Abstract
Objective:
To investigate the longitudinal changes of gut structural damage in chronically untreated HIV infection.
Design:
This is a 96-week prospective, single site, cohort study of antiretroviral therapy (ART)-naïve HIV-infected participants.
Methods:
Intestinal fatty acid-binding proteins (I-FABP) was used as a surrogate marker of gut structural damage. We assessed changes in I-FABP over 96 weeks and examined the associations between I-FABP, HIV variables, and inflammation. Spearman’s correlations and linear mixed effect models were used to study relationships among variables.
Results:
A total of 63 HIV-infected, ART-naïve patients were included in this analysis. At baseline, 76% were male; 62% were African American, with median age and BMI of 40 years and 27kg/m2. Median HIV-RNA and CD4+ T cell counts were 5520 copies/mL and 588 cells/mm3. I-FABP significantly increased from baseline to week 96 (mean change +333.9 pg/mL; p=0.03), and this increase was associated with viral replication (rho=+0.4; p=0.03). I-FABP levels were found to be associated with markers of inflammation: sTNFR-II (rho=0.4, p=0.02) and sVCAM-1 (rho=0.04; p<0.01) at all study time points. Lower baseline CD4+ T cells counts was found to be independently associated with I-FABP progression after adjusting for baseline characteristics variables ( p=0.02).
Conclusion:
Gut structural damage is an ongoing process in the chronic phase of untreated HIV infection, and is largely dependent on viral replication. I-FABP was found to be associated with worse immune function, increase inflammation, and viremia in chronically untreated HIV infection, supporting its role as a biomarker of intestinal barrier dysfunction.
Keywords: I-FABP, Antiretroviral-Naïve, gut integrity
Introduction
It has become increasingly clear that disruption of the gut structural integrity and subsequent microbial translocation contribute to the increased inflammation seen in HIV-infected patients on antiretroviral therapy (ART) 1,2. Despite effective viral suppression with ART, gut damage is not reversed 3,4. Much of the current research effort is looking at therapeutic strategies to restore gut structural integrity, in the hope of reducing non-AIDS related co-morbidities 1,5. The underlying mechanisms of gut structural damage are not completely understood. Once HIV infection occurs, the gut mucosa is dramatically altered in the acute phase of the infection 6; however, the dynamics and the extent to which this damage persists in the chronic stage of the disease are not fully elucidated.
Intestinal fatty acid binding protein (I-FABP) has been identified as a surrogate marker of gut epithelial destruction 7. Most of the existing literature on I-FABP has studied this marker in the setting of acute HIV infection or ART-initiation trials. Although few cross-sectional studies measured I-FABP in the setting of chronic HIV infection2,8,9, no previous data has described the longitudinal changes in I-FABP in untreated chronic infection. Furthermore, I-FABP was found to have different functions in the same type of cells10,11 and conflicting data were found on the role of I-FABP in predicting mortality in the HIV population8,12. In fact, we and others have recently shown13,14 that in HIV-naïve participants initiating treatment, I-FABP increases the first four weeks of treatment and then stabilized for 96 weeks. These findings raise questions as to whether the increase in I-FABP with ART initiation might be due to the beneficial effect of ART on the gut, resulting in epithelial cells regeneration or whether the increase in I-FABP reflects the natural progression of gut barrier dysfunction among HIV-infected individuals13.
In order to better dissect the effects of HIV and ART on gut structural integrity and elucidate mechanistic approaches to restore gut integrity, a better understanding of the dynamics of gut wall impairment in the chronic phase of untreated HIV disease is clearly needed.
The objectives of our study were therefore to assess changes in I-FABP over 96 weeks, in a cohort of chronically HIV-infected participants naïve to ART; and to study the relationships between I-FABP, HIV variables, and inflammation in the chronic stage of untreated infection.
