Abstract
Models to assess the addictive-like properties of nicotine in mice are limited. Therefore, we aimed to characterize and validate an addiction index by using an oral nicotine free-choice paradigm in mice.
Adult C57BL/6J, DBA/2J, or genetically modified mice carrying deletions for nicotinic acetylcholine receptor (nAChR) subunits, (n=8–10/ sex/ group) were given a choice of water or nicotine (10–960 μg/ml) solution using a two-bottle free-choice (2BC) paradigm. In general, oral nicotine intake and preference were higher in female mice compared to males. Absence of nicotine led to withdrawal, and intermittent access resulted in an escalation in consumption and greater nicotine withdrawal than continuous exposure. Additionally, oral nicotine consumption increased nucleus accumbens tyrosine hydroxylase levels. While β2 and α6 KO mice showed a significant decrease in nicotine intake, deletion of α5 nAChRs increased nicotine consumption at high concentrations. Deletion of the α7 subunit altered the observed sex difference in nicotine consumption, with females consuming less than males. The α4β2 partial agonist varenicline decreased oral nicotine consumption. Although addition of quinine to the nicotine solution lowered nicotine intake, mice primed with nicotine did not lower their intake after quinine addition. Nicotine deprivation followed by re-exposure showed increased nicotine consumption, and DBA/2J mice consumed less nicotine compared to C57BL/6J.
We validated the mouse 2BC paradigm to study nicotine’s addictive-like properties including nicotine intake, preference, withdrawal, and escalation of nicotine consumption during binge drinking or after reinstatement of a deprivation period.
Keywords: nicotine, addiction, dependence, two-bottle choice, withdrawal, oral self-administration
1. Introduction
Nicotine is the main active ingredient and primary addictive component in tobacco that leads to and maintains tobacco addiction (Benowitz, 2009). A new generation of smokeless tobacco products such as e-cigarettes, e-hookah, snus, and oral dissolvables have grown in popularity in both the U.S. and world market over the past several years (Popova and Ling, 2013). However, relatively little is known about their dependence liability and long-term health effects (Popova and Ling, 2013; Romito and Saxton, 2014).
Here we adapted the two-bottle free-choice (2BC) paradigm, a technically simple model that does not require surgical manipulation and is amenable to various types of experimental manipulations, to assess oral nicotine consumption in a mouse model. This model has been used to study the biological and behavioral effects of nicotine in rodents (Aschhoff et al., 2000; Dadmarz and Vogel, 2003; Renda and Nashmi, 2014; Rowell et al., 1983). Although previous studies with 2BC failed to observe significant preference for nicotine over water (Klein et al., 2004; Robinson et al., 1996), more recent reports have shown a slight preference for nicotine solutions compared to water in mice and rats (Adriani et al., 2002; Locklear et al., 2012; Nesil et al., 2015, 2013; Peng et al., 2017) and an increase in somatic signs after nicotine withdrawal (Locklear et al., 2012; Nesil et al., 2015). However, it remains unclear to what extent mice undergoing oral nicotine intake in the 2BC paradigm exhibit addiction-like behaviors.
With these previous studies in mind, we designed a series of experiments using the 2BC paradigm in C57BL/6J mice, which have shown higher oral consumption for drugs of abuse relative to other mouse strains (Meliska et al., 1995; Rodgers, 1966). An important aspect the 2BC provides is the opportunity for animals to choose how much nicotine or water to consume, since the loss of control over the ability to choose not to consume a substance of abuse despite being aware of the negative consequences is an important criterion for addiction (American Psychiatric Association., 2013). We examined the impact of sex and strain on nicotine consumption across different concentrations in the 2BC model, followed by exploring the impact of the consumption/withdrawal cycle using an intermittent access protocol. This was done to assess whether oral nicotine can produce escalating binge-like patterns of consumption and withdrawal symptoms during periods of abstinence, which are also indicators of dependence (American Psychiatric Association., 2013). Nicotine withdrawal is a group of symptoms that occur after sudden discontinuation or deprivation in nicotine intake. The primary symptoms associated with nicotine withdrawal are listed in The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as follows: irritability/anger/frustration, anxiety, depressed mood, difficulty concentrating, increased appetite, insomnia, and restlessness (American Psychiatric Association 2013). Moreover, it has been hypothesized that nicotine dependence may result from the motivation to take nicotine to attenuate the negative effects of nicotine abstinence, instead of nicotine’s positive reinforcing properties. It’s possible that nicotine abstinence-induced negative affective states may potentiate the incentive value of nicotine to promote the escalation of compulsive nicotine intake through negative reinforcement mechanisms (Cohen and George, 2013). Therefore, we explored the existence of a nicotine deprivation effect by assessing nicotine consumption upon reinstatement following a period of nicotine deprivation. We then used a pharmacological approach to further validate our 2BC model through antagonism and genetic deletion of known nicotinic receptor (nAChRs) subunits. We evaluated changes in nicotine consumption following administration of varenicline (Chantix®), an FDA approved smoking cessation medication that was reported to reduce nicotine intake in i.v. self-administration models in rodents (George et al., 2011). Subunits of nAChRs that play an essential role in nicotine reward and reinforcement such as β2, α5 and α6 (Exley et al., 2011; Fowler et al., 2013, 2011; Harenza et al., 2014; Jackson et al., 2010; Maskos et al., 2005; Picciotto et al., 1998; Pons et al., 2008; Walters et al., 2006) were also assessed in the 2BC paradigm using various nicotinic knock-out (KO) mice.
Finally, since the dopaminergic system is considered a crucial component of the rewarding and dependence-producing properties of nicotine [see review (Juarez and Han, 2016)], we tested whether oral nicotine intake in the 2BC model stimulates the neuronal mesolimbic dopaminergic system by measuring tyrosine hydroxylase (TH) levels in the nucleus accumbens, an essential neural substrate of nicotine reward.
2. Materials and Methods
2.1. Animals
The study was performed on adult (10–12 weeks of age at the beginning of experiment) female and male C57BL/6J and DBA/2J mice supplied by Jackson Laboratory (Bar Harbor, ME). For the genetically modified animals, mice null (knock-out, KO) for the α5 (Jackson laboratories, Bar Harbor, ME) (Salas et al., 2003), α6 (Institut Pasteur, Paris, France) (Champtiaux et al., 2002), α7 (The Jackson Laboratory), and β2 (Institut Pasteur, Paris, France) (Picciotto et al., 1998) were used. KO and their wildtype (WT) littermates were bred in an animal care facility at Virginia Commonwealth University. All the genetically modified mice used in each experiment were backcrossed at least 10 to 12 generations. Mutant and wild types were obtained from crossing heterozygote mice. This breeding scheme controlled for any irregularities that might occur with crossing solely mutant animals. Mice were housed one per cage with ad libitum access to food in a temperature- and humidity-controlled out-of-the-vivarium space, (21±3ºC, 55±10%) on a 12-h light/dark cycle. While animals were provided free access to food, they received their water as drinking solutions from two bottles at all times. The study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Drugs and chemicals
(−)-Nicotine base [(−)-1-Methyl-2-(3-pyridyl)pyrrolidine, (S)-3-(1-Methyl-2-pyrrolidinyl)pyridine)] liquid (cat no: N3876) and quinine (cat no: 22620) were purchased from Sigma-Aldrich (St. Louis, MO). Nicotine solutions were prepared by dissolving in distilled water. To avoid confounds such as taste factors and the reinforcing effects of sweeteners themselves (Morgan and Sizemore, 2011), mice were provided with unsweetened nicotine solution. All nicotine solutions were prepared fresh every other day. Varenicline (7,8,9,10-tetrahydro- 6,10-methano- 6H-pyrazino (2,3-h)(3) benzazepine) was supplied by the National Institute of Drug Abuse (NIDA Drug Supply Program, Bethesda, MD). Varenicline was dissolved in physiological saline (0.9% sodium chloride) and injected intraperitoneally at a total volume of 1ml/100 g body weight. Quinine was given at a concentration of 30 μM (9.73 mg/ml). All doses are expressed as the free base of the drug.
2.3. Two-bottle free-choice test
Nicotine consumption and preference were measured in mice using the 2BC paradigm. Briefly, mice were individually housed and habituated in cages containing two water bottles (25 ml plastic tubes containing metal spouts) for seven days. Following habituation, one of the water bottles was replaced with a bottle containing nicotine, and mice were given unlimited access to both bottles. Fluid intakes were recorded, and the bottle positions were alternated every day to control for the development of a side preference. Every three days, the concentration of nicotine was increased (5 – 960 μg/ml). Although nicotine does not lose its pharmacological activity for at least 3 days in aqueous solution (Klein et al., 2004), we replaced with fresh solutions every other day. During the period of the test, female and male mice were housed in same room. The volumes of water (ml) and nicotine (ml) were measured daily. Nicotine intake was calculated in mg of nicotine per kilogram (mg/kg) of body weight per day and nicotine preference as the volume of nicotine consumed as a percentage of the total fluid consumed. An empty cage (animal free) with two bottles including test solution and water was used to determine the volume of the leaked/evaporated. The spillage value in in this study was ~0.2 ml/day. To minimize the effect of handling-stress on behavior, the mice were weighed every three day and the average mass as in kg of each mouse was used for their daily nicotine intake dose calculation as mentioned previously (Renda et al., 2016).
2.4. Behavior testing
2.4.1. Nicotine intake and preference in various nicotinic KO mice
First, a dose response curve of nicotine (5 – 960 μg/ml) in 2BC test was determined in C57BL/6J naïve mice. Then, the 2BC test was also carried out in genetically modified mice for β2, α6, α7, and α5 nAChRs to evaluate the possible involvement of these receptor subunits in oral nicotine consumption. For this reason, KO mice for the β2, α6, α7, and α5 subunits were used. WT littermates were used as control for β2 and α7 KO mice. C57BL/6J strain naïve mice were used as controls for α6 and α5 KO mice. These animals were given a range of concentration (10 – 120 μg/ml; for α5 KO and WT the range was 10 – 960 μg/ml) and nicotine intake, preference and total fluid intake were measured as described above. Because previous studies showed an enhancement of nicotine reward and intake with high nicotine concentrations in α5 KO mice in both i.v. self-administration and CPP (Fowler et al., 2013, 2011; Jackson et al., 2010), we used a wider range of nicotine concentrations in α5 KO mice. The numbers of animals used for KO mice and their controls are: β2 (n=17/per group; 9 male and 8 female), α6 (n=16/per group; 8 male and 8 female), α7 (n=16/per group; 8 male and 8 female), and α5 (n=15/per group; 8 male and 7 female) subunits.
