Abstract
OBJECTIVE
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) are suspected endocrine disruptors widely detected across populations. We examine the extent to which PFASs are associated with diabetes incidence and microvascular disease. Secondarily, we tested whether a lifestyle intervention modifies associations and decreases concentrations.
RESEARCH DESIGN AND METHODS
We analyzed data from a prospective cohort of 957 participants from the Diabetes Prevention Program (DPP) trial and Diabetes Prevention Program Outcomes Study (DPPOS). At baseline, participants were randomized to an intensive lifestyle intervention of diet, physical activity, and behavior modification or a placebo medication. We quantified plasma concentrations of six PFASs at baseline and 2 years after randomization. Participants were monitored for ∼15 years, repeatedly tested for diabetes, and evaluated for microvascular disease at the end of the follow-up.
RESULTS
A doubling in baseline branched perfluorooctanoic acid concentration was associated with a 14% increase in diabetes risk for the placebo (hazard ratio [HR] 1.14, 95% CI 1.04, 1.25) but not in the lifestyle intervention group (HR 1.01, 95% CI 0.92, 1.11, Pinteraction = 0.11). Mean change in plasma baseline branched perfluorooctanoic acid concentration was greater for the placebo (0.96 ng/mL; 95% CI 0.71, 1.22) compared with the lifestyle intervention group (0.31 ng/mL; 95% CI 0.14, 0.48) 2 years after randomization. Each doubling in N-ethyl-perfluorooctane sulfonamido acetic acid was associated with 17% greater odds of prevalent microvascular disease (OR 1.17, 95% CI 1.05, 1.31), and a similar association was observed for perfluorodimethylhexane sulfonic acid (OR 1.18, 95% CI 1.04, 1.35), regardless of treatment.
CONCLUSIONS
Some plasma PFASs were associated with diabetes and microvascular disease. Our results suggest that exercise and diet may attenuate the diabetogenic association of PFASs.
Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFASs) are a family of environmentally persistent anthropogenic chemicals. Many are universally detected in the U.S. (>95% of the population) and worldwide as well as in natural ecosystems (1,2). PFASs have been produced since the late 1940s, and because of their unique properties, including thermal stability, hydrophobicity, and oleophobicity, they have been used in nonstick cookware, oil- and water-resistant textiles, greaseproof food packaging, surfactants, fire-fighting foams, pesticides, cosmetics, and construction and electronic products, among other applications (3). Human exposure from direct and indirect sources, including contaminated food and drinking water, is pervasive (4). Some PFASs are readily absorbed in the body, bind to serum proteins, and have small-to-negligible renal clearance, accumulating in organs such as the liver. In humans, half-lives of commonly used PFASs are relatively long and estimated to range from ∼2 to 9 years, depending on the compound (5).
PFASs are suspected endocrine disruptors (6), and laboratory studies have established that some PFASs can interact with multiple nuclear receptors, leading to endocrine and metabolic dysregulation (7,8). In prospective epidemiological studies, circulating PFASs have been associated with weight gain in adults (9,10) and adiposity in adolescents (11). However, reported associations with diabetes are based on cross-sectional or case-control studies, with conflicting findings reporting null, adverse, and even protective associations (12–14).
In a secondary analysis of participants from the Diabetes Prevention Program (DPP) trial and Diabetes Prevention Program Outcomes Study (DPPOS), we evaluated associations of baseline plasma PFAS concentrations with diabetes incidence as well as microvascular disease prevalence at the end of follow-up. The DPP trial and DPPOS follow-up study were designed to test interventions for the prevention of type 2 diabetes. We hypothesized that baseline plasma PFAS concentrations would be associated with diabetes incidence and microvascular disease during 15 years of average follow-up but that the initial lifestyle intervention would attenuate these associations. Our secondary aim was to study the extent to which the lifestyle intervention would reduce plasma PFAS concentration 2 years after randomization.
Research Design and Methods
Study Population
We selected 957 DPP participants with available samples and sufficient plasma volume (≥400 μL) collected at baseline and 2 years after randomization stored at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) repository. The DPP was a randomized clinical trial conducted between July 1996 and May 1999 among adults without diabetes with elevated fasting and postload plasma glucose concentrations recruited from 27 clinical centers across the U.S. (15). Participants were randomized to a lifestyle intervention of exercise and diet, metformin, or a metformin placebo-treated group. To be eligible for DPP, participants needed to be 25 years or older with a BMI of ≥24 kg/m2 (≥22 kg/m2 for Asians), a fasting glucose concentration of 95–125 mg/dL (5.3–6.9 mmol/L), and a 2-h glucose measure of 140–199 mg/dL (7.8–11.0 mmol/L) after a 75-g oral glucose tolerance test (OGTT) (15). DPP was designed to enroll ≥50% women, ≥50% racial and ethnic minorities, and ≥20% of participants 65 years old or older (16).
DPP was terminated early in July 2001 because diabetes incidence was significantly lower in the intervention arms of the trial. A 13-month bridge period separated DPP trial and DPPOS follow-up protocols (17). Study participants were monitored in DPPOS (1 September 2002 to 2 January 2014) for ∼15 years since the initial randomization (18). The institutional review board at each clinical center approved the protocols, and participants provided informed consent. For the current study, the Harvard Pilgrim Health Care Institutional Review Board approved the protocol. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research. We followed the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines for reporting cohort studies.
Interventions
At baseline, DPP participants were randomized to a metformin (not included in this study), a lifestyle intervention, or a medication placebo group using an adaptive randomization strategy stratified by clinic to ensure balance by treatment (15). The original study included a fourth pharmacological intervention (troglitazone) that was discontinued due to potential liver toxicity. In the current study, we only included individuals from the placebo-treated control and the lifestyle intervention groups.
