Abstract
Background
Crohn's disease (CD) is a chronic relapsing inflammatory condition and maintenance of remission is a major issue as many patients fail to achieve remission with medical management and require surgical interventions. Purine analogues such as azathioprine (AZA) and 6‐mercaptopurine (6‐MP) have been used to maintain surgically‐induced remission in CD, but the effectiveness, tolerability and safety of these agents remains controversial.
Objectives
To assess the efficacy and safety of purine analogues (AZA and 6‐MP) for maintenance of surgically‐induced remission in CD.
Search methods
We searched PubMed, MEDLINE, Embase, CENTRAL, and the Cochrane IBD Group Specialized Register from inception to 26 July 2018 (and from inception to 31 July 2019). In addition, we searched reference lists of all included studies and relevant reviews, conference proceedings and trials registers.
Selection criteria
Randomised controlled trials (RCTs) with a duration of at least three months that enrolled adults and children with surgically‐induced remission of CD and compared AZA or 6‐MP to no treatment, placebo or any other active intervention were considered for inclusion.
Data collection and analysis
Two authors independently assessed trial eligibility, extracted data, assessed the risk of bias and assessed the certainty of the evidence using GRADE. The primary outcome was clinical relapse. Secondary outcomes included endoscopic relapse, radiologic and surgical relapse, adverse events (AEs), serious adverse events (SAEs), withdrawal due to AEs and health‐related quality of life.
Main results
Ten RCTs with a total of 928 participants were included. Study participants were adults recruited from university clinics and gastroenterology hospitals who received interventions post‐surgery for a duration between 12 to 36 months. Most study participants were recruited less than three months after surgery in all except one study where participants were recruited between 6 to 24 months post‐surgery. One study was rated as low risk of bias, six studies were rated high risk of bias and three were rated unclear risk of bias.
There was moderate certainty evidence that purine analogues are more efficient for preventing clinical relapse than placebo. At 12 to 36 months, 51% (109/215) of AZA/6‐MP participants relapsed compared to 64% (124/193) of placebo participants (RR 0.79; 95% CI 0.67 to 0.92; 408 participants; 3 studies; I² = 0%; moderate certainty evidence). The certainty of the evidence regarding the efficacy of AZA or 6‐MP for maintaining postoperative clinical remission compared to 5‐ASA compounds was low. At 12 to 24 months , 64% (113/177) of purine analogue participants relapsed compared to 59% (101/170) of 5‐ASA participants (RR 1.05; 95% CI 0.89 to 1.24; 347 participants; 4 studies; I² = 8%; low certainty evidence). The certainty of evidence that purine analogues are inferior for preventing postsurgical clinical relapse compared to tumour necrosis factor alpha agents (anti‐TNF‐α) was very low. At 12 to 24 months, 43% (29/67) of AZA participants relapsed compared to 14% (10/72) of anti‐TNF‐α participants (RR 2.89; 95% CI 1.50 to 5.57; 139 participants; 3 studies; I² = 0%; very low certainty evidence).
The effect of purine analogues compounds on AEs compared to placebo or any active treatment was uncertain, as the quality of evidence ranged from very low to low. After 12 to 24 months, 14% (12/87) of purine analogue participants experienced an AE compared to 10% (8/81) of placebo participants (RR 1.36; 95% CI 0.57 to 3.27; 168 participants; 2 studies; I² = 0%; low certainty evidence). The effect of purine analogues on AEs compared to 5‐ASA agents was uncertain. After 12 to 24 months, 41% (73/176) of purine analogue participants had an AE compared to 47% (81/171) of 5‐ASA participants (RR 0.89; 95% CI 0.74 to 1.07; 346 participants; 4 studies; I² = 15%; low certainty evidence). The effect of purine analogues on AEs in comparison to anti TNF‐α agents was uncertain. At 12 to 24 months, 57% (32/56) of AZA participants had an AE compared to 51% (31/61) of anti‐TNF‐α participants (RR 1.13; 95% CI 0.83 to 1.53; 117 participants; 2 studies; I² = 0%; low certainty evidence). Purine analogue participants were more like than 5‐ASA participants to have a SAE (RR 3.39, 95% CI 1.26 to 9.13, 311 participants; 3 studies; I² = 9%; very low certainty evidence), or to withdraw due to an AE (RR 2.21, 95% CI 1.28 to 3.81; 425 participants; 5 studies; I² = 0%; low certainty evidence). Commonly reported AEs across all studies included leucopenia, arthralgia, abdominal pain or severe epigastric intolerance, elevated liver enzymes, nausea and vomiting, pancreatitis, anaemia, nasopharyngitis and flatulence.
Authors' conclusions
Moderate certainty evidence suggests that AZA and 6‐MP may be superior to placebo for maintenance of surgically‐induced remission in participants with CD. There was no clear difference in the number of clinical relapses when purine analogues were compared with 5‐ASA agents, however this is based on low certainty evidence. There was very low certainty evidence that AZA and 6‐MP are more likely to result in more serious adverse events (SAEs) and withdrawals due to an AE (low certainty) when compared to 5‐ASA agents. Very low certainty evidence suggests that purine analogues may be inferior to anti‐TNF‐α agents, however, no firm conclusions can be drawn. Further research investigating the efficacy and safety of AZA and 6‐MP in comparison to other active medications in surgically‐induced remission of CD is warranted.
Plain language summary
Azathioprine and 6‐mercaptopurine for the maintenance of surgically‐induced remission in Crohn’s disease
What was the aim of this review?
The aim of this review was to understand the benefits and harms of purine analogues (azathioprine (AZA) and 6‐mercaptopurine (6‐MP)) used for maintaining remission following surgery in people with Crohn's disease (CD).
What is Crohn's disease?
Crohn's disease is a chronic disease of the gut. The disease is known to constantly change from periods when sufferers have symptoms (relapse) to periods when the symptoms disappear (remission) for a short time. Symptoms include abdominal pain, diarrhoea and weight loss. People with Crohn's disease may undergo surgery to remove diseased parts of their gut. However, their symptoms can return after a short time. Different drugs can be given to ensure that people with Crohn's disease are free from symptoms for as long as possible. However, there are concerns about possible side effects that may arise. Purine analogues (AZA and 6‐MP) are a group of immunosuppressive drugs which have been used for over five decades to manage Crohn's disease. We researched whether purine antimetabolites can maintain remission in people with Crohn's disease after the diseased portion of their gut has been removed.
What are the main results of the review?
The review authors found 10 relevant studies with a total of 928 participants, conducted across several European countries, Israel and the US. The studies included people with Crohn's disease over 16 years of age who had undergone surgery and were free from symptoms. These studies compared purine analogues with placebo (e.g. a sugar pill), or oral 5‐aminosalicylic acid (5‐ASA) formulations or with anti‐tumour necrosis factor‐alpha (anti‐TNF‐α) drugs. 5‐ASA and anti‐TNF‐ɑ drugs are used reduce inflammation (pain and swelling) in the gut.
One study was high quality, while six studies were of lower quality and three studies did not report enough information to make a judgement on quality. Purine analogues are probably better than placebo for maintaining surgically‐induced remission of Crohn's disease (moderate certainty evidence). The analysis of studies that compared purine antimetabolites to 5‐ASA medications found no difference in the number of people who remained in remission. However, more people who received purine analogues experienced serious side effects or discontinued treatment due to side effects than those who received 5‐ASA (very low and low certainty evidence). The analysis of studies that compared purine analogues to anti‐TNF‐α drugs showed that purine analogues were less effective for maintaining remission of Crohn's disease after surgery. However, the overall certainty of evidence was very low. Well designed studies are needed to better understand the benefits and harms of purine analogues compared with anti‐TNF‐ɑ agents and other active drugs used for Crohn's disease. Due to sparse data and inconsistent reporting across all studies, the effect of purine analogues on side effects compared with placebo, 5‐ASA or biologics was uncertain. Commonly reported side effects across the studies included leucopenia (a reduction in the number of white cells in the blood), pancreatitis (inflamed pancreas), arthralgia (joint pain), abdominal pain or severe epigastric intolerance, elevated liver enzymes, nausea and vomiting, anaemia (low number of red blood cells), nasopharyngitis (common cold) and flatulence (intestinal gas).
How up‐to‐date is this review?
The review authors searched for studies that had been published up to 31 July 2019.
Conclusions
There is moderate certainty evidence that AZA and 6‐MP may be superior to placebo for maintenance of surgically‐induced remission in participants with Crohn's disease. There was no clear difference in the number of clinical relapses when purine analogues were compared with 5‐ASA agents, however this was based on low certainty evidence. There was very low certainty evidence that AZA and 6‐MP are more likely to result in more serious side effects and withdrawals due to side effects when compared to 5‐ASA agents. Very low certainty evidence suggests that purine analogues may be inferior to anti‐TNF‐α agents for preventing relapse, however, no firm conclusions can be drawn. Further research investigating the benefits and harms of AZA and 6‐MP in comparison to other active medications in surgically‐induced remission of CD is warranted.
Summary of findings
Summary of findings for the main comparison. Azathioprine or 6‐mercaptopurine compared to placebo for maintenance of surgically‐induced remission in Crohn's disease.
| Azathioprine or 6‐mercaptopurine compared to placebo for maintenance of surgically‐induced remission in Crohn's disease | ||||||
| Patient or population: People with surgically‐induced remission in Crohn's disease Setting: Outpatient Intervention: Azathioprine (100‐150 mg/day) or 6‐mercaptopurine (1 mg/kg/day ‐ 50 mg/day) Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Azathioprine or 6‐mercaptopurine | |||||
|
Clinical relapse Follow‐up: 12 to 36 months |
Study population | RR 0.79 (0.67 to 0.92) | 408 (3 RCTs) | ⊕⊕⊕⊝ MODERATE1 | Clinical relapse defined as: a CDAI>250 (D'Haens 2008); a CDAI>150 and 100 point increase from baseline (Mowat 2016) or a grading score > 2 (Hanauer 2004). | |
| 642 per 1,000 | 508 per 1,000 (430 to 591) | |||||
|
Endoscopic relapse Follow‐up:12 to 36 months |
Study population | RR 0.85 (0.64 to 1.13) | 321 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 3 | Endoscopic relapse defined as an endoscopic score i ≥2 (D'Haens 2008; Mowat 2016) | |
| 752 per 1,000 | 639 per 1,000 (481 to 849) | |||||
| Radiologic relapse | Outcome not reported | Not reported | ||||
| Surgical relapse | Outcome not reported | Not reported | ||||
|
Adverse events Follow‐up: 12 to 24 months |
Study population | RR 1.36 (0.57 to 3.27) | 168 (2 RCTs) | ⊕⊕⊝⊝ LOW 4 | Reported adverse events include hair loss, leukopenia, diarrhoea, abdominal pain, hepatotoxicity and arthralgia | |
| 99 per 1,000 | 134 per 1,000 (56 to 323) | |||||
|
Serious adverse events Follow‐up:12 to 36 months |
Study population | RR 1.78 (0.39 to 8.18) | 327 (2 RCTs) | ⊕⊕⊝⊝ LOW 5 | Reported serious adverse events include arthralgia, pancreatitis, leucopenia and bowel obstruction | |
| 13 per 1,000 | 23 per 1,000 (5 to 108) | |||||
|
Withdrawal due to adverse events Follow‐up:12 to 36 months |
Study population | RR 0.90 (0.63 to 1.29) | 408 (3 RCTs) | ⊕⊕⊕⊝ MODERATE6 | Adverse events leading to withdrawal included abnormal blood results leading to temporary discontinuation of treatment in 28% of the participants. However, specific details on reasons for discontinuation were not clearly stated | |
| 254 per 1,000 | 228 per 1,000 (160 to 328) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the median risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due serious imprecision (233 events).
2 Downgraded one level due to serious inconsistency ( I² = 64%).
3 Downgraded one level due to serious imprecision (227 events).
4 Downgraded two levels due to very serious imprecision (20 events) and 95% CI which includes appreciable benefit and harm
5 Downgraded two levels due to very serious imprecision (7 events) and 95% CI which includes appreciable benefit and harm
6 Downgraded one level due to serious imprecision (100 events) and 95% CI which includes appreciable benefit and harm
Summary of findings 2. Azathioprine or 6‐mercaptopurine compared to 5‐aminosalicylic acid for maintenance of surgically‐induced remission in Crohn's disease.
| Azathioprine or 6‐mercaptopurine compared to 5‐aminosalicylic acid for maintenance of surgically‐induced remission in Crohn's disease | ||||||
| Patient or population: People with surgically‐induced remission in Crohn's disease Setting: Outpatient Intervention: Azathioprine (2 mg/kg/day) or 6‐mercaptopurine (50 mg/day) Comparison: 5‐aminosalicylic acid | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 5‐aminosalicylic acid | Risk with Azathioprine or 6‐mercaptopurine | |||||
|
Clinical relapse Follow‐up: 12 to 24 months |
Study population | RR 1.05 (0.89, 1.24) | 347 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Clinical relapse defined as a clinical grading score ≥ 2 (Hanauer 2004;Savarino 2013) or CDAI ≥ 200 (Ardizzone 2004) |
|
| 556 per 1,000 | 595 per 1,000 (456 to 773) | |||||
|
Endoscopic relapse Follow‐up: 24 months |
Study population | RR 0.78 (0.52 to 1.17) | 35 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | Endoscopic relapse defined as a Rugeerts score ≥ 2 (Savarino 2013) | |
| 833 per 1,000 | 650 per 1,000 (433 to 975) | |||||
|
Radiologic relapse Follow‐up: 24 months |
Study population | RR 0.92 (0.66 to 1.28) | 35 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | Radiologic relapse defined as a radiographic grading score ≥ 2 (Savarino 2013) | |
| 833 per 1,000 | 767 per 1,000 (550 to 1,000) | |||||
|
Surgical relapse Follow‐up: 24 months |
Study population | RR 0.81 (0.50 to 1.29) | 142 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 5 | Surgical relapse defined as a need for another surgery (Ardizzone 2004) | |
| 366 per 1,000 | 297 per 1,000 (183 to 472) | |||||
|
Adverse events Follow‐up: 12 to 24 months |
Study population | RR 0.89 (0.74 to 1.07) | 346 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 6 | Reported adverse events include leukopenia, abdominal pain, nausea, nasopharyngitis, diarrhoea. and headache | |
| 476 per 1,000 | 424 per 1,000 (353 to 510) | |||||
|
Serious adverse events Follow‐up: 12 to 24 months |
Study population | RR 3.39 (1.26 to 9.13) | 311 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 7 | Reported serious adverse events include postoperative bowel obstruction | |
| 39 per 1,000 | 134 per 1,000 (50 to 360) | |||||
|
Withdrawal due to adverse events Follow‐up: 12 to 24 months |
Study population | RR 2.21 (1.28, 3.81) | 425 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 8 | Adverse events leading to withdrawal include Severe epigastric intolerance, increase in liver function test results, leukopenia, acute pancreatitis | |
| 76 per 1,000 | 172 per 1,000 (93 to 317) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the median risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to high risk of bias
2 Downgraded one level due to serious imprecision (214 events) and 95% CI which includes no effect and appreciable harm
3 Downgraded two levels due to very serious imprecision (26 events) and 95% CI which includes no effect and appreciable benefit
4 Downgraded two levels due to very serious imprecision (28 events) and 95% CI which includes no effect and appreciable benefit
5 Downgraded two levels due to very serious imprecision (47 events) and 95% CI which includes appreciable benefit and harm
6 Downgraded one level due to serious imprecision (154 events) and 95% CI which includes no effect and appreciable benefit
7 Downgraded two levels due to serious imprecision (33 events)
8 Downgraded one level due to serious imprecision (58 events)
Summary of findings 3. Azathioprine or 6‐mercaptopurine compared to anti TNF‐α for maintenance of surgically‐induced remission in Crohn's disease.
| Azathioprine or 6‐mercaptopurine compared to anti‐TNF‐α for maintenance of surgically‐induced remission in Crohn's disease | ||||||
| Patient or population: People with surgically‐induced remission in Crohn's disease Setting: Outpatient Intervention: Azathioprine (2‐2.5 mg/kg/day) or 6‐mercaptopurine (1.5 mg/kg/day) Comparison:Anti‐TNF‐α | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with anti‐ TNF‐α | Risk with Azathioprine or 6‐mercaptopurine | |||||
|
Clinical relapse Follow‐up: 12 to 24 months |
Study population | RR 2.89 (1.50 to 5.57) | 139 (3 RCTs) | ⊕⊕⊝⊝ VERY LOW 1 2 | Clinical relapse defined as: an HBI ≥2 (Armuzzi 2013), a clinical recurrence grading score ≥2 (Savarino 2013) or a CDAI score >200 (Lopez‐Sanroman 2017) |
|
| 139 per 1,000 | 401 per 1,000 (208 to 774) | |||||
| Endoscopic relapse Follow‐up: 12 to 24 months | Study population | RR 3.67 (1.05 to 12.81) | 157 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 4 | Endoscopic relapse defined as a Rugeerts score ≥ 2 (Armuzzi 2013; Lopez‐Sanroman 2017; Savarino 2013; Scapa 2015) | |
| 265 per 1,000 | 973 per 1,000 (278 to 1,000) | |||||
|
Radiologic relapse Follow‐up: 12 to 24 months |
Study population | ‐ | 117 (2 RCTs) | ‐ | Radiologic relapse defined as a radiographic grading score ≥ 2 (Savarino 2013) or magnetic resonance enterography score ≥2 (Lopez‐Sanroman 2017) *due to considerable heterogeneity (I² = 85%), data pooling was not feasible. It is uncertain whether azathioprine leads to a difference in radiologic relapse when compared to infliximab as the certainty of the evidence is very low (RR1.36, 95% CI 0.94 to 1.98; RR 12.24, 95% CI 1.8 to 83.12) |
|
| see comment | see comment | |||||
| Surgical relapse | Outcome not reported | Not reported | ||||
|
Adverse events Follow‐up: 12 to 24 months |
Study population | RR 1.13 (0.83 to 1.53) | 117 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 5 | Adverse events include bronchitis, nasopharyngitis, arthralgia, nausea, abscess were reported in Savarino 2013. Full details were not reported in Lopez‐Sanroman 2017 | |
| 508 per 1,000 | 574 per 1,000 (422 to 778) | |||||
|
Serious adverse events Follow‐up: 12 months |
Study population | RR 0.51 (0.17 to 1.54) | 84 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 6 | Serious adverse events not reported | |
| 200 per 1,000 | 102 per 1,000 (34 to 308) | |||||
|
Withdrawal due to adverse events Follow‐up: 12 to 24 months |
Study population | RR 3.97 (0.92 to 17.22) | 139 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 6 | Adverse events leading to withdrawal included severe nausea, leukopenia, arthralgia, urothelial carcinoma, dyspepsia, dyspnoea, death, atopic dermatitis and abdominal pain with increase in pancreatic enzymes | |
| 28 per 1,000 | 110 per 1,000 (26 to 478) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the median risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to high risk of bias
2 Downgraded two levels due to very serious imprecision (39 events)
3 Downgraded one level due to serious imprecision (65 events)
4 Downgraded one level due to substantial heterogeneity (I² = 64%)
5 Downgraded one level due to serious imprecision (63 events)
6 Downgraded two levels due to very serious imprecision (13 events)
Background
Description of the condition
Crohn’s disease is a chronic idiopathic inflammatory disorder of the gastrointestinal tract with an etiology that combines genetic predisposition, environmental factors and an inappropriate immune response to the gut microbiota which may involve the whole gastrointestinal tract (Abraham 2009). There is no cure for the disease, and management strategies are mainly focused on induction and maintenance of remission. Approximately 75% of patients with Crohn's disease will eventually undergo surgical resection (Bernell 2000), and this can induce remission. However, endoscopic recurrence of disease has been reported to be as high as 73% at one year post surgery (Rutgeerts 1990), and clinical relapse rates have been reported to range from 22 to 55% at five years post surgery (Williams 1990). There is no standard therapy for the prevention of postoperative recurrence in Crohn's disease (Hanauer 2001). A number of agents have been studied, but considerable uncertainty remains as to the efficacy of such treatments.
Description of the intervention
Corticosteroids, the mainstay of treatment of acute exacerbations, have been used extensively as Crohn's disease therapy. However, the chronic use of glucocorticosteroids is limited due to the multiple adverse reactions and the lack of effectiveness for maintaining remission in Crohn's disease (Steinhart 2003). 5‐aminosalicylic acid (5‐ASA) agents have been shown to be safe and may be effective for maintenance of post‐surgical remission, although the existing data suggests that the efficacy of these agents depends on the ability of these drugs to reach the terminal ileum and colon in therapeutic concentrations and may have limited clinical efficacy (Gordon 2011). Probiotics and budesonide do not appear to provide any benefit for maintenance of surgically‐induced remission in Crohn's disease (Benchimol 2009; Doherty 2009; Rolfe 2006). Nitroimidazole antibiotics may reduce the risk of relapse in surgically‐induced remission, however, these agents are not well tolerated and are associated with a higher risk of serious adverse events (Doherty 2009). Studies have demonstrated that tumour necrosis factor‐alpha (TNF‐α) antagonists such as infliximab (Regueiro 2009), or adalimumab (Savarino 2013) may provide a benefit for reducing the risk of relapse in surgically‐induced remission, but these agents are expensive. Purine analogues such as azathioprine (AZA) and 6‐mercaptopurine (6‐MP) have been used in clinical practice for over five decades with a demonstrated efficacy for the long‐term maintenance of remission in both Crohn's disease (Chande 2015), and ulcerative colitis (Timmer 2016), and are relatively inexpensive. Evidence suggests that the effect of thiopurine formulations seem to last for up to five years (Fraser 2002), significantly reducing the risk of perianal and intestinal surgery (Camus 2013).
How the intervention might work
6‐MP and its prodrug AZA which is non‐enzymatically degraded to 6‐MP are purine antimetabolites that reduce cell proliferation and have immune modulating properties. 6‐MP is metabolised to its active component 6‐thioguanine nucleotide which competitively interferes with nucleic acid metabolism by inhibiting the proliferation of T and B lymphocytes and reducing the numbers of cytotoxic T cells and plasma cells (Lennard 1992; Sahasranaman 2008). There are some trial data which suggest that neutrophil count is a predictor of induction and maintenance of remission (Colonna 1994), which may suggest the mechanism of action, although this is not well understood. The major limiting factor for long term use of AZA and 6‐MP agents has been the occurrence of adverse events in approximately 10% of patients leading to withdrawal of therapy (Hafraoui 2002), with dose‐dependent and idiosyncratic adverse events occurring. There is evidence which suggests that thiopurine methyltransferase deficiency accounts for some of the dose and metabolism‐related toxicity to purine analogues including leucopenia, thrombocytopenia and in the long‐term potentially lymphoma and non‐melanoma skin cancer (Axelrad 2016; Gomollon 2017; Lennard 1989; Weinshilboum 1980), while adverse reactions such as arthralgias, pancreatitis, hepatitis, nausea, non‐pancreatic abdominal pain, rush, fever and diarrhoea are attributed to hypersensitivity reactions (Sandborn 1996).
