Epidemiological studies have established that hepatitis C virus (HCV) is associated with diffuse large B‐cell lymphoma (DLBCL). This article reports on patients with HCV‐positive DLBCL treated with direct‐acting antivirals either concurrently or subsequently to a curative‐intent first‐line immunochemotherapy.
Keywords: Diffuse large B‐cell lymphoma, Hepatitis C virus, Direct‐acting antivirals, R‐CHOP
Abstract
Background.
International guidelines suggest hepatitis C virus (HCV) eradication by direct‐acting antivirals (DAAs) after first‐line immunochemotherapy (I‐CT) in patients with HCV‐positive diffuse large B‐cell lymphoma (DLBCL), although limited experiences substantiate this recommendation. Moreover, only a few data concerning concurrent administration of DAAs with I‐CT have been reported.
Subjects, Materials, and Methods.
We analyzed hematological and virological outcome and survival of 47 consecutive patients with HCV‐positive DLBCL treated at 23 Italian and French centers with DAAs either concurrently (concurrent cohort [ConC]: n = 9) or subsequently (sequential cohort [SeqC]: n = 38) to first‐line I‐CT (mainly rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone [R‐CHOP]‐like).
Results.
Median age was 61 years, 89% of patients had stage III/IV, and 25% presented evidence of cirrhosis. Genotype was 1 in 56% and 2 in 34% of cases. Overall, 46 of 47 patients obtained complete response to I‐CT. All patients received appropriate DAAs according to genotype, mainly sofosbuvir‐based regimens (n = 45). Overall, 45 patients (96%) achieved sustained virological response, 8 of 9 in ConC and 37 of 38 in SeqC. DAAs were well tolerated, with only 11 patients experiencing grade 1–2 adverse events. Twenty‐three patients experienced hepatic toxicity (grade 3–4 in seven) following I‐CT in SeqC, compared to only one patient in ConC. At a median follow‐up of 2.8 years, two patients died (2‐year overall survival, 97.4%) and three progressed (2‐year progression‐free survival, 93.1%).
Conclusion.
Excellent outcome of this cohort of HCV‐positive DLBCL suggests benefit of HCV eradication by DAAs either after or during I‐CT. Moreover, concurrent DAAs and R‐CHOP administration appeared feasible, effective, and ideally preferable to deferred administration of DAAs for the prevention of hepatic toxicity.
Implications for Practice.
Hepatitis C virus (HCV)‐associated diffuse large B‐cell lymphomas (DLBCLs) represent a great therapeutic challenge, especially in terms of hepatic toxicity during immune‐chemotherapy (I‐CT) and long‐term hepatic complications. The advent of highly effective and toxicity‐free direct‐acting antivirals (DAAs) created an exciting opportunity to easily eradicate HCV shortly after or in concomitance with first‐line immunochemotherapy (usually R‐CHOP). This retrospective international study reports the real‐life use of the combination of these two therapeutic modalities either in the concurrent or sequential approach (DAAs after I‐CT) in 47 patients. The favorable reported results on long‐term outcome seem to support the eradication of HCV with DAAs in all patients with HCV‐positive DLBCL. Moreover, the results from the concurrent approach were effective and safe and displayed an advantage in preventing hepatic toxicity during I‐CT.
Introduction
In the past two decades, epidemiological studies established that hepatitis C virus (HCV) is consistently associated with diffuse large B‐cell lymphoma (DLBCL), because, at least in high‐prevalence countries like Italy, up to 10%–15% of patients with DLBCL carry HCV‐positive serology [1], [2], [3], [4], [5]. Notably, HCV‐positive DLBCLs frequently display clinical, histological, and molecular features suggesting a possible transformation from previous marginal‐zone lymphomas (MZL) [3], [6], [7], [8], a lymphoma subtype frequently associated with HCV. In contrast, a recent comprehensive analysis also suggested that de novo HCV‐positive DLBCLs carry specific gene expression profiling with respect to HCV‐negative counterpart [9].
The recent advent of interferon (IFN)‐free regimens, represented by different classes of direct‐acting antivirals (DAAs), revolutionized the treatment paradigm of HCV infection, bridging‐up viral eradication rate close to 100% across all genotypes without significant side effects [10]. Recent demonstration of 67% overall response in patients with HCV‐associated indolent lymphoma treated exclusively with DAAs seems to confirm the pathogenetic link between HCV and certain lymphoma subtypes, mainly MZL [11], and paved the way to DAA integration as well in the therapeutic scenario of HCV‐positive DLBCL, as HCV eradication has been suggested to potentially improve long‐term outcome in this setting [12]. Updated international hematology (National Comprehensive Cancer Network; NCCN) and hepatology guidelines (European Association for the Study of the Liver; EASL) recommend HCV eradication by DAAs in patients with HCV‐positive DLBCL achieving complete response (CR) after first‐line immunochemotherapy (I‐CT) [13], [14], although limited data have been reported so far [15]. Moreover, recent experiences evidenced the feasibility and the efficacy of concurrent administration of DAAs with I‐CT in HCV‐positive DLBCL [16], [17], a strategy that may potentially obviate the risk of I‐CT‐related severe hepatic toxicity, reported as high as 27% of cases in previous series [7], [18], [19].
Based on these premises, we collected a large series of consecutive cases of HCV‐positive DLBCL treated with DAAs either concurrently or subsequently to a curative‐intent first‐line I‐CT.
Subjects, Materials, and Methods
Study Design and Patients
We retrospectively selected all the consecutive adult patients affected by DLBCL (de novo or transformed) or grade 3b follicular lymphoma (g3b FL) and infected by HCV, defined as HCV‐RNA positivity, and treated with DAAs during or after first‐line curative‐intent I‐CT within 17 Italian centers of Fondazione Italiana Linfomi and 6 French centers belonging to the ANRS‐CO22 HEPATHER cohort. HIV positivity and central nervous system involvement by lymphoma were exclusion criteria. Three patients have already been described in the literature and were included with updated follow‐up [15], [16].