Methods
This analysis from a 96-week prospective cohort study examines changes over time in the gut integrity marker I-FABP in ART-naïve HIV-infected adults. Eligibility criteria have been previously described 15,16. Briefly, HIV-infected participants >18 years, naïve to ART, and unlikely to require ART for at least 48 weeks based on treatment guidelines at the time (ART initiation at CD4+ T-cells count <350 cells/mm3) and without known cardiovascular diseases, diabetes mellitus, and active infectious or inflammatory conditions were eligible for inclusion. Participants were recruited from University Hospitals Cleveland Medical Center (UHCMC) Cleveland, OH, USA. Participants signed an informed consent before study entry.
Study evaluations
Fasting (>8 hours) blood samples were collected from participants at baseline, weeks 48 and 96, and plasma samples were stored at −70 °C and never thawed until analyzed. Stored plasma was used to test for high sensitivity CRP (hsCRP), fibrinogen, D-dimer, interleukin-6 (IL-6), soluble tumor necrosis factor alpha receptors (sTNFR-I and sTNFR-II), soluble vascular cell adhesion molecule-1 (VCAM-1), and soluble intercellular adhesion molecule-1 (ICAM-1) as previously described 15,16. Levels of I-FABP were measured by ELISA in the Dahms Clinical Research Unit at UHCMC.
Statistical Analysis
Cross-sectional and longitudinal analyses were performed. Non-normally distributed biomarker values were transformed into the logarithmic scale for analysis. Descriptive summaries of absolute levels and changes in I-FABP levels from baseline to weeks 48 and 96 are presented. Wilcoxon Rank-Sum tests or paired t-tests were used to assess changes in biomarkers over time. Associations between gut integrity markers and markers of inflammation were examined with Spearman’s rank-based correlations. Linear mixed effect model was used to examine the association between I-FABP changes with demographic and HIV variables.
Results
A total of 63 participants were eligible for this analysis. At baseline, 76% of participants were male; 62% were African Americans. The median age was 40 years, and BMI was 27 kg/m2; median baseline HIV-1 RNA level was 5520 copies/mL, CD4+ T cell count and nadir CD4+ T cells were 588 and 483 cells/mm3 respectively, and the known median duration of HIV infection was 3.3 years.
Out of the 63 participants included at baseline, 21 were started on ART by their primary care provider before 96-time point and compared with ART-naïve HIV-infected participants who remained until the end of the study period, participants who initiated ART before week 96 had lower baseline CD4+ T cells, and viral load without any significant differences in I-FABP levels.
Longitudinal changes in I-FABP, viremia, and CD4+ T cell count
Changes in markers of inflammation and immune activation overtime have been previously published15,16. Median levels of I-FABP were 1388 pg/mL at baseline. There was no evidence of a change in I-FABP levels over the first 48 weeks (mean increase of +11.1 pg/mL; p=0.2); however, a significant increase from baseline to week 96 was seen (mean increase of +333.9pg/mL; p=0.03). Furthermore, when limiting our analysis to the 42 participants who remained in the study, our results remain unchanged.
After adjusting or controlling for a number of important the confounding risk factors (Table 1), we estimated the longitudinal profile of I-FABP using linear mixed effects model with a random intercept. Coefficients of week 48 is not statistically significant (p=0.934), however, the coefficient of week 96 is marginally significant (p=0.056). This implies that I-FABP did not change significantly between baseline and week 48, but did change significantly between baseline and week 96. We plotted (Figure 1) the estimated longitudinal I-FABP profile over the study period. Interestingly, pattern of HIV-RNA levels change seems similar to the pattern of I-FABP changes over the study period; viral replication progressively increased over time, but this increase was only significant from baseline to week 96 (mean increase of +0.23 log10 copies RNA/mL, p<0.01). On the other hand, CD4+ T cells count significantly decreased over time (mean decrease of −86 CD4+ T cells/mm3 at 48 weeks and of −124.4 cells/mm3 at 96 weeks from baseline; both p<0.05).
