Abstract
BACKGROUND:
The hypothesis that dopamine plays an important role in the pathophysiology of pathological gambling is pervasive. However, there is little to no direct evidence for a categorical difference between pathological gamblers and healthy control subjects in terms of dopamine transmission in a drug-free state. Here we provide evidence for this hypothesis by comparing dopamine synthesis capacity in the dorsal and ventral parts of the striatum in 13 pathological gamblers and 15 healthy control subjects.
METHODS:
This was achieved using [18F]fluoro-levo-dihydroxyphenylalanine dynamic positron emission tomography scans and striatal regions of interest that were hand-drawn based on visual inspection of individual structural magnetic resonance imaging scans.
RESULTS:
Our results show that dopamine synthesis capacity was increased in pathological gamblers compared with healthy control subjects. Dopamine synthesis was 16% higher in the caudate body, 17% higher in the dorsal putamen, and 17% higher in the ventral striatum in pathological gamblers compared with control subjects. Moreover, dopamine synthesis capacity in the dorsal putamen and caudate head was positively correlated with gambling distortions in pathological gamblers.
CONCLUSIONS:
Taken together, these results provide empirical evidence for increased striatal dopamine synthesis in pathological gambling.
Keywords: Addiction, Dopamine, [18F]DOPA, Gambling, Neuroimaging, Reward
Behavioral, cognitive, and neurobiological profiles of individuals with pathological gambling resemble those of individuals with substance use disorder, especially stimulant addiction (1–5). As a consequence, pathological gambling was recently reclassified as an addiction disorder in the DSM-5 (6). However, it is still unclear whether some of the dopamine abnormalities that characterize substance use disorder are also present in pathological gambling. The current study examined the role of dopamine synthesis capacity in pathological gambling. Below, we highlight central fi ndings linking altered dopamine function with substance addiction before reviewing existing evidence of altered dopamine function in pathological gambling.
Converging evidence from various lines of research indicates that substance use disorder is characterized by a decrease in striatal dopamine D2/D3 receptor availability (7,8), even though this reduction is more consistently observed among stimulant users than in individuals with opiate, nicotine, or cannabis dependence (7). In humans, this has been evidenced by cross-sectional studies using [11C]raclopride positron emission tomography (PET) and single-photon emission computed tomography imaging techniques (7–17). In addition, human studies focusing on dopamine synthesis capacity, measured with [18F]fluoro-levo-dihydroxyphenylalanine ([18F]DOPA) PET, have revealed either low or unaltered dopamine synthesis capacity across various substance use disorders (16,18–21).
Whether observed differences in D2/D3 receptor availability are a cause or consequence of drug addiction, and how it interacts with presynaptic dopamine function, is an area of active research. Longitudinal studies in animals have revealed that diminished baseline availability of striatal dopamine D2/D3 receptors is both a predictor and a consequence of continued drug use. For example, lower baseline availability of striatal dopamine D2/D3 receptors in drug-naive monkeys predicts high rates of subsequent cocaine self-administration (22). Longitudinal scanning further reveals reduced D2/D3 receptor ligand binding following repeated drug exposure (23,24). Micro-PET studies in rats have confirmed and extended these findings by showing that high impulsivity traits are associated with low dopamine D2/D3 receptor availability and predispose to the development of drug addiction (25–29). These findings concur with human studies showing that trait impulsivity is a vulnerability marker for addiction (30), although—in contrast to animal research—the direction of the association with dopamine D2/D3 receptors is less clear. Indeed, whereas some studies have reported positive correlations in healthy control subjects (HCs) (31,32), other studies have reported negative correlations in HCs and methamphetamine-dependent users (9,33).
Studies focusing on targets of dopamine functioning have so far led to different results in pathological gambling compared with stimulant dependence. In fact, all PET studies in pathological gambling have failed to reveal abnormal dopamine D2/D3 receptor availability in pathological gamblers (PGs) relative to HCs (34–38). Despite this lack of group differences, two studies found a negative correlation between baseline dopamine D2/D3 receptor binding in the ventral striatum and trait impulsivity in PGs (35,36). Similarly, PET studies investigating gambling-induced dopamine release have failed to reveal overall group differences but have shown correlations with relevant measures related to gambling severity, excitement, and performance (36,38,39). Currently, direct evidence for abnormal dopamine functioning in pathological gambling comes exclusively from studies showing altered responsiveness to dopaminergic drugs (40,41). In particular, PGs were shown to display greater amphetamine-induced dopamine release in the dorsal striatum, as measured with PET imaging using the D3 receptor-preferring radioligand [11C]-(+)-4-Propyl-9-hydroxynaphthoxazine, compared with HCs (40). This increased dopaminergic response echoes a recurrent clinical observation in Parkinson’s disease: following dopaminergic treatment aimed at compensating for dopamine cell loss, a subset of patients with Parkinson’s disease develop gambling disorder symptoms (42). These observations suggest that enhanced dopaminergic transmission may represent a biological substrate of gambling disorder.
Thus, so far nearly all dopamine PET studies on pathological gambling have focused on dopamine D2/D3 receptors, investigating either receptor availability or the effects of dopaminergic drugs and gambling tasks. To date, there has been a paucity of research investigating dopamine synthesis capacity in PGs, with only one recent study reporting no difference with HCs (43). Yet, increased dopamine synthesis capacity has been associated with increased behavioral disinhibition and financial extravagance in healthy subjects and patients with Parkinson’s disease (44,45). Here we used dynamic [18F]DOPA PET imaging to investigate striatal dopamine synthesis capacity in male PGs and HCs matched for age, education, and an estimate of verbal IQ.
METHODS AND MATERIALS
Subjects
In total, 15 PGs and 15 HCs were recruited. All HCs and 13 PGs had also participated in a previous pharmaco-functional magnetic resonance imaging (fMRI) study (41,46). The other 2 PGs were newly recruited. PGs were recruited through advertisement and addiction treatment centers, and they reported not to be medicated or in treatment for their gambling at the time of the PET study. HCs were recruited through advertisement.
