Abstract
Myeloid derived suppressor cells (MDSCs) are immature suppressive cells found in tumors and immunological niches. Here, we highlight the ability of MDSCs to promote IL-17 producing T cells (Th17) and regulatory T cells (Tregs) in addition to suppressing cytotoxic T cells in different tumor models. These interactions between MDSCs and T cells support tumor growth because IL-17 is tumorigenic in many cancer types and Tregs suppress antitumor T cells. Besides T cells, MDSCs promote regulatory B cells (Bregs) and suppress overall B cell function; however, tumor evoked Bregs also regulate MDSC function suggesting cross-regulation between MDSCs and B cells. These multiple functions shed light on how MDSCs dysregulate several arms of host immune response. Moreover, MDSCs promote tumor cell survival and angiogenesis to support tumors. Therefore, the multifunctional feature of MDSCs make them attractive immunotherapeutic targets.
Introduction
MDSCs are gaining importance due to their key role in promoting resistance to current therapies in different types of cancer (1–3). In cancer, tumor derived growth factors and inflammatory cytokines increase proliferation but prevent terminal differentiation of myeloid cells, resulting in increased frequencies of MDSCs (4). This is consistent with their initial characterization as immature cells of monocytic or granulocytic origin. They are distinct from monocytes and neutrophils due to their ability to suppress antigen-specific and non-antigen specific T cells in many tumor models. Historically, MDSCs were considered inhibitory macrophages (5). However, MDSCs are phenotypically distinct from macrophages and dendritic cells (DCs) due to reduced expression of F4/80 (macrophage marker), MHC class II (antigen presenting molecule) and CD11c (DC marker) (6). During their lifespan, some MDSCs differentiate to tumor associated macrophages (TAMs) and DCs and others remain undifferentiated (7, 8) (Fig. 1). Although TAMs suppress T cells, they mainly promote an IL-4 dependent type 2 response, produce epidermal growth factor (EGF), and produce proteases to promote tumors (9). Therefore, MDSCs have a robust immunosuppressive activity among myeloid cells in cancer.
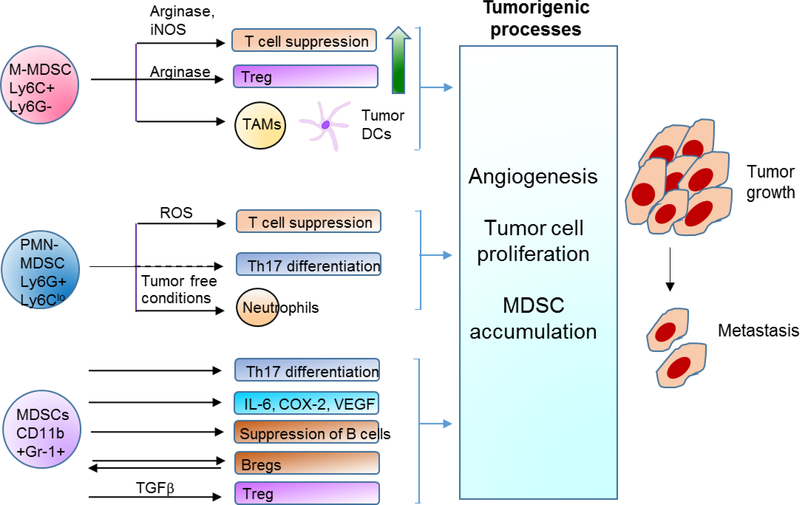
Figure. 1. Diverse functions of MDSCs in cancer.
MDSCs are generated from hematopoietic stem cells from the bone marrow under the influence of growth factors including GM-CSF and G-CSF, and COX-2. They acquire immunosuppressive potential on activation by cytokines including IL-6 and IL-4. COX-2 by itself can expand MDSCs and confer suppressive function. With Ly6C and Ly6G, MDSCs can be separated into M-MDSCs, I-MDSCs, and PMN-MDSCs. They suppress T cell function, promote expansion of Tregs and Th17 cells, modulate B cells by both suppressing B cells and promoting Bregs, and also produce inflammatory cytokines. Overall, MDSCs use these different mechanisms to promote tumor growth, angiogenesis, and metastasis.
MDSCs were originally identified by the Gr-1 antibody (clone RB6–8C5), which recognizes both Ly6C (monocyte marker) and Ly6G (granulocyte marker) antigens (6). However, Gr-1 antibody binds with more affinity to Ly6G than to Ly6C. As a result, CD11b+Gr-1lo cells are identified as monocytic MDSCs (M-MDSCs) and CD11b+Gr-1hi cells as polymorphonuclear MDSCs (PMN-MDSCs) (10). Antibodies specific to Ly6C or Ly6G showed that M-MDSCs are CD11b+Ly6C+Ly6G- and PMN-MDSCs are CD11b+Ly6G+Ly6Clo (11). Recently, a consensus was reached about the criteria for classifying myeloid cells as MDSCs (11). Myeloid cells expressing Ly6C or Ly6G and capable of suppressing T cell proliferation or function are identified as MDSCs. Some studies report the function of total MDSCs (CD11b+Gr-1+) but others delineate subset specific mechanisms of MDSCs (Table I). Evaluating the importance of MDSC subsets in dysregulating antitumor immunity is useful for defining effective treatment strategies. For example, in colon cancer, several reports show that M-MDSCs promote tumor growth by suppressing T cells (12–14) but recent studies show that PMN-MDSCs are also critical for tumor progression (15–17). In addition to M-MDSCs, an intermediate MDSC (I-MDSC) subset was found in spleens of tumor bearing ApcMin/+ mice (18), which have high circulating level of GM-CSF (19). Compared to M-MDSCs, I-MDSCs express intermediate levels of Ly6C and Ly6G suggesting a heterogeneous composition of cells within this subset (20). Consistently, an I-MDSC like subset accumulated in mice with CT26 colon carcinoma overexpressing GM-CSF and in mice with breast tumor cells expressing high GM-CSF (6, 10). In lung cancer, patients with more M-MDSCs experienced disease relapse after treatment (21). However, in murine lung cancer, there is higher accumulation of PMN-MDSCs compared to M-MDSCs and most studies report the suppressive potential of total MDSCs (22–24). Therefore, additional evidence will provide a complete picture of MDSC function in different tumor models.
Table I.
