Abstract
Restrictive eating disorders (ED) are increasing and represent a serious risk to the health of adolescent females. Restrictive ED in youth are often treated through aggressive short-term refeeding. Although evidence supports that this intervention is the “gold standard” for improving ED outcomes in youth, little research has specifically probed appetite and meal-related responses to this type of intensive, short-term refeeding in newly diagnosed individuals. Information about appetite and meal-related dysfunction could provide valuable insights regarding treatment-interfering features of ED in both acute inpatient and longer-term outpatient treatment. The purpose of this study was to evaluate the hunger, fullness, olfactory, and gustatory responses of adolescents with newly-diagnosed restrictive ED and to probe how and when these responses are altered by refeeding. Using a quasi-experimental ecologically valid methodology, this study described and compared profiles of hunger, fullness, olfactory, and gustatory responses in adolescent females (n = 15) with newly diagnosed restrictive ED at hospital admission (i.e., severe malnutrition) and after medical refeeding, in comparison to healthy controls (n = 15). Results showed that newly diagnosed (i.e., malnourished) adolescents with ED showed significantly different meal-related experiences than controls. Refeeding improved some of these differences, but not all. Following refeeding, females with ED continued to show lower hunger, greater fullness, and lower pleasantness of smell ratings compared to controls. Unpleasantness of taste ratings maladaptively increased, such that females who were re-fed reported more aversive scents than pre-treatment. Profiles of meal-related responses were also identified and compared between groups. The applicability of these findings are discussed within the context of critical periods of change during refeeding treatment and potentially promising intervention targets that might enhance treatment outcomes for adolescents with newly onset, restrictive ED.
Eating disorders (ED) are a serious concern among adolescent and young adult women, ranking as the third most common chronic disorder, behind obesity and asthma (Fisher et al., 1995; Golden, 1997; Medicine, 1995a, 1995b; Rohde, Stice, & Marti, 2015; Smink, van Hoeken, & Hoek, 2013). ED rates are on the rise, particularly among children and adolescents, without discrimination of race or social classes, in progressively younger populations throughout the United States and the world (Crago, Shisslak, & Estes, 1996; Forman-Hoffman, 2004; Golden, 1997; Reijonen, Pratt, Patel, & Greydanus, 2003; Walcott, Pratt, & Patel, 2003).
With respect to etiology, interdisciplinary approaches uniquely characterize the onset and maintenance mechanisms of ED as dynamic and multi-systemic. The neurobiological approach is one such theory which conceptualizes ED as an interaction of neurobiology, neuropsychology, and psychopathology (Frampton, Hutchinson, Watkins, & Lask, 2012). Within this model, the etiology of ED is posited as an underlying abnormality, dysfunction or disconnection of brain circuitry that regulates behavior, structural and functional differences in the brain, cognition, emotion, appetite, and visual processing (Frampton et al., 2012; Phillipou, Rossell, & Castle, 2014).
Neurobiological research provides a framework for studying appetite dysfunction in individuals with restrictive ED (e.g., anorexia nervosa) (Roessner, Bleich, Banaschewski, & Rothenberger, 2005). Andersen and colleagues assessed patients with AN-R (anorexia nervosa-restricting type), AN-BP (anorexia nervosa-binge/purge subtype), and BN (bulimia nervosa) on their visual analog ratings of hunger and fullness before and after a meal within one week of admission to an inpatient eating disorders treatment center and at discharge. All ED patients had lower hunger and higher fullness compared to healthy controls. Although their ratings of hunger increased and fullness decreased at discharge, they remained considerably different than healthy controls (Andersen, Stoner, & Rolls, 1996). Significant differences in smell and taste responses are reported in patients with restrictive ED including decreased odor identification and discrimination, and decreased gustatory sensitivity (Aschenbrenner, Scholze, Joraschky, & Hummel, 2008; Schreder et al., 2008). Aschenbrenner and colleagues (2008) found that gustatory and olfactory function, critical elements of pre-digestive functioning, are significantly impaired in individuals with restrictive ED but not in individuals with other types of ED diagnoses (Aschenbrenner et al., 2008). These findings suggest that gustatory and olfactory dysfunction are directly associated with hunger, malnutrition, and low-weight status. (Aschenbrenner et al., 2008; Dazzi, Nitto, Zambetti, Loriedo, & Ciofalo, 2013; Rapps et al., 2010). Subtle differences in dietary restraint have consistently predicted impaired olfactory sensitivity (Stafford, Tucker, & Gerstner, 2013) that is reversible with weight restoration. Yet, because weight restoration is a gradual and often protracted process, taste-related deficits may maintain or compromise ED recovery given that dysfunctional appetite-related sensory processing may negatively influence eating behavior (Aschenbrenner et al., 2008).
Hunger, satiety, olfactory, and gustatory changes associated with restrictive ED may render it difficult for individuals to comply with the considerable dietary intake expected during nutritional restoration. This presents a conflict for the adolescent patient, for whom re-nourishment is the gold standard treatment of new onset ED (Le Grange, Accurso, Lock, Agras, & Bryson, 2014; Lock et al., 2010; Roessner et al., 2005; Vocks, Herpertz, Rosenberger, Senf, & Gizewski, 2011). Disruption of these sensory processes may interfere with the enjoyment and reinforcement value of food, thereby hindering the individuals’ ability to select recovery-promoting foods and consume expected quantities. Whereas previous research supports the use of supplementation-based olfaction enhancement for improved weight restoration (Su & Birmingham, 2002), it is generally agreed upon that the best way to modify olfactory dysfunction is through controlled weight gain (Roessner et al., 2005). However, the treatment implications are that the sensory changes associated with restrictive ED can actively discourage weight gain.
Despite existing findings regarding sensory changes in individuals with ED, questions remain about the impact of these changes on appetite and treatment outcomes for individuals with ED. No study to date has assessed sensory changes in ED over the course of the short-term refeeding process during an inpatient admission. Thus, the purpose of this study was to evaluate the hunger, fullness, smell, and taste responses in adolescents with newly-diagnosed ED. We hypothesized that the meal-related experiences of adolescents with ED would closely approximate those of healthy controls at the conclusion of refeeding.
