Abstract
BACKGROUND:
The OPTN implemented Share35 on 6/18/2013 to broaden deceased-donor liver sharing within regional boundaries. We investigated whether increased sharing under Share35 impacted geographic disparity in deceased-donor liver transplantation (DDLT) across Donation Service Areas (DSAs).
METHODS:
Using SRTR 6/2009-6/2017, we identified 86,083 adult LT candidates and retrospectively estimated MELD-adjusted DDLT rates using nested multilevel Poisson regression with random intercepts for DSA and transplant program. From the variance in DDLT rates across 49 DSAs and 102 programs, we derived the DSA-level median incidence rate ratio (MIRR) of DDLT rates. MIRR is a robust metric of heterogeneity across each hierarchical level; larger MIRR indicates greater disparity.
RESULTS:
MIRR was 2.18 pre-Share35 and 2.16 post-Share35. Thus, two candidates with the same MELD in two different DSAs were expected to have a 2.2-fold difference in DDLT rate driven by geography alone. After accounting for program-level heterogeneity, MIRR was attenuated to 2.10 pre-Share35 and 1.96 post-Share35. For candidates with MELD 15-34, MIRR decreased from 2.51 pre- to 2.27 post-Share35, and for candidates with MELD 35-40, MIRR increased from 1.46 pre- to 1.51 post-Share35, independent of program-level heterogeneity in DDLT. DSA-level heterogeneity in DDLT rates was greater than program-level heterogeneity pre- and post-Share35.
CONCLUSIONS:
Geographic disparity substantially impacted DDLT rates before and after Share35, independent of program-level heterogeneity and particularly for candidates with MELD 35-40. Despite broader sharing, geography remains a major determinant of access to deceased-donor liver transplantation.
INTRODUCTION
The liver allocation system prioritizes candidates by MELD score, and thus, candidates with the same MELD score in different places across the United States should have similar access to deceased donor livers. However, data show persistent geographic disparities in access to deceased-donor liver transplantation (DDLT) across geographic regions and Donation Service Areas (DSAs) through variation in median MELD at transplant,1–3 mean MELD at transplant,4,5 median time on the waitlist,6 and the ratio of candidates to donors within a DSA.7 In one DSA a candidate might expect to receive a transplant at a MELD of 24, while in another DSA they might expect a transplant at a MELD of 35. The probability of death within 90 days on the waitlist also varies geographically for candidates;8 from 20% to 80% for candidates with MELD between 36 to 40.9 Acknowledging this, the Organ Procurement and Transplantation Network (OPTN) resolved in 2012 that “existing geographic disparity in allocation of organs is unacceptably high.”10 After extensive community deliberation,11,12 allocation changes that slightly alter regional boundaries for livers from some donors were approved on December 4, 2017, with implementation expected in November 2018.13
Prior to the 2017 decision, however, the OPTN implemented Share 35 on June 18, 2013, giving priority to regional candidates with MELD≥35 over local candidates with MELD<35 to provide greater access to deceased-donor liver transplant (DDLT) for candidates with the highest risk of death on the waitlist. We previously described national-level changes in distribution and mortality for the initial 12 months of the Share 35 policy, showing an increase in liver transplants for recipients with MELD≥35, decrease in discard rates, decrease in waitlist mortality, and no change in cold ischemia time.14 Edwards et al also observed an increase in geographic disparity as measured by variance of median MELD at transplant following Share 35;3 however, this metric does not account for candidates who did not receive a transplant, the time-varying nature of MELD, accumulated time on the waitlist at a given MELD, nor does it distinguish between program practices and geography.
Our simulation models previously predicted that increased regional sharing under Share 35 would paradoxically increase geographic disparity as measured by the variance of median allocation MELD at transplant per DSA, because the existing regional boundaries are ill-suited to balance supply and demand of livers.1 While Share 35 was implemented to improve access for the sickest candidates on the waitlist, the present study asks how Share 35 impacted geographic disparity in access to DDLT across DSAs, using the median incidence rate ratio (MIRR) of DDLT rates across DSAs as a measure of geographic disparity. MIRR is a robust metric of heterogeneity in DDLT rates across DSAs that accounts for time-varying allocation MELD and characterizes the variation in transplant rates per waitlist-year across DSAs.15,16 MIRR has a natural interpretation as the increase in access to DDLT that candidates could expect if they moved from their DSA to a DSA with higher transplant rates.
Thus, we used eight years of registry data and a robust metric of heterogeneity to characterize changes in geographic disparity in access to DDLT after the implementation of Share 35, accounting for time on the waitlist and program variation.
METHODS
Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR) September 2018 public release. The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.18 The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This study used deidentified data and was exempted by the Johns Hopkins School of Medicine Institutional Review Board (NA_00042871).
