Abstract
Background:
Borderline Personality Disorder (BPD) is a pervasive psychiatric disorder characterized by emotion dysregulation, impulsivity, impaired self-perceptions and interpersonal relationships, and currently affects 1-3% of the US population. (Torgersen et al. 2001; Lenzenweger et al. 2007; Tomko et al. 2014). One major obstacle to our understanding of the neural underpinnings of BPD is a lack of valid animal models that translate the key known features of the disorder to a system that is amenable to study.
Objective:
To summarize the etiology, major symptoms, and symptom triggers of BPD and then propose a blueprint for building an animal model of BPD by choosing key components of the disorder that can be implemented in rodents.
Results:
We identify the role of early life stress and subsequent mild stress in adulthood as contributing etiological factors, and the potential use of altered communication between frontal cortices and the amygdala in extinction and habituation, increased impulsivity, dysregulation of the hypothalamic pituitary axis, and increased neuroinflammation as biological markers of BPD. Building upon these features of BPD, we propose a two-hit animal model that uses maternal abandonment to alter maturation of the HPA axis and mild secondary adult stress to evoke behavioral symptoms such as increased impulsivity, impaired extinction, habituation and social interactions.
Conclusion:
Through exploration of the etiology, symptom presentation, and altered neurological function, we propose an animal model of BPD. We believe that a number of existing animal paradigms that model other mental health disorders should be combined in a unique way to reflect the etiology, symptom presentation, and altered neurological function that is evident in BPD. These models, when compared to available human data, will inform research and treatment in humans for better understanding of systems from the micro-molecular level to more global physiology underlying BPD.
1. Challenges posed by Borderline Personality Disorder Symptomatology
Borderline personality disorder (BPD) is characterized by a pervasive pattern of instability with regards to affect, relationships, impulse control, and sense of self. This pattern causes patients to present with extreme fears of abandonment, emotional dysregulation, recurrent suicidal behaviors or gestures, feelings of emptiness, and difficulty controlling anger (American Psychiatric Association, 2013). Most research into its prevalence has found that between 1 and 3% of community samples meet the criteria for BPD (Torgersen et al. 2001; Lenzenweger et al. 2007; Tomko et al. 2014). This prevalence increases overall to levels of 9.3% to 12.3% within clinical outpatient populations with some studies showing a rate as high as 26.8% within inpatient communities (Koenigsberg et al. 1985; Fossati et al. 2000; Zimmerman et al. 2005).
Those with BPD have severe functional impairment in areas such as employment, interpersonal relationships, and on global assessments of functioning (Skodol et al. 2002; Tomko et al. 2014). This severity in functional impairment is worsened by the likelihood for suicide, as up to 10% of the BPD population dies by suicide, a rate 50 times that of the average population, with individuals on average attempting suicide 3.3 times during their lifetime (Black et al. 2004; Soloff & Chiappetta 2012).
Due to the severity of symptoms, those with BPD tend to receive enormous amounts of psychiatric treatment even in comparison with all other personality disorders (PDs). Almost all BPD patients receive individual therapy and standing medications at some point in their lives, and many are hospitalized multiple times. BPD patients begin using services at a younger age than patients suffering from any other PD, as for example antisocial or narcissistic personality disorder, and most need some form of intervention in the late teens or early twenties. Although treatment utilization decreases over time, it remains significantly higher than in other PDs, incurring high costs over a patient’s lifetime (Zanarini et al. 2001, 2015; Tomko et al. 2014). Several manualized psychotherapies have been shown to be effective in treating BPD. These include treatments focusing on developing skills for emotion regulation, anticipating and coping with interpersonal stresses, and self-soothing (e.g. Dialectical Behavior therapy (DBT); Rudge et al. 2017), using the real-time interpersonal relationship with the therapist to identify maladaptive defensive patterns and to reconstitute an integrated identity (e.g. Transference Focused Psychotherapy (TFP); Yeomans & Levy, 2002), and enhancing the capacity to recognize the mental states in self and other that underlie behavior (e.g. Mentalization-Based Treatment (MBT); Fonagy & Bateman, 2006). Medications, such as antidepressants (e.g. the selective serotonin reuptake inhibitor fluoxetine), mood stabilizers (e.g. the anticonvulsant divalproex Na, Topiramate), and second generation antipsychotics (e.g. aripiprazole/ olanzapine) are minimally effective in the treatment of BPD, but are often employed to treat specific features of BPD (e.g. impulsive aggression, mood lability), or comorbid diagnoses, such as Major Depressive Disorder or Post-Traumatic Stress Disorder (Lieb et al. 2010).
Among outpatient psychiatric patients, borderline patients are twice as likely to have three or more comorbid diagnoses, and nearly four times as likely to have four or more diagnoses over their lifetimes among outpatient psychiatric patients. These comorbidities include Major Depressive Disorder (MDD), Bipolar I or II (BP), Panic Disorder, social and specific phobias, Post-Traumatic Stress Disorder (PTSD), Obsessive Compulsive Disorder (OCD), eating disorders, substance use disorder and somatoform disorder (Zimmerman & Mattia 1999; Hall & Riedford 2017). Many of these disorders show some overlap in symptomatology with BPD, however, the treatments for these disorders are not effective for the majority of BPD patients. A major contributing factor to the shortage of available targeted treatments for BPD is our inadequate understanding of the neurobiological mechanisms underlying this disorder. This problem is exacerbated by the lack of translational animal models that are solely dedicated to creating a comprehensive understanding of BPD.
Whereas there is significant comorbidity between BPD and other disorders of emotion regulation (Table 1) BPD remains diagnostically distinct, and pharmacological treatments developed to ameliorate the respective symptoms of PTSD and MDD remain marginally effective for treating BPD (Lieb et al. 2010). In this review, we examine the etiology, presentation, and current treatment of BPD, and the variety of animal models currently used to develop a neurobiological understanding of mood and emotion regulation. We then propose a guideline for the creation of an animal model that draws from existing paradigms but is dedicated specifically to BPD.
Table 1:
Comparison of symptoms between Borderline Personality Disorder and Disorders with High Comorbidity
| Behavior/Symptoms | BPD | BD | PTSD | MDD | Eating Disorders |
Addiction |
|---|---|---|---|---|---|---|
| Unstable self-image | ✔ | ✔ | ✔ | |||
| Poor impulse control | ✔ | ✔ | ✔ | ✔ | ||
| Affect instability | ✔ | ✔ | ✔ | |||
| Unstable jobs/relationships | ✔ | ✔ | ✔ | ✔ | ||
| Suicidal Behavior | ✔ | ✔ | ✔ | |||
| Feelings of emptiness | ✔ | ✔ | ||||
| Worries of abandonment | ✔ | |||||
| Dissociation/Paranoia | ✔ | ✔ |
We will also draw upon the dimensional construct approach of the Research Domain Criteria (RDoC) framework (https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml). We identify RDoC compatible domains of the model such as Negative Valence Systems, Social Processes, Cognitive Control (e.g. impulsivity), and Arousal Regulatory System. This strategy can foster the identification and study of specific neurobiological phenotypes relevant to BPD (Insel et al., 2010; Willner & Belzung, 2015).
2. Etiology of BPD
2.1. The role of child abuse and maternal abandonment in BPD
While the etiology of BPD is unclear, many theories on the formation of BPD have pointed to early childhood adversity as a major contributing factor (Adler & Buie 1979; Kernberg 1985; Linehan, 1993). Theories regarding the impact of childhood stress are corroborated by research showing that a large proportion of patients with BPD report a history of child abuse or neglect at a higher level than other personality disorders (e.g. antisocial, narcissistic, avoidant, dependent, etc). This research indicates a childhood marked by sexual abuse or increased rates of emotional withdrawal, verbal or emotional abuse, or neglect by the caregiver (Battle et al. 2004; Crowell et al. 2009; Zanarini 2000; Zanarini et al. 2006). The severity of the reported childhood abuse has also been linked to increased impairment in cognitive and interpersonal symptoms of affected BPD patients (Zanarini et al. 2002). Whereas BPD patients without a history of child abuse show a decrease in BPD-related symptoms with age, this pattern is violated in patients with a history of early maternal separation, resulting in high symptom severity throughout the lifespan (Crawford et al. 2009).
Increased symptom presentation after early life stress (ELS) may be attributed to accelerated development of the connectivity between cortical regions, such as the frontal cortices and the insula, with subcortical structures, such as the amygdala and hippocampus, (Callaghan et al. 2014; Gee et al. 2013). Animal work has shown that during development with a non-abusive caregiver, the presence of the caregiver prevents stimulus-evoked increases in corticosterone as well as amygdala activation in the child, whereas early life abuse and neglect by the caregiver negate these protective effects (Santiago et. la. 2017; Moriceau & Sullivan, 2006; Tang et al. 2014). During development glucocorticoid fluctuation helps calibrate hypothalamic pituitary adrenal (HPA) activation in response to external stress, whereas increased levels of glucocorticoids in response to ELS enhances maturation (Tang et al. 2014; Moriceau et al. 2004). Likewise, BPD patients with a self-reported history of child abuse have higher plasma adrenocorticotropic hormone (ACTH) and cortisol concentrations when challenged with an injection of dexamethasone/corticotropin releasing factor (Kaufman et al. 2000; Rinne et al. 2002; Teicher et al. 2003). Additionally, youth who were institutionalized in childhood show higher amygdala reactivity to threat than non-institutionalized youth, and a more adult-like pattern of communication between the prefrontal cortex and the amygdala (Gee et al. 2013).
ELS as an etiology of BPD is a tractable approach for animal models and a number of ELS paradigms are already in use. Animal models using ELS show that abuse in early life dysregulates the stress response, modulates interneuron development in the prefrontal cortex, and drives synaptic plasticity in the threat response system at earlier time points (Roth et al. 2009; Goodwill et al., 2018).
