Abstract
We describe here two approaches introduced by Abrahamsen (1987) that can be used by behavior analysts to interpret neuroscientific data. The first is a “boundary-bridging” approach aimed at understanding the interdisciplinary interactions between the behavioral and the neural levels of analysis while keeping the two domains independent. When presenting the boundary-bridging approach, we describe neuroplasticity, a perspective that describes how changes at the brain level can be understood by examining behavioral factors. In the second part of the paper, we contrast two “boundary-breaking” perspectives: neuropsychology and behavior analytic neuroscience. In neuropsychology, localized brain activation is used to explain behavior. In behavior analytic neuroscience, brain responses are interpreted as behavior. We discuss the conditions under which brain responses can be considered behavior and propose that including brain responses within a behavioral framework may allow carrying out a more sophisticated and temporally detailed behavior analysis.
Keywords: Behaviorism, Neuroscience, Integration, Neuroplasticity, Neuropsychology, Physiology
Some things are easier said than done. There is much talk about the benefits of interdisciplinarity, and behaviorists have spoken of trying to reach out of our traditionally defined domain to interact and fruitfully collaborate with other disciplines (Vyse, 2013). There is not, however, much discussion about the specific ways to pursue interdisciplinarity or the theoretical and philosophical challenges involved in such endeavors. For example, when we talk about collaborations between behavior analysis and neuroscience, we notice from the outset that the units of analysis appear to be different in the two disciplines. Behavioral units are situated acts of the whole organism interacting with and in an environmental context; the power of behavioral formulations comes from the independence of these units from particular topographical constraints (Skinner, 1935). Neural units, on the other hand, seem structurally definite, and there are no obvious analogs of operants or stimulus classes in the neural domain. Given these difficulties, how are behavior analysts supposed to interpret and interact with data from the neural sciences?
Skinner (1938, p. 425) acknowledged the importance of maintaining the independence of a behavioral level of analysis in relation to physiological variables, while concurrently recognizing the importance of neuroscientific research.1 More specifically, Skinner (1988, p. 470) mentioned that “A behavioral analysis has two necessary but unfortunate gaps—the spatial gap between behavior and the variables of which it is a function and the temporal gap between the actions performed upon an organism and the often deferred changes in its behavior. These gaps can be filled only by neuroscience, and the sooner they are filled, the better.” However, are all approaches to neuroscience equally relevant to a science of behavior? Where, in the vast array of methods and findings that characterize the current neuroscientific literature, can behavior analysts find common ground?
In the following, we attempt to begin a discussion around these issues. Without striving here to provide a comprehensive literature review, we identify parts of the neuroscience literature that can be seen as compatible with the theoretical assumptions of behavior analysis, and other parts that might be regarded as incompatible. We also begin a discussion of ways in which neuroscientific tools and methods can enhance our understanding of behavior qua behavior. We frame this discussion in the context of a distinction introduced by Abrahamsen (1987) between “boundary breaking” and “boundary bridging” as we have found the distinction to be a useful way to identify and categorize interactions between formally defined disciplines.
A boundary, in Abrahamsen’s account, represents a conceptual dividing line between the subject matter of one formally defined discipline and another. There may be many ways to characterize boundaries and the reasons they exist (e.g., Bechtel, 2012; Choi & Pak, 2006) but that discussion is outside the scope of this paper, so for present purposes, we assume that what constitutes a boundary is self-evident. The first part of this essay will describe interdisciplinary interactions that can be defined as “boundary bridging” and refer to some possible areas of bridging between a behavioral and a neural perspective, specifically the neuroplasticity approach. An alternative to boundary bridging is presented in the second part of the paper. This second approach, also introduced by Abrahamsen (1987), is named “boundary breaking” in which the traditional ways of delineating disciplinary boundaries itself comes under question. In boundary breaking, the scope of a domain expands to account for data traditionally considered to belong to another domain. Two boundary-breaking approaches, neuropsychology and behavior analytic neuroscience, are presented.
We hasten to qualify our discussion in two important ways. First, in our experience, interactions between defined disciplines can rarely, if ever, be characterized as pure versions of one or the other of these two approaches. Instead, the interactions likely consist of some mix of boundary breaking and boundary bridging. Thus, the two types of interactions are not mutually exclusive. Second, we do not intend to suggest that these two are the only kinds of interactions possible between disciplines. A closer look at varieties of examples of interdisciplinarity may reveal other categories of interaction, so our account should not be viewed as exhaustive.
Boundary Bridging
“Interdisciplinarity” is a synthesis of two or more disciplines, establishing a new level of discourse and integration of knowledge. For example, when nuclear physics is combined with medicine, it leads to new treatments for cancer. When methods from mathematics were transferred to physics, mathematical physics was born, and when they were transferred to meteorological phenomena or stock-market processes, they gave rise to chaos theory. Transferring methods from particle physics to astrophysics produced quantum cosmology, and from the transfer of computer methods to art, computer art was generated. Interdisciplinary efforts can create new disciplines. For instance, quantum information processing amalgamates elements of quantum physics and computer science; bioinformatics combines molecular biology with computer science (Choi & Pak, 2006, p. 355).