Method
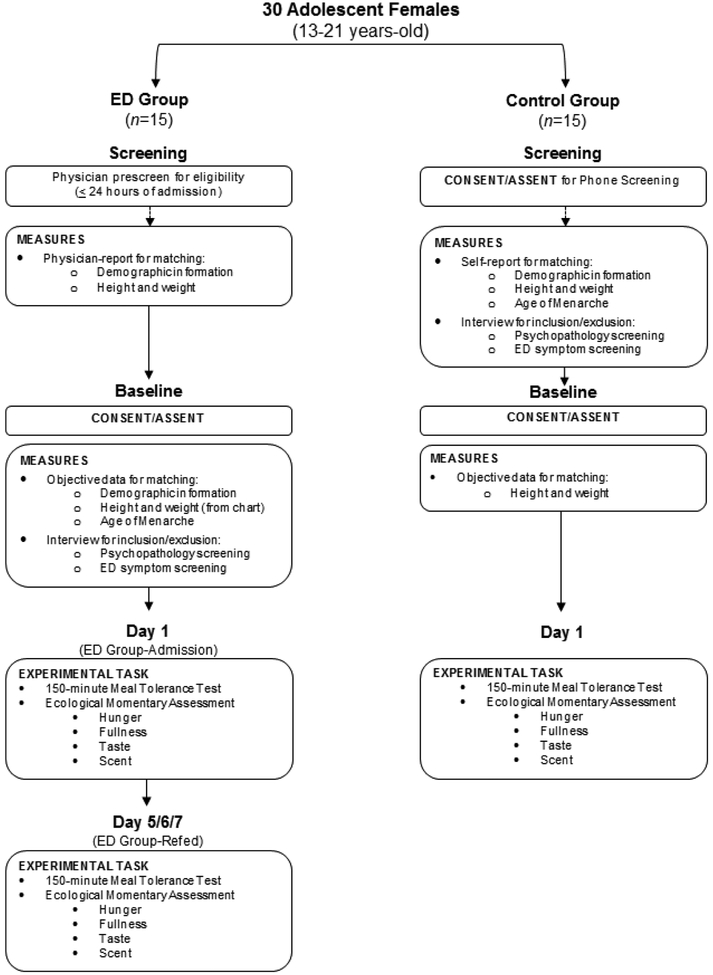
This study was approved by a hospital-based Institutional Review Board and conducted in accordance with current ethical standards for human subjects research. Written consent and assent was obtained from all participants and/or their caregivers before initiation of study procedures and participants were compensated for their time and effort. This study aimed to outline responses to a meal in adolescent females with newly diagnosed restrictive ED at two separate timepoints: 1) admission for newly diagnosed ED [ED-Admission] and 2) after nutritional treatment for malnutrition [ED-Refed] (Figure 1). This research also aimed to evaluate the similarities and/or differences between these profiles for adolescent ED and typical adolescents (i.e., healthy controls).
Figure 1.
Schematic overview of the study. Girls in the ED group were recruited within 24 hours of admission for medical stabilization for a new-onset restrictive eating disorder. The ED group was recruited first; controls were matched to girls in the ED group on age, race, socioeconomic status, age at menarche (±6 months), and BMI percentile (≤20%).
Extending prior research, this study used a quasi-experimental paradigm (i.e., experience sampling; (Trull & Ebner-Priemer, 2009) to evaluate the following meal-related experiences: a) hunger, b) fullness, c) taste, and d) smell. Experience sampling allows the instantaneous assessment of changes in subjective affective, cognitive, and behavioral experiences of participants in response to contextual stimuli (e.g., mealtime) (Trull & Ebner-Priemer, 2009). In contrast to extant cross-sectional investigations, this study measured meal perception profiles over time for each group to examine changes at specific timepoints within each group as well as overall profile differences between groups.
Participants.
Between August 2012 and June 2013, adolescent females aged 13 through 20 with a newly diagnosed restrictive ED (i.e., anorexia nervosa or eating disorder not otherwise specified; ED Group: n = 15) were identified through the inpatient medical team, who asked potentially eligible adolescents and their guardians for permission to contact the study staffa. Study staff then approached patients within 24 hours of admission. Inclusion criteria for the ED group included: 1) diagnosis of a restrictive ED that was confirmed by a valid measure of eating disorder pathology (see Method); 2) ED was newly diagnosed or newly presenting (duration of ≤6 months); 3) patient required hospital admission for medical stabilization related to malnutrition; 4) patient evidenced a significant change in body weight that represents a deviation from prior growth or stable body weight, a significant percentage of body weight lost, and/or change in body mass index (BMI) percentiles for age. Participants in the ED group received inpatient refeeding treatment (American Psychiatric Association, 2006), and gained an average of 0.80 kg after an average of 6.40 days of treatment.
A healthy control group (n = 15) consisting of lean adolescent females was recruited as the study was interested in evaluating group differences that were not confounded by differences in weight/nutritional status. Controls were matched to ED group on age (+/− 6 months ED girls’ age), age at menarche (age at menarche +/− 12 months of ED girl’s age at menarche), race, weight (BMI percentile within 20% of ED match), and socioeconomic status (SES). Inclusion criteria for the control group included: 1) may not be currently diagnosed with an ED (as confirmed by a valid measure of ED pathology (Eating Disorders Diagnostic Scale) (Stice, Telch, & Rizvi, 2000); 2) low BMI percentile for age and height; 3) Stable body weight (no increase or decrease in body weight equal to 5% of current weight or greater during 1-month prior to enrollment).
Exclusion criteria for both study groups included: 1) English not the primary language; 2) significant past medical history (e.g., Type 1 Diabetes Mellitus, inflammatory bowel disease); 3) current panic disorder, posttraumatic stress disorder, bipolar disorder, or thought disorder per screening questions from the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS) (Kaufman et al., 1997); 4) a developmental disorder; 5) Pregnant or delivered a baby in the past year; 6) currently taking a medication that affects the Hypothalamic-Pituitary Axis (e.g., prednisone, metformin, antipsychotics); 7) sibling enrolled in the study; 8) self-reported history of psychiatric or clinical syndrome diagnoses per KSADS (Kaufman et al., 1997).