2.2. Genetics of BPD: Early Stages of Analysis
ELS is not the sole defining etiology of BPD, and not all those who experience ELS go on to develop BPD. Based on twin studies, the genetic heritability of BPD is considered to be. 40, meaning that roughly forty percent of the variability underlying BPD can be accounted for by genetic contribution (Distel et al. 2007; Kendler et al. 2008). As with other psychiatric disorders, BPD is associated with a complex polygenetic background that needs further investigation via genome-wide association studies (GWAS) with large cohorts, as has been the case for other psychiatric conditions (Bassir Nia et al., 2018; Power et al., 2017; Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium, 2011). To date, there is a relative dearth of large GWAS studies in BPD patients, with most findings coming from small sample sizes (for an in-depth review, see Bassir Nia et al., 2018). A handful of these studies have been conducted thus far, with only one study using subjects that met diagnostic criteria for BPD (Lubke et al. 2014; Witt et al. 2017). Overall, genetic analyses of BPD have found a small number of genes that reach significance, but these findings lack specificity as the identified genes are involved in enzymatic activity and exocytosis, and play a role in a variety of mental health disorders including MDD, Bipolar Disorder and Schizophrenia (Witt et al. 2017; Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium, 2011; Ripke et al. 2013; Duan et al. 2014).
Thus far, single candidate gene association studies have seen small effect sizes with no associations surviving meta-analysis or replication (Calati et al. 2013; Amad et al. 2014). This remains true for specific genetic polymorphisms, as many have been identified, particularly within the serotonergic system; however, none are able to survive a meta analytic review (Calati et al. 2013).
One such polymorphism, the serotonin transporter linked polymorphic region (5-HTTLPR), located within the promoter region on chromosome 17, is worth mentioning due to its role in impulsivity and stress reactivity more generally. The 5-HTTLPR coding for the serotonin transporter is tri-allelic with one shorter (S) and two longer (LA and LG) alleles. The LG allele functions similarly in vitro to the S allele, and the two are often be combined into one S’ allele subtype, characterized by less serotonin transporter expression compared to the LA allele. As less serotonin transporter is expressed, serotonin is cleared more slowly from the synapse for individuals with the S’ allele (Collier et al. 1996). Although it has been widely studied, meta analyses have returned mixed results on the relationship of the 5-HTTLPR to depressive and anxious traits in response to stress, possibly due to varied diagnostic inventory use, methods for assessing stress (i.e. questionnaires vs clinical interviews), the types of stressors included in the sample, and genotype characterization between studies (Risch et al., 2009, Karg et al., 2011; Culverhouse et al., 2017; Bleys et al., 2018; Middeldorp et al. 2007; Munafò et al. 2009; Schinka et al. 2004; Sen et al. 2004). 5-HTTLPR has not been shown to have an independent relationship with a risk for BPD but is far more likely to function in a modulatory capacity for other genes, possibly mediating the response to ELS (Ni et al 2006; Pascual et al. 2008). For example, polymorphisms for a longer version of 5-HTTLPR were associated with increased impulsivity in BPD patients that suffered childhood abuse (Wagner et al. 2009). Overall, although the role of genetics is undeniable in the underpinnings of BPD, the lack of known replicable and diagnostically specific genetic risk factors, suggest that at present this route is a poor candidate for a BPD-specific animal model.
3. Daily Life Stress in Adulthood and Symptoms of BPD
While genetic background and ELS can account for the development of a BPD diagnosis, the onset of a particular symptom is typically preceded by a more temporally proximal stressful event. Emotional instability in response to quotidian adult stress is an integral aspect of BPD. Indeed, Dialectical Behavioral Therapy (DBT) is one effective psychotherapy for BPD, which specifically provides BPD patients with the tools to identify and manage daily stress. To test the contingency between a stressful event or emotional state with symptom presentation clinical researchers use experience sampling, an approach where subjects report, in real-time, their daily life experiences (including events, emotions, perceptions, symptoms) throughout the day over a period of one or more days. As a result of this work, BPD subjects were shown to be especially sensitive to daily life stress, with minor stressors perceived as more intense by BPD subjects relative to healthy controls, and their emotional response likewise more intense (Berenson et al. 2011; Myin-Germeys et al. 2005). Since BPD patients are particularly reactive interpersonally, animal models distinguishing between social and non-social stresses may prove valuable.
3.1. Non-social Stress
Patients with psychotic disorders exhibit increased symptoms following minor daily life stress, and this tendency extends to the stress-induced transient psychotic symptoms seen in BPD (Myin-Germeys et al. 2005). In BPD patients, non-social stress, such as a deadline or the memory of a past event, has been associated with the onset of dissociative symptoms, paranoia and hallucinations (Glaser et al. 2010; Stiglmayr et al. 2008). Non-social stress has been modeled in animals by unpredictable and uncontrollable administration of mild shocks (Perova et al., 2015), or via unpredictable restraint stress (Donaldson et al., 2014). Such non-social stress paradigms in animals can be used at reduced levels as minor stress triggers in adulthood to study the onset of symptom presentation in BPD (see Section 5.1).
3.2. Social Stress
Stressors based in social interactions, such as rejection or abandonment, invalidation of one’s experiences, a perceived interpersonal offense or betrayal, have all been linked to symptom onset of BPD. Further, disappointment at having one's self-concept threatened, and being offended, have all been associated with the onset of BPD symptoms. Perceived rejection is associated with intense attempts to avoid abandonment, relationship instability, uncertain self-image, impulsive behavior, unstable mood, feelings of emptiness, dissociation, and intense anger. While anger is a common response to rejection, those with BPD are thought to have a higher rejection sensitivity and therefore respond more intensely to minor rejection (Berenson et al. 2011; Miskewicz et al. 2015). Being alone, another social stressor, has also been linked to intense efforts to avoid abandonment, and impulsive behaviors (Miskewicz et al. 2015). While many of these stressors are difficult to reconstruct in an animal model, aloneness can be replicated via social isolation, and rejection via a mild form of social defeat stress, or introducing a new animal into an established hierarchy (see Section 5.1).
4. Anatomy and Physiology of BPD
An assortment of neuroimaging techniques has been used to study regional gray matter volume, white matter connectivity, and functional connectivity (connectivity implied by the synchronous neural activity of separate brain regions) in BPD patients during specific behavioral tasks. Magnetic resonance imaging (MRI) has revealed differences between healthy control subjects and BPD patients in regional brain volumes and white matter integrity in fronto-limbic regions. BPD patients have decreased gray matter volume of the anterior cingulate cortex (ACC), the hippocampus, medial prefrontal cortex (mPFC), and the dorsolateral frontal cortex (dlPFC), while the findings for the amygdala are mixed (Brambilla et al. 2004; Denny et al., 2016; Hall et al., 2010; Kuhlmann et al., 2013; Minzenberg et al., 2008). Studies using diffusion tensor imaging show that BPD patients present with decreased fractional anisotropy (an indicator of diminished axonal integrity or fiber density) in the fornix, cingulum, corpus callosum, uncinate fasciculus, and prefrontal white matter fasciculi (Carrasco et al. 2012; New et al. 2013; Lischke et al. 2015; Whalley et al. 2015; Gan et al. 2016). Lischke et al. (2015) and Gan et al. (2016) also tested axial and radial diffusivity to gain a better understanding of the integrity of the white matter tracts. Both studies found an increase in radial diffusivity, indicating myelin degeneration rather than axonal injury in these tracts (Song et al. 2002; Sun et al. 2006). These changes may be due to a stress-related inflammatory response, a view that is supported by increased cytokine expression in BPD (Díaz-Marsá et al., 2012)
When viewing emotionally valenced images, BPD patients have a hyperactive response in the amygdala, and a hypoactive response in frontal cortices such as the OFC, and dorsolateral prefrontal cortex (dlPFC, Baczkowski et al. 2017; Herpertz et al. 2001; Koenigsberg et al 2009; Soloff et al. 2015, 2017). There is notable overlap between these findings and changes observed in PTSD patients who present with decreased ventral prefrontal cortical volume and resting state activity, combined with hyperactivity in the ACC and amygdala that have been repeatedly associated with poor extinction learning and recall (Hayes et al., 2012; Stevens et al., 2013; Marin et al., 2016). These findings suggest that we can draw on animal models of PTSD, which have shown that during successful extinction, activity in the ventral mPFC suppresses fear via its communication with downstream targets, including the amygdala, where the vmPFC is thought to shift the excitatory-inhibitory balance towards inhibition, suppressing amygdala output and defensive responding (Quirk et al., 2003; Rosenkranz & Grace, 2001; Berretta et al., 2005; Cho et al., 2013; Bukalo et al., 2015; Maren & Holmes 2016). Thus, we would expect that decreased vmPFC volume should result in diminished suppression of amygdala activity, and contribute to elevated autonomic and behavioral responses associated with aversive experience (Likhtik et al., 2014; Likhtik & Paz, 2015; Maren & Holmes 2016).
In support of this data, BPD patients also show decreased prefrontal function and amygdala hyperactivity that is coupled with impaired extinction and lack of habituation to aversive stimuli (Denny et al. 2018; Hazlett et al. 2012; Krause-Utz et al. 2016; Schulze et al. 2016). For example, work using fMRI during acquisition of differential fear conditioning in female BPD patients shows that there is a lack of habituation to an unpaired stimulus in the amygdala as well as increased activity in the insula compared to healthy controls (Krause-Utz et al. 2016). Likewise, during extinction of conditioned stimuli, BPD patients show delayed recruitment of the prefrontal cortex coupled with continued amygdala activation to unreinforced stimuli (Krause-Utz et al. 2016). BPD patients also show a non-adapting amygdala response during novel and repeated presentations of emotionally salient images (Denny et al. 2018; Hazlett et al. 2012; Koenigsberg et al. 2014). Furthermore, during stressful situations, the inflicting of pain can cause a decrease in amygdala activity associated with a reduction of subjective anxiety for the patient (Niedtfeld et al. 2010; Reitz et. al., 2015). The replicated finding of delayed extinction and impaired habituation in BPD suggests that hyperactivity in the amygdala, the insula and anterior cingulate cortex, combined with a hypoactive mPFC are relevant behavioral and physiological markers for validating an animal model of BPD.
