Graphical abstract
Keywords: Rotenone, C. annuum, Tyrosine hydroxylase, Oxidative stress, Nutraceuticals
Highlights
-
•
Capsicum annuum reverses rotenone-induced reduction tyrosine hydroxylase activity.
-
•
C. annuum potentiates the ameliorative effect of L-DOPA on rotenone-induced motor dysfunction.
-
•
C. annuum attenuates rotenone-induced oxidative stress in the brain.
Abstract
Rotenone is a natural pesticide and environmental neurotoxin which mimics key aspects of Parkinson’s disease. This study evaluated the effect of ethyl acetate extract of Capsicum annuum L. (C. annuum) in rotenone-intoxicated rats. Oral doses of C. annuum extract (50, 100 & 200 mg kg−1) and rotenone (2 mg kg−1 i.p.) were co-administered for 25 days during which rearing behavior was monitored. Biochemical alterations in the levels of tyrosine hydroxylase (TH), monoamine oxidase (MAO), superoxide dismutase (SOD) as well as reduced and oxidized glutathione (GSH) were estimated. Decrease in rearing behavior resulting from rotenone exposure was ameliorated by 200 mg kg−1 of C. annuum. Furthermore, rotenone exposure significantly (P < 0.05) decreased TH and increased MAO levels respectively. Impaired brain antioxidant capacity, typified by significantly (P < 0.05) decreased GSH redox status and SOD levels were also observed in rotenone-treated rats. However, co-administration of C. annuum ameliorated rotenone-induced derangements and potentiated the effect of levodopa. These results taken together suggests that C. annuum protects against rotenone-induced neurotoxicity by modulating dopamine metabolism and GSH redox status in rat brain.
1. Introduction
Parkinson’s disease (PD), the most common neurodegenerative disorder after Alzheimer’s disease [1] is a movement disorder that affects about 7–10 million people worldwide [2]. It is characterized by the selective loss of dopaminergic neurons and low levels of dopamine in the striatum [3,4]. Deficits in dopamine concentrations ultimately result in dysregulation of the basal ganglia circuitries manifested as the clinical motor symptoms which includes bradykinesia, resting tremor, rigidity and postural instability [5]. Though the etiology of PD remains unclear, it could be considered a clinical and pathological syndrome that can result from several distinct—but related—pathogenic processes. Apart from single-gene mutations (cause monogenic forms of PD), there are also genetic risk loci or variants that confer susceptibility to PD development [6].
Rotenone is a commonly used natural pesticide which is also regarded as a neurotoxin. It is highly lipophillic, a property that enables it cross biological membranes independent of transporters [7]. Intracellularly, rotenone acts as a high affinity inhibitor of complex I of the mitochondrial electron transport chain [8], resulting in mitochondria perturbations which forms the basis of its toxicity. Chronic exposure of various experimental animals to sublethal concentrations of rotenone results in symptomatic mimicking of PD including locomotor deficits and selective loss of doperminergic neurons [9,10]. Therefore, rotenone treated-animals are viable models for investigating actions of novel therapeutic agents for the treatment of PD.
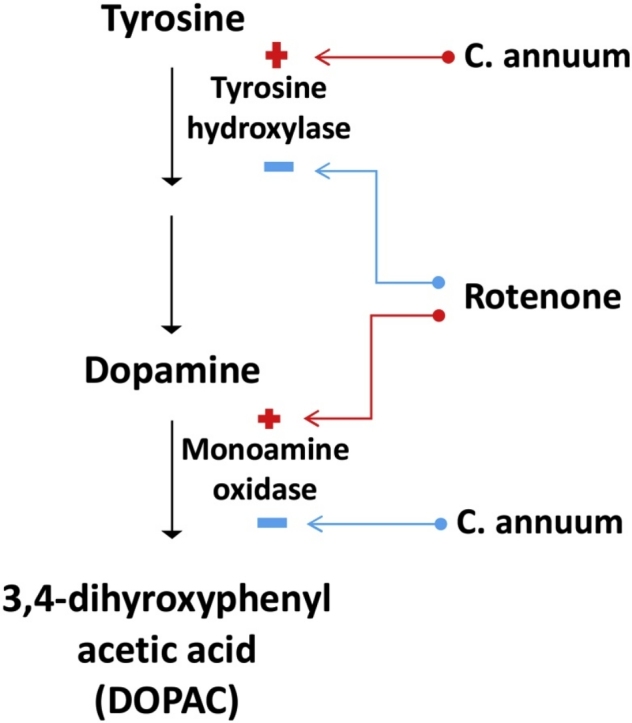
Clinical interventions in PD management is based mainly on maintaining dopamine concentration in affected brain regions. This includes dopamine replacement (oral administration of L-DOPA and dopamine agonists) and inhibitors of dopamine catabolism (monoamine oxidase inhibitors). Monoamine oxidase (MAO) catalyzes the catabolism of biogenic amine neurotransmitters and occurs as two isoforms, MAO-A and MAO-B, with MAO-B being the predominant isoform in the basal ganglia. Inhibitors of MAO-B, such as 1-deprenyl, are often administered as adjuncts in combination with L-DOPA in PD management [11]. Recent pharmacological approaches aim at improving tyrosine hydroxylase (TH) activity since it catalyzes the rate limiting step in the biosynthesis of dopamine and other catecholamines. Moreover, PD is also considered a TH deficiency syndrome of the striatum [[12], [13], [14]]. Oxidative stress is intimately linked to PD pathology. It is thought to be the common underlying mechanism that leads to cellular dysfunction and neurodegeneration in PD [15], justifying the use of antioxidants in the pharmacotherapy of PD [16]. Despite the successes of the available therapeutics, occurrence of motor complications from prolonged use diminishes their clinical effect [17]. Therefore, novel therapeutic agents and approaches are urgently needed to stop or delay the progressive course of the disease [16].
Functional foods are known to contain nutrients and phytochemicals that confer physiological or medical benefits. As such, they are considered viable therapeutic options for neurodegenerative diseases [18]. The multiplicity of phytochemicals makes them particularly atractive in that they are able to target various biomolecules involved in disease pathology. In this study, we investigated the neuroprotective effects of pepper (Capsicum annuum Linn) extract on rotenone-induced neurotoxicity. Capsicum annuum (C. annuum), the second most-consumed vegetable worldwide, is characterized by its high level of phytochemicals [19]. Capsicums have been a part of human diet since the beginning of civilization and are a popular culinary spice valued for their sensory attributes of color, pungency and flavor [20]. They are also a component of many herbal preparations used in the treatment of neurological disorders [21]. Oboh and Ogunruku [22] reported the ability of pepper diet to protect the brain from oxidative damage induced by cyclophosphamide. Furthermore, eating plants belonging to the family Solanacea to which Capsicums belong, have been associated with a 19% lower risk of PD in 500 diagnosed patients [23]. This study particularly investigated actions of C. annuum extracts on activities of enzymes involved in dopamine matabolism and antioxidant enzyme biomarkers with a view to assessing its possible therapeutic utility in PD management.
2. Materials and methods
2.1. Materials
2.1.1. Sample collection
Pepper (Capsicum annuum Linn) fruits were collected from Gbogan area of Osun State, Nigeria around September 2017. Identification and authentication were carried out at the Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria (Voucher number 17533). Fruits were lyophilized and thereafter blended. Samples were stored in a desiccator until used. All reagents were of analytical grade.