Sample characteristics and descriptive statistics for the full sample (N = 30) and each group are outlined in Table 1.
Table 1.
Characteristics of female participants by group. Values are arithmetic means and their standard deviations (N=30).
| Total Sample (N = 30) | ED-Admission (n = 15) | Lean Controls (n = 15) | ||||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |
| Age (years) | 15.27 ± 1.91 | 12.96–20.49 | 15.24 ± 1.95 | 12.96–20.49 | 15.17 ± 2.02 | 13.37–20.46 |
| BMI (kg/m2)* | 17.69 ± 2.28 | 11.30–20.50 | 16.46 ± 2.56 | 11.30–19.80 | 18.93 ± 0.95 | 17.00–20.50 |
| Age at Menarche (years)† | 12.64 ± 0.98 | 11.00–15.00 | 12.88 ± 1.00 | 11.50–15.00 | 12.40 ± 0.92 | 11.00–14.00 |
| Highest Historical Weight (lbs.)** | 117.43 ± 21.57 | 73.00–165.00 | 126.13 ± 25.68 | 73.00–165.00 | 108.73 ± 11.93 | 90.00–130.00 |
| Lowest Historical Weight (lbs.) | 97.55 ± 14.12 | 63.00–122.00 | 97.00 ± 17.00 | 63.00–122.00 | 98.14 ± 10.85 | 80.00–115.00 |
| Current Grade in School | 9.47 ± 1.59 | 8.00–13.00 | 9.53 ± 1.55 | 8.00–13.00 | 9.40 ± 1.68 | 8.00–13.00 |
| N | % | N | % | N | % | |
| White Race | 15 | 100% | 15 | 100% | 15 | 100% |
| Non-Hispanic Ethnicity | 15 | 100% | 15 | 100% | 15 | 100% |
| SES (parental education) | ||||||
| First Head of Household | ||||||
| High School or Equivalent | 3 | 10.00% | 3 | 20.00% | ||
| Partial College | 2 | 6.70% | 2 | 13.30% | ||
| Standard College Or University | 13 | 43.30% | 5 | 33.30% | 8 | 53.30% |
| Graduate Professional Training | 12 | 40.00% | 7 | 46.70% | 5 | 33.30% |
| Second Head of Household | ||||||
| High School or Equivalent | 5 | 16.70% | 3 | 20.00% | 2 | 13.30% |
| Partial College | 5 | 16.70% | 1 | 6.70% | 4 | 26.70% |
| Standard College Or University | 10 | 33.30% | 6 | 40.00% | 4 | 26.70% |
| Graduate Professional Training | 8 | 26.70% | 4 | 26.70% | 4 | 26.70% |
| No Second Head of Household | 2 | 6.70% | 1 | 6.70% | 1 | 6.70% |
Note: 2 participants in each group were pre-menarcheal (n = 4);
significant group difference detected (t(18) = −3.50, p < .01);
significant group difference detected (t(20) = 2.38, p < .05).
Study Procedures.
The ED group completed study procedures in their hospital room on the medical unit. Study visits were conducted in coordination with the hospital stay, such that the study protocol minimized interruption to/deviations from standard care and limited patient distress related to cognitive changes associated with malnutrition (e.g., mental inflexibility) (Tchanturia, Campbell, Morris, & Treasure, 2005). After consent/assent was obtained, data that would be used for matching was collected and participants were informed that study procedures for a Day 1 Visit would commence the following morning at 0800 hours.
The study protocol for the Control group mirrored that of the ED group but did not require overnight hospitalization (Figure 1). The control group completed their study procedures as outpatients within a Clinical and Translational Research Center (CTRC). Potential controls responding to recruitment materials provided phone consent/assent and participated in a phone screening to obtain subjective report of matching criteria. Subsequently, a baseline Study Visit was completed to objectively collect matching data. If baseline visit data confirmed the match, the control participant was scheduled to complete the study protocol within 7 days.
Meal Tolerance Test (MTT) Protocol.
Participants in the ED and lean control groups participated in a meal tolerance test (MTT) protocol involving ecological momentary assessment of psychological states, consumption of a standardized mixed meal (8 oz. milkb and 2.2 oz cereal consisting of 360 total calories [i.e., 20% from fat, 66% from carbohydrate, 14% from protein] (Salbe et al., 2007)), and serial vital signs over the course of 150 minutes. The standardized mixed meal used in the MTT is volumetrically and nutritionally equivalent to what is prescribed by the medical team at this stage of inpatient treatment for the ED group and consistent with an appropriate portion of daily caloric intake and needs for controls. For purposes of experimental control, participants in the control group were instructed to fast starting at 2200 hours the night before the MTT; they arrived at the CTRC at 0730 hours to allow a 30-minute rest period before commencement of the MTT at 0800 hours. For participants in the ED group, the inpatient medical protocol did not permit eating after 2000 hours.
For all participants, the MTT began promptly at 0800 hours at which time the participant completed a series of baseline self-report measures. Next, the participant consumed a standardized mixed nutrient meal. The commencement of eating signaled timepoint zero minutes and participants were permitted 10 minutes to complete the meal. Immediately upon finishing the meal and subsequently at 15 to 30 minute intervals for a total of 150 minutes, participants completed repeated measures assessments of their meal experiences. Vital signs were recorded every 5–30 minutes during the MTT to ensure safety.
For the ED group, the MTT was repeated on the final full day of hospitalization (ED-Refed; Day 5, 6, or 7 [average = 6.40 days], determined by the medical team in accordance with each patient’s medical status).
Perceived Hunger and Fullness.
Hunger and fullness were measured using a visual analog scale called the Satiety Labeled Intensity Magnitude (SLIM) scale (Cardello, Schutz, Lesher, & Merrill, 2005). The SLIM has been shown to be a sensitive, reliable, and easy to use scale for measuring perceived hunger and fullness (Cardello et al., 2005). It was developed to measure perceived satiety responses over time within the same individual. The SLIM utilizes a horizontal, 100 mm, bidirectional hunger/fullness scale anchored by the terms “greatest imaginable fullness” and “greatest imaginable hunger” with a midpoint of “neither hungry nor full.” The participant is directed to mark the scale at any point along the axis corresponding to their level of hunger or fullness “at that very moment.” A rating anywhere above the midpoint of the scale indicates that some degree of fullness is perceived. These line ratings were scored by measuring the 100 mm line to the nearest mm, producing a 100-piont scale. Participant ratings were independently measured by two trained members of the study staff whose scores demonstrated excellent reliability (Hunger: α = .99; Fullness: α = 1.00).
