Can natural selection act on parasites to compromise barriers to cancer?
Characterizing the factors that disrupt the cellular barriers to cancer (e.g., cell-cycle arrest, apoptosis, repression of telomerase, cell adhesion, and asymmetric cell division) and are essential to oncogenesis is necessary to identify targets for therapeutic interventions [1]. Exacerbating causes can contribute to cancer by compromising host restraints on cancer rather than breaking barriers; examples of such exacerbating causes are factors that drive angiogenesis, or increased proliferation during pro-inflammatory responses [1]. All viruses that are recognized by the International Agency for Research on Cancer (IARC) as Group 1 carcinogens, namely human papillomaviruses (HPV), Hepatitis B and C viruses (HBV and HCV), Human Herpes Virus type 8 (HHV-8), and Human T-cell lymphotropic virus type 1 (HTLV-1) [2], break barriers to cancer and therefore generate essential causes of their associated cancers [1]. These may be the result of natural selection. For example, from an evolutionary point of view, it is probably advantageous to a virus that its host cell resists cell death, evades the immune system, and proliferates. Other intracellular organisms (bacteria and unicellular eukaryotes) could similarly benefit from altering the cellular mechanisms that prevent oncogenesis. Indeed, the ability of intracellular bacteria and protozoan parasites to block apoptosis is now broadly recognized [3,4]. Increasing evidence implicates bacteria (certain strains of Escherichia coli, Fusobacterium nucleatum, Salmonella Typhi, Chlamydia trachomatis, and a range of Mycoplasma) and protists (Cryptosporidum parvum, Trichomonas vaginalis, Trypanosoma cruzi, Toxoplasma gondii) in cancer development [2, 5–26].
For extracellular parasites, and in particular helminths, the evolutionary path that could lead them to break the cellular barriers to cancer is more difficult. At present, three multicellular parasites, the trematodes Schistosoma haematobium, Opisthorchis viverrini, and Clonorchis sinensis are recognized as Group 1 carcinogens by IARC, contributing 0.4% to human cancer [2]. However, increased risk of cancer is associated with increasing numbers of other parasites, e.g., species of Echinococcus, Strongyloides, Fasciola, Heterakis, Platynosomum, and Trichuris [27] and is likely broadly underestimated due to the asymptomatic/subclinical nature of some of these infections, wide occurrence among healthcare underserved communities, and long latency between initial infection or exposure and clinical manifestation of cancer. The latest advances in microbiology suggest a new paradigm—multi-microbial factors could explain why and how some parasites breach host barriers to cancer. Herein, we review the literature indicating that microbes can contribute in an essential sense to oncogenesis through their interaction with the host and with parasites.
High prevalence of microbes with oncogenic potential in asymptomatic populations
The composition of the microbiome is thought to result from complex co-evolutionary mechanisms among hosts and microbes. Antagonistic pleiotropy refers to genes that are beneficial early in life, and improve fitness, but become detrimental later in life [28]. Recent studies suggest that microbes and microbial communities can have similar effects: microbiome composition induces a life-history trade-off between life span and reproduction in flies [29]. Thus, natural selection will favor establishment of microbes that are either beneficial to the host or present a low cost of infection early in life, even if these microbes promote oncogenesis later in life. Indeed, the human microbiome includes known and often highly prevalent oncogenic microbes, such as Epstein Barr Virus (EBV), that infect 90% of the human population [30], and many members of the gut microbiota are associated with cancer [6]. Only a small fraction of the population carrying oncogenic microbes develops cancer, suggesting that cofactors that exacerbate the susceptibility to cancer are necessary. Other microbes that are not identified as oncogenic may show high prevalence and present as asymptomatic infection, but may enact essential causes of cancer. Microbial communities previously regarded as commensal may have diverse roles in oncogenesis during the long asymptomatic/subclinical period preceding clinical diagnosis of cancer.
Parasites can modulate the oncogenicity of host-associated microbes
Coinfection by multiple parasites is common in the wild. If we consider that any host-associated microbe can move along the parasitism-mutualism spectrum in a context-dependent manner, coinfection is the norm. Viruses, bacteria, archaea, and eukaryotic parasites have coinhabited the same host lineages for hundreds of millions of years and can either directly interact when they inhabit the same host tissue or indirectly interact via modulation of the host immune system. These interactions can influence tumor development and progression.
A widely appreciated example is the role of Plasmodium falciparum as an indirect risk factor for Burkitt lymphoma, a monoclonal B cells cancer for which EBV infection is generally considered essential [31]. Recent studies have clarified the mechanisms by which P. falciparum contributes to oncogenesis. The immunosuppression associated with P. falciparum malaria leads to an increase in EBV-infected B cells in the germinal center, which dysregulates activation-induced cytidine deaminase expression, leading to DNA damages, including c-myc translocation that should lead to cell apoptosis, but EBV rescues the infected cell by inhibiting apoptosis, effectively leading to Burkitt lymphoma [32–34].
