Abstract
Objective
To determine the rate of progression from acute recurrent pancreatitis (ARP) to chronic pancreatitis (CP) in children and assess risk factors.
Study Design
Data were collected from the INternational Study group of Pediatric Pancreatitis: In search for a cuRE (INSPPIRE) cohort. Kaplan-Meier curves were constructed to calculate duration of progression from initial attack of acute pancreatitis (AP) to CP. Log-rank test was used to compare survival (non-progression) probability distribution between groups. Cox proportional hazard regression models were fitted to obtain hazard ratio (with 95% CI) of progression for each risk variable.
Results
Of 442 children, 251 had ARP, 191 CP. The median time of progression from initial attack of AP to CP was 3.79 years. The progression was faster in those age ≥6 years at the first episode of AP compared to those age <6 years (median time to CP: 2.91 vs 4.92 years; p=0.01). Children with pathogenic PRSS1 variants progressed more rapidly to CP compared to children without PRSS1 variants (median time to CP: 2.52 vs 4.48 years; p=0.003). Within six years after the initial AP attack, cumulative proportion with exocrine pancreatic insufficiency (EPI) was 18.0% (95% CI: 12.4%, 25.6%); diabetes mellitus was 7.7% (95% CI: 4.2%, 14.1%).
Conclusions
Children with ARP rapidly progress to CP, EPI and diabetes. The progression to CP is faster in children who were ≥6 years at the first episode of AP or with pathogenic PRSS1 variants. The factors that impact the aggressive disease course in childhood warrant further investigation.
Keywords: PRSS1, pediatric pancreatitis, diabetes mellitus, pancreatic insufficiency, natural history
INTRODUCTION
Acute pancreatitis (AP), acute recurrent pancreatitis (ARP) and chronic pancreatitis (CP) are thought to be a disease continuum. Although studies have evaluated factors associated with the development of CP, few studies have looked at the time of progression and factors that contribute to this progression. Recent work from our multicenter consortium, the International Study Group for Pediatric Pancreatitis: In search of a cure (INSPPIRE) would suggest that the progression from ARP to CP occurs quickly in children, since the mean age at the diagnosis of ARP was 9.1 years and of CP was 10.2 years.1,2 Rapid pancreatic disease progression in children is of grave concern, since pediatric CP is associated with high disease burden, multiple emergency room visits and hospitalizations, medical, endoscopic and surgical procedures, life-changing operations such as total pancreatectomy with islet autotransplantation (TPIAT) and an increased lifetime risk for pancreatic adenocarcinoma.1,3,4
Pediatric CP is most commonly associated with genetic risk factors including variants in the cationic trypsinogen (PRSS1), serine protease inhibitor Kazal type 1 (SPINK1), cystic fibrosis transmembrane regulator (CFTR), chymotrypsin C (CTRC), and carboxypeptidase A1 (CPA1) genes.5–13 Several genetic variants are associated with early onset disease (PRSS1, CTRC, CPA1).14–16 However, the factors associated with rapid progression from AP to ARP, then CP are largely unknown.
In the current study, we aimed to characterize the rate of progression from the first episode of AP to CP utilizing the INSPPIRE cohort. We also sought to investigate whether genetic associations with pancreatitis, phenotypic or demographic factors were associated with faster progression.
METHODS AND STATISTICS
Study design and participants
Demographic and clinical information was collected on children at the time of enrollment from 18 INSPPIRE institutions. We used the enrollment criteria for ARP and CP as previously described.17–19 All information was collected through standardized patient and physician questionnaire forms and entered into REDCap (Research Electronic Data Capture, Vanderbilt University, Nashville Tennessee) database in all centers from March 2012 to February 2017. All centers obtained institutional review board approval for this study.
AP is defined by typical abdominal pain consistent with pancreatitis and amylase and/or lipase levels greater than 3x upper limits of normal (ULN) or imaging findings compatible with AP. ARP is defined by 2 or more episodes of acute pancreatitis that are at least one month apart with resolution of symptoms between episodes of AP, or complete normalization of pancreatic enzyme levels and complete resolution of clinical symptoms between episodes of AP, irrespective of the time interval between AP episodes.
