Summary
Determining how neurotransmitter input causes various neuronal activities is crucial to understanding neuronal information processing. In Caenorhabditis elegans, AIY interneurons receive several sources of sensory information as glutamate inputs and regulate behavior by integrating these inputs. However, the relationship between glutamate input and the Ca2+ response in AIY under environmental noise, in other words, without explicit stimulation, remains unknown. Here, we show that glutamate-input fluctuations evoke a sporadic Ca2+ response in AIY without stimulation. To ensure that Ca2+ response can be considered AIY output, we show that the membrane-potential depolarization precedes Ca2+ responses in AIY. We used an odor as model stimulation to modulate the sensory inputs. Simultaneous imaging of glutamate input and Ca2+ response, together with glutamate transmission mutants, showed that glutamate-input fluctuations evoke sporadic Ca2+ responses. We identified the input-output relationships under environmental noise in vivo, and our results address the relationship between sensory-input fluctuations and behavioral variability.
Subject Areas: Neuroscience, Systems Neuroscience, Sensory Neuroscience
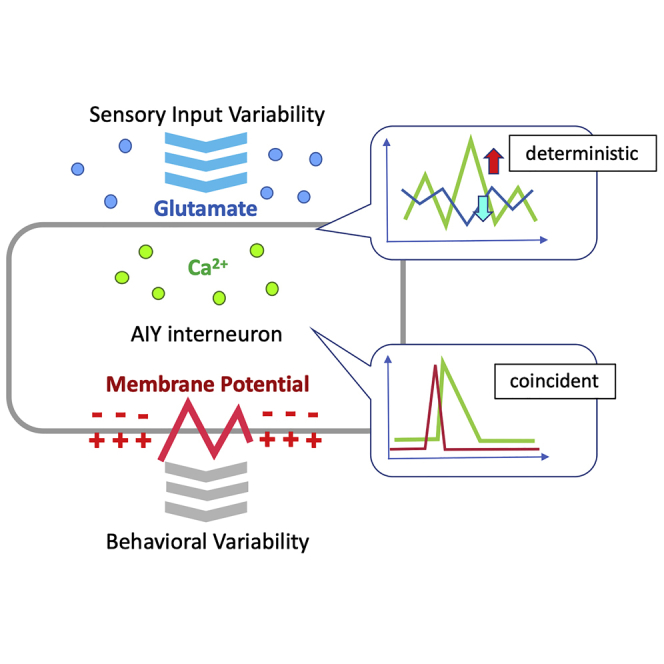
Graphical Abstract
Highlights
-
•
Glutamate-input fluctuation evokes a sporadic Ca2+ response in AIY
-
•
The membrane-potential depolarization precedes Ca2+ responses in AIY
-
•
Ca2+ increases in AIY when the glutamate input decreases, and vice versa
-
•
Mutation of glutamate signaling abolished Ca2+ response in AIY
Neuroscience; Systems Neuroscience; Sensory Neuroscience
Introduction
Information processing from sensory inputs to regulate behavior in noisy environments is a fundamental topic in neuroscience. To improve our understanding of such information processing, the relationship between the noise level of the stimulation and animal perception has been studied in depth (Faisal et al., 2008, Nienborg and Cumming, 2009, Renart and Machens, 2014). However, at the neuronal level these relationships remain largely unclear. Although the input-output relationship in neurons has also been thoroughly investigated (Augustine et al., 2003, Cichon and Gan, 2015, Destexhe, 2011, Frick et al., 2004, Losonczy et al., 2008, Ogawa and Oka, 2015, Stuart and Spruston, 2015), it remains unknown how neurotransmitter inputs affect postsynaptic neuronal activity in vivo in noisy environments, because simultaneous measurement of input and output in vivo is technically difficult due to the complexity of the networks involved (Ogawa and Oka, 2015, Prešern et al., 2015, Stuart and Spruston, 2015).
The nematode Caenorhabditis elegans is a suitable model for investigating this question. It has a simple neuronal circuit (White et al., 1986), as well as optical transparency, making it well suited for visualization with various imaging techniques (Kerr and Schafer, 2006). The AIY interneurons of C. elegans are particularly suitable for this purpose; they receive various sensory inputs, including gustatory, olfactory, and thermal information (Chalasani et al., 2007, Clark et al., 2006, Satoh et al., 2014), which regulate the animal's behavior following integration (Kocabas et al., 2012, Li et al., 2014, Satoh et al., 2014). Furthermore, AIY interneurons show a sporadic Ca2+ response regardless of the presence of explicit stimulation (Chalasani et al., 2007, Clark et al., 2006), whereas sensory neurons show a deterministic Ca2+ response to environmental stimulation (Chalasani et al., 2007, Clark et al., 2006, Kato et al., 2014, Tsukada et al., 2016). Both glutamate receptor mutations and the ablation of sensory neurons have been shown to abolish the Ca2+ response to sensory stimulation in AIY (Chalasani et al., 2007, Clark et al., 2006). This suggests that glutamate input to AIY can evoke the sporadic Ca2+ response as a result of sensory input integration under natural noise.
We used several simultaneous fluorescence imaging techniques to identify the input-output relationship in AIY in a noisy environment in vivo. To ensure that Ca2+ spikes could be considered AIY output, we first showed that depolarization of the membrane potential precedes Ca2+ spikes, by simultaneously imaging both in AIY. We used an odor, isoamyl alcohol (IAA) as model stimulation to modulate the sensory inputs, and simultaneous imaging of glutamate input and the Ca2+ response showed that glutamate input decreases when the Ca2+ spikes occur, with or without odor stimulation, and vice versa. We also investigated these relationships in both the glutamate receptor and glutamate-defective mutants and showed that fluctuations in glutamate input evoke the sporadic Ca2+ response in AIY. As far as we know, this is the first report identifying the input-output relationship for fluctuations under natural environmental noise in vivo. Glutamate inputs represent sensory inputs, and AIY neurons have been reported to regulate behavior; our results thus suggest that fluctuation in sensory input induces behavioral variability.
