Abstract
Background
The prevention of long‐term psychological distress following traumatic events is a major concern. Systematic reviews have suggested that individual psychological debriefing is not an effective intervention at preventing post‐traumatic stress disorder (PTSD). Over the past 20 years, other forms of intervention have been developed with the aim of preventing PTSD.
Objectives
To examine the efficacy of psychological interventions aimed at preventing PTSD in individuals exposed to a traumatic event but not identified as experiencing any specific psychological difficulties, in comparison with control conditions (e.g. usual care, waiting list and no treatment) and other psychological interventions.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO and ProQuest's Published International Literature On Traumatic Stress (PILOTS) database to 3 March 2018. An earlier search of CENTRAL and the Ovid databases was conducted via the Cochrane Common Mental Disorders Controlled Trial Register (CCMD‐CTR) (all years to May 2016). We handsearched reference lists of relevant guidelines, systematic reviews and included study reports. Identified studies were shared with key experts in the field.
We conducted an update search (15 March 2019) and placed any new trials in the 'awaiting classification' section. These will be incorporated into the next version of this review, as appropriate.
Selection criteria
We searched for randomised controlled trials of any multiple session (two or more sessions) early psychological intervention or treatment designed to prevent symptoms of PTSD. We excluded single session individual/group psychological interventions. Comparator interventions included waiting list/usual care and active control condition. We included studies of adults who experienced a traumatic event which met the criterion A1 according to the Diagnostic and Statistical Manual (DSM‐IV) for PTSD.
Data collection and analysis
We entered data into Review Manager 5 software. We analysed categorical outcomes as risk ratios (RRs), and continuous outcomes as mean differences (MD) or standardised mean differences (SMDs), with 95% confidence intervals (CI). We pooled data with a fixed‐effect meta‐analysis, except where there was heterogeneity, in which case we used a random‐effects model. Two review authors independently assessed the included studies for risk of bias and discussed any conflicts with a third review author.
Main results
This is an update of a previous review.
We included 27 studies with 3963 participants. The meta‐analysis included 21 studies of 2721 participants. Seventeen studies compared multiple session early psychological intervention versus treatment as usual and four studies compared a multiple session early psychological intervention with active control condition.
Low‐certainty evidence indicated that multiple session early psychological interventions may be more effective than usual care in reducing PTSD diagnosis at three to six months' follow‐up (RR 0.62, 95% CI 0.41 to 0.93; I2 = 34%; studies = 5; participants = 758). However, there was no statistically significant difference post‐treatment (RR 1.06, 95% CI 0.85 to 1.32; I2 = 0%; studies = 5; participants = 556; very low‐certainty evidence) or at seven to 12 months (RR 0.94, 95% CI 0.20 to 4.49; studies = 1; participants = 132; very low‐certainty evidence). Meta‐analysis indicated that there was no statistical difference in dropouts compared with usual care (RR 1.34, 95% CI 0.91 to 1.95; I2 = 34%; studies = 11; participants = 1154; low‐certainty evidence) .At the primary endpoint of three to six months, low‐certainty evidence indicated no statistical difference between groups in reducing severity of PTSD (SMD –0.10, 95% CI –0.22 to 0.02; I2 = 34%; studies = 15; participants = 1921), depression (SMD –0.04, 95% CI –0.19 to 0.10; I2 = 6%; studies = 7; participants = 1009) or anxiety symptoms (SMD –0.05, 95% CI –0.19 to 0.10; I2 = 2%; studies = 6; participants = 945).
No studies comparing an intervention and active control reported outcomes for PTSD diagnosis. Low‐certainty evidence showed that interventions may be associated with a higher dropout rate than active control condition (RR 1.61, 95% CI 1.11 to 2.34; studies = 2; participants = 425). At three to six months, low‐certainty evidence indicated no statistical difference between interventions in terms of severity of PTSD symptoms (SMD –0.02, 95% CI –0.31 to 0.26; I2 = 43%; studies = 4; participants = 465), depression (SMD 0.04, 95% CI –0.16 to 0.23; I2 = 0%; studies = 2; participants = 409), anxiety (SMD 0.00, 95% CI –0.19 to 0.19; I2 = 0%; studies = 2; participants = 414) or quality of life (MD –0.03, 95% CI –0.06 to 0.00; studies = 1; participants = 239).
None of the included studies reported on adverse events or use of health‐related resources.
Authors' conclusions
While the review found some beneficial effects of multiple session early psychological interventions in the prevention of PTSD, the certainty of the evidence was low due to the high risk of bias in the included trials. The clear practice implication of this is that, at present, multiple session interventions aimed at everyone exposed to traumatic events cannot be recommended. There are a number of ongoing studies, demonstrating that this is a fast moving field of research. Future updates of this review will integrate the results of these new studies.
Keywords: Humans; Cognitive Behavioral Therapy; Cognitive Behavioral Therapy/methods; Desensitization, Psychologic; Psychotherapy; Psychotherapy/methods; Quality of Life; Randomized Controlled Trials as Topic; Stress Disorders, Post‐Traumatic; Stress Disorders, Post‐Traumatic/prevention & control; Time Factors; Waiting Lists
Plain language summary
Multiple session early psychological interventions for prevention of post‐traumatic stress disorder
Why was this review important?
Traumatic events can have a significant effect on the ability of individuals, families and communities to cope. In the past, single session interventions such as psychological debriefing were widely used with the aim of preventing continuing psychological difficulties. However, previous reviews have found that single session individual interventions have not been effective at preventing post‐traumatic stress disorder (PTSD). A range of other forms of intervention have been developed to try to prevent people exposed to trauma from developing PTSD.
Who will be interested in this review?
• People exposed to traumatic events and their loved ones.
• Professionals working in mental health services.
• General practitioners.
• Commissioners.
What questions did this review try to answer?
Are multiple session early psychological interventions (i.e. interventions over two or more sessions beginning within the first three months after the traumatic event) more effective than treatment as usual or another psychological intervention in:
• reducing the number of people diagnosed with PTSD;
• reducing the severity of PTSD symptoms;
• reducing the severity of depressive symptoms;
• reducing the severity of anxiety symptoms;
• improving the general functioning (e.g. social, psychological, occupational and functioning) of recipients of the intervention.
Which studies were included in the review?
We searched for randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) that examined multiple session early psychological interventions in the prevention of PTSD, published between 1970 and March 2018.
We included 27 studies with 3963 participants.
What did the evidence from the review tell us?
• We found low‐certainty evidence that multiple session early psychological interventions may be more effective than treatment as usual in preventing PTSD diagnosis three to six months after receiving the intervention.
• We found very low‐certainty evidence that multiple session early psychological interventions may be neither more nor less effective than treatment as usual in preventing PTSD, either immediately after, or at seven to 12 months after, the intervention. We also found very low‐certainty evidence that multiple session early psychological interventions may be neither more nor less effective than treatment as usual in reducing the severity of PTSD symptoms, either immediately or at subsequent points of follow‐up.
• We found low‐certainty evidence that multiple session early psychological interventions may be associated with a higher dropout rate than other psychological interventions.
• We found low‐certainty evidence that multiple session early psychological interventions may be neither more nor less effective than other psychological interventions in diagnosing PTSD; reducing the severity of PTSD, depression and anxiety; or in maintaining the general functioning of participants receiving the intervention.
• We found no studies that measured adverse effects.
• We found no studies that measured use of health‐related resources.
What should happen next?
The current evidence base is small. However, new studies are being conducted and future updates of this review will incorporate the results of these.
Summary of findings
Summary of findings for the main comparison. Any early psychological intervention compared to waiting list/usual care for the prevention of post‐traumatic stress disorder.
| Any early psychological intervention compared to waiting list/usual care for the prevention of post‐traumatic stress disorder | |||||
|
Patient or population: various Setting: various Intervention: any early psychological intervention Comparison: waiting list/usual care | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with waiting list/usual care | Risk difference with any early psychological intervention | ||||
| PTSD diagnosis: post‐treatment | 556 (5 RCTs) | ⊕⊝⊝⊝ Very lowa,b | RR 1.06 (0.85 to 1.32) | Study population | |
| 283 per 1000 | 17 more per 1000 (42 fewer to 91 more) | ||||
| PTSD diagnosis: 3–6 months | 758 (5 RCTs) | ⊕⊕⊝⊝ Lowc,d | RR 0.62 (0.41 to 0.93) | Study population | |
| 215 per 1000 | 82 fewer per 1000 (127 fewer to 15 fewer) | ||||
| PTSD diagnosis: 7–12 months | 132 (1 RCT) | ⊕⊝⊝⊝ Very lowe,f | RR 0.94 (0.20 to 4.49) | Study population | |
| 47 per 1000 | 3 fewer per 1000 (38 fewer to 164 more) | ||||
| Dropouts from treatment | 1154 (11 RCTs) | ⊕⊕⊝⊝ Lowd,k | RR 1.34 (0.91 to 1.95) | Study population | |
| 125 per 1000 | 43 more per 1000 (11 fewer to 119 more) | ||||
| Severity of PTSD symptoms: 3–6 months | 1921 (15 RCTs) | ⊕⊕⊝⊝ Lowg,h | — | The mean severity of PTSD symptoms: 3–6 months was 0 | SMD 0.1 lower (0.22 lower to 0.02 higher) |
| Severity of depressive symptoms: 3–6 months | 1009 (7 RCTs) | ⊕⊕⊝⊝ Lowg,i | — | The mean severity of depressive symptoms at 3–6 months was 0 | SMD 0.04 lower (0.19 lower to 0.1 higher) |
| Severity of anxiety symptoms: 3–6 months | 945 (6 RCTs) | ⊕⊕⊝⊝ Lowg,j | — | The mean severity of anxiety symptoms at 3–6 months was 0 | SMD 0.05 lower (0.19 lower to 0.10 higher) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aThree of the five studies were at high risk of bias (Brunet 2013; Mouthaan 2013; Rothbaum 2012). bDowngraded two levels for imprecision as the total number of events was fewer than 300 and 95% CI included both little or no effect. cThree of the five studies were at high risk of bias (Jones 2010; Mouthaan 2013; Rothbaum 2012). dDowngraded one level for imprecision as the total number of events was fewer than 300. eThe study was at high risk of bias (Mouthaan 2013). fDowngraded two levels as the total number of events was fewer than 300 and 95% CI included both little or no effect. gDowngraded one level for imprecision as the 95% CI includes both little or no effect. hTwelve of the 15 studies were at high risk of bias (Als 2015; Borghini 2014; Brom 1993; Brunet 2013; Curtis 2016; Holmes 2007; Jensen 2016; Jones 2010; Kazak 2005; Mouthaan 2013; Rothbaum 2012; Zatzick 2001). iSix of the seven studies were at high risk of bias (Als 2015; Curtis 2016; Holmes 2007; Jensen 2016; Mouthaan 2013; Zatzick 2001). jFive of the six studies were at high risk of bias (Als 2015; Curtis 2016; Jensen 2016; Kazak 2005; Mouthaan 2013). kEight of the 11 studies were at high risk of bias (Brom 1993; Brunet 2013; Holmes 2007; Kazak 2005; Rothbaum 2012; Ryding 1998; Ryding 2004; Zatzick 2001).
Summary of findings 2. Any early psychological intervention compared to active control condition for the prevention of post‐traumatic stress disorder.
| Any early psychological intervention compared to active control condition for the prevention of post‐traumatic stress disorder | |||||
|
Patient or population: various Setting: various Intervention: any early psychological intervention Comparison: active control condition | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with active control condition | Risk difference with any early psychological intervention | ||||
| Dropouts from treatment | 425 (2 RCTs) | ⊕⊕⊝⊝ Lowe,f | RR 1.61 (1.11 to 2.34) | Study population | |
| 168 per 1000 | 103 more per 1000 (19 more to 225 more) | ||||
| Severity of PTSD symptoms: 3–6 months | 465 (4 RCTs) | ⊕⊕⊝⊝ Lowc,d | — | The mean severity of PTSD symptoms at 3–6 months was 0 | SMD 0.02 lower (0.31 lower to 0.26 higher) |
| Severity of depressive symptoms: 3–6 months | 409 (2 RCTs) | ⊕⊕⊝⊝ Lowd,e | — | The mean severity of depressive symptoms at 3–6 months was 0 | SMD 0.04 higher (0.16 lower to 0.23 higher) |
| Severity of anxiety symptoms: 3–6 months | 414 (2 RCTs) | ⊕⊕⊝⊝ Lowd,e | — | The mean severity of anxiety symptoms at 3–6 months was 0 | SMD 0 (0.19 lower to 0.19 higher) |
| General functioning: 3–6 months | 239 (1 RCT) | ⊕⊕⊝⊝ Lowg,h | — | The mean general functioning at 3–6 months was 0 | MD 0.03 lower (0.06 lower to 0) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aStudy was at high risk of bias (Gidron 2001). bDowngraded two levels for imprecision as total number of events was fewer than 300 and 95% CI included both little or no effect. cAll four studies were at high risk or bias (Cox 2018a; Gamble 2010; Gidron 2001; Gidron 2007). dDowngraded one level for imprecision as 95% CI included both little or no effect. eBoth studies were at high risk of bias (Cox 2018a; Gamble 2010). fDowngraded one level for imprecision as total number of events was fewer than 300. gStudy was at high risk of bias (Gamble 2010). hDowngraded one level for imprecision as total number of participants was fewer than 400.
Background
Description of the condition
There is now a large body of literature to show that traumatic experience can cause significant psychological difficulties for large numbers of people, through events such as natural disasters (e.g. Berger 2012; Schulz 2013), human made disasters (e.g. Jenkins 2012), military combat (Brunet 2015; Richardson 2019; Stevelink 2018), rape (Dworkin 2017), violent crime (e.g. Lowe 2017; Wilson 2015), and road traffic accidents (Heron‐Delaney 2013). Many individuals show great resilience in the face of such experiences and will manifest short‐lived or subclinical stress reactions that diminish over time, although some will experience delayed onset of symptoms (Bryant 2013). Most people recover without medical or psychological assistance (McNally 2003). Nevertheless, a range of psychological difficulties may develop following trauma in some of those who have been exposed. These include depressive reactions; phobic reactions and other anxiety disorders; alcohol and other substance misuse and less frequently obsessive compulsive disorder, psychotic reactions and conversion symptoms. Some individuals display symptoms consistent with acute stress disorder (ASD) in the early phase after a traumatic event. Post‐traumatic stress disorder (PTSD) is one of the most common enduring mental health problems to occur and has probably received most attention in the research literature.
PTSD is defined by the Diagnostic and Statistical Manual 5th edition (DSM5) as a syndrome which is comprised of four clusters of symptoms: repeated re‐experiencing of the trauma; avoidance of internal and external reminders; negative alterations in cognition and mood; and alterations in arousal and reactivity (APA 2013). For a diagnosis of PTSD to be made, symptoms have to have been present for more than one month. The Diagnostic and Statistical Manual 4th edition (DSM‐IV) used the term 'acute PTSD' to describe PTSD beginning before three months (APA 1994). While this term is no longer used in DSM5, the first three months continues to be seen as the priority period for early intervention. Reported rates of PTSD beginning within 12 months vary across different trauma exposed populations, with an estimated prevalence across studies at around 29% at one‐month post‐trauma and 17% at 12 months (Santiago 2013). Prevalence rates tend to be higher for people who have experienced intentional over non‐intentional trauma (Santiago 2013). Epidemiological research suggests that around 40% of people who develop early onset PTSD go on to develop a chronic disorder (Santiago 2013). The impact on social, interpersonal and occupational functioning for people who develop chronic PTSD can be very significant across the life span (Litz 2004; Kearns 2012).
Description of the intervention
To date, Cochrane Reviews have considered psychological intervention of PTSD (Bisson 2013), and pharmacological treatment of PTSD (Stein 2006). A large number of randomised controlled trials (RCTs) have demonstrated the effectiveness of some psychological interventions in treating chronic PTSD (Bisson 2013; NICE 2018). Trauma‐focused cognitive behavioural therapy (CBT; see Bisson 2013; Bradley 2005), and eye movement desensitisation and reprocessing (EMDR) (NICE 2018), have the strongest evidence base. Evidence‐based interventions are not effective for everyone and many people remain symptomatic, even after treatment is completed (Bradley 2005).
Since the 1990s, clinicians have been increasingly involved in attempts to develop interventions that might mitigate against the effects of trauma and prevent the onset of chronic PTSD. For several years, single session interventions such as psychological debriefing were a widely used and popular form of intervention. Debriefing came under increasing scrutiny in the 1990s and has been the subject of one Cochrane Review first published in 1998 and subsequently updated (Rose 2002). Other reviews reported similar findings (van Emmerik 2002; Bastos 2015). The lack of evidence for the efficacy of single session individual debriefing has therefore led many experts in the field to caution against its use (e.g. NICE 2018).
How the intervention might work
Increasingly the field has turned its attention to other models of intervention (Kearns 2012; Qi 2016). These models have included multiple session interventions aimed at any individual exposed to a traumatic event with the aim of preventing the development of PTSD, interventions aimed at individuals with a known or suspected specific risk factor and interventions aimed at individuals who are clearly symptomatic. For example, psychological first aid has been increasingly prescribed as an initial form of intervention (NCTSN/NCP 2006). Psychological first aid refers to the provision of basic comfort, information, support and attendance to immediate practical and emotional needs. Brief forms of CBT offered from around two weeks' post incident have been proposed as interventions to prevent the onset of PTSD and to treat those who develop symptoms in the early stages after a trauma. Interventions aimed at enhancing social support have also been suggested (Litz 2002; Ormerod 2002). Several recent studies have been conducted to evaluate some of these forms of intervention.
Why it is important to do this review
Some experts in the field (e.g. Bisson 2003; Brewin 2008; Qi 2016) advocate interventions that are targeted at those who are most at risk of continuing psychological difficulty. However, in the immediate aftermath of a traumatic event there is often a strong imperative from healthcare services, the public and politicians to provide psychological intervention to everyone who has been exposed regardless of symptomatology. The issues of who should be offered the intervention, timing of intervention and mode of intervention are at this time still contentious. We previously published a Cochrane Review of multiple session early psychological interventions for the prevention of PTSD (Roberts 2009) in which we found 11 studies of brief psychological interventions aimed at preventing PTSD in individuals exposed to a specific traumatic event. We found no evidence to support the use of these interventions. In a second review, we found evidence for trauma‐focused CBT over a waiting list control and over supportive counselling for individuals who were displaying traumatic stress symptoms (Roberts 2010). Evidence was strongest when individuals met diagnosis for ASD or acute PTSD. This review aims to clarify the current evidence base by conducting an updated review of multiple session early interventions aimed at preventing PTSD in individuals who have been exposed to a traumatic event but have not been identified as experiencing any specific psychological difficulties.
Objectives
To examine the efficacy of psychological interventions aimed at preventing PTSD in individuals exposed to a traumatic event but not identified as experiencing any specific psychological difficulties, in comparison with control conditions (e.g. usual care, waiting list and no treatment) and other psychological interventions.
Methods
Criteria for considering studies for this review
Types of studies
RCTs. Sample size, language and publication status were not used to determine whether or not a study should be included.
Types of participants
Any adult aged 18 years or older, exposed to a traumatic event. When a study included mixed adult and adolescent participants, we attempted to obtain separate data for adults when this was available. If this data was not available we required that at least 80% of the sample was aged 18 or over to include the study. For the purposes of the review, an event was considered traumatic if it was likely to meet criterion A1 of DSM‐IV for PTSD (APA 1994). Therefore, the majority of participants in included studies were considered to have experienced, witnessed, or been confronted with an event or events that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others.
We excluded studies that enlisted participants who met a certain symptom profile (e.g. ASD, acute PTSD, depression) or recruited participants on the basis of responses to a screening measure.
Types of interventions
This review considered any multiple session early psychological intervention designed to prevent symptoms of traumatic stress, and begun within three months of a traumatic incident. We excluded single session interventions because they are the subject of a separate Cochrane Review (Rose 2002). Early psychological interventions aimed at treating individuals who were identified as symptomatic (e.g. with ASD or acute PTSD) is subject to a separate review (Roberts 2010) conducted at the same time as this review.
For the purpose of the review, a psychological intervention included any specified non‐pharmaceutical intervention aimed at preventing the onset of PTSD offered by one or more health professional or lay person, with contact between therapist and participant on at least two occasions. We decided a priori that eligible intervention categories would include forms of psychological therapy based on a specified theoretical model. Potential intervention categories were identified from previous PTSD‐based reviews (Bisson 2013; NICE 2018). These were:
trauma‐focused cognitive behavioural therapy (TF‐CBT) – any psychological intervention that predominantly used trauma‐focused cognitive, behavioural or cognitive‐behavioural techniques. This category included exposure therapy;
stress management/relaxation – any psychological intervention that predominantly taught relaxation or anxiety/stress management techniques;
TF‐CBT group therapy – any approach delivered in a group setting that predominantly used trauma‐focused cognitive, behavioural or cognitive‐behavioural techniques;
CBT – any psychological intervention that predominantly used non‐trauma‐focused cognitive, behavioural or cognitive‐behavioural techniques. This category excluded the use of exposure therapy;
EMDR – any psychological intervention that predominantly used EMDR;
non‐trauma‐focused CBT group therapy – any approach delivered in a group that predominantly used non‐trauma‐focused cognitive, behavioural or cognitive‐behavioural techniques;
other psychological intervention – any psychological intervention that predominantly used non‐trauma‐focused techniques that would not be considered cognitive, behavioural or cognitive‐behavioural techniques. This category included non‐directive counselling, psychodynamic therapy and hypnotherapy.
We also decided a priori that eligible interventions would include non‐pharmaceutical interventions that were not based or only partially based on a specified theoretical model but that nevertheless aimed to reduce symptoms of traumatic stress, to include the following categories.
Education or information giving intervention – any intervention which predominantly provided only education or information about possible future difficulties or offered advice about constructive means of coping, or both.
Stepped care – any a priori specified care plan which offered intervention in a stepped care manner based on the continuing needs of the included participants.
Interventions aimed at enhancing positive coping skills and improving overall well‐being – any non‐pharmaceutical intervention which aimed to improve well‐being such as an occupational therapy intervention, an exercise‐based intervention or a guided self‐help intervention.
We decided a priori that the trials considered would include:
psychological intervention versus waiting list or usual care control;
psychological intervention versus other psychological intervention.
From prior knowledge of the literature, it was clear that a number of different forms of intervention had been evaluated on differing participant groups. Several studies were thought to have offered intervention to all individuals exposed. Others were known to have evaluated interventions for those who met inclusion based on predictors of future risk. We decided to undertake comparison of all interventions together initially and to undertake subanalysis on specific interventions and interventions targeted at individuals meeting specific risk factors as appropriate.
Types of outcome measures
Primary outcomes
Rates of PTSD among those exposed to trauma as measured by a standard classificatory system, assessed using a standardised measure such as the Clinician‐Administered PTSD Scale (CAPS; Blake 1995).
Dropout from treatment.
Secondary outcomes
Severity of traumatic stress symptoms using a standardised measure such as the CAPS (Blake 1995), Impact of Event Scale (Horowitz 1979), the Davidson Trauma Scale (Davidson 1997), or the Post‐traumatic Diagnostic Scale (Foa 1995). In circumstances where an individual study utilised both a clinician‐administered and a self‐reported measure, primacy was given to outcomes using the clinician‐administered measure, as such measures are considered to provide the 'gold standard' in the traumatic stress field (e.g. Foa 1997).
Severity of self‐reported depressive symptoms using a standardised measure such as the Beck Depression Inventory (Beck 1961).
Severity of self‐reported anxiety symptoms using a standardised measure such as the Beck Anxiety Inventory (Beck 1988), or the Spielberger State‐Trait Anxiety Inventory (Spielberger 1970)
Adverse effects.
General functioning including quality of life measures such as the 36‐item Short Form (SF‐36; Ware 1993).
Use of health‐related resources.
Comparisons involving follow‐up data would only be made when outcome data were available for similar time points. These time points were decided a priori as post‐treatment, three to six months post‐trauma, seven to 12 months post‐trauma, one to two years post‐trauma, two years and beyond. Three to six months post‐trauma was the primary outcome period.
Search methods for identification of studies
Electronic searches
Cochrane Common Mental Disorders Controlled Trials Register (CCMD‐CTR)
The Cochrane Common Mental Disorders (CCMD) Group maintains a specialised register of RCTs, the CCMD‐CTR. This register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm and other mental disorders within the scope of this Group. The CCMD‐CTR is a partially studies‐based register with more than 50% of reference records tagged to approximately 12,500 individually PICO‐coded study records. Reports of trials for inclusion in the register are collated from (weekly) generic searches of MEDLINE (from 1950), Embase (from 1974) and PsycINFO (from 1967); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review specific searches of additional databases. Reports of trials are also sourced from international trial registries, drug companies, the handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on the Group's website, with an example of the core MEDLINE search displayed in Appendix 1. The CCMD‐CTR fell out of date in June 2016, with the move of the editorial base from the University of Bristol to York.
Searches for this review were conducted in August 2008, May 2016 and March 2018.
Search one (1 August 2008)
CCMDCTR‐Studies: Diagnosis = "stress disorder*" or PTSD and Intervention = therapy or intervention or counsel* or debriefing and Age‐group = adult or aged or "not stated" or unclear and not Duration of therapy = "1 session"
CCMDCTR‐References: Keyword = "Stress Disorder*" or "Stress‐Disorder*" or Free‐text = PTSD and Free‐text = debrief* or *therap* or intervention* or counsel*
Search two (6 May 2016)
CCMDCTR‐Studies Register: (PTSD or posttrauma* or post‐trauma* or "post trauma*" or "combat disorder*" or "stress disorder*"):sco,stc CCDMDCTR‐References Register: (PTSD or posttrauma* or post‐trauma* or "post trauma*" or "combat disorder*" or "stress disorder*"):ti,ab,kw,ky,emt,mh,mc [Key to field tags. ti:title; ab:abstract; kw:keywords; ky:other keywords; mh:MeSH headings; mc:MeSH check words; emt:EMTREE headings; sco:healthcare condition; stc:target condition]
Search three (3 March 2018)
CCMD's information specialist conducted additional searches on the following bibliographic databases, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
The search was for a suite of PTSD reviews and the search terms were based on population or psychological debriefing (Appendix 2).
The Cochrane Central Register of Controlled Trials (CENTRAL), Issue 2 of 12, February 2018;
Ovid MEDLINE (2014 to 3 March 2018);
Ovid Embase (2014 to 3 March 2018);
Ovid PsycINFO (2014 to 3 March 2018);
Ebsco PILOTS (2014 to 3 March 2018).
Search four (15 March 2019)
In keeping with MECIR conduct standard c37 (searches to be within 12 months of publication), we ran an update search in March 2019. We screened the abstracts and placed any new trials matching our inclusion criteria as 'awaiting classification'. These will be incorporated into the next version of this review, as appropriate.
Searching other resources
Reference lists
We searched reference lists of the National Institute for Health and Care Excellence PTSD guidelines (NICE 2018), and studies identified in the search and of related review articles.
Personal communication
We provided a list of included references on the website of the International Society for Traumatic Stress Studies and contacted the membership to ask them to identify any studies that they thought might be missing.
Data collection and analysis
Selection of studies
Two review authors (NR and CL) independently read the abstracts of all potential trials. If an abstract appeared to represent an RCT, each review author independently read the full report to determine if the trial met the inclusion criteria. When agreement could not be reached about inclusion, we consulted a third review author. The studies excluded on further reading are listed in the Characteristics of excluded studies table, with reasons for their exclusion.
Data extraction and management
We designed a data extraction sheet to capture data that was entered into Review Manager 5 software (Review Manager 2014). Information extracted included demographic details of participants, details of the traumatic event, the randomisation process, the interventions used, dropout rates and outcome data. Two review authors (of NR, NK and JK) independently extracted data. When agreement could not be reached, we discussed the issue a third review author.
Assessment of risk of bias in included studies
Two review authors (of NR, NK and JK) independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions and listed below (Higgins 2011). We resolved conflicts through discussion with a third review author (JB).
Random sequence generation.
Allocation concealment.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias (including baseline imbalances, early termination of the trial, researcher allegiance).
We did not assess blinding of participants and personnel as a double‐blind methodology for studies of psychological treatment is impossible as it is clear to participants what treatment they are receiving.
We judged each potential source of bias as high, low or unclear, and provided a supporting quotation from the study report, together with a justification for the judgement, in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary. When information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account risk of bias for studies that contributed to that outcome.
Measures of treatment effect
Continuous data
We analysed continuous outcomes using mean difference (MD) when all trials had measured outcome on the same scale. When trials measured outcomes on different scales, we used the standardised mean difference (SMD).
Dichotomous data
We used the risk ratio (RR) as the main categorical outcome measure as this is more widely used than odds ratio (OR) in health‐related practice. All outcomes were presented using 95% confidence intervals (CI).
Unit of analysis issues
For trials which had a crossover design, we considered only results from the first randomisation period. If the trial was a three (or more) armed trial, consideration was given to undertaking pair‐wise meta‐analysis with each arm, depending upon the nature of the intervention in each arm and the relevance to the review objectives. Management of cluster randomised trials followed guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
When intention‐to‐treat (ITT) data were available, we reported these in the results. We attempted to access ITT data wherever possible. We used completer‐only data when this was the only type of data available. In cases where there was inadequate information within a particular paper to undertake analysis, we attempted to compute missing data from other information available within the paper, using guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For example, we calculated standard deviations (SDs) for continuous data when only the standard error (SE) or t statistics or P values were reported. When imputation was not possible or when further clarification was required, we attempted to contact the authors to request additional information. In cases where no further useable data were available, the study was not included in further analysis.
Assessment of heterogeneity
We used a visual inspection of the forest plots initially to explore for possible statistical heterogeneity (variation in intervention effects or results). We measured heterogeneity between studies by observing the I2 test and the Chi2 test (P < 0.10). An I2 of less than 30% was considered to indicate that statistical heterogeneity might not be important; an I2 of 30% to 60% to indicate moderate heterogeneity; an I2 of 50% to 90% to indicate substantial heterogeneity and an I2 greater than 75% to indicate considerable heterogeneity. Clinical and methodological heterogeneity reflect issues such as differences in participant populations, intervention types, study design and methodological rigour. We anticipated that included studies would evaluate a range of different interventions in a wide variety of populations. Therefore, we used a random‐effects model for all comparisons.
Assessment of reporting biases
It was decided a priori that if a minimum of 10 studies were available in a meta‐analysis, we would prepare funnel plots and examine them for signs of asymmetry. Where there was asymmetry, we planned to consider other possible reasons for this.
Data synthesis
We pooled data from more than one study using a fixed‐effect model, except where heterogeneity was considered to be present. In these cases, we used a random‐effects model as described below.
Subgroup analysis and investigation of heterogeneity
It was decided a priori that we would explore the following possible causes of clinical heterogeneity if sufficient data allowed.
Number of treatment sessions taken (two to six versus seven or more).
Type of traumatic event (combat‐related trauma versus rape and sexual assault versus other civilian trauma).
Participant characteristics (men versus women)
Sensitivity analysis
It was decided a priori that sensitivity analysis would explore possible causes of methodological heterogeneity if sufficient data allowed. Analysis would be based on the following criteria.
-
Trials considered most susceptible to bias would be excluded based on the following quality assessment criteria:
those with unclear allocation concealment;
high levels of postrandomisation losses (more than 40%) or exclusions;
unblinded outcome assessment or blinding of outcome assessment uncertain.
Use of ITT analysis versus completer outcomes would be undertaken depending on available data.
'Summary of findings' tables
We evaluated the certainty of available evidence using the GRADE approach. We generated 'Summary of findings' tables using GRADEpro GDT software, which imports data from Review Manager 5 (GRADEpro GDT; Review Manager 2014). These tables provided outcome‐specific information concerning the overall certainty of evidence from studies included in the comparison, the magnitude of effect of the interventions examined and the sum of available data on outcomes considered. We included information on the first seven outcomes of our review: PTSD diagnosis (on a clinician‐administered scale), severity of traumatic stress symptoms, severity of self‐reported depressive symptoms, severity of self‐reported anxiety symptoms, dropouts, adverse effects and general functioning. For the primary outcome of PTSD diagnosis, we reported all time points available. For the secondary outcomes, we prioritised the primary end point of three to six months' postintervention.
We assessed the certainty of evidence using five factors.
Limitations in study design and implementation of available studies.
Indirectness of evidence.
Unexplained heterogeneity or inconsistency of results.
Imprecision of effect estimates.
Potential publication bias.
For each outcome, we classified the certainty of evidence according to the following categories.
