Significance
Phospholipid flippases constitute the largest subfamily of P-type ATPases and have in eukaryotic organisms evolved as a central transport system for selective translocation of phospholipids across biological membranes to generate membrane lipid asymmetry, a property essential for numerous cellular processes. The importance of flippases is highlighted by severe neurological disorders and liver diseases caused by flippase dysfunction in humans. The electrogenicity of phospholipid transport by flippases has not previously been explored. We demonstrated that phosphatidylserine translocation by the flippase ATP8A2 generates electrical current, resulting from specific steps in the flippase reaction cycle moving the charged lipid head group between the membrane bilayer leaflets, and that no charged substrate is being countertransported. These findings unravel key features of phospholipid flippases.
Keywords: flippase, phospholipid transport, charge displacement, SSM method, CAMRQ syndrome
Abstract
Phospholipid flippases (P4-ATPases) utilize ATP to translocate specific phospholipids from the exoplasmic leaflet to the cytoplasmic leaflet of biological membranes, thus generating and maintaining transmembrane lipid asymmetry essential for a variety of cellular processes. P4-ATPases belong to the P-type ATPase protein family, which also encompasses the ion transporting P2-ATPases: Ca2+-ATPase, Na+,K+-ATPase, and H+,K+-ATPase. In comparison with the P2-ATPases, understanding of P4-ATPases is still very limited. The electrogenicity of P4-ATPases has not been explored, and it is not known whether lipid transfer between membrane bilayer leaflets can lead to displacement of charge across the membrane. A related question is whether P4-ATPases countertransport ions or other substrates in the opposite direction, similar to the P2-ATPases. Using an electrophysiological method based on solid supported membranes, we observed the generation of a transient electrical current by the mammalian P4-ATPase ATP8A2 in the presence of ATP and the negatively charged lipid substrate phosphatidylserine, whereas only a diminutive current was generated with the lipid substrate phosphatidylethanolamine, which carries no or little charge under the conditions of the measurement. The current transient seen with phosphatidylserine was abolished by the mutation E198Q, which blocks dephosphorylation. Likewise, mutation I364M, which causes the neurological disorder cerebellar ataxia, mental retardation, and disequilibrium (CAMRQ) syndrome, strongly interfered with the electrogenic lipid translocation. It is concluded that the electrogenicity is associated with a step in the ATPase reaction cycle directly involved in translocation of the lipid. These measurements also showed that no charged substrate is being countertransported, thereby distinguishing the P4-ATPase from P2-ATPases.
Asymmetric phospholipid distribution between the leaflets of biological membranes plays a fundamental role in several physiological processes, e.g., blood coagulation, apoptosis, cell and organelle shape determination, intracellular vesicle trafficking, membrane protein regulation, membrane stability and impermeability, cell signaling, and host–virus interactions (1–4). P4-ATPases, also known as flippases, play a central role in phospholipid transport between the membrane bilayer leaflets to generate the membrane lipid asymmetry. P4-ATPases constitute the largest subfamily of the P-type ATPase protein family, which also encompasses the more thoroughly investigated ion transporting P2-ATPases: Ca2+-ATPase, Na+,K+-ATPase, and H+,K+-ATPase. The predicted 3D structure and the conserved key residues involved in phosphorylation and dephosphorylation of P4-ATPases suggest that the transport mechanism of these enzymes is related to the mechanism of the P2-ATPases. It was shown that the mammalian flippase ATP8A2, which is highly expressed in the retina, brain, testis, and spinal cord, undergoes phosphorylation by ATP at the aspartate conserved in all P-type ATPases, as part of a catalytic cycle E1→E1P→E2P→E2 (“P” indicating phosphorylation, Fig. 1A) similar to that known from P2-ATPases (5, 6). ATP8A2 translocates its 2 known substrates phosphatidylserine (PS) and phosphatidylethanolamine (PE) from the exoplasmic leaflet to the cytoplasmic leaflet of the membrane bilayer. The dephosphorylation of the E2P intermediate of ATP8A2, E2P→E2, was found to require activation by the binding of PS or PE, analogous to the activation of dephosphorylation of the Na+,K+-ATPase by K+ binding to E2P from the extracellular side of the plasma membrane (6). Phosphatidylcholine (PC), the predominant phospholipid of the exoplasmic leaflet in eukaryotic membranes, is not a substrate of ATP8A2, and in the presence of PC with PS and PE absent, the phosphoenzyme intermediate is very stable (1, 6), suggesting that in the native membrane, ATP8A2 remains continuously phosphorylated until its exoplasmic surface encounters the appropriate lipid substrate, PS or PE. The stoichiometry of PS or PE molecules translocated per ATP hydrolyzed has not been determined, and it is also unknown whether ATP8A2 or other P4-ATPases countertransport an ion or other substrate from the cytoplasm toward the exoplasm in connection with the E1→E1P→E2P reaction sequence, as seen for the P2-ATPases. In the latter, the binding to the E1 form of the ion to be translocated from the cytoplasm toward the exoplasm (Ca2+, Na+, and H+, for Ca2+-ATPase, Na+,K+-ATPase, and H+,K+-ATPase, respectively) is mandatory for the phosphoryl transfer from ATP, but so far, no ion or other substrate has been shown to play a similar role in the phosphorylation of a P4-ATPase (H+, SO42-, acetate, Cl−, Ca2+, Na+, and K+ are just some of the putative candidates examined, reviewed in ref. 1), thus raising the question whether P4-ATPases are in fact one-directional pumps undergoing spontaneous phosphorylation.