2.1.2. Chemicals
Rotenone was obtained from Chem Cruz, (Santa Cruz Biotechnology Inc., Dallas). Thiobarbituric acid (TBA), benzylamine, semicarbazide, L-tyrosine, sodium periodate, Hepes salt, Tris base, glutathione reductase, iron(II) sulphate, benzene, hydrochloric acid, sodium hydroxide, reduced glutathione, oxidized glutathione, 2-vinylpyridine, triethanolamine, Folin-Ciocalteau’s reagent, trichloroacetic acid, acetic acid, 5,5′-dithio-bis(2-nitrobenzoic) acid (DNTB), hydrogen peroxide, sodium dodecyl sulphate, 2,4-dinitrophenyl hydrazine (DNPH), potassium chloride, calcium chloride, sodium carbonate and tetrahydrobiopterin were procured from Sigma Aldrich, Inc., (St Louis, MO, USA). Except stated otherwise, all other chemicals and reagents were of analytical grade and water was glass distilled.
2.1.3. Sample preparation
A solvent mixture of methanol and water (1:1, v/v) in 0.5 M HCl was used to extract the pepper fruits (1:4 w/v) for 48 h and thereafter filtered through filter paper (Whatman no. 1). The filtrate was reduced under vacuum at 40 °C to obtain a crude extract which was reconstituted in water and partitioned with an equal volume of ethylacetate. The ethylacetate fraction was concentrated in vacuo at 40 °C using a rotary evaporator. The extract obtained was stored at 4 °C until use.
2.1.4. Animals
Wistar strain male albino rats (120 ± 30 g), obtained from the Animal House, College of Health Sciences, Obafemi Awolowo University, Ile Ife, Osun State, Nigeria were acclimatized for two weeks during which they were maintained ad libitum on standard commercial pellet diet and water at 25 °C on a 12 h light/dark cycle. The study was carried out in strict compliance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (NIH Publications No. 8023, revised 1978). All efforts were made to minimize animal suffering.
2.2. Experimental design
A stock concentration of rotenone was prepared by dissolving 375 mg of rotenone in 2.5 ml of dimethyl sulfoxide (DMSO) and was stored at −20 until further use. Before administration, the rotenone stock concentration was diluted in 1 ml of sunflower oil to achieve a concentration of 2 mg kg−1 which was administered interperitoneally once daily for a period of 25 days. C. annuum ethyl acetate extract was dissolved in distilled water and administered daily by oral gavage in graded doses of 50 mg kg−1, 100 mg kg−1 and 200 mg kg−1. Rotenone was administered 4 h after C. annuum extract administration. Levodopa was dissolved in distilled water and administered orally at a concentration of 30 mg kg−1.
The animals were weighed before the commencement of treatments and body weight was measured at 72 h interval throughout the duration of the study with the aim of monitoring the health of the animals. They were randomly divided into seven (7) experimental groups of 6 animals each as follows: Control – received solvent and vehicle (DMSO and sunflower oil); PD – received rotenone only; Group 3, 4 and 5 – received rotenone and 50 mg kg−1, 100 mg kg−1 C. annuum extract and 200 mg kg−1 C. annuum extract respectively; Group 6 – received rotenone and 30 mg kg−1 levodopa; Group 7 – received rotenone, 100 mg kg−1 C. annuum extract and 30 mg kg−1 levodopa.
2.3. Tissue collection and preparation for Biochemical assays
At the end of the experiment, animals were sacrificed by cervical dislocation and the brain tissue was quickly excised, blotted with filter paper, weighed and rinsed in cold saline solution. The tissues were homogenized in phosphate buffered saline. The tissue homogenate was centrifuged at 5000 rpm for 30 min. at 4 . The supernatant was collected and preserved at −20 until further analysis.
2.4. Rearing/cylinder test
Rearing was determined in the final week of the experiment between days 21 - 25. When placed in a clear cylinder, rats engage in exploratory behavior, including rearing. The animals were placed in a clear plexi glass cylinder (height – 30 cm, diameter – 20 cm) for 5 min and the number of rears was quantified [24]. A Rear was classified as the raising of forelimbs above the shoulder level and when contact was made with the walls of the cylinder with either or both forelimbs. Removal of both forelimbs from the cylinder wall and contact with the table surface was required before another rear was scored.
2.5. Biochemical analyses
2.5.1. Tyrosine hydroxylase (TH) activity assay
Tyrosine hydroxylase activity was measured according to the method of Vermeer et al. [25] with slight modifications. Briefly, an assay mixture containing brain supernatant (0.5 mg protein), tetrahydrobiopterin (BH4), and iron (II) sulphate was initially allowed to incubate for 5–10 min on ice. Thereafter, 10 mM HEPES buffer (pH 6.8), tyrosine and sodium periodate were added to obtain final concentrations of 0.25 mM, 62.5 μM, 12.5 mM and 100 μM for BH4, iron, tyrosine and sodium periodate respectively. Rate of formation of L-DOPA cyclized to dopachrome ( = 3700 M−1. cm−1) was monitored at 475 nm and TH activity was expressed as unit/mg protein.
2.5.2. Monoamine oxidase activity assay
Monoamine oxidase (MAO) activity was determined based on modified methods of Green and Haughton [26] and Turski et al. [27] as reported by Gacche et al. [28]. The reaction mixture contained 25 mM phosphate buffer (pH 7), 12.5 mM semicarbazide, 10 mM benzylamine (pH 7) and 50 μl of brain homogenate (0.043–0.054 mg protein) in a total reaction volume of 1 ml. 250 μl of acetic acid was added after 30 min and boiled for 3 min and subsequently centrifuged at 1000 g for 5 min. The resultant supernatant (1 ml) was mixed with an equal volume of 0.05% of 2, 4-DNPH. 1.25 ml of benzene was added after 10 min of incubation at room temperature. The benzene layer was collected and mixed with equal volume of 0.1 M NaOH, the alkaline layer was decanted and heated at 80 for 10 min; the orange-yellow colour developed was read at 450 nm. MAO activity was expressed as unit/mg protein ( = 12,000 M−1. cm−1).
2.5.3. Lipid peroxidation assay (thiobarbituric acid reaction)
Lipid peroxidation assay was carried out using the modified method of Ohkawa et al. [29]. The assay mixture of 300 μl containing 100 μl of brain homogenate (0.107–0.136 mg protein) 30 μl 0.1 M Tris−HCl buffer (pH 7.4) was incubated at 37 for 1 h. This was subsequently followed by the addition of 300 μl of 8.1% sodium dodecyl sulphate, 500 μl of acetic acid/HCl (pH 3.4) mixture and 500 μl 0.8% TBA (Thiobarbituric acid). Colour development was stimulated by incubating the reaction mixture at 100 for 1 h. The absorbance of TBARS (Thiobarbituric acid reactive species) produced was measured at 532 nm. Malondialdehyde (MDA) levels was subsequently calculated and expressed as mol MDA/mg protein ( = 1.56 105 M−1. cm−1).