High certainty : further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty : further research is likely to have an important impact on our confidence in the estimate of effect, and may change the estimate.
Low certainty : further research is very likely to have an important impact on our confidence in the estimate of effect, and is likely to change the estimate.
Very low certainty : we are very uncertain about the estimate.
We downgraded the evidence from high certainty by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
See Figure 1.
1.

PRISMA flow diagram.
The search identified 7380 titles and abstracts and two review authors independently read 297 papers in detail to establish if they met the specified inclusion criteria.
An update search (15 March 2019) identified 781 RCT records. We screened the abstracts and have added 11 new studies to those awaiting classification.
Included studies
Twenty‐seven studies, including 11 identified in Roberts 2009, evaluated brief (two or more sessions) psychological interventions aimed at preventing PTSD in people exposed to a specific traumatic event. Twenty five studies were reported in English, one in French (Andre 1997), and one in Persian (Taghizadeh 2008). The studies are described in the Characteristics of included studies table.
Study design
All included studies were two armed RCTs, apart from one study (Lindwall 2014), which included three arms, one of which evaluated intervention offered to children which was not eligible for inclusion in this review. Participants would not have been blind to their allocation group. Sample size in the included studies varied from 17 (Gidron 2001) to 386 (Jensen 2016) participants. The total number of participants randomised in the 27 studies was 3963.
Participants
Seven studies were conducted in the USA (Biggs 2016; Cox 2018a; Curtis 2016; Kazak 2005; Lindwall 2014; Rothbaum 2012; Zatzick 2001); three in Canada (Brunet 2013; Irvine 2011; Marchand 2006); three in Australia (Gamble 2005; Gamble 2010; Holmes 2007); two in the Netherlands (Brom 1993; Mouthaan 2013); two in Israel (Gidron 2001; Gidron 2007); two in Sweden (Ryding 1998; Ryding 2004); one in France (Andre 1997); one in Iran (Taghizadeh 2008); one in the UK (Als 2015); one in Switzerland (Borghini 2014); one in Denmark (Jensen 2016); one in China (Wang 2015); one in Sri Lanka (Wijesinghe 2015); and one study was conducted in various European countries including Denmark, Italy, Norway, Portugal, Sweden and the UK (Jones 2010).
Five studies evaluated interventions offered to mothers who had experienced traumatic births (Gamble 2005; Gamble 2010; Ryding 1998; Ryding 2004; Taghizadeh 2008), while another study was of mothers of babies born at less than 33 weeks' gestation (Borghini 2014). Five studies included individuals who had been involved in road traffic accidents (Brom 1993; Gidron 2001; Gidron 2007; Wang 2015; Zatzick 2001). In four studies, participants were family members (Als 2015; Curtis 2016; Kazak 2005; Lindwall 2014): one study was in relatives of patients admitted to an intensive care unit (Curtis 2016), one was in parents whose child was newly diagnosed with cancer (Kazak 2005), one was in parents whose child received a stem cell or bone marrow transplant (Lindwall 2014), and one was in parents of a child admitted to a paediatric intensive care unit (Als 2015). Three studies were in individuals who had experienced major physical trauma and had been admitted to a trauma centre (Holmes 2007; Mouthaan 2013; Rothbaum 2012), while another three were in individuals who had been admitted to an intensive care unit (Cox 2018a; Jensen 2016; Jones 2010). One study included individuals who had been exposed to armed robbery, involving acts of violence (Marchand 2006). Participants in this study had to have reported experiencing intense fear, helplessness or horror during or after the robbery for inclusion. One study was conducted in individuals who had been exposed to a life‐threatening event (Brunet 2013), one included bus drivers who had been assaulted (Andre 1997), one was in people who had received an implantable cardioverter defibrillator (ICD) (Irvine 2011), one was on military mortuary workers (Biggs 2016), and one was in snakebite victims (Wijesinghe 2015).
Interventions
Twenty‐one studies compared multiple session early interventions versus treatment as usual. Five studies used an approach which we grouped as "brief individual trauma processing" (Brom 1993; Gamble 2005; Marchand 2006; Rothbaum 2012; Ryding 1998). This subgroup consisted of a number of brief therapies – lasting two or more sessions – that were theoretically diverse but shared similar core treatment components. These included: psychoeducation, therapist directed reliving of the index trauma to promote elaboration of the trauma memory and help to contextualise or reframe aspects of the experience. Two studies used CBT (Andre 1997; Irvine 2011), two used brief dyadic CBT (therapy involving a trauma exposed individual in conjunction with a significant other person, such as a partner, spouse or other family member) (Brunet 2013; Kazak 2005), one used a self‐guided Internet‐based intervention (Mouthaan 2013), one used brief interpersonal therapy (IPT) (Holmes 2007), one used a counselling intervention (Taghizadeh 2008), one used group counselling (Ryding 2004), one used psychological first aid group sessions (Biggs 2016), one used collaborative care (Zatzick 2001), one used intensive care diaries (Jones 2010), one used three‐step early intervention (Borghini 2014), one used supported psychoeducation (Als 2015), one used communication facilitator in an intensive care setting (Curtis 2016), one used nurse‐led intensive care recovery programme (Jensen 2016), and one used creative art (Wang 2015).
Six studies compared a psychological intervention with another intervention. Two studies compared memory structuring intervention with supportive listening (Gidron 2001; Gidron 2007), one study compared CBT with an education programme (Cox 2018a), one study compared a resilience programme with parenting support (Gamble 2010), one study compared a child‐targeted intervention (massage and humour therapy) with child‐targeted plus a parent‐targeted (massage, relaxation, imagery) intervention (Lindwall 2014), and one compared psychological first aid and psychoeducation and CBT versus psychological first aid and psychoeducation (Wijesinghe 2015).
Further information about specific interventions is provided in the Characteristics of included studies table.
Outcomes
Most of the included studies used well validated self‐report measures of PTSD, depression or anxiety as key outcomes. Measures used are listed in the Characteristics of included studies table. The most commonly used tool for measuring PTSD was the Impact of Events Scale (IES) (Als 2015; Andre 1997; Brom 1993; Marchand 2006; Rothbaum 2012; Ryding 1998; Ryding 2004; Taghizadeh 2008), followed by the Impact of Event Scale – Revised (IES‐R) (Brunet 2013; Cox 2018a; Irvine 2011; Kazak 2005; Lindwall 2014; Mouthaan 2013; Wang 2015), Post‐Traumatic Stress Disorder Checklist (PCL) (Biggs 2016; Curtis 2016; Holmes 2007; Zatzick 2001), and CAPS (Brunet 2013; Mouthaan 2013; Wang 2015). Other PTSD scales used less commonly included the Mini‐International Neuropsychiatric Interview for PTSD (MINI‐PTSD) (Gamble 2005), Perinatal Posttraumatic Stress Disorder Questionnaire (PPQ) (Borghini 2014), Harvard Trauma Questionnaire (HTQ) (Jensen 2016), PTSD Symptom Scale – Interview Version (PSS‐I) (Rothbaum 2012), and Post‐traumatic Stress Symptom Scale – Self Report (PSS‐SR) (Wijesinghe 2015).
Other outcomes such as anxiety, depression and quality of life were measured using Hospital Anxiety and Depression Scale (HADS) (Als 2015; Andre 1997; Cox 2018a; Holmes 2007; Irvine 2011; Jensen 2016; Mouthaan 2013; Wang 2015), Patient Health Questionnaire (PHQ‐9) (Biggs 2016; Curtis 2016), SF‐36 (Holmes 2007; Irvine 2011; Jensen 2016; Mouthaan 2013), Generalised Anxiety Disorder Assessment (GAD‐7) (Curtis 2016), World Health Organization Quality of Life Assessment (Biggs 2016), Trauma Symptom Inventory (Brom 1993), Social Constraints Scale (SCS) (Brunet 2013), Social Adjustment Scale by Self‐Report (SAS‐SR) (Brunet 2013), EuroQol (EQ‐5D) (Cox 2018a), Patient‐Reported Outcomes Measurement Information System (PROMIS) (Cox 2018a), Brief Coping Orientation to Problems Experienced (COPE) (Cox 2018a), Edinburgh Postnatal Depression Scale (EPDS) (Gamble 2005; Gamble 2010; Ryding 2004), Depression Anxiety and Stress Scale‐21 (DASS‐21) (Gamble 2005; Gamble 2010), Maternity Social Support Scale (MSSS) (Gamble 2005), and health‐related quality of life (HRQoL) (Gamble 2010).
We used data from a clinician‐administered PTSD severity measure for six studies (Brunet 2013; Gamble 2005; Jones 2010; Marchand 2006; Mouthaan 2013; Rothbaum 2012). Data were only available from self‐report measures from17 trials (Als 2015; Borghini 2014; Brom 1993; Cox 2018a; Curtis 2016; Gamble 2010; Gidron 2001; Gidron 2007; Holmes 2007; Irvine 2011; Jensen 2016; Kazak 2005; Lindwall 2014; Ryding 1998; Ryding 2004; Taghizadeh 2008; Zatzick 2001).
Excluded studies
See Characteristics of excluded studies table.
In this update, we culled the list of excluded studies (previously reported in the first version of this review (Roberts 2009), to those studies which narrowly missed the inclusion criteria, those readers may plausibly expect to see included.
We excluded 38 studies following access of full‐text articles, as they did not satisfy the inclusion criteria. Of these, participants already had PTSD symptoms in 34 trials (Ben‐Zion 2018; Bisson 2004; Bryant 1998; Bryant 1999; Bryant 2003; Bryant 2005; Bryant 2008; Bugg 2009; Cernvall 2015; Echeburua 1996; Ehlers 2003; Foa 2006; Freedman (in preparation); Freedman (submitted); Freyth 2010; Jarero 2011; Jarero 2015; Nixon 2012; Nixon 2016; O'Donnell (in preparation); O'Donnell 2012; Öst unpublished; Shalev 2012; Shapiro 2015; Shapiro 2018; Shaw 2013; Sijbrandij 2007; Skogstad 2015; van Emmerik 2008; Wagner 2007; Wu 2014; Zatzick 2004; Zatzick 2013; Zatzick 2015). Four studies evaluated single session interventions (Resnick 2005; Rose 1999; Rothbaum (submitted); Turpin 2005).
Studies awaiting classification
One study could not be accessed and has been included in studies awaiting classification (Kilpatrick 1984). A further 11 newly completed studies were identified in the most recent search of March 2019 and are awaiting classification (see Characteristics of studies awaiting classification table).
Ongoing studies
For details of ongoing studies, see Characteristics of ongoing studies table.
Risk of bias in included studies
2.
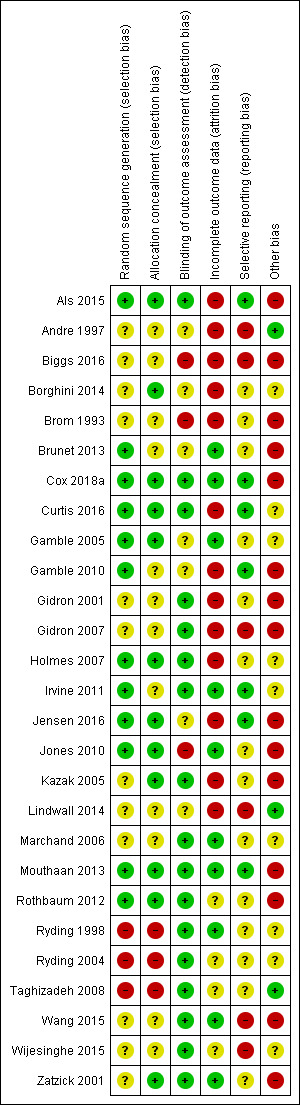
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
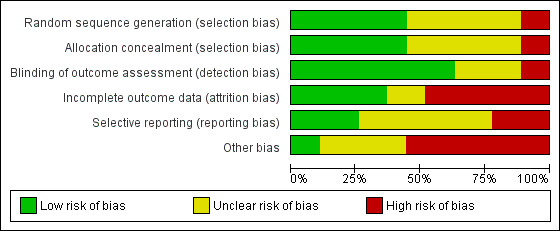
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Methodological quality of many of the included studies was poor. The process of recruitment used by Brom 1993 was of particular concern as recruitment took place prior to invitation to potential participants to join the study. This may lead to significant difference in response rate, dropout rate, other dropout factors and baseline scores between the treatment and control group.
Random sequence generation
Twelve studies provided an adequate description of the randomisation process and were at low risk of selection bias (Als 2015; Brunet 2013; Cox 2018a; Curtis 2016; Gamble 2005; Gamble 2010; Holmes 2007; Irvine 2011; Jensen 2016; Jones 2010; Mouthaan 2013; Rothbaum 2012). Twelve studies did not report the randomisation process and were therefore at unclear risk of selection bias (Andre 1997; Biggs 2016; Borghini 2014; Brom 1993; Gidron 2001; Gidron 2007; Kazak 2005; Lindwall 2014; Marchand 2006; Wang 2015; Wijesinghe 2015; Zatzick 2001). Three studies were at high risk of selection bias as the process was not truly random (Ryding 1998; Ryding 2004: Taghizadeh 2008). Ryding 2004 randomised women who gave birth on approximately 18 predetermined days of the month to the counselling group. Ryding 1998 selected every second emergency caesarean section patient, according to the delivery ward register, for counselling, the remainder were in the comparison group. Taghizadeh 2008 randomised participants by day of the week.
Allocation concealment
Twelve studies reported adequate concealment procedures and were at low risk of selection bias (Als 2015; Borghini 2014; Cox 2018a; Curtis 2016; Gamble 2005; Holmes 2007; Jensen 2016; Jones 2010; Kazak 2005; Mouthaan 2013; Rothbaum 2012; Zatzick 2001). In 12 studies, allocation concealment was unclear or inadequate (Andre 1997; Biggs 2016; Brom 1993; Brunet 2013; Gamble 2010; Gidron 2001; Gidron 2007; Irvine 2011; Lindwall 2014; Marchand 2006; Wang 2015; Wijesinghe 2015). Three studies made no attempt to hide allocation concealment and were at high risk of selection bias (Ryding 1998; Ryding 2004; Taghizadeh 2008).
Blinding of participants and personnel
This was not assessed as a double‐blind methodology for studies of psychological treatment is impossible as it is clear to participants what treatment they are receiving.
Blinding of outcome assessors
Seventeen studies blinded outcome assessors and were at low risk of detection bias (Als 2015; Cox 2018a; Curtis 2016; Gidron 2001; Gidron 2007; Holmes 2007; Irvine 2011; Kazak 2005; Marchand 2006; Mouthaan 2013; Rothbaum 2012; Ryding 1998; Ryding 2004; Taghizadeh 2008; Wang 2015; Wijesinghe 2015; Zatzick 2001). Seven studies did not report this (Andre 1997; Borghini 2014; Brunet 2013; Gamble 2005; Gamble 2010; Jensen 2016; Lindwall 2014), and three studies were at high risk of detection bias (Biggs 2016; Brom 1993; Jones 2010).
Incomplete outcome data
Ten studies fully reported loss to follow‐up and adequately dealt with missing outcome data (Brunet 2013; Cox 2018a; Gamble 2005; Irvine 2011; Jones 2010; Marchand 2006; Mouthaan 2013; Ryding 1998; Wang 2015; Zatzick 2001). Gamble 2005 included follow‐up data from all participants (one participant in the intervention group could not be contacted at initial follow‐up). Marchand 2006 and Zatzick 2001 included withdrawals in analysis by estimation of outcome by the method of 'last observation carried forward'. Thirteen studies did not adequately report missing outcome data and loss to follow‐up and were at high risk of bias (Als 2015; Andre 1997; Biggs 2016; Borghini 2014; Brom 1993; Curtis 2016; Gamble 2010; Gidron 2001; Gidron 2007; Holmes 2007; Jensen 2016; Kazak 2005; Lindwall 2014). Four studies provided data only for treatment completers and recorded withdrawals without reasons by group or the number of withdrawals was not specified and were at unclear risk of bias (Rothbaum 2012; Ryding 2004; Taghizadeh 2008; Wijesinghe 2015).
Selective reporting
Seven studies published protocols and reported on prespecified outcomes and were at low risk of reporting bias (Als 2015; Cox 2018a; Curtis 2016; Gamble 2010; Irvine 2011; Jensen 2016; Mouthaan 2013). Fourteen studies did not publish protocols and therefore it was unclear if they were free from reporting bias (Borghini 2014; Brom 1993; Brunet 2013; Gamble 2005; Gidron 2001; Holmes 2007; Jones 2010; Kazak 2005; Marchand 2006; Rothbaum 2012; Ryding 1998; Ryding 2004; Taghizadeh 2008; Zatzick 2001). One study did not report PTSD diagnosis as a primary outcome and was at high risk of reporting bias (Gidron 2007). Five other studies with null findings were at high risk of selective reporting because they did not provide data that could be used in meta‐analysis (Andre 1997; Biggs 2016; Lindwall 2014; Wang 2015; Wijesinghe 2015).
Other bias
Three studies were at low risk of other bias (Andre 1997; Lindwall 2014; Taghizadeh 2008). Nine studies were at unclear risk of other bias (Borghini 2014; Curtis 2016; Gamble 2005; Holmes 2007; Irvine 2011; Marchand 2006; Ryding 1998; Ryding 2004; Wijesinghe 2015). Fifteen studies were at high risk of other bias (Als 2015; Biggs 2016; Brom 1993; Brunet 2013; Cox 2018a; Gamble 2010; Gidron 2001; Gidron 2007; Jensen 2016; Jones 2010; Kazak 2005; Mouthaan 2013; Rothbaum 2012; Wang 2015; Zatzick 2001). Reasons for studies being judged at high risk of other bias include small sample size (Als 2015; Gidron 2001; Gidron 2007; Kazak 2005; Wang 2015; Zatzick 2001), inadequate description of the intervention (Biggs 2016), evaluation of treatment adherence not reported (Biggs 2016; Gidron 2001; Gidron 2007; Jensen 2016), differences in treatment groups at baseline (Brom 1993; Gamble 2010), study authors affiliated with intervention (Brunet 2013; Gidron 2001; Gidron 2007; Mouthaan 2013; Rothbaum 2012), higher‐than expected attrition rate (Cox 2018a), PTSD diagnosis based on assessor administration of PDS (Jones 2010) and intervention not manualised (Wang 2015; Zatzick 2001).
Effects of interventions
The meta‐analyses included 21 studies with 2721 participants. Results were reported for all available outcome measures specified in the methodology. None of the studies identified reported data on adverse effects or use of health‐related resources. Six studies could not be used in the meta‐analysis as they reported no useable data (Andre 1997; Biggs 2016; Lindwall 2014; Taghizadeh 2008; Wang 2015; Wijesinghe 2015).
Comparison 1: any intervention versus waiting list/usual care
Twenty‐one studies compared a psychological intervention against a waiting list or treatment as usual condition (Als 2015; Andre 1997; Biggs 2016; Borghini 2014; Brom 1993; Brunet 2013; Curtis 2016; Gamble 2005; Holmes 2007; Irvine 2011; Jensen 2016; Jones 2010; Kazak 2005; Marchand 2006; Mouthaan 2013; Rothbaum 2012; Ryding 1998; Ryding 2004; Taghizadeh 2008; Wang 2015; Zatzick 2001).
Primary outcome
1. PTSD diagnosis
Six studies used a clinician‐administered scale to measure PTSD diagnosis and were entered into the meta‐analyses (Brunet 2013; Gamble 2005; Jones 2010; Marchand 2006; Mouthaan 2013; Rothbaum 2012). The remaining 15 studies used a self‐reported measure and were not included in the meta‐analyses (Als 2015; Andre 1997; Biggs 2016; Borghini 2014; Brom 1993; Curtis 2016; Holmes 2007; Irvine 2011; Jensen 2016; Kazak 2005; Ryding 1998; Ryding 2004; Taghizadeh 2008; Wang 2015; Zatzick 2001).
Post‐treatment
Five studies provided data on clinician‐administered diagnosis of PTSD (Brunet 2013; Gamble 2005; Marchand 2006; Mouthaan 2013; Rothbaum 2012). Post‐treatment there was a lack of evidence for difference between intervention and control conditions (RR 1.06, 95% CI 0.85 to 1.32; I2 = 0%; studies = 5; participants = 556; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.
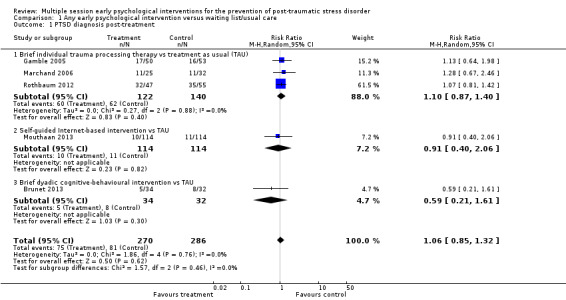
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 1 PTSD diagnosis post‐treatment.
When analysed by type of psychological intervention, there was a lack of evidence for difference between treatment as usual and brief individual trauma processing therapy (RR 1.10, 95% CI 0.87 to 1.40; I2 = 0%; studies = 3; participants = 262). There was uncertainty in estimating differences between treatment as usual and self‐guided Internet‐based interventions (RR 0.91, 95% CI 0.40 to 2.06; studies = 1; participants = 228), brief dyadic CBT (RR 0.59, 95% CI 0.21 to 1.61; studies = 1; participants = 66) reflected by the very wide confidence intervals.
Three to six months' follow‐up
Five studies provided data on clinician‐administered diagnosis of PTSD at three to six months' follow‐up (Gamble 2005; Jones 2010; Marchand 2006; Mouthaan 2013; Rothbaum 2012). Multiple session early psychological interventions were associated with a reduction in PTSD symptoms compared to treatment as usual (RR 0.62, 95% CI 0.41 to 0.93; I2 = 34%; studies = 5; participants = 758; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.
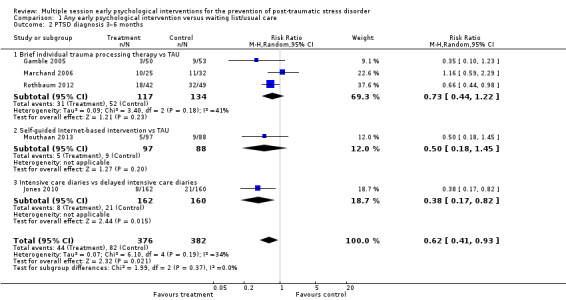
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 2 PTSD diagnosis 3–6 months.
When analysed by type of intervention, meta‐analysis of three studies identified uncertainty for the difference between treatment as usual and brief individual trauma processing therapy reflected in the wide confidence interval (RR 0.73, 95% CI 0.44 to 1.22; I2 = 41%; studies = 3; participants = 251). There was considerable imprecision in estimating the difference between self‐guided Internet‐based interventions and treatment as usual (RR 0.50, 95% CI 0.18 to 1.45; studies = 1; participants = 185). Evidence from one study of 322 participants showed that intensive care diaries were more effective than delayed access to diaries (RR 0.38, 95% CI 0.17 to 0.82).
Seven to 12 months' follow‐up
One study measured clinician‐administered PTSD at seven to 12 months' follow‐up (Mouthaan 2013). There was very high imprecision in estimating differences between self‐guided Internet‐based intervention and treatment as usual (RR 0.94, 95% CI 0.20 to 4.49; studies = 1; participants = 132; very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.
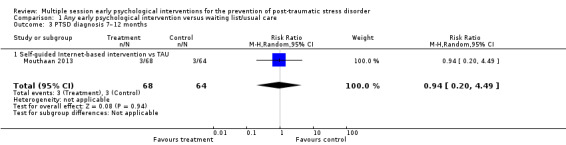
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 3 PTSD diagnosis 7–12 months.
2. Dropout from treatment
Eleven studies provided data on the number of participants who left the study early (Brom 1993; Brunet 2013; Gamble 2005; Holmes 2007; Irvine 2011; Kazak 2005; Marchand 2006; Rothbaum 2012; Ryding 1998; Ryding 2004; Zatzick 2001). Meta‐analysis indicated that there was no significant difference in dropouts (RR 1.34, 95% CI 0.91 to 1.95; I2 = 34%; studies = 11; participants = 1154; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.
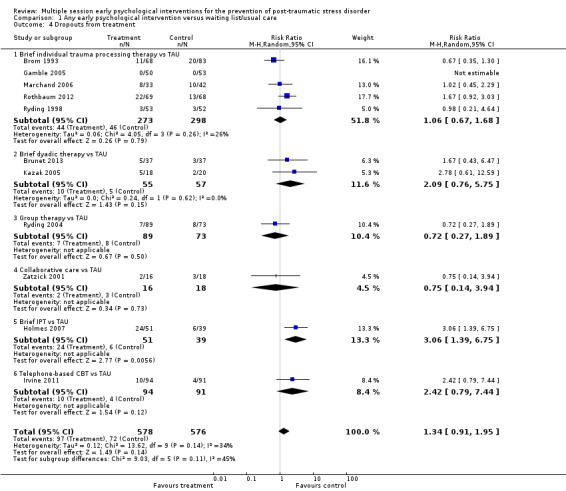
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 4 Dropouts from treatment.
When analysed by type of intervention, evidence from one study indicated brief IPT was associated with a higher dropout rate than TAU (RR 3.06, 95% CI 1.39 to 6.75; participants = 90). There was no significant difference, and wide confidence intervals, in the dropout rates between treatment as usual and brief individual trauma processing therapy (RR 1.06, 95% CI 0.67 to 1.68; I2 = 26%; studies = 5; participants = 571), brief dyadic therapy (RR 2.09, 95% CI 0.76 to 5.75; I2 = 0%; studies = 2; participants = 112), group therapy (RR 0.72, 95% CI 0.27 to 1.89; studies = 1; participants = 162), collaborative care (RR 0.75, 95% CI 0.14 to 3.94; studies = 1; participants = 34), or telephone‐based CBT (RR 2.42, 95% CI 0.79 to 7.44; studies = 1; participants = 185).
Secondary outcomes
1. Severity of PTSD symptoms
Post‐treatment
Nine studies provided data on the severity of PTSD symptoms post‐treatment (Borghini 2014; Brom 1993; Gamble 2005; Jones 2010; Marchand 2006; Mouthaan 2013; Rothbaum 2012; Ryding 2004; Zatzick 2001). Meta‐analysis showed no evidence of a difference between multiple session early psychological interventions and treatment as usual (SMD –0.09, 95% CI –0.29 to 0.12; studies = 9; participants = 1326; Analysis 1.5). There was a high degree of heterogeneity in this result (I2 = 67%).
1.5. Analysis.
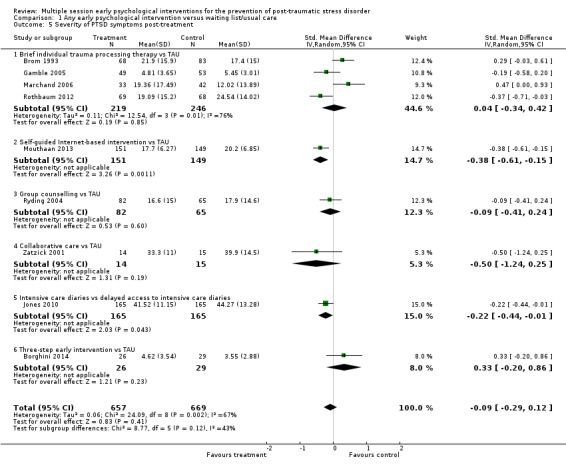
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 5 Severity of PTSD symptoms post‐treatment.
When analysed by type of intervention, evidence from one study of 300 participants indicated that self‐guided Internet‐based CBT may be more effective than treatment as usual (SMD –0.38, 95% CI –0.61 to –0.15). Another study of 330 participants showed that intensive care diaries may be more effective than delayed access to diaries in reducing severity of PTSD symptoms postintervention (SMD –0.22, 95% CI –0.44 to –0.01). There was very high imprecision (wide confidence intervals) in estimating differences between treatment as usual and brief individual trauma processing therapy (SMD 0.04, 95% CI –0.34 to 0.425; I2 = 76%; studies = 4; participants = 46), group counselling (SMD –0.09, 95% CI –0.41 to 0.24; studies = 1; participants = 147), collaborative care (SMD –0.50, 95% CI –1.24 to 0.25; studies = 1; participants = 29), three‐step early intervention (SMD 0.33, 95% CI –0.20 to 0.86; studies = 1; participants = 55).
It is evident that the heterogeneity in the group of participants who received brief individual trauma processing therapy contributed to the overall heterogeneity observed. It was not possible to investigate heterogeneity by number of sessions of the intervention, time between trauma exposure and intervention or type of traumatic event. However, it was possible to undertake a sensitivity analysis to look into gender of participants. Of the four studies on brief individual trauma processing therapy, one was done in women only (Gamble 2005). However, removing this study from the meta‐analysis had little effect on the heterogeneity within this group (I2 = 82%). In the subgroup analysis, there was no evidence (Χ2=0.89, P=0.35) of difference between studies of brief individual trauma processing therapy targeting women only (SMD –0.19, 95% CI –0.58 to 0.20; studies = 1; participants = 102) and studies where gender was mixed (SMD 0.12, 95% CI –0.39 to 0.62; studies = 3; participants = 363). But the small number of studies limits the ability to identify differences between subgroups.
We planned to perform a sensitivity analysis excluding studies with unclear allocation concealment, high levels of post‐randomisation losses (greater than 40%) and unblinded outcome assessment or blinding of outcome assessment uncertain. However, this was not possible as three studies were at unclear risk of selection bias (Brom 1993; Gamble 2005; Marchand 2006), two studies were at high risk of attrition bias (Brom 1993; Rothbaum 2012), and two studies failed to blind or failed to report the blinding of outcome assessors (Brom 1993; Gamble 2005).
One study measured severity of PTSD symptoms using the PCL‐17 (Biggs 2016). Data were not in a usable format for meta‐analysis. Authors reported that the severity of PTSD symptoms did not differ significantly between the intervention and control group at one or two months' post‐treatment.
Three to six months' follow‐up
Fifteen studies provided data on the severity of PTSD symptoms at three to six months' follow‐up (Als 2015; Borghini 2014; Brom 1993; Brunet 2013; Curtis 2016; Gamble 2005; Holmes 2007; Irvine 2011; Jensen 2016; Jones 2010; Kazak 2005; Marchand 2006; Mouthaan 2013; Rothbaum 2012; Zatzick 2001). There was no significant difference in severity of PTSD symptoms between multiple session early psychological interventions and treatment as usual (SMD –0.10, 95% CI –0.22 to 0.02; I2 = 34%; studies = 15; participants = 1921; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 6 Severity of PTSD symptoms: 3–6 months.
When analysed by type of intervention, evidence from one study (300 participants) indicated that self‐guided Internet‐based CBT may be more effective than treatment as usual (SMD –0.27, 95% CI –0.50 to –0.04). Two studies on a total of 103 participants showed that brief dyadic therapy may be more effective in reducing severity of PTSD symptoms three to six months' post‐treatment (SMD –0.41, 95% CI –0.81 to –0.02). None of the other interventions (brief individual trauma processing therapy, collaborative care, brief IPT, intensive care diaries, three‐step early intervention, telephone‐based CBT, supported psychoeducation, communication facilitation in an ICU setting and nurse‐led ICU recovery programme), showed any evidence of effectiveness over treatment as usual in the severity of PTSD symptoms three to six months' postintervention.
One study measured severity of PTSD at six months but could not be entered in the meta‐analysis as authors did not report SDs (Andre 1997). The mean IES in the group receiving CBT decreased from 21.7 to 17.2, while the mean IES in the control group decreased from 15.6 to 12.0. However, data were only available for 39/65 participants in the intervention group and 45/67 in the control group.
One study measured severity of PTSD symptoms using the PCL‐17 (Biggs 2016). Data were not in a usable format for meta‐analysis. Authors reported that the severity of PTSD symptoms did not differ significantly between the intervention and control group at three or four months' post‐treatment.
Seven to 12 months' follow‐up
Four studies provided data on the severity of PTSD symptoms at seven to 12 months' follow‐up (Borghini 2014; Irvine 2011; Jensen 2016; Mouthaan 2013). There was no evidence of difference in severity of PTSD symptoms between multiple session early psychological interventions and treatment as usual (SMD –0.09, 95% CI –0.32 to 0.14; I2 = 57%; studies = 4; participants = 765; Analysis 1.7).