Study population
We identified 141 transplant programs with actively listed DDLT candidates between 6/2009 and 6/2017. Of these programs, 37 programs had performed fewer than one DDLT per year over the study period and two programs had listed fewer than 50 candidates with MELD≥15; these programs were excluded to improve stability of program-level estimates. From the remaining 102 programs in 49 DSAs, we identified 86,083 adult (age ≥ 18) prevalent and incident liver transplant candidates with allocation MELD≥15 who were actively listed in the four years prior to Share 35 (“pre-Share35:” 6/18/2009-6/17/2013) and in the four years following Share 35 (“post-Share35:” 6/18/2013-6/17/2017). We compared age, sex, race, BMI, and primary indication for transplant among active candidates before and after Share 35 using Wilcoxon rank sum tests for continuous variables and χ2-tests for categorical variables. We characterized candidates’ MELD at study entry during both study periods. For pre-Share35 candidates, we reported MELD at 6/18/2009 for prevalent candidates or first active MELD for incident candidates, and for post-Share35, we reported MELD at 6/18/2013 for prevalent candidates or first active MELD for incident candidates in Table 1.
Table 1.
Characteristics of active adult deceased-donor liver transplant (DDLT) in the 4 years pre- and post-Share 35; 6/2009–6/2017.
| Candidates | Pre-Share35 | Post-Share35 | p-value |
|---|---|---|---|
| N | 45195 | 48248 | |
| Age at study entry, median (IQR) | 56 (50, 61) | 58 (51, 63) | <0.01 |
| Female | 34.9% | 35.7% | <0.01 |
| Race/Ethnicity | 0.03 | ||
| Caucasian | 69.9% | 69.9% | |
| African American | 9.4% | 9.0% | |
| Hispanic/Latino | 15.2% | 15.5% | |
| Other | 5.5% | 5.7% | |
| BMI, median (IQR) | 28 (25, 32) | 28 (25, 32) | <0.01 |
| Indication for transplant | <0.01 | ||
| Hepatitis C | 35.5% | 27.9% | |
| Non-alcoholic liver disease | 8.5% | 13.7% | |
| Hepatocellular carcinoma | 10.1% | 10.1% | |
| Alcoholic liver disease | 14.7% | 19.9% | |
| Other | 31.3% | 28.4% | |
| Allocation MELD, median (IQR) | 21 (17, 23) | 22 (17, 26) | <0.01 |
| Status 1 | 3.0% | 2.3% | <0.01 |
Changes in DSA-specific transplant rates
To characterize changes in DDLT rates within DSAs, we defined liver transplant rates per DSA and per allocation MELD as the number of transplants performed for candidates at that MELD divided by the number of active person-years spent waiting at that MELD. Inactive time on the waiting list was excluded because inactive candidates were not in the risk set for receiving a transplant. As an individual candidate’s MELD score varied, each person-day of waiting was counted in different risk sets according to that candidate’s allocation MELD. Candidates were censored at death, waitlist removal for reasons other than DDLT, or administratively on 6/18/2017. We used multilevel Poisson regression with a random intercept framework, to estimate MELD-adjusted deceased-donor liver transplant rates per person-year within each DSA before and after Share 35. To account for non-linearity of allocation MELD in transplant rates and to calculate the expected increase in DDLT rate per MELD increase, MELD was categorized into groups that spanned 3-4 MELD points, plus one category for Status 1 candidates: (15-18, 19-21, 22-24, 25-28, 29-31, 32-34, 35-37, 38-40, and Status 1). The pre- and post-Share35 models were only adjusted for candidate allocation MELD score in order to estimate transplant rates based on allocation priority.
Estimation of median incidence rate ratios
Our objective was to characterize heterogeneity in deceased-donor transplant rates across DSAs and to separate DSA-level variation from program-level variation. From the multilevel Poisson regression model, DSA-level variance and program-level variance were used to estimate two median incidence rate ratios (MIRRs) at the DSA and program levels, respectively. The DSA-level MIRR indicates the extent of heterogeneity in DDLT rates across DSAs (geographic variation) and program-level MIRR indicates the extent of heterogeneity in DDLT rates across transplant programs (program variation).16,19,20 Allowing DDLT rates to vary across transplant programs accounts for program-level variation in DDLT rates, so that the heterogeneity across DSAs can be interpreted as independent of variation induced by individual programs.
A larger MIRR indicates greater geographic disparity.15,19,21 In the context of this study, the DSA-level MIRR indicates the extent to which an active candidate’s DSA determines their relative rate of DDLT, or the expected increase in DDLT rate should a given candidate move to or list in another DSA with a higher DDLT rate.
The MIRRs can also be interpreted as an estimate of between-DSA and between-program incidence rate ratios (IRR) of DDLT rate. Between-DSA IRRs are the pairwise comparisons of DDLT rate between each pair of DSAs. For each DSA pair, the DSA with the higher DDLT rate is compared to the DSA with the lower DDLT rate to estimate the IRR or relative difference in their respective DDLT rates. Thus, all IRRs are greater than or equal to one. The median of these pairwise comparisons is the median of the incidence rate ratios or MIRR. DSA-level MIRR was estimated with and without accounting for program-level variation to illustrate the extent to which geographic variation was explained by program-level variation. Additionally, Share 35 only altered sharing for candidates with MELD 35-40, thus we studied candidates with MELD 35-40 and MELD 15-34 separately before and after Share 35.
Variation in program-specific transplant rates after Share 35
While the goal of the study was to understand geographic variation in DDLT rates, we also calculated the program-specific DDLT rates in the four years after Share 35 to illustrate variation across programs within each DSA, using the program-level and DSA-level random intercepts generated by the multilevel Poisson regression model.