2.5.4. GSH and GSSG measurement
Assays for GSH and GSSG were carried out according to the method of Rahman et al. [30]. Brain tissue was homogenized in 10% (w/v) of 9.1 mM phosphate buffer (pH 7.5) containing 0.45 mM ethylene diethyltetracetic acid (EDTA) and 0.6% sulfosalicylic acid – 0.1% Triton-X solution. The homogenate was centrifuged at 4000 for 10 min at 4 and the supernatant was collected for GSH and GSSG determination. The GSH assay mixture consisted of 9.1 mM sodium phosphate buffer (pH 7.5) (containing 0.45 mM EDTA), brain homogenate, 0.46 mM DTNB and 170 units ml−1 glutathione reductase (GR). After 30 s of incubation, 0.22 mM β-NADPH was added and immediately the absorbance was read at 412 nm at 30 s intervals for 5 min. GSH concentration in the brain tissues was obtained by comparing absorbance with GSH (0.103–26.4 nM) calibration curve and was expressed as nm/mg protein. In order to derivatize GSH (which is critical for GSSG determination) present in the brain tissue supernatant initially obtained, 2 μl of 10% 2-vinylpyridine was added to 100 μl of the supernatant and vortexed before incubation at 25 for 1 h. 6 μl of 15% triethanolamine was then added to neutralize the excess 2-vinylpryridine (final pH 6–7). The tissue homogenate was left to stand for 10 min before GSSG determination. This was then subjected to the assay procedure previously described for GSH. Absorbance obtained was compared with GSSG (0.103–26.4 nM) calibration curve in order to obtain the brain tissue GSSG concentration which was expressed as nm/mg protein.
2.5.5. Catalase activity assay
Catalase activity was determined in brain supernatant as described by Aebi [31]. Briefly, 50 μl of the brain supernatant was added to a cuvette containing 0.1 M phosphate buffer (pH 7.4) and 20 mM of H2O2. Catalase activity was determined as change in absorbance at 240 nm for 1 min and expressed as unit/mg protein i.e. one unit of enzyme activity is 1 mM of H2O2 degraded/min (εH2O2 = 3700 M−1 cm−1).
2.5.6. Superoxide dismutase activity assay
The superoxide dismutase (SOD) activity was determined according to the method of McCord and Fridovich [32]. 20 μl of Brain homogenate was added to a mixture containing 75 mM Tris−HCl buffer (pH 8.2) (containing 30 mM EDTA) and 2 mM of pyrogallol. SOD activity was determined as change in absorbance at 420 nm for 3 min and expressed as unit/mg protein. One unit of SOD activity was given as the amount of SOD necessary to inhibit 50% of pyrogallol oxidation.
2.6. Quantification of compounds by HPLC-DAD
Analytical High Performance Liquid Chromatography (HPLC-DAD) was performed with a Shimadzu Prominence Auto Sampler (SIL-20A) HPLC system coupled with a diode array detector (DAD) (SPD-M20A) while data were evaluated with LC solution 1.22 SP1 software. Separation was carried out under gradient conditions on a C18 column (4.6 mm × 150 mm) packed with 5 μm diameter particles. The mobile phase was water containing 2% acetic acid (A) and methanol (B). The composition gradient was 5% of B until 2 min and changed to obtain 25%, 40%, 50%, 60%, 70% and 100% B at 10, 20, 30, 40, 50 and 60 min respectively. The flow rate was 0.7 mL/min and the injection volume was 40 μL. The chromatography peaks were confirmed by comparing retention time with those of reference standards and by DAD spectra (200–600 nm). All chromatography operations were carried out at ambient temperature and in triplicate.
2.7. Statistical analysis
Data obtained were subjected to statistical analysis using GraphPad® Prism Statistical Package Version 7 and expressed as mean ± standard error of mean (SEM). Data obtained from cylinder test was subjected to two-way analysis of variance (ANOVA) while all other data were compared using one-way ANOVA followed by Tukey test. Significance was accepted at P < 0.05
3. Results
3.1. C. annuum Lin improves motor coordination in rotenone-intoxicated rats and potentiates the effect of levodopa
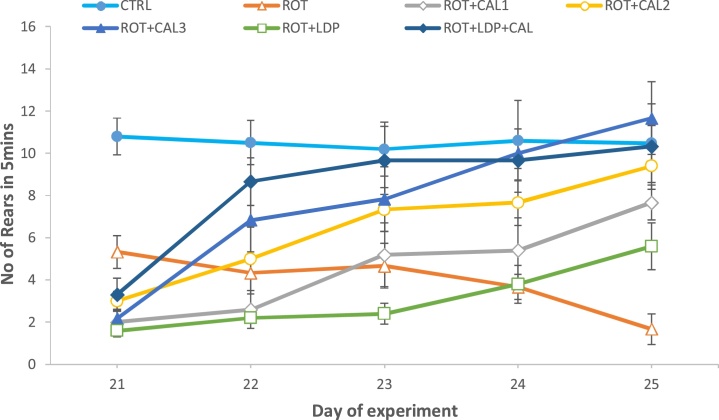
Cylinder test was used to evaluate motor coordination in rats exposed to rotenone for a period of five consecutive days (21st–25th day). There was a significant (P < 0.05) increase in the number of rears/5 min. of non-exposed (control) rats compared to the rotenone-only-exposed (disease group) group (Fig. 1). This indicates motor dysfunction resulting from rotenone exposure. Rats treated with pepper extracts showed improvement in motor skills at extract concentrations of 100 mg kg−1 and 200 mg kg−1. Normal motor skills were restored in these animals by day 25. Interestingly, animals treated with levodopa (30 mg kg−1) exhibited poor performance in the test paradigm employed. However, co-administration of levodopa and C. annuum extract (100 mg kg−1) resulted in a significant (P < 0.05) improvement in motor skills adjudged by the increase in number of rears.
Fig. 1.
Mitigation of rotenone-induced locomotor deficits in rats by Capsicum annuum.
Values are expressed as mean ± SEM (n = 6). CTRL: Basal control; ROT: Disease control (2 mg kg−1 Rotenone); ROT + CAL1: Rotenone (2 mg kg−1) + C. annuum (50 mg kg−1); ROT + CAL2: Rotenone (2 mg kg−1) + C. annuum (100 mg kg−1); ROT + CAL3: Rotenone (2 mg kg−1) + C. annuum (200 mg kg−1); ROT + LDP: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1); ROT + LDP + CAL: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1) + C. annuum (100 mg kg−1).
3.2. C. annuum ameliorates aberrant dopamine metabolism resulting from rotenone exposure
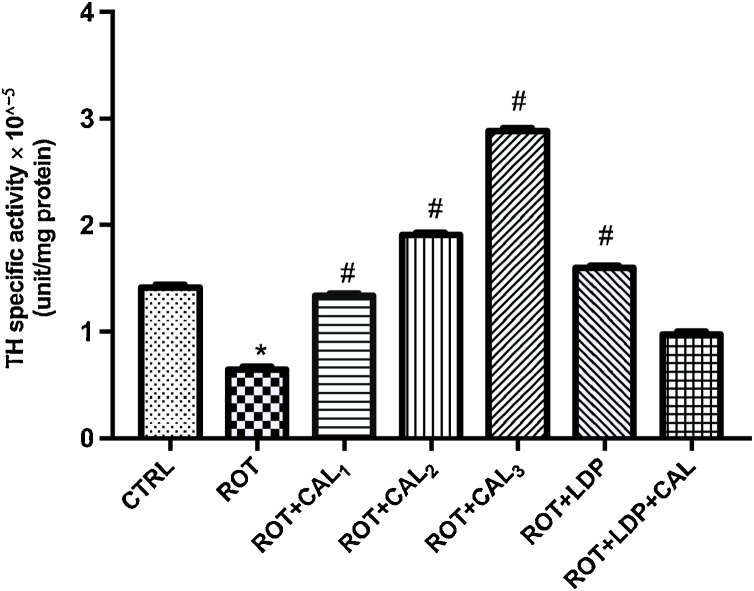
TH activity was significantly (P < 0.05) decreased as a result of rotenone intoxication (Fig. 2). There was no significant difference between the control and ROT + CAL1 groups indicating that C. annuum at its lowest concentration was able to normalize TH activity. Significant (P < 0.05) dose-dependent increase in TH activities was observed with increasing concentrations of C. annuum compared to the basal control, disease control and positive control (rotenone and levodopa treated) groups. Co-administration of C. annuum and levopoda produced no significant effect on the enzyme’s activity.