All subjects who had participated in the pharmaco-fMRI study underwent a structured psychiatric interview [MiniInternational Neuropsychiatric Interview-Plus, (47)] administered by a medical doctor prior to the fMRI study. The 2 PGs who were newly recruited were also assessed with the Mini-International Neuropsychiatric Interview-Plus (47) administered by a clinical psychologist. Subjects were excluded if they had a lifetime history of schizophrenia, bipolar disorder, attention-deficit/hyperactivity disorder, autism, bulimia, anorexia, anxiety disorder, or obsessive-compulsive disorder or if they had a past 6-month history of major depressive episode. Current or past-year substance use disorder was also an exclusion criterion, as assessed at the time of the PET study using the 10-item Drug Abuse Screening Test questionnaire (48). Based on this criterion, data from 2 PGs were not included in the main analyses because of meeting the DSM-IV-TR criteria for cannabis dependence during the past year. As assessed with the Mini-International Neuropsychiatric Interview-Plus interview, 1 of the excluded cannabis-dependent PGs also had a history of cocaine dependence that lasted for 1.5 years and ended 5.5 years prior to the PET study. In addition, 1 included PG had histories of alcohol and cocaine dependence that lasted for 1 year and ended 8 and 15 years prior to the PET study, respectively. None of the other PGs or HCs had a history of substance use disorder. Furthermore, subjects were excluded if they were currently following psychiatric treatment, using (psychotropic) medication, or drank more than four alcoholic beverages daily.
All gamblers qualified as PGs because they met five or more DSM-IV-TR criteria for pathological gambling and were otherwise healthy. Of these subjects, 4 PGs had been in cognitive behavioral treatment for their gambling problems 2 to 6 years before the PET study. The severity of gambling symptoms was assessed using the South Oaks Gambling Screen [SOGS (49)]. All PGs had a minimum lifetime SOGS score of 5 (range = 5–18) when initially included in the pharmaco-fMRI study (41,46), whereas HCs, with the exception of 2 subjects (scoring 1 and 2, respectively), had a SOGS score of 0. The severity of gambling symptoms was measured again at the time of the PET study using past-year and past-3-month versions of the SOGS (see Table 1; past-year SOGS score range = 5–11, past-3-month SOGS score range = 0–10). Frequent forms of gambling were assessed using item 1 of the SOGS and are expressed in terms of the percentage of gamblers playing the following games at least once a week for money: slot machines (53%), card games (46%), casino games (33%), sports betting (40%), lotteries (40%), stock market (7%), and bowling, pool, golf, darts, or the like (7%).
Table 1.
Demographics and Self-Report Measures
| HCs (n =15) |
PGs (n = 13) |
Statistic | |
|---|---|---|---|
| Age, Years | 36.20 (11.63) | 40.92 (6.70) | p = .209 |
| Number of Smokers | 2 | 5 | p = .257 |
| FTND Score in Smokers | 4.00 (1.41) | 5.60 (2.61) | p = .465 |
| Verbal IQ (Based on NART) | 104.93 (9.17) | 101.38 (11.05) | p = .361 |
| Income | 1648.66 (1037.73) | 1650.00 (1038.54) | p = .487 |
| BMI | 23.85 (3.12) | 24.06 (1.90) | p = .840 |
| AUDIT | 6.00 (3.78) | 6.69 (3.86) | p = .636 |
| Number of Subjects Scoring ≥8 on AUDITa | 5 | 5 | p = .549 |
| BIS-11 | 58.07 (8.33) | 73.25 (10.14) | p < .001 |
| SOGS, Past Year | 0.40 (0.83) | 9.15 (1.63) | p < .001 |
| SOGS, Past 3 Months | 0.06 (0.26) | 3.23 (3.39) | p < .001 |
| GBQ-Total | 47.71 (21.59) | 90.77 (30.94) | p < .001 |
| Injected Dose of [18F]DOPA, MBq | 184.20 (14.15) | 188.54 (7.65) | p = .333 |
Values represent mean (SD) or n.
AUDIT, Alcohol Use Disorders Identification Test; BIS-11, revised Barratt Impulsiveness Scale; BMI, body mass index; [18F]DOPA, [18F]fluoro-levo-dihydroxyphenylalanine; FTND, Fagerström Test for Nicotine Dependence; GBQ-total, Gamblers’ Beliefs Questionnaire-total score; HCs, healthy control subjects; NART, National Adult Reading Test (Dutch version); PGs, pathological gamblers; SOGS, South Oaks Gambling Screen.
aNone of the subjects met the DSM-IV criteria for alcohol dependence as assessed with the MINI-Plus. Self-report measures were acquired on the day of the [18F]DOPA PET scan.
Study Procedure
The delay between data collection of the previous pharmaco-fMRI study (41,46) and the PET study ranged from 5 to 29 months (median = 23.5 months). After an initial telephone screening allowing us to verify that the reinvited gamblers were still experiencing gambling problems during the past year, eligible subjects were invited to the Radboud University Medical Centre to participate in the PET study and complete self-report measures. These measures included the past-year and past-3-month versions of the SOGS mentioned above, the Gamblers’ Beliefs Questionnaire (50), and the revised Barratt Impulsiveness Scale (51). Approximately 1 hour before entering the PET scanner, subjects received 150 mg of carbi-dopa and 400 mg of entacapone to reduce peripheral metabolism of [18F]DOPA and increase tracer availability in the brain while having no psychotropic side effects. The subjects further performed computerized tasks not reported here.
MRI Scan
A high-resolution anatomical scan (Tl-weighted magnetization prepared rapid acquisition gradient-echo, repetition time = 2300 ms, echo time = 3.03 ms, 8° flip angle, 192 sagittal slices, slice-matrix size = 256 × 256, voxel size =1 × 1 × 1 mm3) was obtained using a 3T MR Siemens scanner (Erlangen, Germany) at the Donders Centre for Cognitive Neuroimaging and was used for coregistration with the PET data.
