Abstract
Microfluidics is a technique for the handling of small volumes of liquids on the order of picoliters to nanoliters and has impact for miniaturized biomedical science and fundamental research. Because of its multi- and interdisciplinary nature (i.e., combining the fields of biology, chemistry, physics, and engineering), microfluidics offers much potential for educational applications, both at the university level as well as primary and secondary education. Microfluidics is also an ideal “tool” to enthuse and educate members of the general public about the interdisciplinary aspects of modern sciences, including concepts of science, technology, engineering, and mathematics subjects such as (bio)engineering, chemistry, and biomedical sciences. Here, we provide an overview of approaches that have been taken to make microfluidics accessible for formal and informal learning. We also point out future avenues and desired developments. At the extreme ends, we can distinguish between projects that teach how to build microfluidic devices vs projects that make various microscopic phenomena (e.g., low Reynolds number hydrodynamics, microbiology) accessible to learners and the general public. Microfluidics also enables educators to make experiments low-cost and scalable, and thereby widely accessible. Our goal for this review is to assist academic researchers working in the field of microfluidics and lab-on-a-chip technologies as well as educators with translating research from the laboratory into the lecture hall, teaching laboratory, or public sphere.
I. INTRODUCTION
Microfluidics underpins a range of cutting-edge technologies in a wide variety of applications. Single-cell analysis, 1 next generation gene sequencing, 2 and point-of-care diagnostics 3 are examples of technologies that have been enabled by microfluidics. It is a very broad and active research field; a Web of Science search of the topic “microfluidics” returns nearly 20 000 publications, and while it is about to enter its fourth decade, there is insufficient literature on the inclusion of microfluidics in the undergraduate classroom/laboratory or on how to teach it. In fact, at writing, a Web of Science search of “microfluidics” and “education” returned a mere 37 references. Exposing students to microfluidics is not only important for promoting the field, but will also help prepare the next generation of scientists and engineers who use this technology to solve challenges in healthcare, biology, and the environment. No doubt there are many educators and university courses that do include microfluidics in their curricula but are not formally reported in the literature. And as far as we are aware, there are no recent reviews of the literature on this topic, though we direct readers to a 2011 article by Fintschenko, 4 which focuses on practical considerations for teaching microfluidics while highlighting a commercially available teaching pack. There is also an excellent, albeit dated, article by Legge that introduces educators to the idea of a lab on a chip (LOC). 5 We hope to highlight those ideas which have been shared publicly and encourage others to share their teaching activities and innovations. Thus, we have written this review with the aim of inspiring researchers to take their expertise in microfluidics and bring it out of the research laboratory and into the undergraduate classroom.
Microfluidics is a highly interdisciplinary field combing physics, chemistry, biology, and engineering for the manipulation of small volumes of liquids on the order of microliters to attoliters. 6 Microfluidic chips and workstations have been described as tools for mediating interdisciplinary learning in the research laboratory, 7 and there is no reason why this would not extend into the undergraduate classroom. Interdisciplinary teaching is important to prepare students for the world beyond the classroom, as the world is inherently interdisciplinary, and interdisciplinary teaching thus has the potential to provide an opportunity for authentic learning experiences. 8,9 More authentic, inquiry-based learning experiences have been advocated for through national-level initiatives, such as the Next Generation Science Standards in the United States of America. 10 Inquiry-based learning fits within constructivist theories of learning, which suggest that new information is placed within a context of prior knowledge. 11 An interdisciplinary subject like microfluidics provides ample opportunities for learners to connect ideas from one field with another.
In addition to its interdisciplinary nature, microfluidics as a subject is very hands-on. All the examples from the literature included in this review are of practical activities that students can perform. These hands-on activities lend themselves to inquiry-based learning, allowing learners to participate in the same methods and opportunities for decision making as experts in the field. 12 For instance, laboratory courses using a problem-based learning framework have been developed using microfluidics. 13
The hands-on nature also makes microfluidics an engaging tool for science communication and outreach. Many universities and institutes hold showcase events intended to engage with the public on the research being undertaken and its real-world applications; these events may also incorporate activities for schoolchildren. The settings for such activities may be off-University campus, e.g., school visits, stalls, or activities at museums, shopping centres, and Science Festivals as well as activities on-campus through Open Days or Workshops. Introducing schoolchildren to new areas of science beyond their standard curriculum is important in engaging and inspiring potential future researchers in exciting fields. Introducing members of the general public to modern multidisciplinary science helps improve understanding, reduces misconception, and breaks down barriers. 14,15 Microfluidic technology really lends itself beautifully for such outreach activities, as it can be demonstrated with hands-on, visual activities that involve everyday objects such as LEGO® bricks, 16 craft objects, 17 and foodstuff or food dyes, 18 which all help engage the audience and decrease the barrier to communication.
Our goal for this review is to assist academic researchers working in the field of microfluidics and lab-on-a-chip technologies consider how to translate research from the laboratory into the lecture hall, teaching laboratory, or public sphere. We anticipate that our readers are familiar with microfluidics from an academic research perspective and do not provide an overview of the field. However, for nonpractitioners, we recommend Legge’s review of the field 5 which is directed at educators. We begin by reviewing approaches to teaching “about microfluidics.” Next, we highlight examples that use microfluidics and lab-on-a-chip technologies as a means to teach students “about chemistry and biology.” Recognizing that there are informal learning opportunities, we share examples of workshops and activities used in an outreach setting (e.g., Science Festivals, school visits, etc.). We then provide a survey of different fabrication techniques that are suitable for the constraints of the university teaching environment (e.g., cost, complexity, and time). We finish with some personal tips gleaned from classroom and outreach experiences as well as encouragement to those about to integrate the ideas presented here with their own teaching and learning environment.
II. FORMAL TEACHING ABOUT MICROFLUIDICS
The use of microfluidics in teaching can be broadly separated into two categories—teaching about microfluidics or teaching using microfluidics—as helpfully suggested by Fintschenko. 4 In this section, we will focus on reports, mainly of laboratory experiments, that help students learn about microfluidics and the unique physics of fluids under low Reynolds number conditions. We also include reports of activities geared toward younger students and the general public under the banner of “outreach.”
There are a number of topics that can be covered when teaching students about microfluidics; laboratory activities that focus on specific microfluidics concepts 19,20 as well as modules and whole courses on microfluidics 13,21,22 have been reported. The physics of fluids at the microscale is central to microfluidics. A five lesson inquiry-based module for high school students that focused on concepts such as Reynolds number and fluid transport at the microscale was shared in the literature. 19 In this module, students had the opportunity to predict fluid flow (the mixing of two colored solutions) in a variety of pre-made microchannel devices and then verify their predictions through experimentation. Laboratory activities included in the module also focused on the design of simple microchannel schemes, fabrication of devices using PDMS, and application to a problem focused on acid-base chemistry. More importantly, these activities served to increase students’ understanding of qualities and tasks important to scientific practice.
Courses have also been developed at the undergraduate and graduate level. A four-week laboratory module that introduces students to both microscale phenomena and applications of microfluidic technology was developed at the University of Toronto, Canada. 21 The module was implemented in an advanced undergraduate analytical chemistry course and through it students were introduced to concepts in fluid dynamics such as Péclet number, diffusion, electroosmotic flow, and zeta potential. Further, students were given hands-on experience in designing, fabricating, and using microchannel devices, with a final application to electrophoretic separations of DNA. To teach students how to design microfluidic devices and approach problems, Morton and Bridle developed a problem-based learning (PBL) activity for M.Sc. students. 13 By using low-cost and rapid fabrication methods, students got to design and build their own chips to solve one of several assigned problems, for example, focused on separations or on mixing. Taking advantage of low-cost and rapid techniques facilitates the cycle of design-build-test.
III. FORMAL TEACHING WITH MICROFLUIDICS
We now turn our attention to reports of how microfluidics can be used to teach students about chemistry and biology.
A. Chemistry
1. Analytical chemistry
The first microfluidic devices were developed for chemical separations 23,24 and microfluidics continues to have broad use in the field of analytical chemistry. 25 To introduce undergraduate students in an analytical chemistry course to microfluidics and to teach them about electrophoretic separations, Teerasong and McClain 26 developed a 3-h laboratory experiment centered on the separation of food dyes. In the activity, students fabricate a microfluidic capillary electrophoresis chip from a solution of photopolymerizable monomer sandwiched between two glass plates with predrilled holes for inlets. The authors noted that this fabrication was nontrivial and that it could take students several attempts. Students separate a mixture of food dyes (FD&C Blue 1 and FD&C Red 40) and this simple experiment is used as the basis to teach electrophoretic mobility. Given the challenges that could be faced when including chip fabrication, commercial instruments that rely on microfluidics may provide a safer option. Using commercial equipment, such as the Agilent Bioanalyzer, not only introduces students to microfluidics but has the benefit of training students with cutting-edge equipment they might use later on in their professional careers. Motivated by a desire to give this type of practical experience, Chao et al. 27 developed a laboratory experiment for teaching separations using an Agilent Bioanalyzer. The Bioanalyzer uses chip-based capillary gel electrophoresis (CGE) to separate DNA fragments. In this experiment, students extract and amplify DNA from fruit and then use CGE to identify the lengths of the fragments formed. While the Bioanalyzer automatically labels peaks with their corresponding lengths, students prepare a calibration curve and calculate the theoretical plate numbers.
Molecular and biochemical analysis is a prominent application for microfluidics. In this context, Giri et al. 28 reported a laboratory experiment to teach students quantitative analysis with enzyme-linked immunosorbent assays (ELISAs). The major advantages of presenting students with an ELISA in a microfluidic format are the decreased incubation times and decreased sample volumes required over the conventional microplate format. Students are equipped with glass microchannels but perform all of the surface modifications themselves and analysis can be performed with a fluorescence microscope. The learning objectives focused heavily on understanding analytical figures of merit such as sensitivity and limit of detection.
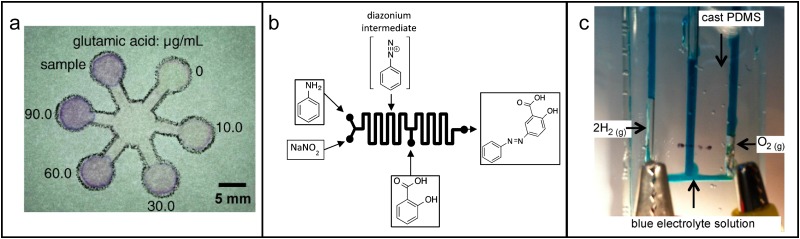
Because of the limited time students and instructors have for undergraduate laboratories, it is best if microfluidic devices can be made in advance. This, however, leaves out valuable hands-on experience in device fabrication. Facile fabrication techniques such as paper devices let students produce devices and run the experimental activity in a short period of time. Cai et al. 29 report a teaching activity for the quantitation of amino acids in green tea extracts. The extracts were prepared by boiling tea leaves for 40 min, and during this time students could make their own microfluidic devices [Fig. 1(a)] using wax pens, filter paper, and an oven. Students performed colorimetric analysis using photographs of their paper fluidic devices and ImageJ software.