Why it is important to do this review
Maintenance of remission in Crohn's disease is a major issue as many patients fail to achieve remission with medical management and require surgical interventions. Purine analogues have been used to maintain surgically‐induced remission in Crohn's disease, but the effectiveness, tolerability and safety of these drugs remains controversial. Relatively few studies have been published that investigate the role of AZA or 6‐MP for maintenance of remission following surgery in patients with Crohn's disease. One multicentre randomised placebo controlled trial involving 81 patients found a significant reduction in endoscopic recurrence when AZA was used in conjunction with metronidazole in comparison to metronidazole alone (D'Haens 2008). In another multicentre randomised controlled trial, it was concluded that 6‐mercaptopurine was more effective than either mesalamine or placebo at preventing postoperative recurrence at 24 months following surgery (Hanauer 2001). However, a single‐centre randomised open‐label trial found no significant difference in clinical relapse rates between AZA and mesalamine (Ardizzone 2004). A previous review by this team in 2014 found evidence that purine analogues may be superior to placebo for maintenance of surgically‐induced remission in patients with Crohn's disease, although this was based on two small studies (Gordon 2014). The results for efficacy outcomes between purine analogues and 5‐ASA agents were uncertain. However, patients taking purine analogues were more likely than 5‐ASA patients to discontinue therapy due to adverse events. No firm conclusions could be drawn from the two small studies that compared AZA to infliximab or adalimumab. Adalimumab seemed superior to AZA but further research was needed to confirm these results. Hence, an up‐to‐date systematic review using the Cochrane Collaboration format was indicated to summarise the current evidence on the use of purine analogues for the maintenance of surgically‐induced remission in Crohn's disease.
Objectives
The primary objective was to evaluate the efficacy and safety of AZA and 6‐MP for maintenance of surgically‐induced remission in Crohn's disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials which compared AZA or 6‐MP agents to either a no treatment control, placebo or any other active intervention, with treatment durations of at least three months were considered for inclusion.
Types of participants
Participants of any age and sex with a diagnosis of Crohn's disease confirmed by any established method who were in remission following surgery were considered for inclusion. Remission could be defined by a recognized Crohn's disease activity index such as the Crohn's disease activity index (CDAI) or endoscopy, or by participants who have undergone a curative surgical resection, or as defined by the authors of the primary studies. were considered for inclusion. Eligible trials could be conducted in any setting (e.g. single centre or multi‐centre).
Types of interventions
The controlled interventions of interest included any randomised controlled trial that compares oral AZA or 6‐MP agents to an no treatment, placebo or another active intervention for maintenance of surgically‐induced remission. Studies that compare AZA or 6‐MP agents to an intervention that focuses on enteral nutrition, oral nutrient supplementation, medical foods, probiotics, parental nutrition or herbal medicines were excluded. We also excluded dose optimisation studies.
Types of outcome measures
Primary outcomes
The primary outcome measure was clinical relapse as defined by the primary studies.
Secondary outcomes
The secondary outcome measures included the proportion of participants who experienced:
Endoscopic relapse;
Radiologic relapse;
Surgical relapse;
Histologic relapse;
Adverse event (as defined by FDA 2018. We also noted where studies failed to provide sufficient information and simply report outcome as ‘adverse event’);
Serious adverse events (as defined by FDA 2018. We also noted where studies failed to provide sufficient information and simply report outcome as ‘serious adverse event’);
Withdrawal due to adverse events; and
Health‐related quality of life (HRQoL).
Adverse events and serious adverse events that are known to be associated with AZA or 6‐MP include:
Bone marrow suppression: pancytopenia, leucopenia, neutropenia, thrombocytopenia;
Hypersensitive reactions: malaise, vomiting, diarrhoea, rash, hypotension;
Malignancy;
Liver function impairment, jaundice;
Pancreatitis;
Pulmonary: pneumonitis; and
Renal: interstitial nephritis.
The outcome measures were reported at the last time point available (assumed to be at the end of follow‐up if not specified) and the time point specified in the methods as being of primary interest (if this was different from the latest time point available). However, it was also indicated when studies reported outcomes at other time points.
Search methods for identification of studies
Electronic searches
For the review update, we searched the following electronic databases from inception to 26 July 2018:
MEDLINE;
Embase;
PubMed;
CENTRAL; and
Cochrane IBD Group Specialized Register.
No restrictions were placed on language. Note that the searches were designed to include RCTs conducted on adults and children participants, but to exclude dose optimisation studies and trials that compare AZA or 6‐MP agents to oral nutrition supplements (enteral nutrition drinks, tube feeds), medical foods, probiotics, parenteral nutrition, herbal medicines or a combination of these modalities. The search strategy was more than one year old prior to publication of the updated review. Thus, we ran another search from inception to 31 July 2019 prior to publication. The search strategies are reported in Appendix 1.
Searching other resources
Reference searching
We searched reference lists from included articles and any existing relevant reviews. We also searched ongoing trials registered on ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform portal.
Abstracts of major gastroenterology meetings
A manual search of abstracts and proceedings submitted to recent major gastroenterology meetings was performed for the following journals to identify more trials:
Gastroenterology (American Gastroenterological Association);
Gut (British Society of Gastroenterology);
American Journal of Gastroenterology (American College of Gastroenterology); and
Journal of Pediatric Gastroenterology and Nutrition (European / North American Society of Paediatric Gastroenterology, Hepatology and Nutrition).
If a relevant abstract was identified, details of the full study methodology and results were requested from the authors in order to allow a thorough assessment of the quality of identified studies.
Data collection and analysis
This updated review was based upon the methods described in the published protocol (Gordon 2014), and in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
The selection of studies included the following steps: title screening, abstract screening and full‐text review. Two authors (MG and TGH) independently reviewed each article at each stage of selection. Included and excluded studies were recorded. The two authors (MG and TGH) independently screened the titles using the titles of the papers that appeared to have even a minor possibility of inclusion. Adjudication did not occur at the title screening stage and studies that were ambiguous were included by default. The two authors (MG and TGH) then independently screened the abstracts of the articles that report studies with a reasonable possibility of inclusion. Differences in assessment for inclusion were resolved by discussion between the two independent investigators (MG and TGH). Adjudication did not occur at the abstract screening state. Lastly, the two authors (MG and TGH) independently screened the full text which involved selection of articles based on careful examination of the full report. Differences in assessment for inclusion were resolved by discussion between the two independent investigators. Adjudication was performed as needed by a third author (ZIE).
Data extraction and management
A data extraction form was developed to extract information on relevant features and results of included studies. Two authors (ZIE and TGH) independently extracted and recorded data on the predefined checklist. Extracted data included the following items:
Study design: type of RCT, setting, number of interventions, year, author's contact;
Population characteristics: age, sex, disease distribution, disease duration, site of disease, medication, type and time since operation, total number of participants originally assigned to each treatment group;
Intervention: type and dose of agent;
Control: no active treatment, placebo, other drugs;
Concurrent medications; and
Outcomes: time of assessment, length of follow up, type of Crohn's disease activity index used, definitions of remission and relapse, site of surgery, relapse rates, adverse events.
Assessment of risk of bias in included studies
Two authors (ZEI and TGH) independently assessed bias using the Cochrane risk of bias tool (Higgins 2011). Adjudication was performed as needed by a third author (MG). Each domain was assessed as having a low, high, or unclear risk of bias. Domains assessed included:
Sequence generation (i.e. was the allocation sequence adequately generated?);
Allocation sequence concealment (i.e. was allocation adequately concealed?);
Blinding (i.e. was knowledge of the allocated intervention adequately prevented during the study?);
Incomplete outcome data (i.e. were incomplete outcome data adequately addressed?);
Selective outcome reporting (i.e. are reports of the study free of suggestion of selective outcome reporting?); and
Other potential sources of bias (i.e. was the study apparently free of other problems that could put it at a high risk of bias?).
Each domain followed standard definitions used for Cochrane systematic reviews (Higgins 2011). Study authors were contacted for further information when insufficient information was provided to determine the risk of bias.
The overall certainty of the evidence was assessed using the GRADE approach (Guyatt 2008; Schünemann 2011). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Randomised trials start as high quality evidence, but may be downgraded due to: risk of bias (methodological quality), indirectness of evidence, unexplained heterogeneity, imprecision (sparse data) and publication bias. The overall quality of the evidence for each outcome was determined after considering each of these factors and graded as:
High ‐ we are very confident that the true effect lies close to that of the estimate of the effect;
Moderate ‐ we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
Low ‐ our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect; or
Very low. We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Measures of treatment effect
For binary outcomes, risk ratio (RR) estimates and associated two‐sided 95% confidence intervals (CI) were calculated. For nominal or ordinal outcomes, we calculated the RR with corresponding 95% for each category relative to a reference category. For continuous outcomes, we calculated the mean difference (MD) and corresponding 95% CI. If studies in future updates report continuous outcomes that have been measured using different scales (e.g. IBDQ and SF‐36), we will calculate the standardised mean difference (SMD) and 95% CI.
Unit of analysis issues
The unit of analysis was the individual participant. We planned to include cross‐over trials if data were available from the first phase of the study (i.e. before cross‐over occurred). For outcomes where events recur (e.g. clinical relapses, adverse events), we calculated the proportion of participants who experienced at least one event, individual events were not counted separately. The studies were otherwise not anticipated to have repeated observations of outcomes or multiple treatment events. If studies had randomised subjects to more than one AZA or 6‐MP treatment arm, these groups would have been combined for the primary analysis.
Dealing with missing data
We collected information on how each trial handled missing data. When a study appeared to collect and not report all primary outcomes of interest, the original investigators were contacted to request missing data. If the original investigators did not provide the data, this would be noted in the systematic review. For studies with missing dichotomous data, an intention‐to‐treat analysis was performed where participants with missing data were assumed to have been treatment failures.
Assessment of heterogeneity
We assessed heterogeneity through visual inspection of the forest plots and by calculating the Chi² and I² statistics (Boreinstein 2009). For studies that had qualitative homogeneity, statistical heterogeneity was assessed using the Chi² test (a P value < 0.10 was considered statistically significant heterogeneity). The degree of heterogeneity across studies was estimated using the I² statistic. An I² of 25% or less was considered low heterogeneity, 26% to 50% was considered moderate heterogeneity, and 50% and greater was considered substantial heterogeneity. Where sufficient data are available, we planned to explore possible explanations for heterogeneity including factors such as participant characteristics (e.g. age, sex), condition severity, treatment type and dose, and healthcare system/country. Where appropriate, these factors were to be investigated further through sub‐group analyses and meta‐regression (Boreinstein 2009). Where sufficient data are available, we planned to use sensitivity analyses to explore possible causes of methodological heterogeneity (Sutton 2000).
Assessment of reporting biases
If there were an appropriate number of studies in a pooled analysis (i.e. > 10 studies), we planed to investigate potential publication bias using funnel plots (trial effects versus trial size) (Egger 2001). However, the number of studies in each comparison group was smaller than 10.
Data synthesis
Data from individual trials were combined for meta‐analysis if the interventions, patient groups and outcomes were sufficiently similar (determined by consensus). We calculated the pooled RR and corresponding 95% CI for dichotomous outcomes. Analyses were grouped by type of intervention treatment (e.g. AZA or 6‐MP versus placebo, AZA or 6‐MP versus 5‐ASA). Where there were multiple studies in an analysis we used a random‐effects model to obtain a more conservative interpretation otherwise, we used a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to assess the impact of potential effect modifiers such as age of participants (paediatric versus adult studies), drug type (azathioprine versus 6‐mercaptopurine) and length of follow‐up (12 months or less versus greater than 12 months). There were no studies on children.
Sensitivity analysis
Sensitivity analyses based on random‐effects versus fixed‐effect models were planned where appropriate data or numbers of studies were available. Sensitivity analysis was also planned to explore possible explanations for significant heterogeneity.
Results
Description of studies
Results of the search
The original search for this update was conducted on 26 July 2018. The search expired before the update could be published, so we ran another search on 31 July 2019. The electronic database search conducted on 31 July 2019 identified 1201 records, while 37 records were found through other sources (See Figure 1). Of the 52 full‐text records assessed for eligibility, 35 reports of 10 studies (928 participants) were included in this systematic review update. Two studies (NCT03185611; NL1344), were classified as ongoing Characteristics of ongoing studies. Eleven studies (15 reports) were excluded for different reasons as presented in the Characteristics of excluded studies table.
1.
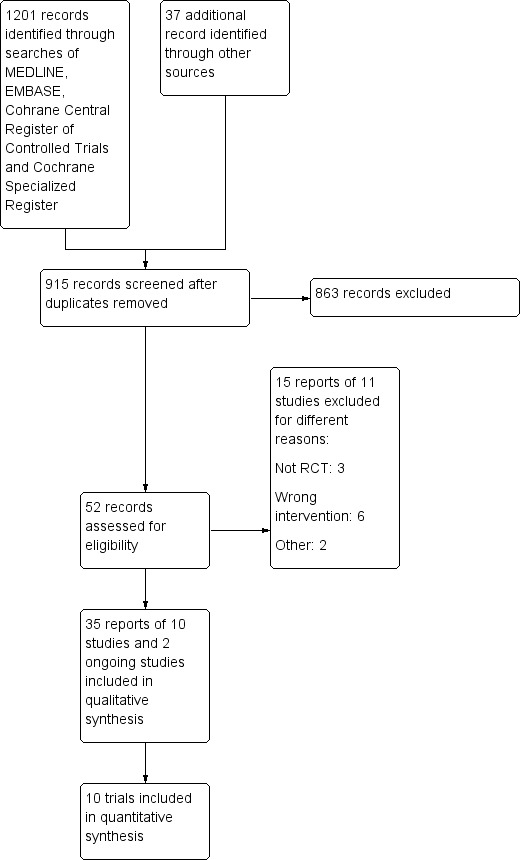
Study flow diagram.
Detailed information about all included studies are presented in the Characteristics of included studies table.
Included studies
Study design and setting
This systematic review includes reports of single centre and multicentre randomised controlled trials with a parallel design with a duration of 52 weeks (Lopez‐Sanroman 2017; Reinisch 2010), to 36 months (Mowat 2016). These studies were all published between 2004 and 2017. There were four single‐centre studies conducted in two different countries: Italy (Ardizzone 2004; Armuzzi 2013; Savarino 2013), and Israel (Scapa 2015). The multicentre studies were conducted across Belqium (D'Haens 2008), Spain (Lopez‐Sanroman 2017, the UK (Mowat 2016), or as a multinational collaboration of several countries across Europe and Israel (Reinisch 2010), and Europe and the USA (Hanauer 2004). Regarding the care setting, three studies were conducted either in gastroenterology hospitals and medical clinics/centres (Lopez‐Sanroman 2017; Reinisch 2010), and secondary and tertiary hospitals (Mowat 2016), or as a collaboration between university clinics and hospitals and medical centres in four studies (Ardizzone 2004; D'Haens 2008; Hanauer 2004; Savarino 2013). The country and care setting were not reported in Herfarth 2006.
Participants
The total number of participants included in nine studies was 928 and ranged from 22 participants (Armuzzi 2013), to 240 (Mowat 2016). Scapa 2015 did not clearly report the number of participants randomised. All participants were adults with Crohn's disease who had undergone a resective surgical procedure to remove macroscopic disease. The majority of these participants were recruited within three months of surgery or before hospital discharge, except the 78 participants in Reinisch 2010 who were enrolled between 6 and 24 months postoperatively. Interventions were conducted on participants with quiescent Crohn's disease and the disease activity prior to enrolment was established by generally accepted endoscopic, histological and radiological criteria. However, it is important to note that Reinisch 2010 included participants in subsequent postoperative clinical remission (CDAI < 200), but with signs of moderate to severe endoscopic recurrence. For this reason we only collected data on adverse events from Reinisch 2010.
The age of participants was reported nine studies and ranged between an average of 32.7 years (Scapa 2015), to 40 years (D'Haens 2008) in seven trials. Two trials reported age as median (Armuzzi 2013; Lopez‐Sanroman 2017). All studies appear to have been conducted on a male and female adult population. None of the studies included paediatric participants. Herfarth 2006 did not report any information about the age of participants.
Interventions
All included studies were parallel two arm trials except for two studies that had three intervention arms (Hanauer 2004; Savarino 2013). The duration of the intervention ranged from 52 weeks (Armuzzi 2013; Herfarth 2006; Lopez‐Sanroman 2017; Reinisch 2010; Scapa 2015), to 24 months (Ardizzone 2004; D'Haens 2008; Hanauer 2004; Mowat 2016; Savarino 2013).
The studies compared the efficacy of AZA or 6‐MP agents with placebo or another active treatment. Table 4 reports a summary of all interventions which are also summarized below:
1. Key definitions and outcomes.
| Comparison | Study ID | Time from surgery till recruitment | Site of surgery % / exclusions | Clinical relapse definition | Endoscopic / surgical/ radiologic/ histological relapse definition/ |
| AZA & 6‐MP versus Placebo | |||||
| AZA versus Placebo (12 months) both arms 750 mg/day metronidazole (3 months) |
D'Haens 2008 | 2 weeks | Perforating disease 48 *Macroscopic evidence for CD proximally or distally to the site of resection or the presence of frank pancolitis or an ileorectal anastomosis, patients with a stoma; operation for fibrostenosis only |
CDAI > 250 | Rutgeerts i ≥ 2 |
| 6‐MP 50 mg/day versus Placebo (24 months) |
Hanauer 2004 | Before postoperative hospital discharge | N/A * Active perianal disease or any active disease in other segments of the intestine |
Clinical recurrence grading > 2 (Hanauer) | Rutgeerts i ≥ 2 Radiographic relapse: Radiographic recurrence grading > 2 |
| 6‐MP 1 mg/kg/day versus Placebo (3 years) |
Mowat 2016 | ≤ 3 months | Ileal 39; Colonic 2; Ileocolonic 59 * Need for further surgery, stricturoplasty alone, formation of a stoma |
CDAI > 150 and a 100‐point increase from baseline | Rutgeerts i ≥ 2 HRQOL: IBDQ scores |
| AZA & 6‐MP vs 5‐ASA | |||||
| AZA 2 mg/kg versus Mesalamine 3 mg/kg (24 months) | Ardizzone 2004 | Maximum 2 weeks | Small bowel only 25.3; Colon 5.6; Small bowel and colon 9.8; upper gastrointestinal tract 16.2 *Surgical procedures other than conservative surgery or for perianal disease only |
CDAI > 200 Surgical relapse: need for another surgical procedure |
n/a |
| 6‐MP 50 mg/day versus Mesalamine 3 g/day (24 months) |
Hanauer 2004 | Before postoperative hospital discharge | N/A * Active perianal disease or any active disease in other segments of the intestine |
clinical recurrence grading > 2 | Rutgeerts i ≥ 2 Radiographic relapse: radiographic recurrence grading > 2 |
| AZA 2 mg/kg/day versus Mesalamine 4 g/day (12 months) |
Reinisch 2010 | 6‐24 months | N/A * Short bowel syndrome, an ileocolonic stoma |
CDAI > 200 | Rutgeerts i ≥ 2 HRQOL: IBDQ |
| AZA 2 mg/kg/day versus Mesalazine 3 g/day (24 months) |
Savarino 2013 | 2‐4 weeks | Ileum 49, Ileocolonic 51. * Fibrostenotic stricture, macroscopically active disease not resected at the time of surgery, and presence of a stoma |
1. ≥ 2 on the clinical recurrence grading scale by Hanauer 2. CDAI > 200 |
Rutgeerts i≥2 Radiologic relapse: ≥ 2 radiographic recurrence grading scale HRQl: IBDQ>170 |
| AZA & 6‐MP vs anti‐TNF‐α | |||||
| AZA 2.5 mg versus Infliximab 5 mg/kg (12 months) |
Armuzzi 2013 | 2‐4 weeks | Not reported *Active perianal disease, presence of stoma |
HBI ≥ 8 | Rutgeerts' score ≥ i2 |
| AZA 2 mg/kg/day versus Adalimumab (24 months) |
Savarino 2013 | 2‐4 weeks | Ileum 49, Ileocolonic 51. * Fibrostenotic stricture, macroscopically active disease not resected at the time of surgery, and presence of a stoma |
1. ≥ 2 on the clinical recurrence grading scale by Hanauer 2. CDAI > 200 |
Rutgeerts i ≥ 2 Radiologic relapse: ≥ 2 radiographic recurrence grading scale HRQl: IBDQ > 170 |
| AZA 2.5 mg/kg/day versus Adalimumab (52 weeks) both arms 750mg/day metronidazole (3 months) |
Lopez‐Sanroman 2017 | 2 weeks | Ileal 58, ileocolonic 41 * Postsurgical stoma, resection for short indolent stenosis, inaccessible anastomosis to endoscopy |
CDAI > 200 | Rutgeerts i ≥ 2 |
| 6‐MP 1.5 mg/kg/day versus Adalimumab (12 months) |
Scapa 2015 | < 45 days | 6‐MP 1.5 mg/kg/day vs Placebo (12 months) | Scapa 2015 | < 45 days |
AZA: azathioprine; 6‐MP: 6‐mecarptopurine; mg: milligram; CD: Crohn's disease; CDAI: Crohn's disease activity index; NA: not applicable; kg: kilogram; g: gram; HRQOL: health related quality of life; IBDQ: inflammatory bowel disease questionnaire; TNF: tumour necrosis factor; HBI: Harvey Bradshaw index; 5‐ASA: 5‐aminosalicylic acid
AZA or 6‐MP versus placebo
AZA versus placebo (D'Haens 2008).
Both intervention arms also received concomitant metronidazole (750 mg/day) therapy for the first three months of the study (D'Haens 2008).
6‐Mercaptopurine versus placebo (Hanauer 2004; Mowat 2016).
AZA or 6‐MP versus oral 5‐ASA agents
AZA versus mesalamine (Ardizzone 2004; Herfarth 2006; Reinisch 2010; Savarino 2013).
6‐MP versus mesalamine (Hanauer 2004).
AZA or 6‐MP versus anti‐TNF‐α
AZA versus infliximab (Armuzzi 2013).
AZA versus adalimumab (Lopez‐Sanroman 2017; Savarino 2013).
In Lopez‐Sanroman 2017 both intervention arms also received concomitant metronidazole (750 mg/day) therapy for the first three months of the study.
6‐Mercaptopurine versus adalimumab (Scapa 2015).
No studies that compared AZA or 6‐MP agents to a no treatment control group were identified.
The use of concurrent treatment was discussed in all but two studies (Savarino 2013; Scapa 2015). In one study all participants were receiving oral metronidazole (500 mg/day) for two weeks after surgery (Armuzzi 2013), while in two studies both intervention arms were administered metronidazole (ornidazole) for the first three months after surgery (D'Haens 2008; Lopez‐Sanroman 2017). In Mowat 2016 any concomitant therapy had to be well documented and there was no reported use of active concomitant treatments. Corticosteroids were allowed to be tapered by standardized stepwise dose reductions in three studies (Ardizzone 2004; D'Haens 2008; Hanauer 2004). Symptomatic treatment with antacids, antidiarrhoeal or spasmolytic medication on demand was permitted in three studies but had to be scrupulously recorded (Ardizzone 2004; D'Haens 2008; Hanauer 2004). D'Haens 2008 permitted topical therapy for perianal disease and cholestyramine for the treatment of bile‐acid diarrhoea. Continous use of nonsteroidal anti‐inflammatory drugs was prohibited and only occasional use of paracetamol and tramadol was allowed in Savarino 2013.