Sensory Characteristics of Food.
A second set of visual analog scales was administered to evaluate hedonic responses to the tasting of foods. These ratings were based upon Williamson and Colleagues’ (2005) methods for measuring sensory characteristics of foods consumed with a focus on the pleasantness and unpleasantness of the taste and smell of the foods (Williamson et al., 2005). Sensory characteristics were evaluated using a horizontal, 100 mm, bidirectional scale anchored by the terms “not at all pleasant” and “extremely pleasant” or “not at all unpleasant” and “extremely unpleasant” (Williamson et al., 2005). The participant was directed to mark the scale at any point along the axis corresponding to their perceptions of the food’s pleasantness of taste and smell and unpleasantness of taste and smell. Because participants’ sensory ratings could only be measured in response to the meal, these measures were not collected prior to meal consumption (i.e., at baseline), but were collected immediately after consumption of the meal and every 15–30 minutes thereafter. These line ratings were scored by measuring the 100 mm line to the nearest mm, producing a 100-piont scale. Participant ratings were independently measured by two trained members of the study staff whose demonstrated excellent reliability (α’s = 1.00).
Data Analysis.
Standardized mean differences (Cohen’s d, effect sizes) and 95% CI error bars were used to illustrate notable differences between ED groups and in comparison to lean controls. Specifically, Cohen’s d values ≥ .20 were considered noteworthy when comparing the same ED participants at admission versus re-fed stages. Cohen’s d values ≥ .50 were considered noteworthy when comparing ED participants at the re-fed stage in comparison to the lean control condition. Selection of these values is based upon existing literature that has determined that effect sizes of differences between ED and healthy controls on neuropsychological tasks tend to range between .16–.73 (Zakzanis, Campbell, & Polsinelli, 2010). Studies evaluating within-group differences in psychological variables for individuals with ED during brief intervals (e.g., 1–4 weeks) show effect sizes ranging from .03–.27 (Hatch et al., 2010; Moser et al., 2003).
All group comparison trajectory plots and descriptive data used to calculate Cohen’s d effect sizes were obtained using IBM SPSS 19 (SPSS Inc., Chicago, IL, USA).
Results
Across meal-related perceptions involving taste and smell, the ratings of ED participants at admission were relatively similar to those of the lean control group. However, group differences were identified for hunger and fullness ratings between ED participants and lean controls as well as meal-related perceptions between the ED participants at admission compared to after refeeding (see Table 2 and Figure 2).
Table 2.
Effect sizes as Cohen’s d for group differences in meal perceptions at meal tolerance test (MTT) assessments
| Meal-Related Perception | Comparison1 | MTT Assessment (Minutes) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| −5 | 0 | 15 | 30 | 60 | 90 | 120 | 150 | ||
|
d 95% Cl |
d 95% Cl |
d 95% Cl |
d 95% Cl |
d 95% Cl |
d 95% Cl |
d 95% Cl |
d 95% Cl |
||
| Hunger | ED-A vs. Controla | −1.01 (−1.94– −0.07) | −0.63 (−1.39–0.13) | −0.29 (−1.15–0.57) | −0.26 (−1.21–0.70) | −0.58 (−1.55–0.39) | −0.65 (−1.54–0.24) | −0.91 (−1.89–0.08) | −1.29 (−2.23– −0.36) |
| ED-A vs. ED-Rb | −0.09 (−1.18–1.00) | 0.15 (−0.55–0.84) | 0.16 (−0.64–0.95) | 0.23 (−0.63–1.10) | 0.21 (−0.66–1.08) | −0.15 (−1.12–0.82) | 0.02 (−1.13–1.18) | 0.29 (−0.87–1.46) | |