FIG. 1.
Microfluidics for chemistry education. (a) Photograph of a paper microfluidic device to quantify amino acids in green tea extracts. Reproduced with permission from Cai et al., J. Chem. Educ. 90(2), 232–234 (2013). Copyright 2013 American Chemical Society. (b) Overview of microfluidic reactor for the two-step synthesis of azo dyes from aniline, sodium nitrite, and salicylic acid. Reproduced with permission from Feng et al., J. Chem. Educ. 92(4), 723–727 (2015). Copyright 2015 American Chemical Society. (c) Photograph of electrolysis of water on a chip. The cast PDMS chip is connected to two platinum wire electrodes. The stoichiometry of hydrogen and oxygen evolution can be visualized. Reproduced from Davis et al, J. Chem. Educ. 92(1), 116–119 (2015). Copyright 2015 Author(s), licensed under a Creative Commons Attribution 4.0 License.
2. Synthetic chemistry
There are only a couple reports of microfluidic chemical synthesis. Traditionally, undergraduate laboratories focus on batch processes, where synthesis occurs in a stirred reaction vessel. However, many industrial syntheses are performed as flow processes. Microfluidics presents an opportunity to conduct synthetic reactions both in flow and on the microscale. Feng et al. 30 prepared a laboratory activity to teach both organic and inorganic flow synthesis. Over two 4-h laboratory periods, students prepare microfluidic chips following the method developed by the Khine Lab 31 and carry out microfluidic synthesis of magnetite particles, gold nanoparticles, and azo dyes. An overview of the dye synthesis is shown in Fig. 1(b). The laboratory exercise seeks to build upon students’ prior knowledge of diffusion and mass transport to demonstrate the difference between flow and batch processes as well as the advantage of microscale reactions. Wanting to simplify microfluidic fabrication for the students, Emmanuel et al. 32 decided to use capillary coils as microreactors for the synthesis of inorganic nanoparticles. With this approach, students could easily assemble their systems using high-performance liquid chromatography (HPLC) connectors and could interchange capillaries of different diameters to study the effect of residence time without changing the mixing efficiency. Students were also able to perform in-line analysis by surface enhanced Raman spectroscopy.
3. Concepts in general chemistry
In addition to analysis and synthesis, there are many concepts from a general chemistry curriculum that can be taught with microfluidics. Laboratory activities that focus on fundamental chemical concepts can serve two purposes: (1) they can be a means to give students practical experience while at the same time reducing reagent volumes (and consequently costs and hazards) or (2) they can be used to introduce students to microfluidics in upper-year courses by using chemistry that is already a part of the students’ prior knowledge. This first point is nicely exhibited by a microfluidic demonstration of the electrolysis of water developed by Davis et al. 33 By using microfluidics, the cost of expensive glassware (Hoffman apparatus) is eliminated and the students can get results within 1 min, visually verifying that water comprises two elements in a 2:1 ratio [Fig. 1(c)]. Polydimethylsiloxane (PDMS) devices were prepared in advance (out of safety concerns of having students work with uncured PDMS), but the students had a chance to fabricate their own microfluidic devices out of gelatin, which worked equally well. The activity was directed at high school students, but would also be appropriate in an introductory undergraduate course. The second purpose of microfluidic activities for general chemistry concepts is neatly demonstrated by a microfluidic acid-base titration experiment developed by Greener et al. 34 in which part of the motivation was to promote microfluidics as a field to students earlier in their career. Acid-base titrations were carried out in a flow reactor with mixing in a serpentine channel. The volumes of acid and base could be changed quickly by adjusting the relatively flow rates and a pH electrode was used to record the pH downstream. Thus, a full titration experiment could be performed under 30 min. Although the experiments were specifically targeted at analytical chemistry students, the rapid generation of titration curves is attractive for the general chemistry classroom as well.
B. Biology/bioengineering
Educational microfluidics that include biology can be divided into projects where a learning technology relies on using microfluidics “inside” as an enabling component that facilitates a biology experiment vs projects were microfluidic devices are actually designed, built, and then tested with biological substrates. Based on our literature search, there currently seem to be significantly fewer educational microfluidics projects that involve biology compared to chemistry.
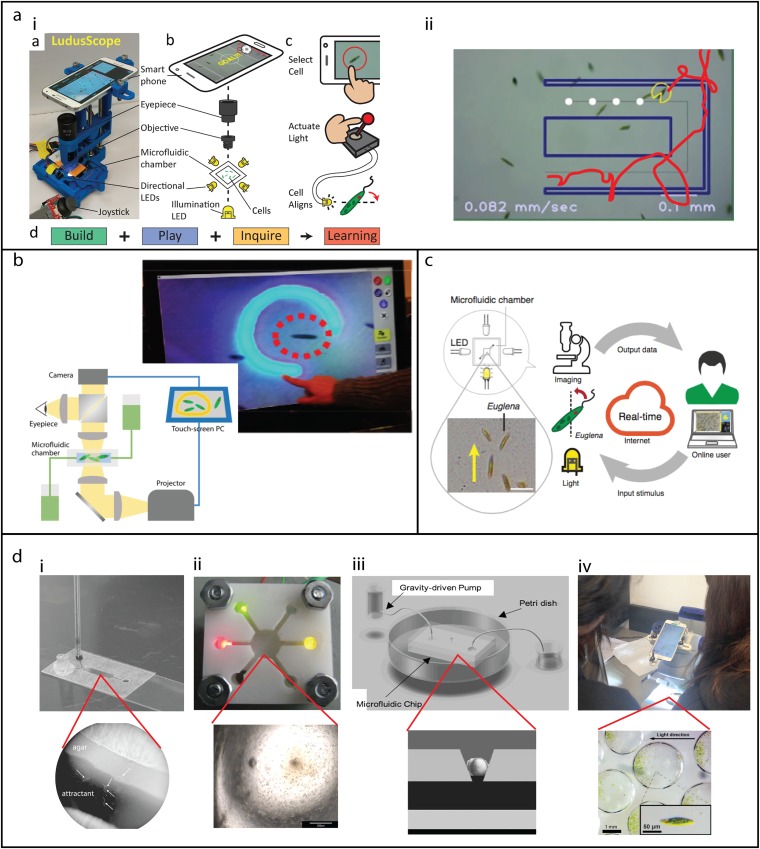
Microfluidics has been utilized to realize devices that make the observation and manipulation of microscopic cells directly accessible to the general public. 35,36 For example, humans can provide physical stimuli (e.g., light, electric fields, chemicals) onto living cells in real time and observe their responses [Fig. 2(a-i)]. These devices then also enabled biotic games, e.g., through overlaying of virtual objects on a screen the user can play games like PAC Man, where phototactic Euglena cells 37 are guided through a simple microfluidic device, and where real time cell tracking enables interaction with virtual objects, i.e., “collecting” these objects [Fig. 2(a-ii)]. 35,38 These concepts were then further developed into an interactive museum exhibit (“Trapit”), where museum visitors of all ages can interact with cells by drawing light patterns on a touch screen, which is then projected onto these cells [Fig. 2(b)]. 39,40 Overall, the design space for such artistic and playful applications—all of which have education potential—has been explored in a number of related design projects. 36,41–44 The vision of this line of research is to ultimately develop interactive devices equivalent to, e.g., the home computer of the 1970s, that are programmable and accessible, and thereby enable many applications in art, entertainment, and education. 35,44
FIG. 2.
Microfluidics in formal and informal biology education: (a) LudusScope (i) DIY smartphone microscopy with integrated microfluidics and directional light emitting diodes (LED) enabling real-time interaction with living, phototactic Euglena cells (ii) Biotic video games can be realized on setups like in (i), where real-time object tracking and virtual overlay turns directional phototaxis experiments into playful games. Reproduced from Kim et al., PLoS One 11(10), e0162602 (2016), Copyright 2016 Author(s), licensed under a Creative Commons Attribution 4.0 License. (b) The interactive museum exhibit “TrapIt” enables visitors of all ages to interrogate the behavior of living cells (housed in a microfluidic device) through a touchscreen, where images drawn by the visitor are projected as light onto cells, which then respond correspondingly—providing a real-time interactive experience with living cells. Reproduced with permission from Lee et al., in Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing (ACM, Seoul, Republic of Korea, 2015), pp. 2593–2602. Copyright 2015 Association for Computing Machinery. (c) Real-time interactive biology experimentation cloud labs utilize microfluidics for long-term stable culturing of cells. Reproduced with permission from Hossain et al., Nat. Biotechnol. 34, 1293–1298 (2016). Copyright 2016 Springer Nature. (d) Examples of integrating microfluidics and biology into K-12 and college education: (di) interrogating nematodes chemotaxis and microecology. Adapted from Stilwell et al., Am. Biol. Teach. 79(9), 753–762 (2017). (dii) Euglena behavior in response to different wavelength of light; Reproduced with permission from Mauk et al., in ASSE Annual Conference and Exposition, Conference Proceedings, (San Antonio, Texas, 2012); (diii) zebrafish development; Reproduced from Shen et al., Zebrafish 6(2), 201–213 (2009). Copyright 2009 Mary Ann Liebert, Inc., licensed under Creative Commons License. (div) capturing Euglena in droplets and interrogating phototaxis. Reproduced with permission from Gerber et al., Biomicrofluidics 9(6), 064105 (2015), Copyright 2015 AIP Publishing LLC.
Related microfluidic devices have formed the foundation for real-time interactive biological cloud labs that enable biophysics experiments at low cost and scale across the world [Fig. 2(c)]. 45–48 In these cloud labs, learners can remotely operate interactive microscopes, and where microfluidics plays a key part in order to keep the biological systems, i.e., phototactic Euglena cells, functional and responsive. These cloud labs have meanwhile been deployed in multiple educational settings from middle to graduate school and also as an open online course. 45 Importantly, experiments take only about 1 min, and cost ∼1 US cent, and scaling to millions of experiments a year—hence an educational impact at global scale—is technologically feasible. 45 User studies with remote participants also demonstrated how key components of scientific inquiry are facilitated, e.g., going from open exploration to hypothesis formulation and robust testing—all through the web. 46 While such cloud technologies so far have focused on Euglena phototaxis, the future extension to many different microfluidic-supported subject areas in chemistry, biology, biophysics and more is apparent.