5. Mapping Current Animal Models of Psychiatric Disorders onto BPD
Given that BPD patients display a constellation of behaviors that are already modeled in animals for other mental health disorders, including PTSD, MDD, and addiction (Table 1), we draw upon relevant paradigms from these models (Table 2). The tests aim to create species-specific and etiologically relevant representations of pathological behaviors seen in humans. We begin by outlining the relevant “toolbox” of strategies that are currently used in animals to model PTSD, MDD, and substance abuse, which can be adapted and recombined for modeling BPD. Then, we propose a combination of paradigms that are most useful for representing BPD-like behavior and physiology in animals. Importantly, the interpretation of why a particular behavior occurs is often associated with more abstract concepts such as reward processing, impulsivity, and anxiety. These concepts, although fraught with anthropomorphic categorizations, nonetheless allow for investigation of brain-specific systems that are translationally relevant and can lead to therapeutic and basic science advances.
Table 2.
Suggested symptom measures for inclusion in an animal model of BPD.
| Symptom | Existing Model? |
Measures | RDoC |
|---|---|---|---|
| Unstable self-image | X | ||
| Poor impulse control | ✔ | Delay Discounting: Choice of small early vs. large later rewards Choice of small frequent vs. large infrequent rewards |
Domain: Positive Valence System Construct: Reward Valuation |
| Affect instability | ✔ | Habituation to stimuli Extinction acquisition Extinction recall |
Domain: Arousal/Regulatory system, Construct: Arousal Domain: Negative Valence Construct: Acute Threat (Fear) |
| Unstable jobs/relationships | ✔ | Social interaction test 3-chamber social interaction |
Domain: Social Processes Constructs: Affiliation, Attachment |
| Suicidal Behavior | X | ||
| Feelings of emptiness | X | ||
| Worries of abandonment | X | ||
| Dissociation/Paranoia | X |
Currently, women are diagnosed with BPD in disproportionately higher numbers than men, a factor that may be more attributable to clinical bias and the reported differences in BPD expression rather than actual rates in the population. For example, men are more likely to report impulsive aggression, while women report greater chronic emptiness, instability, and self-mutilation (Hoertel et al., 2014; Sher et al., 2019). Simultaneous work in human populations and in animal models of BPD would provide more detailed information on sex differences in behavioral responding (e.g. behavioral response by social vs. asocial stressor), and will give novel insights into the underlying neural mechanisms that drive BPD-like behaviors in both sexes.
5.1. Stress-Induction in Modeling Mental Health Disorders
Given that PTSD is defined as a stress and trauma induced disorder (American Psychiatric Association, 2013), and MDD is also stress-responsive, current animal models have been developed to simulate highly stressful or traumatic events in humans, in order to systematically explore the impact of stress on behavior. Therefore, various forms of stress are considered for incorporation into an animal model of BPD.
Early Life Stress:
ELS is an important component for a model of BPD, as most BPD patients have undergone severe stress early in life (see Section 2.1). In rodent models ELS is typically caused by altering the dam-pup relationship through bedding deprivation, maternal separation, or maternal deprivation. The bedding deprivation approach stresses the dam via limited access to bedding materials for nest building, which results in the dam leaving the nest more often in search for materials and increases the dam’s abusive behavior toward her pups (Rice et al. 2008). The repertoire of abusive rodent behavior includes the dam dropping, dragging, roughly handling, or ignoring her pups; all of which are rarely seen in control conditions (Ivy et al., 2008, Rice et al., 2008). In animal models employing this approach to induce ELS, the pups grow up to exhibit an increased corticosterone response to restraint stress, show higher defensive freezing in tests of fear conditioning, and more anxiety-like behavior in assays of innate anxiety (e.g. ELS exposed rats spend less time in the open arms of an Elevated Plus Maze test compared to rats that were raised with adequate care; Dalle Molle et al. 2012; Machado et al. 2013; Walker et al., 2017). Another form of inducing ELS is maternal separation where the mother and pups are separated for a short duration (3-6 hrs) over multiple days or for a longer period of separation (24hrs) for only one session. Notably, depriving pups of their mothers between postnatal days 4 and 20 for an extended period of time (24hrs) results in a hyper-reactive HPA axis in the pups, as assessed via increased levels of corticosterone following stress (Banqueri et al. 2017). Maternal separation is also associated with increased innate anxiety in adulthood (Stanton et al. 1988; Sampath et al. 2014; Sousa et al. 2014).
Social Stress Induction:
Social stress is an important factor in BPD, as social stressors often trigger behavioral dysregulation in BPD (see Section 3.2). Animal models of social stressors include social defeat stress, or housing and social instability stress. In the social defeat stress paradigm, the animal (usually a mouse) is exposed to a larger aggressor who will physically dominate it for a short time (~5 minutes). The subordinate mouse is then housed in the presence of the aggressor, while being separated by divider to prevent physical harm but can still see and smell the aggressor. This paradigm is intensified by adding social instability in the form of switching to a novel aggressor–cohabitant pairing on each day of the paradigm (Golden et al., 2011). Other forms of social stress include mild variable stress, whereby an animal’s living environment is altered in new and unexpected ways every day (including cage tilting, overcrowding, shifted light/dark cycles, Hodes et al., 2015; Menard et al., 2017). Other examples of housing or social instability involve consistently changing the subject’s cage mates or isolating the subject from its cohort and housing it alone. Another, more molecular form of social stress is predator scent stress where a mouse is exposed to a predator smell, such as components of fox or cat urine (Wang et al., 2018).
Non-Social Stress Induction:
Variants of non-social stressors have been used to model PTSD-like trauma and to induce MDD-like behaviors. They may provide a different perspective on the influence of stress in BPD. Stress-enhanced fear learning (SEFL) is incorporated in models of PTSD. This may take the form of exposure to unpredictable shocks in order to simulate a traumatic event (Rau and Fanselow, 2009; Rhajbandari et al., 2018). Notably, this protocol results in enhanced fear learning, and resistance to extinction (Rau et al., 2005; Long and Fanselow, 2012), which are two important behavioral outcomes that overlap between PTSD and BPD. Likewise, long lasting learned helplessness is achieved by repeatedly exposing an animal to an uncontrollable and unpredictable stressor, such as a many repeated presentations of low-intensity foot shock (e.g. 360 shocks over two sessions), followed by continued re-exposure to the stressful context (Landgraf et al., 2015; Maier, 2001). Notably, animals that are exposed to the same number foot shocks but for whom this stressor is controllable (e.g. they find a way to turn it off through a level press), don’t develop depression-like symptoms to conditioned stimuli, suggesting the importance of control over one’s environment in developing symptoms of MDD (Baratta et al., 2007). Other forms of stress induction in animal models include underwater trauma, which involves placing a water-naive rat or mouse into a container of water that is deep enough to prevent the animal from standing and submerging the animal into the water for roughly 30 seconds (Moore et al. 2012). Another form of stress is restraint stress, in which a mouse or rat is immobilized using an apparatus such as a plastic restraint tube for 30 minutes to 2 hours, a paradigm that also leads to impaired extinction (Rahman et al., 2018).
By using a variety of stressors like a foot shock, restraint, or underwater trauma, and presenting them as a series of non-repeating stressors at different times in the light-dark cycle over several days, animals don’t acclimate to the stressors, and show an MDD-like state of anhedonia. Recently, a two-hit model, combining early life stress with social defeat in adulthood was employed for the induction of depressive-like symptoms (Peña et al., 2017). Another two-hit animal model has been used to model schizophrenia, where the “first hit” refers to an in utero/neonatal immune reaction typically brought on by molecules like lipopolysaccharide found in gram negative bacteria membranes (Bayer et al. 1999; Maynard et al. 2001; Feigenson et al. 2014). However, the two-hit approach that is more relevant for BPD does not rely on an in utero manipulation, but rather a combination of ELS and mild daily stress in adults.
5.2. Behavioral Measures Utilized to Examine Stress-Induction Models of Mental Health Disorders
Animal models of MDD incorporate behavioral measures such as anhedonia, social withdrawal, decreased motivation for obtaining reward, and behavioral despair (Peña et al., 2017; Menard et al., 2017; Anacker et al., 2018). These depression-like symptoms are operationalized in the sucrose preference test (modeling anhedonia, Yan et al. 2010; Peña et al., 2017; Menard et al., 2017), forced swim test/tail suspension test (decreased movement is interpreted as loss of motivation or behavioral despair, Nollet et al 2013; Smolinsky et al., 2009), and social interaction test (social withdrawal, Golden et al., 2011; Hodes et al., 2015). If a particular induction method leads to increased anhedonia, weight loss, decreased self-grooming, and social withdrawal, it is considered to be a particularly strong animal model of MDD. The most relevant of these paradigms for modeling BPD is the social interaction test, as unstable relationships is a cardinal feature of BPD diagnosis (American Psychiatric Association, 2013). PTSD-like behavioral responses to stress include heightened anxiety, sensitization to fear learning, and impaired extinction learning (Rau et al., 2005; Long and Fanselow, 2012; Maren & Holmes, 2016; Rahman et al., 2018). Of these, impaired extinction of conditioned fear and heightened anxiety, are useful tests for modeling BPD in animals. Likewise, stress exposure has been associated with increased impulsivity in BPD patients (Krause-Utz et al., 2016; Fields et al., 2014). Behavioral measures of impulsivity using delay-discounting tasks in animals have characterized a variety of traits that can model the BPD-like endophenotype, and are easily integrated with human studies to define the neural mechanisms of impulsivity in BPD. To validate animal models of BPD, we will focus on measures of social interactions (mostly used in MDD research), emotion regulation (used in MDD and PTSD research), and impulsivity (often used in substance abuse research), as the most appropriate outcomes for modeling BPD-like symptoms.
