Abstract
The prevailing dogma is that thermogenic brown adipose tissue (BAT) contributes to improvements in glucose homeostasis in obesogenic animal models, though much of the evidence supporting this premise is from thermostressed rodents. Determination of whether modulation of the BAT morphology/function drives changes in glucoregulation at thermoneutrality requires further investigation. We used loss- and gain-of-function approaches including genetic manipulation of the lipolytic enzyme Pnpla2, change in environmental temperature, and lifestyle interventions to comprehensively test the premise that a thermogenic-like BAT phenotype is coupled with enhanced glucose tolerance in female mice. In contrast to this hypothesis, we found that 1) compared to mice living at thermoneutrality, enhanced activation of BAT and its thermogenic phenotype via chronic mild cold stress does not improve glucose tolerance in obese mice, 2) silencing of the Pnpla2 in interscapular BAT causes a brown-to-white phenotypic shift accompanied with inflammation but does not disrupt glucose tolerance in lean mice, and 3) exercise and low-fat diet improve glucose tolerance in obese mice but these effects do not track with a thermogenic BAT phenotype. Collectively, these findings indicate that a thermogenic-like BAT phenotype is not linked to heightened glucose tolerance in female mice.
Introduction
Brown adipose tissue (BAT) is a highly combustible organ when stimulated, which is largely dependent on its structural morphology. Unlike white adipocytes, brown adipocytes are multilocular with numerous lipid droplets, high abundance of mitochondria with a dense array of cristae, and high expression of uncoupling protein 1 (UCP1) required for nonshivering thermogenesis (NST) (1,2). Principally conserved to BAT, NST is predicated upon the acceleration of oxidative substrate metabolism that is uncoupled from ATP synthesis by the activation of UCP1, resulting in the conversion of stored and circulating energy substrates into heat (1). The activation of NST is largely mediated via the sympathetic nervous system, with environmental cold being the greatest physiological stimulator of adrenergic activation. Enhanced adrenergic signaling in BAT involves a series of intracellular events including stimulation of lipases, such as patatin-like phospholipase domain-containing 2 (PNPLA2), and the hydrolysis of triacylglycerol (TG) to fatty acids that increase UCP1 activity and provides a fuel source for thermogenesis via fatty acid oxidation (1). In contrast to the traditional view that intracellular production of fatty acids by lipolysis is a major driving force for thermogenesis, two recent reports (3,4) published as our studies were concluding showed that PNPLA2 is not obligatory for BAT thermogenesis. Notwithstanding these new and important findings, BAT expression of PNPLA2 is attenuated in obese mice (5) and the physiological implications of this relationship warrant further study.
In the context of obesity, BAT has emerged as an attractive antidiabetes target in recent years, in part due to its high energy combustion that acts as a sink for circulating substrates such as glucose and its identification and functional activity in adult humans (6–10). Epidemiological studies report that BAT is inversely associated with insulin resistance and type 2 diabetes (9,11), and experimental evidence in both animals (12–15) and humans (16–19) identifies BAT as playing a role in regulating glucose metabolism and insulin sensitivity. Recent data demonstrate that even under minimal thermostress (25°C), ablation of UCP1 impairs glucose homeostasis in lean and obese mice (20).
Hence, while the consensus is that BAT is an important regulator of glucose homeostasis, there is some precedence in the literature to suggest that thermogenic BAT may not directly improve glycemic control (21). Thus, whether modulation of BAT morphology/function drives changes in glucoregulation requires further investigation. Herein, we used loss- and gain-of-function approaches including genetic manipulation of Pnpla2, change in environmental temperature, and lifestyle interventions (i.e., diet and physical activity) to test the premise that modulation of BAT function is coupled with changes in glucose tolerance in mice.
Research Design and Methods
Ethics Approval
All procedures were approved in advance and carried out in compliance with the University of Missouri Institutional Animal Care and Use Committee. The University of Missouri is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Animals and Diets
Ninety-five C57BL/6J female mice were purchased from The Jackson Laboratory (000664; Bar Harbor, ME). Female mice were used for all experiments, as females generally exhibit a greater thermogenic BAT phenotype, which is characterized by higher expression of UCP1 and its activity (22), and superior systemic metabolic function compared with males (23–26). In this regard, it has been postulated that improved metabolic function in females may be partly attributable to their enhanced BAT activity (27,28). As such, we reasoned that if a link exists between BAT function and glucose tolerance, this would be most apparent in females. For avoidance of confounding effects of chronic aging on BAT function (29,30), relatively young adult (14 weeks of age) and middle-aged (24 weeks of age) mice were used for all experiments. Mice were housed one to three per cage in an environmentally controlled animal facility maintained at thermoneutrality (28°C) or 20°C (in experimental approach 1) on a 12-h light:dark cycle from 0700 to 1900 h with constant access to fresh water and food ad libitum. Mice were kept at thermoneutrality to study the role of BAT in a physiological setting that is more human relevant (31,32). Mice were fed one of the following diets: normal chow, 3.98 kcal/g food, 13% kcal fat, 58% kcal from carbohydrate, and 29% kcal protein (Laboratory Rodent Diet 5001*; LabDiet); Western diet (WD), 4.73 kcal/g food, 46% kcal from fat, and 36% kcal from carbohydrate (17.5% sucrose and 17.5% high-fructose corn syrup by weight), and 18% kcal from protein (modified 58Y1 [5APC]; TestDiet); or control diet (CD), 3.50 kcal/g food, 10% kcal fat, 72% kcal carbohydrate, and 18% kcal protein (58Y2; TestDiet). Energy content per gram of food was determined via bomb calorimetry as previously described (33,34).
Experimental Design
Experimental Approach 1: Sympathetic Regulation of BAT in Lean and Obese Mice
Experiment 1 was designed to 1) determine the role of sympathetic innervation of BAT at thermoneutrality and 2) examine the extent to which increased sympathetic tone caused by decreased environmental temperature mitigates WD-induced BAT dysfunction and improves glucose tolerance. Nine-week-old female mice (n = 8) underwent unilateral interscapular BAT (iBAT) denervation and were terminated 12 weeks thereafter via CO2 asphyxiation for molecular characterization of iBAT. Another cohort of 5-week-old female mice fed a WD for 9 weeks were either kept under thermoneutral housing conditions (28°C) (n = 13) or exposed to mild cold (i.e., housed at 20°C) (n = 8). A third group of CD-fed mice (n = 10) housed at thermoneutrality were included as reference controls to determine the degree of metabolic dysfunction caused by WD. Mice were euthanized after a 5-h fast via CO2 asphyxiation at 14 weeks of age. The following in vivo assessments were conducted: cold tolerance test (CTT) (at 11 weeks of age), energy expenditure (EE) via metabolic cages (at 12 weeks of age), glucose tolerance test (GTT) (at 13 weeks of age), and EchoMRI (at 14 weeks of age).