In another line of work, there have been multiple projects that actually let students build the microfluidic devices themselves in order to then do biology experiments: (1) An integrated projected that facilitates building, playing, and learning is the LudusScope, which was developed as a DIY device that allows integrated building, experimentation, and learning with/of a smartphone microscope [Fig. 2(a)]. 38 Here, students use/learn about sticker microfluidics, but also CAD, 3D printing, optics, electronics, and biology (see below). (2) A Stanford Bioengineering team developed an introductory, 10-week long course to teach bioengineering undergraduate students the fundamentals of device design. 41 This devices class teaches students microfluidics, CAD, 3D printing, optics, electronics, and biology—while students build a biotic game setup as a final project. The paper provides the complete course material. Bioethical issues could also be incorporated. 49 (3) A low-cost and easy-to-use microfluidics platform for K-12 classrooms was developed and tested that allows the investigation of the predatory nematode chemotaxis behavior and thereby illustrating aspects of microbial symbiosis, pathogenesis, and ecology [Fig. 2(d-i)]. 50 (4) Microfluidic devices were designed for Engineering Technology curricula and high school science classes that include experiments incorporating PCR and phototaxis of algae (Euglena) [Fig. 2(d-ii)]. 51 (5) Over one semester, an undergraduate team in a biomedical engineering class designed, built, and tested a zebrafish microfluidic bioreactor applying microfluidics to study zebrafish development [Fig. 2(d-iii)]. 52 Students learned engineering and biology experimental design, chip microfabrication, mathematical modeling, zebrafish husbandry, principles of developmental biology, fluid dynamics, microscopy, and basic molecular biology theory and techniques. The students also provided weekly written and oral reports and finally presented conference posters. (6) Sticker microfluidics were developed as an inexpensive rapid-prototyping method based on laser-cut acrylic blocks and double-sided tape that allows researchers and children to quickly assemble multilayered microfluidic devices from easily prefabricated building blocks [Fig. 2(d-iv)]. 43 This kit was then integrated and tested in a 90-min lesson plan for children age 12–14 years old, where students experimented with colored fluids, generated water droplets in oil, captured Euglena cells inside these droplets, and then executed basic phototaxis experiments with these cells [Fig. 2(d-iv)]. (7) DNA replication was achieved using a microfluidic convective PCR setup. A portable motion analysis microscope revealed the flow patterns using fluorescent bead tracers; the system was tested and assessed with undergraduate students. 53 (8) We also point to the iGEM projects (International Genetically Engineered Machine) 54,55 as an example of student-driven learning, and where a number of projects actually involve microfluidics, i.e., 11 projects from the years 2008–2016 state in their abstract that they utilize microfluidics; in most cases, microfluidics is used as a supporting platform for the biology experiments. 56
IV. INFORMAL SETTINGS
Of course, teaching about microfluidics is not limited to the university classroom. There has been a realization by researchers as well as funding bodies, governments, and learned societies that communication with the general public is paramount not only to enthuse the next generation of potential scientists, but also to help reduce misconceptions about science and scientists. Many funders now require elements of science outreach and engagement with the general public as part of the grant delivery. Thus, researchers in the microfluidics community are taking part in public science events such as Science Festivals, Nights of Science, Scientific Cafes, Pint of Science, Soapbox Science, to name a few, as well as offering workshops and activities at Open Days and visiting local schools. 18 Furthermore, outreach opportunities such as school visits offer opportunities for academic researchers to support local schools in training young scientists and engineers by exposure to microfluidics. 57
Due to the interdisciplinary nature of most microfluidics research projects, hands-on activities with microfluidic devices can be a great starting point to engage members of the general public in conversations about engineering, physics, and chemistry as well as environmental and life sciences. To be engaging, it is important to keep the main points simple and relevant, and as far as possible, jargon-free, while making them exciting and fun. For those looking for further resources on effective science communication and outreach, we recommend resources such as Christensen’s text 58 and Varner’s framework. 59 Relatively few microfluidics outreach activities are reported in the literature, and hence we include in this review activities demonstrated during a science outreach event held as part of the 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS) in Dublin, Ireland in 2016. Around 25 different hands-on-activities, developed by researchers from academia and industry in Europe, North America, and East Asia were run with 500 local teenage pupils and explanatory posters of these activities are stored in the form of a repository. 60 We further break down categories of topics for outreach to (i) teaching about fabrication of microfluidic devices, (ii) teaching about concepts of flow behavior, (iii) teaching about concepts of physical forces, and (iv) teaching about point-of-care diagnostics and biomedical research.
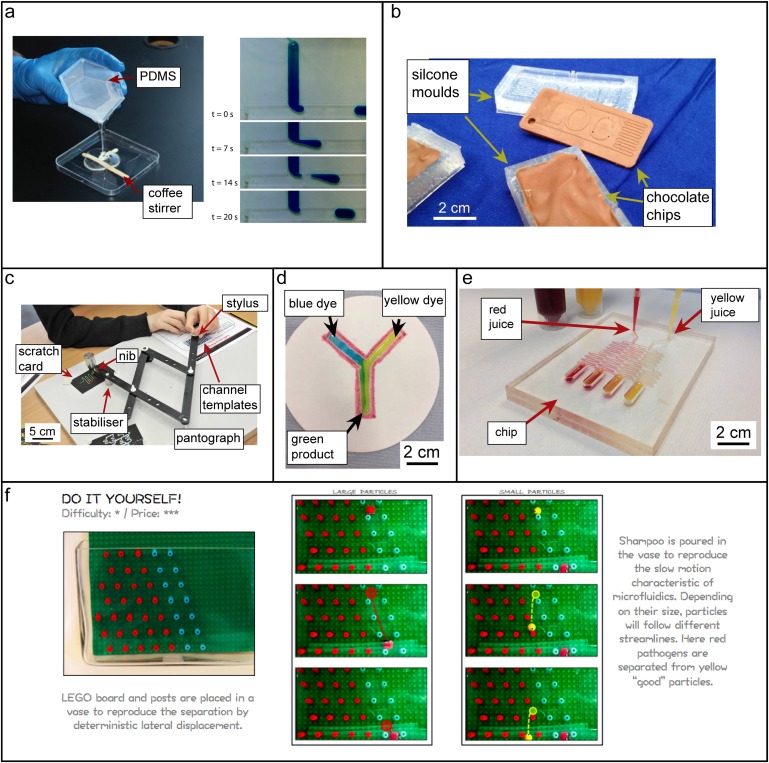
Microchip fabrication commonly involves processes in the dust-free cleanroom environment, complex machinery, or handling potentially harmful chemicals. In outreach settings away from the laboratory, demonstrations of fabrication techniques that use household materials are a good option. These safe materials make it easier for participants to get hands-on experience, rather than just watching a demonstration by a trained professional. For example, soft lithography can be demonstrated using larger molds made from materials such as wooden coffee stirrers or craft sticks and casting with materials such as modeling clays, 17 food-grade gelatin (Jell-O) 61 and, of course, PDMS. 62 Making macroscale channels eliminates the need for microscopy to visualize the chip, and even complex architectures, such as T-junctions for droplet formation, can be realized with simple methods [Fig. 3(a)]. However, long curing times (e.g., overnight and up to two days is recommended for Jell-O chips) can be a disadvantage. Other manufacturing techniques can be demonstrated with a little bit of creativity. For instance, the process of injection molding can be demonstrated by molding chocolate with syringes and plastic molds [Fig. 3(b)]. 18 A technique like computer numerical control (CNC) machining can be demonstrated with a pantograph and a scratch card. Using the pantograph, the student/participant can trace out the design of a microfluidic chip with a stylus (akin to inputting the G-code to a CNC machine) while a nib on the duplicating arm scratches out a facsimile in the scratch card, analogous to the removal of material by the machine bit [Fig. 3(c)]. 18 Paper microfluidic devices are particularly amenable to outreach activities. They can be made and used within minutes with materials as simple as coffee filters and wax crayons [Fig. 3(d)]; several fabrication techniques have been reported for science outreach including paper punching, wax crayons drawing around stencils, or free-hand as well as wax printing. 18,63,64
FIG. 3.
Outreach activities with microfluidics. (a) Droplet generator T-junction made from coffee stirrers and PDMS (left). Photograph of droplet generation using the chip (right). Reproduced with permission from D. Bardin and A. P. Lee, Lab Chip 14(20), 3978–3986 (2014). Copyright 2014 The Royal Society of Chemistry. (b) Chocolate chips formed from silicone molds to demonstrate principles of injection molding. (c) A pantograph mimics a CNC machine. The stylus traces the channel templates and the nib traces out the pattern on a scratch card. (d) Simple paperfluidic device to mix blue and yellow dyes made with filter paper and wax crayons. (e) Laminar flow, diffusion, and mixing are demonstrated with a gradient chip and red and yellow juices. (b)–(e) Reproduced with permission from Esfahani et al., Biomicrofluidics 10(1), 011301 (2016). Copyright 2016 AIP Publishing LLC. (f) A DLD device comprising a LEGO board and pins and shampoo. Such a setup can demonstrate separation of large and small particles. Reproduced from M. Jimenez and H. L. Bridle, Lab Chip 15(4), 947–957 (2015). Copyright 2015 Author(s), licensed under a Creative Commons 3.0 Unported License.
Concepts of fluid behavior, such as laminar flow, diffusion, flow around obstacles, and droplet generation can all be visualized readily and interactively in real time. Many groups have taken the approach of larger-scale demonstrations and activities that do not involve inspection via microscope and these are arguably better suited for the outreach environment. At the macroscale, low Reynolds numbers to demonstrate laminar flow can be achieved in highly viscous liquids. A common demonstration of this is the mixing and unmixing of colored dyes in a Couette cell 65,66 filled with a viscous liquid such as clear corn syrup. A Couette cell can simply be made with concentric beakers with spacers to maintain their relative positions, but there are also plans and commercially available products on the Internet. The “Christmas Tree” channel designs 67 used to generate concentration gradients allow direct interactive visualization of laminar flow and diffusion in microfluidic channels and can be demonstrated with food dye solutions or even fruit juices 18 [Fig. 3(e)]. Owens and Hart have developed LEGO®-style bricks 16 that allow simple interconnecting of fluidic circuits to demonstrate laminar flow, diffusion, and mixing. Laminar flow through capillary forces can also be demonstrated in paperfluidic devices with food dyes. 68 Laminar flow around obstacles is exploited in deterministic lateral displacement (DLD) for the size-based separation of microparticles. This has been scaled up by Jimenez and Bridle, 17 using a LEGO® board with pins as obstacles which is placed into a glass tank filled with relatively viscous clear shampoo [Fig. 3(f)]. Clay balls of different sizes (centimeter scale) made from FIMO® can be placed into the tank by the participant and watched as they travel at an easily observable speed either straight down the pin board or are displaced to the side. They can be elegantly fished out with a magnetic stirrer bar angle to allow repetition of the experiment.