Social interactions:
Social withdrawal is typically measured with tests that assay changes in social interactions after stress exposure (e.g. social defeat stress, chronic variable stress). In this assay, a mouse is given the chance to explore either one of two cups, one that contains a novel mouse and one that is empty. Mice are social animals, however a stressor typically decreases the amount of time mice spend exploring other mice, increasing exploration of the empty cup instead (Menard et al., 2017; Peña et al., 2017; Hodes et al., 2015). In this approach, the pre- to post-stress comparison controls for individual differences in innate anxiety, and sensitivity to social stimuli. In the case of BPD, the social interaction should also be scored for social aggression in addition to social avoidance.
Emotion Regulation:
Emotion regulation is probed via responses to innately anxiogenic stimuli, behavioral responding to stimuli that are conditioned to be associated with a threatening versus a non-threatening outcome, and responses to stimuli that undergo extinction of previously aversive associations. The former addresses emotional reactivity and the latter, one possible mechanism of emotional regulation, both of which are dysregulated in BPD. Tests of innate anxiety reactivity exploit exposure to anxiogenic environments or stimuli that animals try to avoid in the wild (e.g. for rodents this includes large, empty, well lit spaces, for primates this includes snakes). ELS, social stress, and non-social stress increase innate anxiety on measures such as the open field, and EPM, collectively indicating that after stress animals decrease exploration of innately anxiogenic environments (Machado et al. 2013; Walker et al., 2017, Peña et al., 2017; Chattarji et al., 2015). Tests of new learning about an aversive conditioned stimulus (CS) typically rely on classical conditioning by pairing a neutral stimulus (e.g. a light, tone, or the context itself) with an aversive unconditioned stimulus (US, e.g. electric shock to the feet or an air puff to the eye). Prior stress enhances fear expression upon re-exposure to the context or to the stimulus that has been previously associated with the physically aversive event (Rau et al., 2005; Chattarji et al., 2015). One form of emotion regulation may be tested via acquisition and retention of extinction learning, where subjects learn that the previously paired CS no longer predicts the US. The inability to extinguish CS-US associations, and difficulty learning to distinguish stimuli that predict threat from those that do not, closely approximates human behavior in PTSD where patients show persistently high level of fear to trauma-associated cues, even long after the cues stop being associated with the trauma (Marin et al., 2014; 2016; Rabinak et al., 2017). Notably, impaired acquisition and retention of extinction learning has been modeled in animals by via genetic modification as well as stress exposure (Maren & Holmes, 2016; Chauveau et al., 2012; Gunduz-Cinar et al., 2018), which may provide a useful direction for future work in genetic modeling of BPD. Thus impairments in extinction learning and retention are useful behavioral markers of the emotional dysregulation seen in BPD.
Impulsivity
BPD patients manifest impulsivity that is typically self-damaging, such as risky sexual behavior, reckless driving, binge eating, and excessive spending. Such behaviors reflect immediate need for gratification without considering the consequences and can be modeled by delay discounting tasks in animals (Dalley & Robbins, 2017). Animal and human versions of the delay discounting paradigm measure the propensity to choose a small but immediate reward versus a larger delayed reward, where the value of the larger reward becomes discounted relative to smaller one by the longer delay before receipt of the larger reward (Bailey et al., 2018). Likewise, risky choice is a probabilistic form of discounting akin to gambling, where the larger reward is less frequent and is thereby associated with increased risk (Dalley & Robbins, 2017). Notably, damage to the nucleus accumbens core (in the ventral striatum), the medial PFC/ OFC, as well as to PFC-amygdala communication has been associated with increased risky choices (Cardinal et al., 2001; St Onge & Floresco, 2010; St Onge et al., 2012; Stopper et al., 2014; Zeeb et al., 2015). In line with this, recent work shows that BPD patients have decreased ventral striatum activation during choices associated with loss (Herbort et al., 2016), suggesting that there is overlap between animal and human studies in this area. Furthermore, binge eating is considered to be a type of impulsive behavior in BPD, and therefore this behavioral assay could be considered as a secondary outcome measure in an animal model of BPD. However, the effects of stress on food intake are not unidirectional. Whereas chronic stress leads to anhedonia and weight loss, a factor that has been used to model MDD, combining chronic stress with food restriction and refeeding was shown to lead to binge-like eating behavior (Hagan et al., 2002; Artiga et al., 2007). Furthermore, stress exposure via maternal separation during pre-weaning in combination with social isolation stress in juvenile rats was shown to increase bingeing-like behavior (Jang, 2011). Thus, food intake may be pursued as an outcome measure for modeling BPD, but first we suggest that a more detailed analysis of how stress affects feeding is conducted.
6. Dimensional and Categorical Modeling
In this review, we first emphasize categorical modeling -- that is developing an animal model for the diagnostic category of BPD. However, we also propose modeling dimensions of BPD psychopathology that may cut across diagnoses. In this approach, domains of psychopathology relevant to BPD can be studied independently across diagnoses. This approach offers the opportunity to identify important endophenotypes, to understand component mechanisms of psychopathology (and their relationships to normal function), and to target treatment to specific dysfunctions. The NIMH has articulated such an approach in its Research Domain Criteria (RDoC) program. Each strategy has its strengths and weaknesses. The categorical approach tracks current clinical practice, in which treatments are selected based on the specific diagnosed disorder or its predominant symptoms. In this regard, our proposal to develop an animal model for BPD affords the possibility of developing specific treatments for this disorder, and addresses the dearth of animal models for BPD, whereas such models exist (as described above) for depression, PTSD, and substance use among others. The transdiagnostic dimensional approach may more closely expose underlying biological mechanisms. While our principal focus here is upon strategies to design models of the BPD diagnosis per se, below we describe approaches to model individual BPD dimensions. We believe that a combination of categorical and dimensional modeling will have the greatest yield in informing us about BPD psychopathology and treatment approaches.
7. Building an animal model of BPD
We propose an animal model of BPD that integrates stress induction and behavioral outcomes that have proved useful in modeling other mental health disorders, combined in a manner that has face and construct validity that is specific to BPD. We propose that the model is further validated by how well it recapitulates known pathophysiology of BPD (Section 4). Given that there is currently no BPD-specific pharmacological treatment, pharmacologic validation is not currently possible.
7.1. A two hit model: early life stress followed by mild adult stress
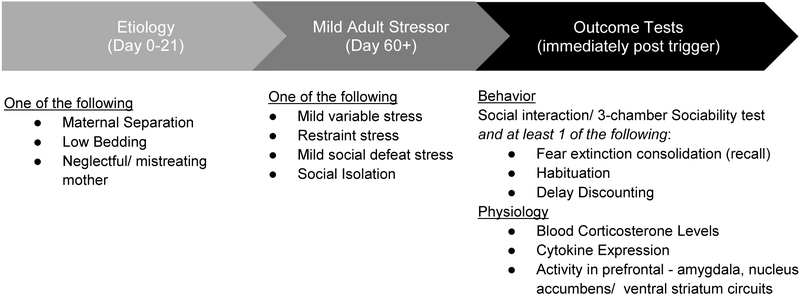
In light of the current lack of knowledge regarding the genetic underpinnings of BPD, efforts to incorporate genetics into the model would be premature. We suggest an etiologic model that combines ELS, seen to be prevalent in BPD (see Section 2.1), with stress exposure in adulthood (i.e. a two-hit model), analogous to the observed development of BPD (see Section 3 and Figure 1). As described in Section 5.1, currently existing ELS paradigms for the pre-weaning period simulate early life etiology of BPD in rodents. These include early maternal separation, the creation of a mother that mistreats or neglects her pups by introducing insufficient bedding or other environmental stressors that increase corticosterone levels in the pups, (Opendak et al., 2017; Flannery et al., 2017; Goodwill et al., 2018). Following ELS, and once the animal has reached adulthood, a mildly stressful version of a variety of stress paradigms can be used to mimic conditions that lead to symptom onset in BPD. The adult stressors may come from social stress paradigms, such as chronic mild variable stress, social isolation, or brief social defeat stress (Nollet et al., 2013; Menard et al., 2017; Peña et al., 2017). Alternatively, ELS may be combined with a non-social stressor, such as repeated and unpredictable restraint stress, shock-evoked stress (e.g. SEFL), or food restriction (Perova et al., 2015; Rajbhandari et al., 2018; Donaldson et al., 2014). Individual parameters within each paradigm (e.g. length or frequency of stress exposure), can be adjusted for mild levels of stress that do not affect behavioral performance in healthy, non-ELS controls.
Figure 1.
A suggested layout for an animal model dedicated to the study of BPD. The timeline refers to development of mice, and confers a model with early life stress as the etiology established pre-weaning, followed by mild stress administration in adulthood, and outcome measures immediately after the trigger. We suggest several possibilities for inducing Early Life Stress, and for Mild Adult Stress. The suggested methods for stress induction and outcome testing are a guide, and the specific constellation of chosen tests will depend on each researcher’s interests. For the most accurate behavioral model of clinical BPD, we recommend that deficits in a test of social interaction or the 3-chamber sociability test are established along with a deficit in at least one of the following behavioral measures: Fear extinction consolidation, Habituation, Delay Discounting (see Section 7.2). Physiology measures have expected outcomes based on previous findings (see Section 7). Repeated mild stressor-outcome measures are appropriate.
7.2. Laboratory Outcome Measures for Model Validation
To best match the time course of symptom onset in BPD, behavioral measures should be conducted immediately post exposure to the minor adult stressor (Miskewicz et al. 2015; Figure 1). Furthermore, we would expect that re-testing for the same behavioral outcomes at time points that are further removed from the mild stress, would demonstrate normalized behavior, as this is the time course of symptoms seen in patients with BPD. However, we would expect to see that another exposure to mild stress, will once again reinstate the tested maladaptive responses. Broad categories of BPD symptoms that lend themselves to animal modeling include affect instability, poor impulse control, and unstable relationships (Section 5.2, Table 2). Below, we outline a series of behavioral and physiological measures that we suggest to model BPD. For each type of behavioral test, we indicate the associated RDoC area (Table 2). We suggest that for the animal model of BPD to be valid, that behavior on the social interaction test, and at least one of the other proposed paradigms shows maladaptive responses to mild stress, and recapitulates BPD-like physiology. This approach is in line with the current practices outlined in DSM-V (American Psychiatric Association, 2013), where a patient must demonstrate disrupted interpersonal communication, and pathological personality traits for a diagnosis of BDP.