Similarly, infection with Strongyloides stercoralis, a common parasitic nematode, shortens the delay between HTLV-1 infection and the occurrence of T-cell leukemia [35, 36]. S. stercoralis benefits HTLV-1 with a higher proviral load in individuals infected by the roundworm, due to the proliferative expansion of HTLV-1–infected cells [37]. By promoting cell proliferation, S. stercolis is an exacerbating cause of cancer. Infection with HTLV-1 results in a suppressed immune response against helminths and in the reduced efficacy of antiparasitic drugs, which lead to higher prevalence of S. stercoralis infection in HTLV-1–infected individuals [38].
Evidence is accumulating that members of the gut, oral, and vaginal microbiomes could initiate or influence the progression of oncogenesis by different processes, including the induction of a chronic inflammatory state or immune response, altering stem cell dynamics, the biosynthesis of toxic and genotoxic metabolites, and affecting host metabolism [6, 39, 40]. Many parasites significantly alter their host microbiome composition [41]. Outcomes would therefore depend on the individual’s microbiome composition at the time of infection, which would render the association between parasite infection and cancer difficult to resolve.
A positive association between the highly prevalent sexually transmitted protozoan parasite T. vaginalis and cervical neoplasia in women and prostate cancer in men has been reported [13–18, 42]. T. vaginalis infection significantly affects the vaginal microbiome with a shift from a lactobacillus-dominated microbiome to a community of bacteria responsible for the widely spread syndrome of bacterial vaginosis [43]. Metabolites released by the parasite, e.g., indole, support the survival of intracellular sexually transmitted bacteria such as C. trachomatis, which has been independently associated with cancer [44]. Given the positive association between bacterial vaginosis and cervical precancerous lesions [45], studies are needed to clarify the role of the microbiota as a cofactor, or essential factor, for T. vaginalis–associated cancer.
S. haematobium is the causative agent of urogenital schistosomiasis (UGS). This trematode parasite is endemic in 76 countries in Africa and the Middle East, but it can also be found in Europe [46, 47]. UGS is a major risk factor for squamous cell carcinoma of the urinary bladder [48, 49]. Early studies have found that UGS promotes bacterial coinfection and is associated with a high concentration of N-nitroso compounds in the urine, suggesting that infection favors nitrate-reducing bacteria that produce the cancer-inducing nitrosamines [50–52]. More recent studies of the microbiome of noninfected and infected patients also revealed marked differences [53, 54]. In addition, schistosomes can directly interact with bacteria, and Salmonella is known to routinely attach to a range of species of schistosomes [55]. HPV, EBV, and BK polyomavirus (BKV) have been found in a minority of bladder cancers by some investigators, although not by others [56]. Given the potential for some of these viruses and bacteria to cause cancer, studies are needed to test their roles as causative agents for urinary bladder cancer associated with UGS.
Parasites can transmit pro-inflammatory or oncogenic microbes
There are numerous compelling examples of parasites that carry microbes that participate in the infectious process, notably the well-documented Wolbachia-filarial nematodes system [57, 58]. There is even evidence that parasites have received genes from prokaryotic symbionts via horizontal gene transfers, including numerous Wolbachia genes in symbiont-free filarial nematodes [59], the thymidine kinase of C. parvum [60], and the N-acetylneuraminate lyase of T. vaginalis [61], further demonstrating the selective advantage that microbial symbionts confer to their parasitic hosts. Microbial symbionts of parasites can be transmitted to the host and be responsible for inflammatory-associated pathogenesis [62, 63]. In addition, the release of Wolbachia from filarial nematodes and of Trichomonavirus (TVV) from T. vaginalis upon parasite death have significant adverse effects that impair treatment efficiency [62, 63]. Similarly, relapse of Salmonella infection can occur in the absence of antischistosomal treatment, probably because of the antibiotic resistance of Salmonella attached to the blood fluke [64, 65]. Could the ability of parasite-associated microbes to infect host cells, and their role in inflammation, be responsible for oncogenesis, in lieu of their parasitic host?
