Abstract
Social animals must detect, evaluate and respond to the emotional states of other individuals in their group. A constellation of gestures, vocalizations, and chemosignals enable animals to convey affect and arousal to others in nuanced, multisensory ways. Observers integrate social information with environmental and internal factors to select behavioral responses to others via a process call social decision-making. The Social Decision Making Network (SDMN) is a system of brain structures and neurochemicals that are conserved across species (mammals, reptiles, amphibians, birds) that are the proximal mediators of most social behaviors. However, how sensory information reaches the SDMN to shape behavioral responses during a social encounter is not well known. Here we review the empirical data that demonstrate the necessity of sensory systems in detecting social stimuli, as well as the anatomical connectivity of sensory systems with each node of the SDMN. We conclude that the insular cortex is positioned to link integrated social sensory cues to this network to produce flexible and appropriate behavioral responses to socioemotional cues.
Keywords: Insular cortex, social decision making, social communication, emotion, sensory integration
GRAPHICAL ABSTRACT
Insular cortex is anatomically positioned to integrate and convey socioemotional cues from the external environment to the social decision-making network, which generates flexible and context-appropriate social behavioral responses.
1. Social Decision Making
It is nearly impossible to overstate the importance of sociality for the survival and success of many species (Wilson, 1992). Social behaviors are tightly interwoven with emotion, and communicating one’s emotional state may evoke adaptive behavioral responses from other group members. Animals detect, appraise and respond to others’ emotional states with flexible and context-appropriate behaviors though a process called social decision-making. This capacity gives rise to fundamental social actions across species, including parenting, mate selection and aggression (Ranote et al., 2004; Rendon et al., 2015), as well as the more complex and multidimensional facets of human social interactions like empathy, perspective-taking and compassion (Shamay-Tsoory, 2011; Tremblay et al., 2017; Sueur & Pelé, 2016; Marsh, 2018;). In humans, impaired emotion recognition and the subsequent inability to generate appropriate social responses may underlie the social impairments characteristic of psychiatric disorders like autism and schizophrenia (Lozier et al., 2014; Edwards et al., 2002). Thus, understanding the neural mechanisms by which social animals detect and respond to socioemotional cues is an important goal of neurobiology.
Emotion, of course, is a complex and multi-faceted construct with varying definitions and applications (Cabanac, 2002). One element of emotion is “core affect,” a moment-to-moment reflection of an individual’s arousal level and hedonic state that is influenced by external stimuli and fluctuations in internal state (Russell, 2003). Core affect falls along two dimensions that span from sedating to arousing and from aversive to pleasing. In humans, emotional labels are used to describe specific combinations along these dimensions. For example, happiness reflects a core affect with modest arousal and pleasure, whereas fear describes a core affect that is aroused and aversive. The ability to communicate one’s core affect likely evolved to elicit myriad social actions that promote individual or group survival and so we are able to use a comparative approach to study the mechanisms underlying these social actions. Throughout this review of social decision-making and behaviors observed primarily in rodents, we will use the term “social affect” to describe the expression of an animal’s core affect. The behavioral responses that result from appraising another’s’ affective state are considered “social affective behaviors.”
Many species transmit their core affect to other members of the group in expressions that include vocalizations, touch, chemosignals, olfactory cues, and behavioral gestures (Darwin, 1976; Insel, 2010), including facial expressions (Eckman & Oster, 1979; Chevalier-Skolnikoff, 1973; Sotocinal et al., 2011). For example, an infant’s cry prompts maternal attention, and a distressed cry or vocalization may elicit help from other members of the species (Marsh, 2018; Ihnat et al., 1995; Brudzynski, 2013). Behavioral responses to social affective cues are generated via social-decision making, defined as the process by which sensory and perceptive systems in the brain appraise and integrate social information, like social affect, with situational factors, past experiences and internal physiology to shape specific, context-appropriate social behaviors. One product of integrating and appraising these factors is that an animal can compute the reward value of possible social behavioral responses (Gil et al., 2013; Weiss et al., 2015; Vanderschuren et al., 2016) which may alter the likelihood an animal will execute certain social behaviors (Caldú & Dreher, 2007). In the brain, a “social decision making network (SDMN)” of interconnected brain regions is the central orchestrator of social behavior (O’Connell & Hofmann, 2011; Fig 1) and this review seeks to understand how social affect might influence this system.
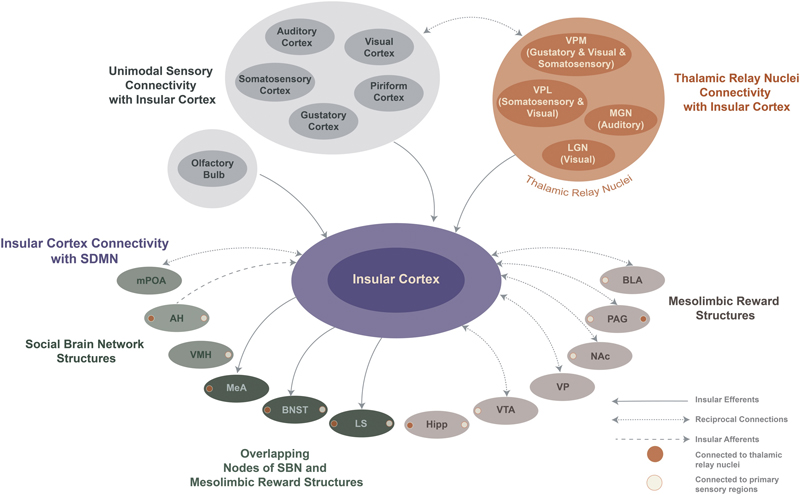
FIGURE 1. Schematic of insula connectivity with unimodal thalamic relay nuclei, primary sensory areas, and the social decision-making network.
Thalamic nuclei encode and relay sensory information that conveys affect from the external environment to primary sensory areas where sensory information is decoded and assigned meaning. This information is also relayed via parallel projections to the insular cortex, where social cues are contextualized with internal physiology and other factors to produce unique output patterns in insula efferents that influence SDMN network activity and generate flexible behaviors. Few connections exist between thalamic nuclei and SDMN nodes, except for the following: medial geniculate nucleus projects to AH, MeA and PAG; ventroposterior medial nucleus projections to BNST and LS; lateral geniculate nucleus projects to Hipp (orange circles) and, likewise, unimodal cortical connectivity with SDMN is sparse (beige circles; see section 3.1 for anatomical connections) so sensory information must reach the SDMN via other projections. Insular cortex is well-connected with thalamic relay nuclei, primary sensory areas, and most SDMN structures, suggesting IC is positioned to integrate sensory cues to influence SDMN activity and, therefore, contribute to the integration of socioemotional information with social behaviors. Abbreviations: MeA: Medial amygdala; AH: Anterior hypothalamus; VMH: Ventromedial hypothalamus; mPOA: Medial preoptic area; BNST: Bed nucleus stria terminalis; LS: Lateral septum; Hipp: Hippocampus; VTA: Ventral tegmental area; VP: Ventral pallidum; NAc: Nucleus accumbens core; PAG: Periaqueductal grey; BLA: Basolateral amygdala; LGN: Lateral geniculate nucleus; MGN: Medial geniculate nucleus; VPL: ventroposterior lateral nucleus of the thalamus; VPM: ventroposterior medial nucleus of the thalamus.
An obstacle to understanding the anatomical correlates underlying social decision-making is its complexity; several parallel neural processes must coordinate across a network of brain regions to produce an observable social behavior. These include salience detection, sensory integration, memory for context and the identity of another individual, motivation, reward, and the motor systems that enable animals to generate social responses to socioemotional cues (Insel & Fernald, 2004; O’Connell & Hofmann, 2011). While these processes are not specific to information processing in the social realm per se, they are necessary to generate flexible, nuanced and context-appropriate social actions.
Human neuroimaging already provides some anatomical insight regarding these processes in social decision-making. In the prefrontal cortex (PFC), functional magnetic resonance imaging (fMRI) identified that activity in the dorsolateral, dorsomedial, ventrolateral and ventromedial prefrontal cortices correlated to regulating selfish versus prosocial actions, trust, risk assessment and reward valuation and emotion recognition, respectively (Rilling & Sanfey, 2011). Beyond PFC, the fusiform face area, anterior cingulate, and ventral striatum and orbitofrontal cortex are implicated in social recognition, inferring others’ intentions, and decoding value signals to inform behavioral responses (Tremblay et al., 2017). Regarding insular cortex (IC), the focus of this review, there is substantial evidence from neuroimaging studies correlating IC activity or connectivity with empathy (Bernhardt & Singer, 2012) and emotion recognition (Green et al., 2016; Yamada et al., 2016). These human neuroimaging studies run parallel to investigations across taxa which investigate the brain systems and neurochemicals that are conserved, or at least have homologs, in mammals, amphibians, reptiles and avian species (reviewed in detail below). These comparative studies, therefore, omit the neocortical structures that are unique to mammals, thus leaving a significant question as to how to relate these lines of research: how do the cortical correlates of social cognition interact with hypothalamic and brainstem systems that we know are essential for many basic social behaviors?
The considerable homology between the rodent and primate brains allows rodent research to identify links between cortical and subcortical brain systems that provide the anatomical basis of social decision-making (Ruff & Fehr, 2014) and in some cases there are examples of functional and anatomical findings in humans that corroborate mechanistic studies in rodents (reviewed in section 4). However, for rodent research to address the gaps between human and comparative research we must first establish that social decision-making in rodents involves the integration of social affective cues. A number of studies demonstrate that social signals emitted by one animal, referred to as the “demonstrator,” can elicit behavioral changes in an “observer.” For example, rats will more readily press a lever to release a squealing rat than a toy block from a harness (Rice & Gainer, 1962) and to spare a conspecific from a painful shock (Green, 1969). More recent findings report that rodents transfer negative affective states, including fear, pain, and stress, which elicit social responses. Observer rats (Knapska et al., 2006), mice (Meyza et al., 2015), and voles (Burkett et al., 2016) spend more time exploring a cagemate that underwent tone-shock conditioning, and observers display increased exploration of the demonstrator (Knapska et al., 2010; Mikosz et al., 2015). Further, observer rats that witness or interact with footshocked or fear conditioned demonstrators show observational fear learning, increased sensitivity to pain, and a greater propensity to avoid noxious stimuli (Jeon et al., 2010; Kim et al., 2010). These phenomena highlight the important role of social affect in rodent prosocial behaviors, and we encourage interested readers to read a comprehensive account of this literature by Meyza and colleagues (2017).
The findings from rodent paradigms are congruent with human work (Von Dawans et al., 2012; Kawamichi et al., 2012; Bouchard et al., 2013; FeldmanHall et al., 2015) which identify that social affective behaviors that result from detecting a stressed demonstrator are influenced by a range of factors including, social rank, age, sex, previous experience with a stressor and familiarity. Regarding age, our lab has reported that adult rats will approach a stressed juvenile, but avoid a stressed adult (Rogers-Carter et al., 2018b). Numerous examples identify familiarity as an important determinant of prosocial behavior in rodent social paradigms: familiarity is either necessary for, or augments, vicarious fear (Jones et al., 2014), pain empathy (Li et al., 2014; Langford et al., 2006), social approach (Rogers-Carter et al., 2018a) and consoling behavior (Burkett et al., 2016). Similarly, rats will work to press a lever to release a restrained conspecific of the same, but not different, strain (Ben-Ami Bartal, Rodgers et al., 2014) but see (Silberberg et al., 2014). Furthermore, several cases of prosocial actions require that the observer had prior exposure to the stressor (Kiyokawa et al., 2014; Ishii et al., 2016; Guzmán et al., 2009), implying a role of mnemonic processes.
While the ability to detect social affective cues is crucial to species success, exactly how this information drives behavioral responses in a social interaction is not completely understood. In the upcoming sections we will articulate how external factors, social cues and physiological state converge to shape social affective behavior from the lens of mechanistic and behavioral studies in rodents. First, we review the existing anatomical models of social behaviors constructed from comparative neurobiology (Newman, 1999; O’Connell & Hofmann, 2011). Next, we discuss the mechanisms by which social affective cues are detected and processed by sensory regions in the rodent brain, while considering the flow of sensory information to social brain structures. We argue that the existing social brain models are incomplete with regard to sensory processing and salience detection. Finally, we synthesize evidence from anatomical, functional and behavioral studies conducted in both humans and rodents that posit the insular cortex (IC) as a site where social sensory stimuli, internal physiological state, and reward value converge. These features position IC to contribute to some of the processes currently unaccounted for in rodent models of social decision-making and bridge findings from human neuroimaging work and mechanistic insights from rodents.
2. Anatomical Substrates of Social Decision-Making
2.1. The Social Brain Network
A network model of brain structures that are able to execute elementary social behaviors in rodents was first introduced in the Social Brain Network (SBN; Newman, 1999). This system includes the LS, mPOA, AH, VMH, PAG, VTA, MeA and BNST (See Fig. 1 caption for list of abbreviations). Homologous networks exist in in birds, bony fish (Goodson, 2005) and reptiles (Crews, 2003). SBN nodes were included in the network model if they were reciprocally connected with the other regions, contained receptors for gonadal hormones, and were implicated in at least one mammalian social behavior. The SBN model posited that specific social behaviors are not mediated by one specific structure, but rather arise from unique patterns of SBN network activity that are elicited during exposure to certain social stimuli. Accordingly, recent reports support the notion that different patterns of activation across the SBN can give rise to a broad repertoire of social behaviors. For example, in the MeA, GABAergic neurons can produce aggressive, grooming, or mounting behavior depending on the strength of optogenetic excitation (Hong et al., 2014). Consistently, the strength of MeA neural activation during a social interaction varied with conspecific sex (Yao et al., 2017). A similar phenomenon is observed in the VMH where estrogen receptor 1 neurons in the VMH modulate behaviors ranging from investigation to attack (Lee et al., 2014), and in the BLA where inputs to the mPFC have bidirectional control to increase or decrease social investigation (Felix-Ortiz et al., 2016). Moreover, the social cues that drive activity patterns in the SBN may induce long-lasting changes in neurons; progesterone-expressing neurons in the VMH mediate aggression in single-housed, but not sociallyhoused male mice (Yang et al., 2017). Consistent with the idea presented in the SBN, unique patterns of activity in response to external stimuli are reported in humans (Smith et al., 2009) and in zebrafish during social interactions (Teles et al., 2015).