Statistical analysis
Analyses were performed using Stata/SE 15.2 (College Station, Texas). For all analyses, p < 0.05 was considered statistically significant. MIRR was calculated using the Stata command xtmrho following a multilevel Poisson regression.
RESULTS
Waitlist candidates
We identified 45,195 and 48,248 candidates who were active on the liver transplant waitlist in the pre- and post-Share35 eras, respectively. Candidates in the post-Share35 era were slightly older, more likely to have non-alcoholic fatty liver disease or alcoholic liver disease as primary indication for transplant, more likely to have had a slightly higher MELD, and less likely to have been Status 1 (Table 1).
DSA-specific deceased donor transplant rates
After adjustment for allocation MELD, pre-Share35 DSA-specific DDLT rates ranged from 0.22-4.19 with median (IQR) 1.07 (0.47-1.98) per person-year (Figure 1a and 2a), and post-Share35 rates ranged from 0.20-3.50 with median (IQR) 1.18 (0.60-2.07) (Figure 1a and 2b). Adjusted DSA-specific DDLT rates were higher after Share 35 for 23 DSAs and lower after Share 35 for 26 DSAs (Figure 1a). DSA-specific changes in DDLT rate following Share 35 varied within and across regions (Figure 1b). DDLT rates remained relatively higher in southeastern DSAs after Share 35 (Figure 2b). The relative rate of DDLT increased with increasing MELD score before and after Share 35 (Table 2). Compared to candidates with MELD 15-18, candidates with MELD 35-37 had a 119-fold higher rate of DDLT pre-Share35 (95% CI 111-128), and a 164-fold higher rate of DDLT after Share35 (95% CI 154-174); this was expected with increased sharing for candidates with the highest MELDs.
Figure 1. (A) DSA-specific DDLT rates before and after Share 35 and (B) change in DSA-specific DDLT rates after Share 35 by OPTN region.
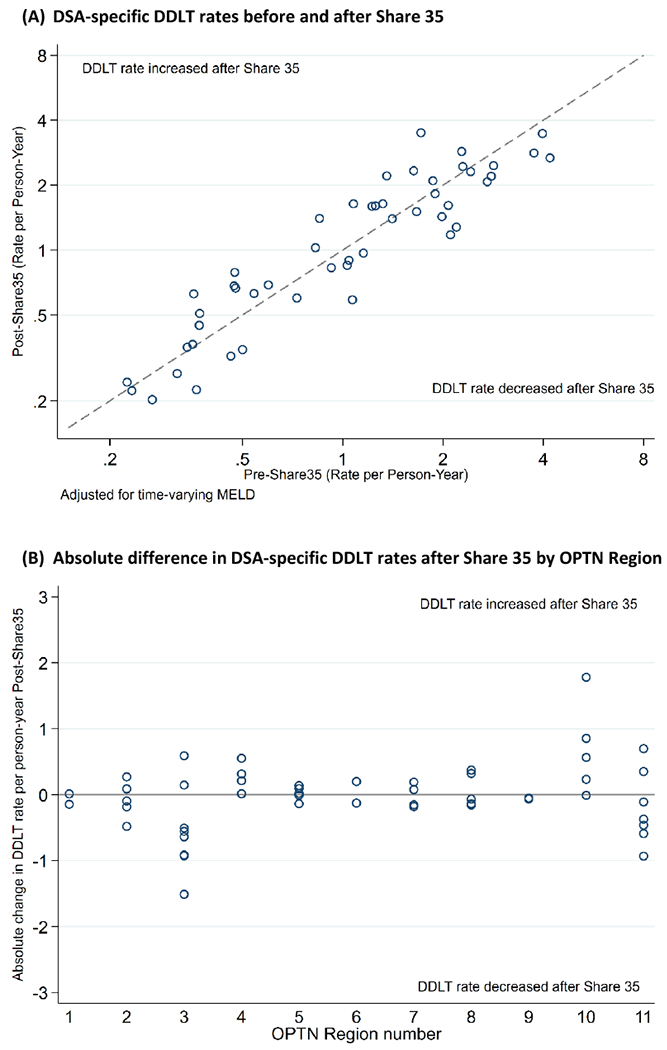
Each dot represents a DSA. Twenty-six DSAs experienced a decrease and 23 experienced an increase in DDLT rate after Share 35. Figure 1a x- and y-axes are on log scale so that small differences at lower DDLT rates can be better observed. Figure 2 illustrates that changes in DDLT rate at the DSA-level after Share 35 varied within and across regions.
Figure 2. MELD-adjusted deceased-donor liver transplant (DDLT) rates per person-year in each Donation Service Area pre- and post-Share 35.