Results
Membrane-Potential Spike Precedes Ca2+ Response in AIY
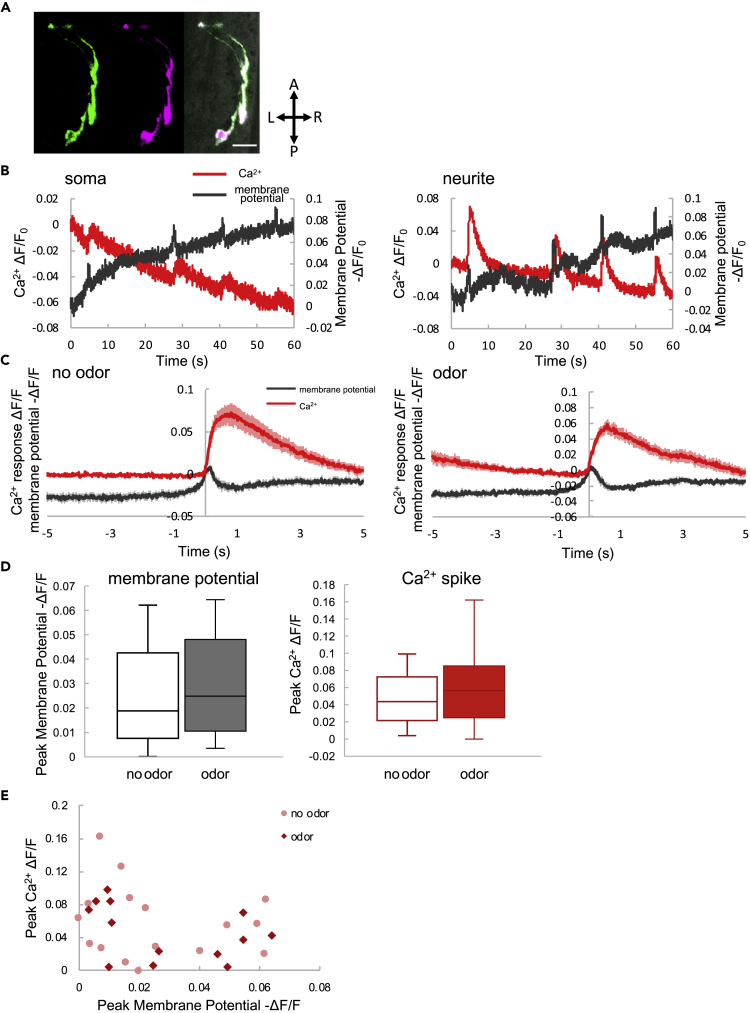
To quantify the input-output relationship in a single neuron in vivo, we investigated AIY interneurons in C. elegans. AIY neurons receive environmental information from sensory neurons via glutamate as a neurotransmitter and show a large sporadic Ca2+ response (hereafter, a “spike”) as a result of sensory integration (Chalasani et al., 2007, Clark et al., 2006). In C. elegans, Ca2+ is thought to be the main charge carrier in membrane-potential changes, due to the lack of voltage-gated Na+ channels in its neurons (Faumont et al., 2011, Faumont et al., 2006, Gao and Zhen, 2011, Goodman et al., 1998, Mellem et al., 2008, Shidara et al., 2013). Recently, the relationship between membrane potential and Ca2+ channels has been thoroughly investigated, and it has been shown that Ca2+ channels are necessary for membrane-potential spikes (Liu et al., 2018, Shindou et al., 2019). Ca2+ responses in AIY regulate the animal's behavior (Li et al., 2014), so the results of information processing should be expressed as membrane-potential changes. However, the relationship between Ca2+ and membrane potential in AIY remains unclear (Liu et al., 2018, Liu et al., 2009, Shidara et al., 2013), because it has not been measured simultaneously in AIY. We therefore measured both simultaneously, using a genetically encoded voltage indicator, ArcLight (Jin et al., 2012), and a genetically encoded Ca2+ indicator, R-GECO (Zhao et al., 2011) (Figure 1A). As previously suggested (Faumont et al., 2012), changes in membrane potential appeared as a spike-like response under ArcLight fluorescence, similar to a Ca2+ spike (Figure 1B). Importantly, these membrane-potential spikes were coincident with Ca2+ spikes. Membrane-potential changes were detected at the neurite and even at the soma, as reported in our earlier study (Shidara et al., 2013).
Figure 1.
Simultaneous Imaging of Membrane Potential and Ca2+ in AIY Interneurons
(A) Confocal image of the voltage indicator (ArcLight: green, left) and Ca2+ indicator (R-GECO magenta, middle). The merged image is on the right. Scale bar, 10 μm. A, anterior; L, left; P, posterior; R, right.
(B) Representative results of Ca2+ (red) and membrane potentials (black) at the soma (left) and neurite (right).
(C) Averaged Ca2+ spike (red) and membrane-potential spike (black) without (left) and with (right) odor. The shadows around the solid lines indicate the standard error of the mean (SEM) (no odor, N = 20, n = 16; odor, N = 10, n = 15).
(D) Membrane potential (left) and Ca2+ spike (right) intensities, with (filled boxes) and without (hollow boxes) odor. Box plots include the median (center line), quartiles (boxes), and range (whiskers). The statistical metrics are as follows: membrane potential, p = 0.38; Ca2+, p = 0.85; paired t tests (no odor, N = 20, n = 16; odor, N = 10, n = 15).
(E) Correlation between Ca2+ spike amplitude and membrane-potential spike amplitude. The red diamonds and pink dots are the responses in the presence and absence of odor stimulation, respectively. The statistical metrics are as follows: no odor, Pearson correlation coefficient (PCC) = −0.19, p = 0.48; odor, PCC = −0.40, p = 0.14 (no odor, N = 20, n = 16; odor, N = 10, n = 15).
To investigate the relationship between membrane-potential spikes and Ca2+ spikes, we collected and summarized the data (Figure 1C). Membrane-potential spikes preceded Ca2+ spikes, and their amplitudes were not modulated by odor presentation (Figure 1D). However, the amplitudes of Ca2+ spikes and membrane-potential spikes were not correlated (Figure 1E). Based on these results, membrane-potential changes precede Ca2+ changes. In subsequent analyses, therefore, we consider Ca2+ spikes to be AIY output.