Patients treated with DAAs after the conclusion of first‐line I‐CT were grouped within the sequential cohort (SeqC), whereas patients who received DAAs concurrently with first‐line I‐CT (started before, in the same time, or after I‐CT initiation but maintaining an overlap in time with it) were included in the concurred cohort (ConC).
Diagnosis of DLBCL or g3b FL was performed according to the World Health Organization classification of 2008 and update of 2016 [20]. Germinal center (GC) or non‐GC subtype was determined according to Hans immunohistochemical algorithm [21], whenever available. Staging included positron emission tomography‐computed tomography evaluation according to the Lugano classification [22].
The decision to treat patients with DAAs and to adopt a concurrent or sequential approach was taken case by case by close interaction between local hepatologists and hematologists. The selection of specific DAA regimen relied on HCV genotype and DAA availability in each country at the time of treatment start and was based on EASL recommendations [13].
Approval for this study, which was based on the use of archival or cohort data, was obtained from the Institutional Review Boards of participating centers. The report was prepared in accordance with the STROBE statement. Data management and analysis were carried out in agreement with the tenets of the Declaration of Helsinki of 1964, as revised in 2000. All patients gave informed consent.
Clinical and Virological End‐Points
The primary end‐points were progression‐free survival (PFS) of DLBCL and sustained virological response (SVR) rate after DAAs. Overall survival (OS), event‐free survival (EFS), and toxicity of DAAs administered either concurrently or subsequently to I‐CT were secondary endpoints. PFS was defined as the time between beginning of treatment and disease progression or relapse or death from any cause (or last follow‐up for censored cases). EFS was defined as time between start of treatment and disease progression, failure of treatment (including end‐stage liver events and hepatocellular carcinoma; HCC), or death as a result of any cause (or last follow‐up for censored cases), whereas OS was defined as the time between DLBCL diagnosis and death from any cause (or last follow‐up for censored cases). Toxicity was classified according to definitions of Common Terminology Criteria for Adverse Event version 4.03. Response evaluation was based on the Lugano classification criteria for lymphomas of 2014 [22]. Response assessment was performed 1 month after the end I‐CT. SVR was defined as undetectable HCV viral load (<15 IU/ml) assessed 12 weeks after completion of DAA therapy.
Hepatological Evaluation and Antiviral Treatments
HCV serology was detected at the time of the initial staging of lymphoma using a second or third generation enzyme‐linked immunosorbent assay (ELISA). HCV immunoreactivity was confirmed by qualitative detection of HCV‐RNA. Serum HCV‐RNA load was determined by quantitative reverse‐transcription polymerase chain reaction (detection value of 12–1 × 108 IU/mL) and performed at DLBCL diagnosis, before the beginning of DAAs, after 4 weeks, at the time of DAAs completion, and after 12 weeks from DAA termination (to evaluate SVR). HCV genotype was determined by molecular assays (genotyping, LIPA). Before the initiation of DAAs, patients were tested for liver fibrosis by transient elastography measurement with FibroScan (EchoSens, Paris, France) and/or liver biopsy, using METAVIR scoring system (F0–F4). Cirrhosis was defined as >12.4 KPa and/or F4.
Statistical Analysis
Quantitative variables were summarized by their median and range. Categorical variables were described by counts and relative frequencies. Association between categorical variables was tested via Fisher's exact test, whereas the comparison of a quantitative variable between two groups of patients was carried out with the Wilcoxon rank‐sum test for unpaired samples. The Kaplan‐Meier product‐limit method was used to estimate survival curves, and the log‐rank test was adopted to evaluate the differences between different groups of patients. Cox regression analysis was then applied (by checking the applicability assumption) to determine the influence of the variables on the survival. The limit of significance for all analyses was defined as p ≤ .05. All statistical analysis were performed using Stata 12.1 (StataCorpLP, College Station, TX).
Results
Patients’ Characteristics
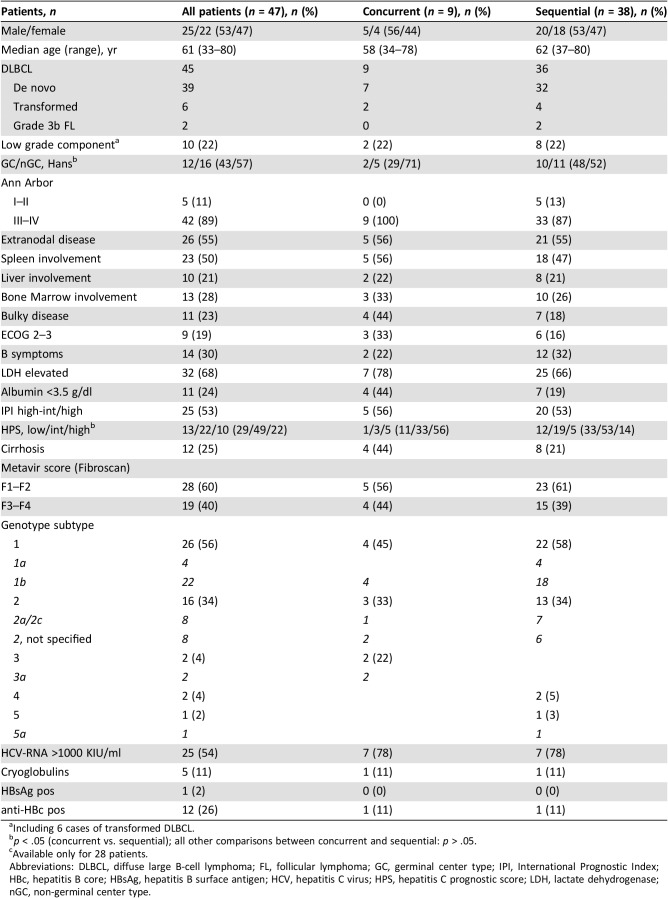
Histological, clinical, and virological features of 47 patients with HCV+ DLBCL or g3b FL treated with DAA regimens either concurrently (ConC: n = 9) or subsequently to first‐line I‐CT (SeqC: n = 38) are summarized in Table 1. Considering SeqC, 24 patients (63%) received DAAs within 2 years from completion of I‐CT (including 17 patients within 1 year), whereas the remaining 14 were treated with IFN‐free antiviral therapy (AVT) beyond 2 years, at the time of DAA approval.