Infection with the liver fluke O. viverrini is recognized as a definitive cause of cancer, as infection often leads to cholangiocarcinoma (bile duct cancer). The factors that lead to cancer development have not yet been clearly identified [66]. One intriguing hypothesis is the potential for O. viverrini to serve as a vector of the oncogenic bacterium Helicobacter pylori and other bacteria into the biliary tree, triggering the malignant transformation of cholangiocytes [67]. Indeed, H. pylori was found within the gut of O. viverrini [68], and coinfection is associated with higher expression of pro-inflammatory cytokines and more severe hepatobiliary morbidity, suggesting that the bacteria contributes to opisthorchiasis-associated cholangiocarcinoma [69, 70]. Sequencing of prokaryotic 16S genes from O. viverrini revealed the presence of diverse bacteria, including Bordetella, Brochothrix, Burkholderia, Leminorella, Pseudomonas, Serratia, and Sphingomonas [71]. The role of other microbes, such as viruses, in the disease cannot be excluded given the ability of O. viverrini to convey bacteria to the biliary tract.
It is now well recognized that T. vaginalis hosts a complex core microbiome composed of double-stranded RNA (dsRNA) endobiont viruses of the genus TVV and eubacterial Mycoplasma species that substantially increase T. vaginalis pathogenicity by up-regulating pro-inflammatory responses [72]. Transmission of M. hominis, a member of the T. vaginalis microbiome, is associated with malignant transformation and genome instability that promote prostate cancer development in men and skew the adaptive immune response towards a T-helper 17 cell phenotype, thus creating a favorable environment for tumor development [19, 72–75]. TVV can trigger endosomal TLR3/TRIF-dependent pathways, which means that it can penetrate human cells and could also cause oncogenic damage [62]. Both TVV and Mycoplasma can resist clearance by the host and antimicrobial therapy, which explains adverse effects of metronidazole treatment [62, 72]. Should the bacterial or viral symbionts of T. vaginalis induce cancer associated with trichomoniasis, novel therapy could be developed to block malignant transformation in both men and women.
For most parasites, the presence of microbes residing in or directly associated with parasites has not been investigated, and when microbes have been observed, their contribution to oncogenesis has not been assessed. For example, and focusing on parasites listed above that have been linked to cancer prevalence, virus-like particles have been observed in T. cruzi [76], a dsRNA virus has been found in Cryptosporidium and virus load correlates with parasite fecundity [8, 77], Heterakis gallinarum is a vector of the pathogenic bacterium Histomonas meleagridis [78, 79], Trichuris muris hosts a complex bacterial microbiome [80], Schistosoma mansoni might be a vector of HCV [81], and genome sequencing of Fasciola hepatica revealed the presence of the endobacterium, Neorickettsia [82]. In view of these examples, the general lack of information highlights the value of a comprehensive characterization of the viral and bacterial communities associated with parasites [83], and of epidemiological studies that assess the presence of parasites, the prevalence of known microbes, and the transmission to the host, to identify prospective microbial cofactors of oncogenesis.
Conclusions
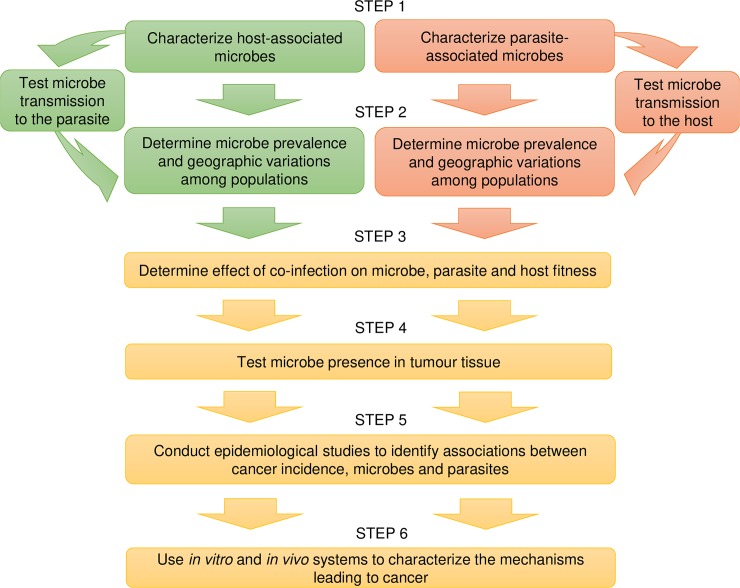
Interindividual variations in microbial communities associated with the host or with the parasite at the time of infection could explain apparently contradictory results in parasite association with cancer among studies due to variations in microbe prevalence among populations. Variations in microbial communities could also explain why some patients, but not others, develop cancer. The task of identifying the contribution of parasites and microbes to cancer can appear overwhelming, but causal inference is feasible with a combination of experimental and epidemiological studies (Fig 1). Following the systematic characterization of microbes associated with parasites, as proposed by the Parasite Microbiome Project [83], and by leveraging the findings from other projects such as the Human Microbiome project [84, 85], epidemiological and clinical studies of cancer could investigate the potential for coinfection by different parasites and microbes, and investigate their interacting effects. Testing for the role of microbes in cancer attributed to parasites has the potential to propel the field forward by revealing cofactors that contribute to the development of precancerous lesions and to the transition from benign to malignant cancer. The presence of newly identified microbes in archived cancer tissues should also be tested to assess their potential role. The payoff for identifying microbial factors that contribute to oncogenesis would be self-evident and compelling with respect to new leads for clinical intervention and prevention. In particular, if a virus plays a causal role or exacerbates cancer progression, vaccine development would be justified, as demonstrated by the protection against both infection and infection-associated cancers delivered by the acclaimed HBV and HPV vaccines [86, 87].