Experimental Approach 2: Silencing of Pnpla2 in iBAT in Chow-Fed Mice Living at Thermoneutrality
Experiment 2 was designed to test the hypothesis that silencing of Pnpla2 causes BAT dysfunction and associated glucose intolerance. Thirteen-week-old female mice (n = 8) underwent bilateral silencing of Pnpla2 in iBAT (i.e., ∼25% of all BAT depots) using adeno-associated virus serotype 8 (AAV8) with shRNA targeting Pnpla2 (Pnpla2-shRNA), whereas control mice (n = 8) received bilateral iBAT injections encoding a scrambled sequence AAV8 (GFP-scrmb-shRNA) with a GFP reporter (cat. no: 7045; Vector Biolabs, Malvern, PA). The titer for each virus was 1013 gene copies/mL. The following validated shRNA sequence (VBLKO-249774) was constructed by a commercial laboratory (cat. no. shAAV-280982; Vector Biolabs): 5′-CCGG-CATCTCCCTGACTCGTGTTTCCTCGAGGAAACACGAGTCAGGGAGATG-TTTTT-3′. The targeting sequence and hairpin loop sequence are 5′-CATCTCCCTGACTCGTGTTTC-3′ and 5′-CTCGAG-3′, respectively. Targeted microinjections of Pnpla2-shRNA and scrambled vector were delivered to both iBAT lobes under anesthesia as described in Supplementary Data. Animals were euthanized after a 5-h fast via CO2 asphyxiation 8 weeks after injections for molecular characterization of iBAT. Mice consumed normal chow during this entire experiment. The following in vivo assessments were conducted: GTT (at 20 weeks of age) and EchoMRI (at 21 weeks of age).
Experimental Approach 3: Lifestyle Intervention and BAT Phenotype in Mice Living at Thermoneutrality
Experiment 3 was designed to assess the extent to which physiological lifestyle interventions including increased physical activity (i.e., voluntary wheel running) and diet switch (i.e., WD replaced by low-fat CD) improve the BAT phenotype and glucose tolerance. Importantly, head-to-head comparisons of the impact of diet versus increased physical activity on BAT metabolism at thermoneutrality in the context of obesity are lacking (35). To provoke metabolic dysfunction, we fed 5-week-old female mice (n = 18) a WD for 9 weeks. At 14 weeks of age, mice were randomized to one of the following groups (n = 9–11/group) for 10 weeks: 1) continuation of WD, 2) continuation of WD with access to running wheels (WD+WR), and 3) switch from WD to CD (CD). Animals were euthanized at 24 weeks of age after a 5-h fast via CO2 asphyxiation. The following in vivo assessments were conducted: CTT (at 21 weeks of age), EE via metabolic cages (at 22 weeks of age), GTT (at 23 weeks of age), and EchoMRI (at 24 weeks of age).
For extended methods, see Supplementary Data.
Statistical Analysis
Student t tests were run for between-group comparisons. In experiments that contained more than two groups, one-way ANOVA was conducted with pairwise comparisons via Tukey correction. All data are presented as mean ± SE. Significance was accepted at P < 0.05. Statistical analyses were performed with SPSS, version 20.0.
Results
Experimental Approach 1: Sympathetic Regulation of BAT in Lean and Obese Mice
Previous literature indicates that sympathetic innervation of BAT is critical for the maintenance of a “brown” phenotype (1); however, most studies have made this connection in standard ambient housing conditions—where sympathetic tone to BAT is markedly heightened (32). To corroborate that sympathetic innervation of BAT is necessary for the multilocular and thermogenic phenotype of BAT at thermoneutrality, we denervated one iBAT lobe, while maintaining the contralateral side intact, in lean mice fed a chow diet (Supplementary Fig. 1A). Unilateral denervation of iBAT enhanced lipid droplet hypertrophy and led to an approximate twofold reduction in UCP1 and tyrosine hydroxylase protein expression, demonstrating the importance of sympathetic innervation of BAT even under thermoneutral conditions (Supplementary Fig. 1B and D). The within-animal experimental design (i.e., denervation of one iBAT fat pad while using the contralateral side as internal control) precluded any relevant analysis of systemic metabolic function.
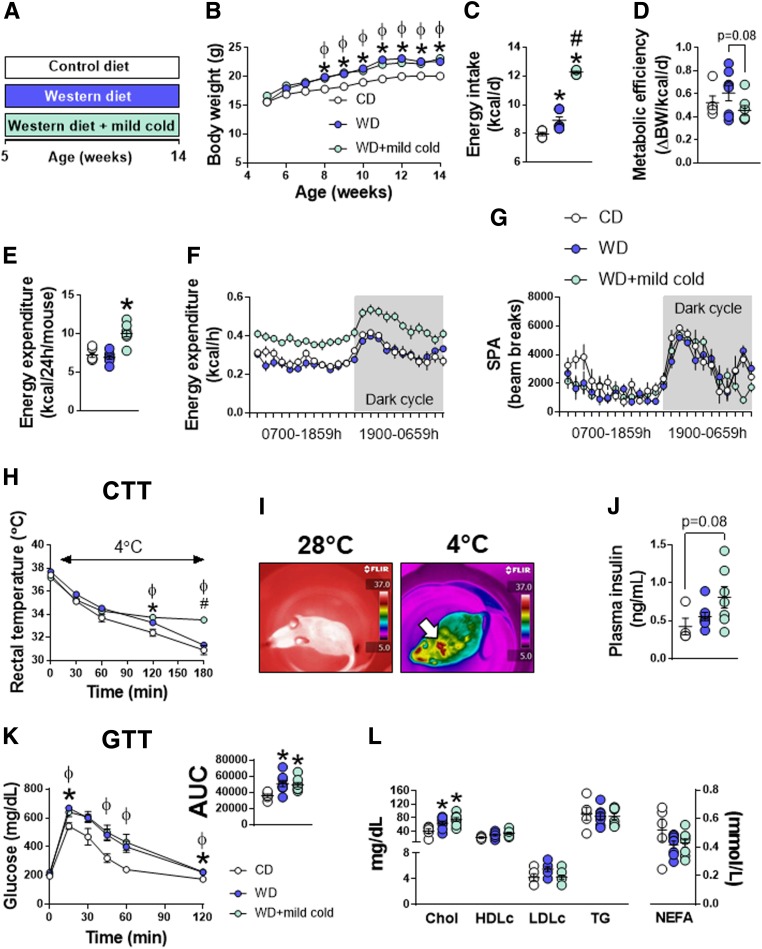
Next, we determined whether enhanced sympathetic activation via mild thermostress (20°C) attenuates WD-induced BAT dysfunction and improves glucose tolerance compared with mice kept in thermoneutral conditions (Fig. 1A). Mild cold exposure did not decrease body weight gain or attenuate adiposity caused by WD feeding (Fig. 1B and Supplementary Table 3). Energy intake and total EE were enhanced with mild cold exposure by 40% and 50%, respectively (Fig. 1C–E). The increase in EE was likely to defend body temperature via NST, given that spontaneous physical activity was not different among groups (Fig. 1E–G). Anecdotally, from 5 to 9 weeks of age, mice exposed to mild cold displayed shivering behavior, whereas no shivering was apparent after 9 weeks of age, suggesting that NST was maintaining body temperature. These observational data are supported by greater cold tolerance in mice exposed to mild thermostress compared with animals living at thermoneutrality (Fig. 1H). Similarly, in a subset of mice, real-time thermal imaging revealed that heat emission was largely conserved to the iBAT region in acute cold-exposed animals with little to no heat emission from surrounding muscle tissue (Fig. 1I). Mild cold exposure failed to correct glucose intolerance caused by WD feeding and did not improve fasting insulin, cholesterol, HDL, or LDL concentrations (Fig. 1J–L).
Figure 1.