Table 1. Histologic, clinical, virological, and hepatological features.
Including 6 cases of transformed DLBCL.
p < .05 (concurrent vs. sequential); all other comparisons between concurrent and sequential: p > .05.
Available only for 28 patients.
Abbreviations: DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; GC, germinal center type; IPI, International Prognostic Index; HBc, hepatitis B core; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HPS, hepatitis C prognostic score; LDH, lactate dehydrogenase; nGC, non‐germinal center type.
Overall, 45 patients had a diagnosis of DLBCL, 39 de novo and 6 transformed from a previous untreated indolent lymphoma (3 splenic MZL, 2 mucosa‐associated lymphoid tissue‐MZL, and 1 follicular lymphoma), while 2 had g3b FL. Notably, 4 out of 39 patients with de novo DLBCL (10%) had a low‐grade component coexisting with DLBCL in the diagnostic biopsy. As a result, 10 patients with DLBCL (22%) had some evidence of evolution from prior indolent lymphoma. Cell‐of‐origin according to Hans algorithm was GC in 43% and non‐GC in 57% of available cases.
Median age was 61 years (range, 33–80). The majority of patients had advanced stage (III/IV) (n = 42, 89%) as well as extranodal localizations (n = 26, 55%), including multiple extranodal sites in eight cases (17%). Splenic and hepatic nodular localizations were detected in 23 (50%) and 10 (21%) patients, respectively. International Prognostic Index (IPI) was high‐intermediate or high in 25 cases (53%).
Concerning virological characteristics, the majority of patients had HCV genotype 1 (n = 26, 56%) followed by genotype 2 (n = 16, 34%). Baseline HCV‐RNA load was >1000 KIU/ml in 25 cases (54%) whereas albumin was <3.5 g/dl in 11 cases (24%). As result, hepatitis C prognostic score for HCV‐positive DLBCL (including low albumin, Eastern Cooperative Oncology Group [ECOG] performance status ≥2, and HCV‐RNA >1000 KIU/ml) [12] was low in 29%, intermediate in 49%, and high in 22% of patients. Only one patient carried hepatitis B surface antigen, whereas 12 (26%) were anti‐HBc‐positive. Before DAA initiation, all patients were evaluated by FibroScan and/or hepatic biopsy: 19 (40%) had advanced fibrosis (F3–F4), whereas 12 (25%) had overt clinical, laboratory, and/or imaging features of cirrhosis.
Treatment
I‐CT regimens and responses are summarized in Table 2. Overall, 39 patients received rituximab, doxorubicin, cyclophosphamide, vincristine and prednisone (R‐CHOP), 6 received rituximab, cyclophosphamide, vincristine, nonpegylated liposomal doxorubicin and prednisone (R‐COMP), and 2 received rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycin and prednisone (R‐ACVBP). In the ConC, R‐CHOP was chosen in eight patients and R‐COMP in one. Anthracycline dose was reduced (median 40% with respect to standard dose) in 15 cases (32%). I‐CT was completed in 43 patients (91%), whereas 4 (all in the SeqC) discontinued prematurely planned I‐CT because of grade [G] 3–4 hematologic (n = 2) or nonhematologic toxicity (n = 2). Radiotherapy consolidation on bulky sites was applied in 7 cases (15%). Overall, 46 out of 47 patients obtained CR after I‐CT (98%), whereas progressive disease was detected in one patient who withdrawn R‐CHOP due to hematologic toxicity. Notably, all nine patients treated with DAAs concurrently with I‐CT obtained CR.
Table 2. Immunochemotherapy regimens and lymphoma responses.
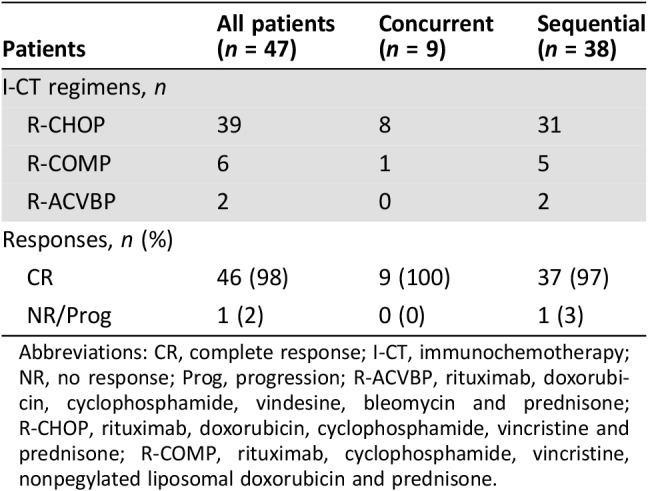
Abbreviations: CR, complete response; I‐CT, immunochemotherapy; NR, no response; Prog, progression; R‐ACVBP, rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycin and prednisone; R‐CHOP, rituximab, doxorubicin, cyclophosphamide, vincristine and prednisone; R‐COMP, rituximab, cyclophosphamide, vincristine, nonpegylated liposomal doxorubicin and prednisone.
