Abstract
Nanocellulose has been subjected to a wide range of chemical modifications towards increasing its potential in certain fields of interest. These modifications either modulated the chemistry of the nanocellulose itself or introduced certain functional groups onto its surface, which varied from simple molecules to polymers. Among many, aliphatic and aromatic mono- and di-isocyanates are a group of chemicals that have been used for a century to modify cellulose. Despite only being used recently with nanocellulose, they have shown great potential as surface modifiers and chemical linkers to graft certain functional chemicals and polymers onto the nanocellulose surface. This review discusses the modification of cellulose and nanocellulose using isocyanates including phenyl isocyanate (PI), octadecyl isocyanate (OI), toluene diisocyanate (TDI), diphenylmethane diisocyanate (MDI), hexamethylene diisocyanate (HMDI), and their derivatives and polymers. It also presents the most commonly used nanocellulose modification strategies including their advantages and disadvantages. It finally discusses the challenges of using isocyanates, in general, for nanocellulose modification.
Keywords: cellulose, nanocellulose, isocyanate, surface, modification, functionalization
1. Introduction
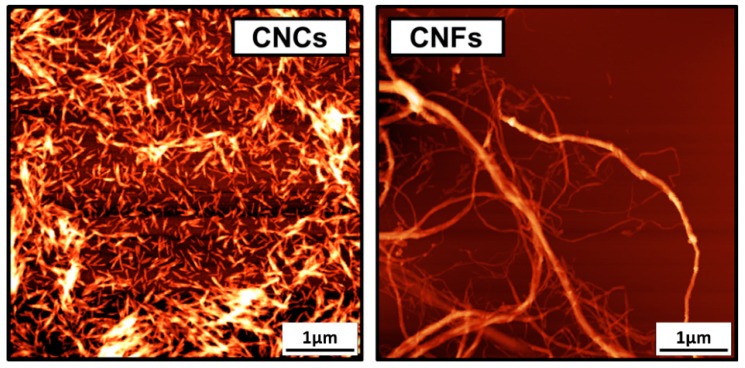
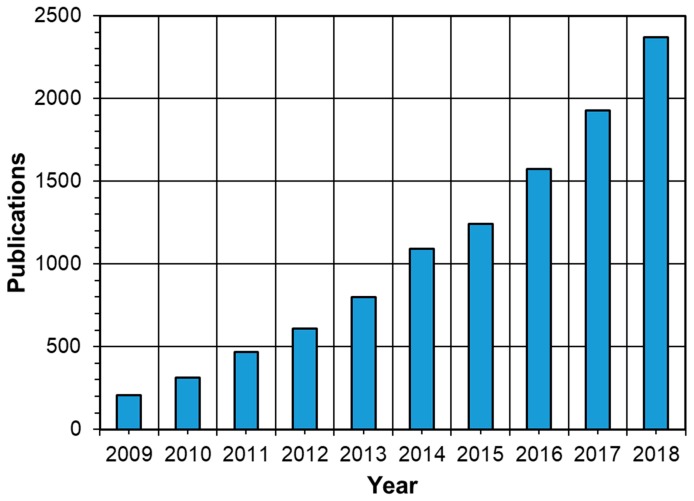
Cellulose is Earth’s most abundant biopolymer. It represents 40–60% of wood mass and can be extracted in the form of 20–40 µm thick fibers upon pulping [1,2]. Apart from its traditional use in the paper and packaging industries and its growing conversion into textile fibers, cellulose can be processed into functional nanoparticles [3]. Among these, cellulose nanocrystals (CNCs) are crystalline nano-rods with a thickness of 3–10 nm and a length of few hundred nanometers (Figure 1) [4]. They are extracted from pulp fibers using an acid-mediated procedure, which has already been industrialized [5]. They can also be extracted directly from wood and lignocelluloses using a variety of reagents and processes [6,7,8]. CNCs with a length in the micrometers can also be obtained from tunicate cellulose [9]. Cellulose nanofibrils (CNFs), another form of nanocellulose, are semi-crystalline spaghetti-like nanoparticles with a thickness of 5–30 nm and a length of few micrometers (Figure 1) [4]. They are produced by the mechanical fibrillation of pulp fibers using a wide range of techniques including microfluidization and homogenization [3]. In addition to their high mechanical properties, biodegradability, and high surface area, CNCs and CNFs are famous for the possibility to modify their surfaces through the abundant hydroxyl groups. They can also orient themselves in 1D, 2D, and 3D nanostructures forming functional liquid crystalline structures [10]. Due to these interesting properties, CNCs and CNFs have shown great potential in a wide range of applications including automotive industry [11], drug delivery [12], tissue engineering [13], packaging [14], and water filtration [15]. As a result, the number of publications and patents on nanocellulose per year increased from 208 in 2009 to 2372 in 2018 (Figure 2). This has also led to the establishment of more than 20 nanocellulose production facilities in the last two decades such as CelluForce, Innventia, and Blue Goose Biorefinaries [3].
Figure 1.
Atomic Force Microscopy images (5 × 5 µm) of CNCs and CNFs. (The images were obtained by the authors using Agilent 5500 AFM (Keysight Technologies, Santa Rosa, CA, USA) for CNC and CNF samples purchased from the University of Maine).
Figure 2.
The number of publications on nanocellulose in the last decade indicating the increasing interest in nanocellulosic materials (Web of Science, July 2019, nanocellulose; cellulose nanocrystals/whiskers/fibers/fibrils; nanocrystalline cellulose; micro/nanofibrillated cellulose).
The surface modification of nanocellulose through its hydroxyl groups has significantly increased its potential. A wide range of chemical functionalities has been placed on nanocellulose surface through simple reactions such as oxidation and acetylation [16,17]. Sometimes, the reaction involved the grafting of functional materials and polymers onto the surface. These modifications aimed at modulating the surface properties of nanocellulose to improve its processing with nonpolar matrices [18,19,20], or to change its affinity to certain polar and nonpolar molecules [21,22]. Other times, the modification aimed at placing functional groups onto the nanocellulose surface to target certain applications [23]. In this case, a chemical linker was often needed to bind these functional groups to nanocellulose, whether they were simple compounds or polymers. Among many modifiers and linkers, aliphatic and aromatic isocyanates have attracted an increasing attention in the recent years for nanocellulose modification although they have been used for a century with cellulose. They are a very interesting group of chemicals that are most famous for making polyurethanes through their reactions with polyols [24].
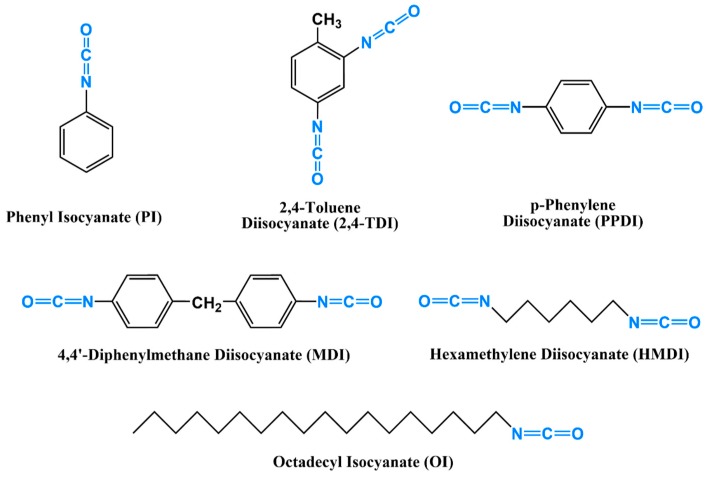
Aliphatic and aromatic mono-isocyanates such as phenyl isocyanate (PI) and n-octadecyl isocyanate (OI), and di-isocyanates such as toluene diisocyanate (TDI), diphenylmethane diisocyanate (MDI), hexamethylene diisocyanate (HMDI), and their polymeric forms have all been used for the modification of cellulose and nanocellulose (Figure 3) [25,26,27,28]. PI and OI were mainly used to decrease the hydrophilicity of cellulose and nanocellulose as they do not have the necessary second isocyanate to function as a linker, while TDI, PPDI, MDI, and HMDI have been used both as surface modifiers and chemical linkers [29,30]. TDI, MDI, and HMDI differ mainly in their molecular rigidity, which is mainly dependent on the benzene rings. MDI is the most rigid because of the two benzene rings while HMDI is the most flexible because of the aliphatic chain. This, for sure, has a significant impact on the mechanical properties of the materials of which they become a part [31]. For instance, the MDI-based polyurethane foams are more rigid than those made using TDI [32].
Figure 3.
The most commonly used aromatic and aliphatic mono- and di-isocyanates for cellulose and nanocellulose modification.
TDI is a very interesting di-isocyanate as the isocyanates of its 2,4-isomer differ in their reactivity. The ortho isocyanate is 5–10 time less reactive than the para isocyanate due to the steric hindrance from the neighboring methyl group [33,34]. This makes 2,4-TDI very advantageous for binding materials to each other as one material can selectively react with the para isocyanate followed by the reaction of the other material with the ortho one. In addition to price, this explains the less frequent use of 2,6-TDI. TDI is often sold as an 80%/20% (2,4/2,6) mixture of its isomers.
To facilitate the reaction between isocyanates and the hydroxyl groups of nanocellulose and the resultant formation of polyurethane bonds, amines such as triethylamine were found to be excellent catalysts although they could also facilitate the self-polymerization of isocyanates as a side reaction [35].