Outcomes
Outcomes were reported at multiple time points in two studies (D'Haens 2008; Mowat 2016), and at a single time point in eight studies (Ardizzone 2004; Armuzzi 2013; Hanauer 2004; Herfarth 2006; Lopez‐Sanroman 2017; Reinisch 2010; Savarino 2013; Scapa 2015). Some studies had followed participants beyond the intervention period, however, outcome data from those time points were not reported in this review.
Outcomes of interest reported across studies included:
Primary outcomes
Clinical relapse (Ardizzone 2004; Armuzzi 2013; D'Haens 2008; Hanauer 2004; Herfarth 2006; Lopez‐Sanroman 2017; Mowat 2016; Reinisch 2010; Savarino 2013).
Secondary outcome
Endoscopic relapse (Armuzzi 2013; D'Haens 2008; Hanauer 2004; Mowat 2016; Reinisch 2010; Savarino 2013; Scapa 2015).
Radiological relapse (Hanauer 2004; Savarino 2013).
Histologic relapse (Armuzzi 2013).
Surgical relapse (Ardizzone 2004).
Adverse events (Ardizzone 2004; D'Haens 2008; Hanauer 2004; Lopez‐Sanroman 2017; Reinisch 2010; Savarino 2013).
Serious adverse events (Ardizzone 2004; Hanauer 2004; Lopez‐Sanroman 2017; Mowat 2016; Reinisch 2010).
Withrawal due to adverse events (Ardizzone 2004; Armuzzi 2013; D'Haens 2008; Hanauer 2004; Herfarth 2006; Lopez‐Sanroman 2017; Mowat 2016; Reinisch 2010; Savarino 2013).
A summary of interventions and outcomes is presented in additional Table 5.
2. Summary of interventions and outcomes.
| Study ID | Group 1 | Group 2 | Group 3 | Relapse |
Quailty of Life |
Adverse Events/ Serious adverse/ Withdrawal due to adverse events |
|
| Ardizzone 2004 | Azathioprine (2mg/kg/day) |
Mesalamine (3g/day) |
Clinical: 32/71 vs 35/71 Surgical: 26/71 vs 21/71 |
n/a |
AE:18/71 vs 27/71 SAE:6/71 vs 15/71 Withdrwal due to AE: 6/71 vs 15/71 |
||
| Armuzzi 2013 | Azathioprine (2.5 mg/kg/day) Infliximab (5 mg/kg/day) |
Infliximab (5 mg/kg/day) |
Clinical: 2/11 vs 1/11 Endoscopic: 5/11 vs 1/11; Histologic: 9/11 vs 2/11 |
n/a | Withdrawal due to AE: 0/11 vs 1/11 | ||
| D'Haens 2008 | Metronidazole (750 mg/day) first 3 mo + Azathioprine (100‐150 mg/day) |
Metronidazole (750mg/day) first 3 months + Placebo |
Clinical: 11/40 vs 19/41 Endoscopic: 22/40 vs 32/41 |
n/a |
AE: 3/40 vs 4/41 Withdrawal due to AE: 3/40 vs 4/41 |
||
| Hanauer 2004 | 6‐Mercaptopurine (50 mg/day) | Mesalamine (3 g/day) | Placebo | Clinical: 32/47 vs 33/44 vs 35/40 | n/a |
AE: 9/47 vs 6/44 vs 4/40 SAE: 2/47 vs 0/44 vs 2/40 Withdrawal due to AE: 9/47 vs 6/44 vs 4/40 |
|
| Lopez‐Sanroman 2017 | Azathioprine (2.5 mg/kg/d) + Metronidazole (750 mg/day) first 3 mo | Adalimumab + Metronidazole (750 mg/day) first 3 mo |
Clinical: 14/39 vs 7/45 Endoscopic: 23/39 vs 19/45 Radiologic: 26/39 vs 22/45 |
n.s. changes between groups |
AE: 20/45 vs 18/39 SAE: 9/45 vs 4/39 Withdrawal due to AE: 1/39 vs 9/45 |
||
| Mowat 2016 | Mercaptopurine (1 mg/kg/day) | Placebo |
Clinical: 66/128 vs 70/112 Endoscopic: 90/128 vs 83/112 |
n.s differences |
SAE: 3/128 vs 2/112 Withdrawal due to AE: 39/128 vs 41/112 |
||
| Reinisch 2010 | Azathioprine (2.0‐2.5 mg/kg/d) + Placebo mesalazine | Mesalazine (4g/d) + Placebo azathioprine |
Not included | Mean IBDQ change |
AE: 34/37 vs 32/41 SAE: 0/37 vs 10/41 Withdrawal due to AE: 1/37 vs 10/41 |
||
| Savarino 2013 | Adalimumab (160‐80 mg 0‐2 weeks and 40 mg/week thereafter) | Azathioprine (2 mg/kg/day) | Mesalamine (3 g/day) |
Clinical by Hanauer score: 2/16 vs 12/17 vs 9/18 Clinical by CDAI: 1/16 vs 12/17 vs 9/18 Endoscopic: 1/16 vs 11/17 vs 15/18 Radiologic: 1/16 vs 13/17 vs 15/18 |
HRQOL (IBDQ >170): 14/16 vs 2/17 vs 3/18 |
AE:11/16 vs 14/17 vs 14/18 Withdrawal due to AE:0/16 vs 1/17 vs 1/18 |
|
| Scapa 2015 | 6‐mercaptopurine (1.5 mg/kg/day) | Adalimumab (160‐80‐40 mg/2 week intervals) | Endoscopic: 4/8 vs 1/11 | n/a | n/a |
mg: milligram; kg: kilogram; g: gram; CDAI: Crohn's disease activity index; NA: not applicable; AE: adverse events; SAE: serious adverse events; HRQOL: health related quality of life; IBDQ: inflammatory bowel disease questionnaire; ns: not significant
Funding and conflict of interest
Four studies were reportedly supported by pharmaceutical companies (Hanauer 2004; Herfarth 2006; Lopez‐Sanroman 2017; Reinisch 2010), but only two declared conflict of interest (Lopez‐Sanroman 2017; Reinisch 2010). The author of one study was contacted to clarify the role of the pharmaceutical company and he confirmed that the company had no role in the study design, data analysis or writing of the paper (Hanauer 2004), whereas the remaining authors did not respond. Two studies were not supported by any grant (Armuzzi 2013; Savarino 2013). Savarino 2013 reported no conflicts of interest. Armuzzi 2013 reported receiving educational grants, and consultancy and lecture fees from a pharmaceutical company. Mowat 2016 was funded by a governmental grant and adequately reported on conflicts of interest. Funding and conflict of interest was not reported in two studies (Ardizzone 2004, D'Haens 2008), but our attempt to clarify this by contacting the authors was unsuccessful.
Excluded studies
Eleven studies (15 reports) were excluded for different reasons. The reasons for exclusion for each study are presented in the Characteristics of excluded studies table and are summarised below:
Three studies were not RCTs (Nos 2000; Reinisch 2013; Robb 2015);
Six studies assessed the wrong intervention (Ferrante 2015; Mañosa 2013; NCT01876264; Wright 2014; Wright 2015; Zhu 2015);
One study was terminated due to slow recruitment (NCT02247258); and
One study assessed the wrong population (Vidigal 2014).
Risk of bias in included studies
The risk of bias was assessed as low in one study (Mowat 2016), high in six studies (Ardizzone 2004; Armuzzi 2013; Herfarth 2006; Lopez‐Sanroman 2017; Savarino 2013; Scapa 2015), and unclear in three (D'Haens 2008; Hanauer 2004; Reinisch 2010). Details of the risk of bias assessment are presented in the Characteristics of included studies tables, and in Figure 2 and Figure 3.
2.
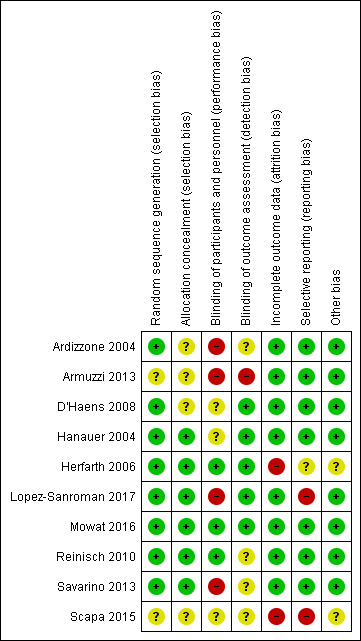
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
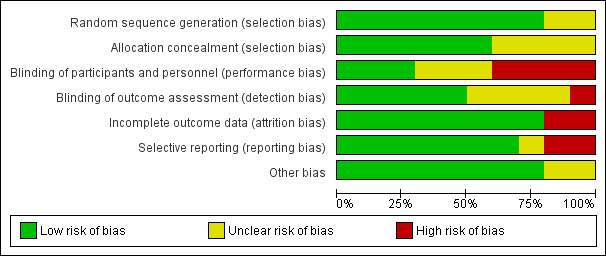
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
In all of the included studies the allocation of participants to an active treatment or placebo was reported as random. Eight studies were judged as being at low risk of bias for random sequence generation as these studies employed computer‐generated randomisation (Ardizzone 2004; D'Haens 2008; Hanauer 2004; Herfarth 2006; Lopez‐Sanroman 2017; Mowat 2016; Reinisch 2010; Savarino 2013). Two studies were judged 'unclear' due to insufficient information on the method of randomisation (Armuzzi 2013; Scapa 2015).
Allocation concealment
In six studies the method of allocation concealment was considered adequate (Hanauer 2004; Herfarth 2006; D'Haens 2008; Lopez‐Sanroman 2017; Reinisch 2010; Savarino 2013). Four studies were judged as unclear risk of bias for allocation concealment as the methods were not adequately described (Ardizzone 2004; Armuzzi 2013; D'Haens 2008; Scapa 2015).
Blinding
Blinding of participants and personnel
Four of the studies included had an open‐label study design and were judged as being at high risk of bias (Ardizzone 2004; Armuzzi 2013; Lopez‐Sanroman 2017; Savarino 2013). All the remaining studies were described as double‐blind. The method of blinding was not adequately described in three studies (D'Haens 2008; Hanauer 2004; Scapa 2015), thus these studies were marked as 'unclear'. Two of these studies failed to describe whether the placebo was sufficiently identical to the intervention to blind study participants (D'Haens 2008; Hanauer 2004), and one study provided insufficient information to make a judgement (Scapa 2015). Due to an adequate description of blinding methods, three studies were assessed as having low risk of performance bias (Herfarth 2006; Mowat 2016; Reinisch 2010).
Blinding of outcome assessment
We assessed one study as having a high risk of detection bias (Armuzzi 2013). Four studies were marked as 'unclear' for detection bias, having failed to adequately describe blinding of outcome assessors (Ardizzone 2004; Reinisch 2010; Savarino 2013; Scapa 2015). The rest of the studies were judged as having a low risk of detection bias, for clearly describing the methods regarding blinding of outcome assessment (D'Haens 2008; Hanauer 2004; Herfarth 2006; Lopez‐Sanroman 2017; Mowat 2016).
Incomplete outcome data
All except two studies reported data fully and documented dropouts and reasons for withdrawals. Incomplete outcome data in these studies was due to study termination (Herfarth 2006), and failure to report the number of randomised and withdrawn participants and reasons for withdrawal (Scapa 2015). The authors of Scapa 2015 were contacted for clarification, however no additional information was provided, except that the study is under preparation for publication. An 'unclear' judgment for this domain was not made for any of the studies.
Selective reporting
Trial registration was available for four studies (Lopez‐Sanroman 2017; Mowat 2016; Reinisch 2010; Scapa 2015). Seven studies were judged as being at low risk of bias for reporting all outcomes prespecified in the trial registration or in the methods section of the study manuscript (Ardizzone 2004; Armuzzi 2013; D'Haens 2008; Hanauer 2004; Mowat 2016; Reinisch 2010; Savarino 2013). One study was judged to be 'unclear' (Herfarth 2006). Two studies were marked 'high' for reporting bias: Scapa 2015 failed to report outcomes prespecified in the trial registration and Lopez‐Sanroman 2017 did not adequately report on a prespecified outcome.
Other potential sources of bias
Eight studies were judged to be at low risk of bias for other apparent sources of potential bias. Two studies provided insufficient information to enable the reviewers make a judgement and were rated as 'unclear' (Herfarth 2006; Scapa 2015).
Effects of interventions
See: Table 1; Table 2; Table 3
AZA or 6‐MP versus placebo
Three studies that compared AZA (100 to 150 mg/day) or 6‐MP in doses of 50 mg/day and 1 mg/kg/day to placebo were identified (D'Haens 2008; Hanauer 2004; Mowat 2016). In one of these studies all participants were also taking either metronidazole or ornidazole (750 mg/day) for the first three months of intervention (D'Haens 2008).
Primary outcome
Clinical relapse
Three studies reported on clinical relapse, with definitions for clinical relapse varying across studies. D'Haens 2008 defined clinical relapse as a CDAI > 250. Mowat 2016 defined clinical relapse as a CDAI > 150 and a 100 point increase in CDAI from baseline. Hanauer 2004 defined relapse as a clinical reoccurrence grading score > 2. There was moderate certainty evidence that AZA or 6‐MP are more efficient in preventing clinical relapse than placebo (Analysis 1.1; Table 1). After a follow‐up of 12 to 36 months, 51% (109/215) of participants in the AZA/6‐MP group relapsed compared to 64% (124/193) of the placebo group (RR 0.79; 95% CI 0.67 to 0.92; 408 participants; 3 studies; I² = 0%; GRADE moderate certainty evidence). The subgroup analysis found no evidence of a difference in clinical relapse between AZA and 6‐MP (P = 0.34). A subgroup analysis based on length of follow‐up found no evidence of a difference in clinical relapse when measured at 12 months or less or at over 12 months (P = 0.34).
1.1. Analysis.
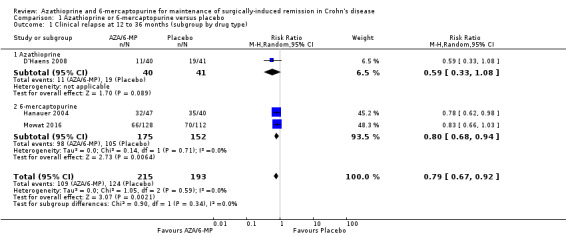
Comparison 1 Azathioprine or 6‐mercaptopurine versus placebo, Outcome 1 Clinical relapse at 12 to 36 months (subgroup by drug type).
Secondary outcomes
The effect on endoscopic relapse rate as well as the tolerability and safety of AZA/6‐MP agents compared to placebo was uncertain, due to very low to low certainty evidence (Table 1).
Endoscopic relapse
Endoscopic relapse (Analysis 1.3), defined as endoscopic score i ≥ 2, was reported in two studies (D'Haens 2008; Mowat 2016). During a follow‐up period of 12 to 36 months, 67% (112/168) of AZA/6‐MP participants relapsed endoscopically, compared to 75% (115/153) of placebo participants (RR 0.85; 95% CI 0.64 to 1.13; 321 participants; 2 studies; I² = 62%; GRADE low certainty evidence). We found no evidence of a difference in endoscopic relapse rates between AZA and 6‐MP (P = 0.11). A subgroup analysis based on length of follow‐up found no evidence of a difference in endoscopic relapse rates measured at different times (P = 0.11). Hanauer 2004 compared endoscopic relapse rates (defined as i ≥ 2) between the 6‐MP (16%; 95% CI 7% to 35%) and placebo (42%; 95% CI 21% to 70%) groups at 24 months (HR 0.48; reported P = 0.13; 87 participants). However, these data were insufficiently reported to be included in the meta‐analysis.
1.3. Analysis.
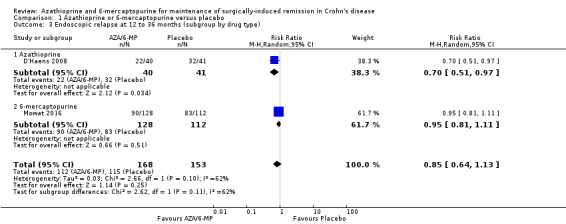
Comparison 1 Azathioprine or 6‐mercaptopurine versus placebo, Outcome 3 Endoscopic relapse at 12 to 36 months (subgroup by drug type).
Radiologic relapse
Radiologic relapse rate defined as a radiographic recurrence grading score ≥ 2 was reported in Hanauer 2004. After 24 months, 33% (95% CI, 19% to 54%) of participants treated with 6‐MP had radiologic relapse compared to 49% (95% CI, 30% to 72%) in the placebo group (HR, 0.61; reported P = 0.19; 84 participants).
Adverse events, serious adverse events and withdrawal due to adverse events
There was no clear difference in the number of participants who experienced adverse events, serious adverse events or withdrawal due to adverse events when AZA/6‐MP drugs are compared to placebo. Adverse events (Analysis 1.5) were reported in two studies ( D'Haens 2008; Hanauer 2004). For the follow‐up period of 12 to 24 months, 14% (12/87) of AZA/6‐MP participants experienced at least one adverse event that was possibly related to treatment compared to 10% (8/81) of the placebo participants (RR 1.36; 95% CI 0.57 to 3.27; 168 participants; 2 studies; I² = 0%; GRADE low certainty evidence). The subgroup analysis found no evidence of a difference in adverse events when AZA and 6‐MP were compared (P = 0.32). A subgroup analysis based on length of follow‐up found no evidence of a difference in adverse events when measured at 12 months or less and over 12 months (P = 0.32). Commonly reported adverse events included hair loss, leukopenia, diarrhoea, abdominal pain, hepatotoxicity and arthralgia.
1.5. Analysis.
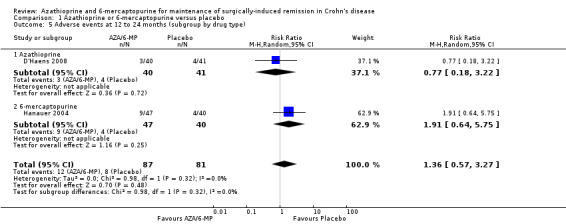
Comparison 1 Azathioprine or 6‐mercaptopurine versus placebo, Outcome 5 Adverse events at 12 to 24 months (subgroup by drug type).
Two studies (Hanauer 2004; Mowat 2016), reported on serious adverse events (Analysis 1.7), and three studies reported on the number of withdrawn participants as a result of adverse reactions to the treatment (D'Haens 2008; Hanauer 2004; Mowat 2016). About 3% (5/175) of AZA/6‐MP participants and 1% (2/152) of placebo participants experienced serious adverse events during 12 to 36 months of intervention follow‐up (RR 1.78; 95% CI 0.39 to 8.18; 327 participants; 2 studies; I² = 0%; GRADE low certainty evidence). During the same period, almost an equal proportion of AZA/6‐MP (24%; 51/215) and placebo (25%; 49/193) participants were withdrawn from the study due to adverse events (RR 0.90; 95% CI 0.63 to 1.29; 408 participants; 3 studies; I² = 3%; GRADE moderate certainty evidence). A subgroup analysis found no evidence of a difference in withdrawal due to adverse events when AZA and 6‐MP were compared (P = 0.69). A subgroup analysis based on length of follow‐up found no evidence of a difference in withdrawals due to adverse events when measured at 12 months or less or at over 12 months (P = 0.69). Commonly reported serious adverse events included arthralgia, pancreatitis, leucopenia and bowel obstruction. Adverse events leading to withdrawal from the study included abnormal blood results leading to led to temporary discontinuation of treatment in 28% of the participants. However, specific details on reasons for discontinuation were not clearly reported.
1.7. Analysis.
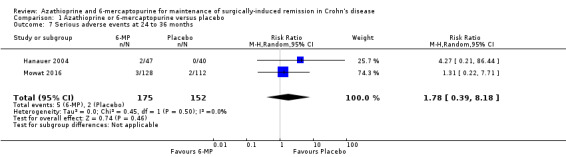
Comparison 1 Azathioprine or 6‐mercaptopurine versus placebo, Outcome 7 Serious adverse events at 24 to 36 months.
Health related quality of life
HRQoL was reported in one study with no difference in IBDQ scores between the two treatments group. However these data were insufficiently reported for inclusion in the analysis (Mowat 2016).
AZA or 6‐MP versus 5‐ASA
A total of five studies compared the efficacy of AZA (2 to 2.5 mg/day) or 6‐MP (50 mg/day) to mesalamine (dose 3 to 4 g/day) (Ardizzone 2004; Hanauer 2004; Herfarth 2006; Reinisch 2010; Savarino 2013). Due to the specific inclusion/exclusion criteria in Reinisch 2010 (Characteristics of included studies), the clinical and endoscopic relapse data from this study were not included in meta‐analyses.
Primary outcome
Clinical relapse
Clinical relapse defined as a clinical recurrence grading score ≥ 2 (Hanauer 2004; Savarino 2013), or a CDAI ≥ 200 (Ardizzone 2004), was reported in four studies. There was low certainty evidence on the efficacy of AZA or 6‐MP for maintaining postoperative clinical remission in comparison to 5‐ASA compounds (Analysis 2.1; Table 2). At the end of the 24 month follow‐up, 64% (113/177) of AZA treated participants clinically relapsed compared to 59% (101/170) of 5‐ASA treated ones (RR 1.05, 95% CI 0.89 to 1.24, 347 participants, 4 studies, I² = 8%; GRADE low certainty evidence). We carried out a subgroup analysis and found no evidence of a difference in clinical relapse rates between AZA and 6‐MP (P = 0.18). A subgroup analysis based on length of follow‐up found no evidence of a difference in clinical relapse rates when measured at 12 months or less or at over 12 months (P = 0.97).
2.1. Analysis.
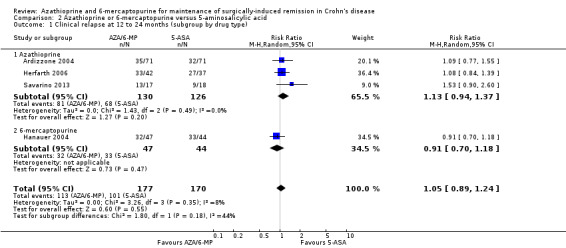
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 1 Clinical relapse at 12 to 24 months (subgroup by drug type).
Secondary outcomes
Endoscopic relapse
Endoscopic relapse was reported in one study (Savarino 2013), and was defined as a Rugeerts score ≥ 2. The efficacy of AZA in comparison to 5‐ASA formulations in preventing endoscopic relapse was uncertain, as the certainty of evidence was very low (Analysis 2.3; Summary of findings table 2). After 24 months, 65% (11/17) of AZA participants relapsed endoscopically compared to 83% (15/18) of 5‐ASA participants (RR 0.78; 95% CI 0.52 to 1.17; 35 participants; 1 study; GRADE very low certainty evidence).
