Abstract
The survival of patients diagnosed with metastatic renal cell carcinoma (RCC) is still limited and the current targeted therapies are only partially effective. Herein, we investigated the clinical value and functions of adiponectin receptors (AdipoR1 and AdipoR2) in metastatic renal cell carcinoma (RCC) patients treated with tyrosine kinase inhibitors (TKIs). A total of 127 mRCC patients treated with first-line TKIs between 2008 and 2017 at a single institution were collected. AdipoR1 and AdipoR2 expression was assessed by immunohistochemistry. AdipoR1 was positively expressed in 87.4% (111/127) of tumors, especially, highly expressed in pulmonary and bone lesions. Patients with low-AdipoR1 expression in primary tumor tissues were more likely to suffer from progressive disease during TKIs treatment (40.0% vs. 11.1%, P = 0 .02), and with decreased progression-free survival (PFS: 19.5 vs. 37.8 mo, P = .001) and overall survival (OS: 62.3 vs 101.1 mo, P = .004) compared to those with high-AdipoR1 expression. Moreover, low-AdipoR1 expression in metastatic tissues was also associated with poor PFS (P = .006) and OS (P = .037). In contrast, AdipoR2 expression was neither associated with sunitinib response nor patient survival. In vitro, we found that adiponectin inhibited migration, invasion and sensitized RCC cells to sunitinib though interacting with AdipoR1, but not AdipoR2. Furthermore, we demonstrated that adiponentin-AdipoR1 axis inhibits tumor cells migration and invasion by blocking the GSK3β/β-Catenin pathway and enhances sunitinib sensitivity via abrogating PI3K/AKT/NF-κB signaling. Our results suggest that adiponentin-AdipoR1 axis may serve as a predictor of TKIs response and could be a potential therapeutic target in the future treatment for metastatic RCC.
Abbreviations: RCC, renal cell carcinoma; mRCC, metastatic renal cell carcinoma; TKIs, tyrosine kinase inhibitors; APN, Adiponectin; PD, progressive disease; PFS, progressive-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; EMT, epithelial–mesenchymal transition
Introduction
Renal cell carcinoma (RCC) constitutes approximately 2–3% of all adult malignancies and its incidence has increased in recent years [1]. Up to 20% of patients have metastatic disease at presentation. Another 10–20% of patients develop metastasis or local recurrence despite curative surgery [2]. Patients with metastatic disease often have poor outcomes. Tyrosine kinase inhibitors (TKIs) are currently a first-line treatment choice for metastatic RCC (mRCC) and show significantly improved overall survival. Unfortunately, resistance to TKIs emerges within 12 months in almost all patients [3], [4]. Moreover, long-term exposure is associated with severe adverse events, dose reductions or interruptions, which result in decreased quality of life. Therefore, the need to identify novel molecular targets and more effective therapeutics for the management of mRCC remains.
Adiponectin (APN) is the most well-known adipocyte-secreted cytokine with anti-diabetic, anti-inflammatory and anti-angiogenic properties [5]. Serum APN level has been confirmed to be inversely associated with tumorigenesis of breast, endometrial, colorectal, gastric and prostate gland tissues [6]. Studies further demonstrated an anti-tumor activity of APN in numerous solid and hematological tumors. To date, studies investigating the association between APN and RCC have mainly focused on localized disease and the results are limited and controversial. In 2008, serum level of APN was reported to be inversely correlated with tumor size and metastasis [7]. However, conflicting results concerning the relationship of serum APN with RCC risk and prognosis have been subsequently reported [8], [9]. Moreover, further investigations attempting to elucidate the potential molecular mechanisms of APN in RCC yielded inconsistent results [10], [11].
APN exerts its action through interacting with AdipoR1 and AdipoR2. AdipoR1 and AdipoR2 activate different signaling pathways and are heterogeneously expressed in varies cancer types [5], [12]. This heterogeneity is closely correlated with the biologic effects of APN in malignancies. However, the functional significance of AdipoR1 and AdipoR2 in RCC has not been defined. In particular, the potential role of APN receptors in mRCC is still unknown. Therefore, the aim of this study is to investigate the biological and clinical involvement of APN receptors in mRCC and to explore its underlying molecular mechanisms.
Materials and Methods
Clinical Samples
One hundred and twenty primary RCC specimens and 30 metastatic specimens were obtained from patients who underwent nephrectomy or metastasectomy between 2008 and 2016 at West China Hospital. All clinical samples were acquired with written informed consents under permission from West China Hospital Ethics Committee (Supplementary Materials and Methods).
Cell Culture
All RCC cell lines used were purchased from ATCC. 293FT cells were a kind gift from Dr. Aiping Tong. 769-P and OS-RC-2 were cultured in RPMI-1640 (Gibco, NY, USA) with 10% FBS (Gibco) and 1% penicillin–streptomycin. A498, ACHN and 293FT were cultured in DMEM (Gibco) with 10% FBS. Caki-1 were cultured in McCOY's 5A (Gibco) with 10% FBS. All cells were maintained in 37 °C in humidified conditions with 5% CO2.
Reagents and Antibodies
Human recombinant full-length adiponectin was procured from AIS (41013, HK, CN). The antibodies used in this study were anti-adiponectin receptor 1 (MABS1007, Millipore, MA, USA), anti-adiponectin receptor 2 (MABS1166, Millipore), anti-β-actin (A2228, Sigma, MO, USA), anti-caspase 3 (9664, CST, MA, USA), anti- Cleaved PARP (5625, CST), anti-GSK3β (12456, CST), anti-phosphor-GSK3β(5558, CST), β-catenin (8480, CST), E-cadherin (ab40772, Abcom, MA, USA), N-cadherin (13116, CST), anti-AKT (9272, CST), anti-phosphor-AKT (4060, CST), anti-IκB (14814, CST), anti-phosphor-IκB (2859, CST), Alexa Fluor 488 (A-11008,Thermo Scientific, NY, USA).
Immunohistochemistry (IHC)
Sections of RCC specimens (including primary and metastatic tumor tissues) were dewaxed, rehydrated, and blocked for endogenous peroxidase activity. After antigen retrieval, sections were incubated with antibodies against AdiponR1 (1:100) and anti-AdiponR2 (1:100) overnight at 4 °C. The sections were subsequently incubated with corresponding secondary antibodies at room temperature for 1 hour, followed by peroxidase-streptavidin-biotin complex and diaminobenzidine to visualize the proteins. The immunostaining of each tissue was scored using the positive intensity (negative, 0; weak, 1; moderate, 2; and strong, 3) of tumor staining. Those with negligible, weak, or moderate staining (0–2) were classified as low staining.
Western Blot Analysis
Western blot was carried out as described previously [13]. Immunodetection was performed using ECL reagent (GE Healthcare Life Sciences, NY, USA) and images were captured with ImageQuant™ LAS 4000 (GE Healthcare).