This review discusses the use of aliphatic and aromatic mono- and di-isocyanates in their molecular and polymeric forms for the surface modification of both cellulose and nanocellulose. For each material, the literature reports will be categorized based on the goal of the surface modification. Some of these reports followed application-oriented approaches, which focused on modifying cellulose and nanocellulose to function in certain applications while other reports were property-oriented, which aimed at improving the properties of cellulose and nanocellulose in general, such as hydrophobicity and thermal stability, without targeting a specific application. Most of the reports; however, focused on the use of isocyanates to facilitate the processing of cellulose and nanocellulose in nonpolar thermoplastic and thermoset matrices to prepare reinforced composites with improved interfacial adhesion.
2. General Scenarios of Nanocellulose Modification Using Isocyanates
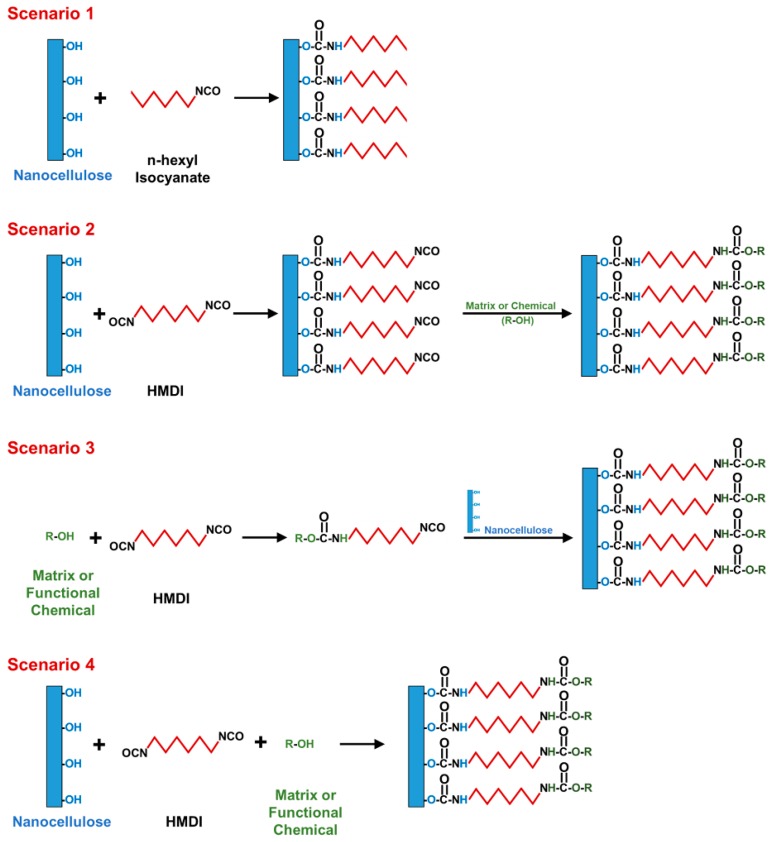
Most of the reported reactions between nanocellulose and isocyanates took place through one of four scenarios. The first scenario represents the reaction of nanocellulose with a mono-isocyanate carrying either a hydrophobic (alkyl or aryl) [36,37] or a functional group [38] (Figure 4). In case of hydrophobization, the modified nanocellulose is usually blended/mixed with a nonpolar matrix to produce a nanocomposite [39,40]. The matrices in this scenario were mainly thermoplastic petroleum-based polymers such as polystyrene, polypropylene, and polyethylene. Sometimes, the reaction between the isocyanate and nanocellulose took place while processing both with the thermoplastic polymer [41]. Scenario 1 was rarely used with di-isocyanates as there is no need for a second isocyanate group in this scenario [42]. Some of those rare cases assumed that both isocyanates reacted with the nanocellulose (cross-linking).
Figure 4.
Overview of the use of isocyanates and di-isocyanates for nanocellulose modification (hexyl isocyanate and HMDI as examples).
Di-isocyanates were mainly used to (1) bind functional groups onto the nanocellulose surface or to (2) support its processing with thermoset matrices. In the first case, two scenarios were used (Figure 4). In Scenario 2, the di-isocyanate is reacted with nanocellulose through one of its isocyanates and the free isocyanate is reacted then with a functional polymer or molecule [43]. Sometimes the free isocyanate is reacted with a non-functional alcohol to obtain a hydrophobic nanocellulose [44]. In Scenario 3, the di-isocyanate is reacted with the functional material or polymer at first then the free isocyanate is reacted with the nanocellulose [45]. This scenario seems to be more efficient than Scenario 2 in general as some reports have shown that di-isocyanates, following Scenario 2, could react with nanocellulose using both of their isocyanates making them dysfunctional for the second stage of the reaction [46,47]. Scenario 4 is the most commonly used for the processing of nanocellulose with thermosets such as polyurethanes (Figure 4). In this scenario, a mixture of the nanocellulose, di-isocyanate, and the polyol are cross-linked together at the same time [48,49]. It is important to mention that these fours scenarios have been used similarly for both cellulose and nanocellulose.
3. Cellulose Modification Using Aliphatic and Aromatic Isocyanates
The first report on the use of isocyanates with cellulose seems to go back to 1920 by Charles [50] followed by other reports focusing on the use of the reaction for textile industry to produce cellulose fibers with improved mechanical and thermal properties [51,52,53]. In 1962 and 1970 the reaction between cellulose and isocyanates was further explored by Ellzey et al. and Ohno et al. respectively, with a focus on PI [54,55]. Ohno later studied the reaction of cellulose with di-isocyanates (2,4-TDI and HMDI) [56,57]. Isocyanates have then become more frequently used to modify cellulose for different purposes (Table 1), which can be categorized into:
Table 1.
Summary of the reports on cellulose modification using mono- and di-isocyanates.
| Category | Cellulose | Isocyanate | Matrix/Chemical | Ref |
|---|---|---|---|---|
| Functional Cellulose | Whatman Powder | 2,4-TDI | - | [58] |
| Cellulose Beads | 2,4-TDI or HMDI | - | [59] | |
| MCC | 2,4-TDI | n-Butanol | [44] | |
| Whatman Paper | Cyclohexyl Isocyanate or PI | - | [60] | |
| Cellulose | Phosphonium-containing Isocyanate | - | [61] | |
| Cotton Cellulose | Sulfopropylbetaine or Quaternary Ammonium Salt with a Reactive Isocyanate | - | [38] | |
| MCC | 2,4-TDI | Amino acids | [62,63] | |
| Plant Cellulose | MDI | - | [64] | |
| Cotton Spheres | HMDI, 2,4-TDI, 1,4-PDI | - | [26] | |
| MCC | Substituted PI | - | [27] | |
| Cellulose Oligomers | 3,5-Dimethylphenyl Isocyanate | - | [28] | |
| Cellulose | 3-Chlorophenyl or 4-Chlorophenyl Isocyanate | Silica | [65] | |
| Improving Cellulose Properties | Cellulose | PI, Cyclohexyl Isocyanate, or HMDI | - | [25] |
| Cellulose | Oxime-blocked Isocyanate Oligomers | - | [66] | |
| Sisal Fibers | MDI | Soy-based Resin | [67] | |
| Six Celluloses | Alkenyl Isocyanates | Styrene or Methylacrylate | [68,69] | |
| Four Celluloses | Isocyanate-containing Polystyrene | - | [70,71] | |
| Cotton Fabric | 2,4-TDI and PEG | - | [72] | |
| Cellophane Sheets | HMDI | Betaines | [73] | |
| Cellulose Fabric | 2-Methacryloyloxyethyl Isocyanate | 2-Methacryloyloxyethyl Phosphoryl Choline | [74] | |
| Cellulose Acetate | Phenyl, Propyl, or Butyl Isocyanate | - | [75,76] | |
| Cellulose Processing with Nonpolar Matrices | Aspen Pulp and Sawdust | Poly(methylene(polyphenyl isocyanate)) | PS | [77,78] |
| Pine Pulp | MDI | PS | [79] | |
| Different Celluloses | Alkyl Isocyanates | PP | [80] | |
| Sisal Fibers | 2,4-TDI | PP | [81] | |
| Whatman Fibers | HMDI | PP | [82] | |
| Bagasse Fibers | Polybutadiene Isocyanate | PP | [41] | |
| Birch Pulp | 2,4-TDI | PP/PLA | [83] | |
| Pineapple Leaf Fibers | Poly(methylene(polyphenyl Isocyanate)) or HMDI | PP and PE | [84] | |
| MCC and Fibers | OI | PE | [69] | |
| Pineapple Leaf Cellulose | Poly(methylene(polyphenyl isocyanate)) | PE | [85,86] | |
| Sisal Fibers | 2,4-TDI-g-Cardanol | PE | [87,88] | |
| Pine Pulp | Derivatives of MDI | PP | [89] | |
| MCC | MDI and PPDI | Natural Rubber | [90] | |
| MCC | OI | Epoxidized Soybean Oil Polymer | [91] | |
| Hemp Fibers | 3-Isopropenyl-dimethylbenzyl Isocyanate | Polyester | [92] | |
| Arbocell Fibers | MDI then Ethanol | Thermoplastic Urethane or a Polyamide | [93] | |
| Kenaf Fibers | Blocked MDI | Polyglycol Polyol, 1,4-Butanediol, and MDI | [94] | |
| Pulp Fibers | HMDI | Castor Oil | [95] | |
| Cellulose Acetate | HMDI | Castor Oil | [96] | |
| Eucalyptus Pulp | OI | Cement | [97,98] | |
| Cellulose/ Matrix Cross-Linking | MCC and Pulp | 2,4-TDI | PCL | [99] |
| Cellulose Diacetate | 2,4-TDI | Poly(caprolactone monoacrylate) | [100] | |
| Cellulose Diacetate | 2,4-TDI | Poly(butylene glycol adipate) | [101] | |
| MCC | MDI | Castor Oil/MDI | [102] | |
| Cotton Cloth | Blocked Isocyanate of 2,4-TDI and Phenol | Lignin | [103] |
3.1. Preparation of Functional Cellulose (Scenario 1 and 2)
Cellulose and its derivatives have been functionalized for a variety of applications including water treatment, chromatography, and biotechnological applications. In terms of the biotechnological applications, Gemeiner et al. used in 1977 the reaction between cellulose and 2,4-TDI to prepare cellulose isothiocyanates, whose binding capacity to thiol and amine compounds was studied [58]. The aim was to explore its potential for enzyme immobilization and for solid-phase sequence analysis of peptides and proteins. In the same year, cellulose beads, upon their reaction with 2,4-TDI or HMDI, were also explored for enzyme immobilization [59]. Dierov et al. developed a hydrophobic cellulose-based sorbent to collect the lipase secreted from a fungus (rhizopus–microporous), by reacting microcrystalline cellulose (MCC) with 2,4-TDI through the para isocyanate followed by reacting the ortho isocyanate with n-butanol [44]. A cellulose-based sorbent was also prepared using cyclohexyl isocyanate or PI to be used for the extraction of natural estrogenic hormones [60]. Pend et al. functionalized cellulose using a phosphonium-containing isocyanate to obtain a product which had both antibacterial and antifungal activities against certain microorganisms [61]. Similarly speaking, the reaction of sulfopropylbetaine or quaternary ammonium salt carrying a reactive isocyanate with cotton cellulose led to a cotton imparting excellent antifouling and bactericidal activity with enhanced hydrophilicity, biocompatibility, and mechanical properties [38].