Fig. 2.
Effect of C. annuum on rotenone-induced changes in Tyrosine Hydroxylase activity of brain tissue in Rats.
Values are expressed as mean ± SEM (n = 6). (*) and (#) represent significant difference at P < 0.05 when compared to the control and disease control groups respectively. CTRL: Basal control; ROT: Disease control (2 mg kg−1 Rotenone); ROT + CAL1: Rotenone (2 mg kg−1) + C. annuum (50 mg kg−1); ROT + CAL2: Rotenone (2 mg kg−1) + C. annuum (100 mg kg−1); ROT + CAL3: Rotenone (2 mg kg−1) + C. annuum (200 mg kg−1); ROT + LDP: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1); ROT + LDP + CAL: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1) + C. annuum (100 mg kg−1).
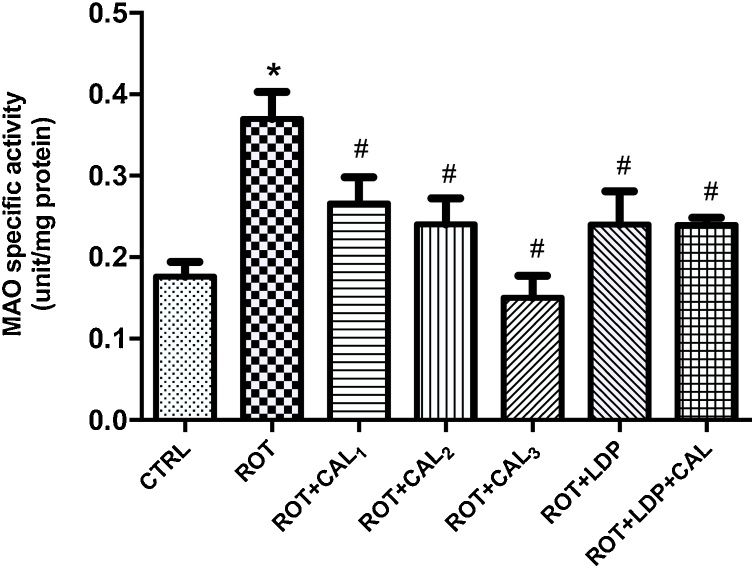
Furthermore, significant (P < 0.05) increase in MAO activity was observed in rotenone-treated rats compared to the basal control (Fig. 3). However, treatment with C. annuum significantly (P < 0.05) decreased MAO activity over a dose range of 50–200 mg kg−1. MAO activity was normalized at the highest dose of C. annuum tested.
Fig. 3.
Effect of C. annuum on rotenone-induced changes in brain monoamine oxidase activity in Rats.
Values are expressed as mean ± SEM (n = 6). (*) and (#) represent significant difference at P < 0.05 when compared to the control and disease control groups respectively. CTRL: Basal control; ROT: Disease control (2 mg kg−1 Rotenone); ROT + CAL1: Rotenone (2 mg kg−1) + C. annuum (50 mg kg−1); ROT + CAL2: Rotenone (2 mg kg−1) + C. annuum (100 mg kg−1); ROT + CAL3: Rotenone (2 mg kg−1) + C. annuum (200 mg kg−1); ROT + LDP: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1); ROT + LDP + CAL: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1) + C. annuum (100 mg kg−1).
3.3. C. annuum suppresses lipid peroxidation and normalizes brain redox status otherwise compromised by rotenone exposure
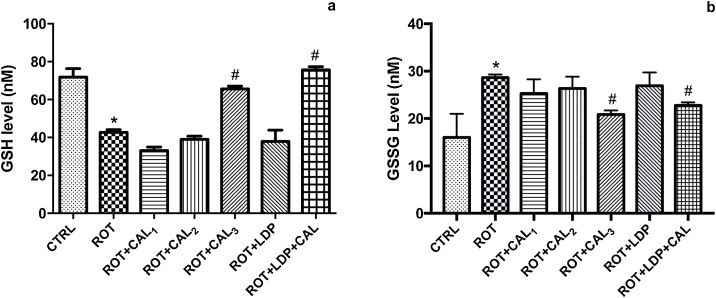
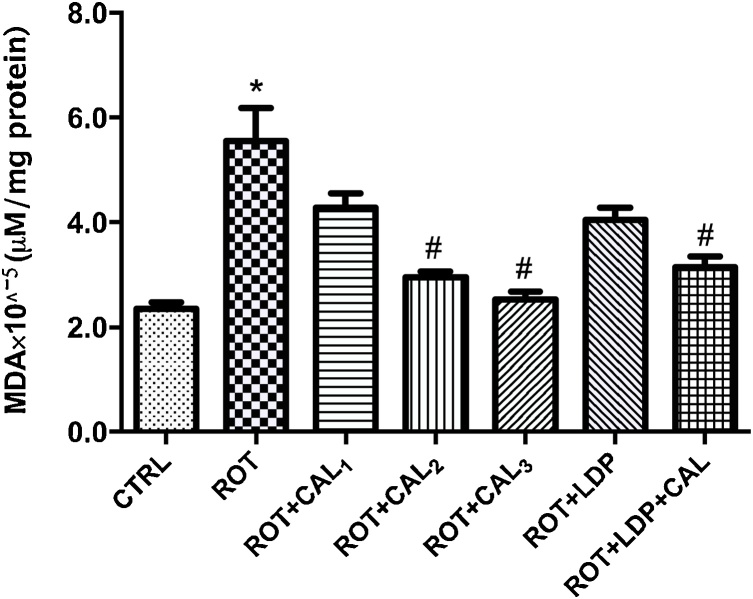
We further sought to investigate if the observed neuroprotection by C. annuum given rotenone exposure is mediated by antioxidant mechanisms. This hypothesis was tested by determining MDA levels (a marker for lipid peroxidation) as well as GSH levels and its oxidized state in the brain. Rotenone exposure resulted in a 40.5% decrease in GSH, 79% increase in GSSG (Fig. 5) and ultimately a decrease (66.8%) in GSH redox status in the brain (Table 1). At lower concentrations, C. annuum was unable to reverse this trend. However, treatment with C. annuum at 200 mg kg−1 significantly improved and normalized the GSH redox status in the brain. Also, co-administration of levodopa and of C. annuum (200 mg kg−1) significantly (P < 0.05) improved GSH redox status in the brain. Decrease in the GSH redox status resulting from rotenone-intoxication corresponded to increase MDA levels in the brain. Rotenone administration resulted in 135.7% increase in brain MDA levels when compared to the basal control (Fig. 4). Treatment with C. annuum attenuated MDA levels in a dose-dependent manner of over a dose range of 50 to 200 mg kg−1.
Fig. 5.
Perturbations in (a) reduced and (b) oxidized glutathione levels in the brain of rotenone-intoxicated Rats.
Values are expressed as mean ± SEM (n = 6). (*) and (#) represent significant difference at P < 0.05 when compared to the control and disease control groups respectively. CTRL: Basal control; ROT: Disease control (2 mg kg−1 Rotenone); ROT + CAL1: Rotenone (2 mg kg−1) + C. annuum (50 mg kg−1); ROT + CAL2: Rotenone (2 mg kg−1) + C. annuum (100 mg kg−1); ROT + CAL3: Rotenone (2 mg kg−1) + C. annuum (200 mg kg−1); ROT + LDP: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1); ROT + LDP + CAL: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1) + C. annuum (100 mg kg−1).