Antiviral Treatment
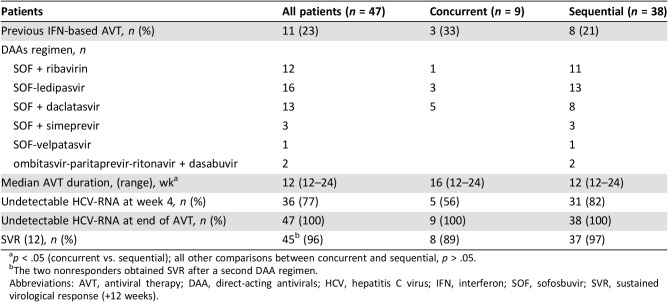
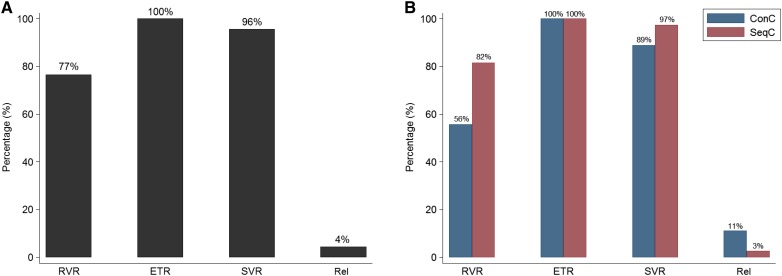
Considering HCV treatment history of the population study, 11 patients (23%) failed a previous course of IFN/ribavirin (RBV)‐based AVT (including first‐generation protease inhibitor boceprevir in two cases) before DLBCL diagnosis. Regarding antiviral regimens (Table 3), all patients received appropriate IFN‐free AVT with DAAs according to genotype: 45 were treated with sofosbuvir (SOF)‐based regimens and 2 were treated with ombitasvir‐paritaprevir‐ritonavir + dasabuvir. In ConC the regimens adopted were SOF + daclatasvir in five cases, ledipasvir (LDV)‐SOF in three cases, and SOF + RBV in one case. Median AVT duration in the whole cohort was 12 weeks (range 12–24), with a longer median duration ConC with respect to SeqC (16 vs. 12 weeks, p < .039). Among patients in ConC, one started DAAs on the same day of I‐CT, four began before (median of 2 weeks), and four began shortly after (median of 1 month). Overall, 45 patients achieved SVR (96%): 8 of 9 (89%) in ConC, and 37 of 38 (97%) in SeqC (Fig. 1A, 1B). Regarding on‐treatment virological responses, 36 patients (77%) had an undetectable viremia at week 4 and 100% at the time of DAA completion. However, HCV‐RNA was again detectable within 12 weeks from the end of treatment in two patients (virological relapsers; Fig. 1). In summary, the first virological relapser was a 77‐year‐old, treatment‐experienced (peg‐IFN + RBV) patient with genotype 1b infection and cirrhosis with stage IV, IPI 5 DLBCL, who was treated concurrently with 12 weeks of LDV‐SOF and R‐CHOP with achievement of CR and HCV‐RNA clearance, although 4 weeks after stopping therapy, HCV‐RNA was again detectable (virological relapse). He received thereafter a second DAA regimen with grazoprevir + elbasvir + SOF + RBV and finally obtained a SVR. The second virological relapser carried a genotype 2 HCV infection (treatment‐naïve, no cirrhosis); after R‐CHOP and achievement of CR, he was treated sequentially with 12 weeks of SOF‐RBV. Similarly, he received a second DAA regimen with 12 weeks of SOF + daclatasvir and also obtained a SVR. In the end, all patients obtained HCV eradication with IFN‐free AVT.
Table 3. DAA regimens and virological responses.
p < .05 (concurrent vs. sequential); all other comparisons between concurrent and sequential, p > .05.
The two nonresponders obtained SVR after a second DAA regimen.
Abbreviations: AVT, antiviral therapy; DAA, direct‐acting antivirals; HCV, hepatitis C virus; IFN, interferon; SOF, sofosbuvir; SVR, sustained virological response (+12 weeks).
Figure 1.
Virological responses. Responses of all patients (n = 47) (A) and according to ConC (n = 9) or SeqC (n = 38) treatment with direct‐acting antivirals with respect to first‐line immunochemotherapy (B).
Abbreviations: ConC, concurrent; ETR, end of treatment response (undetectable HCV‐RNA at treatment completion); Rel, relapsers; RVR, rapid virological response (undetectable HCV‐RNA at week 4); SeqC, sequential; SVR, sustained virological response.
In line with what was previously reported in patients with chronic HCV infection without lymphoma, DAAs were extremely well tolerated in the present series of patients with DLBCL. In particular, no G 3–4 adverse events (AEs) potentially related to DAAs were reported. Eleven patients (23%), five of whom received RBV, experienced 18 G 1–2 AEs: 16 in SeqC (including G1 fatigue in five cases and G2 RBV‐related anemia in three) and only 2 in a patient in the ConC (G1 fatigue and G1 nausea).
Liver Toxicity
Baseline ALT were elevated in 72% of cases, without difference between SeqC (71%) and ConC (75%). In patients treated with DAAs sequentially, namely those receiving I‐CT with active HCV‐related chronic hepatitis, 23 (60%) experienced hepatic toxicity of any grade; severe (grade 3–4 ALT increase) occurred in 7 patients (18%) and mild (grade 1–2) occurred in 16 (42%; Table 4). Conversely, in the ConC, excluding a previously described patient who started LDV‐SOF only after development of G4 ALT increase and HCV‐RNA flare during initial courses of R‐CHOP [16], only one patient (12.5%) experienced liver toxicity (G1; p = .02).
Table 4. Hepatic toxicity of immunochemotherapy.
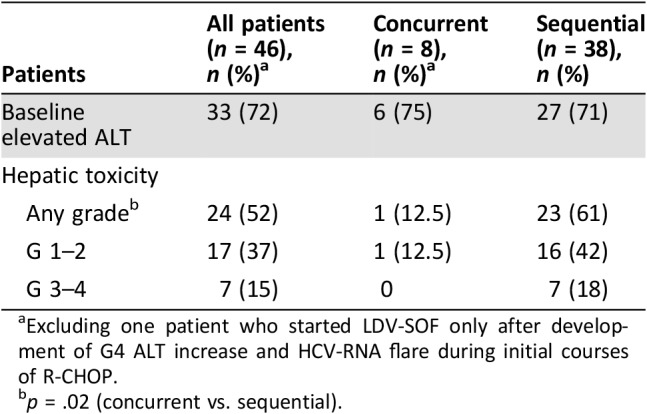
Excluding one patient who started LDV‐SOF only after development of G4 ALT increase and HCV‐RNA flare during initial courses of R‐CHOP.
p = .02 (concurrent vs. sequential).