Effects of mild cold exposure on energy homeostasis and glucose tolerance. A: 5-week-old female mice were randomized to either a CD or WD at thermoneutrality (28°C) for 9 weeks. A third group of animals consumed a WD while housed under mild cold stress conditions (20°C) for 9 weeks. B: Weekly body weights (n = 5–8/group). C and D: Energy intake and metabolic efficiency (i.e., change in body weight over time per energy consumed) (n = 5–8/group). E: Average total EE (n = 5–8/group). F and G: Mean 24-h EE and spontaneous physical activity (SPA) curves (n = 5–8/group). H: Acute CTT. Baseline rectal temperature measurements were recorded in home cages (28°C or 20°C). Thereafter, mice were placed in environmental cold chambers (4°C) and rectal temperature measurements were taken every 30 min for 180 min (n = 5–8/group). I: Representative real-time thermal images captured during the CTT. Identical camera settings were used in ambient (home-cage temperature) and cold (4°C) environments. J: Plasma insulin concentrations (n = 5–8/group). K: GTT (n = 5–8/group). GTTs were performed at the same environmental temperature at which mice were housed. L: Plasma cholesterol and lipid concentrations (n = 5–8/group). Data are mean ± SE. *P < 0.05 vs. CD; #P < 0.05 vs. WD; ɸP < 0.05 CD vs. WD + mild cold. Chol, total cholesterol; d, day; HDLc, HDL cholesterol; LDLc, LDL cholesterol; NEFA, nonesterified fatty acid.
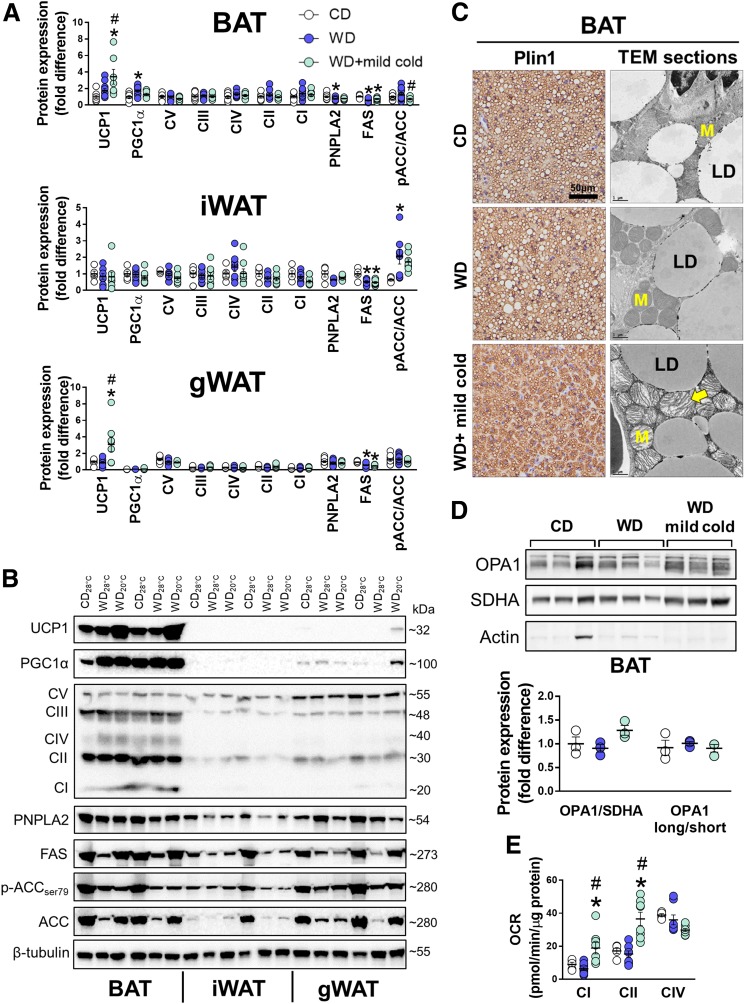
UCP1 protein expression was enhanced by mild cold in BAT and gonadal white adipose tissue (gWAT), but not in inguinal WAT (iWAT), respectively (Fig. 2A and B). Of note, the increase in UCP1 expression in WAT was 11-fold lower than in BAT (i.e., WD + mild cold BAT vs. gWAT). Regardless of temperature, FAS expression was lower in all three adipose tissue depots in WD-fed mice, whereas PNPLA2 was only lower in BAT (Fig. 2A and B). There were no changes in oxidative phosphorylation (OXPHOS) complexes within BAT among groups, whereas WD feeding attenuated complex V in iWAT and gWAT that was not restored by mild cold exposure (Fig. 2A and B). Immunohistochemical evidence demonstrated that compared with thermoneutral animals, BAT from WD + mild cold mice presented with smaller lipid droplets (via Plin1 immunostain) and numerous mitochondria with greater cristae density captured by transmission electron microscopy (Fig. 2C). Relative to mice kept at thermoneutrality, the visual increase in cristae density from mild cold–exposed mice was supported at the molecular level by a tendency for greater expression of total OPA1 (Fig. 2D) (P = 0.11)—a major regulator of mitochondrial fusion and cristae structure—and higher ex vivo complex I and complex II oxygen consumption rates (OCR), whereas OCR via complex IV was decreased (Fig. 2B). Taken together, data from experiment 1 confirm that mild cold exposure enhances thermogenic capacity of BAT in mice with diet-induced obesity but does not improve glucose tolerance.
Figure 2.
Effects of mild cold exposure on the BAT phenotype. A: BAT, iWAT, and gWAT mean protein expression. Data are relative to BAT CD28°C, which is set at 1 (n = 5–13/group). B: Immunoblots from BAT, iWAT, and gWAT run on the same gel; 12 μg protein was loaded per lane. C: Representative Plin1-immunostained and transmission electron microscopy (TEM) images of iBAT. Arrow denotes mitochondrial cristae. TEM section scale bars = 1 μm. LD, lipid droplet; M, mitochondria. D: BAT immunoblots. E: OCR from BAT whole-cell homogenates (n = 5–8/group). Data are mean ± SE. *P < 0.05 vs. CD; #P < 0.05 vs. WD.