Table 1.
Changes in GSH redox status by C. annuum in brain tissue of rotenone-intoxicated rats.
| Treatment groups | Redox status GSH:GSSG |
|---|---|
| Control | 4.494 ± 0.012a |
| Disease control (2 mg kg−1) | 1.492 ± 0.051c |
| Rotenone (2 mg kg−1) + C. annuum (50 mg kg−1) | 1.309 ± 0.095c |
| Rotenone (2 mg kg−1) + C. annuum (100 mg kg−1) | 1.423 ± 0.073c |
| Rotenone (2 mg kg−1) + C. annuum (200 mg kg−1) | 3.144 ± 0.052b |
| Rotenone (2 mg kg−1) + L-DOPA (30 mg kg−1) | 1.596 ± 0.021c |
| Rotenone (2 mg kg−1) + L-DOPA (30 mg kg−1) + C. annuum (100 mg kg−1) | 3.924 ± 0.063a |
Values are presented as mean ± SEM (n = 6); Values with the same superscript letter are not significantly (P<0.05) different.
Fig. 4.
Reduction of Lipid Peroxidation by Capsicum annuum in the brain of rotenone-intoxicated Rats.
Values are expressed as mean ± SEM (n = 6). (*) and (#) represent significant difference at P < 0.05 when compared to the control and disease control groups respectively. CTRL: Basal control; ROT: Disease control (2 mg kg−1 Rotenone); ROT + CAL1: Rotenone (2 mg kg−1) + C. annuum (50 mg kg−1); ROT + CAL2: Rotenone (2 mg kg−1) + C. annuum (100 mg kg−1); ROT + CAL3: Rotenone (2 mg kg−1) + C. annuum (200 mg kg−1); ROT + LDP: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1); ROT + LDP + CAL: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1) + C. annuum (100 mg kg−1).
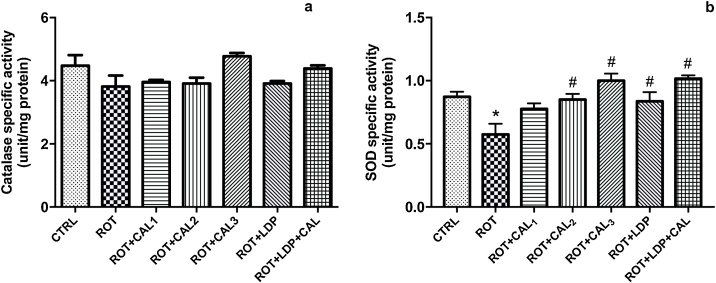
3.4. C. annuum reverses rotenone-induced decrease in brain antioxidant enzymes
Activity of the antioxidant enzymes catalase and superoxide dismutase (SOD) were also measured in the brain. Rotenone intoxication suppressed catalase activity by 14.8% (Fig. 6a), this did not improve when lower concentrations of C. annuum were administered. However, C. annuum at 200 mg kg−1 resulted in a 25.1% increase in catalase activity when compared with the rotenone only treated group. As shown in Fig. 6b, intraperitoneal administration of rotenone resulted in a significant (P < 0.05) reduction in SOD activity when compared with the basal control. Treatment with C. annuum resulted in a dose dependent 35.2%, 48.1% and 74.1% increases in SOD activity at 50 mg kg−1, 100 mg kg−1 and 200 mg kg−1 respectively when compared with the rotenone-intoxicated rats. Furthermore, co-administration of levodopa and extract at 100 mg kg−1 resulted in 74% increase in SOD activity compared with the rats to which only levodopa was administered.
Fig. 6.
Modulatory effect of Capsicum annuum Lin on rotenone-induced changes in antioxidant enzymes (a) catalase (b) superoxide dismutase activities in rats’ brain.
Values are expressed as mean ± SEM (n = 6). (*) and (#) represent significant difference at P < 0.05 when compared to the control and disease control groups respectively. CTRL: Basal control; ROT: Disease control (2 mg kg−1 Rotenone); ROT + CAL1: Rotenone (2 mg kg−1) + C. annuum (50 mg kg−1); ROT + CAL2: Rotenone (2 mg kg−1) + C. annuum (100 mg kg−1); ROT + CAL3: Rotenone (2 mg kg−1) + C. annuum (200 mg kg−1); ROT + LDP: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1); ROT + LDP + CAL: Rotenone (2 mg kg−1) + Levodopa (30 mg kg−1) + C. annuum (100 mg kg−1).
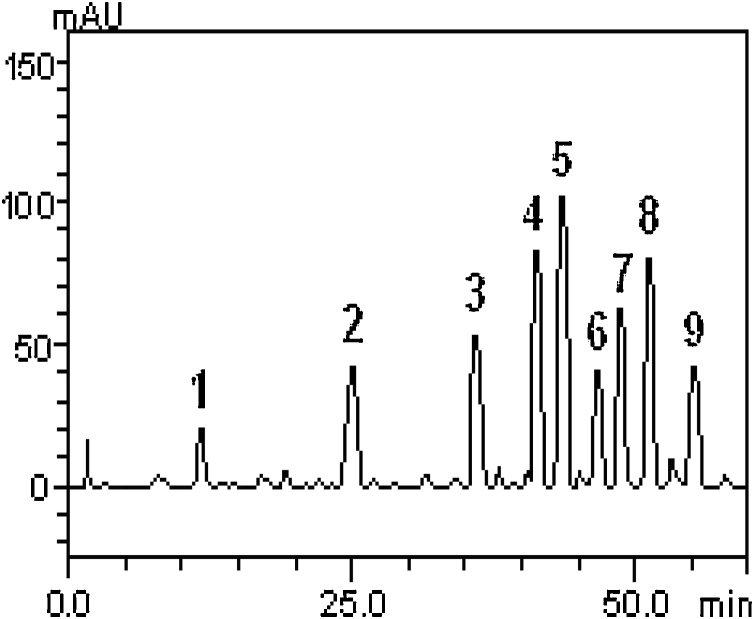
3.5. Qualitative detection of phyto-constituents in C. annuum
Fig. 7 and Table 2 shows some of the phyto-constituents of C. annuum acquired by HPLC-DAD. Nine (9) polyphenolic compounds were identified, these were mostly quercetin and its derivatives. Quercetrin was also shown to be the most abundant of the polyphenols identified.
Fig. 7.
Representative high performance liquid chromatography profile of Capsicum annuum Lin extracts. Gallic acid (peak 1), chlorogenic acid (peak 2), rutin (peak 3), isoquercitrin (peak 4), quercitrin (peak 5), quercetin (peak 6), kaempferol (peak 7), dihydrocapsaicin (peak 8) and capsaicin (peak 9).
Table 2.
Composition of Capsicum annuum Lin.
| Compounds | C. annuum Lin | LOD | LOQ |
|---|---|---|---|
| % | μg/mL | μg/mL | |
| Gallic acid | 1.42 ± 0.1 a | 0.027 | 0.089 |
| Chlorogenic acid | 2.95 ± 0.2 b | 0.031 | 0.035 |
| Rutin | 3.47 ± 0.2 c | 0.008 | 0.026 |
| Isoquercitrin | 4.23 ± 0.3 d | 0.015 | 0.049 |
| Quercitrin | 4.78 ± 0.3 e | 0.034 | 0.113 |
| Quercetin | 2.47 ± 0.4 f | 0.012 | 0.039 |
| Kaempferol | 3.51 ± 0.1 c | 0.019 | 0.062 |
| Dihydrocapsaicin | 4.19 ± 0.5 d | 0.022 | 0.071 |
| Capsaicin | 2.89 ± 0.1 b | 0.037 | 0.124 |
Results are expressed as mean ± standard deviations (SD) of three determinations. Averages followed by different letters differ by Tukey test at p < 0.01.
