Abstract
This study analyzed the expression of membrane OX40 and OX40L (mOX40 and mOX40L) and levels of soluble OX40 and OX40L (sOX40 and sOX40L) in T1D patients to determine their clinical significance. Peripheral blood (PB) was collected from patients with T1D and healthy control participants. Expression of mOX40 and mOX40L on immune cells was detected by flow cytometry. Levels of sOX40 and sOX40L in sera were measured by ELISA. We demonstrated for the first time enhanced sOX40 and sOX40L expression and reduced mOX40 and mOX40L levels in T1D patients which correlated with the clinical characteristics and inflammatory factors. These results suggest that OX40/OX40L signal may be promising biomarkers and associated with the pathogenesis of T1D.
1. Background
Type 1 diabetes (T1D) is a chronic and organ-specific autoimmune disease, is influenced by inherited or environmental factors, is increasing in all age groups, especially examined in children, and leads to the loss of insulin production in beta cells when immune cells invade the pancreatic islets [1–3]. The activation of T cells is mediated by antigen stimulation through T cell receptors and some costimulatory molecules. It has been suggested that CTLA-4 and PD-1 contribute to the development of T1D [4–6]. Multiple clinical immune intervention trials have been provided for the treatment of T1D such as blocking the costimulation of T cells [7–9]. However, the contributions of OX40 and OX40L to the development of T1D remain to be studied.
OX40/OX40L is a pair of important positive costimulatory signal molecules in the second signal system of T cells. OX40 (CD134) and OX40L (CD252), members of the TNF receptor superfamily (TNFRSF) and TNF superfamily (TNFSF), play important roles in T cell expansion in tumors, during infectious inflammation, and in autoimmune diseases [10, 11]. OX40 is expressed in activated T cells [12], while OX40L is mainly expressed in B lymphocytes, dendritic cells, and macrophage cells [12]. OX40/OX40L signaling acts as a key role in the development and differentiation of some immunological cells, especially T cells [13, 14]. Numerous studies have shown the important role of costimulatory molecules in T1D. Blocking costimulatory interactions such as CD28/B7 or CD40/CD40L might be an effective therapy way in NOD mice [15, 16]. Blocking OX40/OX40L interactions at the late age of disease may also suppress diabetes progression [17].
The level of sOX40 and sOX40L is elevated in some tumors and infectious diseases. This suggests that sOX40 and sOX40L act as costimulators to induce immune responses. However, to the best of our knowledge, the significance of membrane and soluble forms of OX40 or OX40L expression and their correlation with clinical parameters in T1D have not been investigated. To characterize OX40 and OX40L expression and determine the role of OX40/OX40L signal in the development and pathogenesis of T1D, we evaluated the expression of sOX40 and sOX40L in the PBMC and sera of patients with T1D and healthy controls (HCs) and analyzed the correlation with clinical and inflammatory indicators.
2. Materials and Methods
2.1. Patients and Controls
In our study, blood samples were collected after overnight fasting from patients (n = 119) at the Endocrinology Department in the Second Affiliated Hospital of Soochow University, Suzhou, China, between 2015 and 2017. The diagnostic criteria for T1D were according to “The T1D Exchange Clinic Registry” [18]. Samples from healthy blood donors (n = 108) were matched to T1D patients with age, gender, and race. In addition, HCs were tested for glucose levels, all T1D autoantibody negative. The study participants were excluded if they had one of the following conditions: acute or chronic inflammatory diseases, infectious diseases, and cancer. For the detection of membrane forms of OX40 and OX40L on immune cells, 46 patients and 44 HCs were enrolled. The approval from the Ethics Review Board of the Second Affiliated Hospital of Soochow University was granted to the patients.
2.2. Antibodies and Flow Cytometry
To assess the expression of mOX40 in CD3+, CD4+, or CD8+ T cells and mOX40L in CD14+ monocyte and CD19+ B cells, peripheral blood mononuclear cell (PBMC) was isolated from T1D patients or healthy donors. Flow cytometry was performed on PBMC incubated with fluorochrome-labeled monoclonal antibodies (mAbs) for 30 min. Anti-CD3-FITC (clone: UCHT1), anti-CD4-FITC (clone: RPA-T4), anti-CD8-FITC (clone: SFCI21), anti-CD14-FITC (clone: RMO52), and anti-CD19-FITC (clone: J3-119) mAbs were from Beckman Coulter. Anti-OX40-PE (clone: ACT35) and anti-OX40L-PE (clone: RM134L) were from eBioscience. Cells were washed and then immediately measured by flow cytometry (Beckman Coulter, CA). Data were analyzed using FlowJo software (Tree Star, OR).
2.3. Soluble OX40 and OX40L Measurement
The samples were centrifuged at 800xg for 8 min, and the sera was stored at -80°C for ELISA. To determine the relationship of sOX40 or sOX40L with the disease activity, serum levels of sOX40 and sOX40L were determined by ELISA using kits obtained from Kalang (Shanghai) and Cusabio (Wuhan), respectively. Sera samples were plated into the 96-well microplates, and ELISA was conducted following the manufacturer's instructions. Plates were read in a microplate reader (Bio-Rad, CA) for the absorbance at 450 nm.
2.4. Cytokine Production
Cytokines interleukin IL-2, IL-4, IL-6, and IL-10, interferon gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and IL-17a were quantified in patients using a cytometric bead array system (CBA) (BD Pharmingen, CA) according to the instructions of the manufacturer. For each reaction, 50 μl sera were mixed with 50 μl beads to each assay tube. 50 μl PE detection reagent was then added, and the samples were incubated for 3 hours at room temperature. Samples were washed with wash buffer, centrifuged, and then run on flow cytometer; data were analyzed using BD™ CBA Software.
