Binodular hepatocellular carcinoma (HCC) are uncommon and underinvestigated. This article evaluates the clinical patterns and outcomes of patients with HCC and presents risk factors associated with recurrence and survival after curative liver resection of binodular HCC.
Keywords: Hepatocellular carcinoma, Hepatectomy, Overall survival, Recurrence‐free survival, Multicentric origin, Intrahepatic metastasis
Abstract
Background.
The long‐term prognosis after liver resection for multinodular (≥3 nodules) hepatocellular carcinoma (HCC) is generally considered to be unfavorable. However, the role of liver resection for binodular HCC is less investigated.
Subjects, Materials, and Methods.
From a multicenter database, consecutive patients who underwent curative‐intent liver resection for binodular HCC and without macrovascular invasion between 2003 and 2015 were retrospectively reviewed. Patients’ clinical variables as well as perioperative and long‐term survival outcomes were analyzed. Univariable and multivariable analyses were performed to identify the risk factors associated with overall survival (OS) and recurrence‐free survival (RFS) after curative resection.
Results.
Of 263 enrolled patients, the perioperative 30‐day mortality and morbidity rates were 1.5% and 28.5%. The 1‐, 3‐, and 5‐year OS and RFS rates were 81.5%, 52.4%, and 39.1% and 57.1%, 35.8%, and 26.6%, respectively. Multivariable Cox‐regression analyses identified preoperative alpha‐fetoprotein level >400 μg/L, tumor size with a sum of two nodules >8 cm, tumor size ratio of large/small nodule >1.5 (asymmetrical proportion), unilateral hemiliver distribution of two nodules, distance of ≤3 cm between two nodules, and microvascular invasion in any nodule as independent risk factors associated with decreased OS and RFS.
Conclusion.
Liver resection was safe and feasible in patients with binodular HCC, with acceptable perioperative and long‐term outcomes. Sum of two tumor sizes, size ratio and distribution, and distance between two nodules were independent risk factors associated with long‐term survival outcomes after surgery. These results may guide clinicians to make individualized surgical decisions and estimate long‐term prognosis for these patients.
Implications for Practice.
Liver resection was safe and feasible in patients with binodular hepatocellular carcinoma, with acceptable perioperative and long‐term outcomes. The sum of two tumor sizes, the size ratio and distribution of the two nodules, and the distance between two nodules were independent risk factors associated with long‐term overall survival and recurrence‐free survival after liver resection. The results of this study may guide clinicians to make individualized surgical decisions, estimate long‐term prognosis, and plan recurrence surveillance and adjuvant therapy for these patients.
摘要
背景。一般认为,多结节(≥3 个结节)型肝细胞癌 (HCC) 肝切除术后的长期预后不佳。然而,对于肝切除术在双结节型HCC中发挥的作用研究较少。
受试者、材料和方法。我们从一个多中心数据库中查找 2003 年至 2015 年间连续接受根治性肝切除术以治疗双结节型HCC且无大血管侵犯的患者,并进行了回顾性分析。我们对患者的临床变量及围手术期和长期生存预后进行了分析。同时,进行了单变量和多变量分析,以确定与根治性切除术后总生存期 (OS) 和无复发生存期 (RFS) 相关的风险因素。
结果。在纳入的 263 例患者中,围手术期 30 天死亡率和病损率分别为 1.5% 和 28.5%。1 年、3 年和 5 年的OS率分别为 81.5%、52.4% 和 39.1%,RFS率分别为 57.1%、35.8% 和
26.6%。在多变量 Cox 回归分析中,将术前 α‐胎蛋白水平大于 400 μg/L、两个结节肿瘤大小之和大于 8 cm、大/小结节肿瘤的大小比大于 1.5(不对称比例)、两个结节单侧半肝分布、两个结节的间距小于(含)3 cm,以及任意结节中存在微血管侵犯作为导致OS和RFS缩短的独立风险因素。
结论。肝切除术在双结节型HCC患者中安全可行,其围手术期和长期预后均可接受。两个肿瘤大小之和、大小比及分布以及两个结节之间的距离是与术后长期生存预后相关的独立风险因素。这些结果可指导临床医师针对每个患者的具体情况做出手术决策,并对这些患者的长期预后进行评估。
实践意义:肝切除术在双结节型肝细胞癌患者中安全可行,其围手术期和长期预后均可接受。两个肿瘤大小之和、大小比及分布以及两个结节之间的距离是与术后长期总生存期和无复发生存期相关的独立风险因素。本研究的结果可指导临床医师针对每个患者的具体情况做出手术决策,并对这些患者的长期预后进行评估,以及为这些患者制定复发监测和辅助治疗方案。
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most prevalent neoplasm and the third leading cause of cancer‐related mortality globally [1], [2]. Survival in patients with HCC is generally poor, with an overall 5‐year survival rate of <15%. Liver resection remains the mainstay of curative treatment for HCC, with survival rates ranging from 40% to 70% at 5 years after resection [3], [4]. The bottleneck that limits long‐term survival outcomes after liver resection of HCC is the high recurrence rate of up to 60%–70% within 5 years after liver resection. Aggressive pathological characteristics of the initial tumor, including large tumor size, incomplete tumor encapsulation, macroscopic and microscopic vascular invasion, and multinodularity, have been associated with postoperative HCC recurrence, especially early recurrence (<2 years after surgery) and worse long‐term overall survival outcomes.
Multinodular HCC may originate from intrahepatic metastasis from a single tumor or multicentric origin from several independent neoplasms [5]. Studies on clonal origin revealed that multinodular HCCs arising from multicentric origin are more likely to benefit from liver resection than those arising from intrahepatic metastasis of a single tumor [6], [7], [8]. Actually, at the time of diagnosis of multinodular HCCs, when the HCC nodules are ≥3, the chance of intrahepatic metastasis is much higher than for those of multicentric origin [9], [10]. Therefore, the long‐term prognosis after liver resection in patients with multinodular HCC of ≥3 nodules is generally unfavorable [11], [12], [13]. Our previous studies have demonstrated that HCC nodules ≥4, total tumor nodule diameter >8 cm, and a ratio of largest/smallest tumor nodule diameter >6 were independently associated with significantly worse long‐term survival outcomes after liver resection for multinodular HCC of ≥3 nodules [14], [15]. Binodular HCC (with two tumor nodules) are uncommon at first diagnosis. However, the clonal origin and the long‐term prognosis after liver resection for binodular HCC have been underinvestigated.