Real-Time Quantitative RT-PCR
Total RNAs were extracted using TRIzol Reagent (ThermoFisher Scientific, MA, USA). Equal amounts of total RNAs were converted into cDNAs using RevertAid First Strand cDNA Synthesis Kit (ThermoFisher Scientific). Quantitative PCR was performed on an ABI Prism® 7500 Sequence Detection System (Applied Biosystems, MA, USA). PCR primer sequences used in this study are listed in Supplementary Table S3.
Migration and Invasion Assay
Migration and invasion assays were performed in transwell system (8-μm pore; Millipore, MA, USA). The polyethylene filters pre-coated with Matrigel Matrix (Corning, NY, USA) were used for invasion assay, and uncoated filters were used for migration assay. Cells (3 × 105) in 300 ml of medium (containing 0.1% FBS) with or without 10 mg/ml APN for 72 h were seeded in the upper chamber and 600 ml of medium with 10% FBS was added to the lower chamber as chemoattractant. After 20 hours, cell that had invaded to the other side of the membrane were fixed in 4% paraformaldehyde and stained with 0.5% crystal. The adherent cells on the membrane were counted under microscopy at ×100 magnification (five fields per insert).
Cell Growth and Cell Viability Assay
Cells seeded in 6-well plates (2.5 × 105 cells/well) were treated with Sunitinib (2 μM), APN (10 μg/ml) or a combination of both. Total cell numbers were counted after 2 days. Cell survival rate (%) = (treatment group cell number/control group cell number) × 100%. Cell viability was determined using MTT assays (Supplementary Materials and Methods).
Colony Formation Assay
Cells were plated at an equal density (1,000 cells/dish) in 60 mm dishes or (500 cells/dish) in 6-well plates for 14 days; the media was changed every 7 days. Colonies were rinsed with PBS, fixed with 4% formaldehyde 0.5%, stained with crystal violet/ and the numbers of colonies were counted.
Plasmid Construction and Lentivirus Transduction
AdipoR1 stable knockdown uses lentiviral short hairpin RNA (pLKO.1 vectors). AdipoR1 and AdipoR2 overexpression uses lentiviral vectors (pCDH-CMV-MCS-EF1-hygromycin). For AdipoR1 stable knockdown, three premade lentiviral AdipoR1 shRNA were constructed and a negative control construct created in the same vector system. For AdipoR1/R2 stable overexpression, human AdipoR1/R2 primers were flanked with restriction sites and the amplified full-length coding sequences were then cloned into lentiviral vectors. ShRNA sequences and human cDNA libraries respectively using primers described in Table S7. One day before transfection, 1.5 × 106 293 T cells were plated in 100-mm dishes. Cells were cotransfected with shRNA constructs or overexpression constructs together with helper plasmids using FuGENE HD (E2311, Promega, WI, USA). Viral stocks were harvested at 48 h and 72 h post-transfection from the culture medium and filtered to remove nonadherent 293FT cells. Cells (1 × 105) were plated on 6-well plate 24 h before transduction then infected with 1.5 ml virus-containing medium with 8 μg/ml polybrene (TR-1003, Sigma). After 12 h, the infectious medium was replaced, and the infection was repeated. Two days after infection, 0.5–2 μg/ml puromycin (P8833, Sigma) or 200–400 μg/ml hygromycin (V900372, Sigma) were used for selection of stable clones. The stably transfected cell lines were confirmed by Western blot and qPCR.
Statistics
SPSS 22.0, GraphPad Prism 6 were applied for data analysis. Continuous parameters were calculated as mean ± SD and were analyzed by Mann–Whitney U test. Categorical parameters were calculated as proportions and were analyzed by chi-square test. Kappa test was used to analyze the consistency of AdipoR1 expression between primary and marched metastatic lesions. Kaplan–Meier method and Cox proportional hazard model were conducted to analyze survival outcomes. Harrell's C-index was used to further assess the discrimination of models calculated by using R software. Statistical significance was set at P < .05.
Results
AdipoR1 and AdipoR2 are disparately expressed in mRCC
A total of 127 mRCC patients treated with sunitinib as first-line therapy between 2008 and 2017 at West China Hospital were included in our study. Baseline clinical and pathological characteristics are shown in Supplementary Table S1.
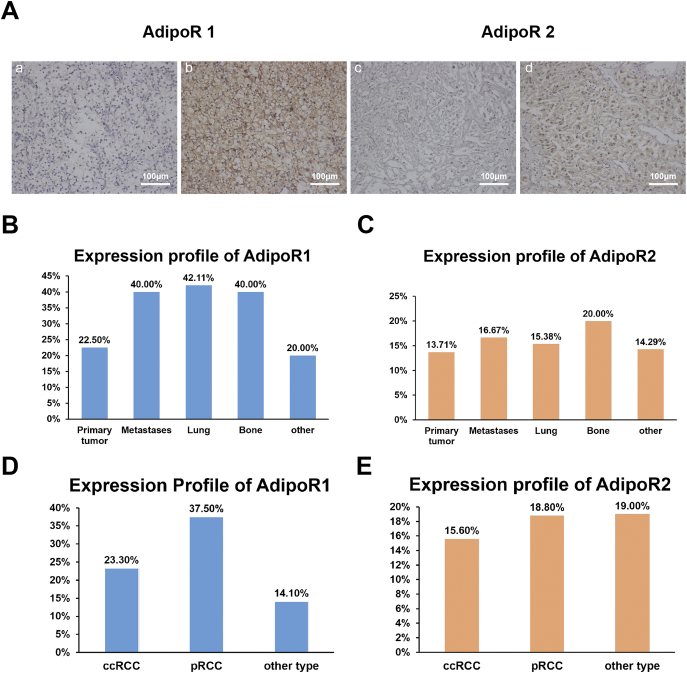
We firstly explored AdipoR1/AdipoR2 expression profiles in primary and metastatic tissues. AdipoR1 and AdipoR2 were differentially expressed in tumor tissues (Figure 1A). AdipoR1 was positively expressed in 87.4% (111/127) of tumors, with 23.6% (30/127) displaying strong positivity in primary and/or metastatic lesions. In contrast, AdipoR2 was expressed in only 16.5% (21/127) of tumors. Of note, AdipoR1 had a heterogeneous staining pattern in different lesions. Overall, metastases had higher AdipoR1 expression compared with primary tumor lesions, (40.0% vs 22.5%, P = .046), particularly in lung and bone lesions (42.1% and 40.0%) (Figure 1B). Moreover, high-AdipoR1 expression was found in 37.5% (6/16) cases of pRCC, 23.3% (21/90) cases of ccRCC and 14.3% (3/21) cases of other pathological types (Figure 1D). However, the expression of AdipoR2 was not associated with tumor sites and pathological subtypes (Figure 1, C and E).
Figure 1.
Expression of AdipoR1 and AdipoR2 in mRCC specimens.
(A) Representative images of immunohistochemical staining of AdipoR1 and AdipoR2 in mRCC specimens. (a) AdipoR1 with low staining. (b) AdipoR1 with high staining. (c) AdipoR2 with negative staining. (d) AdipoR2 with positive staining. Scale bars, 100 μm.
