Abstract
Background
Previous studies have shown that obstructive sleep apnea (OSA) is associated with a higher risk of cognitive impairment or dementia in the elderly, leading to deleterious health effects and decreasing quality of life. This systematic review aims to determine the prevalence of OSA in patients with mild cognitive impairment (MCI) and examine whether an association between OSA and MCI exists.
Methods
We searched Medline, PubMed, Embase, Cochrane Central, Cochrane Database of Systematic Reviews, PsychINFO, Scopus, the Web of Science, ClinicalTrials.gov and the International Clinical Trials Registry Platform for published and unpublished studies. We included studies in adults with a diagnosis of MCI that reported on the prevalence of OSA. Two independent reviewers performed the abstract and full-text screening, data extraction and the study quality critical appraisal.
Results
Five studies were included in the systematic review. Overall, OSA prevalence rates in patients with MCI varied between 11 and 71% and were influenced by OSA diagnostic methods and patient recruitment locations (community or clinic based). Among studies using the following OSA diagnostic measures– self-report, Home Sleep Apnea Testing, Berlin Questionnaire and polysomnography– the OSA prevalence rates in MCI were 11, 27, 59 and 71%, respectively. In a community-based sample, the prevalence of OSA in patients with and without MCI was 27 and 26%, respectively.
Conclusions
Based on limited evidence, the prevalence of OSA in patients with MCI is 27% and varies based upon OSA diagnostic methods and patient recruitment locations. Our findings provide an important framework for future studies to prospectively investigate the association between OSA and MCI among larger community-based cohorts and implement a standardized approach to diagnose OSA in memory clinics.
PROSPERO registration
CRD42018096577.
Electronic supplementary material
The online version of this article (10.1186/s12883-019-1422-3) contains supplementary material, which is available to authorized users.
Keywords: Obstructive sleep apnea, Mild cognitive impairment, Prevalence
Backgroud
Mild cognitive impairment (MCI) and obstructive sleep apnea (OSA) are chronic, debilitating disorders that commonly occur in older individuals and may share a common pathological link [1]. The epidemiology of OSA and MCI is poorly understood and very few studies have assessed their relationship [1, 2].
Different diagnostic criteria for MCI have been proposed over time [3]. With the current criteria [4–9], the prevalence of global MCI in individuals aged ≥60 years is reported to be between 6 and 20% [10, 11] with rates being affected by several modifiable [12] and non-modifiable [12–15] factors.
OSA is a recurrent obstruction of the upper airway during sleep that leads to intermittent hypoxia, high intrathoracic pressure swings and sleep fragmentation that has recently been shown to be associated with a higher risk of MCI or dementia in elderly [1, 16, 17]. Untreated OSA in middle-age causes impairments in attention, vigilance, some aspects of memory, psychomotor performances and executive function [16, 18–20]. Furthermore, associations between OSA and cognition in middle-age and late-life are highly variable and the findings differ based on the definition of apnea hypopnea index (AHI) and setting of the study (clinic vs community). There is evidence suggesting that intermittent hypoxia, which contributes to subsequent oxidative stress and endothelial dysfunction, could be a significant mediator in the deleterious effects of OSA on neurocognitive function [21], but the mechanism(s) involved in this association and the role of sleep fragmentation are unknown [22].
To date, the prevalence of diagnosed or undiagnosed OSA in the MCI population remains unknown. The objectives of this review were to determine the prevalence of OSA in patients with MCI and examine whether an association between OSA and MCI exists. Considering aging of the general population and the increasing prevalence of MCI and dementia, reliably estimating the prevalence of OSA in patients with MCI may guide future health resource planning to diagnose and treat OSA early in the elderly population [13].
Methods
Study design and registration
The protocol of this study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42018096577). We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (Additional file 1: PRISMA) guideline [23].
Inclusion criteria and outcomes
All studies on adults (age > 18 years) that reported the prevalence of OSA (primary outcome of interest) among patients with MCI using established diagnostic methods were included. In particular, the diagnosis of OSA should have been established using sleep studies such as type 1 laboratory polysomnography (PSG), or types 2–4 portable sleep monitors, or sleep questionnaires, or a physician diagnosis. The secondary outcome was the risk of OSA among MCI patients relative to the control population without MCI. From herein we will refer to this population as “controls”. We considered experimental, cohort, cross-sectional and case-control studies and excluded case reports, case series and commentaries. In addition, studies with a mixed population with neurodegenerative disorders such as dementia and defined sleep disorders other than OSA (e.g., central apneas) were also excluded. Only English language articles and human studies were included.