Use of the Social Interaction Test to Assess Social Instability in an Animal Model of BPD Domain - Social Processes, Construct - Affiliation and Attachment
Impairments in interpersonal functioning are a cardinal symptom for a BDP diagnosis. To model unstable relationships, we propose tests of social interaction in dyads and groups to compare animal behavior pre- and post- minor stress triggers, in order to assess interaction patterns such as exploration/sniffing, attack/biting, as well as the three-chamber paradigm to test whether animals will choose a compartment with a familiar or a novel animal. In the animal model of BPD, we would expect that rodents show signs of decreased social exploration after mild adult stress relative to their own pre-stress levels, and we would expect an increase in aggressive rodent behavior, such as increased instances of attacks and biting in dyad interactions.
Habituation and Extinction to Assess Analogs of Emotion Regulation in an Animal Model of BDP Habituation: Domain - Arousal and Regulatory Systems, Construct - Arousal Extinction: Domain - Negative Valence, Construct - Acute Threat (Fear)
To determine the extent to which the animal model captures a phenotype related to affect instability in BPD, we propose assessing fear extinction and startle habituation, both of which are affect related processes that are measurable in animal models by commonly used experimental paradigms. BPD patients show affect instability, which may be assessed by impaired extinction, and enhanced fear conditioned learning (Section 4.0). Maladaptive extinction learning, as observed in BPD patients, is the inability to properly consolidate extinction of the defensive response to stimuli that are continuously presented without the previously accompanying aversive CS. We expect the same impairments in consolidation of extinction learning in animals that are revealed on test of extinction recall. This behavioral approach can be readily combined with an investigation of the physiological and molecular mechanisms of extinction failure.
One well studied aspect of BPD physiology in humans is the dysregulated communication between frontal cortices and the amygdala during habituation and extinction (Koenigsberg et al 2014; see Section 4). In keeping with this, simultaneous physiological recordings in the prefrontal - amygdala circuit during extinction and habituation in animal models of BPD should demonstrate increased amygdala activity during habituation and extinction recall, along with diminished CS-driven prefrontal activation. Likewise, based on human work, a lack of stimulus habituation is predicted to be associated with continuous activation of the amygdala (Koenigsberg et al 2014; Schulze et al., 2016, Krause-Utz et al., 2016, Hazlett et al., 2012). Furthermore, studies in BPD patients show that stress-induced self- infliction of pain downregulates the amygdala (Niedtfeld et al., 2010; Reitz et. al., 2015), suggesting that this reaction to stress may be worth exploring in an animal model as a method of validation. In this regard, following a stressful event, such as social isolation, the rodent amygdala response to a mild form of injury, such as the tail flick or hot plate tests (Illich et al., 1995) should be monitored. The predicted outcome based on observations from work in humans, would be that the stressed animals show faster latency pain responses, and higher amygdala activation. These behavioral analyses can be combined with simultaneous multi-site physiological recordings, immunohistochemistry, or imaging, to evaluate activity within the PFC and amygdala circuit. Likewise, manipulation of PFC-amygdala communication (e.g. via optogenetics) is predicted to either impair extinction recall, if PFC input to the amygdala is inhibited, or improve it, if PFC input to the amygdala is excited. For tests of habituation, amygdala silencing is predicted to repair the response.
Use of Delay Discounting to Assess Impulse Control in an Animal Model of BPD Impulse Control: Domain - Positive Valence System, Construct – Reward Valuation
Impulsive behavior is one of the core symptoms of BPD. To measure impulse control, we propose delayed discounting tests that use choice preference for smaller, more immediate versus larger delayed rewards, and risky choice paradigms that assess choice for smaller and frequently occurring rewards versus larger but infrequent rewards (Table 2). Whereas in non-stressed controls we expect to see behavior that follows low risk options, we expect that in our BPD-like animals will be equally likely to prefer the risky as the non-risky options (Endrass et al. 2016). Physiology and imaging of circuit-level activity during delay discounting tasks are predicted to show decreased dorsal and medial anterior cortex activity (St. Onge & Floresco, 2010; St. Onge et al., 2012; Zeeb et al., 2015), and decreased activity in the nucleus accumbens core (Herbort et al., 2016; Cardinal et al., 2001). We would also expect decreased PFC-amygdala communication (Churchwell et al., 2009).
Hormone and Cytokine Expression to Assess Physiological Responses in an Animal Model of BPD
In conjunction with the behavioral tests we have outlined, physiological measures such as ACTH, corticosterone levels, and cytokine expression should assay stress-related activation and inflammation in control and stressed animals pre- and post- mild stress exposure. We propose that in BPD-like animal’s cytokine and stress-hormone levels will be increased relative to no-stress controls (Rinne et al. 2002; Teicher et al. 2003; Díaz-Marsá et al., 2012).
7.3. Controls
There are multiple points of control that should be used in an animal model of BPD. First, there are built-in controls for each behavioral paradigm described here, whether it be using pups from non-stressed mothers to control for ELS, or unstressed animals in adulthood to control for BPD-like symptom onset. Second, our approach also allows for comparisons between groups that have a background of ELS in the absence of further stress, thereby controlling for the effects of ELS alone on behavior and physiology in comparison to animals that have undergone ELS in addition to mild stress in adulthood, and developed BPD-like symptoms. Third, this approach is readily amenable to comparison between the sexes in order to identify whether female subjects are more susceptible to developing BPD-like symptoms than males.
8. Conclusion
It is our hope that we can make significant progress in understanding the molecular and physiological mechanisms that are specific to BPD by isolating the etiology and symptoms that are tractable with currently established paradigms in animals. Important information regarding network communication and local activity will emerge from physiological recordings or imaging conducted during behavior, and in the slice. Likewise, these approaches will give greater insights into the specifics of BPD-like HPA dysregulation such as glucocorticoid receptor expression or cellular changes across the PFC- amygdala-striatal network. The validation of outcomes based on available human data will pave the way for making new discoveries, from the micro-molecular level to more global physiology that will better inform research and treatment in humans.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Adler G, Buie DH (1979) Aloneness and borderline psychopathology: the possible relevance of child development issues. The International journal of psycho-analysis 60:83–96 [PubMed] [Google Scholar]
- Amad A, Ramoz N, Thomas P, Reviews JR (2014) Genetics of borderline personality disorder: systematic review and proposal of an integrative model [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author. [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R (2018) Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 559:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiga AI, Viana JB, Maldonado CR, Chandler-Laney PC, Oswald KD, Boggiano MM (2007) Body composition and endocrine status of long-term stress-induced binge-eating rats. Physiol Behav 91: 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczkowski BM, Zutphen LV, Siep N, et al. (2017) Deficient amygdala–prefrontal intrinsic connectivity after effortful emotion regulation in borderline personality disorder. European Archives of Psychiatry and Clinical Neuroscience. 267:551–565. doi: 10.1007/S00406-016-0760-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C, Peterson JR, Schnegelsiepen A, Stuebing SL, Kirkpatrick K (2018) Durability and generalizability of time-based intervention effects on impulsive choice in rats. Behav Processes 152: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banqueri M, Méndez M, Arias JL (2017) Behavioral effects in adolescence and early adulthood in two length models of maternal separation in male rats. Behav Brain Res. 324:77–86. doi: 10.1016/j.bbr.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF (2007) Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 146: 1495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassir Nia A, Eveleth M, Gabbay J, Hassan YJ, Zhang B, Perez-Rodriguez MM (2018) Past, present, and future of genetic research in borderline personality disorder. Current Opinion in Psychology. 21:60–68. doi: 10.1016/j.copsyc.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle CL, Shea TM, Johnson DM, Yen S, Zlotnick C, Zanarini MC, et al. (2004) Childhood Maltreatment Associated With Adult Personality Disorders: Findings From the Collaborative Longitudinal Personality Disorders Study. Journal of Personality Disorders. 18:193–211. doi: 10.1521/pedi.18.2.193.32777 [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W (1999) Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “Two hit hypothesis.” Journal of Psychiatric Research 33:543–548. doi: 10.1016/s0022-3956(99)00039-4 [DOI] [PubMed] [Google Scholar]
- Berenson KR, Downey G, Rafaeli E, Coifman KG, Paquin NL (2011) The rejection-rage contingency in borderline personality disorder. J Abnorm Psychol. 120(3):681–90. doi: 10.1037/a0023335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Paré D (2005) Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DW, Blum N, Pfohl B, Hale N (2004) Suicidal Behavior in Borderline Personality Disorder: Prevalence, Risk Factors, Prediction, and Prevention. Journal of Personality Disorders. 18:226–239. doi: 10.1521/pedi.18.3.226.35445 [DOI] [PubMed] [Google Scholar]
- Bleys D, Luyten P, Soenens B, Claes S (2018) Gene-environment interactions between stress and 5-HTTLPR in depression: A meta-analytic update. Journal of Affective Disorders. 226: 339–345. doi: 10.1016/j.jad.2017.09.050 [DOI] [PubMed] [Google Scholar]
