Abstract
ADHD medication is one of the most commonly prescribed medication classes in child and adolescent psychiatry, and its use is increasing rapidly in adult psychiatry. However, major questions and concerns remain regarding the benefits and risks of ADHD medication, especially in real-world settings. We conducted a qualitative systematic review on studies that investigated the effects of ADHD medication on behavioral and neuropsychiatric outcomes using linked prescription databases from the last ten years, and identified 40 studies from Europe, North America, and Asia. Among them, 18 have used within-individual designs to account for confounding by indication. These studies suggested short-term beneficial effects of ADHD medication on several behavioral or neuropsychiatric outcomes (i.e., injuries, motor vehicle accidents, education, substance use disorder), with estimates suggesting relative risk reduction of 9–58% for these outcomes. The within-individuals studies found no evidence of increased risks for suicidality and seizures. Replications studies are needed for several other important outcomes (i.e., criminality, depression, mania, psychosis). The available evidence from pharmacoepidemiology studies on long-term effects of ADHD medication are less clear. We discussed time-varying confounding and other limitations which should be considered when interpreting results from pharmacoepidemiology studies. Further, we highlighted several knowledge gaps to be addressed in future research and implications for research on mechanisms of outcomes of ADHD medications.
Keywords: ADHD medication, short-term outcomes, long-term outcomes, pharmacoepidemiology, within-individual design, real-world evidence
BACKGROUND
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder of childhood that persists into adolescence and adulthood for many individuals (1–3). The prevalence of clinically diagnosed ADHD is increasing in most developed countries (4). For instance, the prevalence among children and adolescents in the US increased from 6.1% in 1997–1998 to 10.2% in 2015–2016 (5). Although these increases appear to reflect changes in diagnostic criteria and awareness of ADHD to a greater extent than they reflect true increases in the prevalence of the condition itself (6), more individuals are nevertheless now being identified worldwide as candidates for ADHD treatment.
Treating ADHD is a major public health issue, as the disorder is associated with high rates of comorbidity (7, 8) and serious outcomes (9–11). Numerous randomized controlled trials (RCTs) have established the efficacy of medications, particularly stimulants, for reducing the core symptoms of ADHD in children, adolescents, and adults, though for some there are side effects and difficulties in tolerating treatment (12). Clinical guidelines now generally recommend stimulants as the first-line pharmacological treatment for school-aged children, adolescents, and adults (13–16). The prevalence of ADHD medication use has increased dramatically in many developed countries during the last decades (17, 18).
Major questions and concerns remain, however, regarding the benefits and risks of ADHD medications (16, 19, 20). Many extant concerns stem from the limitations of the existing research and reflect the inherent constraints of RCTs (21, 22). With respect to ADHD medications, we want to highlight three limitations of RCTs (Table 1). First, the generalizability of findings from existing RCTs to populations with more comorbidity and greater severity is unclear. For example, research has found that individuals excluded from RCTs on ADHD medication show higher rates of comorbidity and lower functioning (23). Second, few RCTs include follow-up evaluations of long-term outcomes. In fact, a recent comprehensive meta-analysis of RCTs did not have sufficient data to inform on outcomes past 12 weeks (12). Third, there is increasing recognition of the need for treatment to address not only the symptoms of ADHD but also ADHD-associated functional impairments (24). And yet, existing RCTs can only provide limited evidence for the effects of pharmacotherapy on risk of rare-but-serious or longer-term outcomes, such as serious injury, suicidality, or other forms of psychopathology. These limitations are compounded by questions of whether ADHD medication may be particularly harmful or helpful for rare outcomes in potentially at-risk subgroups. For example, concerns remain about prescribing ADHD medications to individuals with substance use problems (25, 26) and preschool children (27, 28). Thus, research needs “real-world data” to better provide “real-world evidence” regarding the broader risks and benefits of ADHD medication (29).
Table 1.
Major advantages and disadvantages of randomized clinical trials and pharmacoepidemiology studies.
| Advantages | Disadvantages | |
|---|---|---|
| Randomized clinical trials | • Randomized treatment assignment to balance all potential confounding factors • Both patients and researchers could be blinded from the treatment information |
• Samples may not representative of the real world • Typically short-term • Usually not large enough to study rare outcomes |
| Pharmacoepidemiology studies using population-based prescription databases | • Representative of patients in real-world practice • Long follow-up time • Large sample size |
• Confounding by indication • Misclassification of exposure • Lack of standardized analyses and reporting |
Pharmacoepidemiology research using population-based prescription databases is an increasingly recognized approach for this purpose. These databases are usually linked with other health records or registers and provide individual-level data on diagnosis, prescription, and demographic information (30). As an alternative to RCTs, they have several strengths (Table 1), including data on patients from real-world practice, longer follow-up time, and larger samples. However, unlike RCTs that use randomized treatment assignment to help balance potential confounding factors, whether measured, unmeasured, or unknown, pharmacoepidemiology studies are prone to bias if confounding is not properly addressed (31, 32). A particularly important type of confounding in pharmacoepidemiology studies is “confounding by indication,” which occurs when factors involved in selecting patients into a particular treatment also affect the outcome (33). Within-individual designs, or self-controlled designs, present one approach to account for confounding by indication (34, 35). Instead of comparing medication users and nonusers, within-individual designs compare the risk of outcomes during medicated time periods to that of non-medicated time periods within the same individual. Because the comparison is within the same individual, all confounding from factors that are stable throughout the observed time at risk is eliminated, even if they are unmeasured or unknown.
In the current paper, we reviewed population-based pharmacoepidemiology studies of ADHD medication and behavioral and neuropsychiatric outcomes. These studies address two type of research questions. One question is whether ADHD medications protect against the risk of an outcome, usually a functional or behavioral impairment associated with ADHD (e.g., injuries and accidents, criminality). The second is whether ADHD medications increase the risk of adverse outcomes (e.g., mania, seizures, substance abuse), given concerns from clinical or preclinical studies (36). We highlight the key findings and limitations of these studies. Finally, we discuss remaining knowledge gaps for future studies and investigations of mechanisms of medication effects on ADHD-associated outcomes.