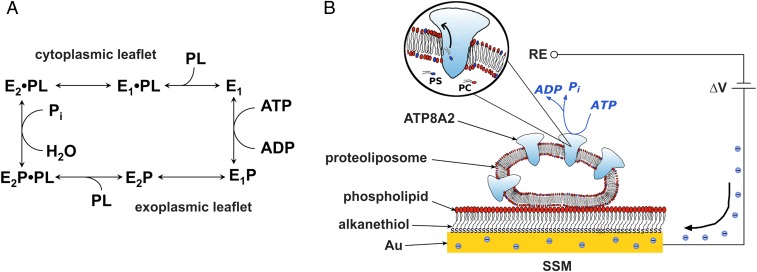
Fig. 1.
Simplified scheme of the ATP8A2 reaction cycle and schematic diagram of the SSM method. (A) The scheme is based on functional similarities to the ion-transporting P2-ATPases (5, 6). E1, E1P, E2P, and E2 are different enzyme conformational states, “P” indicates covalently bound phosphate. The phospholipid substrate, PL (PS or PE), enters the cycle from the exoplasmic leaflet of the lipid bilayer by binding to the E2P phosphoenzyme, thereby stimulating the dephosphorylation and release of the lipid substrate toward the cytoplasmic leaflet as consequence of the E2 to E1 conformational change. (B) Reconstituted proteoliposome containing ATP8A2 adsorbed on the SSM surface and subjected to an ATP concentration jump (not drawn to scale). If the ATP concentration jump induces net charge displacement across the ATPase, a current signal is detected along the external circuit (the blue spheres represent electrons) to keep constant the potential difference (ΔV) applied across the whole system. RE, reference electrode.
Although site-directed mutagenesis and structural modeling studies have provided useful models to describe a lipid translocation pathway in P4-ATPases (7–11), central aspects of the transport pathway and flipping mechanism remain elusive, including the electrogenic properties of the flippases. Electrogenicity is likewise an unexplored feature for other ATP-dependent lipid translocating systems in biological membranes such as ABC transporters. It is unknown whether lipid transfer between membrane bilayer leaflets can lead to displacement of charge across the membrane.
Here, we performed electrical measurements on solid supported membranes (SSMs) to investigate the electrogenic properties of wild-type and mutant forms of the mammalian flippase ATP8A2 with either of its 2 lipid substrates PS and PE. The SSM method (Fig. 1B) has previously been used to study the ion transport mechanism and identify electrogenic steps of P1-ATPase and P2-ATPases (12–17). We observed the generation of a transient electrical current by ATP8A2 in the presence of ATP and PS. The current is associated with a step in the ATPase reaction cycle directly involved in translocation of the lipid: the dephosphorylation, E2P→E2, activated by the lipid substrate, and/or the subsequent E2→E1 conformational transition of the dephosphoenzyme. No charged substrate is being countertransported in connection with the E1→E1P→E2P reaction sequence, thus distinguishing this transport system from the P2-ATPases.
Results
Electrogenic Properties of ATP8A2 in the Presence of PS.
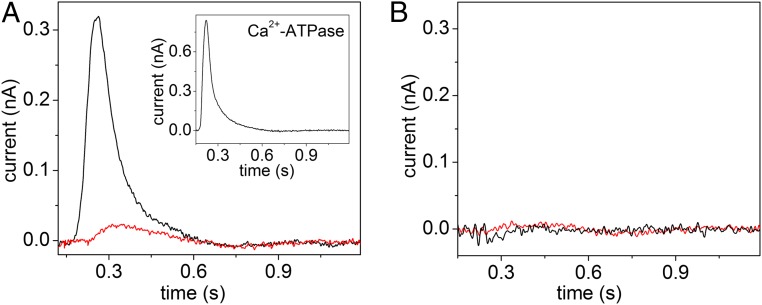
Current measurements were performed on reconstituted proteoliposomes containing purified ATP8A2 together with its accessory CDC50A protein at a 1:1 (wt/wt) lipid-to-protein ratio. The proteoliposomes were adsorbed on an SSM electrode (Fig. 1B) and were perfused with external solutions of various compositions. We activated the adsorbed membrane-bound ATP8A2 by an ATP concentration jump through fast solution exchange (Materials and Methods) and detected the related current signal. It is shown in Fig. 2A that a 100 µM ATP jump (pH 7.5) on ATP8A2 reconstituted into proteoliposomes consisting of a mixture of 90% PC and 10% PS (90PC:10PS proteoliposomes) induced a current transient, which was not observed following addition of ATP to control 90PC:10PS liposomes devoid of ATP8A2 (Fig. 2B). Decreasing the ATP concentration to 50 µM did not reduce the steepness or size of the current transient (SI Appendix, Fig. S1), indicating that saturation of the enzyme with ATP had been reached at this ATP concentration. To confirm that the measured current is actually due to ATP8A2 activity, we performed the ATP jump in the presence of the ATPase inhibitor orthovanadate (50 μM), which caused a strong suppression of the current transient (Fig. 2A).
