Abstract
Vascular endothelial cells (ECs) form a semiselective barrier for macromolecules and cell elements regulated by dynamic interactions between cytoskeletal elements and cell adhesion complexes. ECs also participate in many other vital processes including innate immune reactions, vascular repair, secretion, and metabolism of bioactive molecules. Moreover, vascular ECs represent a unique cell type exposed to continuous, time-dependent mechanical forces: different patterns of shear stress imposed by blood flow in macrovasculature and by rolling blood cells in the microvasculature; circumferential cyclic stretch experienced by the arterial vascular bed caused by heart propulsions; mechanical stretch of lung microvascular endothelium at different magnitudes due to spontaneous respiration or mechanical ventilation in critically ill patients. Accumulating evidence suggests that vascular ECs contain mechanosensory complexes, which rapidly react to changes in mechanical loading, process the signal, and develop context-specific adaptive responses to rebalance the cell homeostatic state. The significance of the interactions between specific mechanical forces in the EC microenvironment together with circulating bioactive molecules in the progression and resolution of vascular pathologies including vascular injury, atherosclerosis, pulmonary edema, and acute respiratory distress syndrome has been only recently recognized. This review will summarize the current understanding of EC mechanosensory mechanisms, modulation of EC responses to humoral factors by surrounding mechanical forces (particularly the cyclic stretch), and discuss recent findings of magnitude-specific regulation of EC functions by transcriptional, posttranscriptional and epigenetic mechanisms using -omics approaches. We also discuss ongoing challenges and future opportunities in developing new therapies targeting dysregulated mechanosensing mechanisms to treat vascular diseases.
Introduction
Mechanical forces associated with cyclic stretch play important roles in the control of vascular functions and pulmonary circulation homeostasis (10, 28, 29, 353). In particular, lung microvascular endothelium is exposed to continuous, time-varying, or cyclic stretch from respiratory cycles during autonomous breathing or mechanical ventilation. While cyclic stretch due to autonomous breathing triggers intracellular signaling pathways to maintain principal endothelial functions such as control of lumen diameter and preservation of monolayer integrity, endothelial cells can sense increased mechanical strain associated with mechanical ventilation and promote inflammation, adhesion, and contractility leading to vascular dysfunction (32, 35). The identification of mechanosensing mechanisms by which endothelial cells convert biomechanical cues to biological responses has been an active research field (83, 95, 127, 140, 349). Regulation of endothelial cells by hemodynamic shear stress has been extensively studied and reviewed by others (67, 72, 83, 84, 127, 140). However, commonalities or differences in molecular mechanisms shared between shear stress and cyclic stretch remains relatively unexplored. The main objectives of this review are (i) to summarize our current knowledge of mechanoreceptors and mechanosensors conducting mechanotransmission and mechanotransduction in vascular endothelium, (ii) to document stretch-induced signal transduction pathways, (iii) to delineate the effect of stretch amplitude in eliciting distinct endothelial responses, and (iv) to discuss ongoing challenges and future opportunities in developing new therapies targeting dysregulated mechanosensing mechanisms to treat vascular diseases. Endothelial responses to physiological stretch have evolved as part of vascular remodeling and homeostasis. Pathological perturbations of normal endothelial stretch-sensing pathways contribute to the etiology of many respiratory disorders. Insights into the stretch-sensing mechanisms at the molecular, cellular, and tissue levels may lead to development of new mechanointerventions that target signaling transduction molecules in vascular endothelium.
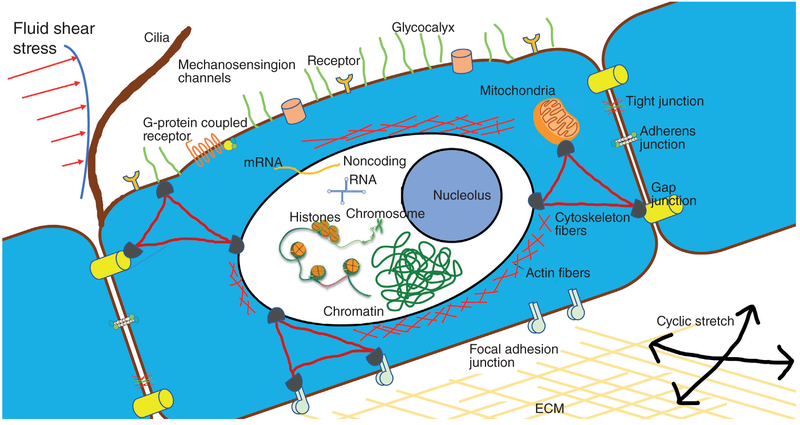
Search for Cellular Mechanical Sensors
Sensing gradients in potential energy—whether magnetic, gravitational, chemical, or mechanical, is a fundamental feature of living cells, and specialized mechanoreceptors have developed in various living systems in response to mechanical forces. Rapidly adapting receptors are a perfect example of specialized mechanoreceptors in the lungs. However, because the majority of cells in the body experience mechanical forces, they also share some basic mechanisms of mechanosensation.
Because cell membranes, cell attachment sites, and cytoskeletal networks directly experience hemodynamic forces, they are considered as primary mechanosensors (83). In addition, cell monolayers such as endothelial cells adhere to neighboring cells and to the extracellular matrix via transmembrane receptors of cadherin (cell-to-cell) and integrin (cell-to-substrate) families. The tensegrity model proposed by Ingber (165) considers sensing of mechanical forces by single cells or cell clusters as a network process. According to this view, cytoskeletal components (microfilaments, microtubules, and intermediate filaments) form an interconnected network, where the microfilaments and intermediate filaments bear tension and the microtubules bear compression. Furthermore, mechanical perturbation of cell monolayers immediately triggers intracellular signaling responses, which become activated by various cell structures acting as mechanosensors. Such putative mechanosensors include mechnosensing ion channels, cell-substrate and cell-cell junctional complexes, and cytoskeleton-associated complexes. Therefore, force transmission by cytoskeletal networks and cell adhesive complexes explains the ability of single cells or cell monolayers to execute complex processes such as spreading, migration, and process mechanical signals applied locally into whole cell responses; cells not only need to sense externally applied forces, but internal mechanical forces as well to drive complex motions (144, 164).
Mechanosensing ion channels
Mechanosensing ion channels represent another example of such mechanosensors (125). Studies suggested that mechanosensitive channels could be tethered to cytoskeletal and external anchors via intracellular and extracellular linkers. Membrane tension may also directly play a role in the ion channel state (178, 220). Disruption of cytoskeletal elements (microfilaments or microtubules), or cell-matrix adhesions inhibits or eliminates the mechanical force-induced increase of intracellular calcium in endothelial cells (5). Thus, mechanical forces transduced to the ion channel via cell adhesions and the cytoskeletal network can affect ion conductivity and activate intracellular signaling in an amplitude-dependent fashion. These observations also indicate that the function of mechanosensitive ion channels is predetermined by the integrity of the cytoskeleton. Two different mechanosensitive channels have been described in vascular cells: shear activated potassium channels and stretch-activated ion channels (108, 258, 326). Mechanically activated potassium and calcium channels, such as inwardly rectifying potassium channels (Kir), transient receptor potential cation channel V4 (TRPV4), and Piezo1 (Fam38a), have been implicated in endothelial responses to blood flow (4, 106, 108, 109, 154, 198, 221, 284). Shear-sensitive channels have been recently reviewed by Gerhold and Schwartz (122). Stretch-activated ionic channels are cation-specific and have an electric activity mainly detectable at the time of their opening. The activation of these channels leads to calcium (Ca2+) influx followed by membrane depolarization. Among the other tissues, stretch-activated ion channel activities have been also described in lung endothelial cells (113, 170). Both of the orientating and elongating responses become inhibited by Gd3+, a potent blocker for the stretch-activated channel (270). We will further discuss the identity of stretch-activated ion channels and their molecular actions related to endothelial function later in the review.
Integrins
Integrins are heterodimers containing two distinct chains, α and β subunits, encoded by 18 and 8 different genes, respectively (160). Both subunits are transmembrane proteins containing small cytoplasmic domains, which interact with focal adhesion proteins talin, paxilin, and others (53, 160). The integrins thus serve to link across the plasma membrane two networks: the extracellular ECM and the intracellular actin filamentous system via multiprotein focal adhesion complexes. Integrins transmit mechanical stretch from the underlying capillary wall to endothelial cells in microvasculatures.
Engagement of integrins in mechanotransduction has been demonstrated experimentally by application of external force to cells using twisting of cell-attached magnetic beads coated with integrin ligand RGD. Such mechanically challenged cells responded to applied deformation by a “stiffening response” (409). Stretch-induced activation of integrins leading to engagement of focal adhesions may be judged by their association with the adaptor protein Shc. This interaction triggers binding of focal adhesion (FA)-associated structural and signaling proteins to Shc, which converts mechanical signal to biochemical cascades (177). Because integrins not only physically connect the cytoskeleton to the extracellular matrix but also function as signaling receptors, they are recognized as the important transmitters of physical forces into chemical signals (189, 228, 269).
Integrin expression itself is controlled by mechanical forces. Uniaxial cyclic stretch upregulates the expression of integrin β3 in endothelial cells, which further enhances cell adhesiveness and resistance of EC monolayer to excessive vessel distension (372). Specific integrins mediate the cyclic stretch-induced endothelial cell reorientation response. Blocking of integrin α2 and β1 subunits by specific antibodies abolished both morphological changes and activation of p38 MAPK in cyclic stretch-exposed endothelial cells. In contrast, blocking α5 and β4 integrin subunits was without effect on cyclic stretch-induced EC reorientation or p38 MAPK activation (151). These findings indicate that specific integrins play a crucial role in the morphological changes and stress signaling in EC exposed to cyclic stretch. In addition, integrins and the associated RhoA small GTPase play a central role in mechanosensing mechanisms by which shear forces are converted to biochemical signaling in vascular endothelium. Molecular insights related to shear-sensing mechanisms mediated by integrins have been comprehensively reviewed by Shyy et al. (349) and Ross et al. (325)
Focal adhesion complexes
FAs are multimolecular complexes consisting of more than 50 different proteins (53). FA form a bidirectional link between the actin cytoskeleton and the cell-extracellular matrix interface and provide additional tethering forces that help maintain endothelial cell barrier integrity.
Mechanical strain or centripetal pulling of the cell by micropipette aspiration causes redistribution of focal adhesions, elongation and increases in size (319, 344). Agonist-induced contraction of endothelial cells attached to the substrate leads to development of tension forces applied to the actomyosin anchoring sites (focal adhesions) [see (27) for review]. Interestingly, increases in focal adhesion size are proportional to the force applied by the cell (19). This process triggers activation of small GTPases, which leads to activation of a Rho kinase-dependent increase in actomyosin contraction (319) and signaling in the focal adhesions (269, 428). Micromanipulation techniques also showed that focal adhesions may function as independent mechanosensors, which via regulation of their size, can also equalize the local balance between the force generated by the cell and extracellular matrix rigidity (27, 121).
What structural focal adhesion proteins mediate stretch-induced signaling and morphological changes? A study by Ngu et al. (274) explored how the cyclic stretch-induced endothelial cell orientation response is regulated by focal adhesion-associated proteins paxillin, focal adhesion kinase (FAK), and zyxin. Inhibition of zyxin expression or overexpression of a mutant lacking a zyxin/alpha-actinin binding site suppresses stretch-induced orientation responses observed in control cells. However, partial inhibition of paxillin and FAK does not significantly affect the degree of cell orientation. Zyxin depletion and the mutation lacking zyxin/alpha-actinin binding also attenuated EC migration and wound closure. These results suggest that zyxin and its interaction with alpha-actinin are important in the regulation of endothelial cell adhesive strength, motility and orientation response to mechanical stretching. Moreover, focal adhesions that contact extracellular matrix and connect to intracellular cytoskeleton also serve as important mechanotransducers to confer and transmit the cell tension in vascular cells exposed to hemodynamic forces (83, 159). Interestingly, distinct FAK phosphorylation and focal adhesion redistribution stimulated by shear stress (15 dyn/cm2) and (18%) cyclic stretch (CS) in endothelial cells have been reported (344).
Emerging evidence suggests that mechanosensitivity of FAC may play a role in agonist-induced signal transduction. Exposure of vascular endothelium to high magnitude cyclic stretch (18% CS) stimulates assembly of FAC signalosome containing paxillin, Erk-1,2, MAP kinase and RhoA-specific guanine nucleotide exchange factor GEF-H1. This complex controls local activation of RhoA signaling by CS itself (119), but also augments agonist-induced permeability response by EC exposed to 18% CS (35, 119). Interestingly, disruption of FAC-associated mechanosensor vinculin attenuated thrombin-induced RhoA activation and EC permeability (41). Other reports demonstrate that agonist-induced cytoskeletal and barrier responses by vascular EC are proportional to a degree of underlying substrate stiffness (44, 241). The data suggest that such “stiffness effect” is due to different extent of FAC mechanical loading in EC attached to high or low compliance substrates and results in different levels of agonist-induced RhoA activation. Collectively, these findings suggest that agonist induced development of actomyosin tension and resulting FAC mechanical loading form a positive feedback loop of RhoA stimulation.
Cell junction molecules
Vascular endothelial specific cadherin, VE-cadherin, is a transmembrane domain that forms homotypic interactions (adherens junctions) between adjacent endothelial cells and links them with cell cytoskeleton via the catenin family of proteins. In contrast to smooth muscle cells, which can respond to stretch in the absence of neighboring cell contact, endothelial cells require cell-cell contact and vascular endothelial cadherin engagement to transduce stretch into proliferative signals (230). A number of studies have suggested the key role of VE-cadherin in activating mechanosensitive signaling pathways in vascular endothelium. A study by Tzima et al. showed that VE-cadherin may serve as an adaptor in endothelial orientation and gene expression response to flow, whereas platelet endothelial cell adhesion molecule-1 (PECAM-1) served as a force transducer leading to activation of signaling by VEGF receptor-2 (VEGFR2) and PI3 kinase (389). This and other studies found PECAM-1 as a mechanosensor located within endothelial cell-cell adhesions. Interestingly, in vitro application of pulling forces directly on endothelial cell surface expressed PECAM-1 using magnetic beads led to Erk activation, which was also observed in flow-exposed EC monolayers. These findings suggest that PECAM-1 may sense mechanical forces generated by both flow-induced shear stress and mechanical stretch (116). Conway et al. recently showed that in addition to interacting with VEGFRs, VE-cadherin can regulate its binding to polarity protein LGN (also known as G-protein-signaling modulator) to confer endothelial responses to shear stress (78).
Gap junctions and their interactions with adherens junctions in mechanosensing
Growing as monolayers in vivo, endothelial cells may sense and transmit mechanical force-induced signals by propagating Ca2 + signaling via gap junctions. Molecular analysis identified Connexin-32 as gap junction proteins specifically involved in mechanically induced propagation of Ca2 + waves in airway epithelial cell monolayers (49). The connexins mediating stretch-induced signal propagation in endothelium remains to be identified. Force application to adherens junction protein N-cadherin in live cells caused activation of stretch-activated calcium-permeable channels and influx of extracellular Ca2 +. Force application to junctional N-cadherin also causes an increase of actin cytoskeleton at intercellular contacts suggesting that cadherins may play a role as intercellular mechanotransducers (196). Large numbers of cells (> 105) form synchronous cell-cell contacts which can transduce Ca2 + signals across the monolayer and require rapid formation of adherens junction-like structures and their colocalization with gap junctional complexes. Thus, dynamic relationships between newly formed adherens junction-like structures and gap junctional complexes [described in fibroblasts (195)] appear to be important for establishing cell-cell communication and may also play an important role in mechanosensing and mechanotransduction by endothelial cells.
Cytoskeleton
The cytoskeletal network plays an essential role in endothelial mechanosensing and mechanotransduction. A “tensegrity” model (165) considers the cytoskeletal components (microfilaments, microtubule, and intermediate filaments) as an interconnected network, where the microfilaments and intermediate filaments bear tension and the microtubules bear compression. This model explains the ability of the cell to execute complex processes such as spreading, migration, and how forces applied locally on the cell result in responses throughout the whole cell.
Intracellular stress transmission through subcellular structural components affects activation of localized mechanosensing sites such as focal adhesions in adherent cells. A study by Deguchi et al. (88) investigated force balance within the basal actomyosin stress fibers and focal adhesion complexes in smooth muscle and endothelial cells. Removal of mechanical restrictions for stress fibers (such as dislodging of cell ends from the substrate) resulted in a decrease in the length of the remaining actin fibers. In addition, a release of the preexisting tension in a single stress fiber was transmitted to another stress fiber physically linked to the former, but not transmitted to the other fibers physically independent of the former. These results suggest that the prestress is balanced in the stress fiber networks that generate basal tension. Consistent with the tensegrity model, disruption of the microtubule network by low doses of either nocodazole or paclitaxel abolishes the cyclic stretch-induced redistribution of RhoA and Rac GTPases critical for actin remodeling and many other functions (305). Similarly, actin disassembly or attenuation of actomyosin assembly and stress fiber formation achieved by either stabilization or depolymerization of F-actin, or Rho kinase inhibition using Y-27632 or activation of protein kinase A (PKA) abolishes cyclic stretch-induced cell reorientation (32, 346), activation of stretch-induced intracellular signaling (6, 32) and cyclic stretch-mediated transcriptional responses (283, 289). We refer the readers to these reviews (29, 46, 141, 176) for the details of the molecular regulation of Rho GTPases and their central roles in cellular mechanotransduction. The tensegrity model can also be used to explain nuclear shape, as disruption of the cell adhesion leads to changes in nuclear ellipticity (80, 192). In addition, tensegrity-based mechanosesnsing mechanisms have been shown to play an important role in gene expression (66), cellular proliferation/differentiation (280), organ development (262), and tumor growth (294). The role of tensegrity in cellular architecture and mechanosensing mechanisms has been comprehensively reviewed by Ingber et al. (163-166).
Cytoskeleton-associated molecular mechanosensors
Even in demembranized cell preparations, that is, in the absence of cell membrane channels and cytosolic regulators, mechanotransduction events, and cyclic stretch induced binding of paxillin, focal adhesion kinase, and p130Cas to the cytoskeleton still occur (331). Transient mechanical stretch also altered enzymatic activity and the phosphorylation status of certain cytoskeleton-associated proteins and enabled these molecules to interact with cytoplasmic proteins added back to the culture system. Thus, the cytoskeleton itself can transduce forces independent of any membrane or membrane-spanning mechanosensors.
A study by Han et al. (143) demonstrated that actin filament-associated protein (AFAP) localized on the actin filaments can directly active c-Src through binding to its SH3 and SH2 domains. Mutations at these specific binding sites on AFAP block mechanical stretch-induced Src activation. These observations led this group to propose a novel mechanism for mechanosenation, by which mechanical stretch-induced cytoskeletal deformation increases the competitive binding between AFAP and c-Src by displacement of SH3 and/or SH2 domains, which in turn induces the configuration change of c-Src and leads to activation of Src and its downstream signaling cascade.
Using a specially developed conformation-specific antibody to p130Cas domain CasSD, Sawada et al. (332) demonstrated physical extension of a specific domain within p130Cas protein in the peripheral regions of intact spreading cells, where higher traction forces are developed and where phosphorylated Cas was detected. These results indicate that the in vitro extension and phosphorylation of CasSD are relevant to physiological force transduction and suggest that Cas acts as a primary force sensor, transducing force into mechanical extension and thereby priming phosphorylation and activation of downstream signaling (332).
Cells that are stimulated by cyclic stretch or shear stress in vitro undergo bimodal cytoskeletal responses that include rapid reinforcement and gradual reorientation of actin stress fibers. Application of cyclic stretch causes thickening of actin stress fibers, which reflects a cellular adaptation to mechanical stress. It also results in robust mobilization of zyxin and zyxin-dependent mobilization of vasodilator-stimulated phosphoprotein from focal adhesions to actin filaments (431). Stretch-induced cytoskeletal reinforcement was abrogated in zyxin-null cells suggesting zyxin as another mechanosensitive protein mediating cyclic stretch-induced mechanosensation and cytoskeletal remodeling in response to mechanical cues.
Mitochondria
Mitochondria may also sense mechanical forces and serve as stress amplifiers; however, their effect may be secondary to sensation through the cytoskeleton. Mitochondria anchor to the cytoskeleton and could function as mechanotransducers by releasing ROS during cytoskeletal strain (6). In mitochondrial deficient HUVEC (ρ0 EC), strain-induced ROS was attenuated by 80%. These ROS were found to be responsible for NF-kB and VCAM-1 mRNA expression. Treatment with cytochalasin D also abrogated strain-induced ROS production, indicating a requirement for the actin cytoskeleton in mitochondrial-dependent ROS (7). Furthermore, VCAM-1 expression was also abrogated in ρ0 EC subjected to cyclic strain. Thus, mitochondria could be key signaling organelles in the setting of cyclic strain. Furthermore, endothelial cells lacking a functional electron transport chain lose the ability to increase oxidant signaling in response to cyclic stretch and fail to activate NF-kB, yet they retain the ability to respond to other stimuli such as lipopolysaccharide (7).
Shear stress is known to stimulate an intracellular free calcium concentration response in ECs. Ca2 + is a key second messenger for signaling that leads to vasodilation and EC survival. EC mitochondria, through Ca2 + uptake/release, regulate the temporal profile of shear-induced ER Ca2 + release (333). EC exposure to steady laminar shear stress results in peroxynitrite (ONOO(−)) formation intramitochondrially with inactivation of the electron transport chain. When exposed to shear stress increased NO and mitochondrial O(2)(*−) production lead to enhanced mitochondrial ONOO(−) formation and suppression of respiration (181). Mechanotransduction of shear forces by the mitochondria is also key for upregulation of antioxidant genes. Shear-induced transient increase in NO-dependent mitochondrial H2O2 mediates HO-1 induction. Under shear, EC mitochondria-derived H2O2 diffuses to the cytosol, where it initiates oxidative signaling leading to hemeoxygenase-1 upregulation and maintenance of the atheroprotective EC status (145).
Nuclear response to mechanotransduction
Increasing evidence suggests that the nucleus is not simply a passive storage house of genetic information, but actively participates in sensing changes in mechanical load. It has long been known that endothelial nuclei undergo shape changes in response to chemical agonists (240), as when they are detached from surfaces (397). Moreover, shear stress causes the height of endothelial cells (dominated by the nucleus) to change: sheared ECs are lower compared to nonsheared ECs (20). Additionally, forces applied to integrins can lead to rapid force transmission to the nucleus in ECs (242). Nuclei have actin stress fibers running down them, which accounts for the nuclear morphology (147, 192, 232, 233, 397). Furthermore, change in nuclear morphology due to mechanical forces or substrate stiffness also results in a change in gene expression (124, 136, 210, 232, 287, 366, 373). Thus, forces are transmitted to the cells through the actin cytoskeleton or microtubules to the nuclear envelope (21, 329), which can lead to gene expression changes. The structure of the nuclear envelope, which mediates force transmission, is complex and beyond this review, but for a good one see (133).
The dominant intermediate filament, which composes the nuclear envelope, is Lamin A. Mutations of lamin cause a subset of diseases called laminopathies, which suggests a crucial role for lamins as load-bearing structure necessary for structural integrity and normal nuclear mechanics. The two best studied are Hutchinson-Gilford Progeria syndrome (abnormal Lamin A), which leads to premature atherosclerosis, and Emery-Dreifuss muscular dystrophy (50). Others include dilated cardiomyopathy and limb-girdle muscular dystrophy (264). However, whether all of these diseases are due to mechanical transduction are unclear.