A boundary-bridging approach entails an interdisciplinary collaboration between scientific domains. Scientists interested in one domain interact with researchers focused on another area, occasionally giving rise to an entirely new, hybrid discipline. As an example, behavioral scientists and neuroscientists have collaborated in trying to understand the relation between behavioral variables and corresponding neural mechanisms, developing the neuroplasticity approach (e.g., Kilgard, 2012). Neuroplasticity is a boundary-bridging perspective consistent with the idea that the brain is a flexible and continuously changing system. More specifically, neuroplasticity research focuses on the relation between changes at the behavioral level and matching changes at the neural level, showing how brain activity and structures can vary over time as a function of exposure to environmental variables. Contingency-related neural changes have been found to involve several brain areas, including the visual and auditory brain cortices, previously considered to be stable after early critical learning windows. For instance, Recanzone, Schreiner, and Merzenich (1993) demonstrated an increase in the proportion of the cerebral cortex responding electrophysiologically to auditory stimuli whose pitch correlated with reinforcement. More specifically, monkeys’ behavioral responses were differentially reinforced in training a discrimination between tones having very similar frequencies. Consequently, the proportion of brain tissue responding to “behaviorally relevant” frequencies expanded compared to that responding to irrelevant frequencies, as measured by implanted electrodes in the auditory cortex. Importantly, experience-dependent plasticity at the brain level is found at all levels of resolution, from the cellular level (e.g., Bliss & Collingridge, 1993) to the macro-structural level. At the macro-level, for example, quantitative structural assessments show how amounts of gray matter change as a function of learning (e.g., Draganski, Gaser, Kempermann, Kuhn, Winkler, Büchel, & May, 2006). Remarkably, Driemeyer, Boyke, Gaser, Büchel, and May (2008, pp. 3–4) showed that “in some cases, dynamic alterations in gray-matter structure can occur very rapidly within a time range of a single week.” It has also become apparent in the past 20 years that, contrary to previous perspectives, new neurons are continuously generated and the rate of neurogenesis is dependent on environmental factors such as the availability of social interactions (Stranahan, Khalil, & Gould, 2006).
The idea that the organization of cortical areas is dynamic is supported by experimental evidence showing that cortical areas can be “instructed” to rewire to perform different kinds of operations. For instance, Roe, Pallas, Hahm, and Sur (1990) rerouted visual connections to the auditory pathway in ferrets to assess whether the auditory cortex would develop the sensitivity to visual orientation of stimuli typical of the visual cortex. The authors reported that, when connected to input from the retina, the auditory cortex can reorganize in a way that resembles the visual cortex. Importantly, rewired organisms emitted quicker fear responses to visual stimuli compared with intact animals (Newton, Ellsworth, Miyakawa, Tonegawa, & Sur, 2004). Those results are consistent with the finding that fear responses are typically faster when organisms respond to auditory stimuli compared with visual stimuli. Researchers concluded that the auditory cortex rewired in a way that resembled the visual cortex while still retaining some features more typical of the auditory cortex (e.g., higher horizontal interconnectivity). Such rewiring might have allowed rewired organisms to emit fear responses at a higher speed compared with intact animals, which were responding to the visual stimuli by using their visual cortex instead of the faster auditory cortex.
Analogous to a behavior analytic approach, a focus of neuroplasticity research involves understanding how environmental variables yield change over time. Also, neuroplasticity and behavior analysis are both compatible with a selectionist framework. Selectionism is an approach originating from evolutionary biology, proposing that biological and behavioral categories can be explained by repeated instances of variation, selection, and replication over time (e.g., Palmer & Donahoe, 1992). Kilgard (2012), a leading neuroscientist focused on neuroplasticity, has explicitly interpreted experience-related changes in cortical maps from a selectionist perspective. Cortical maps are, for instance, the way populations of neurons responding to specific sound frequencies are spatially distributed in the cortex. The auditory cortex is typically organized tonotopically along gradients that gradually shift from low frequencies to high frequencies, but the relative proportion of neurons responding to specific frequencies has been found to vary as a function of reinforcement contingencies. Kilgard argues that “it is surprising that map plasticity has not been seriously entertained as a source of replication with variation upon which reinforcement-based selection could operate as a possible neural basis for adaptive behavior” (Kilgard, 2012, p. 717). Importantly, Kilgard’s statement not only mentions the basic selectionist categories of variation, selection, and replication (see Hull, Langman, & Glenn, 2001) but also includes a specific reference to reinforcement learning, supporting the idea that neuroplasticity research and behavior analysis share a common theoretical framework.
Summarizing, we described neuroplasticity as a boundary-bridging approach that makes sense of changes in neural morphology and activation patterns by taking into account behavioral variables. We also proposed that the neuroplasticity approach is philosophically close to a selectionist perspective, and as such, it can probably be considered an approach compatible with a behavior analytic view.
Boundary Breaking
A second kind of interaction between formally defined disciplines is characterized by Abrahamsen (1987) as boundary breaking. In boundary breaking, the traditionally maintained subdivisions between disciplines are either explicitly challenged or implicitly ignored. That is, the nature of the interaction is one in which one discipline subsumes the subject matter of another discipline as its own. We will examine two antithetical boundary-breaking approaches, the first one existing in the literature and the second one potentially taken by behavior analysts interested in neural variables: neuropsychology and behavior analytic neuroscience.
Neuropsychology
An example of boundary breaking is found in the literature described as neuropsychology. In neuropsychology, order is described at a level of analysis and that information is used to explain (away) the order seen at another level of analysis. More specifically, this literature takes the position that the behavior of organisms is the result or by-product of brain modules and, in its most extreme forms, argues that the behavior will eventually reduce to the action of molecules. For example, certain parts of the neuropsychology literature assume that the distinctions and categories of psychological analyses will be completely explained as the by-product of neural or neuro-chemical processes (e.g., Bickle, 2008). For instance, Bickle (2008, pp. 48–49) states that “real reductionism in genuinely reductionistic neuroscientific practice is a matter of intervening causally, directly into processes at increasing lower levels of biological organization… The cognitive phenomenon reduces to the cellular or molecular mechanisms intervened into, within the anatomical circuits leading ultimately to the motor peripheries generating the measured behaviors.” This approach can be regarded as a form of explanatory reductionism (Thompson, 1984; Schaal, 2003) defined as viewing the subject matter of a discipline as being an index or a by-product of the organization of another subject matter and its own level of analysis.
