Abstract
The germination of Clostridium difficile spores is an important stage of the C. difficile life cycle. In other endospore-forming bacteria, the composition of the medium in which the spores are generated influences the abundance of germination-specific proteins, thereby influencing the sensitivity of the spores towards germinants. In C. difficile media composition on the spores has only been reported to influence the number of spores produced. One of the measures of spore germination is the analysis of the release of DPA from the spore core. To detect DPA release in real time, terbium chloride is often added to the germination conditions because Tb3+ complexes with the released DPA and this can be detected using fluorescence measurements. Although C. difficile spores germinate in response to TA and glycine, recently calcium was identified as an enhancer for spore germination. Here, we find that germination by spores prepared in peptone rich media, such as 70:30, is positively influenced by terbium. We hypothesize that, in these assays, Tb3+ functions similarly to calcium. Although the mechanism(s) causing increased sensitivity of the C. difficile spores that are prepared in peptone rich media to terbium is still unknown, we suggest that the TbCl3 concentration used in the analysis of C. difficile DPA release be carefully titrated so as not to misinterpret future findings.
Introduction
Patients treated with broad-spectrum antibiotics have a disrupted gut microbiome and, as a result, are vulnerable to C. difficile infection (CDI) due to the loss of the colonization resistance that is provided by the microbiota [1]. Upon colonization of the intestinal tract, C. difficile vegetative cells elicit the symptoms of disease through the secretion of two toxins, TcdA (an enterotoxin) and TcdB (a cytotoxin) and their eventual endocytosis by the colonic epithelial cells [2]. C. difficile-infected patients are commonly treated with other antibiotics, such as vancomycin or fidaxomicin, which relieve the primary symptoms of CDI by targeting the actively-growing, toxin-producing, vegetative forms [3]. However, and importantly, these antibiotics also disrupt the gut microbiome and may result in recurring episodes of disease[4].
Though the C. difficile vegetative cells are strictly anaerobic, they form metabolically dormant spores that are resistant to toxic chemicals, high temperature, radiation and oxygen [5–7]. As a result of this dormancy, the spores can survive outside the host in the aerobic environmental setting where the strictly anaerobic, vegetative form cannot. Structurally, endospores are composed of several, well-defined layers [8]. The endospore core contains DNA, RNA, ribosomes and a large amount of dipicolinic acid (pyridine-2-, 6-dicarboxylic acid; DPA), chelated with calcium (CaDPA) that provides heat resistance [6, 9]. The core is surrounded by an inner spore membrane composed of phospholipids, followed by a layer of germ-cell wall (which later becomes the cell wall of the vegetative cell during outgrowth). A thick layer of specialized peptidoglycan, cortex, surrounds the germ-cell wall and protects the spore core against osmolysis. The cortex is surrounded by the outer membrane derived from the mother cell, coat proteins and an exosporium layer [6–8, 10]. When the environmental conditions become favorable, spores germinate and outgrow to the vegetative form [7, 11, 12].
C. difficile spore germination is initiated in response to the combinatorial actions of certain bile acids [e.g., taurocholic acid (TA)] and amino acids (e.g., glycine) [13–17]. In prior work, the bile acid germinant receptor was identified as the germination-specific, subtilisin-like, pseudoprotease, CspC [18]. More recently, we found that a small mutation in the cspA coding region of cspBA resulted in spores that germinate in response to TA alone (without the requirement for an amino acid), suggesting that the CspA protein is the amino acid germinant receptor (Shrestha and Sorg, submitted [19]).
In several endospore-forming bacteria, the abundance of the spore germinant receptors is significantly influenced by the medium used to generate the spores [20]. C. difficile spores are generally prepared in rich media at an optimal temperature and cell density for higher yields [21]. A study by Hornstra et al., demonstrated that in Bacillus cereus ATCC 14579, the composition of the sporulation medium increased the transcription of the seven germinant receptors, and the nutrient-induced spore germination was significantly affected [22]. Similar results were also observed in other spore-forming bacteria (e.g., B. subtilis) [20], however, no studies have been performed in C. difficile on how the medium used to generate spores influences the properties of spore germination; although there are studies that show that high spore yield can be obtained when C. difficile spores are produced in peptone rich medium, such as 70:30 or SMC [21, 23–27]. Here, we report that media composition influences the abundance of the C. difficile spore germinant receptors and find that spores prepared on peptone-rich medium can germinate in response to TA and terbium ions (which may function as a calcium substitute). Because Tb3+ ions are often used to detect the presence of the dipicolinic acid that is released during the early events of spore germination [16, 17, 28–33], these findings could be useful for future analysis of C. difficile spore germination or for the germination of other bacteria that are influenced by calcium ions.
MATERIALS AND METHODS
Growth conditions
C. difficile UK1 (ribotype 027) [18, 34, 35] and M68 (ribotype 078) [17, 35, 36] strains were grown on either BHIS agar medium [Brain heart infusion (Bacto BHI supplemented with 5 g / L yeast extract] or 70:30 agar medium [63 g / L Bacto peptone, 3.5 g / L protease peptone, 11.1 g / L BHI, 1.5 g/ L yeast extract, 1.06 g / L tris base and 0.7 g / L ammonium sulfate (NH4SO4)] or SMC agar medium (90 g / L Bacto peptone, 5 g / L protease peptone, 1 g / L NH4SO4 and 1.5 g / L tris base) [37] or TYG agar medium [(30 g / L Bacto typtone, 20 g / L yeast extract and 10 g / L glucose)] in an anaerobic environment (85% N2, 10% H2, and 5% CO2) (Model B, Coy Laboratories, Grass Lake, MI) at 37 °C. The BHIS, 70:30 and SMC agar media were supplemented with 1 g / L of L-cysteine while TYG agar medium was supplemented with 1 g / L thioglycolate.
