Abstract
Objective:
Jicama (Pachyrhizus erosus) fiber has been documented to exert an immunomodulatory effect both in vitro and in vivo. However, its beneficial effect against metabolic syndrome remains unknown. This study aimed to reveal whether the jicama fiber (JF) could prevent the development of diabetes and obesity caused by a high-sugar diet (HSD).
Materials and Methods:
The JF was isolated from its tuberous part and subsequently used as a supplemental diet for adult male Bagg and Albino (BALB)/c mice fed with a HSD. Four different diet paradigms including normal diet, HSD (30% sucrose), and HSD in combination with 10% and 25% of JF, respectively, were deployed continuously for 8 weeks. Furthermore, the blood glucose level, glucose tolerance, body weight, food and water consumption as well as epididymal white adipose tissue (WAT) and interscapular brown adipose tissue (BAT) mass were determined.
Results:
Our results revealed that supplementation of 25% JF could significantly prevent the blood glucose increase, excessive body weight gain, and glucose intolerance in mice fed with HSD. Moreover, 10% and 25% JF blunted the HSD-induced WAT mass gain but failed to counteract the depletion of BAT mass. Furthermore, the fiber supplementation elicited a minimum effect on rhythm and total food and water intake.
Conclusion:
The JF could effectively sustain blood glucose homeostasis as well as improve body weight and WAT mass profile against the development of diabetes and obesity caused by HSD.
Keywords: Adipose tissue, diabetes, edible fiber, glucose intolerance, obesity
Introduction
Diabetes mellitus and obesity are among the highly prevalent life-threatening metabolic diseases worldwide [1–4]. Such metabolic disorders potently promote the other serious health problems including cardiovascular diseases, hepatic steatosis, chronic kidney diseases, tumor, and cancer [5–10]. It has been well documented that high-sugar diet (HSD) is closely associated with the development of diabetes and obesity [11–14]. However, various sugary foods containing refined carbs and simple sugars are inevitably preferable for both traditional and modern society. Thus, providing an alternative supplemental diet that could preclude the deleterious effect of high sugar consumption is considerably needed.
Jicama (Pachyrhizus erosus, Fabaceae) is one of the potent medicinal plants that is also commonly served as raw or processed edible tuberous dishes [15]. Its tuber contains various bioactive compounds such as inulin, ascorbic acid, flavonoids, choline, saponin, pyridoxine, phytoestrogen, and folic acid [15,16]. Previous reports have demonstrated that the extract of jicama tuber reduced postprandial blood glucose, inhibited the activity of α-glucosidase and α-amylase enzymes involved in carbohydrate digestion, and lowered the glycated hemoglobin in diabetic mice [17]. Another study found that jicama extract could ameliorate insulin sensitivity and intracellular signaling involved in glucose uptake and metabolism in muscle cells [18]. Jicama fresh juice also has been reported to inhibit platelet aggregation in humans, thereby lowers the risk of heart attack and stroke [19]. Therefore, jicama extract exerts various advantageous effects in hampering the development and progression of metabolic diseases.
Despite the pharmacological benefits of jicama tuber’s extract have been extensively elucidated, the effect of another particular substance such as fiber contained by its tuberous part remains less investigated. Jicama tuber contains soluble and insoluble fibers [15,20]. Two previous reports suggested that jicama fiber (JF) could function as an immunomodulator both in vitro and in vivo [20,21]. However, whether the fiber also could elicit the other pharmacological effects particularly against the development of metabolic diseases remains unknown.
In this present study, we aimed to reveal whether the edible fiber isolated from jicama tuber could prevent the development of diabetes and obesity caused by HSD. Some studies have suggested that fibers isolated from plants such as sugarcane [22,23] and bamboo shoot [24,25] could be effectively used to counteract the detrimental effects of high-calorie intake on metabolic homeostasis. We, in this research, performed an experiment using mice to test the hypothesis that JF is also capable to effectively exert such effects.
Materials and Methods
Ethical approval
The animal care and use and the experimental procedures were performed in accordance with the Declaration of Helsinki and standard guideline of animal care and use for experimental purposes established by research committee of Andalas University (No. 03/2-018).
Animals
Healthy adult male Bagg and Albino (BALB)/c mice (2 months old) were purchased from UD Wistar (Yogyakarta, East Java, Indonesia). Upon transffered to the laboratory, the animals were immediately reared in cages (one individual/cage) in an animal room with 12-h-light/dark cycle and regulated RT for a week. Food (standard normal powder diet BP 2; Japfa Comfeed Indonesia Tbk., Jakarta, Indonesia) and drinking water were provided ad libitum. During the rearing period, animals were also habituated for handling every day to minimize the stress response that may occur in the experiment.
Chemicals
Pure sucrose (C12H22O11) was purchased from Himedia Laboratory (Himedia Laboratories, Pennsylvania, USA), D-glucose (C6H12O6) was purchased from Sigma-Aldrich (Sigma-Aldrich Pte Ltd., Singapore), and Formaldehyde (CH2O) was also purchased from Sigma-Aldrich (Singapore).
Isolation of jicama fiber
The fresh tubers of jicama were collected from the local farmers in Kuranji, Padang, West Sumatra Indonesia. The tubers were washed then peeled before grated using a manual grater and stationary blender (Philips HR2116/30, Bogor, Indonesia). Furthermore, the sample was suspended in aquades overnight at RT to separate the fiber from the starch. The supernatant containing crude fiber was collected and filtered before subsequently steamed for 40 min at 100°C. The isolated fiber was dried using an oven (Universal Oven UN30, Memert, Moritz, Australia) at 70°C for 16 h, then ground to be powder. The fiber powder was immediately stored in sterilized jar and sealed before used in the experiment.
