Abstract
Objectives:
Coronary artery bypass grafting (CABG) is associated with better survival than percutaneous coronary intervention (PCI) in patients with mild-to-moderate chronic kidney disease (CKD) and End-Stage Renal Disease (ESRD). However, the optimal strategy for coronary artery revascularization in advanced CKD patients who transition to ESRD is unclear.
Methods:
We examined a contemporary national cohort of 971 US veterans with incident ESRD, who underwent first CABG or PCI up to 5 years prior to dialysis initiation. We examined the association of a history of CABG versus PCI with all-cause mortality following transition to dialysis, using Cox proportional hazards models adjusted for time between procedure and dialysis initiation, socio-demographics, comorbidities and medications.
Results:
582 patients underwent CABG and 389 patients underwent PCI. The mean age was 66±8 years, 99% of patients were male, 79% were white, 19% were African Americans, and 84% were diabetics. The all-cause post-dialysis mortality rates after CABG and PCI were 229/1000 patient-years (PY) [95% CI: 205–256] and 311/1000PY [95% CI: 272–356], respectively. Compared to PCI, patients who underwent CABG had 34% lower risk of death [multivariable adjusted Hazard Ratio (95% CI) 0.66 (0.51-0.86), p=0.002] after initiation of dialysis. Results were similar in all subgroups of patients stratified by age, race, type of intervention, presence/absence of myocardial infarction, congestive heart failure and diabetes.
Conclusion:
CABG in advanced CKD patients was associated lower risk of death after initiation of dialysis compared to PCI.
Keywords: all-cause mortality, coronary artery bypass grafting, percutaneous coronary interventions, chronic kidney disease, end-stage renal disease
Graphical abstract
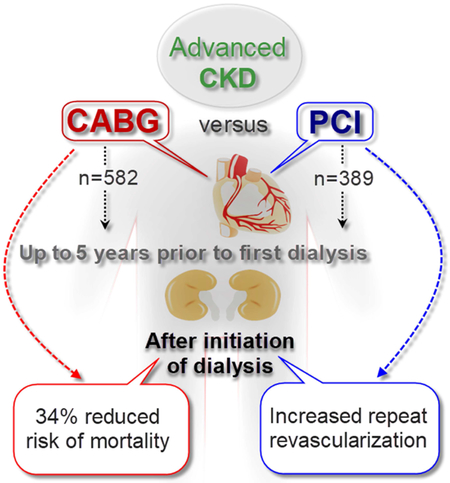
In this large nationally representative cohort of US veterans with incident ESRD, pre-ESRD history of coronary artery revascularization with CABG was associated with a 34% lower multivariable adjusted risk of death after initiation of dialysis compared to PCI.
INTRODUCTION
Patients with chronic kidney disease (CKD) are at increased risk of cardiovascular diseases and related mortality.1-3 In advanced stages of CKD, cardiovascular disease-related mortality rates are much higher than in earliest stages of CKD and in non-CKD populations.1-3 Invasive interventions such as coronary artery bypass grafting (CABG) and percutaneous coronary interventions (PCI) are commonly and successfully used to treat coronary artery disease, but in advanced CKD patients may be an at increased risk of adverse events from these interventions, which may directly impact on their outcomes after transitioning to ESRD.4-8 Physicians should make critical decisions about the optimal strategy of coronary revascularization in this vulnerable group of population with coronary artery disease, balancing their putative benefits with the potential complications associated with the procedures.9-12 Despite advances in coronary revascularization, using second generation drug-eluting stents with radial artery access in PCI and technological developments of CABG surgery, the post-revascularization complications and mortality rate remaining high, especially in CKD population13-18.
Previous randomized controlled trials compared the effects of PCI vs. CABG in patients with coronary artery disease, but typically excluded patients with advanced CKD due to increased risk of major complications (e.g. bleeding, acute kidney injury or short-term risk of death).19-22 Consequently, results from such clinical trials cannot be extrapolated to patients with advanced CKD, in whom the risk-benefit ratio of the two procedures may be shifted because of their different effects on outcomes such as acute kidney injury. Previous retrospective studies in large cohorts reported that compared to PCI, CABG is associated with better survival in mild-to-moderate CKD, in ESRD, and in diabetics.23-26 However, the optimal strategy for coronary artery revascularization in advanced CKD patients is unclear. To address this knowledge gap, we aimed to study post-ESRD all-cause mortality associated with history of CABG versus PCI performed in advanced CKD patients prior to transitioning to ESRD, using a large nationally representative cohort of US veterans with incident ESRD who underwent a first CABG or PCI up to 5 years prior to dialysis initiation. We hypothesized that CABG is associated with reduced mortality compared to PCI in this population.