ECs can also directly sense the direction and strength of blood flow through the hydrodynamic drag applied to their nuclei, independent of cytoskeletal factors. Hydrodynamic drag mechanically displaces the nucleus downstream, inducing planar polarization of ECs (385). In a microbubble study, acute application of a large hydrodynamic force to ECs resulted in an immediate downstream displacement of nuclei and was sufficient to induce persistent polarization. Matrix stiffness dependent expression of nuclear lamin (373) suggests active feedback and matching between substrate mechanical properties and nuclear properties, perhaps as a way to preserve DNA integrity. Functionally, this may also be related to how migrating cells must adapt to their surrounding matrix. As expected, neutrophils have multi-lobed nuclei on histology, which correlates with their need to get into tight spaces, whereas endothelial layers may increase nuclear stiffness to prevent durotaxis of immune cells through endothelial layers (361). External squeezing nucleus through micron-spaced channels causes DNA damage repair enzymes to leak out (92). Certainly, stiffness influences the genotypic profiles of stem cells (105), suggesting that lamin may participate in stiffness sensing based epigenetic changes to gene expression. For examples, in Lamin A knockdowns, chromatin disorganization and histone acetylation are increased, resulting in increased transcriptional activity. Knockdown of Lamin A reduces shear-dependent nuclear translocation of glucocorticoid receptor. Furthermore, shear stress increased HDAC and HAT in control, but not in Lamin A knowndown, suggesting a role for nuclear lamina in regulating chromatin state (273). Modeling studies also suggest that nuclear morphology is crucial for stem cell fate determination, since diffusivity of transcription factors is thought to be a function of local volumetric strain. Therefore, an increase in nuclear spreading might indicate higher flux of transcription factors into the nucleus (272).
However, the precise mechanisms by which nuclear pores and nuclear structure regulate gene expression are unknown and deserve further investigation.
Cilia and glycocalyx
In addition to aforementioned mechanosensors, glycocalyx and primary cilia have emerged as key participants in endothelial mechanosensing mechanisms. Although the putative roles of glycocalyx and primary cilia in stretch-sensing mechanisms remain to be elucidated, a large cohort of studies have demonstrated the importance of glycocalyx and primary cilia in regulating endothelial responses to hemodynamic forces. Here we briefly discussed the molecular insights by which glycocalyx and primary cilia participate in the shear-sensing responses in vascular endothelium, information that could guide future investigations to elucidate the possible role of glycocalyx and primary cilia in endothelial stretch-sensing biology.
Primary cilia (nonmotile, as opposed to motile cilia) act as flow sensors in development, guiding left-right axis specificity during embryogenesis (282); absence of primary cilia results in abnormal valvulogenesis, as primary cilia deflect in response to blood flow, and their degree of deflection is correlated with the amount of intracellular calcium (132). Disruption of inner ear cilia also affects otolith malformation. Furthermore, defective primary cilia also predispose zebrafish embryos to intracranial hemorrhage (183). Primary cilia deflection signal through PKD1 and PKD2, which are mechanosensitive calcium channels (374). PKD1 and PKD2 are localized to the primary cilium and cells deficient in these do not generate calcium upon stimulation by flow (271). Mutations in PKD1 and PKD2 were first identified in humans as the genetic basis for autosomal dominant polycystic kidney disease, a relatively common pathology characterized by the development of multiple renal cysts typically presenting during the third or fourth decade of life. These people are also at risk for pancreatic, hepatic malformations, and intracranial hemorrhage (90), and have early onset hypertension (63). Many patients with PKD mutations exhibit endothelial dysfunction and increased carotid intima-media thickness, both indicators of atherosclerosis, before signs of renal dysfunction or hypertension (103). However, whether primary cilia themselves (and not just the basal bodies) contribute to flow sensing, is more controversial, especially since ECs in culture have been shown to disassemble their primary cilia after a few hours of laminar shear stress (94). Primary cilia were observed in human aorta by electron microscopy (58). In one experiment, primary cilia were not detected in endothelial cells in culture (414) but could be induced with changes in shear stress. Primary cilia, therefore, may have roles in wound repair and signal more under lower shear stress. This would be in accordance with zebrafish studies, given that flow rates in the embryos are much less than in adults. In fact, in vivo primary cilia are present on EC in areas of low or disturbed flow and absent in areas of high flow (168). Furthermore, it was shown in corneal endothelial cells (CECs) that cilia reassembly occurs in response to mechanical injury and precedes basal body polarization and cellular elongation in mature CECs neighboring the wound, which suggests that cilia may be upstream of planar polarization pathway. In contrast, knockdown morphants or mutants of IFT88 (a critical cilia transport protein) demonstrate dysfunctional cilia and show disorganized cellular patterning, mislocalization of junctional markers, and accumulation of cytoplasmic acetylated tubulin (47). Together these studies suggest the intriguing hypothesis that ECs display primary cilia under disturbed or low shear states perhaps as a mechanism to amplify flow sensing, but disassemble them once a critical shear stress has been reached. Further proof that primary cilia localization corresponds with flow states is that cilia were more common on the lesser curvature (more ventral and caudal side) of the aortic arch and less common on the greater curvature (more dorsal and rostral side), where blood flow is less disturbed (94). Another previous study found that cilia are enriched in curved and branched regions of the aorta (391), also where blood flow is more disturbed. These results are consistent with the in vitro data discussed above. Notably, these are the same areas susceptible to atherosclerosis in humans and mouse models (15, 16, 61, 370). Functionally, removing endothelial cilia increased atherosclerosis, increased inflammatory gene expression and decreased eNOS activity in Apoe −/− mice fed a high-fat, high-cholesterol diet, indicating that cilia protect against atherosclerosis.
The glycocalyx (GCX) is a mechanosensor for shear forces on endothelial cells. The major components of the GCX are glycoproteins bearing sialic acids (SA), and proteoglycans (PGs) with associated glycosaminoglycan (GAG) side chains. GAGs are composed of different components such as heparan sulfate (HS), chondroitin sulfate (CS), and hyaluronic acid (HA). These GAGs have been reported to extend from 0.04 to 11 μm above the cell surface (102, 400). HS is the most abundant GAG on the EC surface, and accounts for 50% to 90% of the total GAG pool (285). The membrane-bound glypicans, matrix-associated perlecans, and the transmembrane syndecans are among the three major protein core families of HS proteoglycans (HSPGs) (324). The glycocalyx is critical for normal vascular development (148). For two excellent reviews of function, structure, and components of glycocalyx, see Tarbell et al. (379) or Weinbaum et al. (412). The primary evidence that supports a role for the GCX in mechanotransduction comes from experiments involving use of enzymes to selectively degrade specific components of the GCX, followed by a reassessment of function. In bovine aortic endothelial cells (BAEC), selective degradation of HS with heparinase III could impair shear-induced NO production (114) and inhibits shear-induced increase in hydraulic conductivity of BAEC monolayers. In ex vivo preparations, hyaluronidase treatment reduced flow-induced NO production in isolated canine femoral arteries (257). A similar result for the role of HA (but not HS or SA) was found in porcine superficial femoral arteries (207) and in rat mesenteric arteries (430). Shear stress can alter the distribution of ESG components on the cell surface and their rates of synthesis (123, 200, 201, 429, 434). Furthermore, when heparinase is used to disrupt the GCX, the remodeling of the actin cytoskeleton in response to shear stress was disrupted (381), as was the tendency for BAECs to align with the applied shear direction (261). Transduction from the GCX to the underlying cytoskeleton is an area of active investigation. The syndecans have attachment sites to the cytoskeleton via their cytoplasmic tails and are thought to associate with linker molecules such as ezrin, tubulin, syntenin, syndesmos, dynamin, and α-actinin to distribute force throughout the cell (60, 115, 315, 441). The cytoplasmic domain of syndecans is also linked with G-protein receptors, including those that form a cytoplasmic bond with eNOS (86, 303). This makes the syndecans an ideal candidate both to sense shear stress and transmit these forces into the cell proper. A recent study (101) tested the hypothesis that the transmembrane syndecan-1 (sdc-1) core protein that is linked to the cytoskeleton mediates EC remodeling in response to shear stress. Enzymatic removal of HS that resides on syndecan-1 blocked eNOS activation and EC remodeling. Loss of syndecan-1 induces a proinflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow (402). Syndecan-4 is also critical for mechanotransduction (15). In hypercholesterolemic mice, deletion of syndecan-4 (S4) drastically increased atherosclerotic plaque burden with the appearance of plaque in normally resistant locations and reduces endothelial alignment with direction of flow. There is cross talk between flow state and glycocalyx formation and its location on the cell surface is actively modulated by flow (16) and stiffness (427); following the removal of shear stress, the glycocalyx redistributes and gradually appears in the apical region of the cell membrane. Endothelial glycocalyx is critical in maintaining capillary fluidity and maintaining perfusion homogeneity (248). Various disease states such as sepsis, diabetes, heart failure, and sickle cell disease all present with reduced glycocalyx suggesting a connection between mechanical sensing, nitric oxide production, and microvascular perfusion (59, 248).
In conclusion, mechanical force can be transmitted along the cytoskeleton, and stretch-induced protein conformational changes may control opening of stretch-activated ion channels, modulate interaction between cytoskeletal associated proteins, cell adhesion mechanosensors, and enzymes related to signal transduction, or may even alter enzymatic activities and thus convert physical forces into biochemical reactions.
Stretch-Activated Signaling Pathways in Endothelium
Stretch-activated ion channels
The discovery of the involvement of stretch-activated ion channels in Ca2+ influx and physiologic responses in endothelial cells (359) suggests a possibility of amplitude-dependent regulation of cellular functions by mechanical strain by stretch activated channels. Furthermore, stretch-induced elevation of intracellular Ca2+ is critical for activation of other signaling cascades. Earlier studies Naruse et al. (268, 269) linked stretch-induced endothelial cell orientation with Ca2+ elevations and demonstrated that Ca2+ elevation was necessary for activation of protein tyrosine phosphorylation. Cell treatment with a potent blocker for stretch-activated channels gadolinium Gd3+, or removal of extracellular Ca2+ blocked the tyrosine phosphorylation of Src, focal adhesion-associated signaling proteins paxillin, FAK, and p130CAS.
It became apparent that various stretch-induced responses in vascular endothelium may be mediated by distinct types of stretch-activated channels. Stretch-activated channels may mediate signaling by integrins. Exposure of capillary endothelial cells to cyclic stretch in vitro also activates mechanosen-sitive TRPV4 (transient receptor potential vanilloid 4) ion channels that, in turn, stimulates phosphatidylinositol 3-kinase-dependent activation and engagement of additional beta1 integrin receptors in cell adhesion to the deforming matrices (382). Integrin engagement and enhancement of focal adhesion complexes further promotes cytoskeletal remodeling and cell reorientation. Inhibition of integrin activation by blocking antibodies or knock down of TRPV4 channels abolishes cyclic stretch-induced capillary cell reorientation (382). These findings indicate that force-induced activation of mechanically gated TRPV4 ion channels on the cell surface triggers “integrin-to-integrin” signaling mechanism guiding capillary cell reorientation.
Voltage-gated channels can be also involved in stretch-induced signaling events in endothelial cells, likely as a secondary mechanism triggered by stretch-activated channel opening and membrane depolarization. High magnitudes of cyclic stretch (20% elongation as compared to physiologic levels of 5% elongation or static cells) markedly increased expression of proinflammatory surface molecule P-selectin in jugular vein and tracheal venular endothelial cells (260). Interestingly, such P-selectin upregulation caused by pathologic stretch was augmented by pharmacological and molecular inhibition of Ca(v)3.1 T-type Ca2+ channel. These results suggest that P-selectin expression in systemic venular endothelial cells contributes to a proinflammatory phenotype after mechanical stretch and can be selectively modulated by voltage-gated calcium channel inhibition.
Tight interactions between stretch-activated ion channels and the underlying cytoskeleton appear to be important for physiologic regulation of Ca2+ transients induced by physiological and pathological mechanical strain. TRPV has been identified as a stretch-activated channel, and its involvement in stretch-induced and magnitude-dependent Ca2+-elevations in pulmonary microvascular endothelial cells was confirmed using TRPV inhibitor ruthenium red (169). An important distinction of stretch-activated channels from other Ca2+ influx pathways such as receptor- and storage-operated Ca2+ entry described in lung microvascular endothelial cells is tight regulation by the actin cytoskeleton. Disruption of actin polymerization with cytochalasin D inhibits the stretch-induced [Ca2+]i elevation. In contrast, increases in [Ca2+]i induced by thapsigargin or thrombin are not affected by cytochalasin D. Stimulation of actin polymerization by barrier protective agonist sphingosine-1-phosphate or low dose jasplakinolide enhanced the stretch-induced [Ca2+]i elevation. These results led to the model that actin stress fibers are required for efficient force transmission to open stretch-activated Ca2+ channels. Magnitude-dependent effects of cyclic stretch on endothelial Ca2+ transients suggest that abnormal Ca2+ homeostasis due to excessive mechanical stretch during mechanical ventilation may play a role in ALI/ARDS progression.
Stretch-induced Ca2+ transients may cooperate with other signaling cascades in activation of endothelial functional responses to cyclic stretch. As an example, activation of NO production by cyclic stretch occurs in bi-phasic manner. A potent stretch-activated channel blocker Gd3+ or depletion of external Ca2+ exclusively inhibited the first peak of eNOS and Akt activation but had little effect on the second peak. In turn, the second peak was completely inhibited by PI3K inhibitors wortmannin and LY294002 (376). These results suggest that upregulation of eNOS in response to cyclic stretch was mediated by two distinct pathways: Ca2+ increases via the stretch-activated (SA) channel in an early phase (partially Akt/PKB), and PI3K-Akt/PKB pathways in a late phase.
A study by Amma et al. (9) demonstrated another important link between Ca2+ elevations triggered by stretch-activated ion channels and activation of reactive oxygen species (ROS) production and pathologic ROS signaling (described below). Cyclic stretch-induced activation of ROS lead to generation of lipid terminal peroxidation product 4-hydroxy-2-nonenal (HNE), which modified NFkappaB inhibitory subunit IkappaB and IkappaB kinase (IKK). HNE-mediated modification and phosphorylation of IkappaB and NKK, as well as translocation of pro-inflammatory transcription factor NF-kappaB to the nucleus resulting in COX-2 production were inhibited by extracellular Ca2+ removal or Gd3+ application, as well as by the antioxidants. The stretch-induced Ca2+ increase was inhibited by extracellular Ca2+ removal, or Gd3+ application (9). These studies suggest a scheme in which pathologic cyclic stretch causes enhanced stretch-activated (SA) channel activation leading to pronounced intracellular Ca2+ increase. Such increases lead to increased ROS and generation of lipid peroxidation products, which are potent activators of proinflammatory NFkB signaling.
In addition to magnitude-dependent activation of stretch-sensitive ion channels in healthy endothelium, mechanical stress may be sensed differently by vascular cells at normal or pathologic state. For example, stretch activation of Na+ and Ca2+ channels was greater in VSMCs isolated from spontaneously hypertensive rats (SHR) compared to those from normotensive Wistar Kyoto rats (281). These findings illustrate two major paradigms of mechanotransduction that may be applied in pathologic states: (i) amplitude-dependent effects of mechanical stress on vascular cells and (ii) different responses of healthy and diseased vascular cells to same levels of mechanical stress.
Small GTPases
Rho GTPases are members of the Ras superfamily of monomeric 20 to 30 kDa GTP-binding proteins. The most extensively characterized members are Rho, Rac, and Cdc42, which have distinct effects on actin cytoskeleton, cell adhesions, and cell motility (194, 237, 239, 337, 384). Among 30 potential Rho GTPase effectors identified to date (46), mDia and Rho-associated kinase (Rho-kinase) appear to be required for Rho-induced assembly of stress fibers, MLC phosphorylation and actomyosin-driven cell contraction (120, 189, 393, 401). Rac initiates membrane ruffling, lamellopodia extension, and formation of new adhesions cell-substrate and cell-cell adhesions. Rac effectors, WAVE, WASP, interact with Arp-2/3 complex, and stimulate actin polymerization required for lamellipodia formation and cell motility (51, 65, 265). Consistent with opposing effects of Rac and Rho on endothelial cytoskeletal remodeling and cell adhesion dynamics, activation of Rho is triggered by a number of barrier disruptive agonists such as thrombin, VEGF, TNFα, TGFβ, and microtubule depolymerising agent nocodazole (34, 42, 43, 76, 298, 369, 392), whereas Rac becomes activated in endothelial cells exposed to barrier protective mediators such as sphingosine 1-phosphate, hepatocyte growth factor, oxidized phosphatidylcholine (OxPAPC), and high molecular weight hyaloronan (31, 33, 117, 351, 352). Thus, differential effects of Rac and Rho on endothelial cytoskeleton and permeability suggest that the balance between Rho- and Rac-mediated signaling may be a critical component of pulmonary endothelial barrier regulation in pulmonary endothelium exposed to mechanical and chemical stimulation.
The paradigm of Rac-Rho antagonism in endothelial barrier regulation was also proved to be true in the mechanotransduction arena. A widely accepted notion about beneficial (antiatherogenic and barrier-protective) effects of laminar flow on endothelial monolayers was further confirmed by critical involvement of Rac signaling in the maintenance of endothelial barrier integrity under flow. Laminar flow induces Rac-dependent peripheral translocation of actin polymerization activator cortactin, enhances cortical actin cytoskeleton and peripheral focal adhesions (30, 344, 388, 415), while inhibition of Rac or Rac effector PAK1 prevents shear stress-induced endothelial orientation response (30). Of note, both Rho and Rac1 regulate directionality of flow-induced endothelial cell movement (415). Shear stress-induced activation of Rac signaling, enhancement of peripheral actin cytoskeleton and focal adhesion remodeling have been linked with barrier protective endothelial responses reflected by flow-induced increases in transendothelial electrical resistance (93, 335).
Studies of endothelial cells exposed to cyclic stretch indicate that, in contrast to monophasic mechanism of flow-mediated Rac activation, regulation of Rac, and Rho activities by cyclic stretch is amplitude dependent. Cyclic stretch in the 10% to 20% range activates Rho in pulmonary smooth muscle cells (356), epithelium (383) and endothelium (32, 35, 344). It was also noted, that Rho activation by high cyclic stretch amplitudes is accompanied by reduction of basal Rac activity and inhibition of lamellipodia formation (35, 188). Besides activating Rho, in the nonpulmonary cells, 10% to 15% CS also caused activation of Rac (64, 288). In contrast to 10% to 20% stretch, 5% stretch did not cause marked Rho activation or reduction of Rac activity in endothelial cells (35, 188). Thus, stretch-induced small GTPase signaling and cell responses may be differentially regulated by CS amplitudes and may also be cell type specific.
In comparison to agonist-induced activation of Rho or Rac GTPases, effect of cyclic stretch on these small GTPase activities are transient and of lower magnitude. However, a growing body of evidence suggests that preconditioning of pulmonary endothelial cells at cyclic stretch magnitudes relevant to pathologic or physiologic conditions results in dramatic differences in cell responses to barrier-protective or barrier-disruptive agonists. These differences appear to be due to promotion of barrier-disruptive Rho signaling in endothelial cells preconditioned at high cyclic stretch magnitudes and enhanced barrier-protective Rac signaling in endothelial cells preconditioned at low cyclic stretch magnitudes (32, 35, 39, 40). These differences may be explained in part by increased expression of Rho and other pro-contractile proteins described in EC exposed to high magnitude stretch (32, 40, 62).
It is important to note that stretch-induced activation of Rho may be vital for control of endothelial monolayer integrity in vivo, as it plays a key role in endothelial orientation response to cyclic stretch. Studies of bovine aortic endothelial cells exposed to monoaxial cyclic stretch show that, in contrast to the predominately perpendicular alignment of stress fibers to the stretch direction in untreated cells, the stress fibers in cells with Rho pathway inhibition became oriented parallel to the stretch direction (190). In cells with normal Rho activity, the extent of perpendicular orientation of stress fibers depended on the magnitude of stretch, and orientation response to 3% stretch was absent. Interestingly, activation of Rho signaling by expression of constitutively active RhoV14 mutant enhanced the stretch-induced stress fiber orientation response, which became evident even at 3% stretch. This augmentation of the stretch-induced perpendicular orientation by RhoV14 was blocked by Rho or Rho kinase inhibition (190). These elegant experiments clearly show that the Rho pathway plays a critical role in determining both the direction and extent of stretch-induced stress fiber orientation and endothelial monolayer alignment.
Reactive oxygen species
Pathological elevation of lung vascular pressure or overdistension of pulmonary microvascular and capillary beds associated with regional or generalized lung over-distension caused by mechanical ventilation at high tidal volumes are two major clinical scenarios. Such elevation of tissue mechanical strain increases production of reactive oxygen species (ROS) in endothelial cells (7, 246, 420, 421), vascular smooth muscle cells (135, 167, 275), and fibroblasts (9). In turn, increased ROS production in response to elevated stretch contributes to the onset of ventilation-induced lung injury (VILI) (142, 175, 411) and pulmonary hypertension (135). Superoxide appears to be the initial species generated in these cell types. Potential sources for increased superoxide production in response to mechanical stress, include the NADPH oxidase system (87, 135, 246, 249), mitochondrial production (6, 7, 162), and the xanthine oxidase system (1, 249).
Stretch-induced ROS production in endothelium upregulates expression of cell adhesion molecules and chemokines (70, 421). Several mechanisms of ROS production in EC have been described. Cyclic stretch stimulated ROS production via increased expression of ROS-generating enzymes: NADPH oxidase and NO synthase-3 (eNOS) (13, 14, 152). Kuebler and colleagues reported that circumferential stretch activates NO production in pulmonary EC via activation of PI3K, Akt, and eNOS-dependent signaling cascade (205). Utilization of pulmonary endothelial cells exposed to high magnitude cyclic stretch in vitro and animal models of high tidal volume mechanical ventilation revealed stretch-induced activation of endothelial xantine oxireductase (XOR) that occurred in a p38 and ERK1/2 MAP kinase-dependent fashion. These mechanisms may contribute to the development of VILI (1).
In conditions associated with pulmonary hypertension, high pulmonary vascular pressure results in activation of NFκB pathway, which is triggered by NADPH-dependent ROS production (219, 300). Along with activation of matrix metalloproteases (MMPs) activities, MAP kinase activation, and altered vasomotion also induced by elevated pressure, ROS-dependent NFkB activation leads to propagation of vascular remodeling, enhanced endothelial apoptosis, and endothelial dysfunction associated with pulmonary hypertension.
The other part of stretch-induced modulation of ROS signaling is feedback regulation of antioxidant systems. Although the mechanisms by which cyclic stretch regulates the antioxidant enzyme expression are not well understood, the transcription factor nuclear response factor-2 (Nrf2) appears to be involved in this process (289). The transcription factor Nrf2, via the antioxidant response element (ARE), alleviates pulmonary toxicant- and oxidant-induced oxidative stress by upregulating the expression of several antioxidant enzymes (73). As an additional mechanism, mechanical stretch induces expression of glutathione peroxidase (GPx-1) in endothelial cells, which leads to conversion of hydrogen peroxide to water and attenuation of ROS-dependent upregulation of stretch-induced inflammatory molecules CD40 and THP-1 (403). Cyclic stretch exposure also stimulates ARE-driven transcriptional responses and subsequent expression of antioxidant enzymes such as glutathione peroxidase22, glutamate-cysteine ligase, heme oxygenase-1, and glutamate cysteine ligase in stretched pulmonary EC (289). Cyclic stretch then transactivates epithelial growth factor receptor (EGFR), and the PI3K-Akt pathway acts as the downstream effector of EGFR and regulates cyclic stretch-induced ARE-activation in an oxidative stress-dependent manner. Thus, EGFR-activated signaling and actin remodeling act in concert to regulate the stretch-induced Nrf2-ARE transcriptional response and subsequent antioxidant enzyme expression. It is important to note that NADPH oxidase inhibitor, which inhibits the generation of ROS, N-acetyl cysteine, and other flavoproteins that regulate ROS production blocked cyclic stretch-induced EGFR phosphorylation, suggesting that ROS-mediated signaling regulates EGFR activation in response to cyclic stretch. These mechanisms again reflect tight relations between cyclic stretch and ROS in mechanochemical regulation of vascular function.