Diet paradigms
Four diet paradigms deployed in this study were normal diet (ND) (standard laboratory ND), HSD (30% sucrose), and HSD in combination with 10% and 25% (w/w) of JF. The justification of fiber dose referred to previous studies [22,24,25]. The composition of diet for each paradigm is shown in Table 1. The diets were provided ad libitum for animals and changed daily to ensure its quality. Each group of treatment consisted of five mice housed in individual cages. The treatment using diet paradigms was carried out for 8 weeks continuously.
Table 1. The composition of diets used as the treatment of the experiment in mice.
| Diets | Proportion (%) | |||
|---|---|---|---|---|
| ND | HSD | HSD +JF 10% | HSD + JF 25% | |
| Normal food powder (BP2) | 100 | 70 | 60 | 45 |
| Sucrose powder | 0 | 30 | 30 | 30 |
| JF’ powder | 0 | 0 | 10 | 25 |
Blood glucose measurements
Routine blood glucose measurement: blood glucose level was measured weekly at 09:00 by using standardized automated blood glucose test meter (AGM-4000 Allmedicus, Anyang, Gyeonggi-do, South Korea) [26]. The measurement was performed by following the recommended protocol [27]. Glucose tolerance test: the intraperitoneal glucose tolerance test (ipGTT) was conducted at the end of treatment by following the standard procedure as described in another report [28]. A single dose of glucose (2 gm/kg bw) was injected intraperitoneally for each mouse. The area under curve (AUC) data were subsequently calculated from the blood glucose levels in ipGTT.
Measurement of food and water intake
Food intake in the dark phase (DP, at 19:30) and light phase (LP, at 07:30) was measured by following the previously described protocol [27]. Water intake was also measured at the same time as food intake measurement by determining the change of water volume indicated by the volume scale in the drinking bottle. The measurements were performed continuously for 7 days at the latest week of treatment.
Body weight and adipose tissue measurements
Body weight was measured weekly using a digital balance specified for animals (UW/UX 321-62150 balance, Shimadzu, Kyoto, Japan). At the end of treatment, the animals were sacrificed by vertebrae cervical dislocation. Furthermore, the epididymal white adipose tissue (WAT) and interscapular brown adipose tissue (BAT) were isolated and weighted using analytical balance (Shimadzu Analytical ATX324; Shimadzu, Kyoto, Japan) before being fixed in 10% formaldehyde as the experimental specimens.
Statistical analysis
All data were displayed as mean ± SE. An analysis of variance (ANOVA) followed by Bonferroni post-hoc test was employed to justify the significant differences among groups of treatment. The p < 0.05 was set as a significant value for all analysis.
Results
Effect of jicama fiber on blood glucose homeostasis
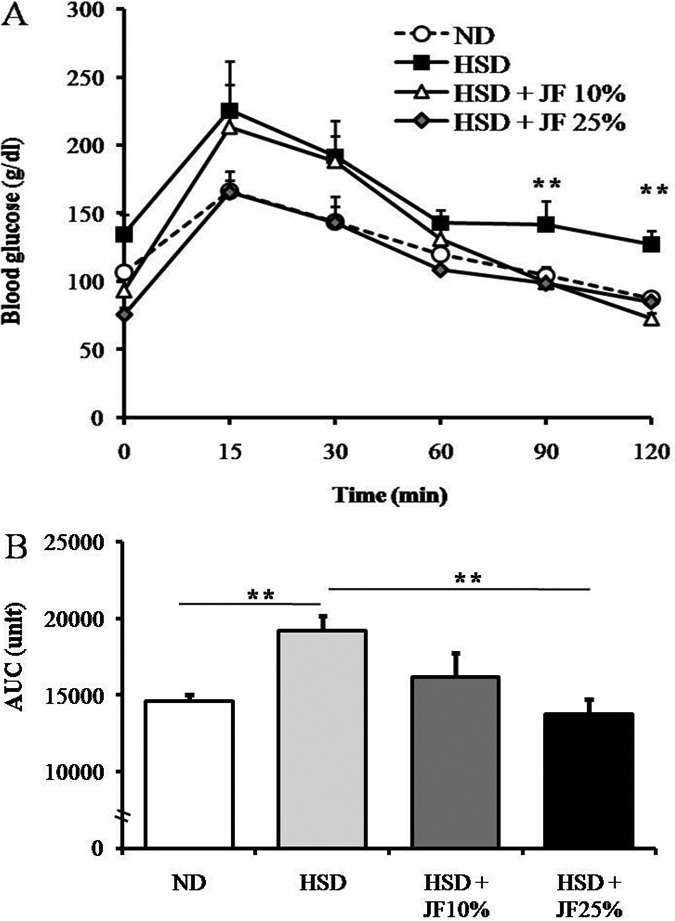
In this present research, we first investigated the effectivity of JF in hampering the development of diabetic symptoms induced by HSD. We measured blood glucose and subsequently conducted GTT in order to evaluate the blood glucose homeostasis. The blood glucose monitoring performed weekly during the treatment revealed that blood glucose level was significantly lower in JF groups (HSD + JF 10%, and HSD + JF 25%) as compared with HSD group, starting at week fourth of treatment (p < 0.05) (Fig. 1). Moreover, the blood glucose in JF groups was comparable with ND group (p > 0.05), showing a normoglycemic state. In line with blood glucose data as measured weekly, the result of GTT conducted at the end of treatment also demonstrated that blood glucose levels of JF groups tended to be lower than the HSD group almost at all the time points after an i.p injection of 2 gm/kg bw glucose (Fig. 2A). The blood glucose levels were significantly lower in JF groups as compared with HSD group particularly at 90 and 120 min after glucose injection (p < 0.01). Furthermore, the AUC data derived from the GTT data (Fig. 2B) depicted that the AUC of JF 25% group, but not JF 10% group, was significantly lower as compared with HSD group (p < 0.01). The AUC of JF 10% and 25% groups were comparable with ND group (p > 0.05).
Figure 1. JF sustained a normoglycemic state in HSD-fed mice. ND (as control), HSD, JF. *p < 0.05 by ANOVA and subsequent Bonferroni post-hoc test. Bars represent mean ± SE. n = 5 for each group.
