Abstract
Introduction
Increased carotid-femoral pulse wave velocity (cf-PWV) in end-stage renal disease (ESRD) indicates enhanced aortic stiffness and mortality risk. We conducted a systematic review and meta-analysis of nonpharmacologic interventions in adults with ESRD to determine their effects on cf-PWV, systolic blood pressure (SBP), and intervention-associated adverse events.
Methods
MEDLINE, EMBASE, and EBM databases were searched. Study screening, selection, data collection, and methodological quality assessments were performed by 2 independent reviewers. Pooled-effect estimates from mean differences and 95% confidence intervals (CIs) were calculated using random effect models.
Results
A total of 2166 subjects with ESRD from 33 studies (17 randomized; 16 nonrandomized) were included. Four intervention-comparator pairs were meta-analyzed. Quality of evidence ranged from very low to moderate. Kidney transplantation decreased cf-PWV (−0.70 m/s; CI: –1.3 to −0.11; P = 0.02) and SBP (−8.3 mm Hg; CI: −13.2 to −3.3; P < 0.001) over pretransplantation. In randomized trials, control of fluid overload by bio-impedance reduced cf-PWV (−1.90 m/s; CI: −3.3 to −0.5); P = 0.02) and SBP (−4.3 mm Hg; CI: −7.7 to −0.93); P = 0.01) compared with clinical assessment alone. Cross-sectional studies also demonstrated significantly lower cf-PWV and SBP in normovolemia compared with hypervolemia (P ≤ 0.01). Low calcium dialysate decreased cf-PWV (−1.70 m/s; CI: −2.4 to −1.0; P < 0.00001) without affecting SBP (−1.6 mm Hg; CI: −8.9 to 5.8; P = 0.61). Intradialytic exercise compared with no exercise reduced cf-PWV (−1.13 m/s; CI: −2.2 to −0.03; P = 0.04), but not SBP (+0.5 mm Hg; CI: −9.5 to 10.4); P = 0.93).
Conclusions
Several nonpharmacologic interventions effectively decrease aortic stiffness in ESRD. The impact of these interventions on cardiovascular outcomes and mortality risk reduction in ESRD requires further study.
Keywords: aortic stiffness, end-stage renal disease, pulse wave velocity, renal dialysis, renal transplantation, vascular stiffness
Atherosclerosis and arteriosclerosis are both prominent in ESRD, and represent important risk factors for the high incidence of cardiovascular disease deaths in this population.1, 2, 3 Arteriosclerosis, in particular, is associated with increased aortic stiffness due to enhanced fibrosis, loss of elastic fibers, and extensive vessel wall calcification.3, 4, 5 In ESRD, elevated aortic stiffness increases SBP and pulse pressure, promoting left ventricular hypertrophy and reduced coronary perfusion.5, 6 Because the aorta is the principal capacitive element of the arterial tree, measurements of cf-PWV accurately reflect the central effects of increased aortic stiffness.5, 6, 7 Indeed, increased cf-PWV is strongly associated with adverse outcomes in ESRD,6, 7 with a rise of 1 m/s increasing adjusted nonfatal cardiovascular events and overall mortality rate by 15%.7 Dialysis patients with cf-PWV greater than 12.0 m/s are nearly 2 times more likely to die and/or develop nonfatal cardiovascular events compared with patients with values less than 8.8 m/s.7
Traditional cardiovascular risk factors, such as age, hypertension, diabetes mellitus, and arteriosclerosis, are overrepresented in chronic kidney disease and are considered to play an important role in the progression of aortic stiffness before development of ESRD.2, 8 Additional risk factors, however, that are unique to ESRD may account for the increase in aortic stiffness during the course of dialysis therapy.3, 4, 5, 6, 8 In chronic dialysis patients, chronic exposure to the effects of uremic toxins, fluid excess, abnormalities in bone mineral metabolism, and limited physical activity also may contribute to progression of aortic stiffness.4, 5, 6, 7, 8 Accordingly, in ESRD, nonpharmacologic strategies aimed at either restoration of renal function (i.e., kidney transplantation), strict control of fluid volume, correction of abnormalities in bone mineral metabolism, and enhanced physical activity have been adopted to decrease progression of aortic stiffness, using cf-PWV to monitor these responses.6, 7, 8, 9 We conducted a systematic review and meta-analysis of studies in ESRD to evaluate the effect of nonpharmacologic interventions that target aortic stiffness on cf-PWV. Second, we determined effects on SBP and intervention-associated adverse events.
Material and Methods
Data Sources and Search Strategy
The review was conducted in accordance with the Cochrane Collaboration methods, Systematic Reviews standards,10 and PRISMA guidelines.11 The study protocol has been previously published12 and registered in PROSPERO (www.crd.york.ac.uk/prospero) (CRD42016033463). A comprehensive, systematic search strategy (Supplementary Appendix S1) was implemented using MEDLINE, EMBASE, Cochrane Central databases, Cochrane Register of Controlled Trials, Cochrane Methodology Register, Health Technology Assessment Database, “Grey Matters Light” of the Canadian Agency for Drugs and Technologies in Health, OVID, EBM Reviews, and grey literature for studies published between January 1965 and March 2018. The original search strategy aimed to capture both pharmacologic and nonpharmacologic interventions that targeted aortic stiffness.
Study Screening, Inclusion, and Exclusion
All abstracts and titles were screened by 2 independent reviewers using prespecified criteria. Abstract selection was restricted to those published in English, French, Italian, or Spanish. Nonhuman, in vitro, modeling and pediatric studies or systematic/narrative reviews were excluded. Full-text eligible reports underwent screening of the Materials and Methods section to confirm that adult patients with ESRD (>18 years) were included, that cf-PWV was incorporated, and that an intervention on aortic stiffness was tested. One of the reviewers screened all full-text copies while a second reviewer randomly verified 75% of all reports. Selected reports underwent full-text review by 2 reviewers for final inclusion-decision using prespecified criteria (Supplementary Appendix S2). Eligible studies were then abstracted by 2 independent reviewers using a piloted, standardized electronic form. All disagreements were resolved by consensus and consultation with a third independent reviewer. If data from selected studies were incomplete, attempts were made to contact the principal study author.
Randomized controlled trials and nonrandomized studies (cohort, case-control, cross-sectional, and single cohorts with before-and-after design) involving adults (>18 years) with ESRD of any duration, receiving or not renal replacement therapy (hemodialysis, peritoneal dialysis, transplantation) were included provided that 10 or more participants received the intervention and its effect was assessed by cf-PWV. We distinguished between pharmacologic and nonpharmacologic interventions. The impact of pharmacologic interventions on cf-PWV will be the subject of a separate review and analysis. Kidney transplantation as nonpharmacologic intervention was studied when it was compared with dialysis therapy in before-and-after study designs and/or prospective cohort or cross-sectional studies.
Outcomes
The primary outcome was reduction in cf-PWV by the nonpharmacologic intervention, and secondary outcomes included effects on SBP and incidence of intervention-associated adverse events.
Methodological Quality
The risk of bias was evaluated by 2 independent reviewers using the Cochrane Collaboration tool in randomized studies.10 For nonrandomized studies, we used “SIGN50” for cohort and case-control studies13 and the National Institutes of Health Quality assessment tool for cross-sectional studies and single cohorts with before/after design.14 Specific coding instructions were provided to reviewers and were piloted before implementation.