Based on a large multicenter database, the aims of this study are (a) to evaluate the clinical patterns as well as the perioperative and long‐term survival outcomes and (b) to elucidate independent risk factors associated with long‐term recurrence and survival after curative liver resection of binodular HCC. This study may provide useful guidance for individual surgical decision‐making, planning recurrence surveillance, and adjuvant therapy.
Subjects, Materials, and Methods
Patient Selection
A multicenter database of consecutive patients who underwent curative‐intent liver resection for binodular HCC from January 2003 to December 2015 at eight Chinese hospitals was retrospectively reviewed. The eight hospitals included the Eastern Hepatobiliary Surgery Hospital, Tongji Hospital, Ziyang First People's Hospital, Pu'er People's Hospital, Liuyang People's Hospital, Fourth Hospital of Harbin, Mengchao Hepatobiliary Hospital, and Meizhou People's Hospital. In this study, binodular HCC was defined as two HCC nodules of any size but >2 cm from each other in distance, or two HCC nodules of >2 cm in size but within 2 cm from each other in distance. The inclusion criteria for this study were patients (a) with binodular HCC confirmed by preoperative imaging and histopathological examination of the resected specimens; (b) who underwent curative liver resection, which was defined as complete resection of all macroscopic tumors with microscopically clear resection margins in the resected specimen (R0 resection); (c) with no macroscopic vascular invasion; (d) without any previous anti‐HCC treatment before resection; and (e) who had a complete record on all essential prognostic variables. The exclusion criteria were patients (a) ≤18 years of age; (b) with a single nodule or multiple nodules of HCC (≥3 nodules) by both preoperative imaging and postoperative histopathological examination; (c) with recurrent HCC; (d) with a combined HCC‐cholangiocarcinoma; (e) with macroscopic vascular invasion or who underwent palliative liver resection, that is, microscopically positive (R1 resection) or grossly positive (R2 resection) resection margins, considering that macrovascular invasion and positive margins have the highest risks of long‐term prognosis, which would weaken the exposure of the prognostic effects from those tumor characteristics from binodular HCC; (f) who had missing data on essential prognostic variables or follow‐up information; and (g) who had an inconsistent nodular number between preoperative imaging and postoperative pathological examination. This study was censored on August 31, 2018. Informed consent for the data to be used for clinical researches was obtained from all the enrolled patients. This study was approved by the Institutional Review Board and Ethics Committee of each of the eight hospitals. Informed consent was obtained from all the enrolled patients, and all analyses were performed in accordance with the ethical guidelines for clinical studies of these involved hospitals.
Clinicopathological Variables
The patients’ demographic characteristics included sex, age, diabetes mellitus, history of alcohol intake, preoperative body mass index, and American Society of Anesthesiologists score. The clinicopathological characteristics included etiologies of liver disease, cirrhosis, portal hypertension, Child‐Pugh grading and preoperative serum alpha‐fetoprotein (AFP) levels within 1 week before surgery, tumor sizes of the two nodules, distribution of the two nodules, distance between the two nodules, microvascular invasion, satellite lesions, and tumor differentiation. Portal hypertension was diagnosed when there was presence of either esophageal varices or splenomegaly with a low platelet count (≤100 × 109/L). Satellite lesions were defined as tumors of <2 cm in diameter and located <2 cm from the main tumor [16]. When two nodules were in the same hemiliver (right or left), it was defined as unilateral hemiliver in distribution; meanwhile, if the two nodules were in different hemilivers or either of the two nodules were at the junction of the right and left hemilivers, this was defined as bilateral hemiliver in distribution.
The operative variables consisted of intraoperative blood loss, intraoperative blood transfusion, extent of hepatectomy, and type of resection. Major hepatectomy involved resection of three or more Couinaud liver segments, whereas minor hepatectomy involved resection of fewer than three segments. The definition of anatomical resection was based on the Brisbane 2000 Nomenclature of Liver Anatomy and Resections [17], and nonanatomical resection indicated wedge/limited resection.
Perioperative outcomes consisted of perioperative 30‐day mortality and morbidity rates. Perioperative mortality was defined as death within 30 days of surgery or before discharge from hospital. Complications that occurred during this period were considered as perioperative morbidity and were classified according to the Clavien‐Dindo system [18]. Major morbidity was defined as Clavien‐Dindo grade III–IV, whereas minor morbidity was grade I–II.
Follow‐Up
Patients were followed up at each of the participating hospitals. The postoperative surveillance strategy for HCC recurrence consisted of a physical examination, serum AFP, and ultrasonography or contrast‐enhanced computed tomography (CT) scan or magnetic resonance imaging (MRI) of the chest and abdomen at 2‐month intervals for the first 6 months, 3‐month intervals thereafter for the next 18 months, and once every 6 months thereafter. Tumor recurrence was defined as appearance of new intra‐ or extrahepatic tumor nodule(s), with or without a rise in serum AFP level, and the intrahepatic nodules had the typical imaging features of HCC on contrast‐enhanced CT or MRI examinations. HCC recurrences were treated with re‐resection; transcatheter arterial chemoembolization (TACE); local ablation; radiation; molecular targeted drugs or supportive therapy, depending on the pattern of recurrence; liver functional reserve; and general conditions of the patients. The dates of recurrence, last follow‐up, and death during follow‐up were recorded.