(B) Expression profiles of AdipoR1 in different metastatic sites.
(C) Expression profiles of AdipoR2 in different metastatic sites.
(D) Expression profiles of AdipoR1 in different pathological subtypes.
(E) Expression profiles of AdipoR2 in different pathological subtypes.
AdipoR1 Expression is Associated with Sunitinib Response and Survival in mRCC
Next, we determine the association of AdipoR1 and AdipoR2 expression with clinical characteristics and survival. Patients with high-AdipoR1 expression tended to be younger (P = .016) and had favorable IMDC scores (P = .065) (Supplementary Table S2). AdipoR2 expression was not related to any clinical/ pathological factors (data not shown).
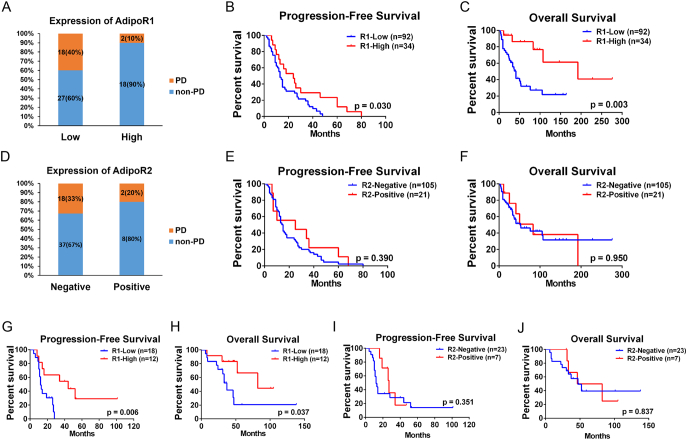
The median follow-up time was 64.1 months (range 20–124 months). At the time of cut-off point, the occurrence rate of disease progression and all cause of mortality were 79.5% (101/127) and 46.4% (59/127), respectively. As shown in Figure 2A, patients with low-AdipoR1 expression were more likely to suffer from progressive disease (PD) during TKIs therapy than those with high-AdipoR1 expression (PD: 40.0% vs. 11.1%, P = .02). Moreover, Kaplan–Meier analysis showed AdipoR1 expression was significantly associated with progressive-free survival (PFS) and overall survival (OS). In comparison to those with low-AdipoR1 expression, patients with high-AdipoR1 expression had both prolonged PFS (19.5 vs. 37.8 mo, P = .001) and OS (62.3 vs 101.1 mo, P = .004) (Figure 2B, C). Cox regression analysis further revealed that high-AdipoR1 expression was an independent prognosticator for mRCC patients with TKIs treatment (PFS: HR = 0.547, 95% CI: 0.306–0.978, P = .042 and OS: HR = 0.310, 95% CI: 0.121–0.789, P = .014) (Table 1). Furthermore, high-AdipoR1 expression in metastatic tissues was also associated with favorable PFS and OS (PFS: 15.9 vs. 45.2 mo, P = .006 and OS: 52.7 vs. 76.7 mo, P = .037) Figure 2, G and H). In contrast, AdipoR2 expression was associated with neither sunitinib response nor patient survival Figure 2, D,E,–F, I and J).
Figure 2.
The relationship between AdipoR1/R2 expression in tumor tissues and sunitinib response and patient survival in mRCC.
(A) The expression levels of AdipoR1 in mRCC patients with PD (n = 20) or non-PD (n = 45) during sunitinib therapy (P = .020).
(B) Kaplan–Meier curves of the progression-free survival of mRCC patients (n = 127), with regard to the expression levels of AdipoR1.
(C) Kaplan–Meier curves of the overall survival of mRCC patients, with regard to the expression levels of AdipoR1.
(D) The expression levels of AdipoR2 (n = 127) in mRCC patients with PD or non-PD during sunitinib therapy (P = .711).
(E) Kaplan–Meier curves of the progression-free survival of mRCC patients, with regard to the expression levels of AdipoR2.
(F) Kaplan–Meier curves of the overall survival of mRCC patients, with regard to the expression levels of AdipoR2.
(G) Kaplan–Meier curves of the progression-free survival of mRCC patients (n = 30), with regard to the expression levels of AdipoR1 in metastatic lesions.
(H) Kaplan–Meier curves of the overall survival of mRCC patients, with regard to the expression levels of AdipoR1 in metastatic lesions.
(I) Kaplan–Meier curves of the progression-free survival of mRCC patients (n = 30), with regard to the expression levels of AdipoR2 in metastatic lesions.
(J) Kaplan–Meier curves of the overall survival of mRCC patients, with regard to the expression levels of AdipoR2 in metastatic lesions.
Table 1.
Cox regression analysis for the identification of the predictors for PFS and OS after TKIs therapy.
| Variable |
PFS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR(95%CI) | P value | HR(95%CI) | P value | HR(95%CI) | P value | HR(95%CI) | P value | |
| Age (<50 vs. ≥50) | 1.874 (1.197–2.993) | .006 | 1.356 (0.808–2.276) | .250 | 1.511 (0.825–2.768) | .181 | -- | -- |
| ECOG score (<2 vs. ≥2) | 1.985 (1.229–3.206) | .005 | 1.639 (0.946–2.840) | .078 | 2.437 (1.323–4.487) | .004 | 2.044 (0.981–4.259) | .056 |
| T stage (<3 vs. ≥3) | 1.142 (0.709–1.839) | .584 | -- | -- | 2.209 (1.230–3.965) | .008 | 2.275 (1.012–5.113) | .047 |
| Primary Tumor Size (<10 cm vs. ≥10 cm) | 0.731 (0.345–1.549) | .414 | -- | -- | 0.988 (0.847–1.152) | .877 | -- | -- |
| Pathology type | 1.233 (0.732–2.075) | .431 | -- | -- | 1.194 (0.609–2.339) | .606 | -- | -- |
| ISUP grade (<3 vs. ≥3) | 0.618 (0.372–1.007) | .053 | -- | -- | 0.662 (0.350–1.254) | .206 | -- | -- |
| Necrosis (Without vs. with) | 1.115 (0.655–1.900) | .688 | -- | -- | 1.083 (0.548–2.138) | .819 | -- | -- |
| Sarcomatoid (Without vs. With) | 1.198 (0.437–3.286) | .726 | -- | -- | 0.840 (0.204–3.461) | .809 | -- | -- |
| Metastatic sites (Lung vs. Other) | 0.685 (0.449–1.046) | .080 | -- | -- | 1.069 (0.628–1.820) | .807 | -- | -- |
| Number of metastases (<2 vs. ≥2) | 2.548 (1.286–3.217) | .015 | 1.421 (0.972–3.247) | .079 | 1.423 (1.023–3.574) | .079 | -- | -- |
| IMDC score⁎ | 1.763 (1.094–2.814) | .020 | 0.956 (0.513–1.781) | .887 | 3.095 (1.724–5.557) | <.001 | 2.660 (0.737–9.597) | .135 |
| MSKCC score ⁎ | 1.357 (0.816–2.258) | .239 | -- | -- | 2.073 (1.114–3.856) | .021 | 0.478 (0.118–5.113) | .303 |
| Nephrectomy | 0.426 (0.212–0.854) | .016 | 0.241 (0.111–0.524) | <.001 | 0.208 (0.095–0.454) | <.001 | 0.131 (0.046–0.374) | <.001 |
| Experience of Metastatectomy | 0.595 (0.398–0.889) | .011 | 0.603 (0.378–0.963) | .034 | 0.508 (0.294–0.877) | .015 | 0.551 (0.271–1.118) | .099 |
| Timing of metastasis# | 1.463 (0.983–2.179) | .061 | -- | -- | 1.840 (1.076–3.147) | .026 | 1.550 (0.766–3.136) | .223 |
| AdipoR1 (Low vs. High) | 0.414 (0.246–0.697) | <.001 | 0.547 (0.306–0.978) | .042 | 0.314 (0.246–0.697) | .002 | 0.310 (0.121–0.789) | .014 |
| AdipoR2 (Negative vs. Positive) | 1.231 (0.738–2.054) | .426 | -- | -- | 1.371 (0.738–2.548) | .318 | -- | -- |
ISUP, International Society of Urological Pathology; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; MSKCC, Memorial Sloan Kettering Cancer Center; PFS, progressive-free survival; OS, overall survival; TKIs, tyrosine kinase inhibitors.