2.5. Statistical Analysis
Statistical analysis was performed using the IBM SPSS statistic 22 (Chicago, IL, USA). All the quantitative data was presented as the mean ± standard deviation (SD). For statistical analysis, differences in continuous variables between two independent samples were evaluated by the Mann-Whitney U test. Dichotomous variables were compared by the χ 2 test. Association between continuous variables was assessed by means of Spearman and partial correlation. Pearson's rank correlation analysis was used to evaluate the associations between dichotomous variables and protein levels. Covariance analysis was used. The statistical software GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA) and IBM SPSS statistic 22 (Chicago, IL, USA) were used for graph creation.
3. Results
3.1. Reduced mOX40 and mOX40L Expression in T1D Patients
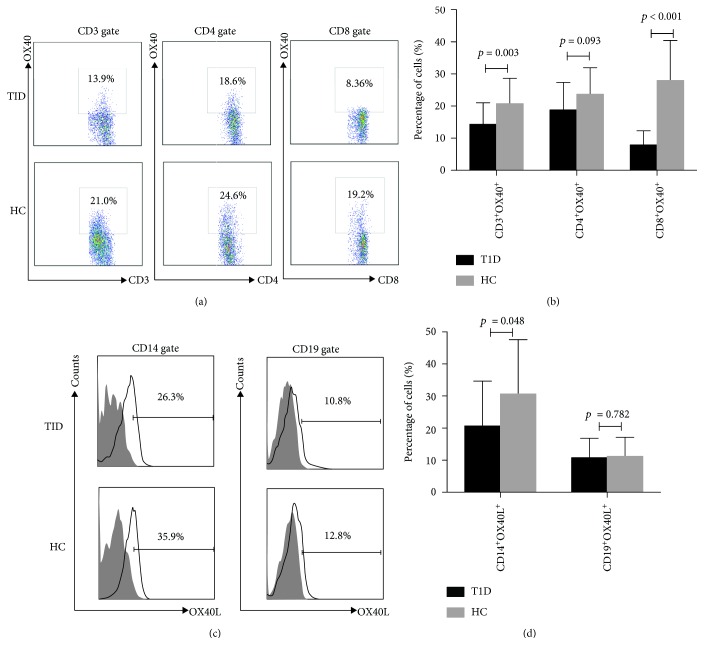
The clinical characteristics of the study population are summarized in Table 1. To investigate the expression of mOX40 and mOX40L in T1D patients, blood specimens were collected from 46 T1D patients and 44 HCs. Flow cytometry analyses demonstrated that OX40 expression on CD3+, CD4+, and CD8+ T cells in PBMC samples was less frequent in patients with T1D than HCs (14.34 ± 1.02% vs. 22.47 ± 1.87%, p = 0.003; 18.78 ± 1.31% vs. 24.85 ± 1.87%, p = 0.093; and 7.94 ± 0.66% vs. 15.9 ± 1.87%, p < 0.001; respectively) (Figures 1(a) and 1(b)). Furthermore, the expression of OX40L on CD14+ monocytes was also decreased significantly in PBMC samples of patients with T1D compared to HCs (28.73 ± 3.69% vs. 38.87 ± 5.46%, p = 0.048). However, there was no significant difference in mOX40L on CD19+ B cells between T1D patients and HC (10.76 ± 0.93% vs. 12.02 ± 1.62%, p = 0.782) (Figures 1(c) and 1(d)).
Table 1.
Clinical features of the study population.
| Type 1 diabetes (n = 119) | Healthy controls (n = 108) | p valuea | |
|---|---|---|---|
| Age (years) | 28.5 (10-53) | 29.4 (24-40) | 0.75 |
| Gender, female (n%) | 57 (47.9%) | 51 (46.8%) | 0.97 |
| Duration (years) | 7 (0-39) | — | — |
| Fasting venous blood glucose (mmol/l) | 11.9 (3.99-22.3) | 5.2 (3.8-6.4) | 0.01 |
| Cr (μmol/l) | 54.2 (26-98) | 82.3 (44-112) | 0.04 |
| BUN (mmol/l) | 4.4 (1.4-8.4) | — | — |
| UA (μmol/l) | 275.7 (81-540) | — | — |
| ALT (IU/l) | 18.9 (2-92) | — | — |
| AST (IU/l) | 20.0 (8-62) | — | — |
| TC (mmol/l) | 4.4 (0.8-7.6) | — | — |
| TG (mmol/l) | 1.1 (0.4-5.7) | — | — |
| LDL (mmol/l) | 2.1 (1.1-5.4) | — | — |
| HDL (mmol/l) | 1.4 (0.7-2.4) | — | — |
| Fasting C-peptide (ng/ml) | 1.6 (<0.01-35) | — | — |
| Anti-ICA positive (%)b | 11 (27%) | — | — |
| Anti-GAD positive (%)b | 17 (41%) | — | — |
| Anti-IAA positive (%)b | 3 (7%) | — | — |
| HbA1c (%) | 9.12% (5.3-17.1%) | — | — |
| Ketosis | 17 (41%) | — | — |
| IFN-γ (pg/ml) | 1.5 (0-17.2) | — | — |
| IL-2 (pg/ml) | 2.7 (0.4-11.9) | — | — |
| IL-4 (pg/ml) | 2.1 (0-14.1) | — | — |
| IL-6 (pg/ml) | 4.2 (0-15.6) | — | — |
| IL-10 (pg/ml) | 4.7 (0.7-20.4) | — | — |
| TNF-α (pg/ml) | 3.1 (0-17.2) | — | — |
| IFN-γ (pg/ml) | 3.1 (0.4-19.3) | — | — |
| IL-17a (pg/ml) | 26.0 (0-159.9) | — | — |
a p value is based on the statistical analysis by the Mann-Whitney U test or the chi-square test assessing overall group differences. bNot all the patients have the antibody results, only 52/119 patients received an autoantibody screening. cAbbreviations: Cr: creatinine; BUN: urea; UA: uric acid; ALT: alanine aminotransferase; AST: aspartate transferase; TC: cholesterol; TG: triglycerides; LDL: low-density lipoprotein; HDL: high-density lipoprotein; IAA: insulin autoantibody.