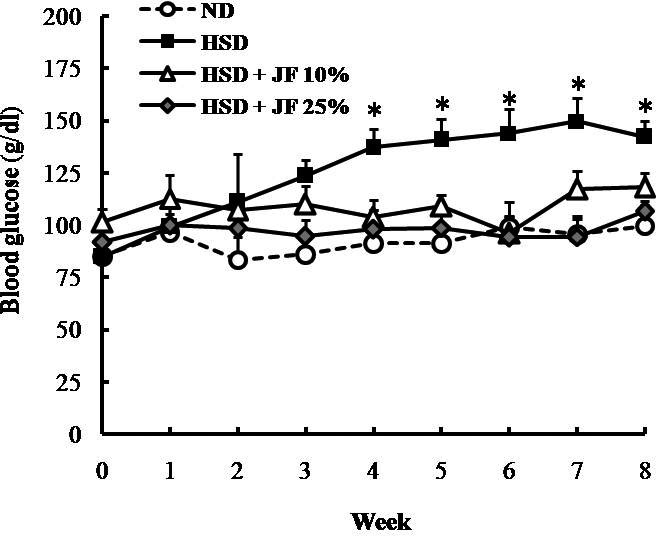
Figure 2. JF sustained glucose tolerance in HSD-fed mice. (A) The blood glucose levels after an intraperitoneal injection of glucose 2 gm/kg bw determined in GTT. (B) The AUC derived from blood glucose level data. ND (as control), HSD, JF, AUC. **p < 0.01 by ANOVA and subsequent Bonferroni post-hoc test. Bars represent mean ± SE. n = 5 for each group.
Effect of jicama fiber on body weight and adipose tissue weight
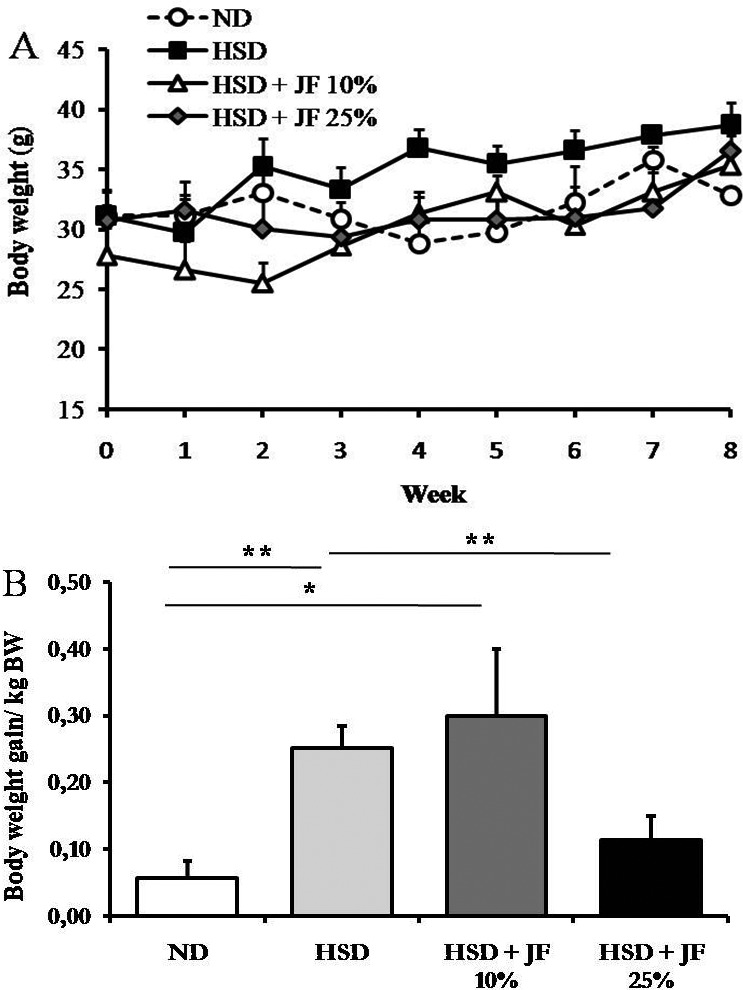
We also measured the body weight and white and BAT weight to reveal the effectivity of JF in hampering the development of HSD-induced obesity. The result showed that body weight tended to be lower in JF groups and ND group as compared with HSD group (Fig. 3A), but it was statistically insignificant (p > 0.05). However, the body weight gain was significantly lower in JF 25% group, but not in JF 10% group, as compared with HSD group (p < 0.01; Fig. 3B) and it was comparable with ND group (p < 0.05).
Figure 3. JF counteracted the excessive body weight gain in HSD-fed mice. (A) Body weight measured weekly for 8 weeks. (B) Body weight gain/kg bw determined at the end of treatment. ND (as control), HSD, JF. **p < 0.01, *p < 0.05 by ANOVA and subsequent Bonferroni post-hoc test. Bars represent mean ± SE. n = 5 for each group.