1.7. Analysis.
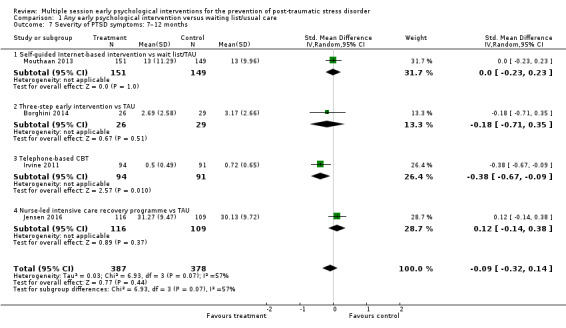
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 7 Severity of PTSD symptoms: 7–12 months.
It was not possible to investigate heterogeneity by number of sessions of the intervention, time between trauma exposure and intervention, or type of traumatic event. One study was conducted in women only, but excluding this from the meta‐analysis had little effect on the heterogeneity (I2 = 70%) (Borghini 2014). We planned to perform a sensitivity analysis excluding studies at high risk of bias. However, this was not possible as three of the four studies which measured this outcome at seven to 12 months were at unclear risk of selection bias (Irvine 2011), unclear risk of detection bias (Borghini 2014; Jensen 2016), and high risk of attrition bias (Borghini 2014; Jensen 2016).
When analysed by type of intervention, evidence from one study (185 participants) indicated that telephone‐based CBT may be more effective than treatment as usual (SMD –0.38, 95% CI –0.67 to –0.09). For all other comparisons, confidence intervals were wide reflecting uncertainty in differences between treatment as usual and self‐guided Internet‐based CBT (SMD 0.00, 95% CI –0.23 to 0.23; studies = 1; participants = 300), three‐step early intervention (SMD –0.18, 95% CI –0.71 to 0.35; studies = 1; participants = 55), or nurse‐led intensive care recovery programme (SMD 0.12, 95% CI –0.14 to 0.38; studies = 1; participants = 225).
The study by Wang 2015 measured severity of PTSD symptoms at 12 months' follow‐up. Authors did not report SDs and, therefore, it could not be used in a meta‐analysis. There were no significant differences between group in terms of PTSD severity as measured by the CAPS score (P = 0.74) or the IES‐R score (P = 0.68).
One study measured severity of PTSD symptoms using the PCL‐17 (Biggs 2016). Data were not in a usable format for meta‐analysis. Authors reported that the severity of PTSD symptoms did not differ significantly between the intervention and control group at seven or 10 months' post‐treatment.
2. Severity of depressive symptoms
Post‐treatment
Five studies reported data on severity of depressive symptoms post‐treatment (Holmes 2007; Mouthaan 2013; Rothbaum 2012; Ryding 2004; Zatzick 2001). Meta‐analysis indicated a lack of evidence of difference between multiple session early psychological interventions and treatment as usual at postintervention (SMD –0.19, 95% CI –0.40 to 0.01; I2 = 35%; studies = 5; participants = 671; Analysis 1.8).
1.8. Analysis.
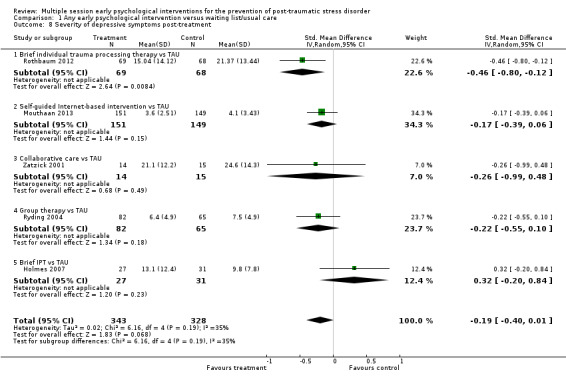
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 8 Severity of depressive symptoms post‐treatment.
When analysed by type of intervention, evidence from one study (137 participants) indicated that brief individual trauma processing therapy may be more effective than treatment as usual (SMD –0.46, 95% CI –0.80 to –0.12). There was a lack of evidence of a difference between treatment as usual and self‐guided Internet‐based CBT (SMD –0.17, 95% CI –0.39 to 0.06; studies = 1; participants = 300), group therapy (SMD –0.22, 95% CI –0.55 to 0.10; studies = 1; participants = 147). There were very wide confidence intervals when comparing treatment as usual with collaborative care (SMD –0.26, 95% CI –0.99 to 0.48; studies = 1; participants = 29), or brief IPT (SMD 0.32, 95% CI –0.20 to 0.84; studies = 1; participants = 58).
One study measured severity of depression using the PHQ‐9 (Biggs 2016). Data were not in a usable format for meta‐analysis. Authors reported that the severity of PTSD symptoms did not differ significantly between the intervention and control group at one or two months' post‐treatment.
Three to six months' follow‐up
Seven studies reported data on severity of depressive symptoms at three to six months' follow‐up (Als 2015; Curtis 2016; Holmes 2007; Irvine 2011; Jensen 2016; Mouthaan 2013; Zatzick 2001). Meta‐analysis indicated no difference between multiple session early psychological interventions and treatment as usual (SMD –0.04, 95% CI –0.19 to 0.10; I2 = 6%; studies = 7; participants = 1009; low‐certainty evidence; Analysis 1.9).
1.9. Analysis.
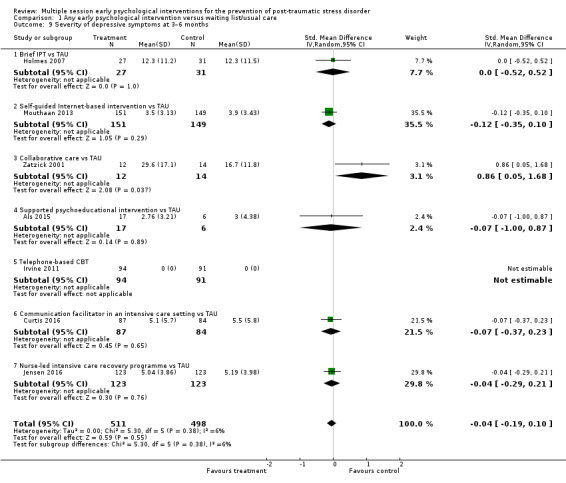
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 9 Severity of depressive symptoms at 3–6 months.
When analysed by type of intervention, there was no statistically significant difference between any specific intervention and treatment as usual.
One study measured depression at six months (Andre 1997). However, SDs were not reported and the study could not be entered in the meta‐analysis. Authors reported that the mean change in HAD score was 3.2 in the intervention group and 3.3 in the control group. There was a large loss to follow‐up in this study; data were only available for 39/65 participants in the intervention group and 45/67 in the control group.
One study measured severity of depression using the PHQ‐9 (Biggs 2016). Data were not in a usable format for meta‐analysis. Authors reported that the severity of PTSD symptoms did not differ significantly between the intervention and control groups at three or four months' post‐treatment.
Seven to 12 months' follow‐up
Three studies reported data on severity of depressive symptoms at seven to 12 months' follow‐up (Irvine 2011; Jensen 2016; Mouthaan 2013). Meta‐analysis indicated no evidence of difference between multiple session early psychological interventions and treatment as usual (SMD 0.01, 95% CI –0.14 to 0.15; I2 = 0%; studies = 3; participants = 745; Analysis 1.10).
1.10. Analysis.
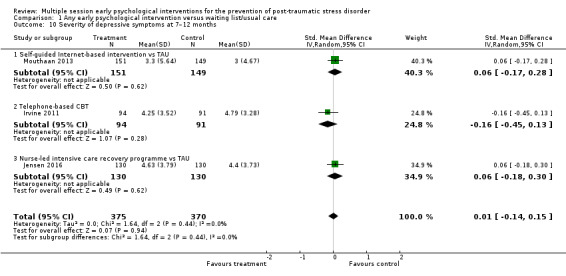
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 10 Severity of depressive symptoms at 7–12 months.
When analysed by type of intervention, there was no difference between any specific intervention and treatment as usual.
One study measured severity of depression at 12 months' follow‐up (Wang 2015). Authors did not report SDs and, therefore, it could not be used in a meta‐analysis. The mean change in depression according to HADS was 3.29 in the treatment and 3.15 in the control group (P = 0.64).
One study measured severity of depression using the PHQ‐9 (Biggs 2016). Data were not in a usable format for meta‐analysis. Authors reported that the severity of PTSD symptoms did not differ significantly between the intervention and control group at seven or 10 months' post‐treatment.
3. Severity of anxiety symptoms
Post‐treatment
Three studies provided data on the severity of anxiety symptoms post‐treatment (Holmes 2007; Kazak 2005; Mouthaan 2013). Meta‐analysis indicated no statistically significant difference between groups in the severity of anxiety symptoms postintervention (SMD –0.41, 95% CI –0.98 to 0.16; I2 = 65%; studies = 3; participants = 358; Analysis 1.11).
1.11. Analysis.
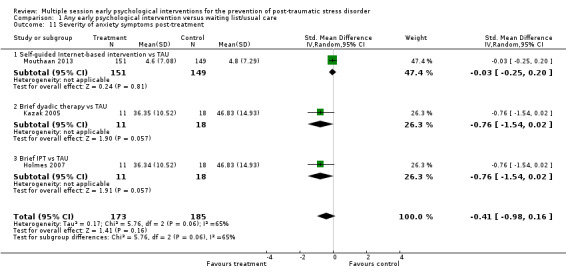
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 11 Severity of anxiety symptoms post‐treatment.
It was not possible to investigate heterogeneity by number of sessions of the intervention, time between trauma exposure and intervention, type of traumatic event or gender of participants. We planned to perform a sensitivity analysis excluding studies at high risk of bias but this was not possible as two studies were at high risk of attrition bias (Holmes 2007; Kazak 2005), and two were at high risk of other bias (Kazak 2005; Mouthaan 2013).
When analysed by type of intervention, there was no statistically significant difference between treatment as usual and self‐guided Internet‐based therapy (SMD –0.03, 95% CI –0.25 to 0.20; I2 = 0%; studies = 1; participants = 300). Although compared with treatment as usual there was evidence of potential benefit for brief dyadic therapy (SMD –0.76, 95% CI –1.54 to 0.02; studies = 1; participants = 29) or brief IPT (SMD –0.76, 95% CI –1.54 to 0.02; studies = 1; participants = 29) confidence intervals were too wide to rule out no difference.
Three to six months' follow‐up
Six studies provided data on the severity of anxiety symptoms at three to six months' follow‐up (Als 2015; Curtis 2016; Irvine 2011; Jensen 2016; Kazak 2005; Mouthaan 2013). Meta‐analysis indicated no difference between multiple session early psychological interventions and treatment as usual (SMD –0.05, 95% CI –0.19 to 0.10; I2 = 2%; studies = 6; participants = 945; low‐certainty evidence; Analysis 1.12).
1.12. Analysis.
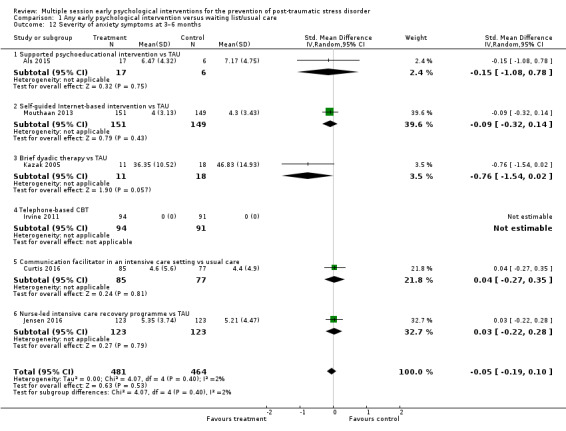
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 12 Severity of anxiety symptoms at 3–6 months.
When analysed by type of intervention, there was no significant difference between any multiple session early psychological treatment and treatment as usual.
One study measured anxiety at six months (Andre 1997). However, SDs were not reported and the study could not be entered in the meta‐analysis. Authors reported that the mean change in HAD score was 6.2 in the intervention group and 6.8 in the control group. There was a large loss to follow‐up in this study; data were only available for 39/65 participants in the intervention group and 45/67 in the control group.
Seven to 12 months' follow‐up
Three studies provided data on the severity of anxiety symptoms at seven to 12 months' follow‐up (Irvine 2011; Jensen 2016; Mouthaan 2013). Meta‐analysis indicated no statistically significant difference between multiple session early psychological interventions and treatment as usual (SMD –0.04, 95% CI –0.27 to 0.18; I2 = 58%; studies = 3; participants = 746; Analysis 1.13).
1.13. Analysis.
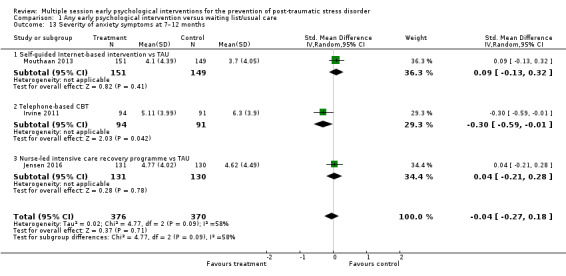
Comparison 1 Any early psychological intervention versus waiting list/usual care, Outcome 13 Severity of anxiety symptoms at 7–12 months.
It was not possible to investigate heterogeneity by number of sessions of the intervention, time between trauma exposure and intervention, type of traumatic event or gender of participants. We planned to perform a sensitivity analysis excluding studies at high risk of bias. However, this was not possible as two of the three studies which measured this outcome at seven to 12 months, were at unclear risk of selection bias (Irvine 2011), unclear risk of detection bias (Jensen 2016), and high risk of attrition bias (Jensen 2016).
When analysed by type of intervention, evidence from one study of 185 participants indicated that telephone‐based CBT may be more effective than treatment as usual (SMD –0.30, 95% CI –0.59 to –0.01). It was unclear if self‐guided Internet‐based CBT (SMD 0.09, 95% CI –0.13 to 0.32; studies = 1; participants = 300), or nurse‐led intensive care diaries (SMD 0.04, 95% CI –0.21 to 0.28; studies = 1; participants = 261) were more or less effective than treatment as usual.
One study measured severity of anxiety at 12 months' follow‐up (Wang 2015). Authors did not report SDs and, therefore, it could not be used in a meta‐analysis. The mean change in anxiety according to HADS was 4.10 in the treatment group and 3.85 in the control group (P = 0.36).
4. Adverse effects
No studies provided data on adverse effects.
5. General functioning
One study measured quality of life using the WHOQOL‐BREF (Biggs 2016). Data were not in a usable format for meta‐analysis. Authors reported that the severity of PTSD symptoms did not differ significantly between the intervention and control groups at one, two, four, seven or 10 months' post‐treatment.
6. Use of health‐related resources
No studies provided data on use of health‐related resources.
Comparison 2: any intervention versus other psychological intervention
Six studies compared a psychological intervention against another active intervention (Cox 2018a; Gamble 2010; Gidron 2001; Gidron 2007; Lindwall 2014; Wijesinghe 2015). However, two of the studies provided no useable data and, therefore, could not be included in the meta‐analyses (Lindwall 2014; Wijesinghe 2015).
Primary outcome
1. PTSD diagnosis
One study measured PTSD diagnosis using the Post‐traumatic Stress Symptom Scale. As this is a self‐reported rather than clinician‐administered tool, this study was not included in the meta‐analysis (Wijesinghe 2015).
Post‐treatment
No study measured PTSD diagnosis post‐treatment.
Three to six months' follow‐up
No study measured PTSD diagnosis post‐treatment using a clinician‐administered tool.
Seven to 12 months' follow‐up
No study measured PTSD diagnosis at seven to 12 months' follow‐up.
2. Dropouts from treatment
Two studies provided data on number of dropouts for any reason,showing participants in early intervention groups were more likely to drop out than active controls (RR 1.61, 95% CI 1.11 to 2.34; I2 = 0%; studies = 2; participants = 425; low‐certainty evidence; Analysis 2.1) (Cox 2018a; Gamble 2010). When analysed by type of intervention, there may be higher drop out for brief individual trauma processing therapy compared with parenting support (RR 1.78, 95% CI 1.12 to 2.84; studies = 1; participants = 262). It was uncertain if there was a difference in drop out between guided self‐help and physical education (RR 1.33, 95% CI 0.71 to 2.50; studies = 1; participants = 163).
2.1. Analysis.
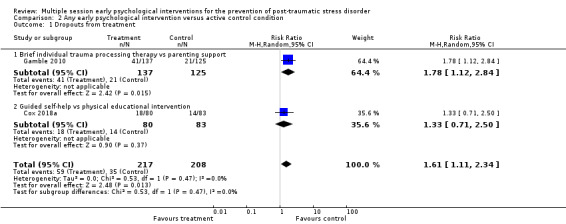
Comparison 2 Any early psychological intervention versus active control condition, Outcome 1 Dropouts from treatment.
Secondary outcomes
1. Severity of PTSD symptoms
Five studies provided data on severity of PTSD symptoms (Cox 2018a; Gamble 2010; Gidron 2001; Gidron 2007; Lindwall 2014), but only four could be entered in a meta‐analysis (Cox 2018a; Gamble 2010; Gidron 2001; Gidron 2007).
Post‐treatment
Two studies provided data on severity of PTSD symptoms post‐treatment (Cox 2018a; Gamble 2010). Meta‐analysis showed a lack of evidence for difference between treatments (SMD 0.13, 95% CI –0.06 to 0.33; I2 = 0%; studies = 2; participants = 392; Analysis 2.2). When analysed by type of intervention, there was imprecision (wide confidence intervals) in estimating differences between brief individual trauma processing therapy and parenting support (SMD 0.14, 95% CI –0.13 to 0.41; studies = 1; participants = 217), or guided self‐help and physical education (SMD 0.13, 95% CI –0.17 to 0.42; studies = 1; participants = 175).
2.2. Analysis.
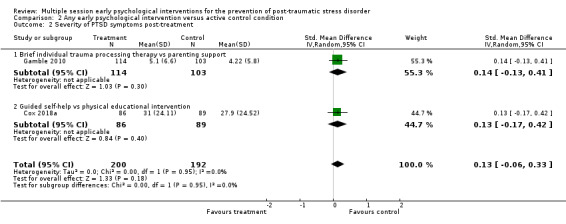
Comparison 2 Any early psychological intervention versus active control condition, Outcome 2 Severity of PTSD symptoms post‐treatment.
Three to six months' follow‐up
Four studies provided data on severity of PTSD symptoms at three to six months' follow‐up (Cox 2018a; Gamble 2010; Gidron 2001; Gidron 2007). There was a wide confidence interval indicating uncertainty on differences between treatments (SMD –0.02, 95% CI –0.31 to 0.26; I2 = 43%; studies = 4; participants = 465; low‐certainty evidence; Analysis 2.3).
2.3. Analysis.
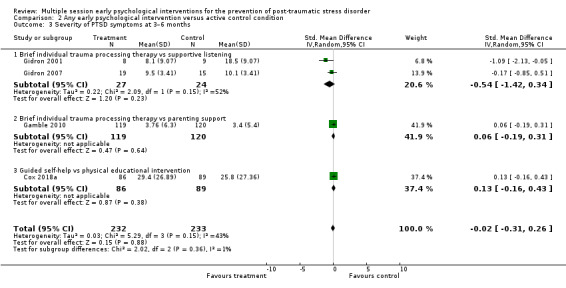
Comparison 2 Any early psychological intervention versus active control condition, Outcome 3 Severity of PTSD symptoms at 3–6 months.
It was not possible to investigate heterogeneity by number of sessions of the intervention, time between trauma exposure and intervention, type of traumatic event or gender of participants. We planned to perform a sensitivity analysis excluding studies at high risk of bias. However, this was not possible as three of the four studies which measured this outcome at three to six months were at unclear risk of selection bias (Gamble 2010; Gidron 2001; Gidron 2007), unclear risk of detection bias (Gamble 2010), and high risk of attrition bias (Gamble 2010; Gidron 2001; Gidron 2007).
When analysed by type of intervention, confidence intervals were wide reflecting a lack of certainty on differences between brief individual trauma processing therapy and supportive listening (SMD –0.54, 95% CI –1.42 to 0.34; I2 = 52%; studies = 2; participants = 51), brief individual trauma processing therapy and parenting support (SMD 0.06, 95% CI –0.19 to 0.31; studies = 1; participants = 239), or guided self‐help and physical education (SMD 0.13, 95% CI –0.16 to 0.43; studies = 1; participants = 175).
One study measured severity of PTSD symptoms in parents of children undergoing stem cell transplantation (Lindwall 2014). A child‐targeted intervention (massage and humour therapy) was compared with a child‐targeted plus a parent‐targeted intervention (massage, relaxation, imagery). Data could not be included in the meta‐analysis. Authors reported no significant differences between interventions (P = 0.45), but that PTSD did decrease significantly from baseline to six months for the two intervention groups as a whole (P < 0.001).
Seven to 12 months' follow‐up
One study provided data on severity of PTSD symptoms at seven to 12 months' follow‐up (Gamble 2010). There was no evidence of a significant difference between brief individual trauma processing therapy and parenting support (MD 1.27, 95% CI –0.60 to 3.14; studies = 1; participants = 200; Analysis 2.4),
2.4. Analysis.
Comparison 2 Any early psychological intervention versus active control condition, Outcome 4 Severity of PTSD symptoms at 7–12 months.
2. Severity of depressive symptoms
Three studies measured severity of depressive symptoms (Cox 2018a; Gamble 2010; Lindwall 2014), but only two provided data that could be used in a meta‐analysis (Cox 2018a; Gamble 2010).
Post‐treatment
Two studies provided data on severity of depressive symptoms post‐treatment (Cox 2018a; Gamble 2010). There was no evidence of a significant difference between treatments (SMD 0.12, 95% CI –0.08 to 0.32; I2 = 0%; studies = 2; participants = 392; Analysis 2.5). When analysed by type of intervention, there was a wide confidence interval when comparing brief individual trauma processing therapy and parenting support (SMD 0.06, 95% CI –0.21 to 0.33; studies = 1; participants = 217) suggesting a lack of certainty on group differences. Although the direction of effect favoured physical education over guided self help the difference wasn't statistically significant (SMD 0.19, 95% CI –0.11 to 0.49; studies = 1; participants = 175).
2.5. Analysis.
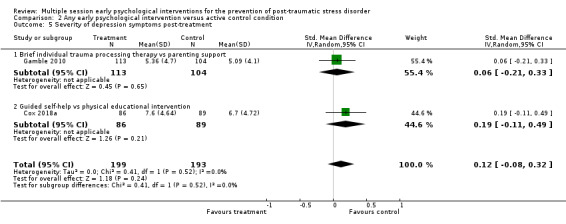
Comparison 2 Any early psychological intervention versus active control condition, Outcome 5 Severity of depression symptoms post‐treatment.
Three to six months' follow‐up
Two studies provided data on severity of depressive symptoms at three to six months' follow‐up (Cox 2018a; Gamble 2010). There was no evidence of a difference between treatments (SMD 0.04, 95% CI –0.16 to 0.23; I2 = 0%; studies = 2; participants = 409; low‐certainty evidence; Analysis 2.6). When analysed by type of intervention, there was no evidence of a difference between brief individual trauma processing therapy and parenting support (SMD 0.09, 95% CI –0.17 to 0.34; studies = 1; participants = 234) or guided self‐help and physical education (SMD –0.04, 95% CI –0.33 to 0.26; studies = 1; participants = 175).
2.6. Analysis.
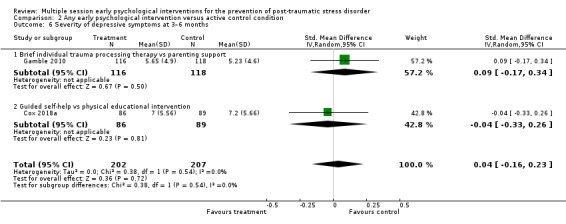
Comparison 2 Any early psychological intervention versus active control condition, Outcome 6 Severity of depressive symptoms at 3–6 months.
One study measured depression in parents of children undergoing stem cell transplantation using the CES‐D scale (Lindwall 2014). Data could not be entered into a meta‐analysis but authors reported no significant differences between groups a child‐targeted intervention (massage and humour therapy) with child‐targeted plus a parent‐targeted (massage, relaxation, imagery) intervention (P = 0.63).
Seven to 12 months' follow‐up
One study provided data on severity of depressive symptoms at seven to 12 months' follow‐up (Gamble 2010). There was no evidence of a difference between brief individual trauma processing therapy and parenting support (MD 0.79, 95% CI –0.66 to 2.24; studies = 1; participants = 198; Analysis 2.7).
2.7. Analysis.
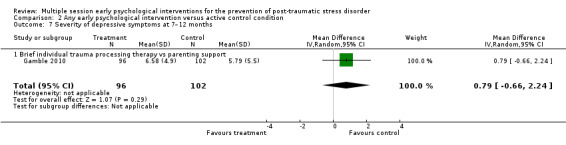
Comparison 2 Any early psychological intervention versus active control condition, Outcome 7 Severity of depressive symptoms at 7–12 months.
3. Severity of anxiety symptoms
Post‐treatment
Two studies provided data on severity of anxiety symptoms post‐treatment (Cox 2018a; Gamble 2010). There was no evidence of a significant difference between treatments (SMD 0.08, 95% CI –0.12 to 0.28; I2 = 0%; studies = 2; participants = 392; Analysis 2.8). When analysed by type of intervention, there was no evidence of a significant difference between brief individual trauma processing therapy and parenting support (SMD 0.10, 95% CI –0.17 to 0.37; studies = 1; participants = 217) or guided self‐help and physical education (SMD 0.05, 95% CI –0.24 to 0.35; studies = 1; participants = 175).
2.8. Analysis.
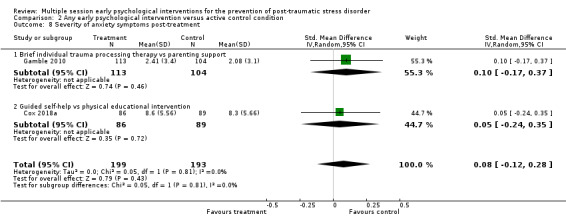
Comparison 2 Any early psychological intervention versus active control condition, Outcome 8 Severity of anxiety symptoms post‐treatment.
Three to six months' follow‐up
Two studies provided data on severity of anxiety symptoms at three to six months' follow‐up (Cox 2018a; Gamble 2010). There was no evidence of a difference between treatments (SMD 0.00, 95% CI –0.19 to 0.19; I2 = 0%; studies = 2; participants = 414; low‐certainty evidence; Analysis 2.9). When analysed by type of intervention, there was no evidence of a difference between brief individual trauma processing therapy and parenting support (SMD 0.03, 95% CI –0.22 to 0.28; studies = 1; participants = 239) or guided self‐help and physical education (SMD –0.04, 95% CI –0.33 to 0.26; studies = 1; participants = 175).
2.9. Analysis.
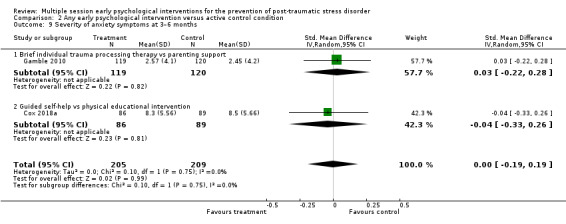
Comparison 2 Any early psychological intervention versus active control condition, Outcome 9 Severity of anxiety symptoms at 3–6 months.
Seven to 12 months' follow‐up
One study provided data on severity of anxiety symptoms at seven to 12 months' follow‐up (Gamble 2010). There was a very wide confidence interval when comparing brief individual trauma processing therapy with parenting support (MD –0.07, 95% CI –1.58 to 1.44; studies = 1; participants = 199; Analysis 2.10).
2.10. Analysis.
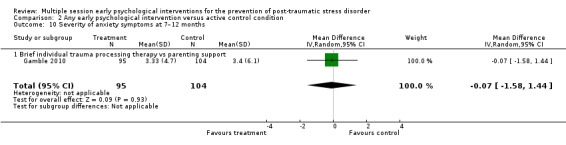
Comparison 2 Any early psychological intervention versus active control condition, Outcome 10 Severity of anxiety symptoms at 7–12 months.
4. Adverse effects
No studies provided data on adverse effects. One study reported interactions between initial traumatic stress symptoms, psychiatric history, past psychiatric diagnosis and IPT intervention in predicting worse PTSD symptoms at six months (Holmes 2007).
5. General functioning
Post‐treatment
One study measured general functioning using the HRQoL (Gamble 2010). There was no difference between counselling aimed at promoting resilience and parenting support (MD –0.02, 95% CI –0.05 to 0.01; studies = 1; participants = 218; Analysis 2.11).
2.11. Analysis.
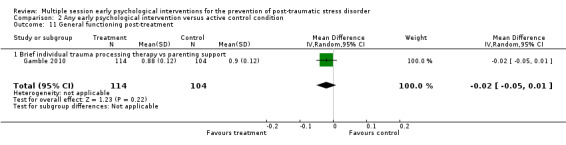
Comparison 2 Any early psychological intervention versus active control condition, Outcome 11 General functioning post‐treatment.
Three to six months' follow‐up
One study measured general functioning at three to six months' post‐treatment (Gamble 2010). There was no significant difference between counselling aimed at promoting resilience and parenting support (MD –0.03, 95% CI –0.06 to 0.00; studies = 1; participants = 239; low‐certainty evidence; Analysis 2.12).
2.12. Analysis.
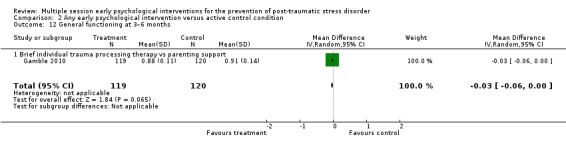
Comparison 2 Any early psychological intervention versus active control condition, Outcome 12 General functioning at 3–6 months.
Seven to 12 months' follow‐up
One study found no difference in general functioning at seven to 12 months' post‐treatment (MD 0.01, 95% CI –0.03 to 0.05; studies = 1; participants = 199; Analysis 2.13) (Gamble 2010).
2.13. Analysis.
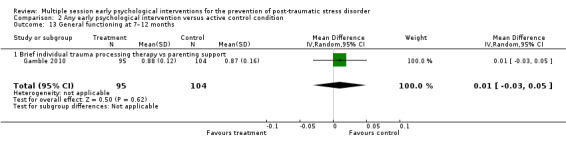
Comparison 2 Any early psychological intervention versus active control condition, Outcome 13 General functioning at 7–12 months.
6. Use of health‐related resources
No studies provided data on use of health‐related resources.
Publication bias
All of the studies identified for this review were published, apart from one (Gamble 2010). Only two comparisons were sufficiently large for us to be able to conduct funnel plots (Analysis 1.6; Analysis 1.4). Visual inspection of these plots suggested that there was no indication of asymmetry (Figure 4; Figure 5). We were unable to investigate the plots by intervention.
4.
Funnel plot of comparison: 1 Any early psychological intervention versus waiting list/usual care, outcome: 1.5 Severity of PTSD symptoms: 3–6 months. TAU: treatment as usual.
5.
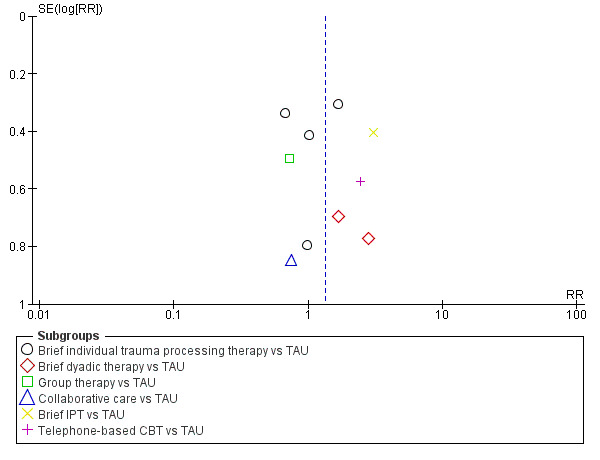
Funnel plot of comparison: 1 Any early psychological intervention versus waiting list/usual care, outcome: 1.13 Dropouts for any reason. CBT: cognitive behavioural therapy; IPT: interpersonal therapy; TAU: treatment as usual.
Discussion
Summary of main results
We identified 27 RCTs (3963 participants) of early multiple session psychological interventions starting within three months of a traumatic event that were designed to prevent traumatic stress symptoms. Twenty‐one studies of 2721 participants provided usable data and were included in a meta‐analysis.