Experimental Approach 2: Silencing of Pnpla2 in iBAT in Chow-Fed Mice Living at Thermoneutrality
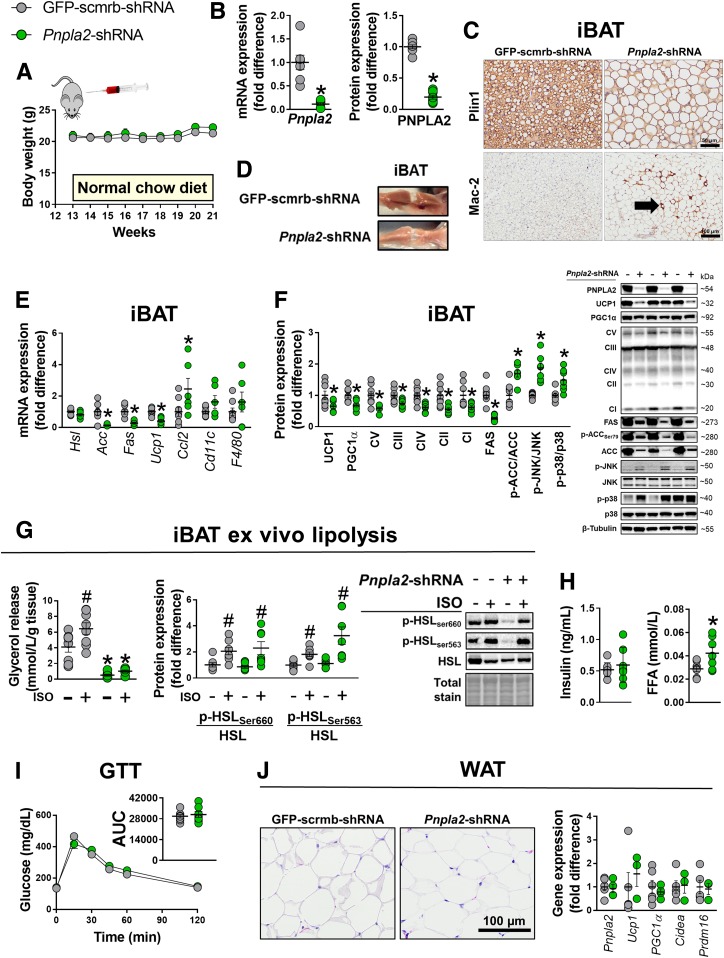
To determine whether loss of iBAT function perturbs glucose tolerance, we silenced Pnpla2 within iBAT (bilateral microinjections) using an AAV8 shRNA in female mice at 13 weeks of age. Compared with the scrambled vector (GFP-scrmb-shRNA), Pnpla2-shRNA virus led to an approximate ninefold and sixfold reduction in iBAT Pnpla2 mRNA and PNPLA2 protein expression, respectively (Fig. 3B), that produced pronounced steatotic hypertrophy (Fig. 3C and D). iBAT mass was 1.4-fold greater in Pnpla2-silenced mice compared with GFP-scrmb-shRNA controls, respectively—an effect that was independent of body weight (Fig. 3A). Downregulation of Pnpla2 suppressed UCP1 mRNA and protein expression, which was also accompanied by an attenuation in the abundance of OXPHOS complexes CV–CI and enhanced inflammatory gene transcripts (Fig. 3E and F). In concert with an upregulation of inflammatory gene expression, Mac-2 immunohistochemical staining revealed increased abundance in positive-stained area as well as increased expression of proteins involved in inflammatory signaling (i.e., phosphorylated (p-)JNK/JNK and p-p38/p38) in Pnpla2-shRNA relative to GFP-scrmb-shRNA mice (Fig. 3C and F). Lipogenic mRNA and protein expression was attenuated in Pnpla2-shRNA mice despite gross lipid droplet expansion (Fig. 3E and F).
Figure 3.
Effects of Pnpla2 silencing in BAT on BAT function and glucose tolerance. A: Weekly body weights. iBAT transfections (i.e., GFP-scrmb-shRNA vs. Pnpla2-shRNA) occurred in 13-week-old mice (n = 8/group). B: Pnpla2 mRNA and protein (ATGL) expression in iBAT (n = 7–8/group). C and D: Representative Plin1 and Mac-2 immunostained images and excised iBAT from a single animal receiving either Pnpla2-shRNA or scrambled-GFP AAV8 vectors directly injected into iBAT. E: iBAT mRNA expression (n = 7–8/group). F: iBAT protein expression with representative immunoblots (n = 7–8/group). G: Lipolysis assays and protein expression of iBAT explants from 20-week-old mice. (− and + symbols indicate that explants were treated, respectively, without or with 10 μmol/L isoproterenol [ISO]) (n = 8/group). H: Fasting insulin and free fatty acid (FFA) concentrations (n = 7–8/group). I: GTTs were performed at 19 weeks of age or 7 weeks after iBAT transfection (n = 8/group). J: Representative hematoxylin-eosin images of gWAT and mRNA expression (n = 4–6/group). Data are means ± SE. *P < 0.05 vs. Pnpla2-scrmb-shRNA; #P < 0.05 vs. unstimulated.
Functionally, Pnpla2 suppression in iBAT abolished isoproterenol-induced glycerol release in iBAT lysates that was independent of hormone-sensitive lipase (HSL) phosphorylation (Fig. 3G), such that phosphorylated HSLSer660 and HSLSer563 were similarly enhanced by isoproterenol in both groups, indicating that Pnpla2 is critical for stimulated intracellular lipolysis in iBAT. Despite the steatotic hypertrophic iBAT phenotype in Pnpla2-shRNA mice, glucose tolerance and fasting insulin concentrations were not different from GFP-scrmb-shRNA controls; however, free fatty acid concentrations were increased in Pnpla2-shRNA mice (Fig. 3H and I). Some evidence suggests that Pnpla2 suppression may lead to compensatory browning of WAT (4), which could contribute to preservation of glucose tolerance. However, expression levels of thermogenic genes (Ucp1, Pgc1α, Cidea, and Prdm16) in WAT were not different between GFP-scrmb-shRNA versus Pnpla2-silenced mice (Fig. 3J). Collectively, data from experiment 2 indicate that downregulation of Pnpla2 in iBAT causes stark adverse morphological and functional changes that do not impair glucose tolerance.
Experimental Approach 3: Lifestyle Intervention and BAT Phenotype in Mice Living at Thermoneutrality
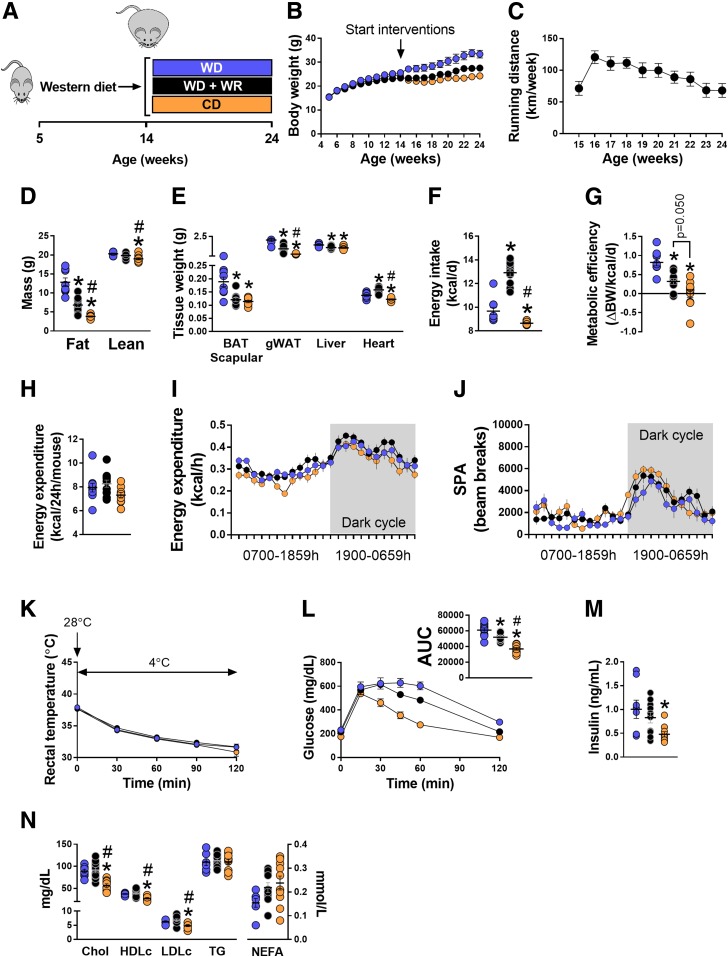
Diet and exercise are well-known strategies to improve obesity-induced metabolic dysfunction (36). Thus, to determine the extent to which improved glucose tolerance tracks with a thermogenic BAT phenotype, we randomized mice with diet-induced obesity (i.e., after 9 weeks of WD feeding) to one of the following treatments for 10 weeks (Fig. 4A): WD, WD+WR, and WD switched to CD. WD+WR and CD significantly attenuated weight gain compared with WD (Fig. 4B). On average, WD+WR mice ran ∼13 km/day (Fig. 4C). Relative to WD mice, fat mass was lower in both WD+WR and CD groups; however, CD had greater reductions than WD+WR (Fig. 4D). BAT mass was greater in WD animals, with no differences between WD+WR and CD groups (Fig. 4E). Compared with WD mice, gWAT weight was attenuated in both WD+WR and CD, but to a greater extent in CD, and as expected, heart weight was increased in WD+WR animals compared with WD and CD (Fig. 4E). Energy intake was significantly greater in WD+WR animals, with no differences between WD and CD, whereas, metabolic efficiency showed a stepwise reduction, WD > WD+WR > CD (Fig. 4F and G), suggesting that WD-fed animals are more efficient at storing energy than CD mice. Total EE and spontaneous physical activity were not different among groups (Fig. 4H–J). In addition, no group differences in rectal temperature measurements during an acute CTT were detected (Fig. 4K). CD mice exhibited 66% and 40% lower glucose area under the curve (AUC) during GTT compared with WD and WD+WR, respectively (Fig. 4L and M). Glucose AUC in WD+WR animals was 19% lower versus WD (Fig. 4L). Fasting insulin concentrations were lower in CD mice compared with WD animals, with no difference compared with WD+WRs (Fig. 4M). Relative to both WD groups, total cholesterol, HDL cholesterol, and LDL cholesterol were significantly lower in CD mice (Fig. 4N).