Tyrosine kinases
The role of integrins in mechanical sensing has been discussed earlier. Stimulation of vascular cells by mechanical stretch results in tyrosine phosphorylation of multiple cellular protein targets. At focal adhesions, where integrins interact with intracellular focal adhesion proteins, FAK and paxillin form a complex with c-Src tyrosine kinase and participate in integrin-mediated mechanotransduction as the Src substrate (56). In endothelial cells, platelet endothelial cell-adhesion molecule-1 (PECAM-1), which is a 130-kDa type I transmembrane glycoprotein, is also localized to focal adhesions and undergoes tyrosine phosphorylation upon mechanical stimulation of endothelial cells (116). Another Src family substrate p130Cas may act as a primary force sensor, transducing force into mechanical extension (332). Exposure to cyclic stretch triggers tyrosine phosphorylation at intracellular focal contacts throughout the cells. Tyrosine phosphorylation signals are predominantly localized to focal contacts (187). Identification of tyrosine phosphorylated proteins revealed FAK, PECAM-1, p130Cas andpaxillin as focal adhesion molecules phosphorylated in response to stretch. Tyrosine phosphorylation at focal contacts therefore appears to be central to the signal transduction pathways and changes in actin organization in endothelial cells that are induced by stretching (187).
Src is a tyrosine kinase associated with the membrane, which plays a role in the stretch-mediated signal transduction. Following activation by stretch, c-Src translocates to the focal contacts (334), where it interacts with an autophosphorylation site on FAK and creates an acceptor for the Src-homology-2 (SH2) domain of Grb2 and thus supports association of FAK with paxillin-Src complex. Pharmacological inhibition of Src abolishes stretch-induced cell orientation response (268). Stretch-induced activation of FAK may also activate RhoA; however, precise mechanism is not well understood. Although several candidate proteins associated with focal adhesions (including paxillin) may also be involved in mechanotransduction, the role for FAK in this context is best studied.
FAK is activated in stretched pulmonary vessels (378), and in cultured endothelial cells exposed to cyclic stretch (344). The recruitment of integrins to focal adhesion sites is mediated by their cytoplasmic domains, which bind proteins of the cytoskeleton.
In proposed mechanism of stretch induced signal transduction leading to cell remodeling (358), activation of stretch-activated ion channels leads to elevation of intracellular Ca2 + that stimulates Src activity leading to protein tyrosine phosphorylation, rearrangement of cytoskeletons and focal adhesions, and ultimately cell remodeling. Other mechanism of stretch-induced FAK tyrosine phosphorylation is via stretch-induced mitochondrial ROS signaling (6).
Studies of pulmonary endothelial cells isolated from lungs ventilated at low (LV) or high (HV) tidal volumes show that HV enhanced tyrosine phosphorylation of focal adhesion protein paxillin, increased focal adhesion formation, and increased surface expression of PECAM1 in isolated endothelial cells. These results show amplitude-dependent, stretch-induced regulation of tyrosine phosphorylation of cytoskeletal and cell contact proteins in the vascular cells, which may reflect enhancement of cell mechanical and adhesive properties to withstand increased mechanical load.
Growth factor receptors represent a family of receptor tyrosine kinases, which upon ligation of appropriate growth factor become activated and phosphorylate their specific downstream targets. Growth factor receptors appear also to be involved in mechanotransduction and may become trans-activated by cell-cell contact. Stretching of VSMCs induces a rapid phosphorylation of PDGF receptor α (PDGFRα) in a magnitude-dependent fashion (157). This stretch-induced PDGFRα phosphorylation is not affected by PDGF blocking antibody, and conditioned medium from the stretched cells does not cause PDGFRα phosphorylation in static VSMCs (157). Similarly, cyclic stretch also induced phosphorylation of PDGFRβ in a magnitude-dependent fashion, and neutralizing antibody against PDGF-BB did not block the PDGFRβ phosphorylation. These results suggest that cyclic stretch activates PDGFRα and PDGFRβ in a ligand-independent manner (345). These results also indicate that the stretch-induced PDGFR activation is not the result of the paracrine or autocrine release of its ligand PDGF. Similar to PDGFR, stretch also induces the phosphorylation of EGF receptor (EGFR) and its recruitment of adaptor proteins Shc and Grb2, which in turn lead to ERK1/2 activation (171). Mechanisms of such growth factor receptor transactivation by mechanical forces are not completely clear, but may involve formation of molecular scaffolds containing cell-cell or cell-substrate receptors linked to receptor tyrosine kinases via adapter proteins such as Shc, which is an adaptor protein containing a C-terminal SH2 domain. Tyrosine-phosphorylated Shc becomes associated with the cognate receptor tyrosine kinases through SH2 binding and mediates the integrin-induced signal transduction caused by mechanical strain. Thus, transactivation of receptor tyrosine kinases by mechanical strain may not only mediate stretch-induced mechanotransduction and immediate cell responses such as permeability, contraction, or secretion, but also control vascular remodeling, cell proliferation, and cell survival. These processes are critical for pulmonary vascular repair during recovery after ALI. Observed upregulation of the key tyrosine kinase receptors Flk-1, Tie-2, and Tie-1 in cyclic stretch-stimulated vascular EC (438) further increases the EC sensitivity to growth factors and therefore facilitates angiogenesis and tissue repair.
Cyclic stretch and MAP kinases
Mitogen-activated protein kinases (MAPK) are a family of Ser/Thr kinases that are activated through a cascade of dualspecificity MAPK kinases in response to distinct extracellular stimuli. Many activities stimulated by growth factors and other mitogens are mediated through so-called extracellular signal-regulated kinases (Erk) belonging to MAPK family. Parallel to the Erk pathway, two MAPK pathways, the p38 MAP kinase and c-Jun NH2-terminal (JNK) kinase pathways become activated in response to many cellular stress stimuli, including cyclic stretch. JNK is also called stress-activated protein kinase (SAPK).
Stretch-induced activation of Erk, p38, and JNK MAPK cascades is a common cellular response to mechanical strain or flow-induced shear stress and has been demonstrated in many cell types (139, 229). Several review articles summarize basic aspects of MAPK signaling and regulation by mechanical forces (116, 139, 216, 229) and propose the mechanism by which mechanical stress activates the FAK and its association with adaptor protein Grb2. This rapid and transient interaction then leads to the mechanical stress-induced Erk2 and JNK1 activation (223). A study by Shi et al. demonstrated that phosphorylation of Erk-1,2 caused by mechanical stretch is independent of Erk-1,2 canonical upstream activator MEK, suggesting a direct mechanism other than Ras-Raf-MEK-ERK cascade (343). This study also showed that endothelial cells exposed to continuous mechanical stimulation are capable of downregulating ERK phosphorylation in a cyclic stretch- and tyrosine phosphatase-dependent manner. However, frequent changes in stretch regimen constitutively activated this ability, suggesting a role of ERK activation status in endothelial cell adaptation to changing cyclic stretch magnitudes in vivo.
The complexity of signaling pathways activated by mechanical stress suggests potential involvement of multiple mechanosensors in MAPK activation. For example, stretch-induced activation of MAP kinase in myocytes requires tyrosine kinase, protein kinase C activities, and elevation of intracellular Ca2+ (425). On the other hand, stretch-induced SAPK activity in rat cardiac myocytes is not dependent on secreted angiotensin II, PKC, or Ca2+ (199).
Shear stress-induced Erk activation in endothelial cells depends on Gi-2 protein, Ras, and protein tyrosine kinase activities (180). As mentioned earlier, cholesterol-sensitive microdomains in the plasma membrane, such as caveolae-like domains, play a critical role in differential activation of ERK and JNK by shear stress (290) implicating caveolae role as mechanosensors. The VE-cadherin role in stretch-induced proliferative signals implies cell-cell junctions in MAPK mechanoregulation (230). Some effects of mechanical stress on MAPK activation are indirect and involve paracrine mechanisms. For example, mechanical stretch-induced Erk activation vascular smooth muscle cells is mediated via angiotensin and endothelin systems (155).
MAPK activation by mechanical stress associated with extensive lung mechanical ventilation plays a critical role in the pathogenesis of pulmonary edema associated with VILI. The following examples support this point. Inhibition of stretch-induced production of inflammatory cytokine IL-8 by bronchial epithelial cells is achieved by pharmacological blockade of p38 MAPK (286). Pharmacologic inhibition of JNK, p38 MAPK, or apoptosis signal related kinase (ASK), a member of the MAPK kinase-kinase family, attenuates high tidal volume ventilation-induced cytokine production, neutrophil migration into the lung, and vascular leak (222). Activation of p38 and Erk MAPKs in pulmonary endothelial cells by mechanical stress increases xanthine oxydoreductase activity and exacerbates oxidative stress involved in VILI-associated pulmonary edema (1). The role of mechanical stress in vascular dysfunction associated with VILI will be discussed in more detail in the following sections.
In summary, mechanical stretch activates multiple signaling pathways to affect different molecules in the MAPK family, leading to the activation of various transcription factors, for example, c-myc, c-fos, and c-jun to modulate VSMC gene expression. Available data indicate that the particular cell type as well as amplitude and frequency of applied mechanical stimulation dictate which particular member MAPK family will be activated and whether this activation will be sustained or transient. These parameters eventually determine the specificity of cellular response to a particular mechanical stimulus.
PI3K/Akt signaling
Phosphoinositide 3-kinase (PI3K) and its downstream target kinase Akt participate in cellular signaling in response to growth factors directed to cell survival, proliferation, and migration responses. In addition to activation by agnoists, PI3K is also involved in stretch-induced signal transduction. Cyclic stretching of vascular endothelial or smooth muscle cells causes a rapid PI3K/Akt activation, which can be inhibited by pre-treatment with N-acetylcysteine, a scavenger of reactive oxygen species (ROS) (440). In endothelial cells, stretch-activated PI3K-Akt signaling leads to upregulation of endothelial specific NO synthase (eNOS) (376), which is important for regulation of local hemodynamics. Interestingly, cyclic stretch-induced activation of PI3K and Akt in mesangial cells does not require signaling by integrins or cytoskeleton, but is triggered by transactivation of EGF receptor (EGFR) and Src-mediated signaling in caveolae. The downstream effect of such PI3K/Akt activation in vascular smooth muscle cells is increased type 1 collagen production (436). Of note, stretch-induced PI3K/Akt signaling in endothelium is independent of signaling activated by stretch-induced intracellular Ca2 + elevation, but cooperates with Ca2 + -dependent mechanisms in biphasic activation of eNOS upregulation caused by cyclic stretch.
Apoptosis pathway
Apoptosis, or programmed cell death, contributes in vascular dysfunction and hyperpermeability caused by VILI-related inflammatory mediators such as TNFα (296, 297). However, pathologic mechanical forces may directly stimulate apoptosis in vascular cells. Cyclic stretch with an area change of 25% increased apoptosis in vascular smooth muscle cells, which was accompanied by sustained activation of c-Jun NH(2)-terminal kinases (JNK) and the mitogen-activated protein kinase p38. In contrast, cyclic stretch with an area change of 7% had no such effect (364). Stretch-induced apoptosis is possibly regulated by GADD153, a growth arrest and DNA damage-inducible gene (71). Interestingly, involvement of beta1-integrin signaling pathway in the mechanical stretch-induced apoptosis in smooth muscle cells (413) suggests a role of mechanosensing via cell-cell contacts in the amplitude-dependent control of vascular cell apoptosis by cyclic stretch. In endothelial cells, application of moderate, physiologic levels of cyclic stretch (6%-10% at 1Hz) inhibited apoptosis in vascular endothelial cells. This antiapoptotic effect was dependent on the activation of phosphatidylinositol 3-kinase and associated with the activation of Akt and the phosphorylation of BAD. In turn, a higher, potentially pathologic level of cyclic stretch (20% at 1 Hz) stimulated EC apoptosis. Increasing the magnitude (from 10% to 15.6%) or frequency (from 60 to 100 cpm) of strain caused earlier phosphorylation of AKT-mediated phosphorylation of GSK-3beta. AKT, glycogen synthase kinase (GSK)-3beta, BAD, and cleaved caspase-3 were also activated by cyclic stretch. In contrast, inhibition of AKT not only prevented AKT, GSK-3beta, and BAD phosphorylation but also inhibited the cyclic stretch-induced increase in cell number as well as the cyclic stretch-induced protection against apoptosis (277). Apoptosis in human pulmonary microvascular endothelial cells exposed to high-amplitude mechanical stretch was reduced by treatment with mTOR inhibitor rapamycin. Such reduction is due to rapamycin-induced actin cytoskeletal changes and increased cellular F-actin content leading to increased endothelial cell mechanical stability (311).
Apoptotic signals detected in endothelial cells subjected to high amplitude cyclic stretch may arise from stretch-induced modulation of ceramide and its metabolites, suggesting that ceramide signaling may affect the maintenance of a viable vascular endothelium during disease, vein grafting, and tissue engineering applications. Mass spectrometry analysis of endothelial cells exposed to 3%, 6%, 10%, or 12% cyclic strain at 1 Hz for up to 72 shows that ceramide levels are elevated in response to cyclic mechanical strain, especially at or above 10% strain intensity (161). These data show that ceramide regulation is fine-tuned to 6% strain, which represents physiological magnitude. Following cessation of strain, ceramide levels quickly return to basal levels, suggesting that strain-related ceramide increases require continued application of strain.
Mechanical strain and angiogenic signals
Mechanical strain applied via the endothelial cell substrate upregulates a spectrum of secreted bioactive molecules. This issue is particularly important in the context of lung angiogenesis and vascular remodeling, as each of these processes occurs concurrently with localized increases in strain and marked changes in molecules secreted by adjacent cells. Excessive mechanical strain stimulates both endothelial cell secretion of latent matrix metalloprotease-2 and multicellular networks in a time- and strain-dependent manner (347). These results indicate that elevated local stress may directly affect new capillary growth (angiogenesis) toward growing tumors, points of increased tissue stress, such as fibrotic sites in the lung and at capillary wall defect sites.
In vitro, cyclic strain significantly increases EC network formation on Matrigel, which reflects an index of angiogenesis. In addition, cyclic stretch triggers expression of angiogenic factors Angiopoietin 1 (Ang1), Tie1, and Tie2, involved in cyclic strain-induced endothelial network formation (263). Exposure of human endothelial cells (ECs) to cyclic stretch (10%) causes temporal upregulation of Notch receptors (1 and 4) at the mRNA and protein level. Knockdown of Notch 1 and 4, or inhibition of Notch mediated gene expression causes a significant decrease in cyclic strain-induced endothelial network formation, and Tie1 and Tie2 mRNA expression. Notch1 was recently shown to contribute to the mechanosensing responses in adult vascular endothelium exposed to hemodynamics (238). Prolonged stretching of microvascular endothelial cells also significantly increases levels of proangiogenic factors MMP-2 and VEGF through respective JNK- and ERK-dependent pathways (255). Other report shows that lung stretch associated with mechanical ventilation of developing lungs caused approximately 50% reduction in endothelial surface area, more than fivefold increase in apoptosis, 50% decrease in lung VEGF-R2 protein, fourfold increase of pSmad2 protein, and >50% increase in lung elastin, which was distributed throughout alveolar walls rather than at septal tips (259). These results show that prolonged mechanical ventilation of developing lungs, even without associated hyperoxia, can inhibit alveolar septation and angiogenesis and increase apoptosis, findings that could reflect stretch-induced changes in VEGF and TGFβ signaling.
In summary, current studies indicate multiple regulatory mechanisms elicited by cyclic stretch on endothelial angiogenic responses at different levels ranging from assembly of endothelial networks, production of angiogenic factors and suppression of apoptotic signals, and morphogenic signals including activation of matrix metalloproteinases (MMPs) and pleiotropic growth factors.
Transcription factors
Transcription factors are a unique group of proteins that control the rate of gene expression through their ability to bind DNA and other proteins, by which the efficiency of RNA polymerase-dependent transcription is enhanced or blocked (365). Activation of specific transcription factors is one of the major molecular mechanisms in determining gene expression output, which allows complex and precise patterns of transcriptomes in hundreds of phenotypically distinct cell types sharing a single genome. It is notable that transcriptional regulation is highly dynamic in responding to biochemical and biomechanical stimuli, controlling diverse biological processes such as cell cycle, intracellular metabolic balance, cell differentiation, and cell proliferation (395). Numerous diseases arise from dysregulation of transcription factors, such as overexpression of oncogene MYC that induces cellular proliferation in acute myeloid leukemia, breast cancer, pancreatic cancer, retinoblastoma, and small cell lung cancer (82). Here, we outline the key transcription factors in the context of endothelial functions related to cyclic stretch. It is important to note that cyclic stretches are heterogeneous with respect to frequencies, magnitudes, and causes under in vivo conditions and various devices have been developed to recapitulate the in vivo circumferential stains in vitro. Therefore, caution needs to be taken when interpreting the pathophysiological consequences of coding and non-coding genes responding to cyclic stretch. For example, arterial endothelial cells are exposed to continuous circumferential stretch as the result of blood pressure-induced mechanical strain while pulmonary microvascular endothelial cells are subjected to cyclic stretch owing to transluminal pressure changes during alveolar inflation. Meanwhile, a wide range of in vitro stretch conditions (e.g., uniaxial/biaxial/equiaxial, magnitudes, waveforms, frequencies, durations, etc.) have been applied to cells and tissues aimed at mimicking in vivo condition (318). Table 1 lists a cohort of mechanosensitive transcription factors and their functional roles in regulating endothelial homeostasis and dysfunction. The molecular actions of these transcription factors are described in detail later.
Table 1.
Mehanosensitive Transcription Factors and Their Functions in Regulating Vascular Endothelial Homeostasis
| Mechanosensitive transcription factors in vascular endothelium |
Biomechanical stimuli | Functions in endothelium |
|---|---|---|
| Kruppel-like factor (KLF) 2 and 4 | Cyclic stretch (158); shear stress (89,214,292) |
Vasodilatation; monolayer integrity; anti-inflammation; antithrombosis; anticoagulation |
| Hypoxia-inducible factor 1-alpha (HIF-1α) | Cyclic stretch (224,255); shear stress (112,418) |
Metabolism; angiogenesis; growth; inflammation |
| Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) | Cyclic stretch (97,197,421); shear stress (191,212) |
Inflammation; adhesion; chemokine and cytokine production; monolayer permeability; apoptosis |
| Focal adhesion/cytoskeleton-associated protein Zyxin | Cyclic stretch (371,416) shear stress (431) |
Inflammation; chemokine and cytokine production; vasoconstriction |
| Activator protein-1 (AP-1) | Cyclic stretch (404,421); shear stress (212) |
Inflammation; adhesion; oxidative stress |
| Yes-associated protein (YAP)/transcriptional coactivator with a PDZ-binding domain (TAZ) | Shear stress (407,408); Extracellular matrix (ECM) stiffness (100) |
Inflammation; adhesion proliferation |
Kruppel-like factors (KLFs) are zinc finger family DNA binding proteins that emerged as major molecular switches in regulating endothelial homeostasis (12). Erythroid Kruppel-like factor (EKLF/KLF1) was the first mammalian Kruppel-like factor to be identified in red blood cells in 1993 (256), followed by the discovery of additional 16 mammalian KLFs (KLF2-17). All KLFs contain three Cys2/His2 zinc fingers in the C terminus that determine the DNA binding specificity. The significance of KLF in regulating cellular functions is highlighted by the fact that KLF4 was identified as one of the four Yamanaka factors (375), transcription factors that are highly expressed in embryonic stem cells and can induce pluripotency in somatic cells. Later studies reported that KLF2 or KLF5 can replace KLF4 to initiate and sustain cellular pluripotency (424). Regulation of KLF2 and KLF4 by mechanical stimuli, particularly blood flow (89, 214, 292), has been well described in vascular endothelium but the stretch-mediated endothelial KLF2 expression was only recently reported (158). A large cohort of studies demonstrated that unidirectional flow, when compared to disturbed flow or static conditions, significantly induces KLF2 and KLF4 in vascular endothelium (89, 292, 339). Indeed, KLF2 and KLF4 are proposed as master transcriptional regulators that mediate the vasodilatory, anti-inflammatory, antithrombotic, anticoagulant properties of quiescent endothelium (12). In contrast, lower expression of KLF2 and KLF4 was detected in vascular endothelium subjected to disturbed flow in arterial regions prone to atherosclerosis (89, 107, 252, 399). Reduced expression of KLF2 or KLF4 has been mechanistically linked to decreased expression of thrombomodulin (TM), endothelial nitric oxide synthase (eNOS), and phospholipid phosphatase 3 (PLPP3) as well as increased expression of endothelin-1 (ET-1), E-selectin (ESEL), and vascular cell adhesion protein 1 (VCAM-1) (225, 226, 292, 342, 399, 417, 419). In addition to shear stress, simvastatin and resveratrol also induce endothelial expression of KLF2 and KLF4 (293, 340, 399). MEK5/MEF2 and miR-92a are common upstream regulators of KLF2 and KLF4 in vascular endothelium (107, 292, 419). Although KLF2 was first cloned from lung tissues and is also known as lung Kruppel like factor (LKLF), stretch-regulation of endothelial KLF2, and its role in lung pathophysiology was only recently described (158). Significant reduction (~50%) of KLF2 was detected in human microvascular human pulmonary microvascular cells subjected to 18% circumferential stretch in comparison with cells under static condition or 5% stretch. Consistent with this in vitro observation, in mouse lungs subjected to high tidal volume ventilation, KLF2 is significantly reduced leading to endothelial barrier disruption. KLF2 overexpression significantly ameliorates LPS-induced lung injury in mice. The protective role of KLF2 is mediated by its regulation of a cohort of genes associated with cytokine storm, oxidation, and coagulation; many of them have been implicated in human acute respiratory distress syndrome (ARDS) by genome-wide association studies (GWAS). In addition, KLF2 mediates endothelial monolayer integrity by transcriptionally activating the Rap guanine nucleotide exchange factor 3/exchange factor cyclic adenosine monophosphate (RAPGEF3/EPAC1) that activates small GTPase Ras-related C3 botulinum toxin substrate 1 (Rac1) (158).
Hypoxia-inducible factor 1-alpha (HIF-1α) is a subunit of the heterodimeric transcription factor hypoxia-inducible factor 1 (HIF1) that recognizes and bind to hypoxia response elements (HREs) in the genome in response to hypoxic stress (338). HIF-1α regulates critical vascular functions such as angiogenesis, metabolism, cell growth, metastasis, and apoptosis (338). Although hypoxia is the main stimulator of HIF activity, emerging evidence suggests biomechanical stimuli are important regulators of HIF. HIF-1α mRNA is increased after prolonged stretch in rat skeletal muscle capillary beds (255) and the inferior vena cava (224). In vascular endothelium, disturbed blood flow significantly stabilizes HIF-1α under normoxia by increasing NAD(P)H oxidase-4 (NOX4)-derived reactive oxygen species (ROS) (418) or activating deubiquitinating enzyme Cezanne (112). Increased HIF-1α stabilization was shown to reprogram endothelial metabolism and activate vascular inflammation by promoting glycolysis and reducing mitochondrial respiratory capacity (112, 418).
