Abstract
Selective estrogen receptor modulators (SERMs) are potentially useful in treating various endometrial disorders, including endometrial cancer, as they block some of the detrimental effects of estrogen. It remains unclear whether each SERM regulates a unique subset of genes and, if so, whether the combination of a SERM and 17β-estradiol has an additive or synergistic effect on gene expression. We performed microarray analysis with Affymetrix Mouse Genome 430 2.0 short oligomer arrays to determine gene expression changes in uteri of ovariectomized mice treated with estradiol (low and high dose), methyl-piperidino-pyrazole (MPP), ICI 182 780, raloxifene, and combinations of high dose of estradiol with one of the SERM and dimethyl sulfoxide (DMSO) vehicle control. The nine treatments clustered into two groups, with MPP, raloxifene, and high dose of estradiol in one, and low dose of estradiol, ICI + estradiol, ICI, MPP + estradiol, and raloxifene + estradiol in the second group. Surprisingly, combining a high dose of estradiol with a SERM markedly increased (P<0.02) the number of regulated genes compared with each individual treatment. Analysis of expression for selected genes in uteri of estradiol and SERM-treated mice by quantitative (Q)RT-PCR generally supported the microarray results. For some cancer-associated genes, including Klk1, Ihh, Cdc45l, and Cdca8, administration of MPP or raloxifene with estradiol resulted in greater expression than estradiol alone (P<0.05). By contrast, IC1182 780 suppressed more genes governing DNA replication compared with MPP and raloxifene treatments. Therefore, IC1182 780 might be superior to MPP and raloxifene to treat estrogen-induced endometrial cancer in women.
Introduction
Estradiol has pleiotropic effects in the uterus (Galand et al. 1971, Lee 1980, Yamashita et al. 1990). Overall, itfunctions to prepare the organ for conceptus implantation by promoting proliferation of the endometrial lining and endometrial glands, uterine edema and hyperemia, angiogenesis, and endometrial remodeling. These events are especially evident in species that have an intrusive form of placentation in which trophoblast and maternal stromal and vascular cells form an intimate association (Lubahn et al. 1993, Krege et al. 1998). Estrogen also up-regulates uterine estrogen receptor expression (Yama-shita et al. 1990) and promotes secretion of proteins, such as lactoferrin (Pentecost & Teng 1987), complement component 3 (C3; Sundstrom et al. 1989), and mucin-1 (MUC1; Surveyor et al. 1995). Microarray studies performed on uterine tissues have revealed that estrogen influences the expression of large numbers of genes, including those considered to be ‘early’ and others whose regulation occurs several hours to days after administration of the hormone (Hewitt et al. 2003). Regulated genes include ones involved with transcription regulation, proteolysis, regulation of cell cycle and proliferation, tissue remodeling (including endometrial basement membrane breakdown), immune modulation, metabolism, detoxification, and stress responses (Fertuck et al. 2003, Hewitt et al. 2003, Khalyfa et al. 2003, Watanabe et al. 2003a,b, 2004, Naciff et al. 2005, Punyadeera et al. 2005, Yanaihara et al. 2005, Hong et al. 2006).
Selective estrogen receptor modulators (SERMs) have been employed to provide more specific information on the differential effects of estrogen acting through ESR1 or ESR2 (Schafer et al. 1999, Spencer et al. 1999). Additionally, these SERM hold therapeutic promise in treating various uterine pathologies, including endometrial cancer and endometriosis (Chan 2002, Jordan 2003). While some SERMs, such as tamoxifen, raloxifene, and ICI 182 780, bind to both ESR1 and ESR2, others are more selective and only bind in an agonistic or antagonistic manner to one ESR form. Methyl-piperidino-pyrazole (MPP) binds selectively to ESR1 rather than ESR2 (e.g. Sun et al. 2002, Harrington et al. 2003). However, we recently demonstrated that MPP demonstrates both agonistic and antagonistic actions on cultured target cells of uterine origin, including ones derived from tumors, as well as in the uteri of mice (Davis et al. 2006). In contrast to MPP, ICI 182 780 is considered to be a pure estrogen receptor antagonist (Dukes et al. 1993, Howell et al. 2000), presumably by enhancing the degradation of one or both ESRs (Wittmann et al. 2007). Raloxifene exerts agonistic effects in the bones and cardiovascular system but antagonistic effects in the uterus, mammary glands, and brain through its actions on both estrogen receptors (Gustafsson 1998, Cano & Hermenegildo 2000, Snyder et al. 2000, Saitta et al. 2001, Stygar et al. 2003, Zheng et al. 2004, Davis et al. 2006).
Numerous microarray studies have been performed to test the effects of estradiol in the rodent and human uterus (Fertuck et al. 2003, Hewitt et al. 2003, Khalyfa et al. 2003, Watanabe et al. 2003a,b, 2004, Naciff et al. 2005, Punyadeera et al. 2005, Yanaihara et al. 2005, Hong et al. 2006), but few have reported on global gene expression changes in the uterus in response to SERMs, particularly MPP, whose actions are still not well understood. However, MPP might hold promise in treating certain gynecological cancers and disorders through its ESR1-selective antagonist actions. Studies on the effects of a combination of 17β-estradiol and a SERM are also sparse. As many women with estrogen-responsive endometrial cancer and other gynecological disorders are treated with a SERM, it is essential to establish the global network of gene changes that occur in response to a SERM either alone or in combination with estradiol. Moreover, it has recently been proposed that one route to treat menopausal symptoms and prevent osteoporosis in women is to partner estrogenic compounds with a SERM (tissue-selective estrogen combinations (TSECs); Komm et al. 2007). Only a few studies to date have examined in detail the global gene expression changes in the uteri of mice or rats in response to an ESR-specific SERM (Green et al. 2001, Hewitt et al. 2003, Helvering et al. 2005, Fong et al. 2007). In the latter study, wild-type and estrogen receptor-β knockout mice were treated with 17β-estradiol + ICI 182 780. The results suggested that ICI 182 780 prevented uterine gene expression changes that occur in response to 17β-estradiol alone.
By combining 17β-estradiol and one of the SERMs, we hypothesized that either the SERM would mitigate the estrogen-induced gene expression changes, as observed above, or a combinatorial effect might result, as has been described previously for select gene expression patterns when estrogen was used in association with a SERM or phytoestrogen (Willard & Frawley 1998, Diel et al. 2001, Kaye et al. 2001, Tanos et al. 2002, Mai et al. 2007, van Meeuwen et al. 2007, Wong et al. 2007). However, these studies did not employ SERMs that selectively bind ESR1 or ESR2, and the synergistic effects might result from the combination of homo- and heterodimers of ESR-bound estrogen and SERM-bound ESR forms (Kaye et al. 2001). Consequently, we sought to perform a comprehensive analysis of chronic, 24–48 h post-treatment, gene changes in the uteri of mice treated with the ESR1-selective antagonist, MPP, raloxifene, ICI 182 780, low and high doses of 17β-estradiol, and the combination of a SERM + β-estradiol.
Materials and methods
Animals
All of the animal experiments were performed in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals (1996 (7th ed.) Washington, DC, USA: National Academy Press) and were approved by the University of Missouri Animal Care and Use Committee. CF1 female mice that weighed ~ 33–35 g were employed for these studies. They were ovariectomized at 5–7 weeks of age, and a week later they were injected i.p. two times, 24 h apart, with one of the following treatments: 1 μg β-estradiol (n = 3), 50 μg β-estradiol (n = 5), 50 μg MPP (n=3), 50 μg raloxifene (n=3), 50 μg ICI 182 780 (n=3), 50 μg β-estradiol +50 μg MPP (n=3), 50 μg β-estradiol +50 μg raloxifene (n = 3), 50 μg β-estradiol + 50 μg ICI 182 780 (n = 5), and DMSO vehicle control (n=3). These doses were chosen based on past studies that tested the effects of β-estradiol and SERMs in mice (Jones & Bern 1977, Carthew et al. 1999, Gutman et al. 2002, Hewitt et al. 2003, McDougall et al. 2003, Chin et al. 2005, Davis et al. 2006, Hatsumi & Yamamuro 2006, Yamamoto et al. 2006, Chen et al. 2007, Glidewell-Kenney et al. 2007, Gresack et al. 2007, Thakur & Sharma 2007). In our previous study, we tested several doses of the β-estradiol, MPP, and raloxifene and found the 50 μg dose of β-estradiol to induce the maximal uterotropic response (Davis et al. 2006). On day 3, the mice were euthanized and their uteri were harvested and stored in RNAlater (Ambion, Austin, TX, USA) for future experiments.
