Abstract
Nature, a vast reservoir of pharmacologically active molecules, has been most promising source of drug leads for the cure of various pathological conditions. Formononetin is one of the bioactive isoflavones isolated from different plants mainly from Trifolium pratense, Glycine max, Sophora flavescens, Pycnanthus angolensis, and Astragalus membranaceus. Formononetin has been well-documented for its anti-inflammatory, anticancer, and antioxidant properties. Recently anticancer activity of formononetin is widely studied. This review aims to highlight the pharmacological potential of formononetin, thus providing an insight of its status in cancer therapeutics. Formononetin fights progression of cancer via inducing apoptosis, arresting cell cycle, and halting metastasis via targeting various pathways which are generally modulated in several cancers. Although reported data acclaims various biological properties of formononetin, further experimentation on mechanism of its action, medicinal chemistry studies, and preclinical investigations are surely needed to figure out full array of its pharmacological and biological potential.
1. Introduction
Natural products have served as an infinite reservoir of various diversified chemical compounds, driving pharmaceutical industries for many years [1]. In the discipline of cancer therapeutics, natural products hold a great potential. It has been reported that, from 1981 to 2014, about 1562 drugs were approved out of which 1211 drugs were derived from small molecules that are nonsynthetic [2]. About 50% of the medicines and 48.6% of anticancer drugs are derivatives of natural products [3]. Chinese encyclopedia that dates back to 2000 BC has reported that 1898 herbal products have been used as medicines [4]. In accordance to World Health Organization, about 80% of population depends upon plant-derived traditional medicines. This drug discovery approach exhibits far-reaching domain where large-scale research could provide novel leads against cellular or molecular targets [5].
Different pharmacological studies on natural products have proclaimed their authenticity as potent anticancer [6], antioxidant [7], and anti-inflammatory agents [8].
Triterpenoid saponins and isoflavones belong to family of amphiphilic glycosides which are naturally present in medicinal plants, herbs, and marine organisms. Saponins and isoflavones have major role in folk medicine due to their biological and pharmacological properties [9]. These secondary metabolites possess various anticancer, anti-inflammatory, and antioxidant properties. Epidemiological studies recommend that intake of food enriched with isoflavonoids reduces the risk of various cancers [9].
Formononetin, an isoflavone isolated from soy bean and red clover, has been known to be endowed with numerous pharmacological attributes such as anticancer [10], anti-inflammatory, [11], and antioxidant attributes [12].
To date, there is no review on the biological potential of formononetin. This article intends to focus on the researches relevant to the biological and pharmacological activities of Formononetin. The literature is searched via different e-sites like PubMed, Elsevier Science Direct, Springer Link, and related journals. Key words which are used for searching are “Formononetin”, “Formononetin and its biological activities”, “anticancer”, and “natural products”.
2. Natural Sources of Formononetin
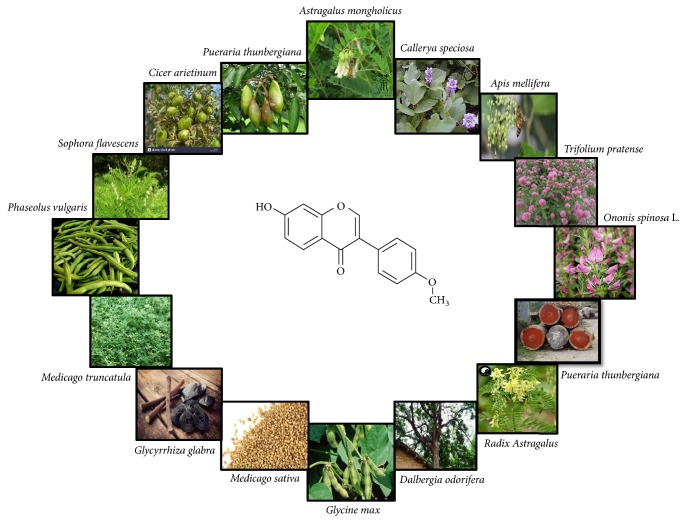
Formononetin (Figure 1) has been reported to be isolated from different plants of bean family “Fabaceae” which is the 3rd largest family of land plants. Genus Trifolium (Fabaceae) contains 250 species, most of which have been documented as rich source of formononetin [13]. In addition to this family, formononetin is also present in different plants including Trifolium pratense [14], Glycine max [15, 16], Sophora flavescens [17], Pycnanthus angolensis [18], Spatholobus suberectus [19], Cicer arietinum [20], Dalbergia odorifera, Pueraria thunbergiana [21], Actaea racemosa [22], Ononis spinosa L. [9], Dalbergia ecastaphyllum [23], Callerya speciosa [24], Astragalus membranaceus [25], and Astragalus mongholicus as shown in Figure 1. The list of plants having Formononetin, their parts, and pharmacological properties are elaborated in Table 1.
Figure 1.
Natural sources of Formononetin.
Table 1.
List of plants containing Formononetin and its biological activities.
