Abstract
A strategy for the installation of small alkyl fragments onto pharmaceutically relevant aliphatic structures has been established via metallaphotoredox catalysis. Herein, we report that tris(trimethylsilyl)silanol can be employed as an effective halogen abstraction reagent that, in combination with photoredox and nickel catalysis, allows a generic approach to Csp3─Csp3 cross-electrophile coupling. In this study, we demonstrate that a variety of aliphatic drug-like groups can be successfully coupled with a number of commercially available small alkyl electrophiles, including methyl tosylate and strained cyclic alkyl bromides. Moreover, the union of two secondary aliphatic carbon centers, a long-standing challenge for organic molecule construction, has been accomplished with a wide array of structural formats. Last, this technology can be selectively merged with Csp2─Csp3 aryl–alkyl couplings to build drug-like systems in a highly modular fashion.
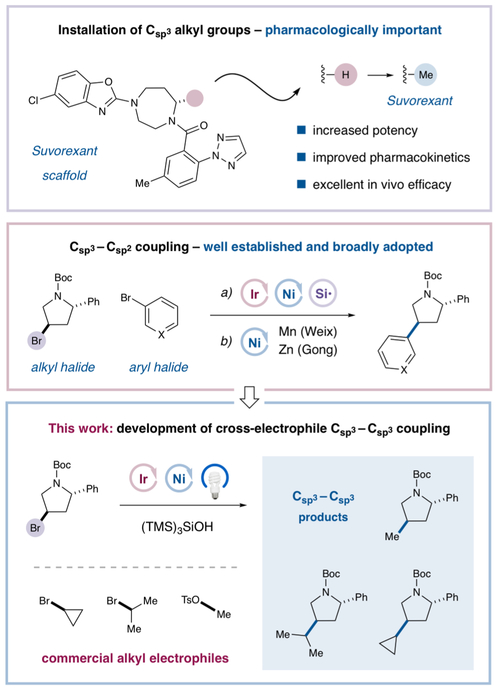
Recently, it has been reported that the clinical success of small molecule therapeutics can be correlated with increasing levels of Csp3 incorporation within the carbon framework of medicinal agents.1 In this regard, small alkyl moieties, and in particular methyl groups, have proven to be of significant value in medicinal chemistry due to their capacity to induce conformational constraints on aliphatic ring systems while decreasing the available sites for P450 metabolism.2 This was demonstrated in the case of the drug Suvorexant, in which installation of the aliphatic C-7 methyl group led to improved potency and pharmacokinetic properties (Scheme 1).3 As such, new methods for the modular installation of small alkyl groups are highly desirable, and the pioneering work of Knochel,4 Fu,5 and others6,7 has established that the heterocoupling of Csp3 centers can be accomplished using organometallic alkyl nucleophiles. However, strategies for the reductive cross-coupling of two alkyl electrophiles have been slower to develop,8,9 and general methods for the pairing of two secondary alkyl centers remain extremely rare.10,11
Scheme 1.
Small Alkyl Group Installation via Halide Coupling
Metallaphotoredox catalysis has become a prominent synthetic strategy in medicinal chemistry for the coupling of complex molecular fragments via C─C, C─N, C─S, and C─O bond formation.12,13 In 2016, our laboratory reported a novel metallaphotoredox pathway to achieve the reductive cross-coupling of aromatic Csp2-halides with aliphatic Csp3-bromides via the catalytic production and application of silyl radicals in combination with nickel catalysis.14 We recently questioned whether it would be possible to employ the same halogen abstraction mechanism to achieve selective Csp3─Csp3 cross-coupling between two discrete alkyl bromides, a pathway that might allow the modular installation of small alkyl groups onto complex drug-like architectures. Among a number of objectives, we hoped to achieve the union of two secondary aliphatic carbon centers, a long-standing challenge for all areas of organic molecule construction (total synthesis, medicinal, process chemistry, etc.),10 given the associated issues involving oxidative addition of hindered alkyl–nickel or alkyl–palladium species into secondary aliphatic Csp3-halide bonds. In particular, we hoped that a halogen abstraction/radical-nickel recombination mechanism might bypass this oxidative addition problem, thereby rendering a novel cross-coupling pathway for the construction of Csp3─Csp3 architectures. As an important design criterion, we recognized that the use of alkyl halides for both reaction partners would remove the requirement for substrate prefunctionalization (e.g., as Grignard, organozinc, or borate salts), thereby reducing operational complexity while expanding the scope of available structural fragments. Furthermore, the reduction in step count would allow for streamlined synthetic sequences and decreased costs.