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a proinflammatory transcription factor that is activated by cyclic stretch in endothelial cells. NF-κB functions as a nuclear effector of signaling pathways emanating from membrane receptors such as Toll-like receptor superfamilies (153). Biomechanical forces including shear stress and circumferential strain are also critical regulators of NF-κB-dependent signaling transduction. Activation of NF-κB, demonstrated by electromobility shift assays, was detected as early as 2 h in endothelial cells after subjected to 10% average strain at 60 cycles/min (97). In addition, Kobayashi et al reported that 25-50% cyclic uniaxial stretch can activate IκB kinase (a positive regulator of NF-κB signaling) in human umbilical vein endothelial cells within 5 min and the activation peaked at 15 min (197). Stretch-activated NF-κB signaling is mechanistically linked to the inflammatory and adhesive endothelial phenotypes associated with mechanical strain. 12% cyclic stretch prompts endothelial NF-κB activation, which stimulates expression of MCP-1, a potent chemoattractant for monocytes (421); 25% uniaxial stretch activates IκB kinase and promotes NF-κB nuclear translocation leading to the IL-6 elevation and secretion (197). Moreover, NF-κB signaling contributes to the elevation of cyclooxygenase-2 (COX-2) in human umbilical endothelial cells exposed to cyclic stretch (437). Mechanistically, strain-induced activation of IκB kinase /NF-κB requires outside-in signaling via integrins that trigger a phosphatidylinositol 3-kinase (PI3-kinase)/phospholipase C (PLC)/protein kinase C (PKC) signaling cascade (330).
Focal adhesion/cytoskeleton-associated protein Zyxin, an atypical transcription factor, was recently proposed as one of the critical mechanosensors to convert mechanical strain into biological signaling in vascular cells (371, 416). Zyxin is composed of N-terminal proline-rich sequences, a nuclear export signal motif, and three copies of C-terminal motif (Lin-11 Isl-1 Mec-3/LIM, a double zinc-finger domain) (236). Zyxin was first identified as a focal adhesion protein that directly interacts with cytoskeletal proteins and actin filaments. Later studies reported the presence of Zyzin in adhesions junctions and in the nucleus as well (23, 150). Attached to the cytoskeleton and enriched in adhesion and junction structures, Zyxin is part of the cellular machinery that senses environmental cues through mechanotransduction. For example, force-induced recruitment of Zyxin in focal adhesions is critical for actin polymerization in adhesion sites stimulated by the mechanical forces (149). Likewise, Zyxin also facilitates actin polymerization at force-bearing cell-cell junctions (355). Besides organizing the dynamics of actin fibers, Zyxin is proposed as a key sensor of mechanical stretch given the ability of Zyxin to shuttle between cytoplasm and nucleus and moreover, the transcriptional capacity of the LIM domains within it. Wojtowicz et al. reported that in human umbilical vein endothelial cells, 10% cyclic stretch (0.5 Hz, 6h) leads to Zyxin redistribution from focal adhesions/stress fibers to the nucleus where Zyxin functions as a transcription factor to regulate genes such as interleukin-8 and chemokine ligand 1 (CXCL1) (416). Further genome-wide transcriptome analyses demonstrated that Zyxin may regulate more than 60% of CS-sensitive genes in human umbilical vein endothelial cells subjected to cyclic stretch. Mechanistically, it is suggested that cyclic stretch activates transient receptor potential channel 3 (TRPC3) in endothelial cells, leading to the release of vasoconstrictor peptide endothelin-1 (ET-1) and stimulation of B-type receptor, resulting in ANP receptor guanylyl cyclase A (GC-A) activation and subsequent Zyxin phosphorylation (mediated by protein kinase G), consequently triggering Zyzin nuclear translocation (371).
Activator Protein-1 (AP-1) is among one of the first mammalian transcription factors to be identified (11). c-Fos and c-Jun are major components of heterodimeric transcription factor AP-1. In addition to the Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra1, and Fra2) subfamilies, activating transcription factor proteins and Maf transcription factors can also contribute to the formation of active AP-1 dimeric complex which regulates a variety of cellular processes including cell proliferation, death, survival and differentiation (341). AP-1 transcription factors have been shown to trans-activate ICAM-1, tissue factor (295), endothelin-1 (215, 322), CXCL-1 (231), VEGFD (243), and MCP-1 (91, 244), which are important molecules in regulating endothelial functions such as inflammation, adhesion, angiogenesis, hemostasis, and vascular tone. Consistent with its pro-inflammatory function, AP-1 activation contributes to the elevation of MCP-1, MMP-2, and MMP-14 in endothelial cells subjected to cyclic stretch (404, 421). Although AP-1 is associated with increased vascular inflammation in most scenarios, deletion of AP-1 family JunD was shown to induce oxidative stress and drive endothelial dysfunction, implying the elasticity of AP-1 in transcriptional activation and target gene specificity due to the choice of dimerization partner (18). Stretch-stimulated AP-1 activity is not limited in vascular endothelia and has been reported in various cell types including cardiomyocytes (328), smooth muscle cells (91, 208, 291, 377), epithelial cells (363), osteoblastic cells (299), fibroblasts (202), mesenchymal cells (138), and myometrial cells (363).
Noncoding RNA
Noncoding RNAs (ncRNAs) have recently emerged as a new class of gene regulators in eukaryotic biology (309). ncRNAs represent multiple classes of functional RNA transcripts with various lengths and characteristics that are not transcribed into proteins but carry out regulatory functions of gene expression such as epigenetic modification, mRNA stability, and translational control. Recent studies demonstrated that non-coding RNAs contribute to the majority of mammalian transcriptional output, consistent with the view that more than 50% of human genome is transcribed but protein-coding genes only represent less than 2% of the total genome sequence (26). Nonprotein-coding genes are broadly divided in two major categories according to their size. Small ncRNAs are typically defined as those less than 200 nucleotides, such as microRNAs, small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), ribosomal RNAs (rRNAs), transfer RNA (tRNAs), and piwi-interacting RNAs (piRNAs). Long noncoding RNAs (lncRNAs) include all ncRNA transcripts greater than 200 bp whose sizes can range up to hundreds or thousands of nucleotides in length with complex secondary structures that might be important to their diverse regulatory functions. As of now, the most studied ncRNAs are microRNAs (miRNAs) whose function in mechanobiology was recently unveiled. miRNAs are highly conserved small RNAs of 19 to 26 nucleotides that posttranscriptionally suppress their target genes (8). Although cyclic stretch-induced endothelial miRNAs and their putative roles in vascular pathophysiology are still poorly understood, two recent studies provided the first line of evidence implicating that mechanosensitive miRNAs actively contribute to the pathogenesis of pulmonary vascular diseases associated with aberrant mechanical stimuli. Garcia and colleagues reported that miR-374a and miR-568 are significantly suppressed by 18% cyclic stretch in pulmonary endothelial cells when compared to cells under static condition (2, 3). Functionally, overexpression of miR-374a attenuates 18% CS-stimulated elevation of nonmuscle myosin light chain kinase isoform that drives compromised endothelial barrier function (3). Furthermore, forced expression of miR-568 in pulmonary endothelial cells mitigates 18% CS-induced increase of pre-B-cell colony enhancing factor (PBEF) (2), a proinflammatory cytokine and nicotinamide adenine dinucleotide biosynthetic enzyme whose augmentation is associated with inflammatory lung diseases. These results collectively indicate stretch-sensitive miRNAs are potential therapeutic targets to prevent or treat vascular diseases. Consistent with the proposed roles of mechanosensitive miRNAs in vascular functions, a cohort of flow-regulated miRNAs was recently identified and implicated in the cardiovascular pathophysiology in relation to endothelial dysfunction driven by disturbed hemodynamics. Endothelial miR-92a is increased in arterial regions susceptible to atherosclerosis where complex hemodynamic conditions of disturbed flow are prevalent (107, 234); complementary in vitro flow experiments demonstrated that disturbed flow elevates miR-92a that suppresses anti-inflammatory transcription factors KLF2 and KLF4 (107, 419). The therapeutic potential of managing miR-92a expression was tested in atherogenic LDLR−/− mice, which demonstrated reduced endothelial inflammation and decreased atherosclerotic lesion size as the result of systemic delivery of antagomirs targeting miR-92a (234). In addition to miR-92a, miR-663 and miR-712 are activated by disturbed flow-associated endothelial activation (276, 360), while miR-10a, miR-19a, and miR-23b are stimulated by unidirectional flow-associated endothelial quiescence (110, 307, 406).
Epigenome
Epigenetic signatures describe the non-genetic modifications to the genome by chemical modification of DNA and its associated proteins such as DNA methylation and histone modifications (174, 320). Epigenome refers to a map of the genome-wide modifications made to DNA and the protein scaffold that supports it (323). A cell’s epigenome determines when genes can be expressed. For instance, almost every cell in the human body arises from the progeny of a single fertilized egg but the same DNA sequence is transcribed uniquely in different cells. Unlike the stable DNA code, the epigenome is dynamically regulated in spatiotemporal fashion and plays a central role in determining phenotypic plasticity of cells. Aberrant epigenetic changes are involved in a variety of human diseases (111, 247). Although still in its infancy, the study of the epigenome may provide a new layer of molecular control in mechanosensing mechanisms related to vascular pathophysiology. A few recent studies demonstrated that mechanical forces are critical regulators of epigenetic mechanisms in vascular endothelium (99, 179, 204, 439).
Methylation of DNA is an epigenetic mechanism that replaces a hydrogen atom by a methyl group at the five carbon of cytosine residues. DNA methylation is a widespread gene regulatory mechanism in vertebrates in which more than half of the genes contain short cytosine-phosphate-guanine dinucleotides (CpG islands) in promoters (182). Methylation of these CpG islands at or near the promoter region typically results in inhibition of gene transcription. DNA methylation induces transcriptional silencing in three ways. First, methylation can directly prevent the binding of transcription factors to promoters. Second, methylated DNA can recruit methyl-CpG-binding domain (MBD) proteins that prevent RNA polymerase from binding to the promoter. Third, DNA methylation has been shown to elicit heterochromatin formation via histone deacetylation, methylation, and local chromatin compaction. DNA methylation plays a central role in mammalian development and normal functioning of the adult organism such as genomic imprinting, X-chromosome inactivation, suppression of repetitive element transcription, and transposition. Aberrant DNA methylation and consequent changes of cellular homeostasis contribute to a variety of human diseases such as cancer, lupus, and multiple sclerosis. DNA methylation is catalyzed by three types of DNA methyltransferases (DNMTs) while cytosine demethylation is mediated by pathways regulated by a cohort of methyl-cytosine dioxygenases known as ten-eleven translocation (TET) proteins. Recent studies employing genome-wide DNA methylation sequencing demonstrated that vascular endothelial cells exhibit distinct methylomes related to the biomechanical environments (99, 179, 439). For instance, application of disturbed oscillatory flow in vitro induces genome-wide hypermethylation in cultured endothelial cells. In agreement with these results, key DNA methyltransferases such as DNMT1 and DNMT3A are shown to be upregulated in vascular endothelia under disturbed flow (99, 179, 439). It remains to be explored whether cyclic stretch regulates DNA methylation.
Histone modifications play a key role in the cell-type-specific and spatiotemporal interpretation of genomic information (323). Histones are a family of nuclear proteins that package, condense, and coil the DNA into nucleosomes, a repeating structural and functional unit of chromatin. A nucleosome is composed of 145-147 bp of DNA wrapped around a histone octameric core that contains two copies of each histone monomer (H2A, H2B, H3, and H4) (380). The nucleosomes and chromosomes not only compact the linear DNA in the nuclei but also govern the accessibility of transcriptional regulators to cis-DNA binding domains. N-terminal “tails” of the histone proteins projecting from the nucleosome are subjected to more than 130 posttranslational modifications (PTMs), such as methylation, acetylation, phosphorylation, sumoylation, ubiquitination, and deamination (320). Histone tail modifications influence nucleosome dynamics and chromatin compaction and control the activation or inactivation of nearby genes by determining the cis-DNA accessibility to chromatin remodeling complexes, transcription factors, and transcriptional coactivators/cosuppressors (435). Histone tail modifications, primarily acetylation/deacetylation and methylation of lysine residues, have been associated with selective accessibility of transcription machinery to specific genomic elements such as open reading frames, promoters, enhancers, silencers, and insulators. For instance, promoters typically exhibit higher trimethylation of histone H3 at Lysine 4 (H3K4me3) while enhancers largely display trimethylation of histone H3 at Lysine 4 (H3K4me1) and acetylation of histone H3 at Lysine 27 (H3k27ac) (130). In contrast, trimethylation of H3 at Lysine 27 (H3K27me3) and H4 at Lysine 20 (H4K20me3) are associated with transcranial repression. The histone codes are dynamically interpreted/regulated by specific enzymes that function as writers (proteins that add PTMs to histones), erasers (enzymes that remove specific PTNM from histone substrates), and readers (proteins that recognize specific histone marks or a combination of marks) (68, 126). The major histone writers are histone acetyltransferases (HATs) and histone methyl transferases (HMTs) whereas histone deacetylases (HDACs) and histone demethylases are key histone erasers. The actions of PTMs to govern transcription are mediated by histone readers, of which chromatin remodeler complex SWI/SNF (switching defective/sucrose nonfermenting) and bromodomain and extraterminal domain family of adaptor proteins (BETs) are the most extensively studied. A few studies have pointed to a role of mechanical forces in regulating histone modifications in vascular endothelium. For instance, disturbed flow was shown to activate a cohort of Class I and Class II HDACs (213) while unidirectional flow induces Sirtuin 1 (69), a NAD-dependent Class III HDAC. Moreover, global changes of the enhancer landscape in endothelium under hemodynamics were recently reported (204). Although recent profiling approaches have determined the genome-wide methylome and histone codes of the epigenome in vascular endothelium, what remain virtually unknown are the changes in DNA methylation and histone modifications in endothelial cells under well-defined stretch conditions, an emerging research direction that may have profound impact on our understanding of the complex stretch-sensing mechanisms.
Physiologic and Pathophysiologic Stretch-Induced Responses in Endothelium
Amplitude dependent regulation of endothelial cell phenotype
Although marked effects of mechanical stimulation on gene expression have been described in many cell systems, the important points regarding the role of mechanical strain magnitude, duration of cyclic stretch, and type of mechanical strain in control of specific endothelial cell functions such as permeability, inflammatory signaling, angiogenesis, survival, or endothelial phenotype in general remain unclear. It is now well recognized that physiologic levels of cyclic stretch and intraluminal pressure are essential for the maintenance of endothelial functions and regulation of mass transport across the vessel wall (217). Cell studies revealed molecular mechanisms of such stretch-induced effects. Endothelial cell preconditioning to 24 h of physiologically relevant 5% cyclic stretch increases protein expression of tight junction proteins occludin and ZO-1 in parallel with their increased localization to the cell-cell border (77). Such enhancement of tight junction complexes by physiologic cyclic stretch reduces transendothelial permeability to FITC-dextran suggesting enhancement of endothelial barrier. Application of uniaxial cyclic stretch also up-regulates the expression of integrin-β3 in endothelial cells, which further enhances the cell adhesiveness and resistance of EC monolayer to hemodynamic forces or excessive vessel distension (372). Long-term preconditioning at physiological 5% cyclic stretch amplitude also causes phenotypic changes in pulmonary endothelial cells leading to reduced permeability responses to barrier-disruptive agonists (40).
In contrast, chronic cyclic stretch preconditioning at pathologic amplitude (18% equibiaxial cyclic stretch) increases expression of contractile and actin binding proteins: endothelial MLCK, MLC, Rho, ZIP-kinase, caldesmon, and HSP27 as well as PAR1 and PAR2 receptors mediating thrombin-induced permeability (32, 40). High magnitude cyclic stretch also elevates the mRNA levels of specific smooth muscle markers, SM22-α, α-smooth muscle actin (α-SMA), caldesmon-1, smooth muscle myosin heavy chain (SMMHC), and calponin-1 in endothelial cells (62). These findings led to speculation that excessive hemodynamic forces may play an important role in modulating endothelial phenotype and even induce a possible endothelial cell to SMC trans-differentiation in response to cyclic strain, which may have another pathological implication in development of pulmonary hypertension.
Pathologic effects of high magnitude stretch
High magnitude endothelial stretch and inflammation
Mechanical ventilation, an indispensable therapeutic modality for the treatment of respiratory failure, can also lead to a number of serious complications, including initiation or exacerbation of underlying lung injury. Inflammatory response is one of the major lung reactions to overinflation. Injurious ventilation increases levels of tumor necrosis factor (TNF)-α, interleukins IL-1β, IL-6, and IL-10, macrophage inflammatory protein-2, and interferon-γ in lavage fluid (25), which may contribute to acute lung injury and the development of multiple organ dysfunction syndrome. The role of stress kinases in cyclic stretch-induced gene expression was already discussed above. These responses to excessive mechanical strain may be also reproduced in the cultures of lung cells exposed to high magnitude cyclic stretch in vitro. Among stretch-induced activation of TNFα, IL-8, and IL-6 production by other lung cells including resident macrophages (304) and alveolar type II epithelial cells (96), exposure of lung endothelium to 20% cyclic stretch triggered IL-8 production, with marginal changes in response to 5% stretch (170). Other studies also show that exposure of endothelial cells to cyclic stretch at 15% elongation increases production of IL-8, whereas 6% cyclic stretch has no effect (283). Cyclic stretching of aortic valve endothelial cells at 20% showed a significant up-regulation of the membrane-bound VCAM-1-, ICAM-1-, and E-selectin, compared to 10% strain and controls (254). Interestingly, in these cells 5% strain also upregulates inflammatory surface receptor expression, as compared to 10% strain, which may be explained by narrow physiological range of stretch amplitude experienced by these cells in vivo. These findings demonstrate proinflammatory responses developed by endothelial cells exposed to excessive mechanical stretch, which may activate neutrophilic inflammation, and contribute to VILI.
Production of prostaglandins and isoprostanes
Isoprostanes are the products of lipid oxidation, which resemble prostanoid structures and have been identified in the alveolar fluid and exhaled air of VILI/ARDS patients. These compounds may further promote lung inflammation and are now used as valuable biomarkers reflecting severity of VILI/ARDS (316, 317). Exposure of human umbilical vein endothelial cells to 10% cyclic stretch increases mRNA levels of two genes controlling synthesis of proinflammatory prostanoids: cyclooxygenase-2 and thromboxane A(2) synthase (TXAS) without effect on cyclooxygenase-1 expression (437). In contrast, expression of prostaglandin synthase was downregulated. Stretch-induced upregulation of COX-2 is mediated by activation of the NFκB signaling pathway indicating cell preconditioning at pathologic cyclic stretch amplitudes. Other study also shows that 10% cyclic stretch is a weak inducer of prostacyclin synthase expression in endothelial cells (336). However, therapeutic elevation of prostacyclin levels significantly attenuates pulmonary vascular endothelial leak associated with mechanical ventilation at high tidal volume and protects barrier function of pulmonary endothelial cells exposed to high magnitude cyclic stretch and thrombin stimulation in vitro (37).
Effects of cyclic stretch on surrounding matrix and smooth muscle
Ventilator-induced lung injury is accompanied by an increased release and activation of MMPs. A total of 18% cyclic stretch applied in vitro increases release and activation of MMP-2 and MMP-1 in human microvascular endothelial cells isolated from the lung (146). Thus, pathologic cyclic stretch of lung endothelial cells may play a role in the release and activation of MMPs during lung injury. Low and high mechanical strains alternatively regulate matrix metalloproteinase-1 expression suggesting amplitude-dependent mechanosensitive regulation of vascular remodeling (426). A study by Young et al. showed that uniaxial cyclic stretch upregulated the secretion of angiopoietin (Ang)-2 and PDGF, and enhanced endothelial migration and sprout formation by human umbilical vein endothelial cells. Endothelial cell migration occurred in a direction perpendicular to the stretch vector, and migration velocities of cells exposed to 13% strain were twofold higher to cells exposed to 7% strain (433). These data show that a singular mechanical cue (cyclic tensile strain) can trigger a cascade of autocrine and paracrine signaling events between ECs and SMCs critical to the angiogenic process. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-beta signaling pathway (17). Exposure to mechanical strain increased the paracrine inhibition of vascular smooth muscle cells (VSMCs) by endothelial cells. Mechanical regulation of perlecan expression in endothelial cells was governed by a mechanotransduction pathway requiring autocrine transforming growth factor beta (TGF-beta) signaling and intracellular signaling through the ERK pathway. These findings suggest a feedback control mechanism in which net arterial remodeling to increased mechanical strain is controlled by a dynamic interplay between growth stimulatory signals from VSMCs and growth inhibitory signals from endothelial cells.
Amplitude dependent effects on redox balance, survival, and apoptosis
ROS signaling is regulated by cyclic stretch in an amplitude-dependent manner and plays a critical role in various endothelial cell responses to cyclic stretch. Long-term application of 5% to 12% cyclic stretch caused magnitude-dependent downregulation of Nox4 expression and ROS formation, as well as decreased Cu/Zn SOD, MnSOD, and catalase expression, which was dependent on stretch-induced NO regulation (131). These results indicate that physiological levels of cyclic strain downregulate Nox4 expression and superoxide anion formation. This mechanism may contribute to a vasoprotective balance between NO and superoxide anions in response to physiological mechanical stimulation of endothelial cells. Cyclic stretching of endothelial cells affects their survival and angiogenesis by producing ROS via NAD(P)H oxidase. Exposure to 5% cyclic stretch increased, while 20% cyclic stretch decreased cell viability index in endothelial cells with inhibited p22phox (203). This study also revealed complex biphasic effects of cyclic stretch on endothelial survival signaling with amplitude- and time-dependent fluctuations of Akt and eNOS phosphorylation, although ROS production was stimulated progressively by strain via the p22phox pathway. Other studies addressing effects of cyclic stretch on endothelial apoptosis have been already discussed earlier in the article and also suggest amplitude-dependent mechanisms activating Akt, mTOR, GSK-3beta, caspase signaling, and ceramide production (161, 277, 311), although the nature of amplitude-dependence in endothelial cell responses remains to be investigated.
In summary, a major focus in current studies has been made so far to characterize endothelial responses to pathologically relevant patterns of mechanical strain. Although these data convincingly show that mechanical stimulation alone has profound effects on endothelial cell phenotype, remodeling, release of bioactive mediators, and other cell responses, the potential interactions between stretch-induced mediators and ongoing mechanical stimulation in modulation of endothelial cell permeability, motility, angiogenesis, or other functions remain obscure. The following sections will discuss crosstalk between cyclic stretch at pathologic or physiologic amplitudes and signaling by bioactive molecules in pulmonary endothelial barrier regulation.
Amplitude-dependent effects of cyclic stretch on agonist-induced regulation of endothelial permeability
The vascular endothelium forms a selective permeable barrier between the blood and the interstitial space of all organs and participates in the regulation of macromolecule transport and blood cell trafficking through the vessel wall. Increased paracellular permeability is result of formation of gaps between adjacent endothelial cells leading to extravasation of water and macromolecules in the lung tissue. A working model of paracellular EC barrier regulation (98, 250) suggests that paracellular gap formation is regulated by the balance of competing contractile forces imposed by actomyosin cytoskeleton, which generate centripetal tension, and adhesive cell-cell and cell-matrix tethering forces imposed by focal adhesions and adherens junctions, which together regulate cell shape changes. Increased EC permeability in response to agonist stimulation is associated with activation of myosin light chain kinase, RhoA GTPase, MAP kinases, and tyrosine kinases, which trigger actomyosin cytoskeletal rearrangement, phosphorylation of regulatory myosin light chains (MLC), activation of EC contraction, destabilization of intercellular (adherens) junctions, and gap formation (250). Barrier disruptive agonists, such as thrombin, TGFβ1, and TNFα, activate Rho and Rho-associated kinase, which may directly catalyze MLC phosphorylation, or act indirectly by inactivating myosin light chain phosphatase (34, 42, 298, 393). In turn, EC barrier enhancement induced by barrier protective factors, such as platelet-derived phospholipid sphingosine-1 phosphate, oxidized phospholipids, HGF, or simvastatin also requires actomyosin remodeling, including formation of a prominent cortical actin rim, disappearance of central stress fibers, and peripheral accumulation of phosphorylated MLC, which is regulated by Rac-dependent mechanisms (31, 117, 173, 227). Thus, the balance between Rho- and Rac-mediated signaling may be a critical component of EC barrier regulation.