Statistical Analysis
Patients’ clinical variables and perioperative outcomes were summarized using frequencies and percentages for categorical variables and mean ± SD or median (range) for continuous variables. Categorical variables were compared using the χ2 test with the Yates correction or Fisher's exact test. Continuous variables were compared using the Student's t test or Mann‐Whitney ranked U test. The cutoff values of the continuous variables were based on either those used commonly in reported studies or the largest Youden index for prognostic prediction. The endpoints of this study were overall survival (OS) and recurrence‐free survival (RFS). OS was defined as the interval between the date of operation and the patient's death or last follow‐up, and RFS was the interval between the date of operation and the date when HCC recurrence or new tumor occurrence were diagnosed, or between surgery and death or last follow‐up for those recurrence‐free patients. Kaplan‐Meier method and log‐rank test were used to estimate and compare the OS and RFS rates. Uni‐ and multivariable Cox regression analyses were used to identify any independent risk factors associated with decreased OS and RFS after liver resection. The statistical analyses were performed using the SPSS software version 25.0 (SPSS, Chicago, IL). All tests were two‐tailed, with a statistically significant p value <.05.
Results
Clinical Variables and Perioperative Outcomes
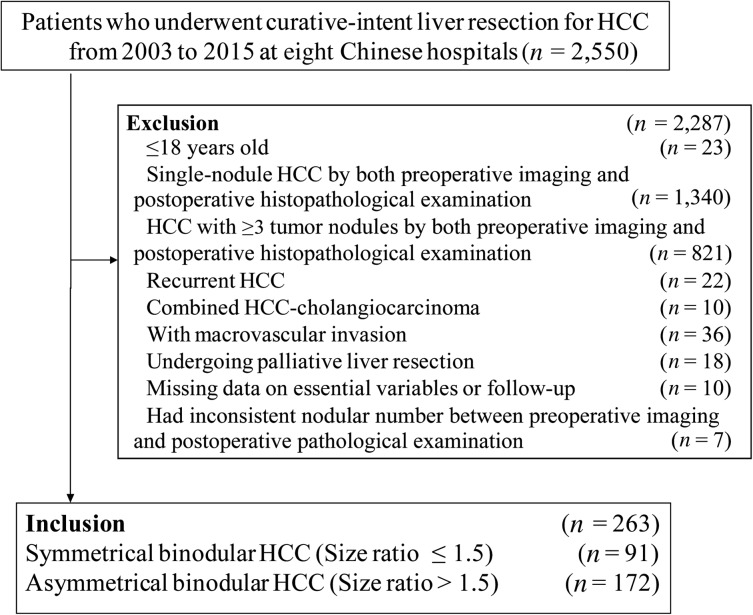
During the study period, 263 patients with binodular HCC who underwent curative liver resection were included in the multicenter database. These patients formed the analytic cohort (Fig. 1). There were 236 men (89.7%) and 27 women (10.3%). The perioperative 30‐day mortality and morbidity rates were 1.5% and 28.5%, respectively, and the minor and major morbidity rates were 21.3% and 7.2%, respectively.
Figure 1.
Selection of the study population.
Abbreviation: HCC, hepatocellular carcinoma.
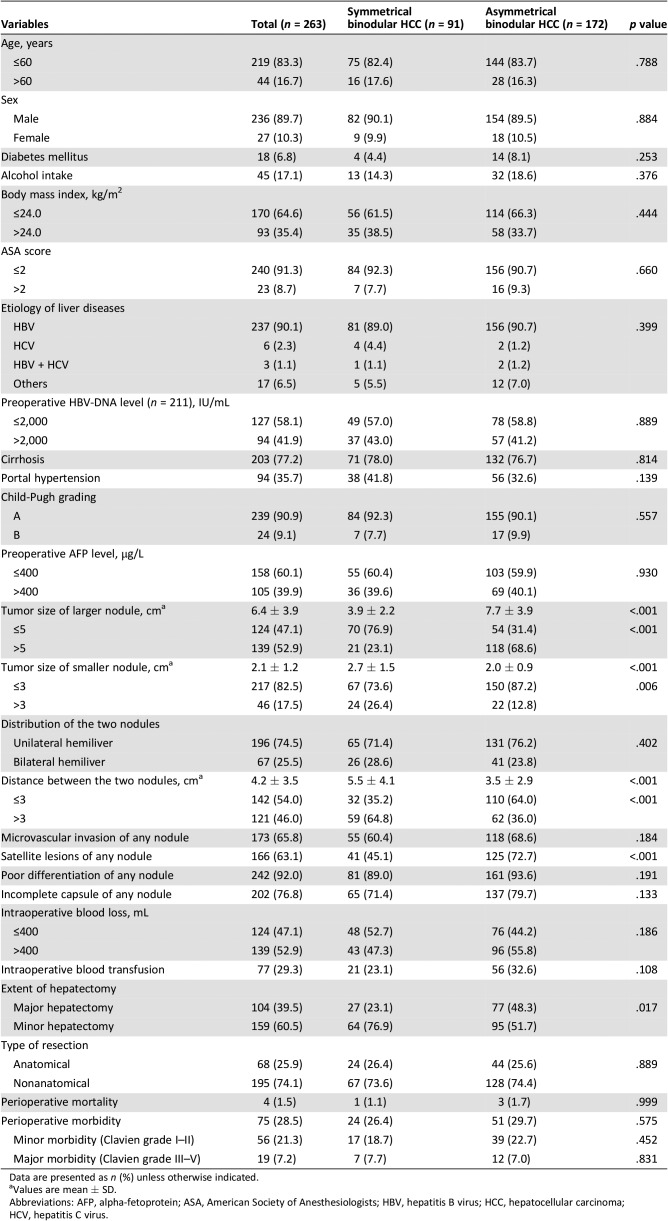
Using the size ratio of the large/small nodules, the tumors were classified as symmetrical (size ratio ≤1.5, n = 91, 34.6%) and asymmetrical (size ratio >1.5, n = 172, 65.4%) HCCs. The patients’ characteristics, operative variables, and perioperative outcomes between these two groups are shown in Table 1. When compared with the symmetrical HCCs, the asymmetrical HCCs had larger tumor sizes of both the large and small nodules, longer distance between the two nodules, a higher percentage of satellite lesions, and a higher rate of minor hepatectomy (all p < .05). There were no significant differences in the perioperative mortality and morbidity rates (for both minor or major liver resection) between patients with symmetrical and asymmetrical HCCs.