Children with irreversible structural changes in the pancreas with or without abdominal pain; OR with exocrine pancreatic insufficiency; OR with diabetes are classified as CP.19 Irreversible structural changes are listed below:
*Ductal calculi, dilated side branches, parenchymal calcifications found in any imaging (abdominal ultrasound, magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP), computerized tomography (CT), endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasound (EUS).
Ductal obstruction or stricture/dilatation/irregularities that are persistent (for >2 months) on any imaging.
Parenchymal atrophy, irregular contour, accentuated lobular architecture, cavities alone are not diagnostic findings for CP.
Surgical or pancreatic biopsy specimen demonstrating histopathologic features compatible with CP (acinar atrophy, fibrosis, protein plugs, infiltration with lymphocytes, plasma cells, macrophages).
Genetic risk factors included mutations in cystic fibrosis transmembrane conductance regulator (CFTR), cationic trypsinogen (PRSS1), pancreatic secretory trypsin inhibitor (SPINK1), chymotrypsin C (CTRC), ordered by treating clinicians. Toxic/Metabolic factors included medication use known to cause pancreatitis, passive or active smoking exposure, hypertriglyceridemia, hypercalcemia, chronic kidney disease, or alcohol consumption. Obstructive risk factors included pancreatitis due to gallstones, pancreaticobiliary malunion, choledochal cyst, functional pancreatic sphincter dysfunction, pancreas divisum, or annular pancreas.
Statistical analysis
Kaplan-Meier curve for the outcome of progression to the diagnosis of CP from the first documented AP attack was constructed showing the product-limit estimate of the cumulative probability of survival (non-progression to CP) over the course of disease duration. The median time to diagnosis of CP (with interquartile range, IQR) was also calculated from the Kaplan-Meier curve. For this analysis, time to CP diagnosis was calculated from date of documented first acute attack to date of diagnosis of CP for those with CP. Those with ARP not diagnosed with CP, were considered as censored observations with follow-up time calculated from date of first acute attack to last follow-up date. In addition, Kaplan-Meier curves of progression to CP for various risk factor groupings (sex, ethnicity, family history of CP, genetic mutations, and anatomic disease) were also constructed and compared using the log-rank test, with p-value <0.05 considered as statistically significant. Cox proportional hazard regression model was also fitted to obtain an estimate of the hazard ratio (with 95% CI) of progression to CP for each risk variable. Multi-factor Cox proportional hazard regression analysis was performed that included risk factors that were found to be statistically significant (p<0.05) into a single model. To avoid exclusion of a large number of observations due to not having genotyping done (n>100), a third category of “not done” was added for the genetic variant risk factor for use in fitting the multi-factor model. All statistical analyses were performed using SAS software (version 9.4).
RESULTS
Demographics and Clinical Characteristics
A total of 442 children were enrolled during the study period. 251 (57%) had a diagnosis of ARP without progression to CP; 191 (43%) had a diagnosis of CP. The demographics and clinical characteristics are shown in Table 1. Toxic-metabolic factors (mostly medication usage) was found in 25.1%; alcohol, smoking, renal failure and hypercalcemia were very rare. The median [IQR] duration from the first AP attack to the last follow-up date was 2.1 [0.8–4.5] years.
Table 1.
Demographics and characteristics of children in progression to CP.