The pathologic mechanical forces experienced by lung tissues during mechanical ventilation at high tidal volume may be a key mechanism propagating VILI and pulmonary edema (314, 387, 398). As already discussed in previous sections, pathologic cyclic stretch induces secretion of various proinflammatory molecules and also activates intracellular stress signaling, which may further exacerbate effects of circulating inflammatory and edema-genic mediators. On the other hand, endothelial cell preconditioning at physiologically relevant cyclic stretch magnitudes promotes cell survival and may protect pulmonary endothelial barrier from effects of edema-genic and inflammatory agents. These interactions between pathophysiologic mechanical stimulation and bioactive molecules in regulation of endothelial functions will be discussed later.
Thrombin
Thrombin is a potent agonist that causes rapid endothelial permeability increases. Similar to other barrier disruptive agents such as TGFb, nocodazole, or TNFa, thrombin stimulates actomyosin contraction, cell retraction, and formation of intercellular gaps, the process mainly regulated by myosin light chain kinase, RhoGTPase, and Rho-associated kinase, which may directly catalyze MLC phosphorylation, or act indirectly by inactivating myosin light chain phosphatase. Exposure of pulmonary endothelial cells to pathologically relevant 18% cyclic stretch enhances thrombin-induced gap formation and delays monolayer recovery.
Several mechanisms may be involved in synergistic effects of pathologic CS on the agonist-induced EC contractility and barrier dysfunction. First, stretch-induced Ca2+ influx may cause additional MLC phosphorylation by Ca2+/calmodulin-dependent myosin light chain kinase (357). Second, cyclic stretch-induced activation of signaling serine/threonine- and tyrosine-specific protein kinases (6, 171, 327, 405) may lead to activation of Rho-specific guanine nucleotide exchange factors and trigger Rho pathway of barrier dysfunction. Third, pathologic cyclic stretch triggers generation of ROS, which may function as second messengers in signal transduction cascades, including the Rho pathway (6). Among these potential mechanisms, synergistic action of pathologic cyclic stretch and thrombin on Rho activation leading to enhanced MLC phosphorylation and cell retraction is the best-characterized mechanism, which may be suppressed by inhibition of Rho kinase or inactivation of Rho (32, 35, 344).
In contrast, endothelial cell exposure to physiological cyclic stretch amplitudes (5% elongation) markedly enhances endothelial recovery after thrombin challenge leading to nearly complete monolayer recovery by 50 min of thrombin stimulation, which is accompanied by peripheral redistribution of focal adhesions and activator of actin polymerization cortactin. Consistent with differential effects on monolayer integrity, 5% cyclic stretch promotes activation of Rac GTPase involved in recovery of peripheral actin cytoskeleton and reannealing endothelial cell junctions (35). Rac inhibition suppresses restoration of endothelial monolayer integrity after thrombin challenge. Interestingly, endothelial cell preconditioning at physiologic cyclic stretch levels (5% elongation, 24 h) enhances paracellular gap resolution after stepwise increase to 18% cyclic stretch (30 min) and thrombin challenge. These results indicate a critical role for physiologic cyclic stretch in endothelial barrier improvement in both, chronic and acute scenario of pathologic mechanical perturbations.
Another important point of these studies is differential regulation of Rho and Rac GTPases by physiological and pathologically relevant levels of cyclic stretch (35). Because antagonistic relations between Rho and Rac signaling in regulation of endothelial permeability have been now confirmed by several groups, modulation of Rac or Rho activities by adjusting mechanical forces and/or coadministration of bioactive molecules may be a promising therapeutic approach in treatment of ventilator-induced lung injury. These strategies will be discussed in more detail later.
Hepatocyte growth factor (HGF)
HGF elicits potent angiogenic activities (57, 134) and exhibits sustained barrier protective effects on human pulmonary endothelial cells (ECs) (227). Clinical studies show dramatic (up to 25-fold) elevation of HGF levels in plasma and BAL fluid in patients with ALI/ARDS (308, 367, 396). This elevation may be directly induced by pathologic mechanical stretch associated with mechanical ventilation, since it was demonstrated that exposure of human alveolar epithelial cells (A549) cultured on a silicoelastic membrane to high magnitude cyclic stretch in vitro induces HGF expression and its release (423). Barrier protective effects of HGF against vascular leak have been associated with stimulation of multiple signaling pathways, including small GTPase Rac, Rac activator Tiam1, phosphatidylinositol-3-kinase (PI3-kinase), and its downstream effector GSK-3β (33, 227). HGF-induced barrier protective effects on the pulmonary endothelium also involve remodeling of the actin cytoskeleton and increased interaction between adherens junction proteins α-catenin and VE-cadherin (227).
VEGF
Vascular endothelial growth factor (VEGF) is a potent angiogenic factor, and its presence at threshold concentrations in essential for endothelial cell survival. VEGF production induced by physiological cyclic stretch described in vascular smooth muscle cells (354) may provide an arterial stimulus for maintenance of steady state levels of VEGF essential for endothelial and alveolar epithelial survival. On the other hand, VEGF, originally called VPF or “vascular permeability factor,” also controls lung vascular permeability to water and proteins. VEGF-induced endothelial permeability is mediated by MAP kinases and Rho-dependent signaling (22, 39, 369). VEGF overexpression in the lungs or injection of purified VEGF increases endothelial permeability in vivo (185, 321). In healthy human subjects, VEGF is highly compartmentalized to the lung with alveolar VEGF protein levels 500 times higher than in plasma (184). During excessive lung mechanical stress or injury such as in ALI or VILI, because of anatomic proximity between alveolar epithelial and microvascular endothelial cells, VEGF may literally spill into pulmonary edema (184, 266). Of note, VEGF production by alveolar epithelial cells becomes further boosted by high magnitudes of cyclic stretch (206). VEGF increases in the lung have been reported in several lung pathologies including hydrostatic edema, ARDS, and LPS-induced lung injury (186, 410). High tidal volume ventilation and corresponding high magnitude cyclic stretch of vascular endothelial and smooth muscle cells in vitro also stimulates VEGF and VEGF receptor expression (137, 245, 438). Importantly, only pathologically relevant stretch amplitudes (15%-20% cyclic stretch) applied to endothelial cells in vitro reproduce activation of VEGF expression observed in VILI patients (310).
HGF, VEGF, and cyclic stretch
Analysis of endothelial permeability responses and activation of cell signaling caused by combinations of high/low cyclic stretch magnitudes, VEGF and HGF shows that: (i) 5% cyclic stretch further stimulates HGF-induced Rac signaling and enhances cortical F-actin rim essential for prevention of endothelial monolayer integrity; (ii) 18% cyclic stretch promotes VEGF-induced Rho signaling, gap formation, and EC permeability; and (iii) physiologic cyclic stretch preconditioning combined with HGF treatment reduces the barrier-disruptive effects of VEGF, and this effect is due to downregulation of the Rho pathway (39). These results suggest synergistic effects of HGF and physiologic cyclic stretch in the Rac-mediated mechanisms of EC barrier protection and suggest an importance of physiologic mechanochemical environment in control of ALI/ARDS severity via regulation of lung endothelial permeability by a balance between different Rho family GTPases. These studies propose a paradigm of mechanochemical regulation of pulmonary endothelial barrier in VILI. In contrast to “vicious circles,” the signaling loops resulting in escalation of lung inflammation via stretch-induced production of inflammatory agents, or potentiation of barrier disruptive Rho signaling, stretch-induced HGF production in VILI may represent an autoregulatory mechanism directed at resolution of pathologic condition. Interactions between protective and disruptive bioactive molecules and interplay of circulating protective and disruptive chemical mediators with protective mechanical ventilation regimen may potentiate beneficiary effects of pharmacologic therapies used in the treatment of VILI/ARDS.
Iloprost
Lung injury and increased vascular leakiness caused by HTV and TRAP6 can be partially reversed by iloprost. Protective effects of iloprost against cyclic stretch- and thrombin-induced endothelial barrier disruption are also due to attenuation of Rho signaling manifested by inhibition of Rho-kinase specific MYPT phosphorylation and reduction of phospho-MLC levels (37). Elevated intracellular cAMP concentrations induced by prostacyclin and its stable analogs activate PKA signaling and recently described PKA-independent Epac/Rap1 signaling cascade (45, 52, 79, 251). PKA reduces endothelial myosin light chain kinase activity, which may decrease pool of phosphorylated MLC, and cause relaxation of actomyosin complex, stabilization of F-actin filaments and strengthening of cell-matrix adhesions (45, 211, 306). PKA also affects Rho signaling. One potential mechanism is PKA-mediated phosphorylation of Rho-GDP dissociation inhibitor, a negative regulator of Rho, leading to Rho inactivation (306).
Oxidized phospholipids
One of the major plasma membrane phospholipids is 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC), which upon oxidation (OxPAPC) may propagate chronic vascular inflammatory processes involved in atherogenesis (218, 235), but also exhibit potent anti-inflammatory effects in acute settings (48, 279). Intravenous OxPAPC protects against tissue inflammation, lung vascular barrier dysfunction, and inflammatory cytokine production caused by aerosolized LPS (279). The observation that intravenous injection of OxPAPC significantly attenuated leukocyte extravasation and decreased BAL protein content induced by intratracheal administration of LPS suggested that the in vivo protective effect of OxPAPC may be in part associated with its direct effects on the endothelial barrier. Treatment of pulmonary endothelial cells with OxPAPC in the range of 5 to 30 μg/mL causes dose-dependent enhancement of monolayer barrier, which lasts over 12 h (31). One important feature of OxPAPC is its ability to suppress Rho-dependent elevation of EC permeability induced by inflammatory and edemagenic agents (36, 38). OxPAPC attenuates endothelial permeability caused by thrombin, IL-6, LPS, or exposure of endothelial cells to 18% cyclic stretch and thrombin (36, 278). Treatment with OxPAPC also accelerates the recovery of the compromised EC barrier function (31, 36). VILI-associated EC barrier dysfunction and protective effects of OxPAPC were also reproduced in the in vivo model of ventilator induced lung injury (278). These studies further support a basic mechanism of endothelial barrier protection in VILI via Rac-dependent suppression of Rho signaling.
Concluding Remarks and Emerging Topics
All cells live in a three-dimensional microenvironment in which they not only contribute to but also sense and respond to mechanical forces of varying magnitude, direction, and frequency. Cellular mechanotransduction, the mechanism by which cells convert mechanical cues into biochemical responses, is required for embryogenesis and physiological control of tissue homeostasis (172). On the other hand, abnormal cell response to mechanical forces promotes pathologies associated with numerous human diseases (172). Mechanotransduction studies have focused on identifying key mechanosensors and cellular components in isolation. It remains relatively unexplored how the whole cell and entire tissues process and integrate this molecular scale information and further orchestrate physiologically relevant response in the context of the multiscale architecture of animal bodies. Recent technological advances in systems biology and -omics methods may provide an integrated approach to investigate the dynamic interactions of individual components that operate at multiple spatiotemporal scales to mediate the cellular responses to the mechanical stimuli. The typical approach in systems biology is to perturb a system, record the responses, integrate the data, and formulate mathematical models that describe the system (75). Recent “-omics” strategies allow investigators to monitor cellular responses to mechanical perturbation in a high-throughput fashion. For instance, Next-Generation RNA sequencing can be used to determine the whole-genome transcriptome of mRNAs, microRNAs, lncRNAs, and mitochondrial RNAs (253) as a function of biomechanical stimuli. Mechanoregulation of DNA methylation at a single nucleotide level across the genome (methylome) can be investigated by methylation sequencing (156). Information about whole-genome chromatin accessibility in cells under a given biomechanical environment can be acquired by DNase I hypersensitive sites sequencing (DNase-Seq) (362), Assay for transposase-accessible chromatin using sequencing (ATAC-Seq) (54), Micrococcal nuclease (MNase)-assisted isolation of nucleosomes sequencing (MAINE-Seq) (301), or Formaldehyde-assisted isolation of regulatory elements sequencing (FAIRE-Seq) (129). In addition, chromatin immunoprecipitation with massively parallel DNA sequencing (ChIP-seq) has been employed to gain high-resolution epigamic landscapes of histone modifications (432). Possible mechanoregulation of cellular metabolism can be studied by Mass Spectrometry (MS) and Nuclear Magnetic Resonance (NMR)-based platforms, which systemically identify the low molecular weight metabolites produced by living cells exposed to mechanical forces (104), in addition to the typical bioenergetics functional assays that only enable the measurement of handful metabolic parameters. Mass Spectrometry (MS)-based strategies are also utilized for the proteomics analyses that systemically identify and quantify a large cohort of proteins in greater detail in biological systems. Additional protein modifications can be detected by targeted proteomics analyses such as the phosphotyrosine-proteome, tyrosine-kinome, and tyrosine-phosphatome (386). It is notable that aforementioned genome-scale molecular information may be obtained at individual cell level given the rapidly evolving single-cell analyses that enable detections of cell-to-cell variation (118, 312). For instance, multiple single-cell RNA sequencing techniques have been developed to measure RNA molecules in individual cells with high resolution and on a whole-genome scale (422). Chromatin accessibility and nuclear architecture in individual cells have been determined by single-cell ATAC-seq (55) and single-cell chromatin conformation capture (Hi-C) (368), respectively. Nevertheless, major challenges remain on how to interpret these multi-omics profiling results. First, a lot of such findings by “-omics” approaches are based on correlative analyses and the functional relevance requires further experimental validation. Recent advances in genome-editing techniques such as Zinc-finger nucleases (ZFNs) (390), transcription activator-like effector nucleases (TALENs) (24), and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas9 (313) are ideal complementary approaches to probe the causal mechanisms in vitro and in vivo suggested by the “-omics” experiments. Second, ever-evolving “-omics” techniques posed challenges in pre-processing, analysis, interpretation, and integration of the datasets. Developments of new statistical and computational tools such as machine learning are important to further unravel the flow of biological information from DNA to proteins to metabolites to cell structures to cell interaction in the context of endothelial mechanosensing mechanisms.
Abnormal cell and tissue response to mechanical cues resulting from changes in tissue mechanics, extracellular matrix remodeling, and deregulation of mechanosensing molecules contributes to the etiology of a wide range of human diseases in the pulmonary, cardiovascular, orthopedic, muscular, and reproductive systems (163). Particularly, dysregulation of mechanosensing mechanisms in endothelial cells is implicated in a wide range of vascular diseases (28, 85, 140). Nevertheless, it remains challenging to develop mechanointerventions that actively target dysregulated mechanosensing mechanisms to treat diseased vasculature. In addition, blood vessels cover 43,000 to 75,000 ft2 surface areas in an adult human while vascular disease typically occurs in a very small portion of the vasculature. For instance, local endothelial inflammation induced by local biomechanical stimuli plays an important role in the pathogenesis of ventilator-induced lung injury, pulmonary hypertension, and atherosclerosis (28, 85, 127, 140); however, systemic approaches to reduce vascular inflammation carry the inevitable risk of increased infections and delaying tissue repair since controlled vascular inflammation is important for tissue homeostasis and host defense against microbiological organisms. Moreover, systemic administration of therapeutic nucleotides typically results in predominant accumulation in the liver and unfavorable pharmacokinetic parameters resulting from rapid in vivo degradation and poor cellular uptake of the nucleotides, leading to low bioavailability in target cells and unwanted side effects in non-target tissues (394). Recent advances in nanotechnology and material sciences may provide attractive strategies for future mechanointerventions by which nanocarriers can be developed to target specific molecular/cellular signatures that characterize diseased blood vessels (74, 193, 209, 267, 302, 348). Lipid-polymer nanoparticles have been formulated to effectively deliver small interfering RNAs (siRNAs) to vascular endothelium in mice, accompanied by nonliver silencing (81). In addition, polymeric nanoparticles have been engineered to promote pulmonary endothelial health in vivo via the overexpression of KLF2, an anti-inflammatory transcription factor that is significantly reduced by pathological stretches during acute lung injury/ventilator-induced lung injury (158). To develop approaches that preferentially target inflamed endothelium, phage display assays were conducted to identify short peptides that specifically bind to adhesion molecules that are expressed in activated vasculature but remain low in healthy endothelium. VCAM1-targeting peptides indeed successfully facilitate VCAM1-mediated intracellular internalization of nanocarriers and nucleotides encapsulated within (193, 209, 267). Monocolonal antibodies targeting cell adhesion molecules can also be used to drive active targeting of nanomaterials to vascular endothelium (193, 348). Phage display assays are also handy tools in identifying affinity peptides targeting endothelium of a given vascular bed (350), such as the discovery of lung endothelial cell-binding peptide (128). Integration of our knowledge in mechanosensing mechanisms at the molecular, cellular, and tissue levels in combination with advances in nanotechnology and material sciences may have unique potential to revolutionize future medical practice to treat dysfunctional vasculature that contributes to more human diseases than any other tissue.
Figure 1.
Mehcanoreceptors and sensors that regulate endothelial responses to biomechanical stimuli. Mechanosensors are distributed throughout the endothelium to sense and convert biomechanical cures (cyclic stretch, extracellular matrix stiffness, and fluid shear stress) into biological signaling. We systematically discussed the molecular identify of the putative mechanosensors in vascular endothelium in this review article. Moreover, mechanosensitive signaling pathways in vascular endothelium were also comprehensively described. Briefly, mechanical forces are transmitted and distributed throughout the endothelial cells via cilia, glycocalyx, cytoskeletal elements, nucleus, adhesion junctions, focal adhesions, and extracellular matrix. For instance, glycocalyx and cilia at the luminal surface can be deformed by mechanical load to trigger activation of ion channels, G-protein-coupled receptors, and calcium signaling. Force imbalance at cell-cell contacts and cytoskeletal strain are transmitted to intracellular junctional protein complexes such as tight junctions, adhesion junctions, and gap junctions, leading to conformational changes of PECAM-1 and α-catenin, activation of Rho family of GTPases (Rho, RAc, and Cdc42) and recruitment of vinculin that trigger intracellular biochemical signals such as activation of src-family kinase, PI3-kinase, and MAP kinase. Cytoskeletal forces and extracellular stuffiness are also sensed by focal adhesions that are complex structures of large macromolecular assembles. For instance, transmembrane integrins that bind to extracellular matrix (ECM) can serve as a focus for force transmission and deformation to activate focal adhesion kinase (FAK), src-family kinase, and small GTPase, leading to regulation of transcription factors YAP/TAZ, JNK, and MRTFs. The nucleus has emerged as an important cellular mechanosensor. External mechanical forces can be transmitted to the nucleus through cytoskeletons resulting in nuclear deformation and consequent changes of nuclear envelope structure and chromatin architecture.
Didactic Synopsis.
Major teaching points
Cyclic stretch due to autonomous breathing is important in maintaining normal endothelial functions and the homeostasis of pulmonary circulation.
Endothelial cells respond to increased mechanical strain associated with mechanical ventilation by promoting inflammation, adhesion, and contractility leading to vascular dysfunction and pulmonary disorders.
- A cohort of mechanosensors has been proposed to mediate mechanotransmission and mechanotransduction in vascular endothelium (Fig. 1).
- Mechanotransmission: External forces due to cyclic stretch and shear stress are loaded onto the endothelium, leading to internal tensional changes opposing the external forces. Externally applied forces are transmitted and distributed throughout the endothelial cells via cilia, glycocalyx, cytoskeletal elements, nucleus, adhesion junctions, focal adhesions, and extracellular matrix.
- Mechanotransduction: Transmitted mechanical forces at mechanotransduction sites alter the biochemical activities of specialized mechanosensory molecules resulting in cellular responses. Endothelial cells respond to changes of tension by activating or inactivating signaling pathways mediated by small GTPase, reactive oxygen species, tyrosine kinases, transcription factors, and non-coding RNAs.
Molecular insights related to stretch-sensing mechanisms in vascular endothelium may lead to new therapies treating pulmonary vascular diseases.
Acknowledgement
This work was supported by National Institutes of Health Grants HL138223 (Y. Fang), HL136765 (Y. Fang), T32 HL007605 (D. Wu), T32 F32HL134288 (D. Wu), HL076259 (K. G. Birukov), and HL087823 (K. G. Birukov).