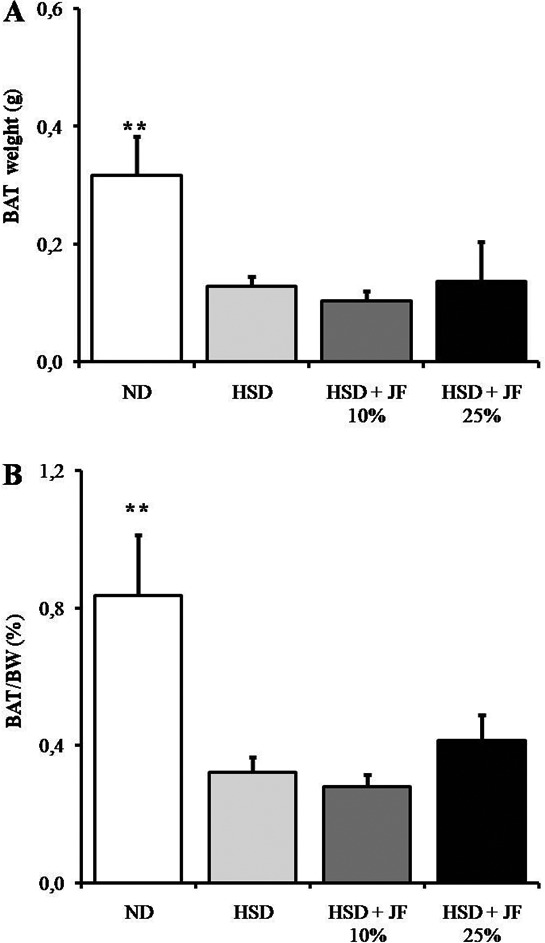
The measurement of epididymal WAT weight at the end of treatment revealed that WAT depot and its proportion to body weight tended to be reduced in JF groups as compared with HSD group (Fig. 4A and B), but it was statistically not significant (p > 0.05). The WAT mass was significantly lower in ND group as compared with HSD group (p < 0.01), but it was comparable with JF groups (p > 0.05). Furthermore, the interscapular BAT weight and its proportion to body weight were significantly lower in JF groups as well as HSD groups as compared with ND group (p < 0.01; Fig. 5A and B).
Figure 4. JF blunted the epididymal WAT increase in HSD-fed mice. (A) WAT weight measured after 8 weeks of treatments. (B) The proportion of WAT to body weight presented in %. ND (as control), HSD, JF. **p < 0.01 by ANOVA and subsequent Bonferroni post-hoc test. Bars represent mean ± SE. n = 5 for each group.
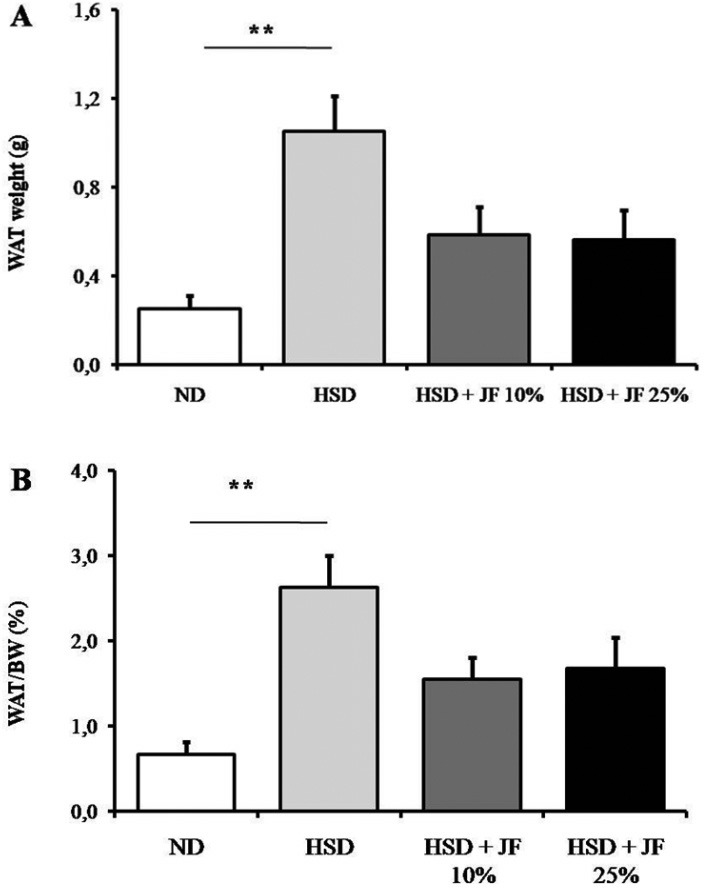
Figure 5. JF failed to counteract BAT decrease in HSD-fed mice. (A) Interscapular BAT weight measured after 8 weeks of treatments. (B) Proportion of BAT against body weight (BW) presented in %. ND (as control), HSD, JF. **p < 0.01 by ANOVA and subsequent Bonferroni post-hoc test. Bars represent mean ± SE. n = 5 for each group.
Effect of jicama fiber on food and water intake
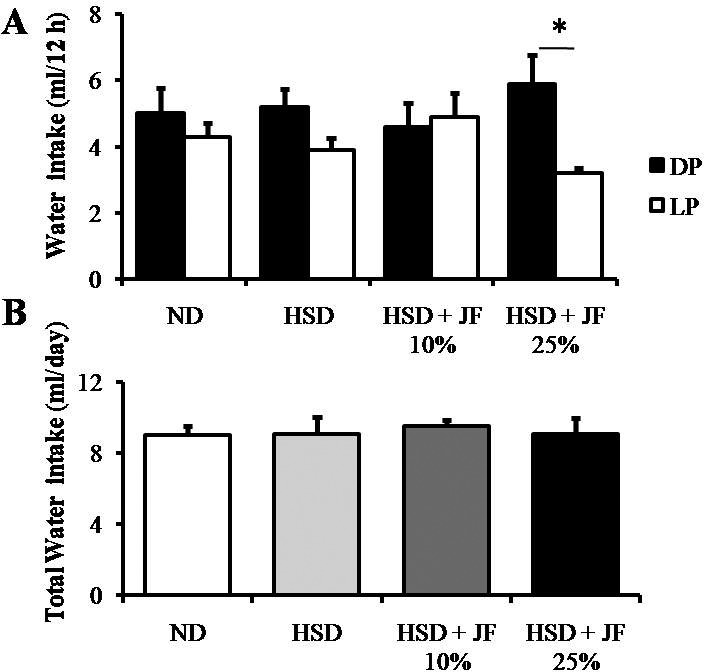
We also carried out food intake and water intake measurements at the latest week of treatment. The rhythm of food intake was comparable among all groups of treatment, showing higher food intake during the DP and lower during the LP (Fig. 6A). Likewise, the total food intake was statistically comparable among all groups of treatment (p > 0.05; Fig. 6B). The rhythm of water intake (Fig. 7A) was changed in JF 25% group as compared with other groups, showing a significant difference between the DP and LP (p < 0.01). However, the total water intake was also comparable among all groups of treatment (p > 0.05; Fig. 7B).