The lifestyle intervention consisted of diet, exercise, and behavior modification with two major goals: achieving a 7% weight loss with subsequent maintenance and a minimum of 150 min/week of physical activity similar in intensity to brisk walking. Dietary changes were initially focused on reducing fat intake and then introducing the maintenance of caloric balance to lose 1–2 lb/week. Trained case managers, or coaches, conducted the lifestyle intervention by meeting with participants individually for the 16 sessions in the first 24 weeks and contacted participants at least monthly thereafter with in-person contact every 2 months. The lifestyle intervention included individualized tailoring of the intervention through a “toolbox” to meet goals as well as frequent contact and feedback from case managers (19). All participants, including the placebo-treated group, received written standard recommendations on the importance of diet and exercise for the prevention of type 2 diabetes and a 20- to 30-min individual session with a case manager. The placebo group took medication placebo twice daily but no motivational counseling was provided (15). A 13-month bridge period separated DPP and DPPOS protocols during which all participants were offered a modified group version of the lifestyle intervention. However, individualized problem solving and behavior change support were not provided in the group setting. Design and detailed description of the interventions have been previously described (15). The rate of participation in the bridge group intervention (≥1 session) was 57% and 40% for the placebo and lifestyle groups, respectively (20).
Plasma PFAS Concentrations
Plasma samples from DPP collected at baseline and during the 2nd year of follow-up stored at the NIDDK repository (https://repository.niddk.nih.gov/home) were shipped to the CDC laboratory for analyses. We analyzed available samples from the placebo and lifestyle intervention groups but did not analyze samples from the metformin group due to unknown interactions with PFASs. We quantified six plasma PFASs using a modification of an online solid-phase extraction–high-performance liquid chromatography–isotope dilution–tandem mass spectrometry method previously developed and used in the National Health and Nutrition Examination Survey (NHANES) (21,22). The limit of detection (LOD) for all analytes was 0.1 ng/mL (Supplementary Table 1). Perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorohexane sulfonic acid (PFHxS), N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA), N-methyl-perfluorooctane sulfonamido acetic acid (MeFOSAA), and perfluorononanoic acid were detected in ≥80% of the samples. We replaced concentrations below the LOD with LOD/√2.
Incident Diabetes and Microvascular Disease
Diabetes incidence was determined based on an annual 75-g OGTT or semiannual fasting plasma glucose tests, according to the 1997 criteria of the American Diabetes Association using a fasting plasma glucose level ≥126 mg/dL (7.0 mmol/L) or 2-h plasma glucose ≥200 mg/dL (11.1 mmol/L) after the OGTT (23). A provisional diagnosis by either test was confirmed with a repeat test within 6 weeks. Hemoglobin A1c (HbA1c) levels were measured at baseline, 6 months after randomization, and annually thereafter by high-performance liquid chromatography but not used for diagnosis of diabetes (18).
As indicated by DPPOS protocol, microvascular disease at the 11th year follow-up visit in DPPOS included the presence or absence of one or more outcomes: nephropathy, retinopathy, or neuropathy (18). Kidney function and neuropathy were measured annually during DPPOS, whereas retinopathy was only measured at the final DPPOS visit. Nephropathy was defined as albuminuria of ≥30 mg/g creatinine in spot urine collected on two consecutive tests, estimated glomerular filtration rate <45 mL/min/1.73 m2 based on annual serum creatinine on two consecutive tests, or renal failure. Retinopathy was diagnosed with seven-field stereoscopic fundus photography if the Early Treatment Diabetic Retinopathy Study (ETDRS) grade was ≥20 in either eye or with treatment of retinopathy. Presence of neuropathy was based on loss of light touch sensation measured with a 10-g Semmes-Weinstein monofilament (18).
Covariates
We obtained deidentified participant data from the NIDDK repository. We adjusted multivariate models for baseline variables selected because they were previously reported to be associated with plasma PFASs in this cohort in univariate associations (10,22). Namely, we adjusted for participant sex, race/ethnicity, baseline age, education, marital status, income, and treatment assignment if not stratified. Smoking history, baseline BMI, and self-reported maternal and paternal diabetes were selected a priori as potential confounders associated with diabetes risk. We had complete information on adjustment variables with the exception of household income, for which some participants refused to answer, and this group was treated as one of the income categories and included in subsequent analyses. One participant was missing information on parental diabetes history and excluded from multivariate analyses. We performed sensitivity analysis further adjusting for baseline caloric intake (kcal/day) measured via Food Frequency Questionnaires among 940 participants with available information.
Statistical Analysis
We report demographic characteristics using frequencies and proportions. Distributions of individual and sum PFAS concentrations were right skewed and thus we log2-transformed them for analyses. We used Cox-proportional hazards models to estimate hazard ratios (HRs) and 95% CIs for the risk of developing diabetes relative to individual and sum plasma PFASs measured at baseline, adjusting for participant sex, race/ethnicity, baseline age, marital status, education, income, smoking history, BMI, maternal diabetes, paternal diabetes, and baseline treatment assignment. The proportional hazards assumption was evaluated by inspecting the Schoenfeld residuals and using a global test of proportional hazards.
We evaluated effect modification by testing the multiplicative interaction (product term) between each baseline plasma PFAS concentration and treatment assignment in multivariate models and present treatment-stratified HRs for diabetes. For any individual PFAS associated with diabetes, we plotted Kaplan-Meier curves of the cumulative incidence by median concentration and estimated associations with changes in HbA1c levels from baseline using longitudinal regression models with random intercepts and slopes. We plotted estimated HbA1c trajectories from baseline for the most frequent covariate categories and report point estimates from adjusted linear regression models at the fifth annual DPPOS visit, ∼9 years after randomization, at which time 83.5% of participants had complete follow-up.