Figure 4.
Effects of voluntary exercise and low-fat diet on energy homeostasis and glucose tolerance in mice with diet-induced obesity. A: Experimental design. Five-week-old mice were fed a WD for 9 weeks. Thereafter, animals were allocated to one of the following groups: WD, WD+WR, and switched from a WD to a CD (n = 9–11/group). B and C: Weekly body weights and weekly running distance (n = 9–11/group). Running wheels were connected to a Sunding bicycle computer (SD-548B; Dongguan Sunding Electron Co., TangXia, DongGuan, China) for determination of weekly running distance. Odometers were checked daily and reset every week. D: Fat mass and lean mass via EchoMRI (n = 9–11/group). E: Organ/tissue weights (n = 9–11/group). F and G: Energy intake and metabolic efficiency (n = 9–11/group). H: Total EE assessed via metabolic cages. Data are presented as kcal/h/mouse (n = 8–11/group). I and J: Mean 24-h EE and spontaneous physical activity (SPA) tracings via metabolic chambers. Dark cycle is shaded in gray (n = 8–11/group). K: Acute CTT. Baseline rectal temperature measurements were recorded in home cages (28°C). Thereafter, mice were placed in environmental cold chambers (4°C) and rectal temperature measurements were taken every 30 min for 120 min (n = 5–9/group). L: GTT with glucose AUC (n = 9–11/group). M and N: Plasma insulin, cholesterol, and lipid concentrations (n = 8–10/group). Data are means ± SE. *P < 0.05 vs. WD; #P < 0.05 vs. WD+WR. BW, body weight; Chol, total cholesterol; d, day; HDLc, HDL cholesterol; LDLc, LDL cholesterol; NEFA, nonesterified fatty acid.
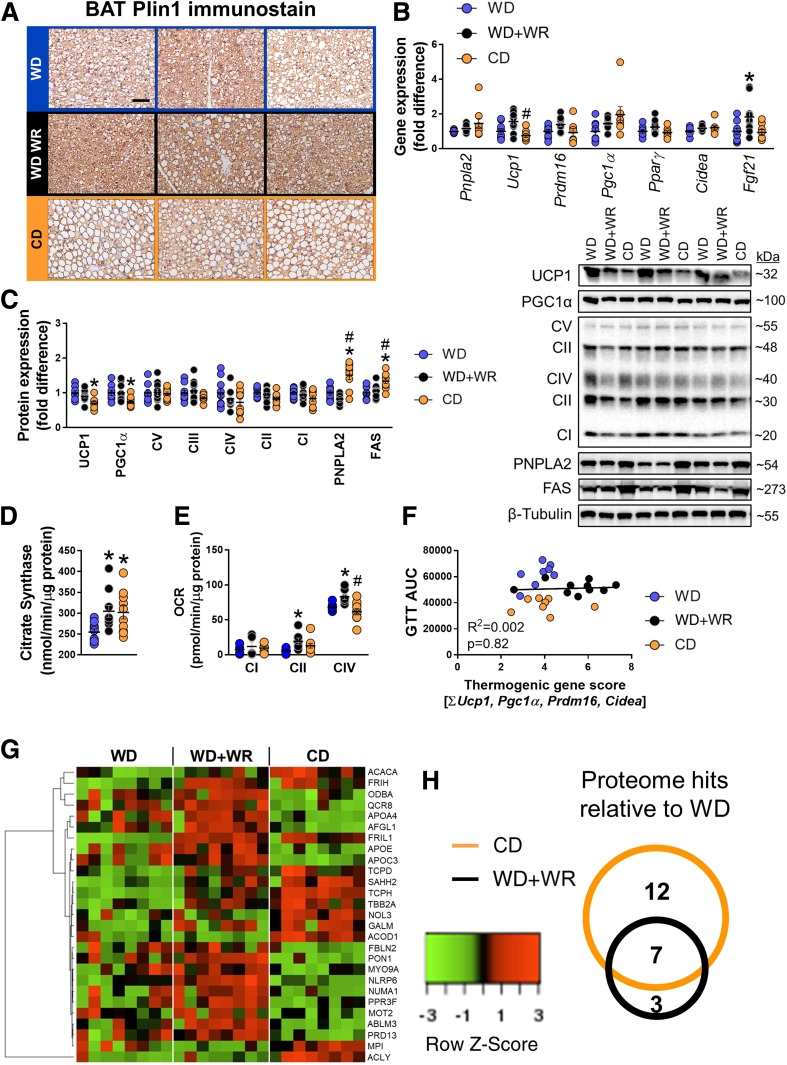
In BAT, Pnpla2 expression was nonsignificantly increased in CD versus WD mice (P = 0.07), with no change in WD+WR mice, whereas UCP1 mRNA expression tended to increase in WD+WR mice and was decreased in CD mice (Fig. 5B). UCP1 and PGC1α protein expression were decreased in CD-fed mice, with no differences between WD-fed groups, while PNPLA2 expression was 50% and 80% greater in CD compared with WD and WD+WRs, respectively (Fig. 5C). OXPHOS complex II was decreased in CD versus WD mice, whereas complexes III, IV, and I were nonsignificantly decreased (Fig. 5C). No differences in OXPHOS complexes were detected between either WD-fed groups. FAS and phosphorylation of ACCSer79 were significantly greater in CD animals with no differences in WD groups (Fig. 5C). Both CD and WD+WR increased BAT citrate synthase activity, while OCR at complex II and complex IV were elevated in WD+WR animals only (Fig. 5D and E). As a marker of BAT thermogenic profile (5), we summed the expression of thermogenic genes and plotted the score as a regression against glucose AUC. There was no association between the thermogenic composite score and glucose tolerance (Fig. 5F).
Figure 5.
Effects of voluntary exercise and diet on BAT function in mice with diet-induced obesity. A: Plin1-immunostained BAT sections from WD, WD+WR, and CD mice. Scale bar, 50 μm. B and C: BAT mRNA and protein expression with representative immunoblots (n = 9–10/group). D: Citrate synthase activity in BAT lysates (n = 9–10/group). E: OCR in BAT lysates (n = 5 pooled samples per group). F: Relationship between GTT AUC and BAT browning score as assessed via the sum of Ucp1, Pgc1α, Cidea, and Prdm16 mRNA expression (n = 8–10/group). G: Heat map summarizing BAT proteome differences between WD, WD+WR, and CD (n = 8/group). H: Number of proteins significantly altered via low-fat diet and physical activity compared with WD. Size of the circles is representative of the number of altered proteins. Overlapping regions indicate proteins that were commonly increased. Data are means ± SE. *P < 0.05 vs. WD; #P < 0.05 vs. WD+WR.