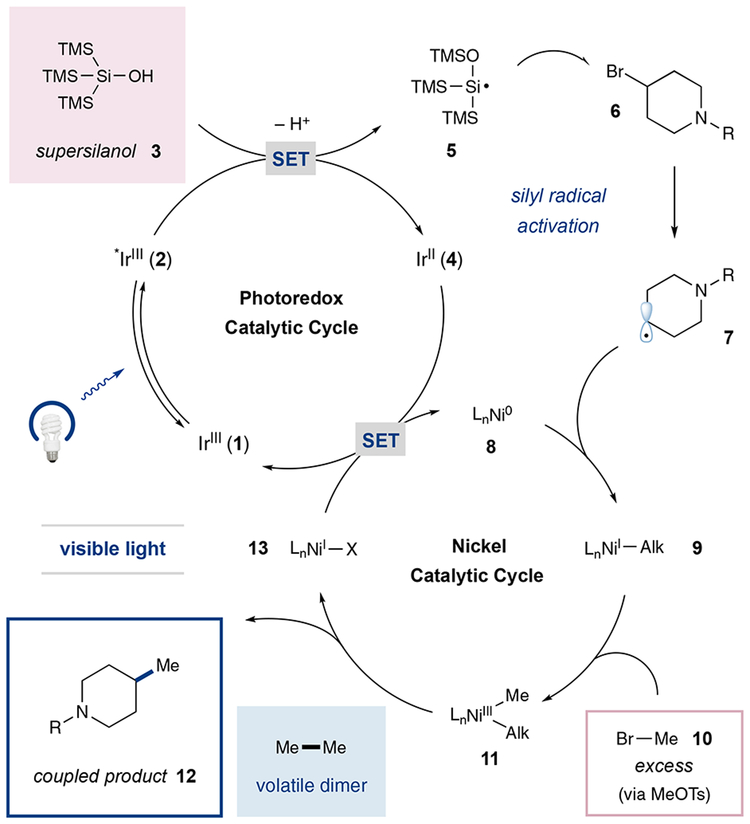
On the basis of recent work from our lab involving (i) nickel catalyzed aryl-alkylation14 and (ii) copper-catalyzed trifluoromethylation,15 we were confident that reductive coupling of two alkyl halide partners should be possible using tris-(trimethylsilyl)silane or the corresponding silanol in combination with nickel and photoredox catalysis. As shown in Scheme 2, we envisioned that visible-light excitation of the Ir(III) photocatalyst Ir[dF(CF3)ppy]2(dtbbpy)PF6 (1) would generate the long-lived (τ = 2.3 μs)16 excited-state *IrIII complex 2. This species is a powerful single-electron oxidant (E1/2red [*IrIII/IrII] = +1.21 V vs SCE in CH3CN),16 and we presumed it would undergo reduction by the silanolate resulting from deprotonation of supersilanol 3 to furnish the reduced Ir(II) catalyst 4.15 The resultant silyloxy-centered radical is known to undergo bond isomerization to produce silyl radical 5,15 which can rapidly17 participate in halogen atom abstraction with alkyl bromides such as 6 to furnish the aliphatic radical 7. At the same time, we anticipated that single-electron reduction of (dtbbpy)Ni(II)Cl2 by the electron-rich Ir(II) form of the photocatalyst 4 would lead to the requisite dtbbpy-ligated Ni(0) complex 8 (E1/2red [IrIII/IrII] = −1.37 V vs SCE in CH3CN, E1/2red [NiII/Ni0] = −1.2 V vs SCE in DMF),16,18 a Ni(0) complex that can readily intercept radical 7.19 Subsequent oxidative addition of this alkyl–Ni(I) species 9 into methyl bromide (10), generated in situ from methyl tosylate, would then lead to the putative dialkyl-organometallic-Ni(III) species 11, which upon reductive elimination would generate the Csp3–Csp3 bond within the desired fragment-coupled adduct 12.20,21 At this stage, both catalytic cycles would converge via SET between the resulting Ni(I) species 13 and the reduced iridium photocatalyst 4 to reestablish both Ir(III) complex 1 and Ni(0) complex 8.16,22 As a key design element, we recognized that the use of excess quantities of the small aliphatic coupling partner (e.g., methyl tosylate) would be operationally viable given that a competitive homodimerization pathway would lead mainly to volatile byproducts (e.g., ethane), thereby allowing facile removal from the desired adduct.
Scheme 2.
Plausible Mechanism for Reductive Methylation
The feasibility of this new approach to Csp3─Csp3 cross-electrophile coupling was first investigated using a drug-like 3-acylpyridinyl piperidine bromide 14 and methyl tosylate in a variety of reaction conditions (see Supporting Information for details). While supersilane was found to be optimal in our previous alkyl-arylation studies,14 we observed that this halogen abstraction agent was generally ineffective in this new Csp3─Csp3 coupling protocol, mainly due to predominant formation of the dehalogenated alkane byproduct. To overcome this halide-reduction problem, we recognized that the use of supersilanol would allow the formation of silyl radicals via a photocatalytic silanolate oxidation/silyl migration sequence, thereby avoiding the use of a Si–H based reagent that can participate in a deleterious hydrogen atom transfer step with alkyl radical intermediates. During the course of reaction optimization, we also found that use of tetrabuty-lammonium bromide as an additive provided superior yields of the desired methylation adduct. We attribute this observation to the importance of rapidly converting methyl tosylate to methyl bromide in situ. Control experiments revealed that excluding light, nickel, or photocatalyst resulted in no product formation (see Supporting Information for details).