RNA isolation and analysis
The murine uteri that were stored in RNAlater (Ambion) were homogenized by using an Ultra-Turrax T25 basic homogenizer (IKA-Works, Inc., Wilmington, NC) for 30–45 s. RNA from each individual uterus was isolated with either TRI Reagent (Sigma) or the RNeasy Mini kit (Qiagen) according to the manufacturer’s protocol. The concentration of the RNA was determined on a NanoDrop ND-1000 spectro-photometer (NanoDrop Technologies, Wilmington, DE, USA) and the quality of the RNA was assessed by using the Experion Automated Electrophoresis System (Bio-Rad Laboratories) at the University of Missouri’s DNA Core Facility. Once the RNA was evaluated as suitable for analysis, it was subsequently used for microarray hybridization.
RNA quality control
Immediately prior to cDNA synthesis, the purity and concentration of RNA samples were re-determined from OD260/280 readings by using a dual beam UV spectrophotometer, and RNA integrity was determined by capillary electrophoresis by using the RNA 6000 Nano Lab-on-a-Chip kit and the Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA), as per the manufacturer’s instructions.
cRNA synthesis and labeling
RNA was processed and labeled according to the standard reverse transcriptase and in vitro transcription methods. First- and second-strand cDNA were synthesized from 2.0 μg total RNA by using oligo-dT24-T7 (5′-GGC CAG TGA ATT GTA ATA CGA CTC ACT ATA GGG AGG CGG-3′) as primer and the Bioarray cDNA Synthesis Kit (ENZO Diagnostics Inc., Farmingdale, NY, USA) according to the manufacturer’s instructions. The cRNA was synthesized and labeled with biotiny-lated UTP and CTP by in vitro transcription for 16 h at 37 °C with the T7 promoter-coupled double-stranded cDNA as template and the Bioarray HighYield RNA Transcript Labeling Kit (ENZO Diagnostics Inc). The labeled cRNA was separated from unincorporated ribonucleotides by passing through a CHROMA SPIN-100 column (Clontech) and ethanol precipitated at − 20 °C for 1 h to overnight.
Oligonucleotide array hybridization and analysis
Labeled cRNA was resuspended in RNase-free H2O and 15.0 μg fragmented by ion-mediated hydrolysis at 95 °C for 35 min in 200 mM Tris-acetate (pH 8.1), 500 mM potassium acetate, and 150 mM magnesium acetate. The fragmented cRNA was hybridized for 16 h at 45 °C to Affymetrix Mouse Genome 430 2.0 short oligomer arrays (Affymetrix, Santa Clara, CA, USA), which detect ~ 44 000 mouse transcripts representing over 34 000 well-characterized mouse genes. Arrays were scanned and analyzed by using the GeneChip Operating System v.1.4 (Affymetrix), GeneMaths XT (for hierarchical clustering, principal component analysis (PCA) and ANOVA), and Webgestalt (for Gene Ontology and pathway analysis).
Quantitative RT-PCR
RNA from each treatment group was reverse transcribed to cDNA using the Superscript III First-Strand Synthesis System (Invitrogen Corp.) or the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Negative control samples included RNA with nuclease-free water in lieu of the RT enzyme. The resulting cDNA concentrations were determined on the NanoDrop (NanoDrop Technologies). The real-time RT-PCR for kallikrein 1 (Klk1; Qiagen, QT01047921), Indian hedgehog (Ihh; Qiagen, QT00096215), complement component 3 (C3; Qiagen, QT00109270), mucin-1 (Muc1; Qiagen, QT00105784), Dnmt1 (Qiagen, QT00157990), Ifrg15 (Qiagen, QT00283472 and realtimeprimers.com), Gli1 (Qiagen, QT00173537), Cebpa (Qiagen, QT00311731), Clusterin (Clu; QT00146174), Cdca8 (QT00119945), Cdc45l (QT00094465), Esr1 (QT01075641), Esr2 (QT00096222), and QuantumRNA Universal 18S Internal Standard (Ambion, AM1718) was performed with the resulting cDNA samples. Catalogue numbers for each of the primers are included, as the sequences are proprietary information of the company. Standard curves were performed for each of the primers to verify their efficiency. The fold change for each gene was determined based on the ΔΔCt method with the 18S reactions serving as the endogenous control (Skern et al. 2005) and DMSO treatment as the calibrator sample. Four replicates were run for each treatment and gene.
Statistical analysis
Dependent variables of fold change for the gene expressions in the quantitative(Q) RT-PCR analyses were analyzed for normality using the Wilk-Shapiro test (SAS Institute, Cary, NC, USA). Fold change of gene expression was logarithmically transformed to approach a normal distribution. Data were analyzed by the general linear model tests of SAS (1988). Differences in gene expression changes among treatment groups were determined by Fisher’s least significant difference with P<0.05 considered statistically significant.
Microarray MAS 5.0 normalized data were analyzed for expression changes by a one-way ANOVA test followed by independent t-tests for each treatment group (versus DMSO control group). Significant changes in gene expression were determined by a ≥1.5-fold change in expression for at least one treatment group (versus control), an ANOVA P≤0.02, a t-test P≤0.02 for at least one treatment group, and MAS 5.0 detection P≤0.065 (M) for all samples within at least one treatment group. Prior to heat map generation and clustering analysis, probe set signal values were log2 transformed and standardized by row mean centering and scaled by S.D. Unsupervised hierarchical clustering was performed in GeneMaths XT (Applied Maths, St Martens-Latem, Belgium) by the unweighted pair-group method by arithmetic averages using Pearson correlation distance as the similarity metric for array clustering and Euclidean distance as the similarity metric for gene clustering. Gene annotation, gene ontology, and biochemical pathway information were obtained by the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov), NetAffX (www.affymetrix.com), Gene Ontology Consortium (http://amigo.geneontology.org), the Kyoto encyclopedia of genes and genomes (KEGG; www.genome.jp/ kegg), and WebGestalt (http://bioinfo.vanderbilt.edu/webgestalt). Significant enrichment of specific gene ontology categories or KEGG pathways were estimated by hypergeometric tests (P≤0.Q1).
Results
Microarray analyses
In these studies, we compared the gene expression patterns in the uteri of ovariectomized mice treated individually with 17β-estradiol, MPP, raloxifene, ICI 182 780, and the combination of 17β-estradiol and one of the SERM for 2 days. Thus, these gene expression changes represent longer term effects. We have determined previously that these doses and times result in significant uterotropic response in 17β-estradiol-treated mice (Davis et al. 2006). A total of 948 probe sets, representing 899 different gene transcripts, exhibited significant differential expression (fold change ≥1.5, P≤0.02) between at least one treatment group and the DMSO vehicle control group. Unsupervised hierarchical clustering (Fig. 1) and PCA (data not shown) showed that samples within each treatment group had the greatest similarity with replicate samples from the same group. Based on the unsupervised hierarchical clustering, a collective heat map was generated where low and high expression values are represented as green and red respectively. Intermediate levels of expression for various probe sets are illustrated as black. Based on this heat map, the nine treatments grouped into two major clusters, with MPP, raloxifene, and the high dose of estradiol clustering together (Fig. 1). The second cluster included low-dose 17β-estradiol, ICI 182 780 + high-dose 17β-estradiol, ICI 182 780, MPP + 17β-estradiol, and raloxifene + 17β-estradiol. In these comparisons, the low-dose 17β-estradiol and the ICI 182 780+ high-dose 17β-estradiol correlated most closely. Based on this result, it would appear that when the high-dose 17β-estradiol is combined with a SERM, in particular ICI 182 780, gene expression changes in the uterus mirror those observed with low-dose 17β-estradiol treatment (Fig. 1). In contrast to previous studies that suggest estradiol and SERMs can modify ESR expression in the uterus and other organs (Borras et al. 1994, Zou & Ing 1998, Nephew et al. 2000, Dardes et al. 2002, Pillai et al. 2002, Martin et al. 2005), none of the treatments significantly affected Esr1 or Esr2 mRNA levels in the microarray studies (data not shown). As indicated below, however, QRT-PCR analysis yielded significant differences in ESR1 and ESR2 in the various treatment groups.
Figure 1.