Figure 1.
Expression of mOX40 and its ligand mOX40L in the PBMC of T1D patients. (a) The expression level of mOX40 was detected by flow cytometry of CD3+, CD4+, and CD8+ T cells from T1D patients and HCs. (b) Percentages of CD3+OX40+, CD4+OX40+, and CD8+OX40+ cells in the PBMC samples of patients with T1D and HCs. (c) The expression level of mOX40L was detected by flow cytometry of monocyte cells from T1D patients and HCs. CD14 and CD19 were used as markers of monocytes and B cells. (d) A histogram representative of a set of CD14+OX40L+ and CD19+OX40L+ is shown in the right figure.
3.2. Correlation between mOX40 and mOX40L Positive Cells and Clinical Parameters of T1D
To determine whether the negative association of mOX40 and mOX40L expression is related to T1D disease activity, we examined the relationship between the presence of mOX40 and mOX40L and the disease state. The statistical analyses of the correlation between clinical features and the expression of mOX40 and mOX40L are presented in Table 2. We found that the expression of glutamic acid decarboxylase (GAD) was negatively correlated with CD8+OX40+ T cells (r = −0.3996, p = 0.0431) and CD19+OX40L+ B cells (r = −0.3789, p = 0.0463). A significant negative correlation between CD3+OX40+ T cells (r = −0.3911, p = 0.0244), CD4+OX40+ T cells (r = −0.4072, p = 0.0187), and creatinine (Cr) was observed; we also found a significant negative correlation between CD3+OX40+ T cells (r = −0.4357, p = 0.0113) and CD4+OX40+ T cells (r = −0.4231, p = 0.0142) with uric acid (UA). However, we did not found any correlation between CD8+OX40+ T cells and Cr or UA (Supplement Figure 1).
Table 2.
Correlation between clinical features and membrane levels of OX40 and OX40L.
| CD4+OX40+ | CD8+OX40+ | CD3+OX40+ | CD19+OX40L+ | CD14+OX40L+ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Ketosis | 0.2692 | 0.2257 | 0.1746 | 0.4301 | 0.2770 | 0.2121 | -0.3000 | 0.1750 | 0.1307 | 0.5619 |
| GAD | -0.0570 | 0.7818 | -0.3996 | 0.0431 | -0.0571 | 0.7816 | -0.3789 | 0.0463 | -0.1401 | 0.4948 |
| ICA | -0.0121 | 0.9529 | -0.1582 | 0.4401 | -0.0365 | 0.8593 | -0.0608 | 0.7677 | 0.1245 | 1.0042 |
| IAA | 0.1467 | 0.4746 | -0.1467 | 0.4746 | 0.1735 | 0.3968 | -0.0133 | 0.9485 | -0.0933 | 0.6502 |
| DKA | 0.4468 | 0.0371 | 0.1043 | 0.6443 | 0.3725 | 0.0878 | -0.0744 | 0.7419 | -0.0148 | 0.9475 |
| HDL | -0.3474 | 0.0555 | -0.2594 | 0.1588 | -0.3528 | 0.0516 | -0.1488 | 0.4245 | -0.1671 | 0.3689 |
| LDL | -0.1773 | 0.3401 | -0.1264 | 0.4979 | -0.2231 | 0.2276 | -0.1470 | 0.4300 | -0.1408 | 0.4501 |
| TC | -0.2280 | 0.2019 | -0.2848 | 0.1081 | -0.2739 | 0.1230 | -0.1321 | 0.4638 | -0.1053 | 0.5597 |
| TG | 0.3627 | 0.0380 | 0.0844 | 0.6404 | 0.2936 | 0.0973 | 0.2199 | 0.2189 | 0.1634 | 0.3637 |
| ALT | -0.0560 | 0.7605 | 0.1015 | 0.5804 | 0.0540 | 0.7688 | 0.0421 | 0.8190 | 0.1166 | 0.5252 |