PET Acquisition
All PET scans were acquired in the Department of Nuclear Medicine at the Radboud University Medical Centre using a Siemens mCT PET/computed tomography (CT) camera (with 40-slice CT, voxel size = 4 × 4 mm in-plane, 5-mm slice thickness). Patients were positioned as comfortably as possible, in a supine position, with the head slightly fixated in a headrest to avoid movement. First, a low-dose CT scan was made for attenuation correction of the PET images, followed by an 89-minute dynamic PET scan. The scan started at the same time as the bolus injection of the [18F]DOPA into an antecubital vein. Images were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation and time-of-flight recovery. They were further scatter corrected and smoothed with a 4-mm full width at half maximum kernel.
Regions of Interest
The regions of interest (ROIs) were hand drawn in native space based on visual inspection of each subject’s structural MRI using Mango software (http://ric.uthscsa.edu/mango/index.html). The dorsal putamen, caudate head, and ventral striatum (including the nucleus accumbens, ventral caudate, and ventral putamen) ROIs were drawn according to previously published guidelines (52). In addition, we drew the caudate body, defined as dorsal caudate posterior to the anterior commissure. The reference region for calculating [18F]DOPA values was cerebellar gray matter delineated by FreeSurfer automatic segmentation (http://surfer.nmr.mgh.harvard.edu). Given the cerebellum’s location posterior and adjacent to the midbrain, and the limited spatial resolution and blurring of PET signal, only the posterior three-fourths of the cerebellum was included in the ROI to avoid contaminating the cerebellar ROI with midbrain [18F]DOPA signal.
PET Analysis
We realigned the [18F]DOPA images to the middle (11th) frame to correct for movement during scanning using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). The mean [18F]DOPA image and the realigned frames were coregistered to the structural MRI scan using SPM8. To create uptake (Ki) images representing the amount of tracer accumulated in the ROIs relative to the cerebellar reference region, we used an in-house graphical analysis program implementing Patlak plotting (53). Ki images were generated from PET frames corresponding to 24 to 89 minutes, which represent the amount of tracer accumulated in the brain relative to the cerebellum. These images are comparable to Ki images obtained using a blood input function but are scaled to tracer volume of distribution in the reference region. Group comparisons of Ki values were conducted using standard repeated-measures analyses of variance with group as a between-subject factor and bilateral (averaged) ROIs as a within-subject factor. A Greenhouse–Geisser correction was applied to correct for violations of sphericity in all analyses of variance. For the post hoc simple main effects of group tested in the various ROIs, we applied a false discovery rate (FDR)-corrected p = .05 to account for multiple comparisons. The same strategy was applied to investigate group differences in the volume of ROIs and their potential role as a confounding variable.
In the PGs, we investigated the relationship between Ki values in the 4 ROIs and measures of gambling severity (SOGS scores), impulsivity (revised Barratt Impulsiveness Scale scores), and gambling cognitive distortions (Gamblers’ Beliefs Questionnaire scores) using Pearson correlations and an FDR-corrected p = .05 to account for multiple comparisons.
RESULTS
Subject Characteristics and Traits
Subject characteristics are summarized in Table 1. The two groups were matched for age, body mass index, net income, and verbal IQ [based on the Dutch version of the National Adult Reading Test (54)]. Measures acquired on the PET testing day indicated that PGs were significantly more impulsive, had higher SOGS scores during the past year and past 3 months, and had more gambling distortions than HCs.
PET Measures
Mean Ki was significantly different between ROIs (F3,78 = 122.95, p < .001, Cohen’s d = 4.34) and groups (F1,26 = 6.01, p = .021, Cohen’s d = 0.965). The group × ROI interaction approached significance (F3,78 = 3.139, p = .051, Cohen’s d = 0.695). Assessing simple main effects of group revealed significantly higher Ki values in PGs in the caudate body (16% higher in PGs: F1,27 = 5.301, pFDR = .040), dorsal putamen (18% higher in PGs: F1,27 = 8.047, pFDR = .012), and ventral striatum (17% higher in pGs: F1,27 = 6.312, pFDR = .025), but not in the caudate head (9% higher in PGs: F1,27 = 1.435, pFDR = .323) (Figure 1).
Figure 1.
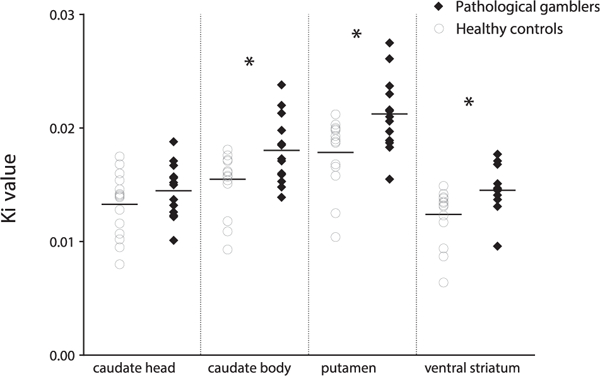
Scatter plot showing Ki values in hand-drawn striatal regions of interest in pathological gamblers and healthy control subjects. *pFDR < −05. FDR, false discovery rate.
We performed a number of additional sensitivity analyses. First, we examined whether the volume of hand-drawn ROIs could have influenced our results. The total number of voxels within all ROIs was not significantly different between groups (F1,25 = 0.174, p = .680), but there was a significant difference between ROIs (F3,78 = 304.39, p < .001) as well as a significant group × ROI interaction (F3,78 = 3.586, p = .028). Assessing simple main effects of group indicated that only the ventral striatum ROI had more voxels in PGs compared with HCs (F1,27 = 8.457, pFDR = .028) (details can be found in Table 2). However, in none of the ROIs were Ki values correlated with ROI volume (all ps > .065), suggesting that group differences in Ki were not driven by differences in volume.
Table 2.
Number of Voxels in Hand-Drawn Brain ROIs
| Number of Voxels in ROI |
HCs (n= 15) |
PGs (n = 13) |
Statistics |
|---|---|---|---|
| Caudate Head | 4057 (696.24) | 3917 (651.03) | F1,27 = 0.229, pFDR = .785 |
| Dorsal Putamen | 6882 (981.28) | 6804 (846.19) | F1,27 = 0.050, pFDR = .825 |
| Ventral Striatum | 2738 (737.08) | 3467 (561.53) | F1,27 = 8.457, pFDR = .028 |
| Caudate Body | 2226 (579.17) | 2003 (320.43) | F1,27 = 1.58, pFDR = .458 |
Values represent mean (SD).