2.4.2. Effects of varenicline on nicotine intake and preference
In order to investigate if varenicline, a partial agonist at the α4β2 nAChRs, reduces oral nicotine consumption, adult male and female C57BL/6J mice (n=8/per sex/per group) were given a choice of water or nicotine (60 μg/ml) solution using a 2BC test in a reversed light cycle room. Every day at 10 am, animals were injected with saline to habituate the animals to intraperitoneal injection. After 3 days, animals were injected with either saline or a 0.3 mg/kg dose of varenicline, a dose that reduced nicotine reward in the mouse conditioned place preference (CPP) test (Bagdas et al., 2018). At twenty-four hours post injection, nicotine intake, preference, and total fluid intake were determined as described above.
2.4.3. Effect of intermittent access on nicotine intake and assessment of spontaneous nicotine withdrawal
Adult male and female C57BL/6J mice had 24 hours of continuous access to nicotine (60 μg/ml) daily for 6 days. On day 6, mice were divided into two groups. One group of mice (n=18; 9 male and 9 female) were given continuous access to nicotine (60 μg/ml) for additional one week. In the second group, mice (n=18; 9 male and 9 female) were given intermittent access to nicotine (60 μg/ml) every other day for one week with food available at all times in a 2BC paradigm. Nicotine and water intake values were recorded after 24 hours of nicotine access on the drinking days. On day 14, nicotine solution in all groups replaced with water and 24 hr later mice were observed for withdrawal signs. To determine the difference from naïve mice, an additional group as given water all the time was added to withdrawal studies. Mice were observed for 30 min for somatic signs, such as paw and body tremors, head shakes, backing, jumps, curls, and ptosis as described previously (Damaj et al., 2003). All studies were performed by an observer blinded to experimental treatment.
2.4.4. Effects of quinine on nicotine intake and preference
To test for compulsive-like nicotine drinking, quinine adulteration was used, which has been used as a measure of compulsive-like responding for alcohol (Vendruscolo et al., 2012). Animals were divided to four groups as follows: nicotine only, nicotine then quinine, nicotine plus quinine, and quinine only. Mice in nicotine only group (6 females and 7 males) received nicotine (60μg/ml) vs water for six consecutive days. Mice in the nicotine then quinine group (7 females and 7 males) received nicotine (60μg/ml) vs water for first three days, then, quinine (30 μM) was added to nicotine solution and mice continued to receive quinine added nicotine solution following three days. Nicotine plus quinine group (7 females and 7 males) was designed to receive the combination of nicotine (60μg/ml) and quinine (30 μM) vs water for all six days of experiment duration. Lastly, quinine only group of mice (6 females and 7 males) were given quinine (30 μM) vs water for six days. The results were presented as the average of data obtained last three days.
2.4.5. Effects of nicotine deprivation on nicotine intake and preference
To test the impact of nicotine deprivation on oral nicotine reinstatement, adult C57BL/6J female and male mice were given a choice of water or nicotine (30 μg/ml) solution in two access models of 2BC: continuous and intermittent access type. In this series of study, intermittent access group received one day nicotine vs water, other day received water vs water by starting the beginning of experiments. After completion 12 session of intermittent access and 24 consecutive days of continuous access exposure, animals were given 48 h water vs water. Nicotine deprivation was stopped and nicotine solution in 2BC paradigm was presented on the cages. Nicotine consumption was presented as pre- (the last day measurement before nicotine deprivation) and post-deprivation (the first day after nicotine deprivation) results.
At the end of nicotine deprivation study, animals were put back on a free choice of water and nicotine for three days. Then, nicotine solution was taken and replaced with water to measure spontaneous nicotine withdrawal. Mice were observed for various withdrawal signs at 6 and 24 hour after nicotine removal. An additional group of naïve mice was given water all the time as a control (nicotine free) group for withdrawal studies. The mice were first evaluated for 5 min in the light dark box test for anxiety-related behavior, followed by a 20-min observation of somatic signs measured as paw and body tremors, head shakes, backing, jumps, curls, and ptosis as described in Damaj et al. (2003). All studies were performed by an observer blinded to experimental treatment.
2.5. Light dark box (LDB) test
The LDB apparatus is composed of a small, enclosed dark or black compartment (36 × 10 × 34 cm) with a passageway (6 × 6 cm) extending to a larger, light or white compartment (36 × 21 × 34 cm). Mice were habituated to the experiment room for 30 minutes before testing. First, mice were placed in the light chamber and allowed to freely explore the apparatus for 5 min. Then the number of entries into the light compartment, the number of transitions and the total time spent in the light compartment were recorded for 5 min by a video monitoring technique and ANY-MAZE software (Stoelting Co., Wood Dale, IL).
2.6. Blood nicotine and cotinine levels measurement
The blood nicotine and cotinine levels were measured in C57BL/6J male and female mice (n=10/per sex/group) under 24 hours of continuous access to nicotine (60 μg/ml) daily for 30 days in the 2BC model. Animals were kept in a switched light cycle which allowed us to collect blood on three time points of Zeitgeber time: 3, 6, and 15 hours after start of dark cycle. The tube position switched daily and nicotine solution and water were replaced with fresh solutions every other day. On day 31, mice were anesthetized with isoflurane and blood samples were taken by intracardiac puncture just before death. Therefore, different cohort mice were used for all time points. The blood was kept in lithium heparin blood-collection tubes and stored at −80 °C until assayed.
2.6.1. Determination of nicotine concentrations in plasma
Plasma samples were collected and stored at −80 °C until analyzed. Aliquots of 0.1 mL of drug free plasma were used to prepare a seven point calibration curves ranging from 1 ng/mL to 100 ng/mL of nicotine and cotinine, a negative control and a blank control for each analytical run. An aliquot of 10 μL of internal standard (ISTD) containing 10 ng of nicotine-d4 and cotinine-d3 in methanol was added each 0.1 mL aliquot of each calibrator, control and specimen except the blank control. These samples were mixed and 100 μL of 5 M ammonium hydroxide was added followed by 2 ml methylene chloride. The samples were mixed for 2 minutes and then centrifuged for 5 minutes at 3000 rpm. The organic layer was transferred to a clean test tube. The aqueous phase was extracted twice more with 2 mL of methylene chloride. The organic phases were combined and 500 μL of 25 mM hydrochloric acid in methanol. Samples were then evaporated to dryness under a gentle stream of nitrogen and reconstituted with 100 μL of mobile phase and placed in auto-sample vials.
An ultra-performance liquid chromatography - tandem mass spectrometer (UPLC-MS/MS) method was used for the identification and quantified of nicotine and its metabolite cotinine. The instrument was a Sciex 6500 QTRAP with an IonDrive Turbo V source for TurbolonSpray® (Ontario, Canada) attached to a Shimadzu UPLC system (Kyoto, Japan) controlled by Analyst software (Ontario, Canada). The chromatographic separation was performed on a Hypersil Gold, 3mm × 50 mm, 5 micron (Thermo Scientific, USA) column with mobile phase containing 10 mM ammonium formate; methanol (10:90 V/V) delivered at a flow rate of 0.5 mL/min. The source temperature was set at 600°C and had a curtain gas at a flow rate of 30 mL/min. The ionspray voltage was 5000 V; with the ion source gases 1 and 2 had flow rates of 50 and 30 mL/min, respectively. The acquisition mode used was multiple reaction monitoring (MRM) in a positive mode. The transition ions were monitored for nicotine (163>130; 163>117), nicotine-d4 (167>134), cotinine (177>80; 177>98) and cotinine-d3 (180>80). The total run time for each sample was 2 minutes. A calibration curve was constructed for each compound based on linear regression using the peak area ratios of the drug to its deuterated ISTD.
2.7. Western Blotting
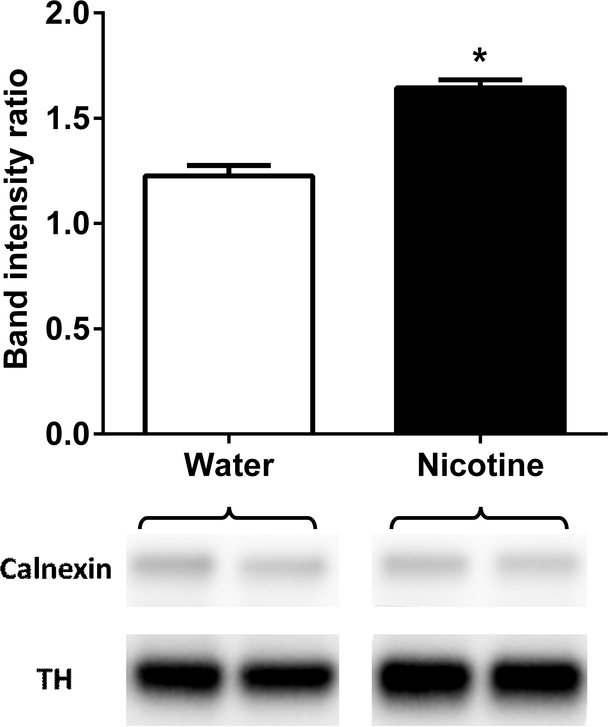
C57BL/6J mice (n=8/per sex/per group) were exposed water vs water or water vs nicotine (60 μg/ml) in 2BC paradigm for a month. At the end of the month, all mice were sacrificed by guillotine and immediately nucleus accumbens (NAc) brain region was collected on ice. The tissue samples were kept in −80°C freezer. Tissue were extracted in RIPA buffer (catalog #J62725, Alfa Aesar, MA, USA). Samples were loaded as 12.5 μg of protein for each well, separated by SDS-PAGE, and transferred to PVDF membranes overnight. The membranes were incubated overnight at 4 °C with anti-TH (1:1000; catalog no #AB152, Millipore, USA) and calnexin (1:20000; catalog no #ab10286, Abcam, UK) antibodies. Then, incubation with peroxidase-conjugated goat anti-rabbit IgG (catalog no #111–035-003, Jackson ImmunoReseach, USA) was performed. ChemiDoc MP Imaging System (Bio-Rad, CA, USA) was used to detect the bands on the blots. The optical densities of the bands were measured using ImageJ software available for free download at http://rsb.info.nih.gov/ij/. TH expression was quantified with respect to the signals of the corresponding calnexin band. Data are expressed the ratio of band intensity.