MDSCs are identified by CD11b+Gr-1+ as total MDSCs or with Ly6C as M-MDSCs or Ly6G as PMN-MDSCs or G-MDSCs. Sources of MDSCs are tumor or bone marrow or spleen or peripheral blood of tumor bearing animals. Most studies were performed in transplanted tumor models and some studies included tumor models with endogenous mutation such as the ApcMin/+ model which carries a common CRC mutation, adenomatous polyposis coli (APC) and in APC-CPC colon tumor model. Recent studies have begun showing that MDSCs also promote tumors through mechanisms regulating B cells, Th17 cells, Tregs, or by direct effects on tumors in addition to suppressing T cells. CT26 colon carcinoma overexpressing GM-CSF; MC38, colon carcinoma; 4T1, breast cancer; DA3, mammary carcinoma; MCA-26 intrahepatic metastatic colon cancer; LLC, lewis lung carcinoma, LLC-OVA, LLC expressing ovalbumin; EL-4 thymoma, DA3 mammary carcinoma, MethA and C3 sarcoma; MCA203, fibrosarcoma; MBL-2, lymphoma; MN-MCA sarcoma; Renca, kidney tumor; pancreatic cancer, KPC; B16, melanoma; B16-IDO, B16 expressing IDO; APC-CPC, colon tumor model; ApcMin/+, intestinal tumor model with adenomatous polyposis coli (APC) mutation; TRAMP-C1, prostate cancer
| MDSC nomenclature and phenotype | Cancer | Source | Function | Mechanism | Tumor-related role | Reference |
|---|---|---|---|---|---|---|
|
Immature Gr-1+ myeloid cells CD11b+Gr-1+ |
MCA-26 | Spleen, BM | MDSCs from BM and spleen suppress proliferation of naive splenocytes stimulated with anti-CD3 and anti-CD28. BM MDSCs also suppressed mixed lymphocyte reaction | Inducible nitric oxide synthase (iNOS) induced nitric oxide (NO) and superoxide dismutase (SOD) mediated superoxides together suppressed non-antigen specific T cell proliferation | Tumor related MDSCs suppressed T cells. | Kusmartsev et al., 2000 |
|
Myeloid suppressor cells (MSC) CD11b+Gr-1+ |
CT26 and C26-GM, MSC-2 cell line | Spleen | Splenic MSCs from mice with C26-GM tumors express arginase and suppress cytolytic activity of allogeneic splenocytes | IL-4 upregulates arginase 1 in MSC-2 to suppress T cells. Arginase 1 activated superoxide radicals induce T cell apoptosis through the iNOS reductase domain. | MSCs were expanded and CD4 and CD8 T cells were reduced. Tumor GM-CSF increased MSCs. | Bronte et al., 2003 |
|
MSC Fr.2 Gr-1+CD115+ (M-MDSCs (monocytic MDSCs)), Fr.2 Gr-1+CD11b+ |
MCA26 | Spleen, BM | M-MDSCs suppress antigen specific CD4 T cells more than total MDSCs | MSCs suppressed antigen specific CD4 T cells by iNOS and promoted Tregs. | Tregs generated by MSCs promote tumor growth | Huang et al., 2006 |
|
MDSC CD11b+Gr-1+ |
CT26, MC38, LLC, EL-4, DA3, MethA and C3 sarcoma | Spleen | MDSCs from mice with EL-4 tumors produces reactive oxygen species (ROS) via nicotinamide adenosine dinucleotide phosphate dehydrogenase (NADPH) oxidase to inhibit IFNγ by antigen specific OT-1 T cells | Stat3 dependent NADPH oxidase regulates ROS in MDSCs. NADPH oxidase deficient MDSCs differentiate into macrophages and DCs | Tumor related MDSCs produced ROS to suppress T cells | Corzo et al., 2009 |
|
MDSCs CD11b+Gr-1+, CD11b+Ly6C+Ly6G-, CD11b+Ly6G+Ly6Clo |
CT26, C26-GM, MBL-2, EL-4, EG7-OVA, MN-MCA, MCA203 | Spleen, BM | BM CD11b+Gr-1+ cells suppress cytotoxicity of antigen specific CD8 T cells. CD11b+Gr-1lo cells are most immunosuppressive followed by CD11b+Gr-1int and CD11b+Gr-1hi. Splenic MDSCs also suppress antigen specific CD8 T cells. |
GM-CSF dependent C/EBPβ is required for the suppressive function of in vitro generated MDSCs and MDSCs from mice with EG-7 tumors. It regulates arginase and nitric oxide synthase 2 (NOS2) expression | Depletion of MDSCs reduced lung metastases but not the growth of primary fibrosarcoma |
Marigo et al., 2010 |
|
MDSCs CD11b+Gr-1+ |
APC-CPC | Tumors | MDSCs and not tumor associated macrophage (TAMs) promoted IL-17 production by CD4 T cells | MDSCs defective in TLR2 failed to promote IL-17 production | Colons tumors were dependent on IL-23 and IL-17 | Grivennikov et al., 2012 |
|
M-MDSCs CD11b+Ly6C+Ly6G- polymorphonuclear MDSCs (PMN-MDSCs) CD11b+LyG+Ly6Clo MDSCs CD11b+Gr-1+ |
EL4, LLC-OVA, KPC, 4T1, CT26 | Blood, spleen, BM | MDSCs express more death receptor 5 (DR5) and undergo cell death; but this results in MDSC expansion | Activating DR5 by agonistic antibody reduces both M-MDSCs and PMN-MDSCs | Tumor reduction after blocking DR5 is dependent on CD8 T cells and increased antigen specific T cell IFNγ. | Condamine et al., 2014 |
|
MDSCs CD11b+Gr-1+ |
B16, B16- indoleamine 2, 3 dioxygenase (IDO), 4T1 | Tumor | CD11b+Gr-1int MDSCs and not CD11b+Gr-1hi MDSCs from B16-IDO tumors suppress polyclonally activated CD8 T cells. CD11b+Gr-1int MDSCs from spleen in this tumor model are not suppressive | CD11b+Gr-1int MDSCs from tumors suppress CD8 T cells by arginase. iNOS, TGFβ, and PD-L1 are also important for suppression in different combinations but not by themselves | Tregs attract MDSCs to the tumor to acquire suppressive function. Treg depletion or IDO inhibition reduces tumor growth. | Holmgaard et al., 2015 |
|
M-MDSCs CD11b+Ly6C+Ly6G- PMN-MDSCs CD11b+LyG+Ly6Clo |