4. Discussion
Systemic rotenone administration has been shown to mimic key aspects of PD pathology such as dopaminergic neurodegeneration in the substantia nigra (SN). This results in depletion of associated neurotransmitters which ultimately results in motor dysfunction [24,33], oxidative stress, neuroinflammation and -synuclein aggregation [11]. Results from this study, in line with previous studies [24,34], showed that chronic exposure to rotenone for a period of 28 days – 60 days impaired locomotion significantly. This study also revealed that such impairments were reversed by the administration of C. annuum alone or in combination with levopoda (an active component of drugs used in the symptomatic management of PD).
Tyrosine hydroxylase (TH) catalyzes the formation of L-DOPA, the rate-limiting step in the biosynthesis of dopamine, and a deficiency in this enzyme has been linked to infantile Parkinsonism [35]. In consonance with results from this study, Alam and Shmidt [36] reported reduction in tyrosine hydroxylase activity in intraperitoneal rotenone-induced PD model. Enhancing tyrosine hydroxylase activity for increased dopamine synthesis could be a strategy for the management of PD. Our results indicate that co-administration of ethylacetate fraction of C. annuum with rotenone resulted in a significant (P < 0.05) increase in tyrosine hydroxylase activity. Evidence from various research demonstrates therapeutic potentials of plant phenolic extracts for the management of PD by maintaining tyrosine hydroxylase activity. For instance, Datla et al. [37] reported the phenols present in orange and lemon peels mitigated the loss of dopaminergic neurons and tyrosine hydroxylase activity in the substantia nigra of PD rat model.
Elevated activity of monoamine oxidase (MAO), which catalyzes the degradation of dopamine in the synaptic cleft, has also been implicated in dopamine depletion associated with PD [38]. Thakur and Nehru [39] reported that rotenone administration resulted in a significant (P < 0.01) increase in monoamine oxidase activity compared with the basal control rats. A similar result was obtained in this study (Fig. 3). Rotenone-treated rats showed a significant increase in MAO activity compared with the basal control rats. Inhibition of MAOs has been linked with therapeutic activities such as neuroprotective properties as well as antidepressant and anti-anxiety functions; suggesting an increase in the level of neurotransmitters in the central nervous system [40]. As such, one of the most sought-after therapeutic strategy for PD management is to slow down dopamine oxidation by reducing MAO activity via its inhibition Also, inhibition of the MAO activity prevents dopamine degradation and release of neurotoxic dopamine metabolites such as ROS and dopamine-derived aldehydes [41,42]. In this study, we have also shown the potential of C. annuum to decrease the activity of MAO in the brain of rotenone intoxicated rats. The ability of C. annuum to both increase TH and decrease MAO activities in the brain of rotenone intoxicated rats alludes to its potential to possibly maintain dopamine levels in PD.
Rotenone toxicity is mediated via inhibition of mitochondrial complex I, limiting oxygen consumption rate with a concomitant decrease in ATP generation. Ultimately, reactive oxygen species (ROS) resulting from the incomplete reduction of molecular oxygen causes mitochondria damage and leaks out into the cytosol where further damage to cellular structures and macromolecules occur. Manjunath [43] reported that rotenone caused marked oxidative stress as evidenced by elevated levels of hydroperoxides, lipid peroxidation, and nitrite concentration in in-vivo models. In PD elevated MAO activities are often accompanied by elevated free radical generation. This derives from oxidation of residual dopamine by MAO during which hydrogen peroxide (H2O2) is produced. H2O2 can readily initiate ROS production in the presence of Fe2+ through the Fenton reaction [38], leading to oxidative stress. Susceptibility of dopaminergic cells to oxidative damage is further advanced by defective iron homeostasis. Iron related genes may either cause or predispose such cells to damage, possibly due to the contribution of iron to the synthesis of tyrosine hydroxylase [44,45]. Also, there is some evidence that there is an increase in iron load in the SN of Parkinson’s disease (PD) patients as compared to controls [44]. Increased lipid peroxidation and reduced antioxidant enzyme activities have also been reported in the brains of post-mortem patients [46], suggesting reduced antioxidant capacity in such patients. This study confirmed rotenone as an oxidative stress inducer as shown by the perturbations in the brain’s GSH redox status and antioxidant enzymes’ activities. It is possible that such perturbations could have contributed to the diminished TH enzyme activity in the rotenone treated group, ROS damage proteins by modifying the side chains of specific amino acid residues into carbonyls. Such carbonyl group formation on proteins may alter expression levels and/or confer a toxic loss or gain of function [47]. It is therefore a possibility that TH catalytic center has been so modified. However, C. annuum proved to be a potent antioxidant (as evidenced by its ability to increase the activities of antioxidant enzymes, prevent lipid peroxidation and modulate the brain’s redox imbalance) and this could be the basis for its observed neuroprotection. Though the exact mechanism underlying the antioxidant action of C. annuum is not fully understood, results of this study suggests that its role could lie in its ability to recycle GSH, which protects cells from oxidative damage and toxicity of xenobiotic electrophiles [48]. In the incidence of oxidative stress, GSH levels are depleted in favour of GSSG. This further exacerbates stress conditions, leaving cellular macromolecules vulnerable to ROS attack. It is therefore interesting to note that C. annuum was able to shift GSH recycling in favour of GSH. Besides inhibition of complex I and oxidative stress, aggregation of -synueclein and polyubiquitin, activation of astrocytes and microglial cells, inflamotory responses, neuronal apoptosis are mechanisms involved in rotenone induced toxicity [49].
Dihydroxyphenylalanine (levodopa) is a "gold standard" and the most effective symptomatic treatment for PD, against which new drugs are compared [17], however, the result of levodopa administration in vivo have been conflicting [50]. Levodopa induces oxidative toxicity, it increases MDA, protein carbonyl content and advanced glycation end products in the healthy mice [17]. In this study, levodopa was unable to attenuate rotenone induced oxidative stress in the brain as evidenced by increased lipid peroxidation, decreased GSH:GSSG ratio coupled with reduced antioxidant enzyme (SOD and catalase) activities. This scenario is similar to oxidative stress conditions in the PD brain [51,52]. However, in the presence of C. annuum, there was an increased antioxidant efficiency which could be attributed to the antioxidant protection conferred on the brain by C. annuum. As stated earlier, there is an increased concentration of iron in the SN of the PD brain, promoting chains of oxidative reactions and a decrease in the concentration of GSH, an important brain antioxidant [46,50]. Oxidative stress in SNpc could result from over activity of surviving neurons with increased H2O2 production [52]. Under these conditions, the addition of levodopa (a dopamine replacement therapy) could add to the pro-oxidant environment in the SNpc by generating reactive oxygen species during levodopa and/or dopamine autooxidation [52]. Furthermore, the reduction in GSH levels could be related to high GSH consumption as well as binding of levodopa derived quinones to the remaining GSH molecules [53]. It is therefore interesting to note that levodopa in combination with the extract at 100 mg/kg BWT resulted in significant (P < 0.05) increase in the redox status of the brain (established by GSH:GSSG ratio) and SOD activity. There was also an increment in catalase activity as well as reduced lipid peroxidation.