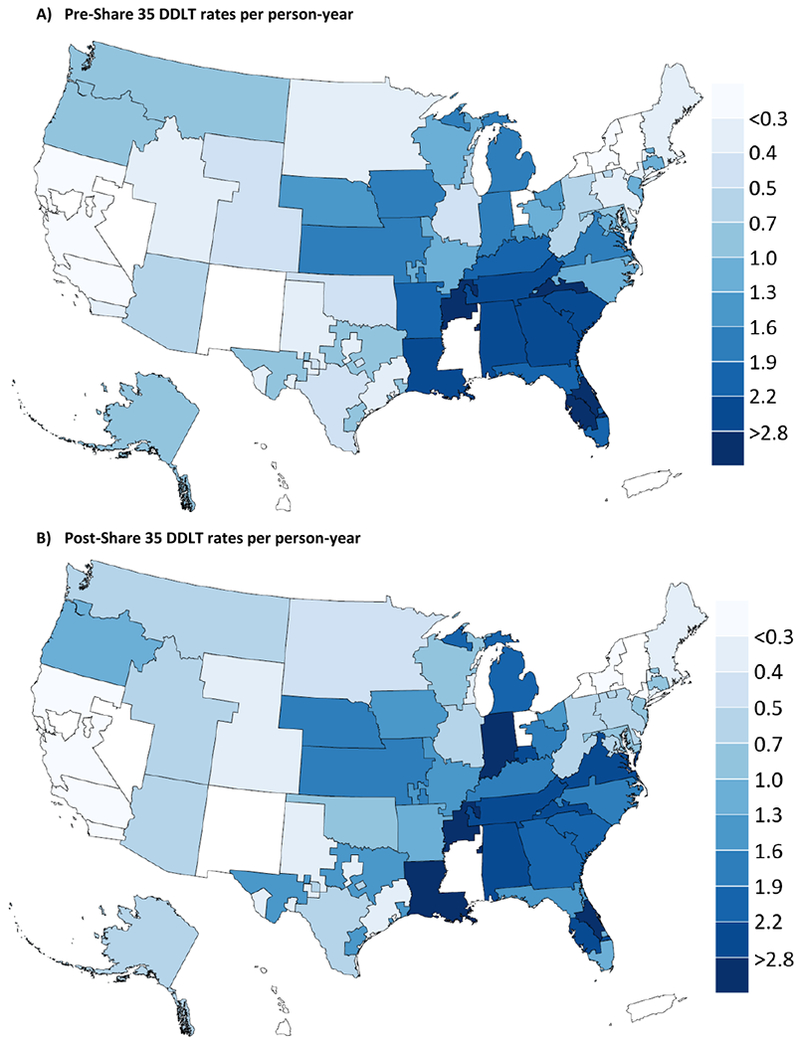
DDLT rates per person-year calculated for candidates with allocation MELD 35-37.
DSAs were excluded (white) if they did not have a liver transplant program during the study period (n=6) or included only programs with low transplant volume (n=3).
Table 2.
Relative rate of receiving a deceased-donor liver transplant (DDLT) associated with each MELD category and the change in relative rate of DDLT with each increase in MELD category (a) pre- and (b) post-Share 35. DSA-level MIRR calculated for each Share 35 era.
| A) Pre-Share 35 (DSA MIRR=2.18) | ||
|---|---|---|
| MELD/Status | Relative Rate of DDLT | Change in Relative Rate per Increase in MELD Category |
| 15-18 | Ref | - |
| 19-21 | 2.52 (2.34-2.71) | 2.52 (2.34-2.71) |
| 22-24 | 6.89 (6.52-7.23) | 2.73 (2.56-2.91) |
| 25-28 | 15.2 (14.4-16.1) | 2.21 (2.12-2.31) |
| 29-31 | 36.6 (34.5-29.0) | 2.41 (2.30-2.53) |
| 32-34 | 84.6 (79.1-90.5) | 2.31 (2.17-2.46) |
| 35-37 | 119.2 (110.8-128.2) | 1.41 (1.31-1.52) |
| 38-40 | 158.0 (148.9-167.7) | 1.32 (1.24-1.42) |
| Status 1 | 454.6 (419.0-493.1) | 2.88 (2.66-3.11) |
| B) Post-Share 35 (DSA MIRR=2.16) | ||
| MELD/Status | Relative Rate of DDLT | Change in Relative Rate per Increase in MELD Category |
| 15-18 | Ref | - |
| 19-21 | 2.32 (2.16-2.49) | 2.32 (2.16-2.49) |
| 22-24 | 4.64 (4.34-4.91) | 2.00 (1.88-2.13) |
| 25-28 | 11.2 (10.6-11.8) | 2.41 (2.30-2.51) |
| 29-31 | 21.2 (20.0-22.5) | 1.90 (1.81-1.98) |
| 32-34 | 47.9 (45.0-51.1) | 2.26 (2.14-2.38)* |
| 35-37 | 164.1 (154.0-174.8) | 3.42 (3.23-3.63) |
| 38-40 | 289.9 (274.1-306.6) | 1.77 (1.68-1.86) |
| Status 1 | 492.4 (452.0-536.4) | 1.70 (1.57-1.84) |
An increase from MELD 29-31 to 32-34 post-Share 35 increases a candidate’s DDLT rate by 2.26-fold, while MIRR of 2.16 indicates that relisting in a different DSA could increase their DDLT rate by 2.16-fold.
DSA MIRR without accounting for program variation
Two candidates with the same MELD in different DSAs were expected to have a 2.18-fold difference in DDLT rate pre-Share35 driven by geographic location alone (DSA MIRR = 2.18). The heterogeneity in DDLT rates across DSAs indicated by DSA MIRR, remained unchanged from 2.18 in the pre-Share 35 era to 2.16 in the post-Share 35 era. The impact of geographic location on DDLT rate can be compared to the impact of MELD on DDLT rate. Post-Share35, an increase in allocation MELD from 29-31 to 32-34 was associated with a 2.26-fold increase in DDLT rate (Table 2B, right-most column), while MIRR indicates that a candidate could increase their DDLT rate by 2.16-fold on average by relisting in a different DSA.