With optimal conditions in hand, we next evaluated the scope of this new Csp3─Csp3 methylation protocol using a wide array of pharmaceutically relevant aliphatic structures. As shown in Table 1, the transformation is effective for heterocyclic bromides such as substituted piperidine (15–17, 70–73% yield) and tetrahydropyran (THP) (18, 70% yield) ring systems, both of which are common motifs within medicinal chemistry.23 Furthermore, functionalized cyclohexane rings are also competent in this reaction manifold, providing the corresponding methyl-bearing adducts in good yield (19 and 20, 63 and 77% yield, respectively). Pyrrolidine, a privileged pharmacophore, can also be alkylated efficiently using this catalytic system (21, 71% yield). Notably, in the case of an indane core, an adjacent protected alcohol is readily tolerated, giving the desired product with excellent diastereoselectivity (22, 42% yield, >20:1 d.r.). Examining more strained systems, we were delighted to find that a number of four-membered rings readily undergo methyl coupling in this transformation, including spirocyclic cyclobutanes and azetidine systems (23–26, 49–68% yield). In the context of acyclic systems, we found that both secondary and primary centers gave the desired products in good yield (27 and 28, 62 and 70% yield, respectively). Surprisingly, anilines such as 16, 25, and 28 are well-tolerated in this protocol, despite their established capacity for amine oxidation under photocatalytic conditions.24
Table 1.
Methylation Scope for the Metallaphotoredox-Catalyzed Coupling of Aliphatic Halidesa
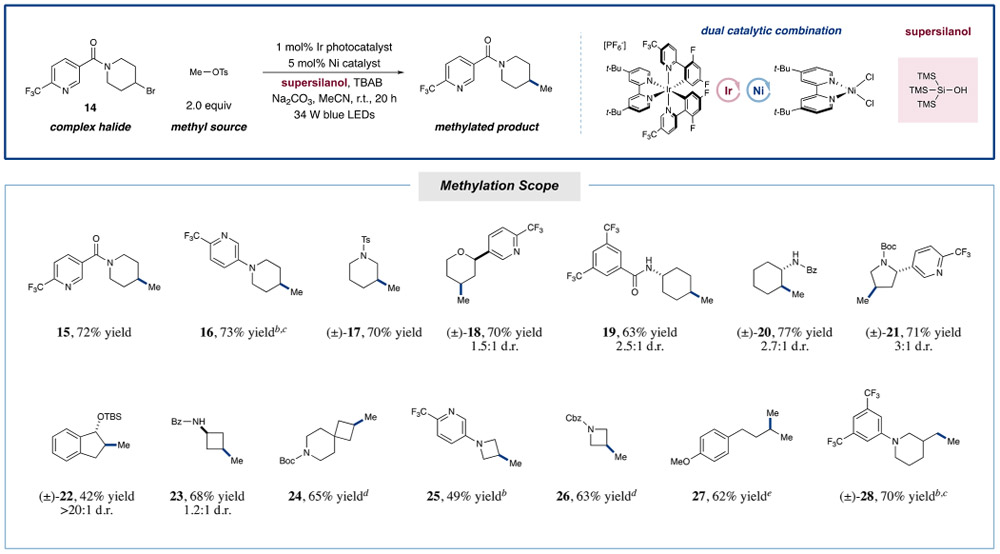 |
Reactions performed with 1.5 equiv supersilanol, 2 equiv Na2CO3, and 2.5 equiv TBAB. Yields isolated unless otherwise noted. Only a small amount (<5%) of homodimerization of the limiting reagent 14 was observed. See SI for experimental details.
Using Ir[dF(Me)ppy]2(dtbbpy)PF6 as photocatalyst.
3 equiv Na2CO3 used.
K3PO4 as base.
GC yield.