EVIDENCE FROM PHARMACOEPIDEMIOLOGY STUDIES ON ADHD MEDICATIONS
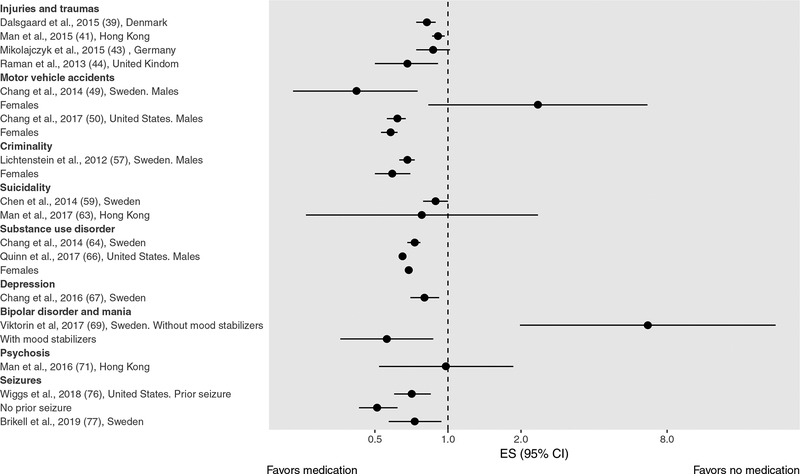
We performed a systematic search in PubMed and Embase for studies that investigated the association between ADHD medications and behavioral or neuropsychiatric outcomes using population-based prescription databases between January 01, 2008 and February 01, 2019, with no language restrictions. We used terms related to ADHD (“Attention-deficit/hyperactivity disorder”, “ADHD”) and medication (“medication”, “stimulant*”, “treatment”) and type of data (“regist*”, “claim*”, “record*”, “population*”) in combination. We followed the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (37) and identified 40 eligible studies (Supplementary Figure 1), encompassing ten different outcomes (Supplementary Table 1). We classified the effects of medication as short-term (e.g., concurrent effects) or long-term (e.g., any extended or accumulated effects). In addition, we distinguished three main types of design/analytical approaches to account for confounding: regression adjustment, propensity-score methods, and within-individual comparisons, and summarized the results separately for those using within-individual comparisons (Figure 1) as they are less biased by unmeasured confounding.
Figure 1. Forest plot of within-individual studies for short-term effects of ADHD medications.
Note: Studies on educational outcomes were not included because they used continuous measures of outcome.
Injuries and traumas
We identified eleven studies on injuries and traumas from Europe, North America, and Asia (38–48). Six studies examined short-term effects, of which four used within-individual designs (39, 41, 43, 44). Four studies, including three within-individual studies, found ADHD medication was significantly associated with 9–32% reduced risk of injury (39–41, 44), with some evidence that results may differ depending on sex (41, 44). No substantial differences were found between studies focusing on stimulants (41, 44, 45) and those considering any ADHD medication (39, 40, 43), while no studies have analyzed the effect of non-stimulant medications separately.
Six studies examined long-term effects, and five of them found a statistically significant negative association between ADHD medication and injuries risk (38, 40, 46–48). Only one study reported a non-statistically significant negative association for medium or high vs low treatment adherence (42). None used a within-individual design.
Motor vehicle accidents
We identified two studies on motor vehicle accidents, one from Sweden and one from the US, and both used within-individual designs (49, 50). When evaluating short-term effects, they reported 38–58% reduced risk of motor vehicle accidents associated with ADHD medication in males (49, 50). However, only one study found a similar result in females (50), while the other found a non-statistically significant positive association in females (49).
One study using a within-individual design also found that ADHD medications were associated long-term reduction in the risk of motor vehicle accidents (50).
Education
We identified seven studies from Europe and the US that examined different aspects of medication use, including timing of medication use, on educational outcomes including changes in test scores, grades, or grade point average (GPA). In terms of short-term effects, two studies used within-individual design and found a statistically significant association between ADHD medication and increased test scores (51) and grade improvement (52), while another study found a non-significant mean difference between current and past users of stimulants (53).
In terms of long-term effects, one study found that late stimulant medication starters (individuals starting stimulants within 12 months from the tests) had higher scores than early starters (individuals starting stimulants earlier than 12 months before the tests) (53), while another study found that late start of stimulant medication was associated with higher risk of academic decline in mathematics, but not in language arts, compared with early start (54). Two studies found consistent treatment with ADHD medication was associated with higher GPA compared with inconsistent treatment (55, 56).
Criminality
We identified two studies on criminality. One study was performed in Sweden using a within-individual design. The study reported 32% and 41% reduced risk of criminality associated with ADHD medication in males and females, respectively, and no significant difference between stimulants and atomoxetine (57). The study also reported a non-statistically significant long-term protective effect (57). The other study was performed in Denmark and reported ADHD medication was associated with significant reduction in both risk of conviction and risk of incarceration (58).
Suicidality
We identified five studies from Europe and Asia that investigated completed suicide or suicide attempts or self-harm (59–63). Three studies investigated short-term effects (59, 62, 63) and two of them adopted within-individual designs (59, 63). None of these studies found evidence for an increased risk of suicidal events, regardless of sex or type of medication (stimulant or non-stimulant).
Three studies investigated long-term effects and found no evidence for an increased risk of suicidal events (60–62). None of them used a within-individual design.
Substance use disorder
We identified three studies on substance use disorder, two from Europe (64, 65) and one from the US (66). Two studies investigating short-term effects using within-individual designs reported that ADHD medication was associated with 27–35% reduced risk of substance use disorder (64, 66).
All three studies investigated long-term effects (64–66). One study adopted a within-individual design and found statistically significant negative associations for previous treatment and treatment duration (66). The other two studies also reported negative associations when comparing treatment to no treatment, although one was not statistically significant (65).
Depression
We identified two studies on risk of depression, one from Sweden and one from Taiwan (67, 68). One study investigating short-term effects using a within-individual design, found ADHD medication was associated with 20% reduced rate of unplanned hospital visits due to depression (67).
Both studies investigated the long-term effect of ADHD medication on depression and found a negative association, but neither used a within-individual design (67, 68).
Bipolar disorder and mania
We identified two studies on bipolar disorder/mania, one from Sweden and one from Taiwan (69, 70). Only the study from Sweden investigated short-term effects and, using a within-individual design, examined the risk of mania associated with treatment with stimulants among patients with bipolar disorder (69). The study found patients on methylphenidate monotherapy had an increased rate of manic episodes within 3 months of medication initiation. By contrast, for patients taking mood stabilizers, the rate of mania was lower after starting methylphenidate.
Both studies investigated the long-term effect of ADHD medication on bipolar disorder (70) and mania (69). The Swedish study found similar results within 3–6 months as results from 0–3 months (69). The Taiwanese study found patients with long-term use of methylphenidate (>365 days) were less likely to be diagnosed with bipolar disorder compared to ADHD patients never taken methylphenidate. The associations for short-term treatment (<= 365 days) or atomoxetine were not statistically significant (70).