Spore purification
C. difficile spores were purified as described previously [16, 17]. Briefly, the C. difficile UK1 and C. difficile M68 strains were grown on either BHIS, 70:30, SMC or TYG agar medium as described above and allowed to grow for 4 days. Cells from each plate were scraped into 1 mL sterile water and incubated at 4 °C overnight. Next, the cells were washed five times with water to remove cell debris and combined into 2 mL total volume. The washed spores were layered on top of 8 mL of 50% sucrose and centrifuged at 4,000 X g for 20 minutes. The spore pellets were separated from the supernatant, and the spores were washed five times with water to remove any sucrose and incubated at 4 °C until use.
Germination of spores isolated from different media
The spores were characterized by measuring DPA release as well as changes to the optical density (OD600) during germination, as described previously [17, 30]. Briefly, spores purified from various media were heat activated at 65 °C for 30 minutes and suspended in water at an OD600 = 50. In order to measure DPA release, the spores were added to final OD600 of 0.25 in 100 μL final volume of buffer (50 mM HEPES, 250 mM NaCl, pH 7.5) supplemented with 250 μM Tb3+ and containing either 10 mM TA or 30 mM glycine or both 10 mM TA and 30 mM glycine in a 96 well black plate. The DPA release was then measured for 2 hours at 37 °C using a SpectraMax M3 (Molecular Devices) plate reader with excitation at 270 nm and emission at 545 nm with a 420 nm cutoff.
In order to measure the germination by optical density, the spores were added to a final OD600 of 0.5 in HEPES buffer supplemented with 30 mM glycine alone or 10 mM TA alone or 10 mM TA and 30 mM glycine in 100 μL final volume with or without 250 μM Tb3+ in 96 well clear plates. OD600 was monitored at 37 °C for 2 hrs. using the plate reader. To determine the influence of metals on C. difficile spore germination, 70:30-prepared spores were incubated with either 10 mM TA or 10 mM TA supplemented with 10 mM or 250 μM of metal chlorides (CaCl2, MnCl2, MgCl2, KCl, LiCl2 or TbCl3). Germination was monitored as above.
Western blot
Samples for SleC activation were prepared by treating UK1 and M68 spores with either 10 mM TA or 30 mM glycine or both 10 mM TA and 30 mM glycine with or without 250 μM Tb3+ and incubated at 37 °C for 2 hours. Soluble protein samples were extracted by boiling the spores in NuPage buffer (Life Technologies) at 95 °C for 20 minutes and centrifuged at 20,000 x g for 10 minutes to separate supernatant from the spore pellet. Solubilized proteins were separated by SDS-PAGE and transferred onto a nitrocellulose membrane for SleC detection by western blot. Similar technique was used to determine the influence of metals on C. difficile spore germination using SleC activation.
In order to quantify amounts of SleC, CspB, and CspC proteins in spores prepared from different media, solubilized proteins were extracted by boiling 2 × 109 spores per mL in NuPAGE (Life Technologies) sample buffer at 95 °C for 20 minutes. Equal volume of spore extracts and recombinant CspB, CspC or SleC standard proteins were separated by SDS-PAGE. Proteins were then transferred onto low-fluorescence polyvinylidene difluoride membrane (PVDF) at 30V for 16 hours. The membrane was then blocked in 10% skimmed milk in TBS (Tris-buffered saline) and washed thrice with TBS containing 0.1% (vol / vol) Tween-20 (TSBT) for 20 minutes each at room temperature. The membranes were then incubated with anti-CspB, anti-CspC or anti-SleC antibodies for 2 hours and washed thrice with TSBT. For the secondary antibody, AlexaFlour 555-labeled donkey anti-rabbit antibody was used to label the membranes for 2 hours in the dark. The membranes were washed again thrice with TBST in the dark, and scanned with a GE Typhoon Scanner using Cy3 setting, an appropriate wavelength for the Alexa Flour 555 fluorophore. The fluorescent bands were analyzed using ImageQuant TL 7.0 image analysis software. Intensity of the extracted protein in each blot was compared to the standard curve that was included on each blot.
Calcium measurement
Calcium concentrations in different media were measured using a Ca2+ detection assay kit (Abcam, ab102505). Standards for Ca2+ were prepared as advised in the kit. The Ca2+ concentration was then measured from BHI, BHIS, 70:30, SMC and TYG medium at OD575 in the plate reader. The OD575 values for the Ca2+ measurement were then converted into μg / L. For the control, 23 mg / L CaCl2 suspended in water was used.
Statistical Analysis
All germination assays were performed in technical triplicate and data points represent the average of three independent experiments. Biological replicates showed the same findings but due to differences in time points for the OD assay and DPA content for the DPA release assays, the data could not be averaged. Calcium concentrations were measured in triplicate. Error bars represent the standard error of the mean. A one-way ANOVA with Tukey’s multiple comparisons test was used to compare the quantified protein amounts. Each blot was loaded with five standard proteins and three spore samples for quantification of proteins.
RESULTS
Tb3+ enhances the germination of C. difficile spores with TA.
Recent work by Kochan et al., on germination by C. difficile spores has shown that calcium plays a role as an enhancer of germination when added with TA [24]. In several studies, terbium has been used to replace calcium, mostly to observe the calcium binding because terbium fluoresces at certain wavelengths and calcium does not [38, 39]. One of the ways germination is observed is through the measurement of the DPA that is released from the spore core. Upon recognition of germinants by C. difficile spores, the cortex layer is degraded by the cortex lytic enzyme (SleC) and, subsequently, DPA is released from the core [40]. In the presence of terbium ions, the released DPA can associate with terbium and the terbium-DPA complex can be detected in a FRET-like assay.