Having examined the scope of Csp3-methylation, we next turned our attention to the capacity of this protocol to introduce a range of small alkyl groups onto the drug-like 3-acylpyridinyl piperidine bromide 14 (Table 2). From the outset, we were pleased to find that ethyl and other primary long-chain aliphatic bromides that incorporate esters, free alcohols, alkenes, epoxides, and basic pyridine moieties could be readily implemented in good to excellent efficiency (29–34, 44–78% yield). Given the importance of small cyclic systems in pharmaceutical synthesis, we were delighted to find that strained cyclic alkanes can be readily employed to forge the desired Csp3─Csp3 bond between two aliphatic ring systems (35 and 36, 40 and 67% yield, respectively). Moreover, functionalized cyclobutanes and four-membered heterocycles can be introduced efficiently, allowing for modular access to a variety of strained Csp3 rich bicyclic motifs (37–40, 50–71% yield). Notably, larger ring systems can be readily installed using this new coupling protocol, including cyclopentyl, piperidinyl, and THP fragments (41–43, 45–57% yield). Perhaps most important, we were able to couple an isopropyl group (44, 42% yield) without the formation of isomeric alkyl products. As a critical aspect of this experiment, the use of a tridentate PyBOX (L1)-ligated Ni(II) catalyst successfully prevented metal–alkyl bond isomerization, which frequently leads to the predominant formation of n-propylated adducts in palladium and nickel catalyzed cross-couplings.25 Indeed, given the difficulty of coupling two secondary aliphatic centers, we were pleased to observe that this catalytic protocol can be extended to a range of methine-bearing halides of varying structural complexity (35–44). As further demonstrated in Table 3, the reaction was generically successful for strained ring systems, such as azetidine and oxetane (45 and 46, 50 and 60% yield, respectively), as well as for larger six- and five-membered rings (47–49, 56–58% yield). Remarkably, coupling of two acyclic secondary centers was also possible using this technology (50, 62% yield). We believe that these results demonstrate the first broadly general approach to crosselectrophile coupling of two secondary aliphatic carbon centers.
Table 2.
Scope of Simple Aliphatic Electrophiles in the Metallaphotoredox-Catalyzed Coupling of Aliphatic Halidesa
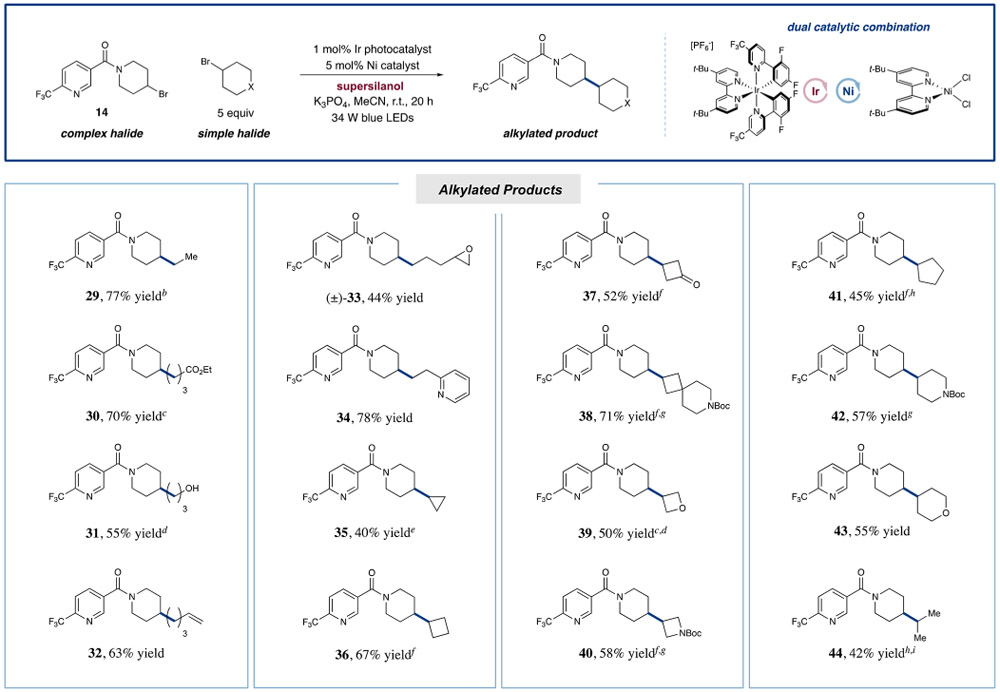 |
Reactions typically performed with 3 equiv supersilanol and 3 equiv K3PO4. See SI for experimental details. Yields isolated unless otherwise noted. Homodimerization of limiting reagent 14 typically between 5 and 10%.
7 equiv small halide.
4 equiv small halide.
UPLC yield.
8 equiv small halide.
Na2CO3 as base.
3 equiv small halide.
Using PyBOX L1 (vide infra) as ligand.
6 equiv small halide.
Table 3.