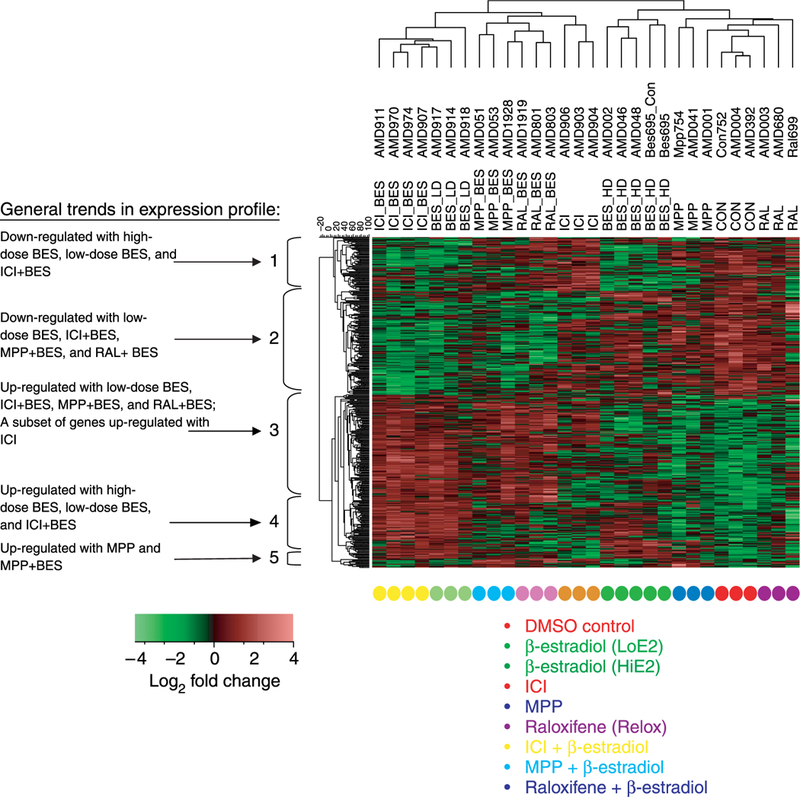
Clustered heatmap of microarray analysis. Probes on the map represent genes regulated at least 1.5-fold changes versus DMSO vehicle control. Red, up-regulated; green, down-regulated; and black, intermediate expression (key in upper left corner); see Materials and methods for a complete description of the analytic methods used to generate this heatmap. Treatments are color coded and listed below the map. In general, the high-dose 17β-estradiol group clustered more closely with the MPP and raloxifene single treatments; whereas the low-dose 17β-estradiol group clustered with ICI 182 780 and combination treatments.
We further analyzed these differences in gene expression by comparing the up- and down-regulated genes in the various groups. Surprisingly, little overlap was evident in the single treatments (Fig. 2, Supplementary Figures 1a–k and 2, see Supplementary data in the online version of the Journal of Molecular Endocrinology at http://jme.endocrinology-journals.org/content/vol40/issue/). However, when 17β-estradiol was combined with one of the SERM, in particular ICI 182 780, a synergistic effect was observed. Specifically, 609 genes were observed to be differentially regulated when 17β-estradiol was combined with ICI 182 780 compared with 178 and 98 genes when the high dose of 17β-estradiol and ICI 182 780 respectively were administered alone. Of the genes altered in the combination treatment, 472 or 78% were unique to the combined treatment and not induced by either of the individual treatments (Fig. 2). Thus, the gene expression responses that occur with the combination of 17β-estradiol and ICI 182 780 and to a lesser extent the other two SERM are not attributed simply to an additive effect.
Figure 2.
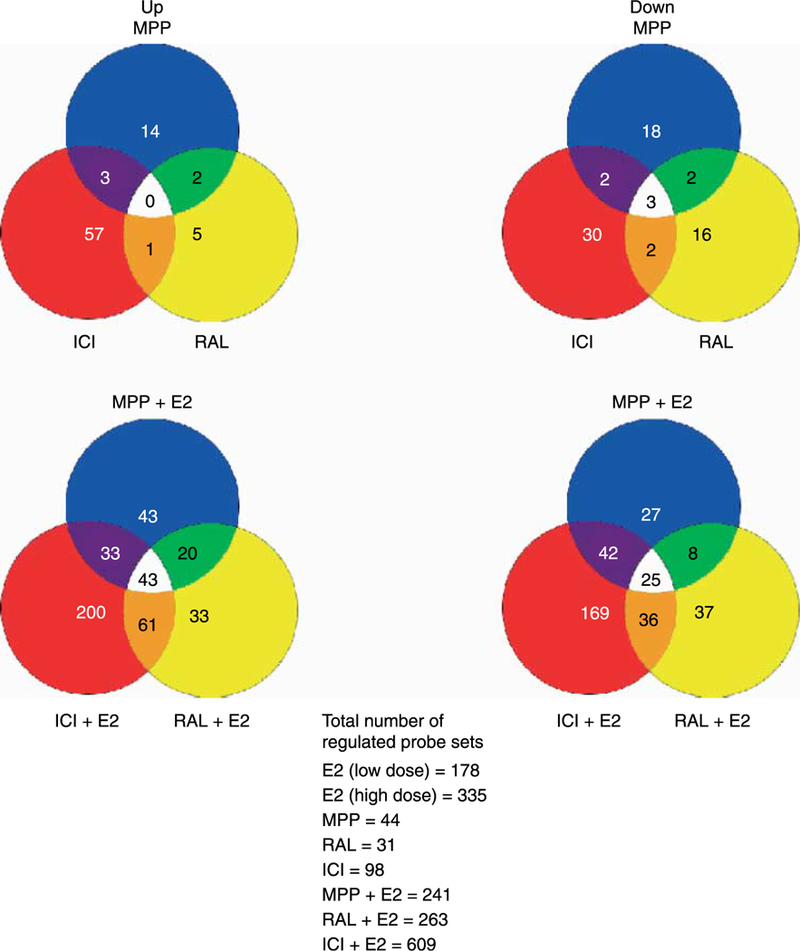
Venn diagrams. The charts compare the up- and down-regulated genes in several of the nine different treatment groups. Intersected areas represent genes that overlap in two or three of the groups. In general, scant gene overlap existed in the individual treatment groups. However, the combination treatment groups of a SERM; in particular, IC1182 780 and 17β-estradiol yielded substantially more gene alterations than each of these individual treatments.
To examine the overall gene trends in the various groups, additional heat maps for various functional categories and the corresponding tables for the fold changes for the various genes within these categories were comprised (Supplementary Figures 1a–k and 2). The observations below are based on these supplementary materials. In general, we observed that the same groups of genes were affected in the low-dose 17β-estradiol group and the ICI 182 780+ high-dose 17β-estradiol treatment group. Genes up-regulated by these two treatments encode a significant number of zinc finger proteins, which in general act as transcriptional repressors; these include Zfp62, Zfp68, Zfp119, Zfp386, Zfp560, Zfp617, Zfp622, Zfp644, Zfp672, and Zfp810. Also up-regulated were a number of genes involved in protein synthesis regulation, such as eukaryotic translation initiation factor 2a (Eif2a), eukaryotic translation initiation factor 4a1 (Eif4a1), mitochondrial ribosomal protein S9 (Mrps9), ribosomal protein S3 (Rps3), ribosomal protein L23 (Rpl23), and the signal transduction component ribosomal protein S6 kinase 1 (Rps6kb1). A number of pro-apoptotic genes, including caspase 4 (Casp4), caspase 12 (Casp12), APAF 1 interacting protein (Apip), programmed cell death 2 (Pcd2), and programmed cell death 10 (Pcd10), were up-regulated, while a number of anti-apopotic genes including clusterin (Clu), apoptosis inhibitor 5 (Api5), Hip1, radixin (Rdx), and synoviolin (Syvn1) were down-regulated, suggesting a trend toward the induction of apoptosis. In addition, several genes involved in glycolysis or gluconeogenesis, including aldolase 1A (Aldoa), phosphoglycerol kinase 1 (Pgk1), and triosephosphate isomerase 1 (Tpi1) were up-regulated in response to these treatments.