2.8. Statistical analysis
The data obtained were analyzed using the GraphPad software, version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. Statistical analysis was done using simply t test (western blotting) or repeated measures (RM) two-way (drinking studies) analysis of variance test (ANOVA), followed by the Sidak post hoc correction. In addition, an ordinary two-way ANOVA followed by the Sidak post hoc correction was used in varenicline and deprivation studies. To test the withdrawal symptoms, plasma nicotine and cotinine levels one-way ANOVA was used with Dunnet and Sidak correction. Before ANOVA, the data were first assessed for the normality of the residuals and equal variance. Variances were similar between groups and were assessed using either the F‐test or the Brown–Forsythe test and the Bartlett’s test. All data passed these tests. The p values < 0.05 were considered significant.
3. Results
3.1. Nicotine intake and preference in C57BL/6J mice
We first determined nicotine consumption and preference in female and male adult C57BL/6J mice exposed to a wide range of nicotine concentrations (5 – 960 μg/ml). While nicotine intake was increased in a dose-response manner, preference score of the drug was decreased at increasing concentrations of nicotine. For example, 60 μg/ml nicotine concentration caused a ~60% of preference in females and ~40% of preference in males, nicotine preference decreased to ~20% at high concentrations (480 and 960 μg/ml). Nicotine intake was significantly higher in female then male mice starting the 60 μg/ml concentration [Fconcentration (9,81) = 185.7, p < 0.001, Fsex (1,9) = 20.51, p < 0.01, and Fconcentration × sex (9,81) = 4.804, p < 0.001; Figure 1A]. Consistently, the nicotine preference score in female mice were significantly higher than males at the 60 μg/ml nicotine concentration [Fconcentration (9,81) = 25.48, p < 0.001, Fsex (1,9) = 2.948, p = 0.1201, and Fconcentration × sex (9,81) = 2.055, p < 0.05; Figure 1B]. Total fluid intake of female and male mice was similar [Fconcentration (9,81) = 3.664, p < 0.001, Fsex (1,9) = 0.5895, p = 0.5895, and Fconcentration × sex (9,81) = 1.16, p = 0.3316; Figure 1C]. In addition, nicotine did not differentially affect body weight gain in female and male mice (Supplementary Figure 1).
Figure 1: Nicotine drinking behavior in adult C57BL/6J mice.
Mice were exposed to either water or nicotine (5 – 960 μg/ml) in two-bottle choice paradigm. Average of nicotine intake (mg/kg/day), preference (%) and total fluid intake (ml/day) over 3 days were calculated for each nicotine concentration in male and female C57BL/6J mice. Significant A) nicotine intake and B) preference were observed in female mice beginning from 60 μg/ml concentration of nicotine in comparison to male counterparts. C) Total fluid intake of both male and female was similar. Data are presented as the mean ± SEM of 10 animals. * indicates p < 0.05.
3.2. nAChRs mediates nicotine intake and preference
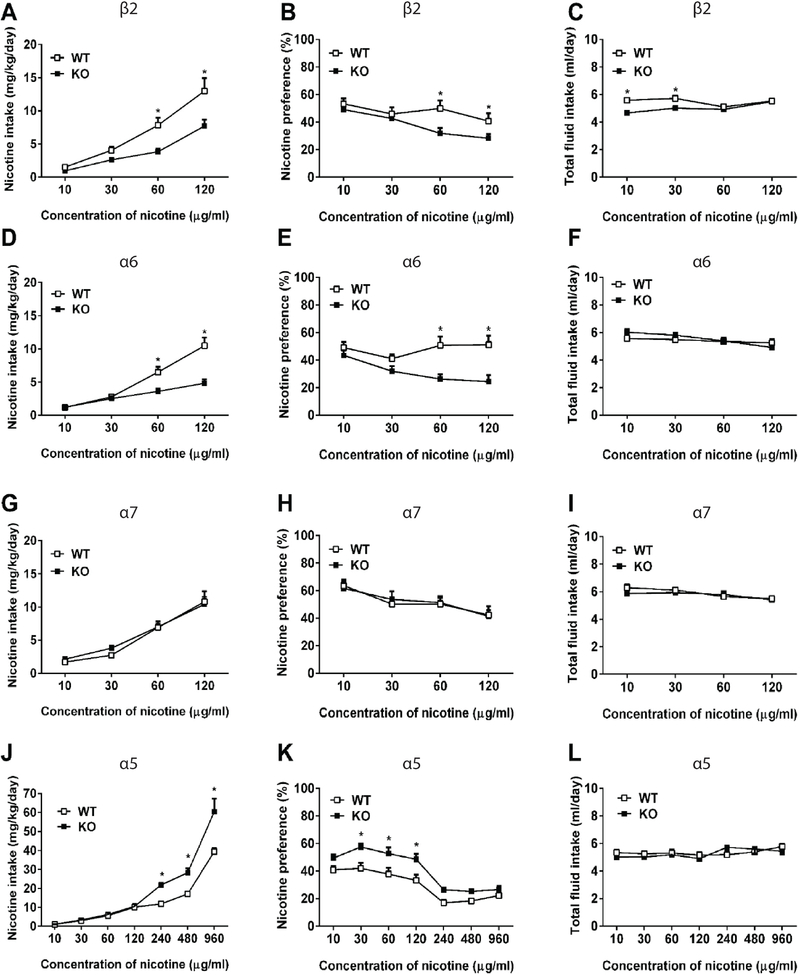
A 2BC test was carried out in genetically modified mice for β2, α6, α7, and α5 nAChRs to evaluate the possible involvement of these receptor subunits in oral nicotine consumption. As seen Figure 2A and B, β2 KO mice showed significantly lower nicotine intake and preference score than their WT littermates at all concentrations except for 10 and 30 μg/ml [Fconcentration (3,51) = 59.48, p < 0.001, Fgenotype (1,17) = 10.46, p < 0.01, and Fconcentration × genotype (3,51) = 7.985, p < 0.001; Figure 2A and Fconcentration (3,51) = 16.56, p < 0.001, Fgenotype (1,17) = 2.778, p < 0.05, and Fconcentration × genotype (3,51) = 6.355, p < 0.01; Figure 2B]. The total fluid intake was significantly higher in WT mice than KO counterparts at the low concentrations of 10 and 30 μg/ml only [Fconcentration (3,51) = 8.831, p < 0.001, Fgenotype (1,17) = 16.49, p < 0.001, and Fconcentration x genotype (3,51) = 7.450, p < 0.01; Figure 2C].
Figure 2: nAChRs mediates nicotine intake and preference.
Mice were exposed to either water or nicotine (10 – 960 μg/ml) in two-bottle choice paradigm. Average of (A, D, G, J) nicotine intake (mg/kg/day), (B, E, H, K) preference (%) and (C, F, I, L) total fluid intake (ml/day) over 3 days were calculated for each nicotine concentration in β2, α6, α7, and α5 WT and KO mice, respectively. Data are presented as the mean ± SEM of 15–18 animals. Equal numbers of animals for each group for β2 (n=17/per group; 9 male and 8 female), α6 (n=16/per group; 8 male and 8 female), α7 (n=16/per group; 8 male and 8 female), and α5 (n=15/per group; 8 male and 7 female) subunits were used for KO mice and their controls. * indicates p < 0.05.
The α6 KO mice also showed a lower nicotine intake and preference score compared to WT (C57BL/6J strain) in different nicotine concentrations [Fconcentration (3,45) = 98.77, p < 0.001, Fgenotype (1,15) = 0.1362, p = 0.7173; Figure 2D and Fconcentration (3, 45) = 33.81, p < 0.001, Fgenotype (1, 15) = 0.0022, p = 0.9628; Figure 2E] without any effect on total fluid consumption [Fconcentration (3, 45) = 7.214, p < 0.001, Fgenotype (1, 15) = 0.3277, p = 0.5755; Figure 2F].
The α7 KO and WT mice also showed similar nicotine intake and preference score at all nicotine concentrations [Fconcentration (3,45) = 98.77, p < 0.001, Fgenotype (1,15) = 0.1362, p = 0.7173; Figure 2G and Fconcentration (3, 45) = 33.81, p < 0.001, Fgenotype (1, 15) = 0.002, p = 0.9628; Figure 2H] without any effect on total fluid consumption [Fconcentration (3, 45) = 7.211, p < 0.001, Fgenotype (1, 15) = 0.3277, p = 0.5755; Figure 2I].
In contrast to β2 and α6 KO mice, α5 KO mice showed higher nicotine intake and preference score than the WT mice in the 2BC test. While α5 KO mice showed similar nicotine consumption compared to WT animals (C57BL/6J strain) at lower concentrations, they significantly consumed more nicotine compared to WT mice at higher concentrations (>240 μg/ml) [Fconcentration (6,84) = 144.1, p < 0.001, Fgenotype (1,14) = 24.37, p < 0.001, and Fconcentration × genotype (6,84) = 10.14, p < 0.001; Figure 2J and Fconcentration (6,84) = 36.07, p < 0.001, Fgenotype (1,14) = 34.74, p < 0.01, and Fconcentration × genotype (6,84) = 1.688 p < 0.001; Figure 2K]. Total fluid intake was similar between α5 KO and WT [Fconcentration (6,84) = 2.673, p < 0.05, Fgenotype (1,14) = 4.044, p = 0.0615, and Fconcentration × genotype (6,84) = 1.614, p = 0.1516; Figure 2L].