METHODS
Study Population
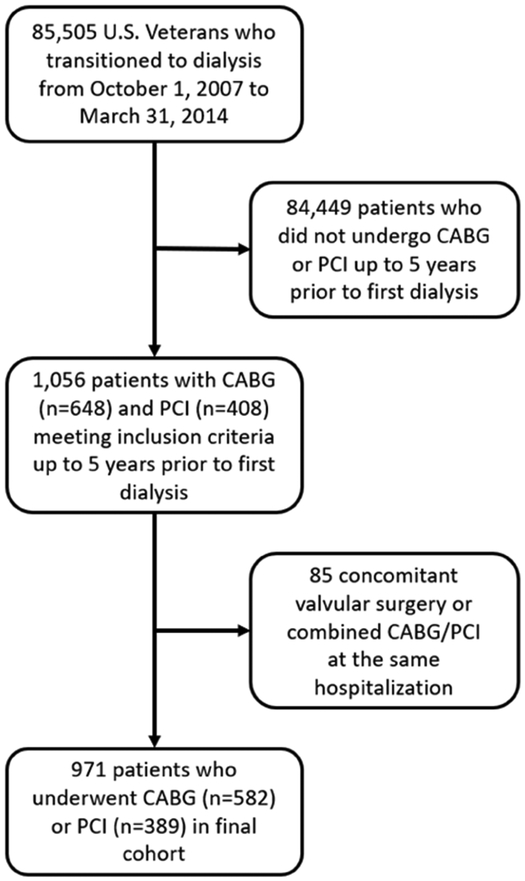
We studied longitudinal data from the Transition of Care in CKD (TC-CKD) study, a historical cohort study examining US veterans with incident ESRD transitioning to dialysis from October 1, 2007 through March 31, 2014.27, 28 A total of 85,505 US veterans were identified from the US Renal Data System (USRDS)29 as a source population. The algorithm for the cohort definition is shown in Figure 1. Patients who did not receive CABG or PCI up to 5 years prior to first dialysis initiation were excluded. For the present study, 1,056 patients who underwent PCI or CABG up to 5 years prior to ESRD (defined as the date of first maintenance dialysis service) were included. Patients receiving both CABG and PCI during the same hospitalization and patients undergoing concomitant ventricular reconstruction or pericardial or valve surgery, were excluded. The final study population consisted of 971 patients, of whom 582 underwent CABG and 389 underwent PCI.
Figure 1.
Flow chart of the patients’ selection.
Abbreviations: CABG, coronary artery bypass grafting; PCI, percutaneous coronary interventions.
Exposures and covariates
A history of CABG surgery and PCI procedure types were determined from International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) procedure codes and Current Procedural Terminology (CPT) procedure codes in the Veterans Affairs (VA) Inpatient or Outpatient Medical SAS Datasets and categorized according to the Clinical Classifications Software procedural classification system (Table E1) Based on CPT and ICD-9 secondary codes, revascularizations were stratified as single- or multivessel procedures. Information about baseline age, race, sex, marital status, per capita income and body mass index (BMI) were obtained from national VA research data files, as previously described.9, 30 Information about comorbidities (diabetes, hypertension, ischemic heart disease, myocardial infarction, cerebrovascular disease, congestive heart failure, peripheral vascular disease, atrial fibrillation, chronic pulmonary disease, connective tissue disease, paraplegia and hemiplegia, hyperlipidemia, liver disease, peptic ulcer disease, depression, dementia, malignancy and anemia) within six months prior to the studied coronary artery revascularization procedure was extracted from VA Inpatient and Outpatient Medical SAS Datasets, and from Centers for Medicare & Medicaid Services (CMS) datasets using diagnostic and procedure codes.31 The Charlson Comorbidity Index score was calculated using the Deyo modification for administrative datasets, without including kidney disease.32 Medication use (angiotensin converting enzyme inhibitor and angiotensin receptor blocker (ACEIs/ARBs), β-blockers, α-blockers, statins, calcium channel blockers, thiazide diuretics, loop diuretics, potassium sparing diuretics, anticoagulants, thrombolytics, aspirin, digitalis, antianginals, vasodilators, antidiabetic agents and radiocontrast material) was determined from VA pharmacy dispensation records in the six months prior to coronary artery revascularization.33
Blood hemoglobin and serum albumin levels were obtained from VA research databases as previously described,9, 30 and their baseline values were defined as the average of each covariate during the six months period preceding CABG or PCI. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation.34, 35 Time to ESRD was defined as the period between the day of coronary revascularization and the first day of dialysis initiation.
Outcome Assessment
The primary outcome of interest was all-cause mortality after dialysis therapy initiation. All-cause mortality data, censoring events, and associated dates were obtained from VA and USRDS data sources.29 The start of the follow-up period was the date of dialysis therapy initiation, and patients were followed up until death or other censoring events, including kidney transplantation, loss of follow-up, or March 31, 2014.
Statistical Analysis
Data are summarized as percentages for categorical variables and as mean ± standard deviation (SD) or median (quartile Q1-Q3), as appropriate. Categorical variables were compared using χ2 tests. Continuous variables were compared using t tests or Mann–Whitney U tests, or ANOVA, as appropriate.
The association of a history of CABG vs. PCI with all-cause mortality was estimated using the Kaplan-Meier method and Cox proportional hazards models. Models were incrementally adjusted for the following potential confounders based on theoretical considerations and their availability in this study: model 1: adjusted for time between procedure and ESRD initiation; model 2: additionally adjusted for demographics (age, sex, race/ethnicity, marital status and income); model 3: additionally adjusted for comorbidities (diabetes, malignancy, liver diseases, hypertension, ischemic heart disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, anemia, atrial fibrillation, depression, hyperlipidemia), eGFR and BMI; model 4: additionally adjusted for medications (anticoagulants, thrombolytic, aspirin, digitalis, beta-blockers, alpha-blockers, CCBs, antianginals, statins, vasodilators, thiazide diuretics, loop diuretics, potassium sparing diuretics, ACEIs/ARBs, radiocontrast and antidiabetics), procedure type (single- vs. multi-vessel) and model 5: additionally adjusted for baseline blood hemoglobin, serum albumin, and systolic and diastolic blood pressure.