Table 1:
Longitudinal analysis of I-FABP using linear mixed effects model
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Risk Factor | Coef. | SE | z | p-value | Lower | Upper |
| HIV RNA/SD* | 0.069 | 0.053 | 1.310 | 0.190 | −0.034 | 0.172 |
| CD4/SD* | −0.126 | 0.056 | −2.240 | 0.025 | −0.236 | −0.016 |
| Age | 0.015 | 0.006 | 2.610 | 0.009 | 0.004 | 0.026 |
| Sex | 0.320 | 0.133 | 2.400 | 0.016 | 0.059 | 0.581 |
| HIV duration | 0.000 | 0.000 | −0.430 | 0.667 | 0.000 | 0.000 |
| Week 48 | −0.005 | 0.059 | −0.080 | 0.934 | −0.120 | 0.111 |
| Week 96 | 0.129 | 0.068 | 1.890 | 0.058 | −0.004 | 0.262 |
Re-scaled by dividing 1-standard deviation for getting tangible and better interpretable regression coefficients.
Figure 1:
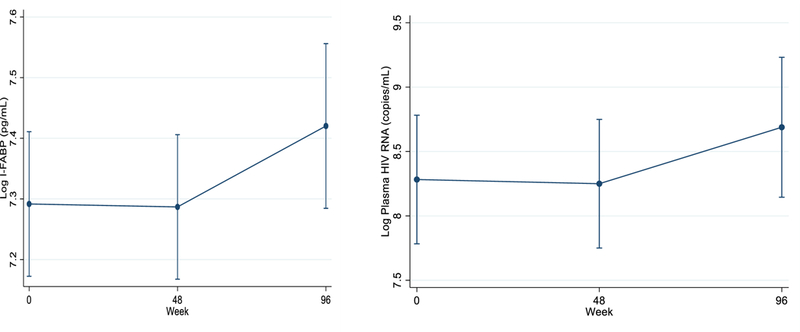
Predicted values of longitudinal changes in I-FABP and HIV-RNA over the study period
Correlations between I-FABP, demographics, viremia, CD4+T cells and inflammatory markers at different time points
At baseline, I-FABP levels significantly correlated with age of participants (rho=0.3, p=0.01), and varied across gender: levels were higher in females compared to males (median I-FABP 1735.7 vs. 1360.3 pg/mL respectively, p=0.04). I-FABP levels were not found to be associated with CD4+T cell count, viral load levels, and nadir CD4+ T cells count at early study time points (baseline and week 48). However, with disease progression, a trend-level correlation between I-FABP and CD4+ T cell (rho= −0.3, p=0.06) was observed at week 96, and I-FABP at week 96 was strongly associated with earlier CD4+T cells count from week 48 (rho=−0.7; p<0.001).
Furthermore, we found that baseline I-FABP levels were significantly associated with sTNFR-II (rho=0.4, p=0.02), and sVCAM-1 (rho=0.4, p<0.01); and these associations remained significant at weeks 48 and 96.
Effect of viremia and CD4+ T cells on I-FABP progression
In an attempt to understand the factors driving the persistent gut structural damage over time in chronically untreated infection, we first explored the association between baseline variables and changes in I-FABP levels, then assessed the correlations between viremia, CD4+ T cells decline and changes in I-FABP levels.
Again, we performed a linear mixed effect model with a random intercept to examine the association between I-FABP progression, baseline demographics and HIV factors. Participants with lower CD4+ T cell count had significantly more increase in their I-FABP levels over time, suggesting that continuous enterocyte damage depends on the initial immune function. Furthermore, enterocyte damage overtime was higher in females and older participants (results presented in Table 1).
Baseline to 96 weeks changes in I-FABP were correlated with baseline to 96 weeks changes in viral load (rho=+0.4; p=0.03) but not with CD4+ T cell count (p=0.7).