We used multivariate logistic regression models to estimate the odds of having any microvascular disease outcome in DPPOS relative to individual and sum plasma PFASs, while adjusting for sex, race/ethnicity, age, marital status, education, income, smoking history, BMI, maternal diabetes, paternal diabetes, baseline treatment assignment, baseline fasting glucose, and HbA1c. We report odds ratios (ORs) and 95% CIs per doubling in individual baseline plasma PFASs among 877 participants with information in at least one of the microvascular outcomes and tested for effect modification by treatment using interactions. We present treatment-stratified models for all associations.
Lastly, we estimated the effect of the lifestyle intervention on mean changes in individual plasma PFASs from baseline to 2 years after randomization compared with placebo using t tests and 95% CIs. Data management and analyses were performed in R 3.5.1 software (The R Foundation for Statistical Computing).
Results
Baseline and year-2 plasma samples were available from 957 participants in DPP, 49.7% randomized to the placebo group and 50.3% to the lifestyle intervention group. Similar to DPP overall, most participants were women (65.3%), Caucasian (57.7%), between 40 and 64 years of age (76.4%), obese (67.7%), college educated (49%), nonsmokers (56.8%), married or cohabitating (67.6%), and with an annual household income ≥$50,000 (41.6%). Baseline characteristics and plasma PFAS concentrations were similar by treatment arm (Table 1).
Table 1.
Baseline demographic characteristics, microvascular disease prevalence, and plasma PFAS concentration among 957 participants, a subset of participants enrolled in the DPP trial and DPPOS
| Characteristics | Overall (N = 957) | Lifestyle (n = 481) | Placebo (n = 476) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Sex | ||||
| Male | 332 (34.7) | 165 (34.3) | 167 (35.1) | |
| Female | 625 (65.3) | 316 (65.7) | 309 (64.9) | |
| Race/ethnicity | ||||
| Caucasian | 552 (57.7) | 274 (57) | 278 (58.4) | |
| African American | 184 (19.2) | 94 (19.5) | 90 (18.9) | |
| Hispanic of any race | 179 (18.7) | 89 (18.5) | 90 (18.9) | |
| All other | 42 (4.4) | 24 (5) | 18 (3.8) | |
| Age, years | ||||
| <40 | 112 (11.7) | 66 (13.7) | 46 (9.7) | |
| 40–44 | 107 (11.2) | 58 (12.1) | 49 (10.3) | |
| 45–49 | 213 (22.3) | 93 (19.3) | 120 (25.2) | |
| 50–54 | 167 (17.5) | 75 (15.6) | 92 (19.3) | |
| 55–59 | 137 (14.3) | 70 (14.6) | 67 (14.1) | |
| 60–64 | 107 (11.2) | 57 (11.9) | 50 (10.5) | |
| ≥65 | 114 (11.9) | 62 (12.9) | 52 (10.9) | |
| BMI categories, kg/m2 | ||||
| <26 | 74 (7.7) | 37 (7.7) | 37 (7.8) | |
| 26 to <28 | 114 (11.9) | 55 (11.4) | 59 (12.4) | |
| 28 to <30 | 128 (13.4) | 72 (15.0) | 56 (11.8) | |
| 30 to <32 | 117 (12.2) | 55 (11.4) | 62 (13.0) | |
| 32 to <34 | 122 (12.7) | 56 (11.6) | 66 (13.9) | |
| 34 to <36 | 117 (12.2) | 65 (13.5) | 52 (10.9) | |
| 36 to <38 | 79 (8.3) | 45 (9.4) | 34 (7.1) | |
| 38 to <40 | 55 (5.7) | 25 (5.2) | 30 (6.3) | |
| 40 to <42 | 53 (5.5) | 23 (4.8) | 30 (6.3) | |
| ≥42 | 98 (10.2) | 48 (10.0) | 50 (10.5) | |
| Education | ||||
| <High school | 45 (4.7) | 19 (4) | 26 (5.5) | |
| High school/GED | 200 (20.9) | 100 (20.8) | 100 (21) | |
| College | 469 (49.0) | 238 (49.5) | 231 (48.5) | |
| Graduate school | 243 (25.4) | 124 (25.8) | 119 (25) | |
| Smoking history | ||||
| Nonsmoker | 544 (56.8) | 279 (58) | 265 (55.7) | |
| Former smoker | 356 (37.2) | 183 (38) | 173 (36.3) | |
| Current smoker | 57 (6.0) | 19 (4) | 38 (8) | |
| Marital status | ||||
| Married/cohabitating | 647 (67.6) | 321 (66.7) | 326 (68.5) | |
| Single | 114 (11.9) | 61 (12.7) | 53 (11.1) | |
| Divorced/separated | 152 (15.9) | 76 (15.8) | 76 (16) | |
| Widowed | 44 (4.6) | 23 (4.8) | 21 (4.4) | |
| Annual household income | ||||
| <$20,000 | 121 (12.6) | 55 (11.4) | 66 (13.9) | |
| $20,000 to <$35,000 | 167 (17.5) | 90 (18.7) | 77 (16.2) | |
| $35,000 to <$50,000 | 194 (20.3) | 103 (21.4) | 91 (19.1) | |
| $50,000 to <$75,000 | 184 (19.2) | 95 (19.8) | 89 (18.7) | |
| >$75,000 | 214 (22.4) | 95 (19.8) | 119 (25) | |
| Refused to answer | 77 (8.0) | 43 (8.9) | 34 (7.1) | |
| Mother has diabetes | ||||
| Yes | 341 (35.6) | 176 (36.6) | 165 (34.7) | |
| No | 580 (60.6) | 290 (60.3) | 290 (60.9) | |
| Don’t know | 35 (3.7) | 14 (2.9) | 21 (4.4) | |
| Missing | 1 (0.1) | 1 (0.2) | 0 | |
| Father has diabetes | ||||
| Yes | 320 (33.4) | 172 (35.8) | 148 (31.1) | |