Fig 1. Flowchart of experimental and epidemiological studies that can be undertaken to assess the role of microbes in oncogenesis.
Funding Statement
Support from the National Institutes of Health (NIH) National Cancer Institute, award CA164719 (PJB), the National Institute of Child Health and Human Development, award R01AI079085 (RNF), and the National Institute of Allergy and Infectious Diseases, awards 1RC1AI086788 (RNF) and 1R56AI091889-01A1 (RNF) is acknowledged. The contents of this article do not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wald PW, Swain Ewald HA. Toward a general evolutionary theory of oncogenesis. Evol Appl, 2013; 6(1): 70–81. 10.1111/eva.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. The Lancet Oncology. 2012;13(6):607–15. 10.1016/S1470-2045(12)70137-7 [DOI] [PubMed] [Google Scholar]
- 3.Gao L-Y, Kwaik YA. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends in Microbiology. 2000;8(7):306–13. 10.1016/S0966-842X(00)01784-4 [DOI] [PubMed] [Google Scholar]
- 4.Carmen JC, Sinai AP. Suicide prevention: disruption of apoptotic pathways by protozoan parasites. Molecular Microbiology. 2007;64(4):904–16. 10.1111/j.1365-2958.2007.05714.x [DOI] [PubMed] [Google Scholar]
- 5.Whitmore SE, Lamont RJ. Oral Bacteria and Cancer. PLoS Pathog. 2014;10(3):e1003933 10.1371/journal.ppat.1003933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulbright LE, Ellermann M, Arthur JC. The microbiome and the hallmarks of cancer. PLoS Pathog. 2017;13(9):e1006480 10.1371/journal.ppat.1006480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang S, Li JY, Wu J, Meng L, Shou CC. Mycoplasma infections and different human carcinomas. World J gastroenterol. 2001;7(2):266–9. Epub 04/15. 10.3748/wjg.v7.i2.266 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins MC, Higgins J, Abrahante JE, Kniel KE, O’Brien C, Trout J, et al. Fecundity of Cryptosporidium parvum is correlated with intracellular levels of the viral symbiont CPV. Int J Parasitol. 2008;38(8):1051–5. 10.1016/j.ijpara.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Shebl FM, Engels EA, Goedert JJ. Opportunistic intestinal infections and risk of colorectal cancer among people with AIDS. AIDS res hum retrovir. 2012;28(9):994–9. 10.1089/AID.2011.0185 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osman M, Benamrouz S, Guyot K, Baydoun M, Frealle E, Chabe M, et al. High association of Cryptosporidium spp. infection with colon adenocarcinoma in Lebanese patients. PLoS ONE. 2017;12(12):e0189422–e. 10.1371/journal.pone.0189422 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Certad G, Benamrouz S, Guyot K, Mouray A, Chassat T, Flament N, et al. Fulminant cryptosporidiosis after near-drowning: a human Cryptosporidium parvum strain implicated in invasive gastrointestinal adenocarcinoma and cholangiocarcinoma in an experimental model. App enviro microbiol. 2012;78(6):1746–51. 10.1128/AEM.06457-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Certad G, Ngouanesavanh T, Guyot K, Gantois N, Chassat T, Mouray A, et al. Cryptosporidium parvum, a potential cause of colic adenocarcinoma. Infect agents cancer. 2007;2:22–. 10.1186/1750-9378-2-22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viiki M. Gynaecological Infections as Risk Determinants of Subsequent Cervical Neoplasia AU—Viikki, Merja. Acta Oncologica. 2000;39(1):71–5. 10.1080/028418600431003 [DOI] [PubMed] [Google Scholar]
- 14.Li C-D, Zhang W-Y, Wu M-H, Zhang S-W, Zhou B-L, Zhu L, et al. [Analysis of high risk factors associated with cervical intraepithelial neoplasia in married women aged 25–54 years in Beijing between 2007–2008]. Zhonghua Fu Chan Ke Za Zhi. 2010;45(10):757–61. . [PubMed] [Google Scholar]
- 15.Yap EH, Ho TH, Chan YC, Thong TW, Ng GC, Ho LC, et al. Serum antibodies to Trichomonas vaginalis in invasive cervical cancer patients. Genit med. 1995;71(6):402–4. 10.1136/sti.71.6.402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutcliffe S, Giovannucci E, Alderete JF, Chang T-H, Gaydos CA, Zenilman JM, et al. Plasma Antibodies against Trichomonas vaginalis and Subsequent Risk of Prostate Cancer. Cancer Epidemiol Biomark Prevent. 2006;15(5):939 10.1158/1055-9965.EPI-05-0781 [DOI] [PubMed] [Google Scholar]