Scope for Coupling of Secondary Aliphatic Centersa
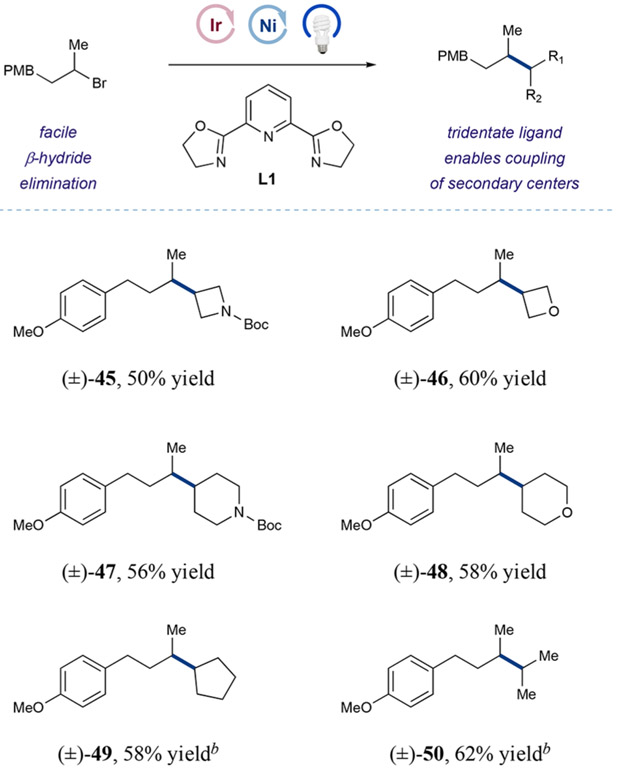 |
Yields isolated unless otherwise noted. Only a small amount (<5%) of homodimerization of the limiting reagent was observed. Reactions performed with 2–5 equiv small halide, 3 equiv Na2CO3 and 2–3 equiv supersilanol. See SI for experimental details.
GC yields.
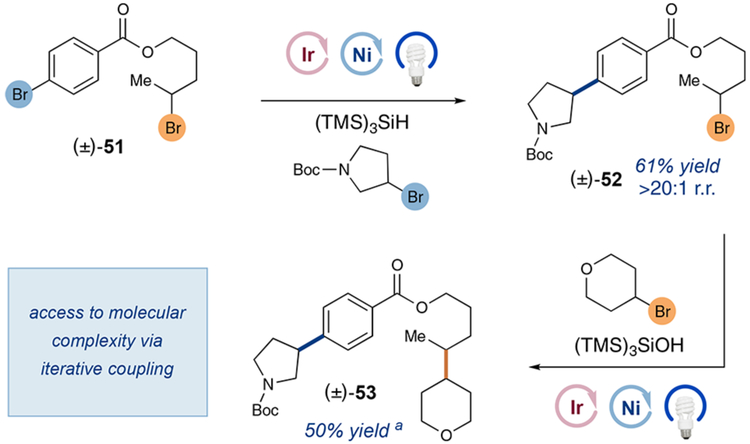
Finally, we sought to investigate the capacity to iteratively build drug-like molecular complexity in a highly expeditious yet modular fashion via the combination of aryl–alkyl and alkyl–alkyl cross-electrophile technologies. To this end, we prepared compound 51, which contains both an aromatic and an aliphatic bromide moiety (Scheme 3). Using the conditions previously published by our lab for metallaphotocatalytic halogen abstraction Csp2─Csp3 coupling,14 it was possible to selectively and efficiently introduce a pyrrolidine ring onto the aromatic ring (compound 52, 61% yield), while leaving the aliphatic bromide group intact. Subjection of the resulting alkyl bromide 52 and 4-bromotetrahydropyran to the protocol outlined herein, resulted in selective Csp3─Csp3 coupling to give the drug-like adduct 53 in useful yield. These results further demonstrate the exquisite functional group tolerance and chemoselectivity of photocatalytic cross-electrophile coupling as well as its potential for application to complex target synthesis.
Scheme 3.
Iterative Coupling Sequence
a Only a small amount (<5%) of homodimerization of the limiting reagent was observed. 1:1 d.r.
Supplementary Material
ACKNOWLEDGMENTS
Financial support provided by the NIHGMS (R01 GM093213) and kind gifts from Merck, BMS, Janssen, and Eli Lilly. This work was supported by Eli Lilly and Company through the Lilly Research Award Program (LRAP).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b12025.