Glutamate Input and Ca2+ Response to Odor Stimulation in AIY Interneurons
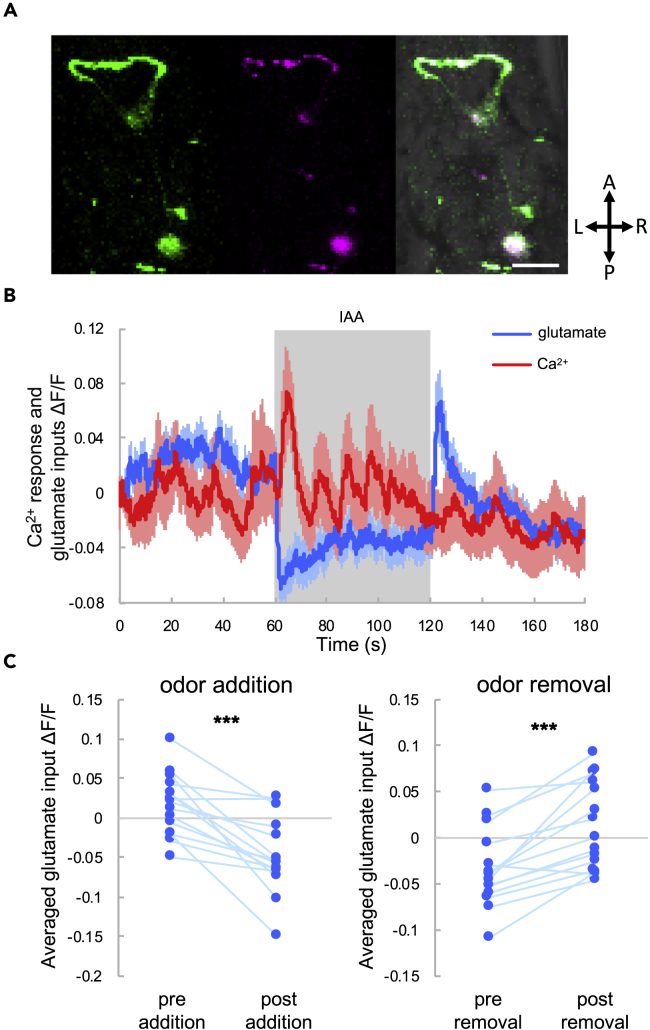
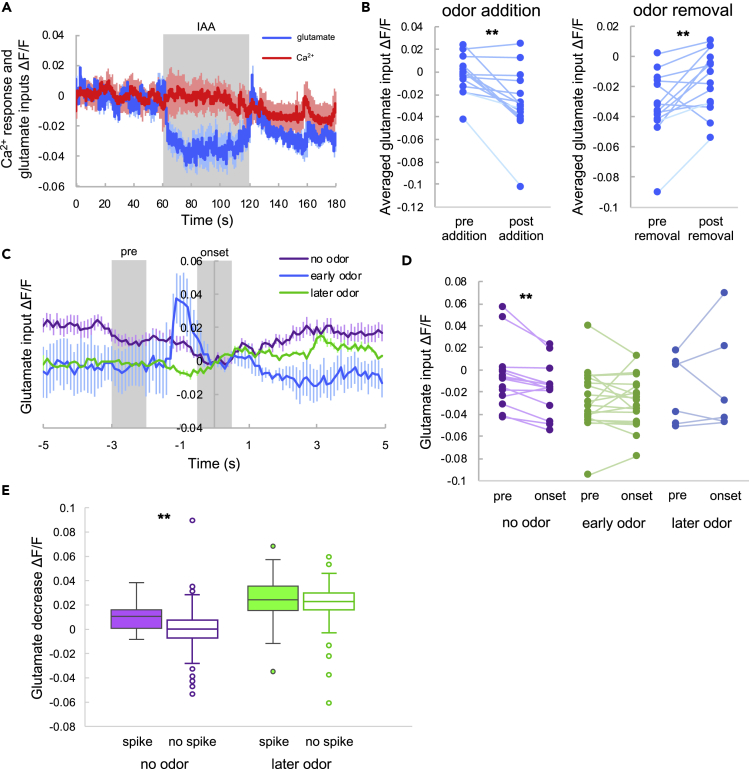
In AIY interneurons, glutamate input reflects environmental information from sensory neurons, and sporadic Ca2+ spikes occur as a result of sensory integration (Chalasani et al., 2007, Clark et al., 2006). These glutamate inputs emanate from gustatory, olfactory, and thermal sensory neurons (Chalasani et al., 2007, Clark et al., 2006, Satoh et al., 2014, White et al., 1986); therefore, in the absence of explicit changes of stimulation, glutamate inputs to AIY presumably reflect environmental noise, such as fluctuations in environmental factors such as temperature, local salt concentration, and olfactory molecules. AIY neurons receive inhibitory glutamate inputs via a glutamate-gated chloride channel, GLC-3 (Clark et al., 2006, Horoszok et al., 2001, Serrano-Saiz et al., 2013). AIY neurons exhibit Ca2+ spikes in response to odor stimulation because sensory neurons reduce their Ca2+ response and neurotransmitter release upon addition of odor (Chalasani et al., 2007, Ventimiglia and Bargmann, 2017) (Figure S1). Mutation of glc-3 or glutamate transmission abolishes Ca2+ spikes in response to odor in AIY neurons (Chalasani et al., 2007). Therefore, sporadic Ca2+ spikes in AIY could occur in response to variable glutamatergic inputs representing environmental noise. To demonstrate this directly, we simultaneously measured glutamatergic inputs and Ca2+ responses in AIY interneurons, using a genetically encoded glutamate indicator, iGluSnFR (Marvin et al., 2013), and a genetically encoded Ca2+ indicator, R-GECO (Zhao et al., 2011), respectively (Figure 2A).
Figure 2.
Simultaneous Imaging of Glutamate Inputs and Ca2+ Responses in AIY Interneurons
(A) Confocal image of the glutamate indicator (iGluSnFR: green, left) and Ca2+ indicator (R-GECO: magenta, middle) in AIY. The merged image is on the right. Scale bar, 10 μm. A, anterior; L, left; P, posterior; R, right.
(B) Averaged glutamate input (blue) and Ca2+ response (red) to odor. The shaded region indicates isoamyl alcohol (IAA) stimulation, and the shadows around the solid lines indicate the SEM.
(C) Averaged glutamate intensities before (pre-addition) and after (post-addition) odor addition (left). Glutamate intensities before (pre-removal) and after (post-removal) odor stimulation removal (right). The statistical metrics are as follows: left, p = 0.00010; right, p = 0.000086; paired t test (N = 15). ***p < 0.001.
As a previous study hypothesized that odor (IAA) stimulation could modify glutamate input to AIY (Chalasani et al., 2007), we selected IAA as model sensory stimulation. We measured glutamate input and the Ca2+ response with and without odor stimulation (Figure 2B). The glutamate input to AIY decreased following odor application and transiently increased following odor removal, in a deterministic manner (Figure 2C). These responses to odor correspond to the responses of sensory neurons (Chalasani et al., 2007, Shidara et al., 2017). Although there were deterministic changes in glutamate input, large Ca2+ spikes were observed even without odor, and often at the onset of odor stimulation, as reported previously (Chalasani et al., 2007). Odor application typically evoked Ca2+ spikes immediately in wild-type animals (see N2 in Figure 6C), suggesting that glutamate decreases, induced by odor application, evoke Ca2+ spikes. Moreover, odor presence increased the frequency of Ca2+ spikes (see N2 in Figure 6D). This suggests that reducing glutamate input via odor application causes Ca2+ spikes.