| ED-R vs. Controla | −1.07 (−1.88– −0.27) | −0.81 (−1.54– −0.09) | −0.46 (−1.28–0.36) | −0.53 (−1.38–0.33) | −0.86 (−1.75–0.03) | −0.42 (−1.48–0.63) | −0.90 (−1.94–0.14) | −1.61 (−2.58– −0.64) | |
| Fullness | ED-A vs. Controla | 0.43 (−0.37–1.23) |
0.68 (0.03–1.34) | 0.56 (−0.14–1.26) | 0.33 (−0.44–1.10) | 0.38 (−0.40–1.17) | 0.36 (−0.50–1.22) | 0.50 (−0.43–1.44) | 0.88 (−0.05–1.80) |
| ED-A vs. ED-Rb | −0.08 (−1.00–0.84) | −0.28 (−0.95–0.38) | −0.10 (−0.93–0.72) | −0.11 (−1.02–0.80) | −0.05 (−0.99–0.90) | −0.05 (−1.13–1.02) | −0.13 (−1.18–0.92) | 0.02 (−1.12–1.16) | |
| ED-R vs. Controla | 0.46 (−0.46–1.38) | 1.09 (0.49–1.68) | 0.69 (−0.03–1.40) | 0.47 (−0.30–1.23) | 0.40 (−0.50–1.28) | 0.39 (−0.55–1.34) | 0.66 (−0.26–1.59) | 0.79 (−0.23–1.80) | |
| Pleasant Taste | ED-A vs. Controla | – | 0.43 (−0.37–1.24) | 0.09 (−0.71–0.90) | −0.03 (−0.80–0.75) | −0.03 (−0.78–0.72) | −0.03 (−0.80–0.73) | −0.07 (−0.93–0.79) | −0.11 (−0.92–0.70) |
| ED-A vs. ED-Rb | – | 0.19 (−0.46–0.84) | 0.11 (−0.65–0.87) | 0.01 (−0.75–0.78) | −0.06 (−0.86–0.74) | 0.00 (−0.83–0.83) | −0.02 (−0.93–0.89) | −0.07 (−0.93–0.79) | |
| ED-R vs. Controla | – | 0.28 (−0.54–1.10) | −0.10 (−0.88–0.86) | −0.03 (−0.91–0.84) | −0.03 (−0.88–0.94) | −0.03 (−0.89–0.83) | −0.05 (−0.93–0.83) | −0.04 (−0.88–0.81) | |
| Unpleasant Taste | ED-A vs. Controla | – | 0.03 (−0.95–1.01) | 0.10 (−0.84–1.05) | 0.14 (−0.84–1.12) | 0.03 (−0.87–0.93) | 0.16 (−0.82–1.14) | 0.25 (−0.74–1.23) | 0.16 (−0.83–1.15) |
| ED-A vs. ED-Rb | – | 0.25 (−0.54–1.04) | 0.39 (−0.44–1.21) | 0.24 (−0.75–1.22) | 0.24 (−0.64–1.12) | 0.10 (−0.86–1.07) | 0.37 (−0.54–1.27) | 0.33 (−0.60–1.25) | |
| ED-R vs. Controla | – | −0.18 (−1.16–0.81) | −0.23 (−1.19–0.73) | −0.09 (−1.15–0.97) | −0.18 (−1.20–0.84) | 0.06 (−0.99–1.10) | −0.09 (−1.05–0.87) | −0.14 (−1.15–0.87) | |
| Pleasant Smell | ED-A vs. Controla | – | −0.04 (−0.53–0.44) | −0.14 (−0.69–0.40) | −0.45 (−1.07–0.17) | 0.00 (−0.48–0.48) | −0.15 (−0.61–0.31) | −0.24 (−0.84–0.36) | −0.41 (−1.01–0.19) |
| ED-A vs. ED-Rb | – | 0.15 (−0.21–0.51) | 0.38 (0.04–0.73) | 0.14 (−0.46–0.75) | 0.56 (0.06–1.05) | 0.46 (−0.05–0.97) | 0.13 (−0.49–0.74) | −0.03 (−0.53–0.48) | |
| ED-R vs. Controla | – | −0.17 (−0.62–0.29) | −0.40 (−0.93–0.13) | −0.59 (−1.22–0.03) | −0.45 (−1.05–0.15) | −0.51 (−1.11–0.08) | -0.39 (−0.97–0.19) | −0.49 (−0.98–0.00) | |
| Unpleasant Smell | ED-A vs. Controla | – | 0.08 (−0.73–0.89) | 0.31 (−0.58–1.21) | 0.50 (−0.36–1.36) | 0.32 (−0.46–1.10) | 0.46 (−0.35–1.28) | 0.66 (−0.18–1.50) | 0.60 (−0.21–1.40) |
| ED-A vs.ED-Rb | – | −0.24 (−0.96–0.48) | −0.12 (−0.87–0.62) | 0.06 (−0.80–0.93) | −0.11 (−0.88–0.66) | 0.07 (−0.67–0.80) | 0.20 (−0.59–0.98) | 0.30 (−0.45–1.05) | |
| ED-R vs.Controla | – | 0.30 (−0.50–1.10) | 0.46 (−0.37–1.29) | 0.41 (−0.52–1.34) | 0.37 (−0.55–1.29) | 0.42 (−0.39–1.22) | 0.49 (−0.36–1.33) | 0.33 (−0.45–1.11) | |
Notes: ED-A = Eating Disorder Group-Admission; ED-R = Eating Disorder Group-Refed; Control = Control Group;
a positive value for Cohen’s d indicates that the second group in the comparison is less than the first whereas a negative value indicates that the second group in the comparison is greater than the first;
Between-group comparisons of importance denoted with boldface in accordance with standard conventions for medium to large effects (d≥.50);
Within-subjects comparisons of importance denoted with boldface in accordance with literature-based expectations such that standardly considered small effects (d≥.20) were considered meaningful (see Analytic Plan for rationale); shaded cells represent key findings.
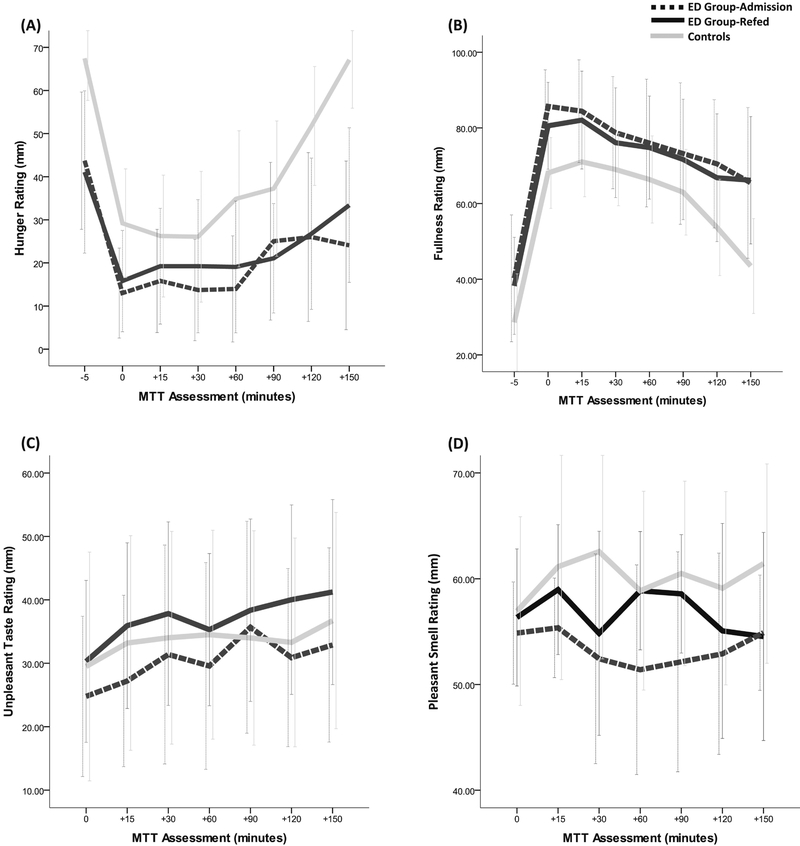
Figure 2.