DSA MIRR after accounting for program variation
Program-level MIRR, or the heterogeneity in DDLT rates across transplant programs, increased from 1.40 to 1.64 post-Share 35 when estimated among all candidates (Table 3). After accounting for program-level variation, heterogeneity across DSAs was slightly attenuated, and decreased minimally after Share 35 from 2.10 to 1.96. The extent of heterogeneity varied by candidate MELD. For candidates with MELD 35-40, program-level MIRR increased from 1.35 to 1.43 post-Share 35 and DSA-level MIRR increased from 1.46 to 1.51. For candidates with MELD 15-34, program-level MIRR increased from 1.54 to 1.83 and DSA-level MIRR decreased from 2.51 to 2.27. For high MELD candidates, relisting in a different DSA with higher transplant rates could improve their DDLT rate by 1.51-fold post-Share35. For lower MELD candidates, geographic disparity across DSAs was greater, and relisting in a different DSA with higher transplant rates could improve their DDLT rate by 2.27-fold post-Share35.
Table 3. Median Incidence Rate Ratio (MIRR) as a measure of heterogeneity in DDLT rates across transplant programs and across DSAs before and after Share 35.
MIRR estimated separately for candidates with allocation MELD 15–34 and 35–40.
| Pre-Share 35 | Post-Share 35 | |||
|---|---|---|---|---|
| Program MIRRa | DSA MIRRa | Program MIRR | DSA MIRR | |
| Overallb | 1.40 | 2.10 | 1.64 | 1.96 |
| MELD 15-34 | 1.54 | 2.51 | 1.83 | 2.27 |
| MELD 35-40 | 1.35 | 1.46 | 1.43 | 1.51 |
Program and DSA MIRR derived from the same nested multilevel Poisson regression model with a random intercept for DSA and a random intercept for transplant programs within each DSA. Program MIRR indicates heterogeneity in DDLT rates across programs independent of geography, and DSA MIRR indicates heterogeneity in DDLT rates across DSAs independent of program-level heterogeneity.
Adjusted for allocation MELD; including Status 1
Variation in program-specific transplant rates after Share 35
In the four years following Share 35, MELD-adjusted program-specific transplant rates ranged from 0.16 to 3.78 with median (IQR) 0.80 (0.37-1.82) per person-year (Figure 3). This variation can be understood in the context of a program-level MIRR of 1.64 after Share 35 (Table 3), which indicates that for any two candidates at two randomly identified programs, we would expect their DDLT rates to vary by an average of 1.64-fold driven by program practices alone. Figure 3 illustrates the variation in DDLT rates across programs without incorporating the additional observed DSA-level variation (Figure 3A) versus the variation in DDLT rates across programs when program-level and DSA-level variation are incorporated (Figure 3B). While variation in DDLT rate across programs was non-trivial (Program MIRR=1.64), DSA-level variation was greater (DSA MIRR=1.96).
Figure 3. Program-specific DDLT rates in the 4 years after Share 35 (A) holding DSA-level variation constant and (B) after including additional variation observed at the DSA-level.
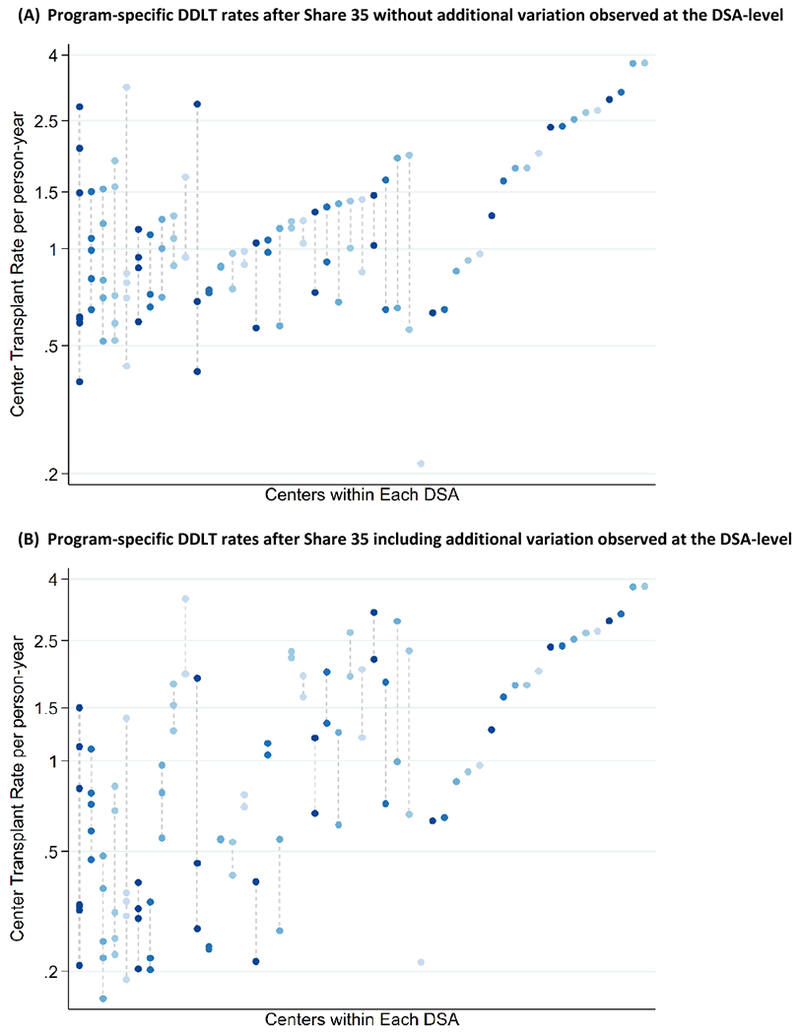
Dots connected with a dashed line are programs within the same DSA. From this figure, the variation driven by programs alone (3A) can be compared to variation driven by program and geographic location (3B).