891 (92%) patients had complete data for analysis in Model 4. In model 5 an additional 420 cases (43% of cohort) had missing variables. Therefore, we regarded model 4 as our main multivariable adjusted model without replacing missing data, but, due to the relatively high proportion of missingness (43%) in model 5, missing covariates were imputed using multiple imputations27.
We conducted several sensitivity analyses to evaluate the robustness of our main findings. The association of a history of CABG and PCI with post ESRD mortality were also examined in subgroups of patients stratified by age (<65 or ≥65 years), race (African-Americans or others), type of intervention (single- or multivessel) and presence/absence of myocardial infarction, congestive heart failure and diabetes. Potential interactions were formally tested by including relevant interaction terms. We also examined the multivariable adjusted (model 4) association of a history of CABG vs. PCI with all-cause mortality in cohorts of patients who underwent these procedures up to one and up to two years prior to dialysis initiation as well as separately in the subgroup of patients who underwent multi-vessel intervention. Additionally, the association of a history of CABG vs. PCI with all-cause mortality was evaluated after 1: 1 propensity score matching in the subgroup of patients who underwent multi-vessel intervention.
P values are two-sided and reported as significant at <0.05 for all analyses. All analyses were conducted using STATA MP Version 15 (STATA Corporation, College Station, TX). The study was approved by the Institutional Review Boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
RESULTS
Baseline characteristics
Patients’ baseline characteristics in the overall cohort and stratified by type of coronary artery revascularization are presented in Table 1. The overall mean±SD age at baseline was 64±8 years; 99% of patients were male; 19% were African American and 84% were diabetics. Patients who underwent PCI (compared to CABG) had higher prevalence of hypertension and chronic pulmonary disease, and lower prevalence of diabetes. Patients who underwent CABG were more likely to use statins, anticoagulants, antidiabetics, aspirin, antianginals, and less likely to use ACEIs/ARBs. In the PCI group compared to CABG group, 73% vs. 12% of patients received single vessel and 29% vs. 88% of patients received multivessel treatments, respectively (p<0.001). The eGFR at the time of procedure and the time to ESRD transition were similar in the PCI and CABG groups, but patients who underwent CABG had significantly lower levels of blood hemoglobin and serum albumin. BMI and systolic and diastolic blood pressure did not differ between the PCI and CABG groups. Patients who underwent PCI, compared to CABG had significantly higher incidence of repeat revascularization (17% versus 4%, p<0.001; Table 1).
Table 1.
Baseline characteristics of patients
| All (n=971) | PCI (n=389) | CABG (n=582) | P value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 64±8 | 65±9 | 64±8 | 0.115 | |
| Gender (male), n (%) | 959 (99) | 384 (99) | 575 (99) | 0.909 | |
| Race, n (%) | 0.576 | ||||
| White | 725 (74) | 292 (75) | 433 (74) | ||
| African-American | 180 (19) | 76 (20) | 104 (18) | ||
| Native American and Asian | 17 (2) | 5 (1) | 12 (2) | ||
| Unknown | 49 (5) | 16 (4) | 33 (6) | ||
| Ethnicity (Hispanic), n (%) | 73 (8) | 23 (6) | 50 (9) | 0.121 | |
| Marital status, n (%) | 0.002 | ||||
| Married | 480 (49) | 216 (55) | 264 (45) | ||
| Single | 86 (9) | 22 (6) | 64 (11) | ||
| Divorced | 318 (33) | 115 (30) | 203 (35) | ||
| Widowed | 87 (9) | 36 (9) | 51 (9) | ||
| Income, $ | 20,225 (10,620-35,028) | 22,788 (11,000-36,276) | 18,741 (10,488-33,876) | 0.207 | |
| Comorbidities at time of procedure | |||||
| Diabetes, n (%) | 773 (80) | 294 (76) | 479 (82) | 0.011 | |
| Hypertension, n (%) | 883 (91) | 368 (95) | 515 (88) | 0.001 | |
| Myocardial Infraction, n (%) | 322 (33) | 119 (31) | 203 (35) | 0.164 | |