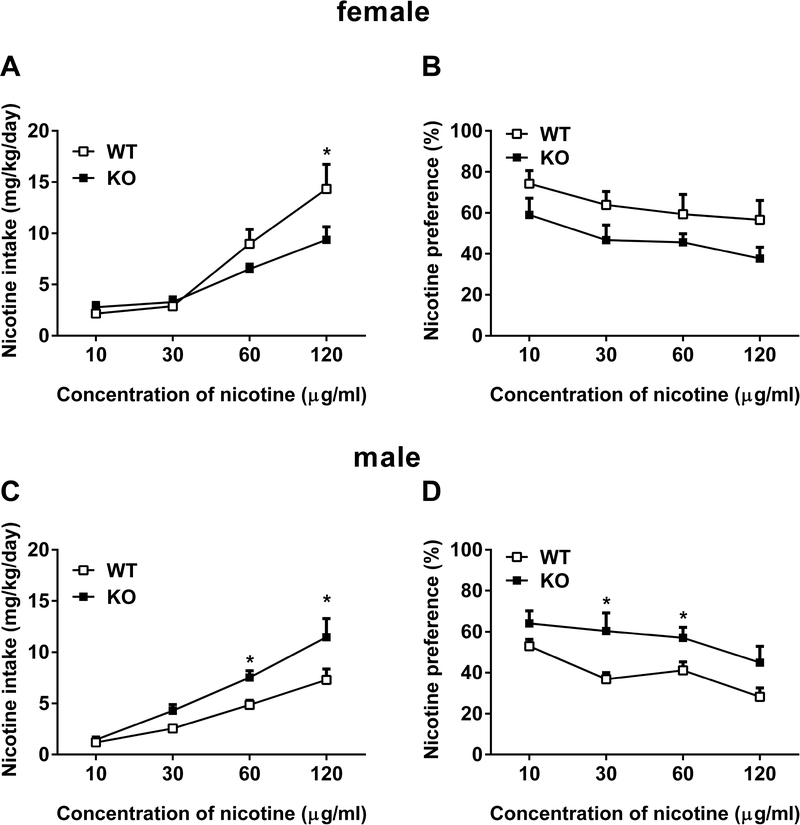
Because there was no significant difference in nicotine intake and preference score between male and female mice, we put all animals together and compared WT vs KO in Figure 2. However, each sex of α7 KO and WT mice showed different nicotine consumption pattern. As seen in Figure 3, female α7 KO mice showed lower intake and preference score compared to their WT counterparts [for nicotine intake, Fconcentration (3,56) = 29.28, p < 0.001, Fgenotype (1,56) = 3.911, p = 0.0529; Fconcentration × genotype (3,56) = 2.657, p = 0.00571; Figure 3A and for nicotine preference, Fconcentration (3, 56) = 2.507, p = 0.068, Fgenotype (1, 56) = 9.767, p < 0.01; Figure 3B]. At concentration of 120 μg/ml, female α7 KO had significantly lower nicotine intake (p < 0.05, Figure 3A). Although nicotine preference score in KO mice were lower than WT mice, it did not reach a significant level at any concentrations (p > 0.05, Figure 3B). Surprisingly, male cohort mice showed a nicotine consumption pattern to opposite direction. The α7 male KO had significantly greater nicotine intake [Fconcentration (3,56) = 35.65, p < 0.01, Fgenotype (1,56) = 14.36, p < 0.001; Fconcentration × genotype (3,56) = 1.97, p = 0.129; Figure 3C] and preference score [Fconcentration (3,56) = 4.981, p < 0.01, Fgenotype (1,56) = 17.46, p < 0.001; Fconcentration × genotype (3,56) = 0.4025, p = 0.7518; Figure 3D] compared to their WT counterparts. The KO male mice had significantly higher nicotine intake than WT littermates at 60 and 120 μg/ml concentrations (p < 0.05). In addition, they had significantly greater nicotine preference score at 30 μg/ml nicotine concentration (p < 0.05).
Figure 3: Oral nicotine consumption pattern is different in female and male α7 nAChRs KO mice.
Mice were exposed to either water or nicotine (10 – 120 μg/ml) in two-bottle choice paradigm. Average of (A, C) nicotine intake (mg/kg/day) and (B, D) preference (%) over 3 days were calculated for each nicotine concentration in α7 KO and WT female and male mice, respectively. Data are presented as the mean ± SEM of 8 animals. * indicates p < 0.05.
3.3. Varenicline, an α4β2 partial agonist, decreases nicotine intake and preference
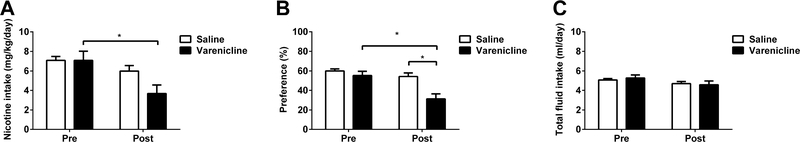
In order to investigate if systemic administration of varenicline, a partial agonist at α4β2 nAChRs, changes oral nicotine consumption, adult male and female C57BL/6J mice (n=8/per sex/per group) were given a choice of water or nicotine (60 μg/ml) solution using a 2BC drinking assay, and after 3 days injected with either saline or an intraperitoneal dose of 0.3 mg/kg of varenicline. At twenty-four hours post injection, there was a significant decrease in both intake and preference score for nicotine in varenicline-injected mice without a considerable effect on total fluid intake. Two-way ANOVA revealed significant effects of varenicline on intake [Ftreatment (1,60) = 2.525, p = 0.1173, Feffect (1,60) = 9.567, p < 0.01, and Finteraction (1,60) = 2.544, p = 0.116; Figure 4A] and preference [Ftreatment (1,60) = 11.94, p < 0.001, Feffect (1,60) = 13.91, p < 0.001, and Finteraction (1,60) = 5.25, p < 0.05; Figure 4B] but not on total fluid intake [Ftreatment (1,60) = 0.0197, p = 0.8888, Feffect (1,60) = 3.409, p = 0.0698, and Finteraction (1,60) = 0.3033, p = 0.5839; Figure 4C]
Figure 4: Varenicline, α4β2 partial agonist, decreases nicotine intake and preference.
Adult C57BL/6J male and female mice were given a choice of water or nicotine (60 μg/ml) solution using a two-bottle choice paradigm. After 3 days, the mice were injected with either saline or a 0.3 mg/kg dose of varenicline. At twenty-four hours post injection the A) nicotine intake, B) preference and C) total fluid intake were measured and compared to pre-injection of varenicline. Varenicline decreased nicotine intake and preference. Data are presented as the mean ± SEM of 16 animals (n=16; 8 male and 8 female). * indicates p < 0.05.
3.4. Intermittent access of nicotine exposure escalated the nicotine intake and preference as well as withdrawal signs of nicotine
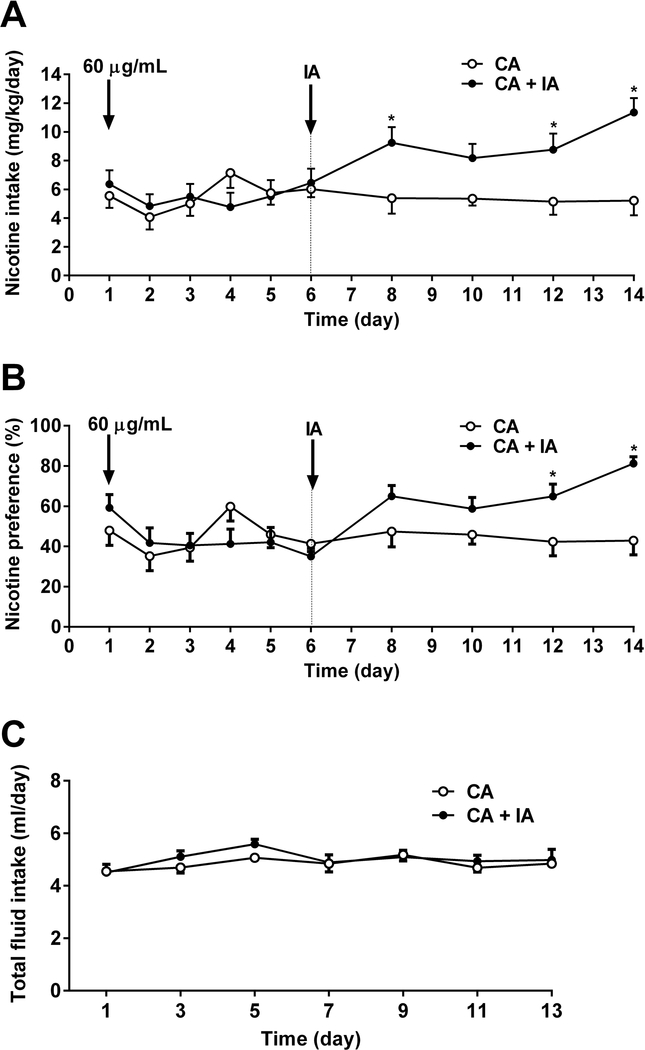
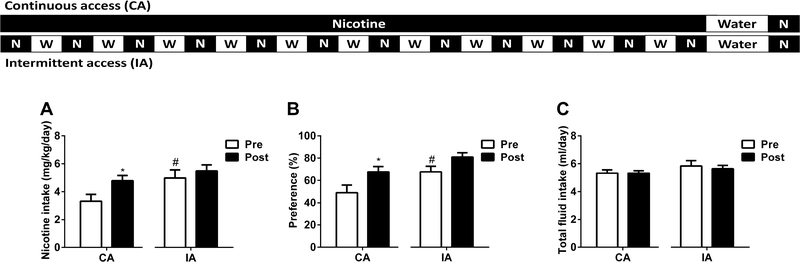
To test the impact of intermittent access on oral nicotine consumption, adult C57BL/6J female and male mice were given a choice of water or nicotine (60 μg/ml) solution. Following six days of continuous water vs nicotine exposure, animals were divided two groups. While one group received intermittent access of nicotine (every other day), the other group received continuous access to nicotine for one more week. As seen in Figure 5, intermittent access to nicotine solution substantially increased nicotine intake [Fintermittent access (9,17) = 4.419, p < 0.05, Ftime (9,153) = 3.809, p < 0.001, and Fintermittent access × time (9,153) = 4.275, p < 0.001; Figure 5A] and preference score [Fintermittent access (9,17) = 2.361, p = 1.428, Ftime (9,153) = 3.725, p < 0.001, and Fintermittent access × time (9,153) = 4.267, p < 0.001; Figure 5B]. Moreover, intermittent access of nicotine did not alter total fluid intake [Fintermittent access (1,17) = 0.9326, p = 0.3477, Ftime (6,102) = 2.33, p < 0.05, and Fintermittent access × time (6,102) = 0.5412, p = 0.7757; Figure 5C]
Figure 5: Intermittent access of nicotine exposure escalated the nicotine intake and preference.
Adult C57BL/6J male and female mice were continuously received either water or nicotine (60 μg/ml) in two-bottle choice experiment for 6 days. Mice then divided into two groups; one underwent intermittent access (IA) and the other had continuous access (CA) to nicotine versus water for one week. A) Significant higher nicotine intake and B) preference were observed in intermittent cohort comparing to the continuous access group. C) Total fluid intake of both cohorts was similar. Data are presented as the mean ± SEM of 18 animals (n=18; 9 male and 9 female). * indicates p < 0.05.