Figure 6. JF did not significantly alter the rhythm (DP vs LP food intake) and total food intake in HSD-fed mice. (A) Food intake measured during the night (DP) and the daytime (LP). (B) Total food intake for 24 h. ND (as control), HSD, JF. **p < 0.01 by ANOVA and subsequent Bonferroni post-hoc test. Bars represent mean ± SE. n = 5 for each group.
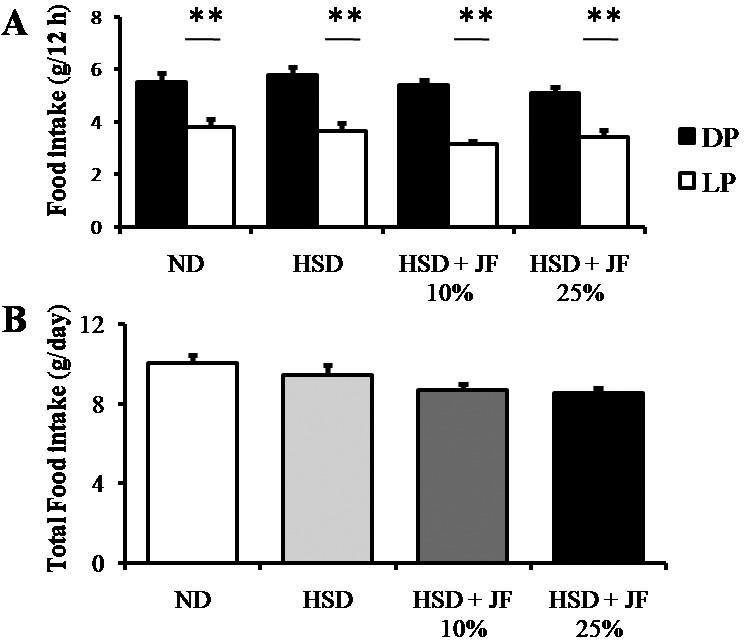
Figure 7. JF altered the rhythm of water intake (DP and LP water intake) but did not affect total water intake in HSD-fed mice. (A) Water intake measured during the night (DP) and day (LP). (B) Total water intake for 24 h. ND (as control), HSD, JF. *p < 0.05 by ANOVA and subsequent Bonferroni-post hoc test. Bars represent mean ± SE. n = 5 for each group.
Discussion
Our current findings revealed that the supplementation of edible fiber isolated from jicama tuber could effectively preclude the dysregulation of blood glucose homeostasis and excessive body weight gain caused by HSD. Importantly, the supplementation of JF exerted a minimum effect on food and water intake.
Consumption of HSD is profoundly implicated in the development of metabolic dysregulations including diabetes and obesity [11,13,14]. In our study, as expected, mice fed with HSD exhibited an apparent hyperglycemic state. Moreover, the HSD-fed mice became intolerant to glucose after 2 months of treatment. This finding might indicate an impaired blood glucose homeostasis toward diabetic development [28]. Otherwise, the supplementation of JF 10% and 25% in HSD could effectively sustain the normoglycemic state. Furthermore, the supplementation of JF 25% notably promoted the glucose tolerance, while the JF 10% only blunted the HSD-induced glucose intolerance. Glucose tolerance reflects the ability of insulin to regulate the glucose homeostasis [28,29]. High sugar intake has been reported to be toxic for pancreatic islet β cells and could reduce insulin sensitivity which subsequently causes glucose intolerance [30]. Therefore, the hampering of hyperglycemia and glucose intolerance by JF supplementation in HSD-fed mice as found in our study could be related to the preventive mechanism of JF on pancreas damage. However, further study concerning the structural changes in the pancreas is required to clarify this speculation.
It also has been documented that HSD could induce an excessive body weight gain toward obesity development in rodents [11]. Previous studies revealed that consuming high-sugar drinks and fast foods frequently could significantly increase the risk of having obesity and diabetes in humans [31,32]. Our present finding also demonstrated that the HSD-fed mice exhibited an excessive body weight gain after 8 weeks of treatment, and it could be hampered by the JF 25% supplementation in their diet. However, at the end of treatment, despite the final body weight of HSD-fed mice was higher; it was statistically comparable with other groups (ND and JF groups). Likely, another study also demonstrated that mice fed with solid sugar diet remained insignificantly different in their body weight as compared with control group, though the fat mass as an indicator of obesity markedly increased [33]. Since our current study also used the solid sugar (sucrose powder) instead of liquid sugar for HSD treatment, thus it might less effective to quickly induce an apparent body weight increase although the adiposity was elevated. We speculate that liquid sugar diet with a prolonged time course of treatment for more than 8 weeks may significantly increase body weight in HSD group.
Our present study also revealed that the supplementation of JF blunted the effect of HSD in increasing epididymal WAT weight. The WAT functions as fat storage or energy depot which commonly increases in obese individuals [34,35]. Thus, the mild decrease of epididymal WAT mass in JF-fed mice could be contributed in lowering body weight gain as found in JF 25% group. However, we observed that in JF 10% group, the mild reduction of WAT mass was not clearly manifested in body weight gain. Even we could not provide the supporting data to explain this discrepancy, but we propose that it is likely due to the increase of lean mass including muscle mass, instead of WAT mass, in JF 10% group. Consequently, the increase of lean mass would contribute to body weight gain. Alternatively, the WAT mass located in other parts such as visceral and subcutaneous WAT could be higher in JF 10% group that would contribute to an excessive weight gain. Further investigation is warranted to explain this discrepancy.