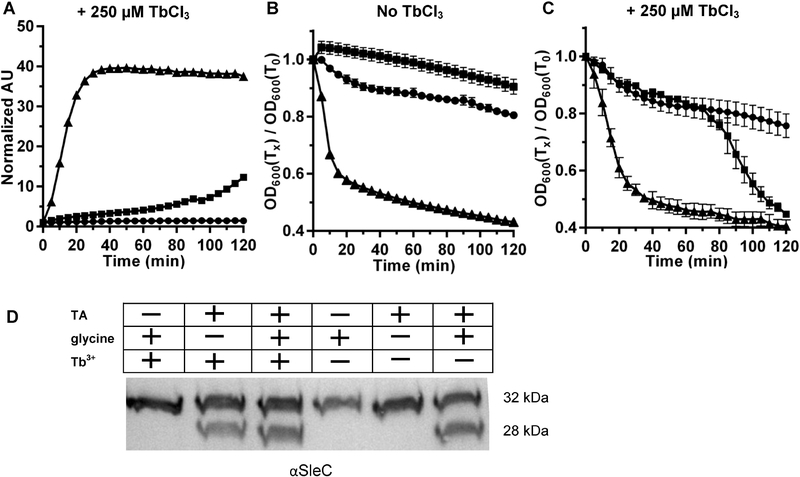
During some of our experiments analyzing the requirements of C. difficile spore germination, we found that, at times, wild type C. difficile spores would seemingly release DPA in response to TA alone. To investigate this phenomenon further, we measured the DPA released from spores that were prepared on BHIS medium. We found that these spores started to release DPA in response to TA alone after 1 hr 30 min incubation in these conditions (Figure 1A). However, when germination was measured by OD at 600 nm, in absence of terbium, the OD did not change with TA alone (Figure 1B). Surprisingly, when the germination was measured by OD in presence of terbium, the OD changed in response to TA alone (Figure 1C) - the germination rate was much faster when glycine was added as a co-germinant. To confirm that our observations were due to the initiation of spore germination and not just an artifact of the assay conditions, we tested the activation of SleC after two hours of incubation in the indicated germination condition. We found that in presence of terbium, SleC is activated when the spores were treated with TA alone, similar to the activation of SleC in response to both TA and glycine (Figure 1D). These results suggest that terbium might be involved in germination of the spores, potentially similar to the response of C. difficile spores to calcium or other metals [23].
Figure 1. Comparison of germination of C. difficile UK1 spores in presence/absence of terbium.
C. difficile UK1 spores were prepared from BHIS medium as described in the materials and methods. (A) CaDPA release from the purified spores was analyzed by suspending the spores in buffer supplemented with 250 μM TbCl3 and (●) 30 mM glycine, (■) 10 mM TA or (▲) 10 mM TA and 30 mM glycine. The extent of germination was also analyzed by determining OD600 over time. Germination by C. difficile spores was analyzed in the absence of TbCl3 (B) or in the presence of 250 μM TbCl3 (C). Data points represent the averages from three independent experiments and error bars represent the standard error of the mean. (D) Equal numbers of spores were incubated with or without 250 μM TbCl3 in 30 mM glycine or 10 mM TA or 10 mM TA and 30 mM glycine for 2 hours and soluble proteins were extracted with NuPAGE buffer and separated by SDS-PAGE followed by immunoblotting with SleC- specific antisera.
The TA-terbium response increased when spores were prepared in peptone rich medium.
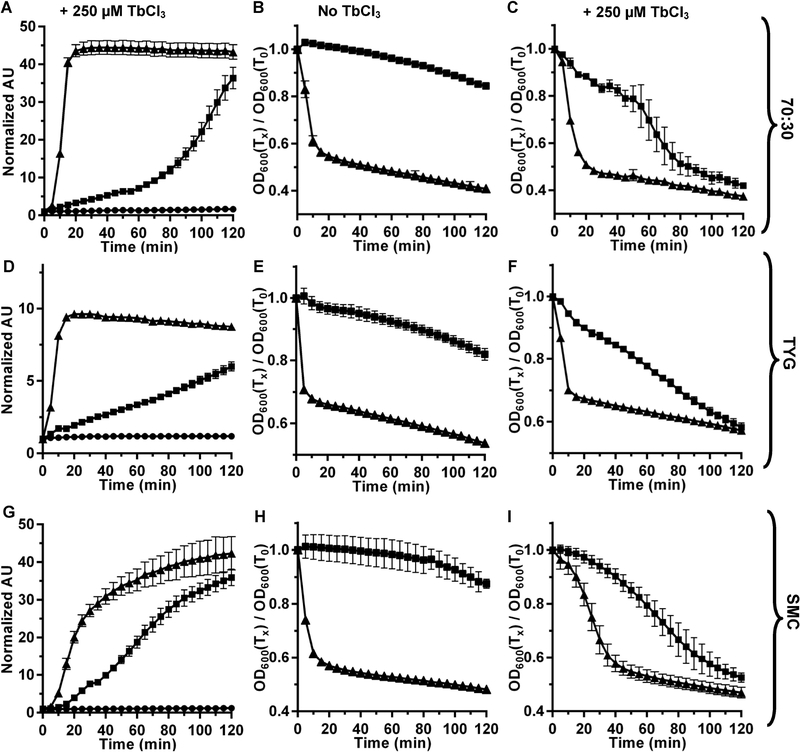
Many C. difficile strains do not efficiently form spores in liquid BHIS medium. Therefore, most studies produce spores on agar medium (e.g., BHIS, 70:30, or SMC) [21].To investigate if our observation that spores germinate in response to TA and Tb3+ was due to how spores were prepared (i.e., BHIS agar medium, on which our laboratory normally produces spores), we prepared spores on BHIS, 70:30, SMC or TYG agar medium and tested how Tb3+ influences germination of these spores. Interestingly, we found that the TA-terbium phenotype increased when spores were prepared in the peptone rich media, when compared to BHIS agar medium. In figure 2A, 2D, and 2G we compared DPA release of wildtype C. difficile UK1 spores prepared from 70:30, TYG or SMC agar medium, respectively. We found that the influence of Tb3+ on C. difficile spore germination was greater in these media compared to BHIS-prepared spores (Figure 1A). Similar to what we observed for BHIS-prepared spores, none of these spores germinated, as measured by changes in OD600, in the absence of Tb3+ (Figure, 2B, 2E, and 2H). However, when terbium was added with TA, the OD of the spore suspension dropped in response to TA and Tb3+ (Figure 2C, 2F and 2I). We also confirmed that the activation of SleC occurred in presence of TA and Tb3+ (Figure S1). These results strongly suggest that the medium used to prepare spores plays a role in the TA-terbium phenotype; the spores become more responsive to terbium when produced on a more peptone rich media (e.g., 70:30, SMC and TYG).
