICH risk might involve both amyloid- and non–amyloid-related mechanisms.
Our study has several strengths and limitations. First, the large number of ICH incident cases in our cohort provides great statistical power to study the LDL-C/ICH relationship, resulting from the unique study setting in the area with a relatively high frequency of ICH. According to the recent findings from the Global Burden of Disease Study, the incidence of hemorrhagic stroke in China ranked the highest globally,5 which might be due to the increased prevalence of risk factors for this stroke type (e.g., high blood pressure and alcohol intake) and an aging population. Second, the availability of repeated measurements for LDL-C concentrations reduced random errors and captured fluctuations and normal changes of LDL-C over time not possible in prior investigations of the topic. We consistently observed a stronger association between cumulative average LDL-C and ICH risk relative to baseline or updated LDL-C. Third, LDL-C concentration was determined directly with the same series of kits consistently throughout the study. Fourth, we had information on the location of hematoma for many incident ICH cases, which provided valuable clues for investigating the underlying mechanisms of the relationship between LDL-C and ICH. Fifth, we collected comprehensive information repeatedly and rigorously such as obesity, hs-CRP and alanine aminotransferase concentrations, eGFR status, and medication use, which could minimize possible residual confounding to the extent possible. Generalizability to other settings and populations (e.g., whites) might be a limitation because the current study was conducted in Tangshan, an industrial city in northern China. In addition, only 54% of patients with incident ICH had clear information on parenchymal location of hematomas. However, we still observed a significant association of LDL-C with both deep and nondeep ICH. Furthermore, the identification of potential ICH with ICD coding might induce misclassification of ICH, especially when ICH was a complication of other disease. However, reviewing medical records by a panel of 3 physicians could reduce the possibility.
In this population-based prospective study, we observed a significant association between lower LDL-C and higher ICH risk when LDL-C was <70 mg/dL, and the risk decreased to a nonsignificant level and stabilized when LDL-C was ≥70 mg/dL. Similar patterns were observed for both deep and nondeep ICH. These results could be important in determining a target of LDL-C range, especially in patients with atherosclerotic disease who might be at higher baseline ICH risk such as aging individuals, those with hypertension, and people with high alcohol drinking. Future studies with large sample sizes with categorical information on parenchymal hematoma location and conducted among other populations would be appropriate to further investigate this association.
Glossary
- ASTEROID
A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- FOURIER
Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- hs-CRP
high-sensitivity C-reactive protein
- ICD-9
International Classification of Diseases, 9th revision
- ICD-10
International Classification of Diseases, 10th revision
- ICH
intracerebral hemorrhage
- IMPROVE-IT
Improved Reduction of Outcomes: Vytorin Efficacy International Trial
- JUPITER
Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin
- LDL-C
low-density lipoprotein cholesterol
- NHANES
National Health and Nutrition Examination Survey
- PCSK9
proprotein convertase subtilisin/kexin type 9
- PROVE-IT
Pravastatin or Atorvastatin Evaluation and Infection Therapy
- REVERSAL
Reversal of Atherosclerosis With Aggressive Lipid Lowering
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Levels
- TC
total cholesterol
Appendix. Authors
Study funding
The study is supported by the start-up grant from the College of Health and Human Development and the Department of Nutritional Sciences, Penn State University, and the Institute for CyberScience Seed Grant Program, Penn State University.
Disclosure
C. Ma reports no disclosures relevant to the manuscript. M. Gurol has received research support from the NIH. Z. Huang, A. Lichtenstein, X. Wang, Y. Wang, S. Neumann, and S. Wu report no disclosures relevant to the manuscript. X. Gao has served on a committee of the Parkinson Study Group and received funding from the NIH/National Institute of Neurological Disorders and Stroke. Go to Neurology.org/N for full disclosures.