- 17.Sutcliffe S, Alderete JF, Till C, Goodman PJ, Hsing AW, Zenilman JM, et al. Trichomonosis and subsequent risk of prostate cancer in the Prostate Cancer Prevention Trial. Inter J cancer. 2009;124(9):2082–7. 10.1002/ijc.24144 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitteregger D, Aberle SW, Makristathis A, Walochnik J, Brozek W, Marberger M, et al. High detection rate of Trichomonas vaginalis in benign hyperplastic prostatic tissue. Medic Microbiol Immunol. 2012;201(1):13–116. [DOI] [PubMed] [Google Scholar]
- 19.Twu O, Dessí D, Vu A, Mercer F, Stevens GC, de Miguel N, et al. Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Nat Acad Sci. 2014;111(22):8179 10.1073/pnas.1321884111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacerdote de Lustig E, Puricelli L, Bal E, Lansetti J. Association of Chagas disease and cancer. Medicina. 1980;40(1):43–6. [PubMed] [Google Scholar]
- 21.Murta E, Oliveira G, Prado Fde O, De Souza M, BM TM, Adad S. Association of uterine leiomyoma and Chagas' disease. Am J Trop Med Hyg. 2002;66(3):321–4. 10.4269/ajtmh.2002.66.321 [DOI] [PubMed] [Google Scholar]
- 22.Oliveira E, Lette M, Ostermayer A, Almeida A, Moreira H. Chagasic megacolon associated with colon cancer. Am J Trop Med Hyg. 1997;56(6):586–98. [DOI] [PubMed] [Google Scholar]
- 23.Manoel-Caetano FdS Borim AA, Caetano A, Cury P, amp x, et al. Cytogenetic alterations in chagasic achalasia compared to esophageal carcinoma. Cancer Gen Cytogen. 2004;149(1):17–22. 10.1016/S0165-4608(03)00274-7 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Li Q, Hu P, Cheng H, Huang G. Two case reports of pituitary adenoma associated with Toxoplasma gondii infection. J clin pathol. 2002;55(12):965–6. 10.1136/jcp.55.12.965 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khurana S, Dubey M, Malla N. Association of Parasitic Infections and Cancers. Ind J Medic Microbiol. 2005;23(2):74–9. 10.4103/0255-0857.16044 [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner M. Theileria annulata promotes Src kinase-dependent host cell polarization by manipulating actin dynamics in podosomes and lamellipodia. Cell Microbiol. 2011;13(4):538–53. 10.1111/j.1462-5822.2010.01553.x [DOI] [PubMed] [Google Scholar]
- 27.Machicado C, Marcos LA. Carcinogenesis associated with parasites other than Schistosoma, Opisthorchis and Clonorchis: A systematic review. Inter J Cancer. 2016;138(12):2915–21. 10.1002/ijc.30028 [DOI] [PubMed] [Google Scholar]
- 28.Williams GC. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution. 1957;11(4):398–411. [Google Scholar]
- 29.Gould AL, Zhang V, Lamberti L, Jones EW, Obadia B, Korasidis N, et al. Microbiome interactions shape host fitness. Proc Nat Acad Sci. 2018;115(51):E11951–E60. 10.1073/pnas.1809349115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzellos S, Farrell PJ. Epstein-Barr Virus Sequence Variation—Biology and Disease. Pathogens. 2012;1(2):156 10.3390/pathogens1020156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, et al. Burkitt's lymphoma. The Lancet. 2012;379(9822):1234–44. 10.1016/S0140-6736(11)61177-X [DOI] [PubMed] [Google Scholar]
- 32.Thorley-Lawson D, Deitsch KW, Duca KA, Torgbor C. The Link between Plasmodium falciparum Malaria and Endemic Burkitt's Lymphoma-New Insight into a 50-Year-Old Enigma. PLoS Pathog. 2016;12(1):e1005331–e. 10.1371/journal.ppat.1005331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robbiani DF, Deroubaix S, Feldhahn N, Oliveira TY, Callen E, Wang Q, et al. Plasmodium Infection Promotes Genomic Instability and AID-Dependent B Cell Lymphoma. Cell. 2015;162(4):727–37. 10.1016/j.cell.2015.07.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torgbor C, Awuah P, Deitsch K, Kalantari P, Duca KA, Thorley-Lawson DA. A Multifactorial Role for P. falciparum Malaria in Endemic Burkitt's Lymphoma Pathogenesis. PLoS Pathog. 2014;10(5):e1004170 10.1371/journal.ppat.1004170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plumelle Y, Gonin C, Edouard A, Bucher B, Thomas L, Brebion A, et al. Effect of Strongyloides stercoralis infection and eosinophilia on age at onset and prognosis of adult T-cell leukemia. Am J Clin Pathol. 1997;107(1):81–7. 10.1093/ajcp/107.1.81 [DOI] [PubMed] [Google Scholar]