References
- 1.Abdulnour RE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, Reddy SP, Watkins JE III, Kayyali US, Garcia JG, Tuder RM, Hassoun PM. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am J Physiol Lung Cell Mol Physiol 291: L345–L353, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Adyshev DM, Elangovan VR, Moldobaeva N, Mapes B, Sun X, Garcia JG. Mechanical stress induces pre-B-cell colony-enhancing factor/NAMPT expression via epigenetic regulation by miR-374a and miR-568 in human lung endothelium. Am J Respir Cell Mol Biol 50: 409–418, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adyshev DM, Moldobaeva N, Mapes B, Elangovan V, Garcia JG. MicroRNA regulation of nonmuscle myosin light chain kinase expression in human lung endothelium. Am J Respir Cell Mol Biol 49: 58–66, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn SJ, Fancher IS, Bian JT, Zhang CX, Schwab S, Gaffin R, Phillips SA, Levitan I. Inwardly rectifying K(+) channels are major contributors to flow-induced vasodilatation in resistance arteries. J Physiol 595: 2339–2364, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res 301: 23–30, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Ali MH, Mungai PT, Schumacker PT. Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells: Role of mitochondrial oxidants. Am J Physiol Lung Cell Mol Physiol 291: L38–L45, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Ali MH, Pearlstein DP, Mathieu CE, Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: Implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287: L486–L496, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V The functions of animal microRNAs. Nature 431: 350–355, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Amma H, Naruse K, Ishiguro N, Sokabe M. Involvement of reactive oxygen species in cyclic stretch-induced NF-kappaB activation in human fibroblast cells. Br J Pharmacol 145: 364–373, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ando J, Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal 15: 1389–1403, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072: 129–157, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res 100: 1686–1695, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Awolesi MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest 96: 1449–1454, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babior BM. The NADPH oxidase of endothelial cells. IUBMB Life 50: 267–269, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Baeyens N, Mulligan-Kehoe MJ, Corti F, Simon DD, Ross TD, Rhodes JM, Wang TZ, Mejean CO, Simons M, Humphrey J, Schwartz MA. Syndecan 4 is required for endothelial alignment in flow and athero-protective signaling. Proc Natl Acad Sci U S A 111: 17308–17313, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai K, Wang W. Shear stress-induced redistribution of the glycocalyx on endothelial cells in vitro. Biomech Model Mechanobiol 13: 303–311, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Baker AB, Ettenson DS, Jonas M, Nugent MA, Iozzo RV, Edelman ER. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-beta signaling pathway. Circ Res 103: 289–297, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakiri L, Matsuo K, Wisniewska M, Wagner EF, Yaniv M. Promoter specificity and biological activity of tethered AP-1 dimers. Mol Cell Biol 22: 4952–4964, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3: 466–472, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Barbee KA, Davies PF, Lal R. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ Res 74: 163–171, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 108: 83–96, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Becker PM, Verin AD, Booth MA, Liu F, Birukova A, Garcia JG. Differential regulation of diverse physiological responses to VEGF in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 281: L1500–L1511, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Beckerle MC. Zyxin: Zinc fingers at sites of cell adhesion. Bioessays 19: 949–957, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG II, Tan W, Penheiter SG, Ma AC, Leung AY, Fahrenkrug SC, Carlson DF, Voytas DF, Clark KJ, Essner JJ, Ekker SC. In vivo genome editing using a high-efficiency TALEN system. Nature 491: 114–118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belperio JA, Keane MP, Lynch JP III, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med 27: 350–364, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57–74, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19: 677–695, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Birukov KG. Cyclic stretch, reactive oxygen species and vascular remodeling. Antioxid Redox Signal 11: 1651–1667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birukov KG. Small GTPases in mechanosensitive regulation of endothelial barrier. Microvasc Res 77: 46–52, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol 26: 453–464, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res 95: 892–901, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced permeability in the human pulmonary endothelial cells by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J 21: 2776–2786, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol 204: 934–947, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 168: 1749–1761, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 292: L924–L935, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Birukova AA, Fu P, Xing J, Cokic I, Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res 155: 44–54, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol 211: 608–617, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 295: L612–L623, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birukova AA, Rios A, Birukov KG. Long-term cyclic stretch controls pulmonary endothelial permeability at translational and post-translational levels. Exp Cell Res 314: 3466–3477, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birukova AA, Shah AS, Tian Y, Gawlak G, Sarich N, Birukov KG. Selective role of vinculin in contractile mechanisms of endothelial permeability. Am J Respir Cell Mol Biol 55: 476–486, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: Role of Rho-dependent mechanisms. J Cell Physiol 201: 55–70, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Birukova AA, Tian X, Cokic I, Beckham Y, Gardel ML, Birukov KG. Endothelial barrier disruption and recovery is controlled by substrate stiffness. Microvasc Res 87: 50–57, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol 215: 715–724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 348(Pt 2): 241–255, 2000. [PMC free article] [PubMed] [Google Scholar]
- 47.Blitzer AL, Panagis L, Gusella GL, Danias J, Mlodzik M, Iomini C. Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proc Natl Acad Sci U S A 108: 2819–2824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bochkov VN, Leitinger N, Birukov KG. Role of oxidized phospholipids in acute lung injury. Curr Resp Med Rev 2: 27–37, 2006. [Google Scholar]
- 49.Boitano S, Dirksen ER, Evans WH. Sequence-specific antibodies to connexins block intercellular calcium signaling through gap junctions. Cell Calcium 23: 1–9, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 21: 285–288, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Borisy GG, Svitkina TM. Actin machinery: Pushing the envelope. Curr Opin Cell Biol 12: 104–112, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Bos JL. Epac proteins: Multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Brown MC, Turner CE. Paxillin: Adapting to change. Physiol Rev 84: 1315–1339, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 10: 1213–1218, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, Chang HY, Greenleaf WJ. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523: 486–490, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 12: 463–518, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 119: 629–641, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bystrevskaya VB, Lichkun VV, Krushinsky AV, Smirnov VN. Centriole modification in human aortic endothelial cells. J Struct Biol 109: 1–12, 1992. [DOI] [PubMed] [Google Scholar]
- 59.Cabrales P, Vazquez BY, Tsai AG, Intaglietta M. Microvascular and capillary perfusion following glycocalyx degradation. J Appl Physiol (1985) 102: 2251–2259, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Carey DJ. Syndecans: Multifunctional cell-surface co-receptors. Biochem J 327(Pt 1): 1–16, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caro CG. Discovery of the role of wall shear in atherosclerosis. Arterioscler Thromb Vasc Biol 29: 158–161, 2009. [DOI] [PubMed] [Google Scholar]
- 62.Cevallos M, Riha GM, Wang X, Yang H, Yan S, Li M, Chai H, Yao Q, Chen C. Cyclic strain induces expression of specific smooth muscle cell markers in human endothelial cells. Differentiation 74: 552–561, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Chapman AB, Stepniakowski K, Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 153–163, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 282: 14–28, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Chen H, Bernstein BW, Bamburg JR. Regulating actin-filament dynamics in vivo. Trends Biochem Sci 25: 19–23, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Chen J, Fabry B, Schiffrin EL, Wang N. Twisting integrin receptors increases endothelin-1 gene expression in endothelial cells. Am J Physiol Cell Physiol 280: C1475–C1484, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem 274: 18393–18400, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Chen T, Dent SY. Chromatin modifiers and remodellers: Regulators of cellular differentiation. Nat Rev Genet 15: 93–106, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A 107: 10268–10273, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng JJ, Wung BS, Chao YJ, Wang DL. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension 31: 125–130, 1998. [DOI] [PubMed] [Google Scholar]
- 71.Cheng WP, Hung HF, Wang BW, Shyu KG. The molecular regulation of GADD153 in apoptosis of cultured vascular smooth muscle cells by cyclic mechanical stretch. Cardiovasc Res 77: 551–559, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Chien S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am J Physiol Heart Circ Physiol 292: H1209–H1224, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 8: 76–87, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Chung EJ, Mlinar LB, Nord K, Sugimoto MJ, Wonder E, Alenghat FJ, Fang Y, Tirrell M. Monocyte-targeting supramolecular micellar assemblies: A molecular diagnostic tool for atherosclerosis. Adv Healthc Mater 4: 367–376, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Civelek M, Lusis AJ. Systems genetics approaches to understand complex traits. Nat Rev Genet 15: 34–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 288: L294–L306, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, Meade G, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: Influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol 26: 62–68, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Conway DE, Coon BG, Budatha M, Arsenovic PT, Orsenigo F, Wessel F, Zhang J, Zhuang Z, Dejana E, Vestweber D, Schwartz MA. VE-cadherin phosphorylation regulates endothelial fluid shear stress responses through the polarity protein LGN. Curr Biol 27: 2727, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955, 2005. [DOI] [PubMed] [Google Scholar]
- 80.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res 102: 1307–1318, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dahlman JE, Barnes C, Khan O, Thiriot A, Jhunjunwala S, Shaw TE, Xing Y, Sager HB, Sahay G, Speciner L, Bader A, Bogorad RL, Yin H, Racie T, Dong Y, Jiang S, Seedorf D, Dave A, Sandu KS, Webber MJ, Novobrantseva T, Ruda VM, Lytton-Jean AKR, Levins CG, Kalish B, Mudge DK, Perez M, Abezgauz L, Dutta P, Smith L, Charisse K, Kieran MW, Fitzgerald K, Nahrendorf M, Danino D, Tuder RM, von Andrian UH, Akinc A, Schroeder A, Panigrahy D, Kotelianski V, Langer R, Anderson DG. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol 9: 648–655, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dang CV. MYC on the path to cancer. Cell 149: 22–35, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 75: 519–560, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: Endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99: 315–327, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davies PF, Mundel T, Barbee KA. A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J Biomech 28: 1553–1560, 1995. [DOI] [PubMed] [Google Scholar]
- 87.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: Role of a superoxide-producing NADH oxidase. Circ Res 82: 1094–1101, 1998. [DOI] [PubMed] [Google Scholar]
- 88.Deguchi S, Ohashi T, Sato M. Intracellular stress transmission through actin stress fiber network in adherent vascular cells. Mol Cell Biomech 2: 205–216, 2005. [PubMed] [Google Scholar]
- 89.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 167: 609–618, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Delmas P, Padilla F, Osorio N, Coste B, Raoux M, Crest M. Polycystins, calcium signaling, and human diseases. Biochem Biophys Res Commun 322: 1374–1383, 2004. [DOI] [PubMed] [Google Scholar]
- 91.Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res 103: 477–484, 2008. [DOI] [PubMed] [Google Scholar]
- 92.Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science 352: 353–358, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DePaola N, Phelps JE, Florez L, Keese CR, Minnear FL, Giaever I, Vincent P. Electrical impedance of cultured endothelium under fluid flow. Ann Biomed Eng 29: 648–656, 2001. [DOI] [PubMed] [Google Scholar]
- 94.Dinsmore C, Reiter JF. Endothelial primary cilia inhibit atherosclerosis. EMBO Rep 17: 156–166, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143, 2005. [DOI] [PubMed] [Google Scholar]
- 96.Dixon DL, Barr HA, Bersten AD, Doyle IR. Intracellular storage of surfactant and proinflammatory cytokines in co-cultured alveolar epithelium and macrophages in response to increasing CO2 and cyclic cell stretch. Exp Lung Res 34: 37–47, 2008. [DOI] [PubMed] [Google Scholar]
- 97.Du W, Mills I, Sumpio BE. Cyclic strain causes heterogeneous induction of transcription factors, AP-1, CRE binding protein and NF-kB, in endothelial cells: Species and vascular bed diversity. J Biomech 28: 1485–1491, 1995. [DOI] [PubMed] [Google Scholar]
- 98.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 99.Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW, Jang I, Son DJ, Kim D, Pan C, Fan Y, Jordan IK, Jo H. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest 124: 3187–3199, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011. [DOI] [PubMed] [Google Scholar]
- 101.Ebong EE, Lopez-Quintero SV, Rizzo V, Spray DC, Tarbell JM. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr Biol (Camb) 6: 338–347, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ebong EE, Macaluso FP, Spray DC, Tarbell JM. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler Thromb Vasc Biol 31: 1908–1915, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol 5: 221–228, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Emwas AH. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol Biol 1277: 161–193, 2015. [DOI] [PubMed] [Google Scholar]
- 105.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006. [DOI] [PubMed] [Google Scholar]
- 106.Fancher IS, Ahn SJ, Adamos C, Osborn C, Oh MJ, Fang Y, Reardon CA, Getz GS, Phillips SA, Levitan I. Hypercholesterolemia-induced loss of flow-induced vasodilation and lesion formation in apolipoprotein E-deficient mice critically depend on inwardly rectifying K(+) channels. J Am Heart Assoc 7: pii: e007430, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol 32: 979–987, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fang Y, Mohler ER III, Hsieh E, Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, Levitan I. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. Circ Res 98: 1064–1071, 2006. [DOI] [PubMed] [Google Scholar]
- 109.Fang Y, Schram G, Romanenko VG, Shi C, Conti L, Vandenberg CA, Davies PF, Nattel S, Levitan I. Functional expression of Kir2.x in human aortic endothelial cells: The dominant role of Kir2.2. Am J Physiol Cell Physiol 289: C1134–C1144, 2005. [DOI] [PubMed] [Google Scholar]
- 110.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A 107: 13450–13455, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature 447: 433–440, 2007. [DOI] [PubMed] [Google Scholar]
- 112.Feng S, Bowden N, Fragiadaki M, Souilhol C, Hsiao S, Mahmoud M, Allen S, Pirri D, Ayllon BT, Akhtar S, Thompson AAR, Jo H, Weber C, Ridger V, Schober A, Evans PC. Mechanical activation of hypoxia-inducible factor 1alpha drives endothelial dysfunction at atheroprone sites. Arterioscler Thromb Vasc Biol 37: 2087–2101, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol 281: L529–L533, 2001. [DOI] [PubMed] [Google Scholar]
- 114.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93: e136–e142, 2003. [DOI] [PubMed] [Google Scholar]
- 115.Fu BM, Tarbell JM. Mechano-sensing and transduction by endothelial surface glycocalyx: Composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med 5: 381–390, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fujiwara K Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J Intern Med 259: 373–380, 2006. [DOI] [PubMed] [Google Scholar]
- 117.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gawad C, Koh W, Quake SR. Single-cell genome sequencing: Current state of the science. Nat Rev Genet 17: 175–188, 2016. [DOI] [PubMed] [Google Scholar]
- 119.Gawlak G, Tian Y, O’Donnell JJ III, Tian X, Birukova AA, Birukov KG. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex. Faseb J 28: 3249–3260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol 13: 584–592, 2001. [DOI] [PubMed] [Google Scholar]
- 121.Geiger B, Bershadsky A. Exploring the neighborhood: Adhesion-coupled cell mechanosensors. Cell 110: 139–142, 2002. [DOI] [PubMed] [Google Scholar]
- 122.Gerhold KA, Schwartz MA. Ion channels in endothelial responses to fluid shear stress. Physiology (Bethesda) 31: 359–369, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giantsos-Adams KM, Koo AJ, Song S, Sakai J, Sankaran J, Shin JH, Garcia-Cardena G, Dewey CF Jr. Heparan sulfate regrowth profiles under laminar shear flow following enzymatic Degradation. Cell Mol Bioeng 6: 160–174, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: Are the pieces now in place? J Cell Biochem 104: 1964–1987, 2008. [DOI] [PubMed] [Google Scholar]
- 125.Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature 413: 194–202, 2001. [DOI] [PubMed] [Google Scholar]
- 126.Gillette TG, Hill JA. Readers, writers, and erasers: Chromatin as the whiteboard of heart disease. Circ Res 116: 1245–1253, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gimbrone MA Jr., Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118: 620–636, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, Fidler IJ, Pasqualini R, Tuder RM, Arap W. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem 283: 29447–29460, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giresi PG, Kim J, McDaniell RM, Iyer VR, Lieb JD. FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin. Genome Res 17: 877–885, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol 17: 26–33, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goettsch C, Goettsch W, Arsov A, Hofbauer LC, Bornstein SR, Morawietz H. Long-term cyclic strain downregulates endothelial Nox4. Antioxid Redox Signal 11: 2385–2397, 2009. [DOI] [PubMed] [Google Scholar]
- 132.Goetz JG, Steed E, Ferreira RR, Roth S, Ramspacher C, Boselli F, Charvin G, Liebling M, Wyart C, Schwab Y, Vermot J. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep 6: 799–808, 2014. [DOI] [PubMed] [Google Scholar]
- 133.Graham DM, Burridge K. Mechanotransduction and nuclear function. Curr Opin Cell Biol 40: 98–105, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A 90: 1937–1941, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92: e80–e86, 2003. [DOI] [PubMed] [Google Scholar]
- 136.Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem Biophys Res Commun 269: 781–786, 2000. [DOI] [PubMed] [Google Scholar]
- 137.Gurkan OU, O’Donnell C, Brower R, Ruckdeschel E, Becker PM. Differential effects of mechanical ventilatory strategy on lung injury and systemic organ inflammation in mice. Am J Physiol Lung Cell Mol Physiol 285: L710–L718, 2003. [DOI] [PubMed] [Google Scholar]
- 138.Haasper C, Jagodzinski M, Drescher M, Meller R, Wehmeier M, Krettek C, Hesse E. Cyclic strain induces FosB and initiates osteogenic differentiation of mesenchymal cells. Exp Toxicol Pathol 59: 355–363, 2008. [DOI] [PubMed] [Google Scholar]
- 139.Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech 40: 947–960, 2007. [DOI] [PubMed] [Google Scholar]
- 140.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10: 53–62, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hall A Rho GTPases and the actin cytoskeleton. Science 279: 509–514, 1998. [DOI] [PubMed] [Google Scholar]
- 142.Hammerschmidt S, Schiller J, Kuhn H, Meybaum M, Gessner C, Sandvoss T, Arnold K, Wirtz H. Influence of tidal volume on pulmonary NO release, tissue lipid peroxidation and surfactant phospholipids. Biochim Biophys Acta 1639: 17–26, 2003. [DOI] [PubMed] [Google Scholar]
- 143.Han B, Bai XH, Lodyga M, Xu J, Yang BB, Keshavjee S, Post M, Liu M. Conversion of mechanical force into biochemical signaling. J Biol Chem 279: 54793–54801, 2004. [DOI] [PubMed] [Google Scholar]
- 144.Han B, Lodyga M, Liu M. Ventilator-induced lung injury: Role of protein-protein interaction in mechanosensation. Proc Am Thorac Soc 2: 181–187, 2005. [DOI] [PubMed] [Google Scholar]
- 145.Han Z, Varadharaj S, Giedt RJ, Zweier JL, Szeto HH, Alevriadou BR. Mitochondria-derived reactive oxygen species mediate heme oxygenase-1 expression in sheared endothelial cells. J Pharmacol Exp Ther 329: 94–101, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haseneen NA, Vaday GG, Zucker S, Foda HD. Mechanical stretch induces MMP-2 release and activation in lung endothelium: Role of EMMPRIN. Am J Physiol Lung Cell Mol Physiol 284: L541–L547, 2003. [DOI] [PubMed] [Google Scholar]
- 147.Hatch EM, Hetzer MW. Nuclear envelope rupture is induced by actin-based nucleus confinement. J Cell Biol 215: 27–36, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Henderson-Toth CE, Jahnsen ED, Jamarani R, Al-Roubaie S, Jones EA. The glycocalyx is present as soon as blood flow is initiated and is required for normal vascular development. Dev Biol 369: 330–339, 2012. [DOI] [PubMed] [Google Scholar]
- 149.Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci 121: 2795–2804, 2008. [DOI] [PubMed] [Google Scholar]
- 150.Hirata H, Tatsumi H, Sokabe M. Zyxin emerges as a key player in the mechanotransduction at cell adhesive structures. Commun Integr Biol 1: 192–195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hirayama Y, Sumpio BE. Role of ligand-specific integrins in endothelial cell alignment and elongation induced by cyclic strain. Endothelium 14: 275–283, 2007. [DOI] [PubMed] [Google Scholar]
- 152.Hishikawa K, Luscher TF. Pulsatile stretch stimulates superoxide production in human aortic endothelial cells. Circulation 96: 3610–3616, 1997. [DOI] [PubMed] [Google Scholar]
- 153.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev 210: 171–186, 2006. [DOI] [PubMed] [Google Scholar]
- 154.Hoger JH, Ilyin VI, Forsyth S, Hoger A. Shear stress regulates the endothelial Kir2.1 ion channel. Proc Natl Acad Sci U S A 99: 7780–7785, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hosokawa H, Aiuchi S, Kambe T, Hagiwara Y, Kubo T. Mechanical stretch-induced mitogen-activated protein kinase activation is mediated via angiotensin and endothelin systems in vascular smooth muscle cells. Biol Pharm Bull 25: 1588–1592, 2002. [DOI] [PubMed] [Google Scholar]
- 156.Hovestadt V, Jones DT, Picelli S, Wang W, Kool M, Northcott PA, Sultan M, Stachurski K, Ryzhova M, Warnatz HJ, Ralser M, Brun S, Bunt J, Jager N, Kleinheinz K, Erkek S, Weber UD, Bartholomae CC, von Kalle C, Lawerenz C, Eils J, Koster J, Versteeg R, Milde T, Witt O, Schmidt S, Wolf S, Pietsch T, Rutkowski S, Scheurlen W, Taylor MD, Brors B, Felsberg J, Reifenberger G, Borkhardt A, Lehrach H, Wechsler-Reya RJ, Eils R, Yaspo ML, Landgraf P, Korshunov A, Zapatka M, Radlwimmer B, Pfister SM, Lichter P. Decoding the regulatory landscape of medulloblastoma using DNA methylation sequencing. Nature 510: 537–541, 2014. [DOI] [PubMed] [Google Scholar]
- 157.Hu Y, Bock G, Wick G, Xu Q. Activation of PDGF receptor alpha in vascular smooth muscle cells by mechanical stress. Faseb J 12: 1135–1142, 1998. [DOI] [PubMed] [Google Scholar]
- 158.Huang RT, Wu D, Meliton A, Oh MJ, Krause M, Lloyd JA, Nigdelioglu R, Hamanaka RB, Jain MK, Birukova A, Kress JP, Birukov KG, Mutlu GM, Fang Y. Experimental lung injury reduces Kruppel-like factor 2 to increase endothelial permeability via regulation of RAPGEF3-Rac1 signaling. Am J Respir Crit Care Med 195: 639–651, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15: 802–812, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Humphries MJ. Integrin structure. Biochem Soc Trans 28: 311–339, 2000. [PubMed] [Google Scholar]
- 161.Hunter O, Maul T, Vorp D, Amoscato AA. Mechanical strain induces ceramide generation in endothelial cells. FASEB J 21: A751-c-, 2007. [Google Scholar]
- 162.Ichimura H, Parthasarathi K, Quadri S, Issekutz AC, Bhattacharya J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J Clin Invest 111: 691–699, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med 35: 564–577, 2003. [DOI] [PubMed] [Google Scholar]
- 164.Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci 116: 1157–1173, 2003. [DOI] [PubMed] [Google Scholar]