Psychosis
We identified two studies on risk of psychotic events (71, 72). One study, from Hong Kong, investigated short-term effects using a within-individual design and did not find evidence for an association between stimulant use and psychotic events among individuals who had a history of psychotic events (71).
The other study, from Taiwan, investigated long-term effects and found a positive association between stimulant use and risk of any psychotic disorders in individuals diagnosed with ADHD, although the association was not statistically significant when considering schizophrenia as the main outcome (72).
Seizures
We identified five studies on seizures, four from the US and one from Sweden, and two of them used a within-individual design (73–77). For short-term effects, none of the five studies found evidence for an increased risk of seizures, regardless of the type of medication (stimulant or non-stimulant) (73–77). Results from the studies that used a within-individual design suggest a possible protective short-term effect of ADHD medication in individuals both with and without a history of seizures (76, 77).
Three out of five studies investigated long-term effects, including one study that used within-individual design (76), and none found evidence for an increased risk of seizures (73, 74, 76).
KEY FINDINGS AND LIMITATIONS
An increasing number of pharmacoepidemiology studies on short-term benefits and risk (18 of 26 studies) have applied within-individual designs to account for confounding factors. The overall pattern of results suggests several short-term benefits and few short-term risks on behavioral and neuropsychiatric outcomes (Figure 1). The available evidence for several important outcomes (e.g. criminality, depression, mania, psychosis) is, however, still scarce, and replications are needed. Consequently, systematic reviews with sufficient data for meta-analyses of short-term effects of ADHD medication have only been applied to injuries as an outcome, finding reduced risk of injuries associated with ADHD medication among individuals with ADHD (78, 79). Quality assessment of the included within-individual studies using the Newcastle-Ottawa Scale suggested high quality (78).
Future within-individual studies of short-term benefits and risks need to consider limitations related to time-varying confounding. Specifically, time-varying factors (e.g., life events, episodes of disease) that motivate individuals to start/stop treatment may simultaneously change their risk of outcomes (e.g., suicidal behavior, substance abuse) and therefore confound the within-individual associations. Several statistical methods have been suggested for appropriate adjustment for time-varying confounding, such as inverse probability of treatment weighting and G estimation, although they also rely on the measurement of time-varying confounders (80). An alternative approach is to use negative controls to detect confounding bias (both time-invariant and time-varying) (81). A drug with similar prescription patterns as the studied medication and with no or negligible causal effects on the studied outcome could be used as a negative control. For example, previous studies have used SSRIs as a negative control in studies of ADHD medications and motor vehicle accidents (49, 50), but they would be unsuitable for studies on suicidality because of the effects of SSRIs on the outcome.
The available evidence from pharmacoepidemiology studies of long-term effects is less clear. There are three main limitations to consider. First, an important problem is that the research question or hypothesis was not clearly defined in many studies. This makes it difficult to disentangle whether a study is addressing the long-term effect of being treated with ADHD medication for a period (e.g., is ADHD medication status at baseline associated with risk of an outcome several years later), or the cumulative effect of being treated for longer periods of time (e.g., is longer duration of ADHD medication treatment associated with higher risk of an outcome). In many available studies, it is also unclear whether these effects are independent from concurrent effect of ADHD medication (e.g., whether the observed long-term effect is explained by exposure to ADHD medication at the time of outcome measure).
Second, some previous studies have used survival analyses on time-to-event outcomes (e.g., injuries, suicide attempts) with duration of treatment defined as time-fixed variables (38, 61). However, duration of treatment depends on length of follow-up time, and time-dependent bias can occur if such variables are analyzed as time-fixed variables (82). To eliminate such bias, duration of treatment should be considered as a time-varying variable, or measured in an equal time window for all participants (66, 67, 72).
Third, few studies of long-term effects have used within-individual designs (3 out of 21 studies), which means that unmeasured confounding factors may explain observed findings. One explanation to why these designs have been less frequently used is that they only allow explorations of certain long-term effects. The few available studies used a lagged approach (50, 66, 76) to test whether medication status at a given time predicted the risk of outcome a few years later. Both concurrent and lagged medication status were included as predictors.
There are several additional, more general, limitations that needs to be considered when interpreting results from both short-term and long-term studies of ADHD medication. First, the use of ADHD medication was measured by filled prescriptions, so the results may only apply to patients who choose to fill prescriptions (83). Further, if patients did not take the medication as indicated, it would introduce misclassification of exposure and bias the results towards the null. Second, unlike double-blinded RCTs, treatment assignment is not masked for either patients or clinicians. It is possible that the same clinician who prescribed ADHD medication also made the outcome assessment, particularly for psychiatric outcomes, which might lead to biased results (32). Third, most of the studies used data from one country/state/region, which may not be generalizable to other settings. For example, there are important cross-national differences in diagnostic and treatment practices of ADHD (17), as well as variation in the validity of ADHD diagnosis from different data sources (32). For studies using insurance claims data, patients with private insurance may be different from those publicly insured or uninsured in terms of access to health care and risks of adverse outcomes. Validation with other samples is necessary.
Suggestions for future studies
First, future studies should clearly state whether the analyses are exploratory or driven by a piori hypotheses about either potential benefit (i.e. a hypothesis about the effectiveness of ADHD medication) or potential risk (a hypothesis about the safety of ADHD medication). Clarity around the type of research question and hypothesis may guide the data analyses and interpretation of the results.
Second, future studies may benefit from greater standardization of analyses and reporting (31, 32). The STROBE statement provides a useful guideline for strengthening the reporting of observational studies in epidemiology, including pharmacoepidemiology studies (84). For studies using within-individual designs, it would be helpful to report the overall number of patients in the study sample, as well as the number that contributes to the within-individual comparison. Improved clarity in reporting is of critical importance for replication studies and meta-analyses.
Third, it would be informative if future studies could, when possible, translate estimates of relative risk into absolute risk for a meaningful period. In many situations, absolute risk gives more relevant information to both patients and clinicians.
Fourth, future studies need to address issues related to selective reporting of findings. A pre-specified study protocol, as it is obligated for RCTs, would be particularly valuable to improve the transparency and interpretation of pharmacoepidemiology studies (31, 32).