FDR, false discovery rate; HCs, healthy control subjects; PGs, pathological gamblers; ROI, region of interest.
An ROI-based partial volume correction (PVC) was performed in native MRI space using individual hand-drawn ROIs and the geometric transfer matrix method (55), resulting in PVC ROIs. A repeated-measures analysis of variance with a Greenhouse–Geisser correction revealed a significant main effect of group (F1,26 = 4.230, p = .050) and PVC ROI (F3,24 = 76.186, p < .001) on Ki values. Moreover, there was a significant Group × PVC ROI interaction (F3,24 = 3.205, p = .041), indicating that the effect of group differed across the striatal subregions. Specifically, post hoc tests showed that Ki values were significantly higher in PGs than in HCs in the dorsal putamen (F1,27 = 7.200, p = .013) but did not differ between groups in the caudate body (F1,27 = 2.390, p = .134), caudate head (F1,27 = 3.128, p = .089), or ventral striatum (F1,27 = 2.905, p = .100).
In the Supplement, we report the results observed when using standard anatomical ROIs (caudate, dorsal putamen, and nucleus accumbens) defined from the Hammersmith anatomical atlas (56). In addition, we report analyses showing that including the 2 PGs with comorbid cannabis dependence leads to qualitatively similar, although weaker, results. Note, however, that the exclusion of these gamblers was precisely motivated by the known confounding effect of drug toxicity on dopaminergic functioning and the suspicion of different dopamine-related abnormalities in drug versus gambling addiction (57).
In addition, there was a significant positive correlation between the level of gambling-related cognitive distortions, measured with the Gamblers’ Beliefs Questionnaire, and Ki values in the dorsal putamen (r = .595, pFDR = .043) (Figure 2) as well as in the caudate head (r = .601, pFDR = .040). There was no significant correlation between gambling severity (measured with the SOGS), or impulsivity (measured with the revised Barratt Impulsiveness Scale), and the Ki values in any of the ROIs.
Figure 2.
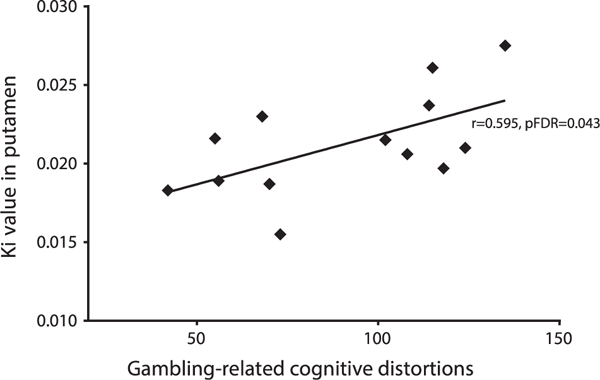
Significant correlation in pathological gamblers between gambling-related cognitive distortions (Gamblers’ Beliefs Questionnaire) and Ki values in the dorsal putamen. FDR, false discovery rate.
DISCUSSION
Our study establishes for the first time a key link between pathological gambling and increased striatal dopamine synthesis capacity. This observation is in line with previous findings showing that dopamine release is increased in the dorsal striatum of PGs following amphetamine administration (40) and is positively correlated with subjective excitement (36,39) and gambling severity (36) in the ventral striatum in the context of gambling. Our results also agree with reports of greater reward-induced dopamine release in Parkinson’s disease patients with treatment-induced pathological gambling symptoms (58,59). Importantly, we also found that higher dopamine synthesis capacity in the dorsal putamen and caudate head was positively correlated with the severity of gambling-related cognitive distortions in PGs. Gambling-related cognitive distortions are a key defining characteristic of pathological gambling (60), predicting not only gambling severity (50,60) but also duration of play (50) and treatment outcome (61–63). Together, these results support the hypothesis that increased dopamine transmission represents an important biological substrate of pathological gambling.
The finding that dopamine synthesis capacity is significantly increased in PGs contrasts remarkably with results from PET studies measuring dopamine synthesis capacity in substance use disorders. Such studies have revealed that substance use disorders are accompanied by either low or unaltered dopamine synthesis capacity (16,18–21), possibly reflecting variability in presynaptic dopamine cell injury due to varying amounts of excessive drug use (17) or differences in drug-induced neuroplasticity (64). We argue that this difference between pathological gambling and substance use disorders might reflect the absence of substance-specific confounds, including toxicity of drugs on the dopamine system, in the case of pathological gambling. This highlights the potential of studying pathological gambling, which does not involve the administration of exogenous substances, for investigating the role of dopamine in addiction. An alternative possibility is that pathological gambling might not be as good a model of addiction as hitherto thought or at least might not be as similar to stimulant addiction as previously hypothesized (5). Importantly, research has shown that various substance use disorders are associated with varying degrees of dopamine abnormality, suggesting that addiction is likely a multiple-neurotransmitter disorder (7).