| Cerebrovascular disease, n (%) | 297 (31) | 108 (28) | 189 (32) | 0.119 | |
| Congestive heart failure, n (%) | 416 (43) | 172 (44) | 244 (42) | 0.480 | |
| Peripheral vascular disease, n (%) | 346 (36) | 130 (33) | 216 (37) | 0.239 | |
| Atrial Fibrillation, n (%) | 65 (7) | 35 (9) | 30 (5) | 0.019 | |
| Chronic pulmonary disease, n (%) | 334 (34) | 154 (40) | 180 (31) | 0.005 | |
| Connective Tissue Disease, n (%) | 24 (3) | 14 (4) | 10 (2) | 0.064 | |
| Paraplegia and Hemiplegia, n (%) | 23 (2) | 10 (3) | 13 (2) | 0.735 | |
| Hyperlipidemia, n (%) | 750 (77) | 303 (78) | 447 (77) | 0.692 | |
| Liver disease, n (%) | 32 (3) | 13 (3) | 19 (3) | 0.947 | |
| Peptic ulcer disease, n (%) | 32 (3) | 13 (3) | 19 (3) | 0.947 | |
| Depression, n (%) | 230 (24) | 100 (26) | 130 (22) | 0.226 | |
| Dementia, n (%) | 8 (0.8) | 7 (2) | 1 (0.2) | 0.006 | |
| Malignancy, n (%) | 123 (13) | 56 (14) | 67 (12) | 0.186 | |
| Anemia, n (%) | 317 (33) | 134 (34) | 183 (31) | 0.328 | |
| Charlson Comorbidity Index | 4(2-5) | 4 (2-5) | 4 (2-5) | 0.675 | |
| Vital parameters at time of procedure | |||||
| Body mass index (kg/m2) | 29.9±5.7 | 30.1±6.0 | 29.8±5.6 | 0.322 | |
| Systolic BP (mmHg) | 144±16 | 144±17 | 144±16 | 0.675 | |
| Diastolic BP (mmHg) | 75±10 | 75±11 | 75±10 | 0.605 | |
| Laboratory parameters at time of procedure | |||||
| Blood hemoglobin, (g/dL) | 11.6±1.7 | 11.9±1.8 | 11.4±1.5 | <0.001 | |
| Serum albumin (g/dL) | 3.43±0.58 | 3.56±0.59 | 3.35±0.56 | <0.001 | |
| eGFR, (ml/min/1.73 m2) | 34 (21-53) | 33 (20-51) | 34 (22-53) | 0.159 | |
| Medications at time of procedure | |||||
| ACEIs/ARBs, n (%) | 741 (24) | 106 (27) | 458 (21) | 0.033 | |
| β-blockers, n (%) | 858 (88) | 335 (86) | 523 (90) | 0.075 | |
| α-blockers, n (%) | 266 (27) | 121 (31) | 145 (25) | 0.034 | |
| Calcium channel blockers, n (%) | 616 (63) | 240 (62) | 376 (65) | 0.356 | |
| Statins, n (%) | 831 (86) | 320 (82) | 511 (88) | 0.016 | |
| Thiazide diuretics, n (%) | 284 (29) | 112 (29) | 172 (30) | 0.798 | |
| Loop diuretics, n (%) | 609 (63) | 245 (63) | 364 (63) | 0.890 | |
| Potassium sparing diuretics, n (%) | 86 (9) | 39 (10) | 47 (8) | 0.295 | |
| Anticoagulants, n (%) | 376 (39) | 135 (35) | 241 (41) | 0.036 | |
| Thrombolytics, n (%) | 5 (0.5) | 0 | 5 (0.8) | 0.067 | |
| Aspirin, n (%) | 712 (73) | 252 (65) | 460 (79) | <0.001 | |
| Digitalis, n (%) | 46 (5) | 23 (6) | 23 (4) | 0.159 | |
| Antianginals, n (%) | 611 (63) | 205 (53) | 406 (70) | <0.001 | |
| Vasodilators, n (%) | 320 (33) | 128 (33) | 192 (33) | 0.978 | |
| Antidiabetics, n (%) | 699 (72) | 223 (57) | 476 (82) | <0.001 | |
| Radiocontrast, n (%) | 5 (0.5) | 2 (0.5) | 3 (0.5) | 0.998 | |
| No. of diseased vessels | <0.001 | ||||
| Single vessel, n (%) | 348 (36) | 277 (71) | 71 (12) | ||
| Multi vessel, n (%) | 621 (64) | 112 (29) | 509 (88) | ||
| Repeat revascularization, n (%) | 94 (10) | 68 (17) | 26 (4) | <0.001 | |
| Repeated CABG, n (%) | 26 (3) | 18 (5) | 8 (1) | ||
| Repeated PCI, n (%) | 68 (7) | 50 (13) | 18 (3) | ||
| Time to ESRD, days | 775 (228-1251) | 769 (222-1252) | 781 (240-1250) | 0.709 | |
Data are presented as mean±SD. median and Q1-Q3 and number of observation and percentage as appropriate
Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BP, blood pressure; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary interventions; ESRD, end-stage renal disease.
All-cause mortality
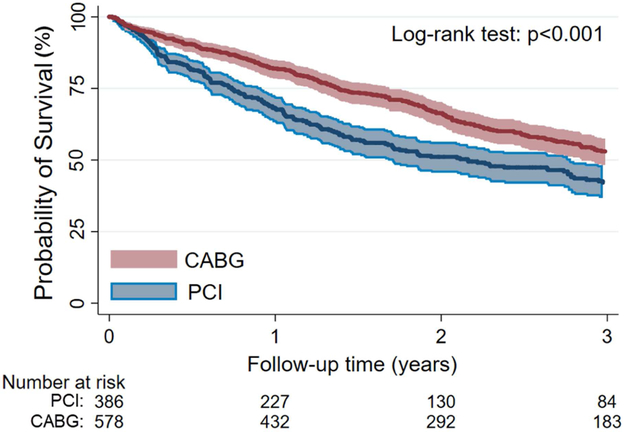
During the median 1.5-year (617 (Q1-Q3: 289-1,155) days) follow-up period after dialysis initiation, there were 523 deaths (54%) in the overall cohort. The crude mortality rate in patients who underwent CABG and PCI were 229/1,000 patient-years [95% Confidence Interval (CI): 205-256] and 311/1,000 patient-years [95% CI: 272–356], respectively. In Kaplan Meier survival analysis (Figure 2), the survival probability was lower in patients who underwent PCI (p<0.001).
Figure 2.
Probability of Survival in the CABG and PCI group in the entire cohort
Abbreviations: CABG, coronary artery bypass grafting; PCI, percutaneous coronary interventions.