Droplet microfluidics is now prominent in next generation sequencing or can be used to make emulsions, smart materials or to produce contrast agents. 69 Interactive droplet or bubble generation experiments can thus offer a great hook to engage members of the public in a discussion about the relevance of microfluidics. This may include manually pumping immiscible sunflower oil and fruit juice through a channel network or controlling droplet formation with hydrostatic pressure by lifting fluid reservoirs and thus observing the droplets in real time. Other approaches have included using a bicycle pump to generate air bubbles, or even by playing with an interactive bubble game. 60
The various physical forces employed in microfluidic systems are also a good topic for outreach activities, as audiences may have some familiarity with these concepts and there are accessible demonstrations. For instance, magnetic particles are often used in microfluidics to capture analytes of interest from a sample. Researchers have tried to illustrate this with large-scale magnetic FIMO balls or ping-pong balls with drawing pins. 60 There are a number of ways centrifugal forces could be demonstrated, though the outreach and demonstrations we have seen require research-grade setups using synchronized strobes and video cameras. 60 One low-cost demonstration could be to adapt the “paperfuge,” a hand-powered spinning cardboard disk with embedded capillary tubes for separating blood plasma, 70 but clearly there is opportunity for other demonstrations to be shared and published. Electrostatic forces, which are used in digital microfluidics to manipulate discrete droplets of polar liquids, can easily be demonstrated with a statically charged balloon or comb and a gentle stream of water. This can be paired with letting students/participants move droplets on a digital microfluidic device connected to a computer-controlled automation system (e.g., DropBot 71 ).
Ping-pong balls can act as readily available and accessible models for biological cells in scaled up demonstrations. For example, a scaled up version of the Di Carlo cell trap 72 is an interactive opener to a discussion about single cell analysis with large numbers of cells. In a further demonstration model, a mixture of blood cells (red and white ping pong balls), a circulating tumor cell (black ping pong ball with Velcro) and a magnetic capture bead (brown ping pong ball with Velcro and drawing pins) have proved a great tool at family events to entertain children as they try to capture the cancer cell while having a discussion with adults about how scientists use molecular recognition in diagnostics and how cancer diagnosis can be integrated and sped up. 60 Researchers have also developed scaled up flow cells which can hold a jelly biopsy, punched out of a molded jelly organ to illustrate the possibilities behind organ-on-a-chip. 60
Finally, to attract an audience more interested in virtual reality rather than hands-on experimentation, Tarn et al. 73 have developed Minecraft Microfluidics. The videogame, Minecraft, lets players generate a world in which they can fabricate structures out of building blocks. Importantly, Minecraft features flowing water and a number of animals/creatures that interact with each other in interesting ways. By exploiting these factors, it is possible to construct models in the game that represent real-world lab-on-a-chip features and operations. Concepts include lithography with masks and sun-light and hammering away structures, concentration gradients illustrated through blue and red cows moving through a channel network and collecting in “pens,” magnetic deflection with a wolf being deflected from a swimming direction as it is attracted to a block with a sheep hidden inside. These analogies represent a fun and entertaining way of explaining the core aspects of commonly used microfluidic methodologies without the need for excessive jargon or background knowledge.
In summary, a wide range of hands-on activities have been developed by researchers across the globe. Different types of activities suit different age ranges, choices also need to be made depending on the type of interaction, i.e., a clearly timed workshop session vs an open-ended interaction at a stall in a Science Fair. Since microfluidics research projects tend to be multidisciplinary, an experiment or demonstration can act as a hook to take conversations into a range of directions such as engineering, physics, chemistry, molecular biology, and biomedical research topics.
V. FABRICATION METHODS
There are a variety of methods for fabricating microfluidic and LOC devices. The teaching environment (university level or otherwise) often poses constraints such as cost, complexity, and time. These constraints are not unique to education and also dictate design considerations for a range of applications of microfluidics. As such, there are many fabrication techniques that were first developed in a research setting that are completely amenable to the classroom. In particular, reports focused on rapid prototyping or potential applications in global health are particularly appropriate for teaching as they exist out of needs for simplicity and minimal costs. With such choice, it should be easy to pick a fabrication method that is appropriate for the activity or application. For instance, if students are expected to fabricate their own devices, then both cost and fabrication time might be driving concerns. Conversely, if time does not allow for students to fabricate their own devices, or if the learning objectives are more squarely focused on using microfluidics merely as a tool to learn something about the natural world, then more expensive fabrication techniques might be chosen to produce reliable and reusable devices. Here, we highlight fabrication techniques for three different regimes of microfluidics (channel, paper, and digital) that are amenable to the classroom.
A. Microchannels
The literature is replete with examples of low-cost channel-based devices, but we have selected some of our favorite examples which we think are broadly accessible and suitable for the classroom and undergraduate laboratory. Microchannels are defined as having a characteristic dimension on the order of tens or hundreds of micrometers. However, many cleanroom-free methods are unable to achieve these finer dimensions, but for most demonstrative or teaching purposes, this should not pose an issue.
1. Shrink film for microfluidics
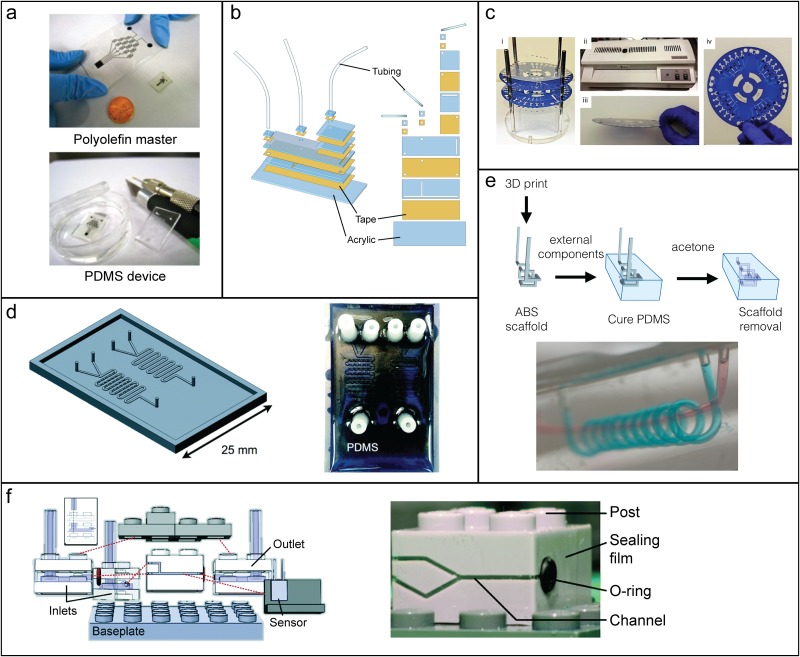
One of the earliest examples of microchannel fabrication specifically targeted toward education is that developed by Khine et al. using prestressed polymer sheets, and is outlined in Fig. 4(a). 74 These prestressed polymers are commonly available as Shrinky Dinks® (polystyrene), though polyolefin 75 was found to have better shrinkage and to warp less. In this method, prestressed polymer sheets are patterned at the macroscale, and then heated, which results in biaxial shrinkage of 60%–95%. By printing on the polymer sheets with a laser printer, positive features are created which, after shrinking, can be used as a master for PDMS chips. 31 Alternatively, engraving the polymer results in negative features, which can be bonded to another sheet of prestressed polystyrene prior to heat shrinking. 76 In terms of equipment, both methods are readily accessible; the former method only requires a laser printer and the latter can be done by hand using razor blades, luer stubs, or syringe needles, though this can be readily adapted to craft cutters and CNC milling machines. The aspect ratio of the channels can be tuned by printing multiple layers of toner (for PDMS molds) or by adjusting the depth of engraving.
FIG. 4.
Microchannel fabrication techniques. (a) Shrink-film generation of masters for PDMS devices. (Top) Laser-printed polyolefin sheets shrink biaxially by 95%. (Bottom) PDMS is cured on the polyolefin master. Adapted with permission from Nguyen et al., Biomicrofluidics 5(2), 022209 (2011). Copyright 2011 AIP Publishing LLC. (b) Laminate microfluidic device from laser-cut tape and acrylic sheets. Acrylic (blue) layers are cut to define the channels and adhesive tape (yellow) is used to laminate the layers together. Reproduced with permission from Gerber et al., Biomicrofluidics 9(6), 064105 (2015), Copyright 2015 AIP Publishing LLC. (c) Print-cut-laminate fabrication method. (i) Polyester sheets with adhesive toner (blue) and hydrophilic valves (black) as well as cut channels are assembled on a guide scaffold. (ii) A conventional office laminator is used to laminate the sheets together into (iii) a single device. (iv) A completed PCL device for centrifugal microfluidics. Adapted from Thompson et al., Nat. Protoc. 10, 875–886 (2015). Copyright 2015 Springer Nature. (d) 3D printed masters for PDMS chips. (Left) A CAD rendering of a chip master showing a serpentine microfluidic channel, posts for tubing connections, and a lip to create a trough for casting PDMS. (Right) A completed PDMS devices. Adapted with permission from Comina et al., Lab Chip 14(2), 424–430 (2014). Copyright 2014 The Royal Society of Chemistry. (e) ESCARGOT method for creating channels in PDMS. (Top) 3D printed ABS scaffolds are submerged in PDMS, which is then cured. Acetone is then used to dissolved the ABS scaffold, resulting in a network of microchannels. (Bottom) Complex architectures such as spirals (blue) around a single channel (red) are possible. Adapted from V. Saggiomo and A. H. Velders, Adv. Sci. 2(9), 1500125 (2015). Copyright 2015 Author(s), licensed under a Creative Commons 4.0 License. (f) Modular LEGO® microfluidic system. (Left) Different building blocks are engraved with different functionalities and can be snapped together on a LEGO® baseplate. (Right) Microfluidic modules are formed from channels milled into the side of a LEGO® brick, which are sealed with a sealing film. An O-ring ensures a tight connection with an adjacent module. The post allows bricks to be snapped together with a third brick or plate. Adapted with permission from C. E. Owens and A. J. Hart, Lab Chip 18(6), 890–901 (2018). Copyright 2018 The Royal Society of Chemistry.
2. Laminated microfluidic devices
Another fabrication approach that is suitable for the university teaching environment is laminated microfluidics. These devices are formed from razor- or laser-cut layers of polymer sheets and/or tape, which are then stacked together. 43,77–79 Channel depth is determined by the thickness of the layers and multiple layers can be stacked for thicker channels. Through this process, complicated multilayer devices like the one shown in Fig. 4(b) are easily assembled. For cell culture experiments, biocompatible tapes are also available. 80
Laminated microfluidic devices can also be formed using printer toner to define channel walls. 81 A pattern of toner is printed onto an overhead transparency sheet (polyester film), which is then laminated using an office laminator to another sheet of transparency. The toner melts between 100 and 150 °C, adhering the two polymer sheets together and also serves to define the channel walls. With this approach, channel depths as small as 6 μm can be achieved. A similar method is print-cut-laminate (PCL) 82 and is capable of greater channel depths. In the PCL method [Fig. 4(c)], toner is used as described above, but a CO2 laser is used to cut out channels within the polyester sheet. This increases channel depths to the thickness of the polyester film (or multiples thereof) and the toner can also be used to create hydrophobic valves. These toner-based devices are robust enough to be used for capillary electrophoresis, 81 as well as survive thermocycling regimes used in PCR. 82,83
3. Other pDMS methods
Soft lithography with PDMS is arguably the most popular method for fabricating microchannels. It has been a very popular choice in the research laboratory because it is relatively cheap and easy to prototype with. However, this is often coupled with the production of a photoresist-on-silicon master by photolithography, which requires access to cleanroom facilities. To overcome this challenge, many methods have been developed to create masters without the need for a cleanroom, though they cannot achieve the same resolutions as photolithography.