Previous work has shown increased gambling-induced dopamine release in the ventral striatum as a function of gambling severity/excitement level (36,39) as well as reduced ventral striatal dopamine D2/D3 receptor availability as a function of sensation seeking/impulsivity (35,36,38). In light of the positive relationship between dopamine synthesis capacity and dopamine release (65,66), on the one hand, and the negative relationship between dopamine synthesis capacity and D2/D3 receptor availability observed in HCs (67), on the other [but see (16,68)], the current results raise the hypothesis that increased striatal dopamine release and reduced D2/D3 receptor availability in pathological gambling may reflect increased dopamine synthesis capacity. However, it is noteworthy that another recent [18F]DOPA PET study did not find evidence of dopamine synthesis abnormality in PGs compared with HCs (43). The basis of this discrepancy is unclear, but we speculate that differences in drug dependence history might play a role, although this information was not provided in the article by Majuri et al. (43). The incidence of smoking was higher in their study compared with the current study (73% vs. 38% in PGs). Given that nicotine/ drugs of abuse can affect dopamine synthesis capacity (18), it is possible that differences in nicotine/drug dependence history between the populations of the two studies contributed to the observed differences in dopamine synthesis levels. Another factor that has been suggested to contribute to mixed results in gambling research is the heterogeneity among PGs (69,70). In particular, it has been proposed that different subtypes of PGs, who gamble for different motives, might be characterized by different underlying brain mechanisms. For example, PGs who gamble to cope with negative affect might be primarily characterized by abnormal functioning of the amygdala circuit, whereas PGs who gamble to enhance positive affect might be primarily characterized by a hyperactive orbitofronto-striatal circuitry (71,72). More research is needed to elucidate the various endophenotypes (including dopamine functioning) underlying pathological gambling subtypes and to assess their validity in research and clinical treatment. Future (longitudinal) studies will be needed to replicate the current findings and better understand the (causal or consequential) interplay among dopamine synthesis capacity, dopamine release, and D2/D3 receptor availability.
The observation that higher dopamine synthesis capacity in PGs was most consistently found in the dorsal parts of the striatum is striking and overlaps with the location of increased striatal dopamine release previously reported as a result of amphetamine administration in the anterior caudate and putamen (40). The dorsal striatum is thought to play a crucial role in habitual control of behavior, as evidenced by dorsolateral striatal lesions disrupting habit formation in animals (73), for instance. Increased dopamine synthesis capacity in the dorsal putamen in PGs also fits with incentive sensitization studies in humans, showing that after repeated exposure to amphetamine the dorsal putamen becomes progressively involved (74,75). Note that such increased dopamine response to rewarding stimuli could also reflect a vulnerability to develop addictive behaviors (76). Indeed, animals with increased addiction vulnerability show increased dopamine release in the nucleus accumbens and dorsomedial striatum following psychostimulant administration, as assessed using voltammetry (77). Thus, increased dopamine synthesis capacity, especially in the dorsal putamen, could be a vulnerability and/or consequence of addictive behavior driving excessive reward-seeking behavior. However, our study was not designed to dissect whether alterations in dopamine synthesis capacity are a direct cause or consequence of pathological gambling, and the origin of this alteration is still speculative. One possibility is that genetic factors affecting components of the dopamine synthesis pathway, such as variants in the dopa decarboxylase gene found to be associated with gambling disorder (78), might play a role there.
Our results should be interpreted with the following limitations in mind. First, the study included a small sample of only male subjects. This strategy led to a homogeneous population without psychiatric comorbidities—in particular, without drug dependence—which enabled us to measure dopamine synthesis capacity in pathological gambling without confounding factors. However, one should note that, with the exception of current and past-year drug dependence history, all other clinical assessments were performed away from the PET study (median = 23.5 months prior to the PET study) on the occasion of a preceding pharmaco-fMRI study. In addition, 5 HCs and 5 PGs scored ≥8 on the Alcohol Use Disorders Identification Test questionnaire, indicating possible alcohol problems. However, because groups did not differ on Alcohol Use Disorders Identification Test scores, it is unlikely that this would have influenced our main finding of enhanced dopamine synthesis capacity in PGs relative to HCs. Interestingly, at the time of the PET scan, only 4 of the 13 gamblers were experiencing acute problems with gambling (as assessed by scores >5 on the past-3-month SOGS). It is unclear how this might have affected our results, but because dopamine synthesis capacity is thought to be a stable measure over long periods of time (79), the increased dopamine synthesis capacity found in our PGs might reflect a vulnerability in the dopamine system rather than a consequence of current gambling problems. Preclinical and longitudinal studies are needed to address whether heightened dopamine synthesis capacity in pathological gambling existed before the onset of problematic gambling or developed as a consequence of the disorder. Another caveat is that although we observed a clearly enhanced dopamine synthesis capacity in PGs compared with HCs in the non-PVC ROIs—specifically in the dorsal putamen, caudate body, and ventral striatum—this group difference was less striking in the PVC ROIs and significant only in the dorsal putamen ROI (which was also the case for atlas-based anatomical ROIs). These differences between non-PVC and PVC results are explained by the incorporation of the size and shape of the ROIs, as well as their proximity to white matter and cerebral spinal fluid, in the latter method. Using PVC ROIs is not always the most sensitive method because PVC methods can amplify the existing noise (80) such as increasing the variance in the time-activity curves (81). Given the scarcity of reports examining [18F]DOPA differences in pathological gambling, we included different analysis methods to comprehensively test the reproducibility of the results. We believe that our analyses reveal robust findings of higher dopamine synthesis capacity in the dorsal putamen in PGs.
At the clinical level, our results suggest that it could be beneficial to reduce dopamine levels in pathological gambling. However, two double-blind, placebo-controlled trials of the atypical antipsychotic olanzapine, a dopamine and serotonin antagonist, have shown no benefit over placebo (82,83), similar to what was found with bupropion, a dopamine and norepinephrine transporter inhibitor (84). Multiple psychopharmacological studies using the dopamine D2/D3 receptor antagonists sulpiride and haloperidol have also yielded inconclusive results regarding the ability of these drugs to normalize reward processing in pathological gambling (41,46,85,86). However, a small single-blind study using the D1 receptor antagonist ecopipam in PGs did lead to significant reductions in gambling severity measures (87). Clearly, more research is needed to assess whether and how striatal dopamine receptor blockade would be effective in treating pathological gambling.
Finally, addiction is a complex mixture of behaviors and cognitions that is reflected in the heterogeneity of the patients and also varies from drug to drug, game to game, and drug to game. As emphasized by Nutt et al. (7), “it is unlikely that a single neurotransmitter could explain every aspect of addiction.”
Supplementary Material
ACKNOWLEDGMENTS
RJvH was supported by a Rubicon grant from the Netherlands Research Organization (Grant No. 446.11.025). GS was supported by a Veni grant from the Netherlands Research Organization (Grant No. 016.155.218). RC was supported by a Vici grant from the Netherlands Research Organization (Grant No. 2015/24762/MaGW) and a James McDonnell scholar award.