Survival and Prognostic Factors
Median follow‐up from DLBCL diagnosis was 2.8 years in all patients (range, 1–10.1), 1.6 years in ConC (1.0–2.9), and 3.9 years in SeqC (1.0–10.1). Since DAA initiation, median observation was 1.5 years (0.4–3.6).
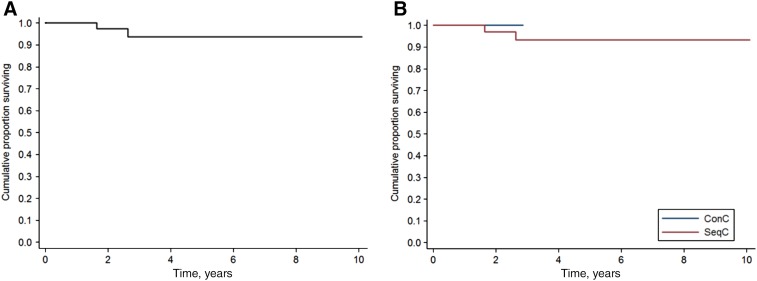
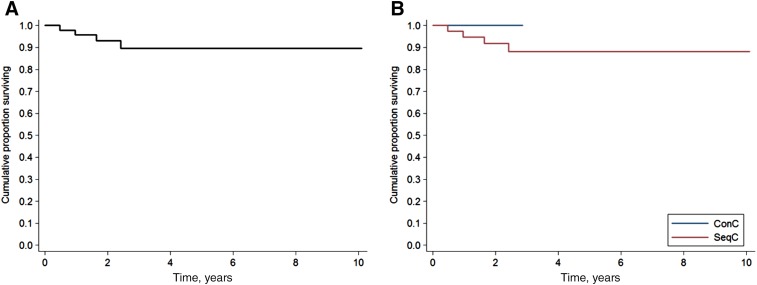
Only two patients died during the follow‐up, both in SeqC: one because of DLBCL progression and one to secondary acute myeloid leukemia while in CR for DLBCL. Two‐year OS was 97.4% in all patients (95% CI, 82.8%–99.6%; Fig. 2), 97.1% in SeqC (95% CI, 80.9%–99.6%), and 100% in ConC (Fig. 2).
Figure 2.
Overall survival. Survival of all patients (n = 47) (A) and according to ConC (n = 9) or SeqC (n = 38) treatment with direct‐acting antivirals with respect to first‐line immunochemotherapy (B).
Abbreviations: ConC, concurrent; SeqC, sequential.
Overall, three patients in SeqC experienced DLBCL progression (all after SVR). In summary, the first was a 63‐year‐old woman who was successfully salvaged with rituximab, dexamethasone, high‐dose cytarabine, cysplatin and autologous hematopoietic stem‐cell transplant (HSCT). The second was a 77‐year‐old man who underwent salvage treatment with rituximab‐lenalidomide, obtaining an ongoing partial response. The last was a 54‐year‐old man with transformed splenic MZL who relapsed 1 year after R‐CHOP and died of progressive disease after failure of rituximab‐bendamustine. As a result, 2‐year PFS in all patients was 93.1% (95% CI, 80.0%–97.7%; Fig. 3A) and 91.9% in SeqC (95% CI, 76.8%–97.3%). Notably, no patient treated concurrently progressed (2‐year PFS in ConC: 100%; Fig. 3B). Among patients in SeqC, excluding patients who received DAAs beyond 2 years from I‐CT completion, 2‐year OS and PFS were 95% and 86.8%, respectively (p = .30 and p = .29 with respect to the entire SeqC).
Figure 3.
Progression‐free survival. Survival of all patients (n = 47) (A) and according to ConC (n = 9) or SeqC (n = 38) treatment with direct‐acting antivirals with respect to first‐line immunochemotherapy (B).
Abbreviations: ConC, concurrent; SeqC, sequential.
Finally, a 77‐year‐old man with cirrhosis in ConC (nonresponder to first DAAs regimen) experienced localized HCC 1 year after completion of R‐CHOP while in CR: he was successfully treated with percutaneous hepatic radio‐frequency ablation and was free from both cancers 1.5 years later. As result, in all patients, 2‐year EFS was 90.6% (95% CI, 76.7%–96.4%; supplemental online Fig. 1).
Although only a few events were registered, we performed an exploratory univariate analysis for prognostic factors. High‐intermediate or high IPI and ≥ 2 extranodal sites retained prognostic significance for PFS and EFS (p < .01). ECOG ≥2 was significantly associated with worse EFS (p = .011) and only with a trend to inferior PFS (p = .09). No other singular element of IPI or hepatitis C prognostic score (HPS) nor HPS itself demonstrated prognostic impact on PFS or EFS. As expected, patients who did not complete planned courses of I‐CT owing to toxicity demonstrated significant lower PFS and EFS (p < .001).
Discussion
In this international retrospective real‐life study, we described the largest series published so far reporting the use of new IFN‐free DAA regimens either sequentially or concurrently with curative‐intent first‐line I‐CT (mainly R‐CHOP) in patients with HCV‐positive DLBCL.
Despite the demonstration of the beneficial activity of viral eradication with IFN‐based AVT in indolent lymphomas [23], only limited attempts to integrate AVT in the treatment algorithm of HCV‐positive DLBCL have been reported in the IFN era. Whereas concurrent administration of IFN and R‐CHOP was associated with excessive hematological toxicity and did not seem to be feasible [24], a few retrospective data suggested a possible improvement in long‐term outcome (disease‐free survival [DFS] and OS) in patients successfully treated with IFN‐based AVT after I‐CT [12], [25], [26]. According to these findings, an interesting retrospective analysis evidenced a favorable impact on OS of successful IFN‐based AVT administered before the DLBCL diagnosis, possibly because of better response to first‐line I‐CT after eradication of chronic HCV infection [27].