Statistical Analyses
Mean differences between end-of-treatment and pretreatment baseline cf-PWV were computed using the reported means and SDs. If different measures of central tendency and distribution were available, means and SD were estimated according to algorithms described by Luo et al.15 and Wan et al.16 Subsequently, weighted mean differences between intervention and comparator were estimated using the final number of participants for each arm of the study. When appropriate, pooled mean differences and 95% CIs were calculated for each intervention using the method of the inverse variance and data were modeled according to the DerSimonian-Laird Method17 (random effects model) (P < 0.05). To reduce “double-counting” error in crossover studies and single cohorts with before/after design, 50% of the total number of study participants were included in each study arm. Statistical heterogeneity was reported by the I2 test. Intergroup differences were analyzed using the Cochrane χ2 test with P ≤ 0.10. Publication bias was investigated if the number of studies per intervention was ≥10. All analyses were performed using RevMan 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). To explore clinical and statistical heterogeneity, effect estimates were reported from different subgroups classified according to mean age and treatment duration. Sensitivity analyses comprised examination of effect model, parameter estimates, study design, and methodological quality.
Quality of Evidence
Two reviewers evaluated quality of evidence according to 5 domains of GRADE recommendations.18 Quality was reported as very low, low, moderate, or high for each of the outcome measures according to each intervention.
Results
Search Results
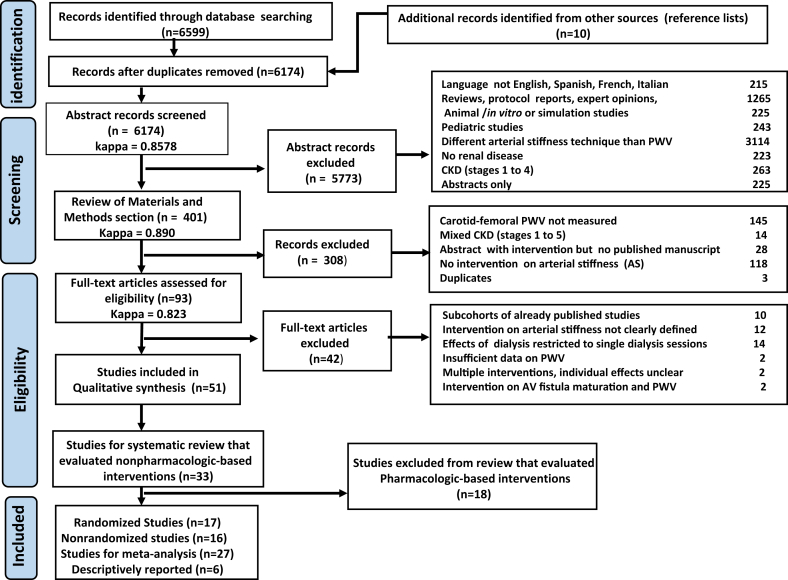
The search strategy identified 6609 citations (Figure 1). After initial screening, 93 reports remained for full-text screening and abstraction. Of these, 51 studies that evaluated at least 1 intervention on cf-PWV were selected, including 33 reports (17 randomized; 16 nonrandomized) associated with nonpharmacologic interventions, and 18 studies (13 randomized and 5 nonrandomized) with pharmacologic interventions. Publication years ranged from 1989 to 2018, with 45 reports (88%) since 2000. Based on clinical and methodological features, 27 of the 33 reports dealing with nonpharmacologic therapies were appropriate for meta-analysis and classified into 4 different interventions (Table 1). Three additional reports were reported descriptively. We did not identify any citation that specifically assessed the long-term effects of frequent hemodialysis on cf-PWV as a strategy to decrease the progression of aortic stiffness in ESRD. In addition, 3 studies that assessed the effects of dialysis modality were not included in the primary intervention groups and are reported descriptively. These citations included 1 randomized study19 reporting that hemodiafiltration (n = 103) did not have a significant effect on cf-PWV compared with hemodialysis (n = 86); a randomized crossover study20 showing that 24 weeks of hemodialysis treatment with high-flux polyamide membranes (n = 23) decreased cf-PWV compared with low-flux polyamide membranes (n = 19); and another randomized trial that found no difference in cf-PWV between low-flux hemodialysis (n = 14) and predilution online hemofiltration (n = 13).21
Figure 1.
PRISMA flowchart. AV, arteriovenous; CKD, chronic kidney disease; PWV, pulse wave velocity.
Table 1.
Studies included in the quantitative analysis classified by contribution to the tested intervention
| Author, reference | Study design | Qualitya | Intervention (n) | Comparator (n) | Age (yr) intervention; comparator |
Exposure (mo) | Dialysis vintage (mo) intervention; comparator |
|---|---|---|---|---|---|---|---|
| 1. Kidney transplantation | |||||||
| Bachelet-Rousseau et al.22 | Cohort, before/after | Acceptableb | KT (n = 39) | Transplant wait-list (on HD or PD) (n = 49) |
55 ± 8; 57 ± 17 |
12 | 21 ± 16; 16 ± 15 |
| Ignace et al.23 | Cohort, before/after | Goodc | KT (n = 52) | Pretransplant HD (48%), PD: 39%, ND: 13% |
50 ± 13 | 3 | 86 ± 74 |
| Kaur et al.24 | Cohort, before/after | Fairc | KT (n = 23) | Pretransplantation HD (86%), PD: 4%, |
36 ± 9 | 3 | 24 ± 19 |
| Zoungas et al.25 | Cohort, before/after | Poorc | KT (n = 31) | Pretransplantation | 46 ± 11 | 12 | 26 ± 28 |
| Stompor et al.26 | Cohort, before/after | Unacceptableb | KT (n = 10) | Transplant wait-list (on PD) (n = 9) |
39 ± 11; 42 ± 9 |
12 | Unreported |
| Keven et al.27 | Cohort, before/after | Unacceptableb | KT (n = 28) | HD (n = 23) | 34 ± 9; 36 ± 11 |
12 | 40 ± 35; 52 ± 20 |
| Hornum et al.28 | Cohort, before/after | Unacceptableb | KT (n = 40) | HD and PD (n = 40) | 38 ± 13; 47 ± 11 |
12 | 28 ± 32; 46 ± 39 |
| Covic et al.29 | Cross sectional | Unacceptableb | KT (n = 20) | HD (n = 41) | 40; 42 |
3 | 39 ± 24; 42 ± 39 |
| Pan et al.30 | Cross sectional | Unacceptableb | KT (n = 20) | HD (n = 20) | 57 ± 11; 43 ± 3 |
12 | 38 ± 4; 39 ± 4 |
| Posadzy- Malaczyñska et al.31 | Cross sectional | Unacceptableb | KT (n = 35)d | HD (n = 35) | 44 ± 2; 43 ± 3e |
44 ± 6 after transplant | 39 ± 3; 38 ± 4e |
| 2. Control of extracellular fluid volume | |||||||
| Onofriescu et al.32 | Parallel RCT (1 center) | Low risk biasf | Bio-electrical impedance guided UF (n = 71) | Clinically guided UF (n = 64) | 52 ± 13 | 12 | 59 ± 60 |
| Hur et al.33 | Parallel RCT (2 centers) | Unclear risk biasf | Bio-electrical impedance guided UF (n = 64) | Clinically guided UF (n = 62) | 51 ± 13; 52 ± 11 |
12 | 64 ± 46; 60 ± 44 |
| Onofriescu et al.34 | Parallel RCT (1 center) | Unclear risk biasf | Bioelectrical impedance guided UF (n = 62) | Clinically guided UF (n = 69) | 52 ± 13; 54 ± 13 |