Figure 2. Comparison of germination of C. difficile UK1 spores prepared in different media.
The germination phenotype of wild type C. difficile UK1 spores prepared from (A, B, C) 70:30 medium, (D, E, F) TYG medium or (G, H, I) SMC medium were analyzed as described in Figure 1 (●) 30 mM glycine, (■) 10 mM TA or (▲) 10 mM TA and 30 mM glycine. Data points represent the averages from three, independent experiments and error bars represent the standard error of the mean.
TA-terbium phenotype is found in a different C. difficile ribotype.
Next, we wanted to test if the TA-terbium phenotype is only true for the C. difficile UK1 strain or if it is observed in another ribotype. In our prior work on C. difficile spore germination, we used the C. difficile M68 strain (ribotype 078) to test the impact of muricholic acids and the effect of different amino acids on C. difficile spore germination [17, 36]. Thus, we used this strain to investigate the impact of Tb3+ on germination by C. difficile spores. As for C. difficile UK1, we prepared C. difficile M68 spores in BHIS, 70:30 or SMC agar medium (M68 strains did not form spores on TYG medium for unknown reasons) and measured DPA release (Figure 3A, 3D and 3G). The M68 strain also germinated in response to TA and Tb3+ and, similar to C. difficile UK1 spores, this phenotype was stronger for spores prepared from 70:30 and SMC compared to spores prepared from BHIS agar medium. Similarly, when we tested germination by OD, in the absence of terbium, spores did not germinate in response to TA alone (Figure 3B, 3E and 3H). However, the addition of Tb3+ to the germination solution resulted in germination (Figure 3F and 3I); BHIS prepared spores (Figure 3C) had a much milder response to TbCl3 addition than did 70:30- or SMC-prepared spores (Figure 3F and 3I). These observations were also confirmed, as for C. difficile UK1, by analyzing SleC activation (Figure S2).
Figure 3. Comparison of germination of C. difficile M68 spores prepared in different media.
The germination phenotype of wild type C. difficile M68 spores prepared from (A, B, C) BHIS medium, (D, E, F) 70:30 medium and (G, H, I) SMC medium were analyzed as described in Figure 1 (●) 30 mM glycine, (■) 10 mM TA or (▲) 10 mM TA and 30 mM glycine. Data points represent the averages from three, independent experiments and error bars represent the standard error of the mean.
The TA-terbium phenotype extends to other metals.
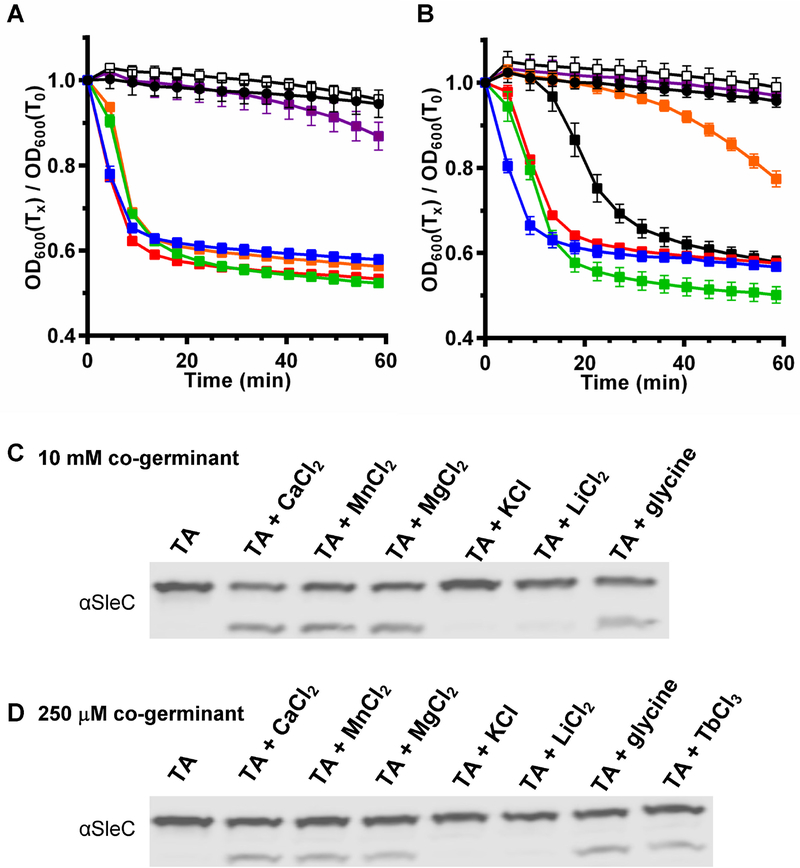
Recent work by Kochan et al. [23] has shown that apart from calcium, germination by C. difficile spores can also be stimulated by other metal ions such as MgCl2 but not with ZnCl2, LiCl2, and KCl. Here, we also tested germination by C. difficile UK1 spores prepared from 70:30 medium, which are sensitive to terbium, with these metal chlorides as enhancers or cogerminants. Similar to the results by Kochan and colleagues [23], we also found that the spores germinated with MgCl2 or CaCl2, but not with LiCl2 nor KCl, at either 10 mM (Figure 4A) or 250 μM (Figure 4B). We also tested MnCl2 and found that at 10 mM MnCl2, the spores germinated similar to CaCl2 or TbCl3 (Figure 4A) and germinated similar to CaCl2 at 250 μM MnCl2 (Figure 4B). The germination was confirmed by analyzing SleC activation. At 10 mM TA and 10 mM CaCl2 or MnCl2 or MgCl2 or glycine, C. difficile spores activated SleC (Figure 4C). A 10 mM TA and 250 μM CaCl2 or MnCl2 or MgCl2 or glycine, C. difficile also activated SleC indicating that these co-germinants are effective at low concentration (Figure 4D). Unfortunately, we could not test ZnCl2. When dissolved, ZnCl2 changed the pH of the solution to pH < 4 and any effort to neutralize the pH resulted in precipitation. Moreover, the effect of pH on C. difficile spore germination has been documented in prior work [23, 41].