Footnotes
DISCLOSURES
WJJ serves as a consultant to Genentech, Novartis, and Bioclinica. All other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2017.06.010.
REFERENCES
- 1.Holden C (2010): Psychiatry: Behavioral addictions debut in proposed DSM-V. Science 327:935. [DOI] [PubMed] [Google Scholar]
- 2.van Holst RJ, van den Brink W, Veltman DJ, Goudriaan AE (2010): Why gamblers fail to win: A review ofcognitive and neuroimaging findings in pathological gambling. Neurosci Biobehav Rev 34:87–107. [DOI] [PubMed] [Google Scholar]
- 3.Potenza MN (2001): The neurobiology of pathological gambling. Semin Clin Neuropsychiatry 6:217–226. [PubMed] [Google Scholar]
- 4.Goudriaan AE, Oosterlaan J, de Beurs E, Van den Brink W (2004): Pathological gambling: A comprehensive review of biobehavioral findings. Neurosci Biobehav Rev 28:123–141. [DOI] [PubMed] [Google Scholar]
- 5.Zack M, Poulos CX (2009): Parallel roles for dopamine in pathological gambling and psychostimulant addiction. Curr Drug Abuse Rev 2: 11–25. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed Washington, DC: American Psychiatric Association. [Google Scholar]
- 7.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PRA (2015): The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci 16:305–312. [DOI] [PubMed] [Google Scholar]
- 8.Ashok AH, Mizuno Y, Volkow ND, Howes OD (2017): Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: A systematic review and metaanalysis. JAMA Psychiatry 74:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. (2009): Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29:14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, et al. :(2004):Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: Relationship with cocaine-seeking behavior. Neuropsychopharmacology 29:1190–1202. [DOI] [PubMed] [Google Scholar]
- 11.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. (2011): Imaging dopamine transmission in cocaine dependence: Link between neurochemistry and response to treatment. Am J Psychiatry 168:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. :Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 58:779–786. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, Fowler JS, Wang GJ (2004): The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology 47(Suppl 1):3–13. [DOI] [PubMed] [Google Scholar]
- 14.Zijlstra F, Booij J, van den Brink W, Franken IHA (2008): Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol 18:262–270. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Han M, Liu X, Deng Y, Li Y, Yuan J, et al. (2013): Dopamine transporter availability in heroin-dependent subjects and controls: Longitudinal changes during abstinence and the effects of Jitai tablets treatment. Psychopharmacology (Berl) 230:235–244. [DOI] [PubMed] [Google Scholar]
- 16.Heinz A, Siessmeier T, Wrase J, Buchholz HG, Gründer G, Kumakura Y, et al. (2005): Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: A combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry 162:1515–1520. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Fowler JS, Wang G-J, Swanson JM, Telang F (2007): Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch Neurol 64:1575–1579. [DOI] [PubMed] [Google Scholar]
- 18.Rademacher L, Prinz S, Winz O, Henkel K, Dietrich CA, Schmaljohann J, et al. (2016): Effects of smoking cessation on pre-synaptic dopamine function of addicted male smokers. Biol Psychiatry 80:198–206. [DOI] [PubMed] [Google Scholar]
- 19.Bloomfield MAP, Morgan CJA, Kapur S, Curran HV, Howes OD (2014): The link between dopamine function and apathy in cannabis users: An [18F]-DOPA PET imaging study. Psychopharmacology (Berl) 231:2251–2259. [DOI] [PubMed] [Google Scholar]
- 20.Bloomfield MAP, Pepper F, Egerton A, Demjaha A, Tomasi G, Mouchlianitis E, et al. (2014): Dopamine function in cigarette smokers: An [18F]-DOPA PET study. Neuropsychopharmacology 39:2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deserno L, Beck A, Huys QJM, Lorenz RC, Buchert R, Buchholz H-G, et al. (2015): Chronic alcohol intake abolishes the relationship between dopamine synthesis capacity and learning signals in the ventral striatum. Eur J Neurosci 41:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. (2002): Social dominance in monkeys: Dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5:169–174. [DOI] [PubMed] [Google Scholar]
- 23.Groman SM, Jentsch JD (2013): Identifying the molecular basis of inhibitory control deficits in addictions: Neuroimaging in non-human primates. Curr Opin Neurobiol 23:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. (2006): PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci 9:1050–1056. [DOI] [PubMed] [Google Scholar]
- 25.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008): High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, et al. (2007): Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME (2005): Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 178:193–201. [DOI] [PubMed] [Google Scholar]
- 28.Poulos CX, Le AD, Parker JL (1995): Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol 6:810–814. [PubMed] [Google Scholar]
- 29.Tournier BB, Steimer T, Millet P, Moulin-Sallanon M, Vallet P, Ibañez V, et al. (2013): Innately low D2 receptor availability is associated with high novelty-seeking and enhanced behavioural sensitization to amphetamine. Int J Neuropsychopharmacol 16:1819–1834. [DOI] [PubMed] [Google Scholar]
- 30.Verdejo-García A, Lawrence AJ, Clark L (2008): Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32:777–810. [DOI] [PubMed] [Google Scholar]
- 31.Reeves SJ, Polling C, Stokes PRA, Lappin JM, Shotbolt PP, Mehta MA, et al. (2012): Limbic striatal dopamine D2/3 receptor availability is associated with non-planning impulsivity in healthy adults after exclusion of potential dissimulators. Psychiatry Res 202:60–64. [DOI] [PubMed] [Google Scholar]