- 36.Montes M, Sawhney C, Barros N. Strongyloides stercoralis: there but not seen. Curr opin infect dis. 2010;23(5):500–4. 10.1097/QCO.0b013e32833df718 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabet A-S, Mortreux F, Talarmin A, Plumelle Y, Leclercq I, Leroy A, et al. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene. 2000;19:4954 10.1038/sj.onc.1203870 [DOI] [PubMed] [Google Scholar]
- 38.Carvalho EM, Da Fonseca Porto A. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Par Immunol. 2004;26(11‐12):487–97. 10.1111/j.0141-9838.2004.00726.x [DOI] [PubMed] [Google Scholar]
- 39.Champer M, Wong A, Champer J, Brito I, Messer P, Hou J, et al. The role of the vaginal microbiome in gynaecological cancer. BJOG: Inter J Obstet Gynaecol. 2018;125(3):309–15. 10.1111/1471-0528.14631 [DOI] [PubMed] [Google Scholar]
- 40.Garrett WS. Cancer and the microbiota. Science (New York, NY). 2015;348(6230):80–6. 10.1126/science.aaa4972 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung JM, Graham AL, Knowles SCL. Parasite-Microbiota Interactions With the Vertebrate Gut: Synthesis Through an Ecological Lens. Front microbiol. 2018;9:843–. 10.3389/fmicb.2018.00843 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Twu O, Dessí D, Vu A, Mercer F, Stevens GC, de Miguel N, et al. <em>Trichomonas vaginalis</em> homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proceedings of the National Academy of Sciences. 2014;111(22):8179 10.1073/pnas.1321884111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onderdonk AB, Delaney ML, Fichorova RN. The Human Microbiome during Bacterial Vaginosis. Clin microbiol rev. 2016;29(2):223–38. Epub 02/10. 10.1128/CMR.00075-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aiyar A, Quayle AJ, Buckner LR, Sherchand SP, Chang TL, Zea AH, et al. Influence of the tryptophan-indole-IFNγ axis on human genital Chlamydia trachomatis infection: role of vaginal co-infections. Front cell infect microbiol. 2014;4:72–. 10.3389/fcimb.2014.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sodhani P, Gupta S, Gupta R, Mehrotra R. Bacterial Vaginosis and Cervical Intraepithelial Neoplasia: Is there an Association or is Co-Existence Incidental? Asia Pac J cancer prevent APJCP. 18(5):1289–92. 10.22034/APJCP.2017.18.5.1289 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berry A, Moné H, Iriart X, Mouahid G, Aboo O, Boissier J, et al. Schistosomiasis haematobium, Corsica, France. Emerg infect dis. 2014;20(9):1595–7. 10.3201/eid2009.140928 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta tropica. 2002;82(2):139–46. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.IARC. Biological agents Volume 100B A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- 49.IARC. Schistosomes, Liver Flukes and Helicobacter Pylori. IARC working group on the evaluation of carcinogenic risks to humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 50.Hicks RM, Walters CL, Elsebai I, Aasser AB, Merzabani ME, Gough TA. Demonstration of nitrosamines in human urine: preliminary observations on a possible etiology for bladder cancer in association with chronic urinary tract infections. Proc Roy Soc Med. 1977;70(6):413–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hicks RM, Walters CL, Elsebai I, Aasser AB, Merzabani ME, Gough TA. Demonstration of nitrosamines in human urine: preliminary observations on a possible etiology for bladder cancer in association with chronic urinary tract infections. Proceedings of the Royal Society of Medicine. 1977;70(6):413–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hicks R, Ismail M, Walters C, Beecham P, Rabie M, El Alamy M. Association of bacteriuria and urinary nitrosamine formation with Schistosoma haematobium infection in the Qalyub area of Egypt. Trans R Soc Trop Med Hyg. 1982;76(4):519–27. 10.1016/0035-9203(82)90153-5 [DOI] [PubMed] [Google Scholar]