Discussion
Gut epithelial dysfunction and subsequent microbial translocation and immune activation in HIV, are the result of complex interactions between the virus and the host response. The initial gut structural damage is known to be one of the main drivers of these interactions; however, the dynamics and the extent to which this damage persists in untreated individuals are not fully elucidated. For the first time in HIV, we measured levels of I-FABP, a plasma marker of intestinal gut damage, over a period of 96 weeks of untreated HIV infection. The key finding in our results was that I-FABP levels increased over time and were associated with viral replication in the chronic phase of untreated infection.
Structural gut damage was shown to occur rapidly and progressively in the acute phase of HIV infection 6, as a result of a direct viral cytotoxic effect, mucosal CD4+T cell depletion, inflammatory, and immune-mediated destruction 9,17. Limited data are available on gut structural damage in the chronic phase of untreated infection. Previous studies have shown that enterocytes loss still occurs in the chronic stage of the disease, and is associated with increased mucosal cytokine production 1,18. Additionally, a recent cross-sectional study by Perkins et al. suggested that although the virus begins and reinforce the vicious cycle of enterocyte damage in the acute phase of the disease, intestinal destruction becomes independent of viral replication in the chronic phase of the infection 19.
The longitudinal aspect of our study allowed us to further look at the dynamics between enterocyte damage and viral replication. In contrast to what has been previously shown 19, we found that viral replication was correlated to enterocyte damage in untreated infection; underscoring the importance of following the evolution of gut damage over time rather than relying on a snapshot to characterize enterocyte damage in chronic infection.
As for the role of CD4+T cells on structural enterocyte damage; we did not find any association between I-FABP and CD4+ T cells counts at baseline (when all participants had by design, a CD4+T cells count >350cells/mm3). However, an inverse association between I-FABP and CD4+T cells was observed at later time points with disease progression. These observations are consistent with previous findings3,19,12 and suggest that CD4+ T cells are associated with increased enterocytes damage in the advanced immune-virological stages of the disease.
While I-FABP has been identified as a useful marker of enterocyte death in many intestinal diseases 20,21; its role in HIV remains unclear. In the acute phase of the infection, I-FABP levels were found to peak two weeks after HIV-RNA detection, then returned to baseline once viral load reached a set point 6,9. Furthermore, in ART-initiation trials, I-FABP levels increased within the first few weeks of treatment and remain elevated despite effective viral suppression 13,14. In our cohort, elevated levels of I-FABP were associated with viral replication, worsening immune function, and systemic markers of inflammation (sTNFR-II and sVCAM-1); supporting the hypothesis that in ART-naïve, chronically HIV-infected patients, circulating levels of I-FABP are associated with a worse gut structural damage and subsequent inflammation, and immune activation. Altogether, these findings suggest that circulating I-FABP might reflect different biological processes in different stages of HIV infection and ART status.
Our study has many advantages, most importantly it presents longitudinal results from a unique cohort of ART-naïve participants. In fact, with the current guidelines recommending the start of antiretroviral therapy regardless of CD4+ T cells count, such a cohort is challenging and almost impossible to follow over time. Several limitations should be noted. Our study is an exploratory analysis and should be used to generate hypothesis to be tested with larger cohorts. Important limitations include the lack of a control group and other measures of gut integrity and microbial translocation.
In conclusion, our study showed that enterocyte damage is a continuous process in the chronic phase of untreated HIV infection and is largely dependent on viral replication. The mechanism underlying these findings is unknown and further studies will be needed to determine whether the virus exerts a direct cytotoxic effect on intestinal gut cells, or whether the destruction occurs as a consequence of virally induced inflammation and immune activation. In the chronic phase of the infection, circulating I-FABP levels were found to be associated with worse immune function, increase inflammation and viremia.
Acknowledgments
The authors would like to thank the patients that participated in this research study.
Vanessa El Kamari helped with study concept and design, performed the statistical analysis and drafted the manuscript. Adbus Sattar helped with statistical analysis and Grace McComsey conceived the study concept and design, obtained funding, oversaw all study procedures and contributed to the manuscript.