| Variables | Count/Sample Size (%) |
|---|---|
| (n=442) | |
| Gender (male) | 191 (43.2) |
| Ethnicity (Hispanic) | 95/408 (23.3) |
| Family history CP, any blood relative | 87/369 (23.6) |
| PRSS1 variants | 91/303 (30.0) |
| CFTR variants | 94/304 (30.9) |
| SPINK1 variants | 94/304 (30.9) |
| Obstructive factors* | 130/437 (29.8) |
| Toxic-metabolic factors** | 101/403 (25.1) |
| Age at first acute attack | (n=419) |
| Median (IQR) | 9.0 (4.8–12.9) |
| <6 years of age at first attack | 147/419 (35.1) |
| Duration of disease, years | 2.1 (0.8–4.5) |
Table showing demographics and clinical characteristics of children with progression to chronic pancreatitis. IQR=Interquartile range= (25th-75th percentile); CP: chronic pancreatitis; CFTR: cystic fibrosis transmembrane conductance regulator; PRSS1: cationic trypsinogen; SPINK1: pancreatic secretory trypsin inhibitor
at least 1 of the following present-- gallstones, pancreaticobiliary malunion, choledochal cyst, functional pancreatic sphincter dysfunction, pancreas divisum or annular pancreas
at least 1 of the following present-- alcohol, smoking (active or passive), hypertriglyceridemia, hypercalcemia, renal failure, medication usage
Risk Factors Associated with Progression to CP
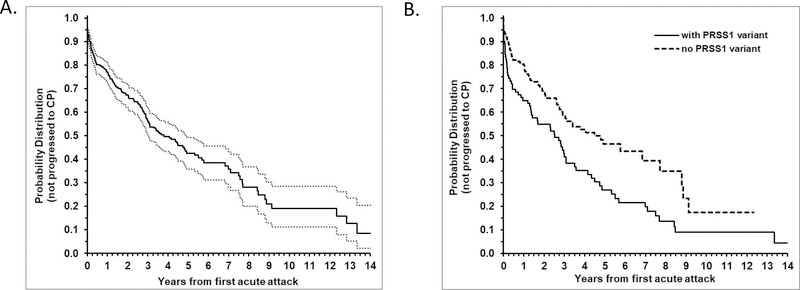
The median [IQR] time of progression from first episode of AP to diagnosis of CP for the entire cohort was 3.79 [1.11–8.46] years (Figure 1a). Of the demographic, genetic, and clinical variables that were examined, having a PRSS1 variant, or absence of a toxic metabolic factor or older age at first episode of AP were found to be significantly associated with a faster rate of progression from AP to CP (Supplemental Table 1). Children with any PRSS1 variant progressed more rapidly to CP compared to children without a PRSS1 variant (median time of progression from first episode of AP to CP: 2.52 [0.25–5.50] years vs 4.48 [1.25–8.87] years, respectively; hazard ratio: 1.68; 95% CI 1.22, 2.32; p=0.001) (Figure 1b). Of 91 patients with PRSS1 variants, 70 had pathogenic or clinically significant variants (53 with CP), and 6 had benign or variants of unknown significance. In 15 patients, no specific PRSS1 mutations were reported. We found that CP progression was significantly shorter in patients with pathogenic PRSS1 mutations, compared to children without PRSS1 mutations 2.52 [0.18–5.50] years vs 4.48 [1.25–8.87] years, respectively; hazard ratio: 1.70; 95% CI 1.20, 2.41; (p=0.003) (Supplemental Table 1). Pathogenic variants of PRSS1 in the INSPPIRE cohort are listed in Supplemental Table 2.
Figure 1. Progression from ARP to CP in the INSPPIRE cohort.
(A) Kaplan-Meier curve (with 95% CI, dashed lines) for the outcome of progression to CP, with estimate of median time to progression of 3.79 (IQR: 1.11–8.46) years. (B) Comparison of Kaplan-Meier of CP progression between those with and without PRSS1 variant showing a significantly faster rate of progression to CP among those with PRSS1 variant with hazard ratio of progression of 1.68; 95% CI 1.22, 2.32; p=0.001). Median time of progression to CP was 2.52 [0.25–5.50] years vs 4.48 [1.25–8.87] years, respectively.
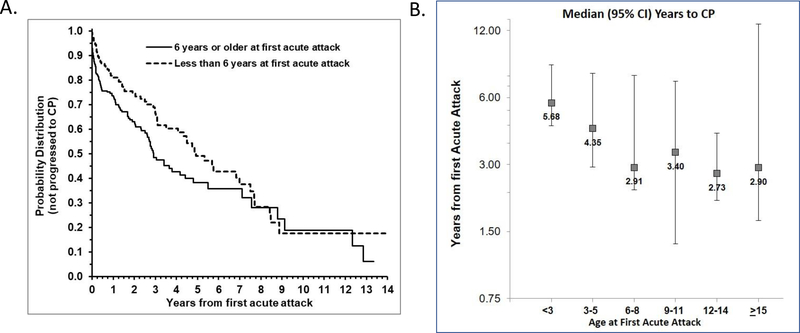
Likewise, children who had their first episode of AP at age 6 years or older had a faster rate of progression to CP compared to those with a first episode at age less than 6 years, with median time of progression of 2.91 [0.86–8.79] years vs 4.92 [1.91–8.46] years, respectively (hazard ratio 1.47; 95% CI: 1.08, 2.01; p=0.01) (Figure 2a). To further confirm the association of older age at presentation (>6 y/o) with a faster rate of progression to CP, the median time to CP was graphed according to age groups (Figure 2b). The youngest age group (<3 y/o with their first AP) had the longest median time to CP (5.68 years) followed by patients with their first AP at age 3 – 5 years (4.35 years). In contrast, children who presented with their first AP at age 6 – 8 years had the 2nd shortest median time to CP (2.91 years) whereas patients with their first AP at age 12 – 14 years had the shortest time (2.73 years). The absence of at least one toxic metabolic risk factor was associated with faster progression to CP: 3.1 [1.07–7.69] years vs 8.87 [1.91–12.33] years, respectively (hazard ratio 0.68; 95% CI: 0.46, 0.99; p=0.046) (Figure S1).