REMAINING KNOWLEDGE GAPS
We see four main knowledge gaps that need to be addressed in future research. First, as highlighted above, more well-conducted pharmacoepidemiology studies are needed to resolve knowledge gaps around the long-term consequences of ADHD medication. This is an important limitation given that many patients may receive medications for years, and some studies suggest that the beneficial effect of ADHD medications may decline two to three years after treatment initiation and that long-term use may be associated with adverse outcomes (85, 86). To address these knowledge gaps, long-term follow-up data (e.g., from childhood to early adulthood) is needed. Data with such time span is increasingly available from administrative healthcare databases, and it should be combined with analytic approaches that handle complex effects of duration, timing, and intensity of medication exposures on outcomes (e.g. time-weighted cumulative exposure models) (87).
One particular important question regarding the long-term effects is whether early exposure to ADHD medication change the developmental trajectories of patients, for example development of substance use problem. Animal studies suggest that repeated exposure to stimulants during the sensitive adolescent period was associated with long-term risk of substance abuse (88). Clinical follow-up studies suggest that ADHD medication neither protects nor increases the risk of later substance use disorders (89, 90), whereas pharmacoepidemiology studies based on prescription databases have found that ADHD medication was associated with lower risk of substance-related events up to three years later (64, 66). These results might be explained by the differences in the research questions and methods of animal, clinical, and pharmacoepidemiology studies. Therefore, more translational research is required to provide practitioners, patients, and their families with this critical information.
Second, although existing studies suggest that ADHD medication, on average in the whole patient population, is associated with short-term benefits and few short-term risks, less is known about benefits and risks in specific sub-groups. For example, older adults are overall underrepresented in existing studies (91, 92). With aging, a series of changes occur that modify the pharmacokinetics and pharmacodynamics of ADHD medication. This may influence the efficacy, tolerability, and safety of ADHD medication treatment (93). Further, many individuals with ADHD present with comorbidities, such as anxiety, personality, and substance use disorders (25, 26). The profile of benefits and risks in individuals with comorbidities may look like the profile in those without, but there are some indications that it may differ. For example, ADHD medication may be less effective among individuals with ADHD and autism spectrum disorders (94). These knowledge gaps need to be addressed.
Third, the high rate of comorbidity and multi-morbidity in individuals with ADHD means that many individuals with ADHD will use medications for other mental/physical health conditions, in addition to ADHD medication. Individuals with ADHD may face problems related to polypharmacy and drug-drug interactions, particularly among older adults with ADHD. Studies on the safety of ADHD medication in combination with other medications are therefore needed (95).
Fourth, in addition to neuropsychiatric outcomes, there are concerns about the safety of ADHD medications for somatic outcomes (e.g., cardiovascular disease, growth delay) (36). However, most of the existing studies are based on clinical samples, and evidence from population-based studies are yet limited (96–98). Future pharmacoepidemiology studies are warranted to investigate both short-term and long-term risks of somatic outcomes associated with ADHD medications and to identify potential at-risk groups.
IMPLICATIONS FOR RESEARCH ON PREDICTORS AND MECHANISMS OF OUTCOMES OF ADHD MEDICATIONS
Experimental trials of pharmacological interventions provide a powerful approach to identify markers of treatment or risk responses and to investigate treatment mechanisms (99). The application of such approaches to pharmacoepidemiological studies is more challenging than experimental trials, because of the need to control for the various confounders discussed above, but can address questions relating to longer term outcomes. One example is the effect of methylphenidate on risk of mania in patients with comorbid and ADHD and bipolar disorder, which is moderated by concomitant use of mood stabilisers (69). This finding has immediate implications for current clinical practice, illustrating the potential for incorporation of predictors into pharmacoepidemiological studies to inform personalised medicine.
Another set of questions relate to the neural and cognitive processes that underpin ADHD itself, and the treatment response to drug treatments. Multiple functional deficits of neural circuitry are associated with ADHD and proposed to mediate causal effects of genes (and environment), as well as treatment effects of medications (100). One approach is to manipulate neural biomarkers with treatments, such as stimulants, and evaluate their role as mediators of the clinical response (101). Such studies are best suited to short-term experimental study designs that can incorporate functional neuroimaging to capture neural/cognitive changes resulting from drug treatments, and link these to changes in clinical symptoms of ADHD.
Although challenging it may also be possible to incorporate such approaches into pharmacoepidemiological studies. Databases may be interrogated for results of neuropsychological and medical test results, and digital health technologies might enable large-scale evaluation of neurocognitive functions, particularly if these became widely used as part of the assessment of patients with neurodevelopmental disorders. It may then be able to infer direct relationships between changes in neurocognitive processes and longer-term ADHD related health outcomes. Overall, these approaches could potentially be used to target causal processes earlier in development and open new avenues for developing novel treatment targeting mediating mechanisms and optimising treatment outcomes.
CONCLUSION
Available pharmacoepidemiology studies suggests short-term beneficial effects of ADHD medications on several behavioral and neuropsychiatric outcomes (e.g., injuries, motor vehicle accidents, education, substance use disorder) and no increased risk for suicidality and seizures. An increasing number of studies have applied within-individual designs, or self-controlled designs, to account for confounding by indication. Replications are needed for several other important outcomes (e.g. criminality, depression, mania, psychosis). The available evidence from pharmacoepidemiology studies of long-term effects are less clear. Time-varying confounding and other limitations have to be considered when interpreting results from pharmacoepidemiology studies. Potential beneficial effects of ADHD medications will have to be carefully weighed against potential harms, including side effects and societal problems (e.g., stimulant misuse and diversion) (102, 103).
Supplementary Material
Funding/Support:
This work was supported by grants from the Swedish Research Council (2013–2280) and the National Institute of Mental Health (R01MH102221). LG is supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 643051. This report reflects only the authors’ views and the European Union is not responsible for any use that may be made of the information it contains. ZC is supported by the Swedish Research Council (2018–02213). PQ is supported by the National Institute on Drug Abuse (R00DA040727). PA is supported by NIHR Biomedical Research Centre for Mental Health, NIHR/MRC (14/23/17) and NIHR senior investigator award (NF-SI-0616–10040).