Table 1. Comparisons of clinical variables and perioperative outcomes between patients with symmetrical and asymmetrical binodular hepatocellular carcinoma according to size ratio between large/small nodule.
Data are presented as n (%) unless otherwise indicated.
Values are mean ± SD.
Abbreviations: AFP, alpha‐fetoprotein; ASA, American Society of Anesthesiologists; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
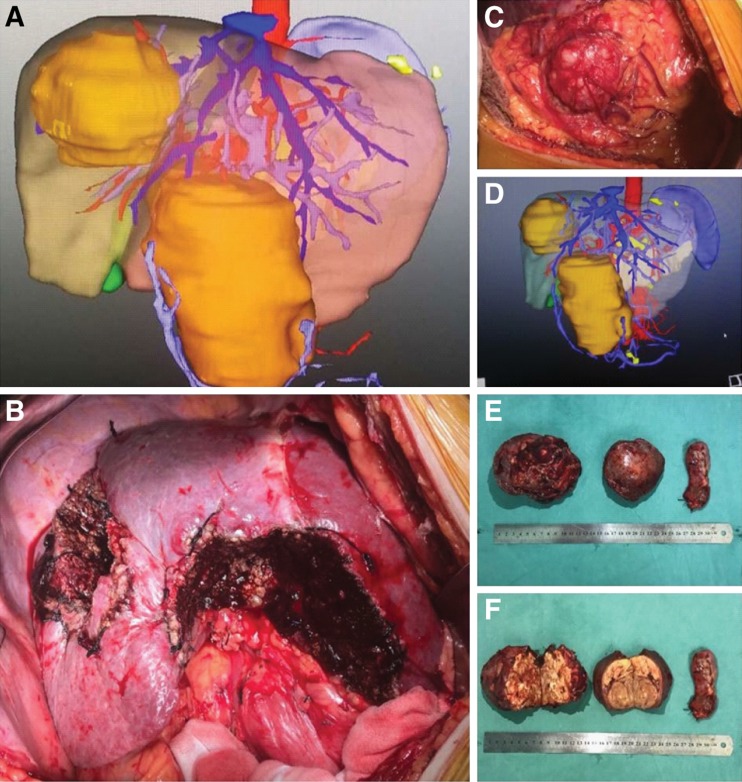
There were 196 patients in the unilateral hemiliver group (74.5%) and 67 in the bilateral hemiliver group (25.5%). Comparisons of the patients’ characteristics, operative variables, and perioperative outcomes between these two groups are summarized in supplemental online Table 1. There were no significant differences in almost all the clinical variables and perioperative outcomes between the two groups, except for the distance between the two nodules (mean ± SD: 3.2 ± 2.8 vs. 7.0 ± 3.8 cm, p < .001). A representative set of the three‐dimensional CT imaging and operative photographs of a 62‐year‐old male with symmetrical HCCs located in bilateral hemilivers are shown in Figure 2.
Figure 2.
A representative set of the three‐dimensional computed tomography (CT) imaging and operative photographs of a 62‐year‐old male with symmetrical HCCs located in bilateral hemilivers. Enhanced CT three‐dimensional imaging (A, D) of a 62‐year‐old man shows two large lesions located in Segments 7 and 8 (right liver, tumor size: 12.0 cm) and Segments 3 and 4 (left liver, tumor size: 12.5 cm), respectively. His preoperative alpha‐fetoprotein level was 825 ng/mL (normal value: ≤20 ng/mL). This patient underwent curative liver resection for symmetrical large binodular hepatocellular carcinomas (B, C, E, F) on October 27, 2015, and was alive and recurrence‐free on the last date of follow‐up on December 1, 2018.
Long‐Term Outcomes
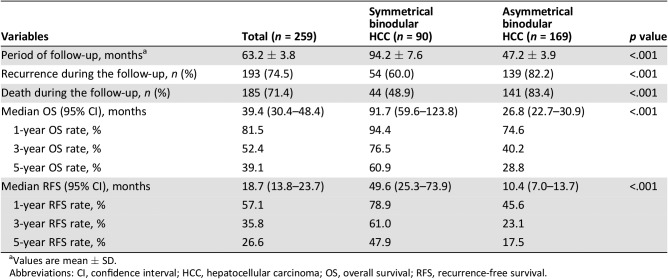
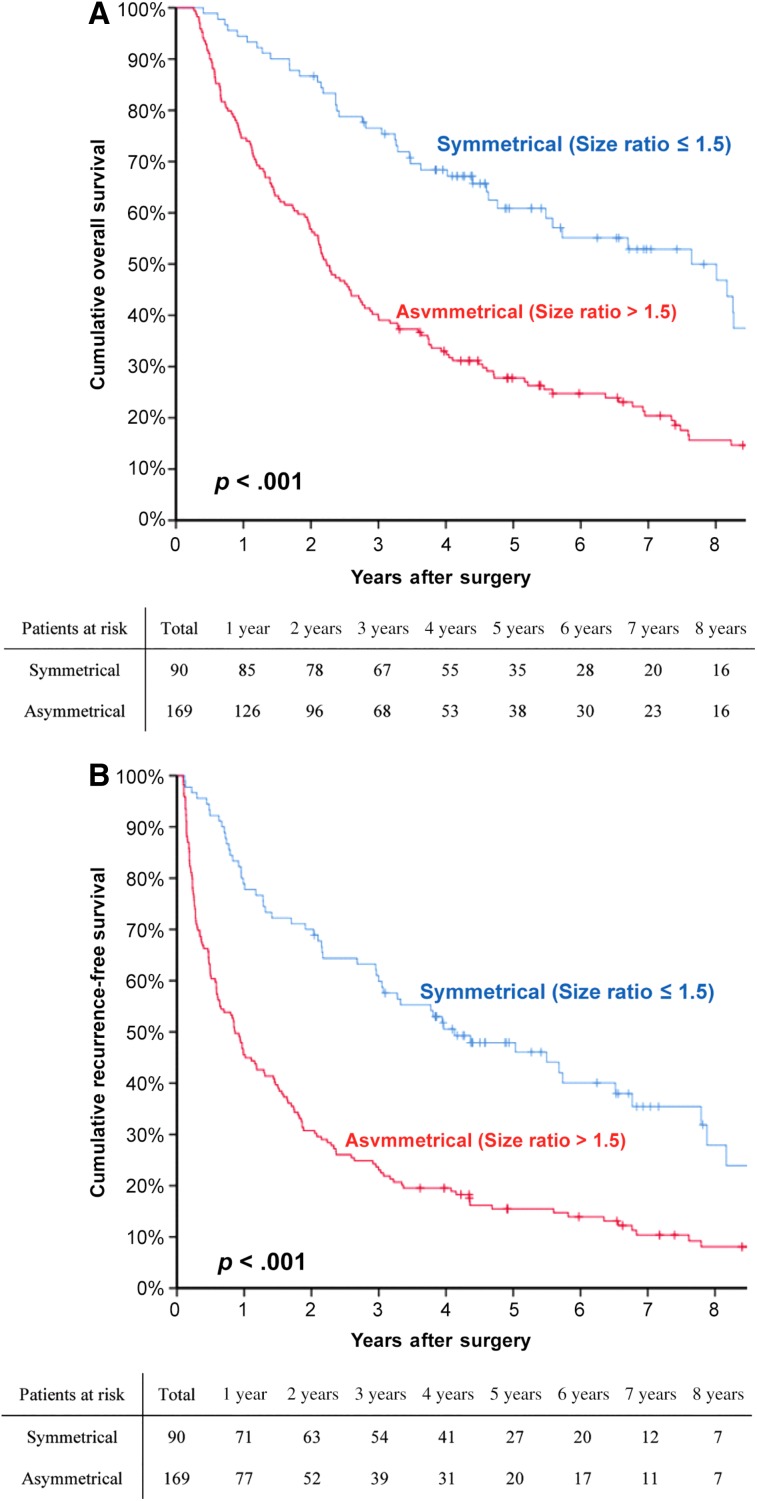
At a median follow‐up of 63.2 months, 193 patients (74.5%) had developed HCC recurrence and 185 patients (71.4%) had died. The 1‐, 3‐, and 5‐year OS rates for the entire cohort were 81.5%, 52.4%, and 39.1%, respectively. The corresponding RFS rates were 57.1%, 35.8%, and 26.6%, respectively (supplemental online Fig. 1). The long‐term outcomes after curative resection between patients with symmetrical and asymmetrical HCC are listed in Table 2. The postoperative recurrence and death rates of the asymmetrical group were significantly higher than those of the symmetrical group (82.2% vs. 60.0%, p < .001, and 83.4% vs. 48.9%, p < .001, respectively). The OS and RFS curves for the symmetrical and asymmetrical groups are depicted in Figure 3. The 5‐year OS and RFS rates of the asymmetrical group were significantly poorer than those of the symmetrical group (28.8% vs. 60.9%, p < .001, and 17.5% vs. 47.9%, p < .001, respectively).