There are two reasons that a neuropsychological reductionist view is incompatible with behavior analytic thinking. Skinner’s earliest and perhaps most important contribution toward the development of a science of behavior was to develop and articulate the position that behavior-environment interactions constituted their own, unique level of analysis that did not need support from analyses at other levels (Skinner, 1935; Branch & Schaal, 1990). Some biologists have echoed a similar stance. For instance, the evolutionary biologist Mayr (1985, p. 58) said, “Systems at each hierarchical level have two characteristics. They act as wholes (as if they were a homogenous entity), and their characteristics cannot (not even in theory) be deduced from the most complete knowledge of the components.” Second, neuropsychology is compatible philosophically with an essentialist perspective; behavior analysis is not. Essentialism is described by Palmer and Donahoe (1992, p. 1345) as “the tendency to view categorical phenomena in nature as reflections of universal, enduring qualities intrinsic to each class or unit.” Neuropsychology relies on the essentialist assumption that there is a process mapped to each brain structure (e.g., episodic remembering is explained by pointing at underlying hippocampal activation; fear processing is explained by pointing at the amygdala), and this process-to-structure mapping is typically considered to be very consistent across organisms of the same species.2
It is possible that an essentialist neuropsychological mapping of particular operations to specific brain areas is likely to occur in some phylogenetically older areas of the brain. For instance, some parts of the so-called reptilian brain (that humans share with reptiles and common ancestors) like the medulla oblongata are relatively conserved across species, and the operations carried out appear to be very similar. For example, the medulla oblongata is involved in regulating a variety of vital functions, including heart rate and blood pressure, in a range of species ranging from fish to humans (e.g., Zillmer, Spiers, & Culbertson, 2007, p. 137). However, phylogenetically younger brain areas—e.g., the neocortex—are typically structured in a way that appears to allow a large number of operations to be carried out. The six-layered structural organization of the neocortex—centered on the cortical column as an operational unit—is, in fact, roughly the same across occipital, temporal, parietal, and frontal areas.
Since neocortical areas within distinct lobes carry out different operations, it is plausible that the neocortex is characterized by significant operational flexibility. A specific cortical area typically described as the prefrontal cortex (PFC) is considered by some researchers (e.g., Gaffan, 2002) a clear example of operational flexibility. The PFC is the most “recent” brain area from a phylogenetic perspective in the human brain and represents about one third of the total neocortex. Interestingly, the PFC has proven to be one of the most difficult parts of the neocortex to characterize. Researchers have struggled to reach a consensus on the operations carried out in the area. Gaffan (2002) has proposed that the lack of specialization of the PFC may point to the possibility that phylogenetic pressures have led to the selection of larger areas that do not carry out specific operations. This lack of specialization might have allowed behavioral flexibility and adaptability.
If this explanation is accurate, it may oppose the perspective that selection of specific operational brain modules (e.g., the Language Acquisition Device; Chomsky, 1985) is responsible for the observed differences between human and nonhuman animals. On the contrary, what appears to characterize phylogenetically recent and more uniquely human areas, such as the PFC, is their lack of operational specificity. Specifically, Gaffan mentions that “according to this view, the prefrontal cortex is not just the apex of a hierarchy of specialized cortical areas but also the level at which the principle of hierarchically organized localized specialization of function is discarded. This explains why the prefrontal cortex occupies such a large area without strongly differentiated functional subdivisions” (Gaffan, 2002, p. 1117). Consistently, neurosurgeons have become increasingly aware of the dynamic nature of the cortex affirming, for example, that
Current developments in functional mapping and neuroimaging techniques have radically changed the classical static view on the functional organization of cortical areas, for a new dynamic perspective on the brain (Duffau, 2005). Indeed, many recent investigations have highlighted the dynamic capability of the brain to reorganize itself, both during everyday life (e.g., learning) and after a pathological event (e.g., stroke or glioma) (Ius, Angelini, de Schotten, Mandonnet, & Duffau, 2011, p. 992).
Summarizing, neuropsychology is an example of boundary breaking close to reductionistic and essentialist perspectives, both of which are incompatible with core behavior analytic assumptions. Another approach—opposite in many respects to neuropsychology—is proposed next: behavior analytic neuroscience.
Behavior Analytic Neuroscience
Another instance of boundary breaking is described here as behavior analytic neuroscience. Behavior analytic neuroscience is non-reductionist (and therefore antithetical to neuropsychology) and is concerned with understanding under what conditions brain responses can be considered behavior and interpreted solely within a behavioral framework. In this boundary-breaking scenario, some neuroscientific measurement systems can be regarded simply as extra tools at the disposal of the behavior analyst. For instance, using implanted electrode arrays, the experimenter can measure the real-time activity of hundreds of neurons and train the operant control of brain responses to move external devices (e.g., Schwarz, Lebedev, Hanson, Dimitrov, Lehew, Meloy, & Nicolelis, 2014). Similarly, a real-time measure of brain activity can be obtained noninvasively in human participants by using measurement tools such as electroencephalography (EEG) and event-related potentials (ERPs; see Ortu, 2012 for a detailed description of the technology). ERPs are a millisecond level resolution measure of the coordinated response (post-synaptic potentials) of thousands to millions cortical neurons.3 The behavior analytic neuroscientist might treat these neural responses similarly to lever presses, key pecks, and other traditional dependent variables, as long as they clearly show sensitivity to antecedent and consequential manipulations. Brain responses in this scenario do not have any kind of special status—they do not differ qualitatively from muscle movements—they instead are responses typically not taken into account because undetected by traditional measurement tools.