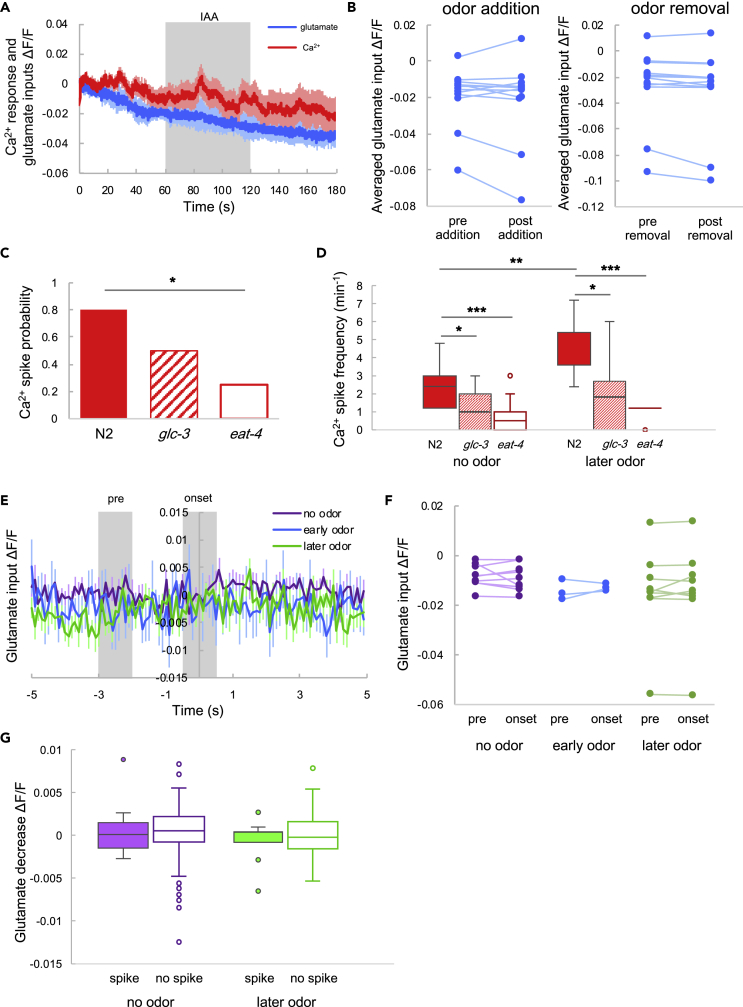
Figure 6.
Ca2+ Responses and Glutamate Inputs in eat-4 Mutants
(A) Averaged glutamate input (blue) and Ca2+ response (red) to odor. The shaded region indicates IAA stimulation and the shadows around the solid lines indicate the SEM (N = 12).
(B) Average glutamate intensity before (pre-addition) and after (post-addition) odor stimulation onset (left), and average intensity before (pre-removal) and after (post-removal) odor stimulation removal (right). Error bars indicate the SEM. The statistical metrics are as follows: left, p = 0.41; right, p = 0.055; paired t test (N = 12).
(C) Ca2+ spike probability following odor addition. Comparison between N2 animals and glc-3 mutants: p = 0.13; comparison between N2 animals and eat-4 mutants: p = 0.014. Fisher's exact test with Holm correction (N2, N = 15; glc-3, N = 12; eat-4, N = 12); *p < 0.05.
(D) Ca2+ spike frequency in N2 (filled), glc-3 (striped), and eat-4 mutants (hollow) with and without odor. Box plots indicate the median (center line), quartiles (boxes), and range (whiskers). The statistical metrics are as follows: N2 data comparison between no odor and later odor, p = 0.0045; glc-3 data comparison between no odor and later odor, p = 0.34; eat-4 data comparison between no odor and later odor, p = 0.34; Wilcoxon signed rank test. No odor data comparison between N2 and glc-3, p = 0.024; no odor data comparison between N2 and eat-4, p = 0.00044; later odor data comparison between N2 and glc-3, p = 0.014; later odor data comparison between N2 and eat-4, p = 0.000015; Wilcoxon rank-sum test. The Holm method was used for the correction (N2, N = 15; glc-3, N = 12; eat-4, N = 12). *p < 0.05, **p < 0.01, ***p < 0.001.
(E) Averaged glutamate responses coincident with Ca2+ spikes. Time 0 indicates the onset of the Ca2+ spike. All glutamate responses were normalized to zero at time 0. The purple, blue, and green lines indicate the responses under the no odor, early odor, and later odor conditions, respectively. The bars indicate the SEM (N = 12, no odor: n = 9; early odor, n = 3; later odor, n = 9).
(F) Glutamate input before Ca2+ spikes and at Ca2+ spike onset. “Pre” indicates the average intensity from −3 to −2 s in (E), and “onset” indicates the average intensity from −0.5 to +0.5 s. The statistical metrics are as follows: no odor: p = 0.60; early odor, p = 0.53; later odor, p = 0.50; paired t test (N = 12; no odor, n = 9; early odor, n = 3; later odor, n = 9).
(G) Averaged glutamate decreases (filled boxes) with and without (hollow boxes) Ca2+ spikes. Box plots indicate the median (center line), quartiles (boxes), and range (whiskers). The statistical metrics are as follows: no odor, p = 0.88; later odor, p = 0.48; Welch's t test (N = 12, no odor, spike, n = 9; no odor, no spike, n = 108; later odor, spike, n = 9; later odor, no spike, n = 100).
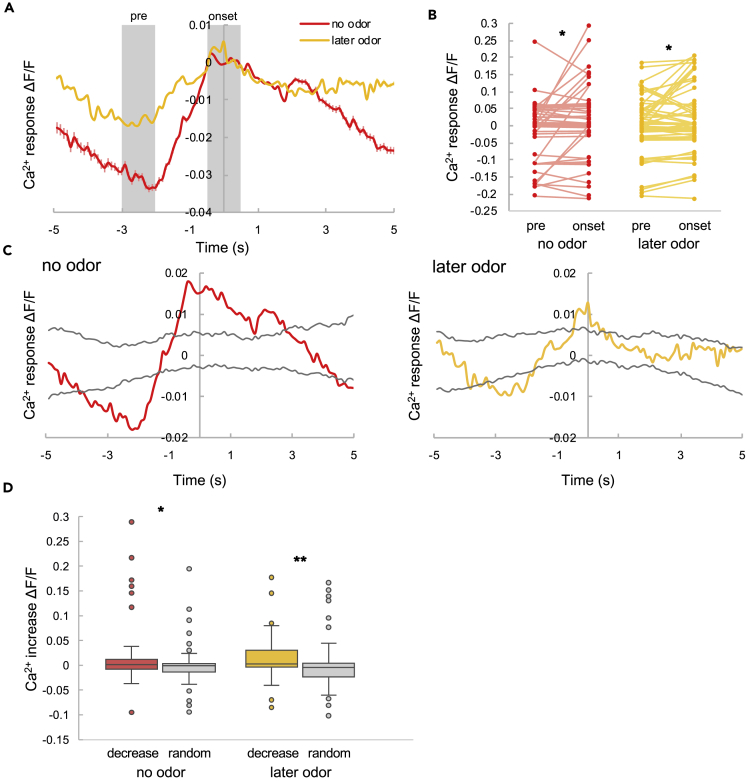
Glutamatergic Input Decreases when the Ca2+ Response Increases in AIY under Natural Noise, and Vice Versa
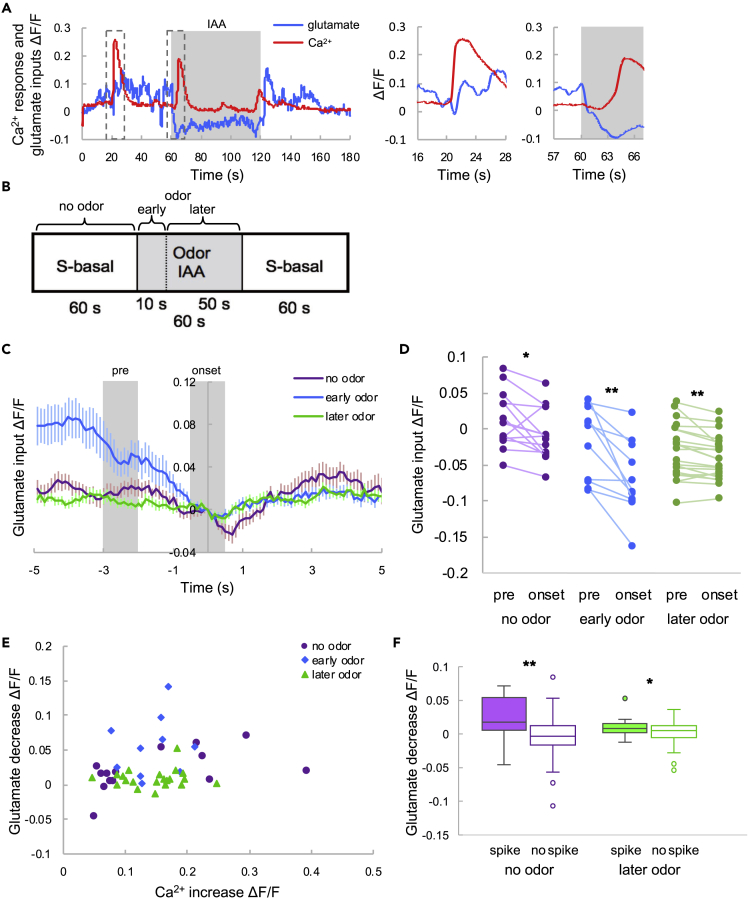
When quantifying the average response of glutamatergic input and Ca2+ in AIY to odor, we found that decreases in glutamatergic input appeared to be related to Ca2+ spikes (Figure 3A). To investigate this relationship further, we classified conditions into three categories: no odor, early odor, and later odor (Figure 3B); glutamatergic inputs were collected and aligned with the timing of Ca2+ spikes (Figure 3C). Glutamatergic inputs decreased before the Ca2+ spikes, regardless of odor presentation (Figure 3D). Decreasing levels of glutamate did not show a strong correlation with Ca2+ spike amplitude (Figure 3E) in any of the three conditions. These results imply that decreases in glutamatergic inputs are necessary to trigger Ca2+ spikes. We also compared the extent of glutamate decreases in the presence or absence of Ca2+ spikes (Figure 3F) and found that glutamate input decreased significantly when Ca2+ spikes occurred, with or without odor, which suggests that decreases in glutamate evoke Ca2+ spikes.
Figure 3.