B16, 4T1 | Spleen, PB | Tumor evoked Bregs (tBregs) promote the suppressive function and metastatic potential of M-MDSCs and PMN-MDSCs | tBregs upregulated arginase, ROS and NOX2, and iNOS genes. However, TGFβ signaling in MDSCs suppresses T cells. | tBreg educated MDSCs increased lung metastases | Bodogai et al., 2015 |
|
M-MDSCs CD11b+Ly6C+Ly6G- granulocytic MDSCs (G-MDSCs) CD11b+LyG+Ly6Clo MDSCs CD11b+Gr-1+ |
4T1 | BM, tumor | Compared to WT MDSCs, CCL5 negative tumor MDSCs poorly suppressed anti CD3/CD28 activated T cells | MDSCs suppressed T cells by NOS2. Absence of CCL5 alters phagocytic capacity of MDSCs, increases MHC ll and CD86, and reduces TAMs. CCL5 deletion inhibits differentiation of BM M-MDSCs to Ly6Ghi cells |
Autocrine CCL5 by myeloid cells appears to regulate CD8 cytotoxic activity and Treg accumulation to promote tumor growth | Ban et al., 2017 |
|
M-MDSCs CD11b+Ly6C+Ly6G- I-MDSCs CD11b+Ly6CintLy6Gint PMN-MDSCs CD11b+LyG+Ly6Clo |
ApcMin/+ | Spleen and LP | Stat6 deletion reduces M-MDSCs, I-MDSCs, and PMN-MDSCs in the spleen and lamina propria (LP) of small intestine | Reduction of MDSCs correlated with increased splenic CD8 cytotoxic cells | Absence of Stat6 reduced tumor growth and MDSCs to enhance antitumor CD8 response. | Jayakumar and Bothwell., 2017 |
|
MDSCs CD11b+Gr-1+ |
4T1 | Spleen | CD19+ B cells and MDSCs accumulate in mice with 4T1 tumors. | MDSCs upregulated PD-L1 on B cells and induced IL-10 and IgA. MDSC educated Bregs suppressed T cells by inducing IL-10 | Shen et al., 2017 | |
|
MDSCs CD11b+Gr-1+ |
LLC | Tumor | Tumor MDSCs reduce frequency and proliferation of B cells | MDSCs suppress B cells in a T cell dependent and independent manner | Wang et al., 2018 | |
|
PMN-MDSCs CD11b+Gr-1+ |
TRAMP-C1 | Tumor | Tumor PMN-MDSCs were increased in mice with castration resistant prostate cancer and produce IL-23. | IL-23 promotes RORγ dependent tumor cell proliferation and Stat3 activation in tumor cells | IL-23 resists survival after surgical removal of prostate tumors | Calcinotto et al., 2018 |
|
M-MDSCs CD11b+Ly6C+Ly6G- G-MDSCs CD11b+LyG+Ly6Clo |
B16-F10 | Tumors, spleen | Autophagy genes in M-MDSCs promote suppressive function | Atg5 deficient MDSCs promote CD4 T cell proliferation and increased CD8 cytotoxicity. Loss of Atg5 also increased MHC ll in these MDSCs. | Deletion of Atg5 in myeloid cells reduced melanoma and lung tumors | Alisaafi et al., 2018 |
MDSCs also express Tie2 and CD31 that are markers of endothelial cells (5, 6). Consistently in a CRC tumor model (MC26), they acquire VEGF receptor and VE-cadherin expression and incorporate into tumor endothelium (25). However, it is unknown if these MDSCs perform the function of endothelial cells. MDSCs from bone marrow (BM) of mice with 4T1 tumors induced bone metastases, suppressed T cells and differentiated to osteoclasts to cause bone degradation (26). Absence of F4/80 showed that these osteoclasts are not macrophages. The ability of MDSCs to differentiate to other cell types is an indication of their plasticity but also suggests a stem cell like feature providing clues about how MDSCs multitask. Stem cells have the ability to differentiate into other cell types. As cells progress to a terminally differentiated state, some genes are silenced to acquire a specific function. In MDSCs, it is likely that the transcriptional program determining whether MDSCs eventually differentiate into DCs or macrophages or neutrophils are all functional, allowing them to perform multiple functions in response to growth factors or cytokines. Apart from immunosuppression, other mechanisms also contribute to the pro-tumor capabilities of MDSCs as previously reviewed (27) (Table I). MDSCs thrive in a tumor environment rich in growth factors, cytokines, and chemokines (28). GM-CSF is a growth factor promoting MDSC accumulation, and it activates the transcription factor CEBP/β to control expansion of MDSC subsets and its ability to suppress antigen specific T cells (10). IL-6 activated Stat3 also contributes to the suppressive potential of MDSCs (29). A combination of growth factors and cytokines can lead to waves of gene expression changes in MDSCs locally in tumors or systemically to, focus their cumulative immunosuppressive strength in preventing cytotoxic T cell expansion and simultaneously perform other tumorigenic functions. The tumor environment could shift the functional direction of each MDSC subset. However, the component of the tumor environment determining this dynamic balance in MDSC function is unclear at present and understanding the underlying mechanisms is valuable. This concept suggests why MDSCs occupy an important niche in promoting tumor development in a variety of cancers and why it is a hurdle for effective immunotherapy.
To consider new strategies for targeting MDSCs in cancer, we aimed to gain better understanding of the functional diversity of MDSCs by evaluating all mechanisms by which MDSCs support tumors. Here we offer our perspective about the diverse roles of MDSC subsets in modulating T and B cell immunity, and other tumorigenic functions.