Favorable vs. intermediate and poor risk.
Synchronous vs. metachronous.
APN Inhibits Migration, Invasion and Sensitizes RCC Cells to Treatment with Sunitinib
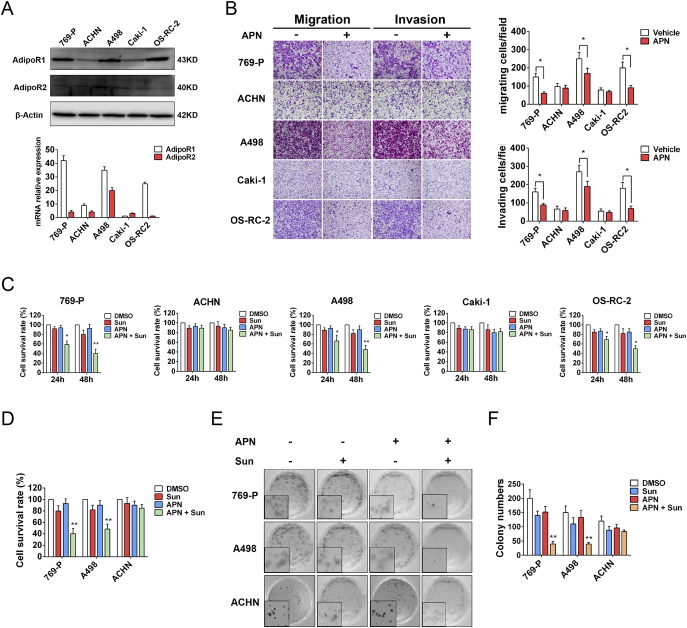
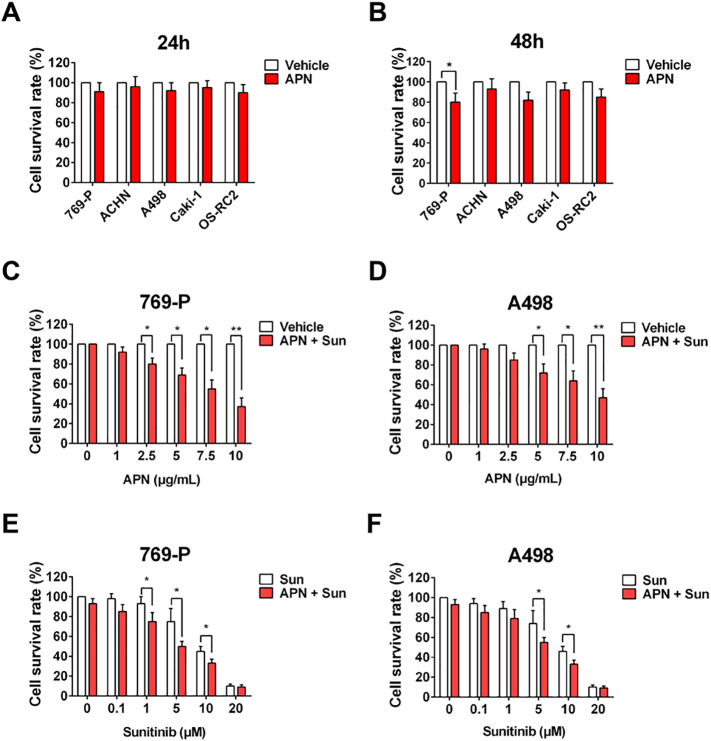
To investigate the mechanism of the relationship between high-AdipoR1 expression and favorable sunitinib response and survival, we firstly evaluated the expression of AdipoR1 and AdipoR2 in several RCC cell lines (Figure 3A). AdipoR1 was highly expressed in 769-P, A498, OS-RC-2, while AdipoR2 was lowly expressed in all cell lines. We next examined the effect of APN on RCC cell proliferation, migration and invasion. Of note, human recombinant full-length APN impeded migration and invasion of cells with high-AdipoR1 expression (769-P, A498, OS-RC-2), but not those with low-AdipoR1 expression (ACHN and Caki-1) (Figure 3B). Administration of APN had no effect on RCC cell proliferation (Supplementary Figure S1, A and B). Moreover, the addition of APN with sunitinib could significantly decrease proliferation and clonogenic potential of cells with high-AdipoR1 expression (Figure 3, C–F). APN also enhanced the anti-proliferation effect of sunitinib in a concentration- and time-dependent manner (Supplementary Figure S1, C–F). These results indicated that APN could inhibit migration and invasion, and sensitize RCC cells to sunitinib.
Figure 3.
APN inhibits migration, invasion and sensitizes RCC cells to sunitinib.
(A) AdipoR1 and AdipoR2 expression in indicated cell lines was evaluated by western blot (up) and qRT-PCR (down).
(B) Invasion and migration of 769-P, ACHN, A498, Caki-1, OS-RC-2 cells were evaluated by Transwell assay after treatment with or without 10 μg/ml recombinant full-length APN for 72 h. Graphs show the relative number of migratory and invasive cells (n = 4).
(C) Proliferation of indicated cells was evaluated by MTT assay after treatment with 2 μM Sunitinib and/or 10 μg/ml APN for 24 h or 48 h (n = 3).
(D) Proliferation of 769-P, A498 and ACHN cells was evaluated by cell growth assay after treatment with Sunitinib (2 μM) and/or APN (10 μg/ml) for 48 h (n = 3).