- Brambilla P, Soloff PH, Sala M, Nicoletti MA, Keshavan S, Soaers JC (2004) Anatomical MRI study of borderline personality disorder patients. Psychiatry Research: Neuroimaging. 131:125–133. doi: 10.1016/j.pscychresns.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Bukalo O, Pinard CR, Silverstein S, Brehm C, Hartley ND, Whittle Net et al. (2015) Prefrontal inputs to the amygdala instruct fear extinction memory formation. Sci Adv.1 pii: e1500251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calati R, Gressier F, Balestri M, Serretti A (2013) Genetic modulation of borderline personality disorder: Systematic review and meta-analysis. Journal of Psychiatric Research. 47:1275–1287. doi: 10.1016/j.jpsychires.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Callaghan B, Sullivan R, Howell B, Tottenham N (2014) The international society for developmental psychobiology Sackler symposium: Early adversity and the maturation of emotion circuits—A cross-species analysis. Dev Psychobiol 56:1635–1650. doi: 10.1002/dev.21260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 292(5526):2499–501. doi: 10.1126/science.1060818 [DOI] [PubMed] [Google Scholar]
- Carrasco J, Tajima-Pozo K, Díaz-Marsá M, Casado A, Lopez-Ibor JJ, Arrazola J, et al. (2012) Microstructural white matter damage at orbitofrontal areas in borderline personality disorder. Journal of Affective Disorders. 139:149–153. doi: 10.1016/j.jad.2011.12.019 [DOI] [PubMed] [Google Scholar]
- Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahmann MM. (2015) Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci 18: 1364–1375. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Lange MD, Jüngling K, Lesting J, Seidenbecher T, Pape HC (2012) Prevention of stress-impaired fear extinction through Neuropeptide S action in the lateral amygdala. Neuropsychopharmacology. 37:1588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Deisseroth K, Bolshakov VY (2013) Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 80:1491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP (2009) Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 123(6): 1185–96. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DA, Stöber G, Li T, Heils A, Catalano M, Bella DD, et al. (1996) A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Molecular psychiatry 1:453–60. [PubMed] [Google Scholar]
- Crawford TN, Cohen PT, Chen H, Anglin DM, Ehrensaft M (2009) Early maternal separation and the trajectory of borderline personality disorder symptoms. Development and Psychopathology. 21:1013–1030. doi: 10.1017/s0954579409000546 [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Linehan MM (2009) A biosocial developmental model of borderline personality: Elaborating and extending Linehan’s theory. Psychol Bull 135:495–510. doi: 10.1037/a0015616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culverhouse RC, Saccone NL, Horton AC, Ma Y, Anstey KJ, Banaschewski T et al. (2017) Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Molecular Psychiatry. 23(1):133. doi: 10.1038/mp.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segal S, Salum GA, et al. (2012) Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry 2:tp2012126. doi: 10.1038/tp.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Robbins TW (2017) Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci. 18(3): 158–171. doi: 10.1038/nrn.2017.8. [DOI] [PubMed] [Google Scholar]
- Denny B, Fan J, Fels S, et al. (2018) Sensitization of the Neural Salience Network to Repeated Emotional Stimuli Following Initial Habituation in Patients With Borderline Personality Disorder. Am J Psychiat 175:657–664. doi: 10.1176/appi.ajp.2018.17030367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Fan J, Liu X, Guerreri S, Mayson S, Rimsky L, et al. (2016) Brain structural anomalies in borderline and avoidant personality disorder patients and their associations with disorder-specific symptoms. Journal of Affective Disorders. 200:266–274. doi: 10.1016/j.jad.2016.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Marsá M, Macdowell KS, Guemes I, Rubio V, Carrasco JL, Leza JC (2012) Activation of the cholinergic anti-inflammatory system in peripheral blood mononuclear cells from patients with Borderline Personality Disorder. Journal of Psychiatric Research. 46:1610–1617. doi: 10.1016/j.jpsychires.2012.09.009 [DOI] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG et al. (2007) Heritability of borderline personality disorder features is similar across three countries. Psychological Medicine. 38:1219–1229. doi: 10.1017/s0033291707002024 [DOI] [PubMed] [Google Scholar]
- Donaldson Z, Piel DA, Santos TL, Richardson-Jones J, Leonardo ED, Beck SG et al. (2014) Developmental effects of serotonin 1A autoreceptors on anxiety and social behaviors. Neuropsychopharmacology 39: 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Shi J, Fiorentino A, Leites C, Chen X, Moy W et al. (2014) A rare functional noncoding variant at the GWAS-implicated MIR137/MIR2682 locus might confer risk to schizophrenia and bipolar disorder. American journal of human genetics. 95:744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Roepke S, Kessler-Scheil S, Kathmann N (2016) Reduced risk avoidance and altered neural correlates of feedback processing in patients with borderline personality disorder. Psychiatry Res.243:14–22. doi: 10.1016/j.psychres.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Feigenson KA, Kusnecov AW, Iverstein S (2014) Inflammation and the two-hit hypothesis of schizophrenia. Neuroscience and biobehavioral reviews 38:72–93. doi: 10.1016/j.neubiorev.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields SA, Lange K, Ramos A, Thamotharan S, Rassu F (2014) The relationship between stress and delay discounting: a meta-analytic review. Behav Pharmacol. 25(5-6):434–44. doi: 10.1097/FBP.0000000000000044. [DOI] [PubMed] [Google Scholar]
- Flannery JE, Gabard-Durnam LJ, Shapiro M, Goff B, Caldera C, Louie J et al. (2017) Diurnal cortisol after early institutional care-Age matters. Dev Cogn Neurosci. June:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonagy P, Bateman A (2006) Mechanisms of change in mentalization-based treatment of BPD. J Clin Psychol 62:411–430. doi: 10.1002/jclp.20241 [DOI] [PubMed] [Google Scholar]
- Fossati A, Maffei C, Bagnato M, Battaglia M, Donati D, Donini M et al. (2000) Patterns of covariation of DSM-IV personality disorders in a mixed psychiatric sample. Comprehensive Psychiatry. 41:206–215. doi: 10.1016/s0010-440x(00)90049-x [DOI] [PubMed] [Google Scholar]
- Gan J, Yi J, Zhong M, Cao X, Jin X, Liu W et al. (2016) Abnormal white matter structural connectivity in treatment-naïve young adults with borderline personality disorder. Acta Psychiatrica Scandinavica. 134:494–503. doi: 10.1111/acps.12640 [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. (2013) Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 110(39): 15638–43. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, Berton O, Russo SJ (2011) A standardized protocol for repeated social defeat stress in mice. Nature protocols, 6(8), 1183–1191. doi: 10.1038/nprot.2011.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser JP, Os VJ, Thewissen V, Myin-Germeys I (2010) Psychotic reactivity in borderline personality disorder. Acta Psychiat Scand 121:125–134. doi: 10.1111/j.1600-0447.2009.01427.x [DOI] [PubMed] [Google Scholar]
- Goodwill HL, Manzano-Nieves G, LaChance P, Teramoto S, Lin S, Lopez C, et al. (2018) Early life stress drives sex-selective impairment in reversal learning by affecting parvalbuim interneurons in orbitofrontal cortex of mice. Cell Reports 25: 2299–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Brockway E, Lederle L, Wilcox T, Halladay LR, Ding Y et al. (2018) Identification of a novel gene regulating amygdala-mediated fear extinction. Mol Psychiatry doi: 10.1038/s41380-017-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K (2002) A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav 77:45–54. [DOI] [PubMed] [Google Scholar]
- Hall J, Olabi B, Lawrie SM, McIntosh AM (2010) Hippocampal and amygdala volumes in borderline personality disorder: A meta-analysis of magnetic resonance imaging studies. Personality and Mental Health. 4:172–179. doi: 10.1002/pmh.128 [DOI] [Google Scholar]
- Hall MA, Riedford KM (2017) Borderline Personality Disorder: Diagnosis and Common Comorbidities. The Journal for Nurse Practitioners. 13:e455–e456. doi: 10.1016/j.nurpra.2017.07.012. [DOI] [Google Scholar]
- Hazlett E, Zhang J, New A, Zelmanova Y, Goldstein KE, Haznedar MM et al. (2012) Potentiated Amygdala Response to Repeated Emotional Pictures in Borderline Personality Disorder. Biol Psychiat 72:448–456. doi: 10.1016/j.biopsych.2012.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM (2012) Quantitative meta-analysis of neural activity in posttraumatic stress disorder, doi: 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbort MC, Soch J, Wüstenberg T, Krauel K, Pujara M, Koenigs M, et al. (2016) A negative relationship between ventral striatal loss anticipation response and impulsivity in borderline personality disorder. Neuroimage Clin. 12:724–736. doi: 10.1016/j.nicl.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K et al. (2001) Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry. 50:292–298. doi: 10.1016/s0006-3223(01)01075-7 [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ et al. (2015) Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci 35, 16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Peyre H, Wall MM, Limosin F, Blanco C (2014) Examining sex differences in DSM-IV borderline personality disorder symptom expression using Item Response Theory (IRT). J Psychiatr Res. 59:213–9. doi: 10.1016/j.jpsychires.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Illich PA, King TA, Grau JW (1995) Impact of shock on pain reactivity: I. Whether hypo- or hyperalgesia is observed depends on how pain reactivity is tested. J Exp Psychol Anim Behav Process 21:331–47. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P (2010) Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am J Psychiatry. 167:7. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ (2008) Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 154:1132–42. doi: 10.1016/j.neuroscience.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JW (2011) An animal model of eating disorders associated with stressful experience in early life. Hom Behav. 59: 213–220. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S (2011) The Serotonin Transporter Promoter Variant (5-HTTLPR), Stress, and Depression Meta-analysis Revisited: Evidence of Genetic Moderation. Archives of General Psychiatry. 68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS (2000) Effects of early adverse experiences on brain structure and function: clinical implications. Biological Psychiatry. 48:778–790. doi: 10.1016/s0006-3223(00)00998-7 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Czajkowski N, Roysamb E, Tambs K, Torgersen S et al. (2008) The structure of genetic and environmental risk factors for DSM-IV personality disorders: a multivariate twin study. Archives of general psychiatry. 65:1438–46. doi: 10.1001/archpsyc.65.12.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernberg O (1985) Borderline Conditions and Pathological Narcissism. Rowman & Littlefield Publishers, Inc [Google Scholar]