| Plants name | Part used/Extract | Yield of Formononetin | Functions | References | |
|---|---|---|---|---|---|
| Botanical name | Common name | ||||
| Astragalus mongholicus | Milk vetch | Roots | 10 mg /200 mg of crude extract % yield = 5 % |
Anti-tumor, Anti-oxidant, Antiviral, Anti-proliferative | [25–27] |
|
| |||||
| Astragalus membranaceus | Goat's horn | Flowers | 10 mg /200 mg of crude extract % yield = 5 % |
Anti-tumor, Anti-oxidant, Antiviral, Anti-proliferative | [25–27] |
|
| |||||
| Dalbergia ecastaphyllum | Brazilian red propolis | - - | 44.14 μg/ mg (Acetate fraction) % yield = 4.414 % |
Anti-tumor | [23, 28] |
|
| |||||
| Trifolium pratense | Red clover | Above ground parts, flower heads |
0.21–0.59 % (Above ground parts) 0.047–0.12% (Flower heads) |
Anti-proliferative | [23, 29] |
|
| |||||
| Ononis spinosa L. | Spiny rest harrow | Root | 113.622mg/ 100 g dry plant extract % yield = 0.133 % |
Beneficial for urinary and bladder infection | [9, 30] |
|
| |||||
| Glycine max | Soya bean | - - | - - | Antioxidant, Anti-inflammatory, Anti-microbial | [15] |
|
| |||||
| Radix astragalus | Yellow leader | Roots | 0.191 μg/mg % yield = 0.019 % |
Osteogenic activity, Prevent postmenopausal osteoporosis, Anti-oxidant | [16, 31] |
|
| |||||
| Glycyrrhiza glabra | Mulethi | Roots | 27.856mg/ 100g % yield = 0.027 % |
Anti-viral, Hepatoprotective | [30] |
|
| |||||
| Glycyrrhiza echinata | Chinese licorice | Roots | 5.218 mg/ 100 g % yield = 0.005 % |
- - | [30] |
|
| |||||
| Cicer arietinum | Chickpea | Seeds | 14.2 mg/ 150mg % yield = 9.46 % |
- - | [20] |
|
| |||||
| Sophora flavescens | Shrubby | Roots | 5mg/ 628g ether-soluble fraction % yield = 0.00079 % |
Immuno-enhancement effects | [17, 32] |
| Pycnanthus angolensis | African nutmeg | Bark | 16.2mg/180 g (n-hexane-EtOAc fraction) % yield = 0.009 % |
Apoptosis inducer | [18, 33] |
|
| |||||
| Spatholobus suberectus | Millettia | Stem | 87.5 mg/25.5 g crude extract % yield = 0.343 % |
Proteasome inhibitory activity | [19] |
|
| |||||
| Actaea racemosa | Black cohosh | Rhizome | - - | - - | [22] |
3. Biological Activities of Formononetin and Mechanisms of Its Action
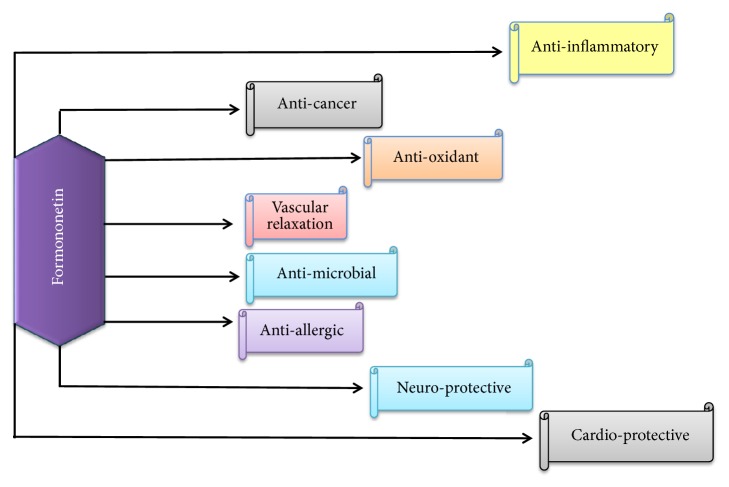
The biological and pharmacological activities of bioactive compound “Formononetin” are well-documented as antitumor activity, [23] antiproliferative activity [14], growth inhibitory activity [55], vasorelaxant action [56], neuroprotective effect [57], antiapoptotic activity [48], cardioprotective activity [58], mammary gland proliferative activity [59], antioxidant activity [60], antimicrobial activity, and anti-inflammatory activity [15] as provided in Figure 2. Numerous in vivo/in vitro investigations have been done on Formononetin to uncover its biological attributes and mechanisms of actions.
Figure 2.
Biological activities of Formononetin.
3.1. Anticancer Activity
Cancer is uncontrolled proliferation of cells which occurs due to genetic or epigenetic modifications and signaling defects in cells [61]. Different synthetic drugs are used for the treatment of this deadly disease [2]. Due to the limited success of synthetic drugs, it is necessary to identify novel natural products having the ability to induce apoptotic cell death and arrest cell cycle in tumor cells without toxic effect on normal cells [62]. Various studies have declared that formononetin can block, delay, or inhibit the initiation and progression of cancer. This review intends to focus on the studies relevant to anticancer potential of Formononetin which will allow the researchers to further investigate this novel chemical entity as a potential anticancer drug candidate.
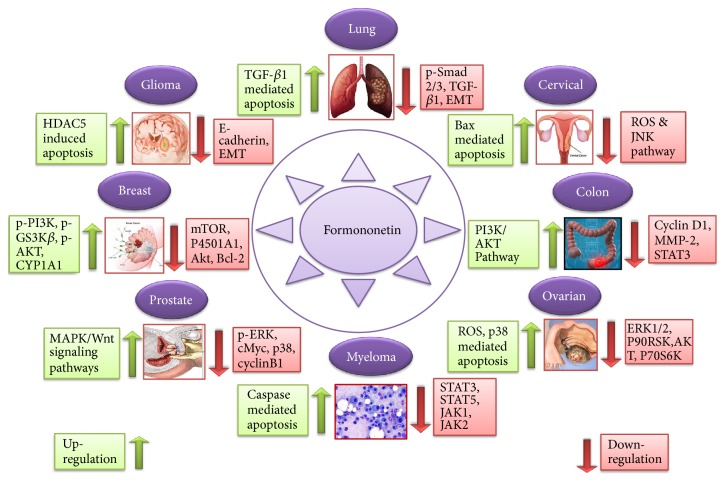
Currently, it is documented that about 60% drugs that are used for cure of cancer are derived from natural sources [63]. Secondary metabolites isolated from natural products encompassing terpenes, alkaloids, isoflavones, and polyphenols have been reported as anticancer agents [64, 65]. Anticancer properties of formononetin have been documented against several types of cancer [66] such as breast [67], colon [41], glioma [54], osteosarcoma [68], multiple myeloma [52], adrenal medulla [51], nasopharyngeal [50], prostate [49], bladder [45], laryngeal [23], lung [43], and cervical cancer [42] (Figure 3).
Figure 3.
Formononetin cytotoxic effects against various cancer types via different signaling pathways.
3.1.1. Formononetin and Cell Cycle Arrest
Uncontrolled divisions of cells are key trait of cancer cells [69]. Natural compounds have ability to prohibit the functions of cyclin dependent kinases and various other cell cycle regulatory proteins, thus causing cell cycle arrest [70].
Formononetin has been documented to arrest the cell cycle at various stages [44]. In human ovarian cancer, Formononetin leads cancer cells towards apoptosis and arrested cell cycle at G0/G1 phase in ES2 and OV90 cell lines [10]. Formononetin downregulated cyclin D1 which further arrested the cell cycle at the phase of G0/G1 in HCT-116 cells [41]. Formononetin arrested cells at G0/G1 phase in HepG2 cancerous cells [23]. In lung cancer cells, it caused the arresting of cell cycle at G1 phase via alteration of the p21, cyclin A, and cyclin D expression [44]. In PC-3 and DU145 cells, this compound has the ability to arrest the cells at G0/G1 phase via reducing AKT/cyclin D1/CDK4 expression [49] (Figure 4).