One option for cleanroom-free fabrication of masters is to use a 3D printer. Two-dimensional networks of channels can be formed in a relatively straightforward fashion by curing PDMS onto a 3D-printed mold and then peeling off the cured PDMS, as depicted in Fig. 4(d). 84 Depending on the material of the mold, the unreacted monomer (from the 3D printer resin) can interfere with PDMS curing and coating 84 or a cleaning procedure needs to be employed. 85 More complex, nonplanar microfluidic devices can be produced from 3D printed molds as well, which are not accessible through photolithographic methods. For instance, Hwang et al. 86 demonstrated that helical and corkscrew patterns can be produced from reusable 3D-printed molds. Care and sometimes creativity are required for removing these molds, as they do not come out in a single layer and may require twisting 86 or the mold can be removed when the PDMS is partially cured, with final curing sealing up any cracks. 85 These methods for nonplanar 3D channel networks can be quite tedious, and so another option is to dissolve the 3D-printed mold after PDMS curing. Saggiomo and Velders developed an elegant method for creating complex 3D channel networks in PDMS, such as the one shown in Fig. 4(e), which they termed ESCARGOT (for Embedded SCAffold RemovinG Open Technology). 87 Molds are printed from acrylonitrile butadiene styrene (ABS), which can then be dissolved in acetone. However, the dissolution process can be quite lengthy, taking up to 12 h.
4. Modular methods and kits
The last category of microchannel fabrication techniques we wish to highlight are modular approaches that allow students to snap together preformed microfluidic elements (e.g., mixers, junctions, etc.). The “plug-n-play” nature of these systems allows students to quickly assemble and reconfigure microfluidic systems, which helps with the discovery process in student learning. Module-based systems hold a lot of promise, and with standardization, they may become the go-to toolset for teaching microfluidics. While many of these examples currently require in-house fabrication, we share them to highlight what we hope could be possible for commercially available microfluidic teaching kits.
Many modular microfluidic systems have used the LEGO® building block system as an inspiration or analogy. There are two reports that use truly LEGO®-like blocks—one which uses PDMS blocks containing microfluidic channels as well as the iconic studs for snapping blocks together 88 and another which makes use of actual LEGO® bricks for the modular components. 16 In the latter method, highlighted in Fig. 4(f), Owens and Hart used a desktop CNC milling machine to mill channels into the sides of the bricks and an adhesive polyethylene film (PCR sealing film) was used to seal the channels. The channels wrap around the corner of the brick where rubber O-rings are used to secure connections between bricks. While this system is not commercially available, the authors have shared their design files. Morgan et al. 89 developed a series 3D-printed modular microfluidic components, although these are custom bricks and do not interface with LEGO® in the same manner as the previous example. One last modular system that has been likened to LEGO® (although bearing no resemblance to the iconic bricks) was developed by Bhargava et al. 90 and uses 3D-printed cubes with microchannel structures embedded internally. Cubes can contain straight channels, mixing elements, T-junctions, and corners (among many other elements) that can be snapped together to create quite complex and nonplanar 3D microfluidic systems.
Po Ki Yuen at Corning Inc. developed two modular microfluidic systems. The first system, 91 termed SmartBuild, is based around a motherboard for snapping in microfluidic components. SmartBuild uses two different types of microfluidic components—there are modular elements (e.g., mixers, reactors, junctions, etc.) with integrated miniature male luer fittings and fluidic connectors with integrated miniature female luer fittings. The modular elements fit into the fluidic connectors, which then snap into the motherboard. A second system 92 forgoes the motherboard and microfluidic “sticks” snap together using magnetic junctions. This allows users to quickly assemble and reassemble microfluidic systems without having to first plan out the underlying geometry on a motherboard. Both of these modular systems rely on 3D-printed componentry, though the magnetic system can also be used with PDMS parts.
B. Paperfluidics
Paperfluidic devices are an attractive option for educational applications. They are inherently low cost and can be made from a variety of readily available materials found both in and outside the research laboratory. Liquid can be guided by either cutting the paper substrate to a specific design 68 or by impregnating the paper with hydrophobic barriers such as wax. 93,94 We recommend the review by Cate et al., 95 which includes a substantial discussion on a range of different paperfluidic fabrication techniques. While many different techniques exist, we highlight those which do not require highly specialized equipment or facilities.
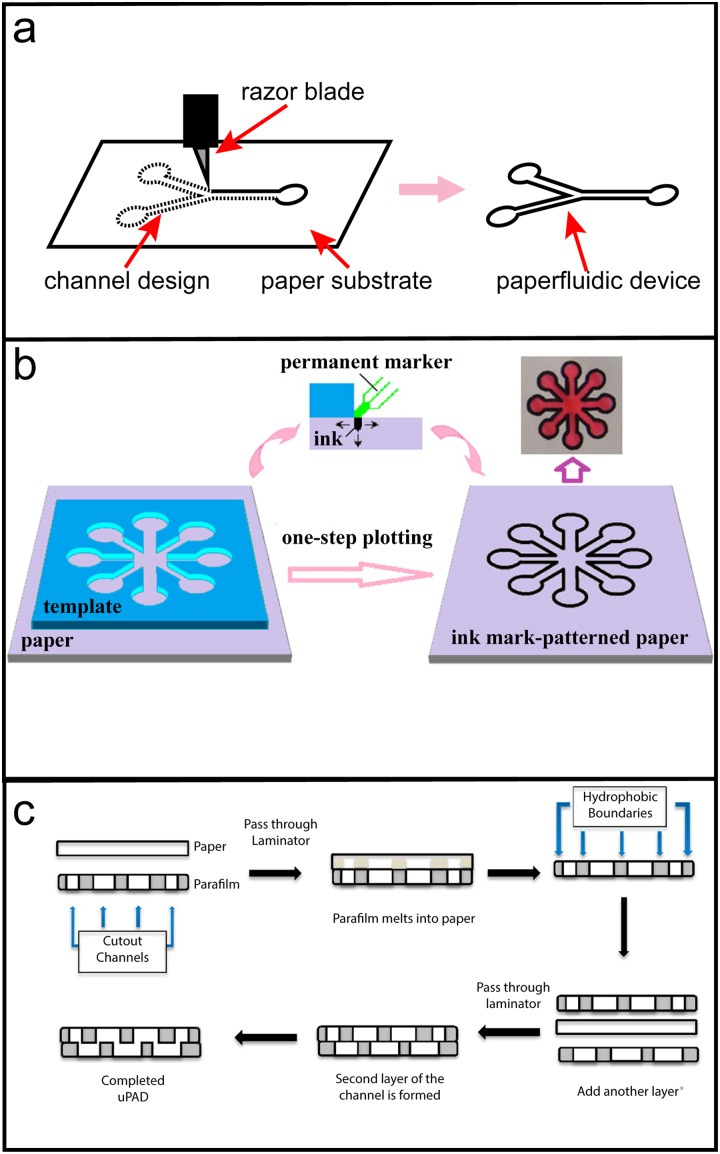
Paperfluidic devices formed by cutting the substrate to a two-dimensional shape can be made with the assistance of a computer-controlled cutter [e.g., a craft cutter equipped with a razor blade, as in Fig. 5(a) or laser cutter] or done by hand. While benchtop CO2 laser cutters are expensive ($400–2000+ USD) and perhaps not as common, craft cutters are more affordable ($150–250 USD) and more widely available. Automated cutters are best suited for devices with complex designs or very narrow channel widths or if mass-production is required. If the devices are too small or too fragile, they can be mounted onto glass or polymer substrates using double-sided tape. 96 Hand-cut devices can obviously be made by freestyle cutting of paper with scissors or a razor blade. For greater precision and reproducibility, stencils that students can place on the paper substrate and cut around with a razor or scalpel could be used.
FIG. 5.
Paperfluidic fabrication methods. (a) Cartoon illustrating method for fabricating cutout paperfluidic devices. A computer-controlled razor blade (i.e., craft cutter) cuts the channel design out of a paper substrate resulting in a paperfluidic device. (b) One-step plotting of paperfluidic devices with a (hydrophobic) permanent marker, template, and paper substrate. Reproduced with permission from Nie et al., Anal. Chem. 84(15), 6331–6335 (2012). Copyright 2012 American Chemical Society. (c) Method for impregnating paper with wax using parafilm and a laminator. The channel design is cut out of the parafilm, matted to the paper substrate, and passed through a laminator. The result is paper with impregnated hydrophobic barriers from the parafilm wax. The process can be repeated several times to create a multilayer device. Reproduced with permission from Koesdjojo et al., J. Chem. Educ. 92(4), 737–741 (2015). Copyright 2015 American Chemical Society.
Patterning hydrophobic barriers within porous paper media is another approach to fabricating paperfluidic devices. Unlike those formed from cutting, patterned devices are not as fragile since structural material is not removed during fabrication. One of the simplest fabrication techniques is to use permanent markers, which typically have water-repellant ink, to draw barriers on filter paper. 97 Tracing a stencil provides a certain level of precision in reproducing paperfluidic devices [Fig. 5(b)]. Like cut devices, computer-controlled and handmade options exist. Wax printers, which rely on transferring melted wax onto paper substrates, are a popular method for paperfluidic fabrication. 94 With these printers, a computer design can turn into a functional device in very few steps: the wax pattern is printed and the substrate is then heated to impregnate it with the wax. Despite the prevalence of reports using solid wax printers, the manufacturer (Xerox) has discontinued production. Printers and wax ink are still commercially available. An alternative, computer-aided method to wax printing is paraffin lamination. 64 A craft cutter is used to cut out designs in Parafilm, which is then melted into the paper substrate using an office laminator. With this method, multilayered devices can be formed from stacking multiple single layer devices and passing the collection through the laminator where residual wax anneals the layers together, as outlined in Fig. 5(c).
C. Digital microfluidics
Digital microfluidics (DMF) is perhaps the most esoteric of the three microfluidic formats we discuss here, and as such, we are unaware of any peer-reviewed reports of applying it explicitly for teaching purposes, though it has been used for outreach events 60 and even games have been created for the format. 98 Historically, DMF devices required access to cleanroom facilities for fabrication and elaborate equipment for their operation. These barriers have only recently come down with the development of cleanroom-free fabrication methods 99–101 and open-source 71,102 or commercial instrumentation.
DMF devices comprise a generic array of “driving” electrodes covered by a layer of dielectric insulator (e.g., Parylene-C, Si3N4, SU-8) and a hydrophobic coating (e.g., Teflon AF, Fluoropel, Cytop). 103 These electrodes have traditionally been formed by photolithography of metal-coated substrates. A second plate (often termed “top plate”), bearing a ground electrode, can be mated to the electrode array with spacers, typically double-sided tape, and this format is termed a “two-plate” device, as shown in Fig. 6(a-i). Alternatively, the ground electrode can be coplanar with the driving electrodes, forming a “one-plate” device. Droplets of polar or conducting liquids are moved by actuating driving electrodes adjacent to the droplet; application of a voltage between the driving and ground electrodes generates and attractive force.