2.2. The Social Decision-Making Network
While the SBN captures the essential anatomical loci underlying many social behaviors in non-human mammals, this network lacks the structures needed to detect and appraise potentially rewarding aspects of social interactions. Indeed, reward signaling motivates social interactions, which are then reinforced by the mesolimbic dopamine system in non-human animals (Dölen et al., 2013b; Hung et al., 2017; Ramos et al., 2015; Smith et al., 2018) and humans (Chelnokova et al., 2014). To this end, O’Connell and Hofmann (2011, 2012), using an evolutionary biology approach, made the keen observation that anatomical nodes of the mesolimbic dopamine reward system evolved to express many of the same receptors and genes that mediate social behaviors that were used to define the SBN across a range of non-human species, including teleosts, amphibians, reptiles, and avian and mammalian species. To account for the requisite reward signaling in social behavior, O’Connell and Hofmann (2011) merged the SBN with the mesolimbic reward system to form the social decision-making network (SDMN).
The SDMN consists of the SBN structures and the NAc, VP, BLA, Hipp, and VTA, with LS, BNST, and MeA (see Fig. 1 caption for abbreviations) as overlapping nodes in both the mesolimbic reward system and SBN. Each mesolimbic structure executes processes that culminate in an animal’s motivation to respond to their environment. It is not fully understood how social behaviors arise from this coordinated activity, but the known roles of each structure allow for speculation: first, social cues may be detected as salient and trigger the VTA (Root et al., 2018; Bromberg-Martin et al., 2010). This information may be integrated with valence in the BLA (LeDoux, 2000; Beyeler et al., 2018) and then contextualized by Hipp, which can place social information into memory and identify familiar conspecifics (Okuyama, 2018; Kogan et al., 2000), and by the NAc, which computes reward value (Higa et al., 2017). Reward value may be broadcast to SDMN network nodes, including the LS and BNST, both of which are implicated in rodent goal-directed actions (Faser et al., 1991), sexual behaviors (Gulia et al., 2002; Maejima et al., 2015; Petrulis, 2013), and aggression (Wong et al., 2016; Consiglio et al., 2005). Valence is integrated with other information in the SDMN to drive approach or avoidance (Hamel et al., 2017; Hoebel et al., 2007) or other social behavioral responses through connections with limbic and motor structures (Mogenson & Yang, 1991; Smith et al., 2009). Importantly, the mesolimbic system accounts for an animal’s ability and motivation to respond to the external environment, whereas the SBN executes specific behaviors in response to mesolimbic inputs.
Mesolimbic structures also express genes associated with dopaminergic signaling, gonadal hormones, and oxytocin (OT) and vasopressin (AVP), all of which regulate behaviors and decision-making across many mammalian species (O’Connell & Hofmann, 2011; Creutz & Kritzer, 2004; Zhang et al., 2008). While sensitivity to OT and AVP were not initial requirements for inclusion in the SDMN, these social nonapeptides are now implicated in myriad social behaviors across species (Donaldson & Young, 2008; Caldwell, 2017) and mechanistic experiments in rodents that causally implicate SDMN nodes to social behaviors have done so via manipulations to, or examination of, OT and AVP signaling (Johnson & Young, 2015; Ophir, 2017). Many social behaviors are age- and sex-specific, which can be explained by differences in OT and AVP activity or receptor expression. For example, in rats, age negatively correlates with propensity to engage in social play, a naturally rewarding behavior, and consistently, juvenile rats have greater OT and AVP receptor binding density in reward-related regions than adults (Smith et al., 2017). Regarding sex, females maintain social recognition longer than males, which is mediated by differences in the AVP system (Bluthe & Dantzer, 1990). Further, the MeA exhibits robust sex differences in AVP binding (DiBenedictis et al., 2017) and promotes maternal behaviors in female rats, but infanticidal behavior in males (Chen et al., 2019). Lesioning AVP cells in the BNST reduced social investigation in males, but increased copulatory behaviors in females (Rigney et al., 2019), and social investigation positively correlated with OT receptor binding the in medial amygdala in males, but not females (Dumais et al., 2013). Further, receptor expression for OT and AVP varies with social experience as pregnant prairie voles exhibited greater AVP receptor expression in the BNST, MeA, VP, NaC and Hipp than non-pregnant voles (Zheng et al., 2013). While not meant to be a comprehensive review of nonapeptides in social behavior, these findings illustrate how investigations of OT and AVP enable causal examination of the SDMN in rodents.
Despite the huge impact of the SDMN on our understanding of the anatomy and signaling systems that underlie social decision-making, this model was developed using a comparative approach and therefore omitted systems that are not conserved widely across species, such as cortical sensory processing regions. However, the SDMN does not capture how social affective sensory cues converge with internal physiology and external environmental factors, or how this information interacts with the neural systems that are directly attributed to social decision-making. Evidence from human studies implicates the cerebral cortex (Adolphs, 2001; Amodio & Frith, 2006b; Hiser & Koenigs, 2018) which receives social information from primary sensory relays (i.e. thalamic nuclei) and integrates this information to produce higher-order representations of sensory information through cortico-cortical network interactions. Anatomists identified the brain regions and tracts by which primary sensory processing structures project to associative “multisensory” structures and ultimately fully integrated structures in the cortex that might serve as the functional anatomic networks that provide the necessary sensory sophistication to inform decision making and cognition (Mesulam, 1998 and see Man et al., 2013, for an historical review of the origins of hypothetical functional networks based on neuroanatomical and mechanistic studies). The next section will review some of the evidence from mammalian research that implicates cortical structures in social decision-making to identify anatomical tracts to connect the cortex to the SDMN.
2.3. The Cortex in Socioemotional Behaviors
In humans, the cortex is implicated in myriad social processes: distinguishing self from other, deciding one’s motivation to engage in a social interaction, and deciphering the intention of another’s motives, among other social cognitions (Amodio & Frith, 2006a). The specific cortical areas most studied in human social cognition that are also present in rodents are the medial prefrontal cortex (mPFC) the orbitofrontal cortex (OFC), insular cortex (IC), and anterior cingulate cortex (ACC). Other areas, such as the fusiform face area and the extrastriate body area enable face and body detection in humans (Arioli et al., 2018) but will not be discussed here as their rodent homologues are unknown.
While not a complete account of the literature examining the human cortex in social cognition, this subset of findings demonstrates the myriad social processes that have cortical correlates. mPFC activity correlates with social status (Mason et al., 2014), perspective-taking (Frith & Frith, 2003), action-outcome prediction (Alexander & Brown, 2011), pair-bonding (Bales et al., 2007), cooperation, social reward, social punishment, and motivation (Bicks et al., 2015). Similarly, ACC activity correlates with others’ social motivation and tracks prediction errors for behavior during a social interaction (Apps et al., 2018; Balsters et al., 2017). Regarding the OFC, activity here correlates with self-other distinction, and OFC neurons preferentially respond to faces and emotional expressions (Barat et al., 2018), signal social reward value, and track if a reward was received by oneself or another (Azzi et al., 2012). In non-human primates, OFC is also implicated in deciphering threatening versus affiliative social cues (Machado & Bachevalier, 2007) and lesions to the OFC reduce freezing to threatening stimuli (Kalin et al., 2007) and produced aberrant social approach and facial expressions (Babineau et al., 2011). Further, OFC can decide behavioral responses to ambiguous contexts based on the reward value of external stimuli (Elliott, 2000). Regarding IC, neural activity is correlated with emotion processing (Boucher et al., 2015), emotion recognition (Green et al., 2016; Yamada et al., 2016), cooperation (Rilling et al., 2008), fairness Gabay et al., 2014; Guo et al., 2013), empathy for pain in others (Bernhardt & Singer, 2012; Gu et al., 2012), social motivation of others (Schreuders, 2018) and emotional awareness (Gu et al., 2013). However, IC is one of the most consistently-activated regions in emotion-related neuroimaging research (Phan et al., 2002) and neuroimaging research generally (Kurth et al., 2010) which suggests that IC is engaged by processes that are important for cognition more broadly than socioemotional decision-making.
To determine the extent that the correlates of cortical activity during human social cognition play mechanistic roles in social decision-making (Lee & Harris, 2013) requires translational research in rodent models where circuit-specific tools allow precise investigation of social behaviors. Indeed, recent findings implicate several of these cortical structures in social cognition. In OFC, subpopulations of neurons respond to social cues and inhibit feeding, which demonstrates this region can mediate internally-motivated behaviors in response to external social stimuli (Jennings et al., 2019). Our lab recently reported that approach or avoidance of stressed conspecifics depends on IC (Rogers-Carter et al., 2018), and mPFC neurons show increased firing during social approach (Lee et al., 2016) and strength of synaptic efficacy positively correlates with social rank in mice (Wang et al., 2011). In voles that interact with a recently stressed cagemate, elevated Fos expression is observed in ACC, and ACC activation by oxytocin is necessary for social consoling behavior (Burkett et al., 2016). A common denominator of these findings is that social responses are the product of detecting external stimuli, and as discussed above, existing social brain network models do not account for this prerequisite process. The goal of the next section is to examine how cortical structures are necessary for detecting and responding to social cues, and their anatomical connectivity with the SDMN. This will lay the foundation to speculate how sensory information is conveyed to the SDMN so that behavioral responses are specific to external information.
3. Primary loci of sensory processes related to social affect in rodents
3.1. Unimodal Sensory Processes
3.1.1. Odor and Piriform Cortex
Odor communication is vital for rodent social behaviors (Johnston, 2003) and olfactory cues and chemosignals communicate one’s, species, sex, and reproductive state, social status, sickness, and familiarity (Arakawa et al., 2008; Liberles, 2014). Exposure to specific odors elicits myriad social behaviors in rodents, such as mating (Coria-Avila et al., 2005; Sakuma, 2008), aggression (Stowers et al., 2013), social learning (Choe et al., 2015), maternal behavior (Fleming & Rosenblatt, 1974), and social buffering of fear (Kiyokawa et al., 2012; Takahashi et al., 2013). Odor information is processed in the piriform cortex, which receives primary afferents from the olfactory bulb and amygdala, and it is modulated by cortical structures including the orbitofrontal and entorhinal cortices (Illig & Wilson, 2009). This anatomical arrangement distinguishes piriform from the other unimodal sensory cortices, which are primarily innervated by thalamic relay nuclei. The piriform cortex can distinguish the identity, concentration and intensity of odors (Bolding & Franks, 2017) and accordingly, is implicated in odor-driven social behaviors. Mice that scent-mark in the presence of a conspecific expressed elevated Fos immunoreactivity in the piriform cortex (Borelli et al., 2009), and female mice exposed to juvenile play sessions with a lemon-scented partner later preferred to mate with a lemon-scented male, over an almond-scented male, and expressed greater piriform cortex Fos immunoreactivity than females that underwent the experiment without odor-pairing (Paredes-Ramos et al., 2014). Piriform cortex also receives oxytocinergic inputs from the paraventricular nucleus (Mitre et al., 2016), which contribute to enhanced discrimination and behavioral responses to odors by increasing the signal-to-noise ratio of social olfactory cues and chemosignals (Oettl & Kelsch, 2017). Piriform activation during social tasks likely reflects the detection of social odors specifically, rather than general olfactory information, because piriform can distinguish social from non-social odors; pharmacological and optogenetic inhibition of piriform oxytocin receptors abolished learning of both appetitive and aversive social, but not non-social, stimuli suggesting a role for this region in distinguishing social from non-social odors (Choe et al., 2015).
Piriform cortex has dense reciprocal connectivity with the MeA, BLA, Hipp and the VTA (Cádiz-Moretti et al., 2016; Coolen & Wood, 1998; Johnson et al., 2000; Aransay et al., 2015; Wyss, 1981; De La Rosa-Prieto et al., 2015) but connectivity with other SDMN nodes is sparse since known projections include only the BNST, NAc and Hipp (Weller & Smith, 1982; Brog et al., 1993; Dong & Swanson, 2006), and SDMN inputs to piriform are only found in AH and LS (Roeling et al., 1994; Siegel & Tassoni, 1971). Whether these connections are sufficient to integrate piriform-mediated odor processing to social decision-making is currently unknown. However, several studies demonstrate an interplay between olfactory cues and other sensory inputs in regard to mediating behavior (Rossier & Schenk, 2003; Moyaho et al., 2015), suggesting that separate anatomical regions capable of multimodal integration combine odor with other social stimuli and contextual factors to drive social behaviors.
3.1.2. Tactile Contact and Somatosensory Cortex
Tactile information is represented in the somatosensory cortex. In rodents, the majority of somatosensory information is detected via the whiskers, which relay physical touch to the ventral posterior medial and posterior medial thalamic nuclei (Erzurumlu & Gaspar, 2012; Viaene et al., 2011). These regions send efferents to barrel cortex, a distinct somatosensory cortex subregion that reflects the somatotopic organization of whisker positioning on the face (Li & Crair, 2011). Barrel cortex neurons respond to tactile behaviors observed during rodent social interactions including pinning, anogenital sniffing, allogrooming, and nape-biting. Following rough and tumble play behavior, juvenile rats express elevated fos immunoreactivity in the barrel cortex (Gordon et al., 2002) and pharmacological blockade of the barrel cortex during social interaction reduced pinning behavior (Charles et al., 2008). Rodents also frequently engaged in “social face touch,” a form of head-to-head contact utilizing the whiskers (Wolfe et al., 2011), and the necessity of both whiskers and barrel cortex are well-documented in this behavior. Whisker activity changes as a function of distance from a conspecific (Wolfe et al., 2011), and during bouts of social face touch, at least 40% of barrel cortex neurons increased firing at a rate significantly higher than that observed during contact with non-social stimuli (Bobrov et al., 2014). Moreover, social isolation, and therefore lack of social contact, results in impaired whisker sensitivity and disrupted synaptic plasticity in the barrel cortex (Miyazaki et al., 2012). Neonatal mice with trimmed whiskers performed poorly on a tactile sensitivity assay and displayed aberrant social behaviors in adulthood (Soumiya et al., 2016). Whisker-driven neural activation in barrel cortex was disrupted in NMDA receptor knock-out mice and correlated with social behavioral deficits (Arakawa et al., 2014), and de-whiskered pups were less successful at nursing than whisker-intact littermates, and were less active during social interactions with siblings (Sullivan et al., 2003). These findings signify that somatosensory cortex contains distinct subregions that are capable of uniquely responding to social stimuli.