| No | 570 (59.6) | 281 (58.4) | 289 (60.7) | |
| Don’t know | 66 (6.9) | 27 (5.6) | 39 (8.2) | |
| Missing | 1 (0.1) | 1 (0.2) | 0 | |
| n/N (%) | n/N (%) | n/N (%) | ||
| Microvascular disease prevalence | ||||
| Any microvascular disease | 252/877 (28.7) | 111/441 (32.6) | 141/436 (32.3) | |
| Nephropathy | 110/871 (12.6) | 52/437 (11.9) | 58/434 (13.4) | |
| Retinopathy | 98/801 (12.2) | 43/395 (10.9) | 55/406 (13.5) | |
| Neuropathy | 88/713 (12.3) | 34/352 (9.7) | 54/361 (15.0) |
| <LOD, n (%) | GM (IQR) | GM (IQR) | GM (IQR) | |
|---|---|---|---|---|
| Baseline PFAS concentration (ng/mL) | ||||
| Sum PFAS | 0 | 38.43 (28.6) | 39.59 (30.10) | 37.30 (27.40) |
| PFOS | 0 | 26.38 (22.8) | 27.17 (23.50) | 25.60 (20.90) |
| n-PFOS | 0 | 18.42 (16.90) | 19.13 (17.10) | 17.74 (15.75) |
| Sm-PFOS | 0 | 7.32 (6.50) | 7.43 (6.40) | 7.21 (6.60) |
| Sm2-PFOS | 564 (58.9) | 0.13 (0.23) | 0.13 (0.23) | 0.14 (0.23) |
| PFOA | 0 | 4.82 (3.20) | 4.90 (3.12) | 4.74 (3.12) |
| n-PFOA | 0 | 4.29 (2.90) | 4.37 (2.80) | 4.21 (2.80) |
| Sb-PFOA | 159 (16.6) | 0.44 (0.50) | 0.44 (0.60) | 0.44 (0.50) |
| PFHxS | 1 (0.1) | 2.41 (2.40) | 2.51 (2.70) | 2.30 (2.0) |
| EtFOSAA | 32 (3.3) | 1.13 (1.50) | 1.14 (1.50) | 1.11 (1.40) |
| MeFOSAA | 29 (2.6) | 0.94 (1.10) | 0.95 (1.20) | 0.93 (1.20) |
| PFNA | 65 (6.8) | 0.53 (0.40) | 0.54 (0.40) | 0.52 (0.40) |
LOD was 0.1 ng/mL for all analytes. PFOS = n-PFOS + Sm-PFOS + Sm2-PFOS; PFOA = n-PFOA + Sb-PFOA. GM, geometric mean; IQR, interquartile range; n-PFOA, linear PFOA, n-PFOS, linear PFOS; Sm-PFOS, perfluoromethylheptane sulfonic acids.
Diabetes developed in 507 participants (52.9%) during a median follow-up time of 8.9 years (range 0.22–7 years) for diabetes incidence. In our sample, diabetes incidence was reduced by 28% for the lifestyle intervention group (HR 0.72; 95% CI 0.61, 0.86) compared with placebo in DPPOS, consistent with overall cohort findings previously reported.
In unadjusted models, we observed an association between baseline branched PFOA (Sb-PFOA) plasma concentration and diabetes incidence (HR 1.07; 95% CI 1.01, 1.13). This association remained consistent after adjusting for sex, race/ethnicity, and baseline age, marital status, education, income, smoking history, BMI, maternal diabetes, paternal diabetes, and treatment assignment (HR 1.08; 95% CI 1.01, 1.15). The observed relationship differed somewhat by baseline treatment assignment (Pinteraction = 0.11). In stratified analyses, each doubling in Sb-PFOA concentration was associated with an adjusted 14% increase in the risk of developing diabetes for the placebo group (HR 1.14; 95% CI 1.04, 1.25) but was attenuated for the lifestyle intervention group (HR 1.01; 95% CI 0.92, 1.11) (Table 2). Modeling PFAS by tertiles yielded consistent results (Supplementary Table 2). Adjustment for baseline caloric intake among 940 participants with available dietary data did not influence results (data not shown).
Table 2.
Adjusted HRs and 95% CIs for diabetes incidence during follow-up relative to baseline plasma PFAS concentration overall and stratified by baseline treatmenta
| Overall N = 956 (507 cases) |
Placebo n = 476 (272 cases) |
Lifestyle n = 480 (235 cases) |
Treatment∗PFAS interaction P |
|
|---|---|---|---|---|
| Baseline log2-scale PFAS concentration | HR (95% CI) | HR (95% CI) | HR (95% CI) | Pinteraction |
| Sum PFAS | 0.97 (0.86, 1.08) | 0.94 (0.80, 1.10) | 1.00 (0.85, 1.19) | 0.49 |
| PFOS | 0.94 (0.85, 1.04) | 0.92 (0.80, 1.07) | 0.96 (0.82, 1.12) | 0.58 |
| n-PFOS | 0.96 (0.87, 1.05) | 0.93 (0.82, 1.07) | 0.98 (0.84, 1.14) | 0.49 |
| Sm-PFOS | 0.93 (0.84, 1.02) | 0.93 (0.81, 1.07) | 0.92 (0.80, 1.06) | 0.79 |
| Sm2-PFOS | 0.98 (0.91, 1.06) | 1.03 (0.93, 1.14) | 0.93 (0.82, 1.04) | 0.19 |
| PFOA | 1.05 (0.94, 1.18) | 1.08 (0.91, 1.29) | 1.03 (0.87, 1.22) | 0.77 |
| n-PFOA | 1.03 (0.91, 1.16) | 1.04 (0.87, 1.24) | 1.03 (0.86, 1.22) | 0.99 |
| Sb-PFOA | 1.08 (1.01, 1.15) | 1.14 (1.04, 1.25) | 1.01 (0.92, 1.11) | 0.11 |
| PFHxS | 0.97 (0.89, 1.05) | 0.90 (0.79, 1.02) | 1.05 (0.93, 1.19) | 0.08 |
| EtFOSAA | 1.03 (0.96, 1.09) | 0.99 (0.90, 1.09) | 1.05 (0.95, 1.15) | 0.38 |
| MeFOSAA | 1.00 (0.92, 1.08) | 0.97 (0.87, 1.09) | 1.03 (0.92, 1.16) | 0.24 |
| PFNA | 1.01 (0.93, 1.10) | 1.00 (0.89, 1.12) | 1.02 (0.90, 1.15) | 0.96 |
PFOS = n-PFOS + Sm-PFOS + Sm2-PFOS. PFOA = n-PFOA + Sb-PFOA. n-PFOA, linear PFOA; n-PFOS, linear PFOS; Sm-PFOS, perfluoromethylheptane sulfonic acids.