Genes suppressed by the low-dose 17β-estradiol group and the ICI 182 780+ high-dose 17β-estradiol treatment groups are related to cell-cycle regulation, DNA replication, mitosis, and cytokinesis including aurora kinase A (Aurka), cyclin A2 (Ccna2), cyclin D1 (Ccnd1), cell division cycle 7 (Cdc7), ligase I (Lig1), ribonucleotide reductase M1 (Rrm1), thymidine kinase 1 (Tk1), and S-phase kinase-associated protein 2 (Skp2). These two treatments down-regulated a number of genes involved in the categories of focal adhesion, extracellular matrix, cell migration, and cytoskeletal function ( Cdh5, Col3a1, Col4a1, Dbn1, Fn1, Lox1, Nav1, Nrp1, P4ha1, Parvb, Rdx, Thbs2, Tnc, and Tns1), in the Wnt/hedgehog/smoothened signaling pathway (dapper homology 1 (Dact1), patched homolog 2 (Ptch2), and secreted frizzled-related protein 1 (Sfrp1),Wnt inhibitory factor 1 (Wif1), SUMO/sentrin specific peptidase 2 (Senp2), and suppressor of fused homology (Sufu)), and in the categories of transcriptional regulation, mRNA processing and mRNA nuclear transport, including ATF6, Runx1, Foxk2, E2F3, Ets1, Etv5, Gli1, Ep400, Nr4a2, and Nr4a1. Deoxynucleotide terminal transferase interacting protein 2 (Dnttip2), also known as estrogen receptor binding protein (Erbp), was suppressed by the combination of ICI + high-dose 17β-estradiol but not by the low-dose 17β-estradiol.
The combinations of MPP + high dose of 17β-estradiol and raloxifene + high dose of 17β-estradiol produced similar expression patterns, although their respective responses appeared less robust than the gene alterations observed with the low-dose 17β-estradiol and the combination of ICI + high-dose 17β-estradiol. In particular, a number of genes related to apoptosis (as detailed above) had similar expression patterns to that observed with the latter treatments. MPP or raloxifene with high doses of 17β-estradiol routinely up-regulated a number of genes in functional categories related to protein synthesis, such as translation activators, ribosomal protein genes, and tRNA modification genes (e.g., eukaryotic translation initiation factor 2a (Eif2a), eukaryotic translation initiation factor 4a1 (Eif4a1), mito-chondrial ribosomal protein S9 (Mrps9), ribosomal protein S3 (Rps3), ribosomal protein L23 (Rpl23) and ribosomal protein S6 kinase 1 (Rps6kb1), or zinc finger proteins (similar to those induced by the low-dose 17β-estradiol and ICI +17β-estradiol treatments). Similar to low-dose 17β-estradiol, ICI 182 780 Chigh-dose 17β-estradiol down-regulated genes involved in Wnt/hedge-hog/smoothened signaling pathway, (as detailed above); in addition, MPP + 17β-estradiol markedly suppressed Wnt11. Other genes down-regulated by these two combination treatments are in functional categories related to focal adhesion, extracellular matrix, cell migration, cytoskeletal function, or cell-cycle progression, DNA replication, and mitosis. The developmental factor, Runxl, was inhibited by both combination treatments, whereas estrogen receptor binding protein (Erbp) was suppressed by MPP + 17β-estradiol but not by raloxifene + 17β-estradiol.
The high dose of 17β-estradiol had a much less robust effect on gene expression than the combination treatments or low-dose 17β-estradiol. High-dose 17β-estradiol up-regulated some genes (Eif2ak2, Qars, and Rps3) but down-regulated others (Rps6ka2 and Rps24) involved in protein synthesis. This treatment also induced select genes regulating glycolysis and gluconeogenesis (Aldoa, Gba, Pgkl, and Tpil) and down-regulated several genes related to cell cycle/DNA replication/mitosis (Ccndl, Gas6, Skp2), a number of genes related to focal adhesion/ECM/cell migration/-cytoskeletal function (Daaml, Dbnl, Nelll, Parvb, Synpo, and Tnc), several genes in the Wnt/hedgehog or smoothened signaling pathway (Ptch2, Sfrpl, and Wntll), and a number of growth-associated transcription factors (E2f2, Etsl, Etv5, Hdac4, and Nr4a2). A few pro-apoptotic (E2f3 and Dapkl) and anti-apoptotic (Api5, Syvnl, and Nfatc2ip) genes were also down-regulated by the high dose of 17β-estradiol.
While the individual SERM treatments did not yield much overlap in gene expression changes or as marked response in gene expression as the combination treatments, some of the common genes induced by MPP, raloxifene, and ICI 182 780 are involved in transcription regulation, the Wnt signaling pathway, and focal adhesion (Supplementary Tables 1 and 2, see Supplementary data in the online version of the Journal of Molecular Endocrinology at http://jme.endo-crinology-journals.org/content/vol40/issue/). IL-7 receptor a (IL7rα) expression was up-regulated in response to MPP or raloxifene treatment, but not by ICI 182 780. Genes down-regulated by these individual treatments include the anti-apoptotic gene, clusterin (Clu), and the focal adhesion/ECM gene β-parvin (Parvb); MPP also down-regulated Wnt11. Surprisingly, in an estrogen-free background, ICI 182 780 treatment alone altered the expression of 98 gene probe sets, which suggests that this compound might independently regulate gene expression either through its effects on ESR1 and ESR2 or by some other unknown mechanism. However, in line with its antagonistic actions, ICI 182 780 suppressed more genes (16/38 down-regulated genes) involved in mitosis and cytokinesis compared with the MPP and raloxifene treatments (Supplementary Figures 1a–k and 2). This compound also suppressed the Runx 1 transcription factor and one Wnt pathway gene, suppressor of fused homology (Sufu) but up-regulated Wnt inhibitory factor 1 (Wif1). ICI 182 780 also up-regulated Erbp, several zinc finger proteins (Zfp62, Zfp119, and Zfp263) and genes regulating protein translation, including Eif4a1, Rpl23, and Rps6kb1.
Quantitative RT-PCR
Based on the microarray results herein and previous microarray studies (Fertuck et al. 2003, Hewitt et al. 2003, Khalyfa et al. 2003, Watanabe et al. 2003a,b, 2004, Naciff et al. 2005, Punyadeera et al. 2005, Yanaihara et al. 2005, Hong et al. 2006), eight genes that demonstrated expression changes in the 17β-estradiol treatment groups were further analyzed by QRT-PCR. Other studies have shown that estradiol governs the expression of Klk1, Muc1, C3, and Ihh in murine uterine tissues and implied that these gene expression changes might correlate with Type I endometrial cancer development in women (Clements & Mukhtar 1997, Castro-Rivera & Safe 1998, Sivridis et al. 2002, Paszkiewicz-Gadek et al. 2005, Katayama et al. 2006). Thus, we sought to examine how the SERMs and 17β-estradiol alter the expression of the above genes in the uterus of wild-type mice. In general, the QRT-PCR results mirrored the microarray analyses. In the high-dose 17β-estradiol treatment group, Klk1, Muc1, and C3 were the only transcripts that were up-regulated compared with the DMSO vehicle control group; the others showed little change in expression. However, compared with all the individual and combination treatment groups, MPP resulted in a dramatic increase in Klk1 expression (Fig. 3b). By contrast, Ihh was up-regulated in the raloxifene treatment group (P<0.05; Fig. 3a).
Figure 3.
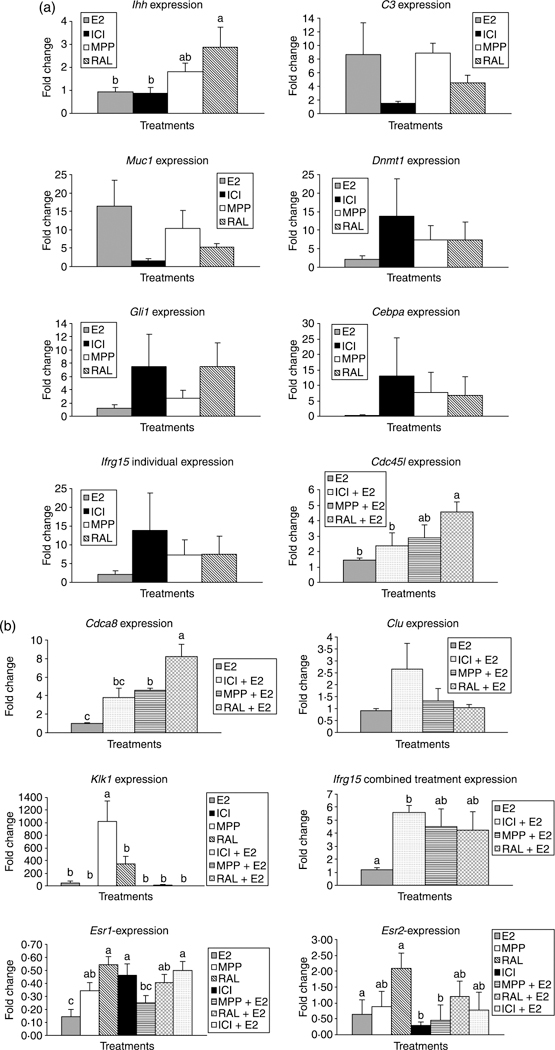
Based on the microarray results, several genes were selected for analysis by quantitative real-time PCR (QRT-PCR). (a) Bars with varying letters indicate gene expression differences (P<0.05). The two genes that showed differences in expression for the various groups were Klk1 and Ihh with MPP and raloxifene respectively inducing the greatest changes in these genes. (b) Quantitative real-time PCR was also performed for selecting genes that differed in the microarray results between 17β-estradiol and the combination of 17β-estradiol with one of the SERM. The 18s gene served as the endogenous control and the DMSO treatment as the calibrator sample. Bars with varying superscripts indicate that the expression for this gene is significantly different for these treatment groups (P<0.001). Error bars, Standard errors of the mean (S.E.M). n=4 for each of the treatments and primers tested.