- Koenigsberg H, Denny B, Fan J, et al. (2014) The Neural Correlates of Anomalous Habituation to Negative Emotional Pictures in Borderline and Avoidant Personality Disorder Patients. Am J Psychiat 171:82–90. doi: 10.1176/appi.ajp.2013.13070852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Siever L, Lee H, et al. (2009) Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res Neuroimaging 172:192–199. doi: 10.1016/j.pscychresns.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Kaplan RD, Gilmore MM, Cooper AM (1985) The relationship between syndrome and personality disorder in DSM-III: experience with 2,462 patients. The American journal of psychiatry. 142:207–12. doi: 10.1176/ajp.142.2.207 [DOI] [PubMed] [Google Scholar]
- Krause-Utz A, Keibel-Mauchnik J, Ebner-Priemer U, Bohus M, Schmahl C (2016) Classical conditioning in borderline personality disorder: an fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 266:291–305. doi: 10.1007/s00406-015-0593-1 [DOI] [PubMed] [Google Scholar]
- Kuhlmann A, Bertsch K, Schmidinger I, Thomann PA, Herpertz SC (2013) Morphometric differences in central stress-regulating structures between women with and without borderline personality disorder. J Psychiatry Neurosci. 38(2): 129–37. doi: 10.1503/jpn.120039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Long J, Der-Avakian A, Streets M, Welsh DK (2015) Dissociation of learned helplessness and fear conditioning in mice: a mouse model of depression. PLoS One. 10(4):e0125892. doi: 10.1371/journal.pone.0125892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF, Lane MC, Loranger AW, Kessler RC (2007) DSM-IV Personality Disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 62:553–564. doi: 10.1016/j.biopsych.2006.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb K, Völlm B, Rücker G, Timmer A, Stoffers JM (2010) Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. The British Journal of Psychiatry. 196:4–12. doi: 10.1192/bjp.bp.108.062984 [DOI] [PubMed] [Google Scholar]
- Likhtik E, Paz R (2015) Amygdala–prefrontal interactions in (mal)adaptive learning. Trends in Neurosciences 38:158–166. doi: 10.1016/j.tins.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA (2014) Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 17(1): 106–13. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan M (1993) Cognitive Behavioral Treatment for Borderline Personality Disorder. Gilford, New york [Google Scholar]
- Lischke A, Domin M, Freyberger HJ, Grabe HJ, Mentel R, Bernheim D, et al. (2015) Structural alterations in white-matter tracts connecting (para-)limbic and prefrontal brain regions in borderline personality disorder. Psychological Medicine. 45:3171–3180. doi: 10.1017/S0033291715001142 [DOI] [PubMed] [Google Scholar]
- Long VA, Fanselow MS (2012) Stress-enhanced fear learning in rats is resistant to the effects of immediate massed extinction. Stress. 15(6):627–36. doi: 10.3109/10253890.2011.650251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubke GH, Laurin C, Amin N, Hottenga J, Willemsen G, Grootheest G et al. (2014) Genome-wide analyses of borderline personality features. Molecular psychiatry 19:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado T, Molle R, Laureano D, Portella A, Werlang I, Benetti C et al. (2013) Early life stress is associated with anxiety, increased stress responsivity and preference for “comfort foods” in adult female rats. Stress. doi: 10.3109/10253890.2013.816841 16:549–556 [DOI] [PubMed] [Google Scholar]
- Maier SF (2001) Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol Psychiatry. 49(9):763–73. [DOI] [PubMed] [Google Scholar]
- Maren S, Holmes A (2016) Stress and Fear Extinction. Neuropsychopharmacology 41:58–79. doi: 10.1038/npp.2015.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MF, Camprodon JA, Dougherty DD, Milad MR (2014) Device-based brain stimulation to augment fear extinction: implications for PTSD treatment and beyond. Depress Anxiety. 2014 April;31(4):269–78. [DOI] [PubMed] [Google Scholar]
- Marin MF, Song H, VanElzakker MB, Staples-Bradley LK, Linnman C, Pace-Schott EF, et al. (2016) Association of Resting Metabolism in the Fear Neural Network With Extinction Recall Activations and Clinical Measures in Trauma-Exposed Individuals. Am J Psychiatry 173: 930–938. doi: 10.1176/appi.ajp.2015.14111460. [DOI] [PubMed] [Google Scholar]
- Maynard T, Sikich L, Lieberman J, LaMantia A (2001) Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophrenia bulletin 27:457–76 [DOI] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. (2017) Social stress induces neurovascular pathology promoting depression. Nat Neuro 20: 1752–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp CM, Geus EJC, Beem LA, Lakenberg N, Hottenga JJ, Slagboom EP, et al. (2007) Family Based Association Analyses between the Serotonin Transporter Gene Polymorphism (5-HTTLPR) and Neuroticism, Anxiety and Depression. Behavior Genetics 37:294–301. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ (2008) Frontolimbic structural changes in borderline personality disorder. J. Psychiatr. Res 42(9): 727–733. doi: 10.1016/j.jpsychires.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskewicz K, Fleeson W, Arnold E, Law M, Mneimne M, Furr MR (2015) A Contingency-Oriented Approach to Understanding Borderline Personality Disorder: Situational Triggers and Symptoms. J Pers Disord 29:486–502. doi: 10.1521/pedi.2015.29.4.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore NLT, Gauchan S, Genovese RF (2012) Differential severity of anxiogenic effects resulting from a brief swim or underwater trauma in adolescent male rats. Pharmacology, Biochemistry and Behavior 102:264–268. doi: 10.1016/j.pbb.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM (2004) Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci 22:415–22. doi: 10.1016/j.ijdevneu.2004.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM (2006) Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience 9:1004–1006. doi: 10.1038/nn1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, et al. (2009) 5-HTTLPR genotype and anxiety-related personality traits: A meta-analysis and new data. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 150B:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul P, Os JV (2005) Behavioural sensitization to daily life stress in psychosis. Psychological Medicine 35:733–741. doi: 10.1017/s0033291704004179 [DOI] [PubMed] [Google Scholar]
- New AS, Carpenter DM, Perez-Rodriguez MM, Ripoll LH, Avedon J, Patil U et al. (2013) Developmental differences in diffusion tensor imaging parameters in borderline personality disorder. Journal of Psychiatric Research. 47:1101–1109. doi: 10.1016/j.jpsychires.2013.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Chan K, Bulgin N, Sicard T, Bismil R, McMain S et al. (2006) Association between serotonin transporter gene and borderline personality disorder. J Psychiat Res 40:448–453. doi: 10.1016/j.jpsychires.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Niedtfeld I, Schulze L, Kirsch P, Herpertz SC, Bohus M, Schmahl C (2010) Affect Regulation and Pain in Borderline Personality Disorder: A Possible Link to the Understanding of Self-Injury. Biol Psychiat 68:383–391. doi: 10.1016/j.biopsych.2010.04.015 [DOI] [PubMed] [Google Scholar]
- Nollet M, Guisquet AM, Belzung C (2013). Models of Depression: Unpredictable Chronic Mild Stress in Mice Current protocols in pharmacology. Chapter 5. Unit 5.65. 10.1002/0471141755.ph0565s61. [DOI] [PubMed] [Google Scholar]
- Opendak M, Gould E, Sullivan R (2017) Early life adversity during the infant sensitive period for attachment: Programming of behavioral neurobiology of threat processing and social behavior. Dev Cogn Neurosci. 25:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual J, Soler J, Barrachina J, Campins JM, Alvarez E, Perez V et al. (2008) Failure to detect an association between the serotonin transporter gene and borderline personality disorder. J Psychiat Res 42:87–88. doi: 10.1016/j.jpsychires.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Peña CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, et al. (2017) Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. 356:1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perova Z, Delevich K, Li B (2015) Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J Neurosci 35:3201–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RA, Tansey KE, Buttenschøn HN, Cohen-Woods S, Bigdeli T, Hall LS, et al. (2017) Genome-wide Association for Major Depression Through Age at Onset Stratification: Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Biol Psychiatry. 81 (4):325–335. doi: 10.1016/j.biopsych.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D (2003) Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci, ;23:8800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Mori S, Lyons M, Milad MR, Phan KL (2017) Acquisition of CS-US contingencies during Pavlovian fear conditioning and extinction in social anxiety disorder and posttraumatic stress disorder. J Affect Disord. 207:76–85. doi: 10.1016/j.jad.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Shukla A, Chattarji S (2018) Extinction recall of fear memories formed before stress is not affected despite higher theta activity in the amygdala. Elife. 7 pii: e35450. doi: 10.7554/eLife.35450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbhandari AK, Gonzalez ST, Fanselow MS (2018) Stress-Enhanced Fear Learning, a Robust Rodent Model of Post-Traumatic Stress Disorder. J Vis Exp. doi: 10.3791/58306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS (2005) Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 29(8): 1207–23. doi: 10.1016/j.neubiorev.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Rau V, Fanselow MS (2009) Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 12(2): 125–33. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Reitz S, Kluetsch R, Niedtfeld I, Knorz T, Lis S, Paret C et al. (2015) Incision and stress regulation in borderline personality disorder: neurobiological mechanisms of self-injurious behaviour. Br J Psychiatry 207:165–172. doi: 10.1192/bjp.bp.114.153379 [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ (2008) A Novel Mouse Model for Acute and Long-Lasting Consequences of Early Life Stress. Endocrinology. 149:4892–4900. doi: 10.1210/en.2008-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne T, Kloet ER, Wouters L, Goekoop JG, DeRijk RH, Brink WND (2002) Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biological Psychiatry. 52:1102–1112. doi: 10.1016/s0006-3223(02)01395-1 [DOI] [PubMed] [Google Scholar]