aAdjusted for participant sex, race/ethnicity, and baseline age, marital status, education, income, smoking history, BMI, maternal diabetes, paternal diabetes, and treatment assignment if not stratified.
Statistically significant results appear in boldface type.
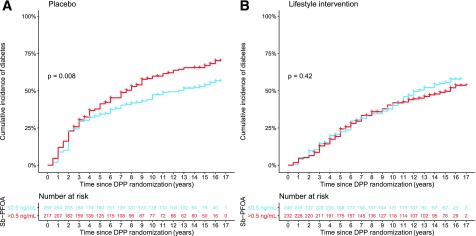
In the placebo group, unadjusted diabetes incidence increased by 37% (HR 1.37; 95% CI 1.08, 1.73) for participants with a plasma Sb-PFOA concentration above the median compared with participants with a Sb-PFOA concentration at or below median levels (≤0.5 ng/mL). No association was observed for the lifestyle intervention group (HR 0.89; 95% CI 0.69, 1.15) by median Sb-PFOA levels. Kaplan-Meier curves by median levels of Sb-PFOA stratified by treatment and log-rank test P values are shown in Fig. 1. Among participants randomized to placebo with a plasma Sb-PFOA concentration above the median, the time at which half developed diabetes (median diabetes time), was 7.5 years (95% CI 6, 9), and this was substantially shorter relative to participants with Sb-PFOA plasma concentration at or below median concentration, 12.5 years (95% CI 9.6, >16). This association was also observed after excluding participants who developed diabetes during DPP (Supplementary Fig. 2).
Figure 1.
Cumulative incidence of diabetes for participants with plasma Sb-PFOA at or below median concentration (≤0.5 ng/mL) or above median Sb-PFOA concentration (>0.5 ng/mL) for the placebo-treated group (A) and the lifestyle intervention group (B).
Baseline plasma Sb-PFOA concentration was associated with changes in HbA1c levels from baseline in the placebo group (Supplementary Fig. 3). For example, at the fifth annual DPPOS visit, ∼9 years after randomization, each doubling in the baseline plasma Sb-PFOA concentration was associated with an absolute increase in HbA1c from baseline of 0.05% (95% CI 0.003, 0.10) in the placebo group but not in the lifestyle intervention group (β = 0.00002%, 95% CI −0.04, 0.04).
Prevalent microvascular disease, defined as the presence of nephropathy, retinopathy, or neuropathy at the end of follow-up among 877 participants, was 28.7%. In models adjusted for sex, race/ethnicity, and baseline age, marital status, education, income, smoking history, BMI, maternal diabetes, paternal diabetes, baseline treatment assignment, baseline fasting glucose, and HbA1c, we observed that each doubling in baseline plasma perfluorodimethylhexane sulfonic acid (Sm2-PFOS) concentration was associated with 18% greater odds of any microvascular disease (OR 1.18, 95% CI 1.04, 1.35). However, this analyte was below the LOD for 58.9% of participants, and thus, these results should be interpreted with caution. In addition, a doubling in plasma concentration of EtFOSAA was associated with 17% greater odds of any microvascular disease (OR 1.17, 95% CI 1.05, 1.31). Plasma EtFOSAA was detected in >96% of all plasma samples at baseline. No significant effect modification by treatment was observed, but some results were stronger among participants randomized to the lifestyle intervention (Table 3). The association with Sm2-PFOS was stronger for retinopathy (OR 1.23; 95% CI 1.03, 1.47) (Supplementary Table 3).
Table 3.
Adjusted ORs for microvascular disease prevalence at the end of follow-up overall and stratified by treatment assignmenta
| Baseline log2-scale PFAS concentration | Overall N = 877 (252 cases) |
Placebo n = 436 (141 cases) |
Lifestyle n = 441 (111 cases) |
Treatment∗PFAS interaction P |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | Pinteraction | |
| Sum PFAS | 1.19 (0.98, 1.45) | 1.10 (0.83, 1.44) | 1.40 (1.00, 1.93) | 0.24 |
| PFOS | 1.17 (0.98, 1.39) | 1.07 (0.84, 1.36) | 1.37 (1.04, 1.84) | 0.25 |
| n-PFOS | 1.16 (0.98, 1.37) | 1.05 (0.84, 1.33) | 1.40 (1.06, 1.86) | 0.17 |
| Sm-PFOS | 1.14 (0.97, 1.35) | 1.11 (0.88, 1.40) | 1.24 (0.96, 1.61) | 0.62 |
| Sm2-PFOS | 1.18 (1.04, 1.35) | 1.16 (0.97, 1.39) | 1.25 (1.02, 1.54) | 0.62 |
| PFOA | 1.04 (0.85, 1.27) | 1.07 (0.80, 1.43) | 1.05 (0.78, 1.42) | 0.84 |
| n-PFOA | 1.04 (0.84, 1.28) | 1.04 (0.76, 1.41) | 1.09 (0.79, 1.49) | 0.68 |
| Sb-PFOA | 1.06 (0.95, 1.19) | 1.13 (0.96, 1.32) | 0.99 (0.84, 1.18) | 0.53 |
| PFHxS | 1.07 (0.92, 1.23) | 0.99 (0.79, 1.23) | 1.17 (0.95, 1.45) | 0.13 |
| EtFOSAA | 1.17 (1.05, 1.31) | 1.18 (1.01, 1.39) | 1.14 (0.96, 1.36) | 0.62 |
| MeFOSAA | 0.99 (0.86, 1.14) | 0.89 (0.74, 1.07) | 1.10 (0.89, 1.38) | 0.08 |
| PFNA | 1.05 (0.91, 1.21) | 1.02 (0.83, 1.25) | 1.08 (0.86, 1.36) | 0.46 |
PFOS = n-PFOS + Sm-PFOS + Sm2-PFOS. PFOA = n-PFOA + Sb-PFOA. n-PFOA, linear PFOA; n-PFOS, linear PFOS; Sm-PFOS, perfluoromethylheptane sulfonic acids.