Additionally, we measured transcript concentrations of a few select genes, Clu and Cdc45l, which our microarray studies revealed were dramatically altered in the uteri of mice treated with a combination of 17β-estradiol plus a SERM. For Cdc45l and Cdca8, the QRT-PCR analysis reflected the microarray results, with raloxifene + 17β-estradiol considerably up-regulating the expression of these genes compared with high-dose 17β-estradiol treatment alone (P<0.001; Fig. 3b). Cdca8 and Ifrg15 gene expression were significantly up-regulated in response to the co-treatments of MPP + 17β-estradiol and ICI 182 780 + 17β-estradiol respectively relative to the high-dose 17β-estradiol treatment (P<0.001; Fig. 3b).
Finally, we measured the expression of ESR1 and ESR2 in the single and combination treatments to determine whether QRT-PCR analysis might detect differences in these genes that the microarray analyses were not sensitive enough to distinguish. Indeed, the QRT-PCR revealed that expression differences between ESR1 and ESR2 existed amongst the various treatment groups. Specifically, high-dose 17β-estradiol resulted in the greatest suppression of ESR2 compared with all of the other treatments (Fig. 3b). This treatment also down-regulated ESR2 compared with MPP, raloxifene, raloxifene + 17β-estradiol, and ICI + 17β-estradiol. By contrast, raloxifene treatment led to the greatest increase in ESR2. Additionally, administration of a SERM alone or in combination with 17β-estradiol inhibited ESR1 to varying extents. The ICI and MPP + 17β-estradiol treatment groups also significantly suppressed ESR2 (Fig. 3b).
Discussion
We have compared the global gene expression changes in the uterus in response to individual and combined treatments of 17β-estradiol and the SERMs (MPP, raloxifene, and ICI 182 780) 48 h and 24 h after the first and second treatments respectively. Thus, our results pertain to the longer term effects of the hormone and coincide approximately with peak uterine edema (Davis et al. 2006). Of the three SERMs examined, ICI 182 780 is considered to be a pure estrogen receptor antagonist, with no estrogenic effects, as it competitively binds to ESR1 and ESR2 in all organs tested to date (Dukes et al. 1993, Howell et al. 2000) and appears to diminish estrogen-induced genes in the murine uterus (Hewitt et al. 2003). Curiously, gene expression changes in response to ICI 182 780 were similar enough to those caused by the low dose of 17β-estradiol to indicate that this supposed anti-estrogen might have partial agonistic effects. For example, the clustering analysis placed ICI 182 780 closer in its gene expression profile to low-dose 17β-estradiol treatment than the two other SERMs. Based on QRT-PCR results, Klk1 and Ihh expression changes in the ICI 182 780-treated mice also echoed those in mice treated with high-dose 17β-estradiol (Fig. 3a).
These results suggest that ICI 182 780 should not be considered a pure estrogen antagonist, even though the compound was able to temper many of the effects of high-dose 17β-estradiol and bring them into the range of those changes occurring in response to the low dose. Past studies in rodents and fish support the notion that ICI 182 780 is a partial agonist/antagonist (Robertson et al. 2001, Wu et al. 2005, Pinto et al. 2006, Zhao et al. 2006). One microarray study that compared the expression changes in ZR-75 breast cancer cells treated with raloxifene, ICI 182 780, and 4-hydroxytamoxifen revealed that all three of these SERM exerted both agonistic and antagonistic effects, and each SERM governed unique gene responses in these cells (Sismondi et al. 2007). In line with its antagonistic effects, ICI treatment suppressed more genes underpinning mitosis and cytokinesis than MPP and raloxifene treatments.
In contrast to these unique and previously unnoticed effects of ICI 182 780, the gene expression changes induced by MPP and raloxifene were somewhat similar, although clearly not identical to each other and clustered separately from those of the 17β-estradiol and ICI 182 780 groups. In the endometrium, MPP is presumed to be a selective ESR1 antagonist (Sun et al. 2002, Harrington et al. 2003) and raloxifene an ESR1 and ESR2 antagonist (Schafer et al. 1999, Spencer et al. 1999). It should be recalled that ESR1 is considered to be the dominating ESR in the uterus, as estradiol fails to induce an increase in uterine wet weight and causes scant alterations in gene expression in the uterus of aERKO mice (Lubahn et al. 1993, Shughrue et al. 1998, Dupont et al. 2000, Hewitt et al. 2003). However, estradiol is able to increase uterine wet weight in PERKO-treated mice and causes similar gene expression changes as in WT mice (Krege et al. 1998, Hewitt et al. 2003). Therefore, the gene expression changes observed in the uterus in response to MPP and raloxifene treatment are likely to be governed primarily by their actions on ESR1 although we cannot rule out the possibility that some downstream actions are mediated through interaction with ESR2, particularly in mice subjected to the co-treatments. While the microarray studies failed to reveal any disparities in Esr1 and Esr2 mRNA between individual treatments, the QRT-PCR results indicate that the raloxifene treatment dramatically increased the expression of ESR1 and ESR2 compared with the other treatments. In contrast, 17β-estradiol yielded the greatest suppression of ESR1 and suppressed ESR2 compared with the majority of the treatments tested. Others have observed similar down-regulation of uterine ESR, particularly in uterine epithelium, by 17β-estradiol treatment (Borras et al. 1994, Nephew et al. 2000, Pillai et al. 2002).
While many of the genes, including Klk1, displayed similar expression patterns in the microarray studies and the QRT-PCR, notable exceptions included Clu, Gli1, Cdc45l, Esr1 and Esr2. The microarray studies did not result in any statistical differences between the groups in the ESR1 and ESR2, the QRT-PCR data yielded dramatic differences, as indicated above. Presumably, these contrasting results between the microarray and QRT-PCR results might be accounted for by differences in sensitivity between the assays. Additionally, different statistical methods were employed for each of these studies, and the rigorous statistics employed with the microarray studies might have filtered out subtle differences between the groups.
While the overall gene expression changes in the uteri of MPP- and raloxifene-treated mice were similar, notable differences were evident, particularly based on the QRT-PCR results. Compared with the individual and combination treatments, MPP yielded the most dramatic up-regulation of Klk1 expression. Klk1 has previously been shown to be up-regulated in uteri of mice treated with estradiol (Rajapakse et al. 2007), and this gene is abundantly expressed in estrogen-induced endometrial cancer cells (Clements & Mukhtar 1997). Thus, our initial hypothesis that the SERM, in particular MPP, would suppress Klk1 expression proved incorrect. Instead, the massive up-regulation of Klk1 observed in response to MPP treatment might be due to its exclusive agonistic effects through uterine ESR1. As a serine protease, Klk1 might be responsible for the breakdown of the surrounding basement membrane and promote cancer cell invasion into the surrounding stroma and blood vessels. Similar to MPP, raloxifene treatment alone and in combination with 17β-estradiol substantially up-regulated known cancer associated genes, including Cdc45l, Cdca8, and Ihh.