Figure 4. C. difficile spores are sensitive to other metals.
C. difficile UK1 spores were prepared from 70:30 medium and purified as described in the materials and methods. Purified spores were incubated with 10 mM TA and the indicated concentration of metals or co-germinant. Germination was assayed by OD for 1 hr at 37 °C in the presence 10 mM TA alone (black circles) or with 10 mM TA and (A) 10 mM or (B) 250 μM metals or co-germinants. CaCl2 (blue), MnCl2 (green), MgCl2 (orange), KCl (open), LiCl2 (purple), glycine (red), TbCl3 (black squares). Respective SleC activation is shown in (C) and (D).
Medium composition influences the abundance of C. difficile spore germinant receptors but does not explain TA-Tb3+ phenotype.
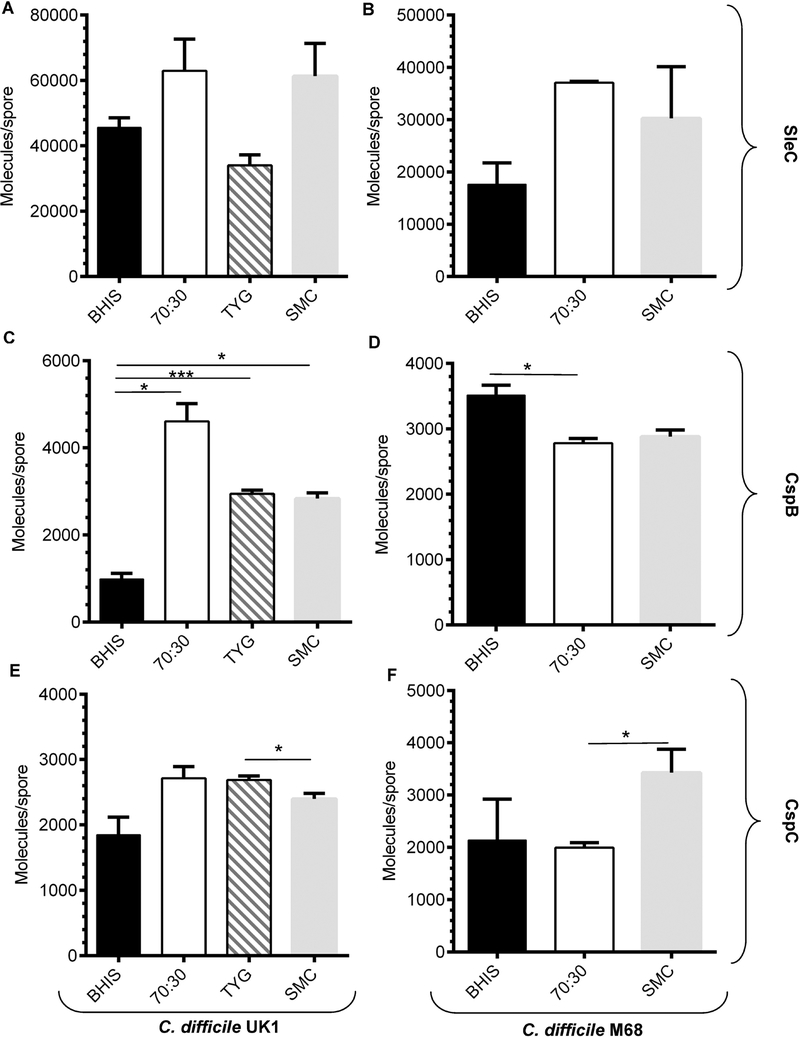
In B. subtilis, it was shown that spores prepared on different media resulted in changes to the amount of Ger proteins found within the spores [20]. We hypothesized that spores prepared in peptone rich media may have increased amounts of the germinosome proteins (i.e., CspB, CspC or SleC) which could influence the sensitivity of spores to terbium. To test this hypothesis, we quantified the amount of CspB, CspC and SleC proteins purified from the different media mentioned above using a previously described technique (due to the quality of our CspA antibody, we could not quantify the abundance of CspA in the spores). We found that there was not a significant increase in SleC levels for C. difficile UK1 or C. difficile M68 spores that were purified from different media (Figure 5A and 5B, respectively). Though the differences in SleC abundance appear striking, they did not meet statistical significance. We observed an increase in CspB protein purified from 70:30, TYG or SMC media compared to BHIS-prepared medium for spores derived from the C. difficile UK1 strain (Figure 5C). However, for C. difficile M68, spores prepared from BHIS contained a larger amount of CspB than did spores prepared on 70:30 or SMC media (Figure 5D). We also observed small differences in abundance of CspC in both the strains purified from different media (Figure 5E, 5F). But, because CspC abundance in C. difficile UK1 SMC-prepared spores was lower than in TYG and C. difficile M68 SMC-prepared spores was more abundant the abundance of CspC is not likely to contribute to the observed phenotypes. These results suggest that producing C. difficile spores on different media can influence the abundance of some spore proteins. However, we did not observe a correlation between medium and the Tb3+ phenotype observed in Figures 1, 2 and 3.