Glutamate Input When the Ca2+ Spike Occurs
(A) Representative responses of Ca2+ and glutamate input (left). Magnifications of the dotted boxes showing the responses without (middle) and with (right) odor stimulation. The blue and red lines are the glutamate input and Ca2+ response, respectively. The shaded region indicates IAA stimulation.
(B) Definition of the response categories.
(C) The averaged glutamate responses coincident with Ca2+ spikes. Time 0 indicates the onset of Ca2+ spikes. All glutamate responses were normalized to zero at time 0. The purple, blue, and green lines indicate the responses under the no odor, early odor, and later odor conditions, respectively (defined in B). The error bars indicate the SEM (N = 15; no odor, n = 15; early odor, n = 11; later odor, n = 21).
(D) Glutamate inputs before the Ca2+ spike and at spike onset. “Pre” indicates the average intensity from −3 to −2 s in (C), and “onset” indicates the average intensity from −0.5 to +0.5 s. The statistical metrics are as follows: no odor, p = 0.010; early odor, p = 0.0024; later odor, p = 0.0037; paired t test (N = 15; no odor, n = 15; early odor, n = 11; later odor, n = 21). *p < 0.05, **p < 0.01.
(E) Correlation between glutamate decreases and Ca2+ increases. The purple dots, blue diamonds, and green triangles are correlations under the no odor, early odor, and later odor conditions, respectively. The statistical metrics are as follows: no odor, PCC = 0.58, p = 0.022; early odor, PCC = 0.080, p = 0.81; later odor, PCC = 0.069, p = 0.77 (no odor, n = 15; early odor, n = 11; later odor, n = 21).
(F) Glutamate decreases with and without Ca2+ spikes. The filled and hollow box plots indicate decreases with and without a spike, respectively. Box plots indicate the median (center line), quartiles (boxes), and range (whiskers). The statistical metrics are as follows: no odor, p = 0.0062; later odor, p = 0.037; Welch's t test (N = 15; no odor, spike, n = 15; no odor, no spike: n = 89; later odor, spike, n = 21; later odor, no spike, n = 69) *p < 0.05, **p < 0.01.
To support the idea that decreases in glutamate induce a Ca2+ response, we investigated whether Ca2+ increases when glutamate decreases. We collected and aligned Ca2+ traces with the timing of decreases in glutamatergic inputs (Figure 4A) and found that Ca2+ reliably increases when glutamatergic input decreases, with or without odor (Figure 4B). Moreover, the degree of increase was significantly larger than in the randomly resampled data (Figures 4C and 4D). These findings show that Ca2+ increases when glutamate decreases and support the idea that decreases in glutamate induce a Ca2+ response.
Figure 4.
Ca2+ Response to Decreases in Glutamate Input
(A) Ca2+ response to decreases in glutamate input. Time 0 indicates the time when the glutamate decreases. All Ca2+ responses were normalized to zero at time 0. The red and yellow lines indicate the response under the no odor and later odor conditions, respectively. The shadows indicate the SEM (for the Ca2+ response under later odor condition, the shadows are too small to see; N = 15; no odor, n = 43; later odor, n = 61).
(B) Ca2+ intensities before (“pre”; averaged from −3 to −2 s in A) and after (“onset”; averaged from −0.5 to +0.5 s in A) glutamate decrease. The statistical metrics are as follows: no odor, p = 0.014; later odor, p = 0.014; paired t test (N = 15; no odor, n = 43; later odor, n = 61). *p < 0.05.
(C) Ca2+ responses to decreases in glutamate input relative to randomly selected data with (right) and without odor (left). The gray lines indicate the averaged randomly selected data ± 3 SD (see Methods).
(D) Ca2+ increases with glutamate decreases (colored boxes) and by random selection (gray boxes). Box plots indicate the median (center line), quartiles (boxes), and range (whiskers). The statistical metrics are as follows: no odor, p = 0.045; later odor, p = 0.0015. Wilcoxon rank-sum test (N = 15; no odor, decrease, n = 43; no odor, random, n = 100; later odor, decrease, n = 61; later odor, random, n = 100). *p < 0.05, **p < 0.01.