Multiple session early psychological interventions may be more effective than treatment as usual in reducing the number of people diagnosed with PTSD three to six months after receiving the intervention. However, there was no significant difference between the two groups immediately post‐treatment or at seven to 12 months' follow‐up. Evidence was not strong enough to suggest that early psychological interventions may be more effective than treatment as usual in reducing severity of PTSD symptoms, depression and anxiety at any time point. Differences in dropouts were not significant. In terms of specific intervention types, intensive care diaries may be more effective than treatment as usual in reducing the number of people diagnosed with PTSD based on data from one study (Jones 2010), but we found no difference in terms of PTSD severity; self‐guided Internet‐based intervention may be more effective than treatment as usual at reducing PTSD severity based on data from one study (Mouthaan 2013); brief dyadic therapy may be more effective than treatment as usual at reducing PTSD severity based on data from two studies (Brunet 2013; Mouthaan 2013). We found no evidence of effect for any other intervention.
There were no significant differences between multiple session early psychological interventions and active control interventions in terms of PTSD diagnosis, reducing the severity of depression and anxiety, or improving general functioning of the recipient either postintervention or at three to 12 months' follow‐up. However, participants who received multiple session interventions were more likely to stop the intervention early.
Overall completeness and applicability of evidence
The studies included in this review directly addressed the primary review question. It was possible to perform meta‐analyses of RCTs of multiple session psychological interventions aimed at preventing PTSD in individuals who had been exposed to a traumatic event but had not been identified as experiencing any specific psychological difficulties. However, meta‐analyses could not be performed for all outcomes and the limited number of studies, their small sample sizes and heterogeneity (see below) complicated interpretation. The question of how interventions fare against another psychological interventions could only be answered for memory structuring and not for other interventions. The number of new studies identified in our most recent search (see Characteristics of studies awaiting classification table) suggests that this is a quickly moving field, particularly in terms of early psychological intervention in medical settings and that a further updated review will be required in the near future.
Given the prominence of cognitive behavioural interventions in the evidence base for psychological therapies it is surprising that only three of the studies identified in this review evaluated a CBT intervention (Andre 1997; Irvine 2011; Rothbaum 2012), although another seven studies included CBT components in their interventions (Brunet 2013; Cox 2018a; Kazak 2005; Marchand 2006; Mouthaan 2013; Wijesinghe 2015; Zatzick 2001). Andre 1997 reported a significant decrease in intrusive symptoms and anxiety in their treatment group. Unfortunately, data provided in their paper did not permit inclusion in a meta‐analysis. Brief individual trauma processing therapies which were based on several different theoretical models but shared active treatment components were the most frequently evaluated interventions. The evidence reviewed did not provide any support for this approach as a preventive intervention.
Participants in the studies included in the review were exposed to single individual traumatic events and there were no studies of larger‐scale traumatic events such as disasters or wars, which limits the external validity of the results across the full range of traumatic events.
Unfortunately, other than the studies where participants were critically ill and at high risk of mortality (Irvine 2011; Jensen 2016; Jones 2010), only one study considered adverse effects (Holmes 2007), and it was unclear whether or not any adverse effects occurred in the other studies. The absence of tolerability assessment (an evaluation of the extent to which patients can endure the unwanted adverse effects of an intervention) is a key shortcoming in the RCTs identified and one that has previously been noted in psychological treatment studies of chronic PTSD (Bisson 2007).
Quality of the evidence
Heterogeneity
There was evidence of both clinical and statistical heterogeneity in the included studies. Although all the trials attempted to prevent PTSD symptoms, the nature of the interventions included in the meta‐analyses was quite diverse. The interventions included in the primary meta‐analysis were two sessions of brief dyadic CBT (Brunet 2013), two sessions of counselling (one face‐to‐face and one by telephone) (Gamble 2005), two sessions of adapted critical incident stress debriefing (Marchand 2006), sharing of an intensive care diary (Jones 2010), brief self‐guided Internet‐based intervention (Mouthaan 2013), and three sessions of modified prolonged exposure (Rothbaum 2012). It was very difficult to compare such trials and there did appear to be some differences in outcomes, for example Jones 2010 appeared to be more effective than Marchand 2006.
There were also differences in the clinical populations which included motor vehicle accident victims, victims of armed robbery or aggression, military mortuary attendants, people in ICUs, women who had emergency caesarean sections or had premature babies, parents of children who were in ICU or had recently been diagnosed with cancer. Unfortunately, the limited number of trials meant that sensitivity analyses could not be performed in a meaningful way to explore these issues further. Statistical heterogeneity was apparent in several analyses, the I2 value demonstrating inconsistencies in the outcomes of some trials that were grouped. When there was statistical heterogeneity, we used a random‐effects model as opposed to a fixed‐effect model to calculate more conservative CIs. We concluded that all trials were essentially trying to measure the same outcome and that it was worthwhile summarising their combined results, but the variation meant that caution should be applied when interpreting the results (Fletcher 2007).
Methodological certainty
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome, and to draw conclusions about the certainty of evidence within the text of the review. One of the main considerations for downgrading GRADE judgements was risk of bias. Concerns over the certainty of the evidence also limit the extent to which conclusions can be generalised. For details of the risk of bias judgements for each study, see the Characteristics of included studies table and the graphical representation of risk of bias presented in Figure 2 and Figure 3.
There were several issues that were problematic in several studies including the randomisation process, incomplete reporting of dropouts and absence of a manualised, replicable specific treatment. As with all psychological treatment trials there are issues with the control groups. This is particularly important in early intervention research where a reduction in symptoms over the duration of the trial would be expected, given the natural course of traumatic stress reactions. The development of a psychological treatment placebo is very difficult, if not impossible, as is blinding of participants and therapists. Some of the wait list/usual care groups may have received some form of intervention by virtue of contact through symptom monitoring, but this was not properly evaluated and it is not possible to determine what, if any, impact on outcomes this would have had. The four studies that did have an active control group showed no difference between that and multiple session early psychological interventions (Cox 2018a; Gamble 2010; Gidron 2001; Gidron 2007).
The sample sizes of most of the studies were also an important limitation. However, the intervention and control groups in most studies appeared well matched at baseline, reducing the risk of the reported unadjusted mean outcomes being influenced by baseline differences. We had intended to undertake subgroup analyses investigating heterogeneity in terms of the type of trauma and the number of sessions of intervention, but there were not enough studies for us to do this.
Potential biases in the review process
This review adhered strictly to the Cochrane guidelines (Higgins 2011). Two review authors independently screened the abstracts identified by the literature search; read all potentially relevant studies; assessed each study against the inclusion criteria; extracted data from the written reports and rated each study for risk of bias. We discussed any disagreements with a third review author, and reached unanimous decisions for inclusion and classification. We carefully followed guidelines set out by Cochrane on statistical methods and used GRADE to assess the certainty of evidence (Higgins 2011). Following these procedures minimised the potential for bias, but some unavoidable issues remained.
All studies included in the review apart from Gamble 2010 were published, which led to the possibility of publication bias. We were unable to undertake investigate for possible publication bias for our primary outcome, but we were able to generate funnel plots for two comparisons and found no evidence to suggest that publication bias was indicated.
We systematically searched numerous online databases for potentially relevant studies, and scrutinised reference lists of included studies. We also contacted experts in the field requesting help to identify missed studies or ongoing work. Nonetheless, we could not fully eliminate the possibility of overlooked RCTs.
There was considerable statistical heterogeneity in four of the pooled comparisons. In circumstances where heterogeneity was thought to be potentially problematic, we used a random‐effects model. Heterogeneity was also a factor that was taken into consideration in downgrading the certainty of the evidence with GRADE.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the only specific systematic review of multiple session interventions to prevent PTSD. The results are consistent with the findings of the previous version of this review (Roberts 2009). Our findings are also consistent with other systematic reviews that have included studies in this area (NICE 2018), and also with prevailing guidance regarding how best to respond following a traumatic event (ACPMH 2007; NICE 2018).
Authors' conclusions
Implications for practice.
These results suggest that at this time there is very limited evidence to support the use of psychological intervention for routine use following traumatic events. We found some contradictory evidence for the use of intensive care diaries in intensive care units and some evidence to suggest the benefit of brief dyadic cognitive behavioural therapy (CBT)‐based approaches and self‐guided Internet‐based intervention, but we did not feel that the available evidence was strong enough to recommend regular use of these interventions without further evaluation.
Implications for research.
The number of ongoing studies reflects that this a fast moving field of research. Future updates of this review will incorporate the results of these studies currently underway. Further well‐designed randomised controlled trials of interventions that appeared to show promise could be subjected to further evaluation (e.g. Brunet 2013; Jones 2010; Kazak 2005; Mouthaan 2013). However, it may be that these interventions would demonstrate greater effect when targeted at individuals who are symptomatic (Roberts 2010), given that many individuals will gradually recover without the need of any intervention (Bryant 2013). We note that preventive cognitive behavioural interventions have not been adequately investigated. However, the absence of effect for brief individual trauma processing therapies, which share some features of trauma‐focused cognitive behavioural therapies, suggests that models that are not targeted on those with traumatic stress symptoms may not be the most fruitful means of intervention delivery. Current evidence favours approaches that focus intervention on people who are symptomatic or have been diagnosed with acute stress disorder or acute post‐traumatic stress disorder (PTSD) (Roberts 2010). Given the positive findings for brief dyadic therapy, it would be of interest to evaluate forms of familial and community intervention and interventions aimed at improving coping skills and enhancing positive and helpful behaviours (Ruzek 2007). Internet‐ and app‐based interventions potentially offer relatively cheap and accessible ways of offering interventions to large numbers of people and should be investigated further. The findings of Mouthaan 2013 demonstrate some support for this approach and there are now a number of studies that have demonstrated the effectiveness of Internet‐based CBT for PTSD (Lewis 2018). Future research should also explore the optimal time to intervene, consider adverse events and tolerability of treatment, and carefully control for additional intervention.
What's new
| Date | Event | Description |
|---|---|---|
| 7 August 2019 | New citation required and conclusions have changed | Review updated. New studies added. |
| 7 August 2019 | New search has been performed | We updated the searches on 3 March 2018; we identified 16 new studies. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 24 February 2010 | Amended | Minor changes to contact details of three authors (including contact author) |
| 17 February 2010 | Amended | Search strategy amended; review link updated; contact author's email address updated; co‐author's name and title corrected |
| 1 February 2009 | Amended | Converted to new review format. |
| 1 October 2008 | Amended | The decision was taken to split the research question proposed in the original protocol 'Multiple session early psychological interventions for prevention and treatment of post‐traumatic stress disorder' into two reviews (one on prevention, and one on treatment). The title of this review changed at that time. The original protocol remains on the Cochrane Library until such time as the review on treatment is published |
Acknowledgements
We wish to express our thanks to Melissa Alderfer, Randall Curtis, Jessica Ford, Jenny Gamble, Elena Garralda, Janet Jensen, Christina Jones, Joanne Mouthaan, Elsa Lena Ryding and Doug Zatzick for providing additional unpublished data. We also thank Sarah Dawson for her help with searches. Finally, thanks to those experts who responded with additional suggestions and contacts during the search process.
The authors and the Cochrane Common Mental Disorders Editorial Team, are grateful to the following peer reviewers and editors for their time and comments: Bradley Belsher, Nick Meader, Karen Morley, and Barbara Rothbaum. They would also like to thank copy editor, Anne Lawson.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. CCMDCTR core MEDLINE search
The search strategy listed below is the weekly Ovid Medline search which was used to inform the Group's specialised register to June 2016. It is based on a list of terms for all conditions within the scope of the Cochrane Common Mental Disorders Group plus a sensitive RCT filter.
Ovid MEDLINE search strategy, used to inform the Cochrane Common Mental Disorders Group's Specialised Register A weekly search alert based on condition + RCT filter only 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/ 2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf. 3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.) 4. (1 and 2 and 3)
Records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs were tagged to the appropriate study record. Similar weekly search alerts were also conducted on OVID Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 2. Database searches (March 2018/2019)
Date of search: 3 March 2018 Date limits: 2014 onwards Database hits:
MEDLINE (1742)
Embase (3319)
CENTRAL (2028)
PsycINFO (1449)
PILOTS (879)
Total=9417 Duplicates removed=4620 Studies screened for RCTs=4797 Records excluded=3632
RCT records identified=1165
Databases: CENTRAL Host: Wiley interface Data Parameters: Cochrane Central Register of Controlled Trials : Issue 2 of 12, February 2018 Date Searched: Monday, March 3rd 2018 Searched by: Chris Cooper Hits: 2028 IDSearchHits #1 MeSH descriptor: [Stress Disorders, Post‐Traumatic] this term only (1492) #2 (PTSD or ((posttrauma* or post‐trauma* or post trauma*) near/3 (stress* or disorder* or psych* or symptom*)) or acute stress disorder* or combat disorder* or war neuros*) (5065) #3 (((acute or traumatic) near/1 stress*) and (expos* or psyc*)) (1525) #4 (traumatised near/1 (victim* or survivor*)) 2 #5 (traumatized near/1 (victim* or survivor*)) 4 #6 (trauma* near/2 (event* or memor* or flashback* or nightmare*)) 553 #7 ((trauma* or posttrauma* or post‐trauma* or victim* or survivor*) and (exposure near/3 (therap* or psychotherap* or training or counsel*))) 417 #8 MeSH descriptor: [Crisis Intervention] this term only 166 #9 (critical incident near/1 (stress or debrief* or de‐brief*)) 24 #10 (debriefing or de‐briefing) 328 #11 (crisis intervention* or CISD) 1003 #12 ((stress or group* or psychological or crisis) near/3 (debrief* or de‐brief*)) 107 #13 (trauma* near/2 (event* or memor* or flashback* or nightmare*)) 553 #14 (EMDR or (eye movement desensitization and reprocessing)) 225 #15 (EMDR or (eye movement desensitisation and reprocessing)) 197 #16 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15) Publication Year from 2014 to 2018 (2893) File: VO1 CENTRAL n2028.txt
Databases: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present Host: OVID Data Parameters: 1946‐Current Date Searched: Monday, March 3rd 2018 Searched by: Chris Hits: 1742 #SearchesResults 1 Stress Disorders, Post‐Traumatic/ 27503 2 (PTSD or ((posttrauma* or post‐trauma* or post trauma*) adj3 (stress* or disorder* or psych* or symptom?)) or acute stress disorder* or combat disorder* or war neuros*).ti,ab,kf. 31111 3 (((acute or traumatic) adj stress*) and (expos* or psyc*)).ti,ab,kf. 10567 4 (traumati#ed adj (victim? or survivor?)).ti,ab,kf. 34 5 (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab,kf. 8174 6 ((trauma* or posttrauma* or post‐trauma* or victim* or survivor?) and (exposure adj3 (therap* or psychotherap* or training or counsel*))).ti,ab,kf,hw. 901 7 Crisis Intervention/ 5457 8 (critical incident adj (stress or debrief* or de‐brief*)).ti,ab,kf. 223 9 (debriefing or de‐briefing).ti,kf. 577 10 (crisis intervention? or CISD).ti,ab,kf.1744 11 ((stress or group? or psychological or crisis) adj3 (debrief* or de‐brief*)).ti,ab,kf. 406 12 (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,kf. 1150 13 (EMDR or (eye movement desensiti#ation and reprocessing)).ti,ab,kf,sh. 510 14 (1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13) 52168 15 randomized controlled trial.pt. 454849 16 controlled clinical trial.pt. 92204 17 randomized.ab. 404382 18 placebo.ab. 186843 19 clinical trials as topic.sh. 182777 20 randomly.ab. 285994 21 trial.ti. 178689 22 (15 or 16 or 17 or 18 or 19 or 20 or 21) 1136215 23 (14 and 22) 4000 24 (2014* or 2015* or 2016* or 2017* or 2018*).yr,dt,ed,ep. 5444042 25 (23 and 24) 1742
Databases: Embase Host: OVID Data Parameters: 1974 to 2018 March 02 Date Searched: Monday, March 3rd 2018 Searched by: Chris Cooper Hits: 3319 #Searches Results 1 posttraumatic stress disorder/ 48854 2 "trauma and stressor related disorders"/ 34962 3 combat disorders/ 26663 4 psychological trauma/ 5351 5 stress disorders, post‐traumatic/ 16743 6 stress disorders, traumatic, acute/ 751 7 (PTSD or ((posttrauma* or post‐trauma* or post trauma*) adj3 (stress* or disorder* or psych* or symptom?)) or acute stress disorder* or combat disorder* or war neuros*).ti,ab,kw. 39945 8 (((acute or traumatic) adj stress*) and (expos* or psyc*)).ti,ab,kw. 15122 9 (traumati#ed adj (victim? or survivor?)).ti,ab,kw. 51 10 (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab,kw. 10514 11 (EMDR or (eye movement desensiti#ation and reprocessing)).ti,kw. 527 12 ((trauma* or posttrauma* or post‐trauma* or victim* or survivor?) and (exposure adj3 (therap* or psychotherap* or training or counsel*))).ti,ab,kw. 1096 13 (critical incident adj (stress or debrief* or de‐brief*)).ti,ab,kw. 275 14 (debriefing or de‐briefing).ti,ab,kw. 4133 15 (crisis intervention? or CISD).ti,ab,kw. 2273 16 ((stress or group? or psychological or crisis) adj3 (debrief* or de‐brief*)).ti,ab,kw. 602 17 (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab,kw. 10514 18 (1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17) 74063 19 crossover‐procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/ or (random* or factorial* or crossover* or cross over* or placebo* or (doubl* adj blind*) or (singl* adj blind*) or assign* or allocat* or volunteer*).tw. 1970074 20 (18 and 19) 7601 21 (2014* or 2015* or 2016* or 2017* or 2018*).yr,dc. 7084132 22 (20 and 21) 3319
Databases: PsycINFO Host: OVID Data Parameters: 1806 to February Week 4 2018 Date Searched: Monday, March 3rd 2018 Searched by: Chris Hits: 1449 #Searches Results 1 posttraumatic stress disorder/ or complex ptsd/ or desnos/ or acute stress disorder/ or combat experience/ or "debriefing (psychological)"/ or emotional trauma/ or post‐traumatic stress/ or exp stress reactions/ or traumatic neurosis/ 50806 2 exp disasters/ 8186 3 (PTSD or ((posttrauma* or post‐trauma* or post trauma*) adj3 (stress* or disorder* or psych* or symptom?)) or acute stress disorder* or combat disorder* or war neuros*).ti,ab. 38985 4 (((acute or traumatic) adj stress*) and (expos* or psyc*)).ti,ab. 16755 5 (traumati#ed adj (victim? or survivor?)).ti,ab. 68 6 (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab. 11819 7 (EMDR or (eye movement desensiti#ation and reprocessing)).ti,ab. 1640 8 ((trauma* or posttrauma* or post‐trauma* or victim* or survivor?) and (exposure adj3 (therap* or psychotherap* or training or counsel*))).ti,ab. 1086 9 crisis intervention/ 3314 10 (critical incident adj (stress or debrief* or de‐brief*)).ti,ab. 443 11 (debriefing or de‐briefing).ti,ab. 2186 12 (crisis intervention? or CISD).ti,ab. 3505 13 ((stress or group? or psychological or crisis) adj3 (debrief* or de‐brief*)).ti,ab. 596 14 (trauma* adj2 (event? or memor* or flashback* or nightmare?)).ti,ab. 11819 15 (1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14) 80813 16 clinical trials.sh. 10820 17 (randomi#ed or randomi#ation or randomi#ing).ti,ab,id. 72509 18 (RCT or at random or (random* adj3 (assign* or allocat* or control* or crossover or cross‐over or design* or divide* or division or number))).ti,ab,id. 82020 19 (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,id,hw.25590 20 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,id. 24054 21 trial.ti. 25583 22 placebo.ti,ab,id,hw. 37267 23 treatment outcome.md. 18762 24 treatment effectiveness evaluation.sh. 21858 25 mental health program evaluation.sh. 2028 26 (16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25) 169119 27 (15 and 26) 4124 28 (2014* or 2015* or 2016* or 2017* or 2018*).yr,dc,mo. 782907 29 (27 and 28) 1449
Database: PILOTS: Published International Literature On Traumatic Stress Host: Pro Quest Data Parameters: 1871‐Current Date Searched: Monday, March 3rd 2018 Searched by: Chris Hits: 879 Search Strategy Set#: S1 Searched for: ti((posttrauma* near/4 (stress* or disorder* or psych* or symptom*))) OR ab((posttrauma* near/4 (stress* or disorder* or psych* or symptom*))) Results: 16999* Set#: S2 Searched for: ti((post‐trauma* near/4 (stress* or disorder* or psych* or symptom*))) OR ab((post‐trauma* near/4 (stress* or disorder* or psych* or symptom*))) Results: 6647° Set#: S3 Searched for: ti((post trauma* near/4 (stress* or disorder* or psych* or symptom*))) OR ab((post trauma* near/4 (stress* or disorder* or psych* or symptom*))) Results: 7214° Set#: S4 Searched for: ti((PTSD or acute stress disorder* or combat disorder* or war neuros*) ) OR ab((PTSD or acute stress disorder* or combat disorder* or war neuros*) ) Results: 30435* Set#: S5 Searched for: ti((((acute or traumatic) near/2 stress*) and (expos* or psyc*)) ) OR ab((((acute or traumatic) near/2 stress*) and (expos* or psyc*)) ) Results: 2341° Set#: S6 Searched for: ti((traumatised near/2 (victim* or survivor*)) ) OR ab((traumatised near/2 (victim* or survivor*)) ) Results: 84° Set#: S7 Searched for: ti((trauma* near/3 (event* or memor* or flashback* or nightmare*)) ) OR ab((trauma* near/3 (event* or memor* or flashback* or nightmare*)) ) Results: 6974° Set#: S8 Searched for: ti(((trauma* or posttrauma* or post‐trauma* or victim* or survivor*) and (exposure near/4 (therap* or psychotherap* or training or counsel*))) ) OR ab(((trauma* or posttrauma* or post‐trauma* or victim* or survivor*) and (exposure near/4 (therap* or psychotherap* or training or counsel*))) ) Results: 787° Set#: S9 Searched for: ti((critical incident near/2 (stress or debrief* or de‐brief*)) ) OR ab((critical incident near/2 (stress or debrief* or de‐brief*)) ) Results: 385° Set#: S10 Searched for: ti((debriefing or de‐briefing)) OR ab((debriefing or de‐briefing)) Results: 685° Set#: S11 Searched for: ti((crisis intervention* or CISD)) OR ab((crisis intervention* or CISD)) Results: 784° Set#: S12 Searched for: ti(((stress or group* or psychological or crisis) near/4 (debrief* or de‐brief*)) ) OR ab(((stress or group* or psychological or crisis) near/4 (debrief* or de‐brief*)) ) Results: 464° Set#: S13 Searched for: ti((trauma* near/3 (event* or memor* or flashback* or nightmare*)) ) OR ab((trauma* near/3 (event* or memor* or flashback* or nightmare*)) ) Results: 6974° Set#: S14 Searched for: ti((EMDR or (eye movement desensitisation and reprocessing))) OR ab((EMDR or (eye movement desensitisation and reprocessing))) Results: 888° Set#: S15 Searched for: ti((EMDR or (eye movement desensitiZation and reprocessing))) OR ab((EMDR or (eye movement desensitiZation and reprocessing))) Results: 888° Set#: S16 Searched for: (s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15) Results: 36840* Set#: S17 Searched for: MAINSUBJECT.EXACT("Randomized Clinical Trial") Results: 1210° Set#: S18 Searched for: ab((randomized or randomised or placebo or randomly)) Results: 2931° Set#: S19 Searched for: ti(trial) Results: 784° Set#: S20 Searched for: (S17 or S18 or S19) Results: 3226° Set#: S21 Searched for: S16 and s20 Results: 2654° Set#: S22 Searched for: (S16 and s20) AND pd(20140101‐20180301) Results: 879° * Duplicates are removed from your search, but included in your result count. ° Duplicates are removed from your search and from your result count.
*********************************************************************************************************************
PTSD update search (15 March 2019):
CLib:CENTRAL (Issue 3 of 12, March 2019, date limited 2018 onwards), n=514 (116 of these are from ClinicalTrials.gov)
Ovid MEDLINE (2018 to 15‐Mar‐2019), n=599
Ovid Embase (2018 to 15‐Mar‐2019), n=1035
Ovid PsycINFO (2018 to 15‐Mar‐2019), n=445
Proquest PTSDpubs, (2018‐03‐01 to 2019‐03‐15) n=197
Total=2790 Duplicates removed, n=1178 Records to screen, n=1612 RCTs, n=781 Reviews, n=157 Irrelevant, n=674
Data and analyses
Comparison 1. Any early psychological intervention versus waiting list/usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PTSD diagnosis post‐treatment | 5 | 556 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.85, 1.32] |
| 1.1 Brief individual trauma processing therapy vs treatment as usual (TAU) | 3 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.87, 1.40] |
| 1.2 Self‐guided Internet‐based intervention vs TAU | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.40, 2.06] |
| 1.3 Brief dyadic cognitive‐behavioural intervention vs TAU | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.21, 1.61] |
| 2 PTSD diagnosis 3–6 months | 5 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.41, 0.93] |
| 2.1 Brief individual trauma processing therapy vs TAU | 3 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.44, 1.22] |
| 2.2 Self‐guided Internet‐based intervention vs TAU | 1 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.18, 1.45] |
| 2.3 Intensive care diaries vs delayed intensive care diaries | 1 | 322 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.17, 0.82] |
| 3 PTSD diagnosis 7–12 months | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.20, 4.49] |
| 3.1 Self‐guided Internet‐based intervention vs TAU | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.20, 4.49] |
| 4 Dropouts from treatment | 11 | 1154 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.91, 1.95] |
| 4.1 Brief individual trauma processing therapy vs TAU | 5 | 571 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.67, 1.68] |
| 4.2 Brief dyadic therapy vs TAU | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [0.76, 5.75] |
| 4.3 Group therapy vs TAU | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.27, 1.89] |
| 4.4 Collaborative care vs TAU | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.14, 3.94] |
| 4.5 Brief IPT vs TAU | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [1.39, 6.75] |
| 4.6 Telephone‐based CBT vs TAU | 1 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [0.79, 7.44] |
| 5 Severity of PTSD symptoms post‐treatment | 9 | 1326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.29, 0.12] |
| 5.1 Brief individual trauma processing therapy vs TAU | 4 | 465 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.34, 0.42] |
| 5.2 Self‐guided Internet‐based intervention vs TAU | 1 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.61, ‐0.15] |
| 5.3 Group counselling vs TAU | 1 | 147 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.41, 0.24] |
| 5.4 Collaborative care vs TAU | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.24, 0.25] |
| 5.5 Intensive care diaries vs delayed access to intensive care diaries | 1 | 330 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.44, ‐0.01] |
| 5.6 Three‐step early intervention vs TAU | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.20, 0.86] |
| 6 Severity of PTSD symptoms: 3–6 months | 15 | 1921 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.22, 0.02] |
| 6.1 Brief individual trauma processing therapy vs TAU | 4 | 466 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.41, 0.30] |
| 6.2 Self‐guided Internet‐based intervention vs TAU | 1 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.50, ‐0.04] |
| 6.3 Brief dyadic therapy vs TAU | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.81, ‐0.02] |
| 6.4 Collaborative care vs TAU | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.37, 1.19] |
| 6.5 Brief interpersonal therapy (IPT) vs TAU | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.42, 0.61] |
| 6.6 Intensive care diaries vs delayed access to intensive care diaries | 1 | 322 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.22, 0.22] |
| 6.7 Three‐step early intervention vs TAU | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.61, 0.45] |
| 6.8 Telephone‐based cognitive behavioural therapy (CBT) vs TAU | 1 | 185 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.49, 0.09] |
| 6.9 Supported psychoeducation vs TAU | 1 | 23 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.61, 0.30] |
| 6.10 Communication facilitator in an intensive care setting vs usual care | 1 | 168 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.29, 0.32] |
| 6.11 Nurse‐led intensive care recovery programme vs TAU | 1 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.29, 0.25] |
| 7 Severity of PTSD symptoms: 7–12 months | 4 | 765 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.32, 0.14] |
| 7.1 Self‐guided Internet‐based intervention vs wait list/TAU | 1 | 300 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 7.2 Three‐step early intervention vs TAU | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.71, 0.35] |
| 7.3 Telephone‐based CBT | 1 | 185 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.67, ‐0.09] |
| 7.4 Nurse‐led intensive care recovery programme vs TAU | 1 | 225 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.14, 0.38] |
| 8 Severity of depressive symptoms post‐treatment | 5 | 671 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.40, 0.01] |
| 8.1 Brief individual trauma processing therapy vs TAU | 1 | 137 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.80, ‐0.12] |
| 8.2 Self‐guided Internet‐based intervention vs TAU | 1 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.39, 0.06] |
| 8.3 Collaborative care vs TAU | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.99, 0.48] |
| 8.4 Group therapy vs TAU | 1 | 147 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.55, 0.10] |
| 8.5 Brief IPT vs TAU | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.20, 0.84] |
| 9 Severity of depressive symptoms at 3–6 months | 7 | 1009 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.10] |
| 9.1 Brief IPT vs TAU | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.52, 0.52] |
| 9.2 Self‐guided Internet‐based intervention vs TAU | 1 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.35, 0.10] |
| 9.3 Collaborative care vs TAU | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 0.86 [0.05, 1.68] |
| 9.4 Supported psychoeducational intervention vs TAU | 1 | 23 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [1.00, 0.87] |
| 9.5 Telephone‐based CBT | 1 | 185 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 Communication facilitator in an intensive care setting vs TAU | 1 | 171 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.37, 0.23] |
| 9.7 Nurse‐led intensive care recovery programme vs TAU | 1 | 246 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.29, 0.21] |
| 10 Severity of depressive symptoms at 7–12 months | 3 | 745 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.14, 0.15] |
| 10.1 Self‐guided Internet‐based intervention vs TAU | 1 | 300 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.17, 0.28] |
| 10.2 Telephone‐based CBT | 1 | 185 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.45, 0.13] |
| 10.3 Nurse‐led intensive care recovery programme vs TAU | 1 | 260 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.18, 0.30] |
| 11 Severity of anxiety symptoms post‐treatment | 3 | 358 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.98, 0.16] |
| 11.1 Self‐guided Internet‐based intervention vs TAU | 1 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.25, 0.20] |
| 11.2 Brief dyadic therapy vs TAU | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.54, 0.02] |
| 11.3 Brief IPT vs TAU | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.54, 0.02] |
| 12 Severity of anxiety symptoms at 3–6 months | 6 | 945 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.19, 0.10] |
| 12.1 Supported psychoeducational intervention vs TAU | 1 | 23 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐1.08, 0.78] |
| 12.2 Self‐guided Internet‐based intervention vs TAU | 1 | 300 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.32, 0.14] |
| 12.3 Brief dyadic therapy vs TAU | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.54, 0.02] |
| 12.4 Telephone‐based CBT | 1 | 185 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 Communication facilitator in an intensive care setting vs usual care | 1 | 162 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.27, 0.35] |
| 12.6 Nurse‐led intensive care recovery programme vs TAU | 1 | 246 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.22, 0.28] |
| 13 Severity of anxiety symptoms at 7–12 months | 3 | 746 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.27, 0.18] |
| 13.1 Self‐guided Internet‐based intervention vs TAU | 1 | 300 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.13, 0.32] |
| 13.2 Telephone‐based CBT | 1 | 185 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.59, ‐0.01] |
| 13.3 Nurse‐led intensive care recovery programme vs TAU | 1 | 261 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.21, 0.28] |
Comparison 2. Any early psychological intervention versus active control condition.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts from treatment | 2 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.11, 2.34] |
| 1.1 Brief individual trauma processing therapy vs parenting support | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.12, 2.84] |
| 1.2 Guided self‐help vs physical educational intervention | 1 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.71, 2.50] |
| 2 Severity of PTSD symptoms post‐treatment | 2 | 392 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.06, 0.33] |
| 2.1 Brief individual trauma processing therapy vs parenting support | 1 | 217 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.13, 0.41] |
| 2.2 Guided self‐help vs physical educational intervention | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.17, 0.42] |
| 3 Severity of PTSD symptoms at 3–6 months | 4 | 465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.31, 0.26] |
| 3.1 Brief individual trauma processing therapy vs supportive listening | 2 | 51 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.42, 0.34] |
| 3.2 Brief individual trauma processing therapy vs parenting support | 1 | 239 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.19, 0.31] |
| 3.3 Guided self‐help vs physical educational intervention | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.16, 0.43] |
| 4 Severity of PTSD symptoms at 7–12 months | 1 | 200 | Mean Difference (IV, Random, 95% CI) | 1.27 [‐0.60, 3.14] |
| 4.1 Brief individual trauma processing therapy vs parenting support | 1 | 200 | Mean Difference (IV, Random, 95% CI) | 1.27 [‐0.60, 3.14] |
| 5 Severity of depression symptoms post‐treatment | 2 | 392 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.08, 0.32] |
| 5.1 Brief individual trauma processing therapy vs parenting support | 1 | 217 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.21, 0.33] |
| 5.2 Guided self‐help vs physical educational intervention | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.11, 0.49] |
| 6 Severity of depressive symptoms at 3–6 months | 2 | 409 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.16, 0.23] |
| 6.1 Brief individual trauma processing therapy vs parenting support | 1 | 234 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.17, 0.34] |
| 6.2 Guided self‐help vs physical educational intervention | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.33, 0.26] |
| 7 Severity of depressive symptoms at 7–12 months | 1 | 198 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐0.66, 2.24] |
| 7.1 Brief individual trauma processing therapy vs parenting support | 1 | 198 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐0.66, 2.24] |
| 8 Severity of anxiety symptoms post‐treatment | 2 | 392 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.12, 0.28] |
| 8.1 Brief individual trauma processing therapy vs parenting support | 1 | 217 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.17, 0.37] |
| 8.2 Guided self‐help vs physical educational intervention | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.24, 0.35] |
| 9 Severity of anxiety symptoms at 3–6 months | 2 | 414 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.19, 0.19] |
| 9.1 Brief individual trauma processing therapy vs parenting support | 1 | 239 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.22, 0.28] |
| 9.2 Guided self‐help vs physical educational intervention | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.33, 0.26] |
| 10 Severity of anxiety symptoms at 7–12 months | 1 | 199 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐1.58, 1.44] |
| 10.1 Brief individual trauma processing therapy vs parenting support | 1 | 199 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐1.58, 1.44] |
| 11 General functioning post‐treatment | 1 | 218 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 11.1 Brief individual trauma processing therapy vs parenting support | 1 | 218 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 12 General functioning at 3–6 months | 1 | 239 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.06, 0.00] |
| 12.1 Brief individual trauma processing therapy vs parenting support | 1 | 239 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.06, 0.00] |
| 13 General functioning at 7–12 months | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 13.1 Brief individual trauma processing therapy vs parenting support | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Als 2015.