Figure 2. Faster progression to CP in children with later onset disease.
(A) Kaplan-Meier curve of progression to CP showing children who had their first episode of AP at 6 years or older had a faster rate of progression to CP compared to those with first episode at age less than 6 years, with median time of progression of 2.91 [0.86–8.79] years vs 4.92 [1.91–8.46] years, respectively (hazard ratio 1.47; 95% CI: 1.08, 2.01; p=0.01) (B) Graph demonstrating children who had their first episode of AP at the age intervals 6 years or older had shorter median number of years to CP compared to children with first episode less than 3 years of age and between 3 – 5 years of age.
When combined into a single model, Cox proportion hazard regression model that included PRSS1 variants, age at first AP episode, and toxic metabolic factor as independent variables showed that PRSS1 variants (hazard ratio 1.86; 95% CI: 1.31, 2.65; p=0.0006) and first episode of AP at age 6 years and older (hazard ratio 1.73; 95% CI: 1.24, 2.42; p=0.001) were still statistically significant. However, the effect of toxic metabolic factor was lessened with hazard ratio of 0.73 (95% CI: 0.49, 1.10; p=0.13). This reduction in the effect of toxic metabolic factor after adjusting for two other risk factors may be due to the inverse association between toxic metabolic factors and PRSS1 variants. PRSS1 variants were present in 34% (76/221) of those without toxic metabolic factor compared to 15% (9/60) of those with toxic metabolic risk factor (p=0.004).
The other factors including CFTR and SPINK1 genetic variants, ethnicity, obstructive and anatomic factors (such as pancreas divisum), and family history of CP, showed no significant association with rate of progression to CP (Supplemental Table 1). Although patients with a family history of CP had an increased hazard ratio (1.33; 95% CI: 0.94, 1.87) that suggested a faster progression from ARP to CP, this was not statistically significant (p=0.10). Limiting the family history to only first-degree relatives with CP compared to no family history of CP showed similar results (hazard ratio 1.25; 95% CI: 0.85, 1.85; p=0.26).
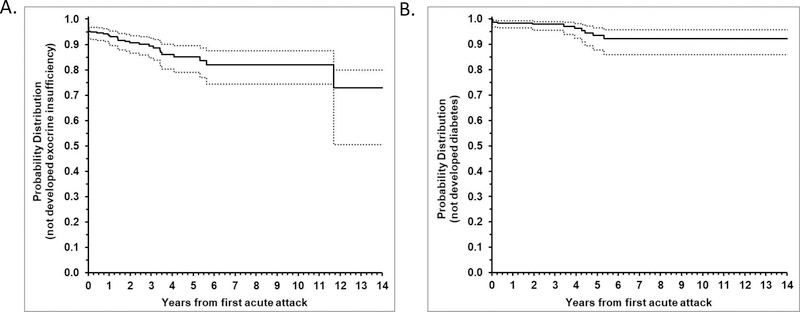
Kaplan-Meier curve in Fig 3a shows the rate of diagnosis of exocrine pancreatic insufficiency (EPI) after initial AP attack. This analysis was based on n=354 ARP or CP patients of which 38 were diagnosed with EPI after AP with known date of diagnosis. There were 24 patients with EPI that were not included because date of EPI diagnosis was not known. An additional 4 patients with EPI prior to AP were also not included in the analysis because the time interval between diagnosis of EPI and CP was longer than a few weeks. From the KM curve, 6.6% (95% CI: 4.4%, 9.9%) were diagnosed with EPI within 1 year of initial AP attack. Within 2 years, there were 9.4% (95% CI: 6.5%, 13.4%) diagnosed with EPI. By 5 years, cumulative proportion with EPI was 14.9% (95% CI: 10.5%, 20.9%). Within 6 years (through 12 years) from initial AP, cumulative proportion with EPI was 18.0% (95% CI: 12.4%, 25.6%). Because 24 with EPI and unknown date of diagnosis were not included in the analyses, these estimates may be biased lower than true rate.