- 165.Ingber DE. Tensegrity: The architectural basis of cellular mechanotransduction. Annu Rev Physiol 59: 575–599, 1997. [DOI] [PubMed] [Google Scholar]
- 166.Ingber DE, Wang N, Stamenovic D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep Prog Phys 77: 046603, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Inoue N, Kawashima S, Hirata KI, Rikitake Y, Takeshita S, Yamochi W, Akita H, Yokoyama M. Stretch force on vascular smooth muscle cells enhances oxidation of LDL via superoxide production. Am J Physiol 274: H1928–H1932, 1998. [DOI] [PubMed] [Google Scholar]
- 168.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol 164: 811–817, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ito S, Suki B, Kume H, Numaguchi Y, Ishii M, Iwaki M, Kondo M, Naruse K, Hasegawa Y, Sokabe M. Actin cytoskeleton regulates stretch-activated Ca2+ influx in human pulmonary microvascular endothelial cells. Am J Respir Cell Mol Biol 43: 26–34, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Iwaki M, Ito S, Morioka M, Iwata S, Numaguchi Y, Ishii M, Kondo M, Kume H, Naruse K, Sokabe M, Hasegawa Y. Mechanical stretch enhances IL-8 production in pulmonary microvascular endothelial cells. Biochem Biophys Res Commun 389: 531–536, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Iwasaki H, Eguchi S, Ueno H, Marumo F, Hirata Y. Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. Am J Physiol Heart Circ Physiol 278: H521–H529, 2000. [DOI] [PubMed] [Google Scholar]
- 172.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol 10: 63–73, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol 30: 662–670, 2004. [DOI] [PubMed] [Google Scholar]
- 174.Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet 33(Suppl): 245–254, 2003. [DOI] [PubMed] [Google Scholar]
- 175.Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology 9: 43–53, 2004. [DOI] [PubMed] [Google Scholar]
- 176.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269, 2005. [DOI] [PubMed] [Google Scholar]
- 177.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A 98: 1042–1046, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jia Z, Ikeda R, Ling J, Viatchenko-Karpinski V, Gu JG. Regulation of Piezo2 mechanotransduction by static plasma membrane tension in primary afferent neurons. J Biol Chem 291: 9087–9104, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang YZ, Jimenez JM, Ou K, McCormick ME, Zhang LD, Davies PF. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circ Res 115: 32–43, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. J Biol Chem 272: 1395–1401, 1997. [DOI] [PubMed] [Google Scholar]
- 181.Jones CI III, Han Z, Presley T, Varadharaj S, Zweier JL, Ilangovan G, Alevriadou BR. Endothelial cell respiration is affected by the oxygen tension during shear exposure: Role of mitochondrial peroxynitrite. Am J Physiol Cell Physiol 295: C180–C191, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jones PA. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat Rev Genet 13: 484–492, 2012. [DOI] [PubMed] [Google Scholar]
- 183.Kallakuri S, Yu JA, Li J, Li Y, Weinstein BM, Nicoli S, Sun Z. Endothelial cilia are essential for developmental vascular integrity in zebrafish. J Am Soc Nephrol 26: 864–875, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kaner RJ, Crystal RG. Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mol Med 7: 240–246, 2001. [PMC free article] [PubMed] [Google Scholar]
- 185.Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 22: 657–664, 2000. [DOI] [PubMed] [Google Scholar]
- 186.Karmpaliotis D, Kosmidou I, Ingenito EP, Hong K, Malhotra A, Sunday ME, Haley KJ. Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 283: L585–L595, 2002. [DOI] [PubMed] [Google Scholar]
- 187.Katanosaka Y, Bao JH, Komatsu T, Suemori T, Yamada A, Mohri S, Naruse K. Analysis of cyclic-stretching responses using cell-adhesion-patterned cells. J Biotechnol 133: 82–89, 2008. [DOI] [PubMed] [Google Scholar]
- 188.Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA. Effects of cell tension on the small GTPase Rac. J Cell Biol 158: 153–164, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem 279: 12001–12004, 2004. [DOI] [PubMed] [Google Scholar]
- 190.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci U S A 102: 15895–15900, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Khachigian LM, Resnick N, Gimbrone MA Jr., Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest 96: 1169–1175, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC, Hodzic D, Wirtz D. A perinuclear actin cap regulates nuclear shape. Proc Natl Acad Sci U S A 106: 19017–19022, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kheirolomoom A, Kim CW, Seo JW, Kumar S, Son DJ, Gagnon MK, Ingham ES, Ferrara KW, Jo H. Multifunctional nanoparticles facilitate molecular targeting and miRNA delivery to inhibit atherosclerosis in ApoE(−/−) mice. ACS Nano 9: 8885–8897, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. J Cell Biol 147: 831–844, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ko K, Arora P, Lee W, McCulloch C. Biochemical and functional characterization of intercellular adhesion and gap junctions in fibroblasts. Am J Physiol Cell Physiol 279: C147–C157, 2000. [DOI] [PubMed] [Google Scholar]
- 196.Ko KS, Arora PD, McCulloch CA. Cadherins mediate intercellular mechanical signaling in fibroblasts by activation of stretch-sensitive calcium-permeable channels. J Biol Chem 276: 35967–35977, 2001. [DOI] [PubMed] [Google Scholar]
- 197.Kobayashi S, Nagino M, Komatsu S, Naruse K, Nimura Y, Nakanishi M, Sokabe M. Stretch-induced IL-6 secretion from endothelial cells requires NF-kappaB activation. Biochem Biophys Res Commun 308: 306–312, 2003. [DOI] [PubMed] [Google Scholar]
- 198.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26: 1495–1502, 2006. [DOI] [PubMed] [Google Scholar]
- 199.Komuro I, Kudo S, Yamazaki T, Zou Y, Shiojima I, Yazaki Y. Mechanical stretch activates the stress-activated protein kinases in cardiac myocytes. Faseb J 10: 631–636, 1996. [DOI] [PubMed] [Google Scholar]
- 200.Koo A, Dewey CF Jr., Garcia-Cardena G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am J Physiol Cell Physiol 304: C137–C146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Koo A, Nordsletten D, Umeton R, Yankama B, Ayyadurai S, Garcia-Cardena G, Dewey CF Jr. In silico modeling of shear-stress-induced nitric oxide production in endothelial cells through systems biology. Biophys J 104: 2295–2306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kook SH, Jang YS, Lee JC. Involvement of JNK-AP-1 and ERK-NF-kappaB signaling in tension-stimulated expression of type I collagen and MMP-1 in human periodontal ligament fibroblasts. J Appl Physiol (1985) 111: 1575–1583, 2011. [DOI] [PubMed] [Google Scholar]
- 203.Kou B, Zhang J, Singer DR. Effects of cyclic strain on endothelial cell apoptosis and tubulogenesis are dependent on ROS production via NAD(P)H subunit p22phox. Microvasc Res 77: 125–133, 2009. [DOI] [PubMed] [Google Scholar]
- 204.Krause MD, Huang RT, Wu D, Shentu TP, Harrison DL, Whalen MB, Stolze LK, Di Rienzo A, Moskowitz IP, Civelek M, Romanoski CE, Fang Y. Genetic variant at coronary artery disease and ischemic stroke locus 1p32.2 regulates endothelial responses to hemodynamics. Proc Natl Acad Sci U S A 115: E11349–E11358, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kuebler WM, Uhlig U, Goldmann T, Schael G, Kerem A, Exner K, Martin C, Vollmer E, Uhlig S. Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am J Respir Crit Care Med 168: 1391–1398, 2003. [DOI] [PubMed] [Google Scholar]
- 206.Kuhn H, Kruger S, Hammerschmidt S, Wirtz H. High concentrations of vascular endothelial growth factor reduce stretch-induced apoptosis of alveolar type II cells. Respirology 15: 343–348, 2010. [DOI] [PubMed] [Google Scholar]
- 207.Kumagai R, Lu X, Kassab GS. Role of glycocalyx in flow-induced production of nitric oxide and reactive oxygen species. Free Radic Biol Med 47: 600–607, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kumar A, Knox AJ, Boriek AM. CCAAT/enhancer-binding protein and activator protein-1 transcription factors regulate the expression of interleukin-8 through the mitogen-activated protein kinase pathways in response to mechanical stretch of human airway smooth muscle cells. J Biol Chem 278: 18868–18876, 2003. [DOI] [PubMed] [Google Scholar]
- 209.Kuo CH, Leon L, Chung EJ, Huang RT, Sontag TJ, Reardon CA, Getz GS, Tirrell M, Fang Y. Inhibition of atherosclerosis-promoting microRNAs via targeted polyelectrolyte complex micelles. J Mater Chem B Mater Biol Med 2: 8142–8153, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol 170: 781–791, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lampugnani MG, Giorgi M, Gaboli M, Dejana E, Marchisio PC. Endothelial cell motility, integrin receptor clustering, and microfilament organization are inhibited by agents that increase intracellular cAMP. Lab Invest 63: 521–531, 1990. [PubMed] [Google Scholar]
- 212.Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun 201: 950–956, 1994. [DOI] [PubMed] [Google Scholar]
- 213.Lee DY, Lee CI, Lin TE, Lim SH, Zhou J, Tseng YC, Chien S, Chiu JJ. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc Natl Acad Sci U S A 109: 1967–1972, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell 11: 845–857, 2006. [DOI] [PubMed] [Google Scholar]
- 215.Lee ME, Dhadly MS, Temizer DH, Clifford JA, Yoshizumi M, Quertermous T. Regulation of endothelin-1 gene expression by Fos and Jun. J Biol Chem 266: 19034–19039, 1991. [PubMed] [Google Scholar]
- 216.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med 259: 381–392, 2006. [DOI] [PubMed] [Google Scholar]
- 217.Lehoux S, Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension 32: 338–345, 1998. [DOI] [PubMed] [Google Scholar]
- 218.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci U S A 96: 12010–12015, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lemarie CA, Tharaux PL, Esposito B, Tedgui A, Lehoux S. Transforming growth factor-alpha mediates nuclear factor kappaB activation in strained arteries. Circ Res 99: 434–441, 2006. [DOI] [PubMed] [Google Scholar]
- 220.Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife 4: pii: e12088, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad RK, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature 515: 279–282, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li LF, Liao SK, Lee CH, Tsai YH, Huang CC, Quinn DA. Ventilation-induced neutrophil infiltration and apoptosis depend on apoptosis signal-regulated kinase 1 pathway. Crit Care Med 33: 1913–1921, 2005. [DOI] [PubMed] [Google Scholar]
- 223.Li S, Kim M, Hu YL, Jalali S, Schlaepfer DD, Hunter T, Chien S, Shyy JY. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem 272: 30455–30462, 1997. [DOI] [PubMed] [Google Scholar]
- 224.Lim CS, Qiao X, Reslan OM, Xia Y, Raffetto JD, Paleolog E, Davies AH, Khalil RA. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J Vasc Surg 53: 764–773, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA Jr., Balasubramanian V, Garcia-Cardena G, Jain MK. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res 96: e48–e57, 2005. [DOI] [PubMed] [Google Scholar]
- 226.Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, Atkins GB, Nayak L, Cui Y, Finigan JH, Jain MK. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol 30: 1952–1959, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: Potential role of glycogen synthase kinase-3beta. FASEB J 16: 950–962, 2002. [DOI] [PubMed] [Google Scholar]
- 228.Liu M, Qin Y, Liu J, Tanswell AK, Post M. Mechanical strain induces pp60src activation and translocation to cytoskeleton in fetal rat lung cells. J Biol Chem 271: 7066–7071, 1996. [PubMed] [Google Scholar]
- 229.Liu M, Tanswell AK, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol 277: L667–L683, 1999. [DOI] [PubMed] [Google Scholar]
- 230.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res 101: e44–e52, 2007. [DOI] [PubMed] [Google Scholar]
- 231.Lo HM, Lai TH, Li CH, Wu WB. TNF-alpha induces CXCL1 chemokine expression and release in human vascular endothelial cells in vitro via two distinct signaling pathways. Acta Pharmacol Sin 35: 339–350, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem 286: 26743–26753, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lovett DB, Shekhar N, Nickerson JA, Roux KJ, Lele TP. Modulation of nuclear shape by substrate rigidity. Cell Mol Bioeng 6: 230–238, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia P, Maccario J, Boulanger CM, Mallat Z, Tedgui A. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res 114: 434–443, 2014. [DOI] [PubMed] [Google Scholar]
- 235.Lusis AJ. Atherosclerosis. Nature 407: 233–241, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Macalma T, Otte J, Hensler ME, Bockholt SM, Louis HA, Kalff-Suske M, Grzeschik KH, von der Ahe D, Beckerle MC. Molecular characterization of human zyxin. J Biol Chem 271: 31470–31478, 1996. [DOI] [PubMed] [Google Scholar]
- 237.Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol 138: 913–926, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mack JJ, Mosqueiro TS, Archer BJ, Jones WM, Sunshine H, Faas GC, Briot A, Aragon RL, Su T, Romay MC, McDonald AI, Kuo CH, Lizama CO, Lane TF, Zovein AC, Fang Y, Tarling EJ, de Aguiar Vallim TQ, Navab M, Fogelman AM, Bouchard LS, Iruela-Arispe ML. NOTCH1 is a mechanosensor in adult arteries. Nat Commun 8: 1620, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285: 895–898, 1999. [DOI] [PubMed] [Google Scholar]
- 240.Majno G, Shea SM, Leventhal M. Endothelial contraction induced by histamine-type mediators: An electron microscopic study. J Cell Biol 42: 647–672, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mambetsariev I, Tian Y, Wu T, Lavoie T, Solway J, Birukov KG, Birukova AA. Stiffness-activated GEF-H1 expression exacerbates LPS-induced lung inflammation. PLoS One 9: e92670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Maniotis. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A 94: 849–854, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Marconcini L, Marchio S, Morbidelli L, Cartocci E, Albini A, Ziche M, Bussolino F, Oliviero S. c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc Natl Acad Sci U S A 96: 9671–9676, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Martin T, Cardarelli PM, Parry GC, Felts KA, Cobb RR. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kappa B and AP-1. Eur J Immunol 27: 1091–1097, 1997. [DOI] [PubMed] [Google Scholar]
- 245.Mata-Greenwood E, Grobe A, Kumar S, Noskina Y, Black SM. Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-beta1 and reactive oxygen species: A requirement for NAD(P)H oxidase. Am J Physiol Lung Cell Mol Physiol 289: L288–L289, 2005. [DOI] [PubMed] [Google Scholar]
- 246.Matsushita H, Lee Kh K, Tsao PS. Cyclic strain induces reactive oxygen species production via an endothelial NAD(P)H oxidase. J Cell Biochem 81: 99–106, 2001. [DOI] [PubMed] [Google Scholar]
- 247.Maunakea AK, Chepelev I, Zhao K. Epigenome mapping in normal and disease States. Circ Res 107: 327–339, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.McClatchey PM, Schafer M, Hunter KS, Reusch JE. The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. Am J Physiol Heart Circ Physiol 311: H168–H176, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003. [DOI] [PubMed] [Google Scholar]
- 250.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 251.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: Opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277: 11497–11504, 2002. [DOI] [PubMed] [Google Scholar]
- 252.Methe H, Balcells M, Alegret Mdel C, Santacana M, Molins B, Hamik A, Jain MK, Edelman ER. Vascular bed origin dictates flow pattern regulation of endothelial adhesion molecule expression. Am J Physiol Heart Circ Physiol 292: H2167–H2175, 2007. [DOI] [PubMed] [Google Scholar]
- 253.Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet 11: 31–46, 2010. [DOI] [PubMed] [Google Scholar]
- 254.Metzler SA, Pregonero CA, Butcher JT, Burgess SC, Warnock JN. Cyclic strain regulates pro-inflammatory protein expression in porcine aortic valve endothelial cells. J Heart Valve Dis 17: 571–577; discussion 578, 2008. [PubMed] [Google Scholar]
- 255.Milkiewicz M, Mohammadzadeh F, Ispanovic E, Gee E, Haas TL. Static strain stimulates expression of matrix metalloproteinase-2 and VEGF in microvascular endothelium via JNK- and ERK-dependent pathways. J Cell Biochem 100: 750–761, 2007. [DOI] [PubMed] [Google Scholar]
- 256.Miller IJ, Bieker JJ. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol Cell Biol 13: 2776–2786, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 285: H722–H726, 2003. [DOI] [PubMed] [Google Scholar]
- 258.Mohler ER III, Fang Y, Shaffer RG, Moore J, Wilensky RL, Parmacek M, Levitan I. Hypercholesterolemia suppresses Kir channels in porcine bone marrow progenitor cells in vivo. Biochem Biophys Res Commun 358: 317–324, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mokres LM, Parai K, Hilgendorff A, Ertsey R, Alvira CM, Rabinovitch M, Bland RD. Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol 298: L23–L35, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Moldobaeva A, Jenkins J, Wagner E. Effects of distension on airway inflammation and venular P-selectin expression. Am J Physiol Lung Cell Mol Physiol 295: L941–L948, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Moon JJ, Matsumoto M, Patel S, Lee L, Guan JL, Li S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol 203: 166–176, 2005. [DOI] [PubMed] [Google Scholar]
- 262.Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn 232: 268–281, 2005. [DOI] [PubMed] [Google Scholar]
- 263.Morrow D, Cullen JP, Cahill PA, Redmond EM. Cyclic strain regulates the Notch/CBF-1 signaling pathway in endothelial cells: Role in angiogenic activity. Arterioscler Thromb Vasc Biol 27: 1289–1296, 2007. [DOI] [PubMed] [Google Scholar]
- 264.Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, de Visser M, Schwartz K. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum Mol Genet 9: 1453–1459, 2000. [DOI] [PubMed] [Google Scholar]
- 265.Mullins RD. How WASP-family proteins and the Arp2/3 complex convert intracellular signals into cytoskeletal structures. Curr Opin Cell Biol 12: 91–96, 2000. [DOI] [PubMed] [Google Scholar]
- 266.Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol 97: 1605–1617, 2004. [DOI] [PubMed] [Google Scholar]
- 267.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 114: 1504–1511, 2006. [DOI] [PubMed] [Google Scholar]
- 268.Naruse K, Sai X, Yokoyama N, Sokabe M. Uni-axial cyclic stretch induces c-src activation and translocation in human endothelial cells via SA channel activation. FEBS Lett 441: 111–115, 1998. [DOI] [PubMed] [Google Scholar]
- 269.Naruse K, Yamada T, Sai XR, Hamaguchi M, Sokabe M. Pp125FAK is required for stretch dependent morphological response of endothelial cells. Oncogene 17: 455–463, 1998. [DOI] [PubMed] [Google Scholar]
- 270.Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol 274: H1532–H1538, 1998. [DOI] [PubMed] [Google Scholar]
- 271.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. [DOI] [PubMed] [Google Scholar]
- 272.Nava MM, Fedele R, Raimondi MT. Computational prediction of strain-dependent diffusion of transcription factors through the cell nucleus. Biomech Model Mechanobiol 15: 983–993, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Nayebosadri A, Ji JY. Endothelial nuclear lamina is not required for glucocorticoid receptor nuclear import but does affect receptor-mediated transcription activation. Am J Physiol Cell Physiol 305: C309–C322, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ngu H, Feng Y, Lu L, Oswald SJ, Longmore GD, Yin FC. Effect of focal adhesion proteins on endothelial cell adhesion, motility and orientation response to cyclic strain. Ann Biomed Eng 38: 208–222, 2010. [DOI] [PubMed] [Google Scholar]
- 275.Nguyen KT, Frye SR, Eskin SG, Patterson C, Runge MS, McIntire LV. Cyclic strain increases protease-activated receptor-1 expression in vascular smooth muscle cells. Hypertension 38: 1038–1043, 2001. [DOI] [PubMed] [Google Scholar]
- 276.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol 300: H1762–H1769, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Nishimura K, Li W, Hoshino Y, Kadohama T, Asada H, Ohgi S, Sumpio BE. Role of AKT in cyclic strain-induced endothelial cell proliferation and survival. Am J Physiol Cell Physiol 290: C812–C821, 2006. [DOI] [PubMed] [Google Scholar]
- 278.Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care 12: R27, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Nonas SA, Miller I, Kawkitinarong K, Chatchavalvanich S, Gorshkova I, Bochkov VN, Leitinger N, Natarajan V, Garcia JG, Birukov KG. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med 173: 1130–1138, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Numaguchi Y, Huang S, Polte TR, Eichler GS, Wang N, Ingber DE. Caldesmon-dependent switching between capillary endothelial cell growth and apoptosis through modulation of cell shape and contractility. Angiogenesis 6: 55–64, 2003. [DOI] [PubMed] [Google Scholar]
- 281.Ohya Y, Adachi N, Nakamura Y, Setoguchi M, Abe I, Fujishima M. Stretch-activated channels in arterial smooth muscle of genetic hypertensive rats. Hypertension 31: 254–258, 1998. [DOI] [PubMed] [Google Scholar]
- 282.Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet 38: 1316–1322, 2006. [DOI] [PubMed] [Google Scholar]
- 283.Okada M, Matsumori A, Ono K, Furukawa Y, Shioi T, Iwasaki A, Matsushima K, Sasayama S. Cyclic stretch upregulates production of interleukin-8 and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol 18: 894–901, 1998. [DOI] [PubMed] [Google Scholar]
- 284.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature 331: 168–170, 1988. [DOI] [PubMed] [Google Scholar]
- 285.Oohira A, Wight TN, Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem 258: 2014–2021, 1983. [PubMed] [Google Scholar]
- 286.Oudin S, Pugin J. Role of MAP kinase activation in interleukin-8 production by human BEAS-2B bronchial epithelial cells submitted to cyclic stretch. Am J Respir Cell Mol Biol 27: 107–114, 2002. [DOI] [PubMed] [Google Scholar]
- 287.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A 104: 15619–15624, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pan J, Singh US, Takahashi T, Oka Y, Palm-Leis A, Herbelin BS, Baker KM. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol 202: 536–553, 2005. [DOI] [PubMed] [Google Scholar]
- 289.Papaiahgari S, Yerrapureddy A, Hassoun PM, Garcia JG, Birukov KG, Reddy SP. EGFR-activated signaling and actin remodeling regulate cyclic stretch-induced NRF2-ARE activation. Am J Respir Cell Mol Biol 36: 304–312, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Park H, Go YM, St John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem 273: 32304–32311, 1998. [DOI] [PubMed] [Google Scholar]
- 291.Park JM, Adam RM, Peters CA, Guthrie PD, Sun Z, Klagsbrun M, Freeman MR. AP-1 mediates stretch-induced expression of HB-EGF in bladder smooth muscle cells. Am J Physiol 277: C294–C301, 1999. [DOI] [PubMed] [Google Scholar]
- 292.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA Jr., Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest 116: 49–58, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA Jr., Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem 280: 26714–26719, 2005. [DOI] [PubMed] [Google Scholar]
- 294.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005. [DOI] [PubMed] [Google Scholar]
- 295.Pendurthi UR, Williams JT, Rao LV. Inhibition of tissue factor gene activation in cultured endothelial cells by curcumin. Suppression of activation of transcription factors Egr-1, AP-1, and NF-kappa B. Arterioscler Thromb Vasc Biol 17: 3406–3413, 1997. [DOI] [PubMed] [Google Scholar]
- 296.Petrache I, Birukov K, Zaiman AL, Crow MT, Deng H, Wadgaonkar R, Romer LH, Garcia JG. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. FASEB J 17: 407–416, 2003. [DOI] [PubMed] [Google Scholar]
- 297.Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol 28: 574–581, 2003. [DOI] [PubMed] [Google Scholar]
- 298.Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun 306: 244–249, 2003. [DOI] [PubMed] [Google Scholar]
- 299.Peverali FA, Basdra EK, Papavassiliou AG. Stretch-mediated activation of selective MAPK subtypes and potentiation of AP-1 binding in human osteoblastic cells. Mol Med 7: 68–78, 2001. [PMC free article] [PubMed] [Google Scholar]
- 300.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res 89: 453–460, 2001. [DOI] [PubMed] [Google Scholar]
- 301.Ponts N, Harris EY, Prudhomme J, Wick I, Eckhardt-Ludka C, Hicks GR, Hardiman G, Lonardi S, Le Roch KG. Nucleosome landscape and control of transcription in the human malaria parasite. Genome Res 20: 228–238, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Poon C, Chowdhuri S, Kuo CH, Fang Y, Alenghat FJ, Hyatt D, Kani K, Gross ME, Chung EJ. Protein mimetic and anticancer properties of monocyte-targeting peptide amphiphile micelles. ACS Biomater Sci Eng 3: 3273–3282, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch 440: 653–666, 2000. [DOI] [PubMed] [Google Scholar]
- 304.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol 275: L1040–L1050, 1998. [DOI] [PubMed] [Google Scholar]
- 305.Putnam AJ, Cunningham JJ, Pillemer BB, Mooney DJ. External mechanical strain regulates membrane targeting of Rho GTPases by controlling the state of microtubule polymerization. Am J Physiol Cell Physiol 284: C627–C639, 2003. [DOI] [PubMed] [Google Scholar]
- 306.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: A protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 284: L972–L980, 2003. [DOI] [PubMed] [Google Scholar]