| Methods | Design: RCT | |
| Participants |
Setting: PICU Type of trauma exposure: parents of children aged 4–16 years admitted to PICU. Inclusion criteria: parents with a child aged 4–16 years admitted to the PICU for ≥ 12 hours. Exclusion criteria: child death prior to discharge; discharge to palliative care; planned admissions; history of PICU admission; overseas address or insufficient English to complete study questionnaires. Sample size: 442 individuals assessed for eligibility; 31 randomised. Mean age: 41.1 (SD not reported) years where reported. Gender: 5 (16.1%) men; 26 (83.9%) women Ethnicity: 12 participants (38.7%) were identified as "white UK". The ethnicity of other participants was not reported. Country: UK |
|
| Interventions |
Group 1: supported psychoeducational intervention: n = 22 2 phases: the first phase (receipt of the psychoeducational tool), was planned to occur within 7 days of discharge from hospital and the second phase (receipt of the telephone call), within 14 days of receiving the tool. The psychoeducational tool consisted of a handbook developed by mental health and paediatric experts and parents with experience of having a child in PICU. The handbook covered 3 main areas: emotional recovery, behavioural recovery and getting back to normal learning. The first section included a description of common emotional reactions in children, their siblings and parents following discharge from PICU, with advice regarding their management. It also included an outline of when recovery became stalled by the development of PTSD, its manifestations, what treatments are available and their rationale. The second section gave more detailed advice to parents about managing behavioural problems in children following hospital discharge. The third section addressed possible learning difficulties (e.g. slowed information processing, memory and attention problems) in the aftermath of the child's admission and provided guidance on how to support affected children. There was an additional section containing a list of contacts of possible sources of further support and advice. The telephone call, conducted by the researcher, was used to discuss each family's post‐PICU experience, reinforce the material in the handbook (thus ensuring all families were exposed to the information), and support families in putting into practice the advice given, if appropriate. Group 2: TAU: n = 9 |
|
| Outcomes |
PTSD: IES Other: HADS; PSS‐PICU Follow‐up: 3–6 months' postdischarge from PICU |
|
| Notes | Primary aim was to evaluate the feasibility of the intervention and procedures to evaluate it through a larger trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to the intervention or TAU using a computer‐generated list of random numbers prepared by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was concealed from the researcher enrolling and assessing participants and was stored with an administrator who had no other involvement in the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Baseline and 3‐ to 6‐month follow‐up questionnaires were posted to families and returned using stamped addressed envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data were assessed on an ITT basis and involved all parent–child pairs randomly assigned and providing follow‐up data. However, outcome data were only available for 17 ( 77.8%) of the intervention group and 6 (66.6%) of the TAU group. |
| Selective reporting (reporting bias) | Low risk | A study protocol was published at the beginning of the trial. The reported plan was to assess changes in mental health outcomes from baseline to follow‐up across both groups. However, the research group was unable to collect baseline data within the specified time frame, and they, therefore, focused solely on the 3‐ to 6‐month outcome data. We did not judge this to constitute a significant reporting bias. |
| Other bias | High risk | Small sample size |
Andre 1997.
| Methods | Design: RCT | |
| Participants |
Setting: community Type of trauma exposure: victims of aggression Inclusion criteria: bus drivers who were victims of aggression Exclusion criteria: not reported Sample size: 132 randomised: 65 to intervention, 67 to control Mean age: 35.2 (SD not reported) years Gender: not reported Ethnicity: not reported Country: France |
|
| Interventions |
Group 1: CBT intervention with 1–6 sessions, 45–60 min each. Mean 2.3 sessions per participant Group 2: Standard care (medical/social care provided by company) |
|
| Outcomes |
PTSD: IES Other: HAD anxiety; number of days off work Follow‐up: 6 months' postintervention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition: intervention (40%), control (33%) Adherence to intervention not reported |
| Selective reporting (reporting bias) | High risk | No protocol identified. The study authors reported no statistically significant differences between intervention and control groups, which meant that it was not possible to include this study in meta‐analyses. This has the potential to lead to an overestimate of effectiveness for those outcomes. |
| Other bias | Low risk | Appeared free from other sources of bias. |
Biggs 2016.
| Methods | Design: longitudinal RCT | |
| Participants |
Setting: participants were recruited from military MAs returning from deployment to the Middle East. Type of trauma exposure: military mortuary work Inclusion criteria: all returning MAs were offered the opportunity to take part. Exclusion criteria: none reported Sample size: 362 soldiers assessed for eligibility, 126 randomised Mean age: 28.1 (SD not reported) years Gender: 85 (67.5%) men; 41 (32.5%) women Ethnicity: 57.6% white, the ethnicity of other participants not reported. Country: USA |
|
| Interventions |
Group 1: Troop Education for Army Morale (TEAM): n = 68 Intervention based on the principles of PFA and included 4 × 2‐hour interactive group sessions held approximately 2, 3, 4 and 7 months' postdeployment, informational handouts, access to a dedicated website, e‐mail and telephone line service. 2 trained psychologists or psychiatrists facilitated intervention sessions. Group 2: assessment only: n = 58 |
|
| Outcomes |
PTSD: PCL Other: PHQ‐9 depression; WHOQOLBREF Follow‐up: 2, 3, 4, 7 and 10 months' postdeployment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Intervention group completed surveys before the start of each intervention session. We judged that this may have influenced responses. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The longitudinal effect of treatment on outcome measures was derived from linear mixed models using total score as the dependent variable and time (baseline and 5 follow‐ups), treatment (intervention vs comparison), and the interaction between time and treatment as independent variables. The way in which missing data were handled was not reported and dropout was high. |
| Selective reporting (reporting bias) | High risk | No study protocol identified. The study authors reported no statistically significant differences between intervention and control groups, which meant that it was not possible to include this study in meta‐analyses. This has the potential to lead to an overestimate of effectiveness for those outcomes. |
| Other bias | High risk | Intervention only described very briefly. No description of an intervention manual and evaluation of treatment adherence was not reported. |
Borghini 2014.
| Methods | Design: RCT | |
| Participants |
Setting: participants recruited from a NICU. Type of trauma exposure: mothers of preterm infants born at < 33 weeks' gestation. Inclusion criteria: mothers of infants born at < 33 weeks' gestation. Exclusion criteria: death of the neonate, parents who were not fluent in French, infant malformation, periventricular leukomalacia grade ≥ 2 or intraventricular haemorrhage, parental mental illness and neonatal abstinence syndrome. Participants were also excluded at 6 months' corrected age (6 months after 40 weeks' gestation), if an infant had severe mental or psychomotor delays during the paediatric appointment. Sample size: 242 mothers eligible for inclusion; 62 met initial inclusion criteria, agreed to take part and were randomised; 2 infants developed severe mental or psychomotor delays during the paediatric appointment at 6 months and the mother was removed from the study, leaving a final sample of 60. Mean age: 33 (SD 4.1) years Gender: 60 (100%) women Ethnicity: not reported. 45/55 completing participants were reported to be of Swiss nationality. Country: Switzerland |
|
| Interventions |
Group 1: 3‐step early intervention: n = 30 Aimed at 3 main dimensions: parental support, parent–infant relationship support and infant development support. Intervention had 3 phases occurring at 33 weeks after conception, 42 weeks after conception and 4 months after the theoretical term of 40 weeks after conception. At 33 weeks after conception, the intervention consisted of a joint observation, including the mother, a nurse and a therapist, of the infant's reactions and adjustments to various stimuli and his/her interaction abilities during a standard care procedure in the NICU. The observation lasted 30–60 min and was videotaped. Parents were encouraged to comment on their infants' behaviours and on their own emotions. At 42 weeks after conception, the intervention consisted of an assessment using the NBAS, which identified infants' stress reactions and self‐regulation capacities. Each NBAS was videotaped. At the infant's 4‐month corrected age, the intervention consisted of 3 sessions, 1 week apart, of a 10‐min mother–infant free play. The interactions were videotaped and used later by the examiner in the interaction guidance phase with the mothers. Each session could last 40–60 min and aimed at promoting the mother's caregiving qualities and sensitivity to the infant through the careful observation of the infant's reactions, needs, competences and vulnerabilities. Group 2: TAU: n = 30 At 33 weeks' conception, families in the preterm group without intervention met in the NICU to obtain information about their infant and their relationship with him or her. There was no joint observation of the infant. At 42 weeks after conception the preterm group without intervention also had the NBAS assessment, but the NBAS was followed by a semi‐structured interview with the parents based on the Clinical Interview for Parents of High‐Risk Infants which allowed mothers to express much of their emotional experiences with their infants at birth and during their subsequent hospitalisation. At the infant's 4‐month corrected age, the preterm group without intervention met for a single appointment. Mothers were asked to play freely with their infant for 10 min and the interaction was videotaped, but there was no interaction guidance afterwards. |
|
| Outcomes |
PTSD: PPQ Other: CARE‐Index (3rd revision) was used to code the quality of video‐recorded mother–infant interactions. Follow‐up: 42 weeks' postconception, and 4 and 12 months' corrected infant birth. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Randomised using a sealed envelope procedure. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | PTSD outcomes assessed via a self‐report measure which appeared to have been administered by the treating clinician. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis was based on completers. 4 (13.3%) participants dropped out of the intervention group; 1 (3.3%) dropped out of the TAU group. There was no explanation of the causes of dropout or a consort diagram. It was unclear how many participants contributed to each follow‐up point. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | Unclear risk | No indication that treatment adherence was assessed. |
Brom 1993.
| Methods | Design: RCT | |
| Participants |
Setting: Participants were recruited from police records of MVA and seen on an outpatient basis. Type of trauma exposure: MVA Inclusion criteria: exposure to an MVAs of moderately serious‐to‐serious severity Exclusion criteria: not specified Sample size: 738 people randomised and written to by the police and invited to take part in study; 151 agreed to take part in the study; 83 (36%) agreed to take part in the monitoring group and 68 (13%) took up the offer of intervention. Mean age: 37.6 (SD 16.6) years Gender: 89 (58.9%) men; 62 (41.1%) women Ethnicity: not reported Country: Netherlands |
|
| Interventions |
Group 1: counselling intervention: n = 68 Counselling intervention lasted 3–6 sessions depending on need. Components of the intervention included: practical help and information on procedures following an MVA and general information about psychological reactions; support to recognise that the event was over, to explore the experience and related emotions and to mobilise support networks; reality testing and confronting emotional reactions; confronting the memory of the experience; ongoing contact at least 2–3 months after the accident; referral for psychotherapeutic treatment where indicated. Group 2: monitoring group: n = 83 Monitoring group were unaware that they were part of a comparison study and received assessments only. |
|
| Outcomes |
PTSD: IES Other: Trauma Symptom Inventory Follow‐up: 1 and 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported but outcomes were likely to be measured by the therapists. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The plan of analysis and management of dropout is not reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | High risk | Randomisation occurred prior to potential participants agreeing to take part in the study and participants were only aware that they were either invited to participate in a secondary prevention programme or that they were invited to participate in a research project. The response rate for the monitoring group was 36%, for the intervention group 13%. Symptom scores on the IES were not matched at baseline. Groups were not matched for marital status and income. Individuals in the monitoring group were more likely to be married and had a statistically significant higher level of income. |
Brunet 2013.
| Methods | Design: RCT | |
| Participants |
Setting: emergency departments of 2 public hospitals Type of trauma exposure: exposure to a life‐threatening event Inclusion criteria: experienced a life‐threatening event in the last 10 days that elicited a peritraumatic reaction of fear, helplessness or horror. Exclusion criteria: did not speak either French or English; had or were suspected of having a traumatic brain injury; had a lifetime diagnosis of psychosis, substance or alcohol dependence, bipolar disorder or mental retardation; had been clinically depressed in the last 2 years; were taking psychotropic medication at the onset of the study; were injured to the extent that they could not participate in the study; lived outside the Montreal metropolitan area; did not have a significant other (a friend, spouse or another family member) to bring to the therapy session; or did not succeed in making an appointment with the therapist within 30 days after trauma exposure. Sample size: 90 people eligible to take part; 83 randomised; 74 completed baseline measures and were identified as the ITT sample; 5 individuals were subsequently withdrawn for protocol violation. Mean age: 36.29 (SD 11.05) years Gender: 40 (54.1%) men; 34 (45.9%) women Ethnicity: 85.1% white; 14.9% other Country: Canada |
|
| Interventions |
Group 1: brief dyadic CBT intervention: n = 37 2‐session dyadic intervention included elements of psychoeducation and motivational interviewing, and targeted communication between the patient and significant other, aiming to facilitate support, promote bidirectional disclosure, reduce disclosure constraining behaviours and improve coping. It promoted the disclosure of thoughts and emotions about the trauma in the natural environment of the dyad while attempting to reduce social constraints on disclosure and negative social support interactions. Group 2: waiting list: n = 37 Waiting list group filled out and returned the questionnaires. |
|
| Outcomes |
PTSD: IES‐R; CAPS Other: SCS; SAS‐SR Follow‐up: postintervention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation undertaken through randomly permuted blocks. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | IES‐R (self‐report measure) was the primary outcome. We judged the risk of detection bias to be low using this measure. PTSD diagnosis was made using the CAPS. It was unclear whether assessor were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis undertaken using ITT principles. 4 (5.4%) individuals were removed from the study for protocol violation and not included in follow‐up analyses. A further 3 (4.1%) participants did not complete outcomes. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | High risk | Authors affiliated with experimental intervention. |
Cox 2018a.
| Methods | Design: multisite RCT | |
| Participants |
Setting: hospital ICUs Type of trauma exposure: patients admitted to an ICU who had been in receipt of mechanical ventilation for > 48 consecutive hours. Inclusion criteria: aged ≥ 18 years of age and in receipt of mechanical ventilation for > 48 consecutive hours, and successful extubation before discharge. Exclusion criteria: pre‐existing or current cognitive impairment; treatment for severe mental illness during the 6 months preceding admission (e.g. active psychosis, suicidality); residence at a location other than home immediately before admission,; poor English fluency; ICU attending physician's expectation of patient survival < 3 months; inability to complete study procedures as determined by study staff; and failure to return home from either a hospital or postacute care facility within 3 months after discharge. Exclusion criteria for family members included history of cognitive impairment and poor English fluency. Sample size: 2002 patients assessed for eligibility; 175 randomised Mean age: 51.73 (SD 13.76) years Gender: 100 (57.1%) men; 75 (42.9%) women Ethnicity: 21.7% African‐American; 70.1% white; 1.1% Asian; 1.1% American Indian/Alaska Native; 0.6% Native Hawaiian or other Pacific Islander; 1.1% Hispanic; 0.6% did not know or did not wish to answer. Country: USA |
|
| Interventions |
Group 1: telephone‐ and web‐based CBT‐based coping skills training: n = 86 CBT intervention delivered in 6 weekly telephone sessions, each lasting approximately 30 min, that addressed the following: introduction and relaxation exercise; progressive muscle relaxation; pleasant activities and activity–rest cycle; communication; cognitive restructuring and pleasant imagery; review and planning for sustainability. Psychologists taught each skill by providing a description and rationale for its use, leading participants through practice with feedback in the context of any self‐reported ongoing stressors, helping participants plan how to apply the skill in real life, and highlighting relevant web‐based content. In addition to learning skills themselves, family members coached patients in applying skills and using the web content on a day‐to‐day basis. Group 2: education programme: n = 89 An education programme was designed with the help of a stakeholder group to address poor comprehension of critical illness, omitting any mention of post‐ICU psychological distress. Programme consisted of 6 informational videos with accompanying web‐based content. Study staff with content expertise in critical illness conducted 2 × 30‐min telephone calls with participants during the 6‐week intervention period to review materials and answer related questions. |
|
| Outcomes |
PTSD: IES‐R Other: HADS; EQ‐5D; global mental and physical health status assessed with the PROMIS; adaptive coping behaviours measured by the Brief COPE. Follow‐up: 3 months' postbaseline (immediately post‐treatment), and 6 months' postbaseline (3 months' post‐treatment). |
|
| Notes | Family members of participants were also included and randomised to both interventions. The data reported here are for patient participants only. Eight participants died during the study. Patients were, on average, middle‐aged and severely ill, with an expected hospital mortality of approximately 50%. Approximately one‐third of patients had been treated for depression, anxiety or PTSD in the 3 months preceding admission. Readmission occurred among 43 (25%) patients during follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken via a password protected computerised system allocated patient–family member dyads at a 1:1 treatment group ratio with blocks of 4, stratifying by 3 factors to ensure balance. |
| Allocation concealment (selection bias) | Low risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained research coordinators blinded to treatment group collected clinical data. Participants completed study surveys by telephone with treatment group‐blinded University coordinators or by a password‐protected electronic patient‐reported outcomes system at baseline and follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses were conducted according to the ITT principle. Primary and secondary survey outcome analyses were conducted with full likelihood methods. |
| Selective reporting (reporting bias) | Low risk | Results reported as described in preregistered protocol. |
| Other bias | High risk | Study conducted with a severely ill population with high levels of premorbid mental health problems. The authors observed higher‐than‐expected attrition, attributable primarily to patients' serious illnesses and occurring after consent but before randomisation. Adherence to the interventions was fairly low and partly attributed to illness. |
Curtis 2016.
| Methods | Design: RCT | |
| Participants |
Setting: 5 ICUs in 2 hospitals Type of trauma exposure: family members of patients in ICU with high risk of mortality. Inclusion criteria: predicted mortality ≥ 30% and a surrogate decision maker. Eligibility criteria for patients included: in ICU for > 24 hours; aged > 18 years; mechanically ventilated at enrolment; SOFA score ≥ 6 or diagnostic criteria predicting a ≥ 30% risk of hospital mortality; legal surrogate decision maker to consent for patient participation; and a family member able to come to the hospital. Eligibility criteria for family members included aged > 18 years and able to complete consent process and questionnaires in English. Exclusion criteria: none. Sample size: 2209 ICU patients screened for eligibility. Family members for 488 patients approached for participation and 170 enrolled; 2 family members withdrew before randomisation. The 168 randomised patients had 268 family members who participated. Mean age of family member participants where available: 50.85 (SD 13.12) years Gender (family members): 79 (29.5%) men; 189 (70.5%) women. Ethnicity where available: 4.8% African‐American; 84.8% white; 2.2% Asian; 3.9% Native American; 0.4% Native Hawaiian; 3.9% other or mixed Country: USA |
|
| Interventions |
Group 1: communication facilitator: n = 137 Communication facilitators assisted families of patients by providing communication support during the ICU stay with the aim to increase families' and clinicians' self‐efficacy expectations about communication in the ICU. Intervention included: interviews by facilitators with family to understand the family's concerns, needs and communication characteristics; meetings by facilitators with physicians, nurses or other clinicians offering brief summary of family concerns, needs and communication characteristics; provision of communication and emotional support adapted to the family member's attachment style; facilitator participation in family conferences and 24‐hour follow‐up with the family after discharge to acute care. Group 2: usual care: n = 131 |
|
| Outcomes |
PTSD: PCL Other: PHQ‐9; GAD‐7; length of stay in ICU; cost of care Follow‐up: symptoms were assessed 3 and 6 months after the patient died in, or was discharged from, the ICU. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified by hospital in block sizes of six, with results provided to study staff in sealed, opaque, consecutively numbered envelopes". Comment: information in the study paper implied computer‐based randomisation. |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up surveys assessing depression, anxiety and PTSD were mailed to family members' homes at follow‐up points. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up was high. Analysis was based on 133 (49.6%) of those randomised at 3 months and 122 (45.5%) at 6 months. |
| Selective reporting (reporting bias) | Low risk | Results reported as described in preregistered protocol. |
| Other bias | Unclear risk | Eligibility criteria were changed during the trial to improve recruitment: the required SOFA score was lowered from ≥ 10 to ≥ 6. |
Gamble 2005.
| Methods | Design: RCT | |
| Participants |
Setting: antenatal clinics of 3 maternity teaching hospitals. Type of trauma exposure: postpartum women at risk of developing psychological trauma symptoms. Inclusion criteria: aged > 18 years, in the last trimester of pregnancy, expected to give birth to a live infant, and able to complete questionnaires and interviews in English and met criterion A of DSM‐IV for PTSD. Exclusion criteria: women experiencing stillbirth or neonatal death. Sample size: 348 women screened for birth‐related trauma exposure, 103 met inclusion criteria and were randomised. Mean age: 28 (SD 6.04) years Gender: 103 (100%) women Ethnicity: 93.2% white/European; 0.97% Asian; 2.9% other Country: Australia |
|
| Interventions |
Group 1: midwife‐led brief counselling intervention: n = 50 Counselling began within 72 hours of birth on the postnatal ward and again by telephone at 4–6 weeks' postpartum. Counselling duration 40–60 min. The counselling process incorporated elements of critical stress debriefing and issues pertinent to the childbearing context. Intervention included building a therapeutic relationship, accepting and working with the mother's perception of their experience, supporting expression of feelings, filling in missing pieces of the account, connecting the event with the mother's emotions and behaviour, reviewing labour management, enhancing social support, reviewing positive approaches to coping, exploring further solutions. Group 2: TAU: n = 53 Standard postnatal care |
|
| Outcomes |
PTSD: MINI‐PTSD Other: EPDS; DASS‐21; MSSS Follow‐up: 4–6 weeks' postpartum and 3 months' postpartum |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised using sealed, opaque envelopes containing computer‐generated, random allocations. |
| Allocation concealment (selection bias) | Low risk | Participants randomised using sealed, opaque envelopes containing computer‐generated, random allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Appeared that assessor who completed outcome assessments at 4–6 weeks was also providing intervention and was not blind to allocation. A second assessor who completed assessments at 3 months was reported to be blind to allocation. Outcomes at 4–6 weeks appeared at high risk of detection bias and outcomes at 3 months appeared at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study retention rate was 100% at 3 months. 1 individual could not be contacted at 4–6 weeks. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | Unclear risk | Treatment adherence not reported. |
Gamble 2010.
| Methods | Design: RCT | |
| Participants |
Setting: 4 hospital‐based antenatal clinics Type of trauma exposure: women with traumatic birth/EmCS. Inclusion criteria: women in third trimester of pregnancy expecting a full term, normal birth, able to understand sufficient English to discuss their experiences meeting DSM‐IV criterion A for PTSD. Exclusion criteria: pre‐existing mental illness, or expecting an adverse birth outcome. Sample size: 890 women screened for inclusion and 262 randomised Mean age: 30 (SD not specified) years Gender: 262 (100%) women Ethnicity: not specified Country: Australia |
|
| Interventions |
Group 1: PRIME: n = 137 PRIME refers to a treatment condition for distressed postpartum women. Counselling was delivered at 72 hours after birth in person and at 6 weeks' postpartum by telephone. PRIME aims to support the expression of feelings and provide a framework for women to identify and work through distressing elements of childbirth (Gamble 2009). Women were provided with an opportunity to review the birth and gain a realistic perception of events. Focussed on developing individual situational supports for the present and near future, affirming negative things can be managed and developing a simple plan for achieving this. Group 2: parenting support: n = 125) Support focused on parenting concerns at 72 hours after birth and 6 weeks' postpartum by telephone. Midwives provided information on managing crying, feeding, sleeping and other parenting issues. Both intervention were delivered in 2 sessions at 72 hours' and 6 weeks' postpartum by telephone. Group 3: control: n = 138 Matched control group who were not traumatised were matched for age, parity and education. This group received standard maternity care as provided by their nominated facility. We have not included data from this group. |
|
| Outcomes |
PTSD: PDS Other: EPDS; DASS; HRQoL; mother–child relationship; parenting satisfaction; sense of competence; parenting stress Follow‐up: 6 weeks', 6 months', 12 months' postpartum |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mothers screening positive were randomly allocated using computer‐generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis based on responses available at each follow‐up point. Proportion of experimental group available at 6 and 12 months lower than control group. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified in protocol. |
| Other bias | High risk | Numerically, more participants indicating they received previous mental health help were in PRIME (40%) compared to the parenting group (29%; P = 0.054). No indication that this was controlled for in analyses. |
Gidron 2001.
| Methods | Design: pilot RCT | |
| Participants |
Setting: hospital emergency department Type of trauma exposure: MVA Inclusion criteria: survived an MVA within the last 24 hours; discharged from hospital within 24 hour after admission, indicating minor injury only and had heart rate > 95 beats/min upon admission into the emergency department Exclusion criteria: brain damage Sample size: number of patients approached to take part not reported; outcomes for 17 participants who completed the study were reported. Mean age: 38.0 (SD not specified) years Gender: 9 (52.9%) men; 8 (47.1%) women Ethnicity: not specified Country: Israel |
|
| Interventions |
Group 1: MSI: n = 8 2‐session intervention delivered over the telephone based on research about the nature of memory processing following trauma, specifically research suggesting that trauma memories are recalled as sensory, affective and fragmented information. Features included focus on time sections of the traumatic event; listening and clarifying details; memory structuring by having the therapist repeat the trauma narrative in an organised, labelled and logical manner, adding initial implications for the patient's life; patient described the traumatic event in the same structured, labelled and logical manner as the therapist did; between session practice of structured description; repeated practice of the new structure in the second session. Finally, the patient was taught about the importance of, and asked about, his/her social support. Group 2: supportive listening: n = 9 Patients telephoned twice, and invited to describe the event to the counsellor. The counsellor provided supportive listening and informed controls about the availability of treatment from the PTSD unit in the hospital. This condition attempted to control for therapist contact, attention to the problem, and simple disclosure and ventilation without guidance. |
|
| Outcomes |
PTSD: PDS Other: none Follow‐up: 3–4 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At outcome assessment, participants were contacted by telephone by a researcher blind to their group status. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study did not include a participant flowchart and the recruitment procedure and process of analysis was only briefly described. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | High risk | Authors affiliated with experimental intervention. Small sample size. Study report not detailed. Therapist training and treatment adherence not reported. |
Gidron 2007.
| Methods | Design: RCT | |
| Participants |
Setting: university medical centre Type of trauma exposure: traffic accident Inclusion criteria: traffic accident 24–48 hours before entry to study; mild injuries; spent up to 24 hours in the hospital; aged 18–60 years and pulse rate of at least 95 beats/min. Exclusion criteria: brain injury or loss of consciousness Sample size: number screened and randomised not reported. Outcomes for 34 participants who completed the study reported. Mean age: 28.3 (SD 10.0) years Gender: 16 (%) men; 18 (%) women Ethnicity: not specified Country: Israel |
|
| Interventions |
Group 1: MSI: n = 19 2‐session intervention delivered by telephone which is based on research about the nature of memory processing following trauma, specifically research suggesting that trauma memories are recalled as sensory, affective and fragmented information. Features of intervention included focus on time sections of the traumatic event; listening and clarifying details; memory structuring by having the therapist repeats the trauma narrative in an organised, labelled and logical manner, adding initial implications for the patient's life; patient described the traumatic event in the same structured, labelled and logical manner as the therapist; between session practice of structured description; repeated practice of the new structure in the second session. Finally, the patient was taught about the importance of, and asked about, his/her social support. Group 2: supportive listening: n = 15 2 telephone calls that provided supportive listening. Included asking patients how they felt, asking whether they wanted to share the details of the event, listening to their story and telling them how to obtain further treatment should they feel such a need. The therapist provided only empathy without structuring patients' reports and without formal debriefing. |
|
| Outcomes |
PTSD: PDS Other: none Follow‐up: 3 months |
|
| Notes | Study reported that it was hypothesised that the MSI would result in fewer symptoms of PTSD than a supportive‐listening control condition, and that these effects may depend on participants' gender. The authors reported positive outcomes for PTSD for the experimental intervention for women only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At follow‐up, participants evaluated by telephone for their PTSD symptoms by a researcher blind to their group status. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study did not include a participant flowchart and the recruitment procedure and process of analysis was only briefly described. The findings reported only fully participating patients and dropouts were not reported. |
| Selective reporting (reporting bias) | High risk | No study protocol identified. Study aimed to replicate findings from Gidron 2001. Gidron 2001 reported PTSD diagnosis as an outcome and it would have been reasonable to expect this study to do the same. |
| Other bias | High risk | Authors affiliated with experimental intervention. Small sample size. Study report not detailed. Therapist training and treatment adherence not reported. |
Holmes 2007.
| Methods | Design: RCT | |
| Participants |
Setting: 2 level 1 trauma centres Type of trauma exposure: major physical trauma. Approximately two‐thirds of patients (43.9%) had received their injuries in road traffic accidents, falls or collisions (12.3%) or through non‐accidental injury (9.6%). The cause of trauma for the remaining participants was not reported. Inclusion criteria: aged > 18 years, had experienced major physical trauma. Exclusion criteria: major head injury or injury was due to self‐harm; current psychotic illness. Sample size: 964 cases of major trauma screened; 146 patients eligible to take part in study; 114 recruited and 90 completed initial assessments and were randomised. Mean age: 37.0 (SD 14.7) years of those recruited Gender: 63 (70%) men; 27 (30%) women Ethnicity: not reported Country: Australia |
|
| Interventions |
Group 1: IPC: n = 51 IPC is a brief universal psychological intervention aimed at addressing the physical and functional consequences of serious injury. The aim of the intervention was to promote adaptation to injury, reduce depressive and PTSD and prevent the emergence of new episodes of psychiatric disorder. As part of the therapy, the impact of the injury on interpersonal issues predating the injury were identified and explored. Interpersonal issues arising after the trauma that were addressed included the degree to which needs were communicated with and responded to by family, health professionals and, in some cases, insurers. The domain of role transition was expanded to include the transition from health to injury. Issues of grief and loss arising from the injury were explored and related to preinjury issues where appropriate. Strategies to enhance adaptation to these issues were outlined in therapy and practised by the patient between sessions. Number of sessions available not reported. Mean number of sessions for those completing therapy 5.9 (SD 1.1). Group 2: TAU: n = 39 Participants were informed following randomisation that they would not be receiving the IPC and would be reassessed at 3 and 6 months. In the case of psychological distress, they were recommended to seek assessment through their primary practitioner, but were also able to contact the study co‐ordinator. |
|
| Outcomes |
PTSD: PCL Other: SCID; BDI; HADS; AUDIT; SF‐36 Follow‐up: 3 and 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation involved the initial assessor contacting a research officer who made a blinded selection from a box of mixed envelopes. |
| Allocation concealment (selection bias) | Low risk | Randomisation involved the initial assessor contacting a research officer who made a blinded selection from a box of mixed envelopes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measures completed at 3 months were self‐reported. At 6 months, a repeat SCID was conducted by an assessor blinded to the treatment condition and the previous assessment results. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study only presented data for those who completed the IPC intervention (52.9% of those allocated to this arm), although the participant flowchart suggests that 92.2% of those randomised to IPC completed the 3‐month assessment and 90.2% completed the 6‐month assessment. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | Unclear risk | Treatment adherence was only evaluated for cases of completed therapy. |
Irvine 2011.