The gene expression profiles suggest that a dose-response curve exists in response to 17β-estradiol that peaks with the low dose and declines in response to the high dose of estradiol. The guiding hypothesis of this work was that when 17β-estradiol was combined with one of the SERMs, the latter would largely eliminate the 17β-estradiol effects, as was observed in WT and bERKO mice co-treated with 17β-estradiol and ICI 182 780 (Hewitt et al. 2003). Additionally, other studies suggest that these SERMs antagonize the effects of 17β-estradiol (Andrade et al. 2002, Zheng et al. 2004, Davis et al. 2006). Instead, the combination of 17β-estradiol and a SERM, in particular ICI 182 780, resulted in a greater number of novel gene expression changes than the sum of the individual treatments. Of the total probe sets altered by the combination treatments, 78% of these were only induced when the two compounds were administered together. Thus, the effects observed when 17β-estradiol is combined with ICI 182 780 are clearly synergistic and not just additive in nature. Moreover, for select genes, such as Cdc45l, Cdca8, and Ifrg15, the combination of 17β-estradiol and one of the SERM resulted in dramatic up-regulation compared with the mice subjected to 17β-estradiol treatment alone (Fig. 3b). Although somewhat surprising, these data are consistent with the results of several other groups, who also showed that the combination of estrogenic and anti-estrogenic compounds can lead to an increased number of gene expression changes rather than to a purely antagonistic effect (Willard & Frawley 1998, Diel et al. 2001, Kaye et al. 2001, Tanos et al. 2002, Mai et al. 2007, van Meeuwen et al. 2007, Wong et al. 2007).
Three models, none of which are mutually exclusive, could be considered feasible to explain these complex outcomes. One is that these SERMs act both as a partial agonist able to regulate its own special set of genes, as well as a 17β-estradiol antagonist, competent to impede but not completely block the action of 17β-estradiol. Under this model, the combination of a SERM and 17 β-estradiol will regulate a greater number of genes than either compound alone, i.e., there will be a synergistic effect. A second explanation might relate to the ability of ESR1 and ESR2 to form a complex mixture of homo- and heterodimers according to which ligands bind, thereby modulating the transactivation of different sets of target genes (Forman et al. 1995, Cowley et al. 1997, Kuiper & Gustafsson 1997, Pettersson et al. 1997, Leung et al. 2006). While it is clear that the combination of homo- and heterodimers of ESR1 and ESR2 can yield contrasting gene expression changes (Cowley et al. 1997, Pettersson et al. 1997, Matthews & Gustafsson 2003, Li et al. 2004, Monroe et al. 2005), which depend upon the nature of the ligand bound, elucidating exactly what blend of ligands bind to these dimers and their molar proportions will be a challenge. The arrangement of two receptor forms that can form hetero- and homodimers and two ligands that can act through both receptor forms would theoretically yield 12 combinations of regulatory complexes, each of which might favor distinct sets of genes. Finally, as the analyses were performed 48 h after initial treatment, the expression alterations observed might be governed by downstream ‘late’ genes.
In conclusion, concordant results from microarray and QRT-PCR analysis have revealed that the SERMs, MPP, raloxifene, and ICI 182 780 differentially regulate gene expression in the uteri of treated mice. While ICI 182 780 is considered a pure ESR antagonist, the gene expression changes that occurred in the uteri of mice treated with this compound closely resembles those treated with a low dose of 17β-estradiol. The combination of 17β-estradiol + a SERM, especially ICI 182 780, resulted in a dramatic effect, with more genes changing expression in the combination treatments than when either compound was used alone. These results support the view that the expression of estrogen receptor target genes might be determined by specific combinations of ESR forms and their various interacting ligands. Together, these data buttress the hypothesis that each of the SERMS, including ICI 182 780, are partial agonists with unique downstream consequences, and that the SERM tested can induce greater effects in combination with 17β-estradiol than in its absence. While ICI 182 780 might exert partial agonistic activity in the uterus, these results suggest that of the three SERM tested, this compound might be the most beneficial in treating various estrogen-induced endometrial disorders, including endometrial cancer, as it can partially mitigate the effects of estrogen and concomitantly suppress several genes that are essential for mitosis and cytokinesis. It has been proposed that the best treatment regimen for menopausal symptoms and prevention of osteoporosis in women is to partner estrogenic compounds with a SERM (TSECs; Komm et al. 2007). Similarly, in women with endometrial cancer, the combination of ICI 182 780 and an estrogenic compound might eliminate the systemic estrogenic antagonism that would otherwise occur in response to ICI 182 780 alone.
Supplementary Material
Acknowledgements
The authors would like to thank Genome Explorations for their assistance with the microarray studies, Drs Wade Welshons, Dennis Lubahn, R Michael Roberts, and Eric Antoniou for their insightful comments, Christina M Roberts for her assistance with the QRT-PCR experiments, and Mr Andrew Cardin for his assistance in the manuscript preparation.
Funding
Grant Support: NIH Grant CA96664.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Andrade PM, Silva ID, Borra RC, Lima GR & Baracat EC 2002. Estrogen and selective estrogen receptor modulator regulation of insulin-like growth factor binding protein 5 in the rat uterus. Gynecological Endocrinology 16 265–270. [PubMed] [Google Scholar]
- Borras M, Hardy L, Lempereur F, el Khissiin AH, Legros N, Gol-Winkler R & Leclercq G 1994. Estradiol-induced down-regulation of estrogen receptor. Effect of various modulators of protein synthesis and expression. Journal of Steroid Biochemistry and Molecular Biology 48 325–336. [DOI] [PubMed] [Google Scholar]
- Cano A & Hermenegildo C 2000. The endometrial effects of SERMs. Human Reproduction Update 6 244–254. [DOI] [PubMed] [Google Scholar]
- Carthew P, Edwards RE & Nolan BM 1999. Uterotrophic effects of tamoxifen, toremifene, and raloxifene do not predict endometrial cell proliferation in the ovariectomized CD1 mouse. Toxicology and Applied Pharmacology 158 24–32. [DOI] [PubMed] [Google Scholar]
- Castro-Rivera E & Safe S 1998. Estrogen- and antiestrogen-responsive-ness of HEC1A endometrial adenocarcinoma cells in culture. Journal of Steroid Biochemistry and Molecular Biology 64 287–295. [DOI] [PubMed] [Google Scholar]
- Chan S 2002. A review of selective estrogen receptor modulators in the treatment of breast and endometrial cancer. Seminars in Oncology 29 129–133. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E & Pepling ME 2007. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 148 3580–3590. [DOI] [PubMed] [Google Scholar]
- Chin M, Isono M, Isshiki K, Araki S, Sugimoto T, Guo B, Sato H, Haneda M, Kashiwagi A & Koya D 2005. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. American Journal of Pathology 166 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J & Mukhtar A 1997. Tissue kallikrein and the bradykinin B2 receptor are expressed in endometrial and prostate cancers. Immunopharmacology 36 217–220. [DOI] [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S & Parker MG 1997. Estrogen receptors alpha and beta form heterodimers on DNA. Journal of Biological Chemistry 272 19858–19862. [DOI] [PubMed] [Google Scholar]
- Dardes RC, Schafer JM, Pearce ST, Osipo C, Chen B & Jordan VC 2002. Regulation of estrogen target genes and growth by selective estrogen-receptor modulators in endometrial cancer cells. Gynecologic Oncology 85 498–506. [DOI] [PubMed] [Google Scholar]
- Davis AM, Ellersieck MR, Grimm KM & Rosenfeld CS 2006. The effects of the selective estrogen receptor modulators, methyl-piperidino-pyrazole (MPP), and raloxifene in normal and cancerous endometrial cell lines and in the murine uterus. Molecular Reproduction and Development 73 1034–1044. [DOI] [PubMed] [Google Scholar]
- Diel P, Smolnikar K, Schulz T, Laudenbach-Leschowski U, Michna H & Vollmer G 2001. Phytoestrogens and carcinogenesis-differential effects of genistein in experimental models of normal and malignant rat endometrium. Human Reproduction 16 997–1006. [DOI] [PubMed] [Google Scholar]