The somatosensory cortex does not receive inputs from any SDMN structures but projects to several SDMN structures which provide anatomical pathways for tactile experience to shape social behaviors. Projections from somatosensory cortex to the VMH may code tactile events during mating (Stanzani & Russo, 1980; Angoa-Pérez & Kuhn, 2015), whereas projections to NAc and VTA (Tai & Kromer, 2014; Wilson, 2014; Hunnicutt et al., 2016; Lenschow & Brecht, 2018; Faget et al., 2016) may convey information about social contact or position to inform reward value and reinforcement (Báez-Mendoza & Schultz, 2013; Dölen et al., 2013b). Sparse somatosensory cortex projections to PAG (Lenschow & Brecht, 2018) may allow social touch to inform adaptive and flexible physical responses in a social interaction (Skuse & Gallagher, 2009). However, none of these tracts have been investigated in rodent social behaviors.
3.1.3. Visible Social Cues and Visual Cortex
Rodents display facial and body expressions during social interactions (Brecht & Freiwald, 2012), yet the role of these gestures in coordinating social behavior remains enigmatic. During social interactions and exposure to aversive stimuli, rats displayed tightening of the eyes, welling of the nose and cheeks, and flattening of the ears (Defensor et al., 2012), and changes in ear posture correspond to changes in general arousal state (Lecorps & Féron, 2015). When rats are tickled to induce a positive affect, ears become dark pink and flex at a wider angle (Finlayson et al., 2016), and mice display a “grimace” during negative affective states, like pain (Sotocinal et al., 2011), which correlates to a shorter and more curved spinal stance (Mittal et al., 2016). Facial expressions are observed during social interactions as well. Naked mole rats show aggressive mouth gaping and incisor fencing (Lacey et al., 1991) to establish social dominance (Brecht & Freiwald, 2012), and similarly, mice pluck the whiskers of others, rendering them unable to display aggressive or dominant faces (Sarna et al., 2000). Whisker position also signals if an interaction is aggressive or prosocial: during aggressive bouts, whiskers are protracted and whisk with high amplitude (Wolfe et al., 2011). Moreover, facial displays, like ear wiggling in females, are associated with courting behavior (Vreeburg & Ooms, 1985). Despite a limited understanding of the meaning of rodent visual cues, they appear to be important contributors to social decisions.
To our knowledge there are no neurobiological accounts of visual cortex neural activity during social interaction or exposure to social stimuli, but the importance of social experience on visual cortex development is fairly well-documented. Rats raised in social isolation displayed increased higher-order dendritic branching (Volkmar & Greenough, 1972) and aberrant expression of dendritic spines in visual cortex pyramidal cells (Connor & Diamond, 1982), whereas rearing in an enriched social environment increased synaptic strength in visual cortex (Mainardi et al., 2010). Lastly, in a murine model of autism, social abnormalities are associated with reduced functional connectivity between visual cortex and other sensory cortical areas (Sforazzini et al., 2016). Visual cortex projections synapse in the PAG, NAc, and VTA (Newman et al., 1989; Wilson, 2014; Dinopoulos & Parnavelas, 1991) but do not receive inputs from the SDMN. This connectivity may provide the anatomical framework for visual information to inform reward valuation during a social interaction, and also suggests that corticocortical interactions are necessary to further process social visual cues and relay them to SDMN.
3.1.4. Vocal Communication and Auditory Cortex
Rodents emit audible and ultrasonic vocalizations to convey negative and positive affective states. The acoustic characteristics, functional purpose and neural correlates of ultrasonic vocalizations are reviewed elsewhere (see: Brudzynski, 2013; Portfors & Perkel, 2014). Ultrasonic vocalizations are observed in prosocial (Pultorak et al., 2016; Burke et al., 2017), reproductive (Neunuebel et al., 2015; Matsumoto & Okanoya, 2016) and territorial behaviors (Pasch et al., 2011; Hammerschmidt et al., 2012) and are encoded by auditory cortex. Auditory cortex neurons are active during playbacks of vocalizations (Carruthers et al., 2013). Further, rats expressed elevated Fos in auditory cortex after exposure to recordings of 22kHz calls (Ouda et al., 2016), and calcium imaging revealed a subset of auditory cortex neurons that respond to the frequency sweep rate of modulating calls (Issa et al., 2017). Ultrasonic vocalizations can modulate social behaviors as well; rats displayed approach behavior when played recordings of 55kHz calls and expressed Fos in the auditory cortex (Sadananda et al., 2008), and auditory neurons in female dams undergo plasticity to increase the signal-to-noise ratio of pup calls (Tasaka et al., 2018), which drive maternal behaviors (Valtcheva & Froemke, 2018).
Auditory cortex projects to the PAG, BLA, Hipp, and VTA, which may allow acoustic information to be incorporated into context and reward valuation (Lindvall & Stenevi, 1978; Newman et al., 1989; Romanski & LeDoux, 1993; Budinger et al., 2008). Further, projections from auditory cortex to LS (Lindvall & Stenevi, 1978) and BNST (Shin et al., 2008) may integrate vocal cues with emotional (Singewald et al., 2011) and physiological states (Herman et al., 2003), and vocal inputs may evoke oxytocin release via projections to the PVN during social interaction (Peñagarikano et al., 2015). Interestingly, auditory cortex has reciprocal connectivity with NAc, an important hub for vocal song learning in birds (Prather, 2013), and so this pathway may help encode social sounds for later recognition or performance. Moreover, NAc projections in the visual cortex also project to the lateral amygdala, which may allow for the integration of acoustic inputs with emotional or affective state (LeDoux et al.,1991). However, like several other unimodal sensory cortical regions, none of the projections from auditory cortex to SDMN structures have been causally examined in a social behavior.
3.1.5. Taste Communication and Gustatory Cortex
Evidence for gustatory communication in social behavior comes from social transmission of food preference. Rats will prefer to consume a certain food if they had recently explored a conspecific that consumed the same food (Galef & Wigmore, 1983; Galef et al., 1985). Similarly, hamsters preferred to consume the diet their mother was fed, but failed to show a preference for a diet fed to either a littermate or a novel conspecific, indicating that the features or relationship of the individual transmitting a food preference are important in determining the observer’s behavior (Lupfer et al., 2003). Lastly, in rats, presentation of an olfactogustatory cue prompted nicotine self-administration during social interaction (Chen et al., 2011). Non-volatile chemosignals are detected through sniffing and licking (Lehman et al., 1980), and so it’s possible that chemosensory information involves gustatory processing to some extent. Regarding the SDMN, gustatory cortex is only connected to this network via inputs from the BLA, which may combine taste with emotional state, such as in conditioned taste aversion acquisition (Gallo et al., 1992). However, gustatory cortex is a subregion of IC and anatomical studies of IC may not distinguish gustatory from the broader IC, the connectivity of which with the SDMN will be discussed below.
3.2. Multimodal Sensory Processing of Socioemotional Cues
Concurrent unimodal sensory information must be integrated to assemble a complete representation of the external environment. To do so requires multisensory convergence and multisensory integration, two processes that are observed in both individual neurons and populations of neurons. Multisensory convergence occurs where inputs from multiple sensory modalities overlap in time, and the resulting neural response is a direct sum of the response that each unimodal input evokes individually. Multisensory integration, however, is identified when the neural response to multisensory inputs is distinctly different (i.e. non-linear) than the sum of the neural responses to converging unimodal signals and, therefore, each individual modality cannot be delineated from the resulting activity (Stein et al., 2014). This binding of information via multisensory integration converts simple sensory representations into higher order abstractions that inform behavioral decisions (Siemann et al., 2014). In animal social behaviors, there are several examples in which concurrent cues take on a unique social meaning (Partan & Marler, 1999). For example, dogs signal threat to one another via growling, but growling in combination with a bowed stance initiates play behavior (Bekoff, 1972). Animals trained to approach a visual cue do so more readily if the cue is paired with an auditory stimulus, which indicates an effect of multisensory presentation on attention (Stein et al., 1989). Furthermore, rat mating behavior (Beach, 1942) and maternal behaviors (Beach & Jaynes, 1956; Smotherman et al., 1974) are mediated by combined olfactory, visual, and tactile cues, do not elicit as robust behavioral responses on their own (but see Stern, 1990).
Only a few studies have examined the neurobiology underlying the integration of multimodal social cues in rodents. In the rat auditory cortex, ultrasonic vocalizations emitted during social interactions evoke fast-spiking neural activity that correspond to the start and end of a bout of tactile contact. However, this activity was inhibited by social face touch, which suggests that the salience of auditory information is modulated by somatosensory information (Rao et al., 2014). Similarly, in lactating female rats, pup odor increases auditory tone-evoked activity of neurons in auditory cortex, which may function to increase maternal attention to pups (Cohen et al., 2011). Consistent with the idea that unimodal sensory cues influence neural responses to stimuli of various modalities, several findings report neural activity that reflects multisensory integration in primary sensory areas in rodents (Duhamel, 2002; Kayser & Logothetis, 2007; Samuelsen & Fontanini, 2017; Ma et al., 2016; Maier et al., 2015) which suggests that sensory inputs may already begin to undergo integration in these regions. Second order sensory areas and cortical structures that execute multisensory integration, including the superior colliculus (Stein et al., 1989) and perirhinal (Otto & Eichenbaum, 1992; Furtak et al., 2007), retrosplenial (Vann et al., 2009), orbitofrontal (Schoenbaum et al., 2003), medial prefrontal (Martin-Cortecero & Nuñez, 2016) cortices are also implicated in both multisensory integration and social behaviors. How integrated, multimodal information arrives in the SDMN however, is currently unknown, but the insular cortex (IC) is one region of interest. The next section will discuss how the unique anatomical arrangement and functional role of the insular cortex warrants consideration of this region as a key hub in social decision-making.
4. Insular Cortex in Social Decision-Making: Evidence from Rodents.
4.1. The Insular Cortex Integrates External Sensory Stimuli
The IC is a well-documented site of multisensory processing (Rodgers et al., 2008; Gogolla et al., 2014) and is reciprocally connected with thalamocortical structures (Craig, 2009; Guldin & Markowitsch, 1983), which suggest IC is positioned to integrate sensory information with emotional and motivational states (Damasio & Carvalho, 2013; Krushel & van der Kooy, 1988, Paulus & Stein, 2006). Unimodal visual, sensory, auditory, gustatory and nociceptive inputs first converge in posterior IC by way of dense connections from somatosensory cortices and the suprageniculate, posterior intralaminar, peripeduncular nucleus, medial geniculate, and reticular thalamic nuclei, all of which relay sensory information from spinal afferents to the brain (Figure 1; Shi & Cassell, 1998; Linke & Schwegler, 2000; Hanamori et al., 1998). Posterior IC can then integrate unimodal sensory inputs, which is reflected in supralinear posterior IC activation to multimodal sensory stimuli (Rodgers et al., 2008) mediated, in part, by excitatory inputs from the thalamic reticular nucleus (Hanamori et al., 1998). Similarly, single unit recordings in the insular auditory field show co-responsivity to both forepaw stimulation and white noise bursts (Kimura et al., 2010) and extracellular recordings in posterior IC reveal overlapping neural responses to tail pinch, baroreceptor stimulation, appetitive and aversive tastes, and arterial chemoreceptor stimulation (Hanamori et al., 1998). Human neuroimaging studies identify IC activity in response to facial expressions (Boucher et al., 2015) and voice recognition (Andics et al., 2010). These properties suggest IC has the capacity to encode both internal and external sensory stimuli of various modalities.
4.2. Insular Cortex Integrates External Stimuli with Changes in Physiological State
In addition to integrating external sensory stimuli, IC is thought to be a locus of interoception because inputs from visceral thalamic nuclei allow IC to detect internal physiological changes (Damasio, 2003; Damasio & Carvalho, 2013; Craig, 2009; Craig 2011, Seth, 2013; and Strigo & Craig, 2016). Homeostatic changes profoundly impact brain function, attention, motivation and behavior, and rodent studies of emotion contagion have identified several instances where exposure to a recently stressed demonstrator can cause physiological changes in an observer. In mice, 22kHz alarm calls emitted by demonstrators activate the sympathetic nervous system of observers (Chen et al., 2009), and after exposure to biting horse flies, demonstrator mice display conditioned analgesia, which is transferred to observers (Kavaliers et al., 2001). Further, both observer and demonstrator voles express elevated plasma corticosterone levels (Burkett et al., 2016), and observer mice display potentiation of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus after interaction with a footshocked mouse (Sterley et al., 2018). This begets the question, do observers have a mechanism to detect these bodily changes in order to generate an appropriate social response? Information about internal state is relayed to the brain by visceral afferents via spinal and cranial nerves, which allow the brain to appraise the body’s physiological state and generate subsequent behavioral responses (Critchley & Harrison, 2013). These signals are detected by a “general visceral IC” (Cechetto & Saper, 1987) which receives inputs from ventroposterior medial and ventroposterior parvocellular thalamic nuclei and responds to diverse visceral input from gastric mechanoreceptors, arterial chemoreceptors, cardiovascular baroreceptors, as well as changes in respiration and vagal nerve activity (Barnabi & Cechetto, 2001). Moreover, IC is known to have top-down control of visceral function via efferent projections to the nucleus of the solitary tract, parabrachial nucleus, and ventrobasal and mediodorsal thalamus (Krushel & van der Kooy, 1988), and stimulation of IC neurons that project to the dorsal vagal complex or lateral hypothalamic area increases blood pressure and airflow (Bagaev & Aleksandrov, 2006), and sympathetic factors like heart rate, renal nerve activation, and blood pressure (Cechetto & Chen, 1990), respectively. These findings position the IC to be involved in either the response to, or initiation of, behavioral responses to various stressors and so the IC may detect changes in the internal state caused by exposure to a stressed demonstrator.