At the end of the experiments on day 14, all animals were given only water and after 24 hr mice were observed for withdrawal signs. Significant effects of somatic signs were determined [Fsomatic signs (2,57) = 68.46, p < 0.001; Supplementary Figure 2]. Both continuous and intermittent access groups showed significant number of somatic signs (p < 0.05). In addition, intermittent access group has higher number of somatic signs than continuous access group (p < 0.05).
3.5. Oral nicotine consumption results in an increase of NAc TH levels
To test the impact of oral nicotine consumption on TH levels in the NAc, adult C57BL/6J female and male mice (n=8/per sex/per group) were given a 24-hr access to water or nicotine (60 μg/ml) solution for 30 days in the 2BC paradigm. Results showed that chronic oral nicotine consumption increased NAc TH levels. There was a significant increase in TH levels in nicotine-treated group when compared to water control (t = 6.762, df = 29, p < 0.001; Figure 6).
Figure 6: Oral nicotine consumption resulted in an increase on tyrosine hydroxylase levels in nucleus accumbens.
Mice were exposed to water versus water or water versus nicotine (60 μg/ml) in two-bottle choice paradigm for a month. After nicotine chronic exposure, tyrosine hydroxylase (TH) levels were measured in nucleus accumbens (NAc) region of brain. TH level was higher in nicotine treated group than water treated group. Data are presented as the mean ± SEM of 16 animals (n=8/per sex/per group). * indicates p < 0.05.
3.6. Plasma nicotine and cotinine levels
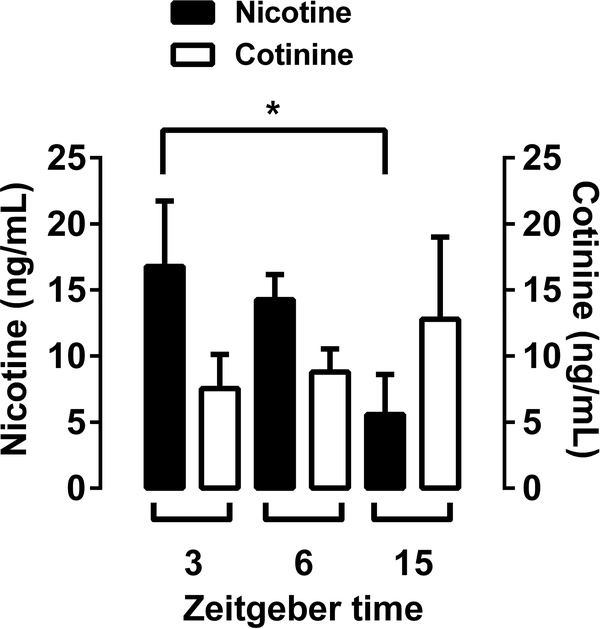
Nicotine and cotinine blood levels were measured at the end of 1-month nicotine (60 μg/ml) exposure in male and female adult C57BL/6J mice. Mice were on a reverse light dark cycle with lights off at 7:00am, initiating the start of their active cycle [zeitgeber (Z) time = 0:00 h]. Because we had previously observed that mice show circadian rhythms in nicotine drinking, we decided to measure nicotine and cotinine plasma levels at three time points corresponding to the start of the active period (3 Z+), midway through the drinking period (6 Z+), and into the light cycle (15 Z+). Although 20 mice per group were provided nicotine 2BC, the final analyses are for fewer subjects because samples were lost due to predictable challenges with blood collection and plasma drug extraction steps. In total, we had n=13–20 mice per group. Overall, one-way ANOVA revealed significant effects for nicotine [Fnicotine (2,52) = 3.163, p < 0.05] but not for cotinine plasma levels [FCotinine (2,43) = 0.408, p = 0.6675; Figure 7]. The results in Figure 7 present a time-related decrease in plasma levels of nicotine. While nicotine plasma levels were approximately 15 ng/ml at 3 Z+, levels were significantly lower (approximately 5 ng/ml) at 15 Z+ (p < 0.05). However, cotinine plasma levels did not show a significant increase or decrease between 3, 6, or 15 Z+ time points.
Figure 7: Plasma nicotine level after oral nicotine consumption.
Adult C57BL/6J male and female mice were exposed to water versus nicotine (60 μg/ml) in two-bottle choice paradigm on a reverse light dark cycle with lights off at 7:00am, initiating the start of their active cycle [zeitgeber (Z) time = 0:00 h] for a period of 30 days. Blood collection was conducted at the end of 1-month nicotine exposure. Nicotine and cotinine plasma levels were measured at three time points corresponding to the start of the active period (3 Z+), midway through the drinking period (6 Z+), and into the light cycle (15 Z+). While a time-related decrease in plasma levels of nicotine was found, cotinine plasma levels did not show an increase or decrease between 3, 6, or 15 Z+ time points.
Data are presented as the mean ± SEM of 13–20 animals. There were n=16 mice for 3 Z+, n=19 mice for 6 Z+, n=20 mice for 15 Z+ in nicotine and n=13 mice for 3 Z+, n=16 mice for 6 Z+, n=17 mice for 15 Z+ in cotinine groups. * indicates p < 0.05.
3.7. Impact of quinine adulteration on aversion-resistant drinking
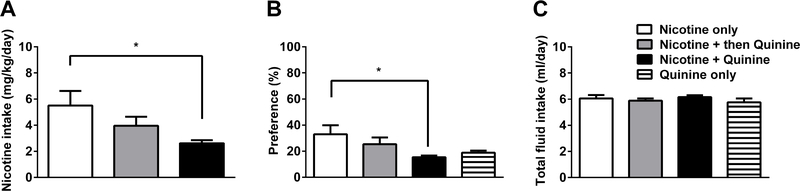
To investigate the voluntary choice to consume nicotine despite adulteration with a bitter taste agent, such as quinine, we used 2BC paradigm and tested nicotine consumption with or without quinine. For this reason, four groups of animals were exposed to either nicotine vs water, nicotine for 7 days then quinine vs water, nicotine plus quinine vs water, or quinine vs water. ANOVA revealed significant effects on nicotine intake [Fintake(2,38) = 3.64, p < 0.05; Figure 8A] and preference score [Fpreference(3,50) = 2.981, p < 0.05; Figure 8B] but not on total fluid intake [Fintake(3,50) = 0.6645, p = 0.5778; Figure 8C]. The mice which received nicotine plus quinine vs water from the beginning to the end of the experiment showed significantly lower nicotine intake and preference score compared to nicotine vs water control group (p < 0.05). However, when animals were first exposed to nicotine itself and after three days given a choice of nicotine plus quinine vs water, mice did not lower their intake and preference score (p > 0.05). Total fluid intake was similar between the groups (p > 0.05).
Figure 8: Quinine addition to nicotine solution fails to decrease nicotine consumption if mice are primed by nicotine.
Adult C57BL/6J male and female mice were given a choice of water or drug: nicotine (60 μg/ml) only, nicotine (60 μg/ml) then quinine (30 μM), nicotine (60 μg/ml) plus quinine (30 μM), and quinine (30 μM) only in two-bottle choice paradigm. While nicotine only, nicotine plus quinine, and quinine only groups were received drug solution vs water for six consecutive days, mice in the nicotine then quinine group received nicotine vs water for first three days, then, mice received quinine added nicotine solution following three days. A) Nicotine intake, B) preference, and C) total fluid intake were measured and compared between the groups. The results were presented as the average of data obtained last three days. Nicotine plus quinine combination resulted in a decrease on nicotine intake and preference. When mice were primed with nicotine vs water exposure before quinine addition, persistent nicotine drinking despite the aversive bitter taste of quinine added to the nicotine solution was observed. Data are presented as the mean ± SEM of 13–14 animals. * indicates p < 0.05.
3.8. Impact of nicotine deprivation on nicotine intake and withdrawal
In order to test the impact of nicotine deprivation on oral nicotine consumption, adult C57BL/6J female and male mice were given a choice of water or nicotine (30 μg/ml) solution in two access models of 2BC: continuous access (24 consecutive days) and intermittent access (12 sessions) type. Following 24 days of continuous or intermittent water vs nicotine exposure, animals were given water vs water for 48 hours. Later, nicotine was given back in 2BC paradigm. ANOVA revealed significant effects of nicotine deprivation on nicotine intake [Faccess (1,62) = 6.215, p < 0.05, Fdeprivation (1,62) = 4.333, p < 0.05, and Finteraction (1,62) = 0.9975, p = 0.3218; Figure 9A] and nicotine preference score [Faccess (1,62) = 9.199, p < 0.01, Fdeprivation (1,62) = 9.19, p < 0.01, and Finteraction (1,62) = 0.2451, p = 0.6223; Figure 9B], but not total fluid intake [Faccess (1,62) = 2.457, p = 0.1221, Fdeprivation (1,62) = 0.1326, p = 0.7170; Figure 8C]. As seen in Figure 8, intermittent access to nicotine solution increased nicotine intake (p < 0.05; Figure 9A) and preference score (p < 0.05; Figure 9B) compared to continuous access exposure before nicotine deprivation. Besides, nicotine deprivation led to an increase on nicotine intake and preference score in continuous access exposure group as seen the results obtained after deprivation (p < 0.05; Figure 9AB). Although an increase was seen in either nicotine intake or preference score in the intermittent access group of mice, results did not reach a significant level in this group (p > 0.05; Figure 9AB). Total fluid intake was similar between the groups and treatments (p > 0.05; Figure 9C).
Figure 9: DBA/2J mice have a lower nicotine consumption compared to C57BL/6J.
Adult DBA/2J and C57BL/6J mice were subjected to either water or nicotine (10 – 960 μg/ml) in two-bottle choice paradigm. A) Nicotine intake, B) preference and C) total fluid intake were seen in DBA/2J male and female mice. D) Nicotine intake, E) preference and F) total fluid intake were observed in DBA/2J and C57BL/6J mice. Data are presented as the mean ± SEM of 16 animals (n=16; 8 male and 8 female). * indicates p < 0.05.