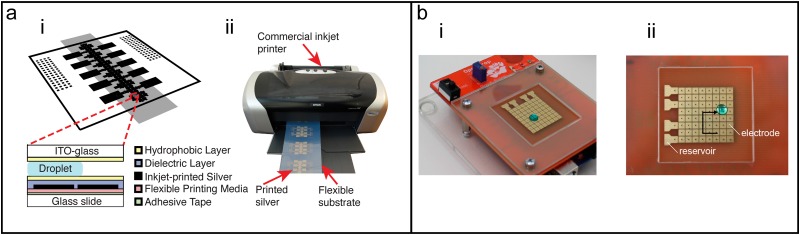
FIG. 6.
Digital microfluidic chip fabrication. (a) Overview of inkjet-printed DMF chips. (i) Two-plate DMF chips comprise a top plate of ITO-glass coated with a hydrophobic layer and a bottom plate of inkjet-printed silver on a flexible printing media which is mounted on a glass slide. (ii) Commercial inkjet printers can be adapted to print silver ink on flexible substrates. Reproduced from Ng et al., Sci. Trans. Med. 10(438), eaar6076 (2018). Copyright 2018 American Association for the Advancement of Science. (b) OpenDrop PCB-based DMF chip and controller. (i) Photograph of the standalone integrated system. (ii) Droplets are manipulated by electrodes patterned in the PCB. Reproduced from M. Alistar and U. Gaudenz, Bioengineering 4(2), 45 (2017). Copyright 2017 Author(s), licensed under a Creative Commons Attribution 4.0 License.
The challenges in developing cleanroom-free fabrication methods are largely focused on (1) patterning metal DMF driving electrodes and (2) identifying dielectric materials that can be easily applied to the bottom plate. There are many different substrates that can be used for the DMF driving electrodes, such as writable compact discs, 104 copper laminate films, 105,106 and commercially sourced printed circuit boards (PCBs). 102 Further, inkjet printers equipped with conductive inks have also been used to form DMF driving electrodes and at low cost [Fig. 6(a-ii)]. 99,100 As for the dielectric layer, there exist a number of alternatives to the typically vapor-deposited materials. For instance, SU8 photoresist, 103,107 thick (∼6%) Teflon AF solutions, 108 and cyanoethyl pullulan 100 can all be spin-coated onto DMF bottom plates. A more readily available option might be to use food wrap 104 or Parafilm 109 films. The hydrophobic layer can be created from commercially available water repellent coatings such as RainX or similar products. 104
Since DMF requires both chips and electronic control systems, it would be ideal for educational purposes to use a system that combines both. The OpenDrop DMF platform [Fig. 6(b)] is an integrated do-it-yourself system with a PCB-based DMF chip which is directly interfaced with the control system. 102 Hydrophobic dielectric films are used to insulate the driving electrodes and the system is operated by computer control, but the most recent versions also include manual joystick control.
VI. SUGGESTIONS AND FINAL THOUGHTS
Taking together the literature discussed and our own experiences as researchers, educators, and communicators, we would like to offer some practical advice for microfluidic researchers looking to incorporate microfluidics into their teaching. A recurring theme in many of the published reports discussed here is contextualization—taking research-level equipment and experience and adapting it to the appropriate learning environment. There are many options for activities, fabrication methods, and lesson ideas to choose from, but it is important to tailor them to both the learning environment and learning outcomes. We highlight different fabrication techniques and activities in the context of different learning environments in Table I. There might be a temptation to merely replicate what is done in the research laboratory, which may not be appropriate for all contexts. We suggest thinking first about the intended audience: are they students training to become experts or an audience of the general public? If the former, then trying to replicate the same methods used in research might be a good idea; if the latter, then it might be better to think about which techniques and materials will help you clearly explain the concept. There might be ways to reproduce the same or similar microscale phenomena but on the macroscale, which is useful if presenting to a crowd. For instance, large-scale models of microfluidic devices can still exhibit laminar flow by using viscous liquids (e.g., shampoo or soap). The location of learning should also be factored in: is it a (well equipped) classroom environment or out in the “playground”? While the ideal is that experiments always work, activities that require more troubleshooting and expert support are best suited to the classroom. For the “playground,” and especially when open-ended free play is desired, the devices and activities should be very robust and risk assessments as well as any required personal protective equipment need to be given consideration. Learning outcomes may vary greatly: is the goal to master a specific technical skill, to learn something about chemistry or biology, or to visualize and understand a concept about liquids on the microscale? For skill mastery or even using microfluidics to learn about the natural world, we recommend minimizing student involvement in design and fabrication, as these processes can be lengthy and have a significant learning curve. There tends to be more room for inquiry-based methods and for students to experiment with design and fabrication when trying to observe and understand microscale physical phenomena.
TABLE I.
Hands-on microfluidic activities for different contexts.
| Context | Time limits | Resources and/or constraints | Fabrication methods | Learning activities | |
|---|---|---|---|---|---|
| Informal learning environment | Drop-in stand (Science Festival, museum, University Open Day, etc.) | 5–10 min |
|
||
| School visit | 30–60 min |
|
|||
| Workshop session on-campus | 30–60 min |
|
Same as above plus: | ||
| Formal learning environment | Introductory undergraduate lab | 1–3 h |
|
|
|
| M.Sc. or specialized undergraduate course | Multiple days |
|
|
Besides the literature we identified and highlighted here, there are likely many more such activities undertaken by university faculty that are not yet published. We, therefore, encourage faculty and other educators to contribute to the literature with examples of microfluidic laboratory activities. Broadly speaking, these activities can be about (i) designing microfluidic devices, (ii) operating microfluidic devices, or (iii) using microfluidics as a convenient platform to experiment and play with fluidic, biological, or chemical phenomena. Analogies between microfluidics and electronics have been previously proposed in terms of design and operation of pressure driven systems, 110 but we think this analogy can be further extended to consider how electronics has become very accessible. The extended set of components (resistors, transistors, integrated circuits, etc.) make circuit design and applications highly accessible for education and do-it-yourself applications. We expect microfluidics to go a similar route by modularity. This modularity can be on the design side, with standard features assembled in a drag-and-drop manner prior to fabrication (similar to integrated circuit design); or there are already modular components which can be assembled together like electronic components on a breadboard. One promising resource we direct readers to is www.Metafluidics.org, an open source repository of microfluidic devices. 111 Though it has not yet led to standardized design components, users can pick and choose from hundreds of shared chips or chip components. When sharing new learning activities in the formal literature, education-focused journals tend to expect evidence of the activity’s impact on student learning, and this should be included in reports submitted to noneducation journals too. This evidence and evaluation could take the form of obtaining participant feedback (students and teachers) or measuring differences in student performance (pre- and postactivity assessment; control and treatment groups), for instance.
Chip fabrication is probably the largest impediment to incorporating teaching with microfluidics. We have presented a number of accessible fabrication techniques, but it would be better if more low-cost components were available. There are a number of companies specialized in microfluidic device fabrication, offering custom chip fabrication as well as stock parts. However, though costs decrease with scale, these chips are still relatively expensive. (e.g., at the time of writing, a simple single channel chip design is listed as €36.20 each from the Microfluidic Chip Shop, www.microfluidic-chipshop.com. This price decreases to only €18.10 per chip for volumes greater than 100 chips). We believe that a larger educational market for DIY components and whole chips could actually drive the market thus increasing accessibility. For educators, adapting to standardized and commercially available microfluidic chips will reduce some flexibility and customization, but it will enable the development and evaluation of new learning activities that can be more easily shared. In this same vein, it is likely more worth developing and sharing new learning activities that take advantage of established fabrication methods, rather than introducing yet another cheap, alternative fabrication technique. Despite this barrier, there is still room for new learning activities in microfluidics and we are hopeful that this review will encourage researchers to contribute to educational applications.
ACKNOWLEDGMENTS
The authors wish to thank M. Jimenez, N. Cira, M. Khine, M. Use, and D. Weibel for helpful conversations and assistance with figures.
Contributor Information
Ingmar H. Riedel-Kruse, Email: , .
Nicole Pamme, Email: .