Fig. 2.
ATP8A2-related current transients in the presence of PS. (A) Current transients observed upon 100 µM ATP concentration jumps on 90PC:10PS proteoliposomes containing ATP8A2 in the absence (black line) or in the presence (red line) of 50 μM orthovanadate in 150 mM NaCl, 1 mM DTT, 5 mM MgCl2, 50 mM Hepes-Tris (“basic medium”), pH 7.5. The average displaced charge, obtained by numerical integration of the ATP-induced current transient in the absence of orthovanadate (Fig. 3B) is 41 ± 2 pC (n = 7); n, number of independent measurements; ±, SEM. Inset shows the average current signal induced by a 100 µM ATP concentration jump on native SR vesicles containing Ca2+-ATPase (present at a concentration of 0.5 mg/mL similar to ATP8A2 during adsorption on the SSM surface) in 100 mM KCl, 1 mM MgCl2, 25 mM Mops (pH 7.0), 0.2 mM DTT, 0.25 mM EGTA, and 0.25 mM CaCl2 (10 µM free Ca2+). The average displaced charge is in this case 84 ± 5 pC (n = 9). (B) Current traces recorded with 90PC:10PS liposomes devoid of ATP8A2 (control liposomes) upon a 100 µM ATP concentration jump (black line) or upon exchange with basic medium without ATP added (red line).
These results suggest that the ATP-induced current signal is related to charge movement across ATP8A2 upon ATP utilization. Because orthovanadate inhibits the ATPase from the cytoplasmic side, the inhibition experiment demonstrates that the enzyme population with the cytoplasmic side facing the external aqueous solution is responsible for the ATP-induced signal (∼70% of the reconstituted proteins according to a previous analysis using proteolytic digestion; ref. 18). We observed that the sign of the ATP8A2-related current is positive. A positive current signal was also observed following ATP concentration jumps on sarcoplasmic reticulum (SR) vesicles containing Ca2+-ATPase adsorbed on a SSM (Fig. 2 A, Inset) and was in this case attributed to translocation and release of Ca2+ ions into the SR vesicle interior (14, 17). The displacement of positive charge in one direction is electrically equivalent to the movement of negative charge in the opposite direction. PS possesses a net negatively charged head group and is a substrate of ATP8A2, whereas PC, also present in the proteoliposomes, is not a substrate and possesses an electrically neutral head group. Thus, the ATP8A2 current signal observed in the presence of PS might result from displacement of negatively charged PS toward the outside of the proteoliposomes (the ATP8A2 cytoplasmic side facing the external aqueous solution) in connection with ATP utilization. The SSM technique allows presteady-state measurements of charge movements within the first transport cycle of the ATPase, while steady-state currents are not measured (14). As shown in Fig. 3B, the average charge displaced by ATP8A2 in the first transport cycle with PS present, obtained by numerical integration of the ATP-induced current transients in several experiments conducted as in Fig. 2A, is 41 ± 2 pC. The corresponding average displaced charge in the native SR vesicles containing Ca2+-ATPase at the same protein concentration (0.5 mg/mL) during adsorption on the SSMs is 84 ± 5 pC (Fig. 2 A, legend), under conditions where the Ca2+-ATPase translocates a net charge of 2 elementary charges in the first cycle (two Ca2+ into the vesicles in exchange for two H+; refs. 13 and 14).
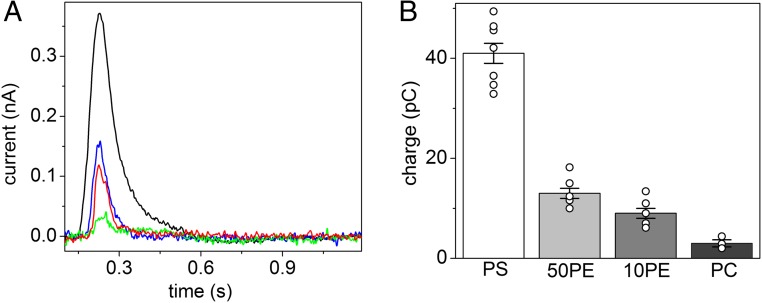
Fig. 3.
Effect of different phospholipids on the ATP8A2-related current transients. (A) Current transients observed upon 100 µM ATP concentration jumps on 90PC:10PS (black line), 90PC:10PE (red line), 50PC:50PE (blue line), and 100PC (green line) proteoliposomes containing ATP8A2 in basic medium, pH 7.5. (B) Average displaced charges obtained by numerical integration of the ATP-induced current transients observed for 90PC:10PS (PS, 41 ± 2 pC, n = 7), 50PC:50PE (50PE, 13 ± 1 pC, n = 5), 90PC:10PE (10PE, 9 ± 1 pC, n = 5), and 100PC (PC, 3.0 ± 0.7 pC, n = 3) proteoliposomes containing ATP8A2; n, number of independent measurements; error bars and ±, SEM. Individual data points are shown by open circles.
The Effect of Lipid Composition on the Electrogenicity of ATP8A2.