Information sources and search strategies
With the help of an information specialist (ME), we conducted a comprehensive search for published and unpublished literature in the following electronic databases: Medline (Ovid), PubMed (non-Medline records only), Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, PsychINFO, Scopus (Elsevier), the Web of Science, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform. The electronic searches were conducted from the date of inception of the databases until May 1, 2018. The search strategy combined MeSH terms and keywords related to OSA with those related to MCI (see Additional file 2 for the search strategy in Medline). In addition, we hand searched the reference list of included full-text articles and review articles to capture studies potentially missed from the original search. The identified citations were imported into an EndNote database and duplicate records were removed.
Study selection and data extraction
Two reviewers (AN and DP) independently screened the titles and abstracts of all studies that resulted from the search to determine eligibility for full-text screening. From these full-text articles, studies were included in the systematic review if the primary outcome was reported. A standardized data extraction list in Excel was used to collect information on study characteristics, participants’ characteristics, details on outcomes and on study quality. Data was extracted from eligible full-text articles independently by two reviewers. Disagreements were resolved by the senior author (FC). When relevant, study authors [2, 24] were contacted for clarification and provision of additional information for the systematic review.
Assessment of study quality
Two reviewers (AN and DP) critically appraised each included study by using the Joanna Briggs Institute critical appraisal checklist for analytical cross-sectional studies [25]. The checklist included the following 7 items: (1) appropriate recruitment of participants; (2) representative sample of the target population; (3) use of objective, standard criteria for ascertaining the exposure (MCI) and the (4) outcome (OSA); (5) identifying and (6) adjusting for confounding factors; and [7] appropriateness of statistical analysis. Items were evaluated using ‘yes’/‘no’/‘unclear’ or ‘not applicable’ options.
Results
Search results
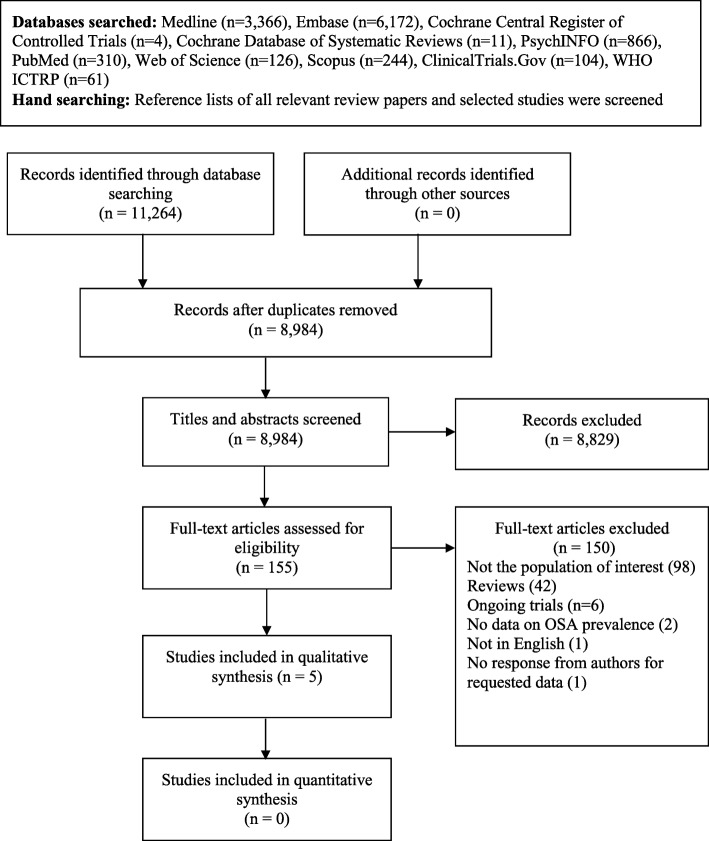
The search returned 11,264 records in total. Of the 155 studies retrieved for full text review, 150 were excluded (Fig. 1). The most common reason for exclusion was having a different study population (n = 98). Five articles were included in the final systematic review [2, 24, 26–28].
Fig. 1.
Flow diagram of study selection process. Abbreviations: WHO ICTRP = World Health Organization International Clinical Trials Registry Platform
Characteristics of selected studies
The characteristics of the included studies are summarized in Table 1. Four studies [24, 26–28] had a cross-sectional design, while one [2] was a retrospective cohort. The studies were conducted in six different countries (Australia, Germany, Italy, South Korea, USA and Canada). The referral population of the five studies included elderly patients, with and without MCI, that were recruited from multiple clinics, including neurology [2, 26, 28] and general practitioner clinics (otherwise called Public Health Centers that are free clinics in South Korea to provide care to the lower socioeconomic classes and consists mostly of elderly patients) [27]. Only one study enrolled a randomly sampled, community-based general population [24]. A control population was included in four studies [2, 24, 27, 28], allowing for between-group comparison.
Table 1.