Figure 5. Quantifying the abundance of CspB, CspC and SleC in C. difficile spores.
2 × 109 spores purified from C. difficile UK1 strain (A, C, E) or the C. difficile M68 strain (B, D, F) that were generated in BHIS medium, 70:30 medium, TYG medium or SMC medium. The proteins were extracted with NuPAGE buffer and samples were separated by SDS-PAGE, transferred to low fluorescence PVDF membranes and blotted with antisera specific to the indicated proteins. Quantified proteins are expressed as molecules / spore and are represented in a bar graph form (A, B) SleC, (C, D) CspB and (E, F) CspC. The data presented represent the averages from three independent experiments and error bars represent the standard error of the mean. Statistical significance was determined using a one-way ANOVA with Tukey’s multiple comparisons test (* p < 0.05; *** p < 0.001).
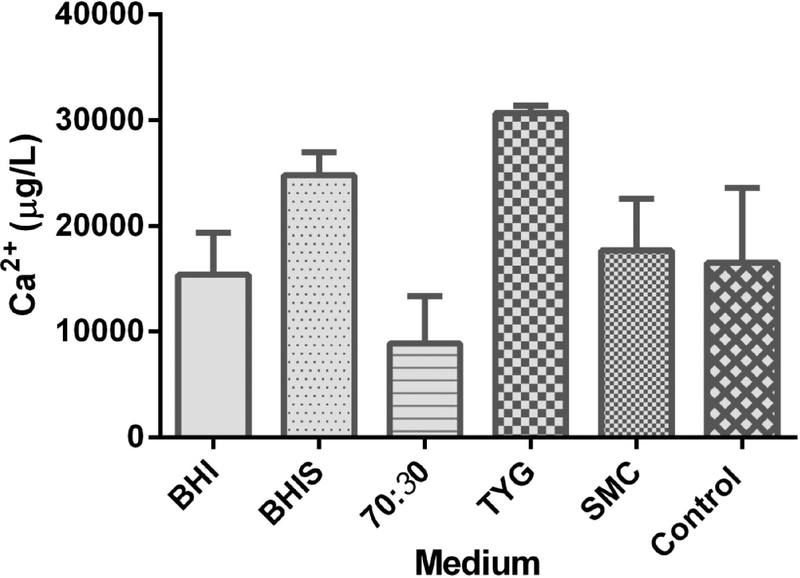
The calcium concentrations are similar in different media.
In several studies, terbium has been used to substitute calcium. Previous studies by Kochan et al. have also shown that calcium is involved in enhancing germination by C. difficile spores in the presence of germinants (TA and glycine) [23, 24]. We hypothesized that TA-terbium is functioning similar to TA-calcium/other metals and that a difference in the calcium concentration in the media used to prepare spores might contribute to spores being sensitive to calcium or terbium in these germination assays. In order to test this hypothesis, we used a colorimetric assay to detect calcium concentration in various media. However, we observed no significant difference in the calcium concentration in BHIS, 70:30, SMC or TYG media (Figure 6). These results suggest that an unidentified factor contributes to the sensitivity to terbium during C. difficile spore germination and that to detect the release of DPA from germination of C. difficile spores, the concentration of terbium should be optimized so as not to influence germination during the assay conditions.
Figure 6. Detection of calcium concentration in different media types.
Bar graph representing the calcium concentration in μg / L from BHI, BHIS, 70:30, TYG and SMC media. 23 mg/mL of calcium chloride dissolved in water is used as a control.
DISCUSSION
Growth media plays a significant role in formation of spores [20–22]. A common medium used for the growth of C. difficile vegetative cells is BHIS agar medium; however, C. difficile spores are frequently produced in / on 70:30 or SMC medium because of the improved spore yield in these media [21, 23–27]. Importantly, the different composition of sporulation media can impact the germination phenotype of the spores. For example, Ramirez-Peralta et al., showed for B. subtilis spores the medium composition had a significant effect on the rate of spore germination [20]. The rate of germination of B. subtilis spores prepared from nutrient-rich, liquid medium was significantly higher compared to nutrient-poor, liquid medium. Similarly, we found that germination of spores prepared under different sporulation conditions (media) resulted in significant differences in germination. Specifically, when we prepared spores from the BHIS medium, at the end of 2 hours germination we observed slight increase in DPA release in presence of TA alone (Figure 1A); the release of DPA corresponds to germination. Oddly, when the OD assay was used to measure the germination, there was no germination in TA alone (Figure 1B). The difference between the assays is the addition of terbium to monitor DPA release. When terbium was added in the OD assay, we observed that, similar to DPA increase in TA alone, germination was observed with OD within 2 hours suggesting that it was terbium, along with TA, that induced the germination of the spores. Although C. difficile is known to germinate in the presence of bile acids (e.g., TA) and amino acids (e.g., glycine), calcium has been identified as an important contributor to spore germination, though its role remains undefined [23, 24, 42]. It is not clear whether calcium functions as a bona fide spore cogerminant, a co-factor for a process essential for spore germination and / or as an enhancer for amino acids. But, removal of calcium by chelation prevents spore germination, indicating that calcium is essential for C. difficile spore germination, and addition of calcium increases the sensitivity of spores to germinants [24].
We found that the TA-terbium germination phenotype increased when spores were prepared from peptone rich medium, such as 70:30, SMC or TYG, and compared to BHIS-prepared spores. As shown in Figure 2, when spores were produced from 70:30, TYG or SMC medium, the spores germinated in response to TA-terbium within 1 hour. The TA-terbium phenotype was stronger in spores prepared from SMC medium followed by TYG, 70:30 then BHIS medium. These results were also true for the C. difficile M68 strain (Figure 3). We found that in wildtype C. difficile UK1 spores, CspB abundance was significantly increased in SMC, 70:30 or TYG medium compared to BHIS medium, but this was not true for C. difficile M68 spores (Figure 5). Unfortunately, we could not analyze the abundance of CspA in this study. It is possible that CspA abundance correlates with the observed phenotype, but we cannot test this directly due to the quality of the CspA antibody.