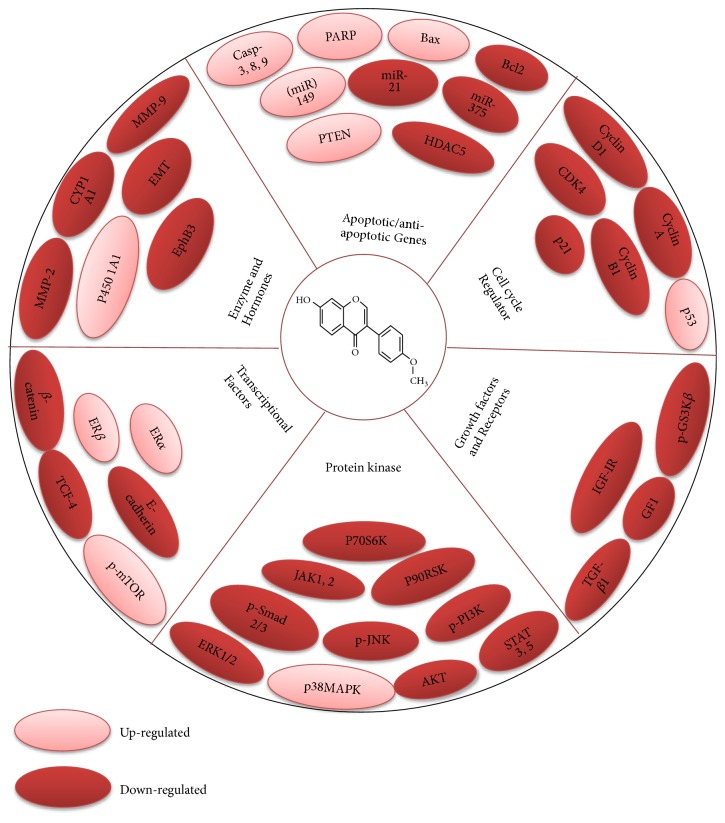
Figure 4.
Diagram presenting mechanism of action and molecular targets for Formononetin resulting in chemotherapeutic activity.
It can be summarized that Formononetin causes the arrest the cells at G0/G1 phase but it needs to be investigated whether it has arrested the cellular cycle at G0 or G1 phase. Furthermore, it is interesting for researchers to investigate whether Formononetin could arrest the cells at G2/M or S-phase. Thus, further mechanistic investigations are yet obligatory to understand the mechanisms by which Formononetin arrests the cell cycle in various cancers.
3.1.2. Formononetin and Apoptosis
Apoptosis is a systematic and synchronized way of cell death which is peculiarized by different morphological features including formation of blebs on cell membrane, condensation of chromosomes, and fragmentation of nucleus [71, 72]. It is reported that certain cellular signals are necessary to regulate the growth of cells but mutation in these signals prevent the cells to undergo apoptosis [73, 74]. Accumulated data indicated that various chemopreventive agents have been identified that can lead cancer cells towards apoptosis [75, 76]. Formononetin has the ability to inhibit or block the growth of cancerous cells via intrinsic or extrinsic apoptosis pathways [49].
Formononetin has emerged as novel agent for its chemotherapeutic activity [52]. Anticancer activity of Formononetin has been known to be associated with induction of apoptosis via activation of caspase family, ROS, activation of Bax, downmodulation of antiapoptotic protein Bcl-2, [53], upregulation of p-AKT [37], inhibiting the activation of NF-κB, reduction of ERK1/2 [10], inhibition of MMP-2/-9, upregulation of p38, p21, and p53 [10, 44], increase of the level of P450 1A1 [36], and blockage of PI3K/AKT, STAT3 signaling pathways [41] (Table 2) (Figure 4).
Table 2.
Molecular targets of Formononetin in different types of cancer.
| Cancer types | Cell lines | Treatment time | IC50 | Molecular targets | Cell cycle arrest | References |
|---|---|---|---|---|---|---|
| Ovarian | ES2,OV90 | 48 h | 40 μM | p38↑, ERK1/2↓, P90RSK↓, AKT↓, P70S6K↓, ROS↑ | G0/G1 | [10] |
| SKOV3 | 24 h | 283.5 μM | caspase3/9↑, Bax/Bcl2↑, MMP-2⊥, MMP-9⊥, p-ERK↓ | - - | [34] | |
| 48 h | 209.3 μM | |||||
| A2780 | 24 h | 310.0 μM | ||||
| 48 h | 186.1μM | |||||
|
| ||||||
| Breast | MDA-MB-231, MCF-7, SK-BR-3 |
24 h | 50 μg/ml | p-GS3Kβ⊥, p-PI3K⊥, p-mTOR↑, p-AKT⊥ | - - | [35] |
| MCF-7 WS8 | 48 h | 10 μg/ml | CYP1A1↓, P450 1A1↑ | - - | [36] | |
| MCF-7 | 24, 48h | 50 μM | ERα↑, miR-375↑, Bcl-2↓, p-AKT↑ | - - | [37] | |
| 4T1, MDA-MB-231 | 24 h | 160 μM | TIMP-2↑, TIMP-1↑, MMP-9↓, MMP-2↓ | - - | [38] | |
| MDA-231, MDA-435 | 24, 48, 72 h | 100 μM | ERβ↑, IGF-1R↓, PARP-1act, miR-375↓ | - - | [39] | |
| MCF-7 | 24, 48, 72 h | 100 μM | GF1/IGF1R-PI3K/ AKTinact, cyclin D1↓, Bax/Bcl-2↑, Ras-p38MAPKact, | - - | [40] | |
|
| ||||||
| Colon | SW1116,HCT116 | 24 h | 200 μM | cyclin D1↓, (MMP)2⊥, MMP9⊥, (miR)149↑, EphB3↓, PI3K/AKT⊥, STAT3⊥ | G0/G1 | [41] |
|
| ||||||
| Hepatoma | HuH-7 | 24 h | 20 μM | - - | - - | [18] |
|
| ||||||
| Cervical | HeLa, SiHa, CaSKi | 24 h | 25 Μm | ROS↓, JNK⊥, caspase-8act, caspase-3act, Bax/Bcl2↑, PI3K/AKT⊥ | - - | [42] |
|
| ||||||
| Laryngeal | Hep-2 | 24, 48 h | 50 and 75 μg/ml | ROS↓, CdCl2⊥ | G0/G1 | [23] |
|
| ||||||
| Lung | A549 | 48 h | 60 mg/ml | p-Smad 2/3↓, TGF-β1⊥, EMT⊥ | - - | [43] |
| A549, NCI-H23 | 24 h | 100 μM | p53↑, p21↑, cyclin A↓, cyclin D1↓, | G1 | [44] | |
|
| ||||||
| Bladder | T24 | 24 h | 200 μM | T24⊥, PTEN↑, p- AKT ↓, miR-21↓ | - - | [45] |
|
| ||||||
| Gastric | MGC-803 | - - | - - | - - | - - | [46] |
|
| ||||||
| Esophagus | EC-109 | - - | - - | - - | - - | [46] |
|
| ||||||
| Prostate | PC3 | 48 h | 1.97 μM | p-ERK↓, p-JNK↓, c-Myc↓ p-p38↓, cyclin B1↓, cyclin A↓, cyclin D1↓, CDK4↓, Axin↑, β-catenin↓, TCF-4↓ | G1 | [46] |
| DU145, PC3 | 48 h | 200 μM | Bcl-2↓, RASD1↑, Bax↑, IGF-1 R↓ | - - | [47] | |
| PC3 | 48 h | >12.5 μM | [48] | |||
| PC-3, DU145 | 48 h | 80μM | AKT /cyclin D1/CDK4↓ | G0/G1 | [49] | |
|
| ||||||
| Nasopharyngeal | CNE2 | 24 h | 1 μM | Bax↓, bcl-2↑, p-ERK1/2↑ | - - | [50] |
|
| ||||||
| Adrenal medulla | PC12 | 24 h | 20 μg/ml | ROS↑, MDA↓, GSH↓ | - - | [51] |
|
| ||||||
| Multiple myeloma | U266, RPMI8226 | 48 h | 100 μM | STAT3↓, STAT5↓, JAK1↓, JAK2↓, cSrc↓, ROS↑, caspase-3act, PARPclevage | - - | [52] |
|
| ||||||
| Osteosarcoma | U2OS | 48 h | 80 μM | Bax↑, Bcl2↓, miR-375↓, caspase-3↑, ERKinact, AKTinact | - - | [53, 54] |
|
| ||||||
| Glioma | U87MG, U251MG, T98G | 24,48 h | 100 μM | E-cadherin↓, HDAC5↑ | - - | [54] |
Downregulation↓, Upregulation ↑, Activation Act, Inhibition⊥, Bax: Bcl-2 associated x protein, Bcl-2: B-cell lymphoma, JNK: c-Jun-N-terminal kinase, MAPK: mitogen activated protein kinase, mTOR: mammalian target of rapamycin, Cdks: cyclin dependent kinases, EKR: extracellular signal-regulated kinase.