Characteristics of studies included in the final review
| Variables | Groups | Dlugaj et al. (2014) [24] | Guarnieri et al. (2012) [26] | Kim et al. (2011) [27] | Osorio et al. (2015) [2] | Wilson et al. (2014) [28] |
|---|---|---|---|---|---|---|
| Referral Population | – | General population (HNR cohort); age 45–75 yrs. | Neurology clinic; age > 60 years | General practitioner clinic (Public health centre); age > 60 years | Multiple clinics (ADNI cohort); age 55–90 yrs. | Neurology clinic; age > 50 years |
| Study design | – | Cross-sectional | Cross-sectional | Cross-sectional | Retrospective Cohorta | Cross-sectional |
| Study country | – | Germany | Italy | South Korea | USA and Canada | Australia |
| MCI diagnosis criteria | – | Petersen (2004) [6] Winblad (2004) [8] | Winblad (2004) [8] | Petersen (1999) [4] | Petersen (2005) [7] | Petersen (2005) [7] |
| OSA diagnosis method | – | ApneaLink™ | History of snoring or sleep apneas & high risk on Berlin Questionnaire | PSG | “Patient reported OSA, followed by physician assessment of diagnosis based on patients’ medical history” | PSG |
| OSA diagnosis criteria | – | AHI ≥ 15 events/hour | NA | AHI ≥ 5 events/hour | NR | AHI ≥ 5 events/hour |
| OSA AHI Indicesb | – |
A: ≥80%, ≥10s H: 50–80%, ≥10s OD: NR |
NA |
A: ≥100%, ≥10s H: ≥50%, ≥10s OD: 3% (or arousal) |
NR |
A: ≥100%, ≥10s H: ≥50%, ≥10s OD: 3% (or arousal) |
| Number of subjects | MCI | 230 | 138 | 30 | 402 | 37 |
| aMCI | 120 | NR | NR | 402 | 10 | |
| naMCI | 110 | 0 | 27 | |||
| Controls | 676 | – | 30 | 607 | 37 | |
| Number of males, n (%) | MCI | 919 (51%) | 50% | 9 (30%) | – | NR |
| Controls | – | 9 (30%) | ||||
| Age (years), mean ± SD | MCI | 63.79 ± 7.45 | 73 ± 9c | 67.97 ± 4.09 | – | 65.5 ± 9.0 |
| Controls | – | 67.37 ± 3.75 | 63.5 ± 8.7 | |||
| BMI (kg/m2), mean ± SD | MCI | 28.06 ± 4.38 | NR | 24.40 ± 3.28 | – | 27.6 ± 5.5 |
| Controls | 24.49 ± 2.75 | 27.1 ± 4 | ||||
| Comorbidities (major) | – | DM (18%), HTN (36%), CAD (7%) | NR | NR | – | NR |
| Education (years), n (%) or mean ± SD | MCI | ≤10: 167 (9%); 11–13: 1002 (56%); ≥14: 624 (35%) | 8.2 ± 4.2c | 6.80 ± 4.67 | – | NR |
| Controls | – | 7.90 ± 5.11 | – | NR | ||
| AHI (events/hour) | MCI | 11.5 ± 11.43 | NR | 13.41 ± 11.61 | NR | 16.4 ± 16d |
| Controls | 15 ± 13.56 | 11.9 ± 10d | ||||
| APOE positive (%) | MCI | 445 (25%) | NR | NR | – | NR |
| Controls | ||||||
| Mini Mental Status Exam, mean ± SD | MCI | NR | 27 ± 2c | NR | NR | 28.1 ± 1.5 |
| Controls | – | 29.2 ± 1.1 |
Abbreviations: A apnea, ADNI Alzheimer’s disease Neuroimaging Initiative, AHI apnea-hypopnea index, APOE Apolipoprotein E, BMI body mass index, CAD coronary artery disease, DM diabetes mellitus, HNR Heinz Nixdorf Recall, HTN hypertension, H hypopnea, MCI mild cognitive impairment, NA not applicable, NR not reported, OD oxygen desaturation, OSA obstructive sleep apnea, PSG polysomnography, SD standard deviation
aData to calculate prevalence and/or odds ratio were provided by the study authors and were taken from baseline measurements of OSA and MCI (cross-sectional)
bThe percentage drop of airflow from baseline for 10 s or more with or without oxygen desaturation
cValues estimated from a bar-graph
dAHI data was available on 24 out of the 37 subjects with MCI and 25 out of the 37 control subjects
The total number of patients with MCI and controls were 837 (range: 30–402) and 1353 (range: 30–676), respectively. The majority of MCI patients, with reported information on the type of MCI, had amnestic MCI (532 aMCI vs. 137 non-amnestic (na)MCI). Whether these individuals had impairments in a single or multiple cognitive domains was not reported. The mean age of patients ranged from 63.8 (CI:63.4–64.1) to 73 (CI:71.5–74.5) years and mean body mass index (BMI) from 24.4 (CI:23.2–25.6) to 28.1 (CI:27.9–28.3) kg/m2.