- 53.Adebayo AS, Survayanshi M, Bhute S, Agunloye AM, Isokpehi RD, Anumudu CI, et al. The microbiome in urogenital schistosomiasis and induced bladder pathologies. PLoS Negl Trop Dis. 2017;11(8):e0005826 10.1371/journal.pntd.0005826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ajibola O, Rowan AD, Ogedengbe CO, Mshelia MB, Cabral DJ, Eze AA, et al. Urogenital schistosomiasis is associated with signatures of microbiome dysbiosis in Nigerian adolescents. Sci Rep. 2019;9(1):829 10.1038/s41598-018-36709-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsiao A, Toy T, Seo HJ, Marks F. Interaction between Salmonella and Schistosomiasis: A Review. PLoS Pathog. 2016;12(12):e1005928 10.1371/journal.ppat.1005928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ewald PW, Swain Ewald HA. Joint Infectious causation of human cancers. Adv Parasitol. 2014; 84. p. 1–26. [DOI] [PubMed] [Google Scholar]
- 57.Fenn K, Blaxter M. Are filarial nematode Wolbachia obligate symbionts? Trends Ecol Evol. 2004;19(4):163–6. 10.1016/j.tree.2004.01.002 [DOI] [PubMed] [Google Scholar]
- 58.Taylor MJ, Bandi C, Hoerauf A. Wolbachia.Bacterial Endosymbionts of Filarial Nematodes. In: Baker JR, Muller R, Rollinson D, editors. Adv Parasitol. 2005. 60. p. 245–84. 10.1016/S0065-308X(05)60004-8 [DOI] [PubMed] [Google Scholar]
- 59.McNulty SN, Foster JM, Mitreva M, Dunning Hotopp JC, Martin J, Fischer K, et al. Endosymbiont DNA in Endobacteria-Free Filarial Nematodes Indicates Ancient Horizontal Genetic Transfer. PLoS ONE. 2010;5(6):e11029 10.1371/journal.pone.0011029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Striepen B, Pruijssers AJP, Huang J, Li C, Gubbels M-J, Umejiego NN, et al. Gene transfer in the evolution of parasite nucleotide biosynthesis. Proc Nat Acad Sci USA. 2004;101(9):3154 10.1073/pnas.0304686101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Koning AP, Brinkman FSL, Jones SJM, Keeling PJ. Lateral Gene Transfer and Metabolic Adaptation in the Human Parasite Trichomonas vaginalis. Mol Biol Evol. 2000;17(11):1769–73. 10.1093/oxfordjournals.molbev.a026275 [DOI] [PubMed] [Google Scholar]
- 62.Fichorova RN, Lee Y, Yamamoto HS, Takagi Y, Hayes GR, Goodman RP, et al. Endobiont viruses sensed by the human host ‚ beyond conventional antiparasitic therapy. PLoS ONE. 2012;7(11):e48418 10.1371/journal.pone.0048418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor MJ, Cross HF, Ford L, Makunde WH, Prasad GBKS, Bilo K. Wolbachia bacteria in filarial immunity and disease. Parasite Immunology. 2001;23(7):401–9. 10.1046/j.1365-3024.2001.00400.x [DOI] [PubMed] [Google Scholar]
- 64.Gendrel D, Richard-Lenoble D, Kombila M, Engohan E, Nardou M, Moussavou A, et al. Schistosoma Intercalatum and Relapses of Salmonella Infection in Children. Am J Trop Med Hyg. 1984;33(6):1166–9. 10.4269/ajtmh.1984.33.1166 [DOI] [PubMed] [Google Scholar]
- 65.Barnhill AE, Novozhilova E, Day TA, Carlson SA. Schistosoma-associated Salmonella resist antibiotics via specific fimbrial attachments to the flatworm. Par Vect. 2011;4(1):123 10.1186/1756-3305-4-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Tong H, Brindley PJ, Meyer CG, Velavan TP. Par Infect Carcinogen Hum Malign. EBioMedicine. 2016;15:12–23. 10.1016/j.ebiom.2016.11.034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chng KR, Chan SH, Ng AHQ, Li C, Jusakul A, Bertrand D, et al. Tissue microbiome profiling identifies an enrichment of specific enteric bacteria In Opisthorchis viverrini associated cholangiocarcinoma. EBioMedicine. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deenonpoe R, Chomvarin C, Pairojkul C, Chamgramol Y, Loukas A, Brindley PJ, et al. The carcinogenic liver fluke Opisthorchis viverrini is a reservoir for species of Helicobacter. APJCP. 2015;16(5):1751–8. 10.7314/apjcp.2015.16.5.1751 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dangtakot R, Pinlaor S, Itthitaetrakool U, Chaidee A, Chomvarin C, Sangka A, et al. Coinfection with Helicobacter pylori and Opisthorchis viverrini Enhances the Severity of Hepatobiliary Abnormalities in Hamsters. Infect Immun. 2017;85(4):e00009–17. 