Table 2 and Table E2 shows the association of a history of CABG (vs. PCI) with all-cause mortality after initiation of dialysis in different models of multivariable Cox regression. In model 1, patients who underwent CABG had a 26% lower risk of death [Hazard Ratio (HR) (95% CI) 0.74 (0.62–0.88), p=0.001] after initiation of dialysis, which remained similar after multivariable adjustments [HR (95% CI) 0.66 (0.51–0.86), p=0.002]. Qualitatively similar results were observed after multiple imputations for model 5 [HR (95% CI): 0.64 (0.50–0.83), p=0.001]. The results also remained similar in the cohort of patients who underwent a first coronary revascularization one or two years prior to the dialysis initiation [HR (95% CI): 0.42 (0.25–0.68), p=0.001 and 0.46 (0.31–0.66), p<0.001, respectively] (Table E3).
Table 2.
Association between post-ESRD all-cause mortality and type of revascularization (CABG vs PCI (reference)) using Cox proportional hazards models
| Models | Patients/Events | HR | 95% CI | p-value |
|---|---|---|---|---|
| Model-1 | 964/523 | 0.74 | 0.62–0.88 | p=0.001 |
| Model-2 | 915/498 | 0.72 | 0.60–0.86 | p<0.001 |
| Model-3 | 893/481 | 0.65 | 0.53–0.80 | p<0.001 |
| Model-4 | 891/480 | 0.66 | 0.51–0.86 | p=0.002 |
Data are presented as odd ratio (95% CI) unless otherwise specified.
Models are as follows:
Model 1: adjusted for time between procedure and ESRD initiation;
Model 2: additionally adjusted for demographics (age, sex, race/ethnicity, marital status and income);
Model 3: additionally adjusted for comorbidities (diabetes, malignancy, liver diseases, hypertension, ischemic heart disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, anemia, atrial fibrillation, depression, hyperlipidemia), eGFR and BMI;
Model 4: additionally adjusted for medications (anticoagulants, thrombolytic, aspirin, digitalis, beta-blockers, alpha-blockers, CCBs, antianginals, statins, vasodilators, thiazide diuretics, loop diuretics, potassium sparing diuretics, ACEIs/ARBs, radiocontrast and antidiabetics), procedure type (single- vs. multi-vessel).
Abbreviations: ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BP, blood pressure; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary interventions; ESRD, end-stage renal disease.
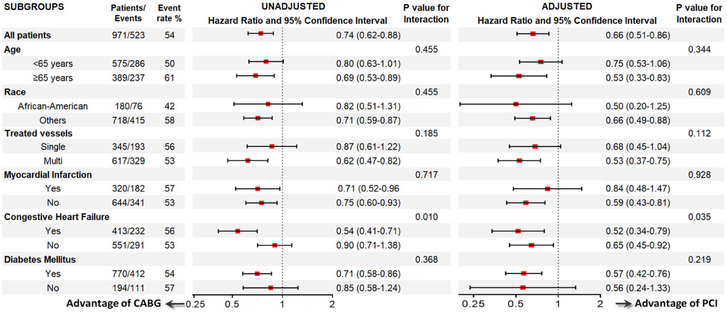
CABG was associated with better survival compared to PCI in all other subgroups (Figure 3). A statistically significant interaction was present for congestive heart failure, with stronger associations of reduced mortality in patients who underwent CABG in the presence of congestive heart failure. CABG was also associated with better survival compared to PCI in the subgroup of patients who underwent multivessel-treatment (Table E4), even after propensity score matching (Table E5 and Figure E1).
Figure 3.
Association between post-ESRD all-cause mortality and type of revascularization (CABG vs PCI (reference)) using Cox proportional hazards models in selected subgroups.
Abbreviations: CABG, coronary artery bypass grafting; PCI, percutaneous coronary interventions; ESRD, end-stage renal disease.
DISCUSSION
In this large nationally representative cohort of US veterans with incident ESRD, we found that pre-ESRD history of coronary artery revascularization with CABG was associated with a 26% lower risk of death after initiation of dialysis compared to PCI. After adjustment for potential cofounders such as demographics, comorbidities, baseline eGFR, BMI, medications, and number of treated vessels, CABG remained associated with 34% lower risk of death (Graphical abstract).
Several randomized clinical trials had compared short-term and long-term outcomes after CABG versus PCI in patients with left main, equivalent to left main, or multivessel coronary artery disease.19, 23,26, 36-39 Some of the clinical trials demonstrated that the rates of death and myocardial infarction were similar between the PCI and CABG groups,36 implementation of PCI with drug-eluting stents were noninferior to CABG regarding major adverse cardiac and cerebrovascular events (MACCE),37, 39 even after 5 years follow-up,19 however, PCI was associated with increased rate of repeat revascularization.36 In contrast to these clinical trials, CABG had significantly reduced rates of death and myocardial infarction, but with a higher rate of stroke, in diabetic patients26 and those with mild to moderate CKD in the same cohort.38 However, these randomized trials did not included patients with advanced CKD, hence more generalizable results for CKD population comes from retrospective cohorts.
The results from retrospective studies in large cohorts involving CKD patients were contradictory.13, 14, 40-43 A few studies reported similar advantages of PCI vs. CABG regarding post procedural mortality,13, 42 whereas, another few studies demonstrated significantly lower rates of death and MACCE associated with CABG compared to PCI.14, 21, 40 Such diverse results of outcomes might be due to investigation of various cohorts consisting of data from different revascularization eras and different proportion of CKD stages, with minority of severe CKD.