In addition to WAT, we also determined the interscapular BAT mass. BAT plays a pivotal role in energy expenditure through the thermogenesis process [36]. It has been reported that HSD could reduce BAT mass particularly the interscapular BAT in rats [12]. Similarly, we also revealed that HSD reduced interscapular BAT mass. However, in our study, a mass reduction of BAT could not be ameliorated by the JF supplementation. This finding may suggest that JF supplementation at the dose used in our study was not effective enough to preclude the structural and functional alterations in BAT caused by HSD.
Although food intake reduction could be associated with a complex hormonal and neuronal pathways involving appetite and satiety modulations [37,38], its outcome simply reduces energy intake that eventually lowers blood glucose and fat mass [38]. Plausibly, the preventive mechanism of a substance against the development of diabetes and obesity could be simply due to the reduction of food intake. However, our finding showed that neither the rhythm nor the quantity of food intake was altered by fiber supplementation. Thus, the prevention of diabetes and obesity symptoms by JF was unlikely to be simply corresponded with reduction of food intake. We also observed that total water intake was unaltered by JF supplementation. However, its rhythm was changed in JF 25% group, showing an apparent increase of water intake during the DP as compared with LP. The increase in water intake reflects the thirst that commonly occurs during ingesting high dietary fiber content with high capacity in binding water [39]. Thus, the change in water intake rhythm in JF 25% group could be attributed to a higher concentration of fiber consumed by mice. Alternatively, the change in the rhythm of water intake might be associated with the change in locomotor activity and other physiological behavior. A study in mice demonstrated that water intake and type of diet are clearly associated with a change in locomotion and exploratory behavior [40]. However, in our present study, neither the locomotor activity nor the exploratory behavior was investigated. Hence, it requires further study to clarify the speculations.
Previous reports have suggested some underlying mechanisms of dietary fibers in regulating metabolic homeostasis. The soluble dietary fibers could reduce the gastric emptying rate that eventually prolongs the satiety state and prevents the spikes of blood glucose upon feeding [41,42]. The insoluble fibers could regulate the movements of the bowel by holding onto the water in the gastrointestinal tract; thereby preclude hyperphagia [41]. The inulin, as a soluble fiber found in jicama, is also effective to improve insulin sensitivity [43]. Furthermore, the fibers could also induce the ghrelin-like peptide 1 secretion by L cells resided in the distal intestine to further increase insulin secretion and its sensitivity and slow down the bowel movements [44]. It also has been revealed that gut microbiota could ferment the fibers to produce short-chain fatty acids (SCFA) including acetate, propionate, and butyrate. The SCFA will subsequently enter the circulatory system and target the metabolic-associated organs including liver, pancreas, adipose tissue, muscle, and brain to elicit its beneficial effects in metabolic process, appetite and satiety regulation, and body mass composition [45,46]. Among such proposed mechanisms, the edible fiber of jicama could be implicated in preventing metabolic diseases including diabetes and obesity through a particular pathway. The revelation of such plausible pathways should be addressed in the future study.
Conclusion
The edible fiber isolated from jicama tuber could effectively sustain a normoglycemic state as well as body weight and adiposity profile against the development of diabetes and obesity caused by HSD in mice. This fiber could be potentially used as a supplemental diet to overcome the metabolic dysregulation.
Acknowledgments
This research was funded by the Basic Research Grant of Faculty of Mathematics and Natural Sciences Andalas University (Contract No.18/UN.16.03.D/PP/FMIPA/2018). The authors would like to thank the Dean of Faculty of Mathematics and Natural Sciences (Prof. Dr. Mansyurdin) for facilitating the grant, and Qonita Fadhilah, Siti Jamalul Insani, and Azki Afidati Putri (Biology Department, Andalas University) for their help in animal maintenance.
Conflict of Interest
The authors declare that they have no conflict of interest.
Authors’ contribution
Putra Santoso, Astri Amelia, and Resti Rahayu designed the experiments. Putra Santoso and Astri Amelia performed the experiments and statistical analysis. Putra Santoso and Resti Rahayu prepared the manuscript. All authors read, revised, and finally approved the manuscript for publication.
References
- [1].Caspard H, Jabbour S, Hammar N, Fenici P, Sheehan JJ, Kosiborod M. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: an analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes Metab. 2018;20(3):667–71. doi: 10.1111/dom.13143. https://doi.org/10.1111/dom.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67(4):862–73. doi: 10.1016/j.jhep.2017.06.003. https://doi.org/10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- [3].Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. https://doi.org/10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Deepa M, Anjana RM, Mohan V. Role of lifestyle factors in the epidemic of diabetes: lessons learnt from India. Eur J Clin Nutr. 2017;71(7):825–31. doi: 10.1038/ejcn.2017.19. https://doi.org/10.1038/ejcn.2017.19. [DOI] [PubMed] [Google Scholar]
- [5].Nurdiantami Y, Watanabe K, Tanaka E, Pradono J, Anme T. Association of general and central obesity with hypertension. Clin Nutr. 2017;S0261-5614(17):30173–5. doi: 10.1016/j.clnu.2017.05.012. https://doi.org/10.1016/j.clnu.2017.05.012. [DOI] [PubMed] [Google Scholar]