The GLC-3 Receptor Primarily Mediates a Sporadic Ca2+ Response in AIY under Odor Stimulation
We demonstrated that glutamate decreases when Ca2+ increases, and vice versa. Previous research suggests that the glutamate-gated chloride channel, GLC-3, is crucial for the sensory-input-evoked Ca2+ response in AIY (Aoki et al., 2017, Chalasani et al., 2007, Horoszok et al., 2001, Ohnishi et al., 2011). Therefore, we investigated the relationship between Ca2+ and glutamate input in a glc-3 mutant. We detected the same type of changes in glutamate input following odor stimulation in glc-3 mutants as in wild-type (N2) animals (Figures 5A and 5B), and found that GLC-3 mutation had a limited effect on the Ca2+ response evoked by odor application (see glc-3 in Figure 6C). However, the Ca2+ spike frequency decreased significantly compared with N2, both with and without odor stimulation (see glc-3 in Figure 6D). Next, glutamatergic inputs were collected and aligned with the timing of Ca2+ spikes, as was done for the N2 animals (Figure 5C). Glutamate decreases were abolished under odor stimulation, but not in the absence of odor stimulation (Figure 5D). The extent of the decrease when accompanied by a Ca2+ spike was not significantly different from that without a Ca2+ spike under the odor stimulation, but was significantly larger in the absence of odor stimulation (Figure 5E). AIY neurons have a metabotropic glutamate receptor, MGL-1 (Jeong and Paik, 2017, Kang and Avery, 2009), which could play a role in initiating Ca2+ spikes. These results suggest that fluctuations in glutamate input via the GLC-3 receptor are important for the sporadic Ca2+ spikes.
Figure 5.
Ca2+ Responses and Glutamate Inputs in glc-3 Mutants
(A) Averaged glutamate inputs (blue) and Ca2+ responses (red) to odor. The shaded region indicates IAA stimulation, and the shadows around the solid lines indicate the SEM (N = 12).
(B) Glutamate intensities before (pre-addition) and after (post-addition) odor stimulation onset (left), and average intensities before (pre-removal) and after (post-removal) odor stimulation removal (right). The statistical metrics are as follows: left, p = 0.0030; right, p = 0.0018; paired t test (N = 12). **p < 0.01.
(C) Averaged glutamate responses coincident with Ca2+ spikes. Time 0 indicates the onset of Ca2+ spikes. All glutamate responses were normalized to zero at time 0. The purple, blue, and green lines indicate the responses under the no odor, early odor, and later odor conditions, respectively. Shadows indicate the SEM (N = 12, no odor, n = 15; early odor, n = 6; later odor, n = 20).
(D) Glutamate input before the Ca2+ spike and at spike onset. “Pre” indicates the average intensity from −3 to −2 s in (C), and “onset” indicates the average intensity from −0.5 to +0.5 s. The statistical metrics are as follows: no odor, p = 0.0012; early odor, p = 0.65; later odor, p = 0.89; paired t test (N = 12, no odor, n = 15; early odor, n = 6; later odor, n = 20). **p < 0.01.
(E) Averaged glutamate decreases with (filled boxes) and without (hollow boxes) Ca2+ spikes. Box plots indicate the median (center line), quartiles (boxes), and range (whiskers). The statistical metrics are as follows: no odor, p = 0.0027; later odor, p = 0.58; Welch's t test (N = 12, no odor, spike, n = 15; no odor, no spike, n = 114; later odor, spike, n = 20; later odor, no spike, n = 82). **p < 0.01.
Glutamate Input Evokes a Sporadic Ca2+ Response in AIY
We thus showed that the GLC-3 receptor plays a crucial role in the sporadic Ca2+ spikes. We used a glutamate-defective mutant, eat-4, to directly reveal the effect of glutamate input on AIY. EAT-4 is a vesicular glutamate transporter, and eat-4 mutants are defective in glutamate transmission (Chalasani et al., 2007, Lee et al., 1999). In contrast to the N2 animals and glc-3 mutants, the glutamate response following odor application and removal was abolished (Figures 6A and 6B). Moreover, the Ca2+ response to odor stimulation was also abolished (Figure 6C), and in fact the Ca2+ spike rarely occurred at all, with or without odor stimulation (Figure 6D). We collected and aligned glutamate input with the timing of Ca2+ spike initiation (Figure 6E), but no decreases were observed (Figure 6F), and the extent of decrease in the presence of a Ca2+ spike did not differ from that without a Ca2+ spike (Figure 6G). These results show that glutamate-input fluctuations evoke sporadic Ca2+ spikes in AIY neurons.
Discussion
In this article, we present two sets of simultaneous imaging investigations into the effects of environmental fluctuations on the interaction between Ca2+ and membrane potentials and Ca2+ and neurotransmitter (glutamate) inputs in AIY interneurons. By combining this approach with the use of mutant strains, we succeeded in determining the input-output relationship. We showed that membrane-potential spikes precede Ca2+ spikes in AIY, a previously unknown relationship. Moreover, decreases in glutamate in response to environmental fluctuations evoke sporadic Ca2+ spikes. We thus succeeded, for the first time, in identifying the relationship between input and output fluctuations in response to environmental fluctuations in vivo.
Glutamate inputs to AIY represent sensory neuronal outputs (Chalasani et al., 2007, Serrano-Saiz et al., 2013, White et al., 1986), and Ca2+ responses in AIY correlate with velocity and turn behavior (Li et al., 2014). Therefore, our results suggest that fluctuations in sensory input induce behavioral variability. It is difficult to address the relationship between sensory input and behaviors under environmental noise, because of the low signal-to-noise ratio (Faumont et al., 2012). Our results thus provide novel insights into the relationship between sensory-input fluctuation and behavioral variability.
In previous research, noise has been applied artificially (Nienborg and Cumming, 2009, Renart and Machens, 2014) and is thus not reflective of real environmental fluctuation. Controlling noise is an appropriate strategy for understanding perception, but it is not suitable for evaluating spontaneous variability. Here, we showed that Ca2+ spikes are caused by glutamate-input fluctuations. AIY neurons play an important role in sensory integration (Chalasani et al., 2007, Clark et al., 2006, Yoshida et al., 2012) and regulate behavior, and Ca2+ responses are correlated with reversal behavior and locomotion speed (Kocabas et al., 2012, Li et al., 2014). Therefore, changing behaviors under natural conditions are presumably caused by fluctuations in sensory input under environmental noise. Our approach of evaluating the input-output relationship in AIY under environmental fluctuations aids understanding of resting-state dynamics in complex neural circuits.