The results of our study, regarding reduced long-term mortality associated with a history of CABG compared to PCI, were comparable with the main results of FREEDOM Trial38 and some retrospective cohort studies.14, 21, 40 However, we examined patients with moderate and advanced CKD, whereas the previous cohort studies included variable proportions of patients with mild-to-moderate CKD. The above-mentioned cohort studies comparing outcomes of CABG vs. PCI in CKD investigated patients with multivessel coronary artery disease. There is a lack of knowledge regarding the ideal intervention strategy in patients with advanced CKD who have single vessel coronary artery disease. In the general population with single vessel coronary artery disease, PCI is considered the optimal revascularization strategy,44 but the risk-benefit ratio may be altered in patients with advanced CKD, who have increased risk of bleeding, fluid overload, AKI, infections and other complications.45 Various risk scores can aid in decision-making about the optimal revascularization strategy in an individual patient,46-48 but these scores haven’t been validated for patients with advanced CKD. In our study, CABG was associated with reduced risk of death compared to PCI in all subgroups, including subgroup of patients with single and multivessel treatments. Results were similar in the subgroup of patients who underwent multi-vessel intervention.
Cardiovascular disease is one of the major causes of early post-ESRD mortality along with infections.29, 49, 50 Sudden cardiac death, heart failure and myocardial infarction are the most frequent cause of cardiovascular mortality during first 5 years after dialysis initiation.51, 52 The mechanisms underlying the higher long-term mortality associated with PCI compared to CABG is not clear. As an exploring mechanisms play role negative vascular remodeling and neointimal cell proliferation of the stented vessels segment, that leads to progression of atherosclerosis and restenosis, following PCI.53 Furthermore, these mechanism could worsened in ESRD patients due to malnutrition, inflammation, atherosclerosis.54
The strengths of this study include its large size, its nationally representative nature, and the examination of patients with advanced CKD. Our study also has limitations that deserve to be mentioned. First, because this was a retrospective observational study, we were unable to collect information on severity and complexity of coronary artery lesions which were used to decide the type of revascularization procedure and its urgent (most likely in PCI) vs. elective (most likely in CABG) nature. Therefore, only associations, but not cause-effect relationships, can be established in this study. Second, most of our patients were male US veterans; hence, the results may not be generalizable to women or other patient populations, in particular those outside the US. Third, due to the observational nature of our study, adjusted analyses were limited to pre-procedural (pre-operative) confounders measured and available in our cohort, and therefore our study may be limited by potential residual confounding from unmeasured intraoperative and postoperative confounders, such as radiocontrast material type and volume, baseline left ventricle ejection fraction, proteinuria and others. Differences in outcomes may be affected by indication bias, as the decision to perform CABG vs. PCI may have been motivated by unmeasured factors in individual patients. Finally, our data was derived from a cohort of incident ESRD patients, hence we could not include information from patients undergoing revascularization procedures with advanced CKD but who died before dialysis or did not initiate dialysis for other reasons. However, to minimize this bias, we provided sensitivity analyses by examining patients who underwent coronary revascularization close to the dialysis start date (within up to one and up to two years), and the results remained similar.
CONCLUSION
In conclusion, CABG in advanced CKD patients was associated with 34% lower risk of death after initiation of dialysis compared to PCI. Decisions about the optimal revascularization strategy needs to balance short-term and long-term risks and benefits of CABG vs. PCI in advanced CKD patients (Video 1).
Supplementary Material
Video 1. Dr Gaipov discussing the background and relevance of the study.
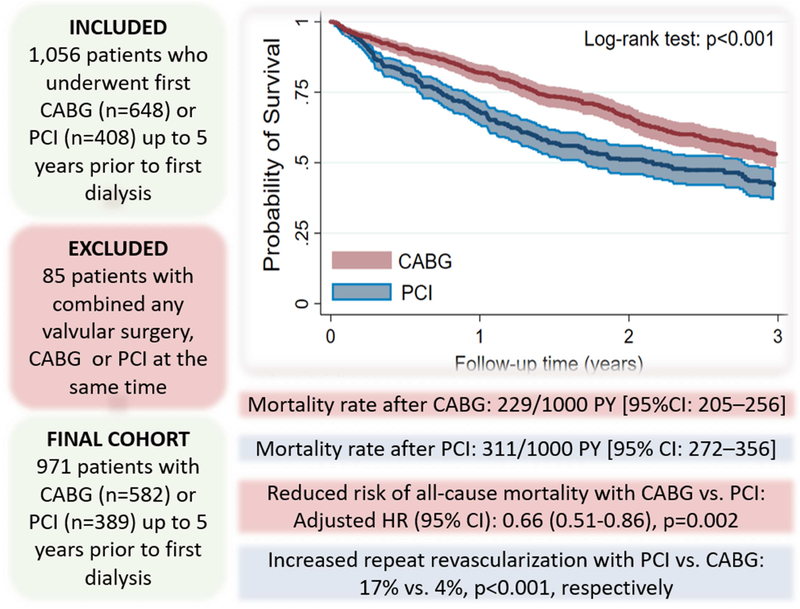
Central picture.
Retrospective cohort study design and main outcomes
Central message.