Regarding social function, visceral vagal inputs are hypothesized to mediate neurophysiological processes underlying stress and subsequent social behaviors (Porges, 2001) and in IC, exposure to stress increases Fos immunoreactivity (Yokoyama & Sasaki, 1999) and induces hypersensitivity to visceral function (Sun et al., 2016). Further, there may be overlapping neural populations in posterior IC that respond to, and therefore integrate, both internal and external information (Shinder & Newlands, 2014), which may give rise to the “self-other” distinction, and subsequent empathic abilities attributed to the IC in human neuroimaging experiments (Uddin et al., 2008; Singer et al., 2009). Consistent with this thought, prior work suggests IC is the region responsible for transforming internal interoceptive signals into representations of an embodied self (Seth, 2013). Thus, if exposure to a stressed demonstrator induces physiological changes in an observer, IC may integrate visceral information with limbic and motivational states via efferents to PFC (Fujita et al., 2010), amygdala (Shi & Cassell, 1998), hypothalamus (Reep & Winans, 1982) and NAc (Wright & Groenewegen, 1996) all of which are implicated in various social behaviors (Yizhar, 2012; for review). Accordingly, IC is proposed to integrate internal autonomic information with external sensory signals to mediate social learning and inform affective decision-making during neurodevelopment (Quattrocki & Friston, 2014).
4.3. Valence coding in the insular cortex
Decisions are influenced by reward value (Ploeger et al., 1991), and IC is implicated in a number of reward-related tasks in rodents. IC neurons respond to cues that predict a water reward, and increase activity after a negative outcome (Jo & Jung, 2016), suggesting IC encodes stimulus value. Consistently, in a conditioned place preference test, rats favored the maze compartment in which they received electrical stimulation of IC (Hurtado & Puerto, 2018), and unilateral stimulation of pIC was sufficient to induce the conditioned place preference (García et al., 2013). Pattij and colleagues (2014) demonstrated that pharmacological blockade of IC dopamine receptors impaired performance in a delay-discounting task. In a rat gambling task, infusion of methamphetamine to IC caused rats to opt for a high-risk, high-reward option (Mizoguchi et al., 2015). Congruent with this evidence, Ishii et al. (2012) observed a decrease in risk-taking during a rat gambling task when IC was pharmacologically inactivated. These findings are consistent with human studies in which insular lesions were associated with impaired risky decision-making (Clark et al., 2008).
The foregoing suggests that IC also contributes to value coding in social settings. A major output of the IC is the NAc, with efferents from anterior IC terminating in the dorsomedial portion of NAc, and efferents from mid and caudal IC terminating in the dorsolateral NAc (Wright & Groenewegen, 1996). NAc encodes reward value and decision-making, and, as noted, both IC and NAc are implicated in rodent and human social behaviors (Lamm & Singer, 2010; Trezza et al., 2011; Singer et al., 2006; Lichtenberg et al., 2018). In one experiment investigating this tract, silencing anterior IC⇢NAc neurons reduced reward seeking behavior (Jaramillo et al., 2018). Interestingly, while glutamatergic inputs to the NAc tend to reinforce instrumental behaviors (Britt et al., 2012), the consequence of NAc glutamate is a product of interactions between glutamate and co-transmitters like dopamine and oxytocin (Dölen et al., 2013b), and the specific postsynaptic NAc cell types (Tye, 2012). Accordingly, glutamatergic inputs to NAc might contribute to both rewarding and aversively motivated social behaviors.
4.4. The Insular Cortex, Rodent Social Behaviors, and Connectivity with SDMN
In light of the description of the sensory connectivity of IC, recent findings implicate IC as a mediator of social behavior in rodents. Our lab has reported that OT receptors in IC mediated both approach toward stressed juvenile rats and avoidance of stressed adult rats (Rogers-Carter et al., 2018b). Further, aberrant GABAergic circuit maturation is observed in the IC of several strains of autism-model mice with known social impairments (Gogolla et al., 2014) and IC microinfusions of a NMDA receptor antagonist are sufficient to decrease wrestling, social exploration, and ultrasonic vocalizations in male rats exposed to alcohol during development (Bird et al., 2017). Regarding social memory, dopaminergic, adrenergic, and serotonergic receptors in IC are involved in social recognition memory (Cavalcante et al., 2017).
These social roles for IC may result from its extensive circuitry with SDMN structures, a feature that is not observed in other higher-order sensory processing sites in the cortex. IC is reciprocally connected with the VTA, BLA, VP, NAc, MeA and PAG (Faget et al., 2016; Reep & Winans, 1982; Dong & Swanson, 2006; Shi & Cassell, 1998; Reynolds & Zahm, 2005; Groenewegen et al., 1993; Wright & Groenewegen, 1996; Cádiz-Moretti et al., 2016; Pardo-Bellver et al., 2012; Neafsey et al., 1986; Herrero et al., 1991), projects to LS, BNST and mPOA (Dong & Swanson, 2004; Dong & Swanson, 2006; Simerly & Swanson, 1986) and receives afferents from AH (Reep & Winans, 1982). The only SDMN structures with which IC lacks connectivity are Hipp and VMH (Figure 1). These connections position IC to integrate internal visceral changes with external sensory information from primary sensory inputs in order to influence SDMN activity, providing the anatomical framework by which external sensory stimuli can drive patterns of activity across the SDMN to elicit specific behavioral responses as hypothesized by Newman (1999). In addition to the anatomical connectivity, Seeley and colleagues (2007) demonstrated that IC is functionally connected to most of the deep brain structures of the SDMN. Specifically, insula BOLD signals were strongly correlated with a “salience network” of ROIs including the ACC, amygdala, VP, thalamus, hypothalamus, PAG, and VTA. This pattern is remarkably congruent to both the rodent anatomy reviewed here and primate insular anatomy (Mesulam & Mufson, 1982; Ongur & Price, 2000). Subsequent neuroimaging research has found that this salience network is involved in many cognitive functions, including socioemotional processes (Toller et al., 2018) and autism (Uddin & Menon, 2009). However, no mechanistic studies of the connections between insula and the SDMN in the context of social affective behavior exist, making this a particularly rich area for discovery.
4.5. Insular Cortex in Social Decision-Making
In the foregoing sections we presented the anatomical foundation to think about the contributions of IC to social cognition in rodents and other non-human mammals. In the extant research, IC activity is correlated with many aspects of social stimuli and behavioral responses possibly because it is positioned to integrate multisensory social stimuli with internal state. In an attempt to place the IC in the information processing stream for social affective behaviors, here we suggest where and how social affect is detected and integrated, leading to social affective behaviors. First, social affective cues from a demonstrator in a negative affective state, that is after a stressor, elicits physiological changes in an observer which reach the IC via thalamic relay nuclei and primary sensory regions. Concurrent input from IC afferents from the basolateral amygdala may convey the valence associated with the cues. IC then binds valence and sensory information to produce a multimodal sensory representation of the social stimulus. It is at this point that IC may attribute affect to the demonstrator. This assessment enables IC to convey information to SDMN structures to generate social behavioral responses. This view leads to the prediction that removing either the BLA or sensory inputs to the IC would render social behavior insensitive to the emotional states of others whereas removal of specific IC projections might selectively impair certain social affective behaviors. In support of this view, network analyses of IC activity and connectivity with somatosensory cortex revealed that the integrity of this circuit is important for social behavior. In healthy mice, somatosensory and IC activity correlated with sociability but in mice treated with valproic acid, a model of autism, decreased functional connectivity associated with social avoidance (Cho et al., 2017). With regard to IC outputs, we found that IC projections to the NAc are selectively active during encounters with stressed juveniles and are necessary for social approach (Rogers-Carter, 2019). We demonstrated that modulating IC activity in the absence of socioemotional manipulations was sufficient to recapitulate either approach or avoidance behaviors (Rogers-Carter & Varela et al., 2018b) which suggests that within IC, ensembles of neurons must contribute in some way to selecting a social behavioral response (i.e. approach or avoid) and this occurs after sensory information arrives.
The anatomical and behavioral evidence reviewed thus far make a strong case that the IC is important to social affective behavior, but does not address why it is important in terms of information processing. Prevailing views of the insular cortex from human neuroimaging studies argue that IC is a locus of salience detection (Seeley et al., 2007 and for review see Menon & Uddin, 2010; Uddin, 2015) and empathic cognition (Bernhardt & Singer, 2012). Considering the IC as a waypoint for multisensory integration likely accounts for the range of cognitive processes that are associated with this region. With regard to salience detection, IC is positioned to respond to meaningful or surprising combinations of internal and external stimuli, and because of its efferent connectivity, the salient events can profoundly shape the locus of neural circuit activity. This is well elaborated and supported by studies wherein salient stimuli trigger a shift from resting state functional connectivity to executive circuit function (Menon & Uddin, 2012). This might be why IC is important to empathy and other social emotional processes: they involve salience detection. In contrast, if the IC is uniquely engaged by social affect or, in other words, plays a unique role in empathic cognition, then one would predict that unique ensembles of IC neurons are active according to specific affective states of other individuals. To better understand what the IC contributes to information processing, future studies should be designed to untangle salience from empathy-specific cognition. Here we suggest a synthesis of the salience and empathy views; perhaps, in response to social affect, the IC may also toggle neural circuit control within the SDMN? The anatomical and behavioral evidence reviewed suggests that IC is engaged during seemingly opposite social behaviors such as both approach and avoidance (Rogers-Carter et al., 2018a, 2018b) but the IC is not needed for typical social behavior between naïve rats (Christianson et al., 2011). Therefore, the sensory social information that precedes these behaviors might drive IC only when it is salient. Specifically, social encounters that involve stress, sex, food might trigger the IC, via its efferents to the SDMN, to toggle network control to yield different social behaviors: avoidance to approach, aggression to submission, and so on.
5. Conclusion
Across species, social decision-making allows for navigating the social world with behavioral flexibility. Behavioral reactions in a social situation reflect the convergence of social information, external stimuli and physiological state to produce appropriate responses. Multimodal sensory integration and convergence allow socioemotional cues to be bound with relevant external cues and internal states. We argue this function is uniquely attributed to IC, which is anatomically positioned to serve as a waypoint for social information to influence activity patterns in the SDMN. From this view, we can better understand why abnormal IC function and connectivity are correlates of a broad range of neuropsychiatric symptoms and should be seen as a focal point for research efforts to better treat, diagnose and prevent mental illness.
HIGHLIGHTS.
Social decision-making requires integration of external and internal sensory cues.
Rodents convey affect via a constellation of multisensory expressions
Current models of the rodent social brain do not fully account for sensory integration.