Regulation of T cell immunity
MDSCs suppress CD4 and CD8 T cells
The immunosuppressive function of MDSCs is critical for their classification and distinction from mature myeloid cells. IL-4 receptor α (IL-4Rα) on MDSCs is linked to their immunosuppressive potential due to IL-4R dependent activation of arginase and inducible nitric oxide synthase (iNOS) (12). Arginase prevents non-antigen specific T cell proliferation by depleting essential nutrients such as arginine (12, 30). iNOS in M-MDSCs produces nitric oxide (NO) to suppress T cells by nitrating the T cell receptor (TCR), which blocks downstream signaling events required for activating T cells (22, 31). IL-4Rα on MDSCs mediates suppression of both antigen specific and non-antigen specific T cells in breast tumor (4T1) and colon cancer (CT26) models (32). In murine melanoma (B16 tumors overexpressing IDO), M-MDSCs rather than PMN-MDSCs from tumors suppress non-antigen specific T cells, express IL-4Rα and arginase, and correlate with tumor growth (30). Loss of autophagy related genes in M-MDSCs increases MHC class ll expression to promote CD8 T cell cytotoxicity and CD4 T cell proliferation in murine melanoma (33). In general, M-MDSCs are more suppressive than PMN-MDSCs (4). PMN-MDSCs primarily suppress antigen specific T cells by producing reactive oxygen species (ROS) (22). NADPH oxidase (NOX2) produces reactive oxygen species (ROS) in MDSCs from colon cancer (CT26 and MC38), lung cancer (LLC), mammary cancer (DA3), and fibrosarcomas (MethA and C3 sarcomas) (23). These findings demonstrate that M-MDSCs and PMN-MDSCs use different mechanisms for suppressing T cells (4). However, in EL-4 tumor bearing mice both MDSC subsets from spleen suppressed antigen specific CD8 T cells similarly and their suppressive function was independent of IL-4Rα (22). Similarly, peripheral blood MDSCs from different tumor models such as 4T1, CT26, and TSA were not dependent on IL-4Rα for suppressing antigen specific T cells (34).
Programmed death ligand 1 (PD-L1) belongs to the B7 family of ligands that regulate T cells. Although blockade of PD-L1 reversed T cell suppression by MDSCs under hypoxic condition (35), PD-L1 on in vitro generated MDSCs or MDSCs from EL-4 tumor bearing mice did not suppress T cells (22, 36). When co-inhibited with other mediators of suppression such as transforming growth factor β (TGFβ) or NO, PD-L1 appears to mediate T cell suppression (30). This suggests that other co-inhibitory ligands in the B7 family such as B7-H3, B7-H4, and V-domain Ig suppressor of T cell activation (VISTA), which are known to inhibit T cell proliferation could mediate the suppressive function of MDSCs (37). Moreover, cyclooxygenase 2 (COX-2) induced prostaglandin 2 (PGE2) in MDSCs promotes immunosuppressive function by upregulating indoleamine 2,3- dioxygenase (IDO) among other mediators of T cell suppression (38). IDO is an important mechanism of immunosuppression preventing antitumor immunity (39). Therefore, more than one mechanism contributes to the suppressive function of MDSCs. A better understanding of mechanisms contributing to MDSC mediated suppression of antitumor cells is vital for improving anticancer therapy and for the design and development of new therapeutics that can have lasting effect on the survival and overall quality of life of cancer patients.
MDSCs support Th17 cell function
In murine colon cancer, MDSCs (Gr-1+ myeloid cells) but not macrophages increase IL-17 production by CD4+ T cells to promote tumor progression (40). Similarly in a carcinoma model, MDSCs differentiated lL-17 producing CD4 T cells whereas TAMs promoted TGFβ producing T cells, which are likely Tregs (41). We showed that I-MDSCs promote the development of IL-17 producing CD4 T cells, which is essential for intestinal tumor progression (20). CD4 T cells differentiate into T helper 1 cells (Th1), T helper 2 cells (Th2), T helper 17 cells (Th17), or T regulatory cells (Tregs) depending on the cytokines they are exposed to. The cytokines IL-1β, IL-23, and IL-6 differentiate CD4 T cells to disease causing (pathogenic) Th17 cells in experimental autoimmune encephalitis (EAE) compared to Th17 cells generated with IL-6 and TGFβ (42). Total MDSCs from ovarian cancer produced IL-1β, IL-23, IL-6, and TGFβ, which upregulate endogenous nitric oxide synthase 2 (NOS2) in T cells to promote IL-17 production (43). Addition of TGFβ to the cytokine cocktail (IL-1β, IL-23, and IL-6) inducing Th17 cells dramatically decreased IL-17 production by CD4 T cells; however, these Th17 cells produced IL-17 than unstimulated T cells. Therefore, MDSCs producing TGFβ promoted some IL-17 production, and the absence of TGFβ possibly increased IL-17 production (43). TGFβ also reduced expression of IL-23 receptor (IL-23R), which suggests that it dampens the responsiveness of Th17 cells to IL-23 (42) and likely reduces the survival of Th17 cells in vivo. This is consistent with the necessity of IL-23 for maintaining pathogenic Th17 cells (44) and with the ability of IL-23 in increasing IL-17 producing CD4 T cells in the brain and spinal cord to promote disease severity in EAE (45). Therefore, MDSCs producing less TGFβ could promote Th17 cells with more disease causing potential. These studies shed light on the ability of MDSCs to generate Th17 cells and suppress antitumor T cells to accomplish the overall aim of promoting tumor growth (Fig.1). Moreover, MDSC like cells regulate Th17 cells by recruiting them to tumors. In murine skin cancer, immature Gr-1+ myeloid cells (IMCs) lacking suppressive function recruited CD4 T cells via CCL4 to promote carcinogen induced skin tumors by IL-17 (46). In castration resistant prostate cancer (CRPC), PMN-MDSCs produced abundant IL-23, which activated the Stat3-RORγ pathway in tumor cells to promote survival (47). IL-23 produced by these PMN-MDSCs could also promote Th17 differentiation, however it is unknown if this mechanism occurs concurrently in CRPC. Together, these studies illustrate the potential of MDSCs to alter the dynamic balance of the tumor immune system to create a self-promoting cycle.
Regulation between MDSCs and Tregs
In mismatch repair proficient (MMR) CRC patients, Tregs were associated with reduced cytotoxic response (48). Tregs were increased in CRC patients before surgery, suppressed antigen specific CD4 T cells, and were associated with tumor recurrence (49). In a colon tumor model, M-MDSCs induced Tregs in vitro and tumor growth was dependent on MDSC induced Tregs (50). This study showed that MDSCs induce Tregs and suppress antigen specific CD4 T cells simultaneously to promote tumors. In a B cell lymphoma model, induction of Tregs was dependent on the arginase activity of MDSCs (51). These tumor-derived MDSCs inhibited antigen specific CD4 T cell proliferation but promoted the expansion of Tregs from naturally occurring Tregs and not from naïve T cells. In this case, inhibition of T cell suppression allowed Treg expansion suggesting that a shift in MDSC function could favor Treg expansion versus suppression of CD4 T cells. In murine melanoma, IDO induced Tregs were critical for recruitment of MDSCs and their suppressive function (30). Together, these studies show that MDSCs promote Tregs and Tregs in turn support MDSC function to promote an immunosuppresive environment conducive for tumor progression.