Conflict of Interest Disclosures:
PA has received funds for consultancy on behalf of KCL to Shire, Eli-Lilly, and Novartis, regarding the diagnosis and treatment of ADHD; educational/research awards from Shire, Eli-Lilly, Novartis, Vifor Pharma, GW Pharma, and QbTech; speaker at sponsored events for Shire, Eli-Lilly, and Novartis. HL reported serving as a speaker for Eli Lilly and Shire and reported receiving a research grant from Shire, all outside of the submitted work. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56:345–365. [DOI] [PubMed] [Google Scholar]
- 2.Lahey BB, Lee SS, Sibley MH, Applegate B, Molina BSG, Pelham WE. Predictors of adolescent outcomes among 4–6-year-old children with attention-deficit/hyperactivity disorder. J Abnorm Psychol. 2016;125:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello EJ, Maughan B. Annual research review: Optimal outcomes of child and adolescent mental illness. J Child Psychol Psychiatry. 2015;56:324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. The Lancet Psychiatry. 2018;5:175–186. [DOI] [PubMed] [Google Scholar]
- 5.Xu G, Strathearn L, Liu B, Yang B, Bao W. Twenty-year trends in diagnosed attention-deficit/hyperactivity disorder among us children and adolescents, 1997–2016. JAMA Network Open. 2018;1:e181471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. International Journal of Epidemiology. 2014;43:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen CM, Steinhausen HC. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. Attention deficit and hyperactivity disorders. 2015;7:27–38. [DOI] [PubMed] [Google Scholar]
- 8.Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, March JS, Arnold LE, Cantwell DP, Conners CK, Elliott GR, Greenhill LL, Hechtman L, Hoza B, Pelham WE, Severe JB, Swanson JM, Wells KC, Wigal T, Vitiello B. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:147–158. [DOI] [PubMed] [Google Scholar]
- 9.Thapar A, Cooper M. Attention deficit hyperactivity disorder. The Lancet. 2016;387:1240–1250. [DOI] [PubMed] [Google Scholar]
- 10.Dalsgaard S, Øtergaard SD, Leckman JF, Mortensen PB, Pedersen MG. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: A nationwide cohort study. Lancet. 2015;385:2190–2196. [DOI] [PubMed] [Google Scholar]
- 11.Hechtman L, Swanson JM, Sibley MH, Stehli A, Owens EB, Mitchell JT, Arnold LE, Molina BS, Hinshaw SP, Jensen PS, Abikoff HB, Perez Algorta G, Howard AL, Hoza B, Etcovitch J, Houssais S, Lakes KD, Nichols JQ, Group MTAC. Functional Adult Outcomes 16 Years After Childhood Diagnosis of Attention-Deficit/Hyperactivity Disorder: MTA Results. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55:945–952 e942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, Atkinson LZ, Tessari L, Banaschewski T, Coghill D, Hollis C, Simonoff E, Zuddas A, Barbui C, Purgato M, Steinhausen H-C, Shokraneh F, Xia J, Cipriani A. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. The Lancet Psychiatry. 2018;5:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pliszka S Practice Parameter for the Assessment and Treatment of Children and Adolescents With Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. [DOI] [PubMed] [Google Scholar]
- 14.Subcommittee on Attention-Deficit/Hyperactivity Disorder: Steering Committee on Quality Improvement and Management. ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AACAP Official Action. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. [DOI] [PubMed] [Google Scholar]
- 16.Chan E, Fogler JM, Hammerness PG. Treatment of attention-deficit/hyperactivity disorder in adolescents: A systematic review. JAMA. 2016;315:1997–2008. [DOI] [PubMed] [Google Scholar]
- 17.Raman SR, Man KKC, Bahmanyar S, Berard A, Bilder S, Boukhris T, Bushnell G, Crystal S, Furu K, KaoYang YH, Karlstad O, Kieler H, Kubota K, Lai EC, Martikainen JE, Maura G, Moore N, Montero D, Nakamura H, Neumann A, Pate V, Pottegard A, Pratt NL, Roughead EE, Macias Saint-Gerons D, Sturmer T, Su CC, Zoega H, Sturkenbroom M, Chan EW, Coghill D, Ip P, Wong ICK. Trends in attention-deficit hyperactivity disorder medication use: a retrospective observational study using population-based databases. The lancet Psychiatry. 2018;5:824–835. [DOI] [PubMed] [Google Scholar]
- 18.Sultan RS, Correll CU, Schoenbaum M, King M, Walkup JT, Olfson M. National Patterns of Commonly Prescribed Psychotropic Medications to Young People. Journal of child and adolescent psychopharmacology. 2018;28:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw P Quantifying the benefits and risks of methylphenidate as treatment for childhood attention-deficit/hyperactivity disorder. JAMA. 2016;315:1953–1955. [DOI] [PubMed] [Google Scholar]
- 20.Storebø OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, Nilausen TD, Magnusson FL, Zwi M, Gillies D, Rosendal S, Groth C, Rasmussen KB, Gauci D, Kirubakaran R, Forsbøl B, Simonsen E, Gluud C. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ. 2015;351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frieden TR. Evidence for Health Decision Making — Beyond Randomized, Controlled Trials. N Engl J Med. 2017;377:465–475. [DOI] [PubMed] [Google Scholar]
- 22.Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surman CBH, Monuteaux MC, Petty CR, Faraone SV, Spencer TJ, Chu NF, Biederman J. Representativeness of participants in a clinical trial for Attention-Deficit/Hyperactivity Disorder? Comparison with adults from a large observational study. Journal of Clinical Psychiatry. 2010;71:1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kollins SH. Moving beyond symptom remission to optimize long-term treatment of attention-deficit/hyperactivity disorder. JAMA pediatrics. 2018. [DOI] [PubMed] [Google Scholar]
- 25.Carpentier PJ, Levin FR. Pharmacological Treatment of ADHD in Addicted Patients: What Does the Literature Tell Us? Harv Rev Psychiatry. 2017;25:50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crunelle CL, van den Brink W, Moggi F, Konstenius M, Franck J, Levin FR, van de Glind G, Demetrovics Z, Coetzee C, Luderer M, Schellekens A, Matthys F. International Consensus Statement on Screening, Diagnosis and Treatment of Substance Use Disorder Patients with Comorbid Attention Deficit/Hyperactivity Disorder. Eur Addict Res. 2018;24:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, McGough J, Wigal S, Wigal TIM, Vitiello B, Skrobala A, Posner K, Ghuman J, Cunningham C, Davies M, Chuang S, Cooper TOM. Efficacy and Safety of Immediate-Release Methylphenidate Treatment for Preschoolers With ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1284–1293. [DOI] [PubMed] [Google Scholar]