In the last four years, the approval of the new IFN‐free AVT with DAAs revolutionized the treatment of chronic HCV infection by enabling achievement of SVR rates that reached peaks of 100% in all viral genotypes and, notably, with almost negligible toxicity [10]. Considering the high‐risk features and frailty of patients with HCV‐positive DLBCL and the detrimental short‐ and long‐term effects of I‐CT on the liver, beginning from 2014 updated international guidelines in hepatology (EASL) and hematology (NCCN) suggested to pursue the eradication of HCV through DAAs once CR was achieved with I‐CT in this setting of patients (sequential approach) [13], [14]. However, very limited clinical data, in the form of case reports [15], [16], have been published so far substantiating this recommendation.
In addition, the absence of significant off‐target hematological and nonhematological toxicity, together with a lack of known predictable drug‐drug interactions with selected chemotherapeutic agents and monoclonal antibodies [28], [29], makes oral DAAs regimens very suitable to be administered concomitantly with I‐CT (concurrent approach). This strategy may present the advantage of potentially preventing the development of severe hepatic toxicity, reported to be as high as 27% during R‐CHOP administration [18], [19]. A recently published pilot prospective experience on 14 patients with newly diagnosed genotype 1b HCV‐positive DLBCL, treated with LDV‐SOF concurrently with R‐CHOP, confirmed the comprehensive safety and the potential additional value in terms of the efficacy of this strategy (1‐year DFS and OS of 95%) [17].
In the present study we had the opportunity to evaluate a large number of patients with HCV‐positive DLBCL or g3b FL treated with DAAs during or after I‐CT and to explore their potential influence on long term outcome. Despite the heterogeneity in the number of patients treated with the two modalities (ConC, n = 9; SeqC, n = 38), the two groups did not display significant differences in baseline characteristics (except higher HPS in ConC), which indeed recapitulated the typical features of HCV‐positive DLBCL (splenic and extranodal involvement, elevated lactate dehydrogenase), similar to previous reported series. Notably, the study included a difficult‐to‐treat population, which presented high‐risk features both in terms of DLBCL prognostic scores (53% high‐intermediate or high IPI; 71% intermediate or high HPS) as well as liver condition (F3–F4 fibrosis in 40%, overt cirrhosis in 25%). The study demonstrated that HCV eradication with appropriate approved DAA regimens is feasible and also successful in a challenging population as HCV‐positive DLBCL patients treated with I‐CT, showing an SVR rate approaching 100% in all genotypes and negligible toxicity (no grade 3–4 AEs, few manageable grade 1–2 AEs, mainly related to RBV). Moreover, although on a small number of patients, we confirmed the absence of significant toxicity issues attributable to DAAs themselves or unexpected drug‐drug interactions in patients treated concurrently with different widely adopted DAA regimens and I‐CT, confirming the findings of the recently published pilot study evaluating contemporary administration of LDV‐SOF and R‐CHOP [17]. Compared to sequential strategy, the concurrent treatment with DAAs and I‐CT exhibited the inherent advantage to be capable to prevent severe hepatic toxicity related to I‐CT‐induced HCV‐RNA flares. In the present study, excluding the previously described patient who started LDV‐SOF only after detection of severe ALT and HCV‐RNA flare, no patient treated concurrently (ConC) developed severe hepatic toxicity, compared with 18% of patients in the SeqC, who instead received I‐CT while on active HCV chronic infection. Moreover, the early achievement of SVR with concurrent approach may also favorably lactate dehydrogenase eventual salvage treatments, including HSCT, in relapsed/refractory patients.
Overall, despite high‐risk baseline features, the present series of HCV‐positive DLBCL patients receiving DAAs during or after I‐CT showed an excellent outcome, with a 2‐year OS and PFS of 97.4% and 93.1%, respectively. Only one patient developed a severe late liver‐related event (HCC, successfully treated), and no patient died due to hepatic failure. In line with our results, a recent Japanese report showed no recurrence or death at 2 years in five patients with genotype 2 HCV‐positive DLBCL treated sequentially with SOF + RBV after achievement of CR with I‐CT [30].
Concerning concurrent DAAs and I‐CT administration, none of the nine patients included in our study progressed or died at a median follow‐up of 1.6 years (2‐year OS and PFS of 100%), in line with remarkable results recently reported by Persico et al. with the same concurrent approach [17].
Taking into account cautions related to cross‐study comparison, the results of our and similar cited studies evaluating administration of DAAs during or after I‐CT seem superior to those reported by previous retrospective series of HCV‐positive DLBCL in the pre‐DAA era (OS, 58%–69% and PFS, 71%–75% at 3 years) [12], [18]. Overall, these findings consistently argue in favor of a positive impact of HCV eradication by DAAs on the long‐term outcome in HCV‐positive DLBCL. The amelioration of hepatic condition associated with HCV clearance, with reduced progression toward cirrhosis and end‐stage hepatic events, may have certainly contributed to improve OS. In a previous large retrospective study on 535 patients, we demonstrated that the prognosis of HCV‐positive DLBCL was independently affected by factors related specifically with HCV, either purely virological (HCV‐RNA viral load) or influenced in part by both HCV chronic infection and lymphoma (albumin and ECOG), which were all included in a specific prognostic score called HPS [12]. It is interesting to note that in the present series neither a HPS model nor any singular HPS element resulted in prognostic relevance, possibly because they have been overcome by HCV eradication through DAAs.
Finally, the surprisingly favorable PFS suggests that HCV eradication may eliminate a possible trigger of relapse that, at least in cases with evidence of histological transformation, may be represented by a postulated residual antigen‐dependent low‐grade clone [31].