- [6].Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular Disease. Circ Res. 2016;118(11):1752–70. doi: 10.1161/CIRCRESAHA.115.306883. https://doi.org/10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- [7].Wicklow B, Wittmeier K, T’Jong GW, McGavock J, Robert M, Duhamel T, et al. Proposed trial: safety and efficacy of resveratrol for the treatment of non-alcoholic fatty liver disease (NAFLD) and associated insulin resistance in adolescents who are overweight or obese adolescents—rationale and protocol. Biochem Cell Biol. 2015;93(5):522–30. doi: 10.1139/bcb-2014-0136. https://doi.org/10.1139/bcb-2014-0136. [DOI] [PubMed] [Google Scholar]
- [8].Lambertz J, Weiskirchen S, Landert S, Weiskirchen R. Fructose: a dietary sugar in crosstalk with microbiota contributing to the development and progression of non-alcoholic liver disease. Front Immunol. 2017;8:1159. doi: 10.3389/fimmu.2017.01159. https://doi.org/10.3389/fimmu.2017.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Subramanian S, Hirsch IB. Diabetic kidney disease: Is there a role for glycemic variability? Curr Diab Rep. 2018;18(3):13. doi: 10.1007/s11892-018-0979-3. https://doi.org/10.1007/s11892-018-0979-3. [DOI] [PubMed] [Google Scholar]
- [10].López-Suárez A. Burden of cancer attributable to obesity, type 2 diabetes and associated risk factors. Metabolism. 2019;92:136–46. doi: 10.1016/j.metabol.2018.10.013. https://doi.org/10.1016/j.metabol.2018.10.013. [DOI] [PubMed] [Google Scholar]
- [11].Torres-Villalobos G, Hamdan-Perez N, Tovar AR, Ordaz-Nava G, Torres N. Combined high-fat diet and sustained high sucrose consumption promotes NAFLD in a murine model. [Nov 25;2018 ];Ann Hepatol. 2015 14(4):540–6. Available via http://www.annalsofhepatology.com/revista/numeros/2015/HP154-14-ombined_oK%20(F_190515m)_PROTEGIDO.pdf. [PubMed] [Google Scholar]
- [12].Velickovic KD, Ukropina MM, Glisic RM, Cakic-Milosevic MM. Effects of long-term sucrose overfeeding on rat brown adipose tissue: a structural and immunohistochemical study. J Exp Biol. 2018;221:jeb166538. doi: 10.1242/jeb.166538. https://doi.org/10.1242/jeb.166538. [DOI] [PubMed] [Google Scholar]
- [13].Lean MEJ, Morenga LT. Sugar and type 2 diabetes. Br Med Bull. 2016;120:43–53. doi: 10.1093/bmb/ldw037. https://doi.org/10.1093/bmb/ldw037. [DOI] [PubMed] [Google Scholar]
- [14].Barrière DA, Noll C, Roussy G, Lizotte F, Kessai A, Kirby K, et al. Combination of high-fat/high fructose diet and low-dose streptozotocin to model long-term type-2 diabetes complications. Sci Rep. 2018;8:424. doi: 10.1038/s41598-017-18896-5. https://doi.org/10.1038/s41598-017-18896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Noman ASM, Hoque MA, Haque MM, Pervin F, Karim MR. Nutritional and anti-nutritional components in Pachyrhizus erosus L. tuber. Food Chem. 2012;102:1112–8. https://doi.org/10.1016/j.foodchem.2006.06.055. [Google Scholar]
- [16].Nursandi F, Machmudi M, Santoso U, Indratmi D. Properties of different aged jicama (Pachyrhizus erozus) plants; IOP Conf Ser Earth Environ Sci; 2017; p. 012003. https://doi.org/10.1088/1755-1315/77/1/012003. [Google Scholar]
- [17].Park CJ, Lee HA, Han JS. Jicama (Pachyrhizus erosus) extract increases insulin sensitivity and regulates hepatic glucose in C57BL/Ksj db/db mice. Clin Biochem Nutr. 2015;58(1):56–63. doi: 10.3164/jcbn.15-59. https://doi.org/10.3164/jcbn.15-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park CJ, Han JS. Hypoglycemic Effect of Jicama (Pachyrhizus erosus) extract on Streptozotocin-induced diabetic mice. Prev Nutr Food Sci. 2015;20(2):88–93. doi: 10.3746/pnf.2015.20.2.88. https://doi.org/10.3746/pnf.2015.20.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thaptimthong T, Kasemsuk T, Sibmooh N, Unchern S. Platelet inhibitory effects of juices from Pachyrhizus erosus L. root and Psidium guajava L. fruit: a randomized controlled trial in healthy volunteers. BMC Complement Altern Med. 2016;16:269. doi: 10.1186/s12906-016-1255-1. https://doi.org/10.1186/s12906-016-1255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kumalasari ID, Nishi K, Harmayani E, Sugahara T. Effect of bengkoang (Pachyrhizus erosus) fiber extract on murine macrophage-like J774.1 cells and mouse peritoneal macrophages. J Funct Foods. 2013;5(2):582–9. https://doi.org/10.1016/j.jff.2012.12.005. [Google Scholar]
- [21].Kumalasari ID, Nishi K, Harmayani E, Raharjo S, Sugahara T. Immunomodulatory activity of Bengkoang (Pachyrhizus erosus) fiber extract in vitro and in vivo. Cytotechnol. 2014;66(1):75–85. doi: 10.1007/s10616-013-9539-5. https://doi.org/10.1007/s10616-013-9539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang ZQ, Yu Y, Zhang XH, Floyd EZ, Bourdreau A, Lian K, et al. Comparing the effects of nano-sized sugarcane fiber with cellulose and psyllium on hepatic cellular signaling in mice. Int J Nanomed. 2012;(7):2999–3012. doi: 10.2147/IJN.S30887. https://doi.org/10.2147/IJN.S30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ellis TP, Wright AG, Clifton PM, Ilag LL. Postprandial insulin and glucose levels are reduced in healthy subjects when a standardised breakfast meal is supplemented with a filtered sugarcanemolasses concentrate. Eur J Nutr. 2016;55(8):2365–76. doi: 10.1007/s00394-015-1043-6. https://doi.org/10.1007/s00394-015-1043-6. [DOI] [PubMed] [Google Scholar]
- [24].Li X, Guo J, Ji K, Zhang P. Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota. Sci Rep. 2016;6:32953. doi: 10.1038/srep32953. https://doi.org/10.1038/srep32953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Luo X, Wang Q, Zheng B, Lin L, Chen B, Zheng Y, et al. Hydration properties and binding capacities of dietary fibers from bamboo shoot shell and its hypolipidemic effects in mice. Food Chem Toxicol. 2017;109(2):1003–9. doi: 10.1016/j.fct.2017.02.029. https://doi.org/10.1016/j.fct.2017.02.029. [DOI] [PubMed] [Google Scholar]