Group profiles of subjective hunger (A), fullness (B), unpleasant taste (C), and pleasant scent (D) made on a 100 mm visual analog scale during a 150-minute meal tolerance test (MTT). Data are means and their 95% Confidence Interval.
As shown in Table 2, refed ED participants show notably lower hunger (d = 0.21 – 0.29) compared to ED participants at admission, but this did not occur during the meal and was only observed at certain time intervals more than 30 minutes after the meal (i.e., 30, 60 and 150 minute intervals). In comparison to lean controls, the hunger of refed ED participants was lower at all but two (15 and 90 minute) time point assessments (see Figure 2A) such that these differences were observed both proximal to the consumption of the meal and up to 150 minutes afterward. Fullness ratings of ED participants when re-fed showed higher feelings of fullness compared to admission (d = −0.28), but only at mealtime (Table 2). In comparison to controls, feelings of fullness for ED participants who are re-fed were higher (d = 0.66 – 1.09) during the meal, shortly after the meal (i.e., 15 minute interval) and well after the meal (i.e., 120 and 150-minute intervals). Interestingly, the fullness trajectories over time for EDs at admission and when re-fed are very similar (see Figure 2B).
No noteworthy effect size differences were detected for any group comparisons involving perceptions of pleasant taste. However, notable effect size differences in unpleasant taste were observed between ED participants at admission and when re-fed. Specifically, ED participants at admission showed notably higher perceptions of unpleasant taste (d = 0.24 – 0.39) at all assessment time points except for 90 minutes. Further, the perceptions of unpleasant taste trajectory for controls lies between the unpleasant taste trajectories for ED participants at admission and when re-fed (see Figure 2C).
With respect to meal perceptions related to smell, refed ED participants show notably higher perceptions of pleasant meal smell compared to ED participants at admission (d = 0.38 – 0.56), but only after the meal (i.e., 15, 60 and 90 minutes post-meal; Table 2 and Figure 2D). In comparison to controls, ED participants when re-fed showed notably lower perceptions of pleasant meal smell (d = 0.51 – 0.59), but again only 30 and 90 minutes post-meal (see Figure 2D). In anticipation of the meal, refed ED participants show notably higher perceptions of unpleasant meal smell in comparison to at admission (d = .24). Then, well after the meal (i.e., 120 and 150 minutes), refed ED participants show notably lower perceptions of unpleasant meal smell in comparison to ED participants at admission (d = 0.20 – 0.30).
Discussion
The goal of the current study was to extend the literature concerning sensory meal-related experiences in adolescents with restrictive ED. Through a rigorous methodology, this research aimed to provide clarity regarding the hunger, fullness, smell, and taste responses to a meal demonstrated by a sample of individuals with newly diagnosed ED within the context of severe malnutrition (i.e., ED-Admission group), following short-term nutritional therapy (i.e., ED-Refed group), and in comparison to healthy controls. Through an ecologically valid, quasi-experimental method, this research sought to identify key changes in meal-related experiences that may be most relevant for understanding and potentially intervening to promote healthy meal-related experiences within the context of ED. Perhaps most notably, our participants experienced significant changes in meal-related sensory experiences (e.g., towards a more normalized response) after just a short period of re-feeding. This is consistent with previous studies. However, despite these changes, our ED participants still had significantly more disrupted meal-related sensory responses as compared to healthy controls (Focker et al., 2012).
Our results regarding key changes in the profiles of meal-related experiences from pre- to post-refeeding provide critical information regarding the ED responses to food consumption. In fact, these findings are the first to report critical periods in meal experiences that could have important clinical implications. For example, our findings provide preliminary support for an improvement in fullness cues for the ED-Refed group compared to the ED-Admission group immediately following a meal, which may afford clinicians an opportunity to combat aversive learning and discomfort associated with fullness. Attempts to intervene upon fullness cues at other timepoints following a meal may be ineffective since the adolescent’s perceptions of fullness would be unchanged. Thus, clinicians may achieve greater success in establishing appetitive learning trials associated with food at 15, 60, and 90 minutes post-meal for the ED-Refed group, since their experiences of pleasant smell related to the food is considerably higher than ED-Admission profiles. This approach has previously been proposed based upon neuroimaging research showing significant cognitive changes with refeeding (Hatch et al., 2010).
Findings that support residual differences between individuals with ED and their healthy counterparts even after short-term, intensive treatment are critical for understanding why treatment outcomes in ED may be underwhelming. A previous study showed considerable differences between individuals with ED and healthy controls even after 115 days of refeeding (Focker et al., 2012). Interestingly, there is evidence that the physiologic impact of ED may not be comparable to other forms of starvation (e.g., cachexia, fasting) (Focker et al., 2012). Therefore, in spite of the low base rate and difficulty associated with recruiting ED samples, our results suggest that the impact of refeeding on physiologic functioning and perception in ED should be investigated using only ED samples and rigorous methods.
In addition to quantifying group differences in meal-related responses, this research also outlined profiles of meal-related experiences in ED. The results identified periods during treatment when re-fed adolescents with ED showed the most demonstrative changes from their meal-related experiences at admission. For instance, the ED-Refed profile of hunger ratings was most different from ED-Admission at 30, 60, and 150 minutes. For fullness ratings, profiles of re-fed adolescents were only improved immediately after the meal. Specifically, fullness was slightly lower immediately following the meal for the ED-Refed versus ED-Admission groups, such that experiences of fullness were otherwise unchanged. Such profile differences were apparent for all variables depicted in Figure 2, which illustrates the uniqueness of each meal experience over time.