| AST | 0.0852 | 0.6426 | 0.2025 | 0.2663 | 0.2056 | 0.2591 | 0.0343 | 0.8519 | 0.0479 | 0.7943 |
| LDH | 0.0260 | 0.9107 | 0.0655 | 0.9978 | 0.1383 | 0.5500 | 0.0416 | 0.8576 | 0.1498 | 0.5168 |
| GGT | -0.0510 | 0.8085 | 0.1969 | 0.3455 | -0.1076 | 0.6086 | 0.0947 | 0.6522 | -0.3137 | 0.1267 |
| Cr | -0.4072 | 0.0187 | -0.1674 | 0.9926 | -0.3911 | 0.0244 | 0.0363 | 0.8410 | -0.2899 | 0.1017 |
| BUN | -0.2858 | 0.1069 | -0.1345 | 0.4557 | -0.2436 | 0.1719 | 0.1462 | 0.4170 | -0.1055 | 0.5589 |
| UA | -0.4231 | 0.0142 | -0.6887 | 0.7033 | -0.4357 | 0.0113 | 0.0885 | 0.6239 | -0.3246 | 0.0653 |
| GLU | 0.1162 | 0.5802 | -0.4501 | 0.8308 | 0.1328 | 0.5268 | 0.0076 | 0.9971 | -0.0561 | 0.7897 |
| C0 | -0.1418 | 0.4238 | -0.1189 | 0.5031 | -0.2069 | 0.2404 | -0.0207 | 0.9072 | 0.1722 | 0.3300 |
| C120 | -0.2638 | 0.2901 | -0.1209 | 0.6328 | -0.3487 | 0.1562 | 0.0830 | 0.7432 | 0.3595 | 0.1429 |
| HbA1c | 0.4666 | 0.0047 | 0.0926 | 0.5966 | 0.3930 | 0.0195 | 0.3994 | 0.0175 | 0.3332 | 0.0504 |
3.3. Higher Serum Levels of sOX40 and sOX40L in T1D Patients
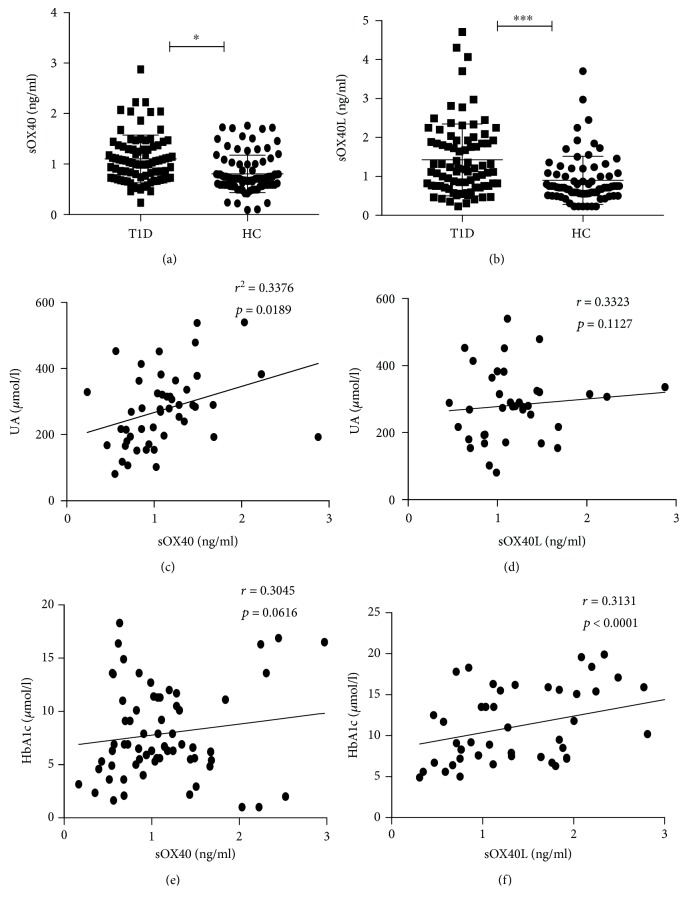
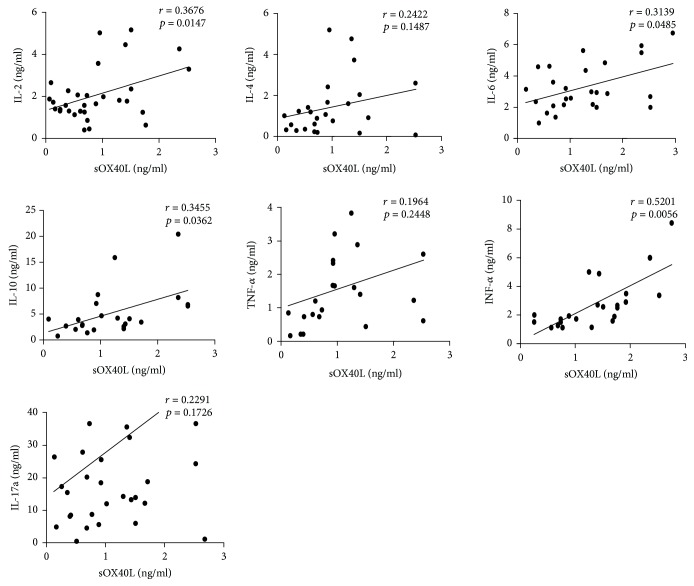
Our previous work demonstrated that in addition to mOX40 and mOX40L, sOX40 and sOX40L are also present in human sera. Serum levels of sOX40 and sOX40L were determined by ELISA. As shown in Figure 2(a), the concentration of sOX40 in the sera of T1D patients was significantly higher than that of HCs (1.08 ± 0.06 ng/ml vs. 0.83 ± 0.08 ng/ml, p = 0.042). Meanwhile, we found that the concentration of sOX40L was also significantly higher than that in HCs (1.42 ± 0.10 ng/ml vs. 0.83 ± 0.08 ng/ml, p < 0.0001) (Figure 2(b)). To explore whether sOX40 and sOX40L are involved in the development of T1D, we further analyzed the relationship between sOX40, sOX40L, and disease activity. We conducted a correlation analysis and found a positive correlation between sOX40 expression and UA (r = 0.3376, p = 0.0189) and hemoglobin A1c (HbA1c) (r = 0.3045, p = 0.0616), that is, T1D patients with higher sOX40 expression levels had higher UA and HbA1c (Figures 2(c) and 2(e)). The correlation between sOX40L and hemoglobin A1c (r = 0.3131, p < 0.0001) was also positive but was not significant with UA (r = 0.3323, p = 0.1127) (Figures 2(d) and 2(f)). These data suggest that the expression of sOX40 and sOX40L correlates with several parameters of disease activity. To evaluate whether OX40 and OX40L play a role in the process of T cell differentiation, we measured the serum levels of some cytokines such as IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α, and IL-17a in T1D patients, finding no significant correlation between sOX40 expressions and cytokines (Table 3). However, sOX40L expression in T1D patients was positively correlated with serum levels of IL-2 (r = 0.3676, p = 0.0147), IL-6 (r = 0.3139, p = 0.0485), IL-10 (r = 0.3455, p = 0.0362), and IFN-γ (r = 0.5201, p = 0.0056) (Figure 3).