| Methods | Design: multisite RCT | |
| Participants |
Setting: 2 hospitals following ICD transplant Type of trauma exposure: surgery for ICD transplant Inclusion criteria: patients who received their first ICD implant for secondary prevention of sudden cardiac death or primary prevention of sudden cardiac death and if their underlying heart disease was hypertrophic cardiomyopathy. Exclusion criteria: insufficient English fluency; medical chart documented evidence of dementia, cognitive impairment or psychosis; ICD implant for primary prevention of sudden cardiac death due to coronary heart disease, Ischaemic cardiomyopathy and congestive heart failure. The latter were excluded because of concerns that their poor and declining heart function might confound evaluation of the CBT. Sample size: 292 patients were eligible to take part in this study, 193 consented and were randomised. Mean age: 64.4 (SD 14.3) years Gender: 159 (82.4%) men; 34 (17.6%) women Ethnicity: not reported Country: Canada |
|
| Interventions |
Group 1: telephone‐based CBT: n = 96 Based on the cognitive theory of anxiety (Salkovskis 1996). Content tailored to specific misconceptions that have been known to cause distress in cardiac and ICD patients and it was informed by our clinical work. It included a therapist manual, 8 telephone counselling sessions, a psychoeducational booklet for the participants and a CD with mindfulness‐based exercises and a progressive muscle relaxation exercise. Ad hoc counselling sessions were offered to participants who experienced an ICD shock. Group 2: TAU: n = 97 Whatever the respective ICD treatment sites routinely offered their patients. All patients received standard educational materials explaining their heart disease and the ICD device. Follow‐up appointments included device interrogation (i.e. to extract arrhythmia events and ICD therapies) and troubleshooting at 6‐month intervals, cardiac care as necessary and non‐systematic supportive reassurance delivered informally in the clinic. Each centre also had access to a cardiac rehabilitation programme and psychiatric consultation as needed. |
|
| Outcomes |
PTSD: IES‐R Other: HADS; phobic anxiety sub scale of the Crown‐Crisp Experiential Index; Physical Component Summary and the Mental Component Summary of the SF‐36 Follow‐up: 6 and 12 months' postbaseline |
|
| Notes | 8 participants died during the study. Missing values were not imputed by the study authors for participants who died over follow‐up, as the cause of death was assumed to be non‐random and potentially related to the phenomenon under study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers program used to randomise participants to the CBT or TAU condition within 4 blocking variables. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up questionnaires were mailed to participants 6 and 12 months after baseline and included a prestamped return envelope. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT approach employed for the treatment outcome analyses. |
| Selective reporting (reporting bias) | Low risk | Results reported as described in preregistered protocol. |
| Other bias | Unclear risk | Evaluation of treatment adherence not reported. |
Jensen 2016.
| Methods | Design: RCT | |
| Participants |
Setting: 10 ICUs Type of trauma exposure: patients admitted to ICU and required mechanical ventilation Inclusion criteria: Danish‐speaking adults aged ≥ 18 years who had been mechanically ventilated ≥ 48 hours. Exclusion criteria: meeting baseline criteria for dementia; patients, who were not oriented in personal data according to the verbal response in Glasgow Coma Score, with detected delirium using the Confusion Assessment Methods for the ICU at randomisation, or enrolled in other follow‐up studies. Sample size: 2105 patients assessed for eligibility; 386 randomised Mean age: 66.8 (SD not reported) years Gender: 229 (59.3%) men; 157 (40.7%) women Ethnicity: not reported Country: Denmark |
|
| Interventions |
Group 1: nurse‐led intensive care recovery programme: n = 235 Individualised ICU recovery programme based on several theoretical approaches towards psychological recovery including Antonovsky's salutogenic model (Antonovsky 1987), illness narratives, person‐centred communication and elements from guided self‐determination and trauma‐focused CBT. Programme consisted of 3 consultations conducted by trained study nurses. Nurse training included 10 workshop days of theory and practice with experts in their field. Included intervention patients received an information pamphlet Life after ICU at randomisation. First consultation conducted at the clinic with the patient and close relative at 1–3 months' post‐ICU. Dialogue focused on past and present as the patient was supported in constructing an illness narrative. A prerequisite for dialogue was the provision of patient photographs taken by ICU nurses during ICU recovery. Second and third consultations at 5 and 10 months' post‐ICU conducted by telephone. Patients prepared by completing "Reflection sheets" indicating issues of importance to the individual. Group 2: standard care: n = 235 Standard care included light sedation, early mobilisation, daily Confusion Assessment Methods for the ICU delirium assessment, written information for visitors, and ICU discharge without follow‐up. ICU diaries were not used, but unplanned ICU visits and access to the medical record after discharge were permitted. Physical training was initiated in the ICU and physical rehabilitation was offered to all patients. |
|
| Outcomes |
PTSD: HTQ‐IV Other: HADS; SF‐36; sense of coherence measured by the Orientation to Life Questionnaire Follow‐up: 3 and 12 months' postdischarge |
|
| Notes | Among randomised patients, 36 (19 %) in intervention group vs 43 (22 %) in standard care group died within the first year post‐ICU. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information in the study paper implied computer‐based randomisation as treatment allocation was concealed by random selection of opaque sealed envelopes in permuted blocks of 6. |
| Allocation concealment (selection bias) | Low risk | Information in the study paper implied computer‐based randomisation as treatment allocation was concealed by random selection of opaque sealed envelopes in permuted blocks of 6. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported questionnaire packages were sent by post at 3 and 12 months' post‐ICU. To increase the response rate, a few patients were assisted by telephone in completing the questionnaires if unable on their own. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Primary analysis was reported to be based on ITT. However, data were only analysed on an ITT basis for 235 participants (67.5 % of participants still alive at 3 months and 76.5% of the participants still alive at 12 months). |
| Selective reporting (reporting bias) | Low risk | Results reported as described in preregistered protocol. |
| Other bias | High risk | Description of an intervention manual and evaluation of treatment adherence not reported. |
Jones 2010.
| Methods | Design: RCT | |
| Participants |
Setting: 6 general district hospitals and 6 university hospitals in 6 European countries. Type of trauma exposure: ICU patients with an ICU stay > 72 hours Inclusion criteria: patients who had been admitted to the ICU and ventilated. Exclusion criteria: patients stayed in the ICU for < 72 hours; ventilated for < 24 hours; were too confused to give informed consent (including severe traumatic brain injury) and had pre‐existing psychotic illness such as schizophrenia and manic depression (a confounding factor for psychological recovery) or diagnosed PTSD. Sample size: 1164 people screened for inclusion and 352 were randomised. Mean age: intensive care diaries: 60 (SD 15.6) years; delayed intensive care diaries: 59 (SD 16) years. Gender: 227 (64.5%) men; 125 (35.5%) women Ethnicity: not reported Country: Denmark, Italy, Norway, Portugal, Sweden, UK |
|
| Interventions |
Group 1: intensive care diaries: n = 177 All patients had an ICU diary written for them while they were in critical care, which the healthcare staff wrote and the family contributed to if they considered they could. Diary was a daily record of the patients' ICU stay, written in everyday language and accompanied by photographs. Memories of the ICU were assessed at 1‐week post‐ICU. Patients received their diary as soon as they wanted following randomisation at 1‐month postdischarge. The diary was introduced to the patient by a research nurse or doctor who ensured that they understood its contents but did not give any advice on what to do with it. Most of the discussions took place in an outpatient setting in a hospital but a small number were in the patients' own homes. In units where the travelling distance was too great for the patient to return to the hospital the discussion of the diary took place over the telephone. Group 2: delayed access to ICU diary: n = 175 Control patients received their diaries after they completed the final follow‐up questionnaires at 3 months. |
|
| Outcomes |
PTSD: PDS administered as a diagnostic interview Other: PTSS‐14, a 14‐item questionnaire that has been validated with ICU patients Follow‐up: 3 months following discharge from ICU |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned to treatment or control group at 1 month using a closed, non‐transparent envelope technique, randomised in blocks of 6 through computerised random number generation. |
| Allocation concealment (selection bias) | Low risk | Patients were assigned to treatment or control group at 1 month using a closed, non‐transparent envelope technique, randomised in blocks of 6 through computerised random number generation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was considered impractical to guarantee blinding of the allocation of the diary as patients would volunteer their use. In order to reduce bias and ensure blinding of the diagnosis of PTSD at the 3‐month follow‐up, the researchers were only trained to interview and administer the PDS but were not made aware of the scoring calculation or in what way each question contributed to the score and final diagnosis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes based on available data at 3‐month follow‐up. Dropout from both groups was balanced and low. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol registered after completion of study. It would make sense to report PTSD severity outcomes (e.g. on the PDS) but these were not reported. |
| Other bias | High risk | The PTSS‐14 has only been subject to preliminary validation in a small UK‐only sample. PTSD diagnosis was based on assessor administration of the PDS. Although the PDS can be used to generate a provisional PTSD diagnosis it is not intended to replace a structured diagnostic interview. A number of participants were excluded retrospectively based on identification of PTSD with onset prior to admission at the 3‐month follow‐up point. |
Kazak 2005.
| Methods | Design: pilot RCT | |
| Participants |
Setting: oncology service within a children's hospital. Type of trauma exposure: parents of children newly diagnosed with cancer. Inclusion criteria: primary caregivers and partner of children aged 0–17 years newly diagnosed with cancer. Exclusion criteria: single caregivers Sample size: 88 families assessed for eligibility; 19 families (38 individuals were randomised) Median age: 37 year for primary caregivers; 42 years for partners Gender: 18 (47.4%) men; 20 (52.6%) women Ethnicity: not reported. Ethnicity for participants' children: 36.8% African‐American; 52.6% white; 5.3% Asian; 5.3% mixed race Country: USA |
|
| Interventions |
Group 1: SCCIP‐ND: n = 9 families, n = 18 individuals SCCIP‐ND is an adaptation of an integrated cognitive behavioural and family therapy intervention developed and tested with adolescent survivors of childhood cancer and their families. SCCIP‐ND is intended for 2 caregivers of a child newly diagnosed with cancer. Caregivers work conjointly to identify beliefs about their experiences during the initial month of treatment, a time in which potentially traumatic events may occur. The focus is on understanding how beliefs about cancer and its treatment influence caregivers and to help family members anticipate the impact of cancer on the family over time. Intervention consisted of 3 × 45‐min sessions of a manualised family intervention, SCCIP‐ND. The goal was to deliver the intervention within the first month after the child's cancer diagnosis. Intervention was facilitated by the use of other families who had experienced cancer. The first session focused on identifying beliefs about cancer, its treatment and the impact on the family using the A‐B‐C Model to examine the relationships between perceptions of cancer‐related events and their feelings, actions and relationships. The second session focused on changing beliefs to enhance family functioning. Caregivers learn how to use reframing to modify their beliefs and subsequently, their emotional, behavioural and interpersonal consequences. Participants are then coached to identify new beliefs that accept the uncontrollable; focus on the controllable; acknowledge their own strengths and use the positive session. The third session addressed family growth and the future through the use of 2 metaphors, "The Family Survival Roadmap" and "Putting Cancer in its Place," to help caregivers recognise their beliefs about the family's future, and share these beliefs with each other. Group2: TAU: n = 10 families, n = 20 individuals Usual psychosocial care. Each family was assigned a social worker who attended the initial family meeting, provided resources and supplemental information about the diagnosis and treatment, and offered support. |
|
| Outcomes |
PTSD: IES‐R Other: STAI Follow‐up: 2 months' post‐treatment |
|
| Notes | Participants were randomised by family dyad, rather than individually. All 19 primary caregivers were mothers. Of the partner caregivers, 18 were fathers and 1 was a grandmother. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation used a predetermined concealed random assignment list maintained by a staff member unaware of patient identity. |
| Allocation concealment (selection bias) | Low risk | Randomisation used a predetermined concealed random assignment list maintained by a staff member unaware of patient identity. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were self‐report questionnaires administered by research assistants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors did not undertake detailed statistical analysis due to the relatively small sample size. Outcome data were reported only for those available to follow‐up, with a larger number of dropouts (including 4 participants withdrawals) from the intervention group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | High risk | Small sample size |
Lindwall 2014.
| Methods | Design: RCT | |
| Participants |
Setting: 4 major paediatric stem cell transplantation centres Type of trauma exposure: participants were parents of children undergoing stem cell or bone marrow transplantation. Inclusion criteria: parent of children aged 6–18 years undergoing stem cell or bone marrow transplantation with an expected stay of 3 weeks were eligible if they were primarily responsible for caring for the child during his/her hospital stay; available to participate throughout the duration of the child's hospitalisation for transplantation; and able to speak and read English fluently. Exclusion criteria: none Sample size: 242 child–parent dyads eligible to take part; 171 were randomised. Mean age: not reported Gender: 59.1% of child–parent dyad men; (40.9%) women Ethnicity: 4.3% Asian; 14.6% black; 5.3% Hispanic; 70.7% white; 5.3% other/unknown Country: USA |
|
| Interventions |
Group 1: child‐targeted intervention involving massage and humour therapy: n = 58 Patients were provided with psychoeducation about the benefits of both massage and humour therapy. Patients were scheduled for 3 massage sessions per week over the course of 4 weeks (i.e. admission through week +3). Humour therapy consisted of providing a "humor cart" that contained enjoyable items such as videos, books and games. Scheduled humour sessions involving an interventionist and the patient occurred once per week. In addition, the humour cart was made available to families ≥ 3 times per week over the course of 4 weeks (i.e. admission through week +3). Group 2: child‐targeted intervention plus a parent‐targeted intervention involving massage and relaxation or imagery (or both): n = 57 Child‐targeted intervention described above plus an additional parent intervention. Parents were provided with psychoeducation about how promoting their own well‐being (i.e. engaging in massage and relaxation training) may also positively benefit their children. Parents were scheduled for a massage session 3 times per week for 4 weeks (i.e. admission through week +3). In addition, parents participated in weekly relaxation training sessions (i.e. admission through week +3) with a member of the research team, which promoted strategies such as muscle relaxation, breathing exercises and guided imagery. Parents were provided with a relaxation tape and player, and they were encouraged to engage in relaxation exercises 15–20 min daily. Group 3: usual care: n = 56 Patient‐parent dyads in the standard care arm of the study did not receive any additional intervention beyond the routine, comprehensive services that are provided for families during the SCT process at these major paediatric SCT centres. |
|
| Outcomes |
PTSD: IES‐R Other: CES‐D for depression Follow‐up: 24 weeks' postadmission |
|
| Notes | Sample characteristics were reported for parents and children in combination and it was not possible to separate them. Data from group 1 was not used in meta‐analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes were completed for 97/167 (58.1%) participants for whom there was available data at baseline. 25 patients died, 11 withdrew (3 withdrew immediately after being randomised to the standard care arm; the remaining 8 withdrew after a period of noncompliance indicating they were no longer interested or felt study procedures were too burdensome), 8 were taken off study for medical reasons (relapse, second transplantation) and 22 failed the week +24 assessment. |
| Selective reporting (reporting bias) | High risk | No study protocol identified. The study authors reported no statistically significant differences between intervention and controls, which meant that it was not possible to include this study in meta‐analyses. This has the potential to lead to an overestimate of effectiveness for those outcomes. |
| Other bias | Low risk | No other biases indicated. |
Marchand 2006.
| Methods | Design: RCT | |
| Participants |
Setting: with the collaboration of a major convenience store chain in the metropolitan area of Montreal. Type of trauma exposure: victims of armed robbery. Inclusion criteria: victim of an armed robbery that included acts of violence ranging from threat of death or injury to physical assault and threat with a weapon. They also had to have reported to the screening interviewer that they experienced intense fear, helplessness or horror during or after the robbery such as described in Criterion A2 of the DSM‐IV PTSD diagnosis. Exclusion criteria: no additional exclusion criteria. Sample size: number screened not reported; 75 individuals were randomised. Mean age: 21.8 (SD = 6.7) years Gender: 36 (48%) men; 39 (52%) women Ethnicity: not reported Country: Canada |
|
| Interventions |
Group 1: CISD‐A: n = 33 In the CISD‐A condition (adapted from Mitchell 1995), debriefings were conducted individually and consisted of 2 × 1‐hour sessions at a 1‐week interval and were conducted by an experienced psychologist. The first debriefing session took place 2–22 days after the robbery and covered the following themes: goals of the session; present tense detailed description of the traumatic event; thoughts and emotions experienced by the participant during and after the event; information about normal stress reactions and the challenge of the participant's irrational beliefs; the stress management techniques; and if necessary, reference to further follow‐up. The second session of debriefing followed the same structure and included a review of reactions, thoughts and emotions experienced during the week following the first session. Group 2: TAU: n = 42 Participants received no interventions and participated only at the assessments. |
|
| Outcomes |
PTSD: PTSD module of the SCID; IES Other: none Follow‐up: 1 and 3 months' postbaseline |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were conducted by graduate students who were blind to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants unavailable for follow‐up was similar for both groups of the study at 1 and 3 months (24%). Completer and ITT analyse using last observation carried forward were undertaken. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | Unclear risk | There was a fairly sizeable difference between the 2 groups for IES scores at baseline. This was not statistically significant but may have contributed to differences at subsequent time points and this was not controlled for in analyses. |
Mouthaan 2013.
| Methods | Design: RCT | |
| Participants |
Setting: 2 level 1 trauma centres Type of trauma exposure: injury patients transported by ambulance or helicopter. Suspected to have experienced possible severe injuries that required specialised acute medical care. Inclusion criteria: aged ≥ 18 years, proficiency in Dutch and having experienced a potential traumatic event (cf. Criterion A1 DSM‐IV PTSD diagnosis). Exclusion criteria: injury resulting from deliberate self‐harm; organic brain condition, psychotic disorder, bipolar disorder or depression with psychotic features; moderate‐to‐severe traumatic brain injury or permanent residency outside the Netherlands. Sample size: 1807 individuals considered; 1032 assessed for eligibility and 300 randomised Mean age: self‐guided Internet‐based intervention: 44.18 (SD 15.76) years; no intervention: 43.49 (SD 16.00) years Gender: 180 (60%) men; 120 (40%) women Ethnicity: not reported. 249 (83%) reported to be of Dutch cultural background. Country: Netherlands |
|
| Interventions |
Group 1: self‐guided Internet‐based intervention: n = 151 Based on established CBT techniques (Trauma TIPS, which is based on CBT techniques of psychoeducation, stress management/relaxation techniques and in vivo exposure). It consists of 6 steps, including introduction to the programme and basic operating instructions; assessments of acute anxiety and arousal using VAS at pre‐ and postintervention; video features of the trauma centre's surgical head explaining the procedures at the centre and the purpose of the programme, and of 3 patient models sharing their experiences after their injury; a short textual summary of 5 coping tips for common physical and psychological reactions after trauma; audio clips with instructions for stress management techniques; contact information for program assistance or professional help for enduring symptoms; and a Web forum for peer support. Group 2: TAU: n = 149 Care as usual, available to patients from both groups, consisted of incidental, non‐structured talks with trauma centre staff or with a patient's general practitioner (GP), either directly following injury or during the course of the trial. |
|
| Outcomes |
PTSD: CAPS; IES‐R Other: HADS; MINI‐Plus, version 5.0 to diagnose major depressive disorders and other anxiety disorders; mental healthcare utilisation using the Trimbos/Institute for Medical Technology Assessment questionnaire and the Short Form Health and Labour Questionnaire. Follow‐up: 1, 3, 6 and 12 months' postinjury |
|
| Notes | The authors noted that participants were reluctant to use the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed by a research member independent of data collection in a 1:1 ratio by a computerised program using random block sizes stratified by study centre. |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by a research member independent of data collection in a 1:1 ratio by a computerised program using random block sizes stratified by study centre. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients were asked not to share information about the randomisation to the assessors, to ensure that they were blind to the allocated interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT and completer‐only analysis was undertaken using mixed‐model analysis. Modest loss to follow‐up at 1 and 3 months. However, missing data > 50% at 6 and 12 months. |
| Selective reporting (reporting bias) | Low risk | Outcomes were as reported in the study protocol. |
| Other bias | High risk | Authors affiliated with experimental intervention. |
Rothbaum 2012.
| Methods | Design: RCT | |
| Participants |
Setting: participants were recruited from a hospital trauma centre. Type of trauma exposure: patients admitted to the emergency department experiencing a DSM‐IV criterion A trauma. Inclusion criteria: adults aged 18–65 years who had experienced a traumatic event within 72 hours of presentation in the emergency department; afraid that they might be killed or seriously injured during the event; able to be contacted following discharge and to return for follow‐up appointments; and alert and oriented and able to provide informed consent. Exclusion criteria: non‐English speaking; lost consciousness > 5 min during the event; history of a serious mental illness; currently suicidal or reported current substance dependence. Sample size: 5608 individuals assessed for eligibility; 4219 did not meet inclusion criteria; 1249 refused to take part or were excluded for other reasons; 137 randomised. Mean age: 31.5 (SD = 11.6) years Gender: 48 (35.0%) men; 89 (65.0%) women Ethnicity: 13.1% black; 78.8% white; 1.5% Native American; 6.6% other Country: USA |
|
| Interventions |
Group 1: modified PE: n = 69 Patients received 3 × 1‐hour sessions of a modified PE intervention, distributed 1 week apart. PE consisted of imaginal exposure, processing time, breathing retraining, psychoeducation and homework tasks (including addressing in vivo exposure goals). Group 2: assessment only: n = 68 |
|
| Outcomes |
PTSD: PTSD PSS‐I; PDS Other: BDI‐II, Additional Treatment Inventory; Standardized Trauma Interview Follow‐up: 4 and 12 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes containing computer‐generated patient random assignments (either to immediate intervention or assessment only) were given to the patient and their nurse after the initial evaluation to ensure that assessors remained blind. |
| Allocation concealment (selection bias) | Low risk | Envelopes containing computer‐generated patient random assignments (either to immediate intervention or assessment only) were given to the patient and their nurse after the initial evaluation to ensure that assessors remained blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data handled with multiple imputation with mixed‐effect models being used to obtain predicted mean values for outcomes at each assessment point. There was a disproportionately higher level of missing data in the intervention group at initial outcome (31.9% compared to 19.1%) and 12 weeks' follow‐up (39.1% compared to 27.9%). |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were not reported in study protocol. |
| Other bias | High risk | Authors affiliated with experimental intervention. |
Ryding 1998.
| Methods | Design: RCT | |
| Participants |
Setting: hospital obstetrics and gynaecology department Type of trauma exposure: EmCS Inclusion criteria: Swedish‐speaking women, subjected to an EmCS delivering a live infant. Exclusion criteria: no additional criteria specified. Sample size: 111 patients screened; 106 randomised and 105 agreed to take part. Mean age: 30 (SD not reported) years Gender: 99 (100%) women Ethnicity: not reported Country: Sweden |
|
| Interventions |
Group 1: counselling: n = 53 Intervention delivered by an obstetrician with a primary psychotherapy qualification. After informed consent, the counsellor booked the participant for a consultation at the maternity ward as soon as practicable. First, the participant was asked to tell her own story about the delivery including the EmCS. Then her thoughts and feelings during 6 phases of the delivery experience (arrival at the unit to sight of the infant for the first time) were explored. The aim of the first consultation was to establish contact and to collect relevant information. The participant was asked what she needed help with. A second consultation took place before the woman was discharged from hospital. The participant was encouraged to talk about her worst memories of and feelings about the delivery. Questions about risks to the life or health of the woman or her baby were dealt with. Existential issues, such as the insecurity of life, were discussed if relevant. The participant was assured that her possible symptoms of post‐traumatic stress reactions were normal under the circumstances. She was instructed how to 'dose' frightening memories when at home. She was encouraged in her role as a new mother. During the third consultation about 2 weeks after delivery, the participant discussed her situation at home and her contact with her baby, her partner and others. Any post‐traumatic stress reactions were again considered. She was given a copy of her complete record, which was examined in detail and explained. Possible feelings of shame or guilt about her performance during or after delivery were discussed, as well as possible feelings of anger or disappointment with the staff. The consultation ended by focusing on a positive memory in relation to the delivery. The fourth consultation took place about 3 weeks after delivery. Thoughts and feelings concerning the delivery experience were again examined. The meaning of the recent EmCS for the individual woman, and what she had learnt from the experience, was discussed. The possibility of another pregnancy was discussed, as well as hopes and misgivings about a future delivery. The participant was given medical advice concerning a possible pregnancy and delivery to come. The first consultation took ≥ 1 hour. The second to fourth meetings were limited to about 45 min. Group 2: TAU: n = 52 A member of the research team contacted participants on the maternity ward. The women completed 3 questionnaires intended to measure the cognitive appraisal of the experienced delivery, the possible presence of post‐traumatic stress reactions and general mental distress. If they had questions regarding the recent delivery or expressed distress of any type, they were encouraged to contact the ward staff. |
|
| Outcomes |
PTSD: IES Other: Symptoms Check List (SCL); Wijma‐Expectancy/Experience Questionnaire Follow‐up: 1 and 6 months' postpartum |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Every second EmCS patient, according to the delivery ward register, was selected for counselling, the remainder being selected for the comparison group. |
| Allocation concealment (selection bias) | High risk | Every second EmCS patient, according to the delivery ward register, was selected for counselling, the remainder being selected for the comparison group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All measures where self‐report which participants completed by post. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout from both groups was low and about equivalent. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | Unclear risk | The study therapist was also the research leader and the study report acknowledges that participant gratitude may have contributed to participant responses. Treatment adherence was not reported. |
Ryding 2004.
| Methods | Design: RCT | |
| Participants |
Setting: hospital obstetrics and gynaecology department Type of trauma exposure: EmCS Inclusion criteria: all Swedish‐speaking women giving birth to a live infant by EmCS at the participating hospital Exclusion criteria: non‐Swedish speakers Sample size: 217 women met the inclusion criteria; 162 were randomised. Mean age: 32 (SD not specified) years, range 19–44 years Gender: 162 (100%) women Ethnicity: not specified Country: Sweden 162 women who had experienced birth by EmCS. Intervention offered to all. |
|
| Interventions |
Group 1: group counselling: n = 89 4 or 5 women were invited to each of the EmCS groups. The group leaders were a maternity and child welfare psychologist and an experienced delivery ward midwife. Consultations lasted for 2 hours, and the groups met twice at a 2‐ to 3‐week interval. The main purpose was to arrange for participants to meet other women who had undergone EmCS so that they could share experiences. It was hoped that the women would be able to discuss both medical procedures (the midwife would have the answers) and psychological matters, such as feelings after a traumatic birth and feelings about motherhood and the baby (the psychologist would be able to provide counselling). It also hoped to identify new mothers in need of individual contact and to provide information about the possibilities of further help. Group 2: TAU: n = 73 Women offered an individual consultation to discuss their recent delivery, if they wished, after completing outcome questionnaires at 6 months' postpartum. Standard care after an EmCS included the midwife and doctor involved in the procedure visiting the mother in the maternity ward so as to exchange information about the experience, although for practical reasons this was not always possible. |
|
| Outcomes |
PTSD: IES Other: Wijma Expectancy/Experience Questionnaire; EPDS Follow‐up: 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women who gave birth on approximately 18 predetermined days of the month were randomised to the counselling group, and the remainder to the control group. |
| Allocation concealment (selection bias) | High risk | Women who gave birth on approximately 18 predetermined days of the month were randomised to the counselling group, and the remainder to the control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All measures where self‐report which participants completed by post. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analyses were based on the number of questionnaires returned. Response rate was high for both groups. Data were reported by median and interquartile range. The reason for this was unclear but may have been because of skew. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | Unclear risk | The group intervention was not manualised and was provided through semi‐structured delivery. Treatment adherence was not reported. |
Taghizadeh 2008.
| Methods | Design: RCT | |
| Participants |
Setting: hospital Type of trauma exposure: traumatic birth Inclusion criteria: women who had experienced a traumatic delivery who had given birth to alive and physically normal baby; could understand and speak Persian; no history of known psychological illnesses or not using psychological drugs; no history of infertility and abortion despite the desire to preserve the foetus Exclusion criteria: mothers who themselves or their infants needed special care; mothers who used external counselling services; mothers who experienced stressful events during the study and ≤ 1 year before childbirth Sample size: 300 women randomised: 150 to each group Mean age: not reported Gender: women Ethnicity: Iranian or who could speak and understand Persian (Farsi) Country: Iran |
|
| Interventions |
Group 1: consultation with midwife: n = 150 The first face‐to‐face consultation session was 72 hours after giving birth. The duration of intervention was 4–6 weeks and each session was 40–60 min. Group 2: TAU |
|
| Outcomes |
PTSD: IES Other: not reported Follow‐up: 3 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | All eligible participants in the first 3 days of week (in Iran it is Sat, Sun, Mon) were assigned to control group and all in other 4 days were assigned to intervention. The next week they exchange the sequence, which means 3 first days of week (in Iran it is Sat, Sun, Mon) were assigned to intervention group and all in other 4 days were assigned to control group. |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trained researcher who was gathering data for postintervention questionnaires was blind about control and intervention groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information on the number of participants who completed treatment and number of dropouts were not reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | Low risk | No other biases indicated. |
Wang 2015.
| Methods | Design: RCT | |
| Participants |
Setting: emergency department of a hospital Type of trauma exposure: severe MVA survivors. Patients were suspected to have experienced possible severe injuries that required specialised acute medical care. Inclusion criteria: aged 18–65 years, and able to communicate in verbal and written Chinese. Exclusion criteria: history of neurological problems, brain surgery, brain damage and spinal cord injuries; current alcohol or drug abuse (or both), and current or past schizophrenic or psychotic disorders. Sample size: 76 individuals assessed for eligibility; 52 randomised. Mean age: 40.3 (SD 14.8) years, based on available data from 46 completers. Gender: 19 (41.3%) men; 27 (58.7%) women, based on available data from 46 completers. Ethnicity: not reported but likely to be Chinese. Country: China |
|
| Interventions |
Group 1: creative arts: n = 26 Intervention delivered in small groups of 4–6 participants in hospital led by a psychologically trained and supervised artist. The primary modalities utilised were creative writing and drawing. Participants were offered 8 weekly 40 min sessions. In the first 2 weeks, participants were requested to perform simple drawing techniques. Weeks 3–5 explored present emotions and issues. Final sessions focused on bringing together resources for moving forward. Group 2: waiting list: n = 26 |
|
| Outcomes |
PTSD: CAPS; IES‐R Other: HADS (depression and anxiety); PTGI Follow‐up: 2, 6 and 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were asked not to share information to the assessors to ensure that they were blind to interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completer‐only analysis, although follow‐up data was available for all participants at 2‐month follow‐up and the majority of participants at subsequent follow‐ups. |
| Selective reporting (reporting bias) | High risk | No study protocol identified. The study authors reported no statistically significant differences between intervention and controls, which meant that it was not possible to include this study in meta‐analysis. This has the potential to lead to an overestimate of effectiveness for those outcomes. |
| Other bias | High risk | Intervention was not manualised so it could not be guaranteed that the therapy was delivered in the same manner to all of the participants. Sample size was small. |
Wijesinghe 2015.