Figure 3. Faster progression to EPI and diabetes mellitus in INSPPIRE cohort.
(A) Kaplan-Meier curve of progression to EPI after initial AP attack is shown. 6.6% (95% CI: 4.4%, 9.9%) were diagnosed with EPI within 1 year of initial AP attack; 9.4% (95% CI: 6.5%, 13.4%) within 2 years, there were diagnosed with EPI; 14.9% (95% CI: 10.5%, 20.9%) by 5 years. Within 6 years (through 12 years) from initial AP attack cumulative proportion with EPI was 18.0% (95% CI: 12.4%, 25.6%). (B) Kaplan-Meier curve of progression to diabetes mellitus after initial AP attack is shown. 2.1% (95% CI: 0.9%, 4.4%) were diagnosed with diabetes mellitus within 2 years of initial AP attack. Overall, 7.7% (95% CI: 4.2%, 14.1%) were diagnosed with diabetes mellitus within 6 years of initial AP attack (through the last follow-up).
The rate of diagnosis of diabetes mellitus after initial AP is shown by Kaplan-Meier curve in Fig 3b. This analysis was based on n=391 ARP or CP patients of which 13 were diagnosed with diabetes after AP. An additional 11 patients that had diabetes prior to AP were not included in the analysis. From the KM curve, 2.1% (95% CI: 0.9%, 4.4%) were diagnosed with diabetes within 2 years of initial AP attack. Overall, 7.7% (95% CI: 4.2%, 14.1%) were diagnosed with diabetes within 6 years of initial AP attack (through the last follow-up).
DISCUSSION
In children, the progression from acute pancreatitis to chronic pancreatitis with exocrine pancreatic insufficiency or diabetes mellitus can occur within 6 years after diagnosis. Pathogenic PRSS1 variants, older age at first episode of acute pancreatitis and absence of toxic metabolic risk factors are associated with an accelerated progression to chronic pancreatitis. Our multicenter study represents the largest cohort of well-characterized children with ARP or CP who have been followed longitudinally for several years. This study highlights the importance of prospective and longitudinal studies to identify the risk factors associated with pancreatic disease progression.
Parniczky et al recognized the importance of genetic susceptibility and family history in the development of pediatric AP, ARP and CP.20 Genetic testing was recommended for children with a 2nd episode of idiopathic acute pancreatitis or a 1st episode of acute pancreatitis with a family history of pancreatitis. No recommendations were given for specific risk factors in progression to CP, EPI or diabetes. Knowing that certain children have an aggressive disease course may help clinicians and families make informed decisions about therapy and have reasonable expectations about the outcome. Children with faster progression to CP, EPI or diabetes may need to be targeted sooner to alter the disease course, prevent or treat complications of CP and provide nutritional support. This work paves the way for developing precision medicine approaches to pancreatic diseases that would account for individual variability in genes, environment and lifestyle of each person. As the data in children continue to emerge, the pediatric pancreatitis guidelines may need to be updated.
A strong association with genetic risk factors and pediatric CP has been well described.1,2,16,21–23 PRSS1 is one of the first reported genes associated with CP.5,24 PRSS1 variants have autosomal dominant inheritance with high penetrance and an increased risk for ARP or CP in the pediatric age group and pancreatic adenocarcinoma later in life.1,2,23,25,26 A strong association of PRSS1 variants with pediatric CP compared to ARP has been observed in the Polish and INSPPIRE cohorts.1,22
Howes et al demonstrated in the EUROPAC cohort that the PRSS1 variants were associated with an earlier presentation of acute pancreatitis with a median age of symptoms at 10 – 14 year, depending on the variant. Although the progression to exocrine or endocrine insufficiency appeared to take longer compared to non-genetic causes of CP, there was a higher cumulative lifetime risk of malabsorption (60.2%) and diabetes mellitus (68.6%) in the hereditary pancreatitis group.23 Pancreatitis starts at a much younger age in our cohort (<6 y/o) and progresses much faster 14, with 18% with EPI and 7.7% diabetes mellitus within 6 years after the initial attack of acute pancreatitis. The cause is unknown, but it was suggested by Howes et al that PRSS1 R122H variant in the US was more aggressive.23 In our cohort, 70 of 76 patients with available PRSS1 mutation profile had a pathogenic mutation, of which 46 had a R122H variant. An aggressive PRSS1 mutation profile may explain rapid progression to CP, then EPI and diabetes in childhood.