DISCUSSION
In this registry-based study of 86,083 adult liver transplant candidates, we measured variation in deceased-donor liver transplant rates across DSAs and reported changes in this variation after Share 35. We used the median incidence rate ratio (MIRR) of DDLT rates, a robust metric that indicates heterogeneity across hierarchical levels of a population. We found significant variation in DDLT rates across transplant programs and across DSAs for candidates with the same MELD score, and no improvement in this variation following the implementation of Share 35 (Pre-Share 35 MIRR = 2.18 and Post-Share 35 MIRR = 2.16). Based on geographic location alone, candidates with MELD 35-40 in two different DSAs had an expected 1.46-fold difference in DDLT rate pre-Share 35 (MIRR=1.46) and a 1.51-fold difference post-Share 35 (MIRR=1.51). The disparity was greater for lower MELD candidates, such that candidates with MELD 15-34 in two different DSAs had an expected 2.51-fold difference in DDLT rate pre-Share 35 (MIRR=2.51) and a 2.27-fold difference post-Share 35 (MIRR=2.27). After accounting for heterogeneity across transplant programs, the heterogeneity in DDLT rate across DSAs was slightly attenuated from 2.16 to 1.96 post-Share 35. Differences in DDLT rate attributable to DSA were greater than differences in DDLT rate attributable to transplant program practices.
While Share 35 was intended to reduce waitlist death rather than reduce geographic disparity, we still might have expected that heterogeneity across Donation Service Areas in access to deceased-donor livers would decrease with broader sharing for high-MELD candidates. Despite this, we observed persistent variation across DSAs for high-MELD candidates. In the recent era, two candidates with MELD 35-40 in two different DSAs had a median 1.5-fold difference in their DDLT rate driven by geography alone. Geographic disparity was even higher for lower MELD candidates, such that two candidates with MELD 15-34 in two different DSAs had a median 2.4-fold difference in their DDLT rate driven by geography alone. This variation is consistent with other studies of geographic disparity across DSAs and regions in access to deceased-donor transplant,4,22,23 organ supply,24,25 and in underlying demand.26,27 However, MIRR allows us to estimate the impact of geographic location on access to transplant for candidates at any MELD independent of program-level decision making.
We were not seeking to isolate the driver of this heterogeneity, but to characterize heterogeneity in DDLT using a robust metric that accounts for variation among transplant programs and Donation Service Areas. Other studies have suggested OPO performance,28 community health scores,27 program acceptance rates,29 and ratio of liver supply to candidate demand7 might drive these disparities. In our models, DSA variation was attenuated after accounting for program-level variation from 2.16 to 1.96 post-Share 35, however geographic disparity persisted for candidates at any MELD and was greater than variation induced by transplant programs (DSA MIRR = 1.96 vs Program MIRR = 1.64). Including other candidate, program, or DSA-specific factors in the model might further minimize the observed heterogeneity across DSAs; however, even if this was the case, these other factors might be driving variation across geographic regions and we do not necessarily want to adjust them away. Regardless of the reason for the disparity, a candidate with a high MELD and a candidate with a lower MELD could relist in a DSA with higher access to transplant and expect their transplant rates to increase by 1.5-fold and 2-fold, respectively. From the candidate’s perspective, the driver of this disparity is less important than the mere fact that it exists.
We reported that geographic disparity in transplant rates as measured by MIRR was unchanged after Share 35. Both in the subgroup of candidates with MELD 35-40 and overall, Share 35 made no observable improvement in DSA-level variation in DDLT rates. This finding was consistent with our prior prediction that increased sharing at the regional level would not improve geographic disparity in DDLT.1 While Edwards et al observed an increase in variation of median MELD at transplant with two years of data following Share 35, and we observed minimal change in MIRR, our conclusions are not dissimilar.3 Both studies found that geographic disparities in access to deceased-donor liver transplant persist despite broader sharing. Stine et al observed persistent disparities in waitlist mortality across regions following Share 35, which is consistent with our observation of continued disparity under broader sharing. However we estimated disparity in transplant rates across DSAs and programs rather than region.5 We found that access to DDLT varies significantly across transplant programs for two candidates with the same MELD score, which is consistent with other studies that have explored program-level variation.23,30 We identified DSA-level and program-level variation in DDLT rates, and consistent with the findings of Wey et al,23 we demonstrated that variation across geographic areas is not fully explained by program-level variation.