Interaction between MDSCs and B cells
The functional relationship between MDSCs and B cells is inter-dependent where MDSCs regulate different B cell functions and vice versa. Bregs are a type of regulatory B cells that primarily produce IL-10, accumulate in various cancers and is one of the immunosuppressive mechanisms supporting tumor progression (52). In MCA205 fibrosarcoma, MDSCs promote Breg like cells (B220+CD138+) that are dependent on IL-10 and TGFβ for producing IgA antibodies (53). These Bregs are similar to regulatory plasma B cells that also produce antibodies and suppress antitumor T cells (54). In a 4T1 breast cancer model, MDSCs appear to expand Bregs with high PD-L1 that produce IL-10 and IgA, and also suppressed T cells by inducing apoptosis (55). MDSCs also non-selectively suppress B cells to disable another arm of antitumor immunity (54). In an orthotopic lung cancer model (LLC), MDSCs suppressed IL-4 and lipopolysaccharide (LPS) activated B cell proliferation in a T cell dependent and independent manner (56). Moreover, MDSCs restrict B cell trafficking to lymph nodes by downregulating L-selectin on B and T cells in 4T1 breast cancer (57). Together, these studies show that MDSCs regulate B cells in tumor systems.
CD19+CD20lo Bregs expanded in mice with 4T1 tumors, in which B cells were depleted with CD20 antibody (58). In this study, Bregs promoted the generation of Tregs to support tumor growth and metastasis. On B cells, CD19 is expressed in early development stages and CD20 is expressed in more differentiated B cells (44). This explains why depleting B cells with CD20 antibody deleted differentiated B cells while allowing less differentiated CD19+ B cells to develop into Bregs (CD19+CD20lo). In B cell deficient mice with breast cancer or melanoma, MDSCs were poorly suppressive (59). In this model, tumor evoked Bregs contributed to the suppressive potential of MDSCs resulting in increased metastasis. These Bregs regulated the immunosuppressive function of MDSC partly through TGFβ signaling (60). Secretory IgM from B cells in mice with chronic lymphocytic leukemia (CLL) and LLC tumors promoted the suppressive function of MDSCs (61). These studies show that in breast cancer and melanoma, Bregs contribute to MDSC function and in fibrosarcoma, lung cancer, and breast cancer, MDSCs promote Bregs, suppress total B cell function, and reduce B cell trafficking. This illustrates the complex role of B cells in dysregulating immunity in cancer.
MDSCs promote tumor cell proliferation
MDSCs produce IL-6, IL-1β, vascular endothelial growth factor (VEGF), and cyclooxygenase 2 (COX-2), all of which promote tumor cell proliferation (4, 62, 63). MDSCs cultured with murine 4T1, CT26, TS/A, or MC38 tumor cells produced more IL-6 compared to tumor cells themselves, showing that MDSCs are a source of IL-6 that supports tumor growth (62). This is consistent with the role of IL-6 induced Stat3 in inhibiting apoptosis to promote tumor cell survival (64). IL-1β promotes tumor cell proliferation by increasing angiogenesis in B16 melanoma and DA/3 mammary adenocarcinoma (65). This is accomplished by inducing chemokines to recruit inflammatory immune cells and promoting VEGF synthesis (66). In this manner, IL-1β activates endothelial cells by VEGF to vascularize tumors resulting in tumor growth. In several cancers, VEGF is critical for developing tumor vasculature to supply additional growth factors to tumors and for migration of inflammatory and suppressor cells to tumors (67). Similarly, COX-2 induced prostaglandin 2 (PGE-2) increases angiogenesis and promotes proliferation of tumor cells (68). From these reports, it appears that besides regulating T cell function, MDSCs also produce cytokines, growth factors, and inflammatory mediators to promote tumor growth by preventing apoptosis, supporting angiogenesis, and expanding MDSCs.
MDSC subsets manipulate tumor immunity
Among the various functions of MDSCs, their immunosuppressive ability stands out as their definitive singular feature. M-MDSCs are most immunosuppressive per cell due to their ability to suppress antigen specific or non-antigen specific T cells. This was demonstrated in B16-IDO, C26-GM, CT26, and MCA26 tumor models and was associated with their ability to express both arginase and iNOS. In 4T1, C26-GM or MCA203 fibrosarcoma tumors, CD11b+Gr-1lo cells (M-MDSCs) are relatively more suppressive than CD11b+Gr-1hi or CD11b+Gr-1int subsets (6). PMN-MDSCs supplement this process by suppressing T cells by producing ROS but their immunosuppressive strength is weaker than M-MDSCs (4, 6, 10). They suppress antigen specific T cells and are more abundant than M-MDSCs in many tumor models (22). In murine colon cancer, total MDSCs promote Th17 cells but in another colon tumor model, M-MDSCs expressing CSF-1R promote Tregs that are critical for tumor progression. It is unknown if PMN-MDSCs promote Tregs. PMN-MDSCs produce tumor-promoting factors such as IL-23 to support resistance by increasing tumor growth after surgical removal of prostate tumors (47). Due to IL-23 production, these PMN-MDSCs could promote Th17 cells. This suggests that MDSC subsets could differ in their ability to promote Tregs and Th17 cells. This also raises the question as to how these two process co-exist in the same cancer type. In cancer, Tregs promote tumors by suppressing T cells, and Th17 cells support tumor progression by promoting cell survival and angiogenesis. Occurrence of these processes in colon cancer demonstrates the multitasking capacity of MDSCs in regulating different arms of T cell immunity. These studies also suggest that different MDSC subsets promote Tregs versus Th17 cells in addition to their overall ability to suppress T cells. Although inhibition of suppressive function may not reduce their ability to promote Tregs or Th17 cells, more evidence is required to substantiate these assumptions conclusively.
By inducing EMT in 4T1 breast tumor cells, M-MDSCs promote the release and migration of tumor cells to metastatic niches (69). In this model, lung infiltrating PMN-MDSCs upregulate genes associated with metastasis. MDSCs also suppressed cytotoxic CD8 T cells to promote lung metastases in 4T1 breast cancer (70). Consistently, lung metastases were reduced after resection of primary 4T1 breast tumors in Stat6 deficient mice, which was dependent on cytotoxic CD8 activity. A recent study showed that MDSCs suppress T cells by arginase activity to promote lung metastases independent of VEGF activity in a 4T1 breast cancer model (71). These reports suggest that MDSCs promote tumor metastasis by suppressing T cells and by promoting metastatic tumor cell transformation.