30 | 107 ± 60; 104 ± 57 |
| Lin et al.35 | Cross sectional | Fairc | Normovolemia ECF/ICF: ≤95th percentile (n = 107) |
Hypervolemia ECF/ICF: > 95th percentile (n = 50) |
56 ± 17; 57 ± 11 |
N/A | 37 ± 42; 64 ± 61 |
| Bia et al.36 | Cross sectional | Poorc | Normovolemia (OH/ECF: <15%) (n = 40) | Hypervolemia (OH/ECF: >15%) (n = 12) | 56 ± 17; 65 ± 12 |
N/A | 72 ± 59; 68 ± 73 |
| Kocyigit et al.37 | Cross sectional | Poorc | Normovolemia FO 10th to 90th percentile (n = 35) |
Hypervolemia FO > 90th percentile (n = 25) |
45 ±11; 49 ±16 |
N/A | 42 ± 33; 39 ± 34 |
| Mitsides et al.38 | Cross sectional | Poorc | Normovolemia OH/ECW < 7% (n = 30) |
Hypervolemia OH/ECW >7% (n = 42) |
53 ± 16; 60 ± 12 |
N/A | 64 ± 66; 79 ± 98 |
| Siriopol et al.39 | Parallel RCT | High-risk biasf | Lung-US + bio-impedance–guided UF (n = 119) | Clinically guided UF (n = 122) | 59 ± 15; 59 ± 13 |
24 | 50 ±54; 48 ± 53 |
| Liu et al.40 | Parallel RCT | Low-risk biasf | Low sodium dialysate (136 mmol/l) (n = 28) | Standard sodium dialysate (138 mmol/l) (n = 29) |
59 ± 10; 57 ± 11 |
12 | 53 ± 65; 63 ± 74 |
| 3. Low calcium dialysate | |||||||
| LeBeouf et al.41 | Random Latin square crossover | Unclear risk biasf | Low calcium (1.0 mmol/l) (n = 18) |
High calcium (1.50 mmol/l) (crossover) |
49 ± 18 | 1 dialysis session × treatment | 20 ± 24 |
| LeBeouf et al.42 | Parallel RCT | Unclear risk biasf | Low calcium (1.12 mmol/l) (n = 14) |
High calcium (1.37 mmol/l) (n = 13) |
68 ± 12; 66 ± 13 |
6 | 6 ± 4; 6 ± 4 |
| Masterson et al.43 | Parallel RCT | Unclear risk biasf | Low calcium (1.3 mmol/l) (n = 22) |
High calcium (1.6 or 1.75 mmol/l) (n = 20) |
53 ± 21; 48 ± 11 |
12 | 25 ± 29g; 16 ± 18g |
| Marchais et al.44 | Parallel RCT | High risk biasf | Low calcium (1.50 mmol/l) (n = 13) |
High calcium (1.75 mmol/l) (n = 13) |
Not reported | Single dialysis | Not reported |
| Moor et al.45 | Randomized crossover | High risk biasf | Low calcium (0.8–1.0 mmol/l) (n = 15) |
High calcium (1.1–1.4 mmol/l) crossover | 54 ± 16 | 1 dialysis session × treatment | 32 ± 37h |
| He et al.46 | Parallel RCT | High risk biasf | Low calcium (1.25 mmol/l) (n = 64) |
High calcium (1.50 mmol/l) (n = 64) |
57 ± 12; 56 ± 12 |
24 | 43 ± 33; 43 ± 42 |
| Kim et al.47 | Cohort, before/after | Fairc | Low calcium (1.5 mmol/l) (n = 20) |
High calcium (1.75 mmol/l) | 63 ± 12 | 6 | 38 ± 10i |
| 4. Intradialytic exercise | |||||||
| Mihaescu et al.48 | Cohort before/after | Acceptableb | Intradialytic exercise (40 min, Borg 12–14) (n = 18) |
No exercise (n = 14) | 56 ± 9; 55 ± 11 |
3 | 54 ± 56; 55 ± 53 |
| Toussaint et al.49 | Randomized crossover | High-risk biasf | Intradialytic exercise (30 min) (n = 18) |
No exercise (crossover) | 68 ± 6i; 65 ± 13i |
3-mo treatment, 1-mo wash-out | 35 ± 31; 72 ± 56 |
| Koh et al.50 | Parallel RCT | Unclear risk biasf | Intradialytic exercise (Borg 12–13, 45 min) (n = 15) |
No exercise (n = 16) | 52 ± 11; 51 ± 14 |
6 | 32 ± 27; 26 ± 22 |
| Cooke et al.51 | Parallel RCT | High risk biasf | Intradialytic exercise (Borg 12–16, 43 min) (n = 10) |
No exercise (n = 10) | 58 ± 17; 53 ± 15 |
4 | Not reported |
ECF/ICF, extracellular fluid to intracellular fluid ratio; FO, absolute fluid overload; HD, hemodialysis; KT, kidney transplantation; NA, not applicable; ND, nondialysis; OH, overhydration; OH/ECF ratio, overhydration to extracellular fluid ratio; OH/ECW, overhydration index to extracellular water content; PD, peritoneal dialysis; RCT, randomized controlled trial; UF, ultrafiltration; US, ultrasonography.
Although tools for observational studies are specific to the methodological design, they are equivalent to the rating level of grading.13, 14
The “SIGN50” tool for assessing methodological quality in cohort studies: Interpretation: high quality (++): Majority of criteria met. Little or no risk of bias. Results unlikely to be changed by further research; Acceptable (+): Most criteria met. Some flaws in the study with an associated risk of bias. Conclusions may change in the light of further studies; Unacceptable = Low quality (0): Either most criteria not met, or significant flaws relating to key aspects of study design. Conclusions likely to change in the light of further studies.
National Institutes of Health quality assessment tool for cross-sectional studies and single cohort before-after (pre-post) studies with no control group. Interpretation: good quality: minimal risk of bias, low risk of measurement errors or other confounding factors that may results from “flaws” in the design or conduct of the study (equivalent to low risk of bias); fair quality; presence of some confounding, selection, information and measurement bias derived from some “flaws” in the design or conduct of the study; there is some doubt about the ability of the study to accurately assess an association between the intervention or exposure and outcome; poor quality: poor internal validity and high risk for “flaws” in the design or execution of the study. There is high doubt about the results reported in the study or the ability of the study to accurately assess an association between the intervention or exposure and the outcome (equivalent to high risk of bias).
Effect size: −0.33; 95% confidence interval: −1.03 to 0.37); P = 0.35.
Values are SE.
The Cochrane collaboration’s tool for assessing risk of bias in RCTs: Interpretation: Low risk of bias: plausible bias unlikely to seriously alter the results; unclear risk of bias: plausible bias that raises some doubt about the results; high risk of bias: plausible bias that seriously weakens confidence in the results.
Estimated from median and interquartile ranges.
Estimated from individual values.
Estimated from median and range values.