Current measurements were also performed on ATP8A2 reconstituted into proteoliposomes of various lipid compositions to evaluate the effect of different phospholipids on the ATP8A2-related current. In addition to 90PC:10PS proteoliposomes, we examined proteoliposomes containing 90% PC and 10% PE (90PC:10PE proteoliposomes), 50% PC and 50% PE (50PC:50PE proteoliposomes), or 100% PC (100PC proteoliposomes). The protein and total lipid concentration were identical for these preparations. In the case of 90PC:10PE and 50PC:50PE proteoliposomes containing ATP8A2, an ATP-induced current transient was observed (Fig. 3A), but this current transient, as well as the related displaced charge (Fig. 3B), was much smaller than the current measured in 90PC:10PS proteoliposomes, although some increase was seen upon increasing the PE content from 10 to 50% (Fig. 3 A and B). Similar to the above-described observations with 90PC:10PS proteoliposomes, the current signal observed with PE-containing proteoliposomes vanished in the presence of 50 µM orthovanadate, and a 100 µM ATP jump on 50PC:50PE liposomes devoid of ATP8A2 likewise yielded no detectable current, thus again attesting that the observed current transient was associated with ATP utilization by ATP8A2.
As further reported in Fig. 3A (green line) and Fig. 3B, no significant current transient was observed for a 100 µM ATP jump on 100PC proteoliposomes containing ATP8A2. Unlike PS and PE, PC is not a substrate of ATP8A2 (18), and PC does not stimulate ATP8A2 dephosphorylation (6). The enzyme is, nonetheless, phosphorylated by ATP in the presence of PC alone (6). Therefore, the experiment with 100PC proteoliposomes shows that the ATP8A2 phosphorylation per se does not generate any current signal.
Determination of the specific ATPase activities of the proteoliposomes yielded (mean ± SEM) 11.3 ± 2.0 (n = 8), 2.9 ± 0.7 (n = 6), 1.6 ± 0.2 (n = 5), and 0.2 ± 0.0 (n = 4) μmol·min−1·mg−1 ATP8A2 protein for 90PC:10PS, 50PC:50PE, 90PC:10PE, and 100PC proteoliposomes, respectively. These differences appear to match the differences in charge displacement fairly well.
To examine the effect of lipid composition on the protein incorporation in the proteoliposomes, the proteoliposome preparations were centrifuged on 55% (wt/wt) sucrose under conditions where only unincorporated protein and protein−lipid complexes of a much lower lipid-to-protein ratio (<0.25:1 [wt/wt]) than the average of 1:1 (wt/wt) are allowed to pellet (Materials and Methods). The pelleted protein fractions were found to constitute (mean ± SEM) 9.6 ± 0.4% (n = 3), 18.3 ± 2.5% (n = 3), and 12.6 ± 2.1% (n = 3) of the total protein in the preparations for 90PC:10PS, 50PC:50PE, and 90PC:10PE proteoliposomes, respectively, thus suggesting that the fraction of unincorporated protein is relatively small, but highest for the 50PC:50PE preparation.
The Effect of pH and Mutations on the Electrogenicity of ATP8A2.
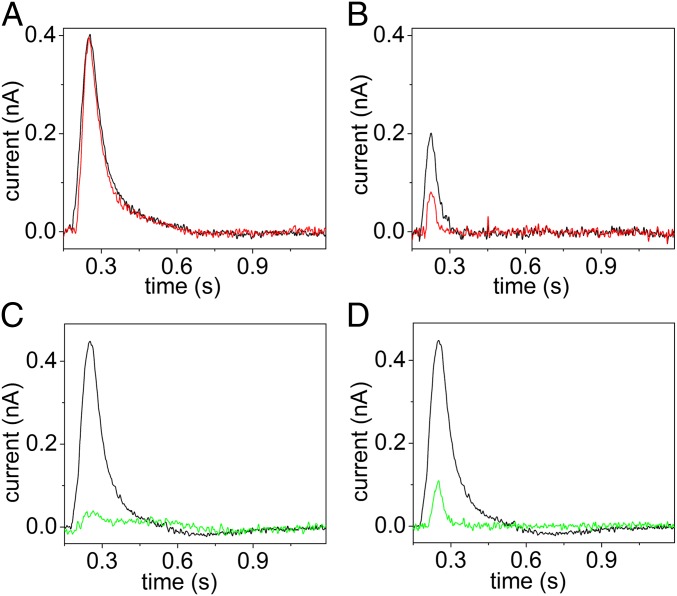
To test the hypothesis that the diminutive current signal observed with PE-containing proteoliposomes is related to the translocation of a small fraction of PE whose head group carries negative charge, the effect of reducing the pH from 7.5 to 6.7 was studied. The ATP concentration jump was performed at either pH value with 90PC:10PS (Fig. 4A) or 50PC:50PE (Fig. 4B) proteoliposomes containing ATP8A2. To ensure pH equilibration between the 2 sides of the membrane, the proteoliposomes were incubated at the given pH for 15 min before carrying out the ATP concentration jump. In the case of 90PC:10PS proteoliposomes, the ATP-induced current transients at pH 7.5 and 6.7 were identical, i.e., no pH effect was observed, whereas for 50PC:50PE proteoliposomes, the ATP-dependent current signal was remarkably reduced at pH 6.7, as expected if the signal were dependent on the ionization of the PE head group. To verify that this result was not caused by a proton gradient created across the membrane, when the pH of the external medium was reduced to 6.7 (the proteoliposomes had been made by dialysis at pH 7.5), the experiments corresponding to Fig. 4 A and B were repeated in the presence of the protonophore 1799. As shown in SI Appendix, Fig. S2, the result obtained in the presence of the protonophore was very similar to that obtained in its absence.