Taken together, M-MDSCs are highly immunosuppressive, induce the generation of Tregs, and promote metastasis. In a cancer setting, this is a potent resistance mechanism for preventing cytotoxic activity of antitumor cells both directly and through Tregs. PMN-MDSCs also suppress T cells, promote tumor growth, and metastasis. Therefore, M-MDSCs and PMN-MDSCs possess multiple mechanisms to promote tumorigenesis supporting the idea that they are versatile and can adapt to suppress the host immune response in a demanding tumor environment (Fig.1).
Impact on cancer immunotherapy
Immunotherapy is an effective strategy for treating most cancers. The success of checkpoint inhibition in increasing survival of patients with aggressive lung cancer and melanoma has provided significant relief from high mortality (72). A subset of CRC patients with high mutation burden are also responsive to checkpoint inhibitor treatment (73). However, efficacy of this treatment is reduced due to the emergence of immunosuppressive mechanisms including MDSCs and Tregs (3, 74).
Existing literature demonstrate the value of targeting MDSCs and possibilities to block MDSC function in cancer to improve treatment are reviewed previously (28, 74). Efforts to inhibit MDSCs are focused on depleting MDSCs, inhibiting their function, or diverting their differentiation to activating DCs or tumoricidal macrophages (4, 28). M-MDSCs retain the potential to differentiate to macrophages or DCs and therefore are amenable to re-education to immunostimulatory myeloid cells that could prime T cells to generate antitumor T cells (22, 75). The close link between CSF-1R+ MDSCs that are similar to M-MDSCs and melanoma and colon cancer progression suggests that blocking CSF-1R could target M-MDSCs by inhibiting their proliferation (1, 50). In a tumor free condition, PMN-MDSCs convert to neutrophils that have a short life span and might not result in long-lasting antitumor immunity unlike antigen presenting cells such as DCs or macrophages. Studies assessing if MDSC depletion reduce tumor growth use the Gr-1 antibody that primarily depletes PMN-MDSCs. Using antibodies that recognize alarmins S100A8 and S100A9, both M-MDSCs and PMN-MDSCs were depleted resulting in greater decease of EL4 tumor growth indicating the importance of M-MDSCs in tumor progression (76). However, these alarmins do not distinguish between PMN-MDSCs and neutrophils. Neutrophils are required for maintaining host immunity, which highlights the importance of finding PMN-MDSC specific targets. A different approach to targeting PMN-MDSCs is by identifying subsets within PMN-MDSCs that are directly related to tumor development. In NSCLC patients, PMN-MDSCs expressing lectin-type oxidized LDL receptor-1 (LOX-1) were more immunosuppressive than total PMN-MDSCs suggesting that LOX-1 could differentiate between PMN-MDSCs and neutrophils (77). This finding suggests that PMN-MDSCs could be specifically depleted using LOX-1 without affecting neutrophils. In CRC, both M-MDSCs and PMN-MDSCs promote tumors. However, the specific mechanisms by which MDSC subsets alter T cells to promote tumor progression in CRC requires further study. Although immunosuppression and induction of Tregs by these subsets could be contributing to disease progression, the association of IL-17 and MDSCs with reduced overall survival and the role of MDSCs in promoting Th17 cells in colon cancer pinpoint the importance of the mechanism by which MDSCs regulate Th17 cells (15). By inhibiting the ability of MDSCs in suppressing T cells, promoting Tregs, and Th17 cells, there is a better likelihood of promoting antitumor immunity. This has significant potential for defining novel therapies for colon cancer by identifying targets in MDSC subsets.
Several clinical trials of commonly used chemotherapeutic and immunotherapeutic agents for targeting MDSC function in various cancers are currently ongoing (74). Despite these intense efforts to divert MDSC function to antitumor activity, their adaptability to support tumor progression is a hurdle that researchers are fighting to overcome. With recent success of immunotherapy in bringing unprecedented improvement in survival, there is widespread interest in finding more translatable therapies to boost efficacy and reduce toxicity. This exemplifies the vital need to target MDSC mechanisms to improve the efficacy of checkpoint inhibitors and surgical removal of tumors. By studying various facets of MDSC function and functional relationship with other immune cell populations, it will be possible to optimally block tumor- promoting function of MDSCs.
Conclusions
We illustrate the multifarious roles of MDSCs in a tumor-promoting environment. Considering that host immune response is protective, MDSCs are a singular example of how tumors dysregulate antitumor immunity using different mechanisms. The immunosuppressive function of MDSCs has the ability to overpower antitumor immunity and is a signature feature of MDSCs. When other mechanisms of MDSCs are taken into account, we get an overall picture of how MDSCs multitask to accomplish the goal of promoting tumor development. The feedback between MDSCs and tumor cells creates a tumor promoting cycle, which can be interrupted to identify immunotherapeutic targets. Several questions arise from this analysis of studies about MDSCs. First, M-MDSCs play a critical role in regulating differentiation of T cells into Tregs in colon cancer model. The relative contribution of M-MDSCs versus PMN-MDSCs in regulating tumor-promoting Tregs is unclear. Second, the critical component or process in MDSCs that promotes regulatory cells such as Tregs and Bregs versus proinflammatory T cells such as Th17 cells is unknown. Third, the interaction between MDSCs and Bregs in breast cancer and melanoma are studied but their role in CRC is unknown. Depending on the type of cancer and related treatment regimens, targeting MDSCs could improve existing treatments and can serve as indicators of disease progression. Knowledge about MDSC mechanisms in each cancer type could provide critical input for devising targeted therapies to improve existing treatments and reduce the intensity of refractory mechanisms.