To our knowledge, this is the first study to use ecological assessment of meal experiences with individuals newly diagnosed with ED. This study is novel as it allowed for comparison of meal-experience profiles of newly diagnosed ED (i.e., starvation phase of the illness) with the medically stabilized (i.e., re-fed) phase of the illness as well as in comparison to a healthy control condition. Yet, results are limited by a small sample size that does not allow for complex statistical analysis and inferential conclusions. The small sample size and exclusion of males does not allow for generalization of results. This study did not address psychological responses to changing meal experiences. It is likely that an adolescent with ED would experience significant distress following a short-term change to her environment and internal cues. Exploration of psychological aspects of treatment that modify meal-related experiences would be valuable, considering that refeeding may “improve” some indicators of illness, while simultaneously disrupting homeostasis that was achieved during gradual starvation (Focker et al., 2012). Such alterations could exacerbate pre-existing psychological dysfunction or stimulate symptomatology as opposed to the expected normalization of functioning. A final limitation is the use of visual analog scales (Williamson et al., 2005; Cardello et al., 2005) to assess meal perceptions. This final limitation might explain the lack of significant differences in ratings of taste and smell between the ED-Admission group and lean controls. This finding was surprising given literature that has identified olfactory deficits in patients with anorexia nervosa (i.e., Rapps et al., 2010; Schecklmann et al., 2012) and may be due to the use of participant ratings of their perceptions of taste and smell rather than chemosensory measures of olfaction.
In spite of these limitations, our study was not limited by convenience sampling, heterogeneous diagnostic groups, varied illness duration, a confounding influence of body weight, and retrospective bias. Additionally, existing results that have demonstrated differences between ED and healthy controls typically evaluate groups after months of nutritional treatment (e.g., 115 days) (Focker et al., 2012). Thus, they do not apply to health systems like that of the United States which provide less intensive treatment. Furthermore, our research involves one of the largest adolescent samples with restrictive ED for which meal experiences are evaluated pre- to post-hospitalization.
Though preliminary and requiring replication, the findings of this research provide novel information concerning the meal-related experiences of adolescent females with restrictive ED. Findings from this research stand to inform expectations of individuals with ED who have completed short-term nutritional treatment. Results are also relevant for purposes of developing intervention paradigms that maximize adaptive changes in meal-related experiences of hunger, fullness, olfaction, and gustatory functioning. Finally, these findings underscore the importance of considering an iatrogenic impact of refeeding, such that normalization of meal experiences may be distressing, cause symptom exacerbation, and act as a barrier to recovery for adolescents with restrictive ED.
Acknowledgements
This project was supported in part by the NIH/ORWH K12 HD051953 to Dr. Abbigail Tissot and NIH/HRSA T32 HP10027–12 (PI: Kristen Copeland, MD); by an Institutional Clinical and Translational Science Award, NIH/NCRR-USPHS Grant Number 1UL1RR026314, to Cincinnati Children’s Hospital Research Foundation; by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), Department of Health and Human Services (DHHS), through a National Research Service Award Training Grant (T32HP10027; PI: Kristen Copeland, M.D.). The information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by the NIH, ORWH, HRSA, DHHS or the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To prevent coercion, members of the study staff were excluded from recruitment when they were active members of the inpatient medical team.
A nutritionally-equivalent lactose-free option (10 oz. soy milk and 2.2 oz cereal; 365 calories with 20% from fat, 65% from carbohydrate, and 15% from protein) was offered to patients who were lactose intolerant.
References
- American Psychiatric A (2006). Treatment of patients with eating disorders,third edition. American Psychiatric Association. Am J Psychiatry, 163(7 Suppl), 4–54. [PubMed] [Google Scholar]
- Andersen AE, Stoner SA, & Rolls BJ (1996). Improved eating behavior in eating-disordered inpatients after treatment: documentation in a naturalistic setting. Int J Eat Disord, 20(4), 397–403. doi: [DOI] [PubMed] [Google Scholar]
- Aschenbrenner K, Scholze N, Joraschky P, & Hummel T (2008). Gustatory and olfactory sensitivity in patients with anorexia and bulimia in the course of treatment. J Psychiatr Res, 43(2), 129–137. doi: 10.1016/j.jpsychires.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Cardello AV, Schutz HG, Lesher LL, & Merrill E (2005). Development and testing of a labeled magnitude scale of perceived satiety. Appetite, 44(1), 1–13. doi: 10.1016/j.appet.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Crago M, Shisslak CM, & Estes LS (1996). Eating disturbances among American minority groups: a review. Int J Eat Disord, 19(3), 239–248. doi: [DOI] [PubMed] [Google Scholar]
- Dazzi F, Nitto SD, Zambetti G, Loriedo C, & Ciofalo A (2013). Alterations of the olfactory-gustatory functions in patients with eating disorders. Eur Eat Disord Rev, 21(5), 382–385. doi: 10.1002/erv.2238 [DOI] [PubMed] [Google Scholar]
- Fisher M, Golden NH, Katzman DK, Kreipe RE, Rees J, Schebendach J, … Hoberman HM (1995). Eating disorders in adolescents: a background paper. J Adolesc Health, 16(6), 420–437. doi: 10.1016/1054-139X(95)00069-5 [DOI] [PubMed] [Google Scholar]
- Focker M, Timmesfeld N, Scherag S, Knoll N, Singmann P, Wang-Sattler R, … Hebebrand J (2012). Comparison of metabolic profiles of acutely ill and short-term weight recovered patients with anorexia nervosa reveals alterations of 33 out of 163 metabolites. J Psychiatr Res, 46(12), 1600–1609. doi: 10.1016/j.jpsychires.2012.08.015 [DOI] [PubMed] [Google Scholar]