Figure 2.
Correspondence correlation between sOX40 and sOX40L levels in HC blood and T1D disease. (a) Increased sOX40 was observed in the sera from T1D patients as compared to HCs. (b) Increased sOX40L levels were observed in the sera from patients with T1D compared with the HC group. (c) Positive correlation between sOX40 and the expression of UA. (d) No significant correlation between sOX40L and the expression of UA. (e) No significant correlation between sOX40 and the expression of HbA1c. (f) Positive correlation between sOX40L and the expression of HbA1c.
Table 3.
Correlation between clinical features and soluble levels of OX40 and OX40L.
| sOX40 | sOX40L | |||
|---|---|---|---|---|
| r | p | r | p | |
| Ketosis | 0.2191 | 0.7217 | -0.0344 | 0.8395 |
| GAD | 0.0289 | 0.3379 | 0.0840 | 0.4954 |
| ICA | 0.0070 | 0.7302 | 0.1846 | 0.1318 |
| IAA | 0.0607 | 0.7442 | 0.1463 | 0.2337 |
| DKA | 0.0962 | 0.6942 | -0.0107 | 0.9448 |
| HDL | 0.1826 | 0.1159 | -0.0511 | 0.6630 |
| LDL | 0.0733 | 0.7493 | 0.1849 | 0.1123 |
| TC | 0.0229 | 0.9884 | 0.1528 | 0.1846 |
| TG | -0.0635 | 0.1745 | 0.0131 | 0.9094 |
| ALT | -0.2506 | 0.7772 | -0.1072 | 0.3632 |
| AST | -0.1859 | 0.6487 | -0.1434 | 0.2228 |
| LDH | -0.2732 | 0.6829 | -0.0224 | 0.8851 |
| GGT | -0.0967 | 0.7239 | -0.142 | 0.3253 |
| Cr | -0.0148 | 0.5676 | -0.1343 | 0.2443 |
| BUN | -0.1330 | 0.1402 | -0.0909 | 0.4317 |
| UA | 0.3376 | 0.0189 | -0.1127 | 0.3323 |
| GLU | 0.1428 | 0.3729 | 0.0974 | 0.4627 |
| C0 | 0.0398 | 0.4693 | 0.1008 | 0.3736 |
| C120 | 0.2641 | 0.4974 | 0.1211 | 0.4952 |
| HbA1c | 0.3045 | 0.0616 | 0.3131 | 0.0001 |
| CD4+OX40+ T | -0.3373 | 0.0225 | — | — |
| CD8+OX40+ T | -0.4733 | 0.7018 | — | — |
| CD3+OX40+ T | -0.3629 | 0.0162 | — | — |
| CD19+OX40L+ B | — | — | 0.2351 | 0.0683 |
| CD14+OX40L+ monocyte | — | — | 0.2499 | 0.0521 |
| IL-2 | 0.2461 | 0.1137 | 0.3676 | 0.0252 |
| IL-4 | 0.1455 | 0.1232 | 0.2422 | 0.1487 |
| IL-6 | 0.0214 | 0.0196 | 0.3139 | 0.0485 |
| IL-10 | -0.1077 | 0.1482 | 0.3455 | 0.0362 |
| TNF-α | 0.4713 | 0.0569 | 0.1964 | 0.2448 |
| IFN-γ | 0.4238 | 0.0922 | 0.5201 | 0.0056 |
| IL-17a | 0.4947 | 0.0535 | 0.2291 | 0.1726 |
Figure 3.
Correlation between sOX40 and sOX40L expression and sera cytokine levels in T1D patients. Positive correlation between sOX40L and the expression of IL-2, IL-6, IL-10, and IFN-γ.
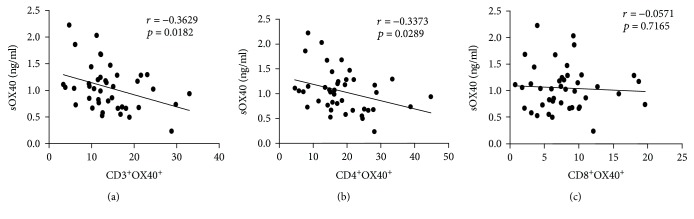
3.4. Negative Correlation between Serum Levels of sOX40 and mOX40 in T1D Patients
Correlation analyses demonstrated that there was a significant negative association between serum OX40 levels and mOX40 expression on CD3+ T cells (r = −0.3629, p = 0.0182) and CD4+ T cells (r = −0.3373, p = 0.0289) in T1D patients (Figure 4). Although not significant, there was a negative association between sOX40 levels and mOX40 expression on CD8+ T cells (r = −0.0571, p = 0.7186) (Table 3).
Figure 4.
Correlation between serum levels of sOX40 and mOX40 in T1D patients. (a) Significant negative correlation between sOX40 and mOX40 expression on CD3+ T cells. (b) Significant negative correlation between sOX40L and mOX40 expression on CD4+ T cells. (c) Negative correlation between sOX40L and mOX40 expression on CD8+ T cells.
4. Discussion
Costimulatory molecules play important roles in regulation of the immune response in autoimmune diseases. Therefore, they may be useful as treatment indicators for the disease [19].