Table 2. Comparisons of long‐term outcomes after curative resection between patients with symmetrical and asymmetrical binodular hepatocellular carcinoma according to size ratio between large/small nodule.
Values are mean ± SD.
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; OS, overall survival; RFS, recurrence‐free survival.
Figure 3.
The overall survival and recurrence‐free survival curves for the symmetrical and asymmetrical groups. Comparisons of overall survival (A) and recurrence‐free survival (B) curves between patients with symmetrical and asymmetrical binodular hepatocellular carcinoma.
The postoperative recurrence and death rates of the unilateral hemiliver group were also significantly higher than those of the bilateral hemiliver group (78.2% vs. 63.4%, p = .019, and 75.1% vs. 60.6%, p = .024, respectively; supplemental online Table 2). The unilateral hemiliver group had significantly poorer 5‐year OS and RFS rates than the bilateral hemiliver group (34.3% vs. 52.6%, p = .019, and 21.1% vs. 42.2%, p = .001, respectively; supplemental online Fig. 2).
The 5‐year OS and RFS rates in patients with a distance of ≤3 cm between the two tumor nodules were significantly worse than those in patients with a distance of >3 cm (20.1% vs. 64.2%, p < .001, and 10.0% vs. 50.3%, p = .001, respectively; supplemental online Fig. 3).
Univariable and Multivariable Analyses of OS and RFS
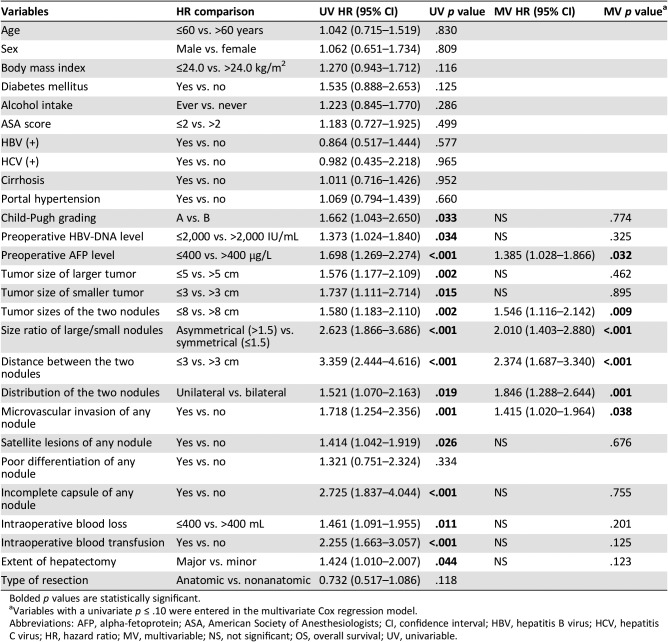
Table 3 shows the results of the univariable and multivariable analyses identifying the risk factors associated with OS after curative liver resection for binodular HCC. Multivariable Cox‐regression analyses demonstrated that preoperative AFP level >400 μg/L (hazard ratio [HR], 1.385; 95% confidence interval [CI], 1.028–1.866; p = .032), sum of tumor size of the two nodules >8.0 cm (HR, 1.546; 95% CI, 1.116–2.142; p = .009), tumor size ratio of large/small nodule >1.5 (asymmetrical proportion; HR, 2.010; 95% CI, 1.403–2.880; p < .001), distance between the two nodules ≤3 cm (HR, 2.374; 95% CI, 1.687–3.340; p < .001), unilateral hemiliver in distribution of the two nodules (HR, 1.846; 95% CI, 1.288–2.644; p = .001), and microvascular invasion of any nodule (HR, 1.415; 95% CI, 1.020–1.964; p = .038) were independent risk factors of worse OS.