Historically, researchers interested in operant conditioning have explored measurement systems that allow recording responses that would not be detected by traditional apparati. For example, Hefferline, Keenan, and Harford (1959) used electromyographic amplification to detect very small muscle twitches below the threshold of awareness (see also Laurenti‐Lions, Gallego, Chambille, Vardon, & Jacquemin, 1985). An escape and avoidance contingency was set up by the experimenters so that an increase in rate of the invisible (and involuntary) muscle twitches would interrupt or postpone aversive noise stimulation. The rate of the measured muscle twitches changed according to the contingencies set up by the experimenter, even if participants were not aware of emitting responses. Analogously, an amplified stethoscope allowed Furman (1973) to measure digestive system activity and arbitrarily increase or decrease the rate of peristaltic movements via delivery of social praise to participants suffering from chronic diarrhea. Along the lines of “untraditional” dependent variables within operant research, researchers Sterman, Wyrwicka, & Roth (1969) and Wyrwicka & Sterman (1968) trained cats to produce a specific brain response (the sensorimotor rhythm, SMR) by delivering a food reward contingent on the brain response. After several weeks of training, the experimenters withheld reinforcement after cats produced the SMR, and experimenters measured a clear extinction burst involving the brain response (see Fig. 1). In the following decades, the whole new field of neurofeedback developed, showing the clear sensitivity of a portion of brain activity to operant contingencies (e.g., Sterman & Egner, 2006).
Fig. 1.
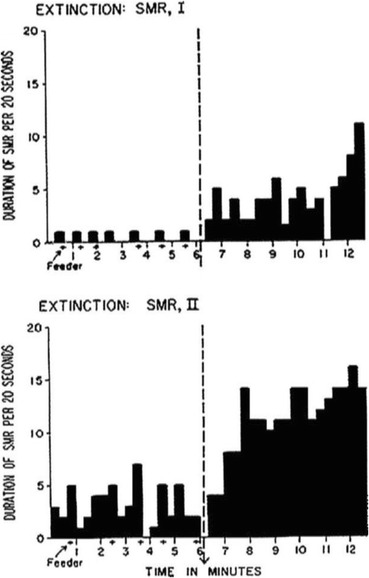
Extinction bursts involving the sensorimotor brain response in cats. The y-axis represents duration of each brain response, and the x-axis represents time in minutes. The dotted vertical line represents the moment in which organisms were put on extinction. Reproduced with permission from Wyrwicka and Sterman (1968)
A framework potentially useful for understanding brain responses from a behavior analytic perspective is the one proposed by Notterman and Mintz (1965) in their operant analysis of subcriterion responses (i.e., responses that do not activate the switch closure but are still above a threshold of detectability by a force transducer). Notterman and Mintz expanded on the traditional digital (binary) nature of operant measurements and introduced an analog analysis based on a continuous measure of response-force, essentially lowering the threshold of response observability. Figure 2 shows a schematic depicting measurement of subcriterion responses. Such measurement captures the moment to moment dynamic nature of the operant, allowing the experimenter to carry out a more sophisticated behavior analysis. Similarly to force transducers, which allow measuring behavioral dynamics that are not captured by the switch closure, we propose here that neuroscientific dependent variables such as EEG and ERPs permit uncovering a further portion of the operant that is not measured by the traditional switch closure or by force transducers. EEG and ERPs, similar to response-force, also represent a continuous measure, and Fig. 3 shows an experimental example of the temporal correlation between response force and an ERP response preceding muscle movement. Figures 1, 3, and 4 also show how traditional dimensions of behavior such as rate/frequency, magnitude, latency, and inter-response time can be measured from brain ERP responses. Interestingly, ERP researchers have used a nomenclature to describe dimensions of brain responses that overlaps considerably with the terminology used by behavioral scientists, especially the ones interested in response force/magnitude. Figure 4 shows specifically how ERP researchers have in fact been measuring peak amplitude of brain responses, peak latency, and inter-peak latency.
Fig. 2.
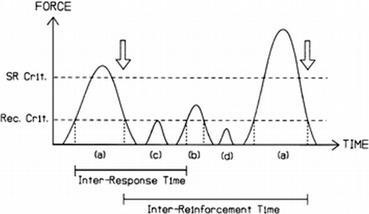
Schematic representing data collection of criterion and subcriterion responses via a force transducer. a Responses exceeding the force requirement indicated by reinforcement criterion (SR Crit.) are scheduled for reinforcement; b, c Responses meeting or exceeding the force requirement indicated by recognition criterion (Rec. Crit.) are recorded as responses but are not scheduled for reinforcement; d Fluctuations below the recognition force criterion are indistinguishable from measurement noise and are not recorded as responses. Reproduced from Brener and Mitchell (1989)
Fig. 3.