(1) Formononetin and MAPK and PI3K/AKT Pathway. MAPK, mitogen-activated protein kinase pathway, performs significant function in cell division, differentiation, proliferation, and cellular apoptosis [77]. MAPK pathway has four distinct signaling domains such as ERK, JNK, BMK-1, and p38 MAPK. Extracellular signals conducted by these kinases regulate the process of cellular apoptosis and growth [78, 79]. MAPK pathway has been reported as novel target to combat cancer. The PI3K/AKT pathway also performs a significant role in tumor development. This activated pathway is affiliated with several cancer types. Therefore, targeting this pathway has the ability to combat cancer [80].
Isoflavonoids have been documented as anticancer agents that inhibit cancer cell proliferation and have antimetastatic effects [35, 81]. Formononetin has ability for the induction of apoptosis in breast cancerous cells via activating Ras/p38 MAPK pathway in a dose dependent mode [40].
Formononetin reduced the p-AKT, p-P90RSK, p-S6, p-ERK1/2, and p-P70S6K proteins as well as enhancing the levels of p-p38 MAPK to mediate cellular proliferation and apoptosis in OV90 and ES2 cells [10]. Treatment with formononetin enhanced the levels of ERα and p-AKT in HUVEC and MCF-7 cancer cells [37]. This natural compound Formononetin suppresses proliferation and invasive capability of cells by inhibiting MMP-2/-9 via inactivation of phosphorylation of AKT and PI3K in HCT-116 and SW1116 cells [37, 41]. Formononetin also exerts its anticancer effects due to downmodulation of p-AKT, miR-21 expression, and upregulation of PTEN in T24 cells [45]. Formononetin inhibited the proliferation via inactivation of PI3K/ AKT pathway, enhancing the Bax, and downmodulating the Bcl-2 expression [68]. Formononetin and its derivatives such as dithiocarbamate with IC50 value of 1.9 μM possess inhibitory potential against PC3 cells [46] (Table 2).
(2) Formononetin and JAK/STAT Signaling Pathway. JAK/STAT pathways mediate the transduction of various signals involving cell division, immunity, tumor development, and cell death. Disruption in JAK/STAT signaling pathways may cause several diseases including cancer and immune disorders [82]. Formononetin decreased the activation of STAT5 and STAT3 by suppressing the nuclear translocation of p-STAT5 and p-STAT3 and also inhibited the activation JAK1 and JAK2 in U266 cells. Formononetin also inhibited the IL-6-induced STAT3 activity which ultimately inhibits the cell viability and activates apoptosis [52]. Formononetin suppresses the cellular invasion and proliferation by inhibition of MMP-2/-9 via inactivation of p-STAT3 pathway in colon carcinoma cells [41].
4. In Vivo Studies and Biosafety Profile
Formononetin with IC50 value 2-6 μM has the ability to promote the expression of p-AKT, miR-375, and Bcl-2 in in vivo mice model [37]. Treatment with Formononetin suppresses growth of tumor in in vivo tumor mouse model at the dose of 60 mg/kg [49]. An in vivo investigation demonstrated that Formononetin combined with other compounds reduced allergic inflammation in mice model via downregulating NF-κB activation [83]. Administration of Formononetin reduced the size, volume, and weight of HeLa tumor in in vivo mice model induced by injection of HeLa cells in the posterior flanks of mice [84]. As Formononetin is the imperative components of Chinese folk medicines Radix Astragalus and Fukeqianjin which are mostly used as antioxidant and anticancer agents, respectively, therefore, it might serve as safe chemotherapeutic drug candidate [85, 86]. Formononetin turns out to be a fascinated bioactive entity as it combines active chemotherapeutic effect with less toxicity in comparison to other isoflavones. However, safe doses and effectiveness of formononetin in targeted therapeutic fields still need to be executed in future.
5. Other Biological Functions
Formononetin is extracted from different plants such as Dalbergia odorifera in which Formononetin along with other compounds showed antiallergic and anti-inflammatory activities [87]. Formononetin together with other known compounds acts as active inhibitor of EV-A71 infection [88]. Brazilian Red propolis extract containing isoflavonoids such as Formononetin exhibits anti-inflammatory potential in a rat model of inflammation [89]. Isoflavones such as Formononetin isolated from soy bean possess antimicrobial and antioxidant activities with IC50 values from 10.6 to 22.6 μg/mL [15]. A study indicates that a methoxy Isoflavone, Formononetin, has potential for bone healing process in mouse model and has promising role in fracture-repair process [90]. Hydroalcoholic extract of Red propolis containing Red propolis has the ability to repair axon after sciatic nerve injury in rat model [91].