- 32.Kim J-H, Son Y-D, Kim H-K, Lee S-Y, Kim Y-B, Cho Z-H (2014): Dopamine D2/3 receptor availability and human cognitive impulsivity: A high-resolution positron emission tomography imaging study with [11C]raclopride. Acta Neuropsychiatr 26:35–2. [DOI] [PubMed] [Google Scholar]
- 33.Caravaggio F, Fervaha G, Chung JK, Gerretsen P, Nakajima S, Plitman E, et al. (2016): Exploring personalitytraits related todopamine D2/3 receptor availability in striatal subregions of humans. Eur Neuropsychopharmacol 26:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boileau I, Payer D, Chugani B, Lobo D, Behzadi A, Rusjan PM, et al. (2013):The D2/3 dopamine receptor in pathological gambling: A positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction 108:953–963. [DOI] [PubMed] [Google Scholar]
- 35.Clark L, Stokes PR, Wu K, Michalczuk R, Benecke A, Watson BJ, et al. (2012): Striatal dopamine D2/D3 receptor binding in pathological gambling is correlated with mood-related impulsivity. NeuroImage 63:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joutsa J, Johansson J, Niemela S, Ollikainen A, Hirvonen MM, Piepponen P, et al. (2012): Mesolimbic dopamine release is linked to symptom severity in pathological gambling. NeuroImage 60: 1992–1999. [DOI] [PubMed] [Google Scholar]
- 37.Linnet J, Peterson E, Doudet DJ, Gjedde A, Moller A (2010): Dopamine release in ventral striatum of pathological gamblers losing money. Acta Psychiatr Scand 122:326–333. [DOI] [PubMed] [Google Scholar]
- 38.Peterson E, Moller A, Doudet DJ, Bailey CJ, Hansen KV, Rodell A, et al. (2010): Pathological gambling: Relation of skin conductance response to dopaminergic neurotransmission and sensation-seeking. Eur Neuropsychopharmacol 20:766–775. [DOI] [PubMed] [Google Scholar]
- 39.Linnet J, MØller A, Peterson E, Gjedde A, Doudet D (2011): Dopamine release in ventral striatum during Iowa Gambling Task performance is associated with increased excitement levels in pathological gambling. Addiction 106:383–390. [DOI] [PubMed] [Google Scholar]
- 40.Boileau I, Payer D, Chugani B, Lobo DSS, Houle S, Wilson AA, et al. (2014):In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: A positron emission tomography study with [11C]-(+)-PHNO. Mol Psychiatry 19:1305–1313. [DOI] [PubMed] [Google Scholar]
- 41.Janssen LK, Sescousse G, Hashemi MM, Timmer MHM, ter Huurne NP, Geurts DEM, et al. (2015): Abnormal modulation of reward versus punishment learning by a dopamine D2-receptor antagonist in pathological gamblers. Psychopharmacology (Berl) 232:3345–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dagher A, Robbins TW (2009): Personality, addiction, dopamine: Insights from Parkinson’s disease. Neuron 61:502–510. [DOI] [PubMed] [Google Scholar]
- 43.Majuri J, Joutsa J, Johansson J, Voon V, Alakurtti K, Parkkola R, et al. (2017): Dopamine and opioid neurotransmission in behavioral addictions: A comparative PET study in pathological gambling and binge eating. Neuropsychopharmacology 42:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence AD, Brooks DJ (2014): Ventral striatal dopamine synthesis capacity is associated with individual differences in behavioral disinhibition. Front Behav Neurosci 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrence AD, Brooks DJ, Whone AL (2013): Ventral striatal dopamine synthesis capacity predicts financial extravagance in Parkinson’s disease. Front Psychol 4:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sescousse G, Janssen LK, Hashemi MM, Timmer MH, Geurts DE, Ter Huurne NP, et al. (2016): Amplified striatal responses to near-miss outcomes in pathological gamblers. Neuropsychopharmacology 41:2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. (1998): The Mini-International Neuropsychiatric Interview(M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20): 22–33. [PubMed] [Google Scholar]
- 48.Yudko E, Lozhkina O, Fouts A, Roy-Byrne P, Gleason J, Back S, et al. (2007): A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat 32:189–198. [DOI] [PubMed] [Google Scholar]
- 49.Lesieur HR, Blume SB (1987): The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. Am J Psychiatry 144:1184–1188. [DOI] [PubMed] [Google Scholar]
- 50.Steenbergh TA, Meyers AW, May RK, Whelan JP (2002): Development and validation of the Gamblers’ Beliefs Questionnaire. Psychol Addict Behav 16:143–149. [PubMed] [Google Scholar]
- 51.Patton JH, Stanford MS, Barratt ES (1995): Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol 51:768–774. [DOI] [PubMed] [Google Scholar]
- 52.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang D-RR, et al. (2001): Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21:1034–1057. [DOI] [PubMed] [Google Scholar]
- 53.Patlak CS, Blasberg RG (1985): Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data: Generalizations. J Cereb Blood Flow Metab 5:584–590. [DOI] [PubMed] [Google Scholar]
- 54.Schmand B, Bakker D, Saan R, Louman J (1991): [The Dutch Reading Test for Adults: A measure of premorbid intelligence level]. Tijdschr Gerontol Geriatr 22:15–19. [PubMed] [Google Scholar]
- 55.Rousset OG, Ma Y, Evans AC (1998): Correction for partial volume effects in PET: Principle and validation. J Nucl Med 39:904–911. [PubMed] [Google Scholar]
- 56.Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. (2003): Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp 19:224–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark L (2014): Disordered gambling: The evolving concept of behavioral addiction. Ann N Y Acad Sci 1327:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Sullivan SS, Wu K, Politis M, Lawrence AD, Evans AH, Bose SK, et al. (2011): Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive-compulsive behaviours. Brain 134(Pt 4): 969–978. [DOI] [PubMed] [Google Scholar]
- 59.Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, et al. (2009): Increased striatal dopamine release in parkinsonian patients with pathological gambling: A [11C]raclopride PET study. Brain 132(Pt 5):1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goodie AS, Fortune EE (2013): Measuring cognitive distortions in pathological gambling: Review and meta-analyses. Psychol Addict Behav 27:730–743. [DOI] [PubMed] [Google Scholar]