2.3. Analysis.
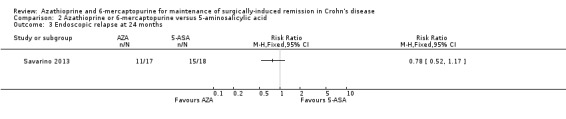
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 3 Endoscopic relapse at 24 months.
Radiologic relapse
Radiologic relapse (follow‐up 24 months), defined as radiographic recurrence grading score ≥ 2, was reported in one study (Savarino 2013) and meta‐analysis was not performed. There is a very low certainty evidence regarding the effect of purine analogues on radiologic relapse rate compared to 5‐ASA drugs (Analysis 2.4). Sixty‐four percent (13/17) of AZA participants experienced radiologic relapse compared to 83% (15/18) of 5‐ASA participants (RR 0.92; 95% CI 0.66 to 1.28; 35 participants; 1 study; GRADE very low certainty evidence).
2.4. Analysis.
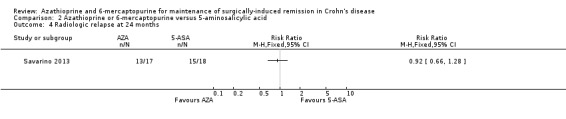
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 4 Radiologic relapse at 24 months.
Endoscopic and radiologic relapse rates were reported by Hanauer 2004, however the results were insufficient to be included in the meta‐analysis. At 24 months (participants 91), reported endoscopic and radiologic relapse rates were 16%; 95% CI 7% to 35% and 33%; 95% CI 19% to 54% in the purine antimetabolites compared to 48%; 95% CI 30% to 70% and 46%; 95% CI 29% to 66% in the mesalamine intervention respectively.
Surgical relapse
Surgical relapse (follow‐up 24 months), defined as the need for another surgery was reported in Ardizzone 2004. The effect of purine analogues compared to 5‐ASA for the maintenance of surgical remission was uncertain, because the quality of evidence was judged as very low (Analysis 2.5; Table 2). During the follow‐up period of two years, the proportion of participants with surgical relapse was 37% (26/71) in the 5‐ASA group versus 30% (21/71) in the purine analogues group (RR 0.81; 95% CI 0.50 to 1.29; 142 participants; 1 study; GRADE very low certainty evidence).
2.5. Analysis.
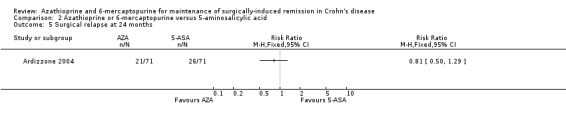
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 5 Surgical relapse at 24 months.
Adverse events, serious adverse events and withdrawal due to adverse events
Adverse events and withdrawal due to adverse events were reported in four studies (Ardizzone 2004; Hanauer 2004; Reinisch 2010; Savarino 2013), and five studies respectively (Ardizzone 2004; Hanauer 2004; Herfarth 2006; Reinisch 2010; Savarino 2013), while serious adverse events were reported in three studies (Ardizzone 2004; Hanauer 2004; Reinisch 2010). The effect of purine analogues when compared to 5‐ASA drugs on adverse events was uncertain, as the quality of evidence was low (Table 2). During a follow‐up of 12 to 24 months, the proportion of participants who experienced at least one adverse event (Analysis 2.6) was 41% (73/176) and 48% (81/170) in the AZA/6‐MP and 5‐ASA groups respectively (RR 0.89; 95% CI 0.74 to 1.07; 346 participants; 4 studies; I² = 15%; GRADE low certainty evidence). We found no evidence of a difference in adverse events when we carried out a subgroup analysis comparing AZA to 6‐MP (P = 0.34). A subgroup analysis based on length of follow‐up found no evidence of a difference in adverse events when measured at 12 months or less or at over 12 months (P = 0.66). Commonly reported adverse events included leukopenia, abdominal pain, nausea, nasopharyngitis, diarrhoea. and headache. During a 12 to 24 months follow‐up, serious adverse events (Analysis 2.8) were experienced by 17% (27/159) of purine analogue participants compared to 4% (6/152) of 5‐ASA participants (RR 3.39; 95% CI 1.26 to 9.13; 311 participants; 3 studies; I² = 9%; GRADE very low certainty evidence). We found no evidence of a difference in serious adverse events when we carried out a subgroup comparing AZA to 6‐MP (P = 1.0). A subgroup analysis based on length of follow‐up found no evidence of a difference in serious adverse events when measured at 12 months or less or at over 12 months (P = 0.19). Commonly reported serious adverse events include postoperative bowel obstruction. The proportion of participants that withdrew from the trial due to an adverse event (Analysis 2.10) during 12 to 24 months follow‐up were 19% (42/218) versus 8% (16/207) in the AZA/6‐MP and 5‐ASA groups respectively (RR 2.21, 95% CI 1.28 to 3.81; participants = 425; studies = 5; I² = 0%; GRADE low certainty evidence). We found no evidence of a difference in withdrawals due to adverse event when AZA and 6‐MP were compared (P = 0.25). A subgroup analysis based on length of follow‐up found no evidence of a difference in withdrawal due to adverse events when measured at 12 months or less or at over 12 months (P = 0.46). Adverse events leading to withdrawal included severe epigastric intolerance, increase in liver function test results, leukopenia and acute pancreatitis.
2.6. Analysis.
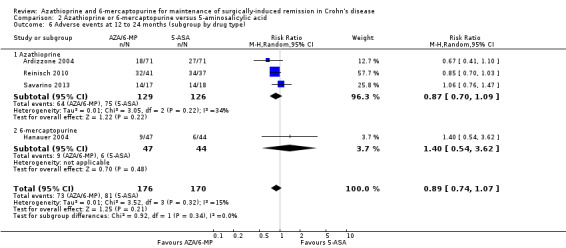
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 6 Adverse events at 12 to 24 months (subgroup by drug type).
2.8. Analysis.
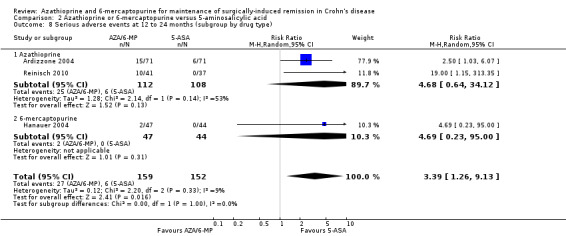
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 8 Serious adverse events at 12 to 24 months (subgroup by drug type).
2.10. Analysis.
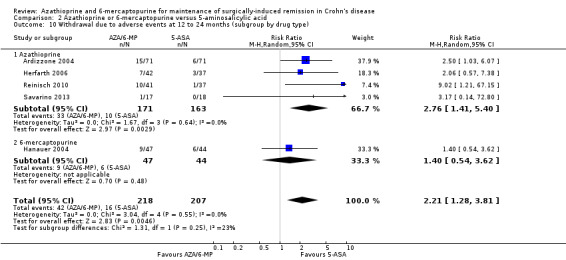
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 10 Withdrawal due to adverse events at 12 to 24 months (subgroup by drug type).
HRQoL
Two studies with a follow‐up of 12 to 24 months reported on HRQoL based on the IBDQ score (Reinisch 2010; Savarino 2013). The effect of 5‐ASA agents on HRQoL was uncertain as a result of serious limitations due to unclear risk of performance and outcome assessment bias and high risk of performance bias and outcome assessment bias in each study respectively and very serious limitations due to sparse data in both studies. Savarino 2013 reported on the proportion of participants with an IBDQ score > 170 (ranging from 32 to 224), which is regarded as a symptomatic remission score (Analysis 2.12). After 24 months of follow‐up, 12% (2/17) of AZA treated participants reported an IBDQ score >170 compared with 17% (3/18) of 5‐ASA participants (RR 0.71; 95% CI 0.13 to 3.72; 35 participants; 1 study; GRADE very low certainty evidence). Reinisch 2010, assessed HRQoL based on the mean change of IBDQ scores compared to baseline (Analysis 2.13.) At 12 months, the mean IBDQ difference compared to baseline was 9 (SD 17.7) in the AZA group versus 5 (SD 27.4) in the 5‐ASA treated group (MD 4; CI 14.36 to ‐6.36; 78 participants; 1 study; GRADE very low certainty evidence).
2.12. Analysis.
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 12 HRQoL ‐ IBDQ > 170 at 24 months.
2.13. Analysis.
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 13 HRQoL ‐ IBDQ at 12 months.
AZA or 6‐MP versus anti‐TNF‐α
Three studies comparing AZA to either infliximab (Armuzzi 2013), or adalimumab (Lopez‐Sanroman 2017; Savarino 2013), and one study comparing 6‐mercaptopurine to adalimumab (Scapa 2015), were identified. For all of the studies, the intervention started within 45 days of surgery. In the Lopez‐Sanroman 2017 study all participants were also administered metronidazole (750 mg/day) for the first three months of the study. The total number of assessed participants was 157.
Primary outcome
Clinical relapse
Clinical relapse (Analysis 3.1) was reported in three studies (Armuzzi 2013; Lopez‐Sanroman 2017; Savarino 2013), and was defined as HBI ≥ 2 (Armuzzi 2013), clinical recurrence grading score ≥2 (Savarino 2013) or CDAI score >200 (Lopez‐Sanroman 2017). The certainty of evidence that AZA is inferior in preventing postsurgical clinical relapse compared to anti‐TNF‐α agents was very low (Table 3). During a follow‐up of 12 to 24 months, 43% (29/67) of participants treated with AZA clinically relapsed compared to 14% (10/72) of participants in the anti‐TNF‐α group (RR 2.89, 95% CI 1.50 to 5.57, 139 participants, 3 studies, I² = 0%; GRADE very low certainty evidence). The subgroup analysis based on length of follow‐up found no evidence of a difference in clinical relapse when measured at 12 months or less or at over 12 months (P = 0.2).
3.1. Analysis.
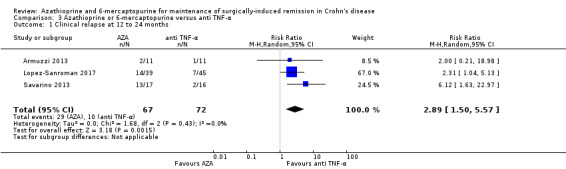
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 1 Clinical relapse at 12 to 24 months.
Secondary outcomes
Endoscopic relapse
Endoscopic relapse was reported in four studies and was defined as Rutgeerts score ≥2. The evidence that purine antimetabolites are less efficient than anti‐TNF‐α for maintaining endoscopic remission was very low (Analysis 3.3; Table 3). The proportion of purine analogue participants with endoscopic relapse was 58% (43/74) compared to 26% (22/83) of anti‐TNF‐α participants (RR 3.67; 95% CI 1.05 to 12.81; 157 participants; 4 studies; I² = 64%; GRADE very low certainty evidence). We found no evidence of a difference in endoscopic relapse rates when we carried out a subgroup analysis to compare AZA to 6‐MP (P = 0.72). A subgroup analysis based on length of follow‐up found a quantitative difference in endoscopic relapse when measured at 12 months or less or at over 12 months (P = 0.2).
3.3. Analysis.
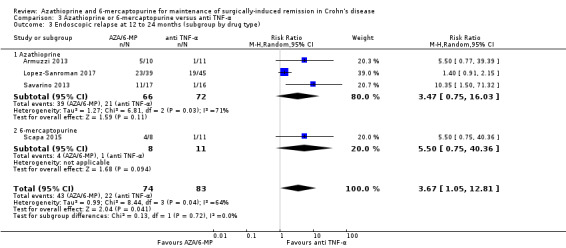
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 3 Endoscopic relapse at 12 to 24 months (subgroup by drug type).
Radiologic relapse
Radiologic relapse was reported in two studies, defined either as radiographic recurrence grading score ≥ 2 (Savarino 2013), or based on magnetic resonance enterography scores MR2 and MR3 (Lopez‐Sanroman 2017). The certainty of evidence that purine analogues are less efficient to prevent endoscopic relapse than adalimumab or infliximab was very low (Analysis 3.5; Table 3). The proportion of participants with endoscopic relapse after 12 to 24 months follow‐up was 69% (39/56) and 38% (23/61) among the purine analogues and anti‐TNF‐α treated groups respectively. Data pooling for Savarino 2013 (RR 1.36, 95% CI 0.94 to 1.98) and Lopez‐Sanroman 2017 (RR 12.24, 95% CI 1.8 to 83.12) was not feasible due to considerable heterogeneity (I² = 85%). The certainty of evidence was rated very low due to high risk of bias and very serious imprecision.
3.5. Analysis.
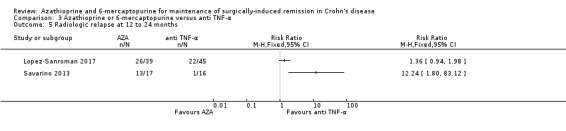
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 5 Radiologic relapse at 12 to 24 months.
Histologic relapse
Histologic relapse (Analysis 3.6), based on the scoring system modified by Regueiro 2009 was reported only in Armuzzi 2013 and a meta‐analysis was not performed. Histologic relapse during 12 months of follow‐up was detected in 82% (9/11) and 18% (2/11) participants of the AZA and infliximab groups respectively (RR 4.50; 95% CI 1.25 to 16.25).
3.6. Analysis.
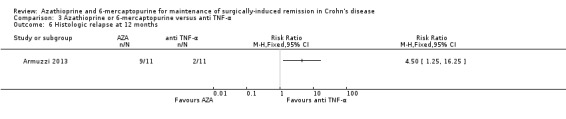
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 6 Histologic relapse at 12 months.
Adverse events, serious adverse events and withdrawal due to adverse events
Adverse events were reported in two studies (Lopez‐Sanroman 2017; Savarino 2013). Withdrawals due to adverse events were reported in three studies ( Armuzzi 2013; Lopez‐Sanroman 2017; Savarino 2013), and serious adverse events were reported in one study (Lopez‐Sanroman 2017). The certainty of evidence regarding tolerability and safety of purine analogues in comparison to anti‐TNF‐α agents ranged from very low to low (Table 3). There was no clear difference in the number of participants who experienced adverse events when AZA/6‐MP was compared to anti‐TNF‐α during the follow‐up of 12 to 24 months (Analysis 3.7). Fifty‐seven per cent (32/56) of AZA/6‐MP participants experienced at least one adverse event compared to 51% (31/61) of the anti‐TNF‐α group (RR 1.13; 95% CI 0.83 to 1.53; 117 participants; 2 studies; I² = 0%; GRADE low certainty evidence). A subgroup analysis based on length of follow‐up found no evidence of a difference in adverse events when measured at 12 months or less or at over 12 months (P = 0.65). Commonly reported adverse events included bronchitis, nasopharyngitis, arthralgia, nausea and abscess (Savarino 2013). Full details concerning adverse events were not reported in Lopez‐Sanroman 2017. The evidence regarding serious adverse events comes from a single study and a meta‐analysis was not performed (Analysis 3.9). The proportion of participants with serious adverse events was 10% (4/39) and 20% (9/45) among the AZA and adalimumab groups respectively (RR 0.51; 95% CI 0.17 to 1.54; 84 participants; 1 study; GRADE very low certainty evidence). The types of serious adverse events were not described. Over a follow‐up that ranged from 12 to 24 months, 16% (11/67) of AZA/6‐MP treated participants were withdrawn from the study due to an adverse event compared to 3% (2/72) of the anti‐TNF‐α treated participants (RR 3.97; 95% CI 0.92 to 17.22; 139 participants; 3 studies; I² = 4%; GRADE low certainty evidence). A subgroup analysis based on length of follow‐up found no evidence of a difference in withdrawals due to adverse events when measured at 12 months or less or at over 12 months (P = 0.24). Adverse events leading to withdrawal included severe nausea, leukopenia, arthralgia, urothelial carcinoma, dyspepsia, dyspnoea, death, atopic dermatitis and abdominal pain with increase in pancreatic enzymes.
3.7. Analysis.
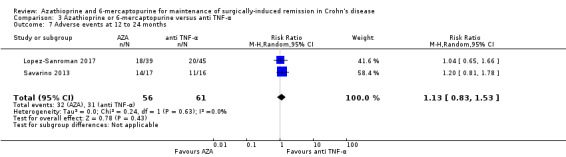
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 7 Adverse events at 12 to 24 months.
3.9. Analysis.
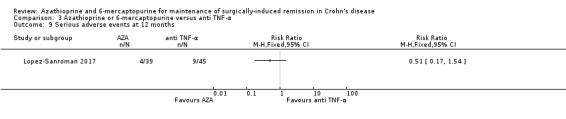
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 9 Serious adverse events at 12 months.
Health related quality of life
A single study assessed health‐related quality of life (Savarino 2013). At 24 months, an IBDQ > 170 was recorded in 12% (2/17) of participants treated with AZA compared to 88% (14/16) treated with adalimumab (RR 0.13; 95% CI 0.04 to 0.50; 34 participants; 1 study). This outcome was also evaluated in Lopez‐Sanroman 2017 using the EuroQol‐5 dimensions questionnaire, however reported data were insufficient to be included in meta‐analysis.
Special safety note
It is worth specifically noting that two adverse events leading to therapy cessation occurred in more than 1% of participants treated with purine analogues. Pancreatitis was a serious adverse event that led to withdrawal across several of the studies. However, the rate of occurrence was almost exclusively in participants receiving purine analogues (11 cases in 354 participants taking purine analogues, 3.1%) compared to no occurrences in any of the comparison groups (i.e. placebo, 5‐ASA or anti‐TNF‐α) in this review. Leucopenia occurred also almost exclusively in participants receiving purine analogues (9 cases in 354 participants, 2.5%) compared to no occurrences in all other groups.
Discussion
Summary of main results
Ten RCTs assessing the effectiveness of AZA and 6‐MP for maintaining surgically‐induced remission in Crohn's disease were studied. The studies recruited people with Crohn's disease over the age of 16 years who were in surgically‐induced remission. The participants were randomised after surgery to received AZA or 6‐MP, placebo, 5‐ASA agents or anti‐TNF‐α agents.
We found moderate certainty evidence that AZA and 6‐MP are superior to placebo for preventing post‐surgical clinical relapse in Crohn's disease. There was low certainty evidence on the safety of AZA and 6‐MP when adverse events and serious adverse events were considered. The certainty of the evidence for withdrawal due to adverse events was moderate. This was due to imprecision resulting from very sparse data.
There was no clear difference clinical relapse when AZA or 6‐MP was compared with 5‐ASA. There are considerable concerns raised with the safety profile of AZA and 6‐MP. For the safety outcome serious adverse events and withdrawals due to adverse events, 5‐ASA was found to be superior to AZA and 6‐MP. However, the certainty of evidence for the efficacy and safety outcomes were low due to high risk of performance bias and sparse data.
There was very low certainty evidence that AZA and 6‐MP may be inferior to anti‐TNF‐α agents for preventing clinical relapse. We also found no clear difference in adverse events, serious adverse events or withdrawals due to adverse events when both outcomes were compared. This was also based on low certainty evidence. The evidence was downgraded due to high risk of performance bias and sparse data, therefore, the results should be interpreted with caution.
Overall completeness and applicability of evidence
We consider the evidence from this review to be applicable to most patients with post‐surgical remission of Crohn's disease. The evidence only assesses AZA or 6‐MP compared with placebo, 5‐ASA or anti‐TNF‐α agents. All outcomes which we had aimed to analyse were reported in the studies. However, other measures of relapse (endoscopic, histologic and surgical) and health related quality of life were sparsely reported. This meant that very little data were available for our analysis. The review found moderate certainty evidence on the efficacy of AZA and 6‐MP compared with placebo. This can be considered complete and not requiring new studies, however, the evidence comparing it with 5‐ASA and anti‐TNF‐α were of very low or low certainty. Therefore, additional studies may change the results. The use of concomitant treatments such as antidiarrhoeal agents, corticosteroids and antibiotics were reported in some studies. The circumstances in which these treatments were given were noted in the studies and judged to be reflective of clinical practice.
Quality of the evidence
The certainty of the evidence was rated very low to moderate. Overall, six studies were at high risk of bias and three were at unclear risk of bias. Downgrading for limitations was mostly due to lack of blinding and this was particularly common with the head to head comparisons of active drugs. For most of the outcomes, there was imprecision due to sparse data as the number of events ranged between seven and 154. For the clinical relapse outcome, the optimal information size was obtained from power calculations in the largest and most recent study (Mowat 2016). Most of the results were consistent except in two instances where there was substantial statistical heterogeneity (I² between 62% and 64%). There was no indirectness as all the studies in the review met the criteria proposed in the scope of the review. We were unable to assess for publication bias due to insufficient data.
Potential biases in the review process
We attempted to reduce potential biases in the review process. A comprehensive literature search was performed to identify all eligible studies. Two review authors independently assessed studies for inclusion, extracted data and assessed study quality.
All analyses were completed using the intention‐to‐treat principle, whereby participants with final missing outcomes were assumed to have relapsed. Given the high attrition rate in the purine analogue groups compared to the 5‐ASA groups, this may have affected the difference in clinical relapse rates between purine analogues and 5‐ASA. However, it is arguably a moot point given that even if purine analogues did have superior efficacy, it is difficult to rationalise the use on the basis of the poor adverse event profile in the published evidence.
One of the included studies administered active concomitant treatments in both intervention arms. This was considered a source of clinical heterogeneity in analyses that included studies which had no concomitant treatments. We did not remove this study from the analyses as a sensitivity analysis showed no difference in the results.
Agreements and disagreements with other studies or reviews
A recent Cochrane review assessing the use of AZA or 6‐MP for maintenance of medically‐induced remission in Crohn's disease revealed that the purine analogues are more effective than placebo, with higher response rates for AZA than 6‐MP (Chande 2015). These findings are mirrored in the four studies comparing purine analogues to placebo. No difference in efficacy was found between AZA or 6‐MP and 5‐ASA. This could be due to lower disease activity following resection of the gut than is achieved in medically‐induced remission of Crohn's disease, so that a milder anti‐inflammatory agent such as 5‐ASA, gives a better risk versus benefit ratio when compared to AZA and 6‐MP. It is also possible that the methodology of the included studies supports this hypothesis, with all but one study recruiting participants in the immediate post‐surgical setting. As such, the participants are potentially at their lowest period of disease activity clinically and microscopically. The findings regarding safety are also consistent with the two most common adverse events noted as pancreatitis and leucopenia.
A Cochrane review looking at the use of 5‐ASA for the maintenance of surgically‐induced remission in Crohn's disease suggests that 5‐ASA may be superior to placebo (Gjuladin‐Hellon 2019). It also suggested that 5‐ASA is a safe and well‐tolerated drug, as the incidence of adverse events was not different in participants receiving 5‐ASA compared to those receiving placebo. The results of this systematic review question the risk versus benefit balance of starting a purine analogue over 5‐ASA in postoperative Crohn's disease.