6. Conclusion and Future Perspectives
In this review article, we have suggested that Formononetin is a good drug candidate with promising pharmacological activities. Various researches have documented the potential applications of Formononetin both in vivo and in vitro. Being an important bioactive constituent of edible foods such as soybean, chickpeas, and alfalfa beans, Formononetin might turn up as a safe chemotherapeutic drug candidate. Many studies have revealed that formononetin is an inducer of apoptosis in many cancerous cells, but still mechanism of its action is not fully clarified. After the analysis of data, we have found that Formononetin is most active against nasopharyngeal cell line CNE2 with IC50 value of 1μM; so, further mechanistic studies should be conducted in future because nasopharyngeal carcinomas are one of the most prevalent cancers in Asia. This review also elucidates the potential role of Formononetin for the cure of several other diseases. Reported studies acclaim multiple biological properties of Formononetin but further experimentations on mechanism of its action, medicinal chemistry, and preclinical researches are yet necessary to figure out full array of its biological and pharmacological potential.
Acknowledgments
This study was supported by Ministry of Science and Technology, China (no. 2016YFE0128500), National Natural Science Foundation of China (no. 31870758), Jilin Provincial Science and Technology Department (20180414057GH, 20170204009YY), Changchun Science & Technology Department, China (17YJ006; 17YJ001), University S & T Innovation Platform of Jilin Province for Economic Fungi (#2014B-1), the Program for Introducing Talents to Universities (no. B07017), The Nagai Foundation Tokyo, Japan (NFT-R-2018), TWAS-COMSTECH Research Grant (no._17-180 RG/PHA/AS_C), and NRPU Research Grants (8381/Punjab/NRPU/R&D/HEC/2017, 8382/Punjab/NRPU/R&D/HEC/2017). The authors would also like to thank Higher Education Commission (HEC), Pakistan, for providing access to related papers from various journals.
Conflicts of Interest
Authors declare that there are no conflicts of interest.
Authors' Contributions
Dongjun Jiang and Rabia Batool made contribution to writing different parts of the manuscript. Azhar Rasul and Ghulam Hussain have made substantial contribution to integration of the data and drafting of the manuscript. Muhammad Mateen Tahir has contributed to acquisition of data. Iqra Sarfraz and Tian Qin designed and generated figures of manuscript. Zeliha Selamoglu and Muhammad Ali have reviewed the manuscript. Xiaomeng Li and Jiang Li have read and approved the final manuscript.
References
- 1.Mishra B. B., Tiwari V. K. Natural products: an evolving role in future drug discovery. European Journal of Medicinal Chemistry. 2011;46(10):4769–4807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 2.Sarfraz I., Rasul A., Jabeen F., et al. Fraxinus: a plant with versatile pharmacological and biological activities. Evidence-Based Complementary and Alternative Medicine. 2017;2017:12. doi: 10.1155/2017/4269868.4269868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman D. J., Cragg G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of Natural Products. 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerwick W. H., Fenner A. M. Drug discovery from marine microbes. Microbial Ecology. 2013;65(4):800–806. doi: 10.1007/s00248-012-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman D. J., Cragg G. M., Snader K. M. Natural products as sources of new drugs over the period 1981–2002. Journal of Natural Products. 2003;66(7):1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 6.Bauereiss N., Obermaier S., Özünal S. E., Baumeister H. Effects of existential interventions on spiritual, psychological, and physical well-being in adult patients with cancer: Systematic review and meta-analysis of randomized controlled trials. Psycho-Oncology. 2018;27(11):2531–2545. doi: 10.1002/pon.4829. [DOI] [PubMed] [Google Scholar]
- 7.Qiu Y. L., Cheng X. N., Bai F., Fang L. Y., Hu H. Z. Aucubin protects against lipopolysaccharide-induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. 2018;106:192–199. doi: 10.1016/j.biopha.2018.05.070. [DOI] [PubMed] [Google Scholar]
- 8.Veeramani C., Alsaif M. A., Al-Numair K. S. Lavatera critica controls systemic insulin resistance by ameliorating adipose tissue inflammation and oxidative stress using bioactive compounds identified by GC–MS. Biomedicine & Pharmacotherapy. 2018;106:183–191. doi: 10.1016/j.biopha.2018.06.121. [DOI] [PubMed] [Google Scholar]
- 9.Gampe N., Darcsi A., Kursinszki L., Béni S. Separation and characterization of homopipecolic acid isoflavonoid ester derivatives isolated from Ononis spinosa L. root. Journal of Chromatography B. 2018;1091:21–28. doi: 10.1016/j.jchromb.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Park S., Bazer F. W., Lim W., Song G. The O-methylated isoflavone, formononetin, inhibits human ovarian cancer cell proliferation by sub G0/G1 cell phase arrest through PI3K/AKT and ERK1/2 inactivation. Journal of Cellular Biochemistry. 2018;119(9):7377–7387. doi: 10.1002/jcb.27041. [DOI] [PubMed] [Google Scholar]
- 11.Wu D., Wu K., Zhu Q., et al. Formononetin administration ameliorates dextran sulfate sodium-induced acute colitis by inhibiting nlrp3 inflammasome signaling pathway. Mediators of Inflammation. 2018;2018 doi: 10.1155/2018/3048532.3048532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin Y.-W., Jung H.-A., Liu Y., et al. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra) Journal of Agricultural and Food Chemistry. 2007;55(12):4691–4697. doi: 10.1021/jf0703553. [DOI] [PubMed] [Google Scholar]
- 13.Dluhošová J., Ištvánek J., Nedělník J., Řepková J. Red clover (Trifolium pratense) and zigzag clover (T. medium) – a picture of genomic similarities and differences. Frontiers in Plant Science. 2018;9:p. 724. doi: 10.3389/fpls.2018.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandli A., Simpson J., Ventura S. Isoflavones isolated from red clover (Trifolium pratense) inhibit smooth muscle contraction of the isolated rat prostate gland. Phytomedicine. 2010;17(11):895–901. doi: 10.1016/j.phymed.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Wang T., Liu Y., Li X., Xu Q., Feng Y., Yang S. Isoflavones from green vegetable soya beans and their antimicrobial and antioxidant activities. Journal of the Science of Food and Agriculture. 2018;98(5):2043–2047. doi: 10.1002/jsfa.8663. [DOI] [PubMed] [Google Scholar]