There are two reports on cellulose functionalization for water filtration. In 1983 Sato et al. used the reaction between cellulose and 2,4-TDI to graft amino acids, such as glycine and serine, onto the cellulose to produce a powder capable of adsorbing heavy metals such as copper, zinc, and cadmium [62,63]. Recently in 2018, cellulose was reacted with MDI to develop a sorbent for the remediation of hydrocarbon-polluted water [64].
To developed chiral stationary phases for liquid chromatography, cotton spheres were reacted with HMDI, 2,4-TDI, 1,4-MDI, or substituted PIs [26,27,28]. Chiroptical cellulose was also obtained upon its reaction with 3-chlorophenyl isocyanate or 4-chlorophenyl isocyanate, which formed with silica chiral nematic mesomorphic structures [65].
3.2. Improving Cellulose Properties (Scenario 1 and 2)
In this category, cellulose was treated with isocyanates to mainly reduce its hydrophilicity or improve its biocompatibility. To reduce it hydrophilicity, cellulose was reacted with PI, cyclohexyl isocyanate, HMDI, and oxime-blocked isocyanate oligomers [25,66,67]. For the same goal, Botaro et al. followed a more complicated approach, by which the cellulose fibers were reacted with alkenyl isocyanate followed by its radical polymerization with other monomers (styrene or methylacrylate) to grow hydrophobic polymeric brushes around the fibers [68,69]. A more-direct approach by Trejo-O’Reilly et al. reacted cellulose with an isocyanate-containing polystyrene [70,71]. Badanova et al. hydrophobized a cellulose fabric by immersing it in an aqueous solution of polyethylene glycol followed by impregnation in an organic solution of 2,4-TDI and thermal pressing [72].
Isocyanates were also used to improve cellulose biocompatibility with human blood. For instance, cellulose sheets were reacted with HMDI through one of its isocyanates followed by the reaction of the other isocyanate with betaine-containing molecules [73]. Similarly, a cellulose fabric was reacted with 2-methacryloyloxyethyl isocyanate to allow a following grafting of phosphoryl choline, which improved the biocompatibility of the fabric to function as a hemodialysis material for blood purification [74]. To improve their mechanical, chemical, and thermal properties, cellulose acetate membranes were reacted with phenyl, propyl and butyl isocyanates [75,76].
3.3. Improving Cellulose Processing and Performance with Nonpolar Matrices (Scenario 1 and 3)
In this category, cellulose was either reacted with the isocyanate prior to mixing/compounding with the nonpolar matrix or the reaction took place during compounding by relying on the heat used in the process. Mono-isocyanates, di-isocyanates, and their polymers have all been used in this category while the matrices were mainly petroleum-based thermoplastic polymers such as polystyrene (PS), polypropylene (PP), and polyethylene (PE). The modification led in most of these reports to a significant improvement in the mechanical properties and a drop in the water uptake of the composite.
Cellulose was modified using poly(methylene(polyphenyl isocyanate)) and MDI to improve the interfacial adhesion between cellulose and PS [77,78,79] while the interfacial adhesion in cellulose/PP composites was improved by reacting cellulose with different alkyl isocyanates [80], 2,4-TDI [81], and HMDI [82]. A PP/PLA composite was reinforced by birch pulp fibers after modifying the fibers with 2,4-TDI. The modification improved the mechanical and thermal properties, water resistance of the composite, and its stability upon weathering [83]. In the case of cellulose/PE composites, cellulose was modified using poly(methylene(polyphenyl isocyanate)), HMDI, and OI before compounding [69,84,85,86]. For the same purpose, sisal fibers were functionalized following Scenario 3 using cardanol and 2,4-TDI before compounding with PE [87,88]. In some cases, the isocyanate is used directly as a compatibilizer. For instance, polybutadiene isocyanate and derivatives of MDI were used as compatibilizers in cellulose/PP composites [41,89]. It is assumed that the isocyanate and cellulose reacted during the processing of the composite.
Cellulose has also been modified before being used to reinforce bio-based matrices such as natural rubber [90], epoxidized soybean oil polymer [91], polyesters [92], thermoplastic polyurethanes and polyamides [93,94], and castor oil [95,96]. In few reports, cellulose was modified using OI to improve its processing and stability in fiber-reinforced cement [97,98].
3.4. Cellulose/Matrix Cross-linking (Scenario 3 and 4)
The reports in this category used di-isocyanates (mainly 2,4-TDI) for cross-linking cellulose with the matrix. In some cases, the matrix was reacted with one of the isocyanates of the di-isocyanate then the cellulose was reacted with the free isocyanate (Scenario 3). For instance, poly(caprolactone) (PCL) was reacted with 2,4-TDI then cellulose was reacted with the free isocyanate to form the composite [99]. Copolymers of cellulose acetate with poly(caprolactone monoacrylate) or poly(butylene glycol adipate) were prepared using the same procedure [100,101]. In other cases, cellulose was reacted with the di-isocyanate then cured together with the matrix, which was made of the same di-isocyanate and a polyol such as lignin or castor oil. For instance, MCC was treated with MDI then added to castor oil and MDI to cure all together for forming the composite [102]. Sometimes the cellulose was added directly without modification to the polyol/di-isocyanate mixture then cured together (Scenario 4) [103]. Clearly, cellulose modification prior to curing improved the mechanical performance of the composite more significantly than without it.
4. Nanocellulose Modification Using Aliphatic and Aromatic Isocyanates
Despite their use for cellulose modification for almost a century, isocyanates were used for the first time in 2008 to modify nanocellulose [104]. Two years earlier isocyanates were used to graft polymers onto the surface of starch nanocrystals [105,106]. Since then, the use of isocyanates for nanocellulose modification has become more common. Unlike cellulose, nanocellulose modification took place only under heterogeneous conditions, which means that it mainly happened on the nanocellulose surface. Nanocellulose has up to 15% of its hydroxyls on the surface (excluding the unreactive C3 hydroxyls) [107,108,109]. This high percentage of surface hydroxyls makes nanocellulose more promising than cellulose for making functional cellulosic materials as it allows a more significant grafting of functional molecules on its surface.
The reports on nanocellulose modification fell in the same four categories mentioned earlier for cellulose but with more focus on nanocellulose processing with thermoplastic and thermoset matrices (Table 2):
Table 2.
Summary of the reports on nanocellulose modification using mono- and di-isocyanates.
| Category | CNCs/CNFs | Isocyanate | Matrix/Chemical | Ref |
|---|---|---|---|---|
| Functional Nanocellulose | CNCs | 3,5-Dimethylphenyl Isocyanate | - | [29] |
| CNCs | OI | - | [30] | |
| CNCs | 2,4-TDI | Photocleavable Polymer | [23] | |
| Improving Nanocellulose Properties | CNFs | OI | - | [110] |
| CNCs | 2,4-TDI | Castor Oil | [45] | |
| CNCs | 2,4-TDI | PHBV | [111] | |
| CNFs | HMDI | Alkyl Diamines | [112] | |
| CNFs | HMDI | - | [113] | |
| Nanocellulose Processing with Nonpolar Matrices | CNCs, CNFs | OI | PCL | [39,40] |
| CNCs | OI | PCL | [114] | |
| CNCs | OI | PCL | [115] | |
| CNCs | 2,4-TDI then PCL diol | PCL | [43] | |
| CNCs | PCL with 2,4-TDI | PCL | [104] | |
| CNCs | OI or 4-Phenylbutyl Isocyanate | PBAT | [36,37] | |
| CNCs | OI | PBAT | [116] | |
| CNCs | OI | PBAT | [117] | |
| CNCs | 2,4-TDI | PLA | [42] | |
| CNCs | OI | PLA | [118] | |
| Nanocellulose Matrix Cross-Linking | CNCs | MDI | Certain Polyols | [48,49] |
| CNCs | HMDI | Polyurethane | [119] | |
| CNFs | Polymeric MDI | Lignin-Soy Polyol with Polymer MDI | [120] | |
| CNCs | MDI | Castor Oil and MDI | [121] | |
| CNCs | Isophorone Diisocyanate | Isophorone Diisocyanate and a Trifunctional Polyether Alcohol | [122] | |
| CNFs | Methylenebis(Cyclohexyl Isocyanate) | Methylenebis(Cyclohexyl Isocyanate) with PEG | [123] | |
| CNFs | MDI | Castor Oil Polyol and MDI | [124] | |
| CNFs | Poly(phenyl Isocyanate) | PEG and Poly(methylene(polyphenyl isocyanate)) | [125] | |
| CNCs | Polymeric MDI | Polyether Polyol and Polymeric MDI | [126] | |
| CNCs | Photocurable Isocyanate (3-isopropenyl-α,α-dimethylbenzyl Isocyanate) | Polyether Polyol and 3-Isopropenyl-α,α-dimethylbenzyl Isocyanate | [127] |
4.1. Preparation of Functional Nanocellulose (Scenario 1 and 3)
There are only three reports on nanocellulose functionalization. Similar to previous reports using cellulose, CNCs were reacted with 3,5-dimethylphenyl isocyanate to function as a stationary phase in liquid chromatography with chiral recognition abilities [29]. CNCs were also reacted with OI to function as a water-in-oil Pickering emulsifier [30]. Both reports followed Scenario 1 in Figure 4. Following Scenario 3, a photocleavable polymer was grafted on the surface of CNCs using 2,4-TDI as a linker [23].