- 28.Charach A, Carson P, Fox S, Ali MU, Beckett J, Lim CG. Interventions for Preschool Children at High Risk for ADHD: A Comparative Effectiveness Review. Pediatrics. 2013. [DOI] [PubMed] [Google Scholar]
- 29.Corrigan-Curay J, Sacks L, Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. 2018;320:867–868. [DOI] [PubMed] [Google Scholar]
- 30.Murray ML, Insuk S, Banaschewski T, Neubert AC, McCarthy S, Buitelaar JK, Coghill D, Dittmann RW, Konrad K, Panei P, Rosenthal E, Sonuga-Barke EJ, Wong IC. An inventory of European data sources for the long-term safety evaluation of methylphenidate. European child & adolescent psychiatry. 2013;22:605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leucht S, Davis JM. Enthusiasm and Skepticism About Using National Registers to Analyze Psychotropic Drug Outcomes. JAMA psychiatry. 2018;75:314–315. [DOI] [PubMed] [Google Scholar]
- 32.Sonuga-Barke EJ. Can Medication Effects Be Determined Using National Registry Data? A Cautionary Reflection on Risk of Bias in “Big Data” Analytics. Biological psychiatry. 2016;80:893–895. [DOI] [PubMed] [Google Scholar]
- 33.Kyriacou DN, Lewis RJ. Confounding by Indication in Clinical Research. Jama. 2016;316:1818–1819. [DOI] [PubMed] [Google Scholar]
- 34.Hallas J, Pottegard A. Use of self-controlled designs in pharmacoepidemiology. J Intern Med. 2014;275:581–589. [DOI] [PubMed] [Google Scholar]
- 35.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ (Clinical research ed). 2016;354:i4515. [DOI] [PubMed] [Google Scholar]
- 36.Graham J, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW, Dopfner M, Hamilton R, Hollis C, Holtmann M, Hulpke-Wette M, Lecendreux M, Rosenthal E, Rothenberger A, Santosh P, Sergeant J, Simonoff E, Sonuga-Barke E, Wong IC, Zuddas A, Steinhausen HC, Taylor E, European Guidelines G. European guidelines on managing adverse effects of medication for ADHD. European child & adolescent psychiatry. 2011;20:17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen VC, Yang YH, Liao YT, Kuo TY, Liang HY, Huang KY, Huang YC, Lee Y, McIntyre RS, Lin TC. The association between methylphenidate treatment and the risk for fracture among young ADHD patients: A nationwide population-based study in Taiwan. PloS one. 2017;12:e0173762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalsgaard S, Leckman JF, Mortensen PB, Nielsen HS, Simonsen M. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. The lancet Psychiatry. 2015;2:702–709. [DOI] [PubMed] [Google Scholar]
- 40.Jacob L, Kostev K. Impact of attention deficit hyperactivity disorder therapy on fracture risk in children treated in German pediatric practices. Osteoporosis International. 2017;28:1265–1269. [DOI] [PubMed] [Google Scholar]
- 41.Man KK, Chan EW, Coghill D, Douglas I, Ip P, Leung LP, Tsui MS, Wong WH, Wong IC. Methylphenidate and the risk of trauma. Pediatrics. 2015;135:40–48. [DOI] [PubMed] [Google Scholar]
- 42.Marcus SC, Wan GJ, Zhang HF, Olfson M. Injury among stimulant-treated youth with ADHD. Journal of attention disorders. 2008;12:64–69. [DOI] [PubMed] [Google Scholar]
- 43.Mikolajczyk R, Horn J, Schmedt N, Langner I, Lindemann C, Garbe E. Injury prevention by medication among children with attention-deficit/hyperactivity disorder: a case-only study. JAMA pediatrics. 2015;169:391–395. [DOI] [PubMed] [Google Scholar]
- 44.Raman SR, Marshall SW, Haynes K, Gaynes BN, Naftel AJ, Sturmer T. Stimulant treatment and injury among children with attention deficit hyperactivity disorder: an application of the self-controlled case series study design. Injury prevention : journal of the International Society for Child and Adolescent Injury Prevention. 2013;19:164–170. [DOI] [PubMed] [Google Scholar]
- 45.van den Ban E, Souverein P, Meijer W, van Engeland H, Swaab H, Egberts T, Heerdink E. Association between ADHD drug use and injuries among children and adolescents. European child & adolescent psychiatry. 2014;23:95–102. [DOI] [PubMed] [Google Scholar]
- 46.Liao YT, Yang YH, Kuo TY, Liang HY, Huang KY, Wang TN, Lee Y, McIntyre RS, Chen VC. Dosage of methylphenidate and traumatic brain injury in ADHD: a population-based study in Taiwan. European child & adolescent psychiatry. 2018;27:279–288. [DOI] [PubMed] [Google Scholar]
- 47.Chien W-C, Chung C-H, Lin F-H, Yeh C-B, Huang S-Y, Lu R-B, Chang H-A, Kao Y-C, Chiang W-S, Chou Y-C, Tsao C-H, Wu Y-F, Tzeng N-S. The risk of injury in adults with attention-deficit hyperactivity disorder: A nationwide, matched-cohort, population-based study in Taiwan. Research in developmental disabilities. 2017;65:57–73. [DOI] [PubMed] [Google Scholar]
- 48.Liou YJ, Wei HT, Chen MH, Hsu JW, Huang KL, Bai YM, Su TP, Li CT, Yang AC, Tsai SJ, Lin WC, Chen TJ. Risk of Traumatic Brain Injury Among Children, Adolescents, and Young Adults With Attention-Deficit Hyperactivity Disorder in Taiwan. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2018;63:233–238. [DOI] [PubMed] [Google Scholar]
- 49.Chang Z, Lichtenstein P, D’Onofrio BM, Sjolander A, Larsson H. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA psychiatry. 2014;71:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang Z, Quinn PD, Hur K, Gibbons RD, Sjolander A, Larsson H, D’Onofrio BM. Association Between Medication Use for Attention-Deficit/Hyperactivity Disorder and Risk of Motor Vehicle Crashes. JAMA psychiatry. 2017;74:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y, Sjolander A, Cederlof M, D’Onofrio BM, Almqvist C, Larsson H, Lichtenstein P. Association Between Medication Use and Performance on Higher Education Entrance Tests in Individuals With Attention-Deficit/Hyperactivity Disorder. JAMA psychiatry. 2017;74:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcus SC, Durkin M. Stimulant adherence and academic performance in urban youth with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:480–489. [DOI] [PubMed] [Google Scholar]
- 53.van der Schans J, Cicek R, Vardar S, Bos JH, de Vries TW, Hoekstra PJ, Hak E. Methylphenidate use and school performance among primary school children: a descriptive study. BMC psychiatry. 2017;17:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoega H, Rothman KJ, Huybrechts KF, Olafsson O, Baldursson G, Almarsdottir AB, Jonsdottir S, Halldorsson M, Hernandez-Diaz S, Valdimarsdottir UA. A population-based study of stimulant drug treatment of ADHD and academic progress in children. Pediatrics. 2012;130:e53–62. [DOI] [PubMed] [Google Scholar]