10.1128/IAI.00009-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deenonpoe R, Mairiang E, Mairiang P, Pairojkul C, Chamgramol Y, Rinaldi G, et al. Elevated prevalence of Helicobacter species and virulence factors in opisthorchiasis and associated hepatobiliary disease. Sci Rep. 2017;7:42744 10.1038/srep42744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plieskatt JL, Deenonpoe R, Mulvenna JP, Krause L, Sripa B, Bethony JM, et al. Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB J. 2013;27(11):4572–84. 10.1096/fj.13-232751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fichorova R, Fraga J, Rappelli P, Fiori PL. Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Res Microbiol. 2017;168(9):882–91. 10.1016/j.resmic.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Namiki K, Goodison S, Porvasnik S, Allan RW, Iczkowski KA, Urbanek C, et al. Persistent Exposure to Mycoplasma Induces Malignant Transformation of Human Prostate Cells. PLoS ONE. 2009;4(9):e6872 10.1371/journal.pone.0006872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan S, Zakariah M, Palaniappan S. Computational prediction of Mycoplasma hominis proteins targeting in nucleus of host cell and their implication in prostate cancer etiology. Tumor Biol. 2016;37(8):10805–13. [DOI] [PubMed] [Google Scholar]
- 75.Alves JJP, De Medeiros Fernandes TAA, De Araújo JMG, Cobucci RNO, Lanza DCF, Bezerra FL, et al. Th17 response in patients with cervical cancer. Oncol lett. 2018;16(5):6215–27. Epub 09/21. 10.3892/ol.2018.9481 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernández-Presas AM, Padilla-Noriega L, Becker I, Robert L, Jiménez JA, Solano S, et al. Enveloped and non-enveloped viral-like particles in Trypanosoma cruzi epimastigotes. Rev Inst Med Trop. 2017;59:e46–e. 10.1590/S1678-9946201759046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nibert ML, Woods KM, Upton SJ, Ghabrial SA. Cryspovirus: a new genus of protozoan viruses in the family Partitiviridae. Arch Virol. 2009;154(12):1959–65. 10.1007/s00705-009-0513-7 [DOI] [PubMed] [Google Scholar]
- 78.Gibbs B. The occurrence of the protozoan parasite Histomonas meleagridis in the adults and eggs of the cecal worm Heterakis gallinae. J Protozool. 1962;9:288–93. [DOI] [PubMed] [Google Scholar]
- 79.Springer WT, Johnson J, Reid WM. Transmission of histomoniasis with male Heterakis gallinarum (Nematoda). Parasitol. 2009;59(2):401–5. Epub 04/06. 10.1017/S0031182000082378 [DOI] [PubMed] [Google Scholar]
- 80.White EC, Houlden A, Bancroft AJ, Hayes KS, Goldrick M, Grencis RK, et al. Manipulation of host and parasite microbiotas: Survival strategies during chronic nematode infection. Sci Adv. 2018;4(3):eaap7399 10.1126/sciadv.aap7399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abou-Zied A, El-Beltagy T, Tantawy H, Soliman R, Badr F. Studies on the genomic association between schistosomiasis and hepatitis C virus infection. Clin Cancer Invest J. 2015;4(3):318–22. 10.4103/2278-0513.151937 [DOI] [Google Scholar]
- 82.McNulty SN, Tort JF, Rinaldi G, Fischer K, Rosa BA, Smircich P, et al. Genomes of Fasciola hepatica from the Americas Reveal Colonization with Neorickettsia Endobacteria Related to the Agents of Potomac Horse and Human Sennetsu Fevers. PLoS Genet. 2017;13(1):e1006537–e. 10.1371/journal.pgen.1006537 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dheilly NM, Bolnick D, Bordenstein SR, Brindley PJ, Figueres C, Holmes EC, et al. Parasite Microbiome Project: Systematic investigation of microbiome dynamics within and across parasite-host interactions. mSystems. 2017;2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.The Integrative Human Microbiome Project: Dynamic Analysis of Microbiome-Host Omics Profiles during Periods of Human Health and Disease. Cell Host & Microbe. 2014;16(3):276–89. 10.1016/j.chom.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. The Lancet. 2009;374(9686):301–14. 10.1016/S0140-6736(09)61248-4 [DOI] [PubMed] [Google Scholar]
- 87.Chang M-H, You S-L, Chen C-J, Liu C-J, Lai M-W, Wu T-C, et al. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology. 2016;151(3):472–80.e1. 10.1053/j.gastro.2016.05.048 [DOI] [PubMed] [Google Scholar]