Kidney Transplantation
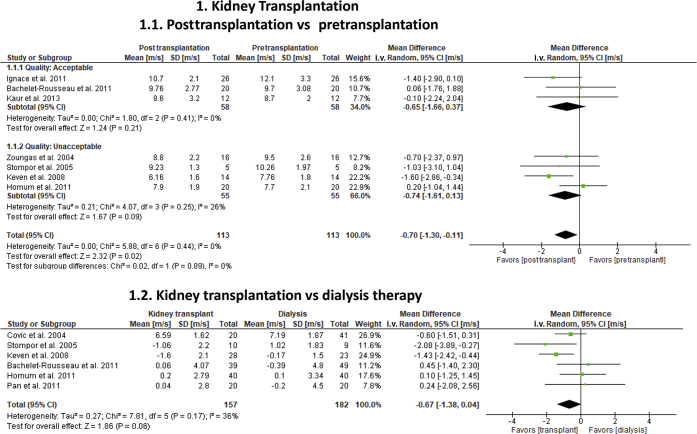
Nine of 10 eligible studies22, 23, 24, 25, 26, 27, 28, 29, 30 provided effect estimates for 2 separate meta-analyses to compare kidney transplantation with dialysis (Table 1; Figure 2). All studies were observational, and quality varied from good (1), to acceptable or fair (2), to unacceptable or poor (7). The first analysis included 223 kidney recipients from 7 transplant cohorts with measurements before and after transplantation (3 to 12 months).22, 23, 24, 25, 26, 27, 28 All studies reported cf-PWV unadjusted for changes in mean blood pressure, except for 2 reports23, 25 that provided both adjusted and unadjusted values. Kidney transplantation significantly decreased cf-PWV (−0.70 m/s; 95% CI: −1.3 to −0.11; P = 0.02) and reduced SBP (−8.3 mm Hg; 95% CI: −13.2 to −3.3; P < 0.001) over pretransplantation. Statistical heterogeneity was low (I2 = 0%) for both outcomes. A sensitivity analysis that included 2 adjusted cf-PWV values23, 25 abolished the effect of transplantation on cf-PWV (−0.35 m/s; 95% CI: −0.94 to 0.23; I2 = 6%; P = 0.23). Forrest plots suggested that there were differences in effect size and directionality between studies. Three studies (90 kidney recipients) showed a benefit of transplantation on cf-PWV (−1.43 m/s; 95% CI: −2.3 to −0.6; P < 0.001),23, 26, 27 whereas 4 others (138 recipients) did not (−0.08 m/s; 95% CI: −0.9 to 0.7; P = 0.84).22, 24, 25, 28 These subgroup differences modified the overall effect estimates (P = 0.03), but variations in study quality (P = 0.89) and time of posttransplant assessment did not (P = 0.63).
Figure 2.
Effect of kidney transplantation on carotid-femoral pulse wave velocity (cf-PWV) in end-stage renal disease. Analysis 1.1 included 113 kidney transplant recipients with cf-PWV measurements before and after transplantation. To reduce “double-counting” error in these studies, 50% of the total number of study participants was included in each comparative arm (before and after transplantation). Analysis 1.2 evaluated the effects of kidney transplantation over dialysis therapy in 157 transplant recipients and 182 dialysis patients matched by age and dialysis vintage. Analysis was stratified according to study quality. All cf-PWV values were nonadjusted for blood pressure. CI, confidence interval.
The second analysis comprised 6 cohort studies with 157 transplant recipients and 182 chronic dialysis subjects matched by age and dialysis vintage.22, 26, 27, 28, 29, 30 Kidney transplantation marginally reduced cf-PWV (−0.67 m/s; 95% CI: −1.4 to 0.1; P = 0.06), but not SBP (−2.4 mm Hg; 95% CI: −7.9 to 3.1; P = 0.39) compared with dialysis. A moderate statistical heterogeneity on cf-PWV (I2 = 36%) was associated with differences in effect size and directionality between studies. Three studies favored transplantation (−1.16 m/s; 95% CI: −1.9 to −0.4; P = 0.003),26, 27, 29 but 3 others did not (+0.22; 95% CI: −0.8 to 1.2; P = 0.66).22, 28, 30 These differences significantly modified overall effect estimates (P = 0.03). An additional report31 excluded from quantitative analyses had longer posttransplant assessments (44.2 ± 2 months) and showed no benefit of transplantation over dialysis (−0.33 m/s; 95% CI: −1.03 to 0.37; P = 0.35).
Control of Extracellular Fluid Volume
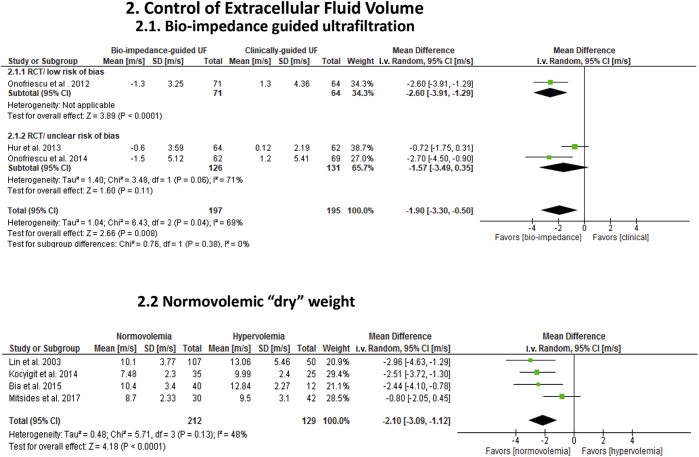
Three randomized trials (1 low risk; 2 unclear risk of bias) in hemodialysis patients evaluated the effect of bio-impedance (n = 197) to control extracellular fluid volume, compared with clinical and radiographic assessment (n = 195) (Table 1; Figure 3).32, 33, 34 Treatment duration varied from 1 year32, 33 to 2.5 years.34 In 2 reports published by a single center,32, 34 use of duplicate patient-specific data between studies could not be verified despite attempts to contact the investigators. These 2 studies were analyzed separately. All individual estimates favored bio-impedance over clinical assessment to improve cf-PWV. Overall, a significant reduction in cf-PWV (−1.90 m/s; 95% CI: −3.3 to −0.5; P = 0.008) and SBP (−4.3 mm Hg; 95% CI: −7.7 to −0.93; P = 0.01) occurred with bio-impedance compared with clinical measures. Statistical heterogeneity, however, was high for cf-PWV (I2 = 69%), and subgroup differences according to study center affected overall effect estimates (P = 0.008). Forrest plots indicated that the effect size was larger and wider in the 2 studies by Onofriescu et al. (−2.63 m/s; 95% CI: −3.7 to −1.6; P = 0.00001)32, 34 compared with Hur et al. (−0.72 m/s; 95% CI: −1.7 to −0.2).33 This variation was attributed to differences in frequency of “dry” weight assessments (2 weeks vs. 3 months) and normovolemic cutoffs (Table 1). The effect by treatment duration, however, was nonsignificant (P = 0.41).
Figure 3.
Effects of interventions to control extracellular fluid volume on carotid-femoral pulse wave velocity (cf-PWV) in end-stage renal disease. Analysis 2.1: Effect of bio-impedance–guided ultrafiltration compared to clinical and radiographic assessment. Analysis 2.2: Effect of normovolemic (n = 212) versus hypervolemic “dry” weight status (n = 129) measured by bio-impedance. All cf-PWV values were nonadjusted for blood pressure. CI, confidence interval; RCT, randomized controlled trial; UF, ultrafiltration.