aAdjusted for participant sex, race/ethnicity, age, and baseline marital status, education, income, smoking history, BMI, maternal diabetes, paternal diabetes, baseline fasting glucose, baseline HbA1c levels, and treatment assignment if not stratified.
Statistically significant results appear in boldface type.
Mean change in total plasma PFOA concentration from baseline to the 2nd year of follow-up differed by treatment. Plasma Sb-PFOA concentration drove this difference. The mean change in Sb-PFOA concentration 2 years after randomization from baseline levels was 0.96 ng/mL (95% CI 0.71, 1.22) in the placebo arm compared with 0.31 ng/mL (95% CI 0.14, 0.48) in the lifestyle intervention group (Supplementary Table 4). This difference remained consistent after adjustment for baseline Sb-PFOA concentration, with a mean difference 0.64 ng/mL lower concentration of plasma Sb-PFOA for the lifestyle compared with the placebo group (95% CI −0.95, −0.34). The proportion of samples with nondetectable Sb-PFOA concentrations was higher for the lifestyle intervention (12.9%) compared with the placebo group (8.2%) 2 years after randomization (Supplementary Table 5).
Conclusions
In this cohort of adults at high risk for diabetes, monitored for ∼15-years, baseline plasma Sb-PFOA concentration was associated with diabetes incidence. This association was attenuated among participants randomized to a lifestyle intervention of exercise and diet compared with those randomized to a placebo. Two other plasma PFASs measured at baseline, Sm2-PFOS and EtFOSAA, were associated with the presence of any microvascular disease at the end of follow-up, regardless of treatment. On average, participants randomized to the lifestyle intervention had a lower increase in plasma Sb-PFOA concentration compared with the placebo group 2 years after randomization. Our findings await further confirmation but provide some evidence that Sb-PFOA might act as endocrine disruptors contributing to diabetes development and that Sm2-PFOS and EtFOSAA could contribute to microvascular disease. Our results also suggest that a lifestyle intervention might attenuate the potential endocrine effect of PFAS.
PFASs are structurally similar to fatty acids and could upregulate fatty acid oxidation pathways, increasing oxidative stress and potentially leading to insulin resistance (24). Our findings are supported by previous toxicological and human studies. In mice, for example, PFOA administration for 28 days led to increased glycogenolysis and hyperglycemia (25). The pancreas might be a target organ for the biological effects of PFASs, because mice exposed to PFOA for 7 days had histopathological changes, increased enzyme secretion, and inflammation in the pancreas (26). Evidence from human studies is limited by study designs, measurement of validated exposure biomarkers, and duration of follow-up. For example, a prospective prepregnancy cohort reported that preconception serum PFOA, but no other PFASs, was associated with a higher risk of developing gestational diabetes (27). Using samples from the Nurses’ Health Study II and a nested case-control design, Sun et al. (14) reported associations for plasma concentration of PFOS and PFOA with incident type 2 diabetes over a mean of ∼6.7 years of follow-up.
We previously reported an association between baseline plasma Sb-PFOA and diabetes incidence in DPP with 2.9 years of median follow-up. However, we did not observe effect modification by baseline treatment during this follow-up period (22). Occupational PFOA-exposed cohorts have reported increased risk of diabetes (28). In a prospective cohort of women with estimated dietary PFOS and PFOA intake and 15 years of follow-up, only PFOA intake was observed to be associated with diabetes (29). A cross-sectional analyses from the NHANES conducted in 2003–2012 reported that only PFOA was associated with prevalent diabetes in men but not women (12). Other cross-sectional studies have not found associations (30). Given the relatively long half-life of PFAS in the human body, long-term prospective studies are required to test for potential associations with health effects. In this study, repeated monitoring for diabetes allowed us to test for prospective associations to capture incident cases. However, we did not account for time-varying confounding, which could have played a role.
We observed attenuation of the diabetogenic Sb-PFOA association with the baseline diet and exercise-based lifestyle intervention. This result is also supported by our previous finding in this cohort in which plasma PFAS concentration was prospectively associated with weight gain for the placebo but not the lifestyle intervention group (10). It is unknown whether PFASs act as endocrine disruptors only in the presence of an unfavorable environment (i.e., high caloric intake/low physical activity) or whether interventions such as diet and exercise mitigate their potential endocrine action and disruption. One randomized crossover trial among adults reported associations of PFOS and perfluorododecanoic acid with insulin resistance and oxidative stress biomarkers that were reduced with a 4-week supplementation of vitamin C (31). A recent study nested within a trial of diet-induced weight loss reported that baseline plasma PFASs were associated with higher weight regain in women, independent of initial dietary interventions (9). Therefore, it is possible that physical activity could have played a role in attenuating associations, potentially modifying the balance of anti- and pro-oxidants in combination with diet. Our finding of plasma PFAS concentration associated with weight gain in the placebo group and the current finding of a stronger association with diabetes incidence in the placebo group suggest long-term benefits of the initial lifestyle intervention. However, in this current study, the statistical test for effect modification was marginal.