Keim et al showed that although patients with either PRSS1 or SPINK1 variants were associated with progression to CP, patients with heterozygous or homozygous SPINK1 variants had a higher frequency and faster progression to CP, including pancreatic calcifications and diabetes.26 In their cohort, patients were diagnosed in their teens and followed into adulthood with some alcohol use allowed. Pancreatic calcifications are rare in pediatric CP 1,2. Although we have previously shown that SPINK1 variants are associated with CP1, we have not demonstrated an association between SPINK1 mutations and faster progression to CP.
In the INSPPIRE cohort, PRSS1 variants were a significant risk factor for pancreatitis in children younger than 6 years of age.14 In contrast, our study further demonstrates the strong association of PRSS1 variants and the more rapid development of CP. Although previous studies have linked genetic variants to the development of CP in children, our study is one of the largest cohorts of pediatric patients screened and tested positive for genetic variants. This study is also unique with its prospective and longitudinal approach that allows for the analysis of disease progression from the first episode of AP to CP.
SPINK1 mutations are significantly associated with pediatric CP in the INSPPIRE cohort.1 In this study, neither SPINK1 nor CFTR variants predicted faster progression to CP. In 68 children with ARP or CP from India, SPINK1 N34S mutation was commonly found, but there was no statistical difference in frequency between children with ARP and CP.27 Similarly, SPINK1 mutations were common in a Polish cohort of children with ARP or CP, with no significant differences between the two groups.22 Two pediatric studies from India implicated SPINK1 mutations in the progression from ARP to CP.28,29 None of these studies have evaluated the time of progression from ARP to CP nor whether certain factors accelerate the progression.
Toxic -metabolic risk factors were found in ~25% of the INSPPIRE cohort. Most of these patients were treated with pancreatitis-associated medications such as azathioprine, 6-mercaptopurine, l-asparaginase, but a clear cause-effect relationship could not be demonstrated. Going forward, mechanisms of drug-induced pancreatitis need to be better investigated, including better documentation of medication dosage and duration of use before the onset of pancreatitis.
Interestingly, we found a negative association between toxic-metabolic risk factors and progression from ARP to CP. Although this seemed as an independent variable, the effect was lessened with positive PRSS1 variants. Larger cohorts and future longitudinal studies are needed to better understand the contribution of environmental factors on disease progression in pediatric pancreatitis.
Our previous study classified acute pancreatitis occurring in children <6 years of age as “early onset acute pancreatitis” 14. In this study, we hypothesized that earlier age of onset would be associated with a faster progression to CP. However, our data have suggested the opposite. The reason for this association is not entirely clear and requires further analysis. One possibility would be the delay in recognition of pancreatic disease despite ongoing symptoms in older children, or perhaps subclinical disease progression. We found a small group of children with EPI diagnosis preceding CP diagnosis, probably because the pediatric CP diagnostic criteria are dependent on the imaging findings. Going forward, we need to develop better methods for the early diagnosis of CP in children.
The strength of the current study is that it is derived from a large cohort of diverse pediatric patients from multiple centers within the US and abroad, who were well-characterized and followed longitudinally. The limitations are that, although the information was collected prospectively, the data were analyzed in a retrospective nature in most. Not all of our patients were followed over the same time period, since the INSPPIRE consortium enrolls on a continuous basis. Some children were followed for only a short period of time (median 2.1 years), therefore, progression to a chronic inflammatory process may still be ongoing. With regard to the genetic variants, not all patients had undergone extensive genotyping or testing for the most common pancreatitis-relevant genes. Even though genetic testing and fecal elastase were not completed in all patients, this remains the largest cohort of children with information on pancreatic disease progression and the impact of genetic factors. There are likely more patients with existing, yet untested genetic variants in our cohort, and the study may be underestimating the role of genetic factors in progression from AP to CP.