While geographic disparity in liver transplantation has been described using the variation in median3 and mean4,5 MELD at transplant, supply-to-demand ratios,7 and other metrics,6 in the present study we have used the median incidence rate ratio (MIRR) of DDLT across DSAs. MIRR has a straightforward interpretation as the expected factor by which DDLT rate would change if the candidate were listed in a different DSA. MIRR is also comparable to the impact of MELD on DDLT rate. We have previously used MIRR to quantify heterogeneity in deceased-donor kidney transplant rates across DSAs.31 This method is increasingly used to illustrate cluster heterogeneity, including across counties,32 neighborhoods,15,33 hospitals,20,34 and even across physicians.34–36 Girotra et al used multilevel logistic regression and the median odds ratio (MOR) to illustrate county-level variation in the odds of survival following out-of-hospital cardiac arrest.32 Austin et al used the median hazard ratio (MHR) to illustrate variation across hospitals in the relative hazard of mortality following acute myocardial infarction.20 Rosana et al used the median incidence rate ratio (MIRR) to illustrate variation in rates of avoidable hospitalization per 1,000 patients across healthcare organizations in Italy.16 Thus, we used multilevel Poisson regression and the MIRR to measure heterogeneity in transplant rates per person-year across DSAs and transplant programs.
The OPTN approved further changes in liver allocation intended to reduce geographic disparities on December 4, 2017. These changes will expand the geographic reach of early tiers of allocation beyond historical region boundaries. Livers recovered within 150 nautical miles of the existing regional boundaries will be shared with some transplant programs outside the region for candidates with MELD≥32. However, allocation order will not change for candidates at programs farther than 150 nautical miles from a regional boundary. The 2017 changes are expected to be implemented in late 2018. Because regional boundaries largely persist in the new allocation system, and because the present study finds that sharing within regional boundaries does not reduce disparity, careful studies of geographic disparity under the new system are warranted when data become available.
The limitations of this study merit consideration. We do not use offer data, so we cannot establish whether variation in transplant rate occurs through variation in liver offers or variation in acceptance practices. However, we have separated the estimation of DSA-level MIRR from the estimation of variation across the program level, which includes program-level decision making at the offer level.29 Furthermore, we were unable to study patients with end-stage liver disease who were not listed for a transplant. While evaluating this population might better characterize demand, these candidates are not in the risk set for receiving DDLT. Living donor liver transplants might also play a role in variation liver transplant rates overall, however, we censored patients who received a living donor transplant because access to living donation is less modifiable through allocation policy. Finally, we adjusted transplant rates for MELD under the assumption that candidates with equal MELD should have similar transplant rates, but it might be the case that candidates in higher-risk communities face excess mortality risk on the waitlist that is not captured by MELD alone.27
We found significant variation in deceased-donor liver transplant rates across DSAs for candidates with similar allocation MELDs, independent of variation across transplant programs. We observed no decrease in between-DSA geographic disparity following increased sharing under Share 35, even for candidates with MELD≥35. Contrary to OPTN aims, a candidate’s transplant program and Donation Service Area continue to be major determinants of access to a liver transplant.
Acknowledgments
FUNDING
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases, R01DK111233 on reducing geographic disparities in kidney and liver allocation. D.L. Segev and A.B. Massie are supported by grant numbers K24DK101828 and K01DK101677 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), respectively.
ABBREVIATIONS
- CI
confidence interval
- CIT
cold ischemia time
- DDLT
deceased-donor liver transplant
- DSA
Donation Service Area
- IRR
incidence rate ratio
- IQR
interquartile range
- LT
liver transplant
- MELD
Model for End-stage Liver Disease
- MIRR
median incidence rate ratio
- SRTR
Scientific Registry of Transplant Recipients
- OPTN
Organ Procurement and Transplantation Network
Footnotes
DISCLOSURE
The authors declare no conflicts of interest. The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, UNOS/OPTN, or the US Government.