The overall neuroprotective effect of C. annuum can not be dissociated from its antioxidant properties. The phytochemical composition of the fruits exhibits a preponderance of polyphenolic compounds particularly quercetin and its derivatives. Interestingly, quercetin has been shown to increase the synthesis of monoamines in the brain [54], inhibit monoamine oxidase [55], up-regulate complex I activity in a rotenone intoxicated brain environment [56] as well as attenuate oxidative stress induced apoptotic cascades mediated by p53 and caspase 3 [57]. Furthermore, these polyphenolic compounds can directly neutralize free radicals by either accepting or donating electrons to eliminate the unpaired state of the radical while they may become new free radicals which are less active and less deleterious. On the other hand, they may neutralize the radical form of other antioxidant molecules such as glutathione radical, regenerating these antioxidants and enhancing their radical scavenging activity. These, in addition to other mechanisms such as double bond conjugation and resonance effect, are at the basis of the antioxidant effect of the polyphenols [58]. Furthermore, the ability of the extract to elevate antioxidant enzymes, inhibit lipid peroxidation and offer membrane stabilizing potential could also be mechanisms underlying C. annuum’s protective effect against rotenone induced toxicity in the brain. This finding is in line with previous findings on the antioxidant potentials of plant phenolics as dietary therapeutic measures in managing neurodegenerative disorders [59]. It is also possible that a synergism between these phytochemicals could have contributed to the marked reduction in oxidative stress in the C. annuum treated groups.
In conclusion, our findings suggest clearly that C. annuum ameliorates rotenone-induced oxidative stress in the brain of rats by modulating GSH redox cycling in favour of GSH and increasing the activity of endogenous antioxidant enzymes. This study also alludes to the potential of C. annuum to modulate dopamine metabolism and improve motor coordination in rats exposed to rotenone. It will however be of further interest to understand the mechanisms underlying the neuroprotective propensity of C. annuum viz. its ability to modulate dopamine metabolism. In all, these findings show that C. annuum could be an adjuvant in the management of neurodegenerative disorders such as PD.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lees J., Hardy J., Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Santiago J.A., Bottero V., Potashkin J.A. Biological and clinical implications of comorbidities in Parkinson’s disease. Front. Aging Neurosci. 2017;9:394. doi: 10.3389/fnagi.2017.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearnley J.M., Lees A.J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1990;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 4.Radad K., Gille G., Rausch W. Dopaminergic neurons are preferentially sensitive to long term rotenone toxicity in in primary cell culture. Toxicol. In Vitro. 2008;22:68–74. doi: 10.1016/j.tiv.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Ji C., Xue G.F., Lijun C., Feng P., Li D., Li L., Li G., Holscher C. A novel dual GLP-1 and GIP receptor agonist is neuroprotective in the MPTP mouse model of Parkinson’s disease by increasing the the expression of BNDF. Brain Res. 2016;634:1–11. doi: 10.1016/j.brainres.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Dardiotis E., Xiromerisiou G., Hadjichristodoulou C., Tsatsakis A.M., Wilks M.F., Hadjigeorgiou G.M. The interplay between environmental and genetic risk factors in Parkinson’s disease susceptibility: the evidence for Pesticides. Toxicology. 2013;307:17–23. doi: 10.1016/j.tox.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Muralidhara C.G. Propensity of Selaginella delicatula aqueous extract to offset rotenone-induced oxidative dysfunctions and neurotoxicity in Drosophila melanogaster: implications for Parkinson’s Disease. Neurotoxicology. 2012;33:444–456. doi: 10.1016/j.neuro.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Greenamyre J.T., Batarbet R., Sherer T. The rotenone model of Parkinson’s disease: genes, environment and mitochondria. Parkinsonism Relat. Disord. 2003;9:59–64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 9.Inden M., Kitamura Y., Abe M., Tamaki A., Takata K., Taniguchi T. Parkinsonian Rotenone Mouse Model: reeavaluation of long-term administration of rotenone in C57BL/6 mice. Biol. Pharm. Bull. 2011;34:92–96. doi: 10.1248/bpb.34.92. [DOI] [PubMed] [Google Scholar]
- 10.Betarbet R., Sherer T.B., Mackenzie G., Garcia-Osuna M., Panov P.A., Greenamyre T. Chronic Systemic Pesticide Exposure Reproduces Features of Parkinson’s disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 11.Youdim M.B.H., Bahkle Y.S. Monoamine Oxidase: isoforms and Inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006;147:287–296. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan M.S., Tabrez S., Priyadarshin M., Priyamuada S., Khan M.M. Targeting parkinson’s – tyrosine hydrolase and oxidative stress as points of interventions. CNS Neurol. Disord. Drug Target. 2012;11:369–380. doi: 10.2174/187152712800792848. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y., Zhang J., Zheng Y. Overview of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol. Disord. Drug Target. 2012;11:305–308. doi: 10.2174/187152712800792901. [DOI] [PubMed] [Google Scholar]
- 14.Haavik J., Toska K. Tyrosine hydroxylase and parkinson’s disease. Mol. Neurobiol. 1998;16:285–309. doi: 10.1007/BF02741387. [DOI] [PubMed] [Google Scholar]
- 15.Hwang O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013;22:11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javed H., Azimullah S., Abdul Khair S.B., Ogha S., Haque M.E. Neuroprotective effect of nerolidol against neuroinflamation and oxidative stress induced by rotenone. BMC Neurosci. 2016;17:58. doi: 10.1186/s12868-016-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolova G., Karamalakova Y., Gadjeva V. Reducing oxidative toxicity of L-dopa in combination with two different antioxidants: an essential oil isolated from Rosa Damascena Mill., and vitamin C. Toxicol. Rep. 2019;6:267–271. doi: 10.1016/j.toxrep.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martirosyan D.M., Singh J. A new definition of functional foods by FFC: what makes a new definition unique? Funct. Foods Health Dis. 2015;5:209–223. [Google Scholar]
- 19.Mateos R.M., Jiminez A., Roman P., Romojaro F., Bacarizo S., Leterrier M., Gomez M., Sevilla F., del Rio L.A., Corpas F.J., Palma J.M. Antioxidant systems from pepper (Capsicum annuum L): involvement in the response to temperature changes in ripe fruits. Int. J. Mol. Sci. 2013;14:9556–9580. doi: 10.3390/ijms14059556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López P., Gorzalczany S., Acevedo C., Alonso R., Ferraro G. Chemical study and anti-inflammatory activity of Capsicum chacoense and C. Baccatum. Braz. J. Pharmacogn. 2012;22:455–458. [Google Scholar]
- 21.Elufioye T.O., Oladele A.T., Cyril-Olutayo C.M., Agbedahunsi J.M., Adesanya S.A. Ethnomedicinal study and screening of plants used for memory enhancement and antiaging in Sagamu, Nigeria. Eur. J. Med. Plants. 2012;2:262–275. [Google Scholar]