Source of Funding
This study was funded by NIH grant DK118757 . The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
GAM has served as consultant for Gilead, Merck, and ViiV/GSK and received grant support to her institution from BMS, Roche, Merck, Astellas, and Gilead.
Footnotes
Conflicts of Interest:
VEK has no potential conflicts.
References
- 1.Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS research and therapy 2016;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelini Z, Baroncelli S, Fantauzzi A, et al. Reduced Plasma Levels of sCD14 and I-FABP in HIV-infected Patients with Mesalazine-treated Ulcerative Colitis. HIV Clin Trials 2016;17(2):49–54. [DOI] [PubMed] [Google Scholar]
- 3.Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Current opinion in HIV and AIDS 2016;11(2):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somsouk M, Estes JD, Deleage C, et al. Gut epithelial barrier and systemic inflammation during chronic HIV infection. Aids 2015;29(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamari VE, Hileman CO, Gholam PM, Kulkarni M, Funderburg N, McComsey GA. Statin Therapy Does Not Reduce Liver Fat Scores in Patients Receiving Antiretroviral Therapy for HIV Infection. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ericsen AJ, Lauck M, Mohns MS, et al. Microbial Translocation and Inflammation Occur in Hyperacute Immunodeficiency Virus Infection and Compromise Host Control of Virus Replication. PLoS pathogens 2016;12(12):e1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelsers MM, Namiot Z, Kisielewski W, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clinical biochemistry 2003;36(7):529–535. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014;210(8):1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allers K, Puyskens A, Epple HJ, et al. Distribution and Activation of CD8+ T Cells in the Duodenal Mucosa before and after HIV Seroconversion. Journal of immunology 2017;198(1):481–491. [DOI] [PubMed] [Google Scholar]
- 10.Makowski L, Hotamisligil GS. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. The Journal of nutrition 2004;134(9):2464S–2468S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottasso Arias NM, Garcia M, Bondar C, et al. Expression Pattern of Fatty Acid Binding Proteins in Celiac Disease Enteropathy. Mediators of inflammation 2015;2015:738563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases 2011;203(6):780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Kamari V, Moser C, Hileman CO, et al. Lower pretreatment gut integrity is independently associated with fat gain on antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sereti I, Krebs SJ, Phanuphak N, et al. Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2017;64(2):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hileman CO, Carman TL, Longenecker CT, et al. Rate and predictors of carotid artery intima media thickness progression in antiretroviral-naive HIV-infected and uninfected adults: a 48-week matched prospective cohort study. Antiviral therapy 2013;18(7):921–929. [DOI] [PubMed] [Google Scholar]
- 16.Hileman CO, Longenecker CT, Carman TL, McComsey GA. C-reactive protein predicts 96-week carotid intima media thickness progression in HIV-infected adults naive to antiretroviral therapy. Journal of acquired immune deficiency syndromes 2014;65(3):340–344. [DOI] [PubMed] [Google Scholar]
- 17.Mudd JC, Brenchley JM. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. The Journal of infectious diseases 2016;214 Suppl 2:S58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epple HJ, Schneider T, Troeger H, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 2009;58(2):220–227. [DOI] [PubMed] [Google Scholar]
- 19.Perkins MR, Bartha I, Timmer JK, et al. The Interplay Between Host Genetic Variation, Viral Replication, and Microbial Translocation in Untreated HIV-Infected Individuals. The Journal of infectious diseases 2015;212(4):578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funaoka H, Kanda T, Fujii H. [Intestinal fatty acid-binding protein (I-FABP) as a new biomarker for intestinal diseases]. Rinsho byori. The Japanese journal of clinical pathology 2010;58(2):162–168. [PubMed] [Google Scholar]
- 21.Wiercinska-Drapalo A, Jaroszewicz J, Siwak E, Pogorzelska J, Prokopowicz D. Intestinal fatty acid binding protein (I-FABP) as a possible biomarker of ileitis in patients with ulcerative colitis. Regulatory peptides 2008;147(1–3):25–28. [DOI] [PubMed] [Google Scholar]