Fig. 4.
Effects of pH and specific mutations on the ATP8A2-related current transients. (A and B) Current transients induced by 100 µM ATP concentration jumps on 90PC:10PS (A) and 50PC:50PE (B) proteoliposomes containing ATP8A2 in basic medium, pH 7.5 (black lines) or 6.7 (red lines). (C and D) Current transients observed following 100 µM ATP concentration jumps on 90PC:10PS proteoliposomes containing ATP8A2 wild-type enzyme (C and D, black lines), mutant E198Q (C, green line), or mutant I364M (D, green line) in basic medium, pH 7.5.
Finally, we examined the effects of specific mutations on the transient currents produced by 90PC:10PS proteoliposomes containing ATP8A2 (Fig. 4 C and D). We first examined the effect of replacing Glu-198 with glutamine. Glu-198 is part of the DGET motif of the cytoplasmic A-domain (TGES in P2-ATPases) that facilitates the dephosphorylation of the phosphointermediate (catalyzes the hydrolysis of E2P liberating Pi and water, cf. Fig. 1A). It was demonstrated that the E198Q mutation allows phosphorylation from ATP but blocks the dephosphorylation with resulting E2P accumulation (6). Interestingly, the E198Q mutant yielded no current transient upon an ATP concentration jump (Fig. 4C). Because the E198Q mutant undergoes the E1P→E2P conformational transition, the absence of the current transient shows that the electrogenicity is not related to this transition. This finding is in line with the absence of a current transient in the experiment with 100PC proteoliposomes, because the phosphorylation of wild-type ATP8A2 in the absence of PS or PE likewise leads to accumulation of E2P formed from E1P (6). Hence, it can be concluded that the electrogenicity is associated with later step(s) in the reaction cycle: the E2P dephosphorylation activated by PS or PE binding and/or the E2→E1 transition.
Mutations in ATP8A2 have been associated with severe neurological disorders in humans (19–22). The isoleucine Ile-364, which is located in the M4 transmembrane segment, is highly conserved among P4-ATPases, and the I364M mutation (I376M in human) was identified as the cause of cerebellar ataxia, mental retardation, and disequilibrium syndrome (20). Recent studies have shown that although I364M was capable of undergoing phosphorylation from ATP, this mutant showed a strongly reduced PS flipping (8). We therefore investigated the effect of the I364M mutation on the electrogenicity of ATP8A2. Notably, this mutation almost completely suppressed the ATP-induced current signal (Fig. 4D), indicating that electrogenic displacement of PS is prevented.
Discussion
In comparison with the ion transporting P2-ATPases Ca2+-ATPase, Na+,K+-ATPase, and H+,K+-ATPase, understanding of P4-ATPases is still very limited. Neither the stoichiometry of phospholipid molecules translocated per ATP hydrolyzed nor the electrogenicity of P4-ATPases has previously been determined. A related issue is whether P4-ATPases countertransport ions in the opposite direction, similar to the P2-ATPases. To address these questions, we here employed a SSM-based electrophysiological technique to investigate the electrogenic properties of the mammalian flippase ATP8A2. Our current measurements have shown that ATP-dependent phospholipid translocation by ATP8A2 can generate a current transient, which is much more prominent with PS as substrate than with PE. The electrogenicity is not associated with the phosphorylation of the enzyme by ATP, forming the E1P intermediate, or with the E1P→E2P conformational transition of the protein, because these steps are allowed in ATP8A2 reconstituted in 100PC proteoliposomes, without PS or PE present, and in the ATP8A2 mutants studied (6, 8), which showed no or only a very small current transient upon addition of ATP. In these enzyme preparations, the dephosphorylation, E2P→E2, is blocked. The electrogenicity must therefore be associated with the dephosphorylation, which normally is activated by the lipid substrate binding from the exoplasmic leaflet, and/or with the ensuing E2→E1 transition of the dephosphoenzyme, thought to release the lipid to the cytoplasmic leaflet (8), i.e., the 2 steps in the transport cycle directly involved in the lipid translocation. Our findings raise the question whether the observed charge displacement results directly from the movement of the charged head group of the phospholipid or is a manifestation of a protein conformational change affecting the electric field intrinsic to the protein, e.g., by movement of a helix dipole. A rough estimate of the number of elementary charges displaced in the first flippase transport cycle can be obtained from the data in Figs. 2 and 3 by comparing the charge displacement by ATP8A2 with that determined for the Ca2+-ATPase under conditions where the Ca2+-ATPase is thought to displace 2 elementary charges per cycle (13, 14). The average displaced charge was for ATP8A2 41 ± 2 pC and for the Ca2+-ATPase 84 ± 5 pC (Fig. 2 A, legend), resulting in an estimate of 41/84 × 2 = 0.98 elementary charges for ATP8A2. Taking into consideration that only 70% of the reconstituted ATP8A2 proteins are believed to expose the cytoplasmic side to the external aqueous solution, thereby having access to ATP (18), whereas all Ca2+-ATPase molecules in SR appear to be oriented right-side-out (23), the calculated number of elementary charges displaced in the first cycle by ATP8A2 would rise to 1.4. Such an extent of charge displacement by ATP8A2 is larger than expected for a protein conformational change and suggests that the movement of the negatively charged head group of the lipid substrate per se contributes to the electrogenicity, perhaps fully accounting for the electrogenicity. Due to their magnitude, the charge displacements determined by the SSM method with the P1-ATPase and P2-ATPase were likewise attributed to the movement of the translocated ions (16, 17). PS in lipid bilayers carries 1 net negative charge at the pH values studied here (pH 7.5 and 6.7) (24). In proteoliposomes, some influence of the specific protein dielectric environment on the ionization of the lipid may be expected. Several lysine and arginine residues are positioned near the proposed lipid translocation pathway of ATP8A2 (1, 6, 8), which might promote deprotonation of the amino group of PS, thus increasing the net negative charge of PS to a value between 1 and 2. With the uncertainties inherent in the calculation, it may be concluded that the measured current transient for PS would be in accordance with a stoichiometry of 1 PS molecule translocated by ATP8A2 in the first cycle.