In summary, the progression from the first episode of pediatric AP to CP with exocrine pancreatic insufficiency or diabetes mellitus can occur within 6 years after diagnosis. Pathogenic PRSS1 variants, older age at the first episode of AP or absence of toxic/metabolic risk factors are associated with more rapid progression to CP. Future studies are needed to evaluate other potential genetic and environmental factors involved in the progression of pancreatitis-related disease utilizing longitudinal and prospective cohorts. This effort is crucial in developing future therapies that will help delay or prevent the progression of CP after the initial attack of AP in susceptible individuals
Supplementary Material
Supplemental Table 1. Analysis of risk factors to assess association with progression to CP
Supplemental Table 2. Pathogenic PRSS1 mutations in INSPPIRE cohort (pathogenic)
Figure S1. Faster progression to CP in children without toxic metabolic risk factors. Comparison of Kaplan-Meier of CP progression between those with and without toxic metabolic risk factors showing a significantly faster rate of progression to CP among those without toxic metabolic risk factors: 3.1 [1.07-7.69] years vs 8.87 [1.91-12.33] years, respectively (hazard ratio 0.68; 95% CI: 0.46, 0.99; p=0.046).
What is known.
Chronic pancreatitis (CP) may present in early childhood with a mean age of diagnosis of approximately 10 years.
Several factors are associated with the development of CP such as genetic variants, anatomical and environmental factors.
What is new.
The progression from acute pancreatitis to chronic pancreatitis with exocrine pancreatic insufficiency or diabetes mellitus can occur within 6 years in children.
Pathogenic PRSS1 variants or an older age at first acute pancreatitis episode are associated with a significantly faster progression to CP in childhood.
Acknowledgments
Funding: Research reported in this publication was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers R21 DK096327, U01 DK108334, R01 DK118752 and National Pancreas Foundation (AU); INSPPIRE registry was developed by CTSA (2UL1 TR000442) and REDCap. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest: Dr. Mark Lowe is on the Board of Directors of the National Pancreas Association; receives royalties from Millipore Inc and UpToDate. Dr. Tanja Gonska received a research grant from Vertex Pharmaceuticals and she is a consultant for Cystic Fibrosis Foundation. Dr. John Pohl is on the speaker’s bureau for Medical Education Resources, Inc.; Dr. Melena Bellin is a consultant for AbbVie Inc and ARIEL Precision Medicine. Dr. Chee Y. Ooi is a consultant for Vertex Pharmaceuticals. Dr. Aliye Uc is a member of American Board of Pediatrics, Subboard of Pediatric Gastroenterology, Associate Editor of Pancreatology, a consultant for Cystic Fibrosis Foundation. The other authors declare no conflicts of interest.
REFERENCES
- 1.Kumar S, Ooi CY, Werlin S, et al. Risk Factors Associated With Pediatric Acute Recurrent and Chronic Pancreatitis: Lessons From INSPPIRE . JAMA Pediatr 2016;170:562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015;166:890–6.e891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 1993;328:1433–7. [DOI] [PubMed] [Google Scholar]
- 4.Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 2002;51:849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 1996;14:141–5. [DOI] [PubMed] [Google Scholar]
- 6.Hassan Z, Mohan V, Ali L, et al. SPINK1 is a susceptibility gene for fibrocalculous pancreatic diabetes in subjects from the Indian subcontinent. Am J Hum Genet 2002;71:964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durie PR. Pancreatitis and mutations of the cystic fibrosis gene. N Eng J Med 1998;339:687–8. [DOI] [PubMed] [Google Scholar]
- 8.Rosendahl J, Witt H, Szmola R, et al. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet 2008;40:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saint-Criq V, Gray MA. Role of CFTR in epithelial physiology. Cell Mol Life Sci 2017;74:93–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharer N, Schwarz M, Malone G, et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. The New England journal of medicine. 1998;339(10):645–652. [DOI] [PubMed] [Google Scholar]
- 11.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nature genetics. 2000;25(2):213–216. [DOI] [PubMed] [Google Scholar]