References
- 1.Feigin VL, Nguyen G, Cercy K, et al. Global Burden of Disease Lifetime Risk of Stroke Collaborators: global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med 2018;379:2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 2016;15:913–924. [DOI] [PubMed] [Google Scholar]
- 3.Krishnamurthi RV, Moran AE, Forouzanfar MH, et al. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart 2014;9:101–106. [DOI] [PubMed] [Google Scholar]
- 4.Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology 2015;45:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013;1:e259-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigin VL, Norrving B, George MG, Foltz JL, Roth GA, Mensah GA. Prevention of stroke: a strategic global imperative. Nat Rev Neurol 2016;12:501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruckdorfer KR, Demel RA, De Gier J, van Deenen LL. The effect of partial replacements of membrane cholesterol by other steroids on the osmotic fragility and glycerol permeability of erythrocytes. Biochim Biophys Acta 1969;183:334–345. [DOI] [PubMed] [Google Scholar]
- 8.Sturgeon JD, Folsom AR, Longstreth WT Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007;38:2718–2725. [DOI] [PubMed] [Google Scholar]
- 9.Noda H, Iso H, Irie F, et al. Low-density lipoprotein cholesterol concentrations and death due to intraparenchymal hemorrhage: the Ibaraki Prefectural Health Study. Circulation 2009;119:2136–2145. [DOI] [PubMed] [Google Scholar]
- 10.Wieberdink RG, Poels MM, Vernooij MW, et al. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam study. Arteriosclerosis, Thromb Vasc Biol 2011;31:2982–2989. [DOI] [PubMed] [Google Scholar]
- 11.Psaty BM, Anderson M, Kronmal RA, et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: the Cardiovascular Health Study. J Am Geriatr Soc 2004;52:1639–1647. [DOI] [PubMed] [Google Scholar]
- 12.Amarenco P, Goldstein LB, Callahan A III, et al. Baseline blood pressure, low- and high-density lipoproteins, and triglycerides and the risk of vascular events in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Atherosclerosis 2009;204:515–520. [DOI] [PubMed] [Google Scholar]
- 13.Glasser SP, Mosher A, Howard G, Banach M. What is the association of lipid levels and incident stroke? Int J Cardiol 2016;220:890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Relationships between lipoprotein components and risk of ischaemic and haemorrhagic stroke in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med 2009;265:275–287. [DOI] [PubMed] [Google Scholar]
- 15.Imamura T, Doi Y, Arima H, et al. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: the Hisayama study. Stroke 2009;40:382–388. [DOI] [PubMed] [Google Scholar]
- 16.Nakaya N, Kita T, Mabuchi H, et al. Large-scale cohort study on the relationship between serum lipid concentrations and risk of cerebrovascular disease under low-dose simvastatin in Japanese patients with hypercholesterolemia: sub-analysis of the Japan Lipid Intervention Trial (J-LIT). Circ J 2005;69:1016–1021. [DOI] [PubMed] [Google Scholar]
- 17.Stoekenbroek RM, Boekholdt SM, Luben R, et al. Heterogeneous impact of classic atherosclerotic risk factors on different arterial territories: the EPIC-Norfolk prospective population study. Eur Heart J 2016;37:880–889. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation Epub 2018 Nov 10.