- Dukes M, Waterton JC & Wakeling AE 1993. Antiuterotrophic effects of the pure antioestrogen ICI 182,780 in adult female monkeys (Macaca nemestrina): quantitative magnetic resonance imaging. Journal of Endocrinology 138 203–210. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P & Mark M 2000. Effect of single and compound knockouts of estrogen receptors alpha (ERa) and beta (ERα) on mouse reproductive phenotypes. Development 127 4277–4291. [DOI] [PubMed] [Google Scholar]
- Fertuck KC, Eckel JE, Gennings C & Zacharewski TR 2003. Identification of temporal patterns of gene expression in the uteri of immature, ovariectomized mice following exposure to ethyny-lestradiol. Physiological Genomics 15 127–141. [DOI] [PubMed] [Google Scholar]
- Fong CJ, Burgoon LD, Williams KJ, Forgacs AL & Zacharewski TR 2007. Comparative temporal and dose-dependent morphological and transcriptional uterine effects elicited by tamoxifen and ethynylestradiol in immature, ovariectomized mice. BMC Genomics 8 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Umesono K, Chen J & Evans RM 1995. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell 81 541–550. [DOI] [PubMed] [Google Scholar]
- Galand P, Leroy F & Chretien J 1971. Effect of oestradiol on cell proliferation and histological changes in the uterus and vagina of mice. Journal of Endocrinology 49 243–252. [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE &Jameson JL 2007. Nonclassical estrogen receptor a signaling mediates negative feedback in the female mouse reproductive axis. PNAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Parrott EL, Butterworth M, Jones PS, Greaves P & White IN Comparisons of the effects of tamoxifen, toremifene and raloxifene on enzyme induction and gene expression in the ovariectomised rat uterus. Journal of Endocrinology 170 555–564. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM & Frick KM 2007. Short-term environmental enrichment decreases the mnemonic response to estrogen in young, but not aged, female mice. Brain Research 1160 91–101. [DOI] [PubMed] [Google Scholar]
- Gustafsson JA 1998. Raloxifene: magic bullet for heart and bone? Nature Medicine 4 152–153. [DOI] [PubMed] [Google Scholar]
- Gutman M, Couillard S, Roy J, Labrie F, Candas B & Labrie C 2002. Comparison of the effects of EM-652 (SCH57068), tamoxifen, toremifene, droloxifene, idoxifene, GW-5638 and raloxifene on the growth of human ZR-75–1 breast tumors in nude mice. International Journal of Cancer 99 273–278. [DOI] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA & Katzenellenbogen BS 2003. Activities of estrogen receptor alpha-and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Molecular and Cellular Endocrinology 206 13–22. [DOI] [PubMed] [Google Scholar]
- Hatsumi T & Yamamuro Y 2006. Downregulation of estrogen receptor gene expression by exogenous 17β-estradiol in the mammary glands of lactating mice. Experimental Biology and Medicine 231 311–316. [DOI] [PubMed] [Google Scholar]
- Helvering LM, Adrian MD, Geiser AG, Estrem ST, Wei T, Huang S, Chen P, Dow ER, Calley JN, Dodge JA et al. 2005. Differential effects of estrogen and raloxifene on messenger RNA and matrix metalloproteinase 2 activity in the rat uterus. Biology of Reproduction 72 830–841. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA & Korach KS 2003. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Molecular Endocrinology 17 2070–2083. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Park SH, Choi KC, Leung PC &Jeung EB 2006. Identification of estrogen-regulated genes by microarray analysis of the uterus of immature rats exposed to endocrine disrupting chemicals. Reproductive Biology and Endocrinology 4 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A, Osborne CK, Morris C & Wakeling AE 2000. ICI 182,780 (Faslodex): development of a novel ‘pure’ antiestrogen. Cancer 89 817–825. [DOI] [PubMed] [Google Scholar]
- Jones LA & Bern HA 1977. Long-term effects of neonatal treatment with progesterone, alone and in combination with estrogen, on the mammary gland and reproductive tract of female BALB/cfC3H mice. Cancer Research 37 67–75. [PubMed] [Google Scholar]
- Jordan VC 2003. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 2. Clinical considerations and new agents. Journal of Medicinal Chemistry 46 1081–1111. [DOI] [PubMed] [Google Scholar]
- Katayama S, Ashizawa K, Gohma H, Fukuhara T, Narumi K, Tsuzuki Y, Tatemoto H, Nakada T & Nagai K 2006. The expression of Hedgehog genes (Ihh, Dhh) and Hedgehogtargetgenes (Ptc1, Gli1, Coup-TfII) is affected byestrogenic stimuli in the uterus of immature female rats. Toxicology and Applied Pharmacology 217 375–383. [DOI] [PubMed] [Google Scholar]
- Kaye AM, Spatz M, Waisman A, Sasson S, Tamir S, Vaya J & Somjen D 2001. Paradoxical interactions among estrogen receptors, estrogens and SERMS: mutual annihilation and synergy. Journal of Steroid Biochemistry and Molecular Biology 76 85–93. [DOI] [PubMed] [Google Scholar]
- Khalyfa A, Klinge CM, Hall WC, Zhao X, Miller MM & Wang E 2003. Transcription profiling of estrogen target genes in young and old mouse uterus. Experimental Gerontology 38 1087–1099. [DOI] [PubMed] [Google Scholar]
- Komm BS, Nagpal S, Chang KC, Berrodin TJ, Freedman LP & McDonell DP 2007. Tissue selective estrogen complexes (TSECs): optimal menopausal therapy. In The Endocrine Society 89th Annual Meeting, Toronto, CA. Abstract # OR38–31. [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA & Smithies O 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. PNAS 95 15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG & Gustafsson JA 1997. The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Letters 410 87–90. [DOI] [PubMed] [Google Scholar]
- Lee AE 1980. The proliferative action of oestriol. Journal of Endocrinology 84 289–294. [DOI] [PubMed] [Google Scholar]
- Leung YK, Mak P, Hassan S & Ho SM 2006. Estrogen receptor (ER)-β isoforms: a key to understanding ER-β signaling. PNAS 103 13162–13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Huang J, Yi P, Bambara RA, Hilf R & Muyan M 2004. Single-chain estrogen receptors (ERs) reveal that the ERα/β heterodimer emulates functions of the ERα dimer in genomic estrogen signaling pathways. Molecular and Cellular Biology 24 7681–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS & Smithies O 1993. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. PNAS 90 11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai Z, Blackburn GL & Zhou JR 2007. Soy phytochemicals synergistically enhance the preventive effect of tamoxifen on the growth of estrogen-dependent human breast carcinoma in mice. Carcinogenesis 28 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LA, Pancholi S, Chan CM, Farmer I, Kimberley C, Dowsett M & Johnston SR 2005. The anti-oestrogen ICI 182,780, but not tamoxifen, inhibits the growth of MCF-7 breast cancer cells refractory to long-term oestrogen deprivation through down-regulation of oestro-gen receptor and IGF signalling. Endocrine-Related Cancer 12 1017–1036. [DOI] [PubMed] [Google Scholar]
- Matthews J & Gustafsson JA 2003. Estrogen signaling: a subtle balance between ER α and ER β. Molecular Interventions 3 281–292. [DOI] [PubMed] [Google Scholar]
- McDougall KE, Perry MJ, Gibson RL, Colley SM, Korach KS & Tobias JH 2003. Estrogen receptor-alpha dependency of estrogen’s stimulatory action on cancellous bone formation in male mice. Endocrinology 144 1994–1999. [DOI] [PubMed] [Google Scholar]
- van Meeuwen JA, van den Berg M, Sanderson JT, Verhoef A & Piersma AH 2007. Estrogenic effects of mixtures of phyto- and synthetic chemicals on uterine growth of prepubertal rats. Toxicology Letters 170 165–176. [DOI] [PubMed] [Google Scholar]
- Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S & Spelsberg TC 2005. Estrogen receptor alpha and beta heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Molecular Endocrinology 19 1555–1568. [DOI] [PubMed] [Google Scholar]
- Naciff JM, Torontali SM, Overmann GI, Carr GJ, Tiesman JP & Daston GP 2005. Evaluation of the gene expression changes induced by 17-α-ethynyl estradiol in the immature uterus/ovaries of the rat using high density oligonucleotide arrays. Birth Defects Research. Part B, Developmental and Reproductive Toxicology 74 164–184. [DOI] [PubMed] [Google Scholar]
- Nephew KP, Long X, Osborne E, Burke KA, Ahluwalia A & Bigsby RM 2000. Effect of estradiol on estrogen receptor expression in rat uterine cell types. Biology of Reproduction 62 168–177. [DOI] [PubMed] [Google Scholar]