REFERENCES
- 1. Heath J. R., Ribas A., and Mischel P. S., Nat. Rev. Drug Discovery 15 , 204 (2015). 10.1038/nrd.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gawad C., Koh W., and Quake S. R., Nat. Rev. Genet. 17 , 175 (2016). 10.1038/nrg.2015.16 [DOI] [PubMed] [Google Scholar]
- 3. Vashist S. K., Luppa P. B., Yeo L. Y., Ozcan A., and Luong J. H. T., Trends Biotechnol. 33 (11), 692–705 (2015). 10.1016/j.tibtech.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 4. Fintschenko Y., Lab Chip 11 (20), 3394–3400 (2011). 10.1039/c1lc90069b [DOI] [PubMed] [Google Scholar]
- 5. Legge C. H., J. Chem. Educ. 79 (2), 173 (2002). 10.1021/ed079p173 [DOI] [Google Scholar]
- 6. Whitesides G. M., Nature 442 , 368–373 (2006). 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- 7. Olsen D. S., Spontaneous Generations: J. History Philos. Sci. 4 (1), 231–254 (2010). 10.4245/sponge.v4i1.11942 [DOI] [Google Scholar]
- 8. Czerniak C. M. and Johnson C. C., Handbook Res. Sci. Educ. 2 395–411 (2014). [Google Scholar]
- 9. Lattuca L. R., Voigt L. J., and Fath K. Q., Rev. Higher Educ. 28 (1), 23–48 (2004). 10.1353/rhe.2004.0028 [DOI] [Google Scholar]
- 10. Schweingruber H., Keller T., and Quinn H., A Framework for K-12 Science Education Practices, Crosscutting Concepts, and Core Ideas (The National Academies Press, Washington, DC, 2012). [Google Scholar]
- 11. Vygotsky L. S., Mind in Society (Harvard University Press, Cambridge, MA, 1978). [Google Scholar]
- 12. Pedaste M., Mäeots M., Siiman L. A., de Jong T., van Riesen S. A. N., Kamp E. T., Manoli C. C., Zacharia Z. C., and Tsourlidaki E., Educ. Res. Rev. 14 , 47–61 (2015). 10.1016/j.edurev.2015.02.003 [DOI] [Google Scholar]
- 13. Morton J. A. S. and Bridle H., Biomicrofluidics 10 (3), 034117 (2016). 10.1063/1.4953448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burns T. W., O’Connor D. J., and Stocklmayer S. M., Public Underst. Sci. 12 (2), 183–202 (2003). 10.1177/09636625030122004 [DOI] [Google Scholar]
- 15. Fischhoff B., Proc. Natl. Acad. Sci. U.S.A. 110 (Suppl. 3), 14033–14039 (2013). 10.1073/pnas.1213273110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Owens C. E. and Hart A. J., Lab Chip 18 (6), 890–901 (2018). 10.1039/C7LC00951H [DOI] [PubMed] [Google Scholar]
- 17. Jimenez M. and Bridle H. L., Lab Chip 15 (4), 947–957 (2015). 10.1039/C4LC00944D [DOI] [PubMed] [Google Scholar]
- 18. Esfahani M. M. N., Tarn M. D., Choudhury T. A., Hewitt L. C., Mayo A. J., Rubin T. A., Waller M. R., Christensen M. G., Dawson A., and Pamme N., Biomicrofluidics 10 (1), 011301 (2016). 10.1063/1.4940884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hemling M., Crooks J. A., Oliver P. M., Brenner K., Gilbertson J., Lisensky G. C., and Weibel D. B., J. Chem. Educ. 91 (1), 112–115 (2014). 10.1021/ed4003018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee S. and Wiener J., J. Chem. Educ. 88 (2), 151–157 (2011). 10.1021/ed100518k [DOI] [Google Scholar]
- 21. Piunno P. A. E., Zetina A., Chu N., Tavares A. J., Noor M. O., Petryayeva E., Uddayasankar U., and Veglio A., J. Chem. Educ. 91 (6), 902–907 (2014). 10.1021/ed400728a [DOI] [Google Scholar]
- 22. Wietsma J. J., van der Veen J. T., Buesink W., van den Berg A., and Odijk M., J. Chem. Educ. 95 (2), 267–275 (2018). 10.1021/acs.jchemed.7b00506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrison D. J., Fluri K., Seiler K., Fan Z., Effenhauser C. S., and Manz A., Science 261 (5123), 895–897 (1993). 10.1126/science.261.5123.895 [DOI] [PubMed] [Google Scholar]
- 24. Harrison D. J., Manz A., Fan Z., Luedi H., and Widmer H. M., Anal. Chem. 64 (17), 1926–1932 (1992). 10.1021/ac00041a030 [DOI] [Google Scholar]
- 25. Ohno K., Tachikawa K., and Manz A., Electrophoresis 29 (22), 4443–4453 (2008). 10.1002/elps.200800121 [DOI] [PubMed] [Google Scholar]
- 26. Teerasong S. and McClain R. L., J. Chem. Educ. 88 (4), 465–467 (2011). 10.1021/ed100717m [DOI] [Google Scholar]
- 27. Chao T.-C., Bhattacharya S., and Ros A., J. Chem. Educ. 89 (1), 125–129 (2012). 10.1021/ed101064p [DOI] [Google Scholar]
- 28. Giri B., Peesara R. R., Yanagisawa N., and Dutta D., J. Chem. Educ. 92 (4), 728–732 (2015). 10.1021/ed4009107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cai L., Wu Y., Xu C., and Chen Z., J. Chem. Educ. 90 (2), 232–234 (2013). 10.1021/ed300385j [DOI] [Google Scholar]
- 30. Feng Z. V., Edelman K. R., and Swanson B. P., J. Chem. Educ. 92 (4), 723–727 (2015). 10.1021/ed5005307 [DOI] [Google Scholar]
- 31. Grimes A., Breslauer D. N., Long M., Pegan J., Lee L. P., and Khine M., Lab Chip 8 (1), 170–172 (2008). 10.1039/B711622E [DOI] [PubMed] [Google Scholar]
- 32. Emmanuel N., Emonds-Alt G., Lismont M., Eppe G., and Monbaliu J.-C. M., J. Chem. Educ. 94 (6), 775–780 (2017). 10.1021/acs.jchemed.6b00899 [DOI] [Google Scholar]
- 33. Davis T. A., Athey S. L., Vandevender M. L., Crihfield C. L., Kolanko C. C. E., Shao S., Ellington M. C. G., Dicks J. K., Carver J. S., and Holland L. A., J. Chem. Educ. 92 (1), 116–119 (2015). 10.1021/ed400757m [DOI] [Google Scholar]
- 34. Greener J., Tumarkin E., Debono M., Dicks A. P., and Kumacheva E., Lab Chip 12 (4), 696–701 (2012). 10.1039/c2lc20951a [DOI] [PubMed] [Google Scholar]
- 35. Riedel-Kruse I. H., Chung A. M., Dura B., Hamilton A. L., and Lee B. C., Lab Chip 11 (1), 14–22 (2011). 10.1039/C0LC00399A [DOI] [PubMed] [Google Scholar]
- 36. Gerber L., Kim H. and Riedel-Kruse I., in DiGRA/FDG ‘16 - Proceedings of the First International Joint Conference of DiGRA and FDG (Dundee, Scotland, 2016), Vol. 13. [Google Scholar]
- 37. Tsang A. C. H., Lam A. T., and Riedel-Kruse I. H., Nat. Phys. 14 (12), 1216–1222 (2018). 10.1038/s41567-018-0277-7 [DOI] [Google Scholar]
- 38. Kim H., Gerber L. C., Chiu D., Lee S. A., Cira N. J., Xia S. Y., and Riedel-Kruse I. H., PLoS One 11 (10), e0162602 (2016). 10.1371/journal.pone.0162602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee S. A., Bumbacher E., Chung A. M., Cira N., Walker B., Park J. Y., Starr B., Blikstein P., and Riedel-Kruse I. H., in Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems (ACM, Seoul, Republic of Korea, 2015), pp. 2593–2602. [Google Scholar]
- 40. Lee S. A., Chung A. M., Cira, N. and Riedel-Kruse I. H., in Proceedings of the Ninth International Conference on Tangible, Embedded, and Embodied Interaction (ACM, Stanford, California, USA, 2015), pp. 273–280. [Google Scholar]
- 41. Cira N. J., Chung A. M., Denisin A. K., Rensi S., Sanchez G. N., Quake S. R., and Riedel-Kruse I. H., PLoS Biol. 13 (3), e1002110 (2015). 10.1371/journal.pbio.1002110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gerber L., Doshi M. C, Kim H., and Riedel-Kruse I., in DiGRA/FDG ‘16-Proceedings of the First International Joint Conference of DiGRA and FDG (Dundee, Scotland, 2016). [Google Scholar]
- 43. Gerber L. C., Kim H., and Riedel-Kruse I. H., Biomicrofluidics 9 (6), 064105 (2015). 10.1063/1.4935593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lam A. T., Samuel-Gama K. G., Griffin J., Loeun M., Gerber L. C., Hossain Z., Cira N. J., Lee S. A., and Riedel-Kruse I. H., Lab Chip 17 (8), 1442–1451 (2017). 10.1039/C7LC00131B [DOI] [PubMed] [Google Scholar]
- 45. Hossain Z., Bumbacher E. W., Chung A. M., Kim H., Litton C., Walter A. D., Pradhan S. N., Jona K., Blikstein P., and Riedel-Kruse I. H., Nat. Biotechnol. 34 , 1293 (2016). 10.1038/nbt.3747 [DOI] [PubMed] [Google Scholar]
- 46. Hossain Z., Bumbacher E., Brauneis A., Diaz M., Saltarelli A., Blikstein P., and Riedel-Kruse I. H., Int. J. Artif. Intell. Educ. 28 (4), 478–507 (2018). 10.1007/s40593-017-0150-3 [DOI] [Google Scholar]
- 47. Hossain Z. and Riedel-Kruse I. H., in Cyber-Physical Laboratories in Engineering and Science Education, edited by Auer M. E., Azad A. K. M., Edwards A., and de Jong T. (Springer International Publishing, Cham, 2018), pp. 271–304. [Google Scholar]
- 48. Washington P., Samuel-Gama K. G., Goyal S., Ramaswami A., and Riedel-Kruse I. H., Proc. Natl. Acad. Sci. U.S.A. 116 (12), 5411–5419 (2019). 10.1073/pnas.1815367116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harvey H., Havard M., Magnus D., Cho M. K., and Riedel-Kruse I. H., Hastings Center Rep. 44 (6), 38–46 (2014). 10.1002/hast.386 [DOI] [PubMed] [Google Scholar]
- 50. Stilwell M. D., Nepper J. F., Clawson E. D., Blair V., Tangen T., and Weibel D. B., Am. Biol. Teach. 79 (9), 753–762 (2017). 10.1525/abt.2017.79.9.753 [DOI] [Google Scholar]
- 51. Mauk M. G., Chiou R., Genis V., Carr M. E., Tadros D., and Sikich C., in ASEE Annual Conference and Exposition, Conference Proceedings (San Antonio, Texas, 2012). [Google Scholar]
- 52. Shen Y.-C., Li D., Al-Shoaibi A., Bersano-Begey T., Chen H., Ali S., Flak B., Perrin C., Winslow M., Shah H., Ramamurthy P., Schmedlen R. H., Takayama S., and Barald K. F., Zebrafish 6 (2), 201–213 (2009). 10.1089/zeb.2008.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Priye A., Hassan Y. A., and Ugaz V. M., Lab Chip 12 (23), 4946–4954 (2012). 10.1039/c2lc40760d [DOI] [PubMed] [Google Scholar]
- 54. Smolke C. D., Nat. Biotechnol. 27 , 1099 (2009). 10.1038/nbt1209-1099 [DOI] [PubMed] [Google Scholar]
- 55. Kelwick R., Bowater L., Yeoman K. H., and Bowater R. P., FEMS Microbiol. Lett. 362 (16), fnv129 (2015). 10.1093/femsle/fnv129 [DOI] [PubMed] [Google Scholar]
- 56.iGEM Team list for all years, see https://igem.org/Team_List?year=all; accessed 21 May 2019.
- 57. Bridle H., Morton J., Cameron P., Desmulliez M. P. Y., and Kersaudy-Kerhoas M., Microfluid. Nanofluidics 20 (7), 103 (2016). 10.1007/s10404-016-1770-x [DOI] [Google Scholar]
- 58. Christensen L. L., The Hands-on Guide for Science Communicators: A Step-by-Step Approach to Public Outreach (Springer Science & Business Media, 2007). [Google Scholar]
- 59. Varner J., BioScience 64 (4), 333–340 (2014). 10.1093/biosci/biu021 [DOI] [Google Scholar]
- 60. Chemical and Biological Microsystems Society, see https://cbmsociety.org/outreach; accessed 7 May 2019.