- 16.Kong X., Wang F., Niu Y., Wu X., Pan Y. A comparative study on the effect of promoting the osteogenic function of osteoblasts using isoflavones from Radix Astragalus. Phytotherapy Research. 2018;32(1):115–124. doi: 10.1002/ptr.5955. [DOI] [PubMed] [Google Scholar]
- 17.Hwang J., Lee S. A., Hong S. S., et al. Monoamine oxidase inhibitory components from the roots ofSophora flavescens. Archives of Pharmacal Research. 2005;28(2):190–194. doi: 10.1007/BF02977714. [DOI] [PubMed] [Google Scholar]
- 18.Mansoor T. A., Ramalho R. M., Luo X., Ramalhete C., Rodrigues C. M. P., Ferreira M.-J. U. Isoflavones as apoptosis inducers in human hepatoma HuH-7 cells. Phytotherapy Research. 2011;25(12):1819–1824. doi: 10.1002/ptr.3498. [DOI] [PubMed] [Google Scholar]
- 19.Shim S. H. 20S proteasome inhibitory activity of flavonoids isolated from Spatholobus suberectus. Phytotherapy Research. 2011;25(4):615–618. doi: 10.1002/ptr.3342. [DOI] [PubMed] [Google Scholar]
- 20.Lv Q., Yang Y., Zhao Y., Gu D., et al. Comparative study on separation and purification of isoflavones from the seeds and sprouts of chickpea by HSCCC. Journal of Liquid Chromatography & Related Technologies. 2009;32(19):2879–2892. doi: 10.1080/10826070903297277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi C. W., Choi Y. H., Cha M.-R., et al. Yeast α-glucosidase inhibition by isoflavones from plants of leguminosae as an in vitro alternative to acarbose. Journal of Agricultural and Food Chemistry. 2010;58(18):9988–9993. doi: 10.1021/jf101926j. [DOI] [PubMed] [Google Scholar]
- 22.Avula B., Wang Y. H., Smillie T. J., Khan I. A. Quantitative determination of triterpenoids and formononetin in rhizomes of black cohosh (Actaea racemosa) and dietary supplements by using UPLC-UV/ELS detection and identification by UPLC-MS. Planta Medica. 2009;75(04):381–386. doi: 10.1055/s-0028-1088384. [DOI] [PubMed] [Google Scholar]
- 23.Frozza C. O. D. S., Santos D. A., Rufatto L. C., et al. Antitumor activity of Brazilian red propolis fractions against Hep-2 cancer cell line. Biomedicine & Pharmacotherapy. 2017;91:951–963. doi: 10.1016/j.biopha.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Qiao F., Jiang X., Cong H., Sun H., Li L., Nick P. Cell shape can be uncoupled from formononetin induction in a novel cell line from Callerya speciosa. Plant Cell Reports. 2018;37(4):665–676. doi: 10.1007/s00299-018-2259-8. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Liu J., Wu K.-X., Guo X.-R., Tang Z.-H. A rapid method for sensitive profiling of bioactive triterpene and flavonoid from Astragalus mongholicus and Astragalus membranaceus by ultra-pressure liquid chromatography with tandem mass spectrometry. Journal of Chromatography B. 2018;1085:110–118. doi: 10.1016/j.jchromb.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 26.Ma X., Tu P., Chen Y., Zhang T., Wei Y., Ito Y. Preparative isolation and purification of two isoflavones from Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao by high-speed counter-current chromatography. Journal of Chromatography A. 2003;992(1-2):193–197. doi: 10.1016/S0021-9673(03)00315-7. [DOI] [PubMed] [Google Scholar]
- 27.Park Y. J., Thwe A. A., Li X., et al. Triterpene and flavonoid biosynthesis and metabolic profiling of hairy roots, adventitious roots, and seedling roots of astragalus membranaceus. Journal of Agricultural and Food Chemistry. 2015;63(40):8862–8869. doi: 10.1021/acs.jafc.5b02525. [DOI] [PubMed] [Google Scholar]
- 28.das Neves M. V., da Silva T. M., Lima E. d., da Cunha E. V., Oliveira E. d. Isoflavone formononetin from red propolis acts as a fungicide against Candida sp. Brazilian Journal of Microbiology. 2016;47(1):159–166. doi: 10.1016/j.bjm.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booth N. L., Overk C. R., Yao P., et al. Seasonal variation of red clover (Trifolium pratense L., Fabaceae) isoflavones and estrogenic activity. Journal of Agricultural and Food Chemistry. 2006;54(4):1277–1282. doi: 10.1021/jf052927u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benedec D., Vlase L., Oniga I., Toiu A., Tǎmaş M., Tiperciuc B. Isoflavonoids from Glycyrrhiza sp. and Ononis spinosa. Farmacia. 2012;60(5):615–620. [Google Scholar]
- 31.Nguyen L. T. H., Nguyen U. T., Kim Y. H., Shin H. M., Yang I. J. Astragali Radix and its compound formononetin ameliorate diesel particulate matter-induced skin barrier disruption by regulation of keratinocyte proliferation and apoptosis. Journal of Ethnopharmacology. 2019;228:132–141. doi: 10.1016/j.jep.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Kyogoku K., Hatayama K., Komatsu M. Constituents of chinese crude drug “kushen” (the root of sophora flavescens ait.). isolation of five new flavonoids and formononetin. Chemical Pharmaceutical Bulletin. 1973;21(12):2733–2738. doi: 10.1248/cpb.21.2733. [DOI] [PubMed] [Google Scholar]
- 33.Nono E. C. N., Mkounga P., Kuete V., Marat K., Hultin P. G., Nkengfack A. E. Pycnanthulignenes A-D, antimicrobial cyclolignene derivatives from the roots of pycnanthus angolensis. Journal of Natural Products. 2010;73(2):213–216. doi: 10.1021/np9007393. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., Liu L., Wang J., Ren B., Zhang L., Li W. Formononetin, an isoflavone from Astragalus membranaceus inhibits proliferation and metastasis of ovarian cancer cells. Journal of Ethnopharmacology. 2018;221:91–99. doi: 10.1016/j.jep.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Zhou R., Chen H., Chen J., Chen X., Wen Y., Xu L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complementary and Alternative Medicine. 2018;18(1):p. 83. doi: 10.1186/s12906-018-2148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunlap T. L., Howell C. E., Mukand N., et al. Red clover aryl hydrocarbon receptor (AhR) and estrogen receptor (ER) agonists enhance genotoxic estrogen metabolism. Chemical Research in Toxicology. 2017;30(11):2084–2092. doi: 10.1021/acs.chemrestox.7b00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J., Zhang X., Wang Y., Ye Y., Huang Z. Formononetin promotes proliferation that involves a feedback loop of microRNA-375 and estrogen receptor alpha in estrogen receptor-positive cells. Molecular Carcinogenesis. 2016;55(3):312–319. doi: 10.1002/mc.22282. [DOI] [PubMed] [Google Scholar]
- 38.Zhou R., Xu L., Ye M., Liao M., Du H., Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Hormone and Metabolic Research. 2014;46(11):753–760. doi: 10.1055/s-0034-1376977. [DOI] [PubMed] [Google Scholar]
- 39.Chen J., Zhao X., Ye Y., Wang Y., Tian J. Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cellular Physiology and Biochemistry. 2013;32(6):1790–1797. doi: 10.1159/000356612. [DOI] [PubMed] [Google Scholar]