During this study, we also found that MnCl2 could function as an enhancer or cogerminant to a similar effect as CaCl2 (Figure 4). Moreover, even though MgCl2 was not as efficient as CaCl2 or MnCl2 at 250 μM (Figure 4B), when tested at 10 mM, it was equally effective. These observations for these three metals are similar to prior work on amino acids as co-germinants [15, 17]. In this prior work, there is a hierarchy of recognition of these amino acids and this hierarchy is largely conserved in another strain. Here, it appears that CaCl2 and MnCl2 are efficiently recognized as enhancer / co-germinants and MgCl2 is weaker. Obviously, there is a growing need to determine the biochemistry of the germinant receptors to determine how they interact with germinants and how these metal ions influences these interactions.
We hypothesized that the calcium concentration in BHIS medium was greater, thus reducing the effect of Tb3+ on germination (i.e., the spore would already be saturated with Ca2+ and be less influenced by Tb3+). However, there were no significant differences in the calcium concentration in different media (Figure 6). Although the mechanism for the increase in terbium sensitivity in spores prepared in different media is not known, there could be various other media components that might relate to the terbium sensitivity. However, we feel that it is important to report the impact of Tb3+ on C. difficile spore germination because analysis of DPA release is a common screening technique in spore germination studies. An increase in sensitivity to terbium may be confused with a bona fide germination phenotype and it is important to confirm the germination phenotype with different assays, such as the OD assay or SleC activation during germination. Finally, we recommend that the Tb3+ concentration used for each C. difficile strain be titered accordingly so as not to influence the germination process.
Supplementary Material
Figure S1. SleC activation with/without terbium for UK1 spores. Equal numbers of C. difficile UK1 spores prepared with 70:30, SMC or TYG medium were incubated with or without 250 μM terbium in 30 mM glycine or 10 mM TA or 10 mM TA and 30 mM glycine for 2 hours and soluble proteins were extracted with NuPAGE buffer and separated by SDS-PAGE followed by immunoblotting with SleC antisera to observe SleC activation.
Figure S2. SleC activation with/without terbium for M68 spores. Equal numbers of C. difficile M68 spores prepared with BHIS, 70:30 or SMC medium were incubated with or without 250 μM terbium in 30 mM glycine or 10 mM TA or 10 mM TA and 30 mM glycine for 2 hours and soluble proteins were extracted with NuPAGE buffer and separated by SDS-PAGE followed by immunoblotting with SleC antisera to observe SleC.
Highlights.
DPA release by germinating endospores is often detected using Tb3+ fluorescence when Tb3+ complexes with the released DPA.
Tb3+ influences C. difficile spore germination, likely functioning as a Ca2+ substitute.
For future analyses, the concentration of TbCl3 used to detect DPA release by germinating C. difficile spore should be titered so as not to influence germination.
Acknowledgments
We thank members of the Sorg lab for critical reading of this manuscript and their helpful comments during this study. We also thank members of Dr. Leif Smith’s laboratory at Texas A&M University for helpful comments during preparation of this manuscript.
This project was supported by awards 5R01AI116895 and 1U01AI124290 to J.A.S. from the National Institute of Allergy and Infectious Disease. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Britton RA, Young VB. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol 20 (2012) 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 18 (2005) 247–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Allen CA, Babakhani F, Sears P, Nguyen L, Sorg JA. Both fidaxomicin and vancomycin inhibit outgrowth of Clostridium difficile spores. Antimicrob Agents Chemother 57 (2013) 664–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers 2 (2016) 16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bhattacharjee D, McAllister KN, Sorg JA. Germinants and their receptors in Clostridia. J Bacteriol 198 (2016) 2767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Paredes-Sabja D, Shen A, Sorg JA. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol 22 (2014) 406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhu D, Sorg JA, Sun X. Clostridioides difficile biology: sporulation, germination, and corresponding therapies for C. difficile infection. Frontiers in Cellular and Infection Microbiology 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Henriques AO, Moran CPJ. Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol 61 (2007) 555–88. [DOI] [PubMed] [Google Scholar]
- [9].Gil F, Lagos-Moraga S, Calderon-Romero P, Pizarro-Guajardo M, Paredes-Sabja D. Updates on Clostridium difficile spore biology. Anaerobe 45 (2017) 3–9. [DOI] [PubMed] [Google Scholar]
- [10].Abhyankar W, Beek AT, Dekker H, Kort R, Brul S, de Koster CG. Gel-free proteomic identification of the Bacillus subtilis insoluble spore coat protein fraction. Proteomics 11 (2011) 4541–50. [DOI] [PubMed] [Google Scholar]
- [11].Olguin-Araneda V, Banawas S, Sarker MR, Paredes-Sabja D. Recent advances in germination of Clostridium spores. Res Microbiol 166 (2015) 236–43. [DOI] [PubMed] [Google Scholar]
- [12].Setlow P. Spore germination. Curr Opin Microbiol 6 (2003) 550–6. [DOI] [PubMed] [Google Scholar]