The international guidance from the European Crohn's and colitis Organisation (ECCO) updated in 2016 is consistent with the findings of this review in that they do indicate a role for purine analogues (Gionchetti 2016), although they do not comment on the limitations of the evidence as identified in this review. Most importantly, the significant safety questions raised in the synthesis performed in this review are not discussed within the ECCO documents in this context (section 8G), although leucopenia is noted in medical induced remission maintenance (6E). The National Insitutue for Clinical and Care Excellence (NICE) in the UK suggests AZA as first line therapy in this context (NICE 2016), citing the previous version of this review in their 2016 guidance (Gordon 2014). Unlike ECCO, the NICE guidance makes no specific mention of safety concerns. Given one of the specific adverse events noted frequently (leucopenia) is recognised in the context of purine analogues, it is important to highlight that the risk of pancreatitis has not been highlighted in either guideline, despite being recognised as the most common serious adverse events in both Cochrane reviews on these medications.
Authors' conclusions
Implications for practice.
Moderate certainty evidence suggests that purine analogues may be superior to placebo for maintenance of surgically‐induced remission in participants with Crohn's disease. There was no clear difference in the number of clinical relapses when purine analogues were compared with 5‐ASA agents, however this is based on low certainty evidence and no firm conclusions can be drawn. However, participants taking purine analogues were more likely than 5‐ASA participants to experience serious adverse events and discontinue therapy due to adverse events. Very low certainty evidence suggests that AZA and 6‐MP may be inferior to anti‐TNF‐α agents, however, no firm conclusions can be drawn.
Implications for research.
Further research investigating the efficacy and safety of AZA and 6‐MP in comparison to other active medications in surgically‐induced remission of Crohn's disease is warranted.
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2019 | New search has been performed | New search and new studies added |
| 31 July 2019 | New citation required and conclusions have changed | Updated review with new authors |
Acknowledgements
Funding for ZIE, TH, and partial funding for MG was provided through a larger NIHR Cochrane Programme Grant in the UK.
Funding for the Cochrane IBD Group (May 1, 2017 to April 30, 2022) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. Search strategy July 26, 2018
PubMed
(randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomised[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab] NOT (animals [mh] NOT humans [mh]))
AND
(Crohn*[tiab] OR IBD tiab] OR Inflammatory bowel disease [tiab] OR [ Regional enteritis [tiab] OR ileitis [tiab])
AND
(surgery[tiab] OR surgic* [tiab] OR post‐surgical [tiab] OR post‐surgery [tiab] OR postoperative [tiab] OR post‐operative [tiab] OR resection [tiab] OR operation [tiab])
AND
(AZA[tiab] OR azathioprine [tiab] OR 6‐mercaptopurine[tiab] OR 6MP[tiab] OR 6‐MP[tiab] OR 6 anti‐metabolite* [tiab] OR antimetabolite* [tiab])
MEDLINE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. randomized controlled trial/
14. or/1‐13
15. exp Crohn disease/ or crohn*.mp.
16. inflammatory bowel disease.mp.
17. IBD.mp.
18. or/15‐17
19. azathioprine.mp. or exp azathioprine derivative/ or exp azathioprine/
20. 6‐mercaptopurine.mp. or exp mercaptopurine/
21. (AZA or 6‐MP or 6MP).mp.
22. exp antimetabolite/ or anti‐metabolite*.mp.
23. antimetabolite*.mp.
24. or/19‐23
25. surgery.mp. or surgery/
26. (surgical or surgically).mp.
27. surgic*.mp.
28. (post‐surgical or post‐surgery).mp.
29. (postoperative or post‐operative).mp.
30. resection.mp. or surgery/
31. operation.mp. or surgery/
32. or/25‐31
33. 14 and 18 and 24 and 32
EMBASE
1 random$.tw.
2 factorial$.tw.
3 (crossover$ or cross over$ or cross‐over$).tw.
4 placebo$.tw.
5 single blind.mp.
6 double blind.mp.
7 triple blind.mp.
8 (singl$ adj blind$).tw.
9 (double$ adj blind$).tw.
10 (tripl$ adj blind$).tw.
11 assign$.tw.
12 allocat$.tw.
13 crossover procedure/
14 double blind procedure/
15 single blind procedure/
16 triple blind procedure/
17 randomized controlled trial/
18 or/1‐17
19. exp Crohn disease/ or crohn*.mp.
20. inflammatory bowel disease.mp.
21. IBD.mp.
22. or/19‐21
23. azathioprine.mp. or exp azathioprine derivative/ or exp azathioprine/
24. 6‐mercaptopurine.mp. or exp mercaptopurine/
25. (AZA or 6‐MP or 6MP).mp.
26. exp antimetabolite/ or anti‐metabolite*.mp.
27. antimetabolite*.mp.
28. or/23‐27
29. surgery.mp. or surgery/
30. (surgical or surgically).mp.
31. surgic*.mp.
32. (post‐surgical or post‐surgery).mp.
33. (postoperative or post‐operative).mp.
34. resection.mp. or surgery/
35. operation.mp. or surgery/
36. or/29‐35
37. 18 and 22 and 28 and 36
CENTRAL
#1 crohn* or "inflammatory bowel disease" or IBD
#2 anti‐metabolite* or antimetabolite*
#3 6‐mercaptopurine or mercaptopurine or 6‐MP or 6MP
#4 AZA or azathioprine
#5 #2 or #3 or #4
# 6 #1 and #5
#7 surgery or surgic* or post‐surgical or post‐surgery or postoperative or post‐operative or resection or operation
#8 #6 and #7
SR‐IBD
Crohn AND 6‐mercaptopurine or 6‐MP or 6MP or azathioprine AND surgery or surgic* or post* or resection or operation (4)
Clinical trials.gov
1. Azathioprine and Crohn’s disease
2. 6‐mercaptopurine and Crohn’s disease
WHO trial registry
1. Azathioprine and Crohn’s disease
2. 6‐mercaptopurine and Crohn’s disease
Data and analyses
Comparison 1. Azathioprine or 6‐mercaptopurine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical relapse at 12 to 36 months (subgroup by drug type) | 3 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.67, 0.92] |
| 1.1 Azathioprine | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.08] |
| 1.2 6‐mercaptopurine | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.68, 0.94] |
| 2 Clinical relapse (subgroup by length of follow‐up) | 3 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.67, 0.92] |
| 2.1 12 months or less | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.08] |
| 2.2 Over 12 months | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.68, 0.94] |
| 3 Endoscopic relapse at 12 to 36 months (subgroup by drug type) | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 3.1 Azathioprine | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.51, 0.97] |
| 3.2 6‐mercaptopurine | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.11] |
| 4 Endoscopic relapse (subgroup by length of follow‐up) | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 4.1 12 months or less | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.51, 0.97] |
| 4.2 Over 12 months | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.11] |
| 5 Adverse events at 12 to 24 months (subgroup by drug type) | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.57, 3.27] |
| 5.1 Azathioprine | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.18, 3.22] |
| 5.2 6‐mercaptopurine | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.64, 5.75] |
| 6 Adverse events (subgroup by length of follow‐up) | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.57, 3.27] |
| 6.1 12 months or less | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.18, 3.22] |
| 6.2 Over 12 months | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [0.64, 5.75] |
| 7 Serious adverse events at 24 to 36 months | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.39, 8.18] |
| 8 Withdrawal due to adverse events at 12 to 36 months (subgroup by drug type) | 3 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.63, 1.29] |
| 8.1 Azathioprine | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.18, 3.22] |
| 8.2 6‐mercaptopurine | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.50, 2.27] |
| 9 Withdrawal due to adverse events (subgroup by length of follow‐up) | 3 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.63, 1.29] |
| 9.1 12 months or less | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.18, 3.22] |
| 9.2 Over 12 months | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.50, 2.27] |
1.2. Analysis.
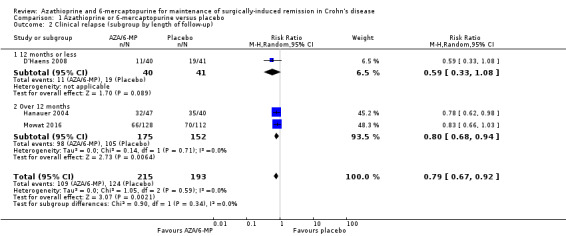
Comparison 1 Azathioprine or 6‐mercaptopurine versus placebo, Outcome 2 Clinical relapse (subgroup by length of follow‐up).
1.4. Analysis.
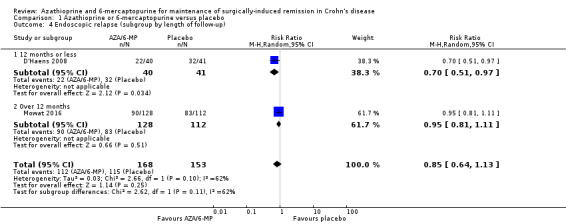
Comparison 1 Azathioprine or 6‐mercaptopurine versus placebo, Outcome 4 Endoscopic relapse (subgroup by length of follow‐up).
1.6. Analysis.
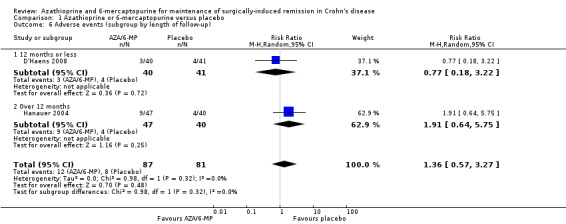
Comparison 1 Azathioprine or 6‐mercaptopurine versus placebo, Outcome 6 Adverse events (subgroup by length of follow‐up).
1.8. Analysis.
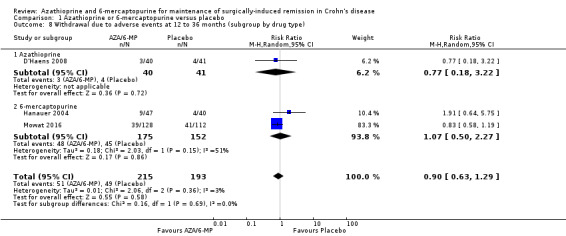
Comparison 1 Azathioprine or 6‐mercaptopurine versus placebo, Outcome 8 Withdrawal due to adverse events at 12 to 36 months (subgroup by drug type).
1.9. Analysis.
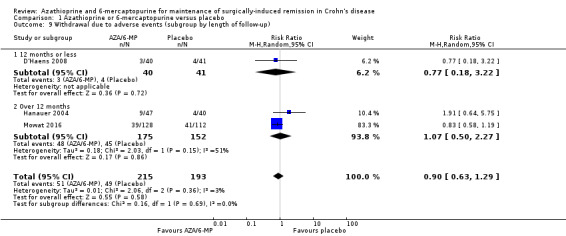
Comparison 1 Azathioprine or 6‐mercaptopurine versus placebo, Outcome 9 Withdrawal due to adverse events (subgroup by length of follow‐up).
Comparison 2. Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical relapse at 12 to 24 months (subgroup by drug type) | 4 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.24] |
| 1.1 Azathioprine | 3 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.94, 1.37] |
| 1.2 6‐mercaptopurine | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.70, 1.18] |
| 2 Clinical relapse (subgroup by length of follow‐up) | 4 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.24] |
| 2.1 12 months or less | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.84, 1.39] |
| 2.2 Over 12 months | 3 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.82, 1.39] |
| 3 Endoscopic relapse at 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Radiologic relapse at 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Surgical relapse at 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse events at 12 to 24 months (subgroup by drug type) | 4 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.07] |
| 6.1 Azathioprine | 3 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.09] |
| 6.2 6‐mercaptopurine | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.54, 3.62] |
| 7 Adverse events (subgroup by length of follow‐up) | 4 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.07] |
| 7.1 12 months or less | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.70, 1.03] |
| 7.2 Over 12 months | 3 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.64, 1.38] |
| 8 Serious adverse events at 12 to 24 months (subgroup by drug type) | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 3.39 [1.26, 9.13] |
| 8.1 Azathioprine | 2 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 4.68 [0.64, 34.12] |
| 8.2 6‐mercaptopurine | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.23, 95.00] |
| 9 Serious adverse events (subgroup by length of follow‐up) | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 3.39 [1.26, 9.13] |
| 9.1 12 months or less | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 19.00 [1.15, 313.35] |
| 9.2 Over 12 months | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [1.12, 6.16] |
| 10 Withdrawal due to adverse events at 12 to 24 months (subgroup by drug type) | 5 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.28, 3.81] |
| 10.1 Azathioprine | 4 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [1.41, 5.40] |
| 10.2 6‐mercaptopurine | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.54, 3.62] |
| 11 Withdrawal due to adverse events (subgroup by length of follow‐up) | 5 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.28, 3.81] |
| 11.1 12 months or less | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 3.54 [0.83, 15.08] |
| 11.2 Over 12 months | 3 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.03, 3.68] |
| 12 HRQoL ‐ IBDQ > 170 at 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 HRQoL ‐ IBDQ at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.2. Analysis.
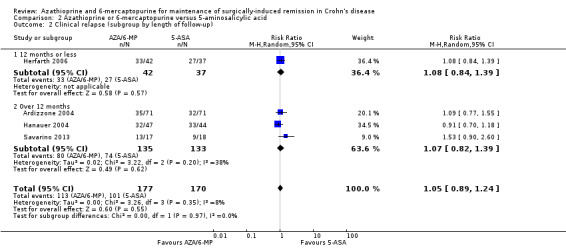
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 2 Clinical relapse (subgroup by length of follow‐up).
2.7. Analysis.
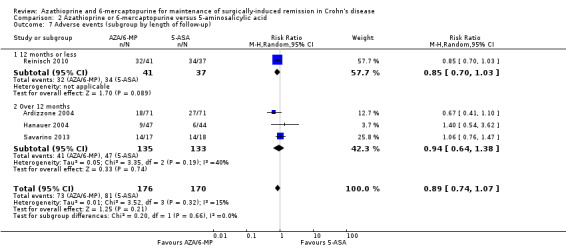
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 7 Adverse events (subgroup by length of follow‐up).
2.9. Analysis.
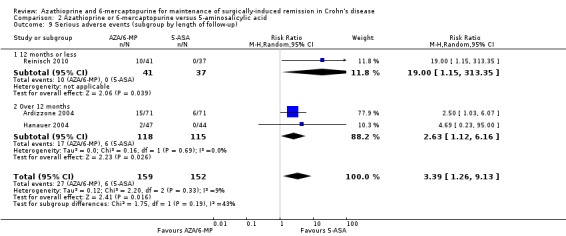
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 9 Serious adverse events (subgroup by length of follow‐up).
2.11. Analysis.
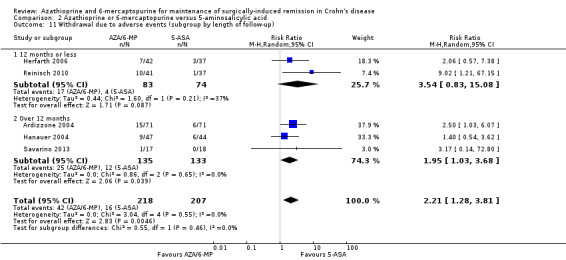
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐aminosalicylic acid, Outcome 11 Withdrawal due to adverse events (subgroup by length of follow‐up).
Comparison 3. Azathioprine or 6‐mercaptopurine versus anti TNF‐α.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical relapse at 12 to 24 months | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.50, 5.57] |
| 2 Clinical relapse (subgroup by length of follow‐up) | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.50, 5.57] |
| 2.1 12 months or less | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 2.27 [1.07, 4.82] |
| 2.2 Over 12 months | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 6.12 [1.63, 22.97] |
| 3 Endoscopic relapse at 12 to 24 months (subgroup by drug type) | 4 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 3.67 [1.05, 12.81] |
| 3.1 Azathioprine | 3 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 3.47 [0.75, 16.03] |
| 3.2 6‐mercaptopurine | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 5.5 [0.75, 40.36] |
| 4 Endoscopic relapse (subgroup by length of follow‐up) | 4 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 3.67 [1.05, 12.81] |
| 4.1 12 months or less | 3 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.83, 7.18] |
| 4.2 Over 12 months | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 10.35 [1.50, 71.32] |
| 5 Radiologic relapse at 12 to 24 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Histologic relapse at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Adverse events at 12 to 24 months | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.83, 1.53] |
| 8 Adverse events (subgroup by length of follow‐up) | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.83, 1.53] |
| 8.1 12 months or less | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.65, 1.66] |
| 8.2 Over 12 months | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.81, 1.78] |
| 9 Serious adverse events at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Withdrawal due to adverse events at 12 to 24 months | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 3.97 [0.92, 17.22] |
| 11 Withdrawal due to adverse events (subgroup by length of follow‐up) | 3 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [0.99, 17.47] |
| 11.1 12 months or less | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 7.17 [1.32, 38.96] |
| 11.2 Over 12 months | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.07, 15.62] |
| 12 HRQoL ‐ IBDQ > 170 at 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.2. Analysis.
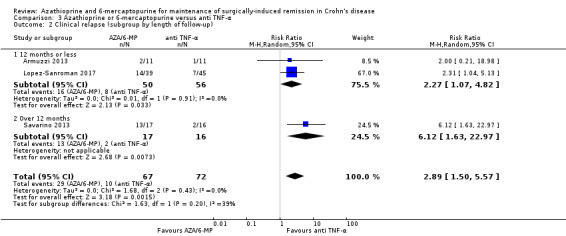
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 2 Clinical relapse (subgroup by length of follow‐up).
3.4. Analysis.
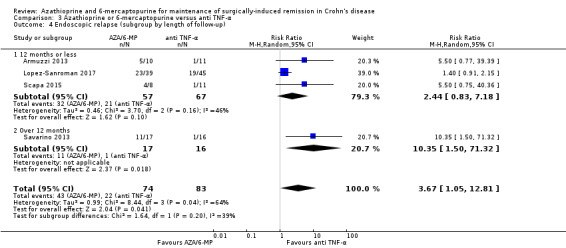
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 4 Endoscopic relapse (subgroup by length of follow‐up).
3.8. Analysis.
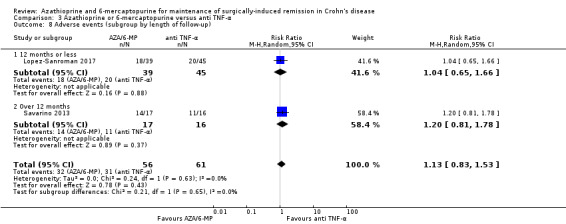
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 8 Adverse events (subgroup by length of follow‐up).
3.10. Analysis.
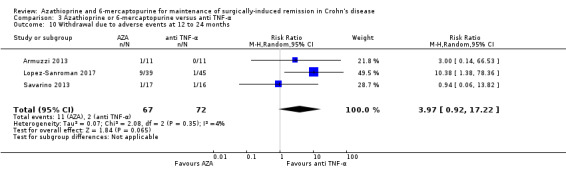
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 10 Withdrawal due to adverse events at 12 to 24 months.
3.11. Analysis.
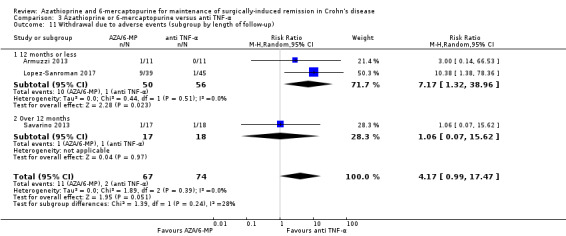
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 11 Withdrawal due to adverse events (subgroup by length of follow‐up).