The current transient generated with PE as substrate was diminutive, compared with the robust transient seen for PS, and almost disappeared when the pH was reduced from 7.5 to 6.7, whereas this pH change did not affect the current transient seen with PS. This is the expected outcome, if the charge displacement results from movement of a charged lipid head group, because only a small fraction of PE is likely to carry negative charge at pH 7.5, and even less at pH 6.7 (24). However, it should also be taken into consideration that the PE-containing proteoliposomes exhibited four- to sevenfold lower specific ATPase activity compared with PS-containing proteoliposomes. The lower ATPase activity of the PE-containing proteoliposomes is in agreement with previous studies of detergent-solubilized ATP8A2 in the presence of a series of different PS and PE concentrations, showing that PE is a much poorer substrate of ATP8A2 than PS, the apparent affinity (as expressed by the reciprocal of Km) for the lipid substrate as well as the Vmax being considerably lower with PE than with PS (8, 18, 25). The 10-fold lower apparent affinity for PE relative to PS (18, 25) might cause lack of saturation of ATP8A2 with the lipid substrate in the PE-containing proteoliposomes, thereby contributing to depletion of the electrogenic signal with PE relative to PS. The observed increase of charge displacement upon increasing the PE content from 10 to 50% (Fig. 3 A and B) indicates that at least in the 90PC:10PE proteoliposomes ATP8A2 is not saturated with PE.
A caveat to the conclusions based on comparison of the signals observed with the proteoliposomes of various lipid compositions is that the efficiency of incorporation of protein in the proteoliposomes might depend on the lipid composition. It can be rationalized that the 90PC:10PS and 90PC:10PE proteoliposomes are rather similar with respect to the number of ATPase molecules per vesicle, due to the fact that the major part of the lipid (90%) is PC in both cases. This consideration is supported by the results of centrifugation of the proteoliposomes on 55% (wt/wt) sucrose, showing that the pelleted protein fraction is relatively small and similar in the 90PC:10PS and 90PC:10PE proteoliposomes (9.6 ± 0.4% and 12.6 ± 2.1%, respectively). Therefore, the reduction of the current transient and ATPase activity observed for 90PC:10PE proteoliposomes relative to 90PC:10PS is not attributable to a reduced protein incorporation in the 90PC:10PE proteoliposomes. This conclusion is in line with the above described finding that the difference between the ATPase activities determined with PS and PE as substrates is not linked to the reconstituted enzyme preparation but is a characteristic of ATP8A2 in the detergent solubilized state as well. The maximum estimate of the unincorporated protein fraction obtained by centrifugation was on the other hand slightly higher for the 50PC:50PE proteoliposomes, amounting to 18.3 ± 2.5%. We cannot exclude that in the 50PC:50PE preparation the number of proteins incorporated in vesicles, and with the cytoplasmic side facing outwards, is less than with the 90PC:10PE preparation. Hence, an increase of the charge displacement per molecule caused by the increase of the PE content from 10 to 50% may not be fully revealed by the results reported in Fig. 3, in which case the lack of saturation with PE would be more evident.
Finally, we wish to emphasize the importance of our findings in relation to the issue of whether countertransport occurs from the cytoplasmic side toward the exoplasm. The absence of a current transient observed with ATP8A2 reconstituted in 100PC proteoliposomes and with the two ATP8A2 mutants indicates that no inorganic ion or other charged substrate is being countertransported in connection with the phosphorylation from ATP, forming E1P, or the E1P→E2P conformational transition. This feature apparently distinguishes this transport system from the P2-ATPases Ca2+-ATPase, Na+,K+-ATPase, and H+,K+-ATPase, which transport ions in both directions. It is thus in good agreement with the present finding that no specific ion seems to be required as trigger of the phosphorylation of flippases (1), unlike the situation with the P2-ATPases.