Table 3. Univariable and multivariable Cox‐regression analyses of overall survival after liver resection for binodular hepatocellular carcinoma.
Bolded p values are statistically significant.
Variables with a univariate p ≤ .10 were entered in the multivariate Cox regression model.
Abbreviations: AFP, alpha‐fetoprotein; ASA, American Society of Anesthesiologists; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; MV, multivariable; NS, not significant; OS, overall survival; UV, univariable.
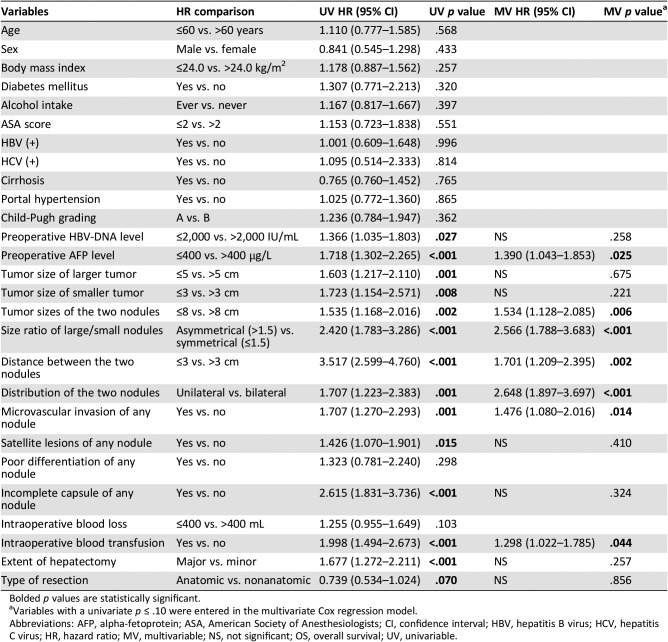
Table 4 depicts the results of the univariable and multivariable Cox‐regression analyses identifying risk factors associated with RFS after curative liver resection for binodular HCC. Multivariable Cox‐regression analyses demonstrated preoperative AFP level >400 μg/L (HR, 1.390; 95% CI, 1.043–1.853; p = .025), sum of tumor size of the two nodules >8 cm (HR, 1.534; 95% CI, 1.128–2.085; p = .006), tumor size ratio of large/small nodule >1.5 (asymmetrical proportion; HR, 2.566; 95% CI, 1.788–3.683; p < .001), distance between the two nodules ≤3 cm (HR, 1.701; 95% CI, 1.209–2.395; p = .002), unilateral hemiliver in distribution of the two nodules (HR, 2.648; 95% CI, 1.897–3.697; p < .001), microvascular invasion of any nodule (HR, 1.476; 95% CI, 1.080–2.016; p = .014), and intraoperative blood transfusion (HR, 1.298; 95% CI, 1.022–1.785; p = .044) were independent risk factors of worse RFS.
Table 4. Univariable and multivariable Cox‐regression analyses of recurrence‐free survival after liver resection for binodular hepatocellular carcinoma.
Bolded p values are statistically significant.
Variables with a univariate p ≤ .10 were entered in the multivariate Cox regression model.
Abbreviations: AFP, alpha‐fetoprotein; ASA, American Society of Anesthesiologists; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; MV, multivariable; NS, not significant; OS, overall survival; UV, univariable.
Discussion
In this multicenter, retrospective cohort study, the clinical patterns as well as perioperative and long‐term survival outcomes of 263 patients who underwent curative liver resection for binodular HCC were evaluated. The results showed that patients with binodular HCC had acceptable short‐ and long‐term outcomes with perioperative mortality and morbidity rates of 1.5% and 28.5%, respectively, and 5‐year OS and RFS rates of 39.1% and 26.6%, respectively. Multivariable Cox‐regression analyses identified preoperative AFP level >400 μg/L, sum of size of the two nodules >8 cm, asymmetrical proportion (size ratio of large/small nodule >1.5), distance between the two nodules ≤3 cm, unilateral hemiliver in distribution of the two nodules, and microvascular invasion of any nodule as the independent risk factors of decreased OS and RFS after liver resection for binodular HCC. To our knowledge, this is the first study to evaluate the long‐term postoperative prognosis and prognostic risk factors exclusively for patients with binodular HCC, which could provide useful guidance for individual surgical decision‐making, planning for recurrence surveillance, and advice on adjuvant therapy for these patients.