3.12. Analysis.
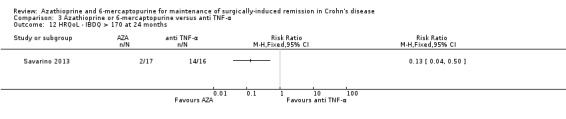
Comparison 3 Azathioprine or 6‐mercaptopurine versus anti TNF‐α, Outcome 12 HRQoL ‐ IBDQ > 170 at 24 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ardizzone 2004.
| Methods |
Study design: RCT, single centre Setting: University “L.Sacco” Hospital (Milan, Italy), 1994 to 2001 |
|
| Participants |
Inclusion: Adult (18 to 70 years) participants who underwent surgery for symptomatic intestinal stenosis or occlusion, which is clinically quiescent (CDAI ≤ 150) able to start oral nutrition and oral medication within the first 2 postoperative weeks Exclusion: Contraindications for use of mesalamine or AZA and pre‐existing hepatic disease, renal dysfunction, clinically important lung disease, systemic infection, short‐bowel syndrome, presence of alcoholic stoma, history of cancer, hypersensitivity to mesalamine or AZA, erythrocyte macrocytosis, use of immunosuppressive drugs in the past 3 months; participants who had received treatment with anti–TNF‐α within the 6 months before surgery; pregnancy/breastfeeding; participants who had undergone surgical procedures other than conservative surgery or for perianal disease only; history of corticosteroid‐dependent disease Age (IG1 / IG2) mean: 38.4 years Sex (M:F): 95: 52 overall; (45:26) versus (50:26) Type of surgery: Stricturoplasty‐ 36; Minimal bowel resection‐ 70; Minimal bowel resection stricturoplasty‐36 Previous surgery (IG1+IG2): 69/142 overall (38/71) versus (31/71) Start of intervention after surgery: < 2 weeks Medication use (IG1+ IG2): Mesalamine or sulphasalazine 62; Corticosteroids 41; Immunosuppressants 9; None 30 Smoker (IG1 / IG2): (28/71) versus (36/71) Number randomised (N = 142): 71 versus 71 Number analysed (N = 138): (69/71) versus (69/71) ‐ ITT; 50/71 versus 61/71 ‐ per protocol Post‐randomisation exclusion (n = 11): (6/71) versus (5/71) (did not start the treatment ‐3 (2 versus 1); lost to follow‐up ‐8 (4 versus 4)) |
|
| Interventions |
Group 1: Azathioprine administered at a dosage of 2 mg/kg/day Group 2: Mesalamine was administered at a dosage of 3 g/day divided into 3 doses All participants: treatment with aminosalicylates, metronidazole, and any other CD‐specific treatment had to be discontinued. Corticosteroids were allowed to be tapered by standardized stepwise dose reductions within 6 weeks after surgery at the latest. Symptomatic treatment with antacids, antidiarrhoeal agents, or spasmolytic agents was allowed but had to be scrupulously recorded. Compliance with treatment was evaluated by a simple questionnaire in which adverse events were also recorded. Participants receiving AZA were regularly assessed by total blood cell count and serum transaminase values to monitor any myelotoxicity and hepatotoxicity of the treatment. Participants were seen at baseline and every 6 months |
|
| Outcomes |
Duration of study: 24 months 1. Clinical relapse defined as the presence of symptoms related to CD, variably associated with radiologic, endoscopic, and laboratory findings, with a CDAI score > 200, which is considered severe enough to warrant treatment with a systemic corticosteroid at a medium‐high dose 2. Surgical Relapse defined as the presence of symptoms refractory to medical treatment or complications requiring another surgical procedure (e.g., occlusive disease, intra‐abdominal abscesses, or high‐flow fistulas) 3. Adverse events |
|
| Notes |
Funding source: Not reported Conflict of interest: Not reported Power calculation: Based on a maximum relapse rate at 2 years of 45% mesalamine, 62 participants per treatment group was considered sufficient to detect a difference of ≥ 25% for the AZA treatment group (type 1 error of 5%). The number of participants in each group was increased to 68 to compensate for an anticipated drop out rate of 10% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “After surgery, patients who met the inclusion criteria and who agreed to enter the study were randomised to receive mesalamine or AZA by a computer‐generated list” and ”Randomization was performed in blocks of 10” Comment: computer generated block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the study is open‐label and blinding was not performed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make judgment, however it is unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: “In the intention‐to‐treat analysis, all randomised patients who received at least one dose of the study drug and were subjected to the baseline evaluation were considered for the analysis.”and “Outcome measures were analysed in all randomised patients who had taken at least one dose of the study medication (intention‐to‐treat population)…” Comment: ITT analysis applied, all withdrawals were low and balanced across groups |
| Selective reporting (reporting bias) | Low risk | Trial registration not available, however all outcomes stated in the method section assessed and reported |
| Other bias | Low risk | Quote: “No significant differences were observed between the 2 treatment groups regarding age, sex, duration of disease, location of disease, fistula and abscess at surgery, surgical procedure, previous operations, and CD therapy during the previous 6 months” Comment: baseline characteristics well balanced across groups |
Armuzzi 2013.
| Methods |
Study design: RCT, single centre Setting: Italy, 2007 to 2011 |
|
| Participants |
Inclusion: Consecutive CD participants who underwent curative ileocolonic resection (all macroscopically inflamed tissues were removed and operative margins were disease‐free at histopathology examination) and considered at “high risk”* of postoperative recurrence were enrolled Exclusion: active perianal disease, presence of stoma, adverse events during previous therapy with infliximab or azathioprine, age > 70 years, surgical complications, active infectious diseases, history of cancer, renal, cardiac or hepatic failure, history of acute or chronic pancreatitis, severe leucopenia (WBC <3000 μu/ml, lymphocyte count <1000 μu/ml) and pregnancy Age (IG1 / IG2) median (range): 32 years (18 to 70 years) Sex (M:F): 15:7 overall; (7:4) versus (8:3) Type of surgery: Not reported Previous surgery (IG1+IG2): Not reported Start of intervention after surgery: 2 to 4 weeks Medication use (IG1+ IG2): Previous treatment with AZA‐5; previous treatment with INF ‐10 Smoker (IG1 / IG2): Not reported Number randomised (N = 22): 11/11 Number analysed (N = 22): (11/11) versus (11/11) Post‐randomisation exclusion (n = 0) |
|
| Interventions |
Group 1: Infliximab (5 mg/kg)at 0, 2 and 6 week and then every 8 weeks for 1 year Group 2: Azathioprine (2.5 mg/kg/day) for 1 year All participants: All participants also received oral metronidazole (500 mg twice daily) for 2 weeks after surgery. No other CD‐related drugs were admitted during the study. Participants were monthly evaluated, according to laboratory tests, the Harvey–Bradshaw Index (HBI) calculation and the adverse event report |
|
| Outcomes |
Duration of study: 12 months and follow‐up at 40 months 1. Clinical recurrence defined by a HBI ≥ 8 2. Endoscopic recurrence defined by a Rutgeerts' score ≥ i2 at 12 months and 40 months (follow‐up) 3. Histologic activity score based on a Histology Score System modified from Regueiro et al 4. Adverse events |
|
| Notes |
Funding source: Not reported Conflict of interest: Authors declare the following conflict of interest: AA received: consultancy from Abbvie, MSD; lecture fees fromAbbvie, MSD, Chiesi, Ferring, Nycomed, Otsuka; educational grants from Abbvie, MSD, Ferring, Nycomed. LG received: educational grants from Abbvie, MSD. CF, AP, MM, DP, GA, FF, IDV, GLR: nothing to declare Power calculation: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were randomised with a simple unblinded 1:1 allocation ratio to receive…” Comment: simple randomisation performed, however insufficient information on the method of randomisation used |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “One unblinded endoscopist (AP) did all the examinations and calculated scores. Two further unblinded endoscopists (IDV and GA) separately reviewed videos and in case of discordance a consensus agreement was reached among the three operators” Comment: blinding of outcome assessors not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “One patient did not tolerate azathioprine because of severe nausea with epigastric pain and withdrew from the study after 5 weeks of treatment” Comment: only one patient withdrew from the study and reason described |
| Selective reporting (reporting bias) | Low risk | Trial registration not available, however all outcome data stated in the method section were reported |
| Other bias | Low risk | Groups well balanced at baseline and no other apparent sources of bias detected |
D'Haens 2008.
| Methods |
Study design: RCT, multicentre Setting: Belgium / University Hospital Leuven and Imelda General Hospital, Bonheiden; 1999 to 2005 |
|
| Participants |
Inclusion: Adult participants (18 to 70 years) who underwent curative ileal or ileocolonic resection with ileocolonic anastomosis for CD with a presence of 1 risk factor for the development of early/severe postoperative recurrence of their CD. Participants had to understand and sign a written informed consent form. Women of childbearing age needed to have a negative pregnancy test and had to use adequate birth control measures during the whole study Exclusion: Presence of macroscopic evidence for CD proximally or distally to the site of resection or the presence of frank pancolitis or an ileorectal anastomosis (ileosigmoidal anastomosis was allowed); participants with a stoma; operation for fibrostenosis only, without evidence of inflammatory activity on histology; former intolerance to metronidazole and/or AZA; who wished to become pregnant; low white blood cell count at inclusion (4000); alcohol or drug abuse; participants who had used AZA in the 2 months before surgery; participants with malignancies and/or ongoing infectious disease (hepatitis, tuberculosis, AIDS) with the exception of herpes simplex infection. Former use of biologicals was not permitted Age (IG1 / IG2) mean: 38.8 years (22 to 67 years) versus 40.0 years (21 to 69 years); overall age not reported Sex (M:F): 44:37 overall; (24:16) versus (20:21) Type of surgery: Not reported Previous surgery (IG1+IG2):Second surgery‐20 (12/8); third surgery‐3 (2/1) Start of intervention after surgery: ≤ 2 weeks Medication use (IG1+ IG2): AZA past use: 5 (3/2); Steroid use at surgery: 21 (12/9) Smoker (IG1 / IG2): (13/40) versus (17/41) Number randomised (N = 81): 40/41 Number analysed (N = 81): (40/40) versus (41/41) Post‐randomisation exclusion (n = 5): (3/40) versus (2/41) (Withdrawal of consent‐5 (3/2) |
|
| Interventions |
Group 1: 3 months of metronidazole therapy at a dose of 250 mg 3 times per day plus AZA depending on body weight. AZA only for the rest of the study. Participants whose body weight was 60 kg received 2 tablets of AZA (100 mg), whereas participants weighing 60 kg received 3 tablets or 150 mg AZA Group 2: 3 months of metronidazole therapy at a dose of 250 mg 3 times per day plus placebo. Placebo only for the rest of the study All participants: Participants intolerant to metronidazole were switched to ornidazole 500 mg twice per day orally. All concomitant anti‐inflammatory medications were discontinued, except for glucocorticosteroids, which were gradually tapered over 6 weeks after surgery. Antibiotics were allowed during the study for concurrent infections, but not for CD. Topical therapy for perianal CD could be continued if necessary. Cholestyramine was allowed for the treatment of bile acid diarrhoea. Participantswere instructed to take their other drugs at least 1 hour after the intake of cholestyramine. Participants underwent clinical evaluation with physical examination and biochemical analysis at baseline and weeks 2, 6, 12, 20, 28, 36, 44, and 52 after randomisation. At week 12 and 52, participants underwent an ileocolonoscopy. Adverse events and concomitant medication were recorded at every scheduled or unscheduled visit.. |
|
| Outcomes |
Duration of study: 12 months 1. Endoscopic recurrence in the neoterminal ileum defined as an endoscopic index ≥ 2 according to Rutgeerts’ endoscopic score 2. Clinical recurrence defined as CDAI > 250 3. Adverse events 4.Withdrawal due to adverse events |
|
| Notes |
Funding source: Not reported Conflict of interest: Not reported Power calculation: It was estimated on the basis of prior recurrence‐prevention studies, that 50‐55% of the participants in the placebo group would have endoscopic recurrence at 1 year. Assuming an efficacy of 65% of AZA, it was calculated that 80 participants were needed to be enrolled in the trial to detect differences in significant endoscopic recurrence among the groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The random allocation sequence was delivered by a randomisation program written in Visual Basic version 6” Comment: Computer generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization took place in the pharmacy of the Leuven University Hospitals within 2 weeks after surgery" Comment: insufficient information to make judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if placebo identical to active drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “At week 12 and 52, an ileocolonoscopy was performed with determination of Rutgeerts’ score for ileal recurrence of CD by an endoscopist who was unaware of treatment assignment” Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Both intention‐to treat and per‐protocol analyses were performed” Comment: ITT analysis applied and attrition rates were similarly low across groups |
| Selective reporting (reporting bias) | Low risk | Trial registration not available, however, all outcomes stated in the method section adequately reported |
| Other bias | Low risk | Quote: “The characteristics of the study populations in the AZA and placebo group were comparable’” Comment: Groups well balanced at baseline, no other apparent sources of bias detected |
Hanauer 2004.
| Methods |
Study design: RCT, multicentre Setting: USA and Belgium / 5 centres; 1992 to 1996 |
|
| Participants |
Inclusion: Participants18 to 65 years of age, with diagnosis of CD for at least 6 months and scheduled for curative ileo‐caecal resection; ability to start oral nutrition within 7 days of operation, need for curative ileo‐caecal resection, and resection margins free of inflammation Exclusion: Active perianal disease or any active disease in other segments of the intestine, anti‐TNF‐α, and/or investigational treatment within 4 months prior to surgery; current treatment with 5‐ASA, azathioprine/6MP, or methotrexate; bowel surgery performed less than 3 months previously; history of colostomy or ileostomy; infections, neoplasia, or uncontrolled diseases; or anticipation of noncompliance with protocols. Subjects who were receiving steroids preoperatively were tapered and weaned according to a strict schedule Age (IG1 / IG2) mean (SD): 34.4 ±11.0 years overall; 34.9 ±11.5 years versus 34.1 ±10.9 years versus 34.2 ±10.9 years Sex (M:F): 60:71 overall; (23:24) versus (19:25) versus (18:22) Type of surgery: Not reported Previous surgery (IG1+IG2): 18 (7/11) Start of intervention after surgery: Therapy initiated before postoperative hospital discharge Medication use (IG1+ IG2): Not reported Smoker (IG1 / IG2): Not reported Number randomised (N = 131): 47/44/40 Number analysed (N = 131): (47/131) versus (44/131) versus (40/131) Post‐randomisation exclusion (n = 27): (12/47) versus (7/44) versus (8/40) (Withdrew consent‐5 (1/2/2); Surgical complication‐3 (2/0/1); Noncompliance‐9 (2/4/3); Lost to follow‐up‐10 (4/2/4) |
|
| Interventions |
Group 1: 50 mg of 6‐mercaptopurine (Purinethol) once daily Group 2: 3 g of Mesalamine (Pentasa); 4 capsules of 250 mg, 3 times daily Group 3: Identical matching placebo All participants: Presurgical therapy, including aminosalicylates, antibiotics, or immunomodulators, was discontinued before surgical resection and was not allowed during the postoperative trial. Preoperative treatment with corticosteroids was completely tapered by 3 months after hospital discharge at a rate determined by the treating physician. No concurrent treatment for Crohn’s disease, aside from topical therapy for perianal disease, was allowed during the duration of the trial. Continuous use of nonsteroidal anti‐inflammatory drugs was not allowed during the study. If the white blood cell count and platelet counts fell below 4500/L or 150,000/L, respectively, the dosage of 6‐MP was reduced by one half |
|
| Outcomes |
Duration of study: 24 months 1. Endoscopic recurrence defined as i≥1 according to the Rutgeerts scoring system: i1‐i2 mild to moderate; i3‐i4 severe. Relapse defined as i≥1 2. Clinical recurrence defined as CDAI > 150 points or an increase in CDAI score of > 70 points or higher from baseline. 3. Histological score assessed by the Geboes scoring system 4.Adverse events 5.Serious adverse events 6.Withdrawal due to adverse events |
|
| Notes |
Funding source: Not reported; However, authors contacted by email on 02/08/2018 and declared none Conflict of interest: Not reported; However, authors contacted by email on 02/08/2018 stating that study was funded by Crohn’s and Colitis Foundation Power calculation: Sample size calculations were performed for the endoscopic criteria, using 2‐sided of 0.05 and 80% power, based on a predicted endoscopic recurrence of 75% at 1 year in the placebo group. A sample size of 50 in each group allows sufficient power to detect a 40% reduction in mild Crohn’s disease lesions and a 75% reduction in more severe lesions at 1 year |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: Patients were randomised by a central computer by permuted blocks of 6 (unknown to investigators) per centre to receive mesalamine (Pentasa; Marion Merrill Dow, Kansas City, MO) 3 g daily, 6‐MP (Purinethol; Burroughs Wellcome, Research Triangle Park, NC) 50 mg daily, or placebo Comment: Computer generated random sequence |
| Allocation concealment (selection bias) | Low risk | Quotes: “Medications were prepared and dispensed by an assigned pharmacist at each site’s investigational pharmacy who was not directly involved in the care of the patients” Comment: Treatment controlled by pharmacies at each centre |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: “Medications were prepared and dispensed by an assigned pharmacist at each site’s investigational pharmacy who was not directly involved in the care of the patients” and “An evaluating (treating) physician followed up each patient and was blinded as to the study drug and laboratory results” Comment: Placebo‐controlled, double‐blind RCT. However, it is unclear whether both study drugs were sufficiently identical with the placebo to blind study participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “Patient evaluation consisted of assessments of clinical, endoscopic, and radiographic disease activity at each study site by the blinded physician” and “Colonoscopic examinations with endoscopic descriptions and photography of the anastomosis and pre‐anastomotic ileum were performed by the blinded investigators (all gastroenterologists) at months 6, 12, and 24“ and “Radiographic interpretations were performed by the blinded inflammatory bowel disease radiologist at each institution” Comment:Assessors blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "The clinical recurrence rates were determined using ITT” Comment: ITT analysis applied, attrition low, similar and balanced across groups |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes stated in the method section reported |
| Other bias | Low risk | Quote: "There were no statistical differences in patient age, sex, disease duration, indications for surgical resection, or preoperative disease activity among the 3 groups” Comment: Groups well balanced at baseline. No other apparent sources of bias detected |
Herfarth 2006.
| Methods |
Study design: Multicentre RCT Setting: Not stated (multicentre RCT) |
|
| Participants |
Inclusion: People with Crohn's who had undergone resective surgery Exclusion: Homozygous TPMT Age: Not reported Sex: Not reported Type of surgery: Not reported Previous surgery: Not reported Start of intervention after surgery: within 2 weeks postoperative Medication use (IG1+ IG2): Smoker (IG1 / IG2): Not reported Number randomised (N = 79): 42/37 Number analysed (N = 37): 18/19 Post‐randomisation exclusion (n = 42) |
|
| Interventions |
Group 1: 2.0 to 2.5 mg/kg body weight/day azathioprine Group 2: 4 g 5‐ASA/day All participants: Not stated |
|
| Outcomes |
Duration of study: 1 year (study was discontinued after one year) 1. Treatment failure (due to severe endoscopic recurrence, lack of efficacy and AE related to study drug) 2. Clinical or severe endoscopic relapse 3. Severe endoscopic relapse 4. Clinical relapse (reviewer calculated: clinical or severe endoscopic relapse minus severe endoscopic relapse) 5. Adverse events 6. Withdrawal due to adverse events |
|
| Notes |
Funding source: Dr. Falk Pharma GmbH, Freiburg, Germany Conflict of interest: Not reported Power calculation: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients in the present study were assigned to one of the two treatment groups (5‐ASA or azathioprine) at random For creation of the randomisation list the programme "Rancode +" (version 3.6) of IDV, Gauting (Germany) was used. The randomisation into two treatment groups was performed in blocks of four. After voluntary written informed consent was obtained and basic selection criteria were checked, the investigator requested the allocation of a unique patient code number (randomisation number, consecutively allocated to each patient), and received medication packs with the randomisation number for the patient" Comment: Confirmed by correspondence from Muller R (2/5/2012) |
| Allocation concealment (selection bias) | Low risk | "The randomization code was prepared and stored by a statistician from a CRO, who was not involved in the conduct nor in the analysis of the study. The Qualified Person of the Sponsor and the contract manufacturer responsible for the preparation of the double‐dummy patients sets received a copy of the randomization list, which was safely stored at both sites, without allowing access by other people. Neither the investigator nor the study team from the clinical operation from the sponsor nor the CRO had access to the random list" Comment: Confirmed by correspondence from Muller R (2/5/2012) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "This was a double‐blind, double‐dummy study. Patients randomized to administer 5‐ASA had to take 5‐ASA VERUM tablets AND azathioprine PLACEBO tablets. Patients randomized to receive azathioprine had to administer azathioprine VERUM tablets AND 5‐ASA PLACEBO tablets Therefore, neither the investigator, nor the patients, nor the sponsor were ware of the TX a patient received until the database was clean, closed, and the code was broken" Comment: Confirmed by correspondence from Muller R (2/5/2012) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "This was a double‐blind, double‐dummy study. Patients randomized to administer 5‐ASA had to take 5‐ASA VERUM tablets AND azathioprine PLACEBO tablets. Patients randomized to receive azathioprine had to administer azathioprine VERUM tablets AND 5‐ASA PLACEBO tablets Therefore, neither the investigator, nor the patients, nor the sponsor were ware of the TX a patient received until the database was clean, closed, and the code was broken" Comment: Confirmed by correspondence from Muller R (2/5/2012) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "The study was stopped prematurely after an interim‐analysis due to a high therapy failure rate. 38 patients (AZA 18 patients; 5‐ASA 20 patients) completed the study and could be evaluated regarding the primary endpoint therapy failure. The other patient terminated the trial prematurely due to the study stop, but were also evaluated for adverse events (AE) and adverse drug reactions (ADR)" Comment: 51% of randomised participants discontinued. High risk for primary outcome and low risk for AE and withdrawal due to AE |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information as trial registration was not available and study was published as abstract |
| Other bias | Unclear risk | Insufficient information as study was published as abstract |
Lopez‐Sanroman 2017.
| Methods |
Study design: RCT, multicentre Setting: Spain, 22 centres; 2012 to 2015 |
|
| Participants |
Inclusion: Adults (18‐70 years) who underwent a resective surgical procedure (radical or non‐radical) for a CD‐specific lesion at 1 of the participating centres; diagnosis of CD established by generally accepted endoscopic, histological, and/or radiological criteria at least 6 months before surgery; evaluation of disease location by a complete investigation of the gastrointestinal tract (gastroscopy, colonoscopy, and small bowel radiography) within a maximum of 1 year before the index surgery; and ability to start oral nutrition (and, thus, oral medication) within the first 10 postoperative days Exclusion: Contraindications for use of mesalamine; pregnancy or intention of pregnancy within the next 18 months; nursing; short bowel syndrome; clinically significant lactase deficiency; any severe additional disease; diagnosis of primary sclerosing cholangitis; presence of an ileocolonic stoma; more than 3 surgeries preceding the index surgery; and failure to obtain informed consent. Age (IG1 / IG2) median [interquartile range]: overall age not reported; 37.00 years [31.00 to 47.00 years] versus 35.00 years [30.0 to 40.0 years] Sex (M:F): 42:42 overall; (23:16) versus (19/26) Type of surgery: not reported Previous surgery (IG1+IG2): 6 (3/3) Start of intervention after surgery: after surgery (consent obtained before surgery) Medication use (IG1+ IG2): Glucocorticoids‐80 (38/42); Immunosuppressants [thiopurines or methotrexate]‐63 (28/35); Anti‐TNF‐α – 49 (21/28) Smoker (IG1 / IG2): 20 (9/11) Number randomised (N = 85): 40/45 Number analysed (N = 84): (39/40) versus (40/40) Post‐randomisation exclusion (n = 3): (1/40) versus (2/45) Consent withdrawal before treatment‐1 (0/1); Loss to follow‐up ‐2 (1/1) |
|
| Interventions |
Group 1: AZA 2.5 mg/kg/day for one year + Metronidazole 250 mg three times a day by mouth was added for the first 3 months Group 2: ADA 160 mg subcutaneously, then 80 mg at Week 2, or 40 mg at Week 4 and every 2 weeks thereafter for one year + Metronidazole 250 mg three times a day by mouth was added for the first 3 months All participants: Adherence to therapy was assessed by direct questioning and by counting of returned medication |
|
| Outcomes |
Duration of study: 52 weeks 1. Endoscopic recurrence defined as i ≥ 2b, 3 and 4 based on Rutgeerts score (24 and 52 weeks) 2. Clinical recurrence defined by 1 of the following: increase in CDAI above 200 (24 and 52 weeks) (CDAI ≥ 200: derived from number randomised ‐ remissions) 3.Radiologic recurrence rate 4.Health Related Quality of Life 5. Adverse events 6.Serious adverse events 7.Withdrawal due to adverse events |
|
| Notes |
Funding source: unrestricted grant from AbbVie [Spanish Working Group on Crohn’s Disease and Ulcerative Colitis] The funding group had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decisions concerning publication. The authors had unrestricted access to the data; the decision to submit the paper for publication was solely and entirely to theirs. Conflict of interest: All authors have declared conflict of interest (mainly grants, personal fees, collaboration with AbbVie outside the submitted work, research funding from AbbVie etc.) Power calculation: The difference in the proportion of endoscopic recurrence between treatment groups was estimated at 35% (10% for ADA + metronidazole and 45% for AZA + metronidazole), considering a type 1 error of 5%, a two‐tailed contrast with Yates' continuity correction, 90% power (1‐type II error), and an allocation ratio of 1:1. Therefore, 38 participants per treatment group would be needed. Withdrawals were estimated at 10%. The minimal sample was estimat3d at 84 evaluable participants.. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“Central randomisation was based on a pre‐generated block randomisation list stratified by centre.” and “Patients were assigned [1:1] to..” Comment:Central randomisation |
| Allocation concealment (selection bias) | Low risk | Quote:“Central randomisation was based on a pre‐generated block randomisation list stratified by centre...Allocation was concealed by means of a computer‐generated randomisation schedule without stratification or block allocation” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:“Neither patients nor investigators were blinded to the administered treatment” Comment: No blinding of personnel and participants performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:“A video recording of the last 15 cm of the neo‐terminal ileum was evaluated by an endoscopist blinded to treatment allocation and experienced in application of the Rutgeerts score [VP]” and “..MRE, which was evaluated centrally by an experienced blinded reader [JR];” Comment:Outcome assessors we blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:“We defined the following populations: 1] the intention‐to‐treat [ITT] population, which included all consenting patients who were randomised and received at least one dose of the study medications” Comment: ITT analysis applied, reasons for withdrawal reported and attrition rates were balanced across groups |
| Selective reporting (reporting bias) | High risk | Trial registration was available (NCT01564823) and all prespecified outcomes were reported in the study except health related quality of life which was only reported as a P‐value in an abstract |
| Other bias | Low risk | Quote:“The groups were similar regarding baseline characteristics, including smoking status, previous resections, CD phenotype, previous perianal disease, and previous drug exposure” Comment: Groups well balanced at baseline. No other apparent sources of bias detected |
Mowat 2016.