This study has many limitations, all related to the multicenter retrospective nature of its design. First, we cannot exclude the influence of selection bias on the prognostic results, as the choice to assign a singular patient to DAAs or not, as well as to adopt a concurrent or sequential approach, was made by local clinicians at each center without homogeneous and predefined criteria. Moreover, in SeqC, there was a high variability in regimens and timing of administration of DAAs after completion of I‐CT, largely related to the approval of DAA regimens. However, also excluding patients who received DAAs beyond 2 years from I‐CT completion, PFS and OS did not significantly change. Second, we did not perform a comparison with a control group of HCV‐positive DLBCL not receiving DAAs. However, the choice of an appropriate control group in a retrospective multicenter study would have been extremely difficult, implying excessive selection bias (inclusion of cases treated in past decades or unfit to receive curative treatment) and making a reliable comparison unlikely.
Third, we did not perform any pathological review or comprehensive gene expression profiling analysis, and consequently, we cannot exclude a particularly favorable prognosis of the present series in terms of molecular features. However, similarly to what was previously reported in HCV‐positive DLBCL [9], according to Hans algorithm, the majority of available cases were non‐GC (57%), which is usually associated with worse prognosis. Moreover, although fluorescence in situ hybridization results for BCL2, BCL6, or MYC were not available for most cases, a recent comprehensive analysis demonstrated that BCL2 translocation was not present in any of the 33 HCV‐positive de novo DLBCL patients assessed, therefore excluding the presence of double‐hit BCL2/MYC lymphomas [9]. In the future, correlative studies re‐assessing the prognostic impact of NOTCH pathway mutations in the context of DAA adjunction to I‐CT would give new interesting clues about HCV‐positive DLBCL pathogenesis and prognosis.
Conclusion
The excellent outcome of this selected retrospective series of consecutive patients with HCV‐DLBCL, treated with DAAs either sequentially or concurrently with R‐CHOP, strongly supports the long‐term benefit of HCV eradication by DAAs in this setting. Our results reinforce the guidelines’ recommendations that DAAs should be advised to all patients with HCV‐positive DLBCL [13], [14], either sequentially or concurrently. At this regard we confirmed in a heterogeneous group of genotypes and with different IFN‐free AVT regimens that concurrent administration of DAAs and R‐CHOP is feasible and effective, by preventing the occurrence of hepatic toxicity [17] and possibly ameliorating the efficacy of I‐CT [27], As a consequence, the concurrent strategy may be ideally preferred to the sequential approach and should be carefully evaluated in every patient with HCV‐positive DLBCL undergoing fist‐line I‐CT.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The Varese group was supported by grants of the Fondazione Regionale Ricerca Biomedica (FRRB), Milan, Italy (FRRB project no. 2015‐0042, genomic profiling of rare hematologic malignancies, development of personalized medicine strategies, and their implementation into the Rete Ematologica Lombarda clinical network), by Fondazione Matarelli (Milano, Italy), Fondazione Rusconi (Varese, Italy) and AIL Varese ONLUS.
Author Contributions
Conception/design: Michele Merli, Luca Arcaini
Provision of study material and patients: Michele Merli, Marco Frigeni, Laurent Alric, Carlo Visco, Caroline Besson, Lara Mannelli, Alice Di Rocco, Angela Ferrari, Lucia Farina, Mario Pirisi, Francesco Piazza, Véronique Loustaud‐Ratti, Annalisa Arcari, Dario Marino, Antonello Sica, Maria Goldaniga, Chiara Rusconi, Massimo Gentile, Emanuele Cencini, Francesco Benanti, Maria Grazia Rumi, Virginia Valeria Ferretti, Paolo Grossi, Manuel Gotti, Roberta Sciarra, Maria Chiara Tisi, Isabel Cano, Valentina Zuccaro, Francesco Passamonti, Luca Arcaini
Collection and/or assembly of data: Michele Merli, Virginia Valeria Ferretti
Data analysis and interpretation: Virginia Valeria Ferretti, Michele Merli
Manuscript writing: Michele Merli, Luca Arcaini
Final approval of the manuscript: Michele Merli, Marco Frigeni, Laurent Alric, Carlo Visco, Caroline Besson, Lara Mannelli, Alice Di Rocco, Angela Ferrari, Lucia Farina, Mario Pirisi, Francesco Piazza, Véronique Loustaud‐Ratti, Annalisa Arcari, Dario Marino, Antonello Sica, Maria Goldaniga, Chiara Rusconi, Massimo Gentile, Emanuele Cencini, Francesco Benanti, Maria Grazia Rumi, Virginia Valeria Ferretti, Paolo Grossi, Manuel Gotti, Roberta Sciarra, Maria Chiara Tisi, Isabel Cano, Valentina Zuccaro, Francesco Passamonti, Luca Arcaini
Disclosures
Laurent Alric: Merck Sharp & Dohme, Abbvie, Gilead Sciences (C/A, RF, H, Other–reimbursed or sponsored travel), Bristol‐Myers Squibb, Janssen (Other–reimbursed or sponsored travel); Véronique Loustaud‐Ratti: Gilead Sciences (SAB), Abbvie, Merck Sharp & Dohme (C/A), Bristol‐Myers Squibb (RF); Paolo Grossi: Merck Sharp & Dohme (RF); Merck Sharp & Dohme, Gilead Sciences, Paratek Pharmaceuticals, Shire, Angelini, Becton Dickinson, Biotest (SAB); Luca Arcaini: Roche, Bayer, Celgene, Sandoz (C/A), Gilead Sciences (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Mele A, Pulsoni A, Bianco E et al. Hepatitis C virus and b‐cell non‐Hodgkin lymphomas: An Italian multicenter case‐control study. Blood 2003;102:996–999. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjose S, Benavente Y, Vajdic CM et al. Hepatitis C and non‐Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol 2008;6:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcaini L, Pascutto C, Passamonti F et al. Bayesian models identify specific lymphoproliferative disorders associated with hepatitis C virus infection. Int J Cancer 2009;124:2246–2249. [DOI] [PubMed] [Google Scholar]