- [26].Ko DH, Lim S, Song SH, Choi SH, Park YJ, Park KU, et al. Performance evaluation of the GlucoDr plus glucometer. Diabetes Technol Ther. 2010;12(4):307–12. doi: 10.1089/dia.2009.0134. https://doi.org/10.1089/dia.2009.0134. [DOI] [PubMed] [Google Scholar]
- [27].Maejima Y, Rita RS, Santoso P, Aoyama M, Hiraoka Y, Nishimori K, et al. Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limitied brain area. Neuroendrocrinology. 2014;101(1):35–44. doi: 10.1159/000371636. https://doi.org/10.1159/000371636. [DOI] [PubMed] [Google Scholar]
- [28].Andrixopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295:E1323–32. doi: 10.1152/ajpendo.90617.2008. https://doi.org/10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- [29].Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, et al. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab. 2008;94:E261–70. doi: 10.1152/ajpendo.00676.2007. https://doi.org/10.1152/ajpendo.00676.2007. [DOI] [PubMed] [Google Scholar]
- [30].Kawahito S, Kitahata K, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009;15(33):4137–42. doi: 10.3748/wjg.15.4137. https://doi.org/10.3748/wjg.15.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Oo SS, Rao USM, Zin T. Prevalence and factors associated with obesity among adult at the kampung kolam, east coast malaysian peninsula-a cross sectional study. Int J Pharm Pharm Sci. 2017;3(9):273–81. https://doi.org/10.22159/ijpps.2017v9i3.16888. [Google Scholar]
- [32].El-Wakkad A, Hassan NE, El-Zayat SR, Sibaii H, El-Masry SAE. Multiple Markers of diabetes in relation to abdominal obesity in obese egyptian adolescent girls. Int J Pharm Pharm Sci. 2012;4(4):276–81. [Google Scholar]
- [33].Vellers HL, Letsinger AC, Walker NR, Granados JZ, Lightfoot JT. High fat high sugar diet reduces voluntary wheel running in mice independent of sex hormone involvement. Front Physiol. 2017;8:628. doi: 10.3389/fphys.2017.00628. https://doi.org/10.3389/fphys.2017.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cummins TD, Holden CR, Sansbury BE, Gibb AA, Shah J, Zafar N, et al. Metabolic remodeling of white adipose tissue in obesity. Am J Physiol Endocrinol Metab. 2014;307(3):E262–77. doi: 10.1152/ajpendo.00271.2013. https://doi.org/10.1152/ajpendo.00271.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Paniagua JA. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes. 2016;7(19):483–514. doi: 10.4239/wjd.v7.i19.483. http://dx.doi.org/10.4239/wjd.v7.i19.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Alcala M, Calderon-Dominguez M, Bustos E, Ramos P, Casals N, Serra D, et al. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci Rep. 2017;(7):16082. doi: 10.1038/s41598-017-16463-6. https://doi.org/10.1038/s41598-017-16463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Santoso P, Maejima Y, Kumamoto K, Takenoshita S, Shimomura K. Central action of ELABELA reduces food intake and activates arginine vasopressin and corticotropin-releasing hormone neurosn in the hypothalamic paraventricular nucleus. Neuroreport. 2015;26(14):820–6. doi: 10.1097/WNR.0000000000000431. https://doi.org/10.1097/WNR.0000000000000431. [DOI] [PubMed] [Google Scholar]
- [38].Benton D, Young HA. Reducing calorie intake may not help you lose body weight. Perspect Psychol Sci. 2017;12(5):703–14. doi: 10.1177/1745691617690878. https://doi.org/10.1177/1745691617690878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tan C, Wei H, Zhao C, Zu C, Peng J. Effects of dietary fibers with high water-binding capacity and swelling capacity on gastrointestinal functions, food intake and body weight in male rats. Food Nutr Res. 2017;61(1):1308118. doi: 10.1080/16546628.2017.1308118. https://doi.org/10.1080/16546628.2017.1308118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Erigbali P, Osim E, Ogregade I. Assessment of body weight change, locomotion and exploration in mice exposed to plantain diet. Gen Med. 2018;(6):306. https://doi.org/10.4172/2327-5146.1000306. [Google Scholar]
- [41].Clark MJ, Slavin JL. The effect of fiber on satiety and food intake: a systematic review. J Am Coll Nutr. 2013;32:200–11. doi: 10.1080/07315724.2013.791194. https://doi.org/10.1080/07315724.2013.791194. [DOI] [PubMed] [Google Scholar]
- [42].Adam CL, Williams PA, Garden KE, Thomson LM, Ross AW. Dose-dependent effects of a soluble dietary fibre (Pectin) on food intake, adiposity, gut hypertrophy and gut satiety hormone secretion in rats. PLoS One. 2015;10(1):e0115438. doi: 10.1371/journal.pone.0115438. https://doi.org/10.1371/journal.pone.0115438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tan S, Caparros-Martin JA, Mattews VB, Koch H, O’Gara F, Croft KD, et al. Isoquercetin and inulin synergistically modulate the gut microbiome to prevent development of the metabolic syndrome in mice fed a high fat diet. Sci Rep. 2018;8(1):10100. doi: 10.1038/s41598-018-28521-8. https://doi.org/10.1038/s41598-018-28521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shah M, Velia A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15(3):181–7. doi: 10.1007/s11154-014-9289-5. https://doi.org/10.1007/s11154-014-9289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Van der Beek CM, Canfora EE, Kip AM, Gorissen SHM, Olde DSWM, van Eijk HM, et al. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism Clin Exp. 2018;87:25–35. doi: 10.1016/j.metabol.2018.06.009. https://doi.org/10.1016/j.metabol.2018.06.009. [DOI] [PubMed] [Google Scholar]
- [46].Morisson DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. doi: 10.1080/19490976.2015.1134082. https://doi.org/10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]