To further explore the impact of extracellular fluid volume on aortic stiffness, we performed a separate analysis of 4 cross-sectional studies that assessed hydration status in dialysis, measured by bio-impedance (Table 1; Figure 3).35, 36, 37, 38 Three studies included subjects on hemodialysis and 1 involved peritoneal dialysis. Study quality ranged from fair (1) to poor (3). Normovolemia (n = 212) was associated with significantly lower cf-PWV (−2.10 m/s; 95% CI: −3.1 to −1.1; P < 0.0001) and SBP (−12.3 mm Hg; 95% CI: −19.5 to −5.1; P = 0.0008) compared with hypervolemia (n = 129). Statistical heterogeneity was moderate (I2: cf-PWV = 48%; SBP = 31%) and this was associated with differences in individual effect sizes. In 1 study,38 mean differences in cf-PWV were smaller and nonsignificant (−0.80 m/s; 95% CI: −2.1 to 0.5; P = 0.21) compared with the other 3 studies (−2.6 m/s; 95% CI: −3.5 to −1.76; P < 0.00001).35, 36, 37 Moreover, 1 study36 demonstrated no effect on SBP (0.0 mm Hg; 95% CI: −16.1 to 16.1), but the other 335,37,38 revealed significant reductions (−13.0 mm Hg; 95% CI: −22.3 to 2.8; P = 0.01). These variations were associated in part with discrepancies in normovolemic cutoffs.
A randomized study39 in hemodialysis patients evaluated the effects of lung ultrasonography followed by bio-impedance (n = −119) versus a clinical method of “dry” weight assessment (n = 122) on cardiovascular outcomes. At 24 months of follow-up, cf-PWV increased significantly (P < 0.001) in both the intervention (+2.87 m/s; 95% CI: 2.57–3.17) and control groups (+2.1 m/s; 95% CI: 1.9–2.3), with no reduction in all-cause mortality or cardiovascular events. In a separate study relevant to control of extracellular fluid volume,40 hemodialysis patients with predialysis plasma sodium concentration higher than 138 mmol/l were randomly assigned to low sodium dialysate (136 mmol/l) or standard dialysate (138 mmol/l). After 12 months of study, there was no significant difference in cf-PWV between the 2 groups (−0.3 m/s; 95% CI: −0.8 to 0.2; P = 0.27).
Low Calcium Dialysate
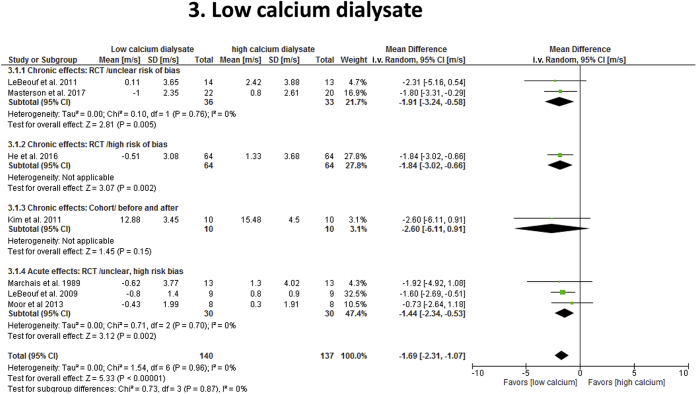
Seven hemodialysis studies41, 42, 43, 44, 45, 46, 47 reported effect estimates on use of low calcium (n = 151) versus high calcium (n = 110) dialysates (2 crossover studies) to reduce cf-PWV (Table 1; Figure 4). Only 5 studies41, 42, 44, 45, 47 reported SBP data. Six studies (3 unclear risk and 3 high risk of bias) were randomized trials and 1 was nonrandomized (fair quality). Calcium dialysate concentrations for low calcium arms ranged from 0.8 to 1.5 mmol/l (mean 1.18 ± 0.25) and between 1.37 to 1.75 mmol/l (mean 1.57 ± 0.16) for high calcium groups. Four studies assessed chronic effects of the intervention (3 to 12 months) and 3 reports evaluated acute effects (<3 weeks).41, 44, 45 Overall, low calcium dialysate was associated with reduction in cf-PWV (−1.70 m/s; 95% CI: −2.4 to −1.0; P < 0.00001) with no effect on SBP (−1.6 mm Hg; 95% CI: −8.9 to 5.8; P = 0.67), compared with high calcium dialysate. Heterogeneity was low (I2 = 0%) and differences in study design and risk of bias did not modify overall effect estimates (cf-PWV: P = 0.89; SBP: P = 0.36). Treatment duration (≤6 months vs. ≥12 months) was not a confounder on effect estimates (P = 0.66) and differences in mean age and dialysis vintage were nonsignificant (P > 0.10). A sensitivity analysis on cf-PWV with and without the 3 acute studies did not change overall effect estimates (P = 0.92).
Figure 4.
Effect of low calcium dialysate on carotid-femoral pulse wave velocity (cf-PWV) in end-stage renal disease. Studies were stratified based on the duration of effects (acute vs. chronic) and study quality or design. To reduce “double-counting” error in crossover studies (LeBeouf et al.41; Moor et al.45) and single cohort studies with before/after design (Kim et al.47), 50% of the total number of study participants was included in each study arm. All cf-PWV values were nonadjusted for blood pressure. CI, confidence interval; RCT, randomized controlled trial.
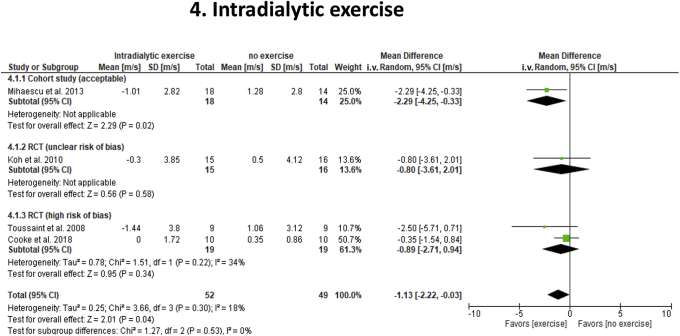
Intradialytic Exercise
Four studies in hemodialysis subjects (1 crossover, 2 parallel trials, 1 cohort) assessed effects of intradialytic exercise (n = 61; 1 crossover study) on cf-PWV and SBP relative to nonexercise (n = 40) (2 high risk of bias; 1 unclear risk; and 1 acceptable) (Table 1; Figure 5).48, 49, 50, 51 Intradialytic exercise for 3 to 6 months decreased cf-PWV (−1.13 m/s; 95% CI: −2.2 to −0.03; P = 0.04) without affecting SBP (+0.5 mm Hg; 95% CI: −9.5 to 10.4; P = 0.93) over no exercise. Overall statistical heterogeneity was low (cf-PWV: I2 = 18%, SBP: 0%), but moderate among studies identified with high-risk bias (I2 = 34%). Although study quality and design did not impact overall effect estimates (cf-PWV: P = 0.53; SBP: P = 0.93), Forrest plots indicated that reports by Mihaescu et al.48 and Toussaint et al.49 showed a large benefit of exercise on cf-PWV (−2.35 m/s; 95% CI: −4.02 to −0.67; P = 0.006), whereas those by Koh et al.50 and Cooke et al.51 did not (−0.42 m/s; 95% CI: −1.51 to 0.68; P = 0.46). These subgroup differences were significant (P = 0.06) and were associated with differences in intensity as measured by Borg scale (12–16), length of training blocks (0.5 to 2 hours), and total duration (3 to 6 months) of exercise.