(E) Clonogenic ability of 769-P, A498 and ACHN cells was evaluated after treatment with Sunitinib (0.5 μM) and/or APN (10 μg/ml).
(F) Graphs show relative colony numbers (n = 2).
Supplementary Figure S1.
Interaction of AdipoR1 Confers Anti-Tumor Effect of APN in RCC Cells
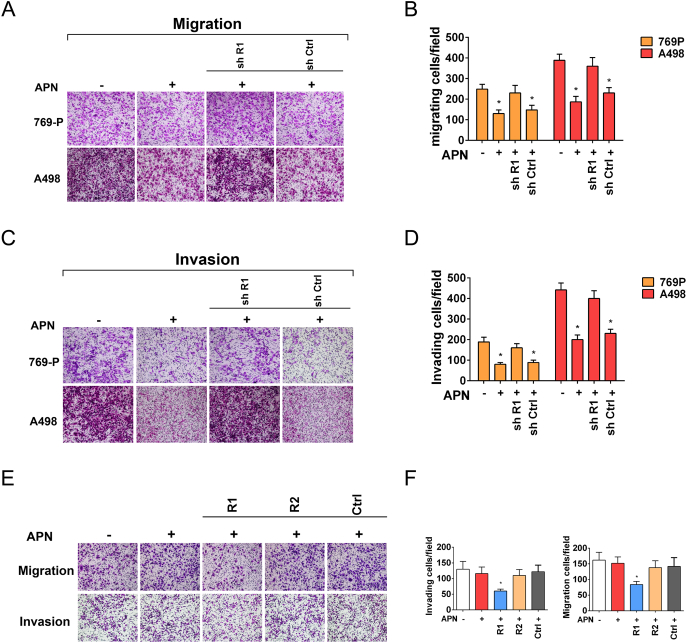
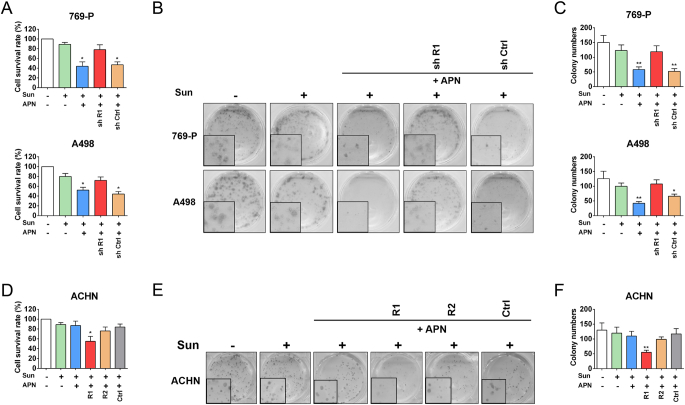
To define whether AdipoR1 or AdipoR2 was responsible for APN-induced anti-tumor effects on RCC cells, we specifically knockdown AdipoR1 in 769-P and A498 cells. Silencing of AdipoR1 impeded APN-induced suppression of migration and invasion (Figure 4, A–D). Depletion of AdipoR1 also desensitized RCC cells to sunitinib confirmed by cell growth and colony formation assay (Figure 5, A–C). Next, we generated ACHN cells with AdipoR1 or AdipoR2 stably overexpressed. AdipoR1 overexpression restored sensitivity of ACHN to APN. APN administration could both hinder invasiveness and sensitize cells to sunitinib (Figure 4, E and F and Figure 5, D–F). However, AdipoR2 overexpression did not augment the anti-tumor properties of APN on ACHN.
Figure 4.
Interaction of AdipoR1 confers migration and invasion inhibitory effect of APN in RCC cells.
(A) Migration of AdipoR1-knockdown and control cells was evaluated by Transwell assay after treatment with APN (10 μg/m) for 72 h.
(B) Graphs show the relative number of migratory cells (n = 4).
(C) Invasion of AdipoR1-knockdown and control cells were evaluated by Transwell assay after treatment with APN (10 μg/ml) for 72 h.
(D) Graphs show the relative number of invasive cells (n = 4).
(E) Invasion and migration of AdipoR1-overexpression, AdipoR2-overexpression and control ACHN cells were evaluated by Transwell assay after treatment with or without 10 μg/ml APN for 72 h.
(F) Graphs show the relative number of migratory and invasive cells (n = 4).
Results are presented as mean ± SD. *P < .05, **P < .001 compared with untreated cells.
Figure 5.
Interaction of AdipoR1 confers sunitinib sensitization effect of APN in RCC cells.
(A) Proliferation of AdipoR1-knockdown and control cells was evaluated by cell growth assay after treatment with Sunitinib (2 μM) and/or APN (10 μg/ml) for 48 h (n = 3).
(B) Clonogenic ability of AdipoR1-knockdown and control cells was evaluated after treatment with Sunitinib (0.5 μM) and/or APN (10 μg/ml).
(C) Graphs show relative colony numbers (n = 2).
(D) Proliferation of AdipoR1-overexpression, AdipoR2-overexpression and control ACHN cells was evaluated by cell growth assay after treatment with Sunitinib (2 μM) and/or APN (10 μg/ml) for 48 h (n = 3).
(E) Clonogenic ability of AdipoR1-overexpression, AdipoR2-overexpression and control ACHN cells was evaluated after treatment with Sunitinib (0.5 μM) and/or APN (10 μg/ml).
(F) Graphs show relative colony numbers (n = 2).
Results are presented as mean ± SD. *P < .05, **P < .001.
Interaction of AdipoR1 with APN impeded migration and invasion through blocking phosphorylation of GSK-3β
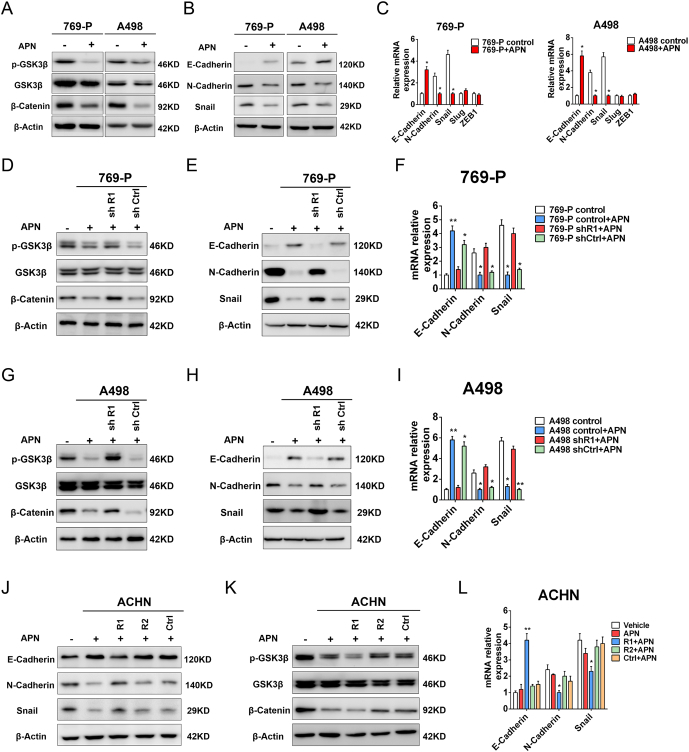
Previous study showed that APN could inhibit phosphorylation of GSK-3β in breast cancer [14]. Therefore, we evaluated whether APN had an effect on GSK-3β signaling pathways in RCC cells. As shown in Figure 6A, APN treatment inhibited phosphorylation of GSK-3β and decreased the accumulation of β-catenin. APN administration also inhibited expression of EMT-related proteins (Figure 6, B and C).