4.2. Improving Nanocellulose Properties (Scenario 1,2, and 3)
Almost all the reports here focused on nanocellulose hydrophobization. CNFs were hydrophobized following Scenario 1 by reacting with OI [110] while CNCs were hydrophobized by reacting with castor oil and 2,4-TDI following Scenario 3 [45] and by reacting with poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and 2,4-TDI following Scenario 4. The modified CNCs showed improved thermal stability and hydrophobicity [111]. CNFs with amine groups on the surface were prepared by reacting the CNFs with HMDI and certain alkyl diamines (Scenario 2) [112]. To improve its mechanical properties, a CNF aerogel was cross-linked with HMDI by immersing it in a solution of HMDI in acetone [113].
4.3. Improving Nanocellulose Processing and Performance with Nonpolar Matrices (Scenario 1, 2, and 3)
To be processed with PCL as a matrix, nanocellulose was modified using different approaches. In one approach, nanocellulose was reacted with OI following Scenario 1 and then mixed with PCL [39,40,114,115]. In the other approaches, PCL was grafted on the nanocellulose surface using 2,4-TDI following either Scenario 2 or 3 and the modified nanocellulose was then mixed with PCL as a matrix [43,104]. Nanocellulose was also processed with poly(butylene adipate-co-terephthalate) (PBAT) upon modification with OI or 4-phenylbutyl isocyanate (Scenario 1) [36,37,116,117]. Following Scenario 1 as well, nanocellulose was processed with PLA upon modification with OI or 2,4-TDI [42,118]. All these reports have shown an improved mechanical or thermomechanical composite performance upon nanocellulose modification due to an improved interfacial adhesion between the nanocellulose and the matrix. In terms of moisture/gas barrier properties and biodegradability, the results varied significantly.
4.4. Nanocellulose/Matrix Cross-Linking (Scenario 4)
The reports in this category cover the use of nanocellulose for reinforcing thermosets of different forms (foams, films, coatings), which were made of lignin, castor oil, poly(ethylene glycol) (PEG), polyether polyols, or other polyols. In some of these reports, the nanocellulose was added to the polyol/di-isocyanate mixture to crosslink all together [48,49,119]. In other reports, the nanocellulose was reacted with the di-isocyanate using one of its isocyanates then cross-linked with a mixture of the polyol with the same di-isocyanate [120,121,122,123,124,125,126,127]. An improvement in the mechanical or thermomechanical properties of the thermoset upon nanocellulose modification was the main outcome of these reports. An increase in the glass transition temperature of the thermoset was also observed.
5. Challenges
It is clear that mono- and di-isocyanates have great potential for nanocellulose modification. However, in addition to their reported toxicity, many challenges exist limiting their use [128,129]. One of the main challenges is that isocyanate reactions must take place in moisture-free environments. Otherwise, the isocyanates will get hydrolyzed and cross-linked to form a polyurea. This also requires the nanocellulose to be solvent exchanged to organic solvents prior to the reaction. This is in general problematic because nanocellulose, especially CNFs, tends to aggregate in these solvents affecting the homogeneity of the reaction. To minimize aggregation, ultrasonication is usually used, which is also problematic as it may, to a certain extent, degrade the nanocellulose and/or alter its surface properties [130].
Another issue is the difficulty of controlling the reaction between di-isocyanates and nanocellulose. Di-isocyanates are supposed to react with the surface hydroxyl groups of nanocellulose only through one of their two isocyanates (see Scenario 2 in Figure 4). In reality, this could be impossible to achieve as a significant fraction of the di-isocyanate reacts using both of its isocyanates becoming dysfunctional for any following grafting. This issue could be more challenging to resolve for HMDI compared to 2,4-TDI as the isocyanates of 2,4-TDI are not equally reactive. Moreover, the molecular flexibility of HMDI compared to 2,4-TDI increases the possibility of both of its isocyanates to react with nanocellulose. In terms of reactivity, the ortho isocyanate of 2,4-TDI is 5-10 times less reactive than the para one due to steric hindrance [33,34]. Despite this difference in reactivity, the reaction parameters have a major impact on para/ortho selectivity. Recently, Abushammala has developed a simple method to quantify the ortho isocyanates on the nanocellulose surface upon its reaction with 2,4-TDI [46]. Using this method, it was possible to optimize the reaction between 2,4-TDI and nanocellulose to obtain a maximum para/ortho selectivity of 93%, which means that 93% of the 2,4-TDI molecules reacted with the nanocellulose surface have their ortho isocyanates available for a following grafting [47]. The study also showed that the reaction temperature had a negative impact on selectivity as it minimizes the difference in the reaction kinetics of para and ortho isocyanates [131]. Another possibility to overcome this challenge is to follow Scenario 3 in Figure 4, which suggests the reaction of the di-isocyanate with the functional material or polymer at first then with the nanocellulose through the free isocyanate. Following this scenario, only the di-isocyanates, which have a free isocyanate, will be able to react with the nanocellulose. Those with two reacted isocyanates will be unreactive and therefore washed away after the reaction.
The final challenge is the self-polymerization of isocyanates whether they are on the nanocellulose surface or in the reaction mixture. Isocyanates, in the presence of a catalyst and heat, can dimerize to uretidinediones and carbodiimides, or trimerize to isocyanurates, or even form larger oligomers. These many possibilities of isocyanates to self-polymerize have a negative impact on the efficiency of nanocellulose modification [132,133,134].
6. Conclusions
A variety of alkyl and aryl mono- and di-isocyanates have been used for the surface modification of nanocellulose. So far, many of these modifications focused on the compatibilization of nanocellulose with nonpolar thermoplastic and thermoset matrices for the fabrication of composites with enhanced interfacial adhesion. The main aim was improving the mechanical properties of the composites. Sometimes the impact of nanocellulose modification on the thermal properties of the composite, such as thermal stability and crystallization kinetics, was investigated. A few other modifications introduced functional groups on the nanocellulose surface to increase its potential in certain applications such as water filtration and biotechnological applications, etc.
The reports on nanocellulose modification took place following one of four scenarios. In the first scenario, the nanocellulose was reacted in one-step with a mono-isocyanate (phenyl isocyanate or octadecyl isocyanate) to reduce the hydrophilicity of the nanocellulose. Sometimes, a di-isocyanate was used for the same purpose. In the second scenario, the nanocellulose was reacted with one of the isocyanates of a di-isocyanate then the free isocyanate was reacted with a functional material or a matrix. This scenario suffers the possibility of the di-isocyanate to react with the nanocellulose using both of its isocyanates becoming dysfunctional for the following step. The third scenario overcame this issue by reacting the di-isocyanate at first with the functional material or matrix using one of its isocyanates then the free isocyanate was reacted with the nanocellulose. The fourth scenario represents mainly the use of nanocellulose to reinforce nonpolar thermosets. In this scenario, the di-isocyanate was reacted with both the nanocellulose and the polyol at the same time.
Despite the reported potential of isocyanate compounds as chemical linkers and surface modifiers, their reactions with nanocellulose face many challenges including the need for moisture-free environment, nanocellulose aggregation during solvent-exchange, reaction controllability, and self-polymerization of isocyanates.