The magnitude of the association observed among participants with above median baseline plasma concentration of Sb-PFOA in the placebo group was substantial, a 37% increase in diabetes incidence after 15 years of follow-up. In addition, only the mean change in plasma Sb-PFOA concentration was lower for the lifestyle intervention compared with the placebo group 2 years after randomization, perhaps because of the relatively shorter half-live of PFOA, ∼2.7 years, compared with other PFASs (e.g., PFOS) (5). In addition, branched isomers of PFOA have lower binding affinity to serum proteins compared with linear isomers (32). Baseline and year-2 plasma Sb-PFOA concentrations were moderately correlated (rs = 0.49) for all participants, similar to other plasma PFASs (Supplementary Table 1). However, the Sb-PFOA plasma concentration was relatively low compared with other PFASs and nondetected in 16.6% and 10.6% of the baseline and year-2 plasma samples, respectively. Although relative small, the increase in concentration 2 years after randomization was significantly lower for participants randomized to the lifestyle intervention compared with placebo, indicating that dietary, lifestyle, and behavior changes may contribute to lowering the plasma Sb-PFOA concentration. Future follow-up in this cohort will help determine whether the lifestyle intervention reduced other plasma PFASs long-term and whether this reduction could be associated with metabolic changes over time.
Studies on PFAS and microvascular disease are limited. In human endothelial cells, PFOS exposure affected cell permeability, and renal microvascular disease has been reported in mice (33,34). A systematic review found toxicological evidence of reduced kidney function, histological/cellular derangements in the proximal tubules, and dysregulation of metabolic pathways linked to kidney disease from PFAS exposure (35). Cross-sectional epidemiological studies have reported associations of PFOS with carotid intima–media thickness (36) and associations between PFOA and PFOS with prevalent chronic kidney disease (37). In NHANES, PFOA was reported to be cross-sectionally associated with cardiovascular disease and also peripheral arterial disease (38,39). The association with Sm2-PFOS and EtFOSAA with subsequent microvascular disease prevalence needs to be cautiously interpreted because Sm2-PFOS concentrations were highly nondetected in 59% of the samples, and although EtFOSAA concentration was detected in >96% of samples, its concentration is relatively low compared with other plasma PFASs. However, prospective studies with repeated objective clinical markers of vascular disease are needed to test this hypothesis and confirm our findings.
Limitations and Strengths
Important limitations of our study include the potential for nongeneralizable results, because participants were overweight/obese with glycemic levels in prediabetes ranges. Sources of PFAS exposure for this sample have not been characterized, but given that individuals were recruited from 27 clinical centers across the U.S., we expect that exposure sources are generalizable to a similar population with prediabetes. We did not measure other environmental contaminants that could also increase the risk for diabetes and potentially correlate with PFAS. Future studies should consider the contribution of multiple endocrine disrupting compounds in the development of diabetes. We conducted multiple tests among several measured PFASs to address our hypotheses, and results could potentially be due to chance. These findings should be corroborated in future studies. Our findings related to diabetes and microvascular disease only show selected plasma PFASs associated with these outcomes. Given that only a few selected associations were observed for some but not all plasma PFAS analytes, further mechanistic and epidemiological studies are needed to confirm these findings.
Our study has important strengths that include long-term follow-up, >80% participant follow-up, regularly scheduled diabetes screenings, high quality of exposure, and covariate measurements. In addition, the successful execution of the lifestyle intervention in DPP provides a unique opportunity to test our hypothesis regarding an interaction between PFAS exposure and lifestyle.
Conclusion
PFASs are persistent environmental contaminants and human exposure is ubiquitous. Our results suggest that they might play a role in the development of diabetes and microvascular disease among adults with prediabetes. However, an intervention of exercise and diet may attenuate the diabetogenic association of PFASs.
Supplementary Material
Article Information
Acknowledgments. The authors thank Kayoko Kato, Jun Ma, Akil Kalathil, and Tao Jia for the quantification of PFASs biomarkers at the CDC as well as Jennifer Thompson and Denise Simon in the Department of Population Medicine at Harvard Pilgrim Health Care Institute for providing valuable logistical support for this project. The Diabetes Prevention Program (DPP) was conducted by the DPP Research Group and supported by the NIDDK, the General Clinical Research Center Program, the National Institute of Child Health and Human Development, the National Institute on Aging, the Office of Research on Women’s Health, the Office of Research on Minority Health, the CDC, and the American Diabetes Association. The data (and samples) from the DPP were supplied by the NIDDK Central Repositories (project number 1X01-DK-104234). X.Y. is deceased.
Funding. This work was supported by National Institutes of Health National Institute of Environmental Health Sciences grants R01-ES-024765 and P3-0ES-000002. A.C., D.R.G., R.H., K.P.K., T.F.W., and E.S.H. received funding (grant R01-ES-024765) and A.F.F. received funding (grants K23-ES-024803 and R01-ES-030101) from the National Institutes of Health during the conduct of the study.
The funders had no role in the analyses, data management, interpretation or preparation, and review or approval of the manuscript and decision to submit for publication.
Duality of Interest. E.S.H. reported receiving personal fees from PTS Diagnostics, Takeda, and Theracos outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.C. wrote the manuscript and performed all statistical analyses. M.-F.H., D.R.G., R.H., K.P.K., P.D.L., A.F.F., A.M.C., X.Y., T.F.W., E.S.H., and E.O. provided critical input in the analyses and writing of the manuscript and were involved in planning the study. A.M.C. and X.Y. performed the chemical analyses. A.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability. Data from DPP and DPPOS data are available in the NIDDK repository (www.niddkrepository.org/home/) and can be requested by any researcher. PFAS plasma concentration data will be released to the NIDDK repository after the completion of this project.