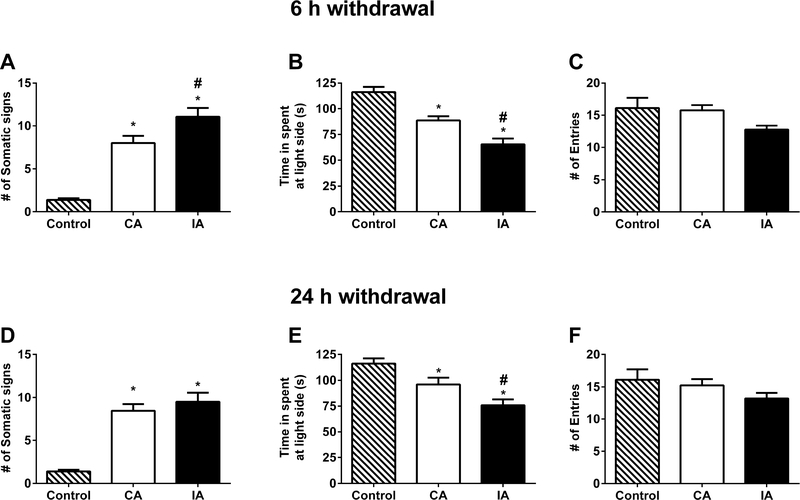
At the end of nicotine deprivation experiments, all animals were given nicotine (30 μg/ml) vs water exposure for three consecutive days and then, nicotine solutions were replaced with water. 6 and 24 hours later, mice were tested for withdrawal signs. Naïve mice under the same room conditions receiving drinking water all the time served as a control group. As shown in Figure 10, nicotine (30 μg/ml) induced robust nicotine withdrawal signs as seen with an increased in anxiety-related behaviors and somatic signs. At 6h withdrawal assay, while both continuous and intermittent exposure of nicotine led to an in increase in somatic signs, the intermittent access group exhibited greater somatic withdrawal signs [Fsomatic signs (2,50) = 48.89, p < 0.001; Figure 10A]. The increase in anxiety-like behavior was also significantly higher in the intermittent access group compared to continuous access group as seen in time spent in light side [Flight side (2,50) = 26.98, p < 0.001; Figure 10B]. However, total entries were similar in all groups at 6h withdrawal [Fentries (2,50) = 2.27, p = 0.1139; Figure 10C], respectively. Moreover, 24h withdrawal assay showed similar results. The increase in somatic signs was significantly higher in continuous and intermittent access groups compared to control mice [Fsomatic signs (2,50) = 39.68, p < 0.001; Figure 10D]. However, intermittent access did not show higher withdrawal signs than continuous access group (p > 0.05). Besides, the increase in anxiety-like behavior was higher in intermittent access group than continuous access group as seen in time spent in light side [Flight side (2,50) = 12.63, p < 0.001; Figure 10E]. The entries were similar between the groups [Fentries (2,50) = 1.343, p = 0.2703; Figure 10F],
Fig. 10. Impact of nicotine deprivation on nicotine withdrawal.
At the end of nicotine deprivation study (Fig. 9), animals were given back a free choice of water and nicotine for three days. Then, nicotine solution was taken and replaced with water to test possible spontaneous nicotine withdrawal following 6th and 24th hour water exposure. A control group, which formed by 10 male and 10 female C57BL/6J mice and received water vs water at all times, was added. A) Somatic signs [Fsomatic signs (2,50) = 48.89, p < 0.001], B) time spent in light side [Flight side (2,50) = 26.98, p < 0.001], and C) number of entries [Fentries (2,50) = 2.27, p = 0.1139] were observed to test 6 hr nicotine withdrawal. Intermittent access group exhibited greater nicotine withdrawal signs compared to continuous access group. Moreover, 24h withdrawal assay showed similar results for D) somatic signs [Fsomatic signs (2,50) = 39.68, p < 0.001], E) time spent in light side [Flight side (2,50) = 12.63, p < 0.001], and F) number of entries [Fentries (2,50) = 1.343, p = 0.2703]. Data are presented as the mean ± SEM of 16–17 animals (8–9 male and 8 female). * indicates p < 0.05 vs control. # indicates p < 0.05 vs continuous access group.
3.9. C57BL/6J mice demonstrate greater nicotine intake and preference than DBA/2J mice
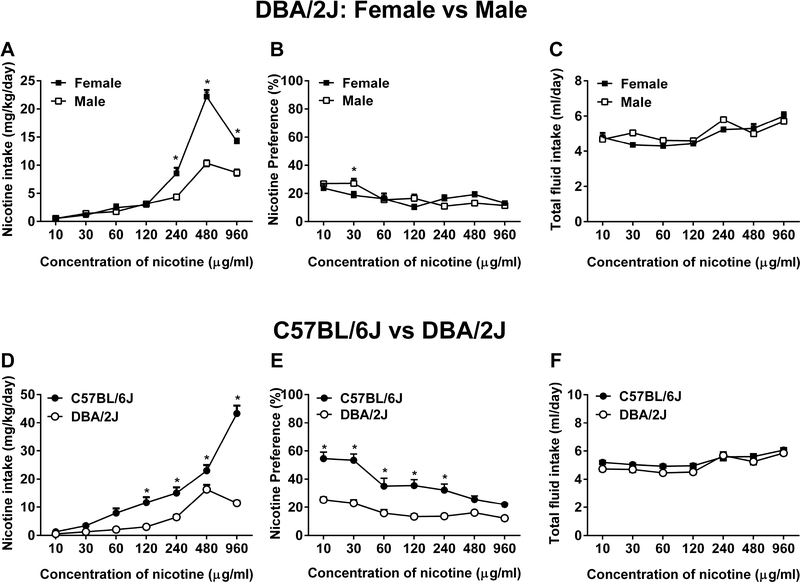
We studied nicotine oral consumption in DBA/2J mice, a strain that diverges from the C57BL/6J strain on the rewarding properties of a variety of drugs of abuse including nicotine (Jackson et al., 2009; Meliska et al., 1995; Rodgers, 1966). DBA/2J and C57BL/6J mice were habituated to the experiment room together and subsequently subjected to a continuous access of nicotine (10 – 960 μg/ml). Nicotine intake up to the concentration 120 μg/ml did not differ between DBA/2J female and male mice. However, nicotine intake was significantly higher in female compared to male mice at higher concentrations (>240 μg/ml) [Fconcentration (6,42) = 320.5, p < 0.001, Fsex (1,7) = 51.40, p < 0.001, and Fconcentration × sex (6,42) = 40.31, p < 0.001; Figure 11A]. Although nicotine preference score in DBA/2J female mice were slightly higher than DBA/2J males at certain concentrations but failed to achieve a level of statistical significance [Fconcentration (6,42) = 12.3, p < 0.001, Fsex (1,7) = 0.0998, p = 0.761, and Fconcentration × sex (6,42) = 4.366, p = 0.0016; Figure 11B]. Total fluid intake of female and male mice was similar [Fconcentration (6,42) = 18.6, p < 0.001, Fsex (1,7) = 1.336, p = .2857, and Fconcentration × sex (6,42) = 2.536, p = 0.0348; Figure 11C]. In addition, nicotine did not differentially affect body weight gain in female and male mice (data not shown).
Fig. 11. DBA/2J mice have a lower nicotine consumption compared to C57BL/6J.
Adult DBA/2J and C57BL/6J mice were subjected to either water or nicotine (10 – 960 μg/ml) in two-bottle choice paradigm. A) Nicotine intake [Fconcentration (6,42) = 320.5, p < 0.001, Fsex (1,7) = 51.40, p < 0.001, and Fconcentration × sex (6,42) = 40.31, p < 0.001], B) preference [Fconcentration (6,42) = 12.3, p < 0.001, Fsex (1,7) = 0.0998, p = 0.761, and Fconcentration × sex (6,42) = 4.366, p = 0.0016], and C) total fluid intake [Fconcentration (6,42) = 18.6, p < 0.001, Fsex (1,7) = 1.336, p = .2857, and Fconcentration × sex (6,42) = 2.536, p = 0.0348] were seen in DBA/2J male and female mice. D) Nicotine intake [Fconcentration (6,90) = 122.3, p < 0.001, Fstrain (1,15) = 92.86, p < 0.001, and Fconcentration × strain (6,90) = 68.14, p < 0.001], E) preference [Fconcentration (6,90) = 15.31, p < 0.001, Fstrain (1,15) = 86.72, p < 0.01, and Fconcentration × strain (6,90) = 5.415, p < 0.001], and F) total fluid intake [Fconcentration (6,90) = 18.27, p < 0.001, Fstrain (1,15) = 10.00, p = 0.0064, and Fconcentration × strain (6,90) = 1.218, p = 0.3044] were observed in DBA/2J and C57BL/6J mice. Data are presented as the mean ± SEM of 16 animals (8 male and 8 female). * indicates p < 0.05.
When compared to C57BL/6J mice, DBA/2J mice showed a lower pattern of oral nicotine consumption. DBA/2J mice significantly consumed less nicotine compared to C57BL/6J mice at most nicotine concentrations [Fconcentration (6,90) = 122.3, p < 0.001, Fstrain (1,15) = 92.86, p < 0.001, and Fconcentration × strain (6,90) = 68.14, p < 0.001; Figure 11D]. Consistently, nicotine preference score of D2 mice was significantly lower than B6J mice [Fconcentration (6,90) = 15.31, p < 0.001, Fstrain (1,15) = 86.72, p < 0.01, and Fconcentration × strain (6,90) = 5.415, p < 0.001; Figure 11E]. Total fluid intake was similar between these strains [Fconcentration (6,90) = 18.27, p < 0.001, Fstrain (1,15) = 10.00, p = 0.0064, and Fconcentration × strain (6,90) = 1.218, p = 0.3044; Figure 11F].
4. Discussion
In the present study, we found that adult C57BL/6J mice will drink nicotine-containing solutions in the two-bottle choice assay, and that nicotine preference is related to nicotine concentration. Our results also show that strain differences and sex influence this behavior. At a concentration of 60 μg/ml, nicotine intake in mice was stable for long periods and resulted in an increase in plasma nicotine and cotinine levels. Nicotine intake was also characterized by insensitivity to taste adulteration with quinine. Importantly, mice subjected to intermittent access (IA) in the two-bottle choice paradigm with 24-h access every other day developed escalation of nicotine intake and high preference. Finally, mice exhibited withdrawal signs and a nicotine deprivation effect after long-term exposure to the drug.