Next, we screened the BAT proteome to determine whether modulation in thermogenic-related proteins by low-fat CD and exercise were related to glucose tolerance. We found 27 proteins that were differentially expressed across groups (Fig. 5G). Relative to WD, WD+WR and CD mice showed an increase in seven common proteins (Fig. 5H). A list of the differentially expressed proteins and their main functions appears in Supplementary Table 4. Interestingly, proteins involved in fatty acid biosynthesis (ACLY and ACACA) were increased in CD but not WD+WR mice, whereas proteins involved in lipid transport/cholesterol metabolism (PON1, APOC3, and APOA4) and a ubiquinol–cytochrome c reductase complex of the mitochondrial respiratory chain were decreased in CD mice (Fig. 5G). Collectively, experiment 3 indicates that changes in the expression of thermogenic genes or UCP1 content in BAT are not related to improvements in glucose tolerance with diet and exercise in obese mice.
Since aging has been implicated as an important factor regulating BAT function (29,30), we also determined whether growth from 14 to 24 weeks of age led to whitening of BAT and concomitant glycemic and metabolic deterioration in CD and WD-fed mice (used in experimental approaches 1 and 3). Compared with mice at 14 weeks, mice at 24 weeks of age had greater body weight, fat mass, and BAT mass regardless of diet (Supplementary Fig. 2). Also independent of the diet, Plin1 immunostaining of BAT revealed that 24-week-old animals had a whitened BAT appearance characterized by larger lipid droplets, as well as impaired cold tolerance, despite no differences in UCP1 protein expression (Supplementary Fig 2). Importantly, however, the effect of increased age on metabolic function was only displayed in WD-fed mice. That is, glucose tolerance was worsened (i.e., 22% increase in glucose AUC) and fasting insulin concentrations were increased by 80% at 24 weeks of age in WD-fed but not CD-fed mice, relative to younger diet-matched mice. Together, these data indicate that 10 weeks of aging in young adult mice causes whitening of BAT in both CD and WD settings, whereas glycemic deterioration only manifests over time when animals are challenged with a WD.
Discussion
In contrast to the prevailing dogma (37–39), we conclude that a thermogenic-like BAT phenotype is not coupled with improved glucose regulation in female mice. Several lines of evidence presented here support this conclusion, including the following: 1) increased sympathetic activation of BAT via chronic mild thermostress (20°C) enhanced BAT function but did not improve glucose tolerance in mice with diet-induced obesity, 2) robust whitening and associated inflammatory milieu in iBAT caused by genetic silencing of Pnpla2 did not impair glucose tolerance in lean mice, and 3) improved glucose tolerance via increased physical activity and low-fat diet in obese mice did not track with a thermogenic BAT phenotype. Taken together, compelling evidence is presented against the hypothesis that BAT is a critical regulator of glucose homeostasis in female mice.
Experimental studies in both animals (12–15,20) and humans (16–19,40) suggest that BAT is an important regulator of glucose metabolism—acting as a substrate “sink”—that has become dogmatic in recent years. These lines of evidence are largely founded on research under environmental (i.e., cold exposure) or pharmacological adrenergic activation (16,41). Thus, whether BAT is crucial for glucose disposal under conditions when thermogenesis is minimal, a setting that is postulated to have greater human relevance (31,32), is less appreciated. Here, under nonthermostressed conditions we show that genetic silencing of Pnpla2, a known regulator of lipid accrual in BAT (42), causes pronounced steatotic hypertrophy and associated inflammation in iBAT of chow-fed mice; however, this does not cause glucose intolerance. This finding is consistent with previous data from our group showing that surgical excision of iBAT is dispensable for metabolic function in lean and obese mice housed at 25°C (5). Furthermore, recent studies indicate that loss of intracellular lipolysis in BAT with use of genetic mouse models does not abolish BAT thermogenesis (3,4) or disrupt glucose metabolism in the setting of mild cold stress (4). Together, these findings suggest that intracellular manipulation of lipolytic programs within BAT does not directly affect glucose homeostasis in mice.
In the current study, we also found a disconnect between thermogenic activation of BAT and enhanced glycemic control in mice with diet-induced obesity. That is, even though mice housed at 20°C exhibited increased cold tolerance, UCP1 expression, and BAT respiratory capacity, relative to thermoneutral mice, they did not display improved glucose tolerance (Figs. 1 and 2). Of note, GTTs were performed at the same temperature at which mice were housed. The lack of improvement in glucose tolerance in animals with “activated” BAT (20°C mice) was accompanied by no changes in body weight and adiposity, suggesting that in a setting of energy balance, augmenting BAT activity does not explicitly improve glucose control. It is most likely that if such enhanced BAT activity would have been accompanied with energy loss (e.g., by restricting the increased energy intake), mice would have revealed an improvement in glucose control (43). Notably, prior investigations showing that cold exposure enhances whole-body glucose uptake in lean and overweight humans were acute in nature (i.e., <12 h of exposure). Thus, while it remains unknown whether chronic cold-induced BAT activation augments glucose control in obese humans, the present data in obese mice suggest that it would not. Indeed, findings presented herein are counter to the idea that it is uncoupled respiration in BAT that contributes to an improvement in glucose homeostasis.
Notably, a mild-to-moderate lipid expansion phenotype in BAT and an associated decrease in thermogenesis have been proposed as an adaptive response that may be beneficial for glucose homeostasis (44). It is plausible that increased glucose uptake in BAT supplies building blocks for glycerol backbone in TG manufacturing and acetyl-coA units for fatty acid biosynthesis in the lipid-expanding tissue. Similarly, an uncoupling between BAT thermogenesis and glycemic control was also demonstrated in male and female mice with brown adipocyte-specific deletion of mitofusin 2, an important component of NST (21). Cold-induced thermogenesis was severely impaired in mitofusin 2–ablated animals despite being protected from glucose dysmetabolism induced by obesity (21). As a corollary, recent human evidence suggests that under minimal thermostress (25°C) BAT glucose utilization occurs mostly via anaerobic or even anabolic pathways (i.e., glyceroneogenesis or fat synthesis) (45), dissociating uncoupled BAT respiration with glucose metabolism. In conjunction with the aforementioned studies, our findings suggest that thermogenic activation of BAT is not directly linked with improved glucoregulation.