The TNF/TNFR family members of costimulatory molecules are critical in regulating T cell responses [20]. Here, we detected the expression of membrane and soluble forms of OX40 and OX40L on immune cells in T1D patients and determined their clinical significance. For the first time, we detected the membrane and soluble forms of OX40 and OX40L expression in the PBMC of T1D patients and analyzed the correlation with disease activity. We found that sOX40 and sOX40L expression in T1D patients was significantly higher compared with the HCs. However, the expression of mOX40 and mOX40L in T1D patients was significantly lower compared with that in HCs. There was no significant correlation of mOX40L of CD19+ B cells in T1D patients and HCs. In addition, sOX40 levels in patients were positively correlated with the disease activity, as indicated by T1D disease activity, and sOX40L levels in the patients were positively correlated with inflammatory cytokines. Our findings suggest that sOX40 and sOX40L might counteract the aberrant immune response and potentially serve as monitoring indicators of disease progression and therapeutic targets in T1D treatment.
Costimulatory molecules can induce the activation of T cells, promote cell survival, support the formation of potential memory T cells, and produce the release of the cytokines [21, 22]. Therefore, the costimulatory molecules may be useful for the diagnosis and treatment of some diseases. Both membrane and soluble forms of costimulatory molecules play important roles in the regulation of immune networks [23]. Costimulatory molecules with soluble forms could be generated from proteolytic cleavage, such as PD-L1, B7H3 [24], ICOS, and ICOSL [25], or could be generated from mRNA splicing like CTLA-4 [26] and PD-1 [27]. It has been found that sOX40 is expressed in patients with amyotrophic lateral sclerosis [28] and chronic lymphocytic leukemia [29], while sOX40L is expressed in the sera of patients with acute coronary syndrome, bronchial asthma (adult), Henoch-Schonlein purpura (children), and rheumatoid arthritis [30–33]. In our study, we observed relatively higher sOX40 and sOX40L levels but lower mOX40 and mOX40L in T1D patients, suggesting that sOX40 and sOX40L might have regulatory functions opposite to mOX40 and mOX40L. High levels of soluble molecule may be the consequence of two different processes, such as high production or decreased depletion. In T1D patients, we speculate that soluble levels increase because mOX40 and mOX40L are cleavaged into the soluble forms, leading to higher sOX40 and sOX40L expression in PBMC compared with the HCs.
Autoantibodies in T1D are risk indicators for the diagnosis and prediction of the disease and surrogate markers for autoimmune diabetes [34–37]. The presence of several autoantibodies such as islet cell autoantibody (ICA) and GAD indicates an autoimmune pathogenic response to beta cells [38]. We measured the membrane variants of OX40 and OX40L in T1D individuals, which were reduced compared to HC. Meanwhile, GAD expression is also associated with mOX40 and mOX40L expression. However, some other indicators such as higher levels of UA are also associated with enlarged risk of the clinical manifestations of diabetic nephropathy in persons with T1D [39, 40]. Moreover, HbA1c is the most common and widely accepted indicator of T1D [41–43]. In our study, we found that T1D patients with higher sOX40 expression levels had higher UA, and higher sOX40L expression levels had higher HbA1c, which means OX40 and OX40L are promising markers for T1D.
The detailed functions of some costimulatory molecules are still controversial. Until now, one of the most attractive approaches to prevent T1D is using islet antigen-specific regulatory T cells (Tregs). Luczynski et al. discovered that the mRNA level of OX40 was lower in Treg cells of children with T1D when compared to the reference patients [2]. However, Szypowska et al. observed that T1D children had higher frequency of CD4+CD25highOX40+ cells than healthy subjects [44]. In our study, we found a reduction of the membrane form of OX40 on CD3+, CD4+, and CD8+ T cells and a reduction of OX40L on CD14+ monocytes in T1D patients correlated with clinical parameters. Bresson et al. have found that OX40 agonist therapy can slow down T1D progression [45]. We speculate that increasing the level of OX40 may be a new therapeutic strategy.
5. Conclusions
Taken together, our study revealed the dissociation between mOX40 and mOX40L expression and sOX40 and sOX40L levels in T1D patients for the first time. We provided evidence for the diagnostic value of OX40 and OX40L in T1D patients. The abnormal expression of OX40 and OX40L molecules appears to be connected with the severity of the disease. Thus, OX40 and OX40L may be promising biomarkers for diagnosis and prognosis of T1D. The in-depth mechanisms of membrane and soluble OX40 and OX40L in T1D remain to be elucidated, and the role of OX40 and OX40L in immune pathogenesis of T1D requires further research.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 81873876, 81600607, 81502454, and 81701596), the Jiangsu Provincial Medical Youth Talent (No. QNRC2016755 and No. QNRC2016770), the Science and Technology Project of Suzhou (No. SYS201740), and the Natural Science Foundation of Jiangsu Province (No. BK20160349).
Abbreviations
- CBA:
Cytometric bead array
- GAD:
Glutamic acid decarboxylase
- HCs:
Healthy controls
- HbA1c:
Hemoglobin A1c
- IFN-γ:
Interferon gamma
- ICA:
Islet cell autoantibody
- mAbs:
Monoclonal antibodies
- PBMC:
Peripheral blood mononuclear cells
- SD:
Standard deviation
- T1D:
Type 1 diabetes
- TNFRSF:
TNF receptor superfamily
- TNFSF:
TNF superfamily
- TNF-α:
Tumor necrosis factor-alpha
- Tregs:
Antigen-specific regulatory T cells
- UA:
Uric acid.
Contributor Information
Chen Fang, Email: afa9911@sina.com.
Cuiping Liu, Email: liucuiping1980@126.com.
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1975 Declaration of Helsinki, as revised in 2008. This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent
Informed consent was obtained from all patients being included in the study.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
Jingnan An and Sisi Ding equally contributed to the study.