Optimal strategy for coronary revascularization in CKD patients who transition to ESRD is proposed. Pre-ESRD coronary revascularization history with CABG vs. PCI had a 34% lower risk of post ESRD death.
Perspective statement.
Decisions about optimal strategy needs to balance risks and benefits of CABG vs. PCI in CKD patients
ACKNOWLEDGMENTS
CPK and KKZ are employees of the Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs. The results of this paper have not been published previously in whole or part. AG was supported by the International Society of Nephrology (ISN) research fellowship program and this work has been made possible through an ISN-funded fellowship. An oral presentation based on this manuscript was presented at ASN Kidney Week 2017 in New Orleans, Louisiana, USA.
Part of this study was presented as an oral presentation at the ASN Kidney Week 2017 (Annual congress of American Society of Nephrology, New Orleans, Oct. 31 to Nov. 5, 2017) and the abstract was selected for webcast in Renal & Urology News. Dr. Kovesdy’s discussion about the background and relevance of our study can be accessed at: http://www.renalandurologynews.com/kidney-week-2017/post-dialysis-survival-odds-better-after-cabg-vs-pci/article/703831/
Funding Support: This study is supported by grant 5U01DK102163 from the National Institute of Health (NIH) to KKZ and CPK, and by resources from the US Department of Veterans Affairs. The data reported here have been supplied in part by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Glossary of Abbreviations:
- BMI
body mass index
- CABG
coronary artery bypass grafting
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- PCI
percutaneous coronary intervention
Footnotes
Disclosures: None of the authors have relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Weiner DE, Tabatabai S, Tighiouart H, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. American journal of kidney diseases. 2006;48:392–401. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003: S24–31. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Pozzoni P, Tentori F, del Vecchio L. Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol Dial Transplant. 2003;18 Suppl 7:vii2–9. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Kovesdy CP, Streja E, et al. Transition of care from pre-dialysis prelude to renal replacement therapy: the blueprints of emerging research in advanced chronic kidney disease. Nephrol Dial Transplant. 2017;32:ii91–ii98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumida K, Kovesdy CP. Disease Trajectories Before ESRD: Implications for Clinical Management. Semin Nephrol. 2017;37:132–143. [DOI] [PubMed] [Google Scholar]
- 6.Arif FM, Sumida K, Molnar MZ, et al. Early Mortality Associated with Inpatient versus Outpatient Hemodialysis Initiation in a Large Cohort of US Veterans with Incident End-Stage Renal Disease. Nephron. 2017;137:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharief S, Hsu CY. The Transition From the Pre-ESRD to ESRD Phase of CKD: Much Remains to Be Learned. Am J Kidney Dis. 2017;69:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iribarne A, DiScipio AW, Leavitt BJ, et al. Comparative Effectiveness of CABG versus PCI in a Real-World STICH Population. The Journal of Thoracic and Cardiovascular Surgery. 2018. [Google Scholar]
- 9.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015; 132:1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigatto C, Komenda P, Tangri N. CABG or PCI: which is better for revascularization of coronary artery disease in chronic kidney disease? Kidney Int. 2016;90:257–259. [DOI] [PubMed] [Google Scholar]
- 11.Shroff GR, Herzog CA. Coronary Revascularization in Patients with CKD Stage 5D: Pragmatic Considerations. J Am Soc Nephrol. 2016;27:3521–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueki C, Miyata H, Motomura N, et al. Off-pump technique reduces surgical mortality after elective coronary artery bypass grafting in patients with preoperative renal failure. J Thorac Cardiovasc Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 13.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Revascularization in Patients With Multivessel Coronary Artery Disease and Chronic Kidney Disease: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. J Am Coll Cardiol. 2015;66:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts JK, Rao SV, Shaw LK, Gallup DS, Marroquin OC, Patel UD. Comparative Efficacy of Coronary Revascularization Procedures for Multivessel Coronary Artery Disease in Patients With Chronic Kidney Disease. Am J Cardiol. 2017;119:1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vora AN, Stanislawski M, Grunwald GK, et al. Association Between Chronic Kidney Disease and Rates of Transfusion and Progression to End-Stage Renal Disease in Patients Undergoing Transradial Versus Transfemoral Cardiac Catheterization-An Analysis From the Veterans Affairs Clinical Assessment Reporting and Tracking (CART) Program. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomai F, Ribichini F, De Luca L, et al. Randomized Comparison of Xience V and Multi-Link Vision Coronary Stents in the Same Multivessel Patient With Chronic Kidney Disease (RENAL-DES) Study. Circulation. 2014;129:1104–1112. [DOI] [PubMed] [Google Scholar]
- 17.Singh A, Schaff HV, Mori Brooks M, et al. On-pump versus off-pump coronary artery bypass graft surgery among patients with type 2 diabetes in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial. Eur J Cardiothorac Surg. 2016;49:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuguchi KA, Huang CC, Shempp I, Wang J, Shekar P, Frendl G. Predicting kidney disease progression in patients with acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:2455–2463 e2455. [DOI] [PubMed] [Google Scholar]
- 19.Ahn JM, Roh JH, Kim YH, et al. Randomized Trial of Stents Versus Bypass Surgery for Left Main Coronary Artery Disease: 5-Year Outcomes of the PRECOMBAT Study. J Am Coll Cardiol. 2015;65:2198–2206. [DOI] [PubMed] [Google Scholar]