At thermoneutrality, sympathetic drive to BAT is minimal, though a recent study revealed that intact innervation of BAT is required for diet-induced thermogenesis at thermoneutrality (46). We corroborate these underappreciated findings that BAT is not dormant at thermoneutrality, evidenced by the fact that unilateral iBAT denervation reduced UCP1 content and increased lipid accrual (Supplementary Fig. 1). Living at thermoneutrality per se may manifest as a shift toward a coupled oxidative phenotype and even increased lipid storage in BAT. While in the setting of obesity the latter may seem unfavorable, we observed at the histological level a well-organized array of lipid droplet distribution in our most metabolically healthy mice (i.e., CD) that appeared larger than in WD-fed animals (Fig. 5A). It has been shown that in the setting of energy deficit, female rodents deactivate BAT thermogenesis to a greater extent than males (47), and this is postulated to be teleologically advantageous for female survival during times of food scarcity (i.e., conserve fat mass). The BAT phenotype from low-fat CD-fed versus WD mice seemed to support this theory, with increased proteomic expression of ACAC (acetyl-CoA carboxylase) and ACLY (ATP-citrate lyase), critical for fatty acid biosynthesis (Fig. 5G). BAT ACLY has been shown to increase in response to cold exposure to fuel NST (48); however, the role of BAT de novo lipogenesis in low-fat diet versus obesogenic diet mice at thermoneutrality is less appreciated (49,50). Notably, lipid storage is bioenergetically expensive and does not necessarily represent an impaired oxidative phenotype, demonstrated previously as the athlete’s paradox in human skeletal muscle (51) and in obese persons with “simple” hepatic steatosis who display increased hepatic fatty acid oxidation (52). Along these lines, a role for adipose de novo lipogenesis in regulating glucose homeostasis during catch-up growth has previously been demonstrated in rats (53). Nonetheless, the metabolic implications for the lipid accretion phenotype in BAT from lean animals living at thermoneutrality require further study.
Diet and increased physical activity are established lifestyle interventions to combat and even correct many metabolic diseases and comorbidities including disruptions to glucose homeostasis (36,54). Here, we confirmed the well-characterized amelioration of obesogenic diet–induced glucose intolerance through increased physical activity and low-fat diet (Fig. 4); however, this improvement did not associate with the induction of classic brown-related mRNA or protein markers of BAT thermogenesis including UCP1 (Fig. 5). Notwithstanding the lack of an association between thermogenic genes and glucose tolerance, we cannot rule out the possibility that diet and/or physical activity may modify the BAT secretome (55) and thus contribute to enhanced glucose handling. In this context, Stanford et al. (56) elegantly demonstrated that a BAT-derived lipokine, 12,13-diHOME, is augmented by exercise in mice and humans and increases fatty acid uptake and fatty acid oxidation in skeletal muscle. This suggests that in addition to its thermogenic function, BAT may be a source of “batokines” or lipokines that are involved in systemic energy homeostasis. Similarly, evidence for a regulatory BAT-muscle axis in exercising mice was recently proposed that appears to mechanistically involve interferon regulatory factor 4 and myostatin (57). Undoubtedly, additional research is needed to understand this tissue cross talk and its biological relevance.
Several aspects of this study require consideration. Only female mice were studied, and thus current findings may not be extrapolable to males. Previous literature indicates that there are sex differences in adipose tissue and lipid metabolism (58,59), where females generally exhibit a greater thermogenic BAT phenotype compared with males, characterized by higher expression of UCP1 and its activity (22). Accordingly, side-by-side comparisons of sex may be warranted in future studies. Moreover, the present experiments were completed with relatively young adult mice. It is known that aging causes BAT involution and dysfunction (60), which is an important consideration when translating experimental findings to humans given that aging humans have less detectable BAT and are more susceptible to metabolic dysfunction than their younger counterparts (61). Of note, we found that compared with young (14 weeks of age) mice, middle-aged (24 weeks of age) animals had greater adiposity and a more whitened BAT phenotype characterized by the appearance of larger lipid droplets and were cold intolerant when fed either a low-fat diet or a WD. Interestingly, glucose intolerance and hyperinsulinemia associated with increased age were unmasked only in mice consuming a WD. These findings suggest that the thermogenic BAT phenotype begins to deteriorate at a relatively young age (24 weeks of age); however, this thermogenic deterioration over time is not sufficient to disturb glucose homeostasis.
In aggregate, we report that a thermogenic-like BAT phenotype does not drive improvements in glucose tolerance in female mice. Accordingly, contrary to a widespread belief, the present data suggest that “uncoupled” BAT may not be vital to maintaining glucose homeostasis even in a setting of mild thermostress accompanied by energy balance.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the following University of Missouri core facilities for their expertise and contributions to this work: Molecular Cytology Core, Veterinary Diagnostic Medical Laboratory, and Charles W. Gehrke Proteomics Center. The authors thank Dr. Alexander Jurkevich and Dr. Frank Baker (Bond Life Sciences Center, University of Missouri, Columbia, MO) for their assistance with confocal microscopy and are thankful for the assistance of Jill Hansen and Dr. Cynthia Besch-Williford (IDEXX Laboratories, Inc.) for their help with immunohistochemistry. The technical assistance from Lisa Watkinson and Terry Carmack (Harry S. Truman Memorial Veterans' Hospital, Columbia, MO) is greatly appreciated.
Funding. This research was supported by the Cardiometabolic Disease Research Foundation (to J.P.) and Sears Trust Research Foundation (to J.P.). J.P. is supported by National Institutes of Health (NIH) grants R01-HL-137769 and K01-HL-125503. J.A.K. is supported by NIH grant R01-DK-101513. R.S.R. is supported by U.S. Department of Veterans Affairs VA-Merit Grant I01BX003271-01. N.C.W. is supported by NIH T32-DK-007563-31. L.A.A. was supported by Initiative for Maximizing Student Diversity EXPRESS Fellows Program R25GM056901. This work was supported in part with resources and the use of facilities at the Harry S. Truman Memorial Veterans’ Hospital.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.C.W. conceived and designed research, performed experiments, interpreted results, and drafted the manuscript. R.A.-P. performed experiments, interpreted results, and edited the manuscript. M.L.W., S.A.H., M.M.H., and L.A.A. performed experiments and edited the manuscript. R.S.R., V.J.V.-P., O.S.S., and J.A.K. interpreted results and reviewed and edited the manuscript. H.S.S. and J.P. conceived and designed research, interpreted the results, and reviewed and edited the manuscript. J.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-1070/-/DC1.