4.1. Characterization of nicotine intake and preference in C57BL/6J mice
To develop a comprehensive analysis of oral nicotine intake and preference in a mouse model, both female and male C57BL/6J mice were subjected to a 2BC test with a wide range of nicotine concentrations. Female mice showed a higher nicotine intake than males in a concentration-dependent manner, with significantly higher intake from 60 to 960 μg/ml and preference ratio at 60 μg/ml, which is consistent with similar concentrations (50 and 75 μg/ml) previously shown (Glatt et al., 2009; O’Rourke et al., 2016). This was not due to a greater total fluid consumption by females, but a larger intake of nicotine relative to water, as can be seen by the total fluid intake. Liking and wanting of nicotine may differ in males and females. Indeed, female rats acquire i.v. nicotine self-administration at lower doses and faster than males (Donny et al., 2000; Rezvani et al., 2008). Sex differences may result from complex reciprocal interactions among genes, gonadal sex, hormonal sex, effects of hormones on the brain [for further read please see review, (Pogun et al., 2017)]. Overall, a nicotine concentration of 60 μg/ml showed the most stable drinking behavior in each sex, with increasing concentrations leading to a decrease in preference, which is consistent with recent work (Peng et al., 2017). This concentration of nicotine in mice exposed to the drug for one month led to an increase in nicotine plasma levels to 15 ng/ml 3 hr into the active cycle and decreases to around 5 ng/ml 15 hr into the light cycle. Our results are consistent with levels shown in previous studies (Adriani et al., 2002; Tammimäki et al., 2008). While brain concentrations of nicotine were not measured, we found an increase in tyrosine hydroxylase levels in the nucleus accumbens of mice exposed to 60 μg/ml nicotine, indicating the activation of catecholamine synthesis and increased dopaminergic activity through oral nicotine consumption in an area implicated in reward and motivation through the mesocorticolimbic dopamine system (Carlezon et al., 2009; Oades and Halliday, 1987). Tyrosine hydroxylase is the rate-limiting enzyme of catecholamine synthesis (Daubner et al., 2011; Dunkley et al., 2004), and DA is the primary neurotransmitter that modulates nicotine reward (Grady et al., 1992).
Using the 2BC paradigm, we observed that mice engaged in nicotine drinking despite pairing with the aversive consequence of bitter-tasting quinine. When mice were primed with nicotine vs water exposure for a week before quinine addition, persistent nicotine drinking despite the additional aversive bitter taste of quinine was observed. In contrast, nicotine plus quinine combination resulted in a decrease on nicotine intake and preference relative to mice that drank nicotine-only. Thus, the same concentration of quinine was shown to be aversive initially but disregarded if mice were exposed a week prior to a nicotine-only solution.
Finally, since the use of inbred mouse strains has proven very useful in examining genetic contributions to drugs of abuse including nicotine, we evaluated nicotine oral consumption in the DBA/2J strain in addition to C57BL/6J mice. These two strains were selected for comparison since they are two of the most commonly used for examining behavioral effects of nicotine and the founders of the recombinant inbred BXD mouse panel. Similar to C57BL/6J mice, nicotine oral consumption was increased in a concentration- and sex-dependent manner in the DBA/2J strain. However, nicotine intake and preference were much lower, with DBA/2J mice failing to show preference to nicotine in comparison to C57BL/6J mice. The difference in nicotine preference between the two strains is similar to that reported in the conditioned place preference test after systemic administration of nicotine (Jackson et al., 2009). Our results are consistent with a previous study where DBA/2J mice showed a lower preference for nicotine than the C57BL/6J mice in the 2BC paradigm at a concentration of 75 μg/ml (Glatt et al., 2009). Similarly, using mouse strains from different providers, DBA/2 strain showed a lower nicotine intake in comparison to the C57BL/6 (Meliska et al., 1995; Robinson et al., 1996). Importantly, the strain differences in nicotine intake was not caused by orosensory factors (Glatt et al., 2009).
4.2. Intermittent access of nicotine and withdrawal symptoms in oral nicotine consumption
The ability to produce escalating patterns of consumption is deemed an indicator for dependence by the American Psychiatric Association (American Psychiatric Association., 2013), and previous studies suggest that intermittency to drugs of abuse increases the amount of drug consumed (Cohen et al., 2012; Dawson et al., 2013; O’Rourke et al., 2016). Cohen et al. (2012) reported that animals given intermittent periods of abstinence with extended access to nicotine self-administration resulted in an escalation of nicotine intake (Cohen et al., 2012). Our results confirmed that intermittent (e.g. 24 h abstinence) access to nicotine in a 2BC model escalates both nicotine intake and preference. The escalation pattern observed is also consistent with previous alcohol intermittent access studies (Dawson et al., 2013). This study is the first to look at the effect of intermittency on oral nicotine consumption. Following chronic intake of nicotine, we observed an increase in somatic signs and anxiety-related behavior 6 and 24 hr after nicotine removal. Intermittent access showed significantly more severe withdrawal signs in comparison to continuous access. The observed somatic signs of withdrawal in our results are consistent with a previous study that used a 2BC oral nicotine (35 μg/ml) with sub-chronic exposure in adult C57BL6J mice (Locklear et al., 2012). This suggests that the 2BC paradigm is capable of inducing nicotine dependence as seen in previously established withdrawal models.
4.3. Characterization of Key nAChRs involved in nicotine intake
We evaluated if the nAChRs subtypes currently known to play an important role in nicotine reward and reinforcement are also involved in nicotine intake in the 2BC paradigm. Both β2 and α6 KO mice showed a significant decrease in nicotine intake and preference. β2 and α6 subunits have been shown to play a crucial role in nicotine reward and reinforcement in the CPP test and intravenous self-administration in rodents (Exley et al., 2011; Jackson et al., 2009; Maskos et al., 2005; Picciotto et al., 1998; Pons et al., 2008; Walters et al., 2006). We further probed the involvement of α4β2* subtypes by testing the effects of the FDA approved smoking cessation medication varenicline (Chantix®) and α4β2* partial agonist in our nicotine 2BC assay (Jorenby et al., 2006; Tonstad et al., 2006). As expected, systemic administration of 0.3 mg/kg dose of varenicline significantly decreased both oral nicotine intake and preference in male and female mice. These results are consistent with varenicline blocking nicotine intake and preference in other rodent models of reward and reinforcement (Bagdas et al., 2018; Biala et al., 2010; George et al., 2011; Rollema et al., 2007).
Interestingly, we observed an increase in nicotine consumption at higher concentrations in α5 KO mice. These results agree with previous studies that showed an enhancement in α5 KO mice of nicotine reward and intake in both i.v. self-administration and CPP (Fowler et al., 2013, 2011; Jackson et al., 2010). The results with α7 KO mice were surprising. While an increase in nicotine intake and preference was seen in male α7 KO mice, the opposite was observed in female KO animals. We have previously reported an enhanced nicotine preference in the CPP test in male α7 KO mice (Harenza et al., 2014). Similarly, pharmacological blockade of α7 nAChRs increased motivation of rats to intravenously self-administer nicotine in rats (Brunzell and McIntosh, 2012). Interestingly, Levin et al., (2009) reported no change in nicotine oral intake in a mixed male and female α7 KO mice early in the 2BC test but saw a gradual decrease in intake after prolong exposure (Levin et al., 2009).
5. Conclusion
In summary, the present findings show that nicotine intake and preference in our 2BC paradigm were sex-, strain- and concentration-dependent without a significant effect on total fluid intake and body weight. Intermittency of nicotine solution led to a significant escalation of nicotine intake and preference, while absence of nicotine resulted in somatic and affective withdrawal signs. Our results show also that β2, α5 and α6 nAChR subunits play a key role in nicotine consumption and showed varenicline to decrease both oral nicotine intake and preference. Our data shows the utility of using an oral nicotine self-administration paradigm in mice to study maladaptive nicotine-taking behaviors and the underlying genetic and neuronal mechanisms.
Supplementary Material
Supplementary Figure 1: Body weight gain over the course of nicotine concentration study
Adult C57BL/6J male and female mice were continuously received either water or nicotine (10 – 960 μg/ml) in two bottle choice experiment in Figure 1. Average body weight of each concentration was presented as the mean ± SEM of 10 animals.
Supplementary Figure 2: Intermittent access of nicotine exposure escalated the withdrawal signs of nicotine
Adult C57BL/6J male and female mice were continuously received either water or nicotine (60 μg/ml) in two bottle choice experiment for 6 days. Mice then divided into two groups; one underwent intermittent access (IA) and the other had continuous access (CA) to nicotine versus water for one week. At the end of study, all animals were given water only. In addition, a group of only water exposed mice was included as control. After 24 hour of water exposure, somatic signs of nicotine withdrawal was seen in both CA and IA access groups compared to control mice. However, IA group showed significantly higher somatic signs than CA group. Data are presented as the mean ± SEM of 18 animals (n=18; 9 male and 9 female). * indicates p < 0.05.
Oral nicotine consumption is sex-, strain- and concentration-dependent
β2, α5 and α6 nAChR subunits play a key role in nicotine consumption
Intermittency of nicotine solution leads to an escalation of nicotine consumption
Absence of nicotine results in somatic and affective withdrawal signs
Acknowledgments
This research was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration, P30 DA033934 and DA 005274 and DA032246 to MID. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
The authors also would like to thank the following people for their invaluable voluntarily support in our studies: Wisam Elia and Elnaz Rahimpour.
