Summary
Background and Aims
Currently temozolomide (TMZ) as a potent agent is widely used to treat the glioblastoma multiforme (GBM), whereas recurrence due to intrinsic or acquired therapeutic resistance often occurs. Combination chemotherapy with TMZ may be a promising therapeutic strategy to improve treatment efficacy.
Methods
Aspirin, TMZ, and aspirin‐/TMZ‐coloaded poly (L‐lactide‐co‐glycolide) (PLGA) microspheres were prepared by spray drying, and cytotoxicities of glioblastoma cells were measured.
Results
Aspirin microsphere treatment induced slight apoptosis and modestly inhibited proliferation of LN229 and U87 cells in vitro and in vivo through inhibition of β‐catenin transactivation. However, aspirin‐/TMZ‐coloaded microspheres presented synergistic antitumor efficacy compared with single TMZ‐loaded microspheres. Aspirin/TMZ microspheres induced more apoptosis and repressed proliferation of LN229 and U87 cells. Corresponding to inhibition of β‐catenin signaling, β‐catenin/TCF4 transcriptional activity and STAT3 luciferase activity were strongly suppressed, and downstream targets expression was decreased. Furthermore, aspirin/TMZ microsphere intratumoral injection downregulated the expression of β‐catenin, TCF4, pAKT, pSTAT3, and PCNA and delayed tumor growth in nude mice harboring subcutaneous LN229 xenografts.
Conclusions
Aspirin sensitized TMZ chemotherapy efficacy through inhibition of β‐catenin transactivation; furthermore, the coloaded microspheres achieved a sustained release action to reduce the TMZ dosage, offering the potential for improved treatment of glioblastomas.
Keywords: Aspirin, Combination chemotherapy, Microspheres, TMZ, β‐catenin
Introduction
To date, localized dual‐drug delivery from biodegradable scaffolds is an important strategy in tissue engineering. Many polymers can be processed into the scaffold as transplant vehicles or delivery carriers for bioactive factors. Among them, poly (L‐lactide‐co‐glycolide) (PLGA) has been widely used in the format of microspheres due to its various characteristics such as biodegradability, biocompatibility, and minimal systemic toxicity as the body deals efficiently with its hydrolysis end products, lactic acid (LA) and glycolic acid (GA) 1. Also, it is easy to formulate into different devices to carry a variety of drug classes 2 and has been approved by the Food and Drug Administration (FDA) for drug delivery 3. More importantly, as a carrier of microparticles to parcel drugs, it can lengthen the sustained release time 4.
Despite major advances in the treatment of glioblastoma multiforme in recent decades by aggressive surgical resection, radiotherapy, and chemotherapy, the prognosis for patients afflicted with this disease has improved little with a median survival time <15 months 5. Temozolomide (TMZ), an alkylating agent administered orally with a favorable toxicity profile, is currently used as a chemotherapeutic agent in the treatment of GBM as it readily crosses the blood–brain barrier and induces apoptosis by damaging the DNA structure 6, 7, 8. Although TMZ is rapidly and completely absorbed after oral administration, the peak plasma concentrations occurs in 1 h, are rapidly eliminated (half‐life of 1.7–1.9 h), and exhibit linear kinetics over a narrow therapeutic dosing range 9. Additionally, tumors can develop resistance to TMZ treatment because of high expression of the DNA repair enzyme O6‐methylguanine‐DNA methyltransferase (MGMT) 10, mutations in p53 or over‐expression of the antiapoptotic protein Bcl‐2 or Bcl‐XL 11. Thus, the short serum half‐life, dose‐limiting side effects, and drug resistance remain the greatest obstacle for successful treatment of glioblastoma by TMZ. To enhance antitumor efficacy and reduce the high‐dose toxicity of TMZ, it is necessary to increase its therapeutic activity through drug combination and the achievement of sustained release time.
Aspirin is one of the oldest and prototypical nonsteroidal antiinflammatory drugs. It inhibits the cyclooxygenase (COX) enzymes COX‐1 and COX‐2, which synthesize the inflammatory mediators, prostaglandins, and thromboxanes 12. In addition, aspirin has a significant antineoplastic effect, which is viewed in the context of the recently discovered role of inflammation in cancer 13. Two recent epidemiological studies demonstrating that regular aspirin use after cancer diagnosis improves outcomes suggest that aspirin plays a role in adjuvant therapy in breast and colorectal cancer 14, 15. Furthermore, our preliminary study showed that aspirin had an antineoplastic action in glioma cells through inhibition of the β‐catenin/TCF signaling pathway 16. Thus, it is necessary to seek a new and safe route of administration to achieve the codelivery of aspirin and TMZ.
In this study, aspirin‐/TMZ‐coloaded PLGA microspheres were prepared for simultaneous delivery of both agents; the combined treatment proposed here showed superior efficacy to cocktail therapy in vitro and in vivo and offered a solution to the limitations of multidrug encapsulation into the same microparticles.
Materials and Methods
Chemicals and Regents
PLGA (LA/GA = 1:1, Mn = 8.79 × 104) was supplied by Chemical Reagent, Tianjin, China. Ethyl acetate and dichloromethane were obtained from Krs Fine Chemical Co., Tianjin, China. Aspirin and TMZ were purchased from Sigma‐Aldrich Co. (St. Louis, MO, USA). FH535 was purchased from Merck (Darmstadt, Germany).
PLGA Microsphere Preparation
Aspirin‐/TMZ‐loaded PLGA microspheres were prepared by spray drying. In brief, 350 mg PLGA, 46.4 mg aspirin, and/or 100 mg TMZ was dissolved in 25 mL ethyl acetate to prepare solutions in of a 14 mg/mL concentration containing 1.86 mg/mL of aspirin and/or 4 mg/mL TMZ (Table 1). The polymer solution was sprayed through the nozzle of a spray‐dryer (Lab Spray‐Dryer L‐117; Beijing Lathing, Beijing, China). The inlet air temperature was 43°C, whereas the outlet air temperature was set at 38°C. Microspheres were collected from the spray‐dryer cyclone and designated as PLGA–aspirin (PLGA‐A), PLGA–TMZ (PLGA‐T), and PLGA–aspirin–TMZ (PLGA‐A‐T), respectively. All microspheres were stored in a desiccator until use.