- 22.Oboh G., Ogunruku O.O. Cyclophosphamide induced oxidative stress in brain: protective effect of hot short pepper (Capsicum frutescens L. Var. abbreviatum) Exp. Toxicol. Pathol. 2010;62:227–233. doi: 10.1016/j.etp.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Neilsen S.S., Franklin G.M., Longstreth W.T., Swanson P.D., Checkoway H. Nicotine from edible Solanaceae and risk of Parkinson’s disease. Ann. Neurol. 2013;74:474–477. doi: 10.1002/ana.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannon J.R., Tapias V., Na H.M., Honick A.S., Drolet R.E., Greenamyre J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009;34:279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermeer L.M., Higgins C.A., Roman D.L., Doon J.A. Real-time monitoring of tyrosine hydroxylase activity using a plate reader assay. Anal. Biochem. 2013;432:11–15. doi: 10.1016/j.ab.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green A.L., Haughton T.M. A colorimetric method for estimation of monoamine oxidase. Biochem. J. 1961;78:172–175. doi: 10.1042/bj0780172. PMID: 13708157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turski W., Turska E., Grass-Bellard M. Modification of the spectrophotometric method of the determination of monoamine oxidase. Enzyme. 1973;14:211–215. [Google Scholar]
- 28.Gacche R.N., Shaikh R.U., Chapole S.M., Jadhav A.D., Jadhav S.G. Kinetics of inhibition of monoamine oxidase using Cymbopogon martini (Roxb.) Wats: a potential antidepressant herbal ingredient with antioxidant activity. Indian J. Clin. Biochem. 2011;26:303–308. doi: 10.1007/s12291-011-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Annu. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 30.Rahman I., Kode A., Biswas S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2007;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 31.Aebi H. Catalase. Meth. Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 32.McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 33.Saravanan K.S., Sindhu K.M., Senthilkumar K.S., Mohanakumar K.P. l-Deprenyl protects against rotenone-induced, oxidative stress-mediated dopaminergic neurodegeneration in rats. Neurochem. Int. 2006;49:28–40. doi: 10.1016/j.neuint.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Maniyath S.P., Solaiappan N., Rathinasamy M. Neurobehavioural changes in Hemiparkinsonian Rat Model induced by roenone. J. Clin. Diagn. Res. 2017;11:AF01–AF05. doi: 10.7860/JCDR/2017/24955.9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colette D.S., Tiffany L., Shanzhi W. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam M., Schmidt W.J. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav. Brain Res. 2002;136:317–324. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 37.Datla K.P., Christidou M., Widmer W.W., Rooprai H.K., Dexter D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport. 2001;12:3871–3875. doi: 10.1097/00001756-200112040-00053. [DOI] [PubMed] [Google Scholar]
- 38.Hauser D.N., Hastings T.G. Mitochondrial oxidative stress in Parkinson’s disease and monogenic Parkinsonism. Neurobiol. Disord. 2013;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thakur P., Nehru B. Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by Sodium salicylate in a chronic rotenone model of Parkinson’s disease. Neuroscience. 2013;231:420–431. doi: 10.1016/j.neuroscience.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Saura J., Luque J.M., Da Cesura A.M., Prada M., Chan-Palay V., Huber G., Loffler J., Richards J.G. Increased monoamine oxidase B activity in plaque associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. 1994;62:15–30. doi: 10.1016/0306-4522(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 41.Thomas T. Monoamine oxidase-B inhibitors in the treatment of Alzheimer’s disease. Neurosci. Aging. 2000;21:343–348. doi: 10.1016/s0197-4580(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 42.Nwanna E.E., Oyeleye S.I., Ogunsuyi O.B., Oboh G., Boligon A.A., Athayde M.L. In vitro neuroprotective properties of some commonly consumed green leafy vegetables in Southern Nigeria. Nutr. Food Sci. J. 2016;2:19–24. [Google Scholar]
- 43.Manjunath M. Effect of Withania somnifera supplementation on rotenone-induced oxidative damage in cerebellum and striatum of the male mice brain. Cent. Nerv. Syst. Agents Med. Chem. 2013;13:43–56. doi: 10.2174/1871524911313010007. [DOI] [PubMed] [Google Scholar]
- 44.Dardiotis E., Siokas V., Sokratous M., Tsouris Z., Michalopoulou A., Andravizou A., Dastamani M., Ralli S., Vinceti M., Tsatsakis A., Hadjigeorgiou G.M. Genetic polymorphisms in amyotrophic lateral sclerosis: evidence for implication in detoxification pathways of environmental toxicants. Environ. Int. 2018;116:122–135. doi: 10.1016/j.envint.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez B., Ferrer I., Gil F., Hilfiker S. Biomonitorization of iron accumulation in the substantia nigra from Lewy body disease patients. Toxicol. Rep. 2017;4:188–193. doi: 10.1016/j.toxrep.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poirier J., Dea D., Baccichet A., Thiffault C. Superoxide dismutase expression in Parkinson’s disease. Ann. N. Y. Acad. Sci. 1994;738:116–120. doi: 10.1111/j.1749-6632.1994.tb21796.x. [DOI] [PubMed] [Google Scholar]
- 47.Sanders L.H., Greenamyre J.T. Oxidative damage to macromolecules in human Parkinson’s disease and the rotenone model. Free Radic. Biol. Med. 2013;62:111–120. doi: 10.1016/j.freeradbiomed.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta R.C., Milatovic D. Insecticides. In: Gupta R.C., editor. Biomarkers of Toxicology. Academic Press; Pasadena, California: 2014. pp. 389–407. [Google Scholar]
- 49.Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurements, and biosynthesis. Mol. Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mytilineou C., Walker R.H., Jnobaptiste R., Olanow C.W. Levodopa is toxic to dopamine neurons in an in vitro but not an in vivo model of oxidative stress. J. Pharmacol. Exp. Ther. 2002;304:792–800. doi: 10.1124/jpet.102.042267. [DOI] [PubMed] [Google Scholar]
- 51.Sian J., Dexter D.T., Lees A.J., Daniel S., Agid Y., Javoy-Agid F., Jenner P., Marsden C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 52.Cohen G. Monoamine oxidase and oxidative stress at dopaminergic synapses. J. Neural Transm. 1990;32:229–238. doi: 10.1007/978-3-7091-9113-2_33. [DOI] [PubMed] [Google Scholar]
- 53.Graham D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- 54.Sarubbo F., Ramis M.R., Kienger C., Aparicio S., Esteban S., Miralles A., Moranta D. Chronic silymarin, quercetin, naringenin treatments increase monoamine synthesis and hippocampal sirt 1 levels improving cognition in aged rats. J. Neuroimmune Pharmacol. 2018;13:24–38. doi: 10.1007/s11481-017-9759-0. [DOI] [PubMed] [Google Scholar]
- 55.Bandaruk Y., Mukai R., Terao J. Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-A in human neuroblastoma SH-SY5Y cells. Toxicol. Rep. 2014;1:639–649. doi: 10.1016/j.toxrep.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karuppagounder S.S., Madathil S.K., Pandey M., Haobam R., Rajamma U., Mohanakumar K.P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 57.Bao D., Wang J., Pang X., Liu H. Protective effect quercetin against oxidative stress-induced cytotoxicity in Rat Pheochromocytoma (PC-12) cells. Molecules. 2017;22:1122. doi: 10.3390/molecules22071122. doi:10.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amic D., Davidovic-Amic D., Beslo D., Trinajstic N. Structure-radical scavenging activity relationship of flavonoids. Croat. Chem. Acta. 2003;76:55–61. [Google Scholar]
- 59.Yilmaz B.S., Altum M.L., Orhan I.E., Ergene B., Citoglu G.S. Enzyme inhibitory and antioxidant activities of Viburnum tinus L. Relevant to its neuroprotective potential. Food Chem. 2013;141:582–588. doi: 10.1016/j.foodchem.2013.03.020. [DOI] [PubMed] [Google Scholar]