- Paszkiewicz-Gadek A, Porowska H, Pietruczuk M, Haczynski J, Kisiel DG & Wolczynski S 2005. Effect of estradiol and raloxifene on MUC1 expression and adhesive properties of Ishikawa cells. Oncology Reports 14 583–589. [PubMed] [Google Scholar]
- Pentecost BT & Teng CT 1987. Lactotransferrin is the major estrogen inducible protein of mouse uterine secretions. Journal of Biological Chemistry 262 10134–10139. [PubMed] [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG & Gustafsson JA 1997. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Molecular Endocrinology 11 1486–1496. [DOI] [PubMed] [Google Scholar]
- Pillai SB, Jones JM & Koos RD 2002 Treatment of rats with 17β-estradiol or relaxin rapidly inhibits uterine estrogen receptor beta1 and β2 messenger ribonucleic acid levels. Biology of Reproduction 67 1919–1926. [DOI] [PubMed] [Google Scholar]
- Pinto PI, Singh PB, Condeca JB, Teodosio HR, Power DM & Canario AV 2006. ICI 182,780 has agonistic effects and synergizes with estradiol-17 β in fish liver, but not in testis. Reproductive Biology and Endocrinology 4 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punyadeera C, Dassen H, Klomp J, Dunselman G, Kamps R, Dijcks F, Ederveen A, de Goeij A & Groothuis P 2005. Oestrogen-modulated gene expression in the human endometrium. Cellular and Molecular Life Sciences 62 239–250. [DOI] [PubMed] [Google Scholar]
- Rajapakse S, Yamano N, Ogiwara K, Hirata K, Takahashi S &Takahashi T 2007. Estrogen-dependent expression of the tissue kallikrein gene (Klk1) in the mouse uterus and its implications for endometrial tissue growth. Molecular Reproduction and Development 74 1053–1063. [DOI] [PubMed] [Google Scholar]
- Robertson JA, Zhang Y & Ing NH 2001. ICI 182,780 acts as a partial agonist and antagonist of estradiol effects in specific cells of the sheep uterus. Journal of Steroid Biochemistry and Molecular Biology 77 281–287. [DOI] [PubMed] [Google Scholar]
- Saitta A, Morabito N, Frisina N, Cucinotte D, Corrado F, D’Anna R, Altavilla D, Squadrito G, Minutoli L, Arcoraci V et al. 2001. Cardiovascular effects of raloxifene hydrochloride. Cardiovascular Drug Reviews 19 57–74. [DOI] [PubMed] [Google Scholar]
- SAS 1988. SAS/STAT User’s Guide, Cary, NC: SAS Institute, Inc. [Google Scholar]
- Schafer JI, Liu H, Tonetti DA & Jordan VC 1999. The interaction of raloxifene and the active metabolite of the antiestrogen EM-800 (SC 5705) with the human estrogen receptor. Cancer Research 59 4308–4313. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Scrimo PJ & Merchenthaler I 1998. Comparative distribution of estrogen receptor-alpha (ER-α) and b (ER-β) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids 63 498–504. [DOI] [PubMed] [Google Scholar]
- Sismondi P, Biglia N, Ponzone R, Fuso L, Scafoglio C, Cicatiello L, Ravo M, Weisz A, Cimino D, Altobelli G et al. 2007. Influence of estrogens and antiestrogens on the expression of selected hormone-responsive genes. Maturitas 57 50–55. [DOI] [PubMed] [Google Scholar]
- Sivridis E, Giatromanolaki A, Koukourakis MI, Georgiou L & Anastasiadis P 2002. Patterns of episialin/MUC1 expression in endometrial carcinomas and prognostic relevance. Histopathology 40 92–100. [DOI] [PubMed] [Google Scholar]
- Skern R, Frost P & Nilsen F 2005. Relative transcript quantification by quantitative PCR: roughly right or precisely wrong? BMC Molecular Biology 6 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder KR, Sparano N & Malinowski JM 2000. Raloxifene hydro-chloride. American Journal of Health-System Pharmacy 57 1669–1675. [PubMed] [Google Scholar]
- Spencer CP, Morris EP & Rymer JM 1999. Selective estrogen receptor modulators: women’s panacea for the next millennium? American Journal of Obstetrics and Gynecology 180 763–770. [DOI] [PubMed] [Google Scholar]
- Stygar D, Muravitskaya N, Eriksson B, Eriksson H & Sahlin L 2003. Effects of SERM (selective estrogen receptor modulator) treatment on growth and proliferation in the rat uterus. Reproductive Biology and Endocrinology 1 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA & Katzenellenbogen BS 2002. Antagonists selective for estrogen receptor alpha. Endocrinology 143 941–947. [DOI] [PubMed] [Google Scholar]
- Sundstrom SA, Komm BS, Ponce-de-Leon H, Yi Z, Teuscher C & Lyttle CR 1989. Estrogen regulation of tissue-specific expression of complement C3. Journal of Biological Chemistry 264 16941–16947. [PubMed] [Google Scholar]
- Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK & Carson DD 1995. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 136 3639–3647. [DOI] [PubMed] [Google Scholar]
- Tanos V, Brzezinski A, Drize O, Strauss N & Peretz T 2002. Synergistic inhibitory effects of genistein and tamoxifen on human dysplastic and malignant epithelial breast cells in vitro. European Journal of Obstetrics, Gynecology and Reproductive Biology 102 188–194. [DOI] [PubMed] [Google Scholar]
- Thakur MK & Sharma PK 2007. Transcription of estrogen receptor alpha and beta in mouse cerebral cortex: effect of age, sex, 17β-estradiol and testosterone. Neurochemistry International 50 314–321. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Suzuki A, Kobayashi M, Lubahn DB, Handa H & Iguchi T 2003a. Similarities and differences in uterine gene expression patterns caused by treatment with physiological and non-physiological estrogens. Journal of Molecular Endocrinology 31 487–497. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Suzuki A, Kobayashi M, Takahashi E, Itamoto M, Lubahn DB, Handa H & Iguchi T 2003b. Analysis of temporal changes in the expression of estrogen-regulated genes in the uterus. Journal of Molecular Endocrinology 30 347–358. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Suzuki A, Goto M, Lubahn DB, Handa H & Iguchi T 2004. Tissue-specific estrogenic and non-estrogenic effects of a xenoes-trogen, nonylphenol. Journal of Molecular Endocrinology 33 243–252. [DOI] [PubMed] [Google Scholar]
- Willard ST & Frawley LS 1998. Phytoestrogens have agonistic and combinatorial effects on estrogen-responsive gene expression in MCF-7 human breast cancer cells. Endocrine 8 117–121. [DOI] [PubMed] [Google Scholar]
- Wittmann BM, Sherk A & McDonnell DP 2007. Definition of functionally important mechanistic differences among selective estrogen receptor down-regulators. Cancer Research 67 9549–9560. [DOI] [PubMed] [Google Scholar]
- Wong SP, Li J, Shen P, Gong Y, Yap SP & Yong EL 2007. Ultrasensitive cell-based bioassay for the measurement of global estrogenic activity of flavonoid mixtures revealing additive, restrictive, and enhanced actions in binary and higher order combinations. Assay and Drug Development Technologies 5 355–362. [DOI] [PubMed] [Google Scholar]
- Wu J, Liang Y, Nawaz Z & Hyder SM 2005. Complex agonist-like properties of ICI 182,780 (Faslodex) in human breast cancer cells that predominantly express progesterone receptor-β: implications for treatment resistance. International Journal of Oncology 27 1647–1659. [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Hess HA, Guo GL, Gonzalez FJ, Korach KS, Maronpot RR & Negishi M 2006. Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. Journal of Biological Chemistry 281 16625–16631. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Newbold RR, McLachlan JA & Korach KS 1990. The role of the estrogen receptor in uterine epithelial proliferation and cytodifferentiation in neonatal mice. Endocrinology 127 2456–2463. [DOI] [PubMed] [Google Scholar]
- Yanaihara A, Otsuka Y, Iwasaki S, Aida T, Tachikawa T, Irie T & Okai T 2005. Differences in gene expression in the proliferative human endometrium. Fertility and Sterility 83 (Suppl 1) 1206–1215. [DOI] [PubMed] [Google Scholar]
- Zhao L, O’Neill K & Brinton RD 2006. Estrogenic agonist activity of ICI 182,780 (Faslodex) in hippocampal neurons: implications for basic science understanding of estrogen signaling and development of estrogen modulators with a dual therapeutic profile. Journal of Pharmacology and Experimental Therapeutics 319 1124–1132. [DOI] [PubMed] [Google Scholar]
- Zheng H, Kangas L & Harkonen PL 2004. Comparative study of the short-term effects of a novel selective estrogen receptor modulator, ospemifene, and raloxifene and tamoxifen on rat uterus. Journal of Steroid Biochemistry and Molecular Biology 88 143–156. [DOI] [PubMed] [Google Scholar]
- Zou K & Ing NH 1998. Oestradiol up-regulates oestrogen receptor, cyclophilin, and glyceraldehyde phosphate dehydrogenase mRNA concentrations in endometrium, but down-regulates them in liver. Journal of Steroid Biochemistry and Molecular Biology 64 231–237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