- 40.Chen J., Sun L. Formononetin-induced apoptosis by activation of Ras/p38 mitogen-activated protein kinase in estrogen receptor-positive human breast cancer cells. Hormone and Metabolic Research. 2012;44(13):943–948. doi: 10.1055/s-0032-1321818. [DOI] [PubMed] [Google Scholar]
- 41.Wang A. L., Li Y., Zhao Q., Fan L. Q. Formononetin inhibits colon carcinoma cell growth and invasion by microRNA149mediated EphB3 downregulation and inhibition of PI3K/AKT and STAT3 signaling pathways. Molecular Medicine Reports. 2018;17(6):7721–7729. doi: 10.3892/mmr.2018.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H., Lee D., Kang K. S., Song J. H., Choi Y.-K. Inhibition of intracellular ROS accumulation by formononetin attenuates cisplatin-mediated apoptosis in LLC-PK1 cells. International Journal of Molecular Sciences. 2018;19(3) doi: 10.3390/ijms19030813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He X. R., Han S. Y., Li X. H., Zheng W. X., Pang L. N., et al. Chinese medicine Bu-Fei decoction attenuates epithelial-mesenchymal transition of non-small cell lung cancer via inhibition of transforming growth factor beta1 signaling pathway in vitro and in vivo. Journal of ethnopharmacology. 2017;204:45–57. doi: 10.1016/j.jep.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y., Zhao Y., Ai X., Cheng B., Lu S. Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. International Journal of Clinical and Experimental Pathology. 2014;7(12):8453–8461. [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y., Zhang X., Li Z., Yan H., Qin J., Li T. Formononetin inhibits human bladder cancer cell proliferation and invasiveness via regulation of miR-21 and PTEN. Food & Function. 2017;8(3):1061–1066. doi: 10.1039/c6fo01535b. [DOI] [PubMed] [Google Scholar]
- 46.Fu D., Zhang L., Song J., et al. Design and synthesis of formononetin-dithiocarbamate hybrids that inhibit growth and migration of PC-3 cells via MAPK/Wnt signaling pathways. European Journal of Medicinal Chemistry. 2017;127:87–99. doi: 10.1016/j.ejmech.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X., Li Y., Chen Q., Xiao S., Zeng S. Up-regulating of RASD1 and apoptosis of DU-145 human prostate cancer cells induced by formononetin in vitro. Asian Pacific Journal of Cancer Prevention. 2014;15(6):2835–2839. doi: 10.7314/APJCP.2014.15.6.2835. [DOI] [PubMed] [Google Scholar]
- 48.Huang W., Bi L., Li Z., Zhang X., Ye Y. Formononetin induces the mitochondrial apoptosis pathway in prostate cancer cells via downregulation of the IGF-1/IGF-1R signaling pathway. Pharmaceutical Biology. 2013;52(4):466–470. doi: 10.3109/13880209.2013.842600. [DOI] [PubMed] [Google Scholar]
- 49.Li T., Zhao X., Mo Z., et al. Formononetin promotes cell cycle arrest via downregulation of akt/cyclin D1/CDK4 in human prostate cancer cells. Cellular Physiology and Biochemistry. 2014;34(4):1351–1358. doi: 10.1159/000366342. [DOI] [PubMed] [Google Scholar]
- 50.Guo Y. H., Wang Y., Xin M. Low concentration of formononetin stimulates the proliferation of nasopharyngeal carcinoma cell line CNE2 by upregulating bcl-2 and p-ERK1/2 expression. Pharmaceutical Biology. 2016;54(9):1841–1846. doi: 10.3109/13880209.2015.1129546. [DOI] [PubMed] [Google Scholar]
- 51.Hu Q., Yu J., Yang W., et al. Identification of flavonoids from Flammulina velutipes and its neuroprotective effect on pheochromocytoma-12 cells. Food Chemistry. 2016;204:274–282. doi: 10.1016/j.foodchem.2016.02.138. [DOI] [PubMed] [Google Scholar]
- 52.Kim C., Lee S., Yang W. M., et al. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Letters. 2018;431:123–141. doi: 10.1016/j.canlet.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 53.Hu W., Xiao Z. Formononetin induces apoptosis of human osteosarcoma cell line U2OS by regulating the expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cellular Physiology and Biochemistry. 2015;37(3):933–939. doi: 10.1159/000430220. [DOI] [PubMed] [Google Scholar]
- 54.Liu Q., Sun Y., Zheng J.-M., et al. Formononetin sensitizes glioma cells to doxorubicin through preventing EMT via inhibition of histone deacetylase 5. International Journal of Clinical and Experimental Pathology. 2015;8(6):6434–6441. [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J., Ge B., Wang Y., Ye Y., Zeng S., Huang Z. Biochanin a promotes proliferation that involves a feedback loop of microRNA-375 and estrogen receptor alpha in breast cancer cells. Cellular Physiology and Biochemistry. 2015;35(2):639–646. doi: 10.1159/000369725. [DOI] [PubMed] [Google Scholar]
- 56.Wu J.-H., Li Q., Wu M.-Y., et al. Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways. The Journal of Nutritional Biochemistry. 2010;21(7):613–620. doi: 10.1016/j.jnutbio.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Yu D., Duan Y., Bao Y., Wei C., An L. Isoflavonoids from Astragalus mongholicus protect PC12 cells from toxicity induced by l-glutamate. Journal of Ethnopharmacology. 2005;98(1-2):89–94. doi: 10.1016/j.jep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 58.Li G., Yang M., Hao X., Li C., Gao Y., Tao J. Acute toxicity of sodium formononetin-3'-sulphonate (Sul-F) in Sprague-Dawley rats and Beagle dogs. Regulatory Toxicology and Pharmacology. 2015;73(2):629–633. doi: 10.1016/j.yrtph.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 59.Wang W., Tanaka Y., Han Z., Higuchi C. M. Proliferative response of mammary glandular tissue to formononetin. Nutrition and Cancer. 1995;23(2):131–140. doi: 10.1080/01635589509514369. [DOI] [PubMed] [Google Scholar]
- 60.Pool-Zobel B. L., Adlercreutz H., Glei M., et al. Isoflavonoids and lignans have different potentials to modulate oxidative genetic damage in human colon cells. Carcinogenesis. 2000;21(6):1247–1252. doi: 10.1093/carcin/21.6.1247. [DOI] [PubMed] [Google Scholar]
- 61.Khan M., Maryam A., Zhang H., Mehmood T., Ma T. Killing cancer with platycodin D through multiple mechanisms. Journal of Cellular and Molecular Medicine. 2016;20(3):389–402. doi: 10.1111/jcmm.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rasul A., Millimouno F. M., Ali Eltayb W., Ali M., Li J., Li X. Pinocembrin: a novel natural compound with versatile pharmacological and biological activities. BioMed Research International. 2013;2013:9. doi: 10.1155/2013/379850.379850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dall’acqua S. Natural products as antimitotic agents. Current Topics in Medicinal Chemistry. 2014;14(20):2272–2285. doi: 10.2174/1568026614666141130095311. [DOI] [PubMed] [Google Scholar]