- 55.Jangmo A, Stalhandske A, Chang Z, Chen Q, Almqvist C, Feldman I, Bulik CM, Lichtenstein P, D’Onofrio B, Kuja-Halkola R, Larsson H. Attention-Deficit/Hyperactivity Disorder, School Performance, and Effect of Medication. Journal of the American Academy of Child and Adolescent Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keilow M, Holm A, Fallesen P. Medical treatment of Attention Deficit/Hyperactivity Disorder (ADHD) and children’s academic performance. PloS one. 2018;13:e0207905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lichtenstein P, Halldner L, Zetterqvist J, Sjolander A, Serlachius E, Fazel S, Langstrom N, Larsson H. Medication for attention deficit-hyperactivity disorder and criminality. The New England journal of medicine. 2012;367:2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohr-Jensen C, Bisgaard CM, Boldsen SK, Steinhausen HC. Attention-Deficit/Hyperactivity Disorder in Childhood and Adolescence and the Risk of Crime in Young Adulthood in a Danish Nationwide Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2019. [DOI] [PubMed] [Google Scholar]
- 59.Chen Q, Sjolander A, Runeson B, D’Onofrio BM, Lichtenstein P, Larsson H. Drug treatment for attention-deficit/hyperactivity disorder and suicidal behaviour: register based study. BMJ (Clinical research ed). 2014;348:g3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang KL, Wei HT, Hsu JW, Bai YM, Su TP, Li CT, Lin WC, Tsai SJ, Chang WH, Chen TJ, Chen MH. Risk of suicide attempts in adolescents and young adults with attention-deficit hyperactivity disorder: a nationwide longitudinal study. The British journal of psychiatry : the journal of mental science. 2018;212:234–238. [DOI] [PubMed] [Google Scholar]
- 61.Liang SH, Yang YH, Kuo TY, Liao YT, Lin TC, Lee Y, McIntyre RS, Kelsen BA, Wang TN, Chen VC. Suicide risk reduction in youths with attention-deficit/hyperactivity disorder prescribed methylphenidate: A Taiwan nationwide population-based cohort study. Research in developmental disabilities. 2018;72:96–105. [DOI] [PubMed] [Google Scholar]
- 62.Linden S, Bussing R, Kubilis P, Gerhard T, Segal R, Shuster JJ, Winterstein AG. Risk of Suicidal Events With Atomoxetine Compared to Stimulant Treatment: A Cohort Study. Pediatrics. 2016;137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Man KKC, Coghill D, Chan EW, Lau WCY, Hollis C, Liddle E, Banaschewski T, McCarthy S, Neubert A, Sayal K, Ip P, Schuemie MJ, Sturkenboom M, Sonuga-Barke E, Buitelaar J, Carucci S, Zuddas A, Kovshoff H, Garas P, Nagy P, Inglis SK, Konrad K, Hage A, Rosenthal E, Wong ICK. Association of Risk of Suicide Attempts With Methylphenidate Treatment. JAMA psychiatry. 2017;74:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang Z, Lichtenstein P, Halldner L, D’Onofrio B, Serlachius E, Fazel S, Langstrom N, Larsson H. Stimulant ADHD medication and risk for substance abuse. Journal of child psychology and psychiatry, and allied disciplines. 2014;55:878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinhausen HC, Bisgaard C. Substance use disorders in association with attention-deficit/hyperactivity disorder, co-morbid mental disorders, and medication in a nationwide sample. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2014;24:232–241. [DOI] [PubMed] [Google Scholar]
- 66.Quinn PD, Chang Z, Hur K, Gibbons RD, Lahey BB, Rickert ME, Sjolander A, Lichtenstein P, Larsson H, D’Onofrio BM. ADHD Medication and Substance-Related Problems. The American journal of psychiatry. 2017;174:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang Z, D’Onofrio BM, Quinn PD, Lichtenstein P, Larsson H. Medication for Attention-Deficit/Hyperactivity Disorder and Risk for Depression: A Nationwide Longitudinal Cohort Study. Biological psychiatry. 2016;80:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee MJ, Yang KC, Shyu YC, Yuan SS, Yang CJ, Lee SY, Lee TL, Wang LJ. Attention-deficit hyperactivity disorder, its treatment with medication and the probability of developing a depressive disorder: A nationwide population-based study in Taiwan. Journal of affective disorders. 2016;189:110–117. [DOI] [PubMed] [Google Scholar]
- 69.Viktorin A, Ryden E, Thase ME, Chang Z, Lundholm C, D’Onofrio BM, Almqvist C, Magnusson PK, Lichtenstein P, Larsson H, Landen M. The Risk of Treatment-Emergent Mania With Methylphenidate in Bipolar Disorder. The American journal of psychiatry. 2017;174:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang LJ, Shyu YC, Yuan SS, Yang CJ, Yang KC, Lee TL, Lee SY. Attention-deficit hyperactivity disorder, its pharmacotherapy, and the risk of developing bipolar disorder: A nationwide population-based study in Taiwan. Journal of psychiatric research. 2016;72:6–14. [DOI] [PubMed] [Google Scholar]
- 71.Man KK, Coghill D, Chan EW, Lau WC, Hollis C, Liddle E, Banaschewski T, McCarthy S, Neubert A, Sayal K, Ip P, Wong IC. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Translational psychiatry. 2016;6:e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shyu YC, Yuan SS, Lee SY, Yang CJ, Yang KC, Lee TL, Wang LJ. Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: A nationwide population-based study in Taiwan. Schizophrenia research. 2015;168:161–167. [DOI] [PubMed] [Google Scholar]
- 73.Liu X, Carney PR, Bussing R, Segal R, Cottler LB, Winterstein AG. Stimulants Do Not Increase the Risk of Seizure-Related Hospitalizations in Children with Epilepsy. Journal of child and adolescent psychopharmacology. 2018;28:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McAfee AT, Holdridge KC, Johannes CB, Hornbuckle K, Walker AM. The effect of pharmacotherapy for attention deficit hyperactivity disorder on risk of seizures in pediatric patients as assessed in an insurance claims database. Current drug safety. 2008;3:123–131. [DOI] [PubMed] [Google Scholar]
- 75.McAfee AT, Landon J, Jones M, Bangs ME, Acharya N, Hornbuckle K, Wong J. A cohort study of the risk of seizures in a pediatric population treated with atomoxetine or stimulant medications. Pharmacoepidemiology and drug safety. 2013;22:386–393. [DOI] [PubMed] [Google Scholar]