MCI criteria
The inclusion criteria utilized by the studies for diagnosing MCI were largely similar [4, 6–8]. The diagnosis of MCI was made if the patient met the following criteria: 1) cognitive complaint from either the participant and/or family member, 2) objective cognitive impairment not normal for age, 3) preserved activities of daily living and, 4) absence of dementia (does not meet criteria for a dementia syndrome). Participants that met criteria 3 and 4 and had subjective and objective memory complaints were categorized as having aMCI, while those with deficits in cognitive domains other than memory (e.g. language, executive function etc.) were diagnosed with naMCI [24, 28].
OSA criteria
The OSA diagnostic method differed across the selected studies. To diagnose OSA, two studies utilized PSG [27, 28], one used the ApneaLink™ [24] (a portable sleep apnea testing device), one used the Berlin Questionnaire [26], and another used patients’ “self-reported information followed by physician assessment based on patients medical history” [2], which will be referred to “self-report” from herein. The two studies that used PSG considered patients having OSA if they had an AHI ≥ 5 events/hour. The study that used the ApneaLink [24] device, applied an AHI cut-off of ≥15 events/hour to diagnose OSA.
Quality of studies
The critical appraisal of the identified studies is presented in Table 2. All studies defined the inclusion criteria and described the study population in sufficient details, providing references to the original study and cohort from which participants were recruited when relevant. An objective and standard criteria was used to diagnose the exposure/condition, MCI, and appropriate statistical analysis was used. Although confounding factors such as age, sex, type of MCI, level of education or the presence of APOE gene were identified, they were not dealt with in the data analysis for all studies. Finally, only two studies [27, 28] used the current gold standard, PSG, to measure the outcome, OSA.
Table 2.
Quality of included studies
| Author | Criteria for inclusion in the sample clearly defined? | Study subjects and the setting described in detail? | Exposure measured in a valid and reliable way? | An objective, standard criteria used for measurement of the condition? | Confounding factors identified? | Strategies to deal with confounding factors stated? | Outcomes measured in a valid and reliable way? | Appropriate statistical analysis used? |
|---|---|---|---|---|---|---|---|---|
| Dlugaj et al. [24] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Guarnieri et al. [26] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Kim et al. [27] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Osorio et al. [2] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Wilson et al. [28] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
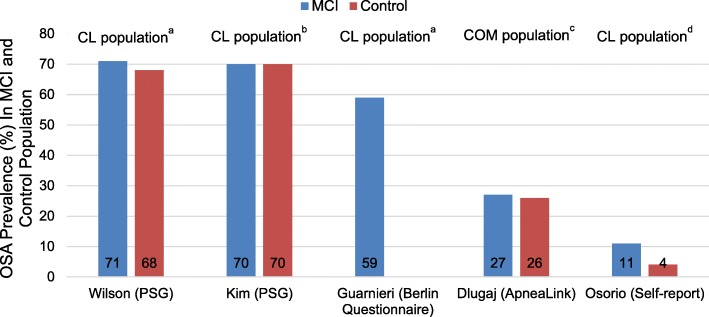
Prevalence of OSA in MCI
Five studies documented the prevalence of OSA in individuals with MCI, of which four included the prevalence of OSA in the control population (Table 3). Overall, results indicated that OSA is present in 11–71% of MCI population compared to 4–70% in controls (Fig. 2). The prevalence of OSA in MCI was the highest among two studies that used PSG to diagnose OSA and recruited elderly patients from a clinic-based sample, 70 and 71%, respectively [27, 28]. In a community-based sample population, the prevalence of OSA in patients with and without MCI was 27 and 26%, respectively [24]. For studies using the following OSA diagnostic measures– self-report [2], ApneaLink [24] and Berlin Questionnaire [26]– the OSA prevalence rates in MCI were 11, 27 and 59%, respectively.
Table 3.