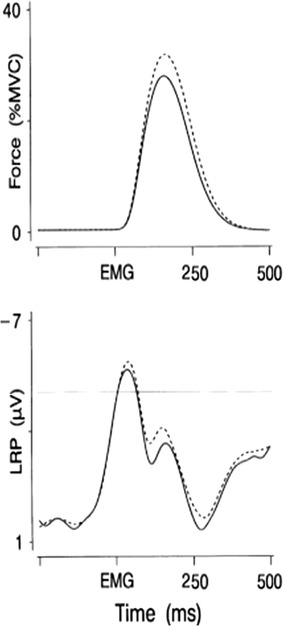
Concurrent brain ERP response (bottom panel) and a muscle EMG response (top panel) are shown. The onset of the brain response is shown preceding the muscle response. Reproduced with permission from van Boxtel, van der Molen, Jennings, and Brunia (2001)
Fig. 4.
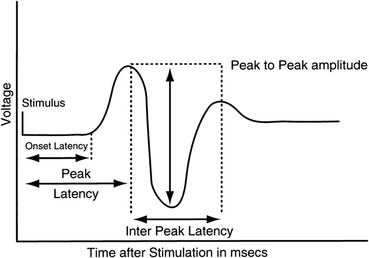
Schematic of a brain response measured through event-related potentials. Highlighted dimensions of the response are peak latency, onset latency, inter-peak latency, and peak-to-peak amplitude. Reproduced with permission from Banoub, Tetzlaff, and Schubert (2003)
As an example of how introducing new measurement tools (including neural measures) may facilitate a more sophisticated behavior analysis, let us consider the traditional ways in which response latency is measured. When considering a response evoked by presentation of a stimulus, occurrence of the switch closure caused by a lever press can be regarded as the onset of the response. The time interval between stimulus delivery and the switch closure is typically described as response latency. However, if we measure initiation of muscle movement preceding the lever press (with electrodes placed on the muscle fiber of interest), we would see that the electromyographic (EMG) response necessarily precedes the lever press. Accordingly, by considering detection of muscle movement as the onset of the response, latency of the response would be shortened. Similarly, brain activity in the motor cortex (measured by EEG) preceding the EMG response and the lever press can occur hundreds of milliseconds before any detectable muscle movement. If the experimenter can measure and describe the reliable temporal sequencing of the three described variables (a lever press preceded by an EMG response preceded by an EEG response), what counts as the moment at which behavior initiates after stimulus presentation? Should the earliest measurable response that is related in an orderly way to antecedent stimulation be considered as the onset of the response? This would allow the possibility of delivering reinforcement accordingly, thereby potentially decreasing the response-reinforcement delay.
A potential advantage of using measurement tools that may allow detecting portions of the operant that are not typically recorded by traditional lever presses or even EMG is that these tools may open the door to measuring occurrence of what have been historically described as covert responses (Skinner, 1953, 1957). Let us consider for instance the case in which a neural response is reinforced before the subsequent motor response; in this situation, the participant may learn not to emit the motor response at all. While this may potentially be deleterious in the case of typically developing participants, measuring and reinforcing neural responses may have a degree of usefulness in paralyzed participants that have lost the availability of some or all motor topographies that are traditionally reinforced. Importantly, brain responses have already been used to create spelling interfaces for patients with neuromuscular disabilities and, in general, brain responses are widely used as behavioral topographies to create brain-computer interfaces (BCIs) with a wide variety of applications (e.g., Wolpaw, Birbaumer, Heetderks, McFarland, Peckham, Schalk, & Vaughan, 2000; Sepulveda, 2011). Moreover, within the neurosciences, researchers have also tried to assess if covertly performing specific motor activities activates brain areas in a similar fashion as behavior carried out via muscle effectors. More specifically, several studies (e.g., Porro, Francescato, Cettolo, Diamond, Baraldi, Zuiani, & Di Prampero, 1996; Schnitzler, Salenius, Salmelin, Jousmäki, & Hari, 1997) compared brain activation in the motor cortices during execution of overt vs. covert motor responses, finding activation in both cases. Importantly, Porro, Francescato, Cettolo, Diamond, Baraldi, Zuiani, and Di Prampero (1996) quantified the difference in magnitude of activation in the motor cortex between covert and overt execution. Activation during motor imagery was 30 % as great as during overt motor performance, suggesting a quantitative, but not qualitative, difference between covert and overt performance. Similarly, a study by Caldara, Deiber, Andrey, Michel, Thut, & Hauert (2004) suggested that the difference between overt and covert responses “consists of a quantitative modulation of the activity of common structures in M1… primary motor structures are involved to the same extent in the actual or imagined execution of a motor act. These findings reinforce and refine the functional-equivalence hypothesis between actual and imagined motor acts.” The functional-equivalence hypothesis states that covert motor behavior can be considered equivalent to overt motor behavior because brain activity measured during covert motor behavior resembles the activity present during overt performance. Importantly, motor imagery can also lead to improvements in overt motor performance, despite the absence of any overt movement (e.g., Driskell, Copper, & Moran, 1994; Feltz & Landers, 1983). Covert practice has been used accordingly to improve overt performance in patients with motor disabilities (Dickstein, Dunsky, & Marcovitz, 2004; Dijkerman, Letswaart, Johnston, & MacWalter, 2004; Johnson-Frey, 2004; Kimberley, Khandekar, Skraba, Spencer, Van Gorp, & Walker, 2006; Stevens & Stoykov, 2003). These results are consistent with behavior analytic theories on the assumption of continuity between overt and covert behavior (e.g., Palmer, 2009).
In sum, in the boundary-breaking approach characterized as neuropsychology neural structures and biochemical factors are seen as having essential and enduring properties that explain the organization of the behavioral phenomenon. Conversely, the approach described as behavior analytic neuroscience is non-reductionist and considers brain responses within an operant framework as behavioral topographies that are undetected by traditional measurement tools.