Figure 6.
Interaction of AdipoR1 with APN impeded migration and invasion though blockade phosphorylation of GSK-3β
(A-B) Western blot analysis of indicated proteins of 769-P and A498 cells treated with or without APN (10 μg/ml) for 48 h.
(C) qRT-PCR analysis of indicated genes of 769-P and A498 cells treated with or without APN (10 μg/ml) for 48 h (n = 3).
(D-E) Western blot analysis of indicated proteins of AdipoR1-knockdown and control 769-P cells treated with or without APN (10 μg/ml) for 48 h.
(F) qRT-PCR analysis of indicated genes of AdipoR1-knockdown and control 769-P cells treated with or without APN (10 μg/ml) for 48 h (n = 3).
(G-H) Western blot analysis of indicated proteins of AdipoR1-knockdown and control A498 cells treated with or without APN (10 μg/ml) for 48 h.
(I) qRT-PCR analysis of indicated genes of AdipoR1-knockdown and control A498 cells treated with or without APN (10 μg/ml) for 48 h (n = 3).
(J-K) Western blot analysis of indicated proteins of AdipoR1-overexpression, AdipoR2-overexpression and control ACHN cells treated with or without APN (10 μg/ml) for 48 h.
(L) qRT-PCR analysis of indicated genes of AdipoR1-overexpression, AdipoR2-overexpression and control ACHN cells treated with or without APN (10 μg/ml) for 48 h (n = 3).
Results are presented as mean ± SD. *P < .05 compared with untreated cells.
Subsequently, we investigated whether interaction with AdipoR1 mediated inactivation of the GSK-3β/β-catenin pathway. We found that depletion of AdipoR1 significantly abrogated APN-mediated downregulation of p-GSK-3β and accumulation of β-catenin in 769-P and A498 cells (Figure 6, D and G). Silencing of AdipoR1 also restored the expression of EMT-related proteins (Figure 6, E and F, H and I). Correlating with these results, AdipoR1 overexpression in ACHN cell suppressed phosphorylation of GSK-3β, decreased the accumulation of β-catenin and restrained expression of EMT-related proteins (Figure 6, J–L). In contrast, overexpression AdipoR2 had no effect on the GSK-3β/β-catenin pathway after treatment with APN.
Interaction of AdipoR1 with APN Sensitizes RCC Cell to Sunitinib through Inhibition PI3K/AKT/NF-κB Pathway
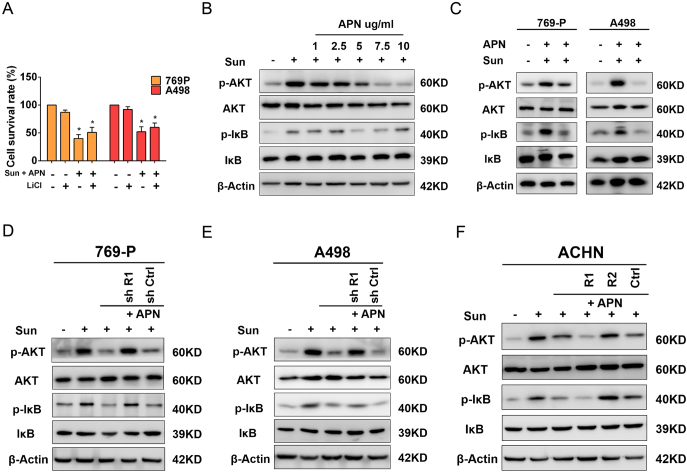
We also explored whether inactivation phosphorylation of GSK-3β could sensitize RCC cells to sunitinib. Unfortunately, treatment with lithium chloride (LiCl), a GSK-3β inhibitor, was unable to affect proliferation of RCC cells when combined with APN and sunitinib treatment (Figure 7A). This result suggested that GSK-3β was not the key mechanism for APN-induced sunitinib sensitization. We, found that low-dose sunitinib treatment elevated phosphorylation of AKT in 769-P and A498 cells and increased phosphorylated-I-κB protein. The addition of APN substantially abrogated sunitinib-induced phosphorylation of AKT and I-κB (Figure 7, B and C). Furthermore, we found APN inhibited AKT and I-κB activation in a dose-dependent manner (Figure 7B). These data indicated that blocking the PI3K/AKT/NF-κB pathway could be involved in APN-induced sunitinib sensitization in RCC cells.
Figure 7.
Interaction of AdipoR1 with APN sensitizes RCC cell to sunitinib through inhibition of the PI3K/AKT/NF-κB pathway
(A) Proliferation of 769-P and A498 cells was evaluated by MTT assay after treatment with Sunitinib (2 μM) and APN (10 μg/ml) with or without 20 mM LiCl for 48 h (n = 3).
(B) Western blot analysis of indicated proteins of 769-P cells treated with Sunitinib (2 μM) and increasing concentrations of APN (0-10 μg/ml) for 48 h.
(C) Western blot analysis of indicated proteins of 769-P and A498 cells treated with Sunitinib (2 μM) with or without APN (10 μg/ml) for 48 h.
(D) Western blot analysis of indicated proteins of AdipoR1-knockdown and control 769 cells treated with Sunitinib (2 μM) with or without APN (10 μg/ml) for 48 h.
(E) Western blot analysis of indicated proteins of AdipoR1-knockdown and control A498 cells treated with Sunitinib (2 μM) with or without APN (10 μg/ml) for 48 h.
(F) Western blot analysis of indicated proteins of AdipoR1-overexpression, AdipoR2-overexpression and control ACHN cells treated with Sunitinib (2 μM) with or without APN (10 μg/ml) for 48 h.
Results are presented as mean ± SD. *P < .05 compared with untreated cells.
We further elucidated whether activation of AdipoR1 was able to sensitize RCC cells to sunitinib. We found that AdipoR1 depletion abrogated APN-induced downregulation of phosphorylation of AKT and I-κB in 769-P and A498 cells (Figure 7, D and E). Moreover, overexpression of AdipoR1, but not AdipoR2, in ACHN reduced phosphorylation of AKT and I-κB following APN treatment (Figure 7F).