Funding
The authors would like to thank the Fraunhofer Institute for Wood Research (WKI) for funding this research work through the Wilhelm-Klauditz Fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sun R. Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels: Chemistry, Extractives, Lignins, Hemicelluloses and Cellulose. 1st ed. Elsevier; Amsterdam, The Netherlands: 2010. [Google Scholar]
- 2.Spence K.L., Venditti R.A., Rojas O.J., Habibi Y., Pawlak J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Water interactions and physical properties for packaging applications. Cellulose. 2010;17:835–848. doi: 10.1007/s10570-010-9424-8. [DOI] [PubMed] [Google Scholar]
- 3.Mao J., Abushammala H., Brown N., Laborie M.-P. Nanocelluloses: Their Preparation, Properties, and Applications, ACS Symposium Series. Volume 1251. ACS Publications; Washington, DC, USA: 2017. Comparative assessment of methods for producing cellulose I nanocrystals from cellulosic sources; pp. 19–53. [Google Scholar]
- 4.Standard Terms and Their Definition for Cellulose Nanomaterial. International Organization for Standardization (ISO); Geneva, Switzerland: 2017. [Google Scholar]
- 5.Bondeson D., Mathew A., Oksman K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose. 2006;13:171. doi: 10.1007/s10570-006-9061-4. [DOI] [Google Scholar]
- 6.Abushammala H., Goldsztayn R., Leao A., Laborie M.-P. Combining steam explosion with 1-ethyl-3-methylimidazlium acetate treatment of wood yields lignin-coated cellulose nanocrystals of high aspect ratio. Cellulose. 2016;23:1813–1823. doi: 10.1007/s10570-016-0911-4. [DOI] [Google Scholar]
- 7.Abushammala H., Krossing I., Laborie M.-P. Ionic liquid-mediated technology to produce cellulose nanocrystals directly from wood. Carbohydr. Polym. 2015;134:609–616. doi: 10.1016/j.carbpol.2015.07.079. [DOI] [PubMed] [Google Scholar]
- 8.Leung C.W., Luong J.H., Hrapovic S., Lam E., Liu Y., Male K.B., Mahmoud K., Rho D. Cellulose nanocrystals from renewable biomass. 8,900,706. U.S. Patent. 2014 Dec 2;
- 9.Sacui I.A., Nieuwendaal R.C., Burnett D.J., Stranick S.J., Jorfi M., Weder C., Foster E.J., Olsson R.T., Gilman J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. Acs Appl. Mater. Interfaces. 2014;6:6127–6138. doi: 10.1021/am500359f. [DOI] [PubMed] [Google Scholar]
- 10.Moon R.J., Martini A., Nairn J., Simonsen J., Youngblood J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011;40:3941–3994. doi: 10.1039/c0cs00108b. [DOI] [PubMed] [Google Scholar]
- 11.Kiziltas A., Erbas Kiziltas E., Boran S., Gardner D.J. Micro-and nanocellulose composites for automotive applications; Proceedings of the SPE Automotive Composites Conference and Exhibition (ACCE); Novi, MI, USA. 11–13 September 2013. [Google Scholar]
- 12.Plackett D., Letchford K., Jackson J., Burt H. A review of nanocellulose as a novel vehicle for drug delivery. Nord. Pulp. Pap. Res. J. 2014;29:105–118. doi: 10.3183/npprj-2014-29-01-p105-118. [DOI] [Google Scholar]
- 13.Dugan J.M., Gough J.E., Eichhorn S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomed. 2013;8:287–298. doi: 10.2217/nnm.12.211. [DOI] [PubMed] [Google Scholar]
- 14.Khan A., Huq T., Khan R.A., Riedl B., Lacroix M. Nanocellulose-based composites and bioactive agents for food packaging. Crit. Rev. Food Sci. Nutr. 2014;54:163–174. doi: 10.1080/10408398.2011.578765. [DOI] [PubMed] [Google Scholar]
- 15.Voisin H., Bergström L., Liu P., Mathew A. Nanocellulose-based materials for water purification. Nanomaterials. 2017;7:57. doi: 10.3390/nano7030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraschini C., Chauve G., Bouchard J. TEMPO-mediated surface oxidation of cellulose nanocrystals (CNCs) Cellulose. 2017;24:2775–2790. doi: 10.1007/s10570-017-1319-5. [DOI] [Google Scholar]
- 17.Wu Z., Xu J., Gong J., Li J., Mo L. Preparation, characterization and acetylation of cellulose nanocrystal allomorphs. Cellulose. 2018;25:4905–4918. doi: 10.1007/s10570-018-1937-6. [DOI] [Google Scholar]
- 18.Yuan H., Nishiyama Y., Wada M., Kuga S. Surface acylation of cellulose whiskers by drying aqueous emulsion. Biomacromolecules. 2006;7:696–700. doi: 10.1021/bm050828j. [DOI] [PubMed] [Google Scholar]
- 19.Salajková M., Berglund L.A., Zhou Q. Hydrophobic cellulose nanocrystals modified with quaternary ammonium salts. J. Mater. Chem. 2012;22:19798–19805. doi: 10.1039/c2jm34355j. [DOI] [Google Scholar]
- 20.Song Z., Xiao H., Zhao Y. Hydrophobic-modified nano-cellulose fiber/PLA biodegradable composites for lowering water vapor transmission rate (WVTR) of paper. Carbohydr. Polym. 2014;111:442–448. doi: 10.1016/j.carbpol.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Cervin N.T., Aulin C., Larsson P.T., Wågberg L. Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose. 2012;19:401–410. doi: 10.1007/s10570-011-9629-5. [DOI] [Google Scholar]
- 22.Laitinen O., Hartmann R., Sirviö J.A., Liimatainen H., Rudolph M., Ämmälä A., Illikainen M. Alkyl aminated nanocelluloses in selective flotation of aluminium oxide and quartz. Chem. Eng. Sci. 2016;144:260–266. doi: 10.1016/j.ces.2016.01.052. [DOI] [Google Scholar]
- 23.Morandi G., Thielemans W. Synthesis of cellulose nanocrystals bearing photocleavable grafts by ATRP. Polym. Chem. 2012;3:1402–1407. doi: 10.1039/c2py20069d. [DOI] [Google Scholar]
- 24.Akindoyo J.O., Beg M., Ghazali S., Islam M., Jeyaratnam N., Yuvaraj A. Polyurethane types, synthesis and applications–a review. RSC Adv. 2016;6:114453–114482. doi: 10.1039/C6RA14525F. [DOI] [Google Scholar]
- 25.Zhang C., Gilbert R., Fornes R. Abstracts of Papers of the American Chemical Society. Volume 203 American Chemical Society; Washington, DC, USA: 1992. Preliminary studies of reduction of moisture absorption of cellulose using masked isocyanates. [Google Scholar]
- 26.Chen W., Bin Q., Bai Z.-W., Zhou X.-P., Xie X.-L. Partial carbamoylation of cellulose microspheres: A new method to prepare adsorbents for liquid chromatography. Chin. J. Polym. Sci. 2013;31:1725–1732. doi: 10.1007/s10118-013-1312-x. [DOI] [Google Scholar]
- 27.Chen W., Zhang M., Feng Y., Wu J., Gao X., Zhang J., He J., Zhang J. Homogeneous synthesis of partially substituted cellulose phenylcarbamates aiming at chiral recognition. Polym. Int. 2015;64:1037–1044. doi: 10.1002/pi.4884. [DOI] [Google Scholar]
- 28.Okada Y., Yamamoto C., Kamigaito M., Gao Y., Shen J., Okamoto Y. Enantioseparation using cellulose tris (3, 5-dimethylphenylcarbamate) as chiral stationary phase for HPLC: Influence of molecular weight of cellulose. Molecules. 2016;21:1484. doi: 10.3390/molecules21111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X., Wang L., Dong S., Zhang X., Wu Q., Zhao L., Shi Y. Nanocellulose 3, 5-Dimethylphenylcarbamate Derivative Coated Chiral Stationary Phase: Preparation and Enantioseparation Performance. Chirality. 2016;28:376–381. doi: 10.1002/chir.22578. [DOI] [PubMed] [Google Scholar]
- 30.Guo J., Du W., Gao Y., Cao Y., Yin Y. Cellulose nanocrystals as water-in-oil Pickering emulsifiers via intercalative modification. Colloids Surf. A Physicochem. Eng. Asp. 2017;529:634–642. doi: 10.1016/j.colsurfa.2017.06.056. [DOI] [Google Scholar]
- 31.Guo Y.-H., Guo J.-J., Li S.-C., Li X., Wang G.-S., Huang Z. Properties and paper sizing application of waterborne polyurethane emulsions synthesized with TDI and IPDI. Colloids Surf. A Physicochem. Eng. Asp. 2013;427:53–61. doi: 10.1016/j.colsurfa.2013.03.017. [DOI] [Google Scholar]
- 32.Mix R., Gähde J., Goering H., Schulz G. Segmented polyurethanes with 4, 4′-bis-(6-hydroxyhexoxy) biphenyl as chain extender. Part 2. Synthesis and properties of MDI-polyurethanes in comparison with 2, 4-TDI-polyurethanes. J. Polym. Sci. Part A Polym. Chem. 1996;34:33–44. doi: 10.1002/(SICI)1099-0518(19960115)34:1<33::AID-POLA3>3.0.CO;2-4. [DOI] [Google Scholar]
- 33.Belgacem M.N., Quillerou J., Gandini A. Urethanes and polyurethanes bearing furan moieties—3. Synthesis, characterization and comparative kinetics of the formation of diurethanes. Eur. Polym. J. 1993;29:1217–1224. doi: 10.1016/0014-3057(93)90151-5. [DOI] [Google Scholar]