Conclusion
We elaborated on two approaches proposed by Abrahamsen (1987) potentially useful to relate a behavior analytic approach to neuroscientific variables. The first one, described as boundary bridging, involves an explicit collaboration between levels of analysis. Within boundary bridging, we have described neuroplasticity, centered on how behavioral factors can influence neural structures. We have argued that the neuroplasticity approach tends to be compatible with the behavior analytic perspective, as both are focused on selection processes occurring over time. In the second part of the paper, we discussed the boundary-breaking approach and contrasted neuropsychology, typically focused on understanding the “essential” role of brain structures in explaining behavioral functions, to behavior analytic neuroscience, a direct application of behavior analytic principles to neural data (i.e., neural responses are interpreted as behavior). When describing behavior analytic neuroscience we outlined the similarities in the measured dimensions of motor and brain responses and suggested that by including brain responses in a behavioral framework it may be possible to uncover portions of operant classes that have so far been undetected.
Although the approaches were presented separately for clarity purposes, there are instances in which they can co-occur. Boundary-breaking and boundary-bridging approaches may coexist: discipline A may try to break a boundary and “conquer” some of discipline B’s “territory,” while discipline B may at the same time take into consideration elements from discipline A in a boundary-bridging fashion, i.e., relating elements from disciplines A and B while keeping their respective independence intact. From the perspective of behavior analysis, both approaches appear to be important and should probably be pursued: the neuroplasticity boundary-bridging approach may lead to a deeper understanding of the conditions under which behavioral factors bring about neural changes, while the boundary-breaking behavior analytic neuroscience may help uncover portions of behavior that had previously gone unnoticed because they were not measured with traditional measurement tools. When investigating the interaction between the neural and behavioral levels in neuroplasticity, all variance in brain activity, at all levels of resolution, can be taken into account, together with structural, neuroanatomical observations. Conversely, when investigating neural responses as behavior in behavior analytic neuroscience, only the portion of an activated brain sensitive to antecedents and consequences is taken into account. Neuroplasticity requires an understanding of what is known about the physiology involved in the operations carried out by brain areas and how sub-areas are organized with regard to each other. Behavior analytic neuroscience, conversely, does not necessarily require an understanding of neuroanatomy or of the physiological processes involved (similarly to how detecting a lever press does not require understanding arm or hand physiology), as the brain responses taken into account are treated equivalently to the dependent variables traditionally examined within a purely behavioral framework.
The neuroplasticity approach has generated a great deal of research in the past 20 years. Regarding behavior analytic neuroscience, the experimental analysis of behavior would undoubtedly benefit from focusing on neural responses. Similarly to how Notterman (1959) showed the dissociability of response force and rate during continuous reinforcement, leading to the possibility that different dimensions of behavior may in principle be independent of one another, introducing brain responses into the picture may, for instance, allow having a more complete understanding of the effects of reinforcement on different dimensions of the operant. Moreover, the temporally detailed richness of brain responses may allow establishing fractally nested levels of resolution in which what behavior analysts have described as molecular behavioral analyses, with the typical temporal resolution of a few seconds, may be broken down into micro-behavioral analyses involving events lasting hundreds or tens of milliseconds. Such analyses may allow behavior analysts to contribute developing brain-computer interfaces to allow disabled patients to interact with the environment by gaining operant control of their brain responses. On a different but not unrelated note, such detailed understanding of portions of the operant that have so far been undetected may eventually allow testing some key unresolved questions regarding complex human behavior. For example, the outstanding question regarding a verbal mediation in the emergence of untrained relations in stimulus-equivalence research could benefit from the possibility of measuring covert verbal responses (Horne & Lowe, 1996). Similarly, can relational behavior be explained satisfactorily as a generalized operant or is there a role played by covert verbal responses in relational tasks (Palmer, 2004)? Can some instances of remembering be characterized as iterative covert problem solving (Palmer, 1991)? Using neuroscientific tools to uncover unobserved behavior may help behavior analysts carry out a better-informed and therefore more sophisticated behavior analysis.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
The corresponding author is funded by the Beatrice H. Barrett endowment for research on neuro-operant relations to the University of North Texas.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
For a recent and comprehensive treatment of Skinner’s approach to neuroscience, refer to Zilio (2016).
A potential explanation for this approach is the early and extensive reliance of cognitive science on the computer metaphor in which every bit of observed functionality is seen to be the result of software or hardware under the surface. This perspective might have resulted in the subsequent attempt within neuropsychology to map each behavioral regularity to a part of the underlying physiological substrate, i.e., the brain.
So far, three kinds of EEG/ERP brain responses have been used successfully to create a real-time interface with external devices that require a minimal amount of averaging. More specifically, steady-state visually evoked potentials (SSVEPs), the P300 response, and the Mu Rhythm (Wolpaw, Birbaumer, Heetderks, McFarland, Peckham, Schalk, & Vaughan, 2000; Pfurtscheller, Brunner, Schlögl, & Da Silva, 2006a, b; Sepulveda, 2011).
References
- Abrahamsen AA. Bridging boundaries versus breaking boundaries: psycholinguistics in perspective. Synthese. 1987;72(3):355–388. doi: 10.1007/BF00413752. [DOI] [Google Scholar]
- Banoub M, Tetzlaff JE, Schubert A. Pharmacologic and physiologic influences affecting sensory evoked potentials: implications for perioperative monitoring. The Journal of the American Society of Anesthesiologists. 2003;99(3):716–737. doi: 10.1097/00000542-200309000-00029. [DOI] [PubMed] [Google Scholar]
- Bechtel W. Integrating scientific disciplines: case studies from the life sciences (vol. 2) New York: Springer Science & Business Media; 2012. [Google Scholar]
- Bickle, J. (2008). Real reduction in real neuroscience: metascience, not philosophy of science (and certainly not metaphysics!). Being reduced: new essays on reduction, explanation, and causation, 34–51.