- 20.Wanha W, Kawecki D, Roleder T, et al. Long-Term Percutaneous Coronary Intervention Outcomes of Patients with Chronic Kidney Disease in the Era of Second-Generation Drug Eluting Stents. Cardiorenal Med. 2017;7:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lautamaki A, Kiviniemi T, Biancari F, Airaksinen J, Juvonen T, Gunn J. Outcome after coronary artery bypass grafting and percutaneous coronary intervention in patients with stage 3b-5 chronic kidney disease. Eur J Cardiothorac Surg. 2016;49:926–930. [DOI] [PubMed] [Google Scholar]
- 22.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296:1377–1384. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, Winkelmayer WC. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol. 2012;23:2042–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charytan DM, Desai M, Mathur M, et al. Reduced risk of myocardial infarct and revascularization following coronary artery bypass grafting compared with percutaneous coronary intervention in patients with chronic kidney disease. Kidney Int. 2016;90:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 27.Molnar MZ, Streja E, Sumida K, et al. Pre-ESRD Depression and Post-ESRD Mortality in Patients with Advanced CKD Transitioning to Dialysis. Clin J Am Soc Nephrol. 2017;12:1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molnar MZ, Sumida K, Gaipov A, et al. Pre-ESRD Dementia and Post-ESRD Mortality in a Large Cohort of Incident Dialysis Patients. Dement Geriatr Cogn Disord. 2017;43:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69:A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovesdy CP, Alrifai A, Gosmanova EO, et al. Age and Outcomes Associated with BP in Patients with Incident CKD. Clin. J. Am. Soc. Nephrol 2016;11:821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Department of Veterans Affairs: VIReC Research User Guide; VHA Medical SAS Inpatient Datasets FY2006–2007 H, IL, VA Information Resource Center; 2007. [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 33.Center VIR. VIReC Research User Guide: VHA Pharmacy Prescription Data, 2nd ed. Hines, IL: US Department of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2008. [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsh J, Seth M, Aronow H, et al. Choice of Estimated Glomerular Filtration Rate Equation Impacts Drug-Dosing Recommendations and Risk Stratification in Patients With Chronic Kidney Disease Undergoing Percutaneous Coronary Interventions. J Am Coll Cardiol. 2015;65:2714–2723. [DOI] [PubMed] [Google Scholar]
- 36.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 37.Stone GW, Sabik JF, Serruys PW, et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med. 2016;375:2223–2235. [DOI] [PubMed] [Google Scholar]
- 38.Baber U, Farkouh ME, Arbel Y, et al. Comparative efficacy of coronary artery bypass surgery vs. percutaneous coronary intervention in patients with diabetes and multivessel coronary artery disease with or without chronic kidney disease. Eur Heart J. 2016;37:3440–3447. [DOI] [PubMed] [Google Scholar]
- 39.Kim YH, Park DW, Ahn JM, et al. Everolimus-eluting stent implantation for unprotected left main coronary artery stenosis. The PRECOMBAT-2 (Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease) study. JACC Cardiovasc Interv. 2012;5:708–717. [DOI] [PubMed] [Google Scholar]
- 40.Chang TI, Leong TK, Kazi DS, Lee HS, Hlatky MA, Go AS. Comparative effectiveness of coronary artery bypass grafting and percutaneous coronary intervention for multivessel coronary disease in a community-based population with chronic kidney disease. Am Heart J. 2013;165:800–808, 808 e801–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashrith G, Lee VV, Elayda MA, Reul RM, Wilson JM. Short- and long-term outcomes of coronary artery bypass grafting or drug-eluting stent implantation for multivessel coronary artery disease in patients with chronic kidney disease. Am J Cardiol. 2010;106:348–353. [DOI] [PubMed] [Google Scholar]
- 42.Wang ZJ, Zhou YJ, Liu YY, et al. Comparison of drug-eluting stents and coronary artery bypass grafting for the treatment of multivessel coronary artery disease in patients with chronic kidney disease. Circ J. 2009;73:1228–1234. [DOI] [PubMed] [Google Scholar]
- 43.Butt JH, Sorensen R, Back C, et al. Short- and long-term cause of death in patients undergoing isolated coronary artery bypass grafting: A nationwide cohort study. J Thorac Cardiovasc Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 44.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan M Preoperative care of patients with kidney disease. Am Fam Physician. 2002;66:1471–1476, 1379. [PubMed] [Google Scholar]
- 46.Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381:639–650. [DOI] [PubMed] [Google Scholar]
- 47.Tsai TT, Patel UD, Chang TI, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. J Am Heart Assoc. 2014;3:e001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–744; 744–735. [DOI] [PubMed] [Google Scholar]
- 49.Lukowsky LR, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins AJ, Foley RN, Gilbertson DT, Chen SC. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011). 2015;5:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69:A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.den Hoedt CH, Bots ML, Grooteman MP, et al. Should we still focus that much on cardiovascular mortality in end stage renal disease patients? The CONvective TRAnsport STudy. PloS one. 2013;8:e61155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elmore JB, Mehanna E, Parikh SA, Zidar DA. Restenosis of the Coronary Arteries: Past, Present, Future Directions. Interv Cardiol Clin. 2016;5:281–293. [DOI] [PubMed] [Google Scholar]
- 54.Dai L, Golembiewska E, Lindholm B, Stenvinkel P. End-Stage Renal Disease, Inflammation and Cardiovascular Outcomes. Contrib Nephrol. 2017;191:32–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Dr Gaipov discussing the background and relevance of the study.