| Methods |
Study design: RCT, multicentre, Setting: UK / 29 secondary and tertiary hospital; 2008 to 2012 |
|
| Participants |
Inclusion:Participants aged at least 16 years (Scotland) or 18 years (England and Wales) who had a diagnosis of Crohn’s disease and an ileocolic or small bowel resection within the preceding 3 months were eligible for inclusion. Patients successfully treated for a malignancy and in remission for at least 5 years were also eligible Exclusion: Residual active Crohn’s disease present after surgery, known intolerance or hypersensitivity to thiopurines, known need for further surgery, stricturoplasty alone, formation of a stoma, active or untreated malignancy, absent thiopurine methyltransferase activity, substantial abnormalities of liver function tests or full blood count, and pregnancy. Patients receiving treatment for active Crohn’s disease at random allocation Age (IG1 / IG2) mean (SD): 38.76 ± 13.1 years overall; 39.2 ± 12.08 years versus 38.21 ± 13.4 years Type of surgery: not reported Previous surgery (IG1+IG2): not reported Start of intervention after surgery: ≤ 3 months Medication use (IG1+ IG2): not reported Smoker (IG1 / IG2): not reported Number randomised (N = 240): 128/112 Number analysed (N = 240): (128/128) versus (112/112) Post‐randomisation exclusion (n = 56): abnormal blood test results‐18 (12/6); early withdrawal – 21 (8/13); loss to follow‐up – 16 (8/7); death‐1 (0/1) |
|
| Interventions |
Group 1: Once daily oral 6‐mercaptopurine, at a dose of 1 mg/kg bodyweight rounded to the nearest 25 mg. Patients with low thiopurine methyltransferase activity were prescribed half the normal dose for 3 years Group 2: Identical matched placebo for 3 years All participants: Blood monitoring was done weekly for the first 6 weeks and thereafter at 6‐weekly intervals. Patients with abnormal results had a dose reduction, temporary cessation, or cessation as per a study algorithm. At each study visit, the following data were collected: CDAI, physical examination, concomitant medications, and patient‐reported outcomes, including the IBDQ |
|
| Outcomes |
Duration of study: 3 years 1. Clinical recurrence defined as CDAI score of over 150 and a 100‐point increase from baseline, and the need for anti‐inflammatory rescue treatment or primary surgical intervention 2. Secondary endpoint of clinical recurrence defined as reaching either of the individual components of the primary outcome (i.e. either a CDAI score of >150 and a 100‐point increase from baseline, or the need for anti‐inflammatory rescue treatment or primary surgical intervention) 3. Endoscopic relapse defined as a Rutgeerts score of ≥ i2 4. Crohn’s Disease Endoscopic Index of Severity 5. Health‐related quality of life 6. Adverse events 7. Severe adverse events 8.Withdrawal due to adverse events |
|
| Notes |
Funding source: Funded by the Efficacy and Mechanism Evaluation programme, a Medical Research Council and National Institute for Health Research (NIHR) partnership. They had no role in study design, data collection, data analysis, data interpretation, or writing of the report Conflict of interest: Authors declare none conflicting interests Power calculation: A sample size of 234 patients was needed to give 80% power to detect a reduction in the frequency of recurrence from 50% in the placebo group to 30% in the treatment group by 3 years at the 5% level of significance |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned (1:1) to mercaptopurine or identical matched placebo using a computer‐generated web‐based randomisation system managed by the Edinburgh Clinical Trials Unit (University of Edinburgh, Edinburgh, UK) Comment:computer‐generated web‐based random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients’ details were entered into the randomisation system before random allocation and were concealed at randomisation” Comment: Web‐based central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Patients and their carers and physicians were masked to the treatment allocation” Comment: The study is placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Blood monitoring results were reviewed by an independent central clinician masked to treatment allocation and to mean corpuscular volume results. To protect masking, investigators were informed that sham dose reductions were planned for patients on placebo. However, on the advice of the data monitoring committee, sham dose reductions did not occur; the investigators were not informed of this” Comment: Outcome assessors were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Analyses were by intention to treat” Comment: ITT analysis applied. Overall attrition rate of 23% when compared with the event risk (30%), was not considered sufficient to lead to bias |
| Selective reporting (reporting bias) | Low risk | Trial registration available (ISRCTN89489788) and all outcomes stated in the method section reported |
| Other bias | Low risk | Quote: “Baseline characteristics were similar between study groups” Comment: Groups well balanced at baseline. No other apparent sources of bias detected |
Reinisch 2010.
| Methods |
Study design: RCT, multicentre Setting: Austria, the Czech Republic, Germany and Israel ; 21 centres, 2002 to 2007 |
|
| Participants |
Inclusion: Male or female patients aged 18‐70 years with a diagnosis of CD confirmed by endoscopy and histology were eligible for screening if they had (1) undergone resection of the terminal ileum and partial colectomy with ileocolonic resection for complications of ileal CD with construction of an ileocolonic anastomosis in the preceding 6‐24 months; (2) not experienced clinical recurrence due to CD since resection; and (3) a Crohn’s disease activity index (CDAI) score <200 in the preceding 1‐2 weeks. Patients with moderate endoscopic recurrence (Rutgeerts grade i2a: >5 aphthous lesions with normal mucosa between the lesions, or skip areas of larger lesions) or severe endoscopic recurrence (i3‐i4: diffuse aphthous ileitis with diffusely inflamed mucosa, or diffuse inflammation with larger ulcers, nodules and/or narrowing) were recruited into the study Exclusion: Patients with a short bowel syndrome, an ileocolonic stoma, a thiopurine methyltransferase genotype, patients who had received treatment with immunosuppressant agents (methotrexate, ciclosporin, 6‐MP, azathioprine or 6‐thioguanine (6‐TG) or anti‐tumour necrosis factor a (TFNa) since resection, corticosteroids or oral antibiotics (e.g. metronidazole or ciprofloxacin) for >4 weeks since resection, non‐steroidal anti‐inflammatory drugs (NSAIDs) within the preceding 2 weeks (other than paracetamol or low‐dose acetylsalicylic acid); patients who currently had stricturoplasty (unless the present stricture plasty macroscopically showed no inflammation at the time of the index operation) or had serum creatinine >130 μmol/l. Patients were excluded if endoscopy revealed no lesions (grade i0), <5 aphthous lesions (grade i1) and/or if lesions were confined to the ileocolonic anastomosis (i.e. <1 cm long) (grade i2b). Patients in the latter category (grade i2b) were excluded since this presentation is associated with a lower risk of clinical recurrence Age (IG1 / IG2) mean: 35.8 ± 12.08 years overall; 35.5 ± 13.6 years versus 36.0 ± 10.7 years Sex (M:F): 44: 34 overall; (24:17) versus (20/17) Type of surgery: not reported Previous surgery (IG1+IG2): 1 or 2 surgeries‐114 (63/51)); >2 surgeries ‐12 (4/8) Start of intervention after surgery: 6 to 24 months Medication use (IG1+ IG2): Mesalazine ‐ 54 (28/26); Sulfasalazine‐ 5 (4/1); Budesonide‐ 22 (9/13); Corticosteroids‐ 39 (23/16); Azathioprine‐ 14 (6/8); Infliximab‐3 (2/1); Other ‐ 12 (6/6) Smoker (IG1 / IG2): 37 (17/20) Number randomised (N = 78): 41/37 Number analysed (N = 78): (41/41) versus (37/37) Post‐randomisation exclusion (n = 9): (4/41) versus (5/37) ; Lack of cooperation‐7 (4/3); lack of efficacy‐2 (0/2) |
|
| Interventions |
Group 1: Azathioprine 2.0 ‐ 2.5 mg/kg/day (Azafalk 50 mg tablets) + placebo mesalazine tablets Group 2: Mesalazine 4 g/ day (Eudragit L‐coated 500 mg tablets (Salofalk)) + placebo azathioprine tablets All participants: Medications prohibited during the study: immunosuppressants other than study drug, allopurinol, oxipurinol or thiopurinol, azathioprine‐containing or mesalazine containing drugs other than study drug, anti‐TNF‐α therapy, oral antibiotics for >4 weeks or more than three cycles of 2 weeks, NSAIDs for >2 weeks, corticosteroids and cimetidine |
|
| Outcomes |
Duration of study: 52 weeks 1. Therapeutic failure (Clinical relapse) defined as CDAI score ≥ 200 and an increase of ≥ 60 points from baseline or study drug discontinuation due to lack of efficacy or an intolerable adverse drug reaction 2. Endoscopic recurrence defined by endoscopic Rutgeerts score ≥ i2 only 3. Health‐related quality of life based on IBDQ score at 12 months 4. Adverse events 5.Clinical recurrence follow‐up defined as a Rutgeerts score between i2‐i4 within 24 months after the 1‐year treatment |
|
| Notes |
Funding source: Dr Falk Pharma GmbH, Freiburg, Germany Conflict of interest: WR has received an unrestricted grant from Dr. Falk Pharma. EFS and KRH have received speaker’s honoraria. KD, RG and RM are employees of Dr. Falk Pharma. SA, WP, OS, ML, SB‐M, AT, ES and MS have no conflicts of interest to declare. In part, AT, ES and MS are supported by the Robert Bosch Foundation, Stuttgart, Germany Power calculation: The sample size calculation for the primary end point estimated that 62 evaluable patients (31 per treatment arm) were needed to have 80% power to detect a difference of 35% in favour of azathioprine versus mesalazine for the reduction in the 1 year therapeutic failure rate (one‐sided α=0.025). To allow for non‐evaluable patients, a population size of 76 patients (38 per treatment arm) was planned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “..a central randomisation was performed via five computer‐generated randomisation lists (using the program ‘Rancode +’ (version 3.6) of IDV, Gauting, Germany), which were generated for the five body weight classes (40‐50 kg, 51‐60 kg, 61‐75 kg, 76‐100 kg and 101‐128 kg), each in blocks of four, with medication distributed to each centre according to this list" Comment: Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Centralized randomisation in blocks of 4 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To maintain investigator and patient blinding, patients randomised to azathioprine received verum azathioprine tablets and placebo mesalazine tablets; those randomised to mesalazine received verum mesalazine tablets and placebo azathioprine tablets" Comment: a double‐blind, double‐dummy RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The intention‐to‐treat (ITT) population was defined as all randomised patients who received 1 dose of study medication" Comment:The intention‐to‐treat (ITT) population was defined as all randomised patients who received 1 dose of study medication |
| Selective reporting (reporting bias) | Low risk | Trial registration available (NCT00946946) and all prespecified outcomes were reported |
| Other bias | Low risk | Quote: "Baseline characteristics were similar between treatment groups apart from a lower mean CDAI value in the azathioprine cohort (70 versus 102 in the mesalazine arm) and a higher proportion of azathioprine patients with a penetrating disease behaviour (66% versus 43%)" Comment: Some differences at baseline; study supported by Falk Pharma but conflict of interest declared. No other apparent sources of bias detected |
Savarino 2013.
| Methods |
Study design: RCT, single Setting: Italy; University Hospital of Genoa; 2008 to 2010 |
|
| Participants |
Inclusion: Adult patients with ileal or ileocolonic CD within 4 weeks of resection of macroscopically diseased bowel with anastomosis between normal ileum and colon. Exclusion: Patients with (i) more than 10 years of CD requiring first resective surgery for short (10 cm) fibrostenotic stricture, (ii) macroscopically active disease not resected at the time of surgery, and (iii) presence of a stoma. Age (IG1 / IG2) median (range): not reported, overall > 18 years; 45 (22 to 66 years) versus 46 (25 to 65 years) Sex (M:F): 25:26 overall; (8:8) versus (9:8) versus (8:10) Type of surgery: not reported Previous surgery (IG1+IG2): one‐40 (12/15/13); two‐9 (3/2/4); three‐2 (1/0/1) Start of intervention after surgery: 2 to 4 weeks Medication use (IG1+ IG2): Not reported Smoker (IG1 / IG2): 19 (9/4/6) Number randomised (N = 51): 16/17/18 Number analysed (N = 51): (16) versus (17) versus (18) Post‐randomisation exclusion (n = 5): (1/16 ) versus (2/17) versus (2/18) (unclear) |
|
| Interventions |
Group 1: Adalimumab subcutaneous injections 160 / 80 mg at 0 and 2 weeks, followed by 40 mg every 2 weeks for 2 years Group 2: Azathioprine (Azafor, Sofar S.P.A., Milan, Italy), at the dose of 2 mg / kg every day for 2 years Group 3: Mesalamine (Pentasa, Ferring S.P.A., Milan, Italy), at the dose of 3 g / day divided in 3 doses for 2 years All participants: Patients on antibiotics or immunomodulators at entry into the study discontinued these medications 12 weeks before surgery. Continuous use of nonsteroidal anti‐inflammatory drugs was not allowed during the study. No other medications were prescribed except for occasional tablets of paracetamol or tramadol. Patients were subjected to endoscopy at 12 and 24 months; small bowel enteroclysis or magnetic resonance imaging at 12 and 24 months; physical examination with interviews, together with an extensive battery of blood tests weekly for the first 4 weeks and then every 2 months, and completed an IBD‐Q at 1 month before surgery and at 12 and 24 months aft er surgery. The CDAI was determined at each study visit. In addition, adverse events were ascertained at each visit |
|
| Outcomes |
Duration of study: 2 years 1. Clinical recurrence d defined as a score of ≥ 2 on the clinical recurrence grading scale 1‐4 proposed by Hanauer et al. 2. Clinical recurrence based on CD activity index (CDAI) was calculated for each patient and recurrence was set in case of a score > 200, whereas clinical remission was defined by a CDAI score of < 150 2. Endoscopic recurrence defined by a Rutgeerts score of ≥ i2 3. Radiologic recurrence defined as a score of ≥ 2 on the radiographic recurrence grading scale (where 1 indicates normal; 2, mucosal edema / aphthoid ulcers; 3, linear ulcers / cobblestoning; and 4, strictures / fistulas / inflammatory mass) 4. Health‐related quality of life 5. Median Lémann Index 5. Adverse events |
|
| Notes |
Funding source: supported by research funds of the university Conflict of interest: Authors declare no conflict of interest Power calculation: we considered reasonable to hypothesize an endoscopic recurrence rate of ˜ 80 % and 15 % and a clinical recurrence rate of ˜ 65 % and 5 % for the mesalamine and ADA groups, respectively, at 2 years of follow‐up. This estimation has been supported by the results shown in previous trials on postoperative CD relapse. Thus, based on these data, 13 patients per treatment group resulted to be sufficient to detect a difference of at least 65 % for endoscopic recurrence and 60 % for clinical recurrence in favour of the ADA group with a power of 80 % (global type I error of 5 % ). Th e number of patients in each group was increased to 16 to compensate for an anticipated dropout rate of 15 % . |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Eligible and consenting patients were assigned randomly using a computer‐generated sequence (www. randomizer.org) to a regimen of…” Comment: Computer generated random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Patient allocation was concealed and performed by an independent nurse not involved with the trial" Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study is open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "“A blinded investigator (P.D.) reviewed each patient's video‐recorded procedure and provided a separate endoscopic score” and “At the conclusion of the study, the principal investigator (E.S.) rescored each patient by re‐reviewing the video recordings in a random and blinded manner” Comment: Assessors were blinded for endoscopic assessments only. However, no information on clinical assessment of relapse |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Statistical analysis was conducted according to the intention‐to‐treat principle.” Comment: ITT analysis applied. Withdrawals and reasons reported |
| Selective reporting (reporting bias) | Low risk | Trial registration not available, however, all outcomes stated in the method section reported |
| Other bias | Low risk | Quote: “Characteristics were similar for sex, age, smoking, duration of CD, disease behavior, disease location, prior medication exposure, including IFX, and prior surgical resection” Comment: Groups well balanced at baseline; no other apparent sources of bias detected |
Scapa 2015.
| Methods |
Study design: RCT; abstract Setting: Tel Aviv, Israel; study period not reported |
|
| Participants |
Inclusion: All CD patients undergoing a first ileocecectomy for inflammatory complications were prospectively recruited to the Post OPerative Adalimumab Recurrence Trial (POPART). Exclusion: Not reported Age (IG1 / IG2) median (SD): overall not reported; 30.5 ± 2.3 years versus 34.4 ± 2.5 years Sex (M:F): not reported Type of surgery: not reported Previous surgery (IG1+IG2): not reported Start of intervention after surgery: < 45 days Medication use (IG1+ IG2): Not reported Smoker (IG1 / IG2): 4 (1/3) Number randomised (N = 19) Number analysed (N = 19): (8) versus (11) Post‐randomisation exclusion (n = ?) |
|
| Interventions |
Group 1: Thiopurine (6‐mercaptopurine 1.5 mg/kg/day) Group 2: Adalimumab 160 mg/ 80 mg and then 40 mg every other week All participants: All patients underwent ileocolonoscopy at 6 and 12 months to asses for endoscopic recurrence as defined by the Rutgeert's score |
|
| Outcomes |
Duration of study: 12 months 1. Endoscopic recurrence defined as a Rutgeert's score of i0‐i1, while advanced lesions were defined as i2 to i4 |
|
| Notes |
Funding source: not reported Conflict of interest: not reported Power calculation: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make judgment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make judgment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make judgment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Nineteen patients have reached the 24‐week time point" Comment: Abstract does not report how many were randomised, the number of withdrawals, no information regarding any adverse event. Authors informed us via correspondence (12/10/2018) that the full trial will be published by end of 2018, but refused to share trial data |
| Selective reporting (reporting bias) | High risk | Trial registration available (NCT01629628), however clinical relapse and adverse events were not reported in the Abstract. Authors informed us via correspondence (12/10/2018) that the full trial will be published by end of 2018, but refused to share trial data |
| Other bias | Unclear risk | Insufficient information to make judgment |
RCT: randomised controlled trial; CD: Crohn's disease; CDAI: Crohn's disease activity index; IG: intervention group; SD: standard deviation; M: male; F: Female; TNF: tumour necrosis factor; AZA: azathioprine; ITT; intention‐to‐treat; mg: milligram; kg: kilogram; g: gram; WBC: white blood cell count; μu/ml: micro units per millilitre; INF: infliximab; 5‐ASA: 5‐aminosalicylic acid; 6‐MP: 6‐mercaptopurine; L: litre; ADA: adalimumab; NSAID; non‐steroidal anti‐inflammatory drugs; IBDQ: inflammatory bowel disease questionnaire; cm: centimetre
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ferrante 2015 | Wrong intervention: all patients received azathioprine Study compared systematic azathioprine therapy to endoscopically driven azathioprine therapy |
| Mañosa 2013 | Wrong intervention: All patients received azathioprine Study compared combination of azathioprine and metronidazole to azathioprine |
| NCT01876264 | Wrong intervention, extended versus conventional resection |
| NCT02247258 | Trial terminated due to slow recruitment |
| Nos 2000 | Not RCT, confirmed after contacting author |
| Reinisch 2013 | Non randomised follow‐up of included study Reinisch 2010 |
| Robb 2015 | Not RCT; letter |
| Vidigal 2014 | Wrong population: not post‐surgical patients |
| Wright 2014 | Wrong intervention, colonoscopy versus no colonoscopy |
| Wright 2015 | Wrong intervention, colonoscopy versus no colonoscopy |
| Zhu 2015 | Wrong intervention; herbal supplement |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT03185611.
| Trial name or title | Effectiveness of Rifaximin Combined With Thiopurine on Preventing Postoperative Recurrence in Crohn's Disease |
| Methods | RCT, parallel design, multi‐centre study, single blinded (Outcomes Assessor); Location: China |
| Participants | 120 participants, aged 18 to 65 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Two arms; Arm 1: Prescribed Rifaximin (600mg, twice daily) combined with Azathioprine (2.0‐2.5mg/kg/day) for 3 months after surgery, and then Azathioprine monotherapy (2.0 to 2.5 mg/kg/day) for the next 3 months and Arm 2: Prescribed Azathioprine (2.0 to 2.5 mg/kg/day) for 6 months after surgery |
| Outcomes | Primary: Incidence of endoscopic recurrence 6 months after surgery Secondary: Adverse effect |
| Starting date | June 14, 2017 |
| Contact information | Xiang Gao, MD, PhD; gaoxiangmed@163.com |
| Notes |
NL1344.
| Trial name or title | Azathioprine maintenance treatment versus infliximab maintenance treatment in Crohn’s disease patients in remission (Azorix trial) |
| Methods | |
| Participants | Inclusion criteria: 1. Age between 18 and 80 years 2. For at least 6 months a stable dose of combination therapy with IFX and AZA or with IFX and 6MP 3. CD in remission for at least 6 months Exclusion criteria: 1. Failed attempt to quit medication during combination therapy before 2. Abdominal abscesses, fistulas and fluid collections 3. Comorbidity or extra‐intestinal complications that require infliximab treatment 4. Crohn’s disease activity of the upper gastrointestinal tract that requires infliximab treatment 5. Legally incompetent patients |
| Interventions | Two arms. Arm 1:Infliximab consisting of infusions of 5 mg/kg. Patients will receive maintenance therapy at intervals of 6 to 12 weeks Arm 2: Azathioprine in a dose of 2.5 mg/kg daily; 6‐MP will be continued in a dose of 1.5 mg/kg daily |
| Outcomes | Primary outcome: The occurrence of relapse ‐ defined as a disease activity with a CDAI score greater than 150 ‐ during the 12 months follow‐up period. Secondary outcome:Mucosal healing at 12 months; Number of treatment failures after 12 months; Time to relapse; HRQOL at 12 months, measured by IBDQ |
| Starting date | 1st August 2008 |
| Contact information | Dr. P.C.F. Stokkers; p.stokkers@amc.nl |
| Notes |
Differences between protocol and review
The scope of this review was expanded to include:
Trials which compare AZA and 6‐MP with no treatment were not included in the previous version of this review. If such trials become available in future updates, we intend to analyse them separately from the placebo trials
Inclusion criteria is limited to studies with minimum of 3 months of treatment versus 6 months in the previous review.
Endoscopic recurrence was a primary outcome in the previous version of the review, however, we decided to report it as a secondary outcome in this review to ensure consistency across the Cochrane IBD group portfolio.
We also reported adverse event data as a composite outcome not individually as proposed in Gordon 2014 to ensure consistency across the Cochrane IBD group portfolio
Radiologic, surgical, histologic relapse and health related quality of life are additional outcomes which were only included in this version of the review to ensure consistency across the Cochrane IBD group portfolio.