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Branch MN, Schaal DW. Roles and characteristics of theory in behavioral pharmacology. Advances in Behavioral Pharmacology. 1990;7:171–196. [Google Scholar]
- Brener J, Mitchell S. Changes in energy expenditure and work during response acquisition in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15(2):166. [PubMed] [Google Scholar]
- Caldara R, Deiber MP, Andrey C, Michel CM, Thut G, Hauert CA. Actual and mental motor preparation and execution: a spatiotemporal ERP study. Experimental Brain Research. 2004;159(3):389–399. doi: 10.1007/s00221-004-2101-0. [DOI] [PubMed] [Google Scholar]
- Choi BC, Pak AW. Multidisciplinarity, interdisciplinarity and transdisciplinarity in health research, services, education and policy: 1. Definitions, objectives, and evidence of effectiveness. Clinical and Investigative Medicine. 2006;29(6):351. [PubMed] [Google Scholar]
- Chomsky N. Recent contributions to the theory of innate ideas. Netherlands: Springer; 1985. pp. 31–40. [Google Scholar]
- Dickstein R, Dunsky A, Marcovitz E. Motor imagery for gait rehabilitation in post-stroke hemiparesis. Physical Therapy. 2004;84(12):1167–1177. [PubMed] [Google Scholar]
- Dijkerman HC, Letswaart M, Johnston M, MacWalter RS. Does motor imagery training improve hand function in chronic stroke patients? A pilot study. Clinical Rehabilitation. 2004;18(5):538–549. doi: 10.1191/0269215504cr769oa. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Büchel C, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of Neuroscience. 2006;26(23):6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A. Changes in gray matter induced by learning—revisited. PLoS ONE. 2008;3(7):e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell JE, Copper C, Moran A. Does mental practice enhance performance? Journal of Applied Psychology. 1994;79:481–492. doi: 10.1037/0021-9010.79.4.481. [DOI] [Google Scholar]
- Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurology. 2005;4:476–486. doi: 10.1016/S1474-4422(05)70140-X. [DOI] [PubMed] [Google Scholar]
- Feltz DH, Landers DM. The effects of mental practice on motor skill learning and performance: a meta-analysis. Journal of Sport Psychology. 1983;5:25–57. doi: 10.1123/jsp.5.1.25. [DOI] [Google Scholar]
- Furman S. Intestinal biofeedback in functional diarrhea: a preliminary report. Journal of Behavior Therapy and Experimental Psychiatry. 1973;4(4):317–321. doi: 10.1016/0005-7916(73)90001-3. [DOI] [Google Scholar]
- Gaffan, D. (2002). Against memory systems. Philosophical Transactions of the Royal Society B: Biological Sciences 357(1424), 1111–1121. [DOI] [PMC free article] [PubMed]
- Hefferline RF, Keenan B, Harford RA. Escape and avoidance conditioning in human subjects without their observation of the response. Science. 1959;130(3385):1338–1339. doi: 10.1126/science.130.3385.1338. [DOI] [PubMed] [Google Scholar]
- Horne PJ, Lowe CF. On the origins of naming and other symbolic behavior. Journal of the Experimental Analysis of Behavior. 1996;65(1):185–241. doi: 10.1901/jeab.1996.65-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull DL, Langman RE, Glenn SS. A general account of selection: biology, immunology, and behavior. Behavioral and Brain Sciences. 2001;24(03):511–528. doi: 10.1017/S0140525X0156416X. [DOI] [PubMed] [Google Scholar]
- Ius T, Angelini E, de Schotten MT, Mandonnet E, Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain”. NeuroImage. 2011;56(3):992–1000. doi: 10.1016/j.neuroimage.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH. Stimulation through simulation? Motor imagery and functional reorganization in hemiplegic stroke patients. Brain and Cognition. 2004;55(2):328–331. doi: 10.1016/j.bandc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends in Neurosciences. 2012;35(12):715–722. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley TJ, Khandekar G, Skraba LL, Spencer JA, Van Gorp EA, Walker SR. Neural substrates for motor imagery in severe hemiparesis. Neurorehabilitation and Neural Repair. 2006;20(2):268–277. doi: 10.1177/1545968306286958. [DOI] [PubMed] [Google Scholar]
- Laurenti‐Lions L, Gallego J, Chambille B, Vardon G, Jacquemin C. Control of myoelectrical responses through reinforcement. Journal of the Experimental Analysis of Behavior. 1985;44(2):185–193. doi: 10.1901/jeab.1985.44-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E. How biology differs from the physical sciences. In: Depew DJ, Weber BH, editors. Evolution at a crossroads. Cambridge: Bradford Books; 1985. pp. 43–63. [Google Scholar]
- Newton JR, Ellsworth C, Miyakawa T, Tonegawa S, Sur M. Acceleration of visually cued conditioned fear through the auditory pathway. Nature Neuroscience. 2004;7(9):968–973. doi: 10.1038/nn1306. [DOI] [PubMed] [Google Scholar]
- Notterman JM. Force emission during bar pressing. Journal of Experimental Psychology. 1959;58(5):341. doi: 10.1037/h0042801. [DOI] [PubMed] [Google Scholar]
- Notterman JM, Mintz DE. Dynamics of response. Hoboken: Wiley; 1965. [Google Scholar]
- Ortu D. Neuroscientific measures of covert behavior. Behavior Analyst. 2012;35(1):75–87. doi: 10.1007/BF03392267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DC. A behavioral interpretation of memory. In: Hayes LJ, Chase PN, editors. Dialogues on verbal behavior. Reno: Context Press; 1991. pp. 261–279. [Google Scholar]