Experimental details and characterization data (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752. [DOI] [PubMed] [Google Scholar]
- (2).Schönherr H; Cernak T Profound Methyl Effects in Drug Discovery and a Call for New C─H Methylation Reactions. Angew. Chem., Int Ed. 2013, 52, 12256. [DOI] [PubMed] [Google Scholar]
- (3).Cox CD; Breslin MJ; Whitman DB; Schreier JD; McGaughey GB; Bogusky MJ; Roecker AJ; Mercer SP; Bednar RA; Lemaire W; Bruno JG; Reiss DR; Harrell CM; Murphy KL; Garson SL; Doran SM; Prueksaritanont T; Anderson WB; Tang C; Roller S; Cabalu TD; Cui D; Hartman GD; Young SD; Koblan KS; Winrow CJ; Renger JJ; Coleman PJ Discovery of the Dual Orexin Receptor Antagonist [(7R)-4-(5-Chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the Treatment of Insomnia. J. Med. Chem 2010, 53, 5320. [DOI] [PubMed] [Google Scholar]
- (4) (a).Devasagayaraj A; Stüdemann T; Knochel P A New Nickel-Catalyzed Cross-Coupling Reaction between sp3 Carbon Centers. Angew. Chem., Int. Ed. Engl 1996, 34, 2723. [Google Scholar]; (b) Giovannini R; Stüdemann T; Dussin G; Knochel P An Efficient Nickel-Catalyzed Cross-Coupling Between sp3 Carbon Centers. Angew. Chem., Int. Ed 1998, 37, 2387. [DOI] [PubMed] [Google Scholar]
- (5) (a).Zhou JS; Fu GC Cross-Couplings of Unactivated Secondary Alkyl Halides: Room-Temperature Nickel-Catalyzed Negishi Reactions of Alkyl Bromides and Iodides. J. Am. Chem. Soc. 2003, 125, 14726. [DOI] [PubMed] [Google Scholar]; (b) Saito B; Fu GC Alkyl–Alkyl Suzuki Cross-Couplings of Unactivated Secondary Alkyl Halides at Room Temperature. J. Am. Chem. Soc 2007, 129, 9602. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cordier CJ; Lundgren RJ; Fu GC Enantioconvergent Cross-Couplings of Racemic Alkylmetal Reagents with Unactivated Secondary Alkyl Electrophiles: Catalytic Asymmetric Negishi α-Alkylations of N-Bocpyrrolidine. J. Am. Chem. Soc 2013, 135, 10946. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schmidt J; Choi J; Liu AT; Slusarczyk M; Fu GC A general, modular method for the catalytic asymmetric synthesis of alkylboronate esters. Science 2016, 354, 1265. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Mu X; Shibata Y; Makida Y; Fu GC Control of Vicinal Stereocenters through Nickel-Catalyzed Alkyl–Alkyl Cross-Coupling. Angew. Chem., Int. Ed 2017, 56, 5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6) (a).Terao J; Watanabe H; Ikumi A; Kuniyasu H; Kambe N Nickel-Catalyzed Cross-Coupling Reaction of Grignard Reagents with Alkyl Halides and Tosylates: Remarkable Effect of 1,3-Butadienes. J. Am. Chem. Soc 2002, 124, 4222. [DOI] [PubMed] [Google Scholar]; (b) Terao J; Todo H; Begum SA; Kuniyasu H; Kambe N Copper-Catalyzed Cross-Coupling Reaction of Grignard Reagents with Primary-Alkyl Halides: Remarkable Effect of 1-Phenylpropyne. Angew. Chem., Int. Ed. 2007, 46, 2086. [DOI] [PubMed] [Google Scholar]; (c) Ren P; Vechorkin O; von Allmen K; Scopelliti R; Hu X A Structure–Activity Study of Ni-Catalyzed Alkyl–Alkyl Kumada Coupling. Improved Catalysts for Coupling of Secondary Alkyl Halides. J. Am. Chem. Soc 2011, 133, 7084. [DOI] [PubMed] [Google Scholar]
- (7).For a recent review of metal-catalyzed Csp3─Csp3 coupling, see: Choi J; Fu GC Transition metal–catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 2017, 356, eaaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Although slower to develop than their aromatic counterparts (vide infra), as well as traditional cross-coupling methodologies, there have been reports of Csp3─Csp3 cross-electrophile coupling. For examples, see:Yu X; Yang T; Wang S; Xu H; Gong H Nickel-Catalyzed Reductive Cross-Coupling of Unactivated Alkyl Halides. Org. Lett 2011, 13, 2138.Xu H; Zhao C; Qian Q; Deng W; Gong H Nickel-catalyzed cross-coupling of unactivated alkyl halides using bis(pinacolato)diboron as reductant. Chem. Sci 2013, 4, 4022.Liang Z; Xue W; Lin K; Gong H Nickel-Catalyzed Reductive Methylation of Alkyl Halides and Acid Chlorides with Methyl p-Tosylate. Org. Lett 2014, 16, 5620.Liu J-H; Yang C-T; Lu X-Y; Zhang Z-Q; Xu L; Cui M; Lu X; Xiao B; Fu Y; Liu L Copper-Catalyzed Reductive Cross-Coupling of Nonactivated Alkyl Tosylates and Mesylates with Alkyl and Aryl Bromides. Chem. - Eur. J 2014, 20, 15334.For an example of a reductive dimerization of alkyl electrophiles, see: Prinsell MR; Everson DA; Weix DJ Nickel-catalyzed, sodium iodide-promoted reductive dimerization of alkyl halides, alkyl pseudohalides, and allylic acetates. Chem. Commun 2010, 46, 5743.