- 34.Semsarzadeh M., Navarchian A. Kinetic Study of the Bulk Reaction Between TDI and PPG in Prescence of DBTDL and FEAA Catalysts Using Quantitative FTIR Spectroscopy. J. Polym. Eng. 2003;23:225–240. doi: 10.1515/POLYENG.2003.23.4.225. [DOI] [Google Scholar]
- 35.Evans R., Wearne R.H., Wallis A.F. Effect of amines on the carbanilation of cellulose with phenylisocyanate. J. Appl. Polym. Sci. 1991;42:813–820. doi: 10.1002/app.1991.070420326. [DOI] [Google Scholar]
- 36.Morelli C.L., Belgacem N., Bretas R.E., Bras J. Melt extruded nanocomposites of polybutylene adipate-co-terephthalate (PBAT) with phenylbutyl isocyanate modified cellulose nanocrystals. J. Appl. Polym. Sci. 2016;133 doi: 10.1002/app.43678. [DOI] [Google Scholar]
- 37.Morelli C.L., Belgacem M.N., Branciforti M.C., Bretas R.E., Crisci A., Bras J. Supramolecular aromatic interactions to enhance biodegradable film properties through incorporation of functionalized cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2016;83:80–88. doi: 10.1016/j.compositesa.2015.10.038. [DOI] [Google Scholar]
- 38.Zhang S., Yang X., Tang B., Yuan L., Wang K., Liu X., Zhu X., Li J., Ge Z., Chen S. New insights into synergistic antimicrobial and antifouling cotton fabrics via dually finished with quaternary ammonium salt and zwitterionic sulfobetaine. Chem. Eng. J. 2018;336:123–132. doi: 10.1016/j.cej.2017.10.168. [DOI] [Google Scholar]
- 39.Siqueira G., Bras J., Dufresne A. New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir. 2009;26:402–411. doi: 10.1021/la9028595. [DOI] [PubMed] [Google Scholar]
- 40.Siqueira G., Bras J., Follain N., Belbekhouche S., Marais S., Dufresne A. Thermal and mechanical properties of bio-nanocomposites reinforced by Luffa cylindrica cellulose nanocrystals. Carbohydr. Polym. 2013;91:711–717. doi: 10.1016/j.carbpol.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 41.Ashori A., Nourbakhsh A. Polypropylene cellulose-based composites: The effect of bagasse reinforcement and polybutadiene isocyanate treatment on the mechanical properties. J. Appl. Polym. Sci. 2009;111:1684–1689. doi: 10.1002/app.29189. [DOI] [Google Scholar]
- 42.Gwon J.-G., Cho H.-J., Chun S.-J., Lee S., Wu Q., Lee S.-Y. Physiochemical, optical and mechanical properties of poly (lactic acid) nanocomposites filled with toluene diisocyanate grafted cellulose nanocrystals. RSC Adv. 2016;6:9438–9445. doi: 10.1039/C5RA26337A. [DOI] [Google Scholar]
- 43.Zoppe J.O., Peresin M.S., Habibi Y., Venditti R.A., Rojas O.J. Reinforcing poly (ε-caprolactone) nanofibers with cellulose nanocrystals. ACS Appl. Mater. Interfaces. 2009;1:1996–2004. doi: 10.1021/am9003705. [DOI] [PubMed] [Google Scholar]
- 44.Dierov Z.K., Tsiomenko A., Davranov K., Kulaev I. Hydrophobic chromatography and characterization of lipases secreted by the fungus rhizopus-microporous UZLT-4B. Biochem. -Mosc. 1993;58:677–683. [Google Scholar]
- 45.Shang W., Huang J., Luo H., Chang P.R., Feng J., Xie G. Hydrophobic modification of cellulose nanocrystal via covalently grafting of castor oil. Cellulose. 2013;20:179–190. doi: 10.1007/s10570-012-9795-0. [DOI] [Google Scholar]
- 46.Abushammala H. A Simple Method for the Quantification of Free Isocyanates on the Surface of Cellulose Nanocrystals upon Carbamation using Toluene Diisocyanate. Surface. 2019;2:444–454. doi: 10.3390/surfaces2020032. [DOI] [Google Scholar]
- 47.Abushammala H. On the Para/Ortho Reactivity of Isocyanate Groups during the Carbamation of Cellulose Nanocrystals Using 2,4-Toluene Diisocyanate. Polymer. 2019;11:1164. doi: 10.3390/polym11071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Ren H., Ragauskas A.J. Rigid polyurethane foam/cellulose whisker nanocomposites: Preparation, characterization, and properties. J. Nanosci. Nanotechnol. 2011;11:6904–6911. doi: 10.1166/jnn.2011.3834. [DOI] [PubMed] [Google Scholar]
- 49.Li Y., Ragauskas A.J. Ethanol organosolv lignin-based rigid polyurethane foam reinforced with cellulose nanowhiskers. RSC Adv. 2012;2:3347–3351. doi: 10.1039/c2ra00646d. [DOI] [Google Scholar]
- 50.Charles G.P.E. Manufacture of new products derived from cellulose. 1,357,450. U.S. Patent. 1920 Nov 2;
- 51.Welch C.M. Process for the reaction of isocyanates with cellulose in the presence of organic phosphites. 2,993,888. U.S. Patent. 1961 Jul 25;
- 52.George M. Structural element made from paper and like sheets. 2,428,979. U.S. Patent. 1947 Nov 14;
- 53.Ellzey S., Jr., Wade C.P., Mack C.H. Part II: Textile Properties of Fabric Modified by Reaction with Phenyl Isocyanate. Text. Res. J. 1962;32:1029–1033. doi: 10.1177/004051756203201211. [DOI] [Google Scholar]
- 54.Ellzey S., Jr., Mack C.H. Reaction of Aryl Isocyanates with Cotton Cellulose: Part I: Variables in the Reaction Using Phenyl Isocyanate. Text. Res. J. 1962;32:1023–1029. doi: 10.1177/004051756203201210. [DOI] [Google Scholar]
- 55.Ohno Y., Uchimoto I. Studies on reaction of cellulose with isocyanate. 1. Reaction of cellulose with phenyl isocyanate. Kog Kagaku Zasshi. 1970;73:2527–2530. doi: 10.1246/nikkashi1898.73.11_2527. [DOI] [Google Scholar]
- 56.Ohno Y., Sato T., Miyamoto K. Studies on reaction of cellulose with isocyanate. 3. Reaction of cellulose with 2, 4-diisocyanatotoluene in n, n-dimethylformamide. Nippon. Kagaku. Kaishi. 1976;3:1300–1303. doi: 10.1246/nikkashi.1976.1300. [DOI] [Google Scholar]
- 57.Sato T., Ohno Y., Tamura T. Studies on reaction of cellulose with isocyanate. 5. Reaction of cellulose with hexamethylene diisocyanate in n, n-dimethylformamide. Nippon. Kagaku. Kaishi. 1978;5:760–764. doi: 10.1246/nikkashi.1978.760. [DOI] [Google Scholar]
- 58.Gemeiner P., Augustin J., Drobnica L. Reactions of cellulose isothiocyanates with thiol and amino compounds. Carbohydr. Res. 1977;53:217–222. doi: 10.1016/S0008-6215(00)88089-1. [DOI] [PubMed] [Google Scholar]
- 59.Chen L.F., Tsao G.T. Chemical procedures for enzyme immobilization on porous cellulose beads. Biotechnol. Bioeng. 1977;19:1463–1473. doi: 10.1002/bit.260191005. [DOI] [PubMed] [Google Scholar]
- 60.Saraji M., Farajmand B. Chemically modified cellulose paper as a thin film microextraction phase. J. Chromatogr. A. 2013;1314:24–30. doi: 10.1016/j.chroma.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Pend X., Sato M., Kawase T., Ikeno K., Sawada H., Hamada N., Wada K., Takahashi Y., Yoshimura T. Synthesis and soil repellent, antibacterial and antifungal properties of blocked isocyanate co-oligomers having cation segments. Sen-I Gakkaishi. 2002;58:163–169. [Google Scholar]
- 62.Sato T., Karatsu K., Kitamura H., Ohno Y. Synthesis of cellulose derivatives containing amino acid residues and their adsorption of metal ions. Sen’i Gakkaishi. 1983;39:T519–T524. doi: 10.2115/fiber.39.12_T519. [DOI] [Google Scholar]
- 63.Sato T., Motomura S., Ohno Y. Adsorption and desorption of metal ions by systems based on cellulose derivatives that contain amino acid residues. Sen’i Gakkaishi. 1985;41:T235–T240. doi: 10.2115/fiber.41.6_T235. [DOI] [Google Scholar]
- 64.Tursi A., Beneduci A., Chidichimo F., De Vietro N., Chidichimo G. Remediation of hydrocarbons polluted water by hydrophobic functionalized cellulose. Chemosphere. 2018;201:530–539. doi: 10.1016/j.chemosphere.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 65.Sato J., Sugimura K., Teramoto Y., Nishio Y. Preparation and chiroptical properties of cellulose chlorophenylcarbamate–silica hybrids having a chiral nematic mesomorphic structure. Polymer. 2019;173:172–181. doi: 10.1016/j.polymer.2019.04.049. [DOI] [Google Scholar]
- 66.Peng X., Kawase T., Sato M., Ikeno K., Sawada H. Surface modification of cellulose and polyester by oligomeric fluoroalkylating agents having oxime-blocked isocyanate groups. Sen-I Gakkaishi. 2002;58:91–97. doi: 10.2115/fiber.58.91. [DOI] [Google Scholar]
- 67.Rajkumar S., Tjong J., Nayak S., Sain M. Wetting behavior of soy-based resin and unsaturated polyester on surface-modified sisal fiber mat. J. Reinf. Plast. Compos. 2015;34:807–818. doi: 10.1177/0731684415580630. [DOI] [Google Scholar]
- 68.Botaro V.R., Gandini A. Chemical modification of the surface of cellulosic fibres. 2. Introduction of alkenyl moieties via condensation reactions involving isocyanate functions. Cellulose. 1998;5:65–78. doi: 10.1023/A:1009216729686. [DOI] [Google Scholar]