| Methods | Design: RCT | |
| Participants |
Setting: district general hospital Type of trauma exposure: snakebite victims Inclusion criteria: snakebite victims admitted to hospital identified as being envenomed and requiring treatment with antivenom. Exclusion criteria: aged <18 years, known mental illness, and without basic fluency in the Sinhala language. Sample size: 225 snakebite victims randomised. Mean age: 42.1 (SD 12.4) years Gender: 168 (74.7%) men; 57 (25.3%) women Ethnicity: 100% Asian Country: Sri Lanka |
|
| Interventions |
Group 1: PFA and psychoeducation and CBT: n = 75 PFA and psychoeducation following hospital treatment (as described below) and a single session of CBT at 1 month. In this session, victims were initially engaged in a focused discussion on how they had functioned in their daily lives after the snakebite and whether they had any ongoing difficulties. A checklist guided the doctors providing the intervention to identify the victims' dysfunctional cognitions related to health, personal life, functional abilities and overall future expectations. If dysfunctional cognitions were elicited, they were reframed in a positive manner. The doctors also encouraged return to work and normal life. In patients who had not gone back to work to an optimal level, activity scheduling was introduced. A phased return to household and occupational activity was suggested and patients were encouraged to return to their hobbies and pleasurable activities. Other maladaptive coping methods such as substance misuse were discussed and counselling provided as needed. In situations where there was a understandable anxiety about returning to work and being bitten again by a snake, practical safety measures such as wearing boots and gloves and carrying a torch and a stick were encouraged. The duration of this intervention was typically 20 min. Group 2: PFA and psychoeducation: n = 75 Discussion with a non‐specialist doctor about the patient's opinion on the causes and consequences of the snake bite. The intervention followed the normal doctor–patient interview style in which patients were initially engaged in open‐ended questions and allowed to express their views. The conversation was then moved into a more structured discussion, using a structured checklist to ensure a degree of standardisation. This list included important thoughts to elicit, such as myths, negative assumptions, and future plans and expectations of the patient. If any erroneous or maladaptive ideas were identified, they were challenged in a non‐confrontational manner and more plausible, evidence‐based alternative views were expressed. Common misbeliefs which have a negative impact on a person's psychological adaptation and subsequent functional level were specifically addressed. Patients were encouraged to engage in a healthy lifestyle following discharge and to avoid the trap of assuming a sick role. Group 3: no intervention: n = 75 |
|
| Outcomes |
PTSD: PSS‐SR Other: HSCL‐25; BDI; Sheehan Disability Inventory Follow‐up: 6 months |
|
| Notes | Psychological interventions were delivered by a non‐specialist doctors involved in the study were trained by a specialist psychiatrist. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients were assessed for presence of psychological morbidity and functional status 6 months following discharge from hospital by a specialist psychiatrist blind to intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analysis was reported for those available to follow‐up. Loss to follow‐up for the study as a whole was low at 8.4%. However, there was proportionately more missing data for no intervention (9.3%) and PFA only (13.3%) compared to CBT (2.6%). A sensitivity analysis assuming including data for individuals who were unavailable was undertaken based on the assumption of worst clinical assumption. However, the details of these analyses were not reported. |
| Selective reporting (reporting bias) | High risk | A study protocol was available but the planned process of analysis was not explained. The study authors reported no statistically significant differences between intervention and controls, which meant that it was not possible to include this study in meta‐analysis. This has the potential to lead to an overestimate of effectiveness for those outcomes. |
| Other bias | Unclear risk | Psychiatric caseness was based on the use of screening instruments. |
Zatzick 2001.
| Methods | Design: RCT – bias unlikely | |
| Participants |
Setting: level 1 hospital trauma centre Type of trauma exposure: injured MVA and assault victims. Inclusion criteria: hospitalised MVA or assault survivors aged 14–65 years, who were English speaking. Exclusion criteria: patients who sustained severe injuries. Sample size: 105 patients assessed for study participation; 57 eligible to take part; 34 randomised. Mean age: 33.8 (SD 12.1) years Gender: 20 (58.8%) men; 14 (41.7%) women Ethnicity: 61.8% white; 38.2% other Country: USA |
|
| Interventions |
Group 1: collaborative care intervention: n = 16 On the surgical ward, each patient was assigned to a trauma support specialist who met each intervention patient at the bedside. The trauma support specialists were instructed to develop a therapeutic relationship and follow patients for 4 months through primary care outpatient appointments and community rehabilitation. To establish a basis for collaborative problem definition and shared patient–provider treatment planning, the trauma support specialists were instructed to elicit and track patients' post‐traumatic concerns. Patients' post‐traumatic concerns were incorporated into joint problem definition and the trauma support specialists were instructed to intervene on behalf of the patients in the resolution of these concerns whenever possible. A psychotherapy module specifically targeting post‐traumatic distress and substance use was also delivered as part of the multifaceted collaborative intervention. A psychoeducational component of the intervention began with a review of the traumatic event, followed by a discussion of related emotions, cognitions and possible future post‐traumatic symptoms, and closed with suggested coping strategies including algorithms for contacting the support specialist. The motivational enhancement techniques focused on the evaluation of readiness to change and implementation of a motivational interview targeting post‐traumatic alcohol and drug use. Group 2: usual care: n = 18 |
|
| Outcomes |
PTSD: PCL‐C Other: CES‐D; ASI; PCS of the Medical Outcomes Study 12‐Item Short‐Form Health Survey Follow‐up: 1 and 4 months |
|
| Notes | Patients aged 14‐65 were eligible for inclusion in this study. We included it as the majority of participants were likely to have been aged 18 and over based on the mean age of participants (33.8; SD 12.1). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | The project co‐ordinator independently randomised new patients in blocks of 6 to the intervention or control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research associates conducting follow‐up telephone interviews remained blinded to patient group assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome analyses were conducted for the both the ITT sample and for those with complete data on each outcome measure at all time points. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified. |
| Other bias | High risk | Sample size was small. The authors also acknowledge that "there were some difficulties implementing the collaborative care principles of continuous case management and active sustained follow‐up. For instance, patients with no insurance often required intensive service coordination efforts targeting linkage with community agencies. Because these activities required time availability and flexibility of scheduling that taxed the abilities of the highly trained, hospital‐based case managers, these efforts to insure continuity of care frequently fell short." The collaborative intervention was not manualised and was likely to be implemented with marked variability across trauma support specialists. |
ASI: Addiction Severity Index; AUDIT: Alcohol Use Disorders Identification Text; BDI: Beck Depression Inventory; CAPS: Clinician‐Administered PTSD Scale; CBT: cognitive behavioural therapy; CES‐D: Center for Epidemiological Studies Depression Scale; CISD‐A: Critical Incident Stress Debriefing – Adapted; DASS‐21: Depression Anxiety and Stress Scale‐21; DSM: Diagnostic and Statistical Manual of Mental Disorders; EmCS: emergency caesarean section; EPDS: Edinburgh Postnatal Depression Scale; EQ‐5D: EuroQol; GAD: generalised anxiety disorder; GAD‐7: Generalised Anxiety Disorder Assessment; HADS: Hospital Anxiety and Depression Scale; HRQoL: health‐related quality of life; HSCL: Hopkins Symptom Checklist depression scale; HTQ‐IV: Harvard Trauma Questionnaire; ICD: implantable cardioverter defibrillator; ICU: intensive care unit; IES: Impact of Events Scale; IES‐R: Impact of Events Scale – Revised; IPC: interpersonal counselling; ITT: intention‐to‐treat; MA: mortuary attendant; min: minute; MINI‐PTSD: Mini‐International Neuropsychiatric Interview – PTSD; MSI: memory structuring intervention; MSSS: Maternity Social Support Scale; MVA: motor vehicle accident; n: number; NBAS: Neonatal Behavioral Assessment Scale; NICU: neonatal intensive care unit; PCL: Post‐Traumatic Stress Disorder Checklist; PCS: Physical Components Summary; PDS: Posttraumatic Diagnostic Scale; PE: prolonged exposure; PFA: psychological first aid; PHQ: Patient Health Questionnaire; PICU: paediatric intensive care unit; PPQ: Perinatal Posttraumatic Stress Disorder Questionnaire; PRIME: Promoting Resilience In Mothers' Emotions; PROMIS: Patient‐Reported Outcomes Measurement Information System; PSS‐PICU: Parental Stressor Scale – Paediatric Intensive Care Unit; PSS‐I: PTSD Symptom Scale – Interview Version; PSS‐SR: Post‐traumatic Stress Symptom Scale – Self Report; PTGI: Posttraumatic Growth Inventory; PTSD: post‐traumatic stress disorder; PTSS: post‐traumatic stress syndrome; RCT: randomised controlled trial; SAS‐SR: Social Adjustment Scale – Self‐Report; SCCIP‐ND: Surviving Cancer Competently Intervention Program – Newly Diagnosed; SCID: Structured Clinical Interview for DSM; SCL: Symptoms Checklist; SCS: Social Constraints Scale; SD: standard deviation; SF‐36: Short‐Form 36; SOFA: Sequential Organ Failure Assessment; STAI: State‐Trait Anxiety Inventory; TAU: treatment as usual; VAS: visual analogue scale; WHOQOLBREF: World Health Organization Quality of Life Assessment – Brief Version.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ben‐Zion 2018 | Types of participants: treatment study of symptomatic individuals |
| Bisson 2004 | Types of participants: treatment study of symptomatic individuals |
| Bryant 1998 | Types of participants: treatment study of symptomatic individuals |
| Bryant 1999 | Types of participants: treatment study of symptomatic individuals |
| Bryant 2003 | Types of participants: treatment study of symptomatic individuals |
| Bryant 2005 | Types of participants: treatment study of symptomatic individuals |
| Bryant 2008 | Types of participants: treatment study of symptomatic individuals |
| Bugg 2009 | Types of participants: treatment study of symptomatic individuals |
| Cernvall 2015 | Types of participants: treatment study of symptomatic individuals |
| Echeburua 1996 | Types of participants: treatment study of symptomatic individuals |
| Ehlers 2003 | Types of participants: treatment study of symptomatic individuals |
| Foa 2006 | Types of participants: treatment study of symptomatic individuals |
| Freedman (in preparation) | Types of participants: treatment study of symptomatic individuals |
| Freedman (submitted) | Types of participants: treatment study of symptomatic individuals |
| Freyth 2010 | Types of participants: treatment study of symptomatic individuals |
| Jarero 2011 | Types of participants: treatment study of symptomatic individuals |
| Jarero 2015 | Types of participants: treatment study of symptomatic individuals |
| Nixon 2012 | Types of participants: treatment study of symptomatic individuals |
| Nixon 2016 | Types of participants: treatment study of symptomatic individuals |
| O'Donnell (in preparation) | Types of participants: treatment study of symptomatic individuals |
| O'Donnell 2012 | Types of participants: treatment study of symptomatic individuals |
| Resnick 2005 | Types of participants: single session intervention |
| Rose 1999 | Types of participants: single session intervention |
| Rothbaum (submitted) | Types of participants: single session intervention |
| Shalev 2012 | Types of participants: treatment study of symptomatic individuals |
| Shapiro 2015 | Types of participants: treatment study of symptomatic individuals |
| Shapiro 2018 | Types of participants: treatment study of symptomatic individuals |
| Shaw 2013 | Types of participants: treatment study of symptomatic individuals |
| Sijbrandij 2007 | Types of participants: treatment study of symptomatic individuals |
| Skogstad 2015 | Types of participants: treatment study of symptomatic individuals |
| Turpin 2005 | Types of participants: single session intervention |
| van Emmerik 2008 | Types of participants: treatment study of symptomatic individuals |
| Wagner 2007 | Types of participants: treatment study of symptomatic individuals |
| Wu 2014 | Types of participants: treatment study of symptomatic individuals |
| Zatzick 2004 | Types of participants: treatment study of symptomatic individuals |
| Zatzick 2013 | Types of participants: treatment study of symptomatic individuals |
| Zatzick 2015 | Types of participants: treatment study of symptomatic individuals |
| Öst unpublished | Types of participants: treatment study of symptomatic individuals |
Characteristics of studies awaiting assessment [ordered by study ID]
Cairns 2018.
| Methods | Pilot RCT |
| Participants | Family members of individuals admitted to ICU. |
| Interventions | Sensation Awareness Focused Training vs control group |
| Outcomes | HADS, IES, PSS |
| Notes |
Cox 2018b.
| Methods | RCT |
| Participants | ICU patients treated for cardiorespiratory failure. |
| Interventions | A self‐directed mobile app‐based mindfulness programme vs a therapist‐led telephone‐based mindfulness programme vs a web‐based crillness education programme. |
| Outcomes | PTSS, PHQ‐9, GAD‐7, EQ‐5D, PHQ‐15, CAMS‐R, Brief COPE |
| Notes |
Guest 2018.
| Methods | RCT |
| Participants | Adult survivor of an MVA who lodged an insurance claim within 4 months of their MVA |
| Interventions | E‐mailed CBT with telephone support vs healthy lifestyle interventions |
| Outcomes | DASS and IES‐R |
| Notes | Some participants in this study were recruited beyond 3 months. Therefore, inclusion of this study will depend on whether it will be possible to access outcome data for those recruited before 3 months only. The reported results are preliminary and the trial is ongoing. |
Kilpatrick 1984.
| Methods | Not stated |
| Participants | Victims of rape |
| Interventions | Repeated assessment vs delayed assessment vs therapy – Brief Behavioral Intervention |
| Outcomes | Not stated |
| Notes | To date, it has not been possible to access this publication |
Kredentser 2018.
| Methods | Pilot RCT |
| Participants | ICU survivors |
| Interventions | Usual care, ICU diary, psychoeducation, or ICU diary and psychoeducation |
| Outcomes | IES‐R, HADS |
| Notes |
Michelson 2018.
| Methods | RCT |
| Participants | Parents of children admitted to PICU, parental anxiety, depression, global health, post‐traumatic stress, care satisfaction, decision regret and team collaboration |
| Interventions | PICU supports, a navigator‐based communication intervention vs informational brochure |
| Outcomes | Family satisfaction |
| Notes | Information based on conference abstract only. |
Navidian 2017.
| Methods | RCT |
| Participants | Mothers after still birth |
| Interventions | Grief counselling vs usual care |
| Outcomes | PPQ |
| Notes |
Nielsen 2019.
| Methods | RCT |
| Participants | ICU patients and their relatives |
| Interventions | Family authored diaries vs usual care |
| Outcomes | PTSS‐14 |
| Notes |
Rodin 2019.
| Methods | Phase II RCT |
| Participants | Adults with acute leukaemia |
| Interventions | EASE plus usual care vs usual care |
| Outcomes | SASRQ, BDI, Physical symptom burden as measured by the MSAS, Brief Pain Inventory, FACITSp |
| Notes |
Sun 2018.
| Methods | RCT |
| Participants | Pregnant women with foetal abnormalities requiring pregnancy termination |
| Interventions | Family‐support programme vs routine care |
| Outcomes | Family APGAR, IES‐R, Edinburgh Postnatal Depression Scale |
| Notes |
Wade 2019.
| Methods | RCT |
| Participants | Newly diagnosed or recently relapsed adults with acute myeloid leukaemia or acute lymphocytic leukaemia within 1 month of inpatient admission to hospital. |
| Interventions | EASE for acute leukaemia plus usual care vs usual care alone |
| Outcomes | SASRQ |
| Notes | SASRQ evaluates symptoms of acute stress disorder but can reasonably be consider a proxy measure for PTSD. |
Wendlandt 2019.
| Methods | RCT |
| Participants | Surrogate decision makers of patients with chronic critical illness |
| Interventions | Structured family informational and emotional support meetings led by palliative care specialists vs usual care. |
| Outcomes | IES‐R |
| Notes |
BDI: Beck Depression Inventory; Brief COPE: Brief Coping Orientation to Problems Experienced Inventory; CAMS‐R: Cognitive and Affective Mindfulness Scale ‐ Revised; CBT: cognitive behavioural therapy; DASS: Depression Anxiety Stress Scales; EASE: Emotion And Symptom‐focused Engagement; FACITSp: Functional Assessment of Chronic Illness Therapy‐Spiritual Well‐Being Scale; Family APGAR: Family Adaptation Partnership Growth Affection and Resolve index; GAD‐7: Generalised Anxiety Disorder Assessment; HADS: Hospital Anxiety and Depression Scale; ICU: intensive care unit; IES: Impact of Events Scale; IES‐R: Impact of Events Scale – Revised; MSAS: Memorial Symptom Assessment Scale; MVA: motor vehicle accident; PHQ‐9: 9‐item Patient Health Questionnaire; PHQ‐15: 15‐item Patient Health Questionnaire; PICU: paediatric intensive care unit; PPQ: Prenatal Posttraumatic Stress Questionnaire; PSS: Perceived Stress Scale; PTSD: post‐traumatic stress disorder; PTSS: post‐traumatic stress syndrome; RCT: randomised controlled trial; SASRQ: Stanford Acute Stress Reaction Questionnaire.
Characteristics of ongoing studies [ordered by study ID]
ISRCTN39318241.
| Trial name or title | Randomized controlled study of Internet‐based cognitive behavioural therapy for women having post‐traumatic stress after childbirth |
| Methods | RCT |
| Participants | Women, aged ≥ 18 years with a negative birth experience or an immediate caesarean section or a major postpartum haemorrhage (or a combination of these) |
| Interventions | iCBT plus TAU vs TAU |
| Outcomes | Traumatic Event Scale, Edinburgh Postnatal Depression Scale, Satisfaction with Life Scale, Ways of Coping Questionnaire, Communication sub scale from the Evaluation and Nurturing Relationship Issues, Communication and Happiness, Revised Dyadic Adjustment Scale, Postpartum Bonding Questionnaire, EQ‐5D 5D, 36‐item Short Form Health Survey, Hospital Anxiety and Depression Scale |
| Starting date | 28 April 2016 |
| Contact information | Prof Agneta Skoog Svanberg: agneta.skoog_svanberg@kbh.uu.se |
| Notes | Recruitment begins around 8 weeks' postpartum. |
NCT02085512.
| Trial name or title | Prevention of PTSD III: neurocognitive training of emotional regulation |
| Methods | RCT |
| Participants | Adult survivors of traumatic events consecutively admitted to a general hospital emergency department |
| Interventions | Neurobehavioural training group vs control for specific neurocognitive tasks vs control for eventual beneficial effect of performing active computer games |
| Outcomes | CAPS‐IV, PCL‐5, SCID, PDI, BDI, neurocognitive measures |
| Starting date | 13 March 2014 |
| Contact information | Naomi Fine: nomsfine@gmail.com |
| Notes |
NCT02591472.
| Trial name or title | An integrated‐delivery‐of‐care approach to improve patient outcomes, safety, well‐being after orthopaedic trauma |
| Methods | RCT |
| Participants | Patients aged 18–85 years; admitted with severe or multiple orthopaedic trauma (any major bone fractures that impairs mobility or participation in activities of daily living and self‐care, or both); and have received or will receive ≥ 1 surgical procedure for their orthopaedic injuries. |
| Interventions | Integrated care, involving acute care therapies, postacute rehabilitation and follow‐up clinic visits after discharge plus simultaneous psychosocial support vs usual care |
| Outcomes | Physical functioning through various means, PCL, BDI, STAI, Tampa Scale of Kinesiophobia‐11 |
| Starting date | 29 October 2015 |
| Contact information | Heather Vincent: vincehk@ortho.ufl.edu |
| Notes |
NCT03438175.
| Trial name or title | Intensiva 2.0: improve the communication towards families of critically ill patients (Intensiva2) |
| Methods | Cluster RCT |
| Participants | Family members of individuals admitted to an intensive care unit |
| Interventions | Enhanced communication by brochure, website and posters plus TAU vs TAU |
| Outcomes | Comprehension of medical information, Hospital Anxiety and Depression Scale, Short Screening Scale for Symptoms of Post‐traumatic Stress Disorder, PCL‐5, Jefferson Scale for Physician Empathy, Maslach Burnout Inventory |
| Starting date | 1 March 2018 |
| Contact information | Giovanni Mistraletti: giovanni.mistraletti@unimi.it |
| Notes |
NCT03496714.
| Trial name or title | Online psychoeducation for the prevention of PTSD |
| Methods | RCT |
| Participants | Adults who have experienced a Criteria A trauma within the past 30 days, as assessed by the Life Events Checklist for DSM‐5 |
| Interventions | Psychoeducation on safety behaviours and how to fade them vs psychoeducation only vs monitoring only |
| Outcomes | PSS‐SR‐5, PSSI‐5, PCL‐5, PTSD Safety Behavior Inventory, Posttraumatic Cognitions Inventory, Trauma Coping Self‐Efficacy scale, Anxiety Sensitivity Index‐3, BDI, AUDIT‐C, PHQ‐9, GAD‐7 |
| Starting date | 12 April 2018 |
| Contact information | Anna Foulser: afoulser@utexas.edu; Michael Telch: telch@austin.utexas.edu |
| Notes |
NCT03652298.
| Trial name or title | Effects of a neuroscience‐based technique on post‐traumatic stress disorder symptoms, inflammation, and survival in cancer patients announced of a palliative disease progression and their partners (NeuroPrevPTSD) |
| Methods | RCT |
| Participants | Adults who received in the last 7 days the diagnosis of metastatic incurable bladder, prostate, kidney, colorectal or sarcoma cancer |
| Interventions | Memory structuring intervention plus vagal breathing vs support and attention (usual care) |
| Outcomes | PCL‐5, EQ‐5D, Brief Approach/Avoidance Coping Questionnaire |
| Starting date | 25 July 2018 |
| Contact information | Yori Gidron: yori.gidron2@univ‐lille3.fr; Georges‐Michel Reich: M‐Reich@o‐lambret.fr |
| Notes |
Wells 2018.
| Trial name or title | Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway using group‐based metacognitive therapy (PATHWAY Group MCT): study protocol for a randomised controlled trial |
| Methods | Multicentre RCT |
| Participants | Heart disease patients |
| Interventions | Group‐based metacognitive therapy plus usual cardiac rehabilitation vs usual cardiac rehabilitation alone |
| Outcomes | HADS, IES‐R, MCQ‐30, EQ‐5D‐5L |
| Starting date | 2015 |
| Contact information | Adrian Wells: adrian.wells@manchester.ac.uk |
| Notes |
AUDIT‐C: Alcohol Use Disorders Identification Test – C; BDI: Beck Depression Inventory; CAPS‐IV: Clinician‐Administered PTSD Scale; DSM‐5: Diagnostic and Statistical Manual of Mental Disorders – 5th edition; EQ‐5D; EuroQol; GAD‐7: Generalised Anxiety Disorder Assessment; HADS: Hospital Anxiety and Depression Scale; iCBT: Internet‐based cognitive behavioural therapy; IES‐R: Impact of Events Scale – Revised; MCQ‐30: Metacognitions Questionnaire 30; PCL‐5: Post‐Traumatic Stress Disorder Checklist; PDI: Peritraumatic Distress Inventory; PHQ‐9: Patient Health Questionnaire; PSS‐SR‐5: Post‐traumatic Stress Symptom Scale – Self Report; PSSI‐5: Posttraumatic Stress Disorder Symptom Scale Interview for DSM‐5; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; STAI: State‐Trait Anxiety Inventory; TAU: treatment as usual.
Differences between protocol and review
In the previous version of this review, we reported severity of PTSD symptoms as measured by a clinician‐administered measure or a self‐report measure separately. Although clinician‐administered measures are considered to be the 'gold standard' in the traumatic stress field many studies only report outcomes for self‐report measures. Therefore, we used both clinician‐administered and self‐report measures as an index of PTSD severity. When a study used both types of outcome, we gave priority to clinician‐administered measures.
Previously we evaluated risk of bias using a 19‐item checklist that we developed for the review. Since publication of this review, the 'Risk of bias' tool has become the established means by which to evaluate study quality and we, therefore, used this in this review.
Previously we reported findings by the follow‐up periods seven to nine months and 10 to 12 months separately. For this update, we decided to combine outcomes for these periods to seven to 12 months. Previously we did not specify the primary outcome period. For this review, we identified this as three to six months' post‐trauma.
In the previous review, we planned to undertake separate ITT and completer‐only analyses. In this review, we endeavoured to use ITT data wherever possible, but given the heterogeneity of interventions and the limited number of studies, we decided not to separate ITT and completer analyses in this version.
Contributions of authors
NPR: writing of the protocol and review. Undertook quality assessment and data entry.
NJK: commentary on the protocol and review. Undertook quality assessment and recording of data.
JK: commentary on the protocol and review. Undertook quality assessment and recording of data.
LR: writing of the review.
CL: screening of titles and abstracts.
JIB: offered supervision of the protocol development and commentary on the protocol and review. Writing of the 'Discussion' section of the review.
Sources of support
Internal sources
No sources of support provided, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
LR is funded by Cochrane Infrastructure funding to the Common Mental Disorders Cochrane Review Group
Declarations of interest
NPR: none.
NJK: none.
JK: none.
LR: none.
CL: none.
JIB: none.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Als 2015 {published and unpublished data}
- Als LC, Nadel S, Cooper M, Vickers B, Garralda ME. A supported psychoeducational intervention to improve family mental health following discharge from paediatric intensive care: feasibility and pilot randomised controlled trial. BMJ Open 2015;5(12):e009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andre 1997 {published data only}
- Andre C, Lelord F, Legeron P, Reignier A, Delattre A. Effectiveness of early intervention on 132 bus drivers victims of aggression: a controlled trial [Etude controlee sur l'efficacite a 6 mois d'une prise en charge precoce de 132 conducteurs d'autobus victimes d'agression]. Encephale 1997;23:65‐71. [PubMed] [Google Scholar]
Biggs 2016 {published data only (unpublished sought but not used)}
- Biggs QM, Fullerton CS, McCarroll JE, Liu X, Wang L, Dacuyan NM, et al. Early intervention for post‐traumatic stress disorder, depression, and quality of life in mortuary affairs soldiers postdeployment. Military Medicine 2016;181(11):e1553‐60. [DOI] [PubMed] [Google Scholar]
Borghini 2014 {published data only}
- Borghini A, Habersaat S, Forcada‐Geux M, Nessi J, Pierrehumbert B, Ansermet F, et al. Effects of an early intervention on maternal post‐traumatic stress symptoms and the quality of mother‐infant interaction: the case of preterm birth. Infant Behavior & Development 2014;37(4):624‐31. [DOI] [PubMed] [Google Scholar]
Brom 1993 {published data only}
- Brom D, Kleber RJ, Hofman M. Victims of traffic accidents: Incidence and prevention of post‐traumatic stress disorder. Journal of Clinical Psychology 1993;49(2):131‐40. [DOI] [PubMed] [Google Scholar]
Brunet 2013 {published data only}
- Brunet A, Groseilliers IB, Cordova MJ, Ruzek JI. Randomized controlled trial of a brief dyadic cognitive‐behavioral intervention designed to prevent PTSD. European Journal of Psychotraumatology 2013;26(4):21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2018a {published data only}
- Cox CE, Carson SS, Hough CL, Kahn J, White DB, Olsen MK, et al. [Coping skills training to improve psychological distress among critical illness survivors: a randomized clinical trial]. American Journal of Respiratory and Critical Care Medicine. Conference: American Thoracic Society International Conference. 2017; Vol. 195:A6732. [DOI: 10.1164/ajrccmconference.2017.C94] [DOI]
- Cox CE, Hough CL, Carson SS, White DB, Kahn JM, Olsen MK, et al. Effects of a telephone‐ and web‐based coping skills training program compared with an education program for survivors of critical illness and their family members a randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2018;197(1):66‐78. [DOI] [PubMed] [Google Scholar]
Curtis 2016 {published and unpublished data}
- Curtis JR, Treece PD, Nielsen EL, Gold J, Ciechanowski PS, Shannon SE, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end‐of‐life care. American Journal of Respiratory and Critical Care Medicine 2016;193(2):154‐62. [DOI: 10.1164/rccm.201505-0900OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamble 2005 {published data only}
- Gamble J, Creedy D, Moyle W, Webster J, McAllister M, Dickson P. Effectiveness of a counseling intervention after a traumatic childbirth: a randomized controlled trial. Birth 2005;32(1):11‐9. [DOI] [PubMed] [Google Scholar]
Gamble 2010 {unpublished data only}
- Creedy DK, Gamble J, Fenwick J. Effect of a midwife‐led counselling on mental health outcomes for women experiencing a traumatic childbirth: a RCT. Archives of Women's Mental Health 2013;Suppl 1:S3. [Google Scholar]
- Fenwick J, Gamble J, Creedy D, Barclay L, Buist A, Ryding EL. Women's perceptions of emotional support following childbirth: a qualitative investigation. Birth 2013;29:217‐24. [DOI] [PubMed] [Google Scholar]
- Gamble J. Do women who have experienced a traumatic birth and are provided with a midwife led counselling intervention compared with parenting support experience lower levels of postnatal distress?. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=320476 (first received 1 February 2010).