- [13].Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 190 (2008) 2505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ramirez N, Liggins M, Abel-Santos E. Kinetic evidence for the presence of putative germination receptors in Clostridium difficile spores. J Bacteriol 192 (2010) 4215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Howerton A, Ramirez N, Abel-Santos E. Mapping interactions between germinants and Clostridium difficile spores. J Bacteriol 193 (2011) 274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bhattacharjee D, Francis MB, Ding X, McAllister KN, Shrestha R, Sorg JA. Reexamining the germination phenotypes of several Clostridium difficile strains suggests another role for the CspC germinant receptor. Journal of Bacteriology 198 (2016) 777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shrestha R, Sorg JA. Hierarchical recognition of amino acid co-germinants during Clostridioides difficile spore germination. Anaerobe 49 (2018) 41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Francis MB, Allen CA, Shrestha R, Sorg JA. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog 9 (2013) e1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shrestha R, Sorg JA. The requirement for the amino acid co-germinant during C. difficile spore germination is influenced by mutations in yabG and cspA. BioRxiv (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ramirez-Peralta A, Zhang P, Li YQ, Setlow P. Effects of sporulation conditions on the germination and germination protein levels of Bacillus subtilis spores. Appl Environ Microbiol 78 (2012) 2689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Putnam EE, Nock AM, Lawley TD, Shen A. SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. J Bacteriol 195 (2013) 1214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hornstra LM, de Vries YP, de Vos WM, Abee T. Influence of sporulation medium composition on transcription of ger operons and the germination response of spores of Bacillus cereus ATCC 14579. Appl Environ Microbiol 72 (2006) 3746–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kochan TJ, Shoshiev MS, Hastie JL, Somers MJ, Plotnick YM, Gutierrez-Munoz DF, et al. Germinant synergy facilitates Clostridium difficile spore germination under physiological conditions. mSphere 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kochan TJ, Somers MJ, Kaiser AM, Shoshiev MS, Hagan AK, Hastie JL, et al. Intestinal calcium and bile salts facilitate germination of Clostridium difficile spores. PLoS Pathog 13 (2017) e1006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Diaz OR, Sayer CV, Popham DL, Shen A. Clostridium difficile lipoprotein GerS is required for cortex modification and thus spore germination. mSphere 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Edwards AN, Nawrocki KL, McBride SM. Conserved oligopeptide permeases modulate sporulation initiation in Clostridium difficile. Infect Immun 82 (2014) 4276–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Childress KO, Edwards AN, Nawrocki KL, Anderson SE, Woods EC, McBride SM. The Phosphotransfer Protein CD1492 Represses Sporulation Initiation in Clostridium difficile. Infect Immun 84 (2016) 3434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hindle AA, Hall EA. Dipicolinic acid (DPA) assay revisited and appraised for spore detection. The Analyst 124 (1999) 1599–604. [DOI] [PubMed] [Google Scholar]
- [29].Shafaat HS, Ponce A. Applications of a rapid endospore viability assay for monitoring UV inactivation and characterizing arctic ice cores. Appl Environ Microbiol 72 (2006) 6808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shrestha R, Lockless SW, Sorg JA. A Clostridium difficile alanine racemase affects spore germination and accommodates serine as a substrate. J Biol Chem 292 (2017) 10735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Broukhanski G, Budylowski P. Laboratory plasticware - Use at your own risk: Suitability of microcentrifuge tubes for spores’ analysis of Clostridium difficile. Anaerobe (2018). [DOI] [PubMed] [Google Scholar]
- [32].Hofstetter S, Gebhardt D, Ho L, Ganzle M, McMullen LM. Effects of nisin and reutericyclin on resistance of endospores of Clostridium spp. to heat and high pressure. Food Microbiol 34 (2013) 46–51. [DOI] [PubMed] [Google Scholar]
- [33].Zhang P, Liang J, Yi X, Setlow P, Li YQ. Monitoring of commitment, blocking, and continuation of nutrient germination of individual Bacillus subtilis spores. J Bacteriol 196 (2014) 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol 192 (2010) 4983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Killgore G, Thompson A, Johnson S, Brazier J, Kuijper E, Pepin J, et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J Clin Microbiol 46 (2008) 431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Francis MB, Allen CA, Sorg JA. Muricholic acids inhibit Clostridium difficile spore germination and growth. PLoS One 8 (2013) e73653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Permpoonpattana P, Tolls EH, Nadem R, Tan S, Brisson A, Cutting SM. Surface layers of Clostridium difficile endospores. J Bacteriol 193 (2011) 6461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brittain HG, Richardson FS, Martin RB. Terbium (III) emission as a probe of calcium(II) binding sites in proteins. J Am Chem Soc 98 (1976) 8255–60. [DOI] [PubMed] [Google Scholar]
- [39].Nelson DJ, Miller TL, Martin RB. Non-cooperative Ca(II) removal and terbium(III) substitution in carp muscle calcium binding parvalbumin. Bioinorg Chem 7 (1977) 325–34. [DOI] [PubMed] [Google Scholar]
- [40].Burns DA, Heap JT, Minton NP. SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. J Bacteriol 192 (2010) 657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wheeldon LJ, Worthington T, Hilton AC, Elliot TS, Lambert PA. Physical and chemical factors influencing the germination of Clostridium difficile spores. Journal of Applied Microbiology 105 (2008) 2223–30. [DOI] [PubMed] [Google Scholar]
- [42].Kochan TJ, Foley MH, Shoshiev MS, Somers MJ, Carlson PE, Hanna PC. Updates to Clostridium difficile spore germination. J Bacteriol 200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. SleC activation with/without terbium for UK1 spores. Equal numbers of C. difficile UK1 spores prepared with 70:30, SMC or TYG medium were incubated with or without 250 μM terbium in 30 mM glycine or 10 mM TA or 10 mM TA and 30 mM glycine for 2 hours and soluble proteins were extracted with NuPAGE buffer and separated by SDS-PAGE followed by immunoblotting with SleC antisera to observe SleC activation.
Figure S2. SleC activation with/without terbium for M68 spores. Equal numbers of C. difficile M68 spores prepared with BHIS, 70:30 or SMC medium were incubated with or without 250 μM terbium in 30 mM glycine or 10 mM TA or 10 mM TA and 30 mM glycine for 2 hours and soluble proteins were extracted with NuPAGE buffer and separated by SDS-PAGE followed by immunoblotting with SleC antisera to observe SleC.