Figure 5.
Effects of intradialytic exercise on carotid-femoral pulse wave velocity (cf-PWV) in end-stage renal disease. Studies were stratified according to the study quality and design. To reduce “double-counting” error in the crossover study (Toussaint et al.49), 50% of the total number of study participants were included in each study arm. All cf-PWV values were nonadjusted for blood pressure. CI, confidence interval; RCT, randomized controlled trial.
Quality of Evidence
Quality of evidence for both cf-PWV and SBP (Supplementary Table S1) ranged from very low to low except for low calcium dialysate, considered of moderate quality.
Adverse Events
Supplementary Table S2 summarizes adverse events for the interventions and comparators reported in 21 of the 27 studies (78%). No intervention was associated with fatal or severe adverse events.
Discussion
We pooled data from 2166 subjects with ESRD included in 33 reports to evaluate the effects of 4 different nonpharmacologic interventions on cf-PWV. Although quality of evidence ranged from very low to moderate, kidney transplantation, bio-impedance–guided control of extracellular fluid volume, low calcium dialysate, and intradialytic exercise were associated with significant improvements in cf-PWV in ESRD. All nonpharmacologic interventions, except for low calcium dialysate and intradialytic exercise reduced SBP. However, because of the limited information available, effects of antihypertensive medications and variations in heart rate on SBP changes were not accounted for.
Kidney transplantation is associated with improved outcomes in ESRD.52 Effective restoration of kidney function leads to improvement of endothelial dysfunction, uremic toxin removal, recovery of abnormal mineral metabolism, and improved blood pressure control.22, 23, 24, 25, 26, 27, 28, 29, 30 Our findings suggest that both cf-PWV and SBP are reduced after kidney transplantation. These results confirm a previous meta-analysis53 that found a reduction in central arterial stiffness posttransplantation. Our analysis, however, indicates that effects on cf-PWV were smaller (−0.70 m/s) than previously reported (−1.20 m/s).53 Several factors may account for this difference. First, we excluded the report by Kovacs et al.54 who obtained estimates of aortic pulse wave velocity from radial artery waveforms, with effects greater than 2 SDs from the pooled mean differences. Second, we included the study by Stompor et al.26 who measured cf-PWV in transplant recipients (before and after transplantation) and in dialysis patients waiting for transplantation. Third, it is important to note that average baseline cf-PWV in transplant studies was lower than those involving other interventions (Supplementary Table S3). This suggests a less advanced degree of vascular stiffness in pretransplant subjects, which may have diminished the impact of transplantation (i.e., “flooring effect”). Transplant recipients also represent a heterogeneous population with varying pre- and posttransplant management, and this might be expected to affect aortic stiffness.25, 27 Furthermore, selection of control groups in comparative cohort analyses is a potential source of confounding that may have decreased the effect of transplantation.30 Finally, the inclusion of cf-PWV values adjusted for blood pressure in our analysis abolished the effects of transplantation, highlighting the importance of blood pressure reduction on aortic stiffness.23, 25 Other risk factors, such as infection, immunosuppression, new-onset diabetes, nonimmunosuppressive drugs, and dyslipidemia were also not accounted for in our analysis, and may have offset the effects of transplantation on aortic stiffness.22, 24, 25, 28
Upper-extremity native arteriovenous fistula creation has been associated with sustained reductions in blood pressure, total peripheral vascular resistance, and cf-PWV within the first 3 months postoperatively.55, 56 However, longer-term adaptive changes that might be associated with arteriovenous fistula use have not been studied. In addition, the persistence of a functioning arteriovenous fistula in kidney transplant recipients has been associated with increased central aortic pulse pressure, and it has been suggested that surgical ligation may lower cardiovascular risk in this population.57 In our study, we did not include arteriovenous fistula creation as an intervention, because this procedure is considered one aspect of standard of care in hemodialysis, and it is not routinely performed with the unique intention to improve arterial stiffness.
Fluid overload in ESRD is typically assessed by indirect methods.32 A more objective assessment involves bio-impedance spectroscopy.32, 33, 34 Our review suggests that strict control of extracellular fluid by bio-impedance decreases aortic stiffness and SBP compared with clinical methods. Remarkably, bio-impedance reduced cf-PWV by approximately 1.90 m/s relative to the conventional method. By implementing bio-impedance measures in ESRD, such reduction could potentially decrease mortality by as much as 28%.7 Because the quality of evidence was “low” for these studies, additional trials are needed to demonstrate the impact of this intervention on cardiovascular outcomes in ESRD. In addition, the use of lung ultrasonography followed by bio-impedance as a combined method for adjustment of “dry” weight may be less sensitive than bio-impedance alone to reduce cf-PWV.39 Thus, it is possible that in the absence of adequate control of overall fluid volume by bio-impedance, there may be no benefit to monitoring of pulmonary congestion by lung ultrasonography.39 Consequently, the use of lung ultrasonography as a tool to improve aortic stiffness will require further study.
Consensus is lacking on the optimal dialysate calcium concentration, although high calcium dialysate may contribute to vascular calcification and aortic stiffness.41, 42, 43, 44, 45, 46, 47 Based on moderate quality of evidence, our findings strongly support use of low calcium dialysate to reduce aortic stiffness in ESRD. This effect was identified in both acute and chronic trial designs. Due to the small number of published reports and wide range of low calcium concentrations, the optimal calcium concentration to decrease aortic stiffness remains to be determined. Our review revealed that there are short- and long-term effects of low calcium dialysate on aortic stiffness. Acute effects appear to be reversible and related to changes in vascular tone from transient variations in calcium flux.41, 42, 43, 44, 45 Long-term effects, however, may be due to structural changes in the vascular wall associated with changes in bone turnover and regression of vascular calcification.41, 42, 46
Physical function and activity are generally low in ESRD, and exercise may improve quality of life.58 Our findings indicate that supervised intradialytic exercise decreases aortic stiffness in dialysis subjects without altering SBP. The effects of exercise are reversible and may relate to improvements in endothelial function, vascular tone, and/or inflammation.49, 51, 58 Because our study identified important differences in intensity, time of exposure, and duration of exercise between studies, standardization should be a priority for future trials.
Additional Sources of Heterogeneity
Several factors may modify the effects of interventions that target aortic stiffness. Because vascular calcification and aortic stiffness have additive prognostic value on cardiovascular outcomes, the beneficial effects of these interventions may be reduced in patients with ESRD with extensive vascular calcification.7 In addition, differences in predialysis cardiovascular function, effects of cointerventions such as antihypertensive medications or phosphate binders, and the role of inflammation, genetic polymorphisms, vitamin K deficiency, or advanced glycation end-product formation cannot be disregarded as potential sources of variability in the individual responses.4, 8, 9, 59, 60
Strengths and Limitations
The strength of our study lies in the rigorous methodology, comprehensive search, and detailed quality assessments, which permitted an extensive review of multiple interventions that target aortic stiffness in ESRD. An additional strength is that our findings were restricted to assessments of aortic stiffness using cf-PWV, which is considered the “gold standard.”5, 6, 7, 8, 9 Although different instruments for measuring cf-PWV were identified among studies included in our search, these devices and their techniques have been validated and standardized,6, 53, 60 and therefore, variability of measurements was minimized. We recognize that quality of evidence is limited by quality and design of studies. Thus, some pooled estimates are hypothesis-generating due to high statistical and methodological heterogeneity, small number of studies, and lack of control for confounders. A few studies were excluded because of insufficient data and lack of author responses to inquiries, but exclusion of these studies is unlikely to affect our conclusions.