The reported prevalence and odds ratio of OSA in MCI population in included studies
| Author | Groups | Total sample | Subjects with OSA, n | Prevalence % (95% CI) | Odds Ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Dlugaj et al. [24] | MCI | 230 | 61 | 27 (21.0–32.8) | 1.03 (0.74–1.45) | 0.84 |
| aMCI | 120 | 32 | 52 (39.4–65.2) | |||
| naMCI | 110 | 29 | 48 (34.8–60.6) | |||
| Controls | 676 | 174a | 26 (22.5–29.2) | |||
| Guarnieri et al. [26] | MCI | 138 | 81 | 59 (50.0–66.9) | – | – |
| aMCI | NR | NR | – | |||
| naMCI | ||||||
| Controls | – | – | – | |||
| Kim et al. [27] | MCI | 30 | 21 | 70 (50.4–84.6) | 1.00 (0.33–3.02) | 1.00 |
| aMCI | NR | NR | – | |||
| naMCI | ||||||
| Controls | 30 | 21 | 70 (50.4–84.6) | |||
| aOsorio et al. [2] | MCI | 402 | 44 | 11 (8.2–14.5) | 3.61 (2.09–6.22) | < 0.0001 |
| aMCI | 402 | 44 | 100 (90–100) | |||
| naMCI | – | – | – | |||
| Controls | 607 | 23 | 4 (2.5–5.7) | |||
| Wilson et al. [28] | MCI | 37 | 17/24b | 71 (48.8–86.6) | 1.14 (0.34–3.86) | 0.83 |
| aMCI | 10 | NR | – | |||
| naMCI | 27 | |||||
| Controls | 37 | 17/25b | 68 (46.5–84.3) |
Abbreviations: CI confidence interval, MCI mild cognitive impairment, aMCI amnestic mild cognitive impairment, naMCI non-amnestic mild cognitive impairment, OSA obstructive sleep apnea
aData provided by study authors
bAHI data was available on 24 out of the 37 subjects with MCI and 25 out of the 37 control subjects
Fig. 2.
Reported OSA prevalence (%) in patients with MCI and Controls. Abbreviations: CL = clinic; COM = community; HNR = Heinz Nixdorf Recall; MCI = mild cognitive impairment; PSG = polysomnography. aIncludes patients recruited from neurology clinics. bIncludes patients recruited from a public health center. cIncludes patients recruited from HNR cohort (community-based sample). dIncludes patients recruited from multiple clinics, including neurology clinics, OSA risk in MCI vs. Controls [OR 3.61 (2.09–6.22), p < 0.0001]
Risk of OSA in MCI
The risk of OSA in patients with MCI among included studies is summarized in Table 3. The risk of OSA in patients with MCI was over 3-fold compared to the control group in one study that recruited patients from multiple memory clinics (OR:3.61; 95% CI:2.09–6.22; p-value< 0.0001) [2]. There were no differences in risk of OSA between the MCI and control group in the rest of included studies with a control population [24, 27, 28].
Discussion
Summary of main results
To our knowledge, this study is the first systematic review evaluating the prevalence and risk of OSA in patients with MCI. In total, we found five studies reporting the prevalence of OSA in MCI showing considerable variations (11–71%). Two studies that used PSG [27, 28] to diagnose OSA showed that the prevalence of OSA in patients with MCI is high, 70%, while there is no significant association between having OSA and MCI. Furthermore, the prevalence rates were influenced by OSA diagnostic method and patient recruitment location (community or clinic based). Our findings suggest that OSA may be prevalent in individuals with MCI. Due to the cross-sectional nature of the included studies, we were unable to evaluate a temporal relationship between the conditions (i.e., the occurrence of OSA before or after the development of MCI in patient population). Nevertheless, the clinical impact inferred due to the additive burden of these two disorders demands a closer look into their relationship.
Population recruitment locations
The studies were conducted in six different countries and enrolled a patient population from a community, general (i.e., public health centers) or specialty neurology clinics that likely contributed to the variations in OSA rates. A clinic-based elderly population is more likely to have individuals with undiagnosed OSA with accompanying major comorbidities that will primarily prompt these patients to seek help. In turn, OSA may remain undiagnosed in this population due to OSA symptoms of, for example [29], memory and concentration being falsely attributed to the aging process or to other disorders by clinicians, hence, transiently decreasing the OSA point prevalence in the clinic-based sample. Alternatively, OSA prevalence rates in MCI are more discernable in a community-based sample and are likely a better representative of the target population. The study utilizing such population reported an OSA prevalence of 27 and 26% in patients with and without MCI, respectively [24]. This rate closely resembled the OSA prevalence (AHI ≥ 5) of 30% estimated in elderly patients between the ages of 50 and 70 years, in a recent epidemiological study [30]. Hence, the high OSA prevalence noted across studies using a clinic-based sample may not be an appropriate representation of the target population (MCI or controls) and may in fact be the result of selection bias.