- 61.Toneatto T, Gunaratne M (2009): Does the treatment of cognitive distortions improve clinical outcomes for problem gambling? J Contemp Psychother 39:221–229. [Google Scholar]
- 62.Sylvain C, Ladouceur R, Boisvert JM (1997): Cognitive and behavioral treatment of pathological gambling: A controlled study. J Consult Clin Psychol 65:727–732. [DOI] [PubMed] [Google Scholar]
- 63.Winfree WR, Ginley MK, Whelan JP, Meyers AW (2015): Psychometric evaluation of the Gamblers’ Beliefs Questionnaire with treatmentseeking disordered gamblers. Addict Behav 43:97–102. [DOI] [PubMed] [Google Scholar]
- 64.Robinson TE, Berridge KC (2001): Incentive-sensitization and addiction. Addiction 96:103–114. [DOI] [PubMed] [Google Scholar]
- 65.Laruelle M (2000): Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab 20:423–451. [DOI] [PubMed] [Google Scholar]
- 66.Verheij MMM, de Mulder ELW, De Leonibus E, van Loo KMJ, Cools AR (2008): Rats that differentially respond to cocaine differ in their dopaminergic storage capacity of the nucleus accumbens. J Neurochem 105:2122–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito H, Kodaka F, Takahashi H, Takano H, Arakawa R, Shimada H, et al. (2011): Relation between presynaptic and postsynaptic dopaminergic functions measured by positron emission tomography: Implication of dopaminergic tone. J Neurosci 31:7886–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kienast T, Siessmeier T, Wrase J, Braus DF, Smolka MN, Buchholz HG, et al. (2008): Ratio of dopamine synthesis capacity to D2 receptor availability in ventral striatum correlates with central processing of affective stimuli. Eur J Nucl Med Mol Imaging 35:1147–1158. [DOI] [PubMed] [Google Scholar]
- 69.Nower L, Blaszczynski A (2010): Gambling motivations, money-limiting strategies, and precommitment preferences of problem versus non-problem gamblers. J Gambl Stud 26:361–372. [DOI] [PubMed] [Google Scholar]
- 70.Blaszczynski A, Nower L (2002): A pathways model of problem and pathological gambling. Addiction 97:487–499. [DOI] [PubMed] [Google Scholar]
- 71.Goudriaan AE, Yücel M, van Holst RJ (2014): Getting a grip on problem gambling: What can neuroscience tell us? Front Behav Neurosci 8:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yücel M, Carter A, Allen AR, Balleine B, Clark L, Dowling NA, et al. (2017): Neuroscience in gambling policy and treatment: An interdisciplinary perspective. Lancet Psychiatry 4:501–506. [DOI] [PubMed] [Google Scholar]
- 73.Yin HH, Knowlton BJ, Balleine BW (2004): Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 19:181–189. [DOI] [PubMed] [Google Scholar]
- 74.Schneck N, Vezina P (2012): Enhanced dorsolateral striatal activity in drug use: The role of outcome in stimulus-response associations. Behav Brain Res 235:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, et al. (2006):Modeling sensitization to stimulants in humans: An [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry 63:1386–1395. [DOI] [PubMed] [Google Scholar]
- 76.Leyton M, Vezina P (2013): Striatal ups and downs: Their roles in vulnerability to addictions in humans. Neurosci Biobehav Rev 37(9 Pt A):1999–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yorgason JT, Calipari ES, Ferris MJ, Karkhanis AN, Fordahl SC, Weiner JL, et al. (2016): Social isolation rearing increases dopamine uptake and psychostimulant potency in the striatum. Neuropharmacology 101:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lind PA, Zhu G, Montgomery GW, Madden PAF, Heath AC, Martin NG, et al. (2013): Genome-wide association study of a quantitative disordered gambling trait. Addict Biol 18:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vingerhoets FJG, Snow BJ, Schulzer M, Morrison S, Ruth TJ, Holden JE, et al. (1994): Reproducibility of fluorine-18-fluorodopa positron emission tomography in normal human subjects. J Nucl Med 35:18–24. [PubMed] [Google Scholar]
- 80.Aston JAD, Cunningham VJ, Asselin M-C, Hammers A, Evans AC, Gunn RN (2002): Positron emission tomography partial volume correction: Estimation and algorithms. J Cereb Blood Flow Metab 22:1019–1034. [DOI] [PubMed] [Google Scholar]
- 81.Rousset OG, Deep P, Kuwabara H, Evans AC, Gjedde AH, Cumming P (2000): Effect of partial volume correction on estimates of the influx and cerebral metabolism of 6-[18F]fluoro-L-dopa studied with PET in normal control and Parkinson’s disease subjects. Synapse 37: 81–89. [DOI] [PubMed] [Google Scholar]
- 82.Fong T, Kalechstein A, Bernhard B, Rosenthal R, Rugle L (2008): A double-blind, placebo-controlled trial of olanzapine for the treatment of video poker pathological gamblers. Pharmacol Biochem Behav 89:298–303. [DOI] [PubMed] [Google Scholar]
- 83.McElroy SL, Nelson EB, Welge JA, Kaehler L, Keck PE (2008): Olanzapine in the treatment of pathological gambling: A negative randomized placebo-controlled trial. J Clin Psychiatry 69: 433–440. [DOI] [PubMed] [Google Scholar]
- 84.Black DW, Arndt S, Coryell WH, Argo T, Forbush KT, Shaw MC, et al. (2007):Bupropion in the treatment of pathological gambling: A randomized, double-blind, placebo-controlled, flexible-dose study. J Clin Psychopharmacol 27:143–150. [DOI] [PubMed] [Google Scholar]
- 85.Porchet RI, Boekhoudt L, Studer B, Gandamaneni PK, Rani N, Binnamangala S, et al. (2013): Opioidergic and dopaminergic manipulation of gambling tendencies: A preliminary study in male recreational gamblers. Front Behav Neurosci 7:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tremblay A-M, Desmond RC, Poulos CX, Zack M (2011): Haloperidol modifies instrumental aspects of slot machine gambling in pathological gamblers and healthy controls. Addict Biol 16: 467–484. [DOI] [PubMed] [Google Scholar]
- 87.Grant JE, Odlaug BL, Black DW, Fong T, Davtian M, Chipkin R, et al. (2014): A single-blind study of “as-needed” ecopipam for gambling disorder. Ann Clin Psychiatry 26:179–186. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