Integration of social sensory information occurs in insular cortex
Insular cortex is positioned to convey social cues to social decision-making network
ACKNOWLEDGEMENTS
M.M.R-C is funded by a National Science Foundation Graduate Research Fellowship. J.P.C is funded by National Institutes of Mental Health Grants MH109545 and MH110907. The authors have no competing financial conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R (2001). The neurobiology of social cognition. Curr Opin Neurobiol, 11(2), 231–239. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11301245 [DOI] [PubMed] [Google Scholar]
- Alexander WH, & Brown JW (2011). Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci, 14(10), 1338–1344. doi: 10.1038/nn.2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, & Frith CD (2006a). Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci, 7(4), 268–277. doi: 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Amodio DM, & Frith CD (2006b). Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci, 7(4), 268–277. doi: 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Andics A, McQueen JM, Petersson KM, Gál V, Rudas G, & Vidnyánszky Z (2010). Neural mechanisms for voice recognition. Neuroimage, 52(4), 1528–1540. doi: 10.1016/j.neuroimage.2010.05.048 [DOI] [PubMed] [Google Scholar]
- Angoa-Pérez M, & Kuhn DM (2015). Neuroanatomical dichotomy of sexual behaviors in rodents: a special emphasis on brain serotonin. Behav Pharmacol, 26(6), 595–606. doi: 10.1097/FBP.0000000000000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Arakawa K, Dunlap C, & Blanchard RJ (2008). Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev, 32(7), 1236–1248. doi: 10.1016/j.neubiorev.2008.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Suzuki A, Zhao S, Tsytsarev V, Lo FS, Hayashi Y, … Erzurumlu RS (2014). Thalamic NMDA receptor function is necessary for patterning of the thalamocortical somatosensory map and for sensorimotor behaviors. J Neurosci, 34(36), 12001–12014. doi: 10.1523/JNEUROSCI.1663-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aransay A, Rodríguez-López C, García-Amado M, Clascá F, & Prensa L (2015). Long-range projection neurons of the mouse ventral tegmental area: a single-cell axon tracing analysis. Front Neuroanat, 9, 59. doi: 10.3389/fnana.2015.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi JC, Sirigu A, & Duhamel JR (2012). Modulation of value representation by social context in the primate orbitofrontal cortex. Proc Natl Acad Sci U S A, 109(6), 2126–2131. doi: 10.1073/pnas.1111715109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babineau BA, Bliss-Moreau E, Machado CJ, Toscano JE, Mason WA, & Amaral DG (2011). Context-specific social behavior is altered by orbitofrontal cortex lesions in adult rhesus macaques. Neuroscience, 179, 80–93. doi: 10.1016/j.neuroscience.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, & Schultz W (2013). The role of the striatum in social behavior. Front Neurosci, 7, 233. doi: 10.3389/fnins.2013.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagaev V, & Aleksandrov V (2006). Visceral-related area in the rat insular cortex. Auton Neurosci, 125(1–2), 16–21. doi: 10.1016/j.autneu.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, & Mendoza SP (2007). Neural correlates of pair-bonding in a monogamous primate. Brain Res, 1184, 245–253. doi: 10.1016/j.brainres.2007.09.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barat E, Wirth S, & Duhamel JR (2018). Face cells in orbitofrontal cortex represent social categories. Proc Natl Acad Sci U S A, 115(47), E11158–E11167. doi: 10.1073/pnas.1806165115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabi F, & Cechetto DF (2001). Neurotransmitters in the thalamus relaying visceral input to the insular cortex in the rat. Am J Physiol Regul Integr Comp Physiol, 281(5), R1665–74. doi: 10.1152/ajpregu.2001.281.5.R1665 [DOI] [PubMed] [Google Scholar]
- Beach FA (1942). Analysis of the stimuli adequate to elicit mating behavior in the sexually inexperienced rat. Journal of Comparative Neurology, 33(2), 163–207. [Google Scholar]
- Beach FA, & Jaynes J (1956). Studies of maternal retrieving in rats. Iii. Sensory cues involved in the lactating female’s response to her young. Behavior, 10(1). [Google Scholar]
- Bekoff M (1972). The development of social interaction, play, and metacommunication in mammals: an ethological perspective. The Quarterly Review of Biology, 47(4), 412–434. [Google Scholar]
- Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, & Mason P (2014). Pro-social behavior in rats is modulated by social experience. Elife, 3, e01385. doi: 10.7554/eLife.01385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, & Singer T (2012). The neural basis of empathy. Annu Rev Neurosci, 35, 1–23. doi: 10.1146/annurev-neuro-062111-150536 [DOI] [PubMed] [Google Scholar]
- Beyeler A, Chang CJ, Silvestre M, Lévêque C, Namburi P, Wildes CP, & Tye KM (2018). Organization of Valence-Encoding and Projection-Defined Neurons in the Basolateral Amygdala. Cell Rep, 22(4), 905–918. doi: 10.1016/j.celrep.2017.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CW, Barto D, Magcalas CM, Rodriguez CI, Donaldson T, Davies S, … Hamilton DA (2017). Ifenprodil infusion in agranular insular cortex alters social behavior and vocalizations in rats exposed to moderate levels of ethanol during prenatal development. Behav Brain Res, 320, 1–11. doi: 10.1016/j.bbr.2016.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, & Dantzer R (1990). Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res, 535(2), 301–304. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1963571 [DOI] [PubMed] [Google Scholar]
- Bobrov E, Wolfe J, Rao RP, & Brecht M (2014). The representation of social facial touch in rat barrel cortex. Curr Biol, 24(1), 109–115.doi: 10.1016/j.cub.2013.11.049 [DOI] [PubMed] [Google Scholar]
- Bolding KA, & Franks KM (2017). Complementary codes for odor identity and intensity in olfactory cortex. Elife, 6. doi: 10.7554/eLife.22630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli KG, Blanchard DC, Javier LK, Defensor EB, Brandão ML, & Blanchard RJ (2009). Neural correlates of scent marking behavior in C57BL/6J mice: detection and recognition of a social stimulus. Neuroscience, 162(4), 914–923. doi: 10.1016/j.neuroscience.2009.05.047 [DOI] [PubMed] [Google Scholar]
- Bouchard S, Bernier F, Boivin E, Dumoulin S, Laforest M, Guitard T, Robillard G, Monthuy-Blanc J, & Renaud P (2013). Empathy toward virtual humans depicting a known or unknown person expressing pain. Cyberpsychology, Behavior, and Social Networking, 16(1), 61–71. Retrieved from https://www.liebertpub.com/doi/pdfplus/10.1089/cyber.2012.1571 [DOI] [PubMed] [Google Scholar]
- Boucher O, Rouleau I, Lassonde M, Lepore F, Bouthillier A, & Nguyen DK (2015). Social information processing following resection of the insular cortex. Neuropsychologia, 71, 1–10. doi: 10.1016/j.neuropsychologia.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Brecht M, & Freiwald WA (2012). The many facets of facial interactions in mammals. Curr Opin Neurobiol, 22(2), 259–266. doi: 10.1016/j.conb.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, & Bonci A (2012). Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron, 76(4), 790–803. doi: 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, & Zahm DS (1993). The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol, 338(2), 255–278. doi: 10.1002/cne.903380209 [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, & Hikosaka O (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron, 68(5), 815–834. doi: 10.1016/j.neuron.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM (2013). Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol, 23(3), 310–317. doi: 10.1016/j.conb.2013.01.014 [DOI] [PubMed] [Google Scholar]
- Budinger E, Laszcz A, Lison H, Scheich H, & Ohl FW (2008). Non-sensory cortical and subcortical connections of the primary auditory cortex in Mongolian gerbils: bottom-up and top-down processing of neuronal information via field AI. Brain Res, 1220, 2–32. doi: 10.1016/j.brainres.2007.07.084 [DOI] [PubMed] [Google Scholar]
- Burke CJ, Kisko TM, Swiftwolfe H, Pellis SM, & Euston DR (2017). Specific 50-kHz vocalizations are tightly linked to particular types of behavior in juvenile rats anticipating play. PLoS One, 12(5), e0175841. doi: 10.1371/journal.pone.0175841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, & Young LJ (2016). Oxytocin-dependent consolation behavior in rodents. Science, 351(6271), 375–378. doi: 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M (2002). What is emotion. Behav Processes, 60(2), 69–83. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12426062 [DOI] [PubMed] [Google Scholar]
- Cádiz-Moretti B, Abellán-Álvaro M, Pardo-Bellver C, Martínez-García F, & Lanuza E (2016). Afferent and Efferent Connections of the Cortex-Amygdala Transition Zone in Mice. Front Neuroanat, 10, 125. doi: 10.3389/fnana.2016.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldú X, & Dreher JC (2007). Hormonal and genetic influences on processing reward and social information. Ann N Y Acad Sci, 1118, 43–73. doi: 10.1196/annals.1412.007 [DOI] [PubMed] [Google Scholar]
- Caldwell HK (2017). Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. Neuroscientist, 23(5), 517–528. doi: 10.1177/1073858417708284 [DOI] [PubMed] [Google Scholar]
- Carruthers IM, Natan RG, & Geffen MN (2013). Encoding of ultrasonic vocalizations in the auditory cortex. J Neurophysiol, 109(7), 1912–1927. doi: 10.1152/jn.00483.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante LES, Zinn CG, Schmidt SD, Saenger BF, Ferreira FF, Furini CRG, … Izquierdo I (2017). Modulation of the storage of social recognition memory by neurotransmitter systems in the insular cortex. Behav Brain Res, 334, 129–134. doi: 10.1016/j.bbr.2017.07.044 [DOI] [PubMed] [Google Scholar]
- Cechetto DF, & Chen SJ (1990). Subcortical sites mediating sympathetic responses from insular cortex in rats. Am J Physiol, 258(1 Pt 2), R245–55. doi: 10.1152/ajpregu.1990.258.1.R245 [DOI] [PubMed] [Google Scholar]
- Cechetto DF, & Saper CB (1987). Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol, 262(1), 27–45. doi: 10.1002/cne.902620104 [DOI] [PubMed] [Google Scholar]
- Charles Lawrence R, Cale Bonner H, Newsom RJ, & Kelly SJ (2008). Effects of alcohol exposure during development on play behavior and c-Fos expression in response to play behavior. Behav Brain Res, 188(1), 209–218. doi: 10.1016/j.bbr.2007.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelnokova O, Laeng B, Eikemo M, Riegels J, Løseth G, Maurud H, … Leknes S (2014). Rewards of beauty: the opioid system mediates social motivation in humans. Mol Psychiatry, 19(7), 746–747. doi: 10.1038/mp.2014.1 [DOI] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, & Wu Q (2011). Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology, 36(13), 2629–2638. doi: 10.1038/npp.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PB, Hu RK, Wu YE, Pan L, Huang S, Micevych PE, & Hong W (2019). Sexually Dimorphic Control of Parenting Behavior by the Medial Amygdala. Cell, 176(5), 1206–1221.e18. doi: 10.1016/j.cell.2019.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Panksepp JB, & Lahvis GP (2009). Empathy is moderated by genetic background in mice. PLoS One, 4(2), e4387. doi: 10.1371/journal.pone.0004387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Skolnikoff S (1973). Facial expression of emotion in nonhuman primates. Darwin and facial expression: A century of research in review, 11–89. [Google Scholar]
- Cho H, Kim CH, Knight EQ, Oh HW, Park B, Kim DG, & Park HJ (2017). Changes in brain metabolic connectivity underlie autistic-like social deficits in a rat model of autism spectrum disorder. Sci Rep, 7(1), 13213. doi: 10.1038/s41598-017-13642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe HK, Reed MD, Benavidez N, Montgomery D, Soares N, Yim YS, & Choi GB (2015). Oxytocin Mediates Entrainment of Sensory Stimuli to Social Cues of Opposing Valence. Neuron, 87(1), 152–163. doi: 10.1016/j.neuron.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Jennings JH, Ragole T, Flyer JG, Benison AM, Barth DS, Watkins LR, & Maier SF (2011) Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry, 70(5):458–64. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, & Robbins TW (2008). Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain, 131(Pt 5), 1311–1322. doi: 10.1093/brain/awn066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Rothschild G, & Mizrahi A (2011). Multisensory integration of natural odors and sounds in the auditory cortex. Neuron, 72(2), 357–369. doi: 10.1016/j.neuron.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Connor JR, & Diamond MC (1982). A comparison of dendritic spine number and type on pyramidal neurons of the visual cortex of old adult rats from social or isolated environments. J Comp Neurol, 210(1), 99–106. doi: 10.1002/cne.902100111 [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GA, & Lucion AB (2005). Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav, 85(3), 354–362. doi: 10.1016/j.physbeh.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Coolen LM, & Wood RI (1998). Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol, 399(2), 189–209. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9721903 [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Ouimet AJ, Pacheco P, Manzo J, & Pfaus JG (2005). Olfactory conditioned partner preference in the female rat. Behav Neurosci, 119(3), 716–725. doi: 10.1037/0735-7044.119.3.716 [DOI] [PubMed] [Google Scholar]
- Craig AD (2009). How do you feel --now? The anterior insula and human awareness. Nat Rev Neurosci, 10(1), 59–70. doi: 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Craig AD (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Ann NY Acad Sci, 1225, 72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Creutz LM, & Kritzer MF (2004). Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol, 476(4), 348–362. doi: 10.1002/cne.20229 [DOI] [PubMed] [Google Scholar]
- Crews D (2003). The development of phenotypic plasticity: where biology and psychology meet. Dev Psychobiol, 43(1), 1–10. doi: 10.1002/dev.10115 [DOI] [PubMed] [Google Scholar]
- Critchley HD, & Harrison NA (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. doi: 10.1016/j.neuron.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Damasio A (2006). Feelings of Emotion and the Self. Ann NY Acad Sci, 253–61. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Damasio A, Carvalho GB (2013). The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neuroscience, 14(2), 143–52. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- Darwin C (1976). The expression of the emotions in man and animals (London: New York: ). [Google Scholar]
- De La Rosa-Prieto C, De Moya-Pinilla M, Saiz-Sanchez D, Ubeda-Banon I, Arzate DM, Flores-Cuadrado A, … Martinez-Marcos A (2015). Olfactory and cortical projections to bulbar and hippocampal adult-born neurons. Front Neuroanat, 9, 4. doi: 10.3389/fnana.2015.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defensor EB, Corley MJ, Blanchard RJ, & Blanchard DC (2012). Facial expressions of mice in aggressive and fearful contexts. Physiol Behav, 107(5), 680–685. doi: 10.1016/j.physbeh.2012.03.024 [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, & Veenema AH (2017). Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol, 525(11), 2549–2570. doi: 10.1002/cne.24216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinopoulos A, & Parnavelas JG (1991). The development of ventral tegmental area (VTA) projections to the visual cortex of the rat. Neurosci Lett, 134(1), 12–16. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1815143 [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, & Malenka RC (2013b). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179–184. doi: 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, & Malenka RC (2013b). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179–184. doi: 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, & Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science, 322(5903), 900–904. doi: 10.1126/science.1158668 [DOI] [PubMed] [Google Scholar]