Materials and Methods
HEK293T cells were cotransfected with wild-type or mutant bovine ATP8A2 cDNA and cDNA encoding the accessory subunit CDC50A, both contained in a pcDNA3 vector (Invitrogen) (6). The cDNA constructs for mutants E198Q and I364M were obtained using the QuikChange mutagenesis kit (Stratagene). Cells were harvested 48 h after transfection and lysed in the presence of CHAPS detergent. The flippase ATP8A2/CDC50A protein complex in detergent solubilized form was immunoaffinity-purified to >95% purity using the Rho 1D4 antibody coupled to Sepharose (6, 8, 18). This antibody specifically recognizes a C-terminal 9 amino acid 1D4 tag on ATP8A2 (26). The Sepharose matrix with bound ATP8A2/CDC50A was washed with buffer containing n-octyl-β-d-glucopyranoside detergent and PC, followed by elution of the protein complex with 1D4 peptide. The detergent solubilized purified wild-type or mutant ATP8A2/CDC50A was mixed with PC, or the indicated mixture of PC with PS or PE, at a 1:1 (wt/wt) ratio of total lipid to protein (i.e., a ratio close to that of the Ca2+-ATPase in SR, with which ATP8A2 is compared in this study), and reconstitution of proteoliposomes was obtained by detergent removal by dialysis (18). Centrifugation, to pellet protein not incorporated in the proteoliposomes, was performed on 55% (wt/wt) sucrose in medium containing 150 mM NaCl, 1 mM DTT, 5 mM MgCl2, 50 mM Hepes-Tris (“basic medium”), pH 7.5, at 100,000 × g for 18 h at 4 °C in 230-μL tubes, using a TLS-55 rotor in a Beckman Coulter Optima Max microultracentrifuge. Protein concentration was measured by the Coomassie dye binding method (27). The specific ATPase activity (µmol Pi·min−1·mg−1 ATP8A2 protein) was determined by following the liberation of Pi colorimetrically (18) at 37 °C for 15 min in the presence of 7.5 mM ATP, 46 mM Hepes-Tris (pH 7.5), 150 mM NaCl, 12 mM MgCl2, and 1 mM DTT. Reproducibility of the reconstituted product was demonstrated by the similar results of ATPase activity and current measurements obtained for different proteoliposome preparations of the same lipid composition.
For measurement of current transients, the proteoliposomes containing wild-type or mutant ATP8A2 enzyme at a protein concentration of 0.5 mg/mL were adsorbed onto a hybrid alkanethiol/phospholipid bilayer supported by a gold electrode (Fig. 1B) during an incubation time of 60 min. The SSM consists of an octadecanethiol monolayer covalently bound to the gold surface via the sulfur atom and a diphytanoylphosphatidylcholine monolayer on top of the thiol layer (28, 29). The proteoliposomes adsorbed on the SSM surface were incubated with basic medium at a given pH for about 15 min to equilibrate the pH between the inside and outside of the proteoliposomes. ATP concentration jumps to register the ATP-induced current signal were then performed by fast solution exchange with basic medium supplemented with 100 μM ATP (50 μM in the control experiment in SI Appendix, Fig. S1). The fast solution exchange was carried out using the SURFE2Rone device (Nanion Technologies), which contains electrophysiological hardware as well as liquid handling components, including an autosampler, allowing the measurement of fast kinetics with a millisecond time resolution (30, 31). The temperature was maintained at 22–23 °C for all of the experiments. If the ATP concentration jump induces a net charge movement across the ATPase, a current transient is detected due to the capacitive coupling between the proteoliposome and SSM (17, 32).
In some experiments, 1 µM protonophore 1799 (13) was included in the media to ensure that no H+ gradient was formed across the proteoliposome membrane (see Results and SI Appendix, Fig. S2). For vanadate inhibition experiments, the proteoliposomes adsorbed on the SSMs were incubated with the basic medium containing 50 μM sodium orthovanadate for 20 min. Then, this solution was replaced with basic medium containing orthovanadate and ATP, and the current signal was registered.
To verify the reproducibility of the current transients on the same SSM, each single measurement was repeated 5 times and then averaged to improve the signal-to-noise ratio. SDs did not exceed 5%. Moreover, each set of measurements was typically reproduced using at least 3 different SSM electrodes.
Supplementary Material
Acknowledgments
F.T.-B. thanks Dr. Gianluca Bartolommei for his help in preparing Fig. 1. This study was funded in part by Danish Council for Independent Research Grant DFF 6110-00271 (to J.P.A.), Novo Nordisk Foundation Grants NNF16OC0022510 and NNF18OC0053086 (to J.P.A.), and Canadian Institutes of Health Research Grant PJT148649 (to R.S.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910211116/-/DCSupplemental.