In summary, several nonpharmacologic interventions may individually reduce aortic stiffness and SBP in ESRD, with a mean reduction in cf-PWV ranging from 0.70 to 2.61 m/s and average SBP decrease from 4.3 to 13 mm Hg. If effective, these interventions could potentially reduce the risk of cardiovascular events and all-cause mortality in ESRD by approximately 11% to 39%.7 Accordingly, future trials should address the impact of these nonpharmacologic interventions on cardiovascular outcomes and mortality risk reduction in ESRD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We acknowledge Becky Skidmore and Raymond Daniel for their help in the literature search.
This study was funded by the Kidney Research Centre, Ottawa Hospital Research Institute, University of Ottawa.
Author Contributions
RAR and KB conceived and designed this study; RAR created the analytical plan and drafting of the manuscript; RAR, RH, MS, and KB participated in study screening, selection, data extraction, and quality assessment; RAR, KB, BS, and MA contributed to study interpretation and manuscript revisions. All authors approved the final version of the manuscript.
Footnotes
Appendix S1. Search strategy.
Appendix S2. Study selection criteria.
Table S1. Quality of evidence.
Table S2. Summary of adverse events.
Table S3. Pretreatment baseline cf-PWV values.
Supplementary Material
References
- 1.Global Burden of Disease 2013 GFR collaborators; CKD Prognosis Consortium; Global burden of Disease Genitourinary Expert Group. Global Cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28:2167–2179. doi: 10.1681/ASN.2016050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risk of death, cardiovascular events and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Blacher J., Guerin A.P., Pannier B. Arterial calcifications, arterial stiffness and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith D., Ritz E., Covic A. Vascular calcification: a stiff challenge for the nephrologists. Does preventing bone disease cause arterial disease? Kidney Int. 2004;66:1315–1333. doi: 10.1111/j.1523-1755.2004.00895.x. [DOI] [PubMed] [Google Scholar]
- 5.Blacher J., Guerin A.P., Pannier B. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 6.Townsend R.R. Arterial stiffness in CKD: a review. Am J Kidney Dis. 2018;73:240–247. doi: 10.1053/j.ajkd.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verbeke F., Van Biesen W., Honkanen E. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the calcification outcome in renal disease (CORD) study. Clin J Am Soc Nephrol. 2011;6:153–159. doi: 10.2215/CJN.05120610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgianos P.I., Pikilidou M.I., Liakopoulos V. Arterial stiffness in end-stage renal disease-pathogenesis, clinical epidemiology, and therapeutic potentials. Hypertens Res. 2018;41:309–319. doi: 10.1038/s41440-018-0025-5. [DOI] [PubMed] [Google Scholar]
- 9.Blacher J., Safar M.E., Guerin A.P. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852–1860. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J., Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0; Cochrane Collaboration; updated March 2011. http://handbook.cochrane.org/v5.0.0/ Available at. Accessed November 2017.
- 11.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez R.A., Shea B., Hae R. The impact of intervention strategies that target arterial stiffness in end-stage renal disease: a systematic review protocol. Syst Rev. 2016;5:118. doi: 10.1186/s13643-016-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Scottish Intercollegiate Guidelines Network (SIGN) SIGN50; 2010; Edinburgh, Scotland: 2012. A guideline developer’s book.https://www.sign.ac.uk/sign-50.html Available at: Accessed January 2018. [Google Scholar]
- 14.National Institutes of Health National Heart, Lung and Blood Institute. Quality assessment tool for before-after (pre-post) studies with no control group. Health-pro. Guidelines. Cohort. Bethesda MD, 2014:1. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools Available at. Accessed July 2017.
- 15.Luo D., Wan X., Liu J. Optimally estimating the sample mean from the sample size, median, mid-range and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 16.Wan X., Wang W., Liu J. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkins D., Briss P.A., Eccles M. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–1494. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mostovaya I.M., Bots M., van den Dorpel M.A. A randomized trial of hemodiafiltration and change in cardiovascular parameters. Clin J Am Soc Nephrol. 2014;9:520–526. doi: 10.2215/CJN.07140713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P.K., Cheng Y.L., Leung C.B. Effect of membrane permeability on inflammation and arterial stiffness: a randomized trial. Clin J Am Soc Nephrol. 2010;5:652–658. doi: 10.2215/CJN.05620809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beerenhout C.H., Luik A.J., Jeuken-Mertens S.G.J. Pre-dilution on-line haemofiltration vs low-flux haemodialysis: a randomized prospective study. Nephrol Dial Transplant. 2005;20:1155–1163. doi: 10.1093/ndt/gfh775. [DOI] [PubMed] [Google Scholar]
- 22.Bachelet-Rousseau C., Kearney-Schwartz A., Frimat L. Evolution of arterial stiffness after kidney transplantation. Nephrol Dial Transplant. 2011;26:3386–3391. doi: 10.1093/ndt/gfr058. [DOI] [PubMed] [Google Scholar]
- 23.Ignace S., Utescu M.S., De Serres S.A. Age-related and blood pressure-independent reduction in aortic stiffness after kidney transplantation. J Hypertens. 2011;29:130–136. doi: 10.1097/HJH.0b013e32833f5e68. [DOI] [PubMed] [Google Scholar]
- 24.Kaur M., Lal C., Bhowmik D. Reduction in augmentation index after successful renal transplantation. Clin Exp Nephrol. 2013;17:134–139. doi: 10.1007/s10157-012-0653-z. [DOI] [PubMed] [Google Scholar]
- 25.Zoungas S., Kerr P.G., Chadban S. Arterial function after successful renal transplantation. Kidney Int. 2004;65:1882–1889. doi: 10.1111/j.1523-1755.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 26.Stompor T., Rajzer M., Kawecka-Jaszcz K. Renal transplantation ameliorates the progression of arterial stiffness in patients treated with peritoneal dialysis. Perit Dial Int. 2005;25:492–496. [PubMed] [Google Scholar]
- 27.Keven K., Calayoglu R., Sengul S. Comparative effects of renal transplantation and maintenance dialysis on arterial stiffness and left ventricular mass index. Clin Transplant. 2008;22:360–365. doi: 10.1111/j.1399-0012.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 28.Hornum M., Clausen P., Idorn T. Kidney transplantation improves arterial function measured by pulse wave analysis and endothelium-independent dilatation in uraemic patients despite deterioration of glucose metabolism. Nephrol Dial Transplant. 2011;26:2370–2377. doi: 10.1093/ndt/gfq704. [DOI] [PubMed] [Google Scholar]
- 29.Covic A., Goldsmith D.J., Florea L. The influence of dialytic modality on arterial stiffness, pulse wave reflections, and vasomotor function. Perit Dial Int. 2004;24:365–372. [PubMed] [Google Scholar]