- Forman-Hoffman V (2004). High prevalence of abnormal eating and weight control practices among U.S. high-school students. Eat Behav, 5(4), 325–336. doi: 10.1016/j.eatbeh.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Frampton I, Hutchinson A, Watkins B, & Lask B (2012). Neurobiological status at initial presentation predicts neuropsychological functioning in early onset anorexia nervosa at four-year follow up. Dev Neuropsychol, 37(1), 76–83. doi: 10.1080/87565641.2011.583301 [DOI] [PubMed] [Google Scholar]
- Golden NH (1997). The adolescent: vulnerable to develop an eating disorder and at high risk for long-term sequelae. Ann N Y Acad Sci, 817, 94–97. [DOI] [PubMed] [Google Scholar]
- Hatch A, Madden S, Kohn MR, Clarke S, Touyz S, Gordon E, & Williams LM (2010). In first presentation adolescent anorexia nervosa, do cognitive markers of underweight status change with weight gain following a refeeding intervention? Int J Eat Disord, 43(4), 295–306. doi: 10.1002/eat.20695 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry, 36(7), 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Le Grange D, Accurso EC, Lock J, Agras S, & Bryson SW (2014). Early weight gain predicts outcome in two treatments for adolescent anorexia nervosa. Int J Eat Disord, 47(2), 124–129. doi: 10.1002/eat.22221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, & Jo B (2010). Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch Gen Psychiatry, 67(10), 1025–1032. doi: 10.1001/archgenpsychiatry.2010.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine S f. A(1995a). Eating disorders in adolescents: A position paper of the Society for Adolescent Medicine. Journal of Adolescent Health, 28, 20–26. [PubMed] [Google Scholar]
- Medicine S f. A (1995b). Eating disorders in adolescents: A position paper of the Society for Adolescent Medicine. Journal of Adolescent Health, 16, 476–480. [PubMed] [Google Scholar]
- Moser DJ, Benjamin ML, Bayless JD, McDowell BD, Paulsen JS, Bowers WA, … Andersen AE (2003). Neuropsychological functioning pretreatment and posttreatment in an inpatient eating disorders program. Int J Eat Disord, 33(1), 64–70. doi: 10.1002/eat.10108 [DOI] [PubMed] [Google Scholar]
- Phillipou A, Rossell SL, & Castle DJ (2014). The neurobiology of anorexia nervosa: a systematic review. Aust N Z J Psychiatry, 48(2), 128–152. doi: 10.1177/0004867413509693 [DOI] [PubMed] [Google Scholar]
- Rapps N, Giel KE, Sohngen E, Salini A, Enck P, Bischoff SC, & Zipfel S (2010). Olfactory deficits in patients with anorexia nervosa. Eur Eat Disord Rev, 18(5), 385–389. doi: 10.1002/erv.1010 [DOI] [PubMed] [Google Scholar]
- Reijonen JH, Pratt HD, Patel DR, & Greydanus DE (2003). Eating disorders in the adolescent population: An overview. Journal of Adolescent Research, 18(3), 209–222. doi: 10.1177/0743558403018003002 [DOI] [Google Scholar]
- Roessner V, Bleich S, Banaschewski T, & Rothenberger A (2005). Olfactory deficits in anorexia nervosa. Eur Arch Psychiatry Clin Neurosci, 255(1), 6–9. doi: 10.1007/s00406-004-0525-y [DOI] [PubMed] [Google Scholar]
- Rohde P, Stice E, & Marti CN (2015). Development and predictive effects of eating disorder risk factors during adolescence: Implications for prevention efforts. Int J Eat Disord, 48(2), 187–198. doi: 10.1002/eat.22270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbe AD, Lindsay RS, Collins CB, Tataranni PA, Krakoff J, & Bunt JC (2007). Comparison of plasma insulin levels after a mixed-meal challenge in children with and without intrauterine exposure to diabetes. J Clin Endocrinol Metab, 92(2), 624–628. doi: 10.1210/jc.2006-1179 [DOI] [PubMed] [Google Scholar]
- Schecklmann M, Pfannstiel C, Fallgatter AJ, Warnke A, Gerlach M, and Romanos M (2012). Olfaction in child and adolescent anorexia nervosa. Eur Soc Clin Neuropharmacol 119, 721–728. doi: 10.1007/s00702-011-0752-0 [DOI] [PubMed] [Google Scholar]
- Schreder T, Albrecht J, Kleemann AM, Schopf V, Kopietz R, Anzinger A, … Wiesmann M (2008). Olfactory performance of patients with anorexia nervosa and healthy subjects in hunger and satiety. Rhinology, 46(3), 175–183. [PubMed] [Google Scholar]
- Smink FR, van Hoeken D, & Hoek HW (2013). Epidemiology, course, and outcome of eating disorders. Curr Opin Psychiatry, 26(6), 543–548. doi: 10.1097/YCO.0b013e328365a24f [DOI] [PubMed] [Google Scholar]
- Stafford LD, Tucker M, & Gerstner N (2013). A bitter sweet asynchrony. The relation between eating attitudes, dietary restraint on smell and taste function. Appetite, 70, 31–36. doi: 10.1016/j.appet.2013.06.084 [DOI] [PubMed] [Google Scholar]
- Stice E, Telch CF, & Rizvi SL (2000). Development and validation of the Eating Disorder Diagnostic Scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess, 12(2), 123–131. doi: 10.1037/1040-3590.12.2.123 [DOI] [PubMed] [Google Scholar]
- Su JC, & Birmingham CL (2002). Zinc supplementation in the treatment of anorexia nervosa. Eat Weight Disord, 7(1), 20–22. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Campbell IC, Morris R, & Treasure J (2005). Neuropsychological studies in anorexia nervosa. Int J Eat Disord, 37 Suppl, S72–76; discussion S87–79. doi: 10.1002/eat.20119 [DOI] [PubMed] [Google Scholar]
- Trull t. J., & Ebner-Priemer UW(2009). Using experience sampling methods/ecological momentary assessment (ESM/EMA) in clinnical assessment and clinical research: Introduction ot the special section. Psychol Assess, 21(4), 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocks S, Herpertz S, Rosenberger C, Senf W, & Gizewski ER (2011). Effects of gustatory stimulation on brain activity during hunger and satiety in females with restricting-type anorexia nervosa: an fMRI study. J Psychiatr Res, 45(3), 395–403. doi: 10.1016/j.jpsychires.2010.07.012 [DOI] [PubMed] [Google Scholar]
- Walcott D, Pratt H, & Patel D (2003). Adolescents and eating disorders: Gender, racial, ethnic, sociocultural, and socioeconomic issues. Journal of Adolescent Research, 18, 223–243. [Google Scholar]
- Williamson DA, Ravussin E, Wong ML, Wagner A, Dipaoli A, Caglayan S, … Licinio J (2005). Microanalysis of eating behavior of three leptin deficient adults treated with leptin therapy. Appetite, 45(1), 75–80. doi: 10.1016/j.appet.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Campbell Z, & Polsinelli A (2010). Quantitative evidence for distinct cognitive impairment in anorexia nervosa and bulimia nervosa. J Neuropsychol, 4(Pt 1), 89–106. doi: 10.1348/174866409X459674 [DOI] [PubMed] [Google Scholar]