- 76.Wiggs KK, Chang Z, Quinn PD, Hur K, Gibbons R, Dunn D, Brikell I, Larsson H, D’Onofrio BM. Attention-deficit/hyperactivity disorder medication and seizures. Neurology. 2018;90:e1104–e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brikell I, Chen Q, Kuja-Halkola R, D’Onofrio BM, Wiggs KK, Lichtenstein P, Almqvist C, Quinn PD, Chang Z, Larsson H. Medication treatment for attention-deficit/hyperactivity disorder and the risk of acute seizures in individuals with epilepsy. Epilepsia. 2019;60:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Man KKC, Ip P, Chan EW, Law SL, Leung MTY, Ma EXY, Quek WT, Wong ICK. Effectiveness of Pharmacological Treatment for Attention-Deficit/Hyperactivity Disorder on Physical Injuries: A Systematic Review and Meta-Analysis of Observational Studies. CNS drugs. 2017;31:1043–1055. [DOI] [PubMed] [Google Scholar]
- 79.Ruiz-Goikoetxea M, Cortese S, Aznarez-Sanado M, Magallon S, Alvarez Zallo N, Luis EO, de Castro-Manglano P, Soutullo C, Arrondo G. Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: A systematic review and meta-analysis. Neuroscience and biobehavioral reviews. 2018;84:63–71. [DOI] [PubMed] [Google Scholar]
- 80.Mansournia MA, Etminan M, Danaei G, Kaufman JS, Collins G. Handling time varying confounding in observational research. BMJ (Clinical research ed). 2017;359:j4587. [DOI] [PubMed] [Google Scholar]
- 81.Arnold BF, Ercumen A, Benjamin-Chung J, Colford JM Jr. Brief Report: Negative Controls to Detect Selection Bias and Measurement Bias in Epidemiologic Studies. Epidemiology (Cambridge, Mass). 2016;27:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Walraven C, Davis D, Forster AJ, Wells GA. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol. 2004;57:672–682. [DOI] [PubMed] [Google Scholar]
- 83.Li X, Cole SR, Westreich D, Brookhart MA. Primary non-adherence and the new-user design. Pharmacoepidemiology and drug safety. 2018;27:361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 85.Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, Elliott GR, Greenhill LL, Newcorn JH, Wells KC, Wigal T, Gibbons RD, Hur K, Houck PR, Group MTAC. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swanson JM, Arnold LE, Molina BSG, Sibley MH, Hechtman LT, Hinshaw SP, Abikoff HB, Stehli A, Owens EB, Mitchell JT, Nichols Q, Howard A, Greenhill LL, Hoza B, Newcorn JH, Jensen PS, Vitiello B, Wigal T, Epstein JN, Tamm L, Lakes KD, Waxmonsky J, Lerner M, Etcovitch J, Murray DW, Muenke M, Acosta MT, Arcos-Burgos M, Pelham WE, Kraemer HC, Group MTAC. Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. Journal of child psychology and psychiatry, and allied disciplines. 2017;58:663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sylvestre MP, Abrahamowicz M. Flexible modeling of the cumulative effects of time-dependent exposures on the hazard. Stat Med. 2009;28:3437–3453. [DOI] [PubMed] [Google Scholar]
- 88.Kantak KM, Dwoskin LP. Necessity for research directed at stimulant type and treatment-onset age to access the impact of medication on drug abuse vulnerability in teenagers with ADHD. Pharmacology, biochemistry, and behavior. 2016;145:24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Humphreys KL, Eng T, Lee SS. Stimulant medication and substance use outcomes: a meta-analysis. JAMA psychiatry. 2013;70:740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molina BS, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, Hoza B, Epstein JN, Wigal T, Abikoff HB, Greenhill LL, Jensen PS, Wells KC, Vitiello B, Gibbons RD, Howard A, Houck PR, Hur K, Lu B, Marcus S, Group MTAC. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mangoni AA, Jansen PA, Jackson SH. Under-representation of older adults in pharmacokinetic and pharmacodynamic studies: a solvable problem? Expert Rev Clin Pharmacol. 2013;6:35–39. [DOI] [PubMed] [Google Scholar]
- 92.Reeve E, Wiese MD, Mangoni AA. Alterations in drug disposition in older adults. Expert opinion on drug metabolism & toxicology. 2015;11:491–508. [DOI] [PubMed] [Google Scholar]
- 93.Alamo C, Lopez-Munoz F, Garcia-Garcia P, Garcia-Ramos S. Risk-benefit analysis of antidepressant drug treatment in the elderly. Psychogeriatrics. 2014;14:261–268. [DOI] [PubMed] [Google Scholar]
- 94.Sturman N, Deckx L, van Driel ML. Methylphenidate for children and adolescents with autism spectrum disorder. The Cochrane database of systematic reviews. 2017;11:Cd011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Torgersen T, Gjervan B, Lensing MB, Rasmussen K. Optimal management of ADHD in older adults. Neuropsychiatric disease and treatment. 2016;12:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O’Duffy A, Connell FA, Ray WA. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, Cheetham TC, Quinn VP, Dublin S, Boudreau DM, Andrade SE, Pawloski PA, Raebel MA, Smith DH, Achacoso N, Uratsu C, Go AS, Sidney S, Nguyen-Huynh MN, Ray WA, Selby JV. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. Jama. 2011;306:2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hennissen L, Bakker MJ, Banaschewski T, Carucci S, Coghill D, Danckaerts M, Dittmann RW, Hollis C, Kovshoff H, McCarthy S, Nagy P, Sonuga-Barke E, Wong IC, Zuddas A, Rosenthal E, Buitelaar JK, consortium A. Cardiovascular Effects of Stimulant and Non-Stimulant Medication for Children and Adolescents with ADHD: A Systematic Review and Meta-Analysis of Trials of Methylphenidate, Amphetamines and Atomoxetine. CNS drugs. 2017;31:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dunn G, Emsley R, Liu H, Landau S. Integrating biomarker information within trials to evaluate treatment mechanisms and efficacy for personalised medicine. Clinical trials. 2013;10:709–719. [DOI] [PubMed] [Google Scholar]
- 100.Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJ, Tannock R, Franke B. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1:15020. [DOI] [PubMed] [Google Scholar]
- 101.Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Molecular psychiatry. 2010;15:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Compton WM, Han B, Blanco C, Johnson K, Jones CM. Prevalence and Correlates of Prescription Stimulant Use, Misuse, Use Disorders, and Motivations for Misuse Among Adults in the United States. American Journal of Psychiatry. 2018;175:741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. Misuse and Diversion of Stimulants Prescribed for ADHD: A Systematic Review of the Literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.