- 12.Wood NJ. Genetics: Global role for CPA1 variants in the pathogenesis of chronic pancreatitis. Nature reviews Gastroenterology & hepatology. 2013;10(10):567. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Sahin-Toth M. Chymotrypsin C mutations in chronic pancreatitis. Journal of gastroenterology and hepatology. 2011;26(8):1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giefer MJ, Lowe ME, Werlin SL, et al. Early-Onset Acute Recurrent and Chronic Pancreatitis Is Associated with PRSS1 or CTRC Gene Mutations. The Journal of pediatrics. 2017;186:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabarczyk AM, Oracz G, Wertheim-Tysarowska K, et al. Chymotrypsinogen C Genetic Variants, Including c.180TT, Are Strongly Associated With Chronic Pancreatitis in Pediatric Patients. Journal of pediatric gastroenterology and nutrition. 2017;65(6):652–657. [DOI] [PubMed] [Google Scholar]
- 16.Witt H, Beer S, Rosendahl J, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nature genetics. 2013;45(10):1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morinville VD, Husain SZ, Bai H, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. Journal of pediatric gastroenterology and nutrition. 2012;55(3):261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morinville VD, Lowe ME, Ahuja M, et al. Design and implementation of INSPPIRE. Journal of pediatric gastroenterology and nutrition. 2014;59(3):360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uc A, Perito ER, Pohl JF, et al. INternational Study Group of Pediatric Pancreatitis: In Search for a CuRE Cohort Study: Design and Rationale for INSPPIRE 2 From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47(10):1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parniczky A, Abu-El-Haija M, Husain S, et al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis . Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al. ]. 2018;18(2):146–160. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Kim KM, Choi JH, Lee BH, Kim GH, Yoo HW. High incidence of PRSS1 and SPINK1 mutations in Korean children with acute recurrent and chronic pancreatitis. Journal of pediatric gastroenterology and nutrition. 2011;52(4):478–481. [DOI] [PubMed] [Google Scholar]
- 22.Sobczynska-Tomaszewska A, Bak D, Oralewska B, et al. Analysis of CFTR, SPINK1, PRSS1 and AAT mutations in children with acute or chronic pancreatitis. Journal of pediatric gastroenterology and nutrition. 2006;43(3):299–306. [DOI] [PubMed] [Google Scholar]
- 23.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2004;2(3):252–261. [DOI] [PubMed] [Google Scholar]
- 24.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology. 2013;144(6):1292–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebours V, Boutron-Ruault MC, Schnee M, et al. The natural history of hereditary pancreatitis: a national series. Gut. 2009;58(1):97–103. [DOI] [PubMed] [Google Scholar]
- 26.Keim V, Witt H, Bauer N, et al. The course of genetically determined chronic pancreatitis. JOP : Journal of the pancreas. 2003;4(4):146–154. [PubMed] [Google Scholar]
- 27.Poddar U, Yachha SK, Mathias A, Choudhuri G. Genetic predisposition and its impact on natural history of idiopathic acute and acute recurrent pancreatitis in children. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2015;47(8):709–714. [DOI] [PubMed] [Google Scholar]
- 28.Poddar U, Yachha SK, Borkar V, Srivastava A. Is acute recurrent pancreatitis in children a precursor of chronic pancreatitis? A long-term follow-up study of 93 cases. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2017;49(7):796–801. [DOI] [PubMed] [Google Scholar]
- 29.Poddar U, Yachha SK, Borkar V, Srivastava A, Kumar S. A Report of 320 Cases of Childhood Pancreatitis: Increasing Incidence, Etiologic Categorization, Dynamics, Severity Assessment, and Outcome. Pancreas. 2017;46(1):110–115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Analysis of risk factors to assess association with progression to CP
Supplemental Table 2. Pathogenic PRSS1 mutations in INSPPIRE cohort (pathogenic)
Figure S1. Faster progression to CP in children without toxic metabolic risk factors. Comparison of Kaplan-Meier of CP progression between those with and without toxic metabolic risk factors showing a significantly faster rate of progression to CP among those without toxic metabolic risk factors: 3.1 [1.07-7.69] years vs 8.87 [1.91-12.33] years, respectively (hazard ratio 0.68; 95% CI: 0.46, 0.99; p=0.046).