- 307.Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YS, Chien S, Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc Natl Acad Sci U S A 107: 3240–3244, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Quesnel C, Marchand-Adam S, Fabre A, Marchal-Somme J, Philip I, Lasocki S, Lecon V, Crestani B, Dehoux M. Regulation of hepatocyte growth factor secretion by fibroblasts in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 294: L334–L343, 2008. [DOI] [PubMed] [Google Scholar]
- 309.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 17: 47–62, 2016. [DOI] [PubMed] [Google Scholar]
- 310.Quinn TP, Schlueter M, Soifer SJ, Gutierrez JA. Cyclic mechanical stretch induces VEGF and FGF-2 expression in pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 282: L897–L903, 2002. [DOI] [PubMed] [Google Scholar]
- 311.Raaz U, Kuhn H, Wirtz H, Hammerschmidt S. Rapamycin reduces high-amplitude, mechanical stretch-induced apoptosis in pulmonary microvascular endothelial cells. Microvasc Res 77: 297–303, 2009. [DOI] [PubMed] [Google Scholar]
- 312.Ramskold D, Luo S, Wang YC, Li R, Deng Q, Faridani OR, Daniels GA, Khrebtukova I, Loring JF, Laurent LC, Schroth GP, Sandberg R. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat Biotechnol 30: 777–782, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8: 2281–2308, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: A randomized controlled trial. Jama 282: 54–61, 1999. [DOI] [PubMed] [Google Scholar]
- 315.Rapraeger AC. Syndecan-regulated receptor signaling. J Cell Biol 149: 995–998, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Reddy SP, Hassoun PM, Brower R. Redox imbalance and ventilator-induced lung injury. Antioxid Redox Signal 9: 2003–2012, 2007. [DOI] [PubMed] [Google Scholar]
- 317.Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J Suppl 42: 2s–9s, 2003. [DOI] [PubMed] [Google Scholar]
- 318.Riehl BD, Park JH, Kwon IK, Lim JY. Mechanical stretching for tissue engineering: Two-dimensional and three-dimensional constructs. Tissue Eng Part B Rev 18: 288–300, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153: 1175–1186, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Rivera CM, Ren B. Mapping human epigenomes. Cell 155: 39–55, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 108(Pt 6): 2369–2379, 1995. [DOI] [PubMed] [Google Scholar]
- 322.Rodriguez-Pascual F, Redondo-Horcajo M, Lamas S. Functional cooperation between Smad proteins and activator protein-1 regulates transforming growth factor-beta-mediated induction of endothelin-1 expression. Circ Res 92: 1288–1295, 2003. [DOI] [PubMed] [Google Scholar]
- 323.Romanoski CE, Glass CK, Stunnenberg HG, Wilson L, Almouzni G. Epigenomics: Roadmap for regulation. Nature 518: 314–316, 2015. [DOI] [PubMed] [Google Scholar]
- 324.Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest 99: 2062–2070, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA. Integrins in mechanotransduction. Curr Opin Cell Biol 25: 613–618, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sackin H Stretch-activated ion channels. Kidney Int 48: 1134–1147, 1995. [DOI] [PubMed] [Google Scholar]
- 327.Sai X, Naruse K, Sokabe M. Activation of pp60(src) is critical for stretch-induced orienting response in fibroblasts. J Cell Sci 112: 1365–1373, 1999. [DOI] [PubMed] [Google Scholar]
- 328.Salameh A, Wustmann A, Karl S, Blanke K, Apel D, Rojas-Gomez D, Franke H, Mohr FW, Janousek J, Dhein S. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ Res 106: 1592–1602, 2010. [DOI] [PubMed] [Google Scholar]
- 329.Salina D, Bodoor K, Eckley DM, Schroer TA, Rattner JB, Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell 108: 97–107, 2002. [DOI] [PubMed] [Google Scholar]
- 330.Sasamoto A, Nagino M, Kobayashi S, Naruse K, Nimura Y, Sokabe M. Mechanotransduction by integrin is essential for IL-6 secretion from endothelial cells in response to uniaxial continuous stretch. Am J Physiol Cell Physiol 288: C1012–C1022, 2005. [DOI] [PubMed] [Google Scholar]
- 331.Sawada Y, Sheetz MP. Force transduction by Triton cytoskeletons. J Cell Biol 156: 609–615, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127: 1015–1026, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Scheitlin CG, Julian JA, Shanmughapriya S, Madesh M, Tsoukias NM, Alevriadou BR. Endothelial mitochondria regulate the intracellular Ca2+ response to fluid shear stress. Am J Physiol Cell Physiol 310: C479–C490, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem 272: 13189–13195, 1997. [DOI] [PubMed] [Google Scholar]
- 335.Seebach J, Dieterich P, Luo F, Schillers H, Vestweber D, Oberleithner H, Galla HJ, Schnittler HJ. Endothelial barrier function under laminar fluid shear stress. Lab Invest 80: 1819–1831, 2000. [DOI] [PubMed] [Google Scholar]
- 336.Segurola RJ Jr., Oluwole B, Mills I, Yokoyama C, Tanabe T, Kito H, Nakajima N, Sumpio BE. Cyclic strain is a weak inducer of prostacyclin synthase expression in bovine aortic endothelial cells. J Surg Res 69: 135–138, 1997. [DOI] [PubMed] [Google Scholar]
- 337.Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol 145: 837–849, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Semenza GL. Hypoxia-inducible factors: Coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J 36: 252–259, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA Jr., Garcia-Cardena G, Jain MK. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 199: 1305–1315, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 112: 720–726, 2005. [DOI] [PubMed] [Google Scholar]
- 341.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136, 2002. [DOI] [PubMed] [Google Scholar]
- 342.Shen B, Smith RS Jr., Hsu YT, Chao L, Chao J. Kruppel-like factor 4 is a novel mediator of Kallistatin in inhibiting endothelial inflammation via increased endothelial nitric-oxide synthase expression. J Biol Chem 284: 35471–35478, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Shi F, Chiu YJ, Cho Y, Bullard TA, Sokabe M, Fujiwara K. Down-regulation of ERK but not MEK phosphorylation in cultured endothelial cells by repeated changes in cyclic stretch. Cardiovasc Res 73: 813–822, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res 304: 40–49, 2005. [DOI] [PubMed] [Google Scholar]
- 345.Shimizu N, Yamamoto K, Obi S, Kumagaya S, Masumura T, Shimano Y, Naruse K, Yamashita JK, Igarashi T, Ando J. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor beta. J Appl Physiol 104: 766–772, 2008. [DOI] [PubMed] [Google Scholar]
- 346.Shirinsky VP, Antonov AS, Birukov KG, Sobolevsky AV, Romanov YA, Kabaeva NV, Antonova GN, Smirnov VN. Mechano-chemical control of human endothelium orientation and size. J Cell Biol 109: 331–339, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Shukla A, Dunn AR, Moses MA, Van Vliet KJ. Endothelial cells as mechanical transducers: Enzymatic activity and network formation under cyclic strain. Mech Chem Biosyst 1: 279–290, 2004. [PubMed] [Google Scholar]
- 348.Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M, Nakada M, Muzykantov VR. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. Faseb J 25: 348–357, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91: 769–775, 2002. [DOI] [PubMed] [Google Scholar]
- 350.Sidhu SS, Koide S. Phage display for engineering and analyzing protein interaction interfaces. Curr Opin Struct Biol 17: 481–487, 2007. [DOI] [PubMed] [Google Scholar]
- 351.Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem 281: 34381–34393, 2006. [DOI] [PubMed] [Google Scholar]
- 352.Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JG. CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J Biol Chem 282: 30643–30657, 2007. [DOI] [PubMed] [Google Scholar]
- 353.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 369: 2126–2136, 2013. [DOI] [PubMed] [Google Scholar]
- 354.Smith JD, Davies N, Willis AI, Sumpio BE, Zilla P. Cyclic stretch induces the expression of vascular endothelial growth factor in vascular smooth muscle cells. Endothelium 8: 41–48, 2001. [DOI] [PubMed] [Google Scholar]
- 355.Smith MA, Blankman E, Gardel ML, Luettjohann L, Waterman CM, Beckerle MC. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev Cell 19: 365–376, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Smith PG, Roy C, Zhang YN, Chauduri S. Mechanical stress increases RhoA activation in airway smooth muscle cells. Am J Respir Cell Mol Biol 28: 436–442, 2003. [DOI] [PubMed] [Google Scholar]
- 357.Smith PG, Tokui T, Ikebe M. Mechanical strain increases contractile enzyme activity in cultured airway smooth muscle cells. Am J Physiol 268: L999–L1005, 1995. [DOI] [PubMed] [Google Scholar]
- 358.Sokabe M, Naruse K, Sai S, Yamada T, Kawakami K, Inoue M, Murase K, Miyazu M. Mechanotransduction and intracellular signaling mechanisms of stretch-induced remodeling in endothelial cells. Heart Vessels (Suppl 12): 191–193, 1997. [PubMed] [Google Scholar]
- 359.Sokabe M, Nunogaki K, Naruse K, Soga H. Mechanics of patch clamped and intact cell-membranes in relation to SA channel activation. Jpn J Physiol 43(Suppl 1): S197–S204, 1993. [PubMed] [Google Scholar]
- 360.Son DJ, Kumar S, Takabe W, Kim CW, Ni CW, Alberts-Grill N, Jang IH, Kim S, Kim W, Won Kang S, Baker AH, Woong Seo J, Ferrara KW, Jo H. The atypical mechanosensitive microRNA-712 derived from preribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun 4: 3000, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Song KH, Lee J, Park H, Kim HM, Park J, Kwon KW, Doh J. Roles of endothelial A-type lamins in migration of T cells on and under endothelial layers. Sci Rep 6: 23412, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Song L, Crawford GE. DNase-seq: A high-resolution technique for mapping active gene regulatory elements across the genome from mammalian cells. Cold Spring Harb Protoc 2010: pdb.prot5384, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Sooranna SR, Lee Y, Kim LU, Mohan AR, Bennett PR, Johnson MR. Mechanical stretch activates type 2 cyclooxygenase via activator protein-1 transcription factor in human myometrial cells. Mol Hum Reprod 10: 109–113, 2004. [DOI] [PubMed] [Google Scholar]
- 364.Sotoudeh M, Li YS, Yajima N, Chang CC, Tsou TC, Wang Y, Usami S, Ratcliffe A, Chien S, Shyy JY. Induction of apoptosis in vascular smooth muscle cells by mechanical stretch. Am J Physiol Heart Circ Physiol 282: H1709–H1716, 2002. [DOI] [PubMed] [Google Scholar]
- 365.Spitz F, Furlong EE. Transcription factors: From enhancer binding to developmental control. Nat Rev Genet 13: 613–626, 2012. [DOI] [PubMed] [Google Scholar]
- 366.Starr DA. A nuclear-envelope bridge positions nuclei and moves chromosomes. J Cell Sci 122: 577–586, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Stern JB, Fierobe L, Paugam C, Rolland C, Dehoux M, Petiet A, Dombret MC, Mantz J, Aubier M, Crestani B. Keratinocyte growth factor and hepatocyte growth factor in bronchoalveolar lavage fluid in acute respiratory distress syndrome patients. Crit Care Med 28: 2326–2333, 2000. [DOI] [PubMed] [Google Scholar]
- 368.Stevens TJ, Lando D, Basu S, Atkinson LP, Cao Y, Lee SF, Leeb M, Wohlfahrt KJ, Boucher W, O’Shaughnessy-Kirwan A, Cramard J, Faure AJ, Ralser M, Blanco E, Morey L, Sanso M, Palayret MGS, Lehner B, Di Croce L, Wutz A, Hendrich B, Klenerman D, Laue ED. 3D structures of individual mammalian genomes studied by single-cell Hi-C. Nature 544: 59–64, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 13: 237–247, 2006. [DOI] [PubMed] [Google Scholar]
- 370.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: Implications for atherogenesis. Arterioscler Thromb Vasc Biol 27: 346–351, 2007. [DOI] [PubMed] [Google Scholar]
- 371.Suresh Babu S, Wojtowicz A, Freichel M, Birnbaumer L, Hecker M, Cattaruzza M. Mechanism of stretch-induced activation of the mechanotransducer zyxin in vascular cells. Sci Signal 5: ra91, 2012. [DOI] [PubMed] [Google Scholar]
- 372.Suzuki M, Naruse K, Asano Y, Okamoto T, Nishikimi N, Sakurai T, Nimura Y, Sokabe M. Up-regulation of integrin beta 3 expression by cyclic stretch in human umbilical endothelial cells. Biochem Biophys Res Commun 239: 372–376, 1997. [DOI] [PubMed] [Google Scholar]
- 373.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341: 1240104, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev 17: 1–6, 2003. [DOI] [PubMed] [Google Scholar]
- 375.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006. [DOI] [PubMed] [Google Scholar]
- 376.Takeda H, Komori K, Nishikimi N, Nimura Y, Sokabe M, Naruse K. Bi-phasic activation of eNOS in response to uni-axial cyclic stretch is mediated by differential mechanisms in BAECs. Life Sci 79: 233–239, 2006. [DOI] [PubMed] [Google Scholar]
- 377.Tamura K, Chen YE, Lopez-Ilasaca M, Daviet L, Tamura N, Ishigami T, Akishita M, Takasaki I, Tokita Y, Pratt RE, Horiuchi M, Dzau VJ, Umemura S. Molecular mechanism of fibronectin gene activation by cyclic stretch in vascular smooth muscle cells. J Biol Chem 275: 34619–34627, 2000. [DOI] [PubMed] [Google Scholar]
- 378.Tanabe Y, Saito M, Ueno A, Nakamura M, Takeishi K, Nakayama K. Mechanical stretch augments PDGF receptor beta expression and protein tyrosine phosphorylation in pulmonary artery tissue and smooth muscle cells. Mol Cell Biochem 215: 103–113, 2000. [DOI] [PubMed] [Google Scholar]
- 379.Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng 16: 505–532, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol 15: 703–708, 2014. [DOI] [PubMed] [Google Scholar]
- 381.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: A “bumper-car” model. Proc Natl Acad Sci U S A 101: 16483–16488, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104: 1123–1130, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Thomas RA, Norman JC, Huynh TT, Williams B, Bolton SJ, Wardlaw AJ. Mechanical stretch has contrasting effects on mediator release from bronchial epithelial cells, with a rho-kinase-dependent component to the mechanotransduction pathway. Respir Med 100: 1588–1597, 2006. [DOI] [PubMed] [Google Scholar]
- 384.Timpson P, Jones GE, Frame MC, Brunton VG. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr Biol 11: 1836–1846, 2001. [DOI] [PubMed] [Google Scholar]
- 385.Tkachenko E, Gutierrez E, Saikin SK, Fogelstrand P, Kim C, Groisman A, Ginsberg MH. The nucleus of endothelial cell as a sensor of blood flow direction. Biol Open 2: 1007–1012, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Tong J, Helmy M, Cavalli FM, Jin L, St-Germain J, Karisch R, Taylor P, Minden MD, Taylor MD, Neel BG, Bader GD, Moran MF. Integrated analysis of proteome, phosphotyrosine-proteome, tyrosine-kinome, and tyrosine-phosphatome in acute myeloid leukemia. Proteomics 17: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99: 944–952, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. Embo J 21: 6791–6800, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005. [DOI] [PubMed] [Google Scholar]
- 390.Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435: 646–651, 2005. [DOI] [PubMed] [Google Scholar]
- 391.Van der Heiden K, Hierck BP, Krams R, de Crom R, Cheng C, Baiker M, Pourquie MJ, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis 196: 542–550, 2008. [DOI] [PubMed] [Google Scholar]
- 392.van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VW. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol 23: 211–217, 2003. [DOI] [PubMed] [Google Scholar]
- 393.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: Role of Rho kinase and protein tyrosine kinases. Circ Res 87: 335–340, 2000. [DOI] [PubMed] [Google Scholar]
- 394.van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: Opportunities and obstacles. Nat Rev Drug Discov 11: 860–872, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Vaquerizas JM, Kummerfeld SK, Teichmann SA, Luscombe NM. A census of human transcription factors: Function, expression and evolution. Nat Rev Genet 10: 252–263, 2009. [DOI] [PubMed] [Google Scholar]
- 396.Verghese GM, McCormick-Shannon K, Mason RJ, Matthay MA. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. Biologic and clinical significance. Am J Respir Crit Care Med 158: 386–394, 1998. [DOI] [PubMed] [Google Scholar]
- 397.Versaevel M, Braquenier JB, Riaz M, Grevesse T, Lantoine J, Gabriele S. Super-resolution microscopy reveals LINC complex recruitment at nuclear indentation sites. Sci Rep 4: 7362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Villar J, Flores C, Mendez-Alvarez S. Genetic susceptibility to acute lung injury. Crit Care Med 31: S272–S275, 2003. [DOI] [PubMed] [Google Scholar]
- 399.Villarreal G Jr., Zhang Y, Larman HB, Gracia-Sancho J, Koo A, Garcia-Cardena G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun 391: 984–989, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 79: 581–589, 1996. [DOI] [PubMed] [Google Scholar]
- 401.Vouret-Craviari V, Boquet P, Pouyssegur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: Role of Rho proteins in endothelial barrier function. Mol Biol Cell 9: 2639–2653, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Voyvodic PL, Min D, Liu R, Williams E, Chitalia V, Dunn AK, Baker AB. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem 289: 9547–9559, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Wagner AH, Kautz O, Fricke K, Zerr-Fouineau M, Demicheva E, Guldenzoph B, Bermejo JL, Korff T, Hecker M. Upregulation of glutathione peroxidase offsets stretch-induced proatherogenic gene expression in human endothelial cells. Arterioscler Thromb Vasc Biol 29: 1894–1901, 2009. [DOI] [PubMed] [Google Scholar]
- 404.Wang BW, Chang H, Lin S, Kuan P, Shyu KG. Induction of matrix metalloproteinases-14 and -2 by cyclical mechanical stretch is mediated by tumor necrosis factor-alpha in cultured human umbilical vein endothelial cells. Cardiovasc Res 59: 460–469, 2003. [DOI] [PubMed] [Google Scholar]
- 405.Wang JG, Miyazu M, Xiang P, Li SN, Sokabe M, Naruse K. Stretch-induced cell proliferation is mediated by FAK-MAPK pathway. Life Sci 76: 2817–2825, 2005. [DOI] [PubMed] [Google Scholar]
- 406.Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, Wang N, Shyy JY, Li YS, Chien S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci U S A 107: 3234–3239, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Wang KC, Yeh YT, Nguyen P, Limqueco E, Lopez J, Thorossian S, Guan KL, Li YJ, Chien S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci U S A 113: 11525–11530, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak KK, Tong KK, Kwan KM, Wang N, Chiu JJ, Zhu Y, Huang Y. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 2016. [DOI] [PubMed] [Google Scholar]
- 409.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124–1127, 1993. [DOI] [PubMed] [Google Scholar]
- 410.Ware LB, Kaner RJ, Crystal RG, Schane R, Trivedi NN, McAuley D, Matthay MA. VEGF levels in the alveolar compartment do not distinguish between ARDS and hydrostatic pulmonary oedema. Eur Respir J 26: 101–105, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Waters CM. Reactive oxygen species in mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287: L484–L485, 2004. [DOI] [PubMed] [Google Scholar]
- 412.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9: 121–167, 2007. [DOI] [PubMed] [Google Scholar]
- 413.Wernig F, Mayr M, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by beta1-integrin signaling pathways. Hypertension 41: 903–911, 2003. [DOI] [PubMed] [Google Scholar]
- 414.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int 20: 73–81, 1996. [DOI] [PubMed] [Google Scholar]
- 415.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol 161: 429–439, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Wojtowicz A, Babu SS, Li L, Gretz N, Hecker M, Cattaruzza M. Zyxin mediation of stretch-induced gene expression in human endothelial cells. Circ Res 107: 898–902, 2010. [DOI] [PubMed] [Google Scholar]
- 417.Wu C, Huang RT, Kuo CH, Kumar S, Kim CW, Lin YC, Chen YJ, Birukova A, Birukov KG, Dulin NO, Civelek M, Lusis AJ, Loyer X, Tedgui A, Dai G, Jo H, Fang Y. Mechanosensitive PPAP2B regulates endothelial responses to atherorelevant hemodynamic forces. Circ Res 117: e41–e53, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wu D, Huang RT, Hamanaka RB, Krause M, Oh MJ, Kuo CH, Nigdelioglu R, Meliton AY, Witt L, Dai G, Civelek M, Prabhakar NR, Fang Y, Mutlu GM. HIF-1alpha is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. Elife 6: pii: e25217, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G Jr., Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-dependent regulation of Kruppel-like factor 2 is mediated by MicroRNA-92a. Circulation 124: 633–641, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Wung BS, Cheng JJ, Chao YJ, Hsieh HJ, Wang DL. Modulation of Ras/Raf/extracellular signal-regulated kinase pathway by reactive oxygen species is involved in cyclic strain-induced early growth response-1 gene expression in endothelial cells. Circ Res 84: 804–812, 1999. [DOI] [PubMed] [Google Scholar]
- 421.Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res 81: 1–7, 1997. [DOI] [PubMed] [Google Scholar]
- 422.Xue Z, Huang K, Cai C, Cai L, Jiang CY, Feng Y, Liu Z, Zeng Q, Cheng L, Sun YE, Liu JY, Horvath S, Fan G. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 500: 593–597, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Yamamoto H, Teramoto H, Uetani K, Igawa K, Shimizu E. Stretch induces a growth factor in alveolar cells via protein kinase. Respir Physiol 127: 105–111, 2001. [DOI] [PubMed] [Google Scholar]
- 424.Yamanaka S A fresh look at iPS cells. Cell 137: 13–17, 2009. [DOI] [PubMed] [Google Scholar]
- 425.Yamazaki T, Tobe K, Hoh E, Maemura K, Kaida T, Komuro I, Tamemoto H, Kadowaki T, Nagai R, Yazaki Y. Mechanical loading activates mitogen-activated protein kinase and S6 peptide kinase in cultured rat cardiac myocytes. J Biol Chem 268: 12069–12076, 1993. [PubMed] [Google Scholar]
- 426.Yang JH, Briggs WH, Libby P, Lee RT. Small mechanical strains selectively suppress matrix metalloproteinase-1 expression by human vascular smooth muscle cells. J Biol Chem 273: 6550–6555, 1998. [DOI] [PubMed] [Google Scholar]
- 427.Yang JJ, Chen YM, Kurokawa T, Gong JP, Onodera S, Yasuda K. Gene expression, glycocalyx assay, and surface properties of human endothelial cells cultured on hydrogel matrix with sulfonic moiety: Effect of elasticity of hydrogel. J Biomed Mater Res A 95: 531–542, 2010. [DOI] [PubMed] [Google Scholar]
- 428.Yano Y, Saito Y, Narumiya S, Sumpio BE. Involvement of rho p21 in cyclic strain-induced tyrosine phosphorylation of focal adhesion kinase (pp125FAK), morphological changes and migration of endothelial cells. Biochem Biophys Res Commun 224: 508–515, 1996. [DOI] [PubMed] [Google Scholar]
- 429.Yao Y, Rabodzey A, Dewey CF Jr. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol 293: H1023–H1030, 2007. [DOI] [PubMed] [Google Scholar]
- 430.Yen W, Cai B, Yang J, Zhang L, Zeng M, Tarbell JM, Fu BM. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS One 10: e0117133, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol 171: 209–215, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, Shen Y, Pervouchine DD, Djebali S, Thurman RE, Kaul R, Rynes E, Kirilusha A, Marinov GK, Williams BA, Trout D, Amrhein H, Fisher-Aylor K, Antoshechkin I, DeSalvo G, See LH, Fastuca M, Drenkow J, Zaleski C, Dobin A, Prieto P, Lagarde J, Bussotti G, Tanzer A, Denas O, Li K, Bender MA, Zhang M, Byron R, Groudine MT, McCleary D, Pham L, Ye Z, Kuan S, Edsall L, Wu YC, Rasmussen MD, Bansal MS, Kellis M, Keller CA, Morrissey CS, Mishra T, Jain D, Dogan N, Harris RS, Cayting P, Kawli T, Boyle AP, Euskirchen G, Kundaje A, Lin S, Lin Y, Jansen C, Malladi VS, Cline MS, Erickson DT, Kirkup VM, Learned K, Sloan CA, Rosenbloom KR, Lacerda de Sousa B, Beal K, Pignatelli M, Flicek P, Lian J, Kahveci T, Lee D, Kent WJ, Ramalho Santos M, Herrero J, Notredame C, Johnson A, Vong S, Lee K, Bates D, Neri F, Diegel M, Canfield T, Sabo PJ, Wilken MS, Reh TA, Giste E, Shafer A, Kutyavin T, Haugen E, Dunn D, Reynolds AP, Neph S, Humbert R, Hansen RS, De Bruijn M, Selleri L, Rudensky A, Josefowicz S, Samstein R, Eichler EE, Orkin SH, Levasseur D, Papayannopoulou T, Chang KH, Skoultchi A, Gosh S, Disteche C, Treuting P, Wang Y, Weiss MJ, Blobel GA, Cao X, Zhong S, Wang T, Good PJ, Lowdon RF, Adams LB, Zhou XQ, Pazin MJ, Feingold EA, Wold B, Taylor J, Mortazavi A, Weissman SM, Stamatoyannopoulos JA, Snyder MP, Guigo R, Gingeras TR, Gilbert DM, Hardison RC, Beer MA, Ren B. A comparative encyclopedia of DNA elements in the mouse genome. Nature 515: 355–364, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Yung YC, Chae J, Buehler MJ, Hunter CP, Mooney DJ. Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc Natl Acad Sci U S A 106: 15279–15284, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Zeng Y, Tarbell JM. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One 9: e86249, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat Struct Mol Biol 20: 259–266, 2013. [DOI] [PubMed] [Google Scholar]
- 436.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal 19: 1690–1700, 2007. [DOI] [PubMed] [Google Scholar]
- 437.Zhao H, Hiroi T, Hansen BS, Rade JJ. Cyclic stretch induces cyclooxygenase-2 gene expression in vascular endothelial cells via activation of nuclear factor kappa-beta. Biochem Biophys Res Commun 389: 599–601, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Zheng W, Christensen LP, Tomanek RJ. Stretch induces upregulation of key tyrosine kinase receptors in microvascular endothelial cells. Am J Physiol Heart Circ Physiol 287: H2739–H2745, 2004. [DOI] [PubMed] [Google Scholar]
- 439.Zhou J, Li YS, Wang KC, Chien S. Epigenetic mechanism in regulation of endothelial function by disturbed flow: Induction of DNA hypermethylation by DNMT1. Cell Mol Bioeng 7: 218–224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Zhou RH, Lee TS, Tsou TC, Rannou F, Li YS, Chien S, Shyy JY. Stent implantation activates Akt in the vessel wall: Role of mechanical stretch in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 23: 2015–2020, 2003. [DOI] [PubMed] [Google Scholar]
- 441.Zimmermann P, David G. The syndecans, tuners of transmembrane signaling. FASEB J 13(Suppl): S91–S100, 1999. [DOI] [PubMed] [Google Scholar]