- 30.Pan C.R., Schmaderer C., Roos M. Comparing aortic stiffness in kidney transplant recipients, hemodialysis patients, and patients with chronic renal failure. Clin Transplant. 2011;25:E463–E468. doi: 10.1111/j.1399-0012.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- 31.Posadzy-Malaczyñska A., Kosch M., Hausberg M. Arterial distensibility, intima media thickness and pulse wave velocity after renal transplantation and in dialysis normotensive patients. Int Angiol. 2005;24:89–94. [PubMed] [Google Scholar]
- 32.Onofriescu M., Mardare N.G., Segall L. Randomized trial of bioelectrical impedance analysis versus clinical criteria for guiding ultrafiltration in hemodialysis patients: effects on blood pressure, hydration status, and arterial stiffness. Int Urol Nephrol. 2012;44:583–591. doi: 10.1007/s11255-011-0022-y. [DOI] [PubMed] [Google Scholar]
- 33.Hur E., Usta M., Toz H. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2013;61:957–965. doi: 10.1053/j.ajkd.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Onofriescu M., Hogas S., Voroneanu L. Bioimpedance-guided fluid management in maintenance hemodialysis: a pilot randomized controlled trial. Am J Kidney Dis. 2014;64:111–118. doi: 10.1053/j.ajkd.2014.01.420. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y.P., Yu W.C., Hsu T.L. The extracellular fluid-to-intracellular fluid volume ratio is associated with large-artery structure and function in hemodialysis patients. Am J Kidney Dis. 2003;42:990–999. doi: 10.1016/j.ajkd.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Bia D., Galli C., Valtuille R. Hydration status is associated with aortic stiffness but not with peripheral arterial stiffness, in chronically hemodialyzed patients. Int J Nephrol. 2015;2015:628654. doi: 10.1155/2015/628654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocyigit I., Sipahioglu M.H., Orscelik O. The association between arterial stiffness and fluid status in peritoneal dialysis patients. Perit Dial Int. 2014;34:781–790. doi: 10.3747/pdi.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsides N., Cornelis T., Broers N.J.H. Extracellular overhydration linked with endothelial dysfunction in the context of inflammation in haemodialysis dependent chronic kidney disease. PLoS One. 2017;12:E0183281. doi: 10.1371/journal.pone.0183281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siriopol D., Onofriescu M., Voroneanu L. Dry weight assessment by combined ultrasound and bioimpedance monitoring in low cardiovascular risk hemodialysis patients: a randomized controlled trial. Int Urol Nephrol. 2017;49:143–153. doi: 10.1007/s11255-016-1471-0. [DOI] [PubMed] [Google Scholar]
- 40.Liu J., Sun F., Ma L.J. Increasing dialysis sodium removal on arterial stiffness and left ventricular hypertrophy in hemodialysis patients. J Ren Nutr. 2016;26:38–44. doi: 10.1053/j.jrn.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 41.LeBeouf A., Mac-Way F., Utescu M.S. Effects of acute variation of dialysate calcium concentrations on arterial stiffness and aortic pressure waveform. Nephrol Dial Transplant. 2009;24:3788–3794. doi: 10.1093/ndt/gfp351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeBoeuf A., Mac-Way F., Utescu M.S. Impact of dialysate calcium concentration on the progression of aortic stiffness in patients on haemodialysis. Nephrol Dial Transplant. 2011;26:3695–3701. doi: 10.1093/ndt/gfr138. [DOI] [PubMed] [Google Scholar]
- 43.Masterson R., Blair S., Polkinghorne K.R. Low versus high dialysate calcium concentration in alternate night nocturnal hemodialysis: a randomized controlled trial. Hemodial Int. 2017;21:19–28. doi: 10.1111/hdi.12452. [DOI] [PubMed] [Google Scholar]
- 44.Marchais S., Guerin A., Safar M. Arterial compliance in uraemia. J Hypertens Suppl. 1989;7:S84–S85. doi: 10.1097/00004872-198900076-00038. [DOI] [PubMed] [Google Scholar]
- 45.Moor M.B., Kruse A., Uehlinger D.E. Arterial stiffness depends on serum ionized calcium levels during dialysis with regional citrate anticoagulation. Artif Organs. 2013;37:467–474. doi: 10.1111/aor.12037. [DOI] [PubMed] [Google Scholar]
- 46.He Z., Cui L., Ma C. Effects of lowering dialysate calcium concentration on carotid intima-media thickness and aortic stiffness in patients undergoing maintenance hemodialysis: a prospective study. Blood Purif. 2016;42:337–346. doi: 10.1159/000450747. [DOI] [PubMed] [Google Scholar]
- 47.Kim J.K., Moon S.J., Park H.C. Effects of lowering dialysate calcium concentrations on arterial stiffness in patients undergoing hemodialysis. Korean J Intern Med. 2011;26:320–327. doi: 10.3904/kjim.2011.26.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mihaescu A., Avram C., Bob F. Benefits of exercise training during hemodialysis sessions: a prospective cohort study. Nephron Clin Pract. 2013;124:72–78. doi: 10.1159/000355856. [DOI] [PubMed] [Google Scholar]
- 49.Toussaint N.D., Polkinghorne K.R., Kerr P.G. Impact of intradialytic exercise on arterial compliance and B-type natriuretic peptide levels in hemodialysis patients. Hemodial Int. 2008;12:254–263. doi: 10.1111/j.1542-4758.2008.00262.x. [DOI] [PubMed] [Google Scholar]
- 50.Koh K.P., Fassett R.G., Sharman J.E. Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis. 2010;55:88–99. doi: 10.1053/j.ajkd.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 51.Cooke A.B., Ta V., Iqbal S. The impact of intradialytic pedaling exercise on arterial stiffness: a pilot randomized controlled trial in a hemodialysis population. Am J Hypertens. 2018;31:458–466. doi: 10.1093/ajh/hpx191. [DOI] [PubMed] [Google Scholar]
- 52.Tonelli M., Wiebe N., Knoll G. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 53.Sidibé A., Fortier C., Desjardins M.P. Reduction of arterial stiffness after kidney transplantation: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kovacs D., Löcsey L., Szabó L. Noninvasive perioperative monitoring of arterial function in patients with kidney transplantation. Transplant Proc. 2013;45:3682–3684. doi: 10.1016/j.transproceed.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Korsheed S., Eldehni M.T., John S.G. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant. 2011;26:3296–3302. doi: 10.1093/ndt/gfq851. [DOI] [PubMed] [Google Scholar]
- 56.Utescu M.S., LeBoeuf A., Chbinou N. The impact of arteriovenous fistulas on aortic stiffness in patients with chronic kidney disease. Nephrol Dial Transplant. 2009;24:3441–3446. doi: 10.1093/ndt/gfp276. [DOI] [PubMed] [Google Scholar]
- 57.Ferro C.J., Savage T., Pinder S.J., Tomson C.R.V. Central aortic pressure augmentation in stable renal transplant recipients. Kidney Int. 2002;62:166–171. doi: 10.1046/j.1523-1755.2002.00407.x. [DOI] [PubMed] [Google Scholar]
- 58.Mustata S., Chan C., Lai V. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol. 2004;15:2713–2718. doi: 10.1097/01.ASN.0000140256.21892.89. [DOI] [PubMed] [Google Scholar]
- 59.Utescu M.S., Couture V., Mac-Way F. Determinants of progression of aortic stiffness in hemodialysis patients. A prospective longitudinal study. Hypertension. 2013;62:154–160. doi: 10.1161/HYPERTENSIONAHA.113.01200. [DOI] [PubMed] [Google Scholar]
- 60.Briett M., Boutouyrie P., Laurent S., London G.M. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:388–400. doi: 10.1038/ki.2012.131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.