- 19.Li W, Jin C, Vaidya A, et al. Blood pressure trajectories and the risk of intracerebral hemorrhage and cerebral infarction: a prospective study. Hypertension 2017;70:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma C, Pavlova M, Liu Y, Liu C, Wu S, Gao X. Probable REM sleep behavior disorder and risk of stroke: a prospective study. Neurology 2017;88:1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin C, Li G, Rexrode KM, et al. Prospective study of fasting blood glucose and intracerebral hemorrhagic risk. Stroke 2018;49:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teo BW, Xu H, Wang D, et al. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis 2011;58:56–63. [DOI] [PubMed] [Google Scholar]
- 23.rCatapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016;253:281–344. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health.. Cholesterol levels: what you need to know. NIH MedlinePlus: 2012;7(2):6–7. [Google Scholar]
- 25.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS LGTPHCURV9 Macro. Boston: Channing Laboratory; 2011. [Google Scholar]
- 26.Anderson KM, Castelli WP, Levy D. Cholesterol and mortality: 30 years of follow-up from the Framingham study. JAMA 1987;257:2176–2180. [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, Navar-Boggan AM, D'Agostino RB, Williams B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370:1422–1431. [DOI] [PubMed] [Google Scholar]
- 28.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care 2006;29:2114–2116. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol 2005;45:1644–1648. [DOI] [PubMed] [Google Scholar]
- 30.Schoenhagen P, Tuzcu EM, Apperson-Hansen C, et al. Determinants of arterial wall remodeling during lipid-lowering therapy: serial intravascular ultrasound observations from the Reversal of Atherosclerosis With Aggressive Lipid Lowering Therapy (REVERSAL) trial. Circulation 2006;113:2826–2834. [DOI] [PubMed] [Google Scholar]
- 31.Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556–1565. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 2009;373:1175–1182. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein LB, Amarenco P, Szarek M, et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels Study. Neurology 2008;70:2364–2370. [DOI] [PubMed] [Google Scholar]
- 34.Fulcher J, O'Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 35.Sabatine MS, Wiviott SD, Im K, Murphy SA, Giugliano RP. Efficacy and safety of further lowering of low-density lipoprotein cholesterol in patients starting with very low levels: a meta-analysis. JAMA Cardiol 2018;3:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milionis H, Barkas F, Ntaios G, Papavasileiou K, Michel P, Elisaf M. Proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors to treat hypercholesterolemia: effect on stroke risk. Eur J Intern Med 2016;34:54–57. [DOI] [PubMed] [Google Scholar]
- 37.Giugliano RP, Wiviott SD, Blazing MA, et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol 2017;2:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 2017;390:1962–1971. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt AF, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, Casas JP. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;4:CD011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 2018;319:1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yano K, Reed DM, MacLean CJ. Serum cholesterol and hemorrhagic stroke in the Honolulu Heart Program. Stroke 1989;20:1460–1465. [DOI] [PubMed] [Google Scholar]
- 42.Yamori Y, Nara Y, Horie R, Ooshima A. Abnormal membrane characteristics of erythrocytes in rat models and men with predisposition to stroke. Clin Exp Hypertens 1980;2:1009–1021. [DOI] [PubMed] [Google Scholar]
- 43.Rosenson RS, Lowe GD. Effects of lipids and lipoproteins on thrombosis and rheology. Atherosclerosis 1998;140:271–280. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman CJ, Lawson WE, Miller RH, Hultin MB. Correlation of vitamin K-dependent clotting factors with cholesterol and triglycerides in healthy young adults. Arterioscler Thromb 1994;14:1737–1740. [DOI] [PubMed] [Google Scholar]
- 45.Lee SH, Bae HJ, Yoon BW, Kim H, Kim DE, Roh JK. Low concentration of serum total cholesterol is associated with multifocal signal loss lesions on gradient-echo magnetic resonance imaging: analysis of risk factors for multifocal signal loss lesions. Stroke 2002;33:2845–2849. [DOI] [PubMed] [Google Scholar]
- 46.Kim DE, Bae HJ, Lee SH, Kim H, Yoon BW, Roh JK. Gradient echo magnetic resonance imaging in the prediction of hemorrhagic vs ischemic stroke: a need for the consideration of the extent of leukoariosis. Arch Neurol 2002;59:425–429. [DOI] [PubMed] [Google Scholar]
- 47.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E ε4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Ann Neurol 1995;38:254–259. [DOI] [PubMed] [Google Scholar]
- 48.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol 2010;68:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phuah CL, Raffeld MR, Ayres AM, et al. APOE polymorphisms influence longitudinal lipid trends preceding intracerebral hemorrhage. Neurol Genet 2016;2:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data are available to researchers on request by contacting with Dr. Wu or Dr. Gao.