Supplementary Materials
Supplement Figure 1: correlation between CD3+OX40+, CD4+OX40+, and CD8+OX40+ and clinic pathological characteristics. Negative correlation between CD3+OX40+ and CD4+OX40+ and the expression of UA and Cr. No correlation between CD8+OX40+ and the expression of UA and Cr.
References
- 1.Battaglia M., Nigi L., Dotta F. Towards an earlier and timely diagnosis of type 1 diabetes: is it time to change criteria to define disease onset? Current Diabetes Reports. 2015;15(12):p. 115. doi: 10.1007/s11892-015-0690-6. [DOI] [PubMed] [Google Scholar]
- 2.Łuczyński W., Stasiak-Barmuta A., Juchniewicz A., et al. The mRNA expression of pro- and anti-inflammatory cytokines in T regulatory cells in children with type 1 diabetes. Folia Histochemica et Cytobiologica. 2010;48(1):93–100. doi: 10.2478/v10042-008-0113-5. [DOI] [PubMed] [Google Scholar]
- 3.Janzen Claude J. A., Hadjistavropoulos H. D., Friesen L. Exploration of health anxiety among individuals with diabetes: prevalence and implications. Journal of Health Psychology. 2014;19(2):312–322. doi: 10.1177/1359105312470157. [DOI] [PubMed] [Google Scholar]
- 4.Zouidi F., Stayoussef M., Bouzid D., et al. Contribution of PTPN22, CD28, CTLA-4 and ZAP-70 variants to the risk of type 1 diabetes in Tunisians. Gene. 2014;533(1):420–426. doi: 10.1016/j.gene.2013.09.112. [DOI] [PubMed] [Google Scholar]
- 5.Colli M. L., Hill J. L. E., Marroquí L., et al. PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-α and-γ via IRF1 induction. EBioMedicine. 2018;36:367–375. doi: 10.1016/j.ebiom.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Momin S., Flores S., Angel B B., Codner D E., Carrasco P E., Perez-Bravo F. Interactions between programmed death 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) gene polymorphisms in type 1 diabetes. Diabetes Research and Clinical Practice. 2009;83(3):289–294. doi: 10.1016/j.diabres.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Nambam B., Haller M. J. Updates on immune therapies in type 1 diabetes. European Endocrinology. 2016;12(2):89–95. doi: 10.17925/EE.2016.12.02.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orban T., Bundy B., Becker D. J., et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. The Lancet. 2011;378(9789):412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orban T., Bundy B., Becker D. J., et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37(4):1069–1075. doi: 10.2337/dc13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei W., Zeng D., Liu G., et al. Crucial role of OX40/OX40L signaling in a murine model of asthma. Molecular Medicine Reports. 2018;17(3):4213–4220. doi: 10.3892/mmr.2018.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb G. J., Hirschfield G. M., Lane P. J. L. OX40, OX40L and autoimmunity: a comprehensive review. Clinical Reviews in Allergy & Immunology. 2016;50(3):312–332. doi: 10.1007/s12016-015-8498-3. [DOI] [PubMed] [Google Scholar]
- 12.Karulf M., Kelly A., Weinberg A. D., Gold J. A. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. The Journal of Immunology. 2010;185(8):4856–4862. doi: 10.4049/jimmunol.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei W., Zeng D. X., Zhu C. H., et al. The upregulated expression of OX40/OX40L and their promotion of T cells proliferation in the murine model of asthma. Journal of Thoracic Disease. 2014;6(7):979–987. doi: 10.3978/j.issn.2072-1439.2014.06.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavanagh M. M., Hussell T. Is it wise to target the late costimulatory molecule OX40 as a therapeutic target? Archivum Immunologiae et Therapiae Experimentalis. 2008;56(5):291–297. doi: 10.1007/s00005-008-0032-3. [DOI] [PubMed] [Google Scholar]
- 15.Irie J., Wu Y., Kachapati K., Mittler R. S., Ridgway W. M. Modulating protective and pathogenic CD4+ subsets via CD137 in type 1 diabetes. Diabetes. 2007;56(1):186–196. doi: 10.2337/db06-0793. [DOI] [PubMed] [Google Scholar]
- 16.Lenschow D. J., Ho S. C., Sattar H., et al. Differential effects of anti-b7-1 and anti-b7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. Journal of Experimental Medicine. 1995;181(3):1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad C. S., Bhattacharya P., Alharshawi K., et al. Age-dependent divergent effects of OX40L treatment on the development of diabetes in nod mice. Autoimmunity. 2016;49(5):298–311. doi: 10.1080/08916934.2016.1183657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck R. W., Tamborlane W. V., Bergenstal R. M., et al. The T1D exchange clinic registry. The Journal of Clinical Endocrinology & Metabolism. 2012;97(12):4383–4389. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 19.Ford M. L., Adams A. B., Pearson T. C. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nature Reviews Nephrology. 2014;10(1):14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doss G. P., Agoramoorthy G., Chakraborty C. TNF/TNFR: drug target for autoimmune diseases and immune-mediated inflammatory diseases. Frontiers in Bioscience. 2014;19(7):1028–1040. doi: 10.2741/4265. [DOI] [PubMed] [Google Scholar]