We included abstracts in this review.
For the previously published version of this review we contacted leaders in the field and drug manufacturers to identify additional studies. We did not do this for the updated review.
Contributions of authors
Morris Gordon provided methodological expertise and performed screening of abstracts and titles, screening of full‐text articles and was involved with adjudication of GRADE analysis, manuscript preparation, critical revision of the manuscript, and approval of the final manuscript.
Anthony K Akobeng provided methodological expertise and was involved with checking the data analyses, data interpretation, manuscript preparation, critical revision for the manuscript, and approval of the final manuscript.
Zipporah Iheozor‐Ejiofor performed adjudication in the screening and data extraction phases, data extraction, communication with primary study authors, risk of bias assessments, GRADE analysis, manuscript preparation, critical revision for the manuscript and approval of the final manuscript.
Teuta Gjuladin‐Hellon performed data extraction, risk of bias assessments, statistical analyses, data interpretation, manuscript preparation, GRADE analysis, critical revision for the manuscript and approval of the final manuscript.
Declarations of interest
Teuta Gjuladin‐Hellon: None known.
Zipporah Iheozor‐Ejiofor: None known.
Morris Gordon: Received travel fees to attend international scientific and training meeting such as DDW, Advances in IBD, ESPGHAN, BSPGHAN and Cochrane focused international events from companies including: Abbott, Nutricia, Biogaia, Ferring, Allergan, and Tillots.
Anthony K Akobeng: None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ardizzone 2004 {published data only}
- Ardizzone S, Maconi G, Sampietro GM, Russo A, Radice E, Colombo E, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. Gastroenterology 2004;127(3):730‐40. [DOI] [PubMed] [Google Scholar]
Armuzzi 2013 {published data only}
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Infliximab is more effective than azathioprine in the long‐term prevention of postoperative recurrence of Crohn's disease. Gastroenterology April 2015;1:S856. [Google Scholar]
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Prevention of postoperative recurrence with azathioprine or anti‐TNF alpha in patients with Crohn's disease: A pilot study. Digestive and Liver Disease 2012;44:S194. [Google Scholar]
- Armuzzi A, Felice C, Marzo M, Pugliese D, Andrisani G, Papa A, et al. Prevention of postoperative recurrence with azathioprine or anti‐TNF alpha in patients with Crohn's disease: An open‐label pilot study. Gastroenterology 2012;142(5 Suppl 1):S780. [DOI] [PubMed] [Google Scholar]
- Armuzzi A, Felice C, Papa A, Marzo M, Pugliese D, Andrisani G, et al. Prevention of postoperative recurrence with azathioprine or infliximab in patients with Crohn's disease: an open‐label pilot study. Journal of Crohn's and Colitis 2013;7(12):e623‐9. [DOI] [PubMed] [Google Scholar]
D'Haens 2008 {published data only}
- D'Haens G, Vermeire S, Assche G, Noman M, Aerden I, Olmen G, et al. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn's disease: a controlled randomized trial. Gastroeneterology 2008;135(4):1123‐9. [DOI] [PubMed] [Google Scholar]
- D'Haens GR, Noman M, Assche GA, Olmen G, Aerden I, Vermeire S, et al. Combination therapy with metronidazole and azathioprine reduces severe postoperative recurrence of Crohn's disease: A double‐blind controlled randomized trial. Gastroenterology 2007 2007;132(4 Suppl 1):A52. [DOI] [PubMed] [Google Scholar]
Hanauer 2004 {published data only}
- Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, et al. Postoperative maintenance of Crohn's disease remission with 6‐mercaptopurine, mesalamine or placebo: a 2‐year trial. Gastroenterology 2004;127(3):723‐9. [DOI] [PubMed] [Google Scholar]
- Korelitz B, Hanauer S, Rutgeerts P, Present D, Peppercorn M. Post‐operative prophylaxis with 6‐MP, 5‐ASA or placebo inCrohn's disease: A 2 year multicenter trial. Gastroenterology 1998;114(4 Part 2):A486. [Google Scholar]
Herfarth 2006 {published data only}
- Dilger K, Schaeffeler E, Lukas M, Strauch U, Herfarth H, Muller R, et al. Monitoring of thiopurine methyltransferase activity in postsurgical patients with Crohn's disease during 1 year of treatment with azathioprine or mesalazine. Therapeutic Drug Monitoring 2007;29(1):1‐5. [DOI] [PubMed] [Google Scholar]
- Herfarth H, Obermeier F, Tjaden C, Lukas M, Serclova Z, Dignass AU, et al. Double‐blind, double dummy, randomized, multicentre, comparative study on the efficacy and safety of azathioprine (AZA) versus mesalazine (5‐ASA) for prevention of postoperative endoscopic recurrence in Crohn’s disease. Gastroenterology 2006;130(4 Suppl 2):A480‐1. [Google Scholar]
- Herfarth H, Tjaden C, Lukas M, Obermeier F, Dilger K, Muller R, et al. Adverse events in clinical trials with azathioprine and mesalamine for the prevention of postoperative recurrence of Crohn's disease. Gut 2006;55(10):1526‐6. [PMC free article] [PubMed] [Google Scholar]
- Muller R, Herfarth H. More information on 2006 study published in Gut. Email to Morris Gordon 2/5/2012.
Lopez‐Sanroman 2017 {published data only}
- Lopez‐Sanroman A, Vera‐Mendoza I, Domenech E, Taxonera C, Vega V, Marin‐Jimenez I, et al. Adalimumab vs azathioprine in the prevention of postoperative Crohn’s disease recurrence. A GETECCU randomised trial. Journal of Crohn's and Colitis 2017;11(11):1293–301. [DOI] [PubMed] [Google Scholar]
- Lopez‐Sanroman A, Vera‐Mendoza I, Domenech E, Taxonera C, Vega V, Marin‐Jimenez I, et al. Apprecia: Adalimumab vs azathioprine in the prevention of Crohn's disease recurrence after surgical resection. A geteccu multicenter randomized trial. United European Gastroenterology Journal 2015;1:A3. [Google Scholar]
- NCT01564823. Adalimumab on Preventing Post‐chirurgic Recurrence on Crohn's Disease (APPRECIA). clinicaltrials.gov/ct2/show/NCT01564823 (accessed 28 March 2012).
- Taxonera C, López‐Sanromán A, Vera‐Mendoza I, Domènech E, Ruiz VV, Marín‐Jiménez I, et al. Quality of life during one year of postoperative prophylactic drug therapy after intestinal resection in Crohn's patients: Results of the APPRECIA trial. Digestive and Liver Disease 2019;51(4):529‐35. [DOI] [PubMed] [Google Scholar]
Mowat 2016 {published data only}
- Arnott I, Mowat C, Ennis H, Keerie C, Lewis S, Cahill A, et al. The toppic trial: A randomised, double‐blind parallel group trial of mercaptopurine vs placebo to prevent recurrence of Crohn's disease following surgical resection in 240 patients. Gut 2016;65(Suppl 1):A43‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott I, Mowat C, Ennis H, Keerie C, Lewis S, Kennedy N, et al. The TOPPIC trial: A randomised, double‐blind parallel‐group trial of mercaptopurine versus placebo to prevent recurrence of Crohn's disease following surgical resection in 240 patients. Journal of Crohn's and Colitis 2016;10(1 Supplement):S21‐S22. [Google Scholar]
- Arnott I, Mowat C, Ennis H, Keerie C, Lewis S, Kennedy N, et al. The toppic trial: A randomized, double‐blind parallel group trial of mercaptopurine vs placebo to prevent recurrence of Crohn's disease following surgical resection in 240 patients. Gastroenterology 2016;150(4 Suppl 1):S182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN89489788. Randomised controlled trial of 6‐Mercaptopurine (6MP) versus placebo to prevent recurrence of Crohn's disease following surgical resection. isrctn.com/ISRCTN89489788 (accessed 22 February 2007).
- Mowat C, Arnott I, Cahill A, Smith M, Ahmad T, Subramanian S, et al. Mercaptopurine versus placebo to prevent recurrence of Crohn's disease after surgical resection (TOPPIC): A multicentre, double‐blind, randomised controlled trial. Lancet 2016;1(4):273‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinisch 2010 {published data only}
- Angelberger S, Schaeffeler E, Teml A, Petritsch W, Shonova O, Lukas M, et al. Mucosal improvement in patients with moderate to severe postoperative endoscopic recurrence of Crohn's disease and azathioprine metabolite levels. Inflammatory Bowel Diseases 2013;19:590‐8. [DOI] [PubMed] [Google Scholar]
- Angelberger S, Schaeffeler E, Teml A, Petritsch W, Shonova O, Lukas M, et al. Relationship between thiopurine metabolite levels and endoscopic improvement in patients with postoperative moderate to severe endoscopic recurrence of Crohn'sdisease. Gastroenterology 2010;138(5 Suppl 1):S685. [DOI] [PubMed] [Google Scholar]
- NCT00946946. Preventing postoperative relapse in Crohn's disease patients at risk: Azathioprine versus mesalazine. clinicaltrials.gov/ct2/show/NCT00946946 (accessed 27 July 2009).
- Reinisch W, Angelberger S, Petritsch W, Herrlinger K, Shonova O, Lukas M, et al. A double‐blind, double‐dummy,randomized, controlled, multicenter trial on the efficacy and safety of azathioprine vs mesalamine for prevention of clinical relapses in Crohn's disease patients with postoperative moderate or severe endoscopic recurrence. Gastroenterology 2008;134(4 Suppl 1):A70. [Google Scholar]
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar‐Meir S, et al. Azathioprine versus mesalazine for the prevention of postoperative clinical recurrence in patients with Crohn's disease with endoscopic recurrence: efficacy and safety results of a randomized, double‐blind, double‐dummy, multicentre trial. Gut 2010;59(6):752‐9. [DOI] [PubMed] [Google Scholar]
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Greinwald R, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in Crohn's disease patients with endoscopic recurrence: Follow‐up data of a randomised, double‐blind, double‐dummy, 1‐year, multicentre trial. Journal of Crohn's and Colitis 2013;7:S254. [DOI] [PubMed] [Google Scholar]
- Reinisch W, Stange EF. Efficacy of azathioprine versus mesalazine in postoperative Crohn's disease ‐ the authors' response. Gut 2011;60(5):739‐40. [DOI] [PubMed] [Google Scholar]
Savarino 2013 {published data only}
- Bodini G, Pellegatta G, Giannini EG, Savarino V, Savarino EV. Adalimumab therapy rather than azathioprine and mesalamine is able to halt Crohn's disease progression after resective surgery and a post‐hoc analysis of a prospective randomized study. Gastroenterology. 2017; Vol. 152, issue 5 Supplement:S774.
- Savarino E, Bodini G, Dulbecco P, Assandri L, Bruzzone L, Mazza F. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease ‐ a randomized trial. Digestive and Liver Disease 2013;45:S94‐5. [DOI] [PubMed] [Google Scholar]
- Savarino E, Bodini G, Dulbecco P, Assandri L, Bruzzone L, Mazza F, et al. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease: a randomized controlled trial. American Journal of Gastroenterology 2013;108(11):1731‐42. [DOI] [PubMed] [Google Scholar]
- Savarino E, Bodini G, Dulbecco P, Marabotto E, Assandri L, Bruzzone L. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease ‐ a randomized trial. Gastroenterology 2013;144(5 Suppl 1):S21. [DOI] [PubMed] [Google Scholar]
Scapa 2015 {published data only}
- NCT01629628. Adalimumab for the Management of Post‐operative Crohn's Disease (CD) POPART. clinicaltrials.gov/ct2/show/NCT01629628 (accessed 27 June 2012).
- Scapa E, Maharshak N, Kariv Y, Ben‐Horin S, White ID, Santo E, et al. Early initiation of adalimumab significantly diminishes post‐operative Crohn's disease recurrence, and is superior to immunomodulator therapy. Preliminary results from the POPART trial. Gastroenterology 2015;148(4 Supplement):S240‐1. [Google Scholar]
References to studies excluded from this review
Ferrante 2015 {published data only}
- Ferrante M, Papamichael K, Duricova D, D'Haens G, Vermeire S, Archavlis E, et al. Systematic versus Endoscopy‐driven Treatment with Azathioprine to Prevent Postoperative Ileal Crohn's Disease Recurrence. Journal of Crohn's and Colitis 2015;9(8):617‐24. [DOI] [PubMed] [Google Scholar]
- Ferrante M, Papamichael K, Duricova D, D'Haens G, Vermeire S, Archavlis E, et al. Systematic versus endoscopy‐driven treatment with azathioprine to prevent postoperative ileal Crohn's disease recurrence. Journal of Crohn's and Colitis 2014;8:S205‐6. [DOI] [PubMed] [Google Scholar]
- Ferrante M, Papamichael K, Duricova D, D'Haens GR, Vermeire S, Archavlis EJ, et al. Systematic versus endoscopy‐driven treatment with azathioprine to prevent postoperative ileal crohn's disease recurrence: Interim results from a randomized, multicenter trial. Gastroenterology 2014;146(5 Suppl 1):S592. [DOI] [PubMed] [Google Scholar]
Mañosa 2013 {published data only}
- Manosa M, Cabre E, Bernal I, Esteve M, Garcia‐ Planella E, Ricart Gomez E. Azathioprine versus azathioprine plus metronidazole for the prevention of postoperative endoscopic recurrence of Crohn's disease: A randomized, placebo controlled trial. Journal of Crohn's and Colitis 2012;6:S93. [DOI] [PubMed] [Google Scholar]
- Mañosa M, Cabré E, Bernal I, Esteve M, Garcia‐Planella E, Ricart E, et al. Addition of metronidazole to azathioprine for the prevention of postoperative recurrence of Crohn's disease: a randomized, double‐blind, placebo‐controlled trial. Inflammatory Bowel Diseases 2013;19(9):1889‐95. [DOI] [PubMed] [Google Scholar]
NCT01876264 {published data only}
- NCT01876264. Crohn's extent of resection trial. clinicaltrials.gov/ct2/show/NCT01876264 (accessed 12 June 2013).
NCT02247258 {published data only}
- NCT02247258. Azathioprine in the prevention of ileal Crohn's disease postoperative recurrence. clinicaltrials.gov/ct2/show /NCT02247258 (accessed 23 September 2014).
Nos 2000 {published data only}
- Nos P, Hinojosa J, Aguilera V, Moles JR, Pastor M, Ponce J, et al. Azathiprine and 5‐ASA in the prevention of postoperative recurrence in Crohn's disease. Gastroenterología y Hepatología 2000;23(8):374‐8. [PubMed] [Google Scholar]
Reinisch 2013 {published data only}
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Greinwald R, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in Crohn's disease patients with endoscopic recurrence: Follow‐up data of a randomised, double‐blind, double‐dummy, 1‐year, multicentre trial. Journal of Crohn's and Colitis 2013;7:S254. [DOI] [PubMed] [Google Scholar]
Robb 2015 {published data only}
- Robb PM, Sorrentino D. Long‐term prevention of postoperative Crohn's disease recurrence with azathioprine: the wolf in the sheep clothing. International Journal of Colorectal Disease 2015;30(2):283‐4. [DOI] [PubMed] [Google Scholar]
Vidigal 2014 {published data only}
- Vidigal FM, Souza GS, Chebli LA, Rocha Ribeiro TC, Furtado MC, Castro AC, et al. Azathioprine is more effective than mesalazine at preventing recurrent bowel obstruction in patients with ileocecal Crohn's disease. Medical Science Monitor 2014;20:2165‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2014 {published data only}
- Wright EK, Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany S, et al. Intestinal resection in Crohn's disease is associated with significant and durable improvement in health related quality of life although to a lesser extent in women and smokers. Results from the POCER study. Gastroenterology 2014;146(5 Suppl 1):S435. [Google Scholar]
Wright 2015 {published data only}
- Wright EK, Kamm MA, Cruz P, Hamilton AL, Ritchie K, Bell SJ, et al. Structured post‐operative treatment and monitoring to prevent Crohn's disease recurrence is cost effective. Results from the POCER study. Journal of gastroenterology and hepatology 2015;30:145. [Google Scholar]
Zhu 2015 {published data only}
- NCT01015391. Efficacy study of T2 versus AZA to maintain clinical and endoscopic remission in postoperative Crohn's disease. clinicaltrials.gov/ct2/show/NCT01015391 (accessed 18 November 2009).
- Zhu W, Li Y, Gong J, Zuo L, Zhang W, Cao L, et al. Tripterygium wilfordii Hook. f. versus azathioprine for prevention of postoperative recurrence in patients with Crohn's disease: a randomized clinical trial. Digestive & Liver Disease 2015;47(1):14‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT03185611 {published data only}
- NCT03185611. Effectiveness of rifaximin combined with thiopurine on preventing postoperative recurrence in Crohn's disease. clinicaltrials.gov/ct2/show/NCT03185611 (accessed 14 June 2017).
NL1344 {published data only}
- NL1344. Azathioprine maintenance treatment versus infliximab maintenance treatment in Crohn’s disease patients in remission (Azorix trial). trialregister.nl/trial/1344 (accessed 1 August 2008).
Additional references
Abraham 2009
- Abraham C, Cho JHN. Inflammatory Bowel Disease. New England Journal of Medicine 2009;361(21):2066‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Axelrad 2016
- Axelrad JE, Roy A, Lawlor G, Korelitz B, Lichtiger S. Thiopurines and inflammatory bowel disease: current evidence and a historical perspective. World Journal of Gastroenterology 2016;22:101‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benchimol 2009
- Benchimol EI, Seow CH, Otley AR, Steinhart AH. Budesonide for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD002913.pub2] [DOI] [PubMed] [Google Scholar]
Bernell 2000
- Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Annals of Surgery 2000;231(1):38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boreinstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta‐analysis. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2009. [Google Scholar]
Camus 2013
- Camus M, Seksik P, Bourrier A, Nion‐Larmurier I, Sokol H. Long‐term outcome of patients with Crohn’s disease who respond to azathioprine. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 2013;11(4):389‐394. [DOI] [PubMed] [Google Scholar]
Chande 2015
- Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6‐mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD000067.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colonna 1994
- Colonna T, Korelitz BI. The role of leukopenia in the 6‐mercaptopurine induced remission of refractory Crohn's disease. American Journal of Gastroenterology 1994;89(3):362‐6. [PubMed] [Google Scholar]
Doherty 2009
- Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post‐operative recurrence of Crohn's disease. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006873.pub2] [DOI] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Davey‐Smith G, Altman D (Editors). Systematic reviews in health care: Meta‐analysis in context. Second Edition. London.: BMJ Publishing Group, 2001. [Google Scholar]
Fraser 2002
- Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut 2002;50(4):485‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gionchetti 2016
- Gionchetti P, Dignass A, Danese S, Dias FJM, Rogler G, Lakatos PL, et al. on behalf of ECCO. 3rd European Evidenced‐based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2; Surgical Management and Special Situations. Journal of Crohn's and Colitis February 2017;11(2):135‐49. [DOI] [PubMed] [Google Scholar]
Gjuladin‐Hellon 2019
- Gjuladin‐Hellon T, Gordon M, Iheozor‐Ejiofor Z, Akobeng AK. Oral 5‐aminosalicylic acid for maintenance of surgically‐induced remission in Crohn's disease. Cochrane Database of Systematic Reviews 2019, Issue 6. [DOI: 10.1002/14651858.CD008414.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomollon 2017
- Gomollón F, Dignass A, Annese V, Tilg H, Assche G, Lindsay JO, et al. 3rd European evidence‐based consensus on the management of Crohn’s disease 2016: Part 1: Diagnosis and medical management.. Journal of Crohn's & Colitis 2017;11:3‐25. [DOI] [PubMed] [Google Scholar]
Gordon 2011
- Gordon M, Naidoo K, Thomas AG, Akobeng AK. Oral 5‐aminosalicylic acid for maintenance of surgically‐induced remission in Crohn's disease. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008414.pub2] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hafraoui 2002
- Hafraoui S, Dewit O, Marteau P, Cosnes J, Colombel JF, Modigliani R, et al. Mycophenolate mofetil in refractory Crohn's disease after failure of treatments by azathioprine or methotrexate [Le mycophénolate mofétil dans les formes chroniques actives de la maladie de Crohn après échec de lazathioprine ou du méthotrexate]. Gastroenterologie Clinique et Biologique 2002;26(1):17‐22. [PubMed] [Google Scholar]
Hanauer 2001
- Hanauer SB, Sandborn W. Management of Crohn’s disease in adults. American Journal of Gastroenterology 2001;96(3):635‐43. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Lennard 1989
- Lennard L, Loon JA, Weinshilboum RM. Pharmacogenitics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clinical Pharmacology and Therapeutics 1989;46(2):149‐54. [DOI] [PubMed] [Google Scholar]
Lennard 1992
- Lennard L. The clinical pharmacology of 6‐mercaptopurine. European Journal of Clinical Pharmacology 1992;43(4):329‐39. [DOI] [PubMed] [Google Scholar]
NICE 2016
- National Institute of Health and Care Excellence. Crohn's Disease: management (CG152). Available: https://www.nice.org.uk/guidance/cg152/evidence/addendum‐pdf‐2489565421 2016.
Regueiro 2009
- Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pasci M, et al. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology 2009;136(2):441–50. [DOI] [PubMed] [Google Scholar]
Rolfe 2006
- Rolfe VE, Fortun PJ, Hawkey CJ, Bath‐Hextall FJ. Probiotics for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004826.pub2] [DOI] [PubMed] [Google Scholar]
Rutgeerts 1990
- Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99(4):956‐63. [DOI] [PubMed] [Google Scholar]
Sahasranaman 2008
- Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. European Journal ofClinical Pharmacology 2008;64:753‐67. [DOI] [PubMed] [Google Scholar]
Sandborn 1996
- Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6‐mercaptopurine, cyclosporine, and methotrexate. American Journal of Gastroenterology 1996;91(3):423‐33. [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Steinhart 2003
- Steinhart AH, Ewe K, Griffiths AM, Modigliani R, Thomsen OO. Corticosteroids for maintenance of remission in Crohn’s disease. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000301] [DOI] [PubMed] [Google Scholar]
Sutton 2000
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta‐analysis in medical research. Chichester, West Sussex, UK: John Wiley & Sons Ltd, 2000. [Google Scholar]
Timmer 2016
- Timmer A, Patton PH, Chande N, McDonald JW, MacDonald JK. Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD000478.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinshilboum 1980
- Weinshilboum RN, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. American Journal of Human Genetics 1980;32(5):651‐62. [PMC free article] [PubMed] [Google Scholar]
Williams 1990
- Williams JG, Wong WD, Rothenberger DA, Goldberg SM. Recurrence of Crohn's disease after resection. British Journal of Surgery 1990;78(1):10‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gordon 2014
- Gordon M, Taylor K, Akobeng AK, Thomas AG. Azathioprine and 6‐mercaptopurine for maintenance of surgically‐induced remission in Crohn's disease. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD010233.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