References
- 1.Andersen J. P., et al. , P4-ATPases as phospholipid flippases–structure, function, and enigmas. Front. Physiol. 7, 275 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevers E. M., Williamson P. L., Getting to the outer leaflet: Physiology of phosphatidylserine exposure at the plasma membrane. Physiol. Rev. 96, 605–645 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Puts C. F., Holthuis J. C., Mechanism and significance of P4 ATPase-catalyzed lipid transport: Lessons from a Na+/K+-pump. Biochim. Biophys. Acta 1791, 603–611 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Hankins H. M., Baldridge R. D., Xu P., Graham T. R., Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic 16, 35–47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan J. H., Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Coleman J. A., Vestergaard A. L., Molday R. S., Vilsen B., Andersen J. P., Critical role of a transmembrane lysine in aminophospholipid transport by mammalian photoreceptor P4-ATPase ATP8A2. Proc. Natl. Acad. Sci. U.S.A. 109, 1449–1454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldridge R. D., Graham T. R., Two-gate mechanism for phospholipid selection and transport by type IV P-type ATPases. Proc. Natl. Acad. Sci. U.S.A. 110, E358–E367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vestergaard A. L., et al. , Critical roles of isoleucine-364 and adjacent residues in a hydrophobic gate control of phospholipid transport by the mammalian P4-ATPase ATP8A2. Proc. Natl. Acad. Sci. U.S.A. 111, E1334–E1343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montigny C., Lyons J., Champeil P., Nissen P., Lenoir G., On the molecular mechanism of flippase- and scramblase-mediated phospholipid transport. Biochim. Biophys. Acta 1861, 767–783 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Jensen M. S., et al. , Phospholipid flipping involves a central cavity in P4 ATPases. Sci. Rep. 7, 17621 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gantzel R. H., et al. , Disease mutations reveal residues critical to the interaction of P4-ATPases with lipid substrates. Sci. Rep. 7, 10418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pintschovius J., Fendler K., Bamberg E., Charge translocation by the Na+/K+-ATPase investigated on solid supported membranes: Cytoplasmic cation binding and release. Biophys. J. 76, 827–836 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadini Buoninsegni F., Bartolommei G., Moncelli M. R., Inesi G., Guidelli R., Time-resolved charge translocation by sarcoplasmic reticulum Ca-ATPase measured on a solid supported membrane. Biophys. J. 86, 3671–3686 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tadini-Buoninsegni F., Bartolommei G., Moncelli M. R., Guidelli R., Inesi G., Pre-steady state electrogenic events of Ca2+/H+ exchange and transport by the Ca2+-ATPase. J. Biol. Chem. 281, 37720–37727 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., et al. , High-yield heterologous expression of wild type and mutant Ca2+ ATPase: Characterization of Ca2+ binding sites by charge transfer. J. Mol. Biol. 391, 858–871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tadini-Buoninsegni F., et al. , ATP dependent charge movement in ATP7B Cu+-ATPase is demonstrated by pre-steady state electrical measurements. FEBS Lett. 584, 4619–4622 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tadini-Buoninsegni F., Bartolommei G., Moncelli M. R., Fendler K., Charge transfer in P-type ATPases investigated on planar membranes. Arch. Biochem. Biophys. 476, 75–86 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Coleman J. A., Kwok M. C., Molday R. S., Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J. Biol. Chem. 284, 32670–32679 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cacciagli P., et al. , Disruption of the ATP8A2 gene in a patient with a t(10;13) de novo balanced translocation and a severe neurological phenotype. Eur. J. Hum. Genet. 18, 1360–1363 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onat O. E., et al. , Missense mutation in the ATPase, aminophospholipid transporter protein ATP8A2 is associated with cerebellar atrophy and quadrupedal locomotion. Eur. J. Hum. Genet. 21, 281–285 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martín-Hernández E., et al. , New ATP8A2 gene mutations associated with a novel syndrome: Encephalopathy, intellectual disability, severe hypotonia, chorea and optic atrophy. Neurogenetics 17, 259–263 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Alsahli S., Alrifai M. T., Al Tala S., Mutairi F. A., Alfadhel M., Further delineation of the clinical phenotype of cerebellar ataxia, mental retardation, and disequilibrium syndrome type 4. J. Cent. Nerv. Syst. Dis. 10, 1179573518759682 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young H. S., Rigaud J.-L., Lacapère J.-J., Reddy L.-G., Stokes D. L., How to make tubular crystals by reconstitution of detergent-solubilized Ca2+-ATPase. Biophys. J. 72, 2545–2558 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh D., “Phospholipid pKas” in Handbook of Lipid Bilayers, Marsh D., Ed. (CRC Press, Boca Raton, FL, ed. 2, 2013), pp. 157–163. [Google Scholar]
- 25.Mikkelsen S. A., et al. , Asparagine 905 of the mammalian phospholipid flippase ATP8A2 is essential for lipid substrate-induced activation of ATP8A2 dephosphorylation. J. Biol. Chem. 294, 5970–5979 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong J. P., Reboul E., Molday R. S., Kast J., A carboxy-terminal affinity tag for the purification and mass spectrometric characterization of integral membrane proteins. J. Proteome Res. 8, 2388–2396 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford M. M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- 28.Pintschovius J., Fendler K., Charge translocation by the Na+/K+-ATPase investigated on solid supported membranes: Rapid solution exchange with a new technique. Biophys. J. 76, 814–826 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tadini-Buoninsegni F., Nassi P., Nediani C., Dolfi A., Guidelli R., Investigation of Na+,K+-ATPase on a solid supported membrane: The role of acylphosphatase on the ion transport mechanism. Biochim. Biophys. Acta 1611, 70–80 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Tadini-Buoninsegni F., Bartolommei G., Electrophysiological measurements on solid supported membranes. Methods Mol. Biol. 1377, 293–303 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Bazzone A., Barthmes M., Fendler K., SSM-based electrophysiology for transporter research. Methods Enzymol. 594, 31–83 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Schulz P., Garcia-Celma J. J., Fendler K., SSM-based electrophysiology. Methods 46, 97–103 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.