The Barcelona Clinic Liver Cancer (BCLC) staging classification, as recommended by the European Association for the Study of the Liver and American Association for the Study of Liver Diseases [19], is commonly used in clinical management of patients with HCC. In the BCLC staging, patients with multiple HCC nodules but without macrovascular invasion or extrahepatic spread are classified to be in intermediate stage of disease, except for patients who are within the Milan criteria (≤3 nodules that are ≤3 cm in diameter). Only TACE is recommended for these patients. Although HCC with multiple nodules is known to be an adverse prognostic factor, in selected patients, liver resection still provides the best chance of a cure [11], [20]. Whether these simultaneously occurring tumor nodules are the results of intrahepatic metastases from a single initial tumor (“the mother‐child type”) or multicentric in origin from several independent neoplasms (“the brother‐brother type”) is the key influencing postoperative prognosis after liver resection. When the nodule number is ≥3, the relationship is most likely to be “the mother‐child type,” instead of “the brother‐brother type.” The greatest difficulty in estimating the relationship is when the number of tumor nodules is two at the time of HCC diagnosis. All the current approaches including genetic mutation detection are inadequate to accurately distinguish the clonal origins of these simultaneous nodules because of the high heterogeneity in the separate tumor nodules [21], [22], [23]. In this study, the clinicopathological characteristics of the two tumor nodules were used to study the relationship of the nodules indirectly as well as to evaluate the long‐term prognosis after liver resection.
Our previous studies demonstrated that for patients with multiple HCC of ≥3 nodules, the size ratio of the large/small nodule was an independent predictor of long‐term survival after curative resection [14], [15]. The results of this study also showed that the size ratio of the large/small nodule was independently associated with OS and RFS after curative liver resection for binodular HCC. This size ratio may represent the originality of the two tumor nodules in patients. A small size ratio or a symmetrical proportion of the two nodules represented an increased probability of multicentric separate occurrence (“the brother‐brother type”), whereas a large ratio represented an asymmetrical proportion of the two nodules with a higher likelihood of origin from intrahepatic metastasis from an initial tumor (“the mother‐child type”). Thus, this variable can be helpful in selecting patients with binodular HCC to get better survival benefits from liver resection [7], [24].
Intrahepatic metastasis is a predominant characteristic that reflects on the high aggressiveness of HCC and provides an explanation for the poor long‐term survival outcomes after liver resection for patients with the “mother‐child” HCC with multiple nodules. For these patients, even after all gross tumor nodules have been eradicated by resection, micro‐metastases are likely to be left in the remaining liver, leading to early HCC recurrence [25], [26]. As intrahepatic metastases of HCC mainly spread through the portal venous system [27], [28], metastatic foci usually spread to the same liver segment, sector, or hemiliver before spreading to the contralateral hemiliver. On the other hand, binodular HCC in different hemilivers are more likely to be “the brother‐brother type” with independent clonal origins. Multivariable analyses of our study demonstrated that the OS and RFS rates in patients with bilateral hemiliver distribution of the two tumor nodules were significantly higher than those in patients with unilateral hemiliver distribution of the two tumor nodules. This is inconsistent with the previous view that multiple HCC nodules with bilateral hemiliver involvement were generally not recommended for surgery [29]. Our data demonstrated that bilateral hemiliver tumor distribution undergoing liver resection was safe and efficacious.
The distance between the two tumor nodules was another important variable to reflect the biological relationship. A short distance means that the two tumors were close to each other, and they are more likely to be “the mother‐child type,” whereas a long distance likely indicates “the brother‐brother type.” In the present study, patients with a distance of >3 cm between the two tumor nodules had significantly better OS and RFS rates than those with a tumor distance of ≤3 cm.
In short, binodular HCC with symmetrical proportion, bilateral hemiliver distribution, or a long distance between the two tumor nodules are more likely to synchronously arise from multicentric origin, which can theoretically be regarded as two single “early‐stage” HCCs. These tumors responded well to surgical resection (Fig. 2) [24], [30]. On the other hand, binodular HCC with asymmetrical proportion, unilateral hemiliver distribution, or a short distance between the two tumor nodules are likely to arise from metastasis from a single initial tumor, which is a late stage of HCC with poor response to liver resection.
In fact, in this cohort of patients with resected binodular HCC, a small number of patients still meet Milan Criteria (i.e., the sum of diameter of the two tumors does not exceed 3 cm). For these patients who are suitable for liver transplantation, whether liver transplantation could lead to a better long‐term prognosis than liver resection is an interesting topic that needs to be further discussed. Prospective observational studies and even randomized controlled trials are warranted for this issue.
The present study has several limitations. First, this is a retrospective study that has the inherent potential to introduce biases. Second, because all the patients enrolled in this study were submitted to surgical interventions with preserved liver function, and patients with macrovascular invasion were excluded, the data may not represent the entire populations of patients with binodular HCC. Third, the majority of patients had a background of hepatitis B virus infection, which is the predominant etiological factor for HCC in China [31]. The conclusions of this study may not be applied to patients coming from North America and European countries. Fourth, this multicenter study did not allow for the standardization of operative technique in liver resection. Lastly, although the overall sample size of this study was close to 300, the study was still relatively small and did not allow subgroup analyses.
Conclusion
The results of the present multicenter cohort study showed that liver resection could be safely performed in patients with binodular HCC, with acceptable perioperative and long‐term outcomes. The sum of two tumor sizes, size ratio, distribution of the two tumor nodules, and the distance between the two tumor nodules were independent risk factors of long‐term OS and RFS outcomes after liver resection. The results of this study may guide clinicians to make individualized surgical decision, estimate long‐term prognosis, and plan recurrence surveillance and adjuvant therapy for these patients.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported in part by National Natural Science Foundation of China (No. 81702334, 81472284, 81802350, and 81672699), Shanghai Pujiang Program (No. 16PJD004), and Shanghai Sailing Program (17YF1424900). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributed equally
Contributor Information
Feng Shen, Email: fengshensmmu@gmail.com.
Tian Yang, Email: yangtianehbh@smmu.edu.cn.