- 64.Rawat D. S., Singh R. Plant derived secondary metabolites as anti-cancer agents. Anti-Cancer Agents in Medicinal Chemistry. 2013;13(10):p. 1551. doi: 10.2174/187152061310131206154445. [DOI] [PubMed] [Google Scholar]
- 65.Yu L., Ma R., Wang Y., Nishino H. Potent anti-tumor activity and low toxicity of tubeimoside 1 isolated from Bolbostemma paniculatum. Planta Medica. 1994;60(03):204–208. doi: 10.1055/s-2006-959459. [DOI] [PubMed] [Google Scholar]
- 66.Navrátilová L., Applová L., Horký P., Mladěnka P., Pávek P., Trejtnar F. Interaction of soy isoflavones and their main metabolites with hOATP2B1 transporter. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2018;391(10):1073–1073. doi: 10.1007/s00210-018-1541-1. [DOI] [PubMed] [Google Scholar]
- 67.Chen J., Zhang X., Wang Y., Ye Y., Huang Z. Differential ability of formononetin to stimulate proliferation of endothelial cells and breast cancer cells via a feedback loop involving MicroRNA-375, RASD1, and ERalpha. Molecular Carcinogenesis. 2018;57(7):817–830. doi: 10.1002/mc.22531. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y., He J., Chen X., et al. The proapoptotic effect of formononetin in human osteosarcoma cells: involvement of inactivation of ERK and Akt pathways. Cellular Physiology and Biochemistry. 2014;34(3):637–645. doi: 10.1159/000363029. [DOI] [PubMed] [Google Scholar]
- 69.Asghar U., Witkiewicz A. K., Turner N. C., Knudsen E. S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nature Reviews Drug Discovery. 2015;14(2):130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailon-Moscoso N., Cevallos-Solorzano G., Romero-Benavides J., Ramirez Orellana M. Natural compounds as modulators of cell cycle arrest: application for anticancer chemotherapies. Current Genomics. 2017;18(2):106–131. doi: 10.2174/1389202917666160808125645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ouyang L., Shi Z., Zhao S., et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Proliferation. 2012;45(6):487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hengartner M. O. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 73.Fulda S. Evasion of apoptosis as a cellular stress response in cancer. International Journal of Cell Biology. 2010;2010:6. doi: 10.1155/2010/370835.370835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fischer U., Schulze-Osthoff K. Apoptosis-based therapies and drug targets. Cell Death & Differentiation. 2005;12(Suppl 1):942–961. doi: 10.1038/sj.cdd.4401556. [DOI] [PubMed] [Google Scholar]
- 75.Rasul A., Bao R., Malhi M., et al. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules. 2013;18(2):1418–1433. doi: 10.3390/molecules18021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasul A., Ding C., Li X., et al. Dracorhodin perchlorate inhibits PI3K/Akt and NF-kappaB activation, up-regulates the expression of p53, and enhances apoptosis. Apoptosis. 2012;17(10):1104–1119. doi: 10.1007/s10495-012-0742-1. [DOI] [PubMed] [Google Scholar]
- 77.Cargnello M., Roux P. P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiology and Molecular Biology Reviews. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burotto M., Chiou V. L., Lee J.-M., Kohn E. C. The MAPK pathway across different malignancies: A new perspective. Cancer. 2014;120(22):3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santarpia L., Lippman S. M., El-Naggar A. K. Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert Opinion on Therapeutic Targets. 2012;16(1):103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faes S., Dormond O. PI3K and AKT: unfaithful partners in cancer. International Journal of Molecular Sciences. 2015;16(9):21138–21152. doi: 10.3390/ijms160921138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun T., Cao L., Ping N., Wu Y., Liu D., Cao Y. Formononetin upregulates nitric oxide synthase in arterial endothelium through estrogen receptors and MAPK pathways. Journal of Pharmacy and Pharmacology. 2016;68(3):342–351. doi: 10.1111/jphp.12519. [DOI] [PubMed] [Google Scholar]
- 82.Schindler C., Levy D. E., Decker T. JAK-STAT signaling: from interferons to cytokines. The Journal of Biological Chemistry. 2007;282(28):20059–20063. doi: 10.1074/jbc.r700016200. [DOI] [PubMed] [Google Scholar]
- 83.Shen D., Xie X., Zhu Z., et al. Screening active components from yu-ping-feng-san for regulating initiative key factors in allergic sensitization. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0107279.e107279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin Y.-M., Xu T.-M., Zhao Y.-H., Wang Y.-C., Cui M.-H. In vitro and in vivo anti-cancer activity of formononetin on human cervical cancer cell line HeLa. Tumor Biology. 2014;35(3):2279–2284. doi: 10.1007/s13277-013-1302-1. [DOI] [PubMed] [Google Scholar]
- 85.Aaronson D. S., Horvath C. M. A road map for those who don't know JAK-STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 86.Wang K. H., Zhang Y. T., Yang X. W., Xu W., Zhang P. Chemical constituents from Fukeqianjin formula. China Journal of Chinese Materia Medica. 2018;43(11):2300–2312. doi: 10.19540/j.cnki.cjcmm.20180408.006. [DOI] [PubMed] [Google Scholar]
- 87.Chan S.-C., Chang Y.-S., Wang J.-P., Chen S.-C., Kuo S.-C. Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera. Planta Medica. 1998;64(2):153–158. doi: 10.1055/s-2006-957394. [DOI] [PubMed] [Google Scholar]
- 88.Li G., Gao Q., Yuan S., et al. Characterization of three small molecule inhibitors of enterovirus 71 identified from screening of a library of natural products. Antiviral Research. 2017;143:85–96. doi: 10.1016/j.antiviral.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Batista C., Alves A., Queiroz L., et al. The photoprotective and anti-inflammatory activity of red propolis extract in rats. Journal of Photochemistry and Photobiology B: Biology. 2018;180:198–207. doi: 10.1016/j.jphotobiol.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 90.Singh K. B., Dixit M., Dev K., Maurya R., Singh D. Formononetin, a methoxy isoflavone, enhances bone regeneration in a mouse model of cortical bone defect. British Journal of Nutrition. 2017;117(11):1511–1522. doi: 10.1017/S0007114517001556. [DOI] [PubMed] [Google Scholar]
- 91.Barbosa R. A., Nunes T. L. G. M., Nunes T. L. G. M., et al. Hydroalcoholic extract of red propolis promotes functional recovery and axon repair after sciatic nerve injury in rats. Pharmaceutical Biology. 2016;54(6):993–1004. doi: 10.3109/13880209.2015.1091844. [DOI] [PMC free article] [PubMed] [Google Scholar]