- 69.Botaro V.R., Gandini A., Belgacem M.N. Heterogeneous chemical modification of cellulose for composite materials. J. Thermoplast. Compos. Mater. 2005;18:107–117. doi: 10.1177/0892705705042600. [DOI] [Google Scholar]
- 70.Trejo-O’Reilly J., Cavaille J.Y., Gandini A. Cationic copolymerization of styrenes with an isocyanate-bearing homologue. React. Funct. Polym. 1997;32:9–19. doi: 10.1016/S1381-5148(96)00065-X. [DOI] [Google Scholar]
- 71.Trejo-O’reilly J.-A., Cavaille J.-Y., Gandini A. The surface chemical modification of cellulosic fibres in view of their use in composite materials. Cellulose. 1997;4:305–320. doi: 10.1023/A:1018452310122. [DOI] [Google Scholar]
- 72.Badanova A.K., Taussarova B.R., Kutzhanova A.Z. Hydrophobic finishing of cellulosic textile material. World. Appl. Sci. J. 2014;30:1409–1416. [Google Scholar]
- 73.Yuan J., Zhang J., Zang X., Shen J., Lin S. Improvement of blood compatibility on cellulose membrane surface by grafting betaines. Colloids. Surf. B Biointerfaces. 2003;30:147–155. doi: 10.1016/S0927-7765(03)00082-1. [DOI] [Google Scholar]
- 74.Furuzono T., Ishihara K., Nakabayashi N., Tamada Y. Chemical modification of silk fibroin with 2-methacryloyloxyethyl phosphorylcholine. II. Graft-polymerization onto fabric through 2-methacryloyloxyethyl isocyanate and interaction between fabric and platelets. Biomaterials. 2000;21:327–333. doi: 10.1016/S0142-9612(99)00177-5. [DOI] [PubMed] [Google Scholar]
- 75.Ghatge N., Sabne M., Gujar K., Mahajan S. Modification of cellulose acetate by aliphatic isocyanates for reverse osmosis studies. Int. J. Polym. Mater. 1984;10:281–291. doi: 10.1080/00914038408078646. [DOI] [Google Scholar]
- 76.Mahajan S., Sabne M., Gujar K., Ghatge N. Selectivity of isocyanate modified cellulose acetate membranes to sugars. Int. J. Polym. Mater. 1985;11:39–45. doi: 10.1080/00914038508078652. [DOI] [Google Scholar]
- 77.Maldas D., Kokta B.V. Effect of Fiber Treatment on the Mechanical Properties of Hybrid Fiber-Reinforced Polystyrene Composites: I. Use of Mica and Wood Pulp as Hybrid Filler. J. Compos. Technol. Res. 1990;12:217–221. [Google Scholar]
- 78.Maldas D., Kokta B. Effect of fiber treatment on the mechanical properties of hybrid fiber reinforced polystyrene composites: IV. Use of glass fiber and sawdust as hybrid fiber. J. Compos. Mater. 1991;25:375–390. doi: 10.1177/002199839102500402. [DOI] [Google Scholar]
- 79.Girones J., Pimenta M., Vilaseca F., De Carvalho A., Mutje P., Curvelo A. Blocked isocyanates as coupling agents for cellulose-based composites. Carbohydr. Polym. 2007;68:537–543. doi: 10.1016/j.carbpol.2006.10.020. [DOI] [Google Scholar]
- 80.Joly C., Kofman M., Gauthier R. Polypropylene/cellulosic fiber composites chemical treatment of the cellulose assuming compatibilization between the two materials. J. Macromol. Sci. Part A Pure Appl. Chem. 1996;33:1981–1996. doi: 10.1080/10601329608011023. [DOI] [Google Scholar]
- 81.Canche-Escamilla G., Cauich-Cupul J., Mendizabal E., Puig J., Vazquez-Torres H., Herrera-Franco P. Mechanical properties of acrylate-grafted henequen cellulose fibers and their application in composites. Compos. Part A Appl. Sci. Manuf. 1999;30:349–359. doi: 10.1016/S1359-835X(98)00116-X. [DOI] [Google Scholar]
- 82.Qiu W., Zhang F., Endo T., Hirotsu T. Isocyanate as a compatibilizing agent on the properties of highly crystalline cellulose/polypropylene composites. J. Mater. Sci. 2005;40:3607–3614. doi: 10.1007/s10853-005-0790-9. [DOI] [Google Scholar]
- 83.Darie R.N., Vlad S., Anghel N., Doroftei F., Tamminen T., Spiridon I. New PP/PLA/cellulose composites: Effect of cellulose functionalization on accelerated weathering behavior. Polym. Adv. Technol. 2015;26:941–952. doi: 10.1002/pat.3506. [DOI] [Google Scholar]
- 84.Suwanruji P., Tuechart T., Smitthipong W., Chollakup R. Modification of pineapple leaf fiber surfaces with silane and isocyanate for reinforcing thermoplastic. J. Thermoplast. Compos. Mater. 2017;30:1344–1360. doi: 10.1177/0892705716632860. [DOI] [Google Scholar]
- 85.George J., Bhagawan S., Thomas S. Improved interactions in chemically modified pineapple leaf fiber reinforced polyethylene composites. Compos. Interfaces. 1997;5:201–223. doi: 10.1163/156855498X00153. [DOI] [Google Scholar]
- 86.George J., Bhagawan S., Thomas S. Thermogravimetric and dynamic mechanical thermal analysis of pineapple fibre reinforced polyethylene composites. J. Therm. Anal. Calorim. 1996;47:1121–1140. doi: 10.1007/BF01979452. [DOI] [Google Scholar]
- 87.Joseph K., Thomas S., Pavithran C. Effect of chemical treatment on the tensile properties of short sisal fibre-reinforced polyethylene composites. Polymer. 1996;37:5139–5149. doi: 10.1016/0032-3861(96)00144-9. [DOI] [Google Scholar]
- 88.Joseph P., Rabello M.S., Mattoso L., Joseph K., Thomas S. Environmental effects on the degradation behaviour of sisal fibre reinforced polypropylene composites. Compos. Sci. Technol. 2002;62:1357–1372. doi: 10.1016/S0266-3538(02)00080-5. [DOI] [Google Scholar]
- 89.Girones J., Pimenta M., Vilaseca F., Carvalho A.J.d., Mutje P., Curvelo A. Blocked diisocyanates as reactive coupling agents: Application to pine fiber–polypropylene composites. Carbohydr. Polym. 2008;74:106–113. doi: 10.1016/j.carbpol.2008.01.026. [DOI] [Google Scholar]
- 90.Ly B., Thielemans W., Dufresne A., Chaussy D., Belgacem M. Surface functionalization of cellulose fibres and their incorporation in renewable polymeric matrices. Compos. Sci. Technol. 2008;68:3193–3201. doi: 10.1016/j.compscitech.2008.07.018. [DOI] [Google Scholar]
- 91.Zhang S., Xia C., Dong Y., Yan Y., Li J., Shi S.Q., Cai L. Soy protein isolate-based films reinforced by surface modified cellulose nanocrystal. Ind. Crop. Prod. 2016;80:207–213. doi: 10.1016/j.indcrop.2015.11.070. [DOI] [Google Scholar]
- 92.Liu W., Chen T., Qiu R. Effect of fiber modification with 3-isopropenyl-dimethylbenzyl isocyanate (TMI) on the mechanical properties and water absorption of hemp-unsaturated polyester (UPE) composites. Holzforschung. 2014;68:265–271. doi: 10.1515/hf-2013-0104. [DOI] [Google Scholar]
- 93.Reulier M., Perrin R., Avérous L. Biocomposites based on chemically modified cellulose fibers with renewable fatty-acid-based thermoplastic systems: Effect of different fiber treatments. J. Appl. Polym. Sci. 2016;133 doi: 10.1002/app.43878. [DOI] [Google Scholar]
- 94.Datta J., Kopczyńska P. Effect of kenaf fibre modification on morphology and mechanical properties of thermoplastic polyurethane materials. Ind. Crop. Prod. 2015;74:566–576. doi: 10.1016/j.indcrop.2015.05.080. [DOI] [Google Scholar]
- 95.Gallego R., Arteaga J., Valencia C., Franco J. Thickening properties of several NCO-functionalized cellulose derivatives in castor oil. Chem. Eng. Sci. 2015;134:260–268. doi: 10.1016/j.ces.2015.05.007. [DOI] [Google Scholar]
- 96.Tenorio-Alfonso A., Sánchez M.C., Franco J.M. Preparation, characterization and mechanical properties of bio-based polyurethane adhesives from isocyanate-functionalized cellulose acetate and castor oil for bonding wood. Polymer. 2017;9:132. doi: 10.3390/polym9040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tonoli G.H.D., Mendes R.F., Siqueira G., Bras J., Belgacem M.N., Savastano H. Isocyanate-treated cellulose pulp and its effect on the alkali resistance and performance of fiber cement composites. Holzforschung. 2013;67:853–861. doi: 10.1515/hf-2012-0195. [DOI] [Google Scholar]
- 98.Tonoli G.H.D., Belgacem M.N., Siqueira G., Bras J., Savastano Jr H., Lahr F.R. Processing and dimensional changes of cement based composites reinforced with surface-treated cellulose fibres. Cem. Concr. Compos. 2013;37:68–75. doi: 10.1016/j.cemconcomp.2012.12.004. [DOI] [Google Scholar]
- 99.Paquet O., Krouit M., Bras J., Thielemans W., Belgacem M.N. Surface modification of cellulose by PCL grafts. Acta Mater. 2010;58:792–801. doi: 10.1016/j.actamat.2009.09.057. [DOI] [Google Scholar]
- 100.Wang D., Xuan Y., Huang Y., Shen J. Synthesis and properties of graft copolymer of cellulose diacetate with poly (caprolactone monoacrylate) J. Appl. Polym. Sci. 2003;89:85–90. doi: 10.1002/app.12033. [DOI] [Google Scholar]