Discussion
Metastatic RCC is a lethal disease due to frequent relapse and aggressive progression. Current treatment options for patients have limited therapeutic efficacy. Our study investigated the expression and function of APN receptors in mRCC. To our knowledge this is the first time that we identify that the expression of AdipoR1 in RCC tissues could serve as an independent predictor for sunitinib response and overall survival. Activating the APN-AdipoR1 axis exerted an anti-tumor effect in RCC via concomitantly triggering dual pathways: one led to the blocking of GSK-3β/β-catenin signaling to inhibit migration and invasion, and the other obstructed the PI3K/AKT/NF-κB pathway to enhance sensitivity of tumor cells to sunitinib. Therefore, our findings demonstrate AdipoR1 as a novel predictor for TKIs response and as a promising therapeutic target to sensitize or even overcome drug resistance in mRCC.
TKIs therapy failed to reach satisfactory efficacy due to the lack of biomarkers predicting TKIs-responders among patients with mRCC. Therefore, the identification of reliable predictive markers for clinical benefits of TKIs is urgently needed. Herein, we showed that high expression of AdipoR1, either in primary or metastatic RCC tissues, was significantly associated with favorable sunitinib response and improved prognosis. Therefore, it is valuable to assess AdipoR1 expression in mRCC tumors to identify optimal candidates who might maximally survive from sunitinib therapy. In terms of treatment-naïve patients with high-AdipoR1 expression, we propose that the combination of sunitinib with APN or AdipoR1 agonist may enhance the therapeutic efficacy of TKIs in mRCC patients.
APN exerts its effects mainly through interacting with AdipoR1 and AdipoR2. Although shared similar structure, AdipoR1 and AdipoR2 elicit different functions via regulating different intracellular signaling pathways [5], [12]. Previous studies have focused on the relationship between serum APN and clinical outcomes in patients with RCC, while the expression and functions of APN receptors in tumor received less attention. Recently, Ito and colleagues evaluated the expression of AdipoR1 and AdipoR2 in localized RCC and found that the expression of AdipoR1 and AdipoR2 was not correlated with clinical outcome [11]. Notably, studies have demonstrated a significantly higher serum APN level in localized disease in contrast to mRCC [7], [9]. For this reason, in localized RCC, the survival benefit of patients with high-AdipoR1 expression might be masked by relatively high plasma APN levels. In contrast, owing to dramatically decreased serum APN levels in mRCC, as we demonstrated, men with high-AdipoR1 expression could experience superior survival than those with low-AdipoR1 expression. Moreover, the expression of AdipoR1 exhibited heterogeneous patterns in different tumor subtypes and tumor locations. Metastases, especially pulmonary and bone lesions, and pRCC had high AdipoR1 expression. Therefore, metastatic lesions and pRCC may be more sensitive to APN treatment. Furthermore, it is useful to identify patients with bone metastases, usually with poor TKIs-response, who might benefit from combination TKIs and APN therapy.
To date, limited studies have investigated the molecular mechanisms of the APN-AdipoR1 axis in RCC. Kleinmann et al. found that APN exerted anti-angiogenic and invasion inhibitory capacities via activating AMPK pathway and inhibiting VEGF, MMP-2 and MMP-9 secretion [10]. However, Ito et al. reported that exogenous APN could promote proliferation of RCC cells through activating anti-apoptotic signaling, but with no effect on cell migration and invasion [11]. It is noteworthy that the dosage of APN in their studies was far below physiological concentrations (5-30 μg/ml). In addition, the role of APN receptors in RCC has not been illuminated. Our study showed that 10 μg/ml APN, a physical concentration level, could inhibit cell migration and invasion, and enhance sensitivity of RCC cells to sunitinib. More importantly, we demonstrated that the anti-tumor effects of APN were predominately mediated by interacting with AdipoR1, but not AdipoR2. These results together with our clinical study confirmed the pivotal role of APN-AdipoR1 axis in mRCC.
Hyperactivation of the canonical β-catenin/Wnt pathway is one of the most common abnormalities in various cancer types [15]. GSK-3β is mainly a negative regulator of Wnt signaling and its dysregulation is involved in carcinogenesis. In breast cancer, APN inhibits proliferation through blocking phosphorylation of GSK-3β, preventing β-catenin activation and nuclear translocalization [14]. In our study, we did not observe an anti-proliferative effect of APN in RCC cells. However, inhibition of p-GSK-3β/β-catenin by APN was involved in reduction of RCC cell motility and invasiveness. Downregulation of phosphorylation of GSK-3β can hinder EMT, a key event directly correlated with tumor invasion, metastasis, and unfavorable prognosis [16], [17]. We also found that activating the APN-AdipoR1 axis could repress the expression of EMT-related proteins. In a recent study, differential modulation of GSK-3β activity was reported to be related to sunitinib response [18]. However, in our study, we found that inhibition of the GSK-3β/β-catenin pathway by APN was not associated with sunitinib response in RCC cells. Thus, the role of GSK-3β in sunitinib response remained to be identified.
NF-κB is a central player in the behavior of cancer cells and constitutively activated in RCC [19]. NF-κB activates several target genes, which subsequently leads to chemo- and radiotherapy resistance [19], [20]. Aberrant PI3K/AKT signaling is widespread in RCC which plays a central role in regulating NF-κB activation [21], [22]. Previous studies have demonstrated that APN could block the PI3K/AKT pathway in solid tumors and multiple myeloma [14], [23], [24]. APN also inhibited inflammatory response through the NF-κB pathway [25], [26]. Our study suggested that activation of the APN-AdipoR1 axis could inactivate NF-κB via blocking the PI3K/AKT pathway, which then sensitized RCC cells to sunitinib treatment in a concentration- and time- dependent manner. In addition, APN exerted anti-angiogenic properties in several solid cancers including RCC [10], [27]. Therefore, TKIs combined with APN could be a promising treatment strategy to maximize the anti-tumor effects on mRCC. Further study is needed to investigate the anti-angiogenic activity of co-administration APN and TKIs in RCC.
Targeting aberrantly activated pathways in tumor cells directly is problematic since these pathways have several important physical functions in normal cells. In our study, APN could inactivate the p-GSK-3β/β-catenin and PI3K/AKT/ NF-κB pathways in RCC tumors at physiological concentrations. At these concentrations, there is little risk for adverse effects on normal cells. Recently, a small molecule agonist of APN receptors has been shown to ameliorate diabetic symptoms in obese mouse models [28]. Furthermore, a novel AdipoR1 agonist has been demonstrated to exert anti-tumor effects in breast and pancreatic cancer [29], [30]. Therefore, APN or APN-AdipoR1 agonists could serve as promising therapeutic targets to abrogate TKIs-resistance and improve mRCC patient survival.
Conclusion
Our study for the first time explored the role of APN receptors in mRCC. We demonstrated that AdipoR1 could be a potential clinical biomarker predicting sunitinib response and prognosis in patients with mRCC, and further revealed the potential mechanism. Therefore, agents that targeting the APN-AdipoR1 axis may become a promising strategy to impede tumor progression, ameliorate TKIs-resistance and lead to a better outcome for mRCC patients.