References
- 1.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 2.Nicholls DG, Bernson VS, Heaton GM. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl 1978;32:89–93 [DOI] [PubMed] [Google Scholar]
- 3.Schreiber R, Diwoky C, Schoiswohl G, et al. . Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab 2017;26:753–763.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin H, Ma Y, Chanturiya T, et al. . Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab 2017;26:764–777.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunewald ZI, Winn NC, Gastecki ML, et al. . Removal of interscapular brown adipose tissue increases aortic stiffness despite normal systemic glucose metabolism in mice. Am J Physiol Regul Integr Comp Physiol 2018;314:R584–R597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter C, Herndon DN, Chondronikola M, et al. . Human and mouse brown adipose tissue mitochondria have comparable UCP1 function. Cell Metab 2016;24:246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijgen GH, Sparks LM, Bouvy ND, et al. . Increased oxygen consumption in human adipose tissue from the “brown adipose tissue” region. J Clin Endocrinol Metab 2013;98:E1230–E1234 [DOI] [PubMed] [Google Scholar]
- 8.Virtanen KA, Lidell ME, Orava J, et al. . Functional brown adipose tissue in healthy adults. N Engl J Med 2009;360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 9.Cypess AM, Lehman S, Williams G, et al. . Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007;293:E444–E452 [DOI] [PubMed] [Google Scholar]
- 11.Ouellet V, Routhier-Labadie A, Bellemare W, et al. . Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011;96:192–199 [DOI] [PubMed] [Google Scholar]
- 12.Bartelt A, Bruns OT, Reimer R, et al. . Brown adipose tissue activity controls triglyceride clearance. Nat Med 2011;17:200–205 [DOI] [PubMed] [Google Scholar]
- 13.Stanford KI, Middelbeek RJ, Townsend KL, et al. . Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013;123:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford KI, Middelbeek RJ, Townsend KL, et al. . A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015;64:2002–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park YM, Rector RS, Thyfault JP, et al. . Effects of ovariectomy and intrinsic aerobic capacity on tissue-specific insulin sensitivity. Am J Physiol Endocrinol Metab 2016;310:E190–E199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chondronikola M, Volpi E, Børsheim E, et al. . Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014;63:4089–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes (Lond) 2014;38:812–817 [DOI] [PubMed] [Google Scholar]
- 18.Iwen KA, Backhaus J, Cassens M, et al. . Cold-induced brown adipose tissue activity alters plasma fatty acids and improves glucose metabolism in men. J Clin Endocrinol Metab 2017;102:4226–4234 [DOI] [PubMed] [Google Scholar]
- 19.Orava J, Nuutila P, Lidell ME, et al. . Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011;14:272–279 [DOI] [PubMed] [Google Scholar]
- 20.Winn NC, Vieira-Potter VJ, Gastecki ML, et al. . Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Regul Integr Comp Physiol 2017;312:R74–R84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahdaviani K, Benador IY, Su S, et al. . Mfn2 deletion in brown adipose tissue protects from insulin resistance and impairs thermogenesis. EMBO Rep 2017;18:1123–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez AM, Quevedo-Coli S, Roca P, Palou A. Sex-dependent dietary obesity, induction of UCPs, and leptin expression in rat adipose tissues. Obes Res 2001;9:579–588 [DOI] [PubMed] [Google Scholar]
- 23.Rudnicki M, Abdifarkosh G, Rezvan O, Nwadozi E, Roudier E, Haas TL. Female mice have higher angiogenesis in perigonadal adipose tissue than males in response to high-fat diet. Front Physiol 2018;9:1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winn NC, Grunewald ZI, Gastecki ML, et al. . Deletion of UCP1 enhances ex vivo aortic vasomotor function in female but not male mice despite similar susceptibility to metabolic dysfunction. Am J Physiol Endocrinol Metab 2017;313:E402–E412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 2015;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes 2012;36:262–272 [DOI] [PubMed] [Google Scholar]
- 27.Valencak TG, Osterrieder A, Schulz TJ. Sex matters: the effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol 2017;12:806–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quarta C, Mazza R, Pasquali R, Pagotto U. Role of sex hormones in modulation of brown adipose tissue activity. J Mol Endocrinol 2012;49:R1–R7 [DOI] [PubMed] [Google Scholar]
- 29.Graja A, Schulz TJ. Mechanisms of aging-related impairment of brown adipocyte development and function. Gerontology 2015;61:211–217 [DOI] [PubMed] [Google Scholar]
- 30.Lecoultre V, Ravussin E. Brown adipose tissue and aging. Curr Opin Clin Nutr Metab Care 2011;14:1–6 [DOI] [PubMed] [Google Scholar]
- 31.Ganeshan K, Chawla A. Warming the mouse to model human diseases. Nat Rev Endocrinol 2017;13:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol Metab 2018;7:161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grobe JL. Comprehensive assessments of energy balance in mice. Methods Mol Biol 2017;1614:123–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibbald IR, Wolynetz MS. Comparison of three methods of excreta collection used in estimation of energy and nitrogen excretion. Poult Sci 1986;65:78–84 [DOI] [PubMed] [Google Scholar]
- 35.Lehnig AC, Stanford KI. Exercise-induced adaptations to white and brown adipose tissue. J Exp Biol 2018;221(Suppl. 1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neufer PD, Bamman MM, Muoio DM, et al. . Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metab 2015;22:4–11 [DOI] [PubMed] [Google Scholar]
- 37.Schrauwen P, van Marken Lichtenbelt WD. Combatting type 2 diabetes by turning up the heat. Diabetologia 2016;59:2269–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poekes L, Lanthier N, Leclercq IA. Brown adipose tissue: a potential target in the fight against obesity and the metabolic syndrome. Clin Sci (Lond) 2015;129:933–949 [DOI] [PubMed] [Google Scholar]
- 39.Poher AL, Altirriba J, Veyrat-Durebex C, Rohner-Jeanrenaud F. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol 2015;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U Din M, Saari T, Raiko J, et al. . Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell Metab 2018;28:207–216.e3 [DOI] [PubMed] [Google Scholar]
- 41.Xiao C, Goldgof M, Gavrilova O, Reitman ML. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22°C. Obesity (Silver Spring) 2015;23:1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotzbeck P, Giordano A, Mondini E, et al. . Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res 2018;59:784–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui X, Nguyen NL, Zarebidaki E, et al. . Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiol Rep 2016;4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duteil D, Tosic M, Lausecker F, et al. . Lsd1 ablation triggers metabolic reprogramming of Brown adipose tissue. Cell Rep 2016;17:1008–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weir G, Ramage LE, Akyol M, et al. . Substantial metabolic activity of human brown adipose tissue during warm conditions and cold-induced lipolysis of local triglycerides. Cell Metab 2018;27:1348–1355.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer AW, Schlein C, Cannon B, Heeren J, Nedergaard J. Intact innervation is essential for diet-induced recruitment of brown adipose tissue. Am J Physiol Endocrinol Metab 2019;316:E487–E503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valle A, Català-Niell A, Colom B, García-Palmer FJ, Oliver J, Roca P. Sex-related differences in energy balance in response to caloric restriction. Am J Physiol Endocrinol Metab 2005;289:E15–E22 [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Gurmaches J, Tang Y, Jespersen NZ, et al. . Brown fat AKT2 is a cold-induced kinase that stimulates chrebp-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab 2018;27:195–209.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooney GJ, Vanner MA, Nicks JL, Williams PF, Caterson ID. Changes in the lipogenic response to feeding of liver, white adipose tissue and brown adipose tissue during the development of obesity in the gold-thioglucose-injected mouse. Biochem J 1989;259:651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saggerson ED, McAllister TW, Baht HS. Lipogenesis in rat brown adipocytes. Effects of insulin and noradrenaline, contributions from glucose and lactate as precursors and comparisons with white adipocytes. Biochem J 1988;251:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001;86:5755–5761 [DOI] [PubMed] [Google Scholar]
- 52.Koliaki C, Szendroedi J, Kaul K, et al. . Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 2015;21:739–746 [DOI] [PubMed] [Google Scholar]
- 53.Marcelino H, Veyrat-Durebex C, Summermatter S, et al. . A role for adipose tissue de novo lipogenesis in glucose homeostasis during catch-up growth: a Randle cycle favoring fat storage. Diabetes 2013;62:362–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Appl Physiol (1985) 2005;98:3–30 [DOI] [PubMed] [Google Scholar]
- 55.Villarroya F, Gavaldà-Navarro A, Peyrou M, Villarroya J, Giralt M. The lives and times of brown adipokines. Trends Endocrinol Metab 2017;28:855–867 [DOI] [PubMed] [Google Scholar]
- 56.Stanford KI, Lynes MD, Takahashi H, et al. . 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake [published correction appears in Cell Metab 2018;27:1111–1120.e3]. Cell Metab 2018;27:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong X, Yao T, Zhou P, et al. . Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab 2018;28:631–643.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex differences in lipid and lipoprotein metabolism. Mol Metab 2018;15:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr 2017;37:225–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller E. Browning and graying: novel transcriptional regulators of brown and beige fat tissues and aging. Front Endocrinol (Lausanne) 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoneshiro T, Aita S, Matsushita M, et al. . Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011;19:1755–1760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.