- Palmer DC. Data in search of a principle: a review of relational frame theory: a post-skinnerian account of human language and cognition. Journal of the Experimental Analysis of Behavior. 2004;81(2):189–204. doi: 10.1901/jeab.2004.81-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DC. The role of private events in the interpretation of complex behavior. Behavior and Philosophy. 2009;37:3–19. [Google Scholar]
- Palmer DC, Donahoe JW. Essentialism and selectionism in cognitive science and behavior analysis. American Psychologist. 1992;47(11):1344–1358. doi: 10.1037/0003-066X.47.11.1344. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlögl A, Da Silva FL. Mu rhythm (de) synchronization and EEG single-trial classification of different motor imagery tasks. NeuroImage. 2006;31(1):153–159. doi: 10.1016/j.neuroimage.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlögl A, Da Silva FL. Mu rhythm (de) synchronization and EEG single-trial classification of different motor imagery tasks. NeuroImage. 2006;31(1):153–159. doi: 10.1016/j.neuroimage.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, et al. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. The Journal of Neuroscience. 1996;16(23):7688–7698. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GA, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. The Journal of Neuroscience. 1993;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe AW, Pallas SL, Hahm JO, Sur M. A map of visual space induced in primary auditory cortex. Science. 1990;250(4982):818–820. doi: 10.1126/science.2237432. [DOI] [PubMed] [Google Scholar]
- Schaal, D. W. (2003). Explanatory reductionism in behavior analysis. In: Behavior theory and philosophy (pp. 83–102). Springer, New York.
- Schnitzler, A., Salenius, S., Salmelin, R., Jousmäki, V., & Hari, R. (1997). Involvement of primary motor cortex in motor imagery: a neuromagnetic study. Neuroimage, 6(3), 201–208. [DOI] [PubMed]
- Schwarz DA, Lebedev MA, Hanson TL, Dimitrov DF, Lehew G, Meloy J, et al. Chronic, wireless recordings of large scale brain activity in freely moving rhesus monkeys. Nature Methods. 2014;11(6):670–676. doi: 10.1038/nmeth.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda F. Brain-actuated control of robot navigation. Rijeka: INTECH Open Access Publisher; 2011. [Google Scholar]
- Skinner BF. The generic nature of the concepts of stimulus and response. Journal of General Psychology. 1935;12:40–65. doi: 10.1080/00221309.1935.9920087. [DOI] [Google Scholar]
- Skinner BF. The behavior of organisms: an experimental analysis. Cambridge: BF Skinner Foundation; 1938. [Google Scholar]
- Skinner BF. Science and human behavior. New York: Simon and Schuster; 1953. [Google Scholar]
- Skinner BF. Verbal behavior. Cambridge: BF Skinner Foundation; 1957. [Google Scholar]
- Skinner BF. Comments and consequences. In: Catania AC, Harnad S, editors. The selection of behavior: the operant behaviorism of B.F. Skinner. New York: Cambridge University Press; 1988. pp. 382–461. [Google Scholar]
- Sterman MB, Egner T. Foundation and practice of neurofeedback for the treatment of epilepsy. Applied Psychophysiology and Biofeedback. 2006;31(1):21–35. doi: 10.1007/s10484-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Sterman MB, Wyrwicka W, Roth SR. Electrophysiological correlates and neural substrates of alimentary behavior in the cat. Annals of the New York Academy of Sciences. 1969;157:723–739. doi: 10.1111/j.1749-6632.1969.tb12916.x. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Stoykov MEP. Using motor imagery in the rehabilitation of hemiparesis. Archives of Physical Medicine and Rehabilitation. 2003;84(7):1090–1092. doi: 10.1016/S0003-9993(03)00042-X. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neuroscience. 2006;9(4):526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. The examining magistrate for nature: a retrospective review of Claude Bernard’s an introduction to the study of experimental medicine. Journal of the Experimental Analysis of Behavior. 1984;41(2):211–216. doi: 10.1901/jeab.1984.41-211. [DOI] [Google Scholar]
- van Boxtel GJ, van der Molen MW, Jennings JR, Brunia CH. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biological Psychology. 2001;58(3):229–262. doi: 10.1016/S0301-0511(01)00117-X. [DOI] [PubMed] [Google Scholar]
- Vyse S. Changing course. Behavior Analyst. 2013;36:123–135. doi: 10.1007/BF03392295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Birbaumer N, Heetderks WJ, McFarland DJ, Peckham PH, Schalk G, et al. Brain-computer interface technology: a review of the first international meeting. IEEE Transactions on Rehabilitation Engineering. 2000;8(2):164–173. doi: 10.1109/TRE.2000.847807. [DOI] [PubMed] [Google Scholar]
- Wyrwicka W, Sterman MB. Instrumental conditioning of sensorimotor cortex EEG spindles in the waking cat. Physiology and Behavior. 1968;3:703–707. doi: 10.1016/0031-9384(68)90139-X. [DOI] [Google Scholar]
- Zilio, D. (2016). Who, what, and when: Skinner’s critiques of neuroscience and his main targets. Behavior Analyst, 1–22. [DOI] [PMC free article] [PubMed]
- Zillmer EA, Spiers MV, Culbertson W. Principles of neuropsychology. Scarborough: Nelson Education; 2007. [Google Scholar]