Table 1.
Composition and some characteristics of the prepared PLGA microspheres
| Microspheres | P (mg) | A (mg) | T (mg) | L.L (%) | E.E (%) | Diameter (μm) |
|---|---|---|---|---|---|---|
| PLGA‐A | 350 | 46.4 | 0 | 10.7 ± 0.84 | 89.1 ± 7.01 | 1.98 ± 0.43 |
| PLGA‐T | 350 | 0 | 100 | 19.6 ± 0.75 | 86.6 ± 3.34 | 2.24 ± 0.29 |
| PLGA‐A‐T | 350 | 46.4 | 100 | 26.0 ± 0.45 | 86.7 ± 1.51 | 2.36 ± 0.65 |
P, A, and T indicate PLGA, aspirin, and TMZ, respectively. L.L: drug loading efficiency. E.E: drug encapsulation efficiency. The molar ratio of Aspirin and TMZ is 1:2.
Characterization of Microspheres
The morphology of the microspheres was observed by SEM (model S‐2250n; Hitachi, Tokyo, Japan). The samples were mounted on a glass stub by using double‐sided tape coated with gold. The size distribution of PLGA microspheres was determined by zeta potential analyzer (Zeta PALS; Brookhaven Instruments, Holtsville, NY, USA) at 25°C in PBS buffer.
To determine aspirin and TMZ loading levels and encapsulation efficiency, a known amount of PLGA–aspirin, PLGA–TMZ, or PLGA–aspirin–TMZ was dissolved in 1 mL of DCM. The aspirin or TMZ concentration was estimated using a UV–VIS spectrophotometer (Perkin Elmer, Norwalk, CT, USA) at 297 and 329 nm. The loading level (LL) and encapsulation efficiency (EE) of aspirin or TMZ were calculated based on following formulas:
where M is the mass of the prepared PLGA–aspirin, PLGA–TMZ, or PLGA–aspirin–TMZ microspheres by spray drying, M0 is the input masses of PLGA and aspirin; or PLGA and TMZ; or PLGA, aspirin plus TMZ in the preparation process, M1 is the actual mass of aspirin, TMZ or aspirin plus TMZ encapsulated in the microspheres, and M2 is the theoretical amount of aspirin, TMZ, or aspirin plus TMZ in the microspheres.
In vitro Release of Microspheres
PLGA microspheres containing aspirin, TMZ, or aspirin/TMZ were dispersed in PBS buffer (pH 7.4) with 0.02% Tween‐80 (2% w/w of microspheres) and placed in a dialysis membrane tube with MWCO of 7000 Da in a shaking water bath at 37°C. The external phase (PBS, pH 7.4, 500 mL) was stirred continuously. At scheduled intervals of time, 500 μL samples were collected from the dialysis bag for absorbance measurement at 297 nm and 329 nm using a spectrophotometer to characterize the concentration of aspirin or TMZ, and the external phase was replaced with 5 mL distilled water to ensure the sink conditions for the drug release until 500 h.
Cell Culture and Treatment
Human glioma cell lines U87 and LN229 were utilized in this study and cultured as reported previously 11. Upon 80% confluency, cells were starved in DMEM with 1% FBS for 12 h. Then, PLGA, PLGA–aspirin, PLAG–TMZ, or PLGA–aspirin–TMZ were added to a final concentration of 0.02, 5, 10, and 10 μm, respectively, and maintained in this low serum condition for another 12 h. Finally, the treated cells were collected for protein or RNA extraction.
Quantitative Real‐time PCR Analysis
The TaqMan assay kit (Applied Biosystems, Foster City, CA, USA) was used to detect gene expression. Relative quantification of gene expression was conducted using amplification efficiencies derived from cDNA standard curves. Data were shown as fold change () and analyzed initially using Opticon Monitor Analysis Software V2.02 software (MJ Research, Waltham, MA, USA). Specific RT‐PCR primers were obtained from Fulen Gene BiolEngineering Inc., Guangdong, China.
Western Blot Analysis
The protein lysates were separated by SDS‐PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membrane was incubated with primary antibodies against β‐catenin, TCF4, pAKT, and pSTAT3 (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by incubation with an HRP‐conjugated secondary antibody (1:1000 dilution; Zhongshan Bio Corp, Beijing, China). GAPDH (1:1000 dilution; Santa Cruz Biotechnology) was set as a control.
Luciferase Reporter Assay
TOP‐FLASH, FOP‐FLASH 16, and a pSTAT3‐TA‐luc 17 plasmid were transfected into treated cells according to the manufacturer's instructions (Millipore). Following 48‐h incubation, luciferase activity was measured using a dual‐luciferase reporter system (Promega, Madison, WI, USA). Renilla luciferase activity was used as an internal control.
Cell Viability Assay
Cell viability was determined using the MTT [3‐(4, 5‐dimethylthiazol)‐2, 5‐diphenyltetrazolium bromide] assay. IC50 values were calculated from the linear regression line of the plot of percent inhibition versus log inhibitor concentration. The results of the combined treatment were analyzed according to the Zheng‐Jun Jin method 18. Briefly, this method supplied a “Q” value, according to which the combination effect between two drugs can be classified as a synergistic effect (Q > 1.15), an additive effect (0.85 < Q < 1.15), or an antagonistic effect (Q < 0.85). The formula is Q = Ea + b/(Ea + Eb – Ea × Eb), where Ea + b is the average effect of the combination treatment, Ea is the effect of the aspirin only, and Eb is the effect of TMZ only.
Cell Cycle, Apoptosis, and Colony Formation Assay
Cell cycle, apoptosis, and colony assay were performed as previous described 19. For colony formation assay, cells were seeded in 6‐well plates (0.5 × 103 cells per well) and cultured for 2 weeks. Colonies were fixed with methanol for 10 min and stained with 1% crystal violet (Sigma) for 1 min. Each group was measured in triplicate.
Animal Studies
An LN229 glioma subcutaneous model was established on BALB/c‐A nude mice at 4 weeks of age, and the mice were randomly divided into four groups (n = 6). PLGA (0.2 μm/kg), PLGA–aspirin (50 μm/kg), PLGA–TMZ (100 μm/kg), and PLGA–aspirin–TMZ (105 μm/kg) in 100 μL DMEM were intratumorally injected on day 1, and the tumor volume was measured every 2 days for 3 weeks. Tumor volume was measured using the formula: volume = length × width2/2. At the end of the experiment, tumor weight was calculated. DMSO group, TMZ group, and TMZ/FH535 group performed with U87 glioma subcutaneous model. 100 μL DMSO/DMEM (1:1), TMZ (100 μm/kg) in 100 μL DMSO/DMEM (1:1), and FH535 (40 μm/kg) plus TMZ (60 μm/kg) in 100 μL DMSO/DMEM (1:1) were intratumorally injected, respectively. All treatments conducted every 2 days for 3 weeks.
Immunohistochemistry Analysis and HE Stain
The paraffin‐embedded tissue sections were used for the examination of β‐catenin, TCF4, pAKT, pSTAT3 and PCNA expression, and HE stain. Sections were incubated with primary antibodies (1:100 dilutions) overnight at 4°C, followed by biotin‐labeled secondary antibody (1:100 dilutions) for 1 h at 37°C, and then incubated with ABC‐peroxidase and diaminobenzidine (DAB), counterstained with hematoxylin, and visualized using light microscope.
Statistical Analysis
SPSS 16.0 (SPSS, Chicago, IL, USA) was used for anova, chi‐square test, or Student's t‐test. All data are represented by mean ± SD. Statistical significance was determined at P < 0.05.
Results
Synthesis and Characterization of Drug Polymer Conjugates
Microspheres of PLGA–aspirin, PLGA–TMZ, and PLGA–aspirin–TMZ were synthesized by spray drying, with a loading efficiency (%) of 10.7 ± 0.84, 19.6 ± 0.75, and 26.0 ± 0.45 and encapsulation efficiency (%) of 89.1 ± 7.01, 86.6 ± 3.34, and 86.7 ± 1.51, respectively (Table 1). Figure 1A shows the SEM micrographs of PLGA–aspirin, PLGA–TMZ, and PLGA–aspirin–TMZ with features described as spheres with a smooth surface and average diameter (μm) of approximately 1.98 ± 0.43, 2.24 ± 0.29, and 2.36 ± 0.65 (Table 1). The release profiles of aspirin and TMZ from the microspheres are shown in Figure 1B. There was a significant burst release for the initial 20 h followed by a slow release subsequently. Approximately 100% of the encapsulated drug was released from the microspheres after 500 h.
Figure 1.
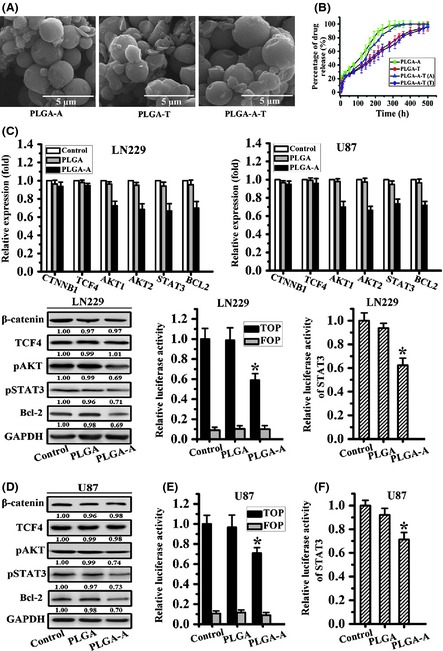
PLGA–aspirin microspheres modestly repressed the Wnt signaling pathway through slight blockage the β‐catenin transactivation. (A) SEM micrographs of PLGA–aspirin, PLGA–TMZ, and PLGA–aspirin–TMZ microspheres. (B) Release profiles of aspirin and TMZ from the PLGA microspheres. (C) CTNNB1 and downstream targets expression were analyzed by quantity real‐time PCR after PLGA or PLGA–aspirin microsphere treatment. (D) β‐catenin and related targets protein expression were assessed by Western blot analysis, while GAPDH serves as an internal. (E, F) β‐catenin transcriptional activity and STAT3 luciferase activity were detected after PLGA and PLGA–aspirin microsphere treatment. All data represented mean ± SD of three individual fields from experiments performed in triplicate for each treatment. *P < 0.05 compared with control and PLGA groups.
Aspirin‐Loaded PLGA Microspheres Repressed β‐catenin Transcriptional Activity
To explore whether PLGA–aspirin microspheres could inhibit Wnt/β‐catenin signaling, the glioma cell lines LN229 and U87 were treated and analyzed for the β‐catenin activity. Real‐time PCR and Western blot assay indicated there was no change of CTNNB1 or TCF4 at mRNA and protein level. However, the downstream targets, AKT1, AKT2, STAT3, and BCL2 expressions were slightly reduced (Figures 1C and D). Furthermore, TOP‐FLASH activity used to assess the β‐catenin/TCF4 transactivation decreased approximately 41% and 35%, respectively, in PLGA–aspirin microsphere‐treated LN229 and U87 cells compared with control and PLGA. There was no change in FOP‐FLASH activity (Figure 1E). STAT3 transcriptional activity was also repressed after PLGA–aspirin microspheres treatment as measured using the STAT3 luciferase reporter assay (Figure 1F). These findings suggested that PLGA–aspirin microspheres could inhibit the Wnt signal pathway, partly by antagonizing β‐catenin/TCF4 transcriptional activity.
PLGA–Aspirin Microspheres Inhibited Glioma Cell Proliferation and Induced Apoptosis through Blockage of β‐catenin Transcriptional Activity
As shown in Figure 2A, in LN229 cells, the G0/G1 phase fractions of the control and PLGA groups were 42.1% and 45%, respectively. However, the PLGA–aspirin microsphere treatment increased this fraction to 57.1%. Similarly, the G2/M phase fraction in the control and PLGA group was 25.2% and 29.7%, and the PLGA–aspirin microsphere treatment reduced it to 17.2%. The S‐phase fraction of Control, PLGA, and PLGA–aspirin‐treated group is 32.7%, 25.4%, and 25.7%, respectively. A similar cell cycle distribution trend was also detected in U87 cells.
Figure 2.
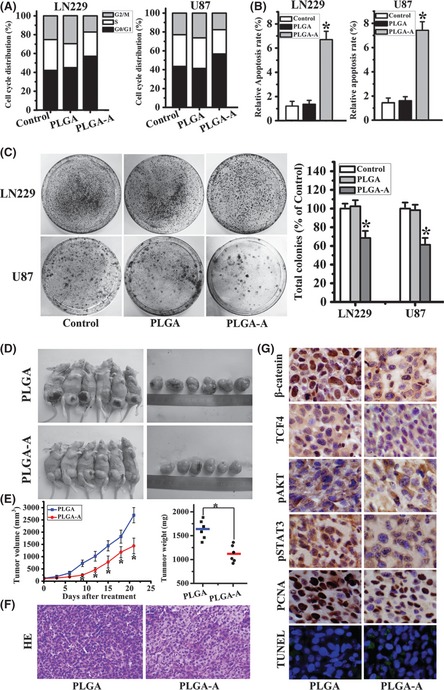
Aspirin‐loaded PLGA microspheres impacted on the glioma malignant progression. (A) LN229 and U87 cell cycle distributions were detected by flow cytometry. Percentages of cells in G0/G1, S, and G2/M phases were shown in both cell lines. (B) Annexin V‐PI assay indicated greater amounts of apoptosis in PLGA–aspirin microsphere group compared with control and PLGA groups. (C) Colony formation assay in LN229 and U87 cell lines. Representative histogram showed total numbers of colony formation by aspirin‐loaded microsphere‐treated cells, which were standardized to control cells (set to 100%). All data represented mean ± SD of three individual fields from experiments performed in triplicate for each treatment. *P < 0.05 compared with control and PLGA groups. (D) Mice and tumor samples obtained from mice bearing LN229/PLGA (n = 6) and LN229/PLGA–aspirin groups (n = 6). (E) Tumor growth and weight evaluation of PLGA–aspirin versus PLGA cells in an in vivo proliferation assay.*P < 0.05 compared with PLGA group. (F) HE stain of tissue samples from PLGA and PLGA–aspirin‐treated groups. (G) Representative photomicrographs of immunohistochemistry for β‐catenin, TCF4, pAKT, and pSTAT3, PCNA as well as TUNEL analysis, on xenograft tumor sections (×200).
After the cells were treated by PLGA–aspirin, PLGA–TMZ, and PLGA–aspirin–TMZ microspheres for 12 h in DMEM with 1% FBS and a 36 h following culture in DMEM with 10% FBS, we conducted apoptosis measurement. The number of annexin V‐positive early‐phase apoptotic cells increased in cells treated with PLGA–aspirin (7.43% for LN229 cells and 6.70% for U87cells) compared with control (1.45% for LN229 cells and 1.21% for U87 cells) and PLGA groups (1.61% for LN229 cells and 1.36% for U87 cells) (Figure 2B). Additionally, LN229 and U87 cells treated by PLGA–aspirin microspheres exhibited a lower colony formation rate (%), 68.5 ± 7.55 and 61.5 ± 7.34, respectively (Figure 2C).
PLGA–Aspirin Microsphere Repressed the Growth of LN229 Glioblastoma Cells in vivo
The in vitro results suggested that aspirin‐loaded PLGA microspheres could suppress glioma tumorigenesis. To confirm this, a proof‐of‐principle experiment was performed using a LN229 glioma xenograft model. On day 9, the tumor size of the PLGA–aspirin microsphere treatment group reached statistical significance compared with the PLGA treatment group. At termination of the study, the tumor volume and weight were significantly different between the PLGA–aspirin treatment group and the PLGA group (Figure 2D,E). Tumors derived from PLGA‐treated cells revealed deeper chromatin staining compared with the PLGA–aspirin‐treated cells, as determined by HE stain (Figure 2F). Meanwhile, nuclear expression of β‐catenin and TCF4, indicating the β‐catenin/TCF4 transcriptional activity, was decreased in tumor specimens from the PLGA–aspirin treatment group. Expressions of pAKT, pSTAT3, and PCNA were also reduced. In addition, the PLGA–aspirin‐treated group had more apoptotic nucleuses compared with the PLGA‐treated group (Figure 2G).
FH535‐Sensitive TMZ Antineoplastic Effect through Blockage Nuclear β‐catenin Translocation in a Xenograft Model
To explore whether FH535, a small molecule inhibitor of β‐catenin, was capable to sensitive TMZ in the combination therapy, we employed a U87 glioma xenograft model. On day 9, a statistical significance of tumor volume emerged between DMSO‐treated group and drugs‐treated group. And then, on day 12, FH535/TMZ treatment group started to show statistically significant difference in size compared with TMZ treatment group. At the termination of the study, tumor volume and weight were significantly different between the TMZ treatment group and the FH535/TMZ treatment group (Figure 3A,B). Nuclear expression of β‐catenin and TCF4 and the expressions of pAKT, pSTAT3, and PCNA in FH535‐/TMZ‐treated group presented pronounced reduction in comparison with DMSO‐ and TMZ‐treated group. Furthermore, more apoptotic nucleuses were also detected in FH535‐/TMZ‐treated group (Figure 3C). The above data suggested a potential mechanism to sensitize TMZ antitumor efficacy, which may partly achieved through blockage β‐catenin transactivation.
Figure 3.
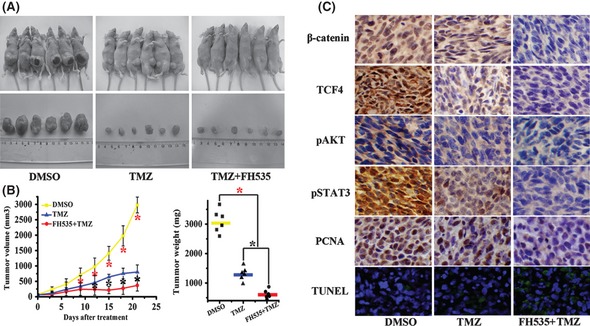
FH535‐sensitive TMZ antitumor activity in U87 xenograft model. (A) Mice and tumor samples from DMSO (n = 6), TMZ (n = 6) and FH535/TMZ combined (n = 6) treatment groups. (B) Tumor growth and weight evaluation of TMZ, FH535‐/TMZ‐ versus DMSO‐treated cells in an in vivo proliferation assay.*P < 0.05 compared with PLGA group. (C) Representative photomicrographs of immunohistochemistry for β‐catenin, TCF4, pAKT, and pSTAT3, PCNA, as well as TUNEL analysis, on xenograft tumor sections (×200).
Aspirin Enhanced TMZ Inhibitory Effect in β‐catenin Transcriptional Activity
In light of the β‐catenin inhibitor such as FH535 could enhance the TMZ treatment efficacy, we assayed whether aspirin could be a TMZ enhancer. Real‐time PCR and Western blot assay demonstrated that after the treatment of PLGA–aspirin–TMZ microspheres, the relative expression of CTNNB1, TCF4, AKT1, AKT2, and STAT3 were reduced in comparison with PLGA–TMZ microsphere treatment group (Figure 4A,B). Meanwhile, β‐catenin and the downstream target STAT3 transcriptional activity were also decreased correspondingly (Figure 4C,D). Thus, we hypothesized that perhaps the lower transcriptional activity of β‐catenin induced by aspirin treatment enhanced the TMZ inhibitory efficacy, which should be further investigated.
Figure 4.
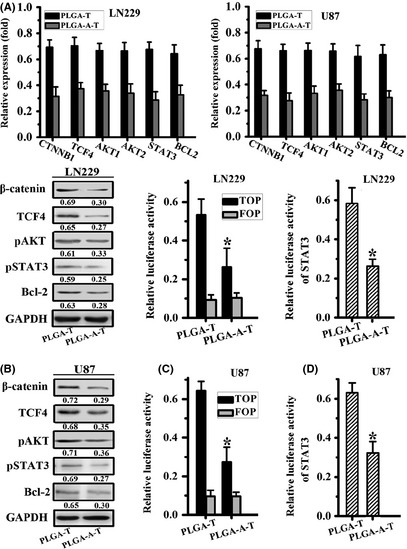
Aspirin enhanced TMZ inhibitory effect in β‐catenin signaling pathway. (A) Quantity real‐time PCR detected the mRNA expression of CTNNB1, TCF4, AKT1, AKT2, STAT3, and BCL2 after the treatment of PLGA–TMZ and PLGA–aspirin–TMZ microspheres. (B) β‐catenin, TCF4, pAKT, pSTAT3, and Bcl‐2 were assayed by Western blot. GAPDH was set as an internal control. (C, D) β‐catenin transcriptional activity and STAT3 luciferase activity detected after PLGA–TMZ and PLGA–aspirin–TMZ microsphere treatment. All data represented mean ± SD of three individual fields from experiments performed in triplicate for each treatment. *P < 0.05 compared with PLGA–TMZ‐treated groups.
Synergistic Effect of combination treatment with Aspirin and TMZ on the Glioma Cell Proliferation and Apoptosis
As indicated, single PLGA–TMZ microsphere treatment exhibited a moderate suppressive effect, and the IC50 value is 18 and 24 μm, respectively, in LN229 and U87, which is not obviously different compared with free TMZ (data not shown), whereas combined treatment with the aspirin yielded a better effect on tumor growth suppression. In the context of lower β‐catenin activation induced by aspirin in LN229 and U87 cell lines, the IC50 was reduced to 5.5 μm and 7.5 μm, respectively (Figure 5A). The combination of aspirin and TMZ also resulted in enhanced efficacy over TMZ alone throughout the 6‐day experiment (Figure 5B).
Figure 5.
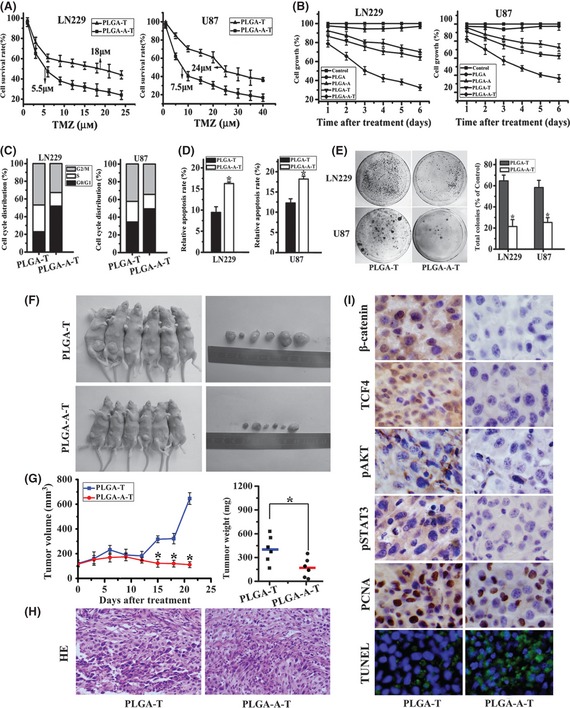
Aspirin‐/TMZ‐coloaded microspheres presented the synergistic antitumor efficacy. (A) MTT assay showed the cell proliferation after the treatment of PLGA–TMZ and PLGA–aspirin–TMZ microspheres in a dose‐dependent manner. (B) An aqueous solution of PLGA–aspirin, PLGA–TMZ, and PLGA–aspirin–TMZ microspheres were incubated with human glioblastoma LN229 and U87 cells for 6 days. Drug induced decrease in cell numbers was measured using the MTT assay. (C) Effect of PLGA–aspirin and PLGA–aspirin–TMZ microspheres on cell cycle distribution in LN229 and U87 cells. (D) Annexin V‐PI assay revealed the apoptosis in PLGA–aspirin–TMZ microsphere treatment groups compared with the PLGA–aspirin treatment groups. (E) Colony formation assay in LN229 and U87 cell lines. Representative histogram showed total numbers of colony formation by aspirin‐/TMZ‐coloaded microsphere‐treated cells, which are standardized to control cells (set to 100%). All data represented mean ± SD of three individual fields from experiments performed in triplicate for each treatment. *P < 0.05 compared with PLGA–TMZ‐treated groups. (F) Mice and tumor samples obtained from mice bearing LN229/PLGA–TMZ (n = 6) and LN229/PLGA–aspirin–TMZ groups (n = 6). (G) Tumor growth and weight evaluation of PLGA–TMZ versus PLGA–aspirin–TMZ treatment group in an in vivo proliferation assay.*P < 0.05 compared with PLGA–TMZ group. (H) HE stain of tissue samples from PLGA–TMZ and PLGA–aspirin–TMZ microsphere treatment groups. (I) Representative photomicrographs of immunohistochemistry for β‐catenin, TCF4, pAKT, and pSTAT3, PCNA, as well as TUNEL analysis, on xenograft tumor sections (×200).
To investigate the nature of the treatment effect for aspirin‐/TMZ‐coloaded PLGA microspheres on LN229 and U87 cells, the Zheng‐Jun Jin method used to evaluate the combination effect among different drugs was performed to analyze the cytotoxicity data for antagonism, additivity, or synergistic after cells are treated for 6 days. The Q value for LN229 and U87 cells was 1.23 and 1.16, respectively, both indicating that the synergistic effects appeared for the administration of the aspirin‐/TMZ‐coloaded PLGA microspheres.
Meanwhile, as shown in Figure 5C, in LN229 and U87 cells, the G0/G1 phase fractions of PLGA–TMZ microsphere‐treated group were 23.0% and 34.6%. However, PLGA–aspirin–TMZ microsphere treatment increased the percentage of cells in G0/G1 phase to 51.9% and 41.4%. Similarly, the S‐phase fraction in PLGA–TMZ‐treated LN229 and U87 cells was 46.8% and 42.3%, respectively, but PLGA–aspirin–TMZ treatment reduced this fraction to 32.8% and 34.3%. The G2/M phase fractions in PLGA–TMZ‐treated LN229 and U87 cells is 46.8% and 42.3%, while reduced to 32.8% and 34.3%, respectively, after the treatment of PLGA–aspirin–TMZ microspheres.
Percentages of apoptotic cells were shown in Figure 5D. Compared with single TMZ treatment in LN229 and U87 cells (12.3% and 9.45%, respectively), the combination of the aspirin and TMZ therapy caused a significantly higher amount (18.2% and 16.3%, respectively) of apoptosis. Additionally, after the treatment of aspirin‐/TMZ‐coloaded microspheres, the percentage of colony formation (%) in LN229 and U87 cell lines was reduced to 21.4 ± 6.54 and 25.2 ± 4.56, in comparison with PLGA–TMZ‐treated group (64.7 ± 5.43 and 58.3 ± 6.76, respectively) (Figure 5E).
Aspirin Enhanced TMZ Antitumor Efficacy in a Xenograft Model in vivo
To further examine whether the combined therapy of aspirin and TMZ had a more notable antitumor effect in vivo, we conducted this using LN229 subcutaneous tumor model. On day 15, a statistically significant difference in tumor volume appeared between PLGA–TMZ and PLGA–aspirin–TMZ microsphere‐treated group (P < 0.05), and the size disparity proceeded until the day 21; at the time, tumor volume and weight were significantly different between the two groups (Figure 5F,G). Compared to PLGA–TMZ‐treated group, samples derived from PLGA–aspirin–TMZ‐treated group presented less numbers of microvessels and weaker nuclei staining, as indicated by HE stain (Figure 5H). Furthermore, expression of nuclear β‐catenin, TCF4, pAKT, pSTAT3, and PCNA was reduced, and TUNEL assay showed that there were more apoptotic nucleuses in the PLGA–aspirin–TMZ‐treated group (Figure 5I).
Discussion
Compelling evidence for the genetic and cellular mechanisms of glioma development, including cell proliferation, apoptosis, and invasion, suggests that the malignant tumor phenotype results from the dysfunction of multiple interrelated growth‐regulatory pathways 20. One of the most important pathways is Wnt/β‐catenin signaling, which is activated by β‐catenin/TCF4 transcriptional complex and functions as a central signaling node for the activation of downstream effectors, such as AKT1 21, AKT2 22, and STAT3 23. Overactivation of β‐catenin signaling not only plays a pivotal role in maintaining the aggressive malignant phenotype of gliomas, but also regulates treatment resistance through promoting cellular survival and inhibiting the induction of apoptosis 24, 25. Thus, β‐catenin becomes an attractive target, and targeted inhibition of β‐catenin transcriptional activity is a rational and promising new approach for the glioma therapy.
TMZ is an effective antiglioma agent, the standard dosage is 150–200 mg/m2 per day for 5 days in a 28‐day cycle 26, yet its main disadvantage is the drug resistance, short serum half‐life, and dose‐limiting side effects. Thus, there is an urgent need to develop promising chemosensitizing strategies, and combination of several anticancer drugs is a proven strategy for effective therapy. Previous studies reported that FH535 could repress the glioma malignant progression through blocking β‐catenin nuclear translocation 27. Here, we confirmed that FH535 could effectively enhance TMZ antitumor efficacy in a xenograft model. Therefore, we speculated that TMZ treatment efficacy could be increased through inhibition of β‐catenin transactivation. In comparison with FH535, aspirin is not only a potent inhibitor of β‐catenin signaling 16, but also a universal antiinflammatory agent that has been widely used in clinical. A recent study has confirmed that daily aspirin use could reduce the long‐term incidence of some adenocarcinomas and provided proof of principle for pharmacological intervention specifically to prevent distant metastasis 28. Given the significance and clinical development potential of aspirin, we combined it with TMZ to assay the treatment efficacy.
PLGA was selected to prepare aspirin, TMZ, and aspirin‐/TMZ‐loaded microspheres as it was widely used as a carrier of microspheres to parcel drugs and to lengthen their sustained release time in the pharmaceutical industry 29, 30, 31. In this study, we compared the antitumor effect of PLGA–aspirin, PLGA–TMZ, and PLGA–aspirin–TMZ microspheres. Our results suggested that aspirin‐/TMZ‐coloaded microspheres presented a synergistic antitumoral efficacy. Furthermore, the most significant advantage of the microspheres is the sustained release action. We performed only once intratumoral injection of aspirin/TMZ microspheres (105 μm/kg), but the antitumoral efficacy is notable. Therefore, we concluded that aspirin‐/TMZ‐coloaded microspheres achieved a proper sustained release action, and the synergistic antitumor efficacy was partly dependent on β‐catenin signaling transactivation.
As a β‐catenin downstream target effector, AKT signaling is directly involved in cancer cells resistance to TMZ cytotoxicity 32, 33, and studies have confirmed that enhanced TMZ therapeutic efficacy can be achieved through blockage PI3K/AKT signaling in glioblastomas and melanomas 34, 35. In the present study, we observed a significant decrease in AKT expression in LN229 and U87 glioma cells after the combined treatment of aspirin and TMZ compared with single aspirin or TMZ treatment. This indicated that the synergistic therapeutic effect of aspirin and TMZ combination treatment could partly be improved through relieving resistance induced by AKT signaling.
STAT3 is a crucial oncogenic transcription factor that is constitutively active and propagates tumorigenesis by preventing apoptosis and enhancing proliferation, angiogenesis, and invasiveness 36. Furthermore, STAT3 is a convergent point of many signaling pathways, and efforts are ongoing to target it for anticancer drug development. A previous study indicated that targeting STAT3 could sensitize malignant glioma cells to alkylating agents such as TMZ, BCNU, and cisplatin 37. We have also found that inhibition of STAT3 was potent in reversing resistance to alkylating agents 24. Therefore, the reduced activity of STAT3 due to inhibition of β‐catenin signaling may also enhance the TMZ antitumor efficacy.
Bcl‐2 is a key regulator of apoptosis. Elevated expression of Bcl‐2 commonly occurs in human malignancies and is related to cancer progression and chemotherapy resistance 38. In the present study, the expression of Bcl‐2 was significantly decreased by the treatment of aspirin‐/TMZ‐coloaded microspheres. Previous reports confirmed that reduced AKT and STAT3 activity could cause inhibition of Bcl‐2 expression 24, 39. Therefore, we concluded that sensitization of glioma cells to combination treatment with aspirin and TMZ ultimately benefited from the inhibitory effects on Bcl‐2 expression (Figure S1).
Taken together, the current study demonstrates a simple and reproducible way to develop a stable release of aspirin‐/TMZ‐coloaded microspheres by PLGA copolymers. Encapsulation of aspirin and TMZ into microspheres not only presents the synergistic antiglioma efficiency, which due to the reduced expression of Bcl‐2 mediated by blockage of β‐catenin signaling transduction pathway, but also achieved a sustained release action to lower the TMZ dosage. However, as a traditional antiinflammatory agent, aspirin is reported to effectively block the NF‐κB signaling pathway 40, which is closely associated with inflammation and tumor microenvironment 41. It is possible that aspirin could interfere with the inflammation and tumor microenvironment through suppressing the NF‐κB pathway. Perhaps, this is another mechanism of aspirin‐sensitive TMZ treatment efficacy. Therefore, the underlining molecular mechanisms of aspirin as a chemotherapeutic enhancer for TMZ and a combined therapeutic strategy using aspirin/TMZ‐containing microsphere delivery system warrant further research.
Conflict of Interest
The authors declare no conflict of interests.
Supporting information
Figure S1. Microparticle‐based aspirin/TMZ delivery effectively lead to the glioma cells apoptosis, which medicated by the reduced β‐catenin/TCF4 transcriptional activity.
Acknowledgments
This work was supported by the China National Natural Scientific Fund (81001128, 81172406 and 51073118), Tianjin Science and Technology Committee (09JCZDJC17600, 10SYSYJC28800), Program for New Century Excellent Talents in University (NCET‐08‐0334), and National High Technology Research and Development Program 863 (2012AA02A508).
Members of the Chinese Glioma Cooperative Group (CGCG) are Z‐D Shi, L Han, K‐L Zhang, L‐Y Chen, J‐X Zhang, P‐Y Pu and C‐S Kang.
References
- 1. Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed 2006;17:247–289. [DOI] [PubMed] [Google Scholar]
- 2. Makadia HK, Siegel SJ. Poly Lactic‐co‐Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011;3:1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang L, Chan JM, Gu FX, et al. Self‐assembled lipid–polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano 2008;2:1696–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zou GK, Song YL, Zhou W, et al. Effects of local delivery of bFGF from PLGA microspheres on osseointegration around implants in diabetic rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2012;114:284–289. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed AU, Tyler MA, Thaci B, et al. A comparative study of neural and mesenchymal stem cell‐based carriers for oncolytic adenovirus in a model of malignant glioma. Mol Pharmaceut 2011;8:1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mrugala MM, Chamberlain MC. Mechanisms of disease: temozolomide and glioblastoma–look to the future. Nat Clin Pract Oncol 2008;5:476–486. [DOI] [PubMed] [Google Scholar]
- 7. Agnihotri S, Gajadhar AS, Ternamian C, et al. Alkylpurine‐DNA‐N‐glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J Clin Invest 2012;122:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Motomura K, Natsume A, Wakabayashi T. Intravenous administration of temozolomide as a useful alternative over oral treatment with temozolomide capsules in patients with gliomas. J Neurooncol 2012;106:209–211. [DOI] [PubMed] [Google Scholar]
- 9. Andrasi M, Bustos R, Gaspar A, Gomez FA, Klekner A. Analysis and stability study of temozolomide using capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:1801–1808. [DOI] [PubMed] [Google Scholar]
- 10. Sarkaria JN, Kitange GJ, James CD, et al. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res 2008;14:2900–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qian X, Ren Y, Shi Z, et al. Sequence‐dependent synergistic inhibition of human glioma cell lines by combined Temozolomide and miR‐21 inhibitor gene therapy. Mol Pharmaceut 2012;9:2636–2645. [DOI] [PubMed] [Google Scholar]
- 12. Dinarello CA. Anti‐inflammatory agents: present and future. Cell 2010;140:935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol 2010;28:1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA 2009;302:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lan F, Yue X, Han L, et al. Antitumor effect of aspirin in glioblastoma cells by modulation of beta‐catenin/T‐cell factor‐mediated transcriptional activity. J Neurosurg 2011;115:780–788. [DOI] [PubMed] [Google Scholar]
- 17. Han L, Yue X, Zhou X, et al. MicroRNA‐21 Expression is regulated by beta‐catenin/STAT3 Pathway and Promotes Glioma Cell Invasion by Direct Targeting RECK. CNS Neurosci Ther 2012;18:573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jin ZJ. About the evaluation of drug combination. Acta Pharmacol Sin 2004;25:146–147. [PubMed] [Google Scholar]
- 19. Chen L, Han L, Zhang K, et al. VHL regulates the effects of miR‐23b on glioma survival and invasion via suppression of HIF‐1alpha/VEGF and beta‐catenin/Tcf‐4 signaling. Neuro Oncol 2012;14:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li C, Zhou C, Wang S, et al. Sensitization of glioma cells to tamoxifen‐induced apoptosis by Pl3‐kinase inhibitor through the GSK‐3beta/beta‐catenin signaling pathway. PLoS ONE 2011;6:e27053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen L, Huang K, Han L, et al. Beta‐catenin/Tcf‐4 complex transcriptionally regulates AKT1 in glioma. Int J Oncol 2011;39:883–890. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Huang K, Shi Z, et al. High beta‐catenin/Tcf‐4 activity confers glioma progression via direct regulation of AKT2 gene expression. Neuro Oncol 2011;13:600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yue X, Lan F, Yang W, et al. Interruption of beta‐catenin suppresses the EGFR pathway by blocking multiple oncogenic targets in human glioma cells. Brain Res 2010;1366:27–37. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Chen L, Bao Z, et al. Inhibition of STAT3 reverses alkylator resistance through modulation of the AKT and beta‐catenin signaling pathways. Oncol Rep 2011;26:1173–1180. [DOI] [PubMed] [Google Scholar]
- 25. Rossi M, Magnoni L, Miracco C, et al. Beta‐catenin and Gli1 are prognostic markers in glioblastoma. Cancer Biol Ther 2011;11:753–761. [DOI] [PubMed] [Google Scholar]
- 26. Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE. A phase II study of extended low‐dose temozolomide in recurrent malignant gliomas. Neuro Oncol 2002;4:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi Z, Qian X, Li L, et al. Nuclear Translocation of beta‐catenin is Essential for Glioma Cell Survival. J Neuroimmune Pharm 2012; DOI: 10.1007/s11481-012-9354-3 [DOI] [PubMed] [Google Scholar]
- 28. Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised led trials. Lancet 2012;379:1591–1601. [DOI] [PubMed] [Google Scholar]
- 29. Sun F, Sui C, Teng L, Liu X, Meng Q, Li Y. Studies on the preparation, characterization and pharmacological evaluation of tolterodine PLGA microspheres. Int J Pharm 2010;397:44–49. [DOI] [PubMed] [Google Scholar]
- 30. Presmanes C, De Miguel L, Espada R, Alvarez C, Morales E, Torrado JJ. Effect of PLGA hydrophilia on the drug release and the hypoglucemic activity of different insulin‐loaded PLGA microspheres. J Microencapsul 2011;28:791–798. [DOI] [PubMed] [Google Scholar]
- 31. Cozar‐Bernal MJ, Holgado MA, Arias JL, et al. Insulin‐loaded PLGA microparticles: flow focusing versus double emulsion/solvent evaporation. J Microencapsul 2011;28:430–441. [DOI] [PubMed] [Google Scholar]
- 32. Caporali S, Levati L, Starace G, et al. AKT is activated in an ataxia‐telangiectasia and Rad3‐related‐dependent manner in response to temozolomide and confers protection against drug‐induced cell growth inhibition. Mol Pharmacol 2008;74:173–183. [DOI] [PubMed] [Google Scholar]
- 33. Gaspar N, Marshall L, Perryman L, et al. MGMT‐independent temozolomide resistance in pediatric glioblastoma cells associated with a PI3‐kinase‐mediated HOX/stem cell gene signature. Cancer Res 2010;70:9243–9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prasad G, Sottero T, Yang X, et al. Inhibition of PI3K/mTOR pathways in glioblastoma and implications for combination therapy with temozolomide. Neuro Oncol 2011;13:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sinnberg T, Lasithiotakis K, Niessner H, et al. Inhibition of PI3K‐AKT‐mTOR signaling sensitizes melanoma cells to cisplatin and temozolomide. J Invest Dermatol 2009;129:1500–1515. [DOI] [PubMed] [Google Scholar]
- 36. Dauer DJ, Ferraro B, Song L, et al. Stat3 regulates genes common to both wound healing and cancer. Oncogene 2005;24:3397–3408. [DOI] [PubMed] [Google Scholar]
- 37. Lo HW, Cao X, Zhu H, Ali‐Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high‐grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin Cancer Res 2008;14:6042–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res 2007;67:2908–2911. [DOI] [PubMed] [Google Scholar]
- 39. Li X, Lu X, Xu H, et al. Paclitaxel/tetrandrine coloaded nanoparticles effectively promote the apoptosis of gastric cancer cells based on “oxidation therapy”. Mol Pharmaceut 2012;9:222–229. [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF‐kappaB pathway in the treatment of inflammation and cancer. J Clin Invest 2001;107:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DiDonato JA, Mercurio F, Karin M. NF‐kappaB and the link between inflammation and cancer. Immunol Rev 2012;246:379–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Microparticle‐based aspirin/TMZ delivery effectively lead to the glioma cells apoptosis, which medicated by the reduced β‐catenin/TCF4 transcriptional activity.