Footnotes
Clinical trial reg. nos. NCT00004992 and NCT00038727, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-2254/-/DC1.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Kannan K, Corsolini S, Falandysz J, et al. . Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 2004;38:4489–4495 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control Prevention (CDC) Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, March 2018, Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control Prevention (CDC), 2018 [Google Scholar]
- 3.Buck RC, Franklin J, Berger U, et al. . Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 2011;7:513–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’eon JC, Mabury SA. Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol 2011;45:7974–7984 [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Fletcher T, Mucs D, et al. . Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 2018;75:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen AA, Leffers H. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 2008;31:161–169 [DOI] [PubMed] [Google Scholar]
- 7.Bjork JA, Butenhoff JL, Wallace KB. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology 2011;288:8–17 [DOI] [PubMed] [Google Scholar]
- 8.Rosenmai AK, Taxvig C, Svingen T, et al. . Fluorinated alkyl substances and technical mixtures used in food paper-packaging exhibit endocrine-related activity in vitro. Andrology 2016;4:662–672 [DOI] [PubMed] [Google Scholar]
- 9.Liu G, Dhana K, Furtado JD, et al. . Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: a prospective study. PLoS Med 2018;15:e1002502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardenas A, Hauser R, Gold DR, et al. . Association of perfluoroalkyl and polyfluoroalkyl substances with adiposity. JAMA Netw Open 2018;1:e181493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: the European Youth Heart Study. Diabetes Care 2016;39:1745–1751 [DOI] [PubMed] [Google Scholar]
- 12.He X, Liu Y, Xu B, Gu L, Tang W. PFOA is associated with diabetes and metabolic alteration in US men: National Health and Nutrition Examination Survey 2003-2012. Sci Total Environ 2018;625:566–574 [DOI] [PubMed] [Google Scholar]
- 13.Lind L, Zethelius B, Salihovic S, van Bavel B, Lind PM. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia 2014;57:473–479 [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma concentrations of perfluoroalkyl substances and risk of type 2 diabetes: a prospective investigation among U.S. women. Environ Health Perspect 2018;126:037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabetes Prevention Program Research Group The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin RR, Fujimoto WY, Marrero DG, et al.; DPP Research Group . The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials 2002;23:157–171 [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venditti EM, Bray GA, Carrion-Petersen ML, et al.; Diabetes Prevention Program Research Group . First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes [published correction appears in Int J Obes (Lond) 2009;33:182]. Int J Obes (Lond) 2008;32:1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 2011;1218:2133–2137 [DOI] [PubMed] [Google Scholar]
- 22.Cardenas A, Gold DR, Hauser R, et al. . Plasma concentrations of per- and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the Diabetes Prevention Program Trial. Environ Health Perspect 2017;125:107001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu W, Jones PD, Celius T, Giesy JP. Identification of genes responsive to PFOS using gene expression profiling. Environ Toxicol Pharmacol 2005;19:57–70 [DOI] [PubMed] [Google Scholar]
- 25.Zheng F, Sheng N, Zhang H, Yan S, Zhang J, Wang J. Perfluorooctanoic acid exposure disturbs glucose metabolism in mouse liver. Toxicol Appl Pharmacol 2017;335:41–48 [DOI] [PubMed] [Google Scholar]
- 26.Kamendulis LM, Wu Q, Sandusky GE, Hocevar BA. Perfluorooctanoic acid exposure triggers oxidative stress in the mouse pancreas. Toxicol Rep 2014;1:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril 2015;103:184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundin JI, Alexander BH, Olsen GW, Church TR. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology 2009;20:921–928 [DOI] [PubMed] [Google Scholar]
- 29.Mancini FR, Rajaobelina K, Praud D, et al. . Nonlinear associations between dietary exposures to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and type 2 diabetes risk in women: findings from the E3N cohort study. Int J Hyg Environ Health 2018;221:1054–1060 [DOI] [PubMed] [Google Scholar]
- 30.Karnes C, Winquist A, Steenland K. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environ Res 2014;128:78–83 [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Park HY, Jeon JD, et al. . The modifying effect of vitamin C on the association between perfluorinated compounds and insulin resistance in the Korean elderly: a double-blind, randomized, placebo-controlled crossover trial. Eur J Nutr 2016;55:1011–1020 [DOI] [PubMed] [Google Scholar]
- 32.Beesoon S, Martin JW. Isomer-specific binding affinity of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) to serum proteins. Environ Sci Technol 2015;49:5722–5731 [DOI] [PubMed] [Google Scholar]
- 33.Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol 2009;56:338–349 [DOI] [PubMed] [Google Scholar]
- 34.Qian Y, Ducatman A, Ward R, et al. . Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: role in endothelial permeability. J Toxicol Environ Health A 2010;73:819–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. Perfluorinated chemicals as emerging environmental threats to kidney health: a scoping review. Clin J Am Soc Nephrol 2018;13:1479–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin C-Y, Lin L-Y, Wen T-W, et al. . Association between levels of serum perfluorooctane sulfate and carotid artery intima-media thickness in adolescents and young adults. Int J Cardiol 2013;168:3309–3316 [DOI] [PubMed] [Google Scholar]
- 37.Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol 2011;174:893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar A, Xiao J, Ducatman A. Perfluorooctanoic acid and cardiovascular disease in US adults. Arch Intern Med 2012;172:1397–1403 [DOI] [PubMed] [Google Scholar]
- 39.Huang M, Jiao J, Zhuang P, Chen X, Wang J, Zhang Y. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national US population. Environ Int 2018;119:37–46 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.