- 4.Nieters A, Kallinowski B, Brennan P et al. Hepatitis C and risk of lymphoma: Results of the European multicenter case‐control study EPILYMPH. Gastroenterology 2006;131:1879–1886. [DOI] [PubMed] [Google Scholar]
- 5.Torres HA, Shigle TL, Hammoudi N et al. The oncologic burden of hepatitis C virus infection: A clinical perspective. CA Cancer J Clin 2017;67:411–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosry J, Miranda RN, Samaniego F et al. Clinicopathologic characteristics and outcomes of transformed diffuse large B‐cell lymphoma in hepatitis C virus‐infected patients. Int J Cancer 2018;142:940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besson C, Canioni D, Lepage E et al. Characteristics and outcome of diffuse large B‐cell lymphoma in hepatitis C virus‐positive patients in LNH 93 and LNH 98 Groupe d'Etude des Lymphomes de l'Adulte programs. J Clin Oncol 2006;24:953–960. [DOI] [PubMed] [Google Scholar]
- 8.Peveling‐Oberhag J, Arcaini L, Hansmann ML et al. Hepatitis C‐associated B‐cell non‐Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol 2013;59:169–177. [DOI] [PubMed] [Google Scholar]
- 9.Visco C, Wang J, Tisi MC et al. Hepatitis C virus positive diffuse large B‐cell lymphomas have distinct molecular features and lack BCL2 translocations. Br J Cancer 2017;117:1685–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang TJ ,Ghany MG. Therapy of hepatitis C‐‐back to the future. N Engl J Med 2014;370:2043–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arcaini L, Besson C, Frigeni M et al. Interferon‐free antiviral treatment in B‐cell lymphoproliferative disorders associated with hepatitis C virus infection. Blood 2016;128:2527–2532. [DOI] [PubMed] [Google Scholar]
- 12.Merli M, Visco C, Spina M et al. Outcome prediction of diffuse large B‐cell lymphomas associated with hepatitis C virus infection: A study on behalf of the Fondazione Italiana Linfomi. Haematologica 2014;99:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017;66:153–194. [DOI] [PubMed] [Google Scholar]
- 14.Zelenetz AD, Gordon LI, Wierda WG et al. Diffuse large B‐cell lymphoma version 1.2016. J Natl Compr Canc Netw 2016;14:196–231. [DOI] [PubMed] [Google Scholar]
- 15.Carrier P, Jaccard A, Jacques J et al. HCV‐associated B‐cell non‐hodgkin lymphomas and new direct antiviral agents. Liver Int 2015;35:2222–2227. [DOI] [PubMed] [Google Scholar]
- 16.Merli M, Frigeni M, Gotti M et al. Direct‐acting antivirals during or after immunochemotherapy in hepatitis C virus‐positive diffuse large B‐cell lymphomas. Hepatology 2017;66:1341–1343. [DOI] [PubMed] [Google Scholar]
- 17.Persico M, Aglitti A, Caruso R et al. Efficacy and safety of new direct antiviral agents in hepatitis C virus‐infected patients with diffuse large B‐cell non‐Hodgkin's lymphoma. Hepatology 2018;67:48–55. [DOI] [PubMed] [Google Scholar]
- 18.Ennishi D, Maeda Y, Niitsu N et al. Hepatic toxicity and prognosis in hepatitis C virus‐infected patients with diffuse large B‐cell lymphoma treated with rituximab‐containing chemotherapy regimens: A Japanese multicenter analysis. Blood 2010;116:5119–5125. [DOI] [PubMed] [Google Scholar]
- 19.Arcaini L, Merli M, Passamonti F et al. Impact of treatment‐related liver toxicity on the outcome of HCV‐positive non‐Hodgkin's lymphomas. Am J Hematol 2010;85:46–50. [DOI] [PubMed] [Google Scholar]
- 20.Swerdlow SH, Campo E, Pileri SA et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hans CP, Weisenburger DD, Greiner TC et al. Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–282. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Fisher RI, Barrington SF et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: The Lugano classification. J Clin Oncol;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arcaini L, Vallisa D, Rattotti S et al. Antiviral treatment in patients with indolent B‐cell lymphomas associated with HCV infection: A study of the Fondazione Italiana Linfomi. Ann Oncol 2014;25:1404–1410. [DOI] [PubMed] [Google Scholar]
- 24.Merli M, Rattotti S, Gotti M et al. Antiviral therapies for managing viral hepatitis in lymphoma patients. Expert Opin Pharmacother 2017;18:363–376. [DOI] [PubMed] [Google Scholar]
- 25.La Mura V, De Renzo A, Perna F et al. Antiviral therapy after complete response to chemotherapy could be efficacious in hcv‐positive non‐Hodgkin's lymphoma. J Hepatol 2008;49:557–563. [DOI] [PubMed] [Google Scholar]
- 26.Michot JM, Canioni D, Driss H et al. Antiviral therapy is associated with a better survival in patients with hepatitis c virus and b‐cell non‐Hodgkin lymphomas, ANRS HC‐13 lympho‐C study. Am J Hematol 2015;90:197–203. [DOI] [PubMed] [Google Scholar]
- 27.Hosry J, Mahale P, Turturro F et al. Antiviral therapy improves overall survival in hepatitis C virus‐infected patients who develop diffuse large B‐cell lymphoma. Int J Cancer 2016;139:2519–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Economides MP, Mahale P, Kyvernitakis A et al. Concomitant use of direct‐acting antivirals and chemotherapy in hepatitis C virus‐infected patients with cancer. Aliment Pharmacol Ther 2016;44:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyvernitakis A, Mahale P, Popat UR et al. Hepatitis C virus infection in patients undergoing hematopoietic cell transplantation in the era of direct‐acting antiviral agents. Biol Blood Marrow Transplant 2016;22:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsutsumi Y, Nakayama C, Kamada K et al. Efficacy and prognosis of antiviral therapy on hepatitis C following treatment of lymphoma in HCV‐positive diffuse large‐cell lymphoma. Ann Hematol 2017;96:2057–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Couronné L, Bachy E, Roulland S et al. From hepatitis C virus infection to B‐cell lymphoma. Ann Oncol 2018;29:92–100. [DOI] [PubMed] [Google Scholar]