The following are the supplementary data related to this article.
Patient's cohort characteristics of the mRCC cohort
Association between AdipoR1 expression and clinicopathologic parameters
Sequences of primers used in this study
Acknowledgments
Acknowledgements
We offer sincere gratitude to Prof. Allen C. Gao from UC Davis Medical Center for their constructive suggestions and careful revision for this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC 81672547), the Science and Technology Support Program of Sichuan Province (2015SZ0230–3) and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
References
- 1.Siegel RL, Miller KD. Jemal A (2018). Cancer statistics. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Sun M, Shariat SF, Cheng C, Ficarra V, Murai M, Oudard S, Pantuck AJ, Zigeuner R, Karakiewicz PI. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011;60:644–661. doi: 10.1016/j.eururo.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 3.Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009;10:992–1000. doi: 10.1016/S1470-2045(09)70240-2. [DOI] [PubMed] [Google Scholar]
- 4.Molina A, Lin X, Korytowsky B, Matczak E, Lechuga M, Wiltshire R, Motzer R. Sunitinib objective response in metastatic renal cell carcinoma: analysis of 1059 patients treated on clinical trials. Eur J Cancer. 2014;50:351–358. doi: 10.1016/j.ejca.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Hebbard L, Ranscht B. Multifaceted roles of adiponectin in cancer. Best Pract Res Clin Endocrinol Metab. 2014;28:59–69. doi: 10.1016/j.beem.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinthus JH, Kleinmann N, Tisdale B, Chatterjee S, Lu JP, Gillis A, Hamlet T, Singh G, Farrokhyar F, Kapoor A. Lower plasma adiponectin levels are associated with larger tumor size and metastasis in clear-cell carcinoma of the kidney. Eur Urol. 2008;54:866–873. doi: 10.1016/j.eururo.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 8.Liao LM, Schwartz K, Pollak M, Graubard BI, Li Z, Ruterbusch J, Rothman N, Davis F, Wacholder S, Colt J. Serum leptin and adiponectin levels and risk of renal cell carcinoma. Obesity (Silver Spring) 2013;21:1478–1485. doi: 10.1002/oby.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Martino M, Leitner CV, Hofbauer SL, Lucca I, Haitel A, Shariat SF, Klatte T. Serum adiponectin predicts cancer-specific survival of patients with renal cell carcinoma. Eur Urol Focus. 2016;2:197–203. doi: 10.1016/j.euf.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Kleinmann N, Duivenvoorden WC, Hopmans SN, Beatty LK, Qiao S, Gallino D, Lhotak S, Daya D, Paschos A, Austin RC. Underactivation of the adiponectin-adiponectin receptor 1 axis in clear cell renal cell carcinoma: implications for progression. Clin Exp Metastasis. 2014;31:169–183. doi: 10.1007/s10585-013-9618-1. [DOI] [PubMed] [Google Scholar]
- 11.Ito R, Narita S, Huang M, Nara T, Numakura K, Takayama K, Tsuruta H, Maeno A, Saito M, Inoue T. The impact of obesity and adiponectin signaling in patients with renal cell carcinoma: A potential mechanism for the "obesity paradox". PLoS One. 2017;12 doi: 10.1371/journal.pone.0171615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28:15–23. doi: 10.1016/j.beem.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Sun GX, Ding R, Li M, Guo Y, Fan LP, Yue LS, Li LY, Zhao M. Ghrelin attenuates renal fibrosis and inflammation of obstructive nephropathy. J Urol. 2015;193:2107–2115. doi: 10.1016/j.juro.2014.11.098. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, Wu D, Cooper GJ, Xu A. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66:11462–11470. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- 15.Nagini S, Sophia J, Mishra R. 2018. Glycogen synthase kinases: Moonlighting proteins with theranostic potential in cancer Seminars in cancer biology. [DOI] [PubMed] [Google Scholar]
- 16.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 17.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 18.Elgendy M, Abdel-Aziz AK, Renne SL, Bornaghi V, Procopio G, Colecchia M, Kanesvaran R, Toh CK, Bossi D, Pallavicini I. Dual modulation of MCL-1 and mTOR determines the response to sunitinib. J Clin Invest. 2017;127:153–168. doi: 10.1172/JCI84386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morais C, Gobe G, Johnson DW, Healy H. The emerging role of nuclear factor kappa B in renal cell carcinoma. Int J Biochem Cell Biol. 2011;43:1537–1549. doi: 10.1016/j.biocel.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Veuger SJ, Hunter JE, Durkacz BW. Ionizing radiation-induced NF-kappaB activation requires PARP-1 function to confer radioresistance. Oncogene. 2009;28:832–842. doi: 10.1038/onc.2008.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo H, German P, Bai S, Barnes S, Guo W, Qi X, Lou H, Liang J, Jonasch E, Mills GB. The PI3K/AKT pathway and renal Ccell carcinoma. J Genet Genomics. 2015;42:343–353. doi: 10.1016/j.jgg.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Network CGAR. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon HS, Chamberland JP, Aronis K, Tseleni-Balafouta S, Mantzoros CS. Direct role of adiponectin and adiponectin receptors in endometrial cancer: in vitro and ex vivo studies in humans. Mol Cancer Ther. 2011;10:2234–2243. doi: 10.1158/1535-7163.MCT-11-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medina EA, Oberheu K, Polusani SR, Ortega V, Velagaleti GV, Oyajobi BO. PKA/AMPK signaling in relation to adiponectin's antiproliferative effect on multiple myeloma cells. Leukemia. 2014;28:2080–2089. doi: 10.1038/leu.2014.112. [DOI] [PubMed] [Google Scholar]
- 25.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 26.Tan PH, Tyrrell HE, Gao L, Xu D, Quan J, Gill D, Rai L, Ding Y, Plant G, Chen Y. Adiponectin receptor signaling on dendritic cells blunts antitumor immunity. Cancer Res. 2014;74:5711–5722. doi: 10.1158/0008-5472.CAN-13-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messaggio F, Mendonsa AM, Castellanos J, Nagathihalli NS, Gorden L, Merchant NB, VanSaun MN. Adiponectin receptor agonists inhibit leptin induced pSTAT3 and in vivo pancreatic tumor growth. Oncotarget. 2017;8:85378–85391. doi: 10.18632/oncotarget.19905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 29.Otvos L, Jr., Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, Lovas S, Nama K, Hoffmann R, Knappe D. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol. 2011;11:90. doi: 10.1186/1472-6750-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akimoto M, Maruyama R, Kawabata Y, Tajima Y, Takenaga K. Antidiabetic adiponectin receptor agonist AdipoRon suppresses tumour growth of pancreatic cancer by inducing RIPK1/ERK-dependent necroptosis. Cell Death Dis. 2018;9:804. doi: 10.1038/s41419-018-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient's cohort characteristics of the mRCC cohort
Association between AdipoR1 expression and clinicopathologic parameters
Sequences of primers used in this study