- 61. Yang C. W. T., Ouellet E., and Lagally E. T., Anal. Chem. 82 (13), 5408–5414 (2010). 10.1021/ac902926x [DOI] [PubMed] [Google Scholar]
- 62. Bardin D. and Lee A. P., Lab Chip 14 (20), 3978–3986 (2014). 10.1039/C4LC00424H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ravgiala R. R., Weisburd S., Sleeper R., Martinez A., Rozkiewicz D., Whitesides G. M., and Hollar K. A., J. Chem. Educ. 91 (1), 107–111 (2014). 10.1021/ed300261a [DOI] [Google Scholar]
- 64. Koesdjojo M. T., Pengpumkiat S., Wu Y., Boonloed A., Huynh D., Remcho T. P., and Remcho V. T., J. Chem. Educ. 92 (4), 737–741 (2015). 10.1021/ed500401d [DOI] [Google Scholar]
- 65. Ohmura N., Kataoka K., Shibata Y., and Makino T., Chem. Eng. Sci. 52 (11), 1757–1765 (1997). 10.1016/S0009-2509(97)00012-2 [DOI] [Google Scholar]
- 66. Piau J. M., Bremond M., Couette J. M., and Piau M., Rheol. Acta 33 (5), 357–368 (1994). 10.1007/BF00366579 [DOI] [Google Scholar]
- 67. Li Jeon N., Baskaran H., Dertinger S. K. W., Whitesides G. M., Van De Water L., and Toner M., Nat. Biotechnol. 20 , 826 (2002). 10.1038/nbt712 [DOI] [PubMed] [Google Scholar]
- 68. Osborn J. L., Lutz B., Fu E., Kauffman P., Stevens D. Y., and Yager P., Lab Chip 10 (20), 2659–2665 (2010). 10.1039/c004821f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shang L., Cheng Y., and Zhao Y., Chem. Rev. 117 (12), 7964–8040 (2017). 10.1021/acs.chemrev.6b00848 [DOI] [PubMed] [Google Scholar]
- 70. Bhamla M. S., Benson B., Chai C., Katsikis G., Johri A., and Prakash M., Nat. Biomed. Eng. 1 , 0009 (2017). 10.1038/s41551-016-0009 [DOI] [Google Scholar]
- 71. Fobel R., Fobel C., and Wheeler A. R., Appl. Phys. Lett. 102 (19), 193513 (2013). 10.1063/1.4807118 [DOI] [Google Scholar]
- 72. Di Carlo D., Wu L. Y., and Lee L. P., Lab Chip 6 (11), 1445–1449 (2006). 10.1039/b605937f [DOI] [PubMed] [Google Scholar]
- 73. Tarn M., Peyman S., Corlyon C., Momtazian H., Smith J., Spencer M., Taylor S., Lorch M., and Pamme N., paper presented at the Proceedings of 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences, μTAS, Dublin, Ireland, 2016. [Google Scholar]
- 74. Nguyen D., McLane J., Lew V., Pegan J., and Khine M., Biomicrofluidics 5 (2), 022209 (2011). 10.1063/1.3576930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nguyen D., Taylor D., Qian K., Norouzi N., Rasmussen J., Botzet S., Lehmann M., Halverson K., and Khine M., Lab Chip 10 (12), 1623–1626 (2010). 10.1039/c001082k [DOI] [PubMed] [Google Scholar]
- 76. Chen C.-S., Breslauer D. N., Luna J. I., Grimes A., Chin W.-c., Lee L. P., and Khine M., Lab Chip 8 (4), 622–624 (2008). 10.1039/b719029h [DOI] [PubMed] [Google Scholar]
- 77. Bartholomeusz D. A., Boutte R. W., and Andrade J. D., J. Microelectromech. Syst. 14 (6), 1364–1374 (2005). 10.1109/JMEMS.2005.859087 [DOI] [Google Scholar]
- 78. Islam M., Natu R., and Martinez-Duarte R., Microfluid. Nanofluidics 19 (4), 973–985 (2015). 10.1007/s10404-015-1626-9 [DOI] [Google Scholar]
- 79. Yuen P. K. and Goral V. N., Lab Chip 10 (3), 384–387 (2010). 10.1039/B918089C [DOI] [PubMed] [Google Scholar]
- 80. Stallcop L. E., Álvarez-García Y. R., Reyes-Ramos A. M., Ramos-Cruz K. P., Morgan M. M., Shi Y., Li L., Beebe D. J., Domenech M., and Warrick J. W., Lab Chip 18 (3), 451–462 (2018). 10.1039/C7LC00724H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lucio do Lago C., Torres da Silva H. D., Neves C. A., Alves Brito-Neto J. G., and Fracassi da Silva J. A., Anal. Chem. 75 (15), 3853–3858 (2003). 10.1021/ac034437b [DOI] [PubMed] [Google Scholar]
- 82. Thompson B. L., Ouyang Y., Duarte G. R. M., Carrilho E., Krauss S. T., and Landers J. P., Nat. Protoc. 10 , 875–886 (2015). 10.1038/nprot.2015.051 [DOI] [PubMed] [Google Scholar]
- 83. Ouyang Y., Duarte G. R. M., Poe B. L., Riehl P. S., dos Santos F. M., Martin-Didonet C. C. G., Carrilho E., and Landers J. P., Anal. Chim. Acta 901 , 59–67 (2015). 10.1016/j.aca.2015.09.042 [DOI] [PubMed] [Google Scholar]
- 84. Comina G., Suska A., and Filippini D., Lab Chip 14 (2), 424–430 (2014). 10.1039/C3LC50956G [DOI] [PubMed] [Google Scholar]
- 85. Chan H. N., Chen Y., Shu Y., Chen Y., Tian Q., and Wu H., Microfluid. Nanofluidics 19 (1), 9–18 (2015). 10.1007/s10404-014-1542-4 [DOI] [Google Scholar]
- 86. Hwang Y., Paydar O. H., and Candler R. N., Sens. Actuators A 226 , 137–142 (2015). 10.1016/j.sna.2015.02.028 [DOI] [Google Scholar]
- 87. Saggiomo V. and Velders A. H., Adv. Sci. 2 (9), 1500125 (2015). 10.1002/advs.201500125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jing N., Qing G., Jing-jiang Q., Miao S., An L., Lei S., Jian-zhong F., Peng Z., and Yong H., Biofabrication 10 (3), 035001 (2018). 10.1088/1758-5090/aaadd3 [DOI] [PubMed] [Google Scholar]
- 89. Morgan A. J. L., Hidalgo San Jose L., Jamieson W. D., Wymant J. M., Song B., Stephens P., Barrow D. A., and Castell O. K., PLoS One 11 (4), e0152023 (2016). 10.1371/journal.pone.0152023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bhargava K. C., Thompson B., and Malmstadt N., Proc. Natl. Acad. Sci. U.S.A. 111 (42), 15013–15018 (2014). 10.1073/pnas.1414764111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yuen P. K., Lab Chip 8 (8), 1374–1378 (2008). 10.1039/b805086d [DOI] [PubMed] [Google Scholar]
- 92. Yuen P. K., Lab Chip 16 (19), 3700–3707 (2016). 10.1039/C6LC00741D [DOI] [PubMed] [Google Scholar]
- 93. Martinez A. W., Phillips S. T., Butte M. J., and Whitesides G. M., Angew. Chem. Int. Ed. 46 (8), 1318–1320 (2007). 10.1002/anie.200603817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Carrilho E., Martinez A. W., and Whitesides G. M., Anal. Chem. 81 (16), 7091–7095 (2009). 10.1021/ac901071p [DOI] [PubMed] [Google Scholar]
- 95. Cate D. M., Adkins J. A., Mettakoonpitak J., and Henry C. S., Anal. Chem. 87 (1), 19–41 (2015). 10.1021/ac503968p [DOI] [PubMed] [Google Scholar]
- 96. Fu E., Liang T., Spicar-Mihalic P., Houghtaling J., Ramachandran S., and Yager P., Anal. Chem. 84 (10), 4574–4579 (2012). 10.1021/ac300689s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nie J., Zhang Y., Lin L., Zhou C., Li S., Zhang L., and Li J., Anal. Chem. 84 (15), 6331–6335 (2012). 10.1021/ac203496c [DOI] [PubMed] [Google Scholar]
- 98.8-bit Frogger game on a digital microfluidics device, see https://blog.arduino.cc/2017/01/29/8-bit-frogger-game-on-a-digital-microfluidics-device/; accessed 11 March 2019.
- 99. Fobel R., Kirby A. E., Ng A. H. C., Farnood R. R., and Wheeler A. R., Adv. Mater. 26 (18), 2838–2843 (2014). 10.1002/adma.201305168 [DOI] [PubMed] [Google Scholar]
- 100. Dixon C., Ng A. H. C., Fobel R., Miltenburg M. B., and Wheeler A. R., Lab Chip 16 (23), 4560–4568 (2016). 10.1039/C6LC01064D [DOI] [PubMed] [Google Scholar]
- 101. Ng A. H. C., Fobel R., Fobel C., Lamanna J., Rackus D. G., Summers A., Dixon C., Dryden M. D. M., Lam C., Ho M., Mufti N. S., Lee V., Asri M. A. M., Sykes E. A., Chamberlain M. D., Joseph R., Ope M., Scobie H. M., Knipes A., Rota P. A., Marano N., Chege P. M., Njuguna M., Nzunza R., Kisangau N., Kiogora J., Karuingi M., Burton J. W., Borus P., Lam E., and Wheeler A. R., Sci. Transl. Med. 10 (438), eaar6076 (2018). 10.1126/scitranslmed.aar6076 [DOI] [PubMed] [Google Scholar]
- 102. Alistar M. and Gaudenz U., Bioengineering 4 (2), 45 (2017). 10.3390/bioengineering4020045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Choi K., Ng A. H. C., Fobel R., and Wheeler A. R., Annu. Rev. Anal. Chem. 5 (1), 413–440 (2012). 10.1146/annurev-anchem-062011-143028 [DOI] [PubMed] [Google Scholar]
- 104. Watson M. W. L., Abdelgawad M., Ye G., Yonson N., Trottier J., and Wheeler A. R., Anal. Chem. 78 (22), 7877–7885 (2006). 10.1021/ac0613378 [DOI] [PubMed] [Google Scholar]
- 105. Abdelgawad M. and Wheeler A. R., Adv. Mater. 19 (1), 133–137 (2007). 10.1002/adma.200601818 [DOI] [Google Scholar]
- 106. Abdelgawad M. and Wheeler A. R., Microfluid. Nanofluidics 4 (4), 349 (2007). 10.1007/s10404-007-0190-3 [DOI] [Google Scholar]
- 107. Dryden M. D. M., Rackus D. D. G., Shamsi M. H., and Wheeler A. R., Anal. Chem. 85 (18), 8809–8816 (2013). 10.1021/ac402003v [DOI] [PubMed] [Google Scholar]
- 108. Yu Y., Chen J., Li J., Yang S., Fan S.-K., and Zhou J., J. Micromech. Microeng. 23 (9), 095025 (2013). 10.1088/0960-1317/23/9/095025 [DOI] [Google Scholar]
- 109. Yafia M., Shukla S., and Najjaran H., J. Micromech. Microeng. 25 (5), 057001 (2015). 10.1088/0960-1317/25/5/057001 [DOI] [Google Scholar]
- 110. Oh K. W., Lee K., Ahn B., and Furlani E. P., Lab Chip 12 (3), 515–545 (2012). 10.1039/C2LC20799K [DOI] [PubMed] [Google Scholar]
- 111. Kong D. S., Thorsen T. A., Babb J., Wick S. T., Gam J. J., Weiss R., and Carr P. A., Nat. Biotechnol. 35 , 523 (2017). 10.1038/nbt.3873 [DOI] [PubMed] [Google Scholar]