Acknowledgments
Funding: NCI diversity re-entry supplement for A.J. to parent R01CA168670-01A1 awarded to A.L.M.B
References
- 1.Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, and Wolchok JD 2016. Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors. EBioMedicine 6: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, and Montero AJ 2009. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber R, Fleming V, Hu X, Nagibin V, Groth C, Altevogt P, Utikal J, and Umansky V 2018. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front Immunol 9: 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marvel D, and Gabrilovich DI 2015. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 125: 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, and Zanovello P 2000. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood 96: 3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 6.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, and Bronte V 2010. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 40: 22–35. [DOI] [PubMed] [Google Scholar]
- 7.Kusmartsev S, and Gabrilovich DI 2003. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol 74: 186–196. [DOI] [PubMed] [Google Scholar]
- 8.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, and Gabrilovich DI 2010. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 207: 2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quail DF, and Joyce JA 2013. Microenvironmental regulation of tumor progression and metastasis. Nat Med 19: 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, and Bronte V 2010. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 32: 790–802. [DOI] [PubMed] [Google Scholar]
- 11.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, and Gabrilovich DI 2016. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7: 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, Colombo MP, and Zanovello P 2003. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol 170: 270–278. [DOI] [PubMed] [Google Scholar]
- 13.Garton AJ, Seibel S, Lopresti-Morrow L, Crew L, Janson N, Mandiyan S, Trombetta ES, Pankratz S, LaVallee TM, and Gedrich R 2017. Anti-KIT Monoclonal Antibody Treatment Enhances the Antitumor Activity of Immune Checkpoint Inhibitors by Reversing Tumor-Induced Immunosuppression. Mol Cancer Ther 16: 671–680. [DOI] [PubMed] [Google Scholar]
- 14.Karakasheva TA, Dominguez GA, Hashimoto A, Lin EW, Chiu C, Sasser K, Lee JW, Beatty GL, Gabrilovich DI, and Rustgi AK 2018. CD38+ M-MDSC expansion characterizes a subset of advanced colorectal cancer patients. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limagne E, Euvrard R, Thibaudin M, Rebe C, Derangere V, Chevriaux A, Boidot R, Vegran F, Bonnefoy N, Vincent J, Bengrine-Lefevre L, Ladoire S, Delmas D, Apetoh L, and Ghiringhelli F 2016. Accumulation of MDSC and Th17 Cells in Patients with Metastatic Colorectal Cancer Predicts the Efficacy of a FOLFOX-Bevacizumab Drug Treatment Regimen. Cancer Res 76: 5241–5252. [DOI] [PubMed] [Google Scholar]
- 16.Chun E, Lavoie S, Michaud M, Gallini CA, Kim J, Soucy G, Odze R, Glickman JN, and Garrett WS 2015. CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function. Cell Rep 12: 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, and Dubois RN 2013. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 24: 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayakumar A, and Bothwell ALM 2017. Stat6 Promotes Intestinal Tumorigenesis in a Mouse Model of Adenomatous Polyposis by Expansion of MDSCs and Inhibition of Cytotoxic CD8 Response. Neoplasia 19: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed A, Janakiram NB, Li Q, Choi CI, Zhang Y, Steele VE, and Rao CV 2011. Chemoprevention of colon and small intestinal tumorigenesis in APC(Min/+) mice by licofelone, a novel dual 5-LOX/COX inhibitor: potential implications for human colon cancer prevention. Cancer Prev Res (Phila) 4: 2015–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayakumar A, and Bothwell ALM 2019. RIPK3-Induced Inflammation by I-MDSCs Promotes Intestinal Tumors. Cancer Res 79: 1587–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamauchi Y, Safi S, Blattner C, Rathinasamy A, Umansky L, Juenger S, Warth A, Eichhorn M, Muley T, Herth FJF, Dienemann H, Platten M, Beckhove P, Utikal J, Hoffmann H, and Umansky V 2018. Circulating and Tumor Myeloid-derived Suppressor Cells in Resectable Non-Small Cell Lung Cancer. Am J Respir Crit Care Med 198: 777–787. [DOI] [PubMed] [Google Scholar]
- 22.Youn JI, Nagaraj S, Collazo M, and Gabrilovich DI 2008. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181: 5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, and Gabrilovich DI 2009. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol 182: 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Amico L, Mahajan S, Capietto AH, Yang Z, Zamani A, Ricci B, Bumpass DB, Meyer M, Su X, Wang-Gillam A, Weilbaecher K, Stewart SA, DeNardo DG, and Faccio R 2016. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J Exp Med 213: 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, and Lin PC 2004. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6: 409–421. [DOI] [PubMed] [Google Scholar]
- 26.Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X, and Ponnazhagan S 2013. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res 73: 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Meir K, Twaik N, and Baniyash M 2018. Plasticity and biological diversity of myeloid derived suppressor cells. Curr Opin Immunol 51: 154–161. [DOI] [PubMed] [Google Scholar]
- 28.Gabrilovich DI, Ostrand-Rosenberg S, and Bronte V 2012. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12: 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Condamine T, Mastio J, and Gabrilovich DI 2015. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol 98: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, Merghoub T, and Wolchok JD 2015. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep 13: 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaraj S, Schrum AG, Cho HI, Celis E, and Gabrilovich DI 2010. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol 184: 3106–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, and Bronte V 2006. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 116: 2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alissafi T, Hatzioannou A, Mintzas K, Barouni RM, Banos A, Sormendi S, Polyzos A, Xilouri M, Wielockx B, Gogas H, and Verginis P 2018. Autophagy orchestrates the regulatory program of tumor-associated myeloid-derived suppressor cells. J Clin Invest 128: 3840–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha P, Parker KH, Horn L, and Ostrand-Rosenberg S 2012. Tumor-induced myeloid-derived suppressor cell function is independent of IFN-gamma and IL-4Ralpha. Eur J Immunol 42: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, and Chouaib S 2014. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211: 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossner S, Voigtlander C, Wiethe C, Hanig J, Seifarth C, and Lutz MB 2005. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol 35: 3533–3544. [DOI] [PubMed] [Google Scholar]
- 37.Ni L, and Dong C 2017. New B7 Family Checkpoints in Human Cancers. Mol Cancer Ther 16: 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, and Kalinski P 2011. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 118: 5498–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prendergast GC, Malachowski WP, DuHadaway JB, and Muller AJ 2017. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res 77: 6795–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, and Karin M 2012. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491: 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee S, Das S, Chakraborty P, Manna A, Chatterjee M, and Choudhuri SK 2013. Myeloid derived suppressor cells (MDSCs) can induce the generation of Th17 response from naive CD4+ T cells. Immunobiology 218: 718–724. [DOI] [PubMed] [Google Scholar]