- Dong HW, & Swanson LW (2004). Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol, 471(4), 396–433. doi: 10.1002/cne.20002 [DOI] [PubMed] [Google Scholar]
- Dong HW, & Swanson LW (2006). Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol, 494(1), 75–107. doi: 10.1002/cne.20790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel JR (2002). Multisensory integration in cortex: shedding light on prickly issues. Neuron, 34(4), 493–495. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12062031 [DOI] [PubMed] [Google Scholar]
- Dumais KM, & Veenema AH (2016). Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol, 40, 1–23. doi: 10.1016/j.yfrne.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, & Pattison PE (2002). Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev, 22(6), 789–832. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12214327 [DOI] [PubMed] [Google Scholar]
- Ekman P, & Oster H (1979). Facial expressions of emotion. Annual review of psychology, 30(1), 527–554. Retrieved from https://www.annualreviews.org/doi/pdf/10.1146/annurev.ps.30.020179.002523 [Google Scholar]
- Erzurumlu RS, & Gaspar P (2012). Development and critical period plasticity of the barrel cortex. Eur J Neurosci, 35(10), 1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget L, Osakada F, Duan J, Ressler R, Johnson AB, Proudfoot JA, … Hnasko TS, (2016). Afferent Inputs to Neurotransmitter-Defined Cell Types in the Ventral Tegmental Area. Cell Rep, 15(12), 2796–2808. doi: 10.1016/j.celrep.2016.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faser KA, Poucet B, Partlow G, & Herrmann T (1991). Role of the medial and lateral septum in a variable goal spatial problem solving task. Physiol Behav, 50(4), 739–744. [DOI] [PubMed] [Google Scholar]
- FeldmanHall O, Raio CM, Kubota JT, Seiler MG, & Phelps EA (2015). The effects of social context and acute stress on decision making under uncertainty. Psychological science, 26(12), 1918–1926. Retrieved from https://journals.sagepub.com/doi/pdf/10.1177/0956797615605807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, & Tye KM (2016). Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience, 321, 197–209. doi: 10.1016/j.neuroscience.2015.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson K, Lampe JF, Hintze S, Würbel H, & Melotti L (2016). Facial Indicators of Positive Emotions in Rats. PLoS One, 11, e0166446. doi: 10.1371/journal.pone.0166446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, & Rosenblatt JS (1974). Olfactory regulation of maternal behavior in rats. II. Effects of peripherally induced anosmia and lesions of the lateral olfactory tract in pup-induced virgins. J Comp Physiol Psychol, 86(2), 233–246. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4590492 [DOI] [PubMed] [Google Scholar]
- Frith U, & Frith CD (2003). Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci, 358(1431), 459–473. doi: 10.1098/rstb.2002.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Adachi K, Koshikawa N, & Kobayashi M (2010). Spatiotemporal dynamics of excitation in rat insular cortex: intrinsic corticocortical circuit regulates caudalrostro excitatory propagation from the insular to frontal cortex. Neuroscience, 165(1), 278–292. doi: 10.1016/j.neuroscience.2009.09.073 [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, & Burwell RD (2007). Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus, 17(9), 709–722. doi: 10.1002/hipo.20314 [DOI] [PubMed] [Google Scholar]
- Gabay AS, Radua J, Kempton MJ, Mehta MA (2014). The Ultimatum Game and the brain: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev, 47, 549–58. [DOI] [PubMed] [Google Scholar]
- Galef BG, Kennett DJ, & Stein M (1985). Demonstrator influence on observer diet preference: Effects of simple exposure and the presence of a demonstrator. Animal Learning & Behavior, 13(1), 25–30. [Google Scholar]
- Galef BG, & Wigmore SW (1983). Transfer of information concerning distant foods: A laboratory investigation of the ‘information-centre’ hypothesis. Anim Behav, 31(3), 748–758. [Google Scholar]
- Gallo M, Roldan G, & Bures J (1992). Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behav Brain Res, 52(1), 91–97. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1335264 [DOI] [PubMed] [Google Scholar]
- García R, Simón MJ, & Puerto A (2013). Conditioned place preference induced by electrical stimulation of the insular cortex: effects of naloxone. Exp Brain Res, 226(2), 165–174. doi: 10.1007/s00221-013-3422-7 [DOI] [PubMed] [Google Scholar]
- Guo X, Zheng L, Zhu L, Li J, Wang Q, Dienes Z, Yang Z. Increased neural responses to unfairness in a loss context (2013). Neuroimage, 77, 246–53. doi: 10.1016/j.neuroimage.2013.03.048. [DOI] [PubMed] [Google Scholar]
- Gil M, Nguyen NT, McDonald M, & Albers HE (2013). Social reward: interactions with social status, social communication, aggression, and associated neural activation in the ventral tegmental area. Eur J Neurosci, 38(2), 2308–2318. doi: 10.1111/ejn.12216 [DOI] [PubMed] [Google Scholar]
- Gogolla N, Takesian AE, Feng G, Fagiolini M, & Hensch TK (2014). Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron, 83(4), 894–905. doi: 10.1016/j.neuron.2014.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL (2005). The vertebrate social behavior network: evolutionary themes and variations. Horm Behav, 48(1), 11–22. doi: 10.1016/j.yhbeh.2005.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, & Panksepp J (2002). Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res Bull, 57(5), 651–659. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11927369 [DOI] [PubMed] [Google Scholar]
- Green JT (1969). Altruistic behavior in the albino rat. Psychonomic Society, 14(1), 47–48. [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, & Dapretto M (2016). Salience Network Connectivity in Autism Is Related to Brain and Behavioral Markers of Sensory Overresponsivity. J Am Acad Child Adolesc Psychiatry, 55(7), 618–626.e1. doi: 10.1016/j.jaac.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, & Haber SN (1993). Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience, 57(1), 113–142. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8278047 [DOI] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, & Fan J (2013). Anterior insular cortex and emotional awareness. J Comp Neurol, 521(15), 3371–3388. doi: 10.1002/cne.23368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldin WO, & Markowitsch HJ (1983). Cortical and thalamic afferent connections of the insular and adjacent cortex of the rat. J Comp Neurol, 215(2), 135–153. doi: 10.1002/cne.902150203 [DOI] [PubMed] [Google Scholar]
- Gulia KK, Kumar VM, & Mallick HN (2002). Role of the lateral septal noradrenergic system in the elaboration of male sexual behavior in rats. Pharmacol Biochem Behav, 72(4), 817–823. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12062571 [DOI] [PubMed] [Google Scholar]
- Guzmán YF, Tronson NC, Guedea A, Huh KH, Gao C, & Radulovic J (2009). Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res, 201(1), 173–178. doi: 10.1016/j.bbr.2009.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel L, Thangarasa T, Samadi O, & Ito R (2017). Caudal Nucleus Accumbens Core Is Critical in the Regulation of Cue-Elicited Approach-Avoidance Decisions. eNeuro, 4(1). doi: 10.1523/ENEURO.0330-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, & Fischer J (2012). The structure and usage of female and male mouse ultrasonic vocalizations reveal only minor differences. PLoS One, 7, e41133. doi: 10.1371/journal.pone.0041133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamori T, Kunitake T, Kato K, & Kannan H (1998). Neurons in the posterior insular cortex are responsive to gustatory stimulation of the pharyngolarynx, baroreceptor and chemoreceptor stimulation, and tail pinch in rats. Brain Res, 785(1), 97–106. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9526057 [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, & Cullinan WE (2003). Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol, 24(3), 151–180. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14596810 [DOI] [PubMed] [Google Scholar]
- Herrero MT, Insausti R, & Gonzalo LM (1991). Cortically projecting cells in the periaqueductal gray matter of the rat. A retrograde fluorescent tracer study. Brain Res, 543(2), 201–212. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2059832 [DOI] [PubMed] [Google Scholar]
- Higa KK, Young JW, Ji B, Nichols DE, Geyer MA, & Zhou X (2017). Striatal dopamine D1 receptor suppression impairs reward-associative learning. Behav Brain Res, 323, 100–110. doi: 10.1016/j.bbr.2017.01.041 [DOI] [PubMed] [Google Scholar]
- Hiser J, & Koenigs M (2018). The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol Psychiatry, 83(8), 638–647. doi: 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, & Rada P (2007). Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol, 7(6), 617–627. doi: 10.1016/j.coph.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Kim DW, & Anderson DJ (2014). Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell, 158(6), 1348–1361. doi: 10.1016/j.cell.2014.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, … Malenka RC, (2017). Gating of social reward by oxytocin in the ventral tegmental area. Science, 357(6358), 1406–1411. doi: 10.1126/science.aan4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ, Zhong H, & Mao T (2016). A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife, 5. doi: 10.7554/eLife.19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado MM, & Puerto A (2018). Tolerance to rewarding brain electrical stimulation: Differential effects of contingent and non-contingent activation of parabrachial complex and lateral hypothalamus. Behav Brain Res, 336, 15–21. doi: 10.1016/j.bbr.2017.08.030 [DOI] [PubMed] [Google Scholar]
- Ihnat R, White NR, & Barfield RJ (1995). Pup’s broadband vocalizations and maternal behavior in the rat. Behav Processes, 33(3), 257–271. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24896999 [DOI] [PubMed] [Google Scholar]
- Illig KR, & Wilson DA (2009). Olfactory Cortex: Comparative Anatomy. Academic Press. [Google Scholar]
- Insel TR (2010). The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron, 65(6), 768–779. doi: 10.1016/j.neuron.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, & Fernald RD (2004). How the brain processes social information: searching for the social brain. Annu Rev Neurosci, 27, 697–722. doi: 10.1146/annurev.neuro.27.070203.144148 [DOI] [PubMed] [Google Scholar]
- Ishii A, Kiyokawa Y, Takeuchi Y, & Mori Y (2016). Social buffering ameliorates conditioned fear responses in female rats. Horm Behav, 81, 53–58. doi: 10.1016/j.yhbeh.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Ishii H, Ohara S, Tobler PN, Tsutsui K, & Iijima T (2012). Inactivating anterior insular cortex reduces risk taking. J Neurosci, 32(45), 16031–16039. doi: 10.1523/JNEUROSCI.2278-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa JB, Haeffele BD, Young ED, & Yue DT (2017). Multiscale mapping of frequency sweep rate in mouse auditory cortex. Hear Res, 344, 207–222. doi: 10.1016/j.heares.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Stewart S, Fortino B, Van Voorhies K, & Besheer J (2018). Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology, 130, 42–53. doi: 10.1016/j.neuropharm.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, … Shin HS (2010). Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci, 13(4), 482–488. doi: 10.1038/nn.2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, & Jung MW (2016). Differential coding of uncertain reward in rat insular and orbitofrontal cortex. Sci Rep, 6, 24085. doi: 10.1038/srep24085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Illig KR, Behan M, & Haberly LB (2000). New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci, 20(18), 6974–6982. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10995842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE (2003). Chemical Communication in Rodents: from Pheromones to Individual Recognition. 84, 4, 1141–1162. [Google Scholar]
- Johnson ZV, & Young LJ (2015). Neurobiological mechanisms of social attachment and pair bonding. Curr Opin Behav Sci, 3, 38–44. doi: 10.1016/j.cobeha.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Riha PD, Gore AC, & Monfils MH (2014). Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Anim Cogn, 17(3), 827–834. doi: 10.1007/s10071-013-0711-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, & Davidson RJ (2007). Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry, 62(10), 1134–1139. doi: 10.1016/j.biopsych.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, & Choleris E (2001). NMDA-mediated social learning of fear-induced conditioned analgesia to biting flies. Neuroreport, 12(4), 663–667. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11277559 [DOI] [PubMed] [Google Scholar]
- Kawamichi H, Tanabe HC, Takahashi HK, & Sadato N (2013). Activation of the reward system during sympathetic concern is mediated by two types of empathy in a familiarity-dependent manner. Social neuroscience, 8(1), 90–100. Retrieved from https://www.tandfonline.com/doi/full/10.1080/17470919.2012.744349 [DOI] [PubMed] [Google Scholar]
- Kayser C, & Logothetis NK (2007). Do early sensory cortices integrate cross-modal information. Brain Struct Funct, 212(2), 121–132. doi: 10.1007/s00429-007-0154-0 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kim ES, Covey E, & Kim JJ (2010). Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One, 5(12), e15077. doi: 10.1371/journal.pone.0015077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Imbe H, & Donishi T (2010). Efferent connections of an auditory area in the caudal insular cortex of the rat: anatomical nodes for cortical streams of auditory processing and cross-modal sensory interactions. Neuroscience, 166(4), 1140–1157. doi: 10.1016/j.neuroscience.2010.01.032 [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Honda A, Takeuchi Y, & Mori Y (2014). A familiar conspecific is more effective than an unfamiliar conspecific for social buffering of conditioned fear responses in male rats. Behav Brain Res, 267, 189–193. doi: 10.1016/j.bbr.2014.03.043 [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Wakabayashi Y, Takeuchi Y, & Mori Y (2012). The neural pathway underlying social buffering of conditioned fear responses in male rats. Eur J Neurosci, 36(10), 3429–3437. doi: 10.1111/j.1460-9568.2012.08257.x [DOI] [PubMed] [Google Scholar]
- Knapska E, Mikosz M, Werka T, & Maren S (2010). Social modulation of learning in rats. Learn Mem, 17(1), 35–42. doi: 10.1101/lm.1670910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, Kaczmarek L, & Werka T (2006). Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc Natl Acad Sci U S A, 103(10), 3858–3862. doi: 10.1073/pnas.0511302103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krushel LA, & van der Kooy D (1988). Visceral cortex: integration of the mucosal senses with limbic information in the rat agranular insular cortex. J Comp Neurol, 270(1), 39–54, 62. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2453537 [DOI] [PubMed] [Google Scholar]
- Lacey EA, Alexander RD, Braude S, Sherman PW, & Jarvis JUM (1991). An ethogram for the naked mole-rat: Nonvocal behaviors In The Biology of the Naked Mole-rat. Princeton, N.J.: Princeton University Press. [Google Scholar]
- Lamm C, & Singer T (2010). The role of anterior insular cortex in social emotions. Brain Struct Funct, 214(5–6), 579–591. doi: 10.1007/s00429-010-0251-3 [DOI] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, … Mogil JS (2006). Social modulation of pain as evidence for empathy in mice. Science, 312(5782), 1967–1970. doi: 10.1126/science.1128322 [DOI] [PubMed] [Google Scholar]
- Lecorps B, & Féron C (2015). Correlates between ear postures and emotional reactivity in a wild type mouse species. Behav Processes, 120, 25–29. doi: 10.1016/j.beproc.2015.08.002 [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annu Rev Neurosci, 23, 155–184. doi: 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, & Romanski LM (1991). Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci Lett, 134(1), 139–144. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1815147 [DOI] [PubMed] [Google Scholar]