- 101.Xu L., Cheng X. Preparation and characterization of cellulose diacetate-graft-poly (butylene glycol adipate) copolymers. Russ. J. Appl. Chem. 2014;87:1763–1772. doi: 10.1134/S1070427214110305. [DOI] [Google Scholar]
- 102.Miao S.D., Liu Y.Y., Wang P., Zhang S.P. Castor oil and microcrystalline cellulose based polymer composites with high tensile strength. Adv. Mater. Res. 2012;399–401:1531–1535. doi: 10.4028/www.scientific.net/AMR.399-401.1531. [DOI] [Google Scholar]
- 103.Cardamone J.M. Reacting cotton cellulose with lignin-based polyurethane. Text. Res. J. 1992;62:371–381. doi: 10.1177/004051759206200702. [DOI] [Google Scholar]
- 104.Habibi Y., Dufresne A. Highly filled bionanocomposites from functionalized polysaccharide nanocrystals. Biomacromolecules. 2008;9:1974–1980. doi: 10.1021/bm8001717. [DOI] [PubMed] [Google Scholar]
- 105.Labet M., Thielemans W., Dufresne A. Polymer grafting onto starch nanocrystals. Biomacromolecules. 2007;8:2916–2927. doi: 10.1021/bm700468f. [DOI] [PubMed] [Google Scholar]
- 106.Thielemans W., Belgacem M.N., Dufresne A. Starch nanocrystals with large chain surface modifications. Langmuir. 2006;22:4804–4810. doi: 10.1021/la053394m. [DOI] [PubMed] [Google Scholar]
- 107.Gu J., Catchmark J.M., Kaiser E.Q., Archibald D.D. Quantification of cellulose nanowhiskers sulfate esterification levels. Carbohydr. Polym. 2013;92:1809–1816. doi: 10.1016/j.carbpol.2012.10.078. [DOI] [PubMed] [Google Scholar]
- 108.Nishiyama Y., Langan P., Chanzy H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002;124:9074–9082. doi: 10.1021/ja0257319. [DOI] [PubMed] [Google Scholar]
- 109.Verlhac C., Dedier J., Chanzy H. Availability of surface hydroxyl groups in Valonia and bacterial cellulose. J. Polym. Sci. Part A Polym. Chem. 1990;28:1171–1177. doi: 10.1002/pola.1990.080280517. [DOI] [Google Scholar]
- 110.Missoum K., Bras J., Belgacem M.N. Organization of aliphatic chains grafted on nanofibrillated cellulose and influence on final properties. Cellulose. 2012;19:1957–1973. doi: 10.1007/s10570-012-9780-7. [DOI] [Google Scholar]
- 111.Yu H.-Y., Qin Z.-Y. Surface grafting of cellulose nanocrystals with poly (3-hydroxybutyrate-co-3-hydroxyvalerate) Carbohydr. Polym. 2014;101:471–478. doi: 10.1016/j.carbpol.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 112.Stenstad P., Andresen M., Tanem B.S., Stenius P. Chemical surface modifications of microfibrillated cellulose. Cellulose. 2008;15:35–45. doi: 10.1007/s10570-007-9143-y. [DOI] [Google Scholar]
- 113.Verdolotti L., Stanzione M., Khlebnikov O., Silant’ev V., Postnova I., Lavorgna M., Shchipunov Y. Dimensionally Stable Cellulose Aerogel Strengthened by Polyurethane Synthesized in Situ. Macromol. Chem. Phys. 2019;220:1800372. doi: 10.1002/macp.201800372. [DOI] [Google Scholar]
- 114.Hassan M.L., Bras J., Hassan E.A., Fadel S.M., Dufresne A. Polycaprolactone/modified bagasse whisker nanocomposites with improved moisture-barrier and biodegradability properties. J. Appl. Polym. Sci. 2012;125:E10–E19. doi: 10.1002/app.36373. [DOI] [Google Scholar]
- 115.Follain N., Belbekhouche S., Bras J., Siqueira G., Chappey C., Marais S., Dufresne A. Tunable gas barrier properties of filled-PCL film by forming percolating cellulose network. Colloids Surf. A Physicochem. Eng. Asp. 2018;545:26–30. doi: 10.1016/j.colsurfa.2018.02.040. [DOI] [Google Scholar]
- 116.Pinheiro I., Ferreira F., Souza D., Gouveia R., Lona L., Morales A., Mei L. Mechanical, rheological and degradation properties of PBAT nanocomposites reinforced by functionalized cellulose nanocrystals. Eur. Polym. J. 2017;97:356–365. doi: 10.1016/j.eurpolymj.2017.10.026. [DOI] [Google Scholar]
- 117.Pinheiro I., Ferreira F., Alves G., Rodolfo A., Morales A., Mei L. Biodegradable PBAT-Based Nanocomposites Reinforced with Functionalized Cellulose Nanocrystals from Pseudobombax munguba: Rheological, Thermal, Mechanical and Biodegradability Properties. J. Polym. Environ. 2019;27:757–766. doi: 10.1007/s10924-019-01389-z. [DOI] [Google Scholar]
- 118.Espino-Pérez E., Bras J., Ducruet V., Guinault A., Dufresne A., Domenek S. Influence of chemical surface modification of cellulose nanowhiskers on thermal, mechanical, and barrier properties of poly (lactide) based bionanocomposites. Eur. Polym. J. 2013;49:3144–3154. doi: 10.1016/j.eurpolymj.2013.07.017. [DOI] [Google Scholar]
- 119.Rueda L., d’Arlas B.F., Zhou Q., Berglund L.A., Corcuera M., Mondragon I., Eceiza A. Isocyanate-rich cellulose nanocrystals and their selective insertion in elastomeric polyurethane. Compos. Sci. Technol. 2011;71:1953–1960. doi: 10.1016/j.compscitech.2011.09.014. [DOI] [Google Scholar]
- 120.Faruk O., Sain M., Farnood R., Pan Y., Xiao H. Development of lignin and nanocellulose enhanced bio PU foams for automotive parts. J. Polym. Environ. 2014;22:279–288. doi: 10.1007/s10924-013-0631-x. [DOI] [Google Scholar]
- 121.Cordero A.I., Amalvy J.I., Fortunati E., Kenny J.M., Chiacchiarelli L.M. The role of nanocrystalline cellulose on the microstructure of foamed castor-oil polyurethane nanocomposites. Carbohydr. Polym. 2015;134:110–118. doi: 10.1016/j.carbpol.2015.07.077. [DOI] [PubMed] [Google Scholar]
- 122.Girouard N.M., Xu S., Schueneman G.T., Shofner M.L., Meredith J.C. Site-selective modification of cellulose nanocrystals with isophorone diisocyanate and formation of polyurethane-CNC composites. ACS Appl. Mater. Interfaces. 2016;8:1458–1467. doi: 10.1021/acsami.5b10723. [DOI] [PubMed] [Google Scholar]
- 123.Ikhwan F., Ilmiati S., Adi H.K., Arumsari R., Chalid M. Novel route of synthesis for cellulose fiber-based hybrid polyurethane; Proceedings of the Innovation in Polymer Science and Technology; Medan, Indonesia. 7–10 November 2016; p. 12019. [Google Scholar]
- 124.Gimenez R.B., Leonardi L., Cerrutti P., Amalvy J., Chiacchiarelli L.M. Improved specific thermomechanical properties of polyurethane nanocomposite foams based on castor oil and bacterial nanocellulose. J. Appl. Polym. Sci. 2017;134 doi: 10.1002/app.44982. [DOI] [Google Scholar]
- 125.Leng W., Li J., Cai Z. Synthesis and characterization of cellulose nanofibril-reinforced polyurethane foam. Polymers. 2017;9:597. doi: 10.3390/polym9110597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kong X., Wolodko J., Zhao L., Curtis J.M. The preparation and characterization of polyurethane reinforced with a low fraction of cellulose nanocrystals. Prog. Org. Coat. 2018;125:207–214. doi: 10.1016/j.porgcoat.2018.08.034. [DOI] [Google Scholar]
- 127.Hubmann M., Kong X., Curtis J.M. Kinetic stabilization of cellulose nanocrystals in a photocurable prepolymer for application as an adhesion promoter in UV-curable coatings. Prog. Org. Coat. 2019;129:101–115. doi: 10.1016/j.porgcoat.2018.12.019. [DOI] [Google Scholar]
- 128.Musk A.W., Peters J.M., Wegman D.H. Isocyanates and respiratory disease: Current status. Am. J. Ind. Med. 1988;13:331–349. doi: 10.1002/ajim.4700130304. [DOI] [PubMed] [Google Scholar]
- 129.Bengtström L., Salden M., Stec A.A. The role of isocyanates in fire toxicity. Fire. Sci. Rev. 2016;5:1–23. doi: 10.1186/s40038-016-0013-2. [DOI] [Google Scholar]
- 130.Marx-Figini M. Studies on the ultrasonic degradation of cellulose macromolecular properties. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1997;250:85–92. doi: 10.1002/apmc.1997.052500106. [DOI] [Google Scholar]
- 131.Aranguren M.I., Williams R.J. Kinetic and statistical aspects of the formation of polyurethanes from toluene diisocyanate. Polymer. 1986;27:425–430. doi: 10.1016/0032-3861(86)90160-6. [DOI] [Google Scholar]
- 132.Buckles R.E., McGrew L. A kinetic study of the dimerization of phenyl isocyanate. J. Am. Chem. Soc. 1966;88:3582–3586. doi: 10.1021/ja00967a021. [DOI] [Google Scholar]
- 133.Schwetlick K., Noack R. Kinetics and catalysis of consecutive isocyanate reactions. Formation of carbamates, allophanates and isocyanurates. J. Chem. Soc. Perkin Trans. 2. 1995;2:395–402. doi: 10.1039/p29950000395. [DOI] [Google Scholar]
- 134.Guo J., He Y., Xie D., Zhang X. Process investigating and modelling for the self-polymerization of toluene diisocyanate (TDI)-based polyurethane prepolymer. J. Mater. Sci. 2015;50:5844–5855. doi: 10.1007/s10853-015-9134-6. [DOI] [Google Scholar]