We identified the relationship between neurotransmitter inputs and the Ca2+ response. In previous research (Branco and Häusser, 2010, Callaway and Katz, 1993, Kramer et al., 2013), caged compounds and the stimulation of presynaptic neurons were used to investigate these relationships. However, such stimulation is often intense and artificial, and neurotransmitter inputs are not directly measurable. To address these shortcomings, we first demonstrated the relationship between neurotransmitter inputs and Ca2+ responses under naturally occurring physiological conditions, and then quantified the input-output relationships using simultaneous imaging of neurotransmitter inputs and neuronal activity.
Limitations of the Study
Here, we identified the relationship between glutamate input and the Ca2+ response. In the eat-4 mutants, however, the Ca2+ responses were rare but were not completely abolished. We acknowledge that other neurotransmitters, such as neuropeptides, also affect the Ca2+ response in AIY (Aoki et al., 2017, Kuhara et al., 2011, Rabinowitch et al., 2016). Although our results show that decreases in glutamate evoke Ca2+ responses in AIY under natural noise, cooperation between several neurotransmitters could modulate the responsiveness of AIY to glutamate inputs, which would modulate sensory information and the behavioral response.
In this study, we revealed the relationships with and without odor condition. However, it is difficult to generalize for the other sensory stimulation, and modulation of the other environmental modalities could have yielded different results. In addition to the different neurotransmitters involved, AIY neurons receive glutamate from several types of sensory neurons (Clark et al., 2006, Kuhara et al., 2011, Kunitomo et al., 2013, Serrano-Saiz et al., 2013, Wang et al., 2017, White et al., 1986). For example, high concentration of salt affects odor sensory neurons (Leinwand and Chalasani, 2013). So, the other sensory stimulation could change the input-output relationship in AIY.
We showed that mutation of glc-3 and eat-4 reduced the frequency of Ca2+ spikes. Because GLC-3 is a glutamate-gated chloride channel (Horoszok et al., 2001), ablation of the glutamate signal should depolarize the membrane potential of AIY neurons. However, shapes of Ca2+ spikes were identical (Figure S2). This indicates that AIY neurons retain the function of Ca2+ spike generation even without glutamate inputs. This suggests that the AIY neurons should have maintained their equilibrium potential even in the mutant animals because depolarization is necessary for generating Ca2+ spikes (Liu et al., 2018, Shindou et al., 2019). In the mutants, glutamate inputs could not cause the depolarization of membrane potential beyond the threshold in response to environmental fluctuations by glutamate inputs, so the Ca2+ spike frequency decreased. However, the mutations could change the physiological property in AIY; we could not dismiss these effects in the mutants.
We also noted that C. elegans does not have voltage-gated Na+ channels in its neurons (Faumont et al., 2011, Faumont et al., 2006, Gao and Zhen, 2011, Goodman et al., 1998, Mellem et al., 2008, Shidara et al., 2013). This fact indicates that the mechanism of spike generation should be different from that of the other animals. Therefore, it is difficult to directly extrapolate our conclusions beyond C. elegans.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Some plasmids for expression for the indicators were provided by a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan. We would like to thank Prof. Loren Looger and HHMI Janelia Farm for providing iGluSnFR plasmids. N2, glc-3, and eat-4 mutant strains were provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We would like to thank Prof. Cornelia I Bargmann at the Rockefeller University for providing strain and plasmid for unc-122::dsRed.
Author Contributions
K.A. designed the research. K.A. performed all experiments and analysis. K.A. wrote the first draft of the manuscript. K.A., K.O., and K. H. edited the manuscript. K.A., K.O., and K. H. contributed to data interpretation. K.O. and K. H. supervised the research.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.07.028.
Supplemental Information
References
- Aoki I., Nakano S., Mori I. Molecular mechanisms of learning in Caenorhabditis elegans. In: Byrne J., editor. Learning and Memory: A Comprehensive Reference. Elsevier; 2017. pp. 415–434. [Google Scholar]
- Augustine G.J., Santamaria F., Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Branco T., Häusser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr. Opin. Neurobiol. 2010;20:494–502. doi: 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Callaway E.M., Katz L.C. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc. Natl. Acad. Sci. U S A. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani S.H., Chronis N., Tsunozaki M., Gray J.M., Ramot D., Goodman M.B., Bargmann C.I. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- Cichon J., Gan W.-B. Branch-specific dendritic Ca2+ spikes cause persistent synaptic plasticity. Nature. 2015;520:180–185. doi: 10.1038/nature14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.A., Biron D., Sengupta P., Samuel A.D.T. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J. Neurosci. 2006;26:7444–7451. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A. Intracellular and computational evidence for a dominant role of internal network activity in cortical computations. Curr. Opin. Neurobiol. 2011;21:717–725. doi: 10.1016/j.conb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Faisal A.A., Selen L.P.J., Wolpert D.M. Noise in the nervous system. Nat. Rev. Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faumont S., Boulin T., Hobert O., Lockery S.R. Developmental regulation of whole cell capacitance and membrane current in identified interneurons in C. elegans. J. Neurophysiol. 2006;95:3665–3673. doi: 10.1152/jn.00052.2006. [DOI] [PubMed] [Google Scholar]
- Faumont S., Lindsay T., Lockery S. Neuronal microcircuits for decision making in C. elegans. Curr. Opin. Neurobiol. 2012;22:580–591. doi: 10.1016/j.conb.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faumont S., Rondeau G., Thiele T.R., Lawton K.J., McCormick K.E., Sottile M., Griesbeck O., Heckscher E.S., Roberts W.M., Doe C.Q., Lockery S.R. An image-free opto-mechanical system for creating virtual environments and imaging neuronal activity in freely moving Caenorhabditis elegans. PLoS One. 2011;6:e24666. doi: 10.1371/journal.pone.0024666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A., Magee J., Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat. Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Gao S., Zhen M. Action potentials drive body wall muscle contractions in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U S A. 2011;108:2557–2562. doi: 10.1073/pnas.1012346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M.B., Hall D.H., Avery L., Lockery S.R. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron. 1998;20:763–772. doi: 10.1016/s0896-6273(00)81014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horoszok L., Raymond V., Sattelle D.B., Wolstenholme A.J. GLC-3: a novel fipronil and BIDN-sensitive, but picrotoxinin-insensitive, L-glutamate-gated chloride channel subunit from Caenorhabditis elegans. Br. J. Pharmacol. 2001;132:1247–1254. doi: 10.1038/sj.bjp.0703937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H., Paik Y.-K. MGL-1 on AIY neurons translates starvation to reproductive plasticity via neuropeptide signaling in Caenorhabditis elegans. Dev. Biol. 2017;430:80–89. doi: 10.1016/j.ydbio.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Jin L., Han Z., Platisa J., Wooltorton J.R.A., Cohen L.B., Pieribone V.A. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 2012;75:779–785. doi: 10.1016/j.neuron.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Avery L. Systemic regulation of starvation response in Caenorhabditis elegans. Genes Dev. 2009;23:12–17. doi: 10.1101/gad.1723409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Xu Y., Cho C.E., Abbott L.F., Bargmann C.I. Temporal responses of C. elegans chemosensory neurons are preserved in behavioral dynamics. Neuron. 2014;81:616–628. doi: 10.1016/j.neuron.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr R.A., Schafer W.R. Intracellular Ca2+ imaging in C. elegans. Methods Mol. Biol. 2006;351:253–264. doi: 10.1385/1-59745-151-7:253. [DOI] [PubMed] [Google Scholar]