- Lee E, Rhim I, Lee JW, Ghim JW, Lee S, Kim E, & Jung MW (2016). Enhanced Neuronal Activity in the Medial Prefrontal Cortex during Social Approach Behavior. J Neurosci, 36(26), 6926–6936. doi: 10.1523/JNEUROSCI.0307-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, … Anderson DJ (2014). Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature, 509(7502), 627–632. doi: 10.1038/nature13169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VK, & Harris LT (2013). How social cognition can inform social decision making. Front Neurosci, 7, 259. doi: 10.3389/fnins.2013.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, & Powers JB (1980). Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science, 210(4469), 557–560. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7423209 [DOI] [PubMed] [Google Scholar]
- Lenschow C, & Brecht M (2018). Physiological and Anatomical Outputs of Rat Genital Cortex. Cereb Cortex, 28(4), 1472–1486. doi: 10.1093/cercor/bhx359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, & Crair MC (2011). How do barrels form in somatosensory cortex. Ann N Y Acad Sci, 1225, 119–129. doi: 10.1111/j.1749-6632.2011.06024.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lu YF, Li CL, Wang Y, Sun W, He T, … Chen J (2014). Social interaction with a cagemate in pain facilitates subsequent spinal nociception via activation of the medial prefrontal cortex in rats. Pain, 155(7), 1253–1261. doi: 10.1016/j.pain.2014.03.019 [DOI] [PubMed] [Google Scholar]
- Liberles SD (2014). Mammalian pheromones. Annu Rev Physiol, 76, 151–175. doi: 10.1146/annurev-physiol-021113-170334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg NT, Lee B, Kashtelyan V, Chappa BS, Girma HT, Green EA, … Roesch MR, (2018). Rat behavior and dopamine release are modulated by conspecific distress. Elife, 7. doi: 10.7554/eLife.38090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, & Stenevi U (1978). Dopamine and noradrenaline neurons projecting to the septal area in the rat. Cell Tissue Res, 190(3), 383–407. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/688339 [DOI] [PubMed] [Google Scholar]
- Linke R, & Schwegler H (2000). Convergent and complementary projections of the caudal paralaminar thalamic nuclei to rat temporal and insular cortex. Cereb Cortex, 10(8), 753–771. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10920048 [DOI] [PubMed] [Google Scholar]
- Lozier LM, Vanmeter JW, & Marsh AA (2014). Impairments in facial affect recognition associated with autism spectrum disorders: a meta-analysis. Dev Psychopathol, 26(4 Pt 1), 933–945. doi: 10.1017/S0954579414000479 [DOI] [PubMed] [Google Scholar]
- Lupfer G, Frieman J, & Coonfield D (2003). Social transmission of flavor preferences in two species of hamsters (Mesocricetus auratus and Phodopus campbelli). J Comp Psychol, 117(4), 449–455. doi: 10.1037/0735-7036.117.4.449 [DOI] [PubMed] [Google Scholar]
- Ma LQ, Ning L, Wang Z, & Wang YW (2016). Visual and noxious electrical stimulus-evoked membrane-potential responses in anterior cingulate cortical neurons. Mol Brain, 9(1), 82. doi: 10.1186/s13041-016-0262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, & Bachevalier J (2007). Measuring reward assessment in a semi-naturalistic context: the effects of selective amygdala, orbital frontal or hippocampal lesions. Neuroscience, 148(3), 599–611. doi: 10.1016/j.neuroscience.2007.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima S, Ohishi N, Yamaguchi S, & Tsukahara S (2015). A neural connection between the central part of the medial preoptic nucleus and the bed nucleus of the stria terminalis to regulate sexual behavior in male rats. Neurosci Lett, 606, 66–71. doi: 10.1016/j.neulet.2015.08.047 [DOI] [PubMed] [Google Scholar]
- Maier JX, Blankenship ML, Li JX, & Katz DB (2015). A Multisensory Network for Olfactory Processing. Curr Biol, 25(20), 2642–2650. doi: 10.1016/j.cub.2015.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainardi M, Landi S, Gianfranceschi L, Baldini S, De Pasquale R, Berardi N, … Caleo M (2010). Environmental enrichment potentiates thalamocortical transmission and plasticity in the adult rat visual cortex. J Neurosci Res, 88(14), 3048–3059. doi: 10.1002/jnr.22461 [DOI] [PubMed] [Google Scholar]
- Man K, Kaplan J, Damasio H, Damasio A (2013). Neural convergence and divergence in the mammalian cerebral cortex: from experimental neuroanatomy to functional neuroimaging. J Comp Neurol, 521(18), 4097–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA (2018). The Caring Continuum: Evolved Hormonal and Proximal Mechanisms Explain Prosocial and Antisocial Extremes. Annu Rev Psychol. doi: 10.1146/annurev-psych-010418-103010 [DOI] [PubMed] [Google Scholar]
- Martin-Cortecero J, & Nuñez A (2016). Sensory responses in the medial prefrontal cortex of anesthetized rats. Implications for sensory processing. Neuroscience, 339, 109–123. doi: 10.1016/j.neuroscience.2016.09.045 [DOI] [PubMed] [Google Scholar]
- Mason M, Magee JC, & Fiske ST (2014). Neural substrates of social status inference: roles of medial prefrontal cortex and superior temporal sulcus. J Cogn Neurosci, 26(5), 1131–1140. doi: 10.1162/jocn_a_00553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto YK, & Okanoya K (2016). Phase-Specific Vocalizations of Male Mice at the Initial Encounter during the Courtship Sequence. PLoS One, 11(2), e0147102. doi: 10.1371/journal.pone.0147102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct, 214(5–6), 655–667. doi: 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1998). From sensation to cognition. Brain, 121(6), 1013–52. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ (1982). Insula of the old world monkey: I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol, 212(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Meyza K, Nikolaev T, Kondrakiewicz K, Blanchard DC, Blanchard RJ, & Knapska E (2015). Neuronal correlates of asocial behavior in a BTBR T (+) Itpr3(tf)/J mouse model of autism. Front Behav Neurosci, 9, 199. doi: 10.3389/fnbeh.2015.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikosz M, Nowak A, Werka T, & Knapska E (2015). Sex differences in social modulation of learning in rats. Sci Rep, 5, 18114. doi: 10.1038/srep18114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, … Froemke RC, (2016). A Distributed Network for Social Cognition Enriched for Oxytocin Receptors. J Neurosci, 36(8), 2517–2535. doi: 10.1523/JNEUROSCI.2409-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A, Gupta M, Lamarre Y, Jahagirdar B, & Gupta K (2016). Quantification of pain in sickle mice using facial expressions and body measurements. Blood Cells Mol Dis, 57, 58–66. doi: 10.1016/j.bcmd.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Takase K, Nakajima W, Tada H, Ohya D, Sano A, … Takahashi T (2012). Disrupted cortical function underlies behavior dysfunction due to social isolation. J Clin Invest, 122(7), 2690–2701. doi: 10.1172/JCI63060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Katahira K, Inutsuka A, Fukumoto K, Nakamura A, Wang T, … Yamada K (2015). Insular neural system controls decision-making in healthy and methamphetamine-treated rats. Proc Natl Acad Sci U S A, 112(29), E3930–9. doi: 10.1073/pnas.1418014112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, & Yang CR (1991). The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol, 295, 267–290. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1776572 [DOI] [PubMed] [Google Scholar]
- Moyaho A, Rivas-Zamudio X, Ugarte A, Eguibar JR, & Valencia J (2015). Smell facilitates auditory contagious yawning in stranger rats. Anim Cogn, 18(1), 279–290. doi: 10.1007/s10071-014-0798-0 [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Hurley-Gius KM, & Arvanitis D (1986). The topographical organization of neurons in the rat medial frontal, insular and olfactory cortex projecting to the solitary nucleus, olfactory bulb, periaqueductal gray and superior colliculus. Brain Res, 377(2), 261–270. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3730872 [DOI] [PubMed] [Google Scholar]
- Neunuebel JP, Taylor AL, Arthur BJ, & Egnor SE (2015). Female mice ultrasonically interact with males during courtship displays. Elife, 4. doi: 10.7554/eLife.06203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DB, Hilleary SK, & Ginsberg CY (1989). Nuclear terminations of corticoreticular fiber systems in rats. Brain Behav Evol, 34(4), 223–64. [DOI] [PubMed] [Google Scholar]
- Newman SW (1999). The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci, 877, 242–257. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10415653 [DOI] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2011). The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol, 519(18), 3599–3639. doi: 10.1002/cne.22735 [DOI] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2012). Evolution of a vertebrate social decision-making network. Science, 336(6085), 1154–1157. doi: 10.1126/science.1218889 [DOI] [PubMed] [Google Scholar]
- Oettl LL, & Kelsch W (2017). Oxytocin and Olfaction. Curr Top Behav Neurosci. doi: 10.1007/7854_2017_8 [DOI] [PubMed] [Google Scholar]
- Okuyama T (2018). Social memory engram in the hippocampus. Neurosci Res, 129, 17–23. doi: 10.1016/j.neures.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex, 10(3), 206–19. [DOI] [PubMed] [Google Scholar]
- Ophir AG (2017). Navigating Monogamy: Nonapeptide Sensitivity in a Memory Neural Circuit May Shape Social Behavior and Mating Decisions. Front Neurosci, 11, 397. doi: 10.3389/fnins.2017.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T, & Eichenbaum H (1992). Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behav Neurosci, 106(5), 762–775. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1445656 [DOI] [PubMed] [Google Scholar]
- Ouda L, Jílek M, & Syka J (2016). Expression of c-Fos in rat auditory and limbic systems following 22-kHz calls. Behav Brain Res, 308, 196–204. doi: 10.1016/j.bbr.2016.04.030 [DOI] [PubMed] [Google Scholar]
- Pardo-Bellver C, Cádiz-Moretti B, Novejarque A, Martínez-García F, & Lanuza E (2012). Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat, 6, 33. doi: 10.3389/fnana.2012.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Ramos P, McCarthy MM, Bowers JM, Miquel M, Manzo J, & Coria-Avila GA (2014). Brain activation by an olfactory stimulus paired with juvenile play in female rats. Physiol Behav, 133, 39–44. doi: 10.1016/j.physbeh.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Partan S, & Marler P (1999). Communication goes multimodal. Science, 283(5406), 1272–1273. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10084931 [DOI] [PubMed] [Google Scholar]
- Pasch B, George AS, Hamlin HJ, Guillette LJ, & Phelps SM (2011). Androgens modulate song effort and aggression in Neotropical singing mice. Horm Behav, 59(1), 90–97. doi: 10.1016/j.yhbeh.2010.10.011 [DOI] [PubMed] [Google Scholar]
- Pattij T, Schetters D, & Schoffelmeer AN (2014). Dopaminergic modulation of impulsive decision making in the rat insular cortex. Behav Brain Res, 270, 118–124. doi: 10.1016/j.bbr.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB (2006). An insular view of anxiety. Biol Psychiatry, 60(4), 383–7. [DOI] [PubMed] [Google Scholar]
- Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H, Lam HA, … Geschwind DH (2015). Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med, 7(271), 271ra8. doi: 10.1126/scitranslmed.3010257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A (2013). Chemosignals and hormones in the neural control of mammalian sexual behavior. Front Neuroendocrinol, 34(4), 255–267. doi: 10.1016/j.yfrne.2013.07.007 [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, & Liberzon I (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage, 16(2), 331–348. doi: 10.1006/nimg.2002.1087 [DOI] [PubMed] [Google Scholar]
- Ploeger GE, Willemen AP, & Cools AR (1991). Role of the nucleus accumbens in social memory in rats. Brain Res Bull, 26(1), 23–27. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1673081 [DOI] [PubMed] [Google Scholar]
- Porges SW (2001). The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol, 42(2), 123–146. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11587772 [DOI] [PubMed] [Google Scholar]
- Portfors CV, & Perkel DJ (2014). The role of ultrasonic vocalizations in mouse communication. Curr Opin Neurobiol, 28, 115–120. doi: 10.1016/j.conb.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF (2013). Auditory signal processing in communication: perception and performance of vocal sounds. Hear Res, 305, 144–155. doi: 10.1016/j.heares.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pultorak JD, Kelm-Nelson CA, Holt LR, Blue KV, Ciucci MR, & Johnson AM (2016). Decreased approach behavior and nucleus accumbens immediate early gene expression in response to Parkinsonian ultrasonic vocalizations in rats. Soc Neurosci, 11(4), 365–379. doi: 10.1080/17470919.2015.1086434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocki E, & Friston K (2014). Autism, oxytocin and interoception. Neuroscience & Biobehavioral Reviews. Retrieved from https://www.sciencedirect.com/science/article/pii/S0149763414002395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Caminer A, Goodwin J, & McGregor IS (2015). Oxytocin and MDMA (‘Ecstasy’) enhance social reward in rats. Psychopharmacology (Berl), 232(14), 2631–2641. doi: 10.1007/s00213-015-3899-9 [DOI] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, & Appleby L (2004). The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport, 15(11), 1825–1829. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15257156 [DOI] [PubMed] [Google Scholar]
- Rao RP, Mielke F, Bobrov E, & Brecht M (2014). Vocalization-whisking coordination and multisensory integration of social signals in rat auditory cortex. Elife, 3. doi: 10.7554/eLife.03185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, & Winans SS (1982). Afferent connections of dorsal and ventral agranular insular cortex in the hamster Mesocricetus auratus. Neuroscience, 7(5), 1265–1288. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7110587 [DOI] [PubMed] [Google Scholar]
- Rendon NM, Keesom SM, Amadi C, Hurley LM, & Demas GE (2015). Vocalizations convey sex, seasonal phenotype, and aggression in a seasonal mammal. Physiol Behav, 152(Pt A), 143–150. doi: 10.1016/j.physbeh.2015.09.014 [DOI] [PubMed] [Google Scholar]
- Reynolds SM, & Zahm DS (2005). Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci, 25(50), 11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICE GE, & GAINER P (1962). “Altruism” in the albino rat. J Comp Physiol Psychol, 55, 123–125. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14491896 [DOI] [PubMed] [Google Scholar]
- Rigney N, Whylings J, Mieda M, de Vries G, & Petrulis A (2019). Sexually Dimorphic Vasopressin Cells Modulate Social Investigation and Communication in Sex-Specific Ways. eNeuro, 6(1). doi: 10.1523/ENEURO.0415-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Goldsmith DR, Glenn AL, Jairam MR, Elfenbein HA, Dagenais JE, Murdock CD, Pagnoni G (2008). The neural correlates of the affective response to unreciprocated cooperation. Neuropsychologia, 46(5),:1256–66. doi: 10.1016/j.neuropsychologia.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Rilling JK, & Sanfey AG (2011). The neuroscience of social decision-making. Annual review of psychology, 62, 23–48. Retrieved from https://www.annualreviews.org/doi/full/10.1146/annurev.psych.121208.131647 [DOI] [PubMed] [Google Scholar]
- Rodgers KM, Benison AM, Klein A, & Barth DS (2008). Auditory, somatosensory, and multisensory insular cortex in the rat. Cereb Cortex, 18(12), 2941–2951. doi: 10.1093/cercor/bhn054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeling TA, Veening JG, Kruk MR, Peters JP, Vermelis ME, & Nieuwenhuys R (1994). Efferent connections of the hypothalamic “aggression area” in the rat. Neuroscience, 59(4), 1001–1024. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8058117 [DOI] [PubMed] [Google Scholar]