Footnotes
Declaration of Interests
None of the other authors declared a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Macr S, Pacifici R, Laviola G, 2002. Restricted daily access to water and voluntary nicotine oral consumption in mice: Methodological issues and individual differences. Behav. Brain Res 134, 21–30. doi: 10.1016/S0166-4328(01)00448-X [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association., 2013. Diagnostic and statistical manual of mental disorders : DSM-5. American Psychiatric Association. [Google Scholar]
- Aschhoff S, Schroff KC, Wildenauer DB, Richter E, 2000. Nicotine consumption of several mouse strains using a two bottle choice paradigm. J. Exp. Anim. Sci 40, 171–177. doi: 10.1016/S0939-8600(00)80009-7 [DOI] [Google Scholar]
- Bagdas D, Alkhlaif Y, Jackson A, Carroll FI, Ditre JW, Damaj MI, 2018. New insights on the effects of varenicline on nicotine reward, withdrawal and hyperalgesia in mice. Neuropharmacology 138, 72–79. doi: 10.1016/j.neuropharm.2018.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, 2009. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol 49, 57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Staniak N, Budzynska B, 2010. Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn. Schmiedebergs. Arch. Pharmacol 381, 361–370. doi: 10.1007/s00210-010-0498-5 [DOI] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM, 2012. Alpha7 Nicotinic Acetylcholine Receptors Modulate Motivation to Self-Administer Nicotine: Implications for Smoking and Schizophrenia. Neuropsychopharmacology 37, 1134–1143. doi: 10.1038/npp.2011.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Thomas MJ, Thomas MJ, 2009. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56 Suppl 1, 122–32. doi: 10.1016/j.neuropharm.2008.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han Z-Y, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux J-P, 2002. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J. Neurosci 22, 1208–1217. doi:22/4/1208 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, George O, 2013. Animal models of nicotine exposure: Relevance to second-hand smoking, electronic cigarette use, and compulsive smoking. Front. Psychiatry 4, 1–21. doi: 10.3389/fpsyt.2013.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O, 2012. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology 37, 2153–2160. doi: 10.1038/npp.2012.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadmarz M, Vogel WH, 2003. Individual self-administration of nicotine by rats. Pharmacol. Biochem. Behav 76, 425–432. doi: 10.1016/j.pbb.2003.08.014 [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR, 2003. Characterization of Spontaneous and Precipitated Nicotine Withdrawal in the Mouse. J. Pharmacol. Exp. Ther 307, 526–534. doi: 10.1124/jpet.103.054908 [DOI] [PubMed] [Google Scholar]
- Daubner SC, Le T, Wang S, 2011. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys 508, 1–12. doi: 10.1016/j.abb.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Miles MF, Damaj MI, 2013. The β2 nicotinic acetylcholine receptor subunit differentially influences ethanol behavioral effects in the mouse. Alcohol 47, 85–94. doi: 10.1016/j.alcohol.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S, 2000. Nicotine selfadministration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 151:392–405. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, Von Nagy-Felsobuki EI, Dickson PW, 2004. Tyrosine hydroxylase phosphorylation: Regulation and consequences. J. Neurochem 91, 1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x [DOI] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux J-P, Maskos U, Cragg SJ, Faure P, 2011. Distinct contributions of nicotinic acetylcholine receptor subunit 4 and subunit 6 to the reinforcing effects of nicotine. Proc. Natl. Acad. Sci 108, 7577–7582. doi: 10.1073/pnas.1103000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ, 2011. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601. doi: 10.1038/nature09797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Tuesta L, Kenny PJ, 2013. Role of α5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology (Berl). 229, 503–513. doi: 10.1007/s00213-013-3235-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF, 2011. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl). 213, 715–722. doi: 10.1007/s00213-010-2024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt AR, Denton K, Boughter JD, 2009. Variation in nicotine consumption in inbred mice is not linked to orosensory ability. Chem. Senses 34, 27–35. doi: 10.1093/chemse/bjn049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, Collins AC, 1992. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J. Neurochem 59, 848–56. [DOI] [PubMed] [Google Scholar]
- Harenza JL, Muldoon PP, De Biasi M, Damaj MI, Miles MF, 2014. Genetic variation within the Chrna7 gene modulates nicotine reward-like phenotypes in mice. Genes, Brain Behav. 13, 213–225. doi: 10.1111/gbb.12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, Damaj MI, 2010. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther 334, 137–46. doi: 10.1124/jpet.110.165738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Mcintosh JM, Brunzell DH, Sanjakdar SS, Damaj MI, 2009. The Role of a6-Containing Nicotinic Acetylcholine Receptors in Nicotine Reward and Withdrawal. J. Pharmacol … 331, 547–554. doi: 10.1124/jpet.109.155457.nAChRs [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Walters CL, Miles MF, Martin BR, Damaj MI, 2009. Characterization of pharmacological and behavioral differences to nicotine in C57Bl/6 and DBA/2 mice. Neuropharmacology 57, 347–355. doi: 10.1016/j.neuropharm.2009.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti N. a, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, 2006. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296, 56–63. doi: 10.1001/jama.296.1.56 [DOI] [PubMed] [Google Scholar]
- Juarez B, Han M-H, 2016. Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure. Neuropsychopharmacology 41, 2424–2446. doi: 10.1038/npp.2016.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM, 2004. Sex differences in voluntary oral nicotine consumption by adolescent mice: A dose-response experiment. Pharmacol. Biochem. Behav 78, 13–25. doi: 10.1016/j.pbb.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, Avery J, Nicholson J, Rose JE, 2009. Nicotinic α7- or β2-containing receptor knockout: Effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav. Brain Res 196, 207–213. doi: 10.1016/j.bbr.2008.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear LL, McDonald CG, Smith RF, Fryxell KJ, 2012. Adult mice voluntarily progress to nicotine dependence in an oral self-selection assay. Neuropharmacology 63, 582–592. doi: 10.1016/j.neuropharm.2012.04.037 [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux J-P, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloëz-Tayarani I, Bemelmans A-P, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux J-P, 2005. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–7. doi: 10.1038/nature03694 [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Bartke A, McGlacken G, Jensen RA, 1995. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol. Biochem. Behav 50, 619–626. doi: 10.1016/0091-3057(94)00354-8 [DOI] [PubMed] [Google Scholar]
- Morgan D, Sizemore GM, 2011. Animal models of addiction: fat and sugar. Curr. Pharm. Des 17, 1168–72. [DOI] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Li MD, Pogun S, 2013. Nine generations of selection for high and low nicotine intake in outbred Sprague-Dawley rats. Behav. Genet 43, 436–444. doi: 10.1007/s10519-013-9605-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Ugur M, Pogun S, 2015. Nicotine withdrawal in selectively bred high and low nicotine preferring rat lines. Pharmacol. Biochem. Behav 131, 91–97. doi: 10.1016/j.pbb.2015.02.009 [DOI] [PubMed] [Google Scholar]
- O’Rourke KY, Touchette JC, Hartell EC, Bade EJ, Lee AM, 2016. Voluntary co-consumption of alcohol and nicotine: Effects of abstinence, intermittency, and withdrawal in mice. Neuropharmacology 109, 236–246. doi: 10.1016/j.neuropharm.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades RD, Halliday GM, 1987. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 434, 117–65. [DOI] [PubMed] [Google Scholar]
- Peng C, Engle SE, Yan Y, Weera MM, Berry JN, Arvin MC, Zhao G, McIntosh JM, Chester JA, Drenan RM, 2017. Altered nicotine reward-associated behavior following α4 nAChR subunit deletion in ventral midbrain. PLoS One 12, 1–22. doi: 10.1371/journal.pone.0182142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP, 1998. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177. doi: 10.1038/34413 [DOI] [PubMed] [Google Scholar]
- Pogun S, Yararbas G, Nesil T, Kanit L, 2017. Sex differences in nicotine preference. J. Neurosci. Res 95, 148–162. doi: 10.1002/jnr.23858 [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W, 2008. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J. Neurosci 28, 12318–27. doi: 10.1523/JNEUROSCI.3918-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L, Ling PM, 2013. Alternative tobacco product use and smoking cessation: a national study. Am. J. Public Health 103, 923–930. doi: 10.2105/AJPH.2012.301070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renda A, Nashmi R, 2014. Chronic nicotine pretreatment is sufficient to upregulate alpha4* nicotinic receptors and increase oral nicotine self-administration in mice. BMC Neurosci. 15, 89. doi: 10.1186/1471-2202-15-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renda A, Penty N, Komal P, Nashmi R, 2016. Vulnerability to nicotine self-administration in adolescent mice correlates with age-specific expression of alpha4* nicotinic receptors. Neuropharmacology 108, 49–59. doi: 10.1016/j.neuropharm.2016.04.019 [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, Horton K, Johnson M, Levin ED 2008. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 154:885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SF, Marks MJ, Collins AC, 1996. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl). 124, 332–9. [DOI] [PubMed] [Google Scholar]
- Rodgers DA, 1966. Factors Underlying Differences in Alcohol Preference Among Inbred Strains of Mice. Psychosom. Med 28, 498–513. [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, Williams KE, 2007. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52, 985–994. doi: 10.1016/j.neuropharm.2006.10.016 [DOI] [PubMed] [Google Scholar]
- Romito L, Saxton MK, 2014. Impact of promotions on awareness, trial, and likelihood of trial of new dissolvable tobacco. Am. J. Heal. Promot 28, 251–258. doi: 10.4278/ajhp.120926-QUAN-469 [DOI] [PubMed] [Google Scholar]
- Rowell PP, Hurst HE, Marlowe C, Bennett BD, 1983. Oral administration of nicotine: its uptake and distribution after chronic administration to mice. J. Pharmacol. Methods 9, 249–61. [DOI] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M, 2003. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol. Pharmacol 63, 1059–66. [DOI] [PubMed] [Google Scholar]
- Tammimäki A, Chistyakov V, Patkina N, Skippari J, Ahtee L, Zvartau E, Männistö PT, 2008. Effect of forced chronic oral nicotine exposure on intravenous self-administration and rewarding properties of acute nicotine. Eur. J. Pharmacol 591, 164–170. doi: 10.1016/j.ejphar.2008.06.081 [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR, 2006. Effect of maintenance therapy with varenicline on smoking cessation: A randomized controlled trial. J. Am. Med. Assoc 296, 64–71. doi: 10.1001/jama.296.1.64 [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, 2012. Corticosteroid-Dependent Plasticity Mediates Compulsive Alcohol Drinking in Rats. J. Neurosci 32, 7563–7571. doi: 10.1523/jneurosci.0069-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI, 2006. The β2 but not α7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl). 184, 339–344. doi: 10.1007/s00213-005-0295-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Body weight gain over the course of nicotine concentration study
Adult C57BL/6J male and female mice were continuously received either water or nicotine (10 – 960 μg/ml) in two bottle choice experiment in Figure 1. Average body weight of each concentration was presented as the mean ± SEM of 10 animals.
Supplementary Figure 2: Intermittent access of nicotine exposure escalated the withdrawal signs of nicotine
Adult C57BL/6J male and female mice were continuously received either water or nicotine (60 μg/ml) in two bottle choice experiment for 6 days. Mice then divided into two groups; one underwent intermittent access (IA) and the other had continuous access (CA) to nicotine versus water for one week. At the end of study, all animals were given water only. In addition, a group of only water exposed mice was included as control. After 24 hour of water exposure, somatic signs of nicotine withdrawal was seen in both CA and IA access groups compared to control mice. However, IA group showed significantly higher somatic signs than CA group. Data are presented as the mean ± SEM of 18 animals (n=18; 9 male and 9 female). * indicates p < 0.05.