REFERENCES
- 1.Gentry SE, Massie AB, Cheek SW, et al. Addressing geographic disparities in liver transplantation through redistricting. Am J Transplant. 2013;13(8):2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heimbach JK, Hirose R, Stock PG, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61(5):1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards EB, Harper AM, Hirose R, Mulligan DC. The impact of broader regional sharing of livers: 2-year results of “Share 35”. Liver Transpl. 2016;22(4):399–409. [DOI] [PubMed] [Google Scholar]
- 4.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91(4):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stine JG, Northup PG, Stukenborg GJ, et al. Geographic variation in liver transplantation persists despite implementation of Share35. Hepatol Res. 2018;48(4):225–232. [DOI] [PubMed] [Google Scholar]
- 6.Volk ML, Choi H, Warren GJ, Sonnenday CJ, Marrero JA, Heisler M. Geographic variation in organ availability is responsible for disparities in liver transplantation between Hispanics and Caucasians. Am J Transplant. 2009;9(9):2113–2118. [DOI] [PubMed] [Google Scholar]
- 7.Rana A, Kaplan B, Riaz IB, et al. Geographic inequities in liver allograft supply and demand: does it affect patient outcomes? Transplantation. 2015;99(3):515–520. [DOI] [PubMed] [Google Scholar]
- 8.Hart A, Schladt DP, Zeglin J, et al. Predicting Outcomes on the Liver Transplant Waiting List in the United States: Accounting for Large Regional Variation in Organ Availability and Priority Allocation Points. Transplantation. 2016;100(10):2153–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massie AB, Caffo B, Gentry SE, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011;11(11):2362–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Executive Summary of the Minutes. OPTN/UNOS Board of Directors Meeting; 2012 November, 12-13, 2012; St. Louis, MO. [Google Scholar]
- 11.Gentry SE, Chow EK, Massie AB, Segev DL. Gerrymandering for Justice: Redistricting U.S. Liver Allocation. Interfaces. 2015;45(5):462–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parent B, Caplan AL. Fair is fair: We must re-allocate livers for transplant. BMC Med Ethics. 2017;18(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. Board approves enhanced liver distribution system [press release] UNOS News Bureau; Organ Procurement and Transplantation Network, US Department of Health and Human Services; 2017. [Google Scholar]
- 14.Massie AB, Chow EK, Wickliffe CE, et al. Early changes in liver distribution following implementation of Share 35. Am J Transplant. 2015;15(3):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosano A, Ricciardi W, van der Zee J. Analysis of the role of General Practice in preventing Avoidable Hospitalisation through a multilevel approach. Epidemiol Biostat Pu. 2016;13(2). [Google Scholar]
- 17.Massie AB, Desai NM, Montgomery RA, Singer AL, Segev DL. Improving distribution efficiency of hard-to-place deceased donor kidneys: Predicting probability of discard or delay. Am J Transplant. 2010;10(7):1613–1620. [DOI] [PubMed] [Google Scholar]
- 18.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med. 2017;36(20):3257–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC, Wagner P, Merlo J. The median hazard ratio: a useful measure of variance and general contextual effects in multilevel survival analysis. Stat Med. 2017;36(6):928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabe-Hesketh SaS A Multilevel and Longitudinal Modeling Using Stata. Stata Press; 2008. [Google Scholar]
- 22.Elwir S, Lake J. Current Status of Liver Allocation in the United States. Gastroenterol Hepatol (N Y). 2016;12(3):166–170. [PMC free article] [PubMed] [Google Scholar]
- 23.Wey A, Gustafson SK, Salkowski N, et al. Program-specific transplant rate ratios: Association with allocation priority at listing and posttransplant outcomes. Am J Transplant. 2018;18(6):1360–1369. [DOI] [PubMed] [Google Scholar]
- 24.Parikh ND, Marrero WJ, Sonnenday CJ, Lok AS, Hutton DW, Lavieri MS. Population-Based Analysis and Projections of Liver Supply Under Redistricting. Transplantation. 2017;101(9):2048–2055. [DOI] [PubMed] [Google Scholar]
- 25.Ladin K, Zhang G, Hanto DW. Geographic Disparities in Liver Availability: Accidents of Geography, or Consequences of Poor Social Policy? Am J Transplant. 2017;17(9):2277–2284. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg DS, French B, Sahota G, Wallace AE, Lewis JD, Halpern SD. Use of Population-based Data to Demonstrate How Waitlist-based Metrics Overestimate Geographic Disparities in Access to Liver Transplant Care. Am J Transplant. 2016;16(10):2903–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross K, Patzer RE, Goldberg DS, Lynch RJ. Sociodemographic Determinants of Waitlist and Posttransplant Survival Among End-Stage Liver Disease Patients. Am J Transplant. 2017;17(11):2879–2889. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg D, Kallan MJ, Fu L, et al. Changing Metrics of Organ Procurement Organization Performance in Order to Increase Organ Donation Rates in the United States. Am J Transplant. 2017;17(12):3183–3192. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg DS, Levine M, Karp S, Gilroy R, Abt PL. Share 35 changes in program-level liver acceptance practices. Liver Transpl. 2017;23(5):604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg DS, French B, Lewis JD, et al. Liver transplant program variability in accepting organ offers and its impact on patient survival. J Hepatol. 2016;64(4):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou S, Massie AB, Luo X, et al. Geographic Disparity in Kidney Transplantation under KAS. Am J Transplant. 2018;18(6):1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girotra S, van Diepen S, Nallamothu BK, et al. Regional Variation in Out-of-Hospital Cardiac Arrest Survival in the United States. Circulation. 2016;133(22):2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: Integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88. [DOI] [PubMed] [Google Scholar]
- 34.Hjerpe P, Ohlsson H, Lindblad U, Bostrom KB, Merlo J. Understanding adherence to therapeutic guidelines: a multilevel analysis of statin prescription in the Skaraborg Primary Care Database. Eur J Clin Pharmacol. 2011;67(4):415–423. [DOI] [PubMed] [Google Scholar]
- 35.Wasfy JH, Hidrue MK, Yeh RW, et al. Differences Among Cardiologists in Rates of Positive Coronary Angiograms. J Am Heart Assoc. 2015;4(10):e002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalemo S, Hjerpe P, Ohlsson H, Eggertsen R, Merlo J, Bostrom KB. Variation in plasma calcium analysis in primary care in Sweden--a multilevel analysis. BMC Fam Pract. 2010;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]