- 42.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, and O’Shea JJ 2010. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467: 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obermajer N, Wong JL, Edwards RP, Chen K, Scott M, Khader S, Kolls JK, Odunsi K, Billiar TR, and Kalinski P 2013. Induction and stability of human Th17 cells require endogenous NOS2 and cGMP-dependent NO signaling. J Exp Med 210: 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul WE 2013. Fundamental immunology. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia. [Google Scholar]
- 45.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, and Cua DJ 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 8: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 46.Ortiz ML, Kumar V, Martner A, Mony S, Donthireddy L, Condamine T, Seykora J, Knight SC, Malietzis G, Lee GH, Moorghen M, Lenox B, Luetteke N, Celis E, and Gabrilovich D 2015. Immature myeloid cells directly contribute to skin tumor development by recruiting IL-17-producing CD4+ T cells. J Exp Med 212: 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, De Bernardis G, Losa M, Mirenda M, Pasquini E, Rinaldi A, Sumanasuriya S, Lambros MB, Neeb A, Luciano R, Bravi CA, Nava-Rodrigues D, Dolling D, Prayer-Galetti T, Ferreira A, Briganti A, Esposito A, Barry S, Yuan W, Sharp A, de Bono J, and Alimonti A 2018. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 559: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Gouvello S, Bastuji-Garin S, Aloulou N, Mansour H, Chaumette MT, Berrehar F, Seikour A, Charachon A, Karoui M, Leroy K, Farcet JP, and Sobhani I 2008. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut 57: 772–779. [DOI] [PubMed] [Google Scholar]
- 49.Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, Kumar M, Jones S, Rees B, Williams G, Gallimore A, and Godkin A 2012. Suppression of tumour-specific CD4(+) T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut 61: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, and Chen SH 2006. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66: 1123–1131. [DOI] [PubMed] [Google Scholar]
- 51.Serafini P, Mgebroff S, Noonan K, and Borrello I 2008. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 68: 5439–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz M, Zhang Y, and Rosenblatt JD 2016. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer 4: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu X, Meng Q, Erben U, Wang P, Glauben R, Kuhl AA, Wu H, Ma CW, Hu M, Wang Y, Sun W, Jia J, Wu X, Chen W, Siegmund B, and Qin Z 2017. Myeloid-derived suppressor cells promote B-cell production of IgA in a TNFR2-dependent manner. Cell Mol Immunol 14: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarvaria A, Madrigal JA, and Saudemont A 2017. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol 14: 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen M, Wang J, Yu W, Zhang C, Liu M, Wang K, Yang L, Wei F, Wang SE, Sun Q, and Ren X 2018. A novel MDSC-induced PD-1(−)PD-L1(+) B-cell subset in breast tumor microenvironment possesses immuno-suppressive properties. Oncoimmunology 7: e1413520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Schafer CC, Hough KP, Tousif S, Duncan SR, Kearney JF, Ponnazhagan S, Hsu HC, and Deshane JS 2018. Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT5. J Immunol 201: 278–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ku AW, Muhitch JB, Powers CA, Diehl M, Kim M, Fisher DT, Sharda AP, Clements VK, O’Loughlin K, Minderman H, Messmer MN, Ma J, Skitzki JJ, Steeber DA, Walcheck B, Ostrand-Rosenberg S, Abrams SI, and Evans SS 2016. Tumor-induced MDSC act via remote control to inhibit L-selectin-dependent adaptive immunity in lymph nodes. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, and Biragyn A 2011. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res 71: 3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, Araki Y, Miyoshi I, Yang L, Trinchieri G, and Biragyn A 2015. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res 75: 3456–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, Gress RE, Chan AC, Hesdorffer C, and Biragyn A 2013. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res 73: 2127–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang CH, Chang S, Hashimoto A, Chen YJ, Kang CW, Mato AR, Del Valle JR, Gabrilovich DI, and Hu CC 2018. Secretory IgM Exacerbates Tumor Progression by Inducing Accumulations of MDSCs in Mice. Cancer Immunol Res 6: 696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beury DW, Parker KH, Nyandjo M, Sinha P, Carter KA, and Ostrand-Rosenberg S 2014. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J Leukoc Biol 96: 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, and Yu H 2008. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest 118: 3367–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu H, Pardoll D, and Jove R 2009. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9: 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, and Apte RN 2003. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A 100: 2645–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voronov E, Carmi Y, and Apte RN 2014. The role IL-1 in tumor-mediated angiogenesis. Front Physiol 5: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goel HL, and Mercurio AM 2013. VEGF targets the tumour cell. Nat Rev Cancer 13: 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stasinopoulos I, Shah T, Penet MF, Krishnamachary B, and Bhujwalla ZM 2013. COX-2 in cancer: Gordian knot or Achilles heel? Front Pharmacol 4: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ouzounova M, Lee E, Piranlioglu R, El Andaloussi A, Kolhe R, Demirci MF, Marasco D, Asm I, Chadli A, Hassan KA, Thangaraju M, Zhou G, Arbab AS, Cowell JK, and Korkaya H 2017. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun 8: 14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ostrand-Rosenberg S, Grusby MJ, and Clements VK 2000. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J Immunol 165: 6015–6019. [DOI] [PubMed] [Google Scholar]
- 71.Secondini C, Coquoz O, Spagnuolo L, Spinetti T, Peyvandi S, Ciarloni L, Botta F, Bourquin C, and Ruegg C 2017. Arginase inhibition suppresses lung metastasis in the 4T1 breast cancer model independently of the immunomodulatory and anti-metastatic effects of VEGFR-2 blockade. Oncoimmunology 6: e1316437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herbst RS, Morgensztern D, and Boshoff C 2018. The biology and management of non-small cell lung cancer. Nature 553: 446–454. [DOI] [PubMed] [Google Scholar]
- 73.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, and Andre T 2017. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 18: 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, Utikal J, and Umansky V 2018. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front Immunol 9: 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, Ochoa AC, Cui Y, Del Valle L, and Rodriguez PC 2014. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity 41: 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, Qian J, Hailemichael Y, Nurieva R, Dwyer KC, Roth J, Yi Q, Overwijk WW, and Kwak LW 2014. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 20: 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, Partlova S, Garfall A, Vogl DT, Xu X, Knight SC, Malietzis G, Lee GH, Eruslanov E, Albelda SM, Wang X, Mehta JL, Bewtra M, Rustgi A, Hockstein N, Witt R, Masters G, Nam B, Smirnov D, Sepulveda MA, and Gabrilovich DI 2016. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol 1. [DOI] [PMC free article] [PubMed] [Google Scholar]