- Kocabas A., Shen C.-H., Guo Z.V., Ramanathan S. Controlling interneuron activity in Caenorhabditis elegans to evoke chemotactic behaviour. Nature. 2012;490:273–277. doi: 10.1038/nature11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R.H., Mourot A., Adesnik H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 2013;16:816–823. doi: 10.1038/nn.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhara A., Ohnishi N., Shimowada T., Mori I. Neural coding in a single sensory neuron controlling opposite seeking behaviours in Caenorhabditis elegans. Nat. Commun. 2011;2:355. doi: 10.1038/ncomms1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitomo H., Sato H., Iwata R., Satoh Y., Ohno H., Yamada K., Iino Y. Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nat. Commun. 2013;4:2210. doi: 10.1038/ncomms3210. [DOI] [PubMed] [Google Scholar]
- Lee R.Y., Sawin E.R., Chalfie M., Horvitz H.R., Avery L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J. Neurosci. 1999;19:159–167. doi: 10.1523/JNEUROSCI.19-01-00159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinwand S.G., Chalasani S.H. Neuropeptide signaling remodels chemosensory circuit composition in Caenorhabditis elegans. Nat. Neurosci. 2013;16:1461–1467. doi: 10.1038/nn.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu J., Zheng M., Xu X.Z.S. Encoding of both analog- and digital-like behavioral outputs by one C. elegans interneuron. Cell. 2014;159:751–765. doi: 10.1016/j.cell.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Hollopeter G., Jorgensen E.M. Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc. Natl. Acad. Sci. U S A. 2009;106:10823–10828. doi: 10.1073/pnas.0903570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Kidd P.B., Dobosiewicz M., Bargmann C.I. C. elegans AWA olfactory neurons fire calcium-mediated all-or-none action potentials. Cell. 2018;175:57–70.e17. doi: 10.1016/j.cell.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Losonczy A., Makara J.K., Magee J.C. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Marvin J.S., Borghuis B.G., Tian L., Cichon J., Harnett M.T., Akerboom J., Gordus A., Renninger S.L., Chen T.-W., Bargmann C.I. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellem J.E., Brockie P.J., Madsen D.M., Maricq A.V. Action potentials contribute to neuronal signaling in C. elegans. Nat. Neurosci. 2008;11:865–867. doi: 10.1038/nn.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienborg H., Cumming B.G. Decision-related activity in sensory neurons reflects more than a neuron’s causal effect. Nature. 2009;459:89–92. doi: 10.1038/nature07821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Oka K. Direction-specific adaptation in neuronal and behavioral responses of an insect mechanosensory system. J. Neurosci. 2015;35:11644–11655. doi: 10.1523/JNEUROSCI.1378-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi N., Kuhara A., Nakamura F., Okochi Y., Mori I. Bidirectional regulation of thermotaxis by glutamate transmissions in Caenorhabditis elegans. EMBO J. 2011;30:1376–1388. doi: 10.1038/emboj.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prešern J., Triblehorn J.D., Schul J. Dynamic dendritic compartmentalization underlies stimulus-specific adaptation in an insect neuron. J. Neurophysiol. 2015;113:3787–3797. doi: 10.1152/jn.00945.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch I., Laurent P., Zhao B., Walker D., Beets I., Schoofs L., Bai J., Schafer W.R., Treinin M. Neuropeptide-driven cross-modal plasticity following sensory loss in Caenorhabditis elegans. PLoS Biol. 2016;14:e1002348. doi: 10.1371/journal.pbio.1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart A., Machens C.K. Variability in neural activity and behavior. Curr. Opin. Neurobiol. 2014;25:211–220. doi: 10.1016/j.conb.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Satoh Y., Sato H., Kunitomo H., Fei X., Hashimoto K., Iino Y. Regulation of experience-dependent bidirectional chemotaxis by a neural circuit switch in Caenorhabditis elegans. J. Neurosci. 2014;34:15631–15637. doi: 10.1523/JNEUROSCI.1757-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Saiz E., Poole R.J., Felton T., Zhang F., De La Cruz E.D., Hobert O. Modular control of glutamatergic neuronal identity in C. elegans by distinct homeodomain proteins. Cell. 2013;155:659–673. doi: 10.1016/j.cell.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara H., Hotta K., Oka K. Compartmentalized cGMP responses of olfactory sensory neurons in Caenorhabditis elegans. J. Neurosci. 2017;37:3753–3763. doi: 10.1523/JNEUROSCI.2628-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara H., Kobayashi J., Tanamoto R., Hotta K., Oka K. Odorant-induced membrane potential depolarization of AIY interneuron in Caenorhabditis elegans. Neurosci. Lett. 2013;541:199–203. doi: 10.1016/j.neulet.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Shindou T., Ochi-Shindou M., Murayama T., Saita E., Momohara Y., Wickens J.R., Maruyama I.N. Active propagation of dendritic electrical signals in C. elegans. Sci. Rep. 2019;9:3430. doi: 10.1038/s41598-019-40158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G.J., Spruston N. Dendritic integration: 60 years of progress. Nat. Neurosci. 2015;18:1713–1721. doi: 10.1038/nn.4157. [DOI] [PubMed] [Google Scholar]
- Tsukada Y., Yamao M., Naoki H., Shimowada T., Ohnishi N., Kuhara A., Ishii S., Mori I. Reconstruction of spatial thermal gradient encoded in thermosensory neuron AFD in Caenorhabditis elegans. J. Neurosci. 2016;36:2571–2581. doi: 10.1523/JNEUROSCI.2837-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventimiglia D., Bargmann C.I. Diverse modes of synaptic signaling, regulation, and plasticity distinguish two classes of C. elegans glutamatergic neurons. Elife. 2017;6 doi: 10.7554/eLife.31234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Sato H., Satoh Y., Tomioka M., Kunitomo H., Iino Y. A gustatory neural circuit of Caenorhabditis elegans generates memory-dependent behaviors in Na+ chemotaxis. J. Neurosci. 2017;37:2097–2111. doi: 10.1523/JNEUROSCI.1774-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.G., Southgate E., Thomson J.N., Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. B Biol. Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Hirotsu T., Tagawa T., Oda S., Wakabayashi T., Iino Y., Ishihara T. Odour concentration-dependent olfactory preference change in C. elegans. Nat. Commun. 2012;3:739. doi: 10.1038/ncomms1750. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Araki S., Wu J., Teramoto T., Chang Y.-F., Nakano M., Abdelfattah A.S., Fujiwara M., Ishihara T., Nagai T., Campbell R.E. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.