Method of OSA diagnosis or screening
The five studies included in this review used different diagnostic methods and criteria to diagnose OSA, which could partially explain the considerable variation in the prevalence rates of OSA. A diagnosis of OSA is made based on an AHI ≥ 5 events/hour for patients reporting symptoms of OSA (e.g. snoring, daytime sleepiness). The prevalence of OSA in MCI for PSG studies using an AHI ≥ 5 was 70 and 71%, respectively [27, 28]. The use of differing definitions for hypopneas in PSG studies has been shown to result in significant variations in the AHI value, which can drastically alter OSA prevalence rates [31]. Both studies, however, had similar apnea and hypopnea definitions according to the American Academy of Sleep Medicine (AASM) guidelines [32]. Diagnostic testing can also be performed using types II-IV portable sleep monitors. One of the five studies used the ApneaLink device and an AHI ≥ 15 events/ hour to diagnose moderate-to-severe OSA demonstrating a prevalence of 27% among the 230 participants with MCI [24]. ApneaLink is a type III portable monitor commonly used for home sleep testing to screen OSA. In adults with moderate-to-high severity of OSA, the ApneaLink has a sensitivity of 75% and specificity of 87% [33]. The specificity of ApneaLink drops to 62% with an AHI cutoff value of 5 that results in mild OSA being undiagnosed [33]. The exclusion of patients with mild OSA in this study population would have resulted in a lower OSA prevalence rate. The Berlin questionnaire [32] is used to screen for high risk patients with OSA and has a pooled sensitivity and specificity of 76 and 45%, respectively, to identify patients with an AHI ≥ 5 events/hour. The study that used the Berlin questionnaire reported a prevalence of 59% among the 138 individuals with MCI [26]. Finally, the study with the lowest prevalence rate employed a patient population with a self-reported OSA diagnosis [2]. The type of OSA diagnostic metric used was not reported. The use of self-reported symptoms would result in significant underestimation of OSA as patients with OSA may be asymptomatic, hence the comparatively low prevalence rate observed in this study. Furthermore, the study used data from the Alzheimer’s disease Neuroimaging Initiative (ADNI) cohort that enrolled only aMCI patients, hence, the associated memory impairments could have partially accounted for the lack in reported information about a previous OSA diagnosis leading to an underestimation of the true prevalence of OSA. Therefore, in memory clinics, a more standardized approach, preferably using objective sleep measurements, needs to be taken when estimating the prevalence of OSA.
Evidence on association between OSA and MCI
Although several prospective cohort studies [1, 16] have demonstrated that patients with OSA have greater neurocognitive deficits, the risk of OSA and subsequent onset of MCI is seldom explored. In the above mentioned ADNI cohort database, patients with OSA had a younger age onset of MCI by a decade compared to those without OSA, even after adjusting for possible confounding variables [2]. Moreover, continuous positive airway pressure (CPAP) therapy conferred a protective effect, essentially delaying the onset of MCI in those individuals being treated for OSA. Similarly, a number of studies have demonstrated a partial reversibility in cognitive dysfunction with CPAP therapy in individuals with OSA, particularly in the domains of attention, vigilance, executive function and memory [34–36]. Finally, a meta-analysis of cross-sectional studies demonstrated that individuals with AD had a 5-fold risk of OSA compared to healthy age-matched controls [17]. Contrary to this, with the exception of one study [2], there were no significant differences in the risk of OSA among individuals with MCI vs. controls. Perhaps, the additive pathological processes and severity of AD makes these individuals prone to OSA development, which may not be present in those with MCI or early AD (i.e. reverse causation). Nonetheless, the results of these studies signify the importance of early recognition and treatment of OSA in possibly diminishing or delaying the future risk of MCI.
Several mechanisms may contribute to the neurocognitive decline in individuals with OSA, including disturbances in oxidative stress, sympathetic activation, endothelial dysfunction, and systemic and vascular inflammation [37, 38]. Long-standing OSA leads to recurrent intermittent hypoxia and alters sleep architecture, which may lead gradually to brain neurodegenerative processes [39]. A recent review proposed several possible mechanisms linking OSA to dementia, highlighting the important roles chronic sleep architecture impairments may play in neurogenesis, synaptic plasticity and memory consolidation [39]. A neuroimaging meta-analysis assessing the neuro-structural differences between patients with OSA and healthy controls reported significant grey matter reductions in the bilateral parahippocampus, left temporal and right frontal lobes of OSA patients [40]. Whilst there is an adverse impact of OSA on the healthy young brain, and this is greater with the aging middle-aged brain [41], the natural assumption is that the additive burden of OSA may exert greater deleterious effects especially to the elderly brain. Untreated OSA can potentially accentuate the progression of MCI and Alzheimer’s disease [2] in cognitively intact individuals due the accumulation of Alzheimer’s disease biomarkers (amyloid beta and tau proteins) [42, 43], through hypoxic insults and/or disrupted sleep architecture [39].
Limitations
Our results should be interpreted with caution since this study has some limitations. First, most of the included studies in our review had a small sample size. A small sampling population can lead to an overestimation of the magnitude of an association and ultimately produce high false-positives. Moreover, it may be difficult to interpret the results from studies with a small sample size due to a wider 95% CI that may lead to an imprecise estimate of the effect. Second, we only looked at studies in English language that may have limited our final study count. Third, with a small number of studies and individuals representing this population, difficulties can arise when attempting to conduct a pooled analysis (i.e. meta-analysis), while adjusting for confounding factors, which can lead to unreliable results. Finally, due to the cross-sectional design of the included studies, evaluating a temporal relationship and associations identified are difficult to interpret.