- 21.Wagner D. H., Jr. Overlooked mechanisms in type 1 diabetes etiology: how unique costimulatory molecules contribute to diabetogenesis. Frontiers in Endocrinology. 2017;8:p. 208. doi: 10.3389/fendo.2017.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maj T., Wei S., Welling T., Zou W. T cells and costimulation in cancer. The Cancer Journal. 2013;19(6):473–482. doi: 10.1097/PPO.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 23.Bour-Jordan H., Bluestone J. A. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunological Reviews. 2009;229(1):41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang G., Hou J., Shi J., Yu G., Lu B., Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated t cells and is detectable in normal human serum. Immunology. 2008;123(4):538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X., Wu J., An J., et al. Development of a novel monoclonal antibody to human inducible co-stimulator ligand (ICOSL): biological characteristics and application for enzyme-linked immunosorbent assay. International Immunopharmacology. 2016;36:151–157. doi: 10.1016/j.intimp.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Magistrelli G., Jeannin P., Herbault N., et al. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. European Journal of Immunology. 1999;29(11):3596–3602. doi: 10.1002/(SICI)1521-4141(199911)29:11<3596::AID-IMMU3596>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen C., Ohm-Laursen L., Barington T., Husby S., Lillevang S. T. Alternative splice variants of the human PD-1 gene. Cellular Immunology. 2005;235(2):109–116. doi: 10.1016/j.cellimm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Ilzecka J. Serum soluble OX40 in patients with amyotrophic lateral sclerosis. Acta Clinica Croatica. 2012;51(1):3–7. [PubMed] [Google Scholar]
- 29.Taylor L., Schwarz H. Identification of a soluble OX40 isoform: development of a specific and quantitative immunoassay. Journal of Immunological Methods. 2001;255(1-2):67–72. doi: 10.1016/S0022-1759(01)00424-0. [DOI] [PubMed] [Google Scholar]
- 30.Lei W., Zhu C. H., Zeng da X. W. Q., et al. SOX40L: an important inflammatory mediator in adult bronchial asthma. Annals of the Academy of Medicine, Singapore. 2012;41(5):200–204. [PubMed] [Google Scholar]
- 31.Yan J., Gong J., Chen G., Liu P., Wang C., Yang P. Evaluation of serum soluble OX40 ligand as a prognostic indicator in acute coronary syndrome patients. Clinica Chimica Acta. 2010;411(21-22):1662–1665. doi: 10.1016/j.cca.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Laustsen J. K., Rasmussen T. K., Stengaard-Pedersen K., et al. Soluble OX40L is associated with presence of autoantibodies in early rheumatoid arthritis. Arthritis Research & Therapy. 2014;16(5):p. 474. doi: 10.1186/s13075-014-0474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin W., Hongya W., Yongjing C., et al. Increased OX40 and soluble OX40 ligands in children with Henoch-Schonlein purpura: association with renal involvement. Pediatric Allergy and Immunology. 2011;22(1, Part I):54–59. doi: 10.1111/j.1399-3038.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- 34.Wenzlau J. M., Hutton J. C. Novel diabetes autoantibodies and prediction of type 1 diabetes. Current Diabetes Reports. 2013;13(5):608–615. doi: 10.1007/s11892-013-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu L., Zhao Z., Steck A. K. T1D autoantibodies: room for improvement? Current Opinion in Endocrinology & Diabetes and Obesity. 2017;24(4):285–291. doi: 10.1097/MED.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampasona V., Liberati D. Islet autoantibodies. Current Diabetes Reports. 2016;16(6):p. 53. doi: 10.1007/s11892-016-0738-2. [DOI] [PubMed] [Google Scholar]
- 37.Fousteri G., Ippolito E., Ahmed R., Rahim Hamad A. Beta-cell specific autoantibodies: are they just an indicator of type 1 diabetes? Current Diabetes Reviews. 2017;13(3):322–329. doi: 10.2174/1573399812666160427104157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lounici Boudiaf A., Bouziane D., Smara M., et al. Could ZnT8 antibodies replace ICA, GAD, IA2 and insulin antibodies in the diagnosis of type 1 diabetes? Current Research in Translational Medicine. 2018;66(1):1–7. doi: 10.1016/j.retram.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Mauer M., Doria A. Uric acid and diabetic nephropathy risk. Contributions to Nephrology. 2018;192:103–109. doi: 10.1159/000484284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hovind P., Rossing P., Tarnow L., Johnson R. J., Parving H. H. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58(7):1668–1671. doi: 10.2337/db09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y., Li S. C., Hu J., et al. Gut microbiota profiling in Han Chinese with type 1 diabetes. Diabetes Research and Clinical Practice. 2018;141:256–263. doi: 10.1016/j.diabres.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Aranda D., Zysberg L., García-Linares E., Castellano-Guerrero A. M., Martínez-Brocca M. A., Gutiérrez-Colosía M. R. Emotional abilities and HbA1c levels in patients with type 1 diabetes. Psychoneuroendocrinology. 2018;93:118–123. doi: 10.1016/j.psyneuen.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Nirantharakumar K., Mohammed N., Toulis K. A., Thomas G. N., Narendran P. Clinically meaningful and lasting HbA1c improvement rarely occurs after 5 years of type 1 diabetes: an argument for early, targeted and aggressive intervention following diagnosis. Diabetologia. 2018;61(5):1064–1070. doi: 10.1007/s00125-018-4574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szypowska A., Stelmaszczyk-Emmel A., Demkow U., Łuczyński W. High expression of OX40 (CD134) and 4-1BB (CD137) molecules on CD4+CD25high cells in children with type 1 diabetes. Advances in Medical Sciences. 2014;59(1):39–43. doi: 10.1016/j.advms.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Bresson D., Fousteri G., Manenkova Y., Croft M., von Herrath M. Antigen-specific prevention of type 1 diabetes in NOD mice is ameliorated by OX40 agonist treatment. Journal of Autoimmunity. 2011;37(4):342–351. doi: 10.1016/j.jaut.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1: correlation between CD3+OX40+, CD4+OX40+, and CD8+OX40+ and clinic pathological characteristics. Negative correlation between CD3+OX40+ and CD4+OX40+ and the expression of UA and Cr. No correlation between CD8+OX40+ and the expression of UA and Cr.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.