- (9).For examples of transition metal-catalyzed reductive Csp2─Csp3 coupling, see:Czaplik WM; Mayer M; Jacobi von Wangelin A Direct Cobalt-Catalyzed Cross-Coupling Between Aryl and Alkyl Halides. Synlett 2009, 2009, 2931.Krasovskiy A; Duplais C; Lipshutz BH Zn-Mediated, Pd-Catalyzed Cross-Couplings in Water at Room Temperature Without Prior Formation of Organozinc Reagents. J. Am. Chem. Soc 2009, 131, 15592.Amatore M; Gosmini C Direct Method for Carbon–Carbon Bond Formation: The Functional Group Tolerant Cobalt-Catalyzed Alkylation of Aryl Halides. Chem. - Eur. J 2010, 16, 5848.Durandetti M; Nédélec J-Y; Périchon J Nickel-Catalyzed Direct Electrochemical Cross-Coupling between Aryl Halides and Activated Alkyl Halides. J. Org. Chem 1996, 61, 1748.Everson DA; Shrestha R; Weix DJ Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc 2010, 132, 920.Everson DA; Jones BA; Weix DJ Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc 2012, 134, 6146.Wang S; Qian Q; Gong H Nickel-Catalyzed Reductive Coupling of Aryl Halides with Secondary Alkyl Bromides and Allylic Acetate. Org. Lett 2012, 14, 3352.Wang X; Wang S; Xue W; Gong H Nickel-Catalyzed Reductive Coupling of Aryl Bromides with Tertiary Alkyl Halides. J. Am. Chem. Soc 2015, 137, 11562.Molander GA; Traister KM; O’Neill BT Reductive Cross-Coupling of Nonaromatic, Heterocyclic Bromides with Aryl and Heteroaryl Bromides. J. Org. Chem 2014, 79, 5771.Bhonde VR; O’Neill BT; Buchwald SL An Improved System for the Aqueous Lipshutz–Negishi Cross-Coupling of Alkyl Halides with Aryl Electrophiles. Angew. Chem., Int. Ed 2016, 55, 1849.
- (10).Jana R; Pathak TP; Sigman MS Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev 2011, 111, 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11) (a).For an example of a general secondary-secondary Kumada coupling, see: Yang C-T; Zhang Z-Q; Liang J; Liu J-H; Lu X-Y; Chen H-H; Liu L Copper-Catalyzed Cross-Coupling of Nonactivated Secondary Alkyl Halides and Tosylates with Secondary Alkyl Grignard Reagents. J. Am. Chem. Soc 2012, 134, 11124. [DOI] [PubMed] [Google Scholar]; (b) For an example of a secondary-secondary Negishi coupling utilizing benzylic halides, see: Binder JT; Cordier CJ; Fu GC Catalytic Enantioselective Cross-Coupling of Secondary Alkyl Electrophiles with Secondary Alkylmetal Nucleophiles: Negishi Reactions of Racemic Benzylic Bromides with Achiral Alkylzinc Reagents. J. Am. Chem. Soc 2012, 134, 17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Twilton J; Le C; Zhang P; Shaw MH; Evans RW; MacMillan DWC The merger of transition metal and photocatalysis. Nat. Rev. Chem 2017, 1, 0052. [Google Scholar]
- (13) (a).Zuo Z; Ahneman DT; Chu L; Terrett JA; Doyle AG; MacMillan DWC Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides. Science 2014, 345, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tellis JC; Primer DN; Molander GA Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science 2014, 345, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kalyani D; McMurtrey KB; Neufeldt SR; Sanford MS Room-Temperature C─H Arylation: Merger of Pd-Catalyzed C─H Functionalization and Visible-Light Photocatalysis. J. Am. Chem. Soc 2011, 133, 18566. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nakajima K; Nojima S; Nishibayashi Y Nickel- and Photoredox-Catalyzed Cross-Coupling Reactions of Aryl Halides with 4-Alkyl-1,4-dihydropyridines as Formal Nucleophilic Alkylation Reagents. Angew. Chem., Int. Ed 2016, 55, 14106. [DOI] [PubMed] [Google Scholar]; (e) Shields BJ; Doyle AG Direct C(sp3)─H Cross Coupling Enabled by Catalytic Generation of Chlorine Radicals. J. Am. Chem. Soc 2016, 138, 12719. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Johnston CP; Smith RT; Allmendinger S; MacMillan DWC Metallaphotoredox-catalysed sp3–sp3 cross-coupling of carboxylic acids with alkyl halides. Nature 2016, 536, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Oderinde MS; Frenette M; Robbins DW; Aquila B; Johannes JW Photoredox Mediated Nickel Catalyzed Cross-Coupling of Thiols With Aryl and Heteroaryl Iodides via Thiyl Radicals. J. Am. Chem. Soc 2016, 138, 1760. [DOI] [PubMed] [Google Scholar]; (h) Corcoran EB; Pirnot MT; Lin S; Dreher SD; DiRocco DA; Davies IW; Buchwald SL; MacMillan DWC Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 2016, 353, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Terrett JA; Cuthbertson JD; Shurtleff VW; MacMillan DWC Switching on elusive organometallic mechanisms with photoredox catalysis. Nature 2015, 524, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Zhang P; Le CC; MacMillan DWC Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc 2016, 138, 8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Le C; Chen TQ; Liang T; Zhang P; MacMillan DWC A radical approach to the copper oxidative addition problem: Trifluoromethylation of bromoarenes. Science 2018, 360, 1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lowry MS; Goldsmith JI; Slinker JD; Rohl R; Pascal RA; Malliaras GG; Bernhard S Single-Layer Electroluminescent Devices and Photoinduced Hydrogen Production from an Ionic Iridium(III) Complex. Chem. Mater 2005, 17, 5712 [Google Scholar]