- Turkstra E, Gamble J, Creedy DK, Fenwick J, Barclay L, Buist A, et al. PRIME: impact of previous mental health problems on health‐related quality of life in women with childbirth trauma. Archives of Women's Mental Health 2013;16(6):561‐4. [DOI] [PubMed] [Google Scholar]
Gidron 2001 {published data only (unpublished sought but not used)}
- Gidron Y, Gal R, Freedman S, Twiser I, Lauden A, Snir Y, et al. Translating research findings to PTSD prevention: results of a randomized‐controlled pilot study. Journal of Traumatic Stress 2001;14(4):773‐80. [DOI] [PubMed] [Google Scholar]
Gidron 2007 {published data only (unpublished sought but not used)}
- Gidron Y, Gal R, Givati G, Lauden A, Snir Y, Binjamin J. Interactive effects of memory structuring and gender in preventing posttraumatic stress symptoms. Journal of Nervous and Mental Disease 2007;195(2):179‐82. [DOI] [PubMed] [Google Scholar]
Holmes 2007 {published data only}
- Holmes A, Hodgins G, Adey S, Menzel S, Danne P, Kossmann T, et al. Trial of interpersonal counselling after major physical trauma. Australian and New Zealand Journal of Psychiatry 2007;41:926‐33. [DOI] [PubMed] [Google Scholar]
Irvine 2011 {published and unpublished data}
- Ford J, Rosman L, Wuensch K, Irvine J, Sears SF. Cognitive‐behavioral treatment of posttraumatic stress in patients with implantable cardioverter defibrillators: results from a randomized controlled trial. Journal of Traumatic Stress 2016;29(4):388‐92. [DOI] [PubMed] [Google Scholar]
- Irvine J, Firestone J, Ong L, Cribbie R, Dorian P, Harris L, et al. A randomized controlled trial of cognitive behavior therapy tailored to psychological adaptation to an implantable cardioverter defibrillator. Psychosomatic Medicine 2011;73:226‐33. [DOI: 10.1097/PSY.0b013e31820afc63] [DOI] [PubMed] [Google Scholar]
- Irvine J, Firestone J, Ong L, Cribbie R, Ritvo P, Katz J, et al. Acceptability of a cognitive behavior therapy intervention to implantable cardioverter defibrillator recipients. Journal of Cognitive Psychotherapy 2010;24:246‐64. [Google Scholar]
Jensen 2016 {published and unpublished data}
- Jensen J, Egerod I, Bestle MH, Christensen, DF, Elklit, A, Hansen Rl, et al. The effectiveness of a recovery program aimed at improving quality of life and sense of coherence in post intensive care patients: a pragmatic multicenter randomized controlled trial, the recovery and aftercare of post intensive care patients (RAPIT) study. Annual Congress of the European Society of Intensive Care Medicine, ESICM; 2016 Oct 1‐5; Milan (Italy). 2016:A38. [DOI: 10.1186/s40635-016-0098-x] [DOI]
- Jensen JF, Egerod I, Bestle MH, Christensen DF, Elklit A, Hansen RL, et al. A recovery program to improve quality of life, sense of coherence and psychological health in ICU survivors: a multicenter randomized controlled trial, the RAPIT study. Intensive Care Medicine 2016;42(11):1733‐43. [DOI: 10.1007/s00134-016-4522-1] [DOI] [PubMed] [Google Scholar]
Jones 2010 {published and unpublished data}
- Jones C, Bäckman C, Capuzzo M, Egerod I, Flaatten H, Granja C, et al. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Critical Care 2010;14(5):R168. [DOI: 10.1186/cc9260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazak 2005 {published data only}
- Kazak AE, Simms S, Alderfer MA, Rourke MT, Crump T, McClure K, et al. Feasibility and preliminary outcomes from a pilot study of a brief psychological intervention for families of children newly diagnosed with cancer. Journal of Pediatric Psychology 2005;30(8):644‐55. [DOI] [PubMed] [Google Scholar]
Lindwall 2014 {published data only}
- Lindwall JJ, Russell K, Huang Q, Zhang H, Vannatta K, Barrera M, et al. Adjustment in parents of children undergoing stem cell transplantation. Biology of Blood and Marrow Transplantation 2014;20(4):543‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchand 2006 {published data only}
- Marchand A, Guay S, Boyer R, Lucci S, Martin A, St‐Hilaire M. A randomized controlled trial of an adapted form of individual critical incident stress debriefing for victims of an armed robbery. Brief Treatment and Crisis Intervention 2006;6:122‐9. [Google Scholar]
Mouthaan 2013 {published and unpublished data}
- Mouthaan J, Sijbrandij M, Vries GJ, Reitsma JB, Schoot R, Goslings JC, et al. Internet‐based early intervention to prevent posttraumatic stress disorder in injury patients: randomized controlled trial. Journal of Medical Internet Research 2013;15(8):e165v. [DOI: 10.2196/jimr.2460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rothbaum 2012 {published data only}
- Malcoun E, Houry D, Arndt‐Jordan C, Kearns MC, Zimmerman L, Hammond‐Susten M, et al. Feasibility of identifying eligible trauma patients for posttraumatic stress disorder intervention.. West Journal of Emergency Medicine 2010;11(3):274‐8. [PMC free article] [PubMed] [Google Scholar]
- Price M, Kearns M, Houry D, Rothbaum BO. Emergency department predictors of posttraumatic stress reduction for trauma‐exposed individuals with and without an early intervention. Journal of Consulting and Clinical Psychology 2014;82(2):336‐41. [DOI: 10.1037/a0035537] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ. Early intervention may prevent the development of posttraumatic stress disorder: a randomized pilot civilian study with modified prolonged exposure. Biological Psychiatry 2012;72(11):957‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Reiser E, Davis JS, Kerley KA, Rothbaum AO, et al. Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. Journal of Clinical Psychiatry 2014;75(12):1380‐7. [DOI: 10.4088/JCP.13m08715] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryding 1998 {published data only}
- Ryding EL, Wijma K, Wijma B. Postpartum counselling after an emergency cesarean. Clinical Psychology and Psychotherapy 1998;5:231‐7. [Google Scholar]
Ryding 2004 {published and unpublished data}
- Ryding EL, Wiren E, Johansson G, Ceder B, Dahlstrom A. Group counselling for mothers after emergency cesarean section: a randomized controlled trial of intervention. Birth 2004;31(4):247‐53. [DOI] [PubMed] [Google Scholar]
Taghizadeh 2008 {published data only}
- Taghizadeh Z, Jafar B, Arbabi M, Faghihzadeh S. The effect of counseling on post traumatic stress disorder after a traumatic childbirth. Hayat 2008;13(4):23‐31. [Google Scholar]
Wang 2015 {published data only (unpublished sought but not used)}
- Wang X, Lan C, Chen J, Wang W, Zhang H, Li L. Creative arts program as an intervention for PTSD: a randomized clinical trial with motor vehicle accident survivors. International Journal of Clinical and Experimental Medicine 2015;8(8):13585‐91. [PMC free article] [PubMed] [Google Scholar]
Wijesinghe 2015 {published data only (unpublished sought but not used)}
- Wijesinghe CA, Williams SS, Kasturiratne A, Dolawaththa N, Wimalaratne P, Wijewickrema B, et al. A randomized controlled trial of a brief intervention for delayed psychological effects in snakebite victims. PLoS Neglected Tropical Diseases 2015;9(8):e0003989. [DOI: 10.1371/journal.pntd.0003989] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zatzick 2001 {published and unpublished data}
- Zatzick DF, Roy‐Byrne P, Russo JE, Rivara FP, Koike A, Jurkovich GJ, et al. Collaborative interventions for physically injured trauma survivors: a pilot randomized effectiveness trial. General Hospital Psychiatry 2001;23:114‐23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ben‐Zion 2018 {published and unpublished data}
- Ben‐Zion Z, Fine NB, Keynan NJ, Admon R, Green N, Halevi M, et al. Cognitive flexibility predicts PTSD symptoms: observational and interventional studies. Frontiers in Psychiatry 2018;9:477. [DOI: 10.3389/fpsyt.2018.00477] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine NB, Achituv M, Etkin A, Merin O, Shalev AY. Evaluating web‐based cognitive‐affective remediation in recent trauma survivors: study rationale and protocol. European Journal of Psychotraumatology 2018;9(1):1442602. [DOI: 10.1080/20008198.2018.1442602] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisson 2004 {published and unpublished data}
- Bisson JI, Shepherd JP, Joy D, Probert R, Newcombe RG. Early cognitive‐behavioural therapy for post‐traumatic stress symptoms after physical injury. British Journal of Psychiatry 2004;184:63‐9. [DOI] [PubMed] [Google Scholar]
Bryant 1998 {published and unpublished data}
- Bryant RA, Harvey AG, Basten C, Dang ST, Sackville T. Treatment of acute stress disorder: a comparison of cognitive‐behavioral therapy and supportive counseling. Journal of Consulting and Clinical Psychology 1998;66(5):862‐6. [DOI] [PubMed] [Google Scholar]
Bryant 1999 {published and unpublished data}
- Bryant RA, Sackville T, Dang ST, Moulds M, Guthrie R. Treating acute stress disorder: an evaluation of cognitive behavior therapy and supportive counseling techniques. American Journal of Psychiatry 1999;156(11):1780‐6. [DOI] [PubMed] [Google Scholar]
Bryant 2003 {published and unpublished data}
- Bryant RA, Moulds M, Guthrie R, Nixon RD. Treating acute stress disorder following mild traumatic brain injury. American Journal of Psychiatry 2003;160:585‐7. [DOI] [PubMed] [Google Scholar]
Bryant 2005 {published and unpublished data}
- Bryant RA, Moulds ML, Guthrie RM, Nixon RD. The additive benefits of hypnosis and cognitive‐behavioral therapy in treating acute stress disorder. Journal of Consulting and Clinical Psychology 2005;73(2):334‐40. [DOI] [PubMed] [Google Scholar]
Bryant 2008 {published and unpublished data}
- Bryant RA, Mastrodomenico J, Felmingham KL, Hopwood S, Kenny L, Kandris E, et al. Treatment of acute stress disorder: a randomized controlled trial. Archives of General Psychiatry 2008;65(6):659‐67. [DOI] [PubMed] [Google Scholar]
Bugg 2009 {published and unpublished data}
- Bugg A, Turpin G, Mason S, Scholes C. A randomised controlled trial of the effectiveness of writing as a self‐help intervention for traumatic injury patients at risk of developing post‐traumatic stress disorder. Behaviour Research and Therapy 2009;47(1):6‐12. [DOI] [PubMed] [Google Scholar]
Cernvall 2015 {published data only}
- Cernvall M, Carlbring P, Ljungman L, Ljungman G, Essen L. Internet‐based guided self‐help for parents of children on cancer treatment: a randomized controlled trial. Psycho‐oncology 2015;24(9):1152‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Echeburua 1996 {published data only}
- Echeburua E, Corral P, Sarasua B, Zubizarreta I. Treatment of acute posttraumatic stress disorder in rape victims: an experimental study. Journal of Anxiety Disorders 1996;10(3):185‐99. [Google Scholar]
Ehlers 2003 {published and unpublished data}
- Ehlers A, Clark DM, Hackmann A, McManus F, Fennell M, Herbert C, et al. A randomized controled trial of cognitive therapy, a self help booklet, and repeated assessments as early interventions for posttraumatic stress disorder. Archives of General Psychiatry 2003;60:1024‐32. [DOI] [PubMed] [Google Scholar]
Foa 2006 {published and unpublished data}
- Foa EB, Zoellner LA, Feeny NC. An evaluation of three brief programs for facilitating recovery after assault. Journal of Traumatic Stress 2006;19(1):29‐43. [DOI] [PubMed] [Google Scholar]
Freedman (in preparation) {unpublished data only}
- Freedman S. Early intervention for preventing posttraumatic stress disorder: an Internet based treatment. Manuscript In preparation. [DOI] [PMC free article] [PubMed]
- Freedman S, Hoffman H. Interrupting traumatic memories: an emergency room virtual reality intervention for pain reduction and the prevention of PTSD. Journal of Cyber Therapy and Rehabilitation 2016;9(1):39. [Google Scholar]
- Freedman SA, Dayan E, Kimelman YB, Weissman H, Eitan R. Early intervention for preventing posttraumatic stress disorder: an Internet‐based virtual reality treatment. European Journal of Psychotraumatology 2015;6:25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freedman (submitted) {unpublished data only}
- Freedman S. Early telephone cognitive behavioral therapy (ET‐CBT) for the prevention of PTSD. Manuscript submitted for peer review.
Freyth 2010 {published and unpublished data}
- Freyth C, Elsesser K, Lohrmann T, Sartory G. Effects of additional prolonged exposure to psychoeducation and relaxation in acute stress disorder. Journal of Anxiety Disorders 2010;24(8):909‐17. [DOI] [PubMed] [Google Scholar]
Jarero 2011 {published data only}
- Jarero I, Artegas L, Luber M. The EMDR protocol for recent critical incidents: application in a disaster mental health continuum of care context. Journal of EMDR Practice and Research 2011;5(3):82‐94. [Google Scholar]
Jarero 2015 {published data only}
- Jarero I, Uribe S, Artigas L, Givaudan M. EMDR protocol for recent critical incidents: a randomized controlled trial in a technological disaster context. Journal of EMDR Practice and Research 2015;9:166‐73. [Google Scholar]
Nixon 2012 {published data only}
- Nixon RD. Cognitive processing therapy versus supportive counseling for acute stress disorder following assault: a randomized pilot trial. Behavior Therapy 2012;43(4):825‐36. [DOI: 10.1016/j.beth.2012.05.001] [DOI] [PubMed] [Google Scholar]
- Nixon RD. Using cognitive processing therapy for assault victims with acute stress disorder. 29th Australian Association for Cognitive and Behaviour Therapy Annual Conference; 2006 Oct 18‐23; Sydney (NSW). 2006:30.
Nixon 2016 {published data only}
- Nixon RD, Best T, Wilksch SR, Angelakis S, Beatty LJ, Weber N. Cognitive processing therapy for the treatment of acute stress disorder following sexual assault: a randomised effectiveness study. Behaviour Change 2016;33(4):232‐50. [Google Scholar]
O'Donnell (in preparation) {unpublished data only}
- O'Donnell M, Lau W, Alkemade N, Fletcher S, Holmes A, Forbes D. The efficacy of telephone delivered cognitive behavioural therapy as an early intervention for high anxiety and affective symptoms after injury. Manuscript in preparation.
O'Donnell 2012 {published data only}
- O'Donnell ML, Lau W, Tipping S, Holmes AC, Ellen S, Judson R, et al. Stepped early psychological intervention for posttraumatic stress disorder, other anxiety disorders, and depression following serious injury. Journal of Traumatic Stress 2012;25(2):125‐33. [DOI] [PubMed] [Google Scholar]
Öst unpublished {unpublished data only}
- Öst LG, Paunovic N, Gillow AM. Cognitive‐behavior therapy in the prevention of chronic PTSD in crime victims. Unpublished manuscript.
Resnick 2005 {published data only}
- Resnick H, Acierno R, Kilpatrick DG, Holmes M. Description of an early intervention to prevent substance abuse and psychopathology in recent rape victims. Behavior Modification 2005;29(1):156‐88. [DOI] [PubMed] [Google Scholar]
Rose 1999 {published data only}
- Rose S, Brewin CR, Andrews B, Kirk M. A randomized controlled trial of individual psychological debriefing for victims of violent crime. Psychological Medicine 1999;29:793‐9. [DOI] [PubMed] [Google Scholar]
Rothbaum (submitted) {unpublished data only}
- Rothbaum BA, Houry D, Heekin M, Leiner AS, Daugherty J, Smith LS, et al. A pilot study of an exposure‐based intervention in the emergency department designed to prevent posttraumatic stress disorder. American Journal of Emergency Medicine 2008;26(3):326‐30. [DOI] [PubMed] [Google Scholar]
Shalev 2012 {published data only}
- Shalev AY, Ankri Y, Gilad M, Israeli‐Shalev Y, Peleg T, Adessky R, et al. Long‐term outcome of early interventions to prevent posttraumatic stress disorder. Journal of Clinical Psychiatry 2016;77(5):580‐7. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Ankri Y, Israeli‐Shalev Y, Peleg T, Adessky R, Freedman S. Prevention of posttraumatic stress disorder by early treatment: results from the Jerusalem trauma outreach and prevention study. Archives of General Psychiatry 2012;69(2):166‐76. [DOI] [PubMed] [Google Scholar]
Shapiro 2015 {published data only}
- Shapiro E, Laub B. Early EMDR intervention following a community critical incident: a randomized clinical trial. Journal of EMDR Practice and Research 2015;9(1):17‐27. [Google Scholar]
Shapiro 2018 {unpublished data only}
- Shapiro E, Laub B, Rozenblat O. Early EMDR Intervention following intense rocket attacks on a town: a randomised clinical trial. Clinical Neuropsychiatry 2018;15(3):194‐205. [Google Scholar]
Shaw 2013 {published data only (unpublished sought but not used)}
- Shaw RJ, John N, Lilo EA, Jo B, Benitz W, Stevenson DK, Horwitz SM. Prevention of traumatic stress in mothers with preterm infants: A randomized controlled trial. Pediatrics 2013;132(4):e886‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, John N, Lilo EA, Jo B, Benitz W, Stevenson DK, et al. Prevention of traumatic stress in mothers of preterms: 6‐month outcomes. Pediatrics 2014;134(2):e481‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sijbrandij 2007 {published and unpublished data}
- Sijbrandij M, Olff M, Reitsma JB, Carlier IV, Vries MH, Gersons BP. Treatment of acute posttraumatic stress disorder with brief cognitive behavioural therapy: a randomised controlled trial. American Journal of Psychiatry 2007;164:82‐90. [DOI] [PubMed] [Google Scholar]
Skogstad 2015 {published data only}
- Skogstad L, Hem E, Sandvik L, Ekeberg O. Nurse‐led psychological intervention after physical traumas: a randomized controlled trial. Journal of Clinical Medicine Research 2015;7(5):339‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turpin 2005 {published data only}
- Turpin G, Downs M, Mason S. Psychological debriefing for preventing post traumatic stress disorder (PTSD). British Journal of Psychiatry 2005;187(1):76‐82. [Google Scholar]
van Emmerik 2008 {published and unpublished data}
- Emmerik AA, Kamphuis JH, Emmelkamp PM. Treating acute stress disorder and posttraumatic stress disorder with cognitive behavioural therapy or structured writing therapy: a randomised controlled trial. Psychotherapy and Psychosomatics 2008;77(2):93‐100. [DOI] [PubMed] [Google Scholar]
Wagner 2007 {published and unpublished data}
- Wagner AW, Zatzick DF, Ghesquiere A, Jurkovoich GJ. Behavioral activation as an early intervention for posttraumatic stress disorder and depression. Cognitive and Behavioral Practice 2007;14(4):341‐9. [Google Scholar]
Wu 2014 {published and unpublished data}
- Wu KK, Li FW, Cho VW. A randomized controlled trial of the effectiveness of brief‐CBT for patients with symptoms of posttraumatic stress following a motor vehicle crash. Behavioural and Cognitive Psychotherapy 2014;42(1):31‐47. [DOI] [PubMed] [Google Scholar]
Zatzick 2004 {published and unpublished data}
- Zatzick D, Roy‐Byrne P, Russo J, Rivara F, Droesch R, Wagner A, et al. A randomized effectiveness trial of stepped collaborative care for acutely injured trauma survivors. Archives of General Psychiatry 2004;61(5):498‐506. [DOI] [PubMed] [Google Scholar]
Zatzick 2013 {published data only}
- Osenbach JE, Lewis C, Rosenfeld B, Russo J, Ingraham LM, Peterson R, et al. Exploring the longitudinal trajectories of posttraumatic stress disorder in injured trauma survivors. Psychiatry 2014;77(4):386‐97. [DOI: 10.1521/psyc.2014.77.4.386] [DOI] [PubMed] [Google Scholar]
- Zatzick D, Jurkovich G, Rivara FP, Russo J, Wagner A, Wang J, et al. A randomized stepped care intervention trial targeting posttraumatic stress disorder for surgically hospitalized injury survivors. Annals of Surgery 2013;257(3):390‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zatzick 2015 {published data only}
- Darnell D, O'Connor S, Wagner A, Russo J, Wang J, Ingraham L, et al. Enhancing the reach of cognitive‐behavioral therapy targeting posttraumatic stress in acute care medical settings. Psychiatric Services 2017;68(3):258‐63. [DOI] [PubMed] [Google Scholar]
- Zatzick D, O'Connor SS, Russo J, Wang J, Bush N, Love J, et al. Technology‐enhanced stepped collaborative care targeting posttraumatic stress disorder and comorbidity after injury: a randomized controlled trial. Journal of Traumatic Stress 2015;28(5):391‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cairns 2018 {published data only}
- Cairns PL. Prevention of post intensive care syndrome‐family with sensation awareness focused training intervention: a randomized controlled trial pilot study. Dissertation Abstracts International: Section B: the Sciences and Engineering 2018;79(10‐B(E)). [Google Scholar]
Cox 2018b {published data only}
- Cox CE, Hough C, Jones D, Ungar A, Reagan W, Key MD, et al. Effect of a self‐directed mobile app mindfulness program for ICU survivors: a pilot RCT. American Journal of Respiratory and Critical Care Medicine 2018;197:A6185. [Google Scholar]
- Cox CE, Hough CL, Jones DM, Ungar A, Reagan W, Key MD, et al. Effects of mindfulness training programmes delivered by a self‐directed mobile app and by telephone compared with an education programme for survivors of critical illness: a pilot randomised clinical trial. Thorax 2018;74(1):33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guest 2018 {published data only}
- Guest R, Tran Y, Gopinath B, Cameron ID, Craig A. Psychological distress following a motor vehicle crash: preliminary results of a randomised controlled trial investigating brief psychological interventions. Trials 2018;19(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilpatrick 1984 {published data only}
- Kilpatrick DG, Veronen LJ. Treatment of rape‐related problems: crisis intervention is not enough. In: Cohen L, Claiborn W, Specter G editor(s). Crisis Intervention: Community‐Clinical Psychology Series. Second Edition. New York (NY): Human Services Press, 1984. [Google Scholar]
Kredentser 2018 {published data only}
- Kredentser MS, Blouw M, Marten N, Sareen J, Bienvenu OJ, Ryu J, et al. Preventing posttraumatic stress in ICU survivors: a single‐center pilot randomized controlled trial of ICU diaries and psychoeducation. Critical Care Medicine 2018;46(12):1914‐22. [DOI] [PubMed] [Google Scholar]
Michelson 2018 {published data only}
- Michelson K, Rychlik K, Ciolino J, Martinez E, Persell S, Fragen P, et al. A randomized trial in the PICU comparing a communication intervention with an informational brochure. Critical Care Medicine 2018;46(Suppl 1):418. [Google Scholar]
Navidian 2017 {published data only}
- Navidian A, Saravani Z, Shakiba M. Impact of psychological grief counseling on the severity of post‐traumatic stress symptoms in mothers after stillbirths. Issues in Mental Health Nursing 2017;38(8):650‐4. [DOI] [PubMed] [Google Scholar]
Nielsen 2019 {published data only}
- Nielsen AH, Angel S, Egerod I, Hansen TB. The effect of diaries written by relatives for intensive care patients on posttraumatic stress (DRIP study): protocol for a randomized controlled trial and mixed methods study. BMC Nursing 2018;17:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen AH, Angel S, Egerod I, Lund TH, Renberg M, Hansen TB. The effect of family‐authored diaries on posttraumatic stress disorder in intensive care unit patients and their relatives: a randomised controlled trial (DRIP‐study). Australian Critical Care 2019;19:19. [DOI] [PubMed] [Google Scholar]
Rodin 2019 {published data only}
- Rodin G, Malfitano C, Rydall A, Schimmer A, Marmar CM, Mah K, et al. Emotion And Symptom‐focused Engagement (EASE): a randomized phase II trial of an integrated psychological and palliative care intervention for patients with acute leukemia. Support Care Cancer 2019 Apr 17 [Epub ahead of print]. [DOI: 10.1007/s00520-019-04723-2] [DOI] [PubMed]
Sun 2018 {published data only}
- Sun S, Li J, Ma Y, Bu H, Luo Q, Yu X. Effects of a family‐support programme for pregnant women with foetal abnormalities requiring pregnancy termination: a randomized controlled trial in China. International Journal of Nursing Practice 2018;24(1):e12614. [DOI] [PubMed] [Google Scholar]
Wade 2019 {published data only}
- Richards‐Belle A, Mouncey PR, Wade D, Brewin CR, Emerson LM, Grieve R, et al. Psychological Outcomes following a nurse‐led Preventative Psychological Intervention for critically ill patients (POPPI): protocol for a cluster‐randomised clinical trial of a complex intervention. BMJ Open 2018;8(2):e020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade DM, Mouncey PR, Richards‐Belle A, Wulff J, Harrison DA, Sadique MZ, et al. Effect of a nurse‐led preventive psychological intervention on symptoms of posttraumatic stress disorder among critically ill patients: a randomized clinical trial. JAMA 2019;321(7):665‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff J, Sadique Z, Grieve R, Howell D, Mouncey P, Wade D, et al. Psychological outcomes following a nurse‐led preventative psychological intervention for critically ill patients trial: statistical and health economic analysis plan. Journal of the Intensive Care Society 2018;19(4):281‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wendlandt 2019 {published data only}
- Carson SS, Cox CE, Wallenstein S, Hanson LC, Danis M, Tulsky JA, et al. Effect of palliative care‐led meetings for families of patients with chronic critical illness: a randomized clinical trial. JAMA 2016;316(1):51‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendlandt B, Ceppe A, Choudhury S, Cox CE, Hanson LC, Danis M, et al. Modifiable elements of ICU supportive care and communication are associated with surrogates' PTSD symptoms. Intensive Care Medicine 2019;45(5):619‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN39318241 {published data only}
- Sjomark J, Parling T, Jonsson M, Larsson M, Skoog Svanberg A. A longitudinal, multi‐centre, superiority, randomized controlled trial of Internet‐based cognitive behavioural therapy (iCBT) versus treatment‐as‐usual (TAU) for negative experiences and posttraumatic stress following childbirth: the JUNO study protocol. BMC Pregnancy and Childbirth 2018;18(1):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02085512 {published data only}
- Fine NB, Achituv M, Etkin A, Merin O, Shalev AY. Evaluating web‐based cognitive‐affective remediation in recent trauma survivors: study rationale and protocol. European Journal of Psychotraumatology 2018;9(1):1442602. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02591472 {published data only}
- Zdziarski‐Horodyski L, Horodyski M, Sadasivan KK, Hagen J, Vasilopoulos T, Patrick M, et al. An integrated‐delivery‐of‐care approach to improve patient reported physical function and mental wellbeing after orthopedic trauma: study protocol for a randomized controlled trial. Trials 2018;19(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03438175 {published data only}
- NCT03438175. Intensiva 2.0: improve the communication towards families of critically ill patients. clinicaltrials.gov/show/NCT03438175 (first received 19 February 2018).
NCT03496714 {published data only}
- NCT03496714. Online psychoeducation for the prevention of PTSD. clinicaltrials.gov/show/NCT03496714 (first received 12 April 2018).
NCT03652298 {published data only}
- NCT03652298. Effects of a neuroscience‐based technique on post‐traumatic stress disorder symptoms, inflammation, and survival in cancer patients announced of a palliative disease progression and their partners. clinicaltrials.gov/show/nct03652298 (first received 29 August 2018).
Wells 2018 {published data only}
- Wells A, McNicol K, Reeves D, Salmon P, Davies L, Heagerty A, et al. Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway using group‐based metacognitive therapy (PATHWAY Group MCT): study protocol for a randomised controlled trial. Trials 2018;19(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACPMH 2007
- Australian Centre for Posttraumatic Mental Health. Australian Guidelines for the Treatment of Adults with Acute Stress Disorder and Posttraumatic Stress Disorder. Brisbane (QLD): ACPMH, 2007. [Google Scholar]
Antonovsky 1987
- Antonovsky A. Unraveling the Mystery of Health: How People Manage Stress and Stay Well. San Francisco (CA): Jossey‐Bass, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington (DC): American Psychiatric Publishing, 2013. [Google Scholar]
Bastos 2015
- Bastos MH, Furuta M, Small R, McKenzie‐McHarg K, Bick D. Debriefing interventions for the prevention of psychological trauma in women following childbirth. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD007194.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56:893‐7. [DOI] [PubMed] [Google Scholar]
Berger 2012
- Berger W, Coutinho ES, Figueira I, Marques‐Portella C, Luz MP, Neylan TC, et al. Rescuers at risk: a systematic review and meta‐regression analysis of the worldwide current prevalence and correlates of PTSD in rescue. Social Psychiatry & Epidemiology 2012;47(6):1001‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisson 2003
- Bisson JI, Roberts N, Macho G. Service innovations: the Cardiff Traumatic Stress Initiative: an evidence‐based approach to early psychological intervention following traumatic events. Psychiatric Bulletin 2003;27(4):145‐7. [Google Scholar]
Bisson 2007
- Bisson JI, Ehlers A, Matthews R, Pilling S, Richards D, Turner S. Psychological treatments for chronic post‐traumatic stress disorder: systematic review and meta‐analysis. British Journal of Psychiatry 2007;190(2):97‐104. [DOI] [PubMed] [Google Scholar]
Bisson 2013
- Bisson JI, Roberts NP, Andrews M, Cooper R, Lewis C. Psychological treatment of post‐traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD003388.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blake 1995
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician administered PTSD scale. Journal of Traumatic Stress 1995;8(1):75‐90. [DOI] [PubMed] [Google Scholar]
Bradley 2005
- Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta‐analysis of psychotherapy for PTSD. American Journal of Psychiatry 2005;162(2):214‐27. [DOI] [PubMed] [Google Scholar]
Brewin 2008
- Brewin CR, Scragg P, Robertson M, Thompson M, d'Ardenne P, Ehlers A. Promoting mental health following the London bombings: a screen and treat approach. Journal of Traumatic Stress 2008;1:3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunet 2015
- Brunet A, Monson E, Liu A, Fikretoglu D. Trauma exposure and posttraumatic stress disorder in the Canadian military. Canadian Journal of Psychiatry 2015;60(11):488‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryant 2013
- Bryant RA, O'Donnell ML, Creamer M, McFarlane AC, Silove D. A multisite analysis of the fluctuating course of posttraumatic stress disorder. JAMA Psychiatry 2013;70(8):839‐46. [DOI] [PubMed] [Google Scholar]
Davidson 1997
- Davidson JR, Book SW, Colket L, Tupler LA, Roth S, David D, et al. Assessment of a new self‐rating scale for posttraumatic stress disorder. Psychological Medicine 1997;27:153‐60. [DOI] [PubMed] [Google Scholar]
Dworkin 2017
- Dworkin ER, Menon SV, Bystrynski J, Allen NE. Sexual assault victimization and psychopathology: a review and meta‐analysis. Clinical Psychology Review 2017;56:65‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 2007
- Fletcher J. What is heterogeneity and is it important?. BMJ 2007;334:94‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foa 1995
- Foa EB. Posttraumatic Stress Diagnostic Scale Manual. Minneapolis (MN): National Computer Systems, Inc, 1995. [Google Scholar]
Foa 1997
- Foa EB, Meadows EA. Psychosocial treatments for posttraumatic stress disorder: a critical review. Annual Review of Psychology 1997;48:449‐80. [DOI] [PubMed] [Google Scholar]
Gamble 2009
- Gamble JA, Creedy DK. A counselling model for postpartum women after distressing birth experiences. Midwifery 2009;25:e21‐30. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Heron‐Delaney 2013
- Heron‐Delaney M, Kenardy J, Charlton E, Matsuoka Y. A systematic review of predictors of posttraumatic stress disorder (PTSD) for adult road traffic crash survivors. Injury 2013;44(11):1413‐22. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Horowitz 1979
- Horowitz MJ, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic Medicine 1979;41:209‐18. [DOI] [PubMed] [Google Scholar]
Jenkins 2012
- Jenkins R, Meltzer H. The Mental Health Impacts of Disasters. Report produced for the Government Office of Science, Foresight project 'Reducing Risks of Future Disasters: Priorities for Decision Makers' 2012.
Kearns 2012
- Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO. Early interventions for PTSD: a review. Depression and Anxiety 2012;29(10):833‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2018
- Lewis C, Roberts NP, Bethell A, Robertson L, Bisson JI. Internet‐based cognitive and behavioural therapies for post‐traumatic stress disorder (PTSD) in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD011710.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Litz 2002
- Litz BT, Gray MJ, Bryant RA, Adler AB. Early intervention for trauma: current status and future directions. Clinical Psychology: Science and Practice 2002;9(2):112‐34. [Google Scholar]
Litz 2004
- Litz BT, Gray MJ. Early intervention for trauma in adults: a framework for first aid and secondary prevention. In: Litz BT editor(s). Early Intervention for Trauma and Traumatic Loss. New York (NY): Guilford Press, 2004. [Google Scholar]
Lowe 2017
- Lowe SR, Galea S. The mental health consequences of mass shootings. Trauma, Violence & Abuse 2017;18(1):62‐82. [DOI] [PubMed] [Google Scholar]
McNally 2003
- McNally RJ, Bryant RA, Ehlers A. Does early psychological intervention promote recovery from posttraumatic stress disorder?. Psychological Science in the Public Interest 2003;4(2):45‐79. [DOI] [PubMed] [Google Scholar]
Mitchell 1995
- Mitchell JT, Everly GS. Critical Incident Stress Debriefing: an Operations Manual for the Prevention of Traumatic Stress Among Emergency Services and Disaster Workers. 2nd Edition. Ellicott City (MD): Chevron, 1995. [Google Scholar]
NCTSN/NCP 2006
- National Child Traumatic Stress Network and National Center for PTSD. Psychological first aid: field operations guide, 2nd edition; 2006. www.nctsn.org/sites/default/files/resources/pfa_field_operations_guide.pdf (accessed prior to 9 July 2019).
NICE 2018
- National Institute for Health and Care Excellence. PTSD: evidence reviews for psychological, psychosocial and other non‐pharmacological interventions for the prevention of PTSD in adults. London (UK): National Institute for Health and Care Excellence, 2018. [PubMed] [Google Scholar]
Ormerod 2002
- Ormerod J. Current research into the effectiveness of debriefing. Psychological Debriefing. Leicester: The British Psychological Society, 2002:8‐17. [Google Scholar]
Qi 2016
- Qi W, Gevonden M, Shalev A. Prevention of post‐traumatic stress disorder after trauma: current evidence and future directions. Current Psychiatry Reports 2016;18(2):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Richardson 2019
- Richardson JD, Thompson A, King L, Ketcheson F, Shnaider P, Armour C, et al. Comorbidity patterns of psychiatric conditions in Canadian armed forces personnel. Canadian Journal of Psychiatry 2019;64(7):501‐10. [DOI: 10.1177/0706743718816057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2009
- Roberts NP, Kitchiner NJ, Kenardy J, Bisson J. Multiple session early psychological interventions for the prevention of post‐traumatic stress disorder. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006869] [DOI] [PubMed] [Google Scholar]
Roberts 2010
- Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. Early psychological interventions to treat acute traumatic stress symptoms. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD007944.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2002
- Rose S, Bisson J, Churchill R, Wessely S. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000560] [DOI] [PubMed] [Google Scholar]
Ruzek 2007
- Ruzek JI. Wanted: a theory of post‐trauma information delivery. Psychiatry 200;71(4):332‐8. [DOI] [PubMed] [Google Scholar]
Salkovskis 1996
- Salkovskis PM, Clark DM, Gelder MG. Cognition‐behaviour links in the persistence of panic. Behaviour Research and Therapy 1996;34:453‐8. [DOI] [PubMed] [Google Scholar]
Santiago 2013
- Santiago PN, Ursano RJ, Gray CL, Pynoos RS, Spiegel D, Lewis‐Fernandez R, et al. A systematic review of PTSD prevalence and trajectories in DSM‐5 defined trauma exposed populations: intentional and non‐intentional traumatic events.. PloS One 2013;8(4):e59236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2013
- Schulz JM, Neria Y, Espinel Z. Psychological impacts of natural disasters. In: Bobrowski PT editor(s). Encyclopedia of Natural Hazards. 1. London (UK): Springer, 2013:779‐91. [Google Scholar]
Spielberger 1970
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the Stait‐Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press, 1970. [Google Scholar]
Stein 2006
- Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD002795.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevelink 2018
- Stevelink SA, Jones M, Hull L, Pernet D, MacCrimmon S, Goodwin L, et al. Mental health outcomes at the end of the British involvement in the Iraq and Afghanistan conflicts: a cohort study.. British Journal of Psychiatry 2018;213(6):690‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Emmerik 2002
- Emmerik AA, Kamphuis JH, Hulsbosch AM, Emmelkamp PM. Single session debriefing after psychological trauma: a meta‐analysis. Lancet 2002;360:766‐71. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36® Health Survey Manual and Interpretation Guide. Boston (MA): New England Medical Center, The Health Institute, 1993. [Google Scholar]
Wilson 2015
- Wilson LC. A systematic review of probable posttraumatic stress disorder in first responders following man‐made mass violence. Psychiatry Research 2015;229(1‐2):21‐6. [DOI] [PubMed] [Google Scholar]