- Ripke A, O’Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S et al. (2013) Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nature genetics. 45:1150–9. doi: 10.1038/ng.2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J et al. (2009) Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression: A Meta-analysis. JAMA 301(23):2462–2471. doi: 10.1001/jama.2009.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA (2001) Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci 21:4090–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T, Lubin F, Funk A, Sweatt D (2009) Lasting Epigenetic Influence of Early-Life Adversity on the BDNF Gene. Biological Psychiatry 65:760–769. doi: 10.1016/j.biopsych.2008.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge S, Feigenbaum J, Fonagy P (2017) Mechanisms of change in dialectical behaviour therapy and cognitive behaviour therapy for borderline personality disorder: a critical review of the literature. J Ment Health 1–11. doi: 10.1080/09638237.2017.1322185 [DOI] [PubMed] [Google Scholar]
- Sampath D, Sabitha KR, Hegde P, Jayakrishnan HR, Kutty BM, Chattarju S et al. (2014) A study on fear memory retrieval and REM sleep in maternal separation and isolation stressed rats. Behavioural Brain Research. 273:144–154. doi: 10.1016/j.bbr.2014.07.034 [DOI] [PubMed] [Google Scholar]
- Santiago A, Aoki C, Sullivan RM (2017) From attachment to independence: stress hormone control of ecologically relevant emergence of infants’ responses to threat. Current Opinion in Behavioral Sciences 14:78–85. doi: 10.1016/j.cobeha.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N (2004) A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Molecular Psychiatry 9:197–202. doi: 10.1038/sj.mp.4001405 [DOI] [PubMed] [Google Scholar]
- Schulze L, Shcmahi C, Niedtfeld I (2016) Neural correlates of disturbed emotion processing in Borderline Personality Disorder: A multi-modal meta analysis. Biol Psych 79: 97–106. doi: 10.1016/j.biopsych.2015.03.027 [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D (2004) Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 127B: 85–89 doi: 10.1002/ajmg.b.20158 [DOI] [PubMed] [Google Scholar]
- Sher L, Rutter SB, New AS, Siever LJ, Hazlett EA (2019) Gender differences and similarities in aggression, suicidal behaviour, and psychiatric comorbidity in borderline personality disorder. Acta Psychiatr Scand. 139(2): 145–153. doi: 10.1111/acps.12981. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Gunderson JG, McGlashan TH, Dyck IR, Stout RL, Bender DS et al. (2002) Functional Impairment in Patients With Schizotypal, Borderline, Avoidant, or Obsessive-Compulsive Personality Disorder. American Journal of Psychiatry. 159:276–283. doi: 10.1176/appi.ajp.159.2.276 [DOI] [PubMed] [Google Scholar]
- Smolinsky A, Bergner C, LaPorte J, Kalueff A (2009) Analysis of Grooming Behavior and Its Utility in Studying Animal Stress, Anxiety, and Depression In: Mood and Anxiety Related Phenotypes in Mice. Humana Press, pp 21–36 [Google Scholar]
- Soloff PH, Abraham K, Ramaseshan K, Burgess A, Diwadkar VA (2017) Hyper-modulation of brain networks by the amygdala among women with Borderline Personality Disorder: Network signatures of affective interference during cognitive processing. Journal of Psychiatric Research. 88:56–63. doi: 10.1016/j.jpsychires.2016.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Chiappetta L (2012) Prospective predictors of suicidal behavior in borderline personality disorder at 6-year follow-up. The American journal of psychiatry. 169:484–90. doi: 10.1176/appi.ajp.2011.11091378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, White R, Omari A, Ramaseshan K, Diwadkar VA et al. (2015) Affective context interferes with brain responses during cognitive processing in borderline personality disorder: fMRI evidence. Psychiatry Research: Neuroimaging. 233:23–35. doi: 10.1016/j.pscychresns.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russel J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 17:1429–36 [DOI] [PubMed] [Google Scholar]
- Sousa VC, Vital J, Costenla A, Batalha VL, Sebastiao AM, Ribeiro JA Lopes LV (2014) Maternal separation impairs long term-potentiation in CA1-CA3 synapses and hippocampal-dependent memory in old rats. Neurobiology of Aging. 35:1680–1685. doi: 10.1016/j.neurobiolaging.2014.01.024 [DOI] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium (2011) Genome-wide association study identifies five new schizophrenia loci. Nature genetics. doi: 10.1038/ng.940 43:969–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S (1988) Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behavioral Neuroscience. doi: 10.1037/0735-7044.102.5.692 102:692. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ (2013) Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatric Res doi: 10.1016/j.jpsychires.2013.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiglmayr CE, Ebner-Priemer UW, Bretz J, Behm R, Mohse M, Lammers CH, et al. (2008) Dissociative symptoms are positively related to stress in borderline personality disorder. Acta Psychiat Scand 117:139–147. doi: 10.1111/j.1600-0447.2007.01126.x [DOI] [PubMed] [Google Scholar]
- St Onge JR, Ahn S, Phillips AG, Floresco SB (2012) Dynamic fluctuations in dopamine efflux in the prefrontal cortex and nucleus accumbens during risk-based decision making. J Neurosci. 32(47): 16880–91. doi: 10.1523/JNEUROSCI.3807-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB (2010) Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 20(8): 1816–28. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Green EB, Floresco SB (2014) Selective involvement by the medial orbitofrontal cortex in biasing risky, but not impulsive, choice. Cereb Cortex. 24(1):154–62. doi: 10.1093/cercor/bhs297. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magnetic resonance in medicine. 55:302–8. doi: 10.1002/mrm.20774 [DOI] [PubMed] [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Romeo RD, Mcewen BS (2014) On the causes of early life experience effects: Evaluating the role of mom. Frontiers in Neuroendocrinology 35:245–251. doi: 10.1016/j.yfrne.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM (2003) The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews. 27:33–44. doi: 10.1016/s0149-7634(03)00007-1 [DOI] [PubMed] [Google Scholar]
- Tomko RL, Trull TJ, Wood PK, Sher KJ (2014) Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. Journal of personality disorders. 28:734–50. doi: 10.1521/pedi_2012_26_093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen S, Kringlen E, Cramer V (2001) The Prevalence of Personality Disorders in a Community Sample. Archives of General Psychiatry. 58:590–596. doi: 10.1001/archpsyc.58.6.590 [DOI] [PubMed] [Google Scholar]
- Wagner S, Baskaya Ö, Lieb K, Dahmen N, Tadic A (2009) The 5-HTTLPR Polymorphism modulates the association of serious life events (SLE) and impulsivity in patients with Borderline Personality Disorder. Journal of Psychiatric Research. 43:1067–1072. doi: 10.1016/j.jpsychires.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Walker CD, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, et al. (2017) Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential, Stress, 20(5):421–448, doi: 10.1080/10253890.2017.1343296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cao L, Lee CY, Matsuo T, Wu K1,2, Asher G, et al. (2018) Large-scale forward genetics screening identifies Trpa1 as a chemosensor for predator odor-evoked innate fear behaviors. NatCommun. 9(1):2041. doi: 10.1038/s41467-018-04324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley HC, Nickson T, Pope M, Nicol K, Romaniuk L, Bastin ME, et al. (2015) White matter integrity and its association with affective and interpersonal symptoms in borderline personality disorder. NeuroImage: Clinical. 7:476–481 doi: 10.1016/j.nicl.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Belzung C (2015) Treatment-resistant depression: are animal models of depression fit for purpose? Psychopharmacology. 232:3473–3495. doi: 10.1007/s00213-015-4034-7 [DOI] [PubMed] [Google Scholar]
- Witt SH, Streit F, Jungkunz M, Frank J, Awasthi S, Reinbold CS, et al. (2017) Genome-wide association study of borderline personality disorder reveals genetic overlap with bipolar disorder, major depression and schizophrenia, doi: 10.1038/tp.2017.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HC, Cao X, Das M, Zhu XH, Gao TM (2010) Behavioral animal models of depression. Neuroscience Bulletin.26:327–337. doi: 10.1007/s12264-010-0323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans F, Levy K (2002) An object relations perspective on borderline personality. Acta Neuropsychiatr 14:76–80. doi: 10.1034/j.1601-5215.2002.140205.x [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Khera GS, Bleichmar J (2001) Treatment histories of borderline inpatients. Comprehensive Psychiatry. 42:144–150. doi: 10.1053/comp.2001.19749 [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Reich BD, Conkey LC, Fizmaurice GM (2015) Treatment Rates for Patients With Borderline Personality Disorder and Other Personality Disorders: A 16-Year Study. Psychiatric Services. 66:15–20. doi: 10.1176/appi.ps.201400055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC (2000) Childhood Experiences Associated with the Development of Borderline Personality Disorder. Psychiatric Clinics of North America. 23:89–101. doi: 10.1016/s0193-953x(05)70145-3 [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Yong L, Frankenberg FR, Hennen J, Reich DB, Marion MF, et al. (2002) Severity of Reported Childhood Sexual Abuse and Its Relationship to Severity of Borderline Psychopathology and Psychosocial Impairment among Borderline Inpatients. The Journal of Nervous and Mental Disease. 190:381. doi: 10.1097/00005053-200206000-00006 [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Williams AA, Lewis RE, Reich RB, Vera SC, Marino MF et al. (2006) Reported pathological childhood experiences associated with the development of borderline personality disorder. American Journal of Psychiatry. 154:1101–1106. doi: 10.1176/ajp.154.8.1101 [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Baarendse PJ, Vanderschuren LJ, Winstanley CA (2015) Inactivation of the prelimbic or infralimbic cortex impairs decision-making in the rat gambling task. Psychopharmacology. 232(24):4481–91. doi: 10.1007/s00213-015-4075-y. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI (1999) Axis I diagnostic comorbidity and borderline personality disorder. Comprehensive Psychiatry. 40(4):245–252 doi: 10.1016/s0010-440x(99)90123-2 [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Rothschild L, Chelminski I (2005) The Prevalence of DSM-IV Personality Disorders in Psychiatric Outpatients. American Journal of Psychiatry. 162:1911–1918. doi: 10.1176/appi.ajp.162.10.1911 [DOI] [PubMed] [Google Scholar]