- (17) (a).Chatgilialoglu C Organosilanes as Radical-Based Reducing Agents in Synthesis. Acc. Chem. Res 1992, 25, 188. [Google Scholar]; (b) Chatgilialoglu C; Lalevée J Recent Applications of the (TMS)3SiH Radical-Based Reagent. Molecules 2012, 17, 527. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chatgilialoglu C Organosilanes in Radical Chemistry; Wiley: Chichester, U.K., 2004. [Google Scholar]; (d) Ballestri M; Chatgilialoglu C; Clark KB; Griller D; Giese B; Kopping B Tris(trimethylsilyl)silane as a Radical-Based Reducing Agent in Synthesis. J. Org. Chem 1991, 56, 678. [Google Scholar]; (e) Chatgilialoglu C; Ferreri C; Landais Y; Timokhin VI Thirty Years of (TMS)3SiH: A Milestone in Radical-Based Synthetic Chemistry. Chem. Rev 2018, 118, 6516. [DOI] [PubMed] [Google Scholar]
- (18).Durandetti M; Devaud M; Périchon J Investigation of the reductive coupling of aryl halides and/or ethyl chloroacetate electrocatalyzed by the precursor NiX2(bpy) with X─ = Cl─, Br─ or MeSO3– and bpy = 2,2′-dipyridyl. New J. Chem 1996, 20, 659. [Google Scholar]
- (19).Gutierrez O; Tellis JC; Primer DN; Molander GA; Kozlowski MC Nickel-Catalyzed Cross-Coupling of Photoredox-Generated Radicals: Uncovering a General Manifold for Stereo-convergence in Nickel-Catalyzed Cross-Couplings. J. Am. Chem. Soc 2015, 137, 4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lin X; Phillips DL Density Functional Theory Studies of Negishi Alkyl–Alkyl Cross-Coupling Reactions Catalyzed by a Methylterpyridyl-Ni(I) Complex. J. Org. Chem 2008, 73, 3680. [DOI] [PubMed] [Google Scholar]
- (21).Breitenfeld J; Ruiz J; Wodrich MD; Hu X Bimetallic Oxidative Addition Involving Radical Intermediates in Nickel-Catalyzed Alkyl–Alkyl Kumada Coupling Reactions. J. Am. Chem. Soc 2013, 135, 12004. [DOI] [PubMed] [Google Scholar]
- (22).We also recognize that an alternative pathway could be operative wherein Ni(0)-mediated oxidative addition and trapping of the alkyl radical 7 by a Ni(II) species could lead to the desired product via the same Ni(III) intermediate depicted in Scheme 2. Furthermore, in the case of small alkyl electrophiles other than methyl tosylate, it is possible that alternative mechanisms involving radical intermediates from both partners may be operative. Either of these potential pathways would alleviate the need for a challenging second oxidative addition, which might explain the ability of this system to couple secondary centers. Studies to elucidate the mechanism(s) of action are currently underway.
- (23) (a).Watson PS; Jiang B; Scott B A Diastereoselective Synthesis of 2,4-Disubstituted Piperidines: Scaffolds for Drug Discovery. Org. Lett 2000, 2, 3679. [DOI] [PubMed] [Google Scholar]; (b) Boivin TLB Synthetic routes to tetrahydrofuran, tetrahydropyran, and spiroketal units of polyether antibiotics and a survey of spiroketals of other natural products. Tetrahedron 1987, 43, 3309. [Google Scholar]
- (24) (a).Noble A; MacMillan DWC Photoredox α-Vinylation of α-Amino Acids and N-Aryl Amines. J. Am. Chem. Soc 2014, 136, 11602. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ahneman DA; Doyle AG C─H functionalization of amines with aryl halides by nickel-photoredox catalysis. Chem. Sci 2016, 7, 7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25) (a).Tamao K; Kiso Y; Sumitani K; Kumada M Alkyl Group Isomerization in the Cross-Coupling Reaction of Secondary Alkyl Grignard Reagents with Organic Halides in the Presence of Nickel-Phosphine Complexes as Catalysts. J. Am. Chem. Soc 1972, 94, 9268. [Google Scholar]; (b) Reger DL; Garza DG; Baxter JC Alkyl Group Isomerization Studies with Unusually Stable Alkylmetal Complexes of Palladium and Platinum. Secondary-Primary Alkyl Isomerization Equilibria In the Absence of Steric Influences from Ancillary Ligands. Organometallics 1990, 9, 873. [Google Scholar]; (c) Breitenfeld J; Vechorkin O; Corminboeuf C; Scopelliti R; Hu X Why Are (NN2)Ni Pincer Complexes Active for Alkyl–Alkyl Coupling: β-H Elimination Is Kinetically Accessible but Thermodynamically Uphill. Organometallics 2010, 29, 3686. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.