- Rogers-Carter MM, Djerdjaj A, Gribbons KB, Varela JA, & Christianson JP (2019) Insular cortex projections to nucleus accumbens core mediate social approach to stressed juvenile rats. bioRxiv 10.1101/544221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Djerdjaj A, Culp AR, Elbaz JA, & Christianson JP (2018). Familiarity modulates social approach toward stressed conspecifics in female rats. PLoS One, 13(10), e0200971. doi: 10.1371/journal.pone.0200971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, & Christianson JP (2018). Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci, 21(3), 404–414. doi: 10.1038/s41593-018-0071-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, & LeDoux JE (1993). Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex, 3(6), 515–532.https://www.ncbi.nlm.nih.gov/pubmed/7511012 [DOI] [PubMed] [Google Scholar]
- Root DH, Estrin DJ, & Morales M (2018). Aversion or Salience Signaling by Ventral Tegmental Area Glutamate Neurons. iScience, 2, 51–62. doi: 10.1016/j.isci.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier J, & Schenk F (2003). Olfactory and/or visual cues for spatial navigation through ontogeny: olfactory cues enable the use of visual cues. Behav Neurosci, 117(3), 412–425. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12802871 [DOI] [PubMed] [Google Scholar]
- Russell JA (2003). Core affect and the psychological construction of emotion. Psychol Rev, 110(1), 145–172. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12529060 [DOI] [PubMed] [Google Scholar]
- Sadananda M, Wöhr M, & Schwarting RK (2008). Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci Lett, 435(1), 17–23. doi: 10.1016/j.neulet.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Sakuma Y (2008). Neural substrates for sexual preference and motivation in the female and male rat. Ann N Y Acad Sci, 1129, 55–60. doi: 10.1196/annals.1417.009 [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, & Fontanini A (2017). Processing of Intraoral Olfactory and Gustatory Signals in the Gustatory Cortex of Awake Rats. J Neurosci, 37(2), 244–257. doi: 10.1523/JNEUROSCI.1926-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna JR, Dyck RH, & Whishaw IQ (2000). The Dalila effect: C57BL6 mice barber whiskers by plucking. Behav Brain Res, 108(1), 39–45. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10680755 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, & Ramus SJ (2003). A systems approach to orbitofrontal cortex function: recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behav Brain Res, 146(1–2), 19–29. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14643456 [DOI] [PubMed] [Google Scholar]
- Schreuders E, Klapwijk ET., Will GJ, Güroğlu B (2018). Friend versus foe: Neural correlates of prosocial decisions for liked and disliked peers. Cogn Affect Behav Neurosci, 18(1), 127–142. doi: 10.3758/s13415-017-0557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci, 27(9), 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK (2013). Interoceptive inference, emotion, and the embodied self. Trends in cognitive sciences, 17(11), 565–573. Retrieved from https://www.sciencedirect.com/science/article/pii/S1364661313002118 [DOI] [PubMed] [Google Scholar]
- Sforazzini F, Bertero A, Dodero L, David G, Galbusera A, Scattoni ML, … Gozzi A (2016). Altered functional connectivity networks in acallosal and socially impaired BTBR mice. Brain Struct Funct, 221(2), 941–954. doi: 10.1007/s00429-014-0948-9 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG (2011). The neural bases for empathy. Neuroscientist, 17(1), 18–24. doi: 10.1177/1073858410379268 [DOI] [PubMed] [Google Scholar]
- Shi CJ, & Cassell MD (1998). Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol, 399(4), 440–468. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9741477 [DOI] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, & Loewy AD (2008). Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol, 511(5), 628–657. doi: 10.1002/cne.21870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinder ME, & Newlands SD (2014). Sensory convergence in the parieto-insular vestibular cortex. J Neurophysiol, 111(12), 2445–2464. doi: 10.1152/jn.00731.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A, & Tassoni JP (1971). Differential efferent projections of the lateral and medial septal nuclei to the hippocampus in the cat. Brain Behav Evol, 4(3), 201–219. doi: 10.1159/000125434 [DOI] [PubMed] [Google Scholar]
- Siemann JK, Muller CL, Bamberger G, Allison JD, Veenstra-VanderWeele J, & Wallace MT (2014). A novel behavioral paradigm to assess multisensory processing in mice. Front Behav Neurosci, 8, 456. doi: 10.3389/fnbeh.2014.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg A, Allouch C, Sandfort S, Kearns D, Karpel H, & Slotnick B (2014). Desire for social contact, not empathy, may explain “rescue” behavior in rats. Anim Cogn, 17(3), 609–618. doi: 10.1007/s10071-013-0692-1 [DOI] [PubMed] [Google Scholar]
- Simerly RB, & Swanson LW (1986). The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol, 246(3), 312–342. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, & Preuschoff K (2009). A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci, 13(8), 334–340. doi: 10.1016/j.tics.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, & Frith CD (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature, 439(7075), 466–469. doi: 10.1038/nature04271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald GM, Rjabokon A, Singewald N, & Ebner K (2011). The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology, 36(4), 793–804. doi: 10.1038/npp.2010.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, & Gallagher L (2009). Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci, 13(1), 27–35. doi: 10.1016/j.tics.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Smith CJW, Mogavero JN, Tulimieri MT, & Veenema AH (2017). Involvement of the oxytocin system in the nucleus accumbens in the regulation of juvenile social novelty-seeking behavior. Horm Behav, 93, 94–98. doi: 10.1016/j.yhbeh.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Smith CJW, Wilkins KB, Li S, Tulimieri MT, & Veenema AH (2018). Nucleus accumbens mu opioid receptors regulate context-specific social preferences in the juvenile rat. Psychoneuroendocrinology, 89, 59–68. doi: 10.1016/j.psyneuen.2017.12.017 [DOI] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, & Berridge KC (2009). Ventral pallidum roles in reward and motivation. Behav Brain Res, 196(2), 155–167. doi: 10.1016/j.bbr.2008.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, & Zachman TA (1974). Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol, 12(1), 55–66. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4429513 [DOI] [PubMed] [Google Scholar]
- Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, … Mogil JS (2011). The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain, 7, 55. doi: 10.1186/1744-8069-7-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumiya H, Godai A, Araiso H, Mori S, Furukawa S, & Fukumitsu H (2016). Neonatal Whisker Trimming Impairs Fear/Anxiety-Related Emotional Systems of the Amygdala and Social Behaviors in Adult Mice. PLoS One, 11(6), e0158583. doi: 10.1371/journal.pone.0158583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzani S, & Russo A (1980). [Afferent connections in the ventromedial hypothalamus of rats demonstrated by retrograde transport of horseradish peroxidase]. Boll Soc Ital Biol Sper, 56(17), 1721–1725. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6161623 [PubMed] [Google Scholar]
- Stein BE, Meredith MA, Huneycutt WS, & McDade L (1989). Behavioral Indices of Multisensory Integration: Orientation to Visual Cues is Affected by Auditory Stimuli. J Cogn Neurosci, 1, 12–24. doi: 10.1162/jocn.1989.1.1.12 [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR, & Rowland BA (2014). Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci, 15(8), 520–535. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25158358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterley TL, Baimoukhametova D, Füzesi T, Zurek AA, Daviu N, Rasiah NP, … Bains JS (2018). Social transmission and buffering of synaptic changes after stress. Nat Neurosci, 21(3), 393–403. doi: 10.1038/s41593-017-0044-6 [DOI] [PubMed] [Google Scholar]
- Stern JM (1990). Multisensory regulation of maternal behavior and masculine sexual behavior: a revised view. Neurosci Biobehav Rev, 14(2), 183–200. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2190118 [DOI] [PubMed] [Google Scholar]
- Stowers L, Cameron P, & Keller JA (2013). Ominous odors: olfactory control of instinctive fear and aggression in mice. Curr Opin Neurobiol, 23(3), 339–345. doi: 10.1016/j.conb.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, & Craig AD (2016). Interoception, homeostatic emotions and sympathovagal balance. Philos Trans R Soc Lond B Biol Sci, 371(1708). doi: 10.1098/rstb.2016.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueur C, & Pelé M (2016). Social network and decision-making in primates: a report on Franco-Japanese research collaborations. Primates, 57(3), 327–332. doi: 10.1007/s10329-015-0505-z [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers MS, Flemming J, Vaught C, Young TA, & Jonathan Polan H (2003). Characterizing the functional significance of the neonatal rat vibrissae prior to the onset of whisking. Somatosens Mot Res, 20(2), 157–162. doi: 10.1080/0899022031000105190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Tan Z, Mensh BD, & Ji N (2016). Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat Neurosci, 19(2), 308–315. doi: 10.1038/nn.4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai AX, & Kromer LF (2014). Corticofugal projections from medial primary somatosensory cortex avoid EphA7-expressing neurons in striatum and thalamus. Neuroscience, 274, 409–418. doi: 10.1016/j.neuroscience.2014.05.039 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, & Mori Y (2013). Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav Brain Res, 240, 46–51. doi: 10.1016/j.bbr.2012.11.017 [DOI] [PubMed] [Google Scholar]
- Tasaka GI, Guenthner CJ, Shalev A, Gilday O, Luo L, & Mizrahi A (2018). Genetic tagging of active neurons in auditory cortex reveals maternal plasticity of coding ultrasonic vocalizations. Nat Commun, 9(1), 871. doi: 10.1038/s41467-018-03183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles MC, Almeida O, Lopes JS, & Oliveira RF (2015). Social interactions elicit rapid shifts in functional connectivity in the social decision-making network of zebrafish. Proc Biol Sci, 282(1816), 20151099. doi: 10.1098/rspb.2015.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Sharika KM, & Platt ML (2017). Social Decision-Making and the Brain: A Comparative Perspective. Trends Cogn Sci, 21(4), 265–276. doi: 10.1016/j.tics.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ, & Vanderschuren LJ (2011). Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci, 31(17), 6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toller G, Brown J, Sollberger M, Shdo SM, Bouvet L, Sukhanov P, Seeley WW (2018). Individual differences in socioemotional sensitivity are an index of salience network function. Cortex, 103, 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM (2012). Glutamate inputs to the nucleus accumbens: does source matter. Neuron, 76(4), 671–673. doi: 10.1016/j.neuron.2012.11.008 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Davies MS, Scott AA, Zaidel E, Bookheimer SY, Iacoboni M, & Dapretto M (2008). Neural basis of self and other representation in autism: an FMRI study of self-face recognition. PLoS One, 3(10), e3526. doi: 10.1371/journal.pone.0003526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Menon V (2009). The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev, 33(8), 1198–203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortex function and dysfunction. Nat Rev Neurosci, 16(1), 55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Valtcheva S, & Froemke RC (2018). Neuromodulation of maternal circuits by oxytocin. Cell Tissue Res. doi: 10.1007/s00441-018-2883-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Achterberg EJ, & Trezza V (2016). The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev, 70, 86–105. doi: 10.1016/j.neubiorev.2016.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, & Maguire EA (2009). What does the retrosplenial cortex do? Nat Rev Neurosci, 10(11), 792–802. [DOI] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, & Sherman SM (2011). Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proc Natl Acad Sci U S A, 108(44), 18156–18161. doi: 10.1073/pnas.1114828108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, & Greenough WT (1972). Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science, 176(4042), 1445–1447. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/5033647 [DOI] [PubMed] [Google Scholar]
- Von Dawans B, Fischbacher U, Kirschbaum C, Fehr E, & Heinrichs M (2012). The social dimension of stress reactivity: acute stress increases prosocial behavior in humans. Psychological science, 23(6), 651–660. Retrieved from https://journals.sagepub.com/doi/pdf/10.1177/0956797611431576 [DOI] [PubMed] [Google Scholar]
- Vreeburg JT, & Ooms MP (1985). Induction of ear wiggling in the estrous female rat by gonadectomized rats treated with androgens and estrogens. Horm Behav, 19(3), 231–236. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4054849 [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, & Hu H (2011). Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science, 334(6056), 693–697. doi: 10.1126/science.1209951 [DOI] [PubMed] [Google Scholar]
- Weiss VG, Hofford RS, Yates JR, Jennings FC, & Bardo MT (2015). Sex differences in monoamines following amphetamine and social reward in adolescent rats. Exp Clin Psychopharmacol, 23(4), 197–205. doi: 10.1037/pha0000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller KL, & Smith DA (1982). Afferent connections to the bed nucleus of the stria terminalis. Brain Res, 232(2), 255–270. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7188024 [DOI] [PubMed] [Google Scholar]
- Wilson CJ (2014). The sensory striatum. Neuron, 83(5), 999–1001. doi: 10.1016/j.neuron.2014.08.025 [DOI] [PubMed] [Google Scholar]
- Wilson EO (1992). The effects of complex social life on evolution and biodiversity. Oikos, 63(1), 13–18. [Google Scholar]
- Wolfe J, Mende C, & Brecht M (2011). Social facial touch in rats. Behav Neurosci, 125(6), 900–910. doi: 10.1037/a0026165 [DOI] [PubMed] [Google Scholar]
- Wong LC, Wang L, D’Amour JA, Yumita T, Chen G, Yamaguchi T, … Lin D (2016). Effective Modulation of Male Aggression through Lateral Septum to Medial Hypothalamus Projection. Curr Biol, 26(5), 593–604. doi: 10.1016/j.cub.2015.12.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, & Groenewegen HJ (1996). Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience, 73(2), 359–373. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8783254 [DOI] [PubMed] [Google Scholar]
- Wyss JM (1981). An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J Comp Neurol, 199(4), 495–512. doi: 10.1002/cne.901990405 [DOI] [PubMed] [Google Scholar]
- Yamada T, Itahashi T, Nakamura M, Watanabe H, Kuroda M, Ohta H, … Hashimoto RI (2016). Altered functional organization within the insular cortex in adult males with high-functioning autism spectrum disorder: evidence from connectivity-based parcellation. Mol Autism, 7, 41. doi: 10.1186/s13229-016-0106-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Yang CF, Chizari MD, Maheswaranathan N, Burke KJ, Borius M, … Shah NM (2017). Social Control of Hypothalamus-Mediated Male Aggression. Neuron, 95(4), 955–970.e4. doi: 10.1016/j.neuron.2017.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Bergan J, Lanjuin A, & Dulac C (2017). Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. Elife, 6. doi: 10.7554/eLife.31373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, & Sasaki K (1999). Regional expressions of Fos-like immunoreactivity in rat cerebral cortex after stress; restraint and intraperitoneal lipopolysaccharide. Brain Res, 816(2), 267–275. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9878776 [DOI] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang C, Jin G, & Zhen X (2008). Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl), 199(4), 625–635. doi: 10.1007/s00213-008-1188-6 [DOI] [PubMed] [Google Scholar]
- Zheng DJ, Larsson B, Phelps SM, & Ophir AG (2013). Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behav Brain Res, 246, 139–147. doi: 10.1016/j.bbr.2013.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]