Conclusions
In summary, the prevalence of OSA in patients with MCI is influenced by OSA diagnostic methods and patient recruitment locations (community or clinic based population). A clinic-based patient population may not appropriately represent general population to estimate OSA prevalence rates. The true OSA prevalence in elderly individuals with MCI may be close to that of the general population with a similar age group, approximately 27%. Longitudinal prospective studies with larger community-based populations and comparable healthy controls, and confirmatory testing are necessary to determine the true prevalence of OSA in MCI. Clinicians caring for patients with OSA and MCI or dementia should consider using standardized methods for diagnosing OSA.
Additional file
PRISMA Checklist. (PDF 398 kb)
Medline (Ovid) Search Terms and Strategy. (DOCX 122 kb)
Acknowledgements
The authors thank Marina Englesakis, HBA, MLIS (Information Specialist, Health Sciences Library, University Health Network, Toronto, ON, Canada) for her assistance with the literature search.
Abbreviations
- ADNI
Alzheimer’s disease Neuroimaging Initiative
- AHI
Apnea Hypopnea Index;
- aMCI
Amnestic Mild Cognitive Impairment
- APOE
Apolipoprotein E
- BMI
body mass index
- CI
Confidence Interval
- CL
Clinic
- COM
Community
- CPAP
Continuous Positive Airway Pressure
- HNR
Heinz Nixdorf Recall
- MCI
Mild Cognitive Impairment
- naMCI
Non-amnestic Mild Cognitive Impairment
- OD
Oxygen desaturation
- OR
Odds Ratio
- OSA
Obstructive Sleep Apnea
- OSAS
Obstructive Sleep Apnea Syndrome
- PSG
Polysomnography
- SD
Standard deviation
- WHO ICTRP
World Health Organization International Clinical Trials Registry Platform
Authors’ contributions
TM, LA and FC contributed to the design of the study and wrote the manuscript. JW, RO and CR contributed to the revision of the manuscript. TM, AN, DP and AA contributed to the data collection, data interpretation and data analysis. All authors read and approved the final manuscript.
Funding
The University Health Network Foundation and the Department of Anesthesia and Pain Medicine, Toronto Western Hospital, University Health Network, University of Toronto.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
J.W- Reports grants from the Ontario Ministry of Health and Long-Term Care, Anesthesia Patient Safety Foundation and Acacia Pharma outside of the submitted work.
F.C- Reports research support from the Ontario Ministry of Health and Long-Term Care, University Health Network Foundation, Acacia Pharma, Medtronics grants to institution outside of the submitted work, Up-to-date royalties, STOP-Bang proprietary to University Health Network.
All other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Talha Mubashir, Email: talmubashir@gmail.com.
Lusine Abrahamyan, Email: lusine.abrahamyan@theta.utoronto.ca.
Ayan Niazi, Email: ayan.niazi.786@gmail.com.
Deween Piyasena, Email: deweenloji@gmail.com.
Abdul A. Arif, Email: abdul.arif@mail.utoronto.ca
Jean Wong, Email: jean.wong@uhn.ca.
Ricardo S. Osorio, Email: Ricardo.OsorioSuarez@nyulangone.org
Clodagh M. Ryan, Email: Clodagh.Ryan@uhn.ca
Frances Chung, Email: frances.chung@uhn.ca.