Author Contributions
Conception/design: Ming‐Da Wang, Chao Li, Jia‐Mei Yang, Feng Shen, Tian Yang
Provision of study material or patients: Ting‐Hao Chen, Ya‐Hao Zhou, Wei‐Min Gu, Hong Wang, Yong‐Yi Zeng, Yao‐Ming Zhang, Jia‐Mei Yang
Collection and/or assembly of data: Ming‐Da Wang, Chao Li, Jun Li, Wan‐Guang Zhang, Wei‐Qin Jiang, Jiong‐Jie Yu, Hao Xing, Han Wu, Jun Han, Zhen‐Li Li, Xin‐Fei Xu, Tian Yang
Data analysis and interpretation: Ming‐Da Wang, Chao Li, Timothy M. Pawlik, Meng‐Chao Wu, Wan Yee Lau, Tian Yang
Manuscript writing: Ming‐Da Wang, Wan Yee Lau, Feng Shen, Tian Yang
Final approval of manuscript: Wan Yee Lau, Meng‐Chao Wu, Jia‐Mei Yang, Feng Shen, Tian Yang
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel R, Ma J, Zou Z et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 2.Gerbes A, Zoulim F, Tilg H et al. Gut roundtable meeting paper: Selected recent advances in hepatocellular carcinoma. Gut 2018;67:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014;63:844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan AC, Poon RT, Ng KK et al. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg 2008;247:666–673. [DOI] [PubMed] [Google Scholar]
- 5.Chianchiano P, Pezhouh MK, Kim A et al. Distinction of intrahepatic metastasis from multicentric carcinogenesis in multifocal hepatocellular carcinoma using molecular alterations. Hum Pathol 2018;72:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano S, Haratake J, Okamoto K et al. Investigation of resected multinodular hepatocellular carcinoma: Assessment of unicentric or multicentric genesis from histological and prognostic viewpoint. Am J Gastroenterol 1994;89:189–193. [PubMed] [Google Scholar]
- 7.Yasui M, Harada A, Nonami T et al. Potentially multicentric hepatocellular carcinoma: Clinicopathologic characteristics and postoperative prognosis. World J Surg 1997;21:860–864; discussion 864–865. [DOI] [PubMed] [Google Scholar]
- 8.Cheung ST, Chen X, Guan XY et al. Identify metastasis‐associated genes in hepatocellular carcinoma through clonality delineation for multinodular tumor. Cancer Res 2002;62:4711–4721. [PubMed] [Google Scholar]
- 9.Lin YW, Lee HS, Chen CH et al. Clonality analysis of multiple hepatocellular carcinomas by loss of heterozygosity pattern determined by chromosomes 16q and 13q. J Gastroenterol Hepatol 2005;20:536–546. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda H, Oda T, Sakamoto M et al. Different pattern of chromosomal allele loss in multiple hepatocellular carcinomas as evidence of their multifocal origin. Cancer Res 1992;52:1504–1509. [PubMed] [Google Scholar]
- 11.Ng KK, Vauthey JN, Pawlik TM et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi‐institutional database. Ann Surg Oncol 2005;12:364–373. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WC, Zhang HB, Yang N et al. Preoperative predictors of short‐term survival after hepatectomy for multinodular hepatocellular carcinoma. World J Gastroenterol 2012;18:3272–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada H, Eguchi H, Noda T et al. Selection criteria for hepatic resection in intermediate‐stage (BCLC stage B) multiple hepatocellular carcinoma. Surgery 2016;160:1227–1235. [DOI] [PubMed] [Google Scholar]
- 14.Yang P, Qiu J, Li J et al. Nomograms for pre‐ and postoperative prediction of long‐term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas. Ann Surg 2016;263:778–786. [DOI] [PubMed] [Google Scholar]
- 15.Yang P, Wu D, Xia Y et al. A prognostic scoring system for patients with multiple hepatocellular carcinomas treated by hepatectomy. Ann Surg Oncol 2015;22:826–833. [DOI] [PubMed] [Google Scholar]
- 16.Yang T, Lu JH, Lau WY et al. Perioperative blood transfusion does not influence recurrence‐free and overall survivals after curative resection for hepatocellular carcinoma: A Propensity Score Matching Analysis. J Hepatol 2016;64:583–593. [DOI] [PubMed] [Google Scholar]
- 17.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333‐39. HPB (Oxford) 2002;4:99; author reply 99–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–1314. [DOI] [PubMed] [Google Scholar]
- 20.Ishizawa T, Hasegawa K, Aoki T et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008;134:1908–1916. [DOI] [PubMed] [Google Scholar]
- 21.El‐Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–2576. [DOI] [PubMed] [Google Scholar]
- 22.Llovet JM, Zucman‐Rossi J, Pikarsky E et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 23.Schulze K, Nault JC, Villanueva A. Genetic profiling of hepatocellular carcinoma using next‐generation sequencing. J Hepatol 2016;65:1031–1042. [DOI] [PubMed] [Google Scholar]
- 24.Utsunomiya T, Shimada M, Taguchi KI et al. Clinicopathologic features and postoperative prognosis of multicentric small hepatocellular carcinoma. J Am Coll Surg 2000;190:331–335. [DOI] [PubMed] [Google Scholar]
- 25.Imamura H, Matsuyama Y, Tanaka E et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200–207. [DOI] [PubMed] [Google Scholar]
- 26.Yang SL, Luo YY, Chen M et al. A systematic review and meta‐analysis comparing the prognosis of multicentric occurrence and vs. intrahepatic metastasis in patients with recurrent hepatocellular carcinoma after hepatectomy. HPB (Oxford) 2017;19:835–842. [DOI] [PubMed] [Google Scholar]
- 27.Sakon M, Nagano H, Nakamori S et al. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: Analysis based on tumor hemodynamics. Arch Surg 2002;137:94–99. [DOI] [PubMed] [Google Scholar]
- 28.Sakon M, Ogawa H, Fujita M et al. Hepatic resection for hepatocellular carcinoma based on tumor hemodynamics. Hepatol Res 2013;43:155–164. [DOI] [PubMed] [Google Scholar]
- 29.Ruzzenente A, Capra F, Pachera S et al. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg 2009;13:1313–1320. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto Y, Fujii H, Matsuda M et al. Multicentric occurrence of hepatocellular carcinoma: Diagnosis and clinical significance. J Hepatobiliary Pancreat Surg 2001;8:435–440. [DOI] [PubMed] [Google Scholar]
- 31.El‐Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–1273.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]