References
- 1.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstenecker A, Mast B. Mild cognitive impairment: a history and the state of current diagnostic criteria. Int Psychogeriatr. 2015;27(2):199–211. doi: 10.1017/S1041610214002270. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Waring S, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Doody R, Kurz A, et al. Current Concepts in Mild Cognitive Impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Morris CJ. Mild Cognitive Impairment as a Clinical Entity and Treatment Target. Arch Neurol. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 8.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 9.Albert M, DeKosky S, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer's Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachdev PS, Lipnicki D, Kochan NA, et al. The prevalence of mild cognitive impairment in diverse geographical and Ethnocultural regions: the COSMIC collaboration. PLoS One. 2015;10(11):1–19. [DOI] [PMC free article] [PubMed]
- 11.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29(4):1–19. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchie K, Carriere I, Ritchie CW, Berr C, Artero S, Ancelin M. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ. 2010;341(c3885):1–9. doi: 10.1136/bmj.c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic study of aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au B, Dale-McGrath S, Tierney M. Sex differences in the prevalence and incidence of mild cognitive impairment: a meta-analysis. Ageing Res Rev. 2017;35:176–199. doi: 10.1016/j.arr.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Luck T, Riedel-Heller SG, Luppa M, et al. Risk factors for incident mild cognitive impairment – results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe) Acta Psychiatr Scand. 2010;121:260–272. doi: 10.1111/j.1600-0447.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 16.Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurology. 2017;74(10):1237–1245. doi: 10.1001/jamaneurol.2017.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emamian F, Khazaie H, Tahmasian M, et al. The association between obstructive sleep apnea and Alzheimer's disease: a meta-analysis perspective. Front Aging Neurosci. 2016;8:78. doi: 10.3389/fnagi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucks RS, Olaithe M, Eastwood PR. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 19.Beebe D, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26(3):298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 20.Ferini Strambi L, Marelli S, Galbiati A, Castronovo C. Effects of continuous positive airway pressure on cognitition and neuroimaging data in sleep apnea. Int J Psychophysiol. 2013;89:203–212. doi: 10.1016/j.ijpsycho.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Li, Chen Ping, Peng Yating, Ouyang Ruoyun. Role of Oxidative Stress in the Neurocognitive Dysfunction of Obstructive Sleep Apnea Syndrome. Oxidative Medicine and Cellular Longevity. 2016;2016:1–15. doi: 10.1155/2016/9626831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496–508. doi: 10.1016/j.jagp.2016.01.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Llberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 24.Dlugaj M, Weinreich G, Weimar C, et al. Sleep-disordered breathing, sleep quality, and mild cognitive impairment in the general population. J Alzheimers Dis. 2014;41(2):479–497. doi: 10.3233/JAD-132132. [DOI] [PubMed] [Google Scholar]
- 25.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer's Manual. Available from https://reviewersmanual.joannabriggs.org/: The Joanna Briggs Institute; 2017.
- 26.Guarnieri B, Adorni F, Musicco M, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord. 2012;33(1):50–58. doi: 10.1159/000335363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(4):374–381. doi: 10.1097/JGP.0b013e3181e9b976. [DOI] [PubMed] [Google Scholar]
- 28.Wilson G, Terpening Z, Wong K, et al. Screening for sleep apnoea in mild cognitive impairment: the utility of the multivariable apnoea prediction index. Sleep Disorders. 2014;2014:945287. doi: 10.1155/2014/945287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11(5):519–524. doi: 10.5664/jcsm.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peppard PE, Young T, Barnet J, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho V, Crainiceanu C, Punjabi NM, Redline S, Gottlieb DJ. Calibration model for apnea-hypopnea indices: impact of alternative criteria for hypopneas. Sleep. 2015;38(12):1887–1892. doi: 10.5665/sleep.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapur V, Auckley D, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho Jae Hoon, Kim Hyun Jun. Validation of ApneaLink™ Plus for the diagnosis of sleep apnea. Sleep and Breathing. 2017;21(3):799–807. doi: 10.1007/s11325-017-1532-3. [DOI] [PubMed] [Google Scholar]
- 34.Pan YY, Deng Y, Xu X, Liu YP, Liu HG. Effects of continuous positive airway pressure on cognitive deficits in middle-aged patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Chin Med J. 2015;128(17):2365–2373. doi: 10.4103/0366-6999.163385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297–1305. doi: 10.5665/sleep.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kylstra W, Aaronson J, Hofman W, Schmand B. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev. 2013;17:341–347. doi: 10.1016/j.smrv.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141(6):1601–1610. doi: 10.1378/chest.11-2214. [DOI] [PubMed] [Google Scholar]
- 38.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93(12):1778–1794. doi: 10.1002/jnr.23634. [DOI] [PubMed] [Google Scholar]
- 39.Gosselin N, Baril AA, Osorio RS, Kaminska M, Carrier J. Obstructive sleep apnea and the risk of cognitive decline in older adults. Am J Respir Crit Care Med. 2019;199(2):142–148. doi: 10.1164/rccm.201801-0204PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng H, Tsai Y-H, Chen C, et al. Mapping gray matter reductions in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Sleep. 2014;37(1):167–175. doi: 10.5665/sleep.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayalon L, Ancoli-Israel S, Drummond SPA. Obstructive sleep apnea and age: a double insult to brain function? Am J Respir Crit Care Med. 2010;182:413–419. doi: 10.1164/rccm.200912-1805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liguori C, Mercuri NB, Izzi F, et al. Obstructive sleep apnea is associated with early but possibly modifiable alzheimer’s disease biomarkers changes. Sleep. 2017;40(5):1–10. [DOI] [PubMed]
- 43.Osorio RS, Ayappa I, Mantua J, et al. Interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer's disease in cognitively normal elderly individuals. Neurobiol Aging. 2014;35(6):1318–1324. doi: 10.1016/j.neurobiolaging.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist. (PDF 398 kb)
Medline